Abstract
Background
Glucagon-like peptide 1 receptor agonists (GLP1RAs) are used in the treatment of diabetes and obesity. Their slowing effect of gastric emptying might change oral drug absorption, potentially affecting pharmacokinetics, particularly in the case of medications with a narrow therapeutic index.
Purpose
The purpose of this systematic review is to summarize data on drug-drug interactions between GLP1RAs and oral drugs.
Data Sources
The PubMed and EMBASE databases were searched up to November, 1st 2023.
Study Selection
We selected pharmacokinetic studies of any injectable GLP1RA given with an oral medication, and product prescribing sheets reporting data without access to the original study.
Data Extraction
Two authors independently extracted the data.
Data Synthesis
Twenty-two reports and six prescribing sheets were included. Treatment with GLP1RAs resulted in unaffected or reduced Cmax and delayed tmax of drugs with high solubility and permeability (warfarin, contraceptive pills, acetaminophen), drugs with high solubility and low permeability (angiotensin converting enzyme inhibitors), drugs with low solubility and high permeability (statins) and drugs with low solubility and permeability (digoxin). However, the use of GLP1RAs did not exert clinically significant changes in the AUC or differences in clinically relevant endpoints.
Limitations
The major limitations of the studies that are included in this systematic review are the enrollment of healthy subjects and insufficient data in conditions that might affect pharmacokinetics (e.g., kidney dysfunction).
Conclusions
To conclude, reduced Cmax and delayed tmax of drugs co-administered with GLP1RAs are consistent with the known delayed gastric output by the latter. Nevertheless, the overall drug exposure was not considered clinically significant. Dose adjustments are probably not required for simultaneous use of GLP1RAs with oral medications. Still, results should be carefully generalized to cases of background kidney dysfunction or when using drugs with narrow therapeutic index. The study is registered in PROSPERO: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022332339.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-023-01392-3.
Key Points
| Pharmacokinetic and pharmacodynamic studies of GLP1RAs generally reported no clinically significant changes in the absorption or action of co-administered oral medications. |
| Dose adjustments are probably not needed for simultaneous use of GLP1RAs with oral medications, but careful attention is still recommended when treating patients with background gastroparesis or kidney dysfunction, or when considering drugs with narrow therapeutic index. |
Introduction
Glucagon-like peptide 1 receptor agonists (GLP1RAs) are widely used in the treatment of diabetes mellitus (DM) and obesity [1, 2]. Beyond glucose control and weight reduction, GLP1RAs were shown to reduce cardiovascular morbidity and mortality and are therefore strongly recommended in early lines of diabetic therapy [3, 4]. Their major mechanisms of action are slowing of gastric emptying, hunger suppression, increased insulin and decreased glucagon secretion from the pancreas.
Glucagon-like peptide 1 receptor agonists do not engage in CYP- or transporter-mediated drug-drug interactions (DDI) [5]. Yet a question remains whether their effect on gastric emptying might modulate the rate of drug absorption (maximal plasma drug concentration, [Cmax], and time to maximal concentration, [tmax]). This may be of clinical significance especially for medications with a narrow therapeutic index, where small differences in blood concentration might lead to therapeutic failure or adverse reactions. Gastroparesis caused by non-pharmacologic etiologies is indeed known to affect drug absorption [6, 7]. However, previous studies on the extent of drug absorption (area under the curve [AUC]) in patients with other gastrointestinal motility disorders reported conflicting results [8, 9]. We aimed to review and harmonize the data available on potential DDIs between GLP1RAs and oral medications.
Materials and Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [10]. The protocol for this systematic review can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022332339.
Sources and Search Strategy
The PubMed and EMBASE electronic databases were comprehensively searched up to November 1st, 2023. The Pubmed database was searched for the following MeSH terms: “glp1”, “glp 1”, “glucagon like peptide 1 receptor agonist”, “exenatide”, “lixisenatide”, “albiglutide”, “liraglutide”, “semaglutide” or “dulaglutide”, and “drug drug interaction” or “pharmacokinetic(s)”. The EMBASE database was searched for the following terms: ‘glucagon like peptide 1 receptor agonist’ OR ‘exenatide’ OR ‘lixisenatide’ OR ‘liraglutide’ OR ‘albiglutide’ OR ‘dulaglutide’ OR ‘semaglutide’ OR ‘exendin 4’ and ‘drug interaction’ OR ‘pharmacokinetics’. The reference lists of all publications were queried to detect studies not identified during the initial search. GLP1RA prescribing sheets were screened for information about DDI not published elsewhere. There was no restriction on population and language.
Data Extraction and Study Selection
The databases were scanned by one author (TDC) and selected data were independently extracted by two authors (TDC, BC). Disagreements were solved by consensus or consultation with a third author (ID). We included all the pharmacokinetic studies conducted in human subjects with any injectable GLP1RA product given concomitantly with an oral medication. Only injectable GLP1RAs were considered eligible (exenatide, lixisenatide, liraglutide, albiglutide, dulaglutide, semaglutide). We excluded studies on drug-drug interactions between GLP1RAs and anti-hyperglycemic medications. Data were extracted from original study manuscripts and from reviews with detailed quantitative results of the pharmacokinetic endpoints of interest. We also extracted pharmacokinetic and pharmacodynamic information that was detailed in the product sheet data of various GLP1RAs. The extracted data included characteristics of the study cohort, study design, details of specific interventions (drugs and dosing) and pharmacokinetic outcomes. Pharmacodynamic outcomes were extracted if available.
Outcomes
The primary pharmacokinetic outcomes were the rate (Cmax, tmax) and the extent (AUC) of drug absorption when an oral drug of interest was given concomitantly with versus without a GLP1RA. Other outcomes were pharmacodynamic parameters including the international normalized ratio (INR) of the prothrombin time (warfarin studies), lipid levels (statin studies) and gonadotropins or progesterone levels (combined oral contraceptive [COC] studies).
Quality Assessment and Risk of Bias
As there are no standard guidelines to assess the risk of bias in pharmacokinetic studies, we applied a previously reported quality rating tool [11].
Data Analysis
Presentation of the data follows the Biopharmaceutics Classification System (BCS) approach [12]. The BCS approach classifies drugs by their aqueous solubility and intestinal permeability into four groups:
Class I: high solubility, high permeability.
Class II: low solubility, high permeability.
Class III: high solubility, low permeability.
Class IV: low solubility, low permeability.
