Abstract
Background
In South Africa, infants who are HIV-exposed are tested for HIV at birth and 10 weeks of age. The COVID-19 pandemic lockdown restrictions resulted in reduced access to healthcare services and uncertain impact on early infant HIV testing.
Objectives
To describe the effects of the COVID-19 pandemic lockdown restrictions on early infant HIV testing and diagnosis in Cape Town, South Africa.
Method
This retrospective cohort study compares HIV-exposed infants born during the first COVID-19 pandemic lockdown (2020) to those born in the same period the year before (2019). Laboratory and other data were abstracted from the Provincial Health Data Centre.
Results
A total of 2888 infants were included: 1474 born in 2020 and 1413 in 2019. Compared to 2019, there was an increase in the 10-week HIV polymerase chain reaction (PCR) uptake in 2020 (71% vs. 60%, P < 0.001). There was also an increase in the proportion of infants who demised without 10-week testing or were lost to follow-up in 2020 compared to 2019 (8% vs. 5%, P = 0.017). Differences detected in birth HIV PCR positivity rates between the two groups (1.1% vs. 0.5%, P = 0.17) did not reach statistical significance; however, a significant increase in vertical transmission of HIV by 10 weeks old was found in the 2020 cohort (1.2% vs. 0.5%. P = 0.046).
Conclusion
Vertical transmission of HIV at 10 weeks increased in the Cape Town Metropolitan during the initial COVID-19 lockdown. There was also an increase in the proportion of deaths without testing by 10 weeks in the 2020 group.
Keywords: vertical transmission prevention, VTP, HIV, COVID-19, early infant diagnosis, vertical transmission, prevention of mother-to-child transmission, PMTCT
What this study adds: Preserved rates of infant HIV testing highlighted the resilience of the South African Vertical Transmission Prevention programme. However, increases in vertical transmission and in the proportion of demise and loss to follow-up in 2020 suggests that the pandemic and lockdown restrictions did have a significant impact on access to healthcare.
Introduction
Untreated HIV infection in infancy results in high mortality and life-long morbidity.1,2,3,4,5 Even short delays in treatment correlate with significant disease progression and poorer immune and clinical recovery when treatment is commenced.4,5,6 Early and repeated testing of HIV-exposed infants is crucial – diagnosis is key to improving access to timely treatment.1,7,8,9,10 The South African Vertical Transmission Prevention (VTP) programme now includes universal HIV polymerase chain reaction (HIV PCR) testing for all HIV-exposed infants at birth and 10 weeks.11,12 This emphasis on infant testing is coupled with rapid treatment initiation targets: 7 days from diagnosis to treatment start, with same-day initiation encouraged in infants who are otherwise well.11,12,13 The VTP programme has become an essential and effective intervention in reducing childhood HIV infections and HIV-associated mortality.14,15,16,17,18
Although universal birth HIV PCR testing has improved infant diagnosis,8,9,19 concerns remain about high attrition rates from the VTP programme with many infants being lost to follow-up or not returning for test results.8,20,21,22,23 Evidence from countries across Africa, including South Africa, identify numerous barriers to care ranging from healthcare infrastructure and system challenges to socio-economic factors and cultural norms.20,21,22,24
On 11 March 2020, the novel coronavirus (SARS-CoV-2) outbreak was declared a global pandemic by the World Health Organization.25 By 23 March 2020, South Africa had 402 confirmed cases and the government announced a strict national lockdown which was enforced 4 days later.26 The lockdown measures included a complete ban of any activity not deemed essential.26,27,28,29,30,31 Despite not being formally suspended, the lockdown led to markedly reduced use of available healthcare services in South Africa.26 Economic hardship, restrictions on the freedom of movement and public transport, as well as psychosocial impacts such as fear of infection and strong stay-at-home messaging, are thought to have contributed to decreased uptake of available healthcare services and exacerbation of pre-existing barriers to care.26,32,33,34,35
Data show that globally, and in South Africa, immunisation rates dropped, the number of child health visits decreased and there were fewer perinatal care enrolments.29,30,36,37,38 Although there was initial evidence of decreased HIV testing nationally and concerns regarding an increase in the absolute number of infants testing HIV-positive at birth,30,33,34,39 these findings were not confirmed in subsequent local studies.37,40,41
To our knowledge there are no studies that looked at the impact of the COVID-19 pandemic on infant HIV diagnostic services in the Western Cape – one of the regions most severely affected by the early waves of the COVID-19 pandemic.28 This study aimed to describe the effects of initial waves of the COVID-19 pandemic and associated lockdown regulations on early infant testing and diagnosis of HIV (EID) in Cape Town, South Africa, compared to the same period the year before.
The objectives were to determine the proportion of HIV-exposed infants born during the COVID-19 lockdown who had birth and 10-week HIV testing compared to infants born in the same period in 2019, before the pandemic, and to determine the proportion of infants testing HIV-positive in each cohort.
Methods
This was a retrospective cohort study of 2888 infants from two regional hospitals in Cape Town, South Africa.
Setting and participants
We included routinely collected data from two level 2 hospitals offering obstetric services in the urban Cape Metro health district: Mowbray Maternity Hospital (MMH) and New Somerset Hospital (NSH).
