Abstract
We have investigated the phosphorylation state of the human cytomegalovirus 86-kDa immediate-early (IE) protein IEP86 from transfected and infected cells. We show that multiple domains of IEP86 are phosphorylated by cellular kinases, both in vitro and in vivo. Our data suggest that serum-inducible kinases play a significant role in cell-mediated IE protein phosphorylation and that a member of the mitogen-activated protein (MAP) kinase (MAPK) family, extracellular regulated kinase 2 (ERK2), phosphorylates several domains of IEP86 in vitro. Alanine substitution mutagenesis was performed on specific serines or threonines (T27, S144, T233/S234, and T555) found in consensus MAP kinase motifs. Analysis of these mutations showed that T27 and T233/S234 are the major sites for serum-inducible kinases and are the major ERK2 sites in vitro. S144 appeared to be phosphorylated in a serum-independent manner in vitro. All of the mutations except T555 eliminated specific phosphorylation in vivo. In transient transfection analyses, IEP86 isoforms containing mutations in S144 and, especially, T233/S234 displayed increased transcriptional activation relative to the wild type, suggesting that phosphorylation at these sites in wild-type IEP86 may result in reduction of its transcriptional activation ability.
The major immediate-early (MIE) gene of human cytomegalovirus (HCMV) gives rise to several transcripts through alternative splicing and polyadenylation of the primary transcript (1, 32, 40, 81, 82, 84, 85, 87) (Fig. 1). Two of these transcripts, expressed with immediate-early (IE) kinetics, encode nuclear phosphoproteins with observed molecular masses of 72 kDa (IEP72; IE1491aa) and 86 kDa (IEP86; IE2579aa). IEP72 and IEP86 function in the temporal transcriptional activation of HCMV early and late viral genes (38, 44, 45, 51, 79, 80). In addition, these proteins are promiscuous transcriptional activators of many cellular genes (8, 29–32, 45, 48, 57, 83, 95, 96). In previous studies, our laboratory and others (9, 11, 20, 30, 42, 45, 47, 49, 50, 74, 77) have shown that activation mediated by IEP86 is correlated with protein-protein interactions involving IEP86 with upstream-bound transcription factors as well as with the basal transcription complex, in particular the TFIID complex. In this regard, both IEP72 and IEP86 appear to function as integral components of the TFIID complex in a manner similarly to TATA-binding protein (TBP)-associated factors (TAFs) (50). Such a role is consistent with the observed promiscuous transcriptional activation mentioned above.
FIG. 1.
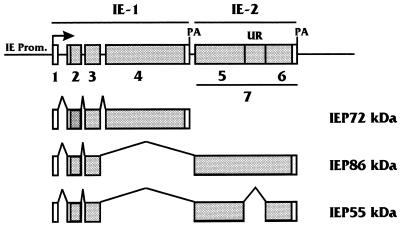
MIE genomic region of HCMV. The diagram shows the MIE gene and the alternatively spliced and polyadenylated (PA) transcripts produced from it which encode the MIE proteins (IEP72, IEP86, and IEP55). The various exons are numbered; open reading frames within exons are shaded. The IE-1 region is composed of exons 1 to 4, and the IE-2 region consists of exon 7. Note that IEP55 differs from IEP86 via an extra splice, resulting in removal of an IEP86 unique region (UR) in exon 7. The IE-2-derived open reading frames flanking the unique region are labeled as exons 5 and 6 specific to IEP55. In some diagrams of the MIE region the exon we have called 7 is numbered as exon 5. Prom., promoter.
The potential role of phosphorylation in the function of the HCMV IE proteins has not been widely addressed. Studies involving a variety of transcription factors have shown that phosphorylation is a major mechanism for controlling the factors’ activities. For example, phosphorylation can affect the ability of transcription factors to bind DNA, to interact with coactivators, and to homodimerize and can alter intramolecular conformation (reviewed in reference 39). In this regard, the herpes simplex virus type 1 (HSV-1) transactivator ICP4, which shows some functional similarities with IEP86, is posttranslationally modified by phosphorylation during the course of infection (53, 61, 90, 92, 93). This may alter the functional properties of ICP4 at different phases of the viral life cycle (76, 92). Similarly, the functions of IEP86 may be affected by its phosphorylation state (32, 67, 72, 77).
One means by which many factors undergo site-specific phosphorylation or dephosphorylation is in response to signal transduction pathways activated by extracellular signals such as growth factors, cytokines, or stress. Kinase pathways activated by such signaling mediate nuclear translocation of members of a family of serine/threonine kinases known as the mitogen-activated protein kinases (MAPKs). Following activation and nuclear entry, the MAPKs can affect the activities of various cellular transcription factors such as c-Jun (17, 70), Elk-1 (89), and c-Myc (15, 27). Other serine/threonine kinases such as cyclic AMP-dependent protein kinase (PKA) and protein kinase C (PKC) also display altered activities as a result of second messenger molecules induced by extracellular signals. Activation of PKA- and PKC-mediated phosphorylation pathways has been shown to affect the activity of cellular transcription factors such as CREB (25) and c-Jun (6), respectively. In addition, PKA appears to play a role in the phosphorylation of HSV-1 ICP4 (92, 93). In this manner, HSV-1 may respond to changes in the extracellular milieu via alterations in the phosphorylation state of ICP4, potentially affecting the balance between latent and lytic infection (76, 92).
In this study, we investigated the phosphorylation state of the HCMV IE proteins, with emphasis on IEP86. We show that multiple domains of IEP86 are phosphorylated by cellular kinases, both in vitro and in vivo. Our data suggest that serum-inducible kinases play a significant role in cell-mediated IE protein phosphorylation. We show that a member of the MAPK family, extracellular regulated kinase 2 (ERK2), phosphorylates several domains of IEP86 in vitro. Alanine substitution mutagenesis of specific serines or threonines found in consensus MAPK motifs of IEP86 was observed to prevent ERK2-mediated phosphorylation of these motifs in vitro, and eliminate specific phosphorylation in vivo, indicating that MAPKs play a significant role in the phosphorylation of IEP86 in vivo. In transient transfection analyses IEP86 isoforms containing mutations in specific MAPK motifs displayed increased transcriptional activation relative to the wild-type (WT) protein. These data suggest that MAPK-mediated phosphorylation of WT IEP86 may result in reduction of its transcriptional activation ability.
MATERIALS AND METHODS
Cells and plasmids.
The HCMV-permissive glioblastoma/astrocytoma cell line U-373 MG was maintained at passage numbers less than 30. Cells were cultured in Dulbecco’s modification of high-glucose Eagle’s medium (HG-DMEM) supplemented with 5% fetal calf serum (FCS).
Plasmid pRSV86 (2, 49) contains a cDNA copy of the IEP86 gene under the control of the Rous sarcoma virus long terminal repeat and was used for expression of IEP86 in mammalian cells. The corresponding control plasmid, containing no cDNA, was pRSV3/BglII (13), and the general DNA filler plasmid was pGEM4 (Promega). IEP86 tagged with six histidine residues was produced in Escherichia coli by using pET28a-86 (50). Fusions of either full-length IEP86, IEP72, or the various IE protein fragments (glutathione S-transferase [GST] fusions [see Fig. 6]) were made with the glutathione binding site of GST contained in pGEX3X (49, 50).
FIG. 6.
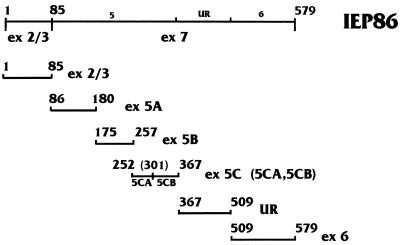
The exonic regions of IEP86 used in GST fusion proteins for phosphorylation studies. First and last amino acids in exonic regions (ex) are numbered above, with corresponding names at the right. UR represents the unique coding region in IEP86 which differentiates it from IEP55 (Fig. 1). GST fusions to full-length IEP86 were made with amino acids 1 to 579, as shown at the top.
Alanine substitution mutations in the IE proteins were constructed by using the splicing overlap extension protocol (37). Appropriate flanking primers were chosen to amplify mutant inserts suitable for swapping into pGEX3X-86, pRSV86, or pET28a-86. Combination mutants were constructed either by swapping mutation-containing restriction fragments from one mutant construct into another or by using single-mutation plasmid constructs as PCR templates for introducing further mutations by splicing overlap extension. All mutant clones were confirmed by DNA sequence analysis.
The promoter of the luciferase reporter plasmid dpm7-LUC contains six copies of the simian virus 40 (SV40) late promoter-derived OCT/TEF element, with point mutations that eliminate OCT-binding but not TEF-1-binding ability, upstream of the β-globin TATA element (24, 86). This promoter was initially removed from pβ6xB20-dpm7 (86) via the SacI and XbaI sites and then inserted between the SacI and XbaI sites of a modified form of pSP72 (Promega) which had a β-globin TATA element between the PstI and HindIII sites, upstream of a chloramphenicol acetyltransferase reporter gene, to form dpm7-CAT (14). The dpm7 promoter and β-globin TATA element were then removed from dpm7-CAT via SacI and HindIII and inserted into pGL2-Promoter (Promega) between the SacI and HindIII sites to form dpm7-LUC (48).
Bacterial fusion protein preparation.
The various GST fusion proteins were made in E. coli HB101 or DH5α, harvested, and purified by batch chromatography with glutathione-agarose beads (Sigma) as previously described (49, 50). Equivalent amounts (1 to 5 μg) of each fusion were used for experiments, with quantitation by Bradford analysis as well as by staining gels with silver, Coomassie blue, or Sypro red (FMC).
WT and mutant forms of histidine-tagged IEP86 were prepared according to procedures supplied by Qiagen, using extracts of E. coli BL21(DE3) which contained pET28a-86 (WT IEP86) or similar plasmids encoding mutant forms of IEP86. The proteins were purified and eluted under native conditions, using Qiagen Ni-nitrilotriacetic acid resin as described by the manufacturer. Eluates were dialyzed into 10 mM Tris (pH 8.0)–5% glycerol. Equivalent amounts of each tagged protein were used for experiments, with quantitation by Bradford analysis as well as by staining gels with silver, Coomassie blue, or Sypro red.
