Abstract
Tick-borne pathogens are transmitted by a wide range of tick species and affect both human and animal health. Understanding the diversity of these pathogens and their co-infection rates in domesticated animals in urban areas is crucial for effective disease management and prevention. In this study, a total of 565 owned dogs in the central region of Thailand were investigated for the infection rate of three genera of Ehrlichia, Hepatozoon, and Babesia infection using multiplex PCR. The results revealed an overall infection rate of 19.1%, with Ehrlichia having the highest infection rate (12.2%), followed by Babesia (2.5%) and Hepatozoon (1.4%). The rate of co-infection was 3%, with mixed infections involving two or three genera. Male dogs exhibited a slightly higher infection rate compared to females, although not statistically significant. Young adult dogs (1–3 years) showed the highest infection rate of both single infections and co-infections. Monthly infection rate indicated variations throughout the year, with co-infection rate significantly associated with overall infection rate. Clinical manifestations in three genera of infected dogs included thrombocytopenia and eosinopenia. The results of this study are useful to design strategies for the management and prevention of tick-borne diseases in the study area.
Keywords: co-infection, Ehrlichia, Hepatozoon, Babesia, prevention of tick-borne diseases
Introduction
Tick-borne diseases in dogs are caused by various etiologic agents that are transmitted to dogs from infected tick. The common tick-borne pathogens that affect dog health include Ehrlichia spp., Anaplasma spp., Hepatozoon spp., and Babesia spp. Ehrlichia is an intracellular bacterium causing canine monocytic ehrlichiosis. Dogs infected with Ehrlichia can develop a range of clinical signs, including fever, loss of appetite, weight loss, joint pain, enlarged lymph nodes, anemia, and bleeding disorders (1). It can be a serious and potentially life-threatening disease. Ehrlichia is transmitted to dogs through saliva of the brown dog tick (Rhipicephalus sanguineus) (2). Hepatozoon is a protozoan parasites. Hepatozoon canis (H. canis) has been reported to infect dogs across inhabited continents (3–6). It is transmitted by ingesting an infected tick (Rhipicephalus sanguineus and possibly other tick species) or by ingesting an animal that contains the larval stages (7). Hepatozoon infections can range from no apparent to severe clinical manifestation, although dogs are most often subclinically or mildly affected. Dogs with compromised immune systems are more likely to develop more severe forms of the disease (8). Clinical signs may include fever, anemia, lethargy, and anorexia, which are commonly observed. Canine babesiosis is caused by an intraerythrocytic protozoan belonging to the Babesia genus. In dogs, Babesia canis (B. canis) (subspecies: canis, vogeli, and rossi), B. gibsoni, B. conradae, and B. microti have been identified (9). Babesia is widely distributed, making it a significant concern for animal health worldwide (10, 11). The geographical distribution of each Babesia spp. varies (9). Babesia is transmitted through the biting of hard tick vector (Ixodidae) species. Infected dogs typically lack obvious clinical symptoms. Clinical manifestation differed depending on the strain of Babesia involved (12). However, all strains could develop hemolysis, thrombocytopenia, and hyperglobulinemia.
Tick-borne disease infections are most commonly associated with warm and humid regions. In endemic areas, infection occurs throughout the year. The prevalence of tick-borne infections in adult dogs is higher than in younger dogs (13). Dogs over 3 years old were reported as a factor associated with Ehrlichia canis (E. canis) infection in dogs (14). Dogs exhibiting thrombocytopenia along with other clinical signs of tick-borne disease were more likely to be infected by tick-borne disease pathogens (15). Co-infection of multiple tick-borne pathogens in dogs has been reported. There is a significant association between exposure to Anaplasma spp. and Babesia spp. in the United States, with 36% of hunting dogs exposed to Anaplasma species infection having been exposed to Babesia spp. (16). Co-infection of E. canis and H. canis or B. canis and H. canis has also been detected in dogs, and they exhibit clinical signs like thrombocytopenia, leukopenia, fever, or lethargy (17). Co-infection with tick-borne pathogens can occur through a single tick that carries multiple pathogens. There have been reports of multiple tick-borne pathogens in individual ticks, with reported rates ranging from 1% to over 10%, with more than one pathogen present (18, 19). The study aims to provide valuable insights into the infection rate, co-infection patterns, risk factors, and potential hematological alterations of three genera of tick-borne pathogens, Ehrlichia, Hepatozoon, and Babesia, in dogs in the central region of Thailand.