The Cape Metropolitan (Metro) health district serves over 4 million people,42 and MMH and NSH manage approximately 11 000 and 6000 deliveries per year, respectively, representing approximately 45% of infants born in the district.43 Both hospitals offer primary level care to women in their immediate geographic service area and secondary level care to women living in a wider catchment area if referred by a primary care facility for a pregnancy or delivery deemed high risk. Estimated antenatal HIV prevalence in this population is between 16% and 20%.44
Immediate post-natal care of mother-infant dyads (including birth HIV PCR testing and initiation of infant chemoprophylaxis) occurs in hospital. Both study sites offer intensive care unit (ICU)-level care to neonates, and even critically unwell infants would undergo birth PCR testing at the birth facility. Follow-up care after discharge is provided in the community at local primary care facilities. In the case of infants who remain hospitalised, chemoprophylaxis, routine HIV testing and antiretroviral treatment (ART) initiation are provided in hospital.
Sample size
All HIV-exposed babies born at the selected facilities between 1 March 2020 and 31 August 2020 (during the first COVID-19 lockdown) were compared to those born during the same period in the preceding year, 1 March 2019 to 31 August 2019. This period spans the initial strictest lockdown levels and the peak of the first wave of the COVID-19 pandemic. Power calculations using estimated sample size, known baseline HIV prevalence, vertical transmission risk and baseline uptake of infant HIV PCR testing predicted the study would have 90% power to detect 5% difference in testing uptake and 80% power to detect 1% difference in positive test results.
Selection criteria
All live-born infants of women living with HIV (WLHIV) at the time of delivery or diagnosed within 7 days of delivery were included. Infants of mothers with negative, discordant, indeterminate or unknown HIV status were excluded. Infants born outside of the study sites (e.g., infants presenting to study sites after delivery) were excluded.
Data sources and collection
De-identified individualised data were requested from the Provincial Health Data Centre (PHDC). As described by Boulle et al. in their 2019 data centre profile, the PHDC consolidates all person-level data from the Department of Health (DOH) using multiple sources including digital hospital information systems, national laboratory result systems and electronic registers for high priority health conditions such as HIV.45 Data are linked through the use of a unique personal identifying folder number which is ubiquitous across the provincial health platforms.45
Data were extracted on infant gender, date of birth, gestational age, facility of birth, date and results of first HIV PCR, as well as dates, facilities and results of any subsequent HIV PCR testing up to 14 weeks of age. Vital status was determined using dates of death, for infants known to have demised, and evidence of healthcare access after 10 weeks of age as a proxy in other infants. Infants with no recorded date of death and no evidence of health-care contact after 10 weeks of age were deemed lost to follow-up. Maternal data were extracted on timing of ART initiation in relation to pregnancy.
Aggregate data were also collected from the hard-copy facility-based birth HIV PCR registers at each study site for comparison with the number of birth HIV PCRs captured in the electronic data set.
Testing definitions
We defined a birth HIV PCR as a test that was taken within 7 days of birth and a 10-week HIV PCR as a test taken between 6 and 14 weeks of birth. Therefore, the age at testing refers to chronological age of the infant.
Data analysis
The data set was prepared and analysed using R.46 Duplicate entries with identical study numbers and birth dates were consolidated. All birth HIV PCR tests were included for analysis. Infants testing HIV-positive at birth were excluded from analysis of 10-week testing. Facility-level data were analysed separately from data obtained from the electronic database to allow for comparative analysis.
Continuous variables were interpreted using means and confidence intervals for normally distributed data or medians and interquartile ranges for non-normally distributed data. Categorical data were described using proportions and frequency tables. The Chi-square test (or, when appropriate, the Fisher’s exact test) was used to test significance and a P-value of < 0.05 was considered statistically significant.
Bias and confounders
To minimise possible selection bias, we included all infants meeting inclusion criteria at both study sites. As this study did not include clinical record review, we could not control for potential confounding variables such as socio-economic circumstances, maternal age, vertical transmission risk stratification, maternal viral load, etc. We aimed to keep groups comparable by keeping the study sites and time periods the same in both years of study. Sample bias may have occurred as all the infants in our cohort were born at regional level referral hospitals in a single health district.
Results
Sample characteristics
We enrolled 2888 live-born infants of WLHIV across the two study sites and periods, 1413 born between 1 March 2019 and 31 August 2019 and 1475 in the same period in 2020, during the COVID-19 pandemic lockdown.
Baseline characteristics of the two cohorts were similar (Table 1). The proportion of deliveries at each facility remained consistent with approximately two-thirds of infants being born at MMH and the remaining third at NSH. Gestational ages of infants at birth were similar in both time periods, with most infants being born at term. There were no significant differences in maternal ART coverage between the two time periods.
TABLE 1.
Baseline characteristics.
| Characteristics | Before lockdown (2019) (n = 1413) |
During lockdown (2020) (n = 1475) |
Total (N = 2888) |
P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Infant gender† | |||||||
| Female | 666 | 47.2 | 749 | 50.8 | 1415 | 49.0 | 0.052 |
| Male | 746 | 52.8 | 726 | 49.2 | 1472 | 51.0 | - |
| Pregnancy outcome facility | |||||||
| Mowbray Maternity Hospital | 934 | 66.1 | 974 | 66.1 | 1908 | 66.1 | 0.970 |
| New Somerset Hospital | 479 | 33.9 | 501 | 33.9 | 980 | 33.9 | - |
| Gestational age (weeks) | |||||||
| < 32 | 39 | 2.8 | 37 | 2.5 | 76 | 2.6 | 0.895 |
| 32–36 | 243 | 17.2 | 250 | 16.9 | 493 | 17.1 | - |
| ≥ 37 | 1131 | 80 | 1188 | 80.5 | 2319 | 80.3 | - |
| Maternal ART status | |||||||
| On ART before pregnancy | 910 | 64.4 | 995 | 67.5 | 1905 | 66 | 0.341 |
| Re-initiated during pregnancy | 425 | 30.0 | 399 | 27.0 | 824 | 28.5 | - |
| Newly initiated in pregnancy | 63 | 4.5 | 65 | 4.4 | 128 | 4.4 | - |
| Initiated after pregnancy | 15 | 1.1 | 16 | 1.1 | 31 | 1.1 | - |
ART, antiretroviral treatment.