The term ‘solubility’ in the BCS report refers to the therapeutic dose of a drug that is soluble in an aqueous media which has pre-specified characteristics (volume, pH and temperature). Assessment of ‘permeability’ in the BCS report is based on the extent of absorption as determined by absolute bioavailability or mass balance. We followed the BCS approach as it provides a surrogate for in vivo bioequivalence studies.
Results
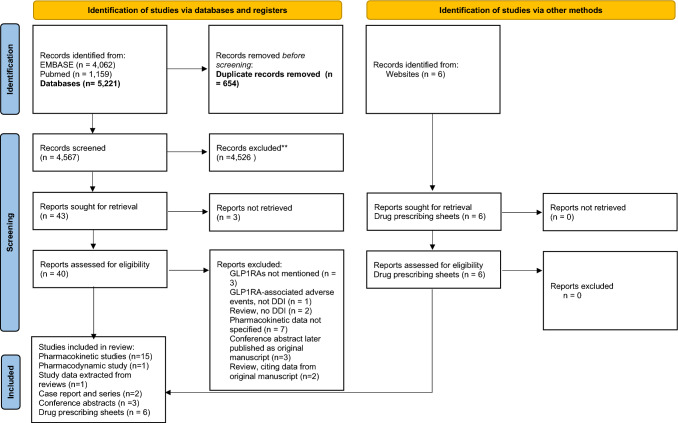
The initial search yielded 5221 potentially relevant references of which 40 records were retrieved for full-text review (Fig. 1). Twenty-two reports were included in the study cohort; these included 15 pharmacokinetic (PK) studies [13–27], one pharmacodynamic (PD) study [28], one review with detailed PK data not elsewhere published [29], three conference abstracts [30–32], one case report [33] and one case series [34] (Fig. 1, Table 1). Prescribing sheets of all GLP1RAs were scanned for PK data not published elsewhere [35–40]. Drug interactions of exenatide were described in eight manuscripts [13, 20–23, 25, 33, 34], liraglutide in five [17, 19, 24, 26, 34], semaglutide in three [16, 18, 27], and lixisenatide, dulaglutide and albiglutide in two each [14, 15, 29–32] and synthetic GLP1 in one manuscript [28]. Three studies enrolled participants with DM [19, 32, 34], and all others enrolled healthy subjects. Two studies on DDI between GLP1RAs and COCs enrolled post-menopausal women [17, 18] and three studies enrolled pre-menopausal women [14, 15, 21].
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers and other sources. From: Page et al. [10]. 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Table 1.
Records included in the systematic review
| Study type/data source | Study design | Investigated drugs | Investigated GLP1RA – dose | Investigated oral drug – dose | Population | Number, gender and age of subjects | |
|---|---|---|---|---|---|---|---|
| Kothare PA 2005 | PK study | Open-label, fixed-sequence | Exenatide – steady state, digoxin – steady state | Exenatide 10 mcg BID | Digoxin 0.25 mg | HS | 23 pts, M, 21–42 y |
| Blase E 2005 | PK study | RCT, single-blind, crossover | Exenatide – steady state, acetaminophen – single dose | Exenatide 10 mcg BID | Acetaminophen 1000 mg | HS | 40 pts, M+F, 18–65 y |
| Soon D 2006 | PK study | Open label, fixed-sequence | Exenatide – steady state, Warfarin – single dose | Exenatide 10 mcg BID | Warfarin 25 mg | HS | 16 pts, M, 22–50 y |
| Kothare PA 2007 | PK study | Open-label, fixed-sequence, | Exenatide – steady state, lovastatin – single dose | Exenatide 10 mcg BID | Lovastatin 40 mg | HS | 22 pts, M+F, 18–66 y |
| Linnebjerg H 2009 | PK study | RCT, double-blind, crossover | Exenatide – 2 doses, lisinopril – steady state | Exenatide 10 mcg BID | Lisinopril 5–20 mg qd | HS | 22 pts, M+F, mean age 60 ± 6.2 y |
| Kothare PA 2012 | PK study | Open-label, randomized, 3-period, crossover | Exenatide – steady state, COC – steady state | Exenatide 10 mcg BID | COC: EE 30 mcg, LV 150 mcg | HS pre-menopause | 38 pts, F, 18–45 y |
| Fujita Y 2013 | Case report | Exenatide | Hydrocortisone | ||||
| Liu YH 2010 | Conference abstract | Open-label, randomized, crossover | Lixisenatide – steady state, warfarin – single dose | Lixisenatide 20 mcg | Warfarin 25 mg | HS | 16 pts, M, age – ND |
| Dahmen R 2011 | Conference abstract | Open-label, randomized, crossover | Lixisenatide and ramipril – steady state | Lixisenatide 20 mcg | Ramipril 5 mg | HS | 30 pts, M+F, age – ND |
| Kapitza C 2011 | PK study | RCT, double-blind, 2-period crossover | Liraglutide – steady state, acetaminophen – single dose | Liraglutide 1.8 mg | Acetaminophen 1000 mg | DM2 | 18 pts, M+F, 48–70 y |
| Jacobsen LV 2011 | PK study | RCT, double-blind, 2-period crossover | Liraglutide – steady state, COC – single dose | Liraglutide 1.8 mg | COC: EE 30 mcg, LV 150 mcg | HS post–menopause | 21 pts, F, 51–71 y |
| Malm–Erjefält M 2015 | PK study | RCT, double-blind, 2-period crossover | Liraglutide – steady state, Atorvastatin, lisinopril and digoxin – single dose | Liraglutide 1.8 mg | Atorvastatin 40 mg, lisinopril 20 mg, digoxin 1 mg, | HS |
M+F, 18–55 y. Atorvastatin, lisinopril: 42 pts. Digoxin: 28 pts |
| Pinelli NR 2013 | PK study | Open-label, fixed-sequence | Liraglutide, tacrolimus – steady state | Liraglutide 1.8 mg | Tacrolimus various doses for goal trough concentration 5–15 ng/mL | Kidney transplant recipients, non-diabetic | 5 pts, M+F, 55.4 ± 8.2 y |
| Nagai Y 2019 | Case series | Liraglutide, exenatide – steady state, dexamethasone – single dose | Liraglutide 0.3–0.9 mg or exenatide 10–20 mcg BID | Dexamethasone 0.5 mg | DM2 | 7 pts | |