Materials and methods
Study design and subject selection
The present study was carried out using the data collected during January to December 2022 on 565 owned dogs that were suspected to be suffering from various tick-borne diseases and treated at Prasu Arthorn Veterinary Teaching Hospital of the Faculty of Veterinary Science, Mahidol University, Thailand. These dogs were investigated for the presence of multiple tick-borne pathogens including Ehrlichia, Hepatozoon, and Babesia, complete blood count (CBC), and blood biochemistry. The tick-borne pathogen detection results of 565 dogs, which are 299 males and 266 females, were gathered from the Monitoring and Surveillance Center for Zoonotic Diseases in Wildlife and Exotic Animals (MoZWE). The CBC and blood biochemistry data of positive tick-borne pathogen dogs were collected from Prasu Arthorn Veterinary Teaching Hospital. All owners completed a consent form authorizing the use of data. In the study of infection rate by age, only 530 dogs could be identified by birth date and were categorized into five age groups: puppies (less than 1 year); young adults (1–3 years); adults (4–6 years); old (7–10 years); and very old (>10 years).
Blood DNA extraction and multiplex-PCR
Two hundred microliters of blood were used to extract DNA using the genomic DNA mini kit® (Geneaid, Taipei, Taiwan). Samples were incubated in lysis buffer (0.1 M NaCl, 10 mM Tris-Cl (pH 8.0), 5% SDS) with 10 μL of proteinase K (10 mg/mL) (minimum 20 min) at 56°C. Purification of DNA was conducted in accordance with the protocol described by the manufacturer. DNA was dissolved in 50 μL of 10 mM Tris–HCl pH 8.0 containing 1 mM EDTA. DNA was kept at −20°C until investigation. Specific three primer pairs of virB9 protein gene of Ehrlichia (forward, Ehr1401F, 5′- CCATAAGCATAGCTG ATAACCCTGTTACAA -3′, and reverse, Ehr1780R, 5′- TGGATAA TAAAACCGTACTATGTATGCTAG -3′) (20), 18S rRNA gene of Hepatozoon (forward, Hep001F, 5′- CCTGGCTATACATGAGCAAA ATCTCAACTT -3′, and reverse, Hep737R, 5′- CCAACTGTCC CTATCAATCATTAAAGC -3′) (20), and the 18S rRNA gene of Babesia (forward, Ba143-167, 5′- CCGTGCTAATTGTAGGGCTAATACA -3′, and reverse, Ba694-667, 5′- GCTTGAAACACTCTARTTTCTCAAAG -3′) (21) were used for Multiplex PCR amplification. A total volume of 50 μL of reaction consists of 5 μL of template DNA, 25 μL of 1x multiplex PCR master mix (QIAGEN, Germany), and 1 μL of each primer at a concentration of 0.2 μM. A C1000 touch thermal cycler (BIO-RAD, Hercules, CA, USA) was used. Amplification conditions were initialed at 95°C 15 min, followed by 35 cycles of 94°C 45 s, 61°C 45 s, 72°C 1 min, and final extension at 72°C 10 min. PCR products were separated on 2.0% agarose gel and stained with GelRed (Bio-tium, Fremont, CA, USA). DNA bands were visualized by UV transluminator. The expected sizes for Ehrlichia, Hepatozoon and Babesia were 380, 737, and 525 bps, respectively.