HCMV infections.
The Towne strain of HCMV (a generous gift of Teresa Compton) was propagated in primary human foreskin fibroblasts. Experimental infections were performed at a multiplicity of infection of 1. The virus inoculum was allowed to adsorb to U-373 MG cells for 1.5 to 2 h at 37°C in HG-DMEM without serum, after which the medium was replaced with HG-DMEM supplemented with 2% FCS.
Transfections.
U-373 MG cells were grown in 100-mm-diameter tissue culture dishes for [32P]orthophosphate labeling studies and in 60-mm-diameter dishes for luciferase reporter assay studies. Cells at 60 to 75% confluence were transfected by the calcium phosphate method as previously described (26). As thoroughly described in reference 26, we do not use internal control plasmids for transfection standardization since the viral activator proteins often affect reporter expression from these plasmids; hence we repeat our transfections multiple times, using different cells and different plasmid preparations.
For 32P labeling, the cells were transfected with 10 μg of pRSV86-based plasmids, expressing either WT or mutant IEP86 cDNAs, or the pRSV3/BglII control plasmid. Total transfected DNA was bought to 20 μg by using pGEM4. For reporter assay studies, cells were transfected with 3 μg of pRSV86-based plasmids and 2 μg of luciferase reporter plasmid, and total DNA was brought to 10 μg by using pGEM4. At 12 to 16 h posttransfection, cells were washed with phosphate-buffered saline, treated with 15% glycerol (26, 49) for 1 min, and then washed twice with phosphate-buffered saline, followed by addition of fresh medium to the cells. Approximately 44 to 48 h after transfection, the cells were either labeled with [32P]orthophosphate (see below) or harvested for reporter assays.
Luciferase reporter assays were performed on transfected cell extracts harvested as instructed by the manufacturer (Promega). Luciferase activity of equal amounts of cell extracts (as determined by Bradford analysis) was measured in a Berthold 9501 Lumat luminometer.
In vivo labeling, harvest, and immunoprecipitation.
Cell labeling and harvest were performed as previously described (75). Prior to the addition of label, cells were rinsed once in Tris-buffered saline and once in phosphate-free HG-DMEM (Gibco). Cells were then labeled for 4 to 5 h at 37°C with 0.4 mCi of [32P]orthophosphate (Amersham) per ml in phosphate-free HG-DMEM supplemented with either 2% (infections) or 5% (transfections) dialyzed FCS. Parallel unlabeled samples were treated in exactly the same fashion except for lack of 32P addition. At harvest, cells were rinsed twice with ice-cold Tris-buffered saline and then lysed in radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors (1 μg of leupeptin per ml, 0.5 mM Nα-p-tosyl-l-lysine chloromethylketone, 0.8 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, (DTT), 2.5 μg of aprotinin per ml, 10 mM sodium fluoride, 10 μM sodium orthovanadate). Lysates were clarified by addition of Pansorbin cells (Calbiochem) prior to centrifugation at 15,000 rpm for 30 min. Equal counts of clarified lysates (1.5 × 109 to 2.0 × 109 cpm) or equal amounts of total protein (1.5 to 2.0 mg of unlabeled samples) were subjected to overnight incubation with 1 μg of monoclonal antibody (MAb) 810 (Chemicon), which recognizes the amino terminus common to both IEP72 and IEP86. During the last hour of incubation, 75 μl of protein A-coated agarose beads (33% [vol/vol] in RIPA buffer; Gibco) was added to precipitate antibody-antigen complexes. The immunoprecipitates were washed three times in RIPA buffer, boiled in 1× Laemmli sample buffer (46), and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). 32P-labeled lanes were fixed in 25% isopropanol–10% acetic acid, dried, and subjected to autoradiography. Unlabeled lanes were transferred to nitrocellulose and then probed with rabbit antisera directed against the amino terminus common to both IEP72 and IEP86 (custom produced by Cocalico Biologicals Inc.) as the primary probe and either 125I-conjugated donkey anti-rabbit immunoglobulin G (Amersham) or horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Cappel) as the secondary probe. Washed blots were either air dried and autoradiographed (125I) or treated with enhanced chemiluminescence reagent (Amersham) and autoradiographed. Where indicated, bands were quantified with a Molecular Dynamics PhosphorImager.
Tryptic phosphopeptide mapping.
Immunoprecipitated, gel-purified 32P-labeled proteins were analyzed by tryptic mapping as previously described (5), using modified trypsin, sequencing grade (Boehringer Mannheim). The peptides were spotted on cellulose thin-layer chromatography (TLC) plates (EM Scientific) and resolved in one dimension by electrophoresis at pH 1.9 and in the second dimension by ascending TLC in phosphochromatography buffer (5).
In vitro phosphorylation assays.
For the preparation of serum-starved whole-cell extracts (WCE), U-373 MG cells were incubated in low serum (0 or 0.5%) for 48 to 72 h prior to extract preparation. For the preparation of serum-stimulated WCE, cells were treated identically except for the addition of medium containing 10% FCS for 15 min prior to extract preparation. Extracts were prepared as previously described (34).
WCE kinase assays were performed by incubating 250 μg of either serum-starved or serum-stimulated WCE with purified, bacterially produced IE protein substrates (GST fusion substrates immobilized on glutathione beads) in the presence of 100 μl of kinase buffer B (20 mM HEPES [pH 8.0], 20 mM MgCl2, 20 mM β-glycerophosphate, 10 μM sodium orthovanadate, 2 mM DTT, 40 μM ATP) (36) containing 5 μCi of [γ-32P]ATP (3 Ci/mmol; Amersham). The reaction mixes were incubated for 30 min with rocking at room temperature. We note that in some experiments the glutathione beads were not added until after the WCE reactions were complete; this resulted in reduction of some background bands. The GST fusion proteins on beads were washed three times with 1 ml of NETN (49) supplemented with protease and phosphatase inhibitors (see above) before boiling in Laemmli sample buffer (46) and separation by SDS-PAGE. Gels were stained with Coomassie blue and dried prior to autoradiography.
WCE kinase assays on His-tagged IEP86 were performed as above, using purified His-tagged IEP86 in solution. To repurify His-tagged substrates from the completed reactions, 10% SDS was added to the reaction mixes to a final concentration of 0.2%. Reaction mixes were incubated at 50°C for 5 min, chilled on ice, and then spun in a microcentrifuge for 5 min at 4°C. Supernatants were transferred to tubes containing 500 μl of RIPA buffer (supplemented with antiproteases and antiphosphatases as described above), 45 μl of protein A-coated agarose beads (33% [vol/vol] in RIPA buffer), and 1 μg of MAb 810. Tubes were incubated at 4°C for 60 to 75 min with rocking. Beads were pelleted by microcentrifugation, washed twice with 1 ml of RIPA buffer, boiled in Laemmli buffer, and separated by SDS-PAGE. Gels were stained with Coomassie blue and dried prior to autoradiography.
The IE substrates were also subjected to in vitro phosphorylation using purified recombinant mouse or rat ERK2 (5 and 60 U/μl, respectively; Calbiochem). The two versions of ERK2 gave similar results. Reactions were performed in 1× Wu kinase buffer (25 mM HEPES [pH 7.5], 10 mM MgCl2, 10 mM β-glycerophosphate, 1 mM DTT, 50 μM ATP, 5 μCi of [γ-32P]ATP [3 Ci/mmol; Amersham]) (91) with 0.5 μl of ERK2 at 30°C for 30 min. The reaction mixes were then boiled in Laemmli buffer and separated by SDS-PAGE. Gels were stained with Coomassie blue and dried prior to autoradiography.
RESULTS
The IE proteins are phosphorylated by cellular kinases in vivo.
Figure 1 shows the map of the HCMV MIE gene. IEP72 and IEP86 contain the identical 85 amino acids at their N termini, derived from two common exons in the IE-1 region. The remainder of the IEP72-encoding transcript is derived entirely from the IE-1 region, giving rise to a 491-residue protein. The remainder of the IEP86-encoding transcript results from a splice into the IE-2 region, producing a distinct carboxy terminus and a total size of 579 amino acids (56, 67, 81, 84).
In a variety of experiments (not shown), we have noted that the migration of IEP86 on denaturing polyacrylamide gels is variable depending on the source of its production. For example, IEP86 produced in human cells migrates significantly more slowly on SDS-PAGE than IEP86 produced in bacteria or rabbit reticulocyte lysates. This suggests differences in posttranslational processing events specific to each source. Such processing appears to be extensive since the observed molecular mass of IEP86 on SDS-PAGE, 82 to 86 kDa, differs markedly from the predicted molecular mass of 64 kDa. In addition to several predicted N-linked glycosylation sites on IEP86, there are a total of over 100 serines, threonines, and tyrosines which could be targets for phosphorylation by protein kinases.