, In the 2019 cohort, one infant had missing gender information.
HIV PCR uptake
There were no significant differences in birth HIV PCR testing uptake which remained over 80% in both years based on the electronic data (Table 2). However, 10-week HIV PCR uptake significantly increased in 2020. In both groups > 90% of infants had at least one HIV PCR within 10 weeks, but significantly more infants had complete testing (i.e. both a birth and a 10-week HIV PCR or a birth HIV PCR only if HIV-positive at birth) in 2020. The mean age at which 10-week HIV PCR was taken remained comparable in both groups: 10.59 weeks in 2019 and 10.68 in 2020 (P = 0.549).
TABLE 2.
HIV PCR uptake.
| HIV PCR uptake | Before lockdown (2019) |
During lockdown (2020) |
Total |
P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Birth HIV PCR done | 1413 | - | 1475 | - | 2888 | - | - |
| No | 215 | 15.2 | 258 | 17.5 | 473 | 16.4 | - |
| Yes | 1198 | 84.8 | 1217 | 82.5 | 2415 | 83.6 | 0.109 |
| 10-week HIV PCR done † | 1407 | - | 1462 | - | 2869 | - | - |
| No | 558 | 39.7 | 432 | 29.5 | 990 | 34.5 | - |
| Yes | 849 | 60.3 | 1030 | 70.5 | 1879 | 65.5 | < 0.001 |
| Overall HIV PCR done | 1413 | - | 1475 | - | 2888 | - | - |
| No recorded testing | 132 | 9.3 | 137 | 9.3 | 269 | 9.3 | 0.999 |
| Complete testing‡ | 705 | 50.0 | 849 | 57.6 | 1554 | 53.8 | < 0.001 |
| Incomplete testing§ | 576 | 40.7 | 489 | 33.1 | 1065 | 36.9 | < 0.001 |
| HIV PCR done between 2 and 5 weeks | 132 | - | 137 | - | 269 | - | - |
| No | 105 | 79.5 | 110 | 80.3 | 215 | 79.9 | - |
| Yes | 27 | 20.5 | 27 | 19.7 | 54 | 20.1 | 0.999 |
HIV PCR, HIV polymerase chain reaction.
, Known HIV-positive infants excluded;
, Both a birth and a 10-week HIV PCR or a birth HIV PCR only if HIV-positive at birth;
, At least one HIV PCR done: missed either birth or 10-week HIV PCR.
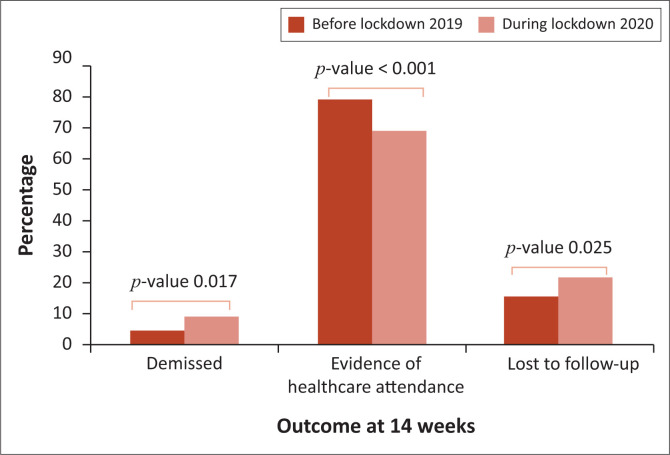
Sub-analysis of infants who did not have a 10-week HIV PCR (Figure 1) showed that most of these infants had evidence of healthcare attendance in the first 14 weeks of age but were not tested when accessing healthcare. Of those without electronic evidence of interaction with health services, significantly more demised in the 2020 period and significantly more were lost to follow-up.
FIGURE 1.
Sub-analysis of infants who did not have 10-week HIV PCR testing.
Vertical transmission of HIV
Although there was doubling in the proportion of infants with a positive HIV PCR at birth and 10 weeks in the 2020 cohort, the difference did not reach statistical significance (Table 3). There was a nearly threefold (2.8-fold) increase in vertical transmission of HIV by 10 weeks of age in 2020 compared to 2019 (P = 0.046).
TABLE 3.
HIV PCR results.
| HIV PCR results | Before lockdown (2019) |
During lockdown (2020) |
Total |
P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Birth HIV PCR result | 1198 | - | 1217 | 2415 | - | - | |
| Negative | 1191 | 99.4 | 1200 | 98.6 | 2391 | 99.2 | - |
| Positive | 6 | 0.5 | 13 | 1.1 | 19 | 0.8 | 0.17 |
| Unknown/inconclusive† | 1 | 0.1 | 4 | 0.3 | 5 | 0.2 | - |
| 10-week HIV PCR result ‡ | 853 | - | 1042 | - | 1895 | - | - |
| Negative | 845 | 99.1 | 1024 | 98.3 | 1869 | 98.6 | - |
| Positive | 0 | 0.0 | 4 | 0.4 | 4 | 0.2 | 0.18 |
| Unknown/inconclusive† | 8 | 0.9 | 14 | 1.3 | 22 | 1.1 | - |
| Overall HIV PCR | 1308 | - | 1365 | - | 2673 | - | - |
| Negative§ | 1302 | 99.5 | 1347 | 98.7 | 2649 | 99.1 | - |
| Positive¶ | 6 | 0.5 | 17 | 1.2 | 23 | 0.9 | 0.046 |
| Unknown/inconclusive† | 0 | 0.0 | 1 | 0.1 | 1 | 0.0 | - |
HIV PCR, HIV polymerase chain reaction.
