Abstract
The transition from childhood to adulthood represents the developmental time frame in which the majority of psychiatric disorders emerge. Recent efforts to identify risk factors mediating the susceptibility to psychopathology have led to a heightened focus on both typical and atypical trajectories of neural circuit maturation. Mounting evidence has highlighted the immense neural plasticity apparent in the developing brain. Although in many cases adaptive, the capacity for neural circuit alteration also induces a state of vulnerability to environmental perturbations, such that early-life experiences have long-lasting implications for cognitive and emotional functioning in adulthood. The authors outline preclinical and neuroimaging studies of normative human brain circuit development, as well as parallel efforts covered in this issue of the Journal, to identify brain circuit alterations in psychiatric disorders that frequently emerge in developing populations. Continued translational research into the interactive effects of neurobiological development and external factors will be crucial for identifying early-life risk factors that may contribute to the emergence of psychiatric illness and provide the key to optimizing treatments.
In recent years, significant interest has been directed to understanding the interplay between the specific neurobiological and behavioral factors that characterize developmental stages. Research in this area has burgeoned in large part as a result of the motivation to identify why particular individuals are susceptible to negative outcomes. Indeed, as illustrated in Figure 1, up to three-quarters of all psychiatric disorders emerge before age 24 (1–3). Moreover, the developmental emergence of psychopathology has been associated with greater severity of symptoms, comorbidities, and higher recurrence rates (4–6). More often than not, cognitive, emotional, and behavioral responses are based on prior experiences that can begin in the earliest stages of life. Thus, a complete understanding of cognitive and emotional functioning must include a deep appreciation of early-life experiences and environments (7). To gain insight into how developing systems function normally, as well as how they may go awry in psychiatric disorders, it is important to delineate how complex developmental trajectories and external factors interact to influence neural circuitry. Neurobiological research has consistently illustrated high conservation in motivated learning and emotion-related systems across species, lending support to the translational value of pre-clinical animal models (7, 8).
FIGURE 1.
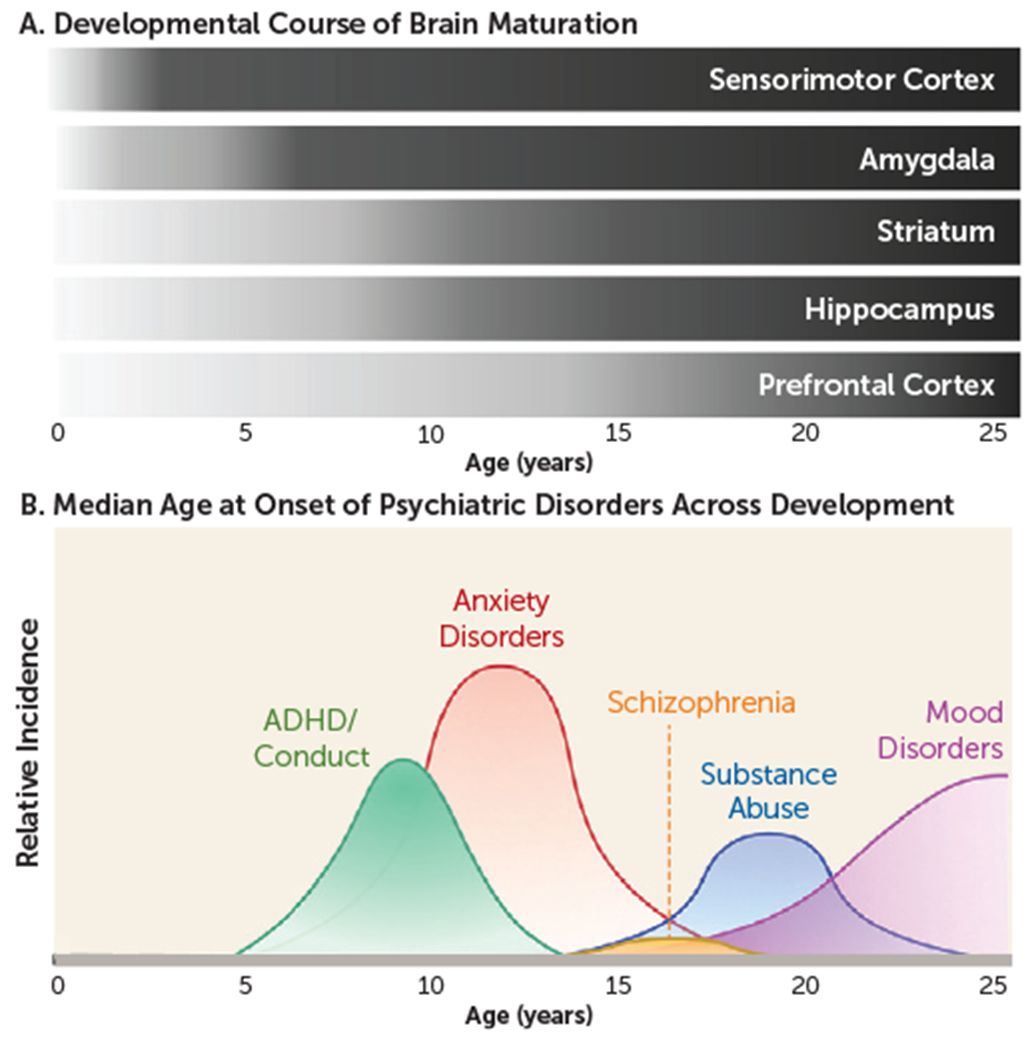
Brain development and emergence of psychiatric disorders
Across species, the developing brain is characterized by a high degree of neural plasticity, which is ecologically advantageous, as it allows for the refinement of neurocircuitry that is specifically tuned to the demands of the surrounding environment. However, the same capacity for neural alteration can make the developing brain particularly vulnerable. Indeed, perturbations to maturing systems can disrupt the refinement of cortical circuits, resulting in long-term consequences for both cognitive and emotional functioning. Moreover, developmental dysregulation of emotional memory systems is a principal component of many psychiatric disorders.
INFANTILE AND CHILDHOOD DEVELOPMENT OF FEAR AND THREAT RESPONDING