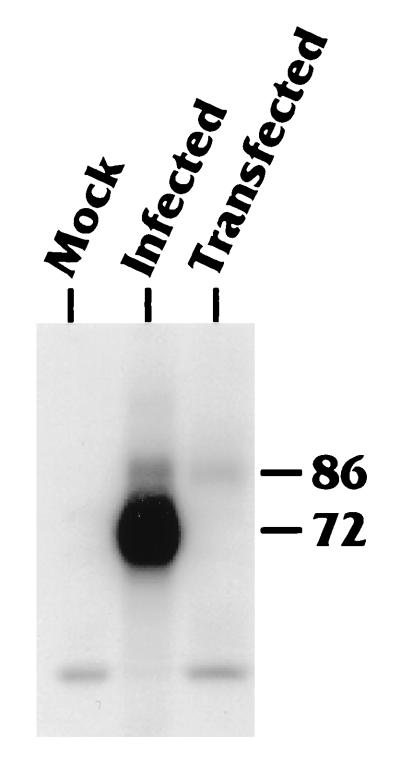
To determine the contributions of cellular, virally encoded, or virally induced kinases to the posttranslational modification of IEP86, we isolated IEP86 from U-373 MG cells which had been (i) transiently transfected with a plasmid which expressed IEP86 from a cDNA or (ii) infected with the Towne strain of HCMV. Cells were labeled for 4 to 5 h with [32P]orthophosphate prior to harvest at 48 h posttransfection or postinfection. Labeled WCE or similar extracts prepared from unlabeled cells were immunoprecipitated with MAb 810, which precipitates both IEP86 and IEP72 (see Materials and Methods). After the washed immunoprecipitates were separated on SDS-PAGE, relative phosphorylation was detected by autoradiography (Fig. 2). Extracts from mock-infected cells gave rise to no specific bands at the expected migration position for either IEP72 or IEP86. As previously observed (22, 32, 60, 69, 72), both IEP72 and IEP86 derived from infected cells were phosphorylated. Western analysis of unlabeled samples prepared in parallel from infected cells demonstrated that IEP72 was consistently expressed at levels 10- to 20-fold higher than levels of IEP86 (not shown). This largely explains the difference in relative intensities of phosphorylation. IEP86 produced from a cDNA in transiently transfected cells was significantly phosphorylated. Hence, cellular kinases are capable of phosphorylating IEP86 independent of viral infection.
FIG. 2.
IEP72 and IEP86 are phosphorylated in both infected and transfected U-373 MG cells. U-373 MG cells were either mock infected, infected with HCMV-Towne at a multiplicity of 1.0, or transiently transfected with a plasmid expressing an IEP86 cDNA, as indicated. Cells were labeled with [32P]orthophosphate from 44 to 48 h postinfection or transfection, just prior to WCE preparation. The extracts were immunoprecipitated with MAb 810, which recognizes the shared amino-terminal end of the IE proteins. Immunoprecipitated complexes were subjected to SDS-PAGE followed by autoradiography to detect phosphorylated proteins. Bands migrating at the expected positions for IEP72 and IEP86 are indicated.
The corresponding unlabeled samples were transferred to nitrocellulose, and relative IE protein expression was determined via Western blotting using an 125I-labeled secondary antibody (not shown). Appropriate bands were quantified with a Molecular Dynamics PhosphorImager. Specific activity was determined by normalizing 32P intensity to 125I intensity for each sample. These data (not shown) indicated that the specific activities of IEP86 isolated from transfected cells and from infected cells were approximately equal at the 48-h time point.
Phosphoamino acid analysis revealed that serine and threonine are the only detectably phosphorylated amino acids in IEP86 produced in either transfected or infected cells (not shown).
Mapping the phosphorylation pattern of IEP86.
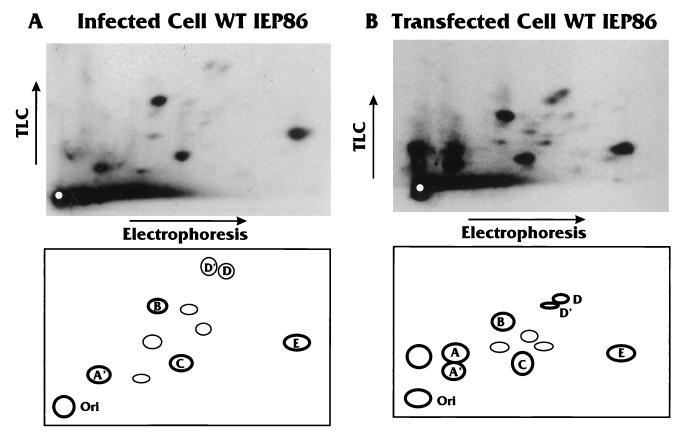
To map the actual phosphorylation patterns for IEP86, 32P-labeled bands were excised from the gels, eluted, and subjected to tryptic digestion and two-dimensional phosphopeptide analysis on TLC plates (5), followed by autoradiography. Figure 3A shows the tryptic phosphopeptides for IEP86 isolated from infected cells; Fig. 3B shows the tryptic phosphopeptides for IEP86 isolated from cDNA-transfected cells where no other viral gene products were present. Schematic diagrams of the phosphopeptide patterns are shown at the bottom of each panel. For reference, we have assigned letter designations to only those peptides which, in the course of this study, showed differences between transfected and infected cells or were affected by specific mutations in IEP86. The results indicate that IEP86 is phosphorylated at multiple sites under both transfection and infection conditions.
FIG. 3.
Tryptic phosphopeptide patterns produced by IEP86 isolated from infected and transfected cells are similar. IEP86 derived from HCMV-infected (A) and cDNA-transfected (B) cells was eluted from excised gel slices and subjected to tryptic digestion and phosphopeptide mapping as described in Materials and Methods. Electrophoresis was performed at pH 1.9, and TLC was conducted in phosphochromatography buffer (5). Typical autoradiograms of the tryptic phosphopeptides are shown at the top. The experiments were repeated several times; despite variations from sample to sample in total radioactivity spotted and differences in overall migration in each dimension, the basic patterns were quite reproducible. In the schematic below each autoradiogram, major phosphopeptides are labeled with letters for reference.
Comparing the major phosphopeptides, the most significant differences between IEP86 from transfected and infected cells were phosphopeptides A/A′ and D/D′. Each of these doublets most likely represents the same phosphopeptide containing different numbers of phosphorylated residues, with the more phosphorylated forms showing decreased migration in both the electrophoresis and chromatography dimensions (5). Infected-cell-derived IEP86 displayed only the more phosphorylated form (A′) of peptide A/A′, whereas transfected-cell-derived IEP86 usually displayed significant amounts of the less phosphorylated form (A) as well. In large part, the patterns of major phosphopeptides from transfected and infected cells are very similar, suggesting that phosphorylation of IEP86 is mediated largely by cellular kinases at this time point of infection (48 h postinfection).
The similarity of the phosphopeptide patterns produced by infected-cell- and transfected-cell-derived IEP86 was confirmed by performing mixing experiments in which tryptic digests of transfected-cell IEP86 were mixed with digests of infected-cell-derived IEP86. All of the major phosphopeptide species derived from each individual source (those lettered in the diagrams in Fig. 3), as well as most of the minor phosphopeptide species, migrated in a completely juxtaposable manner (not shown).
In vitro phosphorylation of IEP86 by serum-starved and serum-stimulated WCE.
To examine which cellular kinases play a role in IEP86 phosphorylation, we used an in vitro assay in which WCE prepared from U-373 MG cells were incubated with bacterially produced IEP86 substrates and [γ-32P]ATP (34, 36). The activities of various kinases in the cell extracts were manipulated by altering the growth conditions before extract preparation. Serum starvation of cells in culture deprives the cells of growth factors and signaling molecules required for continued progression through the cell cycle, causing the cells to arrest in G0 (62, 66). Restimulation with serum leads to transient activation of signaling cascades within the cell. Many of the kinases present in these cascades are dependent on stimulation by c-ras (33, 65). One downstream target of ras is the MAPK family, consisting of serine/threonine protein kinases which translocate into the nucleus upon phosphorylation of conserved TXY motifs (12). To assess the contribution of serum-inducible kinases to the phosphorylation of IEP86, extracts were prepared either from U-373 MG cells which had been serum starved for 48 to 72 hours or from cells which were starved and then stimulated with 10% serum for 15 min prior to harvest. Preliminary experiments (not shown) had indicated that maximal MAPK activation, as judged by the levels of activated ERK1 and ERK2, occurred 15 min after serum stimulation.
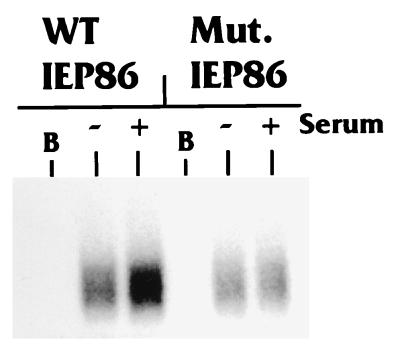
Extracts prepared from starved or stimulated cells, or extract buffer alone, were incubated with [γ-32P]ATP and bacterially produced and purified histidine-tagged IEP86. Figure 4 shows that WT IEP86 incubated in extract buffer alone (lane B) was not phosphorylated, indicating that the bacterially produced IEP86 has no autophosphorylation activity under these conditions. In contrast, WT IEP86 incubated with either the serum-starved or serum-stimulated extracts was significantly phosphorylated, indicating that cellular kinases present in the extracts can phosphorylate IEP86 in vitro. WT IEP86 treated with stimulated extracts exhibited more intense phosphorylation than WT IEP86 treated with starved extracts, suggesting that kinases activated by serum stimulation contribute to the greater phosphorylation of IEP86.
FIG. 4.
Full-length WT IEP86, but not mutant IEP86, is phosphorylated in a serum-inducible manner in vitro. Histidine-tagged full-length IEP86 purified from bacterial extracts was incubated with [γ-32P]ATP plus extract buffer alone (B) or with WCE prepared from U-373 MG cells which had been either serum starved (−) or serum stimulated (+) as described in Materials and Methods. His-tagged IEP86 was repurified and subjected to SDS-PAGE. Relative phosphorylation was detected by autoradiography. Mut. IEP86 represents full-length His-tagged IEP86 containing alanine substitution mutations at Thr27 and Thr233/Ser234.
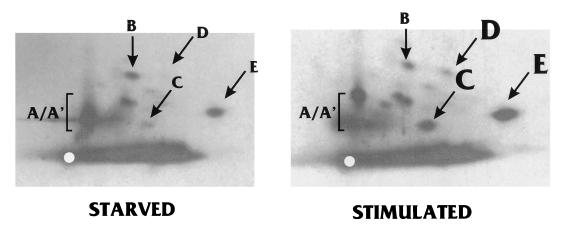
Gel-purified IEP86 was subjected to tryptic phosphopeptide mapping. Autoradiography showed that the phosphopeptide maps of IEP86 phosphorylated by WCE (Fig. 5) were similar to the phosphopeptide maps of IEP86 isolated from transfected and infected U-373 MG cells (Fig. 3). As described earlier, the identities of these peptides were confirmed by experiments where tryptic peptides from each source were mixed and analyzed (not shown). Tryptic phosphopeptides C, D, and E appeared to be more intensely labeled in samples which had been treated with serum-stimulated extracts. Furthermore, tryptic peptides A and A′ have altered mobilities depending on the extract used; this effect is somewhat variable. These data suggest that cellular kinases, particularly serum-inducible kinases, play a significant role in phosphorylation of IEP86.