, ‘unknown’ denotes HIV PCR tests that did not have a documented result and ‘inconclusive’ denotes an equivocal HIV PCR test result;
, Known HIV-positive infants excluded;
, All HIV PCR tests negative;
, Birth or 10 weeks positive HIV PCR test.
Facility-level data
Facility-level data were only available for birth HIV PCR testing. In both years, facility-level data reflected 100% uptake of birth HIV PCR testing. However, 10-week testing and results were not routinely followed up or recorded by either facility. More HIV PCR tests were recorded in facility-level data than were captured in the electronic database in 2019 and 2020. There were 299 additional HIV birth-PCR tests recorded in facility-based registers in 2020 compared to 7 additional tests recorded in 2019 (Table 4). However, as seen in Table 3, the proportion of infants testing HIV-positive in both years remained comparable to the proportions found in the electronic data set.
TABLE 4.
Facility-level data.
| Birth HIV PCR result | Before lockdown (2019) (n = 1205) |
During lockdown (2020) (n = 1516) |
Total (n = 2721) |
P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Negative | 1196 | 99.3 | 1499 | 98.9 | 2695 | 99.0 | 0.425 |
| Positive | 9 | 0.7 | 17 | 1.1 | 26 | 1.0 | - |
HIV PCR, HIV polymerase chain reaction.
Discussion
This study investigated the effects of the early COVID-19 pandemic and associated lockdown regulations on EID in Cape Town, South Africa. We found a statistically significant increase in vertical transmission of HIV in infants born during the COVID-19 pandemic despite unchanged uptake of birth testing and improved uptake of 10-week testing compared with the same period in the year before. Importantly, of the infants who did not undergo a 10-week HIV PCR more infants demised or were lost to follow-up in the 2020 group.
HIV PCR uptake
We found that while the HIV PCR uptake at birth was unchanged during the initial COVID-19 lockdown, the 10-week HIV PCR testing uptake improved compared to the same period in the year before, a testament to the resilience of the South African VTP programme.
A recent study looking at the effect of COVID-19 on HIV, tuberculosis and vertical transmission indicators in Mopani district, Limpopo, South Africa, found significant disruptions in HIV testing, ART initiation, and retention in care among children aged 18 months to 14 years. Interestingly, this study did not show any differences in vertical transmission indicators such as the number of antenatal care visits, number of pregnant WLHIV identified and the number of pregnant WLHIV initiated on ART. The reported HIV PCR tests and HIV PCR positive results were also relatively unaffected during the pandemic. There was, however, a marked increase in antenatal care enrolments in May 2020 postulated to be related to women returning to their rural homes after the initial COVID-19 lockdown.41 Evidence suggests that this migration of pregnant women back to rural provinces was most pronounced in Limpopo, Mpumalanga and the Northern Cape, with metropolitan areas having a significant decrease in initial antenatal care enrolment visits.47
A similar study done in the KwaZulu-Natal province of South Africa, found a small but significant decrease in birth HIV PCR testing during the pandemic but did not investigate differences in vertical transmission.37 In both studies, aggregated data from the District Health Information System (DHIS) were used to analyse the absolute number of HIV PCR tests done in a geographical service area. Our data were specific to individual infants who were HIV exposed and followed up for the first 14 weeks of age. Using de-aggregated data allowed us to highlight proportional differences in testing in a defined population rather than differences in the total number of tests done. Differences in the type of data analysed, as well as intrinsic differences in the populations studied such as pre-existing socio-economic differences48,49 and impacts of the migration of pregnant women after the initial COVID-19 lockdown may explain differences in the study results.
Despite the gains seen in our study in 10-week HIV PCR testing in 2020, the uptake in both 2019 and 2020 was lower than found in previous studies on EID in the Cape Town Metro by Kalk et al. and Dunning et al., between 2014 and 2015.9,19 While being done in policy periods with limited rollout of birth HIV PCR testing, both these studies found the average uptake of HIV PCR testing at 6 to 10 weeks at over 80%. Given the higher proportion of birth HIV PCR testing in our study, this relatively lower uptake of follow-up testing is consistent with their findings reporting that infants who undergo birth HIV PCR testing are less likely to present for follow-up testing.9,19 Kalk et al. also found that infants classified as at high risk for HIV transmission and those born to mothers with inadequate antenatal ART were also less likely to present for follow-up testing – suggesting that pre-existing patterns of poor health-seeking behaviour may contribute to decreased uptake of EID.9
These results differ from a more recently published study which found that having a birth HIV PCR was associated with an increased uptake of repeat HIV PCR testing at 6 or 10 weeks in a cohort of infants born in Khayelitsha, Cape Town.50 While this study spanned a similar time period as the Kalk et al. and Dunning et al. studies, there were several differences in study methodology. Whereas our study, as well as those by Kalk et al. and Dunning et al., analysed routinely collected data and testing results, the Khayelitsha study employed study nurses to take blood samples for both birth HIV PCR samples and point-of-care HIV testing, communicate results, and counsel mothers on the further need for infant testing, prophylaxis and follow-up. A full-time patient tracer also monitored the National Health Laboratory Service (NHLS) database for evidence of 6/10-week HIV PCR testing and if no testing was evident one month after testing was due, patients were actively traced and reminded about follow-up testing.50 These differences in study methodology including additional testing, active counselling and tracing of study participants may explain differences found in uptake of repeat HIV PCR testing.