Determination of hematological and serum biochemical parameters
Blood (1 mL per sample) was collected from the cephalic veins into tubes containing ethylenediamine tetra-acetic acid-coated tubes. CBC was done on an automated Mindray BC-5300vet hematology analyzer (Mindray, Shenzhen, China) within 2 h of sampling.
Total white blood cell count, differential count of leucocytes, red blood cells, hemoglobin concentration, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, RBC distribution width, and platelets were determined.
Non-hemolysed serum was obtained from the blood samples. This serum was used to determine biochemical parameters using the AU-480 chemistry analyzer (Beckman Coulter, Brea, USA).
The parameters measured included total serum proteins and serum albumin.
Statistical analyses
The data were analyzed using the SPSS 27 (IBM, Armonk NY, USA). The study compared the infection rate across various categories. Fisher exact tests were used to examine differences between sex groups. The differences between the five age groups were analyzed using Pearson χ2. The likelihood-ratio G2 was used to assess differences between sampling periods (months). A p value of <0.05 was considered statistically significant. Pearson correlation coefficients were determined to assess the correlation between the monthly infection rate of overall infection and co-infection.
Results
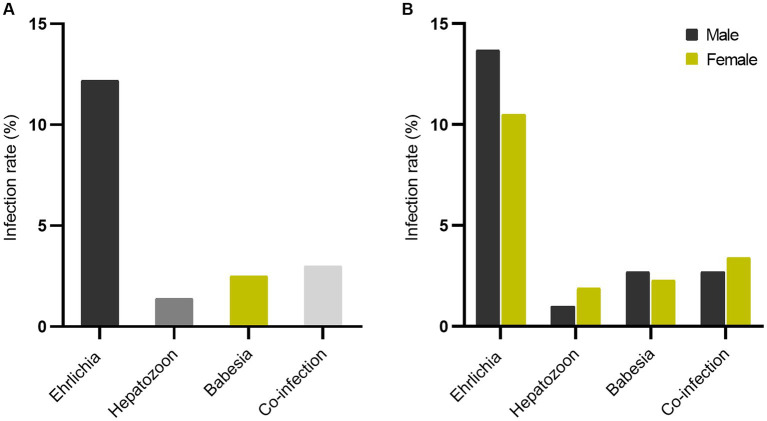
A total of 565 owned dogs were investigated for Ehrlichia, Hepatozoon, and Babesia using multiplex PCR. 108 (19.1%) samples tested were positive for at least one pathogen. The highest infection rate was Ehrlichia infection, 69/565 (12.2%). Hepatozoon infection rate was lowest at 8/565 (1.4%). Babesia infection rate was 14/565 (2.5%). Co-infection rate, where a dog was positive for more than one pathogen, was 17/565 (3%) (Figure 1A). Co-infection of two or three genera was observed. The mixed infections between Ehrlichia and Hepatozoon, Ehrlichia and Babesia, and Hepatozoon and Babesia were found in 3, 8, and 3 samples, respectively. Co-infection involving Ehrlichia, Hepatozoon, and Babesia was detected in 3 samples (Figure 2).
Figure 1.
Tick-borne pathogens: Ehrlichia, Hepatozoon, Babesia, and co-infection rate (A) and sex-separate infection rate (B).
Figure 2.
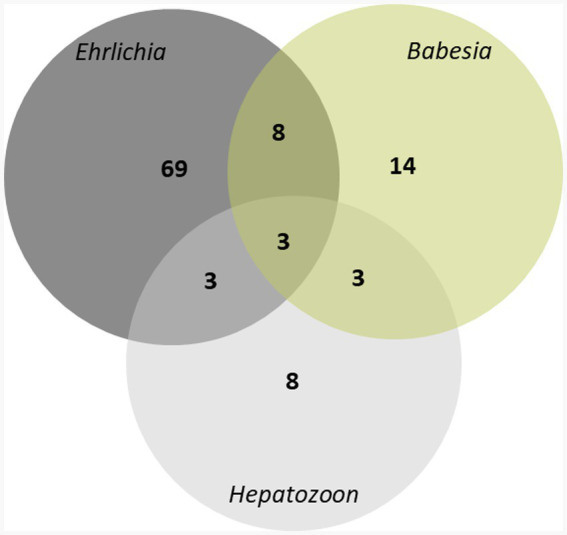
Venn diagram showing the composition of tick-borne pathogens infections, a total of 565 owned dogs were investigated for Ehrlichia, Hepatozoon, Babesia using multiplex PCR.