FIG. 5.
Tryptic phosphopeptide patterns produced by in vitro-phosphorylated IEP86 are similar to those produced by in vivo-phosphorylated IEP86. Histidine-tagged IEP86 phosphorylated by serum-starved WCE (Starved) or serum-stimulated WCE (Stimulated) was eluted from excised gel slices and subjected to tryptic digestion and phosphopeptide mapping. The samples in these autoradiograms were not run as far in the electrophoretic dimension as those shown in Fig. 3. Major phosphopeptides are labeled according to the schematics in Fig. 3. Phosphopeptides which were phosphorylated more strongly by serum-stimulated extracts are labeled with larger letters (C, D, and E).
Mapping the IEP86 domains phosphorylated in vitro by WCE.
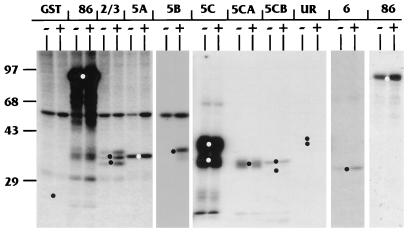
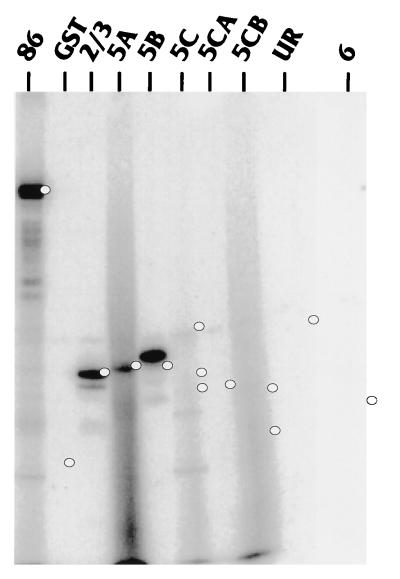
To determine which regions of IEP86 are phosphorylated by cellular kinases, bacterially expressed and purified GST fusions to either full-length IEP86 or various exonic and subexonic fragments (Fig. 6) were incubated with serum-starved or serum-stimulated WCE and [γ-32P]ATP. The repurified fragments were then displayed on SDS-polyacrylamide gels. The results of these experiments are shown in Fig. 7. Lanes are denoted by the specific exonic region in the purified GST fusion used (Fig. 6); lanes marked 86 indicate the GST fusion with full-length wild-type IEP86. The − and + signs indicate serum-starved and serum-stimulated extracts, respectively. The black and white dots indicate the migration of the GST fusion proteins as seen after Coomasie blue or Sypro red staining of the gels. Short exposures of the full-length IEP86 lanes (last set of lanes on the right) agree with results in Fig. 4, showing that serum-stimulated extracts phosphorylate full-length IEP86 more than starved extracts. Individual IEP86 domains phosphorylated more intensely by serum-stimulated extracts included exonic regions 2/3, 5B, and 6; hence, sites within these regions appear to be targeted by serum-inducible kinases.
FIG. 7.
U-373 MG WCE phosphorylate multiple domains of IEP86 in vitro. Purified GST moiety alone or GST fusions to full-length or exonic regions of IEP86 (Fig. 6) were incubated with [γ-32P]ATP and WCE from U-373 MG cells which had been either serum starved (−) or serum stimulated (+). GST fusion substrates were repurified from the reactions, subjected to SDS-PAGE, Coomassie blue stained, and then subjected to autoradiography. The identity of each GST fusion substrate is indicated at the top; 86 represents the fusion with full-length GST-IEP86. The two lanes at the far right marked 86 are from a lighter exposure of the experiment with GST–full-length IEP86. The black and white dots indicate the migration position of each substrate as detected by Coomassie blue staining. Molecular weight markers are indicated on the left in kilodaltons. UR, unique region.
The domain most strongly phosphorylated in vitro was region 5C. Although some WCE preparations suggested that phosphorylation of region 5C may be slightly serum inducible, the majority of extracts indicated that phosphorylation was relatively equal by both starved and stimulated extracts. This phosphorylation appeared to localize to both the amino- and carboxy-terminal portions of region 5C, named 5CA and 5CB, respectively. Region 5A was also phosphorylated relatively equally by both starved and stimulated extracts, while the unique region was very weakly phosphorylated in a serum-independent fashion (visible only in longer exposure than shown in Fig. 7).
We cannot rule out the possibility that other serum-inducible kinases, which had not been induced during the 15-min serum stimulation period, play a role in phosphorylating these domains.
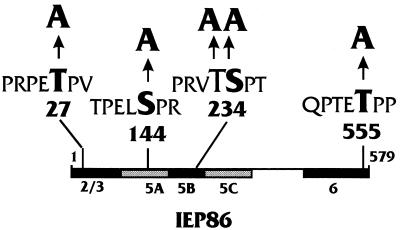
Figure 8 shows a schematic summary of the in vitro WCE phosphorylation results. Amino acid sequence analysis using the ExPASy PROSITE program, as well as a tabulation of kinase site specificity motifs (64), detected the several consensus MAPK phosphorylation motifs shown in the diagram. Each of the regions which showed serum-inducible phosphorylation contains a consensus MAPK motif.
FIG. 8.
Summary of in vitro phosphorylation results, with potential MAPK substrate motifs indicated. Domains of IEP86 which were phosphorylated in vitro under serum-inducible (black) and serum-independent (gray) conditions are indicated. Potential MAPK motifs were determined using the ExPASy PROSITE program as well as a tabulation of kinase site specificity motifs (64). These motifs are shown, with the putative phosphorylated residue(s) highlighted and numbered. As indicated, these motifs were targeted for alanine substitution mutagenesis.
Purified ERK2 phosphorylates IEP86 on several domains in vitro.
To demonstrate that a MAPK could directly phosphorylate IEP86 in vitro, purified recombinant ERK2 and [γ-32P] ATP were incubated with purified bacterially expressed GST fusions to full-length IEP86 or to the various exonic regions. Figure 9 shows that purified ERK2 phosphorylated full-length GST-IEP86 (lane 86). Using the exonic and subexonic IEP86 substrates revealed that the individual domains most efficiently phosphorylated by ERK2 were exonic regions 2/3 (lane 2/3), and 5B (lane 5B). Faint phosphorylation, above a background smear, was reproducibly observed for exonic region 5A (lane 5A). None of the other exonic regions (5C, 5CA, 5CB, unique region, and 6) were significantly phosphorylated by ERK2.
FIG. 9.
Purified ERK2 phosphorylates IEP86 in vitro. Purified GST fusions to full-length or various exonic regions of IEP86 were incubated with [γ-32P]ATP and recombinant rat ERK2 (Calbiochem). Completed reactions were separated by SDS-PAGE, Coomassie blue stained, and subjected to autoradiography. Substrates are labeled at the top as in Fig. 7. The white dots indicate the migration position of each substrate as detected by Coomassie blue staining.
Tryptic phosphopeptide mapping of full-length His-tagged IEP86 phosphorylated by ERK2 in vitro (not shown) indicated that ERK2 phosphorylation gave rise to phosphopeptides which migrate identically to phosphopeptides C and E derived from in vivo-phosphorylated IEP86 (Fig. 3) and from WCE-treated IEP86 (Fig. 5).
Mutation of predicted MAPK sites in IEP86 reduces serum-inducible phosphorylation by WCE and prevents phosphorylation by purified ERK2 in vitro.
Several potential MAPK phosphorylation motifs in IEP86 are shown in Fig. 8; of particular interest are Thr27 (PRPET*P) in exon 2/3, Thr233/Ser234 (PRVTS*P) in exon 5B, and Thr555 (PTET*P) in exon 6, all of which are in domains specifically phosphorylated in vitro in a serum-inducible manner. To determine whether these consensus motifs were utilized, the putative phosphorylated amino acids at positions 27, 233/234, and 555 (marked with asterisks above) were each mutated to alanine (Fig. 8). In addition, an alanine was substituted for Ser144, which lies within a potential MAPK motif that could also be a potent site for PKC and p34cdc2/cyclin B (PELS*PRKK). MAPKs may not be involved in phosphorylation of this motif since levels of in vitro phosphorylation of exonic region 5A, containing Ser144, were similar in serum-starved and stimulated WCE (Fig. 7), and region 5A was not phosphorylated efficiently by purified ERK2 in vitro (Fig. 9).
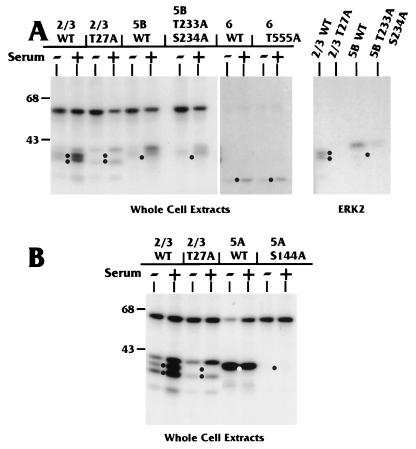
Figure 10 shows the results of in vitro phosphorylation using serum-starved and stimulated extracts with GST fusions to exonic regions containing either WT or mutant MAPK motifs. As previously noted (Fig. 7) and reiterated here (Fig. 10A), wild-type exonic regions 2/3, 5B, and 6 displayed serum-inducible phosphorylation. When the mutant counterparts were similarly tested, we found that phosphorylation of mutant exonic regions 2/3 (T27A) and 5B (T233A/S234A) was dramatically decreased, compared to the WT region, by both the serum-starved and serum-stimulated extracts (Fig. 10A). The mutation in exonic region 6 (T555A) had little effect on in vitro phosphorylation by either extract (Fig. 10A). Thus, the serum-inducible phosphorylation of this region may occur at a site or sites other than Thr555.