Another large study in Johannesburg, Gauteng, South Africa, analysed data extracted from the NHLS database from October 2018 to September 2021 and determined trends in the total number of tests performed and the total number of children with HIV diagnosed, stratified by age, and compared these data to EID indicators collected by the DHIS. During the COVID-19 pandemic they found much higher rates of birth and 10-week HIV PCR uptake than in our study, with the number of HIV PCR tests done at birth and 10 weeks closely approximating the number of live births to WLHIV. While a 6% decrease in 10-week HIV PCR uptake was noted during the second and third quarter of 2020, the period with the strictest COVID-19 lockdown, overall, there was an increase in HIV testing across all age groups over the three 12-month periods analysed.40
Although also using data from a centralised database and analysing routinely collected data, the Johannesburg study had a much broader definition of a birth HIV PCR test (any HIV PCR done from birth up to 6 weeks) and the total number of birth tests performed was found to be higher than the total live births to WLHIV, postulated to be due to a combination of under-reporting of the DHIS indicators and repeat HIV PCR tests performed in the first 6 weeks of life. The absence of unique patient identifiers in the cohort studied may have further impacted on duplication of testing.40
The improved 10-week HIV PCR testing uptake in 2020 found in our study, and the even higher uptake found in Khayelitsha and Johannesburg cohorts, clearly highlight the resilience of the South African VTP programme – even in the face of a global pandemic. Understanding the drivers behind the higher uptake of testing in 2020 could inform interventions to further improve uptake of the follow-up testing and could help to strengthen initiatives targeting uptake of other routine child health services such as childhood immunisation, which suffered severe disruptions during the COVID-19 lockdown.
The increase we found in infant mortality and loss to follow-up in the 2020 group of infants that did not have a 10-week HIV PCR suggests that the COVID-19 pandemic and associated lockdown restrictions did have a significant impact on children’s access to healthcare. However, in both years, most infants who did not have a 10-week HIV PCR did have evidence of healthcare attendance. This finding is equally important and represents critical missed opportunities – perhaps an argument for integrated packages of care with VTP interventions forming part of other routine child health visits.
Vertical transmission
Although there were no statistically significant changes in birth HIV PCR positivity rates between the two groups in our study, overall HIV transmission by 10 weeks of age more than doubled, a notable change from the steady decline in vertical transmission over the last decade.14,15,16,17 In contrast to our findings, the Johannesburg study found there was an overall decrease in positive HIV PCR tests in children < 2 years old in their 3-year study period. Interestingly, they did note some variations during the initial stages of lockdown restrictions; despite no obvious differences in the number of HIV PCR tests performed during the initial strictest COVID-19 lockdown, they found a sudden decrease in the number of positive birth and 10-week HIV PCR tests in that period, followed by a subsequent increase in the number of positive tests when restrictions were relaxed. While the cohort studied was very large and their data sources robust, there were some reported data discrepancies between the two major data sources used in this study that may have impacted on their results. These discrepancies were most notable in relation to the number of positive HIV PCRs found at birth and 10 weeks and were possibly related to the inability to link patient tests accurately as unique patient identifying numbers were not used in that population.40
Our findings of increased vertical transmission of HIV in the first wave of the pandemic coupled with data showing a decrease in antenatal care enrolment29,51,52 and ART collection30,36 suggests service disruption with decreased access and adherence to maternal ART during the COVID-19 lockdown potentially impacting vertical transmission rates. This is a powerful reminder that infant health is inextricably linked with maternal health and that interventions targeting and championing child health also need to consider maternal health. Increased HIV transmission has far-reaching impacts and could undermine hard-won gains in child health and child mortality made over many years. There are also implications for forward planning and the need to increase capacity for healthcare services for children living with HIV.
Differences between electronic data and facility records
In the electronic data analysed, we found that more than 80% of infants in both cohorts underwent birth HIV PCR testing. This is higher than previous estimates of birth testing uptake,9,19 but still falls well below the national 95/95/95 targets. Notably, facility-level data from paper registers did not correspond with the electronic data. Although rates of vertical transmission were comparable, absolute number of birth HIV PCR tests recorded as aggregate data by the birth facilities were higher than the number of birth HIV PCR tests reflected in the electronic data set, and uptake of birth HIV PCR testing was much higher in facility-based registers. The discrepancy was much more pronounced in the 2020 group, which may indicate that electronic data collection was impacted by the COVID-19 pandemic and lockdown disruptions. Exploring these data discrepancies may identify underlying problems in testing or data capture procedures that contribute to reported birth testing rates remaining persistently below national targets. Further research into the impacts of staff re-allocation or absence during the pandemic is warranted to determine if this may have affected data collection accuracy.
Strengths and limitations
Our study was retrospective and thus limited by the accuracy of data collected and entered into existing registers and electronic databases. A recent study evaluating the quality of data from the NHLS data warehouse found poor-quality demographic data, particularly for infants and young children,53 highlighting potential limitations in using electronic databases which may have impacted the study.
The focus of our study was limited to EID and did not explore other aspects of the VTP cascade or linkage to care for HIV-positive infants. The study also did not include data on mode of delivery, predelivery maternal viral load, ART duration and/or interruption which may all impact transmission rates. The single geographic service area data may not be generalisable due to differences in healthcare service infrastructure and accessibility. Although the overall number of deliveries at study facilities remained constant, we did not investigate potential differences in delivery patterns, such as rates of preterm deliveries, caesarean sections, etc. that may have been influenced by the lockdown restrictions. Sample bias may have occurred as all infants in our study were born in regional level referral hospitals within a metropolitan city.