| Bush M 2012 | PK study | Open-label, sequential | Albiglutide – steady state, Digoxin, warfarin – single dose, COC– steady state | Albiglutide 50 mg | Digoxin 0.5 mg, warfarin 25 mg, and COC: EE 35 mcg, NE 500 mcg | HS pre-menopause |
Digoxin: 30 pts, M+F, 18–55 y Warfarin: 16 pts, M, 18–55 y COC: 23 pts, F, 18–40 y |
|
Young MA 2014 |
Review | Review – data extracted on specific DDI | Albiglutide – steady state, Simvastatin – single dose | Albiglutide 50 mg | Simvastatin 80 mg | HS | ND |
|
de la Peña A 2017 |
PK study | Digoxin, atorvastatin: fixed sequence Warfarin: randomized, crossover. COC: two treatment periods | Dulaglutide – single dose, warfarin/ atorvastatin – single dose, digoxin / COC – steady state | Dulaglutide 1.5 mg | Digoxin 0.25 mg, warfarin 10 mg, atorvastatin 40 mg and COC: EE 35 mcg and NG 250 mcg | HS pre-menopause |
Digoxin, warfarin: 16 pts, M+F, 18–65 y. Atorvastatin: 24 pts, M+F, 18–65 y. COC: 14 pts, F, 18–45 y |
| Tham LS 2018 | Conference abstract | Population– exposure–response and physiologic–based pharmacokinetics modeling | Dulaglutide – single dose | Dulaglutide 4.5 mg | Digoxin, warfarin, acetaminophen, lisinopril – doses not specified | DM2 and HS | Computerized modeling, no subjects enrolled |
| Kapitza C 2015 | PK study | Open-label, one-sequence, crossover | Semaglutide – steady state, COC – steady state | Semaglutide 1 mg | COC: EE 30 mcg, LV 150 mcg | HS post-menopause | 43 pts, F, mean age 62.2 ± 6 y |
| Hausner H 2017 | PK study | Open label, one-sequence, crossover | Semaglutide – steady state, warfarin / atorvastatin / digoxin – single dose | Semaglutide 1 mg | Warfarin 25 mg, atorvastatin 40 mg or digoxin 0.5 mg | HS |
M+F, 18–55 y. Warfarin: 23 pts. Atorvastatin, digoxin: 31 pts |
| Langeskov EK 2022 | PK study | Acetaminophen: open label, randomized, cross-over Atorvastatin: open-label, one-sequence, crossover | Semaglutide – steady state, Acetaminophen / atorvastatin – single dose | Semaglutide 1 mg | Acetaminophen 1500 mg, atorvastatin 40 mg |
Acetaminophen: HS obese (average BMI 33.2 kg/m2). Atorvastatin: HS |
M+F Acetaminophen: 29 pts, M+F, 21–65 y. Atorvastatin: 31 pts, M+F, 25–55 y |
| Meier JJ 2005 | PD study | Open-label, single-blinded, randomized, crossover | IV GLP 1, erythromycin – steady state? Metoclopramide, domperidone, cisapride – single dose | Intravenous GLP1 0.8 pmol* kg–1*min–1 for 270 min | Oral metoclopramide 10 mg, domperidone 10 mg, cisapride 10 mg, or IV erythromycin 200 mg/100 mL for 15 min | HS | 9 pts, M, mean age 25 ± 4 y |
BID twice daily, COC combined oral contraceptives, DM2 diabetes mellitus type 2, EE ethinylestradiol, F females, GLP1RA glucagon-like peptide 1 receptor agonists, HS healthy subjects, IV intravenous, LV levonorgestrel, M males, NE norethindrone, NG norelgestromin, PD pharmacodynamic, PK pharmacokinetic, RCT randomized controlled trial
Most studies showed good reporting quality implying an overall low risk of bias (Supplementary table). Fair quality of methodology was considered in studies that enrolled only male subjects (“population” category) [14, 22, 25, 26, 28] or when blood sampling did not coincide with a steady state of an investigated drug [13–17, 19, 24]. The number of subjects was small in one study that was rated poor quality for “sample” [26].
BCS Class I: High Solubility, High Permeability
Warfarin
Warfarin is an anticoagulant with a narrow therapeutic index, assessed by measurement of the INR. The PK and PD studies for assessment of possible DDI between warfarin and GLP1RA were conducted for exenatide [25], lixisenatide [31], albiglutide [14], dulaglutide [15] and semaglutide [16] (Table 2). Specific PK/PD data for liraglutide and warfarin were not identified [37]. Coadministration of warfarin with a GLP1RA resulted in delayed tmax compared to administration of warfarin alone. This result probably reflects a delay in gastric emptying by the GLP1RA. The 90% confidence intervals (CIs) for the AUC and Cmax of warfarin in most studies were generally contained within the pre-specified 0.8–1.25 limits that are used to assess significance of drug-drug interactions. Exceptions were a 19 % and a 22 % reduction in warfarin Cmax when administered with lixisenatide or dulaglutide (both doses of 1.5 mg and 4.5 mg), respectively [15, 31, 32] that were not considered to be of clinical importance. The pharmacodynamic studies demonstrated similar INRAUC and INRmax in subjects on warfarin with or without GLP1RAs. This suggests that the observed minor PK changes were not clinically relevant. There seems to be no need for dose adjustment with the co-administration of warfarin and a GLP1RA.
Table 2.