FIG. 10.
Point mutations at MAPK motifs reduce phosphorylation by U-373 MG WCE in vitro. (A) Purified GST fusions to WT or mutant isoforms of IEP86 serum-inducible phosphorylation domains were tested in the WCE kinase assay as for Fig. 7. WT versions of exonic regions 2/3 and 5B were phosphorylated more strongly than their mutant counterparts by both the starved (−) and stimulated (+) extracts. On the far right, results of incubating WT and mutant versions of GST-exon 2/3 and GST-exon 5B with purified ERK2 are shown. (B) Phosphorylation of WT and mutant versions of GST-exon 5A, a serum-independent phosphorylation domain, was compared to phosphorylation of WT and mutant versions of GST-exon 2/3. Sizes are indicated in kilodaltons.
The substrate containing the mutation in exonic region 5A (S144A) showed no phosphorylation by either the starved or stimulated extracts (Fig. 10B). Thus, Ser144 may be the only residue in this exonic region which is phosphorylated by WCE under these conditions.
To confirm that Thr27 and Thr233/Ser234 were major serum-inducible phosphorylation sites in the context of full-length IEP86, both mutations were put into full-length His-tagged IEP86. The purified His-tagged mutant proteins were assayed with serum-starved and serum-stimulated extracts. The results (Fig. 4) show a dramatic decrease in serum-inducible phosphorylation compared to the analogous WT protein.
In additional studies, we used purified ERK2 for in vitro phosphorylation analysis of the T27A and T233A/S234A mutations. Whereas ERK2 efficiently phosphorylated GST fusions with WT exonic regions 2/3 and 5B, phosphorylation of the mutant counterparts was significantly reduced or eliminated (Fig. 10A).
In summary, the data suggested that much or all of the serum-inducible phosphorylation of IEP86 localizes to Thr27, Thr233/Ser234, and a site(s) in exon 6. The motifs in which these amino acids lie suggest that the kinases primarily responsible for serum-inducible phosphorylation are MAPK family members.
Mutation of MAPK sites affects in vivo phosphorylation of IEP86.
To determine whether the various alanine substitution mutations affect in vivo phosphorylation, plasmids expressing full-length IEP86 with the alanine mutations were transiently transfected into U-373 MG cells. Sets of cells were either labeled with [32P]orthophosphate or mock labeled for the last 4 to 5 h before extraction. Western analysis of immunoprecipitated IEP86 from the mock-labeled cells indicated that WT and mutant forms of IEP86 were expressed to similar levels (data not shown). IE proteins immunoprecipitated from labeled extracts were gel purified and subjected to tryptic phosphopeptide mapping (Fig. 11).
FIG. 11.
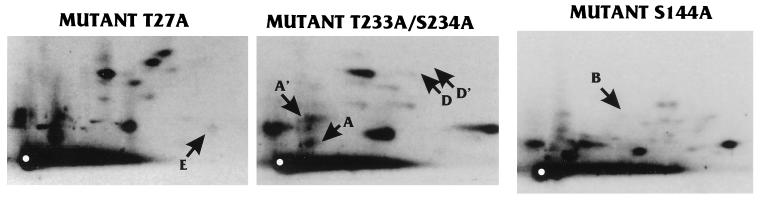
Point mutations at MAPK motifs affect phosphorylation of specific phosphopeptides in vivo. U-373 MG cells were transiently transfected with plasmids expressing cDNAs for IEP86 containing mutations at specific MAPK motifs (Fig. 8). Cells were labeled with [32P]orthophosphate, and IEP86 isoforms were immunoprecipitated as described for Fig. 2. Phosphorylated IEP86 isoforms were eluted from excised gel slices and subjected to tryptic phosphopeptide mapping as for Fig. 3. Shown are typical autoradiograms for IEP86 mutants T27A, S144A, and T233A/S234A. IEP86 mutant T555A was in most respects identical to the WT protein and is not shown; however, some variability in the A/A′ region was occasionally seen, as noted in the text. Specific phosphopeptides affected by the mutations are labeled according to the schematics in Fig. 3.
The mutation of Thr27 resulted in the loss of phosphopeptide E, suggesting that phosphopeptide E represents a predicted tryptic peptide spanning residues 22 to 31 and phosphorylated at Thr27. Likewise, mutation of Ser144 resulted in the loss of phosphopeptide B, suggesting that this spot represented a predicted tryptic peptide spanning residues 117 to 146 and phosphorylated at Ser144. Mutation of Thr233/Ser234 had a more complex effect; phosphopeptides D and D′ were lost, whereas spots A and A′ were reduced in intensity. Interestingly, on one-dimensional SDS-PAGE analysis, the T233A/S234A mutation caused a distinct alteration in mobility compared to the other mutants (not shown). Taken together, these observations suggest that phosphorylation of this motif has a pleiotropic effect, possibly altering the conformation of the protein and affecting phosphorylation at more than one site.
The effects of the mutation at Thr555 were harder to determine. Multiple repetitions indicated that this mutant isoform was, in large part, identical to WT. However, the tryptic phosphopeptide patterns occasionally indicated that peptide A′ was missing (not shown). The location of peptides A and A′, in proximity to the origin, makes them difficult to resolve; hence, the Thr555 mutation requires additional study to definitively map its effect.
Overall, the mutational analyses show that point mutations which affected in vitro phosphorylation, using WCE or in some cases purified ERK2, also affected relevant phosphorylation sites in vivo. Phosphopeptide C, which appears to be strongly affected by serum-inducible kinases (Fig. 5) in vitro and by purified ERK2 (not shown), has yet to be associated with a specific residue in IEP86.
Effects of alanine substitution mutations on transcriptional activation by IEP86.
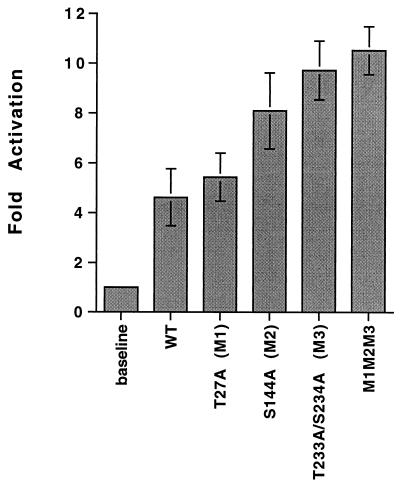
We next examined whether the mutation of specific phosphorylation sites in IEP86 affected its ability to transcriptionally activate a simple promoter in transient transfection analyses. The promoter tested is taken from the SV40 late promoter and has six copies of the OCT/TEF element in which the OCT sites have been mutated, leaving only functional TEF-1 sites (86). These are situated upstream of the β-globin TATA box and the luciferase reporter gene (14, 48). This promoter has previously been shown to be significantly activated by IEP86 (48, 49). Figure 12 shows the results of the transfection analysis. The data show that the mutation of S144 and, especially, T233/S234 caused increased transcriptional activation. Combining the mutations at T27, S144, and T233/S234 resulted in little more activation than with the T233/S234 mutant alone. These results suggest that phosphorylation of WT IEP86 at S144 and, especially, T233/S234 may negatively regulate its ability to transcriptionally activate.
FIG. 12.
Effects of various mutations in phosphorylation sites of IEP86 on transcriptional activation of a simple promoter known to be activated by IEP86 (see text for details). Error bars represent standard error of the mean.
Schematic summary.
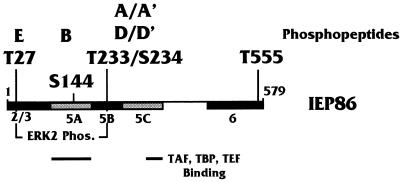
Figure 13 schematically summarizes the data presented in this report. T27 and T233/S234 are the major sites for serum-inducible kinases in vitro and are the major ERK2 sites. The data are correlated with earlier results of protein-protein binding studies between the IE proteins and cellular transcription factors (50). The major protein-protein interaction domains colocalize with domains phosphorylated in a serum-independent manner.
FIG. 13.
Summary of IEP86 phosphorylation data. The schematic of IEP86 indicates the domains phosphorylated (Phos.) in vitro by serum-inducible kinases (black) and under serum-independent conditions (gray). Also shown are the amino acids in MAPK sites which were mutated to alanines. Indicated above each amino acid are the in vivo-labeled tryptic phosphopeptides affected by the alanine mutagenesis. Also indicated below are the regions of IEP86 which have previously been associated with interactions with transcription factors (TAFs, TBP, and TEF-1 [50]).
DISCUSSION
The HCMV IE proteins are the key regulators of the viral life cycle. IE gene expression is required for expression of early and late viral genes and for increased expression of many cellular genes which encode factors necessary for viral replication (35). IEP86 is a major transactivator among the IE gene products. Our lab and others have previously shown that promiscuous transcriptional activation mediated by IEP86 occurs through protein-protein interactions with both upstream-bound transcription factors as well as the basal transcription complex where IEP86 and IEP72 perform TAF-like functions (9, 11, 20, 30, 42, 45, 47, 49, 74, 77). However, this does not appear to be the only mechanism by which IEP86 affects gene expression. IEP86 interacts with a number of cellular regulatory proteins, such as p53 and Rb (28, 41, 58, 78), and thereby affects gene expression by abrogating the normal functions of these proteins. Hence, IEP86, like SV40 T antigen, interacts with multiple cellular proteins to alter cell and viral gene expression via several distinct mechanisms.
Both IEP72 and IEP86 are phosphoproteins; thus, it is highly likely that posttranslational modification by phosphorylation can modulate or direct the various functions of the IE proteins. It is well documented that phosphorylation affects the ability of transcription factors to interact with other proteins, either by conformational shifts, by changes in local charge, or by altered intracellular localization (reviewed in reference 39). In many cases, the regulation of phosphorylation of transcription factors is the result of extracellular signaling activating intracellular kinase cascades, resulting in altered gene expression (39).