Conclusion and recommendations
The uptake of routine HIV PCR testing of HIV-exposed infants increased during the COVID-19 lockdown in 2020, highlighting the resilience of the South African VTP programme – even in the face of a global pandemic. However, higher rates of vertical transmission and the high proportion of loss to follow-up or demise among infants not tested at 10 weeks in 2020, supports the hypothesis that the COVID-19 pandemic lockdown did have an impact on health services and access to care.
Further research to unpack the drivers behind the increased HIV PCR uptake in 2020 could inform interventions for future pandemics and bolster strategies to improve retention of mother-infant dyads in the VTP cascade. More research is also needed to determine if the increases in vertical transmission seen in this study have continued following the lifting of the COVID-19 lockdown restrictions. This would help in interrogating child health service vulnerabilities in times of national crisis.
Acknowledgements
We thank the Western Cape Provincial Health Data Centre (PHDC) for their work and support of this research. We also thank Dr Max Kroon for his contribution to funding open access publishing of this research.
This article is partially based on the author’s thesis entitled ‘Effects of the COVID-19 pandemic on early infant testing and diagnosis of HIV in Cape Town, South Africa’ toward the degree of Master of Medicine in Paediatrics in the https://open.uct.ac.za/home, University of Cape Town, South Africa, with supervisors Dr P. Nongena, Dr E. Kalk and Dr S.M. Kroon, received 24 May 2023, it is available here: https://open.uct.ac.za/home.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
H.v.V. performed this study in completion of a Master of Medicine (MMED) in Paediatrics at the University of Cape Town. As such, H.v.V. was involved in all aspects of the study from conception, data handling, analysis and final write-up. P.N., E.K. and S.M.K. acted as supervisors and were involved in supervising all aspects of the study as well as revision, editing and administration of the study. M.T. cleaned and analysed data and was involved in data visualisation. F.P. and J.E. were involved with data collection and curation. L.F. and N.l.R. were involved with project conceptualisation and data collection.
Ethical considerations
The study was approved by the Faculty of Health Sciences Human Research Ethics Committee at the University of Cape Town in South Africa (174/2021). Permissions were also obtained from the National Health Research Database, the Provincial Department of Health and the management structures of the institutions involved.
As this was a retrospective descriptive study only using routinely collected, de-identified data and did not involve patient contact, a waiver of informed consent was granted. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Data availability
The data were obtained from the PHDC (https://www.westerncape.gov.za/general-publication/provincial-health-data-centre). The PHDC has strict governance structures managing the protection and use of health data and thus the data set and raw data are not available publicly. A formal application and approval process is required for access to data used for this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and are the product of professional research. It does not necessarily reflect the official policy or position of any affiliated institution, funder, agency, or that of the publisher. The authors are responsible for this article’s results, findings, and content.
Funding Statement
Funding information This work was supported by the University’s MMED fund and the Departmental Research Committee.
Footnotes
How to cite this article: Van Vollenhoven H, Kalk E, Kroon SM, et al. Effects of the COVID-19 pandemic on early infant diagnosis of HIV in Cape Town, South Africa. S Afr J HIV Med. 2024;25(1), a1542. https://doi.org/10.4102/sajhivmed.v25i1.1542
References
- 1.Eley B. Care of HIV-exposed and HIV-infected neonates. S Afr J HIV Med. 2015;16(1):360. 10.4102/sajhivmed.v16i1.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23(1):101–106. 10.1097/QAD.0b013e32831c54bd [DOI] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet. 2004;364(9441):1236–1243. 10.1016/S0140-6736(04)17140-7 [DOI] [PubMed] [Google Scholar]
- 4.Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: Are we too late? J Int AIDS Soc. 2014;17(18911):1–6. 10.7448/IAS.17.1.18914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382(9904):1555–1563. 10.1016/S0140-6736(13)61409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatti G, Bock P, Eley B, Mothibi E, Grimwood A. Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: An analysis in four provinces in South Africa, 2004–2009. J Acquir Immune Defic Syndr. 2011;58(3):e60–e607. 10.1097/QAI.0b013e3182303c7e [DOI] [PubMed] [Google Scholar]
- 7.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: A pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40(2):385–396. 10.1093/ije/dyq255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Technau K-G, Kuhn L, Coovadia A, Carmona S, Sherman G. Improving early identification of HIV-infected neonates with birth PCR testing in a large urban hospital in Johannesburg, South Africa: Successes and challenges. J Int AIDS Soc. 2017;20(1):21436. 10.7448/IAS.20.01/21436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalk E, Kroon M, Boulle A, et al. Neonatal and infant diagnostic HIV-PCR uptake and associations during three sequential policy periods in Cape Town, South Africa: A longitudinal analysis. J Int AIDS Soc. 2018;21(e25212):1–8. 10.1002/jia2.25212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman GG. HIV testing during the neonatal period. S Afr J HIV Med. 2015;16(1):a362. 10.4102/sajhivmed.v16i1.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudaly V, Voget J. The Western Cape consolidated guidelines for HIV treatment: Prevention of mother-to-child transmission of HIV (PMTCT), children, adolescents and adults. 2018 (Amended Version). Cape Town: Provincial Government of the Western Cape Department of Health; 2020. [Google Scholar]