One of the earliest examples of functional neurocircuitry commonly studied is the circuitry underlying threat responding. From a very young age, fear and anxiety-related responding can be advantageous. However, numerous psychiatric conditions in which altered fear processing is excessive also emerge during development as the brain is undergoing complex and dynamic changes. Thus, understanding the development of fear and anxiety systems is critical for developing strategies to mitigate fear, stress, and anxiety disorders.
The processing and responding to threats in early life has been shown to differ fundamentally from that observed in adulthood (9–11). In rodents, fear memories acquired before postnatal day 10, during a highly caregiver dependent developmental window of rodent infancy before brief excursions from the nest begin to take place (similar to the first year of human infancy [7]), are not as robust or persistent as those acquired later in life and remain susceptible to forgetting through a process known as infantile amnesia (12–15). Functional emergence of the amygdala during childhood (Figure 1) (and following postnatal day 10 in rodents) coincides with more traditional fear learning to conditioned stimuli (11, 16–18), although retention of fear-related memories is shorter compared with adults (15). Moreover, learned fear associations are not subject to forms of contextually mediated relapse (e.g., renewal, reinstatement, and spontaneous recovery) after extinction (19, 20), which are commonly observed in adults and have been taken to indicate that extinction does not erase a fear memory, because conditioned responding can return after a change in context, reexposure to an aversive outcome, or the passage of time (21). In addition, relative to cued conditioning, contextual conditioning in rodents emerges later in development (22–25).
As additional regions known to be crucial for fear learning are engaged across development, adult-like fear patterns emerge. In particular, circuitry appears to be largely dependent on the amygdala in early life, with the network increasing in complexity by integrating prefrontal and hippocampal connections as these regions and the connections between them develop over the course of childhood. For example, the inverse activity with the amygdala of the prelimbic prefrontal cortex (PFC) for fear expression and the infralimbic PFC for fear attenuation that is observed in adults (26, 27) emerges after the juvenile period (19). This corresponds to the longer-lasting retention of cued fear associations. In addition, both contextual fear memory and contextually mediated relapse emerge with the maturation and integration of hippocampal circuitry (28) (Figure 1).
IMPACT OF STRESS ON INFANTILE AND CHILDHOOD DEVELOPMENT OF FEAR, ANXIETY, AND THREAT RESPONDING
Interestingly, mounting evidence suggests that exposure to stressors during early life can shift the timing of prefrontal and subcortical development (29–33). Because of the high dependence developing individuals have on their caregiver, it is not surprising that deviations in caregiving, including physical and emotional abuse, neglect, parental death or incarceration, and child institutionalization, have been a point of focus for their influence on the development of circuitry underlying emotion and motivated behavior. Although caregivers play a central role in suppressing threat reactivity during infancy and early childhood in rodents, nonhuman primates, and humans (34–37), disruptions to this role can contribute to differential development of fear systems that may increase the propensity for later psychopathology (7, 38–40).