Pharmacokinetic studies with GLP1 receptor agonists and warfarin
| AUC (h·ng/mL)a | Cmax (ng/mL)a | tmax (h) | INRAUCa | INRmaxa | |
|---|---|---|---|---|---|
|
Exenatide 10 mcg BID Soon et al. [25] |
S: 1.06 (1.01, 1.11) R: 1.11 (1.06, 1.17) |
S: 0.97 (0.93, 1.01) R: 1.05 (1.00, 1.09) |
S: W 4 (3–6) W+GLP 6 (4–12) R: W 5 (3–12) W+GLP 6 (5–12) |
0.94 (0.93, 0.96) | 0.88 (0.84, 0.92) |
|
Lixisenatide 20 mcg Liu [31] |
S: 1.03 (0.87, 1.22) | S: 0.81 (0.68, 0.96) |
S: W 1 h W+GLP 8 h |
No effect | No effect |
|
Albiglutide 50 mg Bush et al. [14] |
S: 0.99 (0.95, 1.03) R: 1.02 (0.98, 1.07) |
S: 0.93 (0.87, 0.98) R: 0.94 (0.89, 0.99) |
S: W 1 h W+GLP 1.5 h R: W 1 h W+GLP 1.5 h |
No effect | No effect |
|
Dulaglutide 1.5 mg de la Peña et al. [15] |
S: 0.986 (0.959, 1.01) R: 0.989 (0.958, 1.02) |
S: 0.783 (0.737, 0.833) R: 0.857 (0.817, 0.900) |
S: W 2 (1–4) W+GLP 4 (1–24) R: W 2 (1–4) W+GLP 8 (2–24) |
1.02 (1.01, 1.03) | 1.02 (0.977, 1.07) |
|
Semaglutide 1 mg Hausner et al. [16] |
S: 1.05 (0.99, 1.11) R: 1.04 (0.98, 1.10) |
S: 0.91 (0.85, 0.98) R: 0.93 (0.87, 1.00) |
S+R: W1 h W+GLP 3 h |
1.05 (0.87, 1.28) | 1.04 (0.99, 1.10) |
BID twice daily, Cmax maximum observed plasma concentration, GLP1 glucagon-like peptide 1, INR international normalized ratio, INRmax maximum observed level, R R-warfarin, S S-warfarin, tmax time to maximum observed plasma concentration, W warfarin, W+GLP warfarin administered with a GLP1 receptor agonist
aData are presented as the ratio of the least squares geometric mean (warfarin+GLP1 receptor agonist / warfarin) with a 90% confidence interval for the ratio. The 0.8–1.25 limits are considered for equivalence
Combined Oral Contraceptives
Combined oral contraceptives usually contain estrogen and progesterone. A higher exposure to estrogen and/or progesterone might increase the risk for thromboembolism, and a lower exposure might result in failure of birth control [41]. The Food and Drug Administration (FDA) has issued specific guidance for sponsors of new drugs to encourage evaluation of potential DDI with COCs [42]. Pharmacokinetic studies with exenatide [21], lixisenatide [36], liraglutide [17], albiglutide [14], dulaglutide [15] and semaglutide [18] given concomitantly with COCs are presented in Table 3. Following the FDA guidance, all studies used ethinylestradiol as it is the most commonly used estrogen in COCs [42]. Similarly, norethindrone, norgestimate and levonorgestrel were the progestins of choice for their wide use in the USA. Overall, administration of GLP1RAs resulted in reduced Cmax and delayed tmax of the estrogen and the progesterone elements, consistent with a right shift of the concentration-time curve of drug absorption resulting from the expected prolonged gastric transit time. However, the bioequivalence criterion for the AUC was met for ethinylestradiol in all cases, and the AUC for the progestin component was either unaffected or showed approximately 20% higher mean exposure, which is not considered to affect the safety or efficacy of the combined pills. This attests to an overall unchanged bioavailability of the COCs when given with a GLP1 analogue. Moreover, given the right shift of the concentration-time curve with a similar AUC, it is expected that the minimum concentration of the combined contraceptive pills will not be lower. Indeed, direct measurement of the minimum concentration of ethinylestradiol and norethindrone was similar with or without co-administration of albiglutide [14]. This is especially important with the use of low-dose contraceptives that are dependent on threshold blood levels for birth control. Pharmacodynamic studies were conducted only for albiglutide; the simultaneous administration with COCs did not affect the plasma levels of gonadotropins or progesterone. Given the above and current knowledge, a clinically significant DDI between GLP1RAs and COCs is probably not expected, and there is no recommendation in any GLP1RA prescription sheet for dose adjustment of COCs in this context.
Table 3.
Pharmacological studies with GLP1 receptor agonists and oral medications
| GLP1 agonist | Investigated drug | AUC (h·ng/mL) | Cmax (ng/mL) | tmax (h) | Clinical significance |
|---|---|---|---|---|---|
| Exenatide | Ethinylestradiol | ↔ | ↓ | Delayed | NS |
| Lixisenatide | Ethinylestradiol | ↔ | ↓ | Delayed | NS |
| Liraglutide | Ethinylestradiol | ↔ | ↓ | Delayed | NS |
| Albiglutide | Ethinylestradiol | ↔ | ↔ | ND | NS |
| Dulaglutide | Ethinylestradiol | ↔ | ↓ | Delayed | NS |
| Semaglutide | Ethinylestradiol | ↔ | ↔ | Delayed | NS |
| Exenatide | Levonorgestrel | ↔ | ↓ | Delayed | NS |
| Lixisenatide | Levonorgestrel | ↔ | ↓ | Delayed | NS |
| Liraglutide | Levonorgestrel | ↑ | ↓ | Delayed | NS |
| Albiglutide | Norethindrone | ↔ | ↑↔ | ND | NS |
| Dulaglutide | Norelgestromin | ↔ | ↓ | Delayed | NS |
| Semaglutide | Levonorgestrel | ↑↔ | ↔ | ↔ | NS |
| Exenatide | Acetaminophen | ↓↔ | ↓ | Delayed | NS |
| Lixisenatide | Acetaminophen | ↔ | ↓ | Delayed | NS |
| Liraglutide | Acetaminophen | ↔ | ↓ | Delayed | NS |
| Albiglutide | Acetaminophen | ND | ND | ND | NS |
| Dulaglutide | Acetaminophen | ↔ | ↓ | Delayed | NS |
| Semaglutide | Acetaminophen | ↔ | ↓ | ND | NS |
| Exenatide | Lovastatin | ↓ | ↓ | Delayed | NS |
| Lixisenatide | Atorvastatin | ↔ | ↓ | Delayed | NS |
| Liraglutide | Atorvastatin | ↔ | ↓ | Delayed | NS |
| Albiglutide | Simvastatin | ↓ | ↔ | ↔ | NS |
| Dulaglutide | Atorvastatin | ↓ | ↓ | Delayed | NS |
| Semaglutide | Atorvastatin | ↔ | ↓ | Delayed | NS |
| Exenatide | Lisinopril | ↔ | ↔ | Delayed | NS |
| Lixisenatide | Ramipril | ↑ | ↓ | Delayed | NS |
| Liraglutide | Lisinopril | ↓ | ↓ | Delayed | NS |
| Albiglutide | Lisinopril | ND | ND | ND | NS |
| Dulaglutide | Lisinopril | ↔ | ↔ | Delayed | NS |
| Exenatide | Digoxin | ↔ | ↓ | Delayed | NS |
| Lixisenatide | Digoxin | ↔ | ↓ | Delayed | NS |
| Liraglutide | Digoxin | ↓ | ↓ | Delayed | NS |
| Albiglutide | Digoxin | ↔ | ↑↔ | Delayed | NS |
| Dulaglutide | Digoxin | ↔ | ↓ | Delayed | NS |
| Semaglutide | Digoxin | ↔ | ↔ | ↔ | NS |
AUC area under the curve, Cmax maximum observed plasma concentration, GLP1 glucagon-like peptide 1, ND not determined, NS not significant tmax time to maximum observed plasma concentration
Acetaminophen
Acetaminophen is a widely used over the counter drug for pain control and antipyretic effects. It has also been used in clinical research as a surrogate measure for gastric emptying [43]. Co-administration of exenatide [13], lixisenatide [36], liraglutide [19], dulaglutide [32, 38] or semaglutide [27, 40] resulted in decreased Cmax and delayed tmax of acetaminophen (Table 3). However, the AUC showed bioequivalence with and without the GLP1 agonists. Dose adjustment is probably not needed for the use of acetaminophen in patients treated with GLP1RAs.