- 12.South African National Department of Health . Guideline for the prevention of mother to child transmission of communicable infections (HIV, hepatitis, listeriosis, malaria, syphillis and TB). Pretoria: South African National Department of Health; 2019. [Google Scholar]
- 13.Republic of South Africa National Department of Health . 2019 ART clinical guidelines. Pretoria: Republic of South Africa National Department of Health; 2019. [Google Scholar]
- 14.Lake L, Shung-King M, Hendricks M, et al. Prioritising child and adolescent health: A human rights imperative. In: Shung-King M, Lake L, Sanders D, Hendricks M, editors. South African child gauge 2019. Cape Town: Children’s Institute, University of Cape Town; 2019, p. 30–62. [Google Scholar]
- 15.Woldesenbet S, Kufa T, Lombard C, et al. The 2017 National Antenatal Sentinel HIV Survey Key Findings, South Africa. Pretoria: South African National Department of Health; 2019.. [Google Scholar]
- 16.Goga AE, Dinh TH, Jackson DJ for the SAPMTCTE study group . Evaluation of the effectiveness of the National Prevention of Mother-to-Child Transmission (PMTCT) Programme measured at six weeks postpartum in South Africa, 2010. South African Medical Research Council, National Department of Health of South Africa and PEPFAR/US Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 17.Goga A, Chirinda W, Ngandu NK, et al. Closing the gaps to eliminate mother-to-child transmission of HIV (MTCT) in South Africa: Understanding MTCT case rates, factors that hinder the monitoring and attainment of targets, and potential game changers. S Afr Med J Matern Child Heal Suppl. 2018;108(3 Suppl 1):S17–S24. 10.7196/SAMJ.2017.v108i3b.12817 [DOI] [Google Scholar]
- 18.Health Systems Trust . Infant PCR test positive around 10 weeks rate [homepage on the Internet]. [cited 2020 Nov 03]. Available from: https://www.hst.org.za/healthindicators
- 19.Dunning L, Kroon M, Fourie L, Ciaranello A, Myer L. Impact of birth HIV-PCR testing on the uptake of follow-up early infant diagnosis services in Cape Town, South Africa. Pediatr Infect Dis J. 2017;36(12):1159–1164. 10.1097/INF.0000000000001677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrazo AC, Firth J, Amzel A, Sedillo R, Ryan J, Phelps BR. Interventions to significantly improve service uptake and retention of HIV-positive pregnant women and HIV-exposed infants along the prevention of mother-to-child transmission continuum of care: Systematic review. Trop Med Int Health. 2018;23(2):136–148. 10.1111/tmi.13014 [DOI] [PubMed] [Google Scholar]
- 21.Haas AD, Van Oosterhout JJ, Tenthani L, et al. HIV transmission and retention in care among HIV-exposed children enrolled in Malawi’s prevention of mother-to-child transmission programme. J Int AIDS Soc. 2017;20(1):21947. 10.7448/IAS.20.1.21947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rollins N, Chanza H, Chimbwandira F, et al. Prioritizing the PMTCT implementation research agenda in 3 African countries: INtegrating and Scaling up PMTCT through Implementation REsearch (INSPIRE). J Acquir Immune Defic Syndr. 2014;67(Suppl 2):S108–S113. 10.1097/QAI.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 23.Sibanda EL, Weller IVD, Hakim JG, Cowan FM. The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: A systematic review and meta-analysis. AIDS. 2013;27(17):2787–2797. 10.1097/QAD.0000000000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson L, Grant AD, Watson-Jones D, Kahawita T, Ong’ech JO, Ross DA. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: A systematic review. Trop Med Int Health. 2012;17(5):564–580. 10.1111/j.1365-3156.2012.02958.x [DOI] [PubMed] [Google Scholar]
- 25.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karim SSA. The South African response to the pandemic. N Engl J Med. 2020;382(24):e95. 10.1056/NEJMc2014960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNICEF South Africa . South Africa COVID-19 situation report No. 2 [homepage on the Internet]. Vol. May. 2020. [cited 2020 Nov 20]. Available from: https://www.unicef.org/media/81556/file/South-Africa-COVID19-SitRep-15-May-2020.pdf [Google Scholar]
- 28.UNICEF South Africa . South Africa COVID-19 situation report No. 3 [homepage on the Internet]. Vol. May. 2020. [cited 2020 Nov 20]. Available from: https://www.unicef.org/media/81551/file/South-Africa-COVID19-SitRep-31-May-2020.pdf [Google Scholar]
- 29.UNICEF South Africa . South Africa COVID-19 situation report No. 4 [homepage on the Internet]. Vol. June. 2020. [cited 2020 Nov 20]. Available from: https://www.unicef.org/media/81546/file/South-Africa-COVID19-SitRep-15-June-2020.pdf [Google Scholar]
- 30.UNICEF South Afirca . South Africa COVID 19 situation report No. 5 16–30 June 2020 [homepage on the Internet]. Vol. June. 2020. [cited 2020 Nov 20]. Available from: https://www.unicef.org/media/81541/file/South-Africa-COVID19-SitRep-30-June-2020.pdf [Google Scholar]
- 31.UNICEF South Africa . UNICEF South Africa progress report on the response to the COVID-19 epidemic situation update 01/2020. Vol. 30 April. 2020. [cited 2020 Nov 20]. Available from https://www.unicef.org/southafrica/media/3831/file/ZAF-COVID19-situation-report-1-30%20April-2020.pdf [Google Scholar]