In the case of HSV-1, phosphorylation of the IE viral transactivator ICP4 by PKA has been suggested to play a role in ICP4’s ability to regulate the balance between latent infection and reactivation in neurons (92). Activation of cellular signal transduction pathways, including cyclic AMP-dependent kinase cascades, may induce reactivation in latently infected neurons in culture (76). Infection with HSV-1 engineered to contain an ICP4 isoform missing its major phosphorylated region resulted in a virus with reduced ability to replicate in trigeminal ganglia neurons (92). Correspondingly, WT HSV-1 infection of a cell line deficient in PKA resulted in a much decreased viral replication (92).
We have begun to determine the role that phosphorylation of the HCMV IE proteins may play in the HCMV life cycle by investigating the phosphorylation pattern of IEP86. Using permissive U-373 MG cells, either infected with HCMV or transiently transfected to produce individual IE proteins, we show that IEP86 is phosphorylated on multiple serine and threonine residues (no tyrosines appear to be phosphorylated). The tryptic phosphopeptide pattern of IEP86 isolated from cDNA-transfected cells (48 h posttransfection) is remarkably similar to that of IEP86 isolated from infected cells 48 h postinfection. This finding suggests that cellular kinases may be primarily responsible for phosphorylation of IEP86 at this time point in infection.
Although a number of viral kinase and phosphatase activities have been associated with HCMV (7, 54, 60, 71), it is not surprising that cellular kinases may play a major role in phosphorylating HCMV-encoded proteins. In fact, it is possible that a potentially complex interplay may exist between cellular kinase and phosphatase pathways, virally induced signals, and virally encoded kinases and phosphatases. A number of studies have suggested that such an interplay exists. For example, exposure of cells to HCMV virions is known to induce activation of cellular signal transduction pathways, leading to activation of cellular immediate-early response genes such as c-fos, c-myc, c-jun, and NF-κB (3, 4, 96). Changes in cellular kinase or phosphatase activities upon exposure to HCMV may be involved in viral entry. Keay and Baldwin (43) showed that a 92-kDa HEL cell surface glycoprotein necessary for viral fusion becomes hyperphosphorylated upon exposure to HCMV and mediates increased phosphorylation of cellular proteins through PKC- and tyrosine kinase-mediated pathways. Furthermore, several studies have shown that manipulation of cellular signal transduction pathways near the time of exposure to HCMV can have significant effects on the ability of cells to support HCMV replication (24, 88, 97), as well as the ability of the IE proteins to function as transcriptional activators (63). Use of specific kinase inhibitors has been shown to prevent the effects of these pathways on HCMV.
Several studies have suggested that the IE proteins themselves can alter intracellular signaling pathways in order to activate or repress certain promoters. IEP86 has been shown to interact with the tumor suppressors Rb and p53, causing effects on E2F- and p53-responsive elements, respectively (28, 41, 58, 78). Furthermore, it has been reported that IEP72 interacts with and phosphorylates several members of the E2F and pocket protein families (though not Rb), helping to activate promoters containing E2F elements (52, 60, 69). In addition, previous work from this laboratory has suggested that some of the IE proteins’ transcriptional activation mechanisms reside in an ability to interact with the basal transcription complex, specifically TFIID, and perform a TAF-like function (50). Clearly, the state of phosphorylation of IEP86 or IEP72 could control the efficacy of this interaction. In fact, data presented in this communication suggests that hypophosphorylation may enhance transcriptional activation of simple promoters by IEP86.
We have examined the role of various cellular kinases in phosphorylating the IE proteins by using an in vitro system in which bacterial IE protein substrates were treated with WCE prepared from either serum-starved or serum-stimulated U-373 MG cells. These studies established that while serum-independent kinases mediated significant phosphorylation, serum-inducible kinases contributed to hyperphosphorylation of IEP86. As an initial step in determining the major phosphorylation sites among the more than 100 serine and threonine residues within IEP86, we incubated a variety of exonic and subexonic fragments of IEP86 with the extracts to define major domains of phosphorylation. The region most strongly phosphorylated was exonic region 5C (amino acids 252 to 367), the major protein-protein interaction domain of IEP86. This region has been shown to be involved in interactions with Rb (19, 77) and transcription factors such as TBP, human TAFII130, TFIIB, and TEF-1 (9, 49, 50, 77). Exonic region 5C as well as exonic region 5A (amino acids 85 to 180), another domain involved in protein-protein interactions (50, 77), were both phosphorylated to similar extents by the serum-starved and the serum-stimulated extracts, suggesting that the phosphorylation sites in these regions may be targeted by kinases which are not affected by serum stimulation.
The in vitro analyses indicated that serum-inducible kinases contribute to hyperphosphorylation of three domains of IEP86: the amino-terminal 85 amino acids common to both IEP86 and IEP72 (exonic region 2/3), an 83-residue domain between positions 175 and 257 (exonic region 5B), and the carboxy-terminal 71 amino acids of IEP86 (exonic region 6). Exonic regions 2/3 and 6 contain acidic domains which function as transcriptional activators when fused to the DNA-binding domain of GAL4 (67, 94). Regulated phosphorylation of transcription factor activation domains has been shown in many cases to affect their ability to activate transcription (39). In addition, residues within the exon 2/3 region have been implicated in functional interactions with Rb (19). Exonic region 5B contains both serine-rich and glutamate-rich motifs. Phosphorylation could add to the negatively charged nature of this region, potentially affecting intramolecular conformation or interactions with other proteins or nucleic acids.
Computer analysis revealed that each of these domains (exonic regions 2/3, 5B, and 6) contains consensus MAPK motifs. MAPK family members such as ERK, JNK-1, and p38 are serine/threonine protein kinases which are induced by serum stimulation of cells (12, 15). Experiments with purified ERK2 showed that it could phosphorylate IEP86 in vitro. This was most efficient on two of the serum-inducible phosphorylation domains, exon 2/3 and exon 5B. We have preliminary evidence that purified JNK-1 phosphorylates exon 6 efficiently in vitro, as well as exonic regions 2/3 and 5B (not shown).
The predicted phosphorylated residues within four consensus MAPK motifs of IEP86 were targeted for alanine substitution mutagenesis: (i) Thr27, found within the acidic activation domain of exonic region 2/3 (67, 94); (ii) Ser144, located directly amino terminal to a putative nuclear localization sequence (67) found in the exonic region 5A (Ser144 also lies within overlapping consensus motifs for both PKC and p34cdc2-cyclin B kinase; the number of different kinases which potentially phosphorylate Ser144 may indicate why in vitro phosphorylation of exonic region 5A appears to be serum independent); (iii) Thr233/Ser234, adjacent potential sites found within exonic region 5B; and (iv) Thr555, within the acidic activation domain of exonic region 6 (67, 94).
Results of in vitro phosphorylation using WCE showed greatly reduced serum-inducible phosphorylation of substrates containing alanine substitution mutations at Thr27 and Thr233/Ser234. This finding indicates that the WT forms of these residues serve as sites for serum-inducible kinases. The level of phosphorylation mediated by serum-starved extracts on mutant substrates was also reduced by these mutations. The simplest explanation for this result is that some residual activity of serum-inducible kinases remains in the serum-starved extracts. In support of this hypothesis, Western analysis for activated forms of ERK1 and ERK2 showed a small amount of activated ERK2 present in the serum-starved extracts (not shown). The evidence that these sites were authentic MAPK sites was supported by the very dramatic reduction of phosphorylation of the alanine-mutant substrates by purified ERK2.
The mutant exonic region 5A, containing the Ala substitution of Ser144, displayed no in vitro phosphorylation by either serum-starved or serum-stimulated WCE. This finding suggests that the phosphorylation motif containing Ser144 is the only phosphorylation site in exonic region 5A recognized by kinases in either serum-starved or serum-stimulated conditions. Preliminary data suggest that purified p34cdc2-cyclin B kinase can phosphorylate exonic region 5A on Ser144 in vitro. Finally, mutation of Thr555 in exonic region 6 had little effect on the serum-inducible in vitro phosphorylation of exonic region 6, which suggests that a yet to be defined motif in exonic region 6 is the target for serum-inducible kinases. However, preliminary experiments indicate that the Thr555 mutation does affect the ability of purified JNK-1 to phosphorylate exonic region 6.
The in vivo relevance of these putative phosphorylation sites was demonstrated by two-dimensional tryptic phosphopeptide analysis of WT and mutant forms of IEP86 produced from transfected plasmids. With the exception of Thr555, each mutation resulted in the clear loss or alteration of specific phosphopeptides. Interestingly, mutation of Thr233/Ser234 affected phosphorylation of multiple peptides, suggesting that the phosphorylation state of Thr233/Ser234 may alter the conformation of the protein, resulting in effects on phosphorylation at other sites. Moreover, this mutation is the only individual mutation which caused a mobility shift in IEP86 on SDS-PAGE (not shown). Phosphorylation of specific residues in other viral transactivators such as ICP4 and SV40 large T antigen has been shown to influence the phosphorylation of residues located hundreds of amino acids away (10, 73, 93).
The functional effects of the alanine substitution mutations were tested with respect to transcriptional activation of a simple promoter known to be activated by IEP86 (48, 49). Somewhat surprisingly, transient transfection analyses showed that IEP86 isoforms with these mutations mediated increased activation of the test promoter relative to the WT protein. Mutation of the MAPK motifs at S144 and T233/S234 had the greatest effects of single mutations in terms of increased transcriptional activation. Combining the mutations resulted in no greater activity than the mutation at T233/S234 alone. These results suggest that within U-373 MG cells, phosphorylation of WT IEP86 at S144 and, especially, T233/S234 inhibits transcriptional activation. We reiterate the preceding speculation that phosphorylation at T233/S234 may alter the conformation IEP86. We also note that IEP86 is attributed with a number of other functions which are potentially affected by phosphorylation and remain to be tested.