BCS Class II: Low Solubility, High Permeability
Statins
Statins and GLP1 analogues are often simultaneously prescribed, as dyslipidemia and obesity or diabetes are commonly encountered in the same patient. The PK studies with lixisenatide [36], liraglutide [24] and semaglutide [16, 27] demonstrated reduced Cmax and delayed tmax of atorvastatin, but overall minor changes in the AUC were not considered to be of clinical importance (Table 3). Notably, the efficacy of atorvastatin is poorly correlated with Cmax [44], probably due to complex pharmacokinetics of drug metabolites with longer half-life compared to the parent compound. Treatment with dulaglutide showed similar inhibitory effects on Cmax and tmax, but the AUC of atorvastatin was reduced (0.786, 90% CI 0.752–0.821) [15], as with co-administration of albiglutide [29]. The reduction in AUC was contained within the known >30% PK variability of atorvastatin [45], and was therefore interpreted as non-clinically significant. Likewise, exenatide resulted in reduced Cmax, delayed tmax and reduced AUC of lovastatin [20]. However, an analysis of 966 subjects who received statins in Phase 3 trials of exenatide (51% atorvastatin, 31% simvastatin, 11% pravastatin, 5% lovastatin, 3% fluvastatin) showed comparable lipid-lowering statin effects compared to placebo [20]. The groups also did not differ in the number of subjects who increased their statin dosage. It was concluded that dose adjustments are not expected to be required when prescribing statins with GLP1RAs.
Tacrolimus
Post-transplant DM is seen in 30% of solid organ transplant recipients. Data on the use of GLP1RAs in transplanted patients is scarce. Coadministration of liraglutide and tacrolimus in five kidney transplant recipients resulted in reduced AUC of the latter, but trough levels remained unaltered [26]. There was no change in prescribed doses of tacrolimus or graft rejection. These preliminary results, though reassuring, need validation in larger cohorts, especially due to the narrow therapeutic range of tacrolimus and the risk for graft rejection in undertreatment. Monitoring of drug levels is best advised until further data are published.
BCS Class III: High Solubility, Low Permeability
Angiotensin-Converting Enzyme Inhibitors (ACEI)
Obesity, DM and hypertension constitute three of the criteria for metabolic syndrome. Angiotensin-converting enzyme inhibitors (ACEIs) are widely used as anti-hypertensives and for treatment of diabetic nephropathy. Co-administration of ACEI and GLP1RAs is therefore expected to be highly prevalent. The PK data on ACEI and GLP1 agonist co-treatment showed different effects by different GLP1RAs (Table 3); Still, all changes were interpreted as minor and non-clinically significant [23, 24, 30, 32, 38]. Moreover, the pharmacodynamic response was assessed by a 24-h ambulatory mean blood pressure and was unaffected [23]. Dose adjustment of ACEI in patients treated with GLP1RAs is probably not required; however, blood-pressure monitoring is recommended.
BCS Class IV: Low Solubility, Low Permeability
Digoxin
Diabetes and obesity are risk factors for heart diseases such as atrial fibrillation and congestive heart failure; therefore, digoxin may be given with a GLP1RA in the same individual. Slowing of gastric motility was expected to increase solubility and therefore potentially drug absorption. Accordingly, co-treatment with albiglutide did result in mildly increased Cmax; yet the digoxin blood concentration remained within therapeutic levels and the AUC did not change [14]. The rate or extent of digoxin’s absorption was not affected by coadministration of oral or subcutaneous semaglutide [16]. All other GLP1 agonists resulted in a lower Cmax and a delayed tmax, but the AUC remained unaffected [15, 22, 32, 36]. Liraglutide was an exception as it also decreased the AUC [24]. Nevertheless, in all studies the digoxin concentrations remained within the therapeutic range. Current evidence does not support a need for dose adjustment in coadministration of digoxin and GLP1RAs. However, digoxin is one of the most prevalent pharmacologic causes for hospital admissions for toxicity due to the narrow therapeutic range. We therefore suggest monitoring drug levels at least at the start of cotreatment with GLP1RAs, especially in patients with kidney dysfunction who are at risk for drug accumulation to toxic levels.
Erythromycin
Meier et al. explored a possible pharmacodynamic interaction between GLP1 that decelerates gastric emptying and the prokinetic drugs metoclopramide, domperidone, cisapride and erythromycin in nine healthy males [28]. Erythromycin counteracted the effect of GLP1 on the velocity of gastric emptying and lowered the post-prandial anti-hyperglycemic effect of GLP1. The other prokinetic drugs did not show interaction with GLP1 action.
Discussion
The clinical importance of DDI cannot be overemphasized; DDI that result in drug overdose are responsible for serious and sometimes fatal adverse events. Nonetheless, even a minor adverse event might cause inconvenience and non-adherence or drug discontinuation. Drug-drug interactions that lower drug exposure might result in treatment failure. The FDA and EMA have issued specific guidance to encourage sponsors from the medical industry to assess potential DDI [46, 47]. The studies reviewed in this manuscript align with this guidance.
Only a few cases resulted in reduced Cmax with delayed tmax of the investigated oral drug, findings that are consistent with the known effect of delayed gastric output by GLP1Ras. However, most of the PK studies demonstrated that the absorption characteristics of the oral drugs were not affected, therefore unsurprisingly, the co-exposure to a GLP1RA did not alter the AUC. Moreover, no clinically significant impact was detected even in cases of a decrease in the AUC. Accordingly, PD studies showed no difference in clinically relevant endpoints, further supporting the similar bioavailability of drugs when simultaneously prescribed with a GLP1RA.