- 32.Coker M, Folayan MO, Michelow IC, Oladokun RE, Torbunde N, Sam-Agudu NA. Things must not fall apart: The ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa. Pediatr Res. 2021;89(5):1078–1086. 10.1038/s41390-020-01174-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siedner MJ, Kraemer JD, Meyer MJ, et al. Access to primary healthcare during lockdown measures for COVID-19 in rural South Africa: An interrupted time series analysis. BMJ Open. 2020;10(10):1–10. 10.1136/bmjopen-2020-043763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UNICEF . UNICEF’s HIV programming in the context of COVID-19: Sustaining the gains and reimagining the future for children, adolescents and women. Compendium of innovative approaches in Eastern and Southern Africa, July 2020 UNICEF Eastern and Southern Africa [homepage on the Internet]. 2020;(July):1–24. [cited 2020 Nov 20]. Available from: https://www.unicef.org/esa/media/6621/file/HIVCOVID-19Compendium-July2020.pdf [Google Scholar]
- 35.Nalubega S, Kyenkya J, Bagaya I, et al. COVID-19 may exacerbate the clinical, structural and psychological barriers to retention in care among women living with HIV in rural and peri-urban settings in Uganda. BMC Infect Dis. 2021;21(1):1–10. 10.1186/s12879-021-06684-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization . Access to HIV medicines severely impacted by COVID-19 as AIDS response stalls [homepage on the Internet]. 2020. [cited 2020 Jul 12]. Available from: https://www.who.int/news-room/detail/06-07-2020-who-access-to-hiv-medicines-severely-impacted-by-covid-19-as-aids-response-stalls
- 37.Jensen C, McKerrow NH. Child health services during a COVID-19 outbreak in KwaZulu-Natal Province, South Africa. S Afr Med J. 2020;111(2):13185. 10.7196/SAMJ.2021.v111i2.15243 [DOI] [PubMed] [Google Scholar]
- 38.Burt JF, Ouma J, Lubyayi L, et al. Indirect effects of COVID-19 on maternal, neonatal, child, sexual and reproductive health services in Kampala, Uganda. BMJ Glob Health. 2021;6(8):e006102. 10.1136/bmjgh-2021-006102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorward J, Khubone T, Gate K, et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: An interrupted time series analysis. Lancet HIV. 2021;8(3):e158–e165. 10.1016/S2352-3018(20)30359-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mnyani CN, Smit A, Sherman GG. Infant HIV testing amid the COVID-19 pandemic and evolving PMTCT guidelines in Johannesburg, South Africa. Trop Med Infect Dis. 2022;7(10):302. 10.3390/tropicalmed7100302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutyambizi C, Dunlop J, Maluleke C, et al. Effect of COVID-19 on HIV, tuberculosis, and prevention of mother-to-child transmission of HIV indicators in Mopani district, South Africa. S Afr Med J. 2021;111(12):1181–1189. 10.7196/SAMJ.2021.v111i12.15822 [DOI] [PubMed] [Google Scholar]
- 42.Western Cape Department of Health . Cape Metro District Health Plan 2018/19 – 2020/21 [homepage on the Internet]. 2020, p. 1–34. [cited 2021 Oct 15]. Available from: https://resource.capetown.gov.za/documentcentre/Documents/Citystrategies,plansandframeworks/MetroDistrictHealthPlan_2019-20.pdf [Google Scholar]
- 43.Perinatal Problem Identification Programme (PPIP) . Metro West, Cape Town: (unpublished); 2022. [Google Scholar]
- 44.Woldesenbet SA, Lombard C, Manda S, et al. The 2019 national antenatal HIV sentinel survey [homepage on the Internet]. 2021. [cited 2020 Nov 20] Available from: https://www.ptonline.com/articles/how-to-get-better-mfi-results [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulle A, Heekes A, Tiffin N, et al. Data centre profile: The provincial health data centre of the Western Cape province, South Africa. Int J Popul Data Sci. 2019;4(2):1143. 10.23889/ijpds.v4i2.1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2021. URL https://www.R-project.org/) [Google Scholar]
- 47.Pattinson R, Fawcus S, Gebhardt S, Niit R, Soma-Pillay P, Moodley J. Obstetrics and gynaecology forum – The effect of the first wave of COVID-19 on use of maternal and reproductive health services and maternal deaths in South Africa. Obstet Gynaecol Forum [serial online]. 2020;30(4):38–46. [cited 2023 Aug 25]. Available from: https://journals.co.za/doi/abs/10.10520/ejc-medogv30-n4-a10 [Google Scholar]
- 48.Cooperative Governanace and Traditional Affairs . Mopani district municipality. 2015;3(April):49–58. [cited 2023 Aug 25]. Available from https://www.cogta.gov.za/ddm/wp-content/uploads/2020/11/Mopani-October-2020.pdf [Google Scholar]
- 49.Government of South Africa . Cape Town municipality – Overview [homepage on the Internet]. 2022; [cited 2023 Aug 25]. Available from: https://municipalities.co.za/overview/6/city-of-cape-town-metropolitan-municipality
- 50.Nelson AK, Cassidy T, Trivino Duran L, et al. An analysis of the HIV testing cascade of a group of HIV-exposed infants from birth to 18 months in peri-urban Khayelitsha, South Africa. PLoS One. 2022;17(1):e0262518. 10.1371/journal.pone.0262518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner M, Falcone V, Neururer SB, et al. Perinatal and postpartum care during the COVID-19 pandemic: A nationwide cohort study. Birth. 2022;49(2):243–252. 10.1111/birt.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Townsend R, Chmielewska B, Barratt I, et al. Global changes in maternity care provision during the COVID-19 pandemic: A systematic review and meta-analysis. EClinicalMedicine. 2021;37:100947. 10.1016/j.eclinm.2021.100947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radebe L, Haeri Mazanderani A, Sherman GG. Evaluating patient data quality in South Africa’s National Health Laboratory Service Data Warehouse, 2017–2020: Implications for monitoring child health programmes. BMC Public Health. 2022;22(1):1266. 10.1186/s12889-022-13508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data were obtained from the PHDC (https://www.westerncape.gov.za/general-publication/provincial-health-data-centre). The PHDC has strict governance structures managing the protection and use of health data and thus the data set and raw data are not available publicly. A formal application and approval process is required for access to data used for this study.