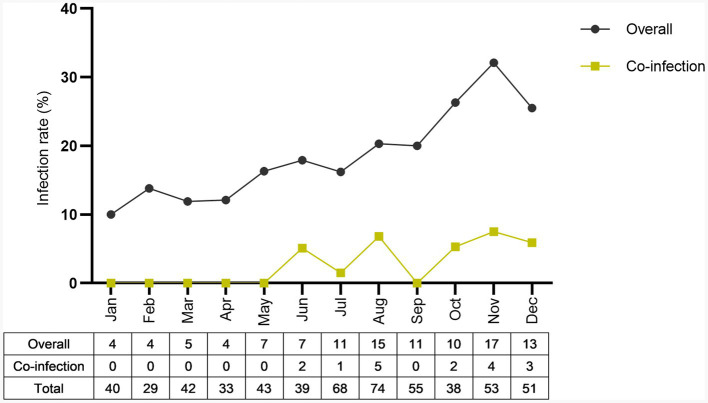
Overall infection rate by sex showed that the infection rate was higher in male dogs (60/299, 20.1%) as compared to female dogs (48/266, 18.0%), but not statistically significant (p = 0.592) (Supplementary Table 1A). The sex difference in infection rate is presented in Figure 1B. The proportion infected by sex is represented in Figure 3. Both sexes had a high infection rate of Ehrlichia, with male dogs exhibiting a higher infection rate than female dogs. Female dogs showed a higher infection rate of Hepatozoon and co-infection than male dogs. The infection rate of Babesia in male dogs was higher than in female dogs. There were no statistically significant differences between sexes in infection rate in all three genera of infection and co-infection (Supplementary Table 1A). Infection rate by age: five age groups were presented (Figure 4). The young adult age group (1–3 years) exhibits the highest infection rate both of overall infection rate (18/74, 24.3%) and co-infection rate (7/74, 9.5%) (Supplementary Table 1B). The co-infection rate was significantly different between age groups (p = 0.022). The infection rate of tick-borne pathogens changes over the course of a year. The monthly infection rate showed that Ehrlichia can be infect throughout the year (Supplementary Table 2). The increase of overall and co-infection rates occurred from June to December (Figure 5). The difference between infection rates by months was significant in co-infection but not overall (p = 0.018, p = 0.232 respectively). Moreover, co-infection rate is associated with overall infection rate (Pearson’s r = 0.81, p < 0.001).
Figure 3.
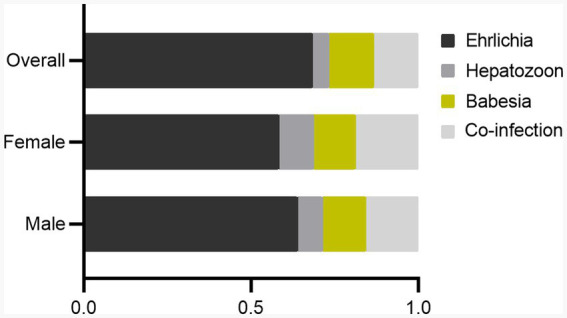
Bar chart showing the proportions of Ehrlichia, Hepatozoon, Babesia, and co-infection infected in male, female, and overall populations (based on infection rate data).
Figure 4.