Gastric emptying is frequently evaluated by the simple and inexpensive paracetamol absorption test; yet the use of the paracetamol test has been criticized, particularly since it cannot assess gastric emptying of solid foods [48, 49]. Scintigraphy is the gold standard technique for measurement of gastric emptying, and the stable isotope breath test is a simpler, less expensive, and well-validated alternative. Studies that used these tests have shown that both short- and long-acting GLP1RAs decelerate the gastric emptying. The slowed gastric motility lowers the duodenal glucose load after a meal, thereby reducing post-prandial plasma glucose excursions [50]. Still, the total glucose uptake remains unchanged [51]. This food kinetic is similar to the drug kinetics described in this review when an oral drug is co-administered with GLP1RAs. Of note, many of the studies used a single-dose administration of GLP1RAs. An open question remains whether chronic use of GLP1RAs will result in tachyphylaxis of the gastric slowing effect, which will mitigate the risk of DDI [51–53]. On the other hand, some data on chronic administration of long acting GLP1RAs have established that deceleration of gastric emptying does continue – albeit less compared to short-acting GLP1RAs. In the same line, many of the drugs that were given simultaneously with the GLP1RAs were administered in a single dose, again raising the question of whether the results can be inferred to the context of chronic use with multiple doses.
The studies on potential DDI with GLP1RAs showed absence of clinically significant effects on the PK and PD of drugs from the four BCS classes. These reassuring results can probably be extrapolated to other drugs from all BCS classes. Nonetheless, the slowed rate of absorption seen in several cases may be important in several scenarios; the first is when a rapid pharmacological effect is required, such as with anti-hyperglycemic drugs that affect immediate post-prandial glucose excursions [48, 54]. Inter-individual variation in gastric emptying is common and is even greater in diabetic patients. Gastroparesis is in fact a major determinant of post-prandial glucose excursions, and a cause for upper gastrointestinal symptoms. Administration of short-acting insulin aims to prevent post-prandial hyperglycemia; its PK characteristics should match the rate of carbohydrate delivery since a mismatch might result in hypoglycemia. Pharmacologically induced slowing of gastric emptying by GLP1RAs should be considered in patients treated with short-acting insulin for appropriate timing of administration of the insulin drug and probable use of rapid rather than ultra-rapid insulin analogues. The rate of gastric emptying is also of clinical importance in the context of advanced bolus programs in insulin pumps. Another example is a potential interaction between dexamethasone and GLP1RAs [34], yielding false positive results of dexamethasone-suppression test. This interaction was reported by Nagai et al in two of seven patients, all presented with no clinical manifestation of Cushing's syndrome. After switching the GLP1RA therapy to insulin administration, a repeat dexamethasone suppression test resulted in an adequate decrease of endogenous cortisol levels. It is suggested that positive dexamethasone suppression test in a patient without high pre-test probability for Cushing's syndrome be repeated without coadministration of GLP1RA or be supported by additional screening tests for Cushing’s syndrome. In the same line, Fujita et al described a patient on chronic hydrocortisone who manifested symptoms of hypocortisolism after the start of exenatide therapy [33]. Pharmacokinetic assessment revealed a decrease in Cmax and delayed tmax of hydrocortisone when administered after a dose of exenatide compared to postprandial administration of hydrocortisone without exenatide. The PK measurements normalized when hydrocortisone was given before a meal and exenatide 1.5 h after that meal. The prolonged retention of drugs in the stomach should also be considered in the context of intoxication; for example, gastrointestinal decontamination with active charcoal is usually recommended if a patient presents within four hours after toxic ingestion of acetaminophen. This timeline may be longer if the patient also receives a GLP1RA. Finally, even minor changes in drug absorption may be clinically significant in the context of drugs with a narrow therapeutic index. The studies on digoxin did not show an effect on time in therapeutic range; but the small study on tacrolimus implied that there might be an effect on the extent of absorption. Special attention and careful monitoring are obviously required in such cases.
Notably, one should also consider PD DDI, as demonstrated in the example of the prokinetic drug erythromycin. Patients who need pro-kinetics are probably not good candidates for GLP1RAs due to potential counteraction of their effect.
One limitation of most PK and PD studies is the enrollment of healthy subjects, whereas the major target population for use of GLP1RAs are patients with diabetes and obesity who may have co-morbidities that might affect the PK results. Kidney dysfunction is one example, as it requires longer duration of PK measurements. Increased prevalence of gastroparesis in diabetic and obese patients is another example [7, 55]. Exenatide prolonged the gastric half-emptying time in all diabetic patients without gastroparesis (n = 20) and worsened the gastric emptying in two of ten diabetics with pre-existing mild gastroparesis [56]. The patients’ reported perception of exenatide’s therapeutic effects was similar in diabetic subjects with versus without gastroparesis at start of exenatide therapy. However, more data are needed to assess whether gastric emptying will be more affected by GLP1RAs in patients with pre-exposure gastric motility disorders, and whether it might result in clinically significant PK changes. Another limitation is the focus on change in drug absorption, whereas other PK parameters can be considered. For example, GLP1RAs-induced weight loss might affect drug distribution; accordingly, Bourron et al reported a case of amiodarone-induced thyrotoxicosis in a patient with 45 % weight loss following Roux-en-Y gastric bypass [57]. We are not aware of similar reports in patients treated by GLP1RAs. However, considering the ongoing development of more potent GLP1RAs and combined GLP-GIP drugs, which will probably induce massive weight losses, awareness should be increased for possible DDI at level of drug distribution.
Conclusion
The known effect of GLP1RAs to slow gastric motility has raised concerns regarding a possible change of the absorption of oral drugs. To date, no clinically significant effect has been observed in any of the investigated oral drugs co-administered with any of the GLP1RAs. These reassuring results underlie the statement in all of the GLP1RAs’ prescribing sheets that dose adjustments are not needed for simultaneous use with oral medications. Still, this recommendation should be carefully generalized to other medicinal products, especially when treating patients with background gastroparesis or kidney dysfunction, or when considering drugs with narrow therapeutic effects.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access funding provided by Tel Aviv University. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of Interest
BC declares that there are no relationships or activities that might bias, or be perceived to bias, her work. ID has received fees from Astra Zeneca, Novo Nordisk, Abbott, Boehringer Ingleheim, Elli Lilly and Sanofi for educational lectures, and participated in advisory boards of Novo Nordisk, Boehringer Ingleheim, Sanofi and Novartis that are outside the scope of this work. ID reports a grant from Astra Zeneca that is outside the scope of the submitted work. DS declares that there are no relationships or activities that might bias, or be perceived to bias, his work. AL declares that there are no relationships or activities that might bias, or be perceived to bias, his work. TDC has received fees from Astra Zeneca for educational lectures that are outside the scope of this work.
Availability of Data and Material
Data are available on request from the authors.
Ethics Approval
Not applicable.
Consent for Publication/Consent to Participate
Not applicable.