ACKNOWLEDGMENTS
We thank the following individuals: Blossom Damania for all of her advice and support, as well as critical review of the manuscript; Tobi Maguire for excellent assistance with virus and cell culture; Robert Netter for important contributions toward experiments for this and future reports; Teresa Compton for HCMV Towne strain stocks and helpful advice; Steven R. Sloan for training and advice in tryptic phosphopeptide mapping techniques; Richard M. Stenberg for originally providing our laboratory with IE protein plasmid constructs; and the members of the Alwine laboratory for support and critical evaluation of the data.
This work was supported by Public Health Service grant CA28379 awarded to J.C.A. by the National Cancer Institute. N.Y.H. was supported by the Medical Scientist Training Program of the University of Pennsylvania School of Medicine. Cheers to all.
REFERENCES
- 1.Akrigg A, Wilkinson G W G, Oram J D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985;2:107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- 2.Baracchini E, Glezer E, Fish K, Stenberg R M, Nelson J A, Ghazal P. An isoform variant of the cytomegalovirus immediate early auto repressor functions as a transcriptional activator. Virology. 1992;188:518–529. doi: 10.1016/0042-6822(92)90506-k. [DOI] [PubMed] [Google Scholar]
- 3.Boldogh I, AbuBakar S, Albrecht T. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science. 1990;247:561–564. doi: 10.1126/science.1689075. [DOI] [PubMed] [Google Scholar]
- 4.Boldogh I, AbuBakar S, Deng C Z, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991;65:1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 6.Boyle W J, Smeal T, Defize L H, Angel P, Woodgett J R, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 7.Britt W J, Auger D. Human cytomegalovirus virion-associated protein with kinase activity. J Virol. 1986;59:185–188. doi: 10.1128/jvi.59.1.185-188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caswell R C, Bryant L, Sinclair J. Human cytomegalovirus immediate-early 2 (IE2) protein can transactivate the human hsp70 promoter by alleviation of Dr1-mediated repression. J Virol. 1996;70:4028–4037. doi: 10.1128/jvi.70.6.4028-4037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caswell R, Hagemeier C, Chiou C-J, Hayward G, Kouzarides T, Sinclair J. The human cytomegalovirus 86K immediate-early (IE) 2 protein requires the basic region of the TATA-box binding protein (TBP) for binding, and interacts with TBP and transcription factor TFIIB via regions of IE2 required for transcriptional regulation. J Gen Virol. 1993;74:2691–2698. doi: 10.1099/0022-1317-74-12-2691. [DOI] [PubMed] [Google Scholar]
- 10.Cegielska A, Moarefi I, Fanning E, Virshup D M. T-antigen kinase inhibits simian virus 40 DNA replication by phosphorylation of intact T antigen on serines 120 and 123. J Virol. 1994;68:269–275. doi: 10.1128/jvi.68.1.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiou C-J, Zong J, Waheed I, Hayward G S. Identification and mapping of dimerization and DNA-binding domains in the C terminus of the IE2 regulatory protein of human cytomegalovirus. J Virol. 1993;67:6201–6214. doi: 10.1128/jvi.67.10.6201-6214.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobb M H, Hepler J E, Cheng M, Robbins D. The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer Biol. 1994;5:261–268. [PubMed] [Google Scholar]
- 13.Damania B, Alwine J C. TAF-like function of SV40 large T antigen. Genes Dev. 1996;10:1369–1381. doi: 10.1101/gad.10.11.1369. [DOI] [PubMed] [Google Scholar]
- 14.Damania, B., and J. C. Alwine. Unpublished results.
- 15.Davis R J. Transcriptional regulation by MAP kinases. Mol Reprod Dev. 1995;42:459–467. doi: 10.1002/mrd.1080420414. [DOI] [PubMed] [Google Scholar]
- 16.DeLuca N A, Schaffer P A. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988;62:732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 18.Dixon R A F, Schaffer P A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein VP175. J Virol. 1980;36:189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortunato E A, Sommer M H, Yoder K, Spector D H. Identification of domains within the human cytomegalovirus major immediate-early 86-kilodalton protein and the retinoblastoma protein required for physical and functional interaction with each other. J Virol. 1997;71:8176–8185. doi: 10.1128/jvi.71.11.8176-8185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furnari B A, Poma E, Kowalik T F, Huong S-M, Huang E-S. Human cytomegalovirus immediate-early gene 2 protein interacts with itself and with several novel cellular proteins. J Virol. 1993;67:4981–4991. doi: 10.1128/jvi.67.8.4981-4991.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghazal P, Young J, Giulietti E, DeMattei C, Garcia J, Gaynor R, Stenberg R M, Nelson J A. A discrete cis element in the human immunodeficiency virus long terminal repeat mediates synergistic trans-activation by cytomegalovirus immediate-early proteins. J Virol. 1991;65:6735–6742. doi: 10.1128/jvi.65.12.6735-6742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson W. Immediate-early proteins of human cytomegalovirus strains AD 169, Davis, and Towne differ in electrophoretic mobility. Virology. 1981;112:350–354. doi: 10.1016/0042-6822(81)90641-3. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert M J, Riddell S R, Plachter B, Greenberg P D. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 24.Gonczol E, Andrews P W, Plotkin S A. Cytomegalovirus replicates in differentiated but not in undifferentiated human embryonal carcinoma cells. Science. 1984;224:159–161. doi: 10.1126/science.6322309. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 26.Gruda M C, Zabolotny J M, Xiao J H, Davidson I, Alwine J C. Transcriptional activation by simian virus 40 large T antigen: interactions with multiple components of the transcription complex. Mol Cell Biol. 1993;13:961–969. doi: 10.1128/mcb.13.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Davis R J. MAP kinase binds to the NH2-terminal activation domain of c-Myc. FEBS Lett. 1994;353:281–285. doi: 10.1016/0014-5793(94)01052-8. [DOI] [PubMed] [Google Scholar]
- 28.Hagemeier C, Caswell R, Hayhurst G, Sinclair J, Kouzarides T. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 1994;13:2897–2903. doi: 10.1002/j.1460-2075.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagemeier C, Walker S M, Sissons P J G, Sinclair J H. The 72K IE1 and 80K IE2 proteins of human cytomegalovirus independently trans-activate the c-fos, c-myc and hsp70 promoters via basal promoter elements. J Gen Virol. 1992;73:2385–2393. doi: 10.1099/0022-1317-73-9-2385. [DOI] [PubMed] [Google Scholar]
- 30.Hagemeier C, Walker S, Caswell R, Kouzarides T, Sinclair J. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J Virol. 1992;66:4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayhurst G P, Bryant L A, Caswell R C, Walker S M, Sinclair J H. CCAAT box-dependent activation of the TATA-less human DNA polymerase α promoter by the human cytomegalovirus 72-kilodalton major immediate-early protein. J Virol. 1995;69:182–188. doi: 10.1128/jvi.69.1.182-188.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermiston T W, Malone C L, Witte P R, Stinski M F. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J Virol. 1987;61:3214–3221. doi: 10.1128/jvi.61.10.3214-3221.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann C, Nassar N. Ras and its effectors. Prog Biophys Mol Biol. 1996;66:1–41. doi: 10.1016/s0079-6107(96)00015-6. [DOI] [PubMed] [Google Scholar]
- 34.Hipskind R A, Baccarini M, Nordheim A. Transient activation of RAF-1, MEK, and ERK2 coincides kinetically with ternary complex factor phosphorylation and immediate-early gene promoter activity in vivo. Mol Cell Biol. 1994;14:6219–6231. doi: 10.1128/mcb.14.9.6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirai K, Watanabe Y. Induction of alpha type DNA polymerases in human cytomegalovirus-infected WI-38 cells. Biochim Biophys Acta. 1976;447:328–339. doi: 10.1016/0005-2787(76)90056-3. [DOI] [PubMed] [Google Scholar]
- 36.Holt K H, Kasson B G, Pessin J E. Insulin stimulation of a MEK-dependent but ERK-independent SOS protein kinase. Mol Cell Biol. 1996;16:577–583. doi: 10.1128/mcb.16.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horton R M, Cai Z, Ho S N, Pease L R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 38.Huang L, Malone C L, Stinski M F. A human cytomegalovirus early promoter with upstream negative and positive-acting elements: IE2 negates the effect of the negative element, and MF-Y binds to the positive element. J Virol. 1994;68:2108–2117. doi: 10.1128/jvi.68.4.2108-2117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–87. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 40.Jahn G, Knust E, Schmolla H, Sarre T, Nelson J A, McDougall J K, Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD169. J Virol. 1984;49:363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jault F M, Jault J-M, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. Cytomegalovirus infection induces high levels of cyclins, phosphorylated RB, and p53, leading to cell cycle arrest. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jupp R, Hoffman S, Stenberg R M, Nelson J A, Ghazal P. Human cytomegalovirus IE86 protein interacts with promoter-bound TATA-binding protein via a specific region distinct from the autorepression domain. J Virol. 1993;67:7539–7546. doi: 10.1128/jvi.67.12.7539-7546.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keay S, Baldwin B R. Evidence for the role of cell protein phosphorylation in human cytomegalovirus/host cell fusion. J Gen Virol. 1996;77:2597–2604. doi: 10.1099/0022-1317-77-10-2597. [DOI] [PubMed] [Google Scholar]
- 44.Kerry J A, Priddy M A, Stenberg R M. Identification of sequence elements in the human cytomegalovirus DNA polymerase gene promoter required for activation by viral gene products. J Virol. 1994;68:4167–4176. doi: 10.1128/jvi.68.7.4167-4176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klucher K M, Sommer M, Kadonaga J T, Spector D H. In vivo and in vitro analysis of transcriptional activation mediated by the human cytomegalovirus major immediate-early proteins. Mol Cell Biol. 1993;13:1238–1250. doi: 10.1128/mcb.13.2.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 47.Lang D, Gebert S, Arlt H, Stamminger T. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J Virol. 1995;69:6030–6037. doi: 10.1128/jvi.69.10.6030-6037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukac, D. M., and J. C. Alwine. Unpublished results.