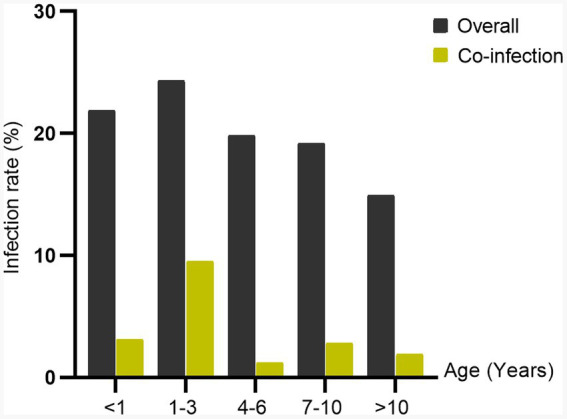
Bar chart demonstrating the infection rate of tick-borne pathogens: overall and co-infection by age group.
Figure 5.
Annual infection rate throughout the year (2022) was presented. Overall and co-infection monthly infection rate was reported as a seasonal trend pattern.
The blood alterations in Ehrlichia infected dogs were thrombocytopenia (57/66), eosinopenia (45/66), hyperproteinemia (43/66), and anemia (23/66). Dogs with Hepatozoon infection had prominent thrombocytopenia (5/7) and hyperproteinemia (4/7). Hematological changes in dogs infected with Babesia were thrombocytopenia (10/11), eosinopenia (9/11), hyperproteinemia (5/11), and anemia (5/11). The hematological alterations in co-infection dogs were observed. All three dogs co-infected with Ehrlichia, Hepatozoon, and Babesia developed leukocytosis (3/3), thrombocytopenia (3/3), eosinopenia (3/3), and hyperproteinemia (2/3) (Supplementary Table 3).
Discussion
The risk of disease transmission from ticks varies based on the geographic region and the specific tick species present there. Hong Kong indicates that the prevalence of Babesia spp. (28.8%) was higher than E. canis (7.4%) (22). In an Ethiopian study of tick-borne infections in dogs, H. canis registered the highest frequency of occurrence (53.8%), with E. canis infection determined at 2.6% (5). Our finding found the highest infection rate was Ehrlichia infection (12.2%). The difference in infection rates for the predominant pathogen may be due to various factors, including tick species, climate and ecology, frequency of tick-host interactions, and control measures (23, 24). Understanding the specific tick species and pathogens present is crucial for effective disease prevention and control. However, our study did not determine the infection rate of pathogens in dog ticks, which is an area that requires further investigation and should be addressed. The study in Northeast Thailand showed the high prevalence of H. canis (65.71%), followed by Babesia spp. (31.43%) and E. canis (30.0%), in brown dog ticks from infected dogs (25). In contrast, another research in same area found that E. canis is the most single pathogen in dogs (64.0%) and ticks (82.0%) (26). It’s essential to consider the possible role of seasonal variation and environmental factors. Our findings illustrate that Ehrlichia infection cases can occur throughout the year. While Babesia and Hepatozoon appear to be more infected rate during the wet season (Supplementary Table 2). The study suggests that climate change is affecting the distribution and infection rate of tick-borne pathogens, with increased rainfall intensity in May leading to an increase in the infection rate of individual pathogens and co-infections (Supplementary Table 2). The observed patterns in monthly infection rate suggest potential fluctuations in pathogen infection rate over time, but the limited duration of our study may not fully reflect the complexity of seasonal dynamics. Future research efforts might benefit from extending the study period across several years in order to completely analyze seasonal fluctuations in tick-borne pathogen co-infection occurrence.