Code Availability
Not applicable.
Author Contributions
Design – BC and TDC. Conduct/data collection – BC and TDC. Analysis – BC, ID, DS, AL and TDC. Writing manuscript – BC, ID, DS, AL and TDC. All authors read and approved the final version.
References
- 1.Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017;19(4):524–36. 10.1111/dom.12849 [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt G, Li J, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. The Lancet. 2022;399(10321):259–69. 10.1016/S0140-6736(21)01640-8 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Professional Practice C, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125–43. 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 4.Mosenzon O, Del Prato S, Schechter M, Leiter LA, Ceriello A, DeFronzo RA, et al. From glucose lowering agents to disease/diabetes modifying drugs: a “SIMPLE” approach for the treatment of type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):92. 10.1186/s12933-021-01281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumiller JJ. Clinical pharmacology of incretin therapies for type 2 diabetes mellitus: implications for treatment. Clin Ther. 2011;33(5):528–76. 10.1016/j.clinthera.2011.04.024 [DOI] [PubMed] [Google Scholar]
- 6.Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–20. 10.1023/A:1016212804288 [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis: diagnosis and management. Drugs. 2009;69(8):971–86. 10.2165/00003495-200969080-00003 [DOI] [PubMed] [Google Scholar]
- 8.Greiff JM, Rowbotham D. Pharmacokinetic drug interactions with gastrointestinal motility modifying agents. Clin Pharmacokinet. 1994;27(6):447–61. 10.2165/00003088-199427060-00004 [DOI] [PubMed] [Google Scholar]
- 9.Hebbard GS, Sun WM, Bochner F, Horowitz M. Pharmacokinetic considerations in gastrointestinal motor disorders. Clin Pharmacokinet. 1995;28(1):41–66. 10.2165/00003088-199528010-00005 [DOI] [PubMed] [Google Scholar]
- 10.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372): n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry-Bibee EN, Kim MJ, Simmons KB, Tepper NK, Riley HE, Pagano HP, et al. Drug interactions between hormonal contraceptives and psychotropic drugs: a systematic review. Contraception. 2016;94(6):650–67. 10.1016/j.contraception.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GUIDELINE IH. FDA M9-Biopharmaceutics-Classification-System-Based-Biowaivers.pdf. 2018. https://www.fda.gov/media/148472/download. Accessed 20 Aug 2023.
- 13.Blase E, Taylor K, Gao HY, Wintle M, Fineman M. Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin-4) in healthy subjects. J Clin Pharmacol. 2005;45(5):570–7. 10.1177/0091270004274432 [DOI] [PubMed] [Google Scholar]
- 14.Bush M, Scott R, Watanalumlerd P, Zhi H, Lewis E. Effects of multiple doses of Albiglutide on the pharmacokinetics, Pharmacodynamics, and safety of Digoxin, Warfarin, or a low-dose oral contraceptive. Postgrad Med. 2012;124(6):55–72. 10.3810/pgm.2012.11.2613 [DOI] [PubMed] [Google Scholar]
- 15.de la Peña A, Cui X, Geiser J, Loghin C. No dose adjustment is recommended for Digoxin, Warfarin, Atorvastatin or a Combination Oral Contraceptive When Coadministered with Dulaglutide. Clin Pharmacokinet. 2017;56(11):1415–27. 10.1007/s40262-017-0531-7 [DOI] [PubMed] [Google Scholar]
- 16.Hausner H, DervingKarsbøl J, Holst AG, Jacobsen JB, Wagner FD, Golor G, et al. Effect of semaglutide on the Pharmacokinetics of Metformin, Warfarin, Atorvastatin and Digoxin in Healthy Subjects. Clin Pharmacokinet. 2017;56(11):1391–401. 10.1007/s40262-017-0532-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobsen LV, Vouis J, Hindsberger C, Zdravkovic M. Treatment with liraglutide-a once-daily GLP-1 analog-does not reduce the bioavailability of ethinyl estradiol/levonorgestrel taken as an oral combination contraceptive drug. J Clin Pharmacol. 2011;51(12):1696–703. 10.1177/0091270010389471 [DOI] [PubMed] [Google Scholar]
- 18.Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel. J Clin Pharmacol. 2015;55(5):497–504. 10.1002/jcph.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapitza C, Zdravkovic M, Hindsberger C, Flint A. The effect of the once-daily human glucagon-like peptide 1 analog liraglutide on the pharmacokinetics of acetaminophen. Adv Ther. 2011;28(8):650–60. 10.1007/s12325-011-0044-y [DOI] [PubMed] [Google Scholar]
- 20.Kothare PA, Linnebjerg H, Skrivanek Z, Reddy S, Mace K, Pena A, et al. Exenatide effects on statin pharmacokinetics and lipid response. Int J Clin Pharmacol Ther. 2007;45(2):114–20. 10.5414/CPP45114 [DOI] [PubMed] [Google Scholar]
- 21.Kothare PA, Seger ME, Northrup J, Mace K, Mitchell MI, Linnebjerg H. Effect of exenatide on the pharmacokinetics of a combination oral contraceptive in healthy women: an open-label, randomised, crossover trial. BMC Clin Pharmacol. 2012. 10.1186/1472-6904-12-8. 10.1186/1472-6904-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kothare PA, Soon DKW, Linnebjerg H, Park S, Chan C, Yeo A, et al. Effect of exenatide on the steady-state pharmacokinetics of digoxin. J Clin Pharmacol. 2005;45(9):1032–7. 10.1177/0091270005278806 [DOI] [PubMed] [Google Scholar]
- 23.Linnebjerg H, Kothare P, Park S, Mace K, Mitchell M. The effect of exenatide on lisinopril pharmacodynamics and pharmacokinetics in patients with hypertension. Int J Clin Pharmacol Ther. 2009;47(11):651–8. 10.5414/CPP47651 [DOI] [PubMed] [Google Scholar]
- 24.Malm-Erjefält M, Ekblom M, Vouis J, Zdravkovic M, Lennernäs H. Effect on the gastrointestinal absorption of drugs from different classes in the biopharmaceutics classification system, when treating with liraglutide. Mol Pharm. 2015;12(11):4166–73. 10.1021/acs.molpharmaceut.5b00278 [DOI] [PubMed] [Google Scholar]
- 25.Soon D, Kothare PA, Linnebjerg H, Park S, Yuen E, Mace KF, et al. Effect of exenatide on the pharmacokinetics and pharmacodynamics of warfarin in healthy Asian men. J Clin Pharmacol. 2006;46(10):1179–87. 10.1177/0091270006291622 [DOI] [PubMed] [Google Scholar]
- 26.Pinelli NR, Patel A, Salinitri FD. Coadministration of liraglutide with tacrolimus in kidney transplant recipients: a case series. Diabetes Care. 2013;36(10):e171–2. 10.2337/dc13-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langeskov EK, Kristensen K. Population pharmacokinetic of paracetamol and atorvastatin with co-administration of semaglutide. Pharmacol Res Perspect. 2022;10(4): e00962. 10.1002/prp2.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier JJ, Kemmeries G, Holst JJ, Nauck MA. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes. 2005;54(7):2212–8. 10.2337/diabetes.54.7.2212 [DOI] [PubMed] [Google Scholar]
- 29.Young MA, Wald JA, Matthews JE, Scott R, Hodge RJ, Zhi H, et al. Clinical pharmacology of albiglutide, a glp-1 receptor agonist. Postgrad Med. 2014;126(7):84–97. 10.3810/pgm.2014.11.2836 [DOI] [PubMed] [Google Scholar]
- 30.Dahmen R, Steinstraesser A, Poitiers F, Ozoux ML, Pinquier JL. Interaction of subcutaneous lixisenatide 20 lg QD on pharmacokinetic and pharmacodynamic properties of oral ramipril 5 MG QD in. Basic Clin Pharmacol Toxicol. 2011;109:89. [Google Scholar]
- 31.Liu YH, Ruus P, Steinstraesser A, Teichert L. Effect of the GLP-1 agonist lixisenatide on the pharmacokinetics of warfarin. In: Abstract conference: 70th scientific sessions of the American Diabetes Association. 2010.