- 49.Lukac D M, Manuppello J R, Alwine J C. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J Virol. 1994;68:5184–5193. doi: 10.1128/jvi.68.8.5184-5193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukac D M, Harel N Y, Tanese N, Alwine J C. TAF-like functions of human cytomegalovirus immediate-early proteins. J Virol. 1997;71:7227–7239. doi: 10.1128/jvi.71.10.7227-7239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malone C L, Vesole D H, Stinski M F. Transactivation of a human cytomegalovirus early promoter by gene products from the immediate-early gene IE2 and augmentation by IE1: mutational analysis of the viral proteins. J Virol. 1990;64:1498–1506. doi: 10.1128/jvi.64.4.1498-1506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margolis M J, Pajovic S, Wong E L, Wade M, Jupp R, Nelson J A, Azizkhan J C. Interaction of the 72-kilodalton human cytomegalovirus IE1 gene product with E2F1 coincides with E2F-dependent activation of dihydrofolate reductase transcription. J Virol. 1995;69:7759–7767. doi: 10.1128/jvi.69.12.7759-7767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michael N, Spector D, Mavromara-Nazos P, Kristie T M, Roizman B. The DNA-binding properties of the major regulatory protein α4 of herpes simplex viruses. Science. 1988;239:1531–1534. doi: 10.1126/science.2832940. [DOI] [PubMed] [Google Scholar]
- 54.Michelson S, Tardy-Panit M, Barzu O. Catalytic properties of a human cytomegalovirus-induced protein kinase. Eur J Biochem. 1985;149:393–399. doi: 10.1111/j.1432-1033.1985.tb08938.x. [DOI] [PubMed] [Google Scholar]
- 55.Mocarski E S. Evidence for posttranscriptional modulation of human cytomegalovirus gene expression. In: Wagner E K, et al., editors. Herpesvirus transcription and its control. Boca Raton, Fla: CRC Press; 1991. pp. 287–300. [Google Scholar]
- 56.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 57.Monick M M, Geist L J, Stinski M F, Hunninghake G W. The immediate early genes of human cytomegalovirus upregulate expression of the cellular genes myc and fos. Am J Respir Cell Mol Biol. 1992;7:251–256. doi: 10.1165/ajrcmb/7.3.251. [DOI] [PubMed] [Google Scholar]
- 58.Muganda P, Mendoza O, Hernandez J, Qian Q. Human cytomegalovirus elevates levels of the cellular protein p53 in infected fibroblasts. J Virol. 1994;68:8028–8034. doi: 10.1128/jvi.68.12.8028-8034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Netter, R. C., N. Y. Harel, and J. C. Alwine. Unpublished results.
- 60.Pajovic S, Wong E L, Black A R, Azizkhan J C. Identification of a viral kinase that phosphorylates specific E2Fs and pocket proteins. Mol Cell Biol. 1997;17:6459–6464. doi: 10.1128/mcb.17.11.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papavassiliou A G, Wilcox K W, Silverstein S J. The interaction of ICP4 with cell/infected-cell factors and its state of phosphorylation modulate differential recognition of leader sequences in herpes simplex virus DNA. EMBO J. 1991;10:397–406. doi: 10.1002/j.1460-2075.1991.tb07961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 63.Paya C V, Virelizier J-L, Michelson S. Modulation of T-cell activation through protein kinase C- or A-dependent signalling pathways synergistically increases human immunodeficiency virus long terminal repeat induction by cytomegalovirus immediate-early proteins. J Virol. 1991;65:5477–5484. doi: 10.1128/jvi.65.10.5477-5484.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson R B, Kemp B E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 65.Peeper D S, Bernards R. Communication between the extracellular environment, cytoplasmic signalling cascades and the nuclear cell-cycle machinery. FEBS Lett. 1997;410:111–116. doi: 10.1016/s0014-5793(97)00319-0. [DOI] [PubMed] [Google Scholar]
- 66.Peeper D S, van der Eb A J, Zantema A. The G1/S cell-cycle checkpoint in eukaryotic cells. Biochim Biophys Acta. 1994;1198:215–230. doi: 10.1016/0304-419x(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 67.Pizzorno M C, Mullen M-A, Chang Y-N, Hayward G S. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J Virol. 1991;65:3839–3852. doi: 10.1128/jvi.65.7.3839-3852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pizzorno M C, O’Hare P, Sha L, LaFemina R L, Hayward G S. Transactivation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988;62:1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poma E E, Kowalik T F, Zhu L, Sinclair J H, Huang E-S. The human cytomegalovirus IE1-72 protein interacts with the cellular p107 protein and relieves p107-mediated transcriptional repression of an E2F-responsive promoter. J Virol. 1996;70:7867–7877. doi: 10.1128/jvi.70.11.7867-7877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 71.Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986;59:714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samaniego L A, Tevethia M J, Spector D J. The human cytomegalovirus 86-kilodalton immediate-early 2 protein: synthesis as a precursor polypeptide and interaction with a 75-kilodalton protein of probable viral origin. J Virol. 1994;68:720–729. doi: 10.1128/jvi.68.2.720-729.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheidtmann K H, Buck M, Schneider J, Kalderon D, Fanning E, Smith A E. Biochemical characterization of phosphorylation site mutants of simian virus 40 large T antigen: evidence for interaction between amino- and carboxy-terminal domains. J Virol. 1991;65:1479–1490. doi: 10.1128/jvi.65.3.1479-1490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scully A L, Sommer M H, Schwartz R, Spector D H. The human cytomegalovirus IE2 86-kilodalton protein interacts with an early gene promoter via site-specific DNA binding and protein-protein interactions. J Virol. 1995;69:6533–6540. doi: 10.1128/jvi.69.10.6533-6540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sefton B M. Labeling cultured cells with 32Pi and preparing cell lysates for immunoprecipitation. In: Coligan J E, et al., editors. Current protocols in protein science, Suppl. 9. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 13.2.1–13.2.7. [Google Scholar]
- 76.Smith R L, Pizer L I, Johnson E M, Jr, Wilcox C L. Activation of second messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 77.Sommer M H, Scully A L, Spector D H. Transactivation by the human cytomegalovirus IE2 86-kilodalton protein requires a domain that binds to both the TATA box-binding protein and the retinoblastoma protein. J Virol. 1994;68:6223–6231. doi: 10.1128/jvi.68.10.6223-6231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Speir E, Modali R, Huang E-S, Leon M B, Sahwl F, Finkel T, Epstein S E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- 79.Staprans S I, Rabert D K, Spector D H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988;62:3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stasiak P C, Mocarski E S. Transactivation of the cytomegalovirus ICP36 gene promoter requires the beta gene product TRS1 in addition to IE1 and IE2. J Virol. 1992;66:1050–1058. doi: 10.1128/jvi.66.2.1050-1058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stenberg R M, Depto A S, Fortney J, Nelson J A. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989;63:2699–2708. doi: 10.1128/jvi.63.6.2699-2708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stenberg R M, Thomsen D R, Stinski M F. Structural analysis of the major immediate-early gene of human cytomegalovirus. J Virol. 1984;49:190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stenberg R M, Fortney J, Barlow S W, Magrane B P, Nelson J A, Ghazal P. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J Virol. 1990;64:1556–1565. doi: 10.1128/jvi.64.4.1556-1565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stenberg R M, Witte P R, Stinski M F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985;56:665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stinski M F, Thomsen D R, Stenberg R M, Goldstein L C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983;46:1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka M, Grossaiklaus V, Herr W, Hernandez N. Activation of the U2 SnRNA promoter by the octamer motif defines a new class of RNA poly II enhancer element. Genes Dev. 1988;2:1764–1778. doi: 10.1101/gad.2.12b.1764. [DOI] [PubMed] [Google Scholar]
- 87.Wathen M W, Thomsen D R, Stinski M F. Temporal regulation of human cytomegalovirus transcription at immediate-early and early times after infection. J Virol. 1981;38:446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weinshenker B G, Wilton S, Rice G P. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988;140:1625–1631. [PubMed] [Google Scholar]
- 89.Whitmarsh A J, Yang S H, Su M S, Sharrocks A D, Davis R J. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilcox K W, Kohn A, Sklyanskaya E, Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity of DNA. J Virol. 1980;33:167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, Harrison J K, Dent P, Lynch K R, Weber M J, Sturgill T W. Identification and characterization of a new mammalian mitogen-activated protein kinase kinase, MKK2. Mol Cell Biol. 1993;13:4539–4548. doi: 10.1128/mcb.13.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xia K, Knipe D M, DeLuca N A. Role of protein kinase A and the serine-rich region of herpes simplex virus type 1 ICP4 in viral replication. J Virol. 1996;70:1050–1060. doi: 10.1128/jvi.70.2.1050-1060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia K, DeLuca N A, Knipe D M. Analysis of phosphorylation sites of herpes simplex virus type 1 ICP4. J Virol. 1996;70:1061–1071. doi: 10.1128/jvi.70.2.1061-1071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yeung K C, Stoltzfus C M, Stinski M F. Mutations of the human cytomegalovirus immediate-early 2 protein define regions and amino acid motifs important in transactivation of transcription from the HIV-1 LTR promoter. Virology. 1993;195:786–792. doi: 10.1006/viro.1993.1431. [DOI] [PubMed] [Google Scholar]
- 95.Yoo Y D, Chiou C-J, Choi K S, Yi Y, Michelson S, Kim S, Hayward G S, Kim S-J. The IE2 regulatory protein of human cytomegalovirus induces expression of the human transforming growth factor-β1 gene through an Egr-1 binding site. J Virol. 1996;70:7062–7070. doi: 10.1128/jvi.70.10.7062-7070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yurochko A D, Kowalik T F, Huong S-M, Huang E-S. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zerbini M, Musiani M, La Placa M. Stimulating effect of heat shock on the early stage of human cytomegalovirus replication cycle. Virus Res. 1986;6:211–216. doi: 10.1016/0168-1702(86)90070-5. [DOI] [PubMed] [Google Scholar]