This study aimed to identify tick-borne pathogens in dogs of different ages and allow a comprehensive understanding of age-related variations in these infections. Our findings reveal that co-infection can occur in any age group. However, co-infection of Ehrlichia, Hepatozoon, and Babesia was detected in dogs under 3 years. Dogs aged between 1and 3 years had the highest overall and co-infection rate. This suggests that younger age group is more susceptible to these infections compared to other age groups. The study in Costa Rica identified that the age of dogs, between 2 and 7 years, significantly influences the risk factors associated with E. canis seropositivity (27). Young age, less than 1 year, was identified as a risk factor for the co-infection prevalence of tick-borne pathogens in South India (28). The difference between susceptible age groups could be due to various factors, such as regional differences or variations in the study populations. Our findings emphasize that the age of dogs can play a significant role in their susceptibility to tick-borne infections and co-infections. Understanding these age-related variations is essential for effective prevention and management of tick-borne diseases in dogs.
Multiple infections caused by tick-borne pathogens are common in tropical and endemic areas where vectors are abundant. Rhipicephalus sanguineus is the main vector of E. canis, H. canis, and B. canis with different transmission route. Concurrent infections can have significant implications for disease pathogenesis and clinical symptoms. Co-infection has been reported to cause hematological alterations, including severe thrombocytopenia, anemia, and hypoalbuminemia (29). Another study through clinical pathological changes in co-infection of tick-borne pathogens in dogs identified anemia, thrombocytopenia, hypoalbuminemia, increased β2 and γ globulin fractions, and an increase in C-reactive protein concentrations (30). Our study found that co-infection in dogs can cause an increase in thrombocytopenia (Supplementary Table 3). In addition, leukocytosis, thrombocytopenia, eosinopenia, and hyperproteinemia were observed during the three pathogen infections. The pathogenicity of co-infections requires further investigation of how these co-infections interact and influence each other. Understanding how these co-infections interact with each other is necessary for both diagnosis and treatment.
Conclusion
The study provides valuable data for disease management and prevention related to tick-borne diseases in urban areas. The study suggests that young adult dogs (aged 1–3 years) had the highest infection rate of both single infections and co-infections. The rate of the co-infection of tick-borne pathogens exhibits an occurrence time interval. Understanding the infection rate and risk factors, such as age and seasonality, is crucial for implementing effective prevention and control measures.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because this study did not directly use animal samples; it only used data from animals that had previously been treated and diagnosed in an animal hospital. The researcher contacted the animal owners to explain the scope of the research and request permission to use animal data in this research. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
TC: Data curation, Writing – review & editing. AS: Conceptualization, Formal analysis, Visualization, Writing – original draft. CM: Investigation, Writing – original draft. LS: Validation, Writing – original draft. ST: Writing – original draft.
Acknowledgments
We are grateful for the help and expertise of staff at Prasu Arthorn Veterinary Teaching Hospital and the Monitoring and Surveillance Center for Zoonotic Diseases in Wildlife and Exotic Animals (MoZWE), Faculty of Veterinary Science, Mahidol University, for data collection. Without your support, this work could not have been undertaken.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Faculty of Veterinary Science, Mahidol University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1341254/full#supplementary-material
References