- 32.Tham LS, Schneck KB, Geiser JS, Posada M, Dickinson G. Integration of population exposure-response and physiological based pharmacokinetics modeling approaches to evaluate gastric-emptying induced drug interaction risks for dulaglutide. J Pharmacokinet Pharmacodyn. 2018;45:S53–4. [Google Scholar]
- 33.Fujita Y, Kitamura T, Otsuki M, Tamada D, Tabuchi Y, Kozawa J, et al. Exenatide alters absorption of hydrocortisone in a diabetic patient with panhypopituitarism: Iatrogenic adrenal insufficiency. Diabetes Care. 2013;36(1): e8. 10.2337/dc12-1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagai Y, Mukai K, Otsuki M, Kimura T, Kozawa J, Nishizawa H, et al. Suppression failure of cortisol secretion by dexamethasone may occur in glucagon-like peptide-1 receptor agonist-treated patients with diabetic autonomic neuropathy. Intern Med. 2019;58(7):949–53. 10.2169/internalmedicine.1585-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA. Exenatide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf
- 36.FDA. Lixisenatide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471orig1s000lbl.pdf
- 37.FDA. Liraglutide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf
- 38.FDA. Dulaglutide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125469s007s008lbl.pdf
- 39.FDA. Albiglutide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s019lbl.pdf
- 40.FDA. Semaglutide prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf
- 41.Stegeman BH, de Bastos M, Rosendaal FR, van Hylckama VA, Helmerhorst FM, Stijnen T, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;12(347): f5298. 10.1136/bmj.f5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Industry FGf. Clinical drug interaction studies with combined oral contraceptives. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-combined-oral-contraceptives-guidance-industry. Accessed 20 Aug 2023.
- 43.Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci. 2001;46(10):2256–62. 10.1023/A:1011935603893 [DOI] [PubMed] [Google Scholar]
- 44.Cilla DD Jr, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60(6):687–95. 10.1016/S0009-9236(96)90218-0 [DOI] [PubMed] [Google Scholar]
- 45.Narwal R, Akhlaghi F, Asberg A, Hermann M, Rosenbaum SE. Development of a population pharmacokinetic model for atorvastatin acid and its lactone metabolite. Clin Pharmacokinet. 2010;49(10):693–702. 10.2165/11535980-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 46.EMA. Investigation of drug interactions. https://www.ema.europa.eu/en/investigation-drug-interactions
- 47.FDA. Drug Interactions | Relevant regulatory guidance and policy documents. https://www.fda.gov/drugs/drug-interactions-labeling/drug-interactions-relevant-regulatory-guidance-and-policy-documents
- 48.Jalleh RJ, Jones KL, Rayner CK, Marathe CS, Wu T, Horowitz M. Normal and disordered gastric emptying in diabetes: recent insights into (patho)physiology, management and impact on glycaemic control. Diabetologia. 2022;65(12):1981–93. 10.1007/s00125-022-05796-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz M, Rayner CK, Marathe CS, Wu T, Jones KL. Glucagon-like peptide-1 receptor agonists and the appropriate measurement of gastric emptying. Diabetes Obes Metab. 2020;22(12):2504–6. 10.1111/dom.14166 [DOI] [PubMed] [Google Scholar]
- 50.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273(5):E981–8. [DOI] [PubMed] [Google Scholar]
- 51.Smits MM, Tonneijck L, Muskiet MH, Kramer MH, Cahen DL, van Raalte DH. Gastrointestinal actions of glucagon-like peptide-1-based therapies: glycaemic control beyond the pancreas. Diabetes Obes Metab. 2016;18(3):224–35. 10.1111/dom.12593 [DOI] [PubMed] [Google Scholar]
- 52.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. The Lancet. 2008;372(9645):1240–50. 10.1016/S0140-6736(08)61206-4 [DOI] [PubMed] [Google Scholar]
- 53.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60(5):1561–5. 10.2337/db10-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horowitz M, O’Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med. 2002;19(3):177–94. 10.1046/j.1464-5491.2002.00658.x [DOI] [PubMed] [Google Scholar]
- 55.Xing J, Chen JD. Alterations of gastrointestinal motility in obesity. Obes Res. 2004;12(11):1723–32. 10.1038/oby.2004.213 [DOI] [PubMed] [Google Scholar]
- 56.Beti C, Stratmann B, Bokman G, Dreier J, Hauber M, Lee-Barkey YH, et al. Exenatide delays gastric emptying in patients with type 2 diabetes mellitus but not in those with gastroparetic conditions. Horm Metab Res. 2019;51(4):267–73. 10.1055/a-0818-6374 [DOI] [PubMed] [Google Scholar]
- 57.Bourron O, Ciangura C, Bouillot JL, Massias L, Poitou C, Oppert JM. Amiodarone-induced hyperthyroidism during massive weight loss following gastric bypass. Obes Surg. 2007;17(11):1525–8. 10.1007/s11695-008-9415-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.