- 1.Komnenou AA, Mylonakis ME, Kouti V, Tendoma L, Leontides L, Skountzou E, et al. Ocular manifestations of natural canine monocytic ehrlichiosis (Ehrlichia canis): a retrospective study of 90 cases. Vet Ophthalmol. (2007) 10:137–42. doi: 10.1111/j.1463-5224.2007.00508.x, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Mathew JS, Ewing SA, Barker RW, Fox JC, Dawson JE, Warner CK, et al. Attempted transmission of Ehrlichia canis by Rhipicephalus sanguineus after passage in cell culture. Am J Vet Res. (1996) 57:1594–8. doi: 10.2460/ajvr.1996.57.11.1594, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Allen KE, Johnson EM, Little SE. Hepatozoon spp infections in the United States. Vet Clin North Am Small Anim Pract. (2011) 41:1221–38. doi: 10.1016/j.cvsm.2011.08.006, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Otranto D, Dantas-Torres F, Weigl S, Latrofa MS, Stanneck D, Decaprariis D, et al. Diagnosis of Hepatozoon canis in young dogs by cytology and PCR. Parasit Vectors. (2011) 4:55. doi: 10.1186/1756-3305-4-55, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadesse H, Grillini M, Simonato G, Mondin A, Dotto G, Frangipane di Regalbono A, et al. Epidemiological survey on tick-borne pathogens with zoonotic potential in dog populations of southern Ethiopia. Trop Med Infect Dis. (2023) 8:102. doi: 10.3390/tropicalmed8020102, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin F, Guo C, Li D, Tian Z, Li F. Molecular survey and genetic characteristics of vector-borne pathogens in domestic dogs from four regions of China. Animals. (2023) 13:1867. doi: 10.3390/ani13111867, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baneth G, Samish M, Alekseev E, Aroch I, Shkap V. Transmission of hepatozoon canis to dogs by naturally-fed or percutaneously-injected Rhipicephalus sanguineus ticks. J Parasitol. (2001) 87:606–11. doi: 10.1645/0022-3395(2001)087[0606:TOHCTD]2.0.CO;2, PMID: [DOI] [PubMed] [Google Scholar]
- 8.O'Dwyer LH. Brazilian canine hepatozoonosis. Rev Bras Parasitol Vet. (2011) 20:181–93. doi: 10.1590/s1984-29612011000300002, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Panti-May JA, Rodríguez-Vivas RI. Canine babesiosis: a literature review of prevalence, distribution, and diagnosis in Latin America and the Caribbean. Vet Parasitol Reg Stud Reports. (2020) 21:100417. doi: 10.1016/j.vprsr.2020.100417, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Cacciò SM, Antunovic B, Moretti A, Mangili V, Marinculic A, Baric RR, et al. Molecular characterisation of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet Parasitol. (2002) 106:285–92. doi: 10.1016/s0304-4017(02)00112-7, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Obeta SS, Ibrahim B, Lawal IA, Natala JA, Ogo NI, Balogun EO. Prevalence of canine babesiosis and their risk factors among asymptomatic dogs in the federal capital territory, Abuja, Nigeria. Parasite Epidemiol Control. (2020) 11:e00186. doi: 10.1016/j.parepi.2020.e00186, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baneth G. Antiprotozoal treatment of canine babesiosis. Vet Parasitol. (2018) 254:58–63. doi: 10.1016/j.vetpar.2018.03.001, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Aktas M, Özübek S, Altay K, Ipek N, Balkaya İ, Utuk A, et al. Molecular detection of tick-borne rickettsial and protozoan pathogens in domestic dogs from Turkey. Parasit Vectors. (2015) 8:157. doi: 10.1186/s13071-015-0763-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ojeda-Chi MM, Rodriguez-Vivas RI, Esteve-Gasent MD, Pérez de León AA, Modarelli JJ, Villegas-Perez SL. Ehrlichia canis in dogs of Mexico: prevalence, incidence, co-infection and factors associated. Comp Immunol Microbiol Infect Dis. (2019) 67:101351. doi: 10.1016/j.cimid.2019.101351, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Hii SF, Traub RJ, Thompson MF, Henning J, O'Leary CA, Burleigh A, et al. Canine tick-borne pathogens and associated risk factors in dogs presenting with and without clinical signs consistent with tick-borne diseases in northern Australia. Aust Vet J. (2015) 93:58–66. doi: 10.1111/avj.12293, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Mahachi K, Kontowicz E, Anderson B, Toepp AJ, Lima AL, Larson M, et al. Predominant risk factors for tick-borne co-infections in hunting dogs from the USA. Parasit Vectors. (2020) 13:247. doi: 10.1186/s13071-020-04118-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabsley MJ, McKibben J, Macpherson CN, Cattan PF, Cherry NA, Hegarty BC, et al. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Vet Parasitol. (2008) 151:279–85. doi: 10.1016/j.vetpar.2007.11.008, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Namina A, Capligina V, Seleznova M, Aleinikova D, Krumins R, Kivrane A, et al. Tick-borne pathogens in ticks collected from dogs, Latvia, 2011-2016. BMC Vet Res. (2019) 15:398. doi: 10.1186/s12917-019-2149-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeb J, Song B, Khan MA, Senbill H, Aziz MU, Hussain S, et al. Genetic diversity of vector-borne pathogens in ixodid ticks infesting dogs from Pakistan with notes on Ehrlichia canis, Rickettsia raoultii and Dirofilaria immitis detection. Parasit Vectors. (2023) 16:214. doi: 10.1186/s13071-023-05804-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kledmanee K, Suwanpakdee S, Krajangwong S, Chatsiriwech J, Suksai P, Suwannachat P, et al. Development of multiplex polymerase chain reaction for detection of Ehrlichia canis, Babesia spp and Hepatozoon canis in canine blood. Southeast Asian J Trop Med Public Health. (2009) 40:35–9. PMID: [PubMed] [Google Scholar]
- 21.Spolidorio MG, Torres MM, Campos WN, Melo ALT, Igarashi M, Amude AM, et al. Molecular detection of Hepatozoon canis and Babesia canis vogeli in domestic dogs from Cuiabá, Brazil. Rev Bras Parasitol Vet. (2011) 20:253–5. doi: 10.1590/s1984-29612011000300015, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Muguiro DH, Nekouei O, Lee KY, Hill F, Barrs VR. Prevalence of Babesia and Ehrlichia in owned dogs with suspected tick-borne infection in Hong Kong, and risk factors associated with Babesia gibsoni. Prev Vet Med. (2023) 214:105908. doi: 10.1016/j.prevetmed.2023.105908, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Estrada-Peña A, Ayllón N, de la Fuente J. Impact of climate trends on tick-borne pathogen transmission. Front Physiol. (2012) 3:64. doi: 10.3389/fphys.2012.00064, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden NH, Lindsay LR. Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol. (2016) 32:646–56. doi: 10.1016/j.pt.2016.04.015, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Eamudomkarn C, Pitaksakulrat O, Boueroy P, Thanasuwan S, Watwiengkam N, Artchayasawat A, et al. Prevalence of Ehrlichia-, Babesia-, and Hepatozoon-infected brown dog ticks in Khon Kaen Province, Northeast Thailand. Vet World. (2022) 15:1699–705. doi: 10.14202/vetworld.2022.1699-1705, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juasook A, Siriporn B, Nopphakhun N, Phetpoang P, Khamyang S. Molecular detection of tick-borne pathogens in infected dogs associated with Rhipicephalus sanguineus tick infestation in Thailand. Vet World. (2021) 14:1631–7. doi: 10.14202/vetworld.2021.1631-1637, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrantes-González AV, Jiménez-Rocha AE, Romero-Zuñiga JJ, Dolz G. Serology, molecular detection and risk factors of Ehrlichia canis infection in dogs in Costa Rica. Ticks Tick Borne Dis. (2016) 7:1245–51. doi: 10.1016/j.ttbdis.2016.07.006, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Kopparthi J, Chennuru S, Rayulu Vukka C, Nalini Kumari K, Rani PD. Co-infections of major tick-borne pathogens of dogs in Andhra Pradesh, South India. Vet Res Forum. (2023) 14:295–9. doi: 10.30466/vrf.2022.546812.3351, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gal A, Harrus S, Arcoh I, Lavy E, Aizenberg I, Mekuzas-Yisaschar Y, et al. Coinfection with multiple tick-borne and intestinal parasites in a 6-week-old dog. Can Vet J. (2007) 48:619–22. PMID: [PMC free article] [PubMed] [Google Scholar]
- 30.Asawakarn S, Dhitavat S, Taweethavonsawat P. Evaluation of the hematological and serum protein profiles of blood parasite coinfection in naturally infected dogs. TJVM. (2021) 51:723–8. doi: 10.56808/2985-1130.3171 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.