A compelling series of studies has led to the suggestion that early-life stress may actually initiate precocious structural and connectivity profiles of the fear neurocircuitry (29, 30, 41, 42) that have been associated with adult-like patterns of behavior (41, 43). While these changes may initially be adaptive for meeting the needs of the developing organism in an adverse environment (30, 44), long-term consequences may also arise from altered developmental trajectories. Indeed, changes to the brain following early-life stress have been associated with psychopathology, including symptoms of depression and anxiety as well as substance use disorders (5, 29, 45–47).
ADOLESCENT DEVELOPMENT OF MOTIVATED RESPONDING
While robust structural changes occur within specific brain regions during infancy and childhood, the refinement of connectivity within and between brain regions has been shown to play a central role in adolescent development (48, 49). Notably, a shift from predominant connectivity between anatomically proximal regions to functional inter-connectivity, especially between distributed networks, increases as the adolescent period progresses (50, 51), and this has been correlated with greater efficiency of cortical processing (52). Moreover, heightened plasticity and the formation of integrated circuitry allows adolescents to interpret the demands of complex and variable environments and responding accordingly, making the adolescent brain well suited to forms of learning that occur in uncertain or changing environments as the individual establishes an independent life (53–56).
At the same time, adolescence reflects a “sensitive window” during which circuit-level formation is highly responsive to environmental information. For example, the characteristic features of the adolescent brain, such as a predominance of subcortical regions over the prefrontal cortex, promote heightened sensitivity to both appetitive and aversive emotionally salient cues. Moreover, states of heightened emotional arousal disrupt deliberative executive functioning and inhibitory control to a greater extent during adolescence than in other age period (57–61). Although the capacity for encoding an appetitive memory is apparent from early in infancy (62), interactions between reward circuitry and behavior have been a preeminent focus of research during later childhood and adolescence, because of an apparent hypersensitivity to reinforcers (61, 63, 64) and even cues signaling a potential reinforcer (55, 57). Sensitivity to threat is also a marked characteristic of adolescence. Indeed, recent evidence from both human and animal studies has indicated that adolescents exhibit an increased acquisition of threat responding (65, 66) as well as diminished extinction learning and extended fear retention relative to younger and older individuals (67–71). This is particularly interesting given that it occurs despite apparent adult-like patterns of fear responding in the juvenile period immediately preceding adolescence (23, 67).
Peaks in sensation seeking during adolescence are often attributed to immaturities in frontostriatal circuitry (72, 73), with improvements in cognitive control coinciding with continued development of this circuitry across the adolescent period (74). On the other hand, substantial changes in the reactivity and connectivity of the amygdala, hippocampus, and PFC are believed to mediate altered fear learning during this time (23, 67, 75–77). Indeed, development of the PFC is protracted relative to most other brain regions, continuing well into adolescence (78–80). Meanwhile, the earlier maturation of subcortical limbic regions (e.g., the nucleus accumbens and amygdala) can result in disproportionately higher activity emerging in subcortical regions relative to the PFC during adolescence (64) (Figure 1). Notably, experimentally inducing a functional imbalance of this kind between the late-developing PFC and earlier-developing subcortical limbic regions has been shown to disrupt performance in an inhibitory learning task (81), indicating that this characteristic of adolescent brain development may be a critical determinant of the capacity for behavioral regulation (82).
With regard to fear neurocircuitry, amygdala projections to the cortex undergo significant development during adolescence (83–85), with bidirectional prelimbic-amygdala synapses maturing earlier than infralimbic-amygdala synapses (86). In addition, during early adolescence, connectivity between basolateral amygdala and prelimbic cortex as well as ventral hippocampus and prelimbic cortex actually appears to be increased (75). Connectivity between prelimbic cortex and amygdala has been linked to the expression of fear, while connectivity between infralimbic cortex and amygdala is critical for attenuating fear (87, 88). Thus, the protracted development of the latter circuit, combined with an overall increase in amygdala signaling, may explain a range of adolescent behaviors indicative of threat sensitivity. In addition, an inability to retrieve contextual fear memories has been observed specifically during adolescence and has been linked to a lack of memory retrieval–associated signaling in the hippocampus (23).
Similarly, in humans, both functional connectivity and the degree to which prefrontal activity synchronizes with regions such as the amygdala or hippocampus increase from childhood to adulthood (76, 89, 90). Moreover, connectivity between amygdala and PFC appears to become more negative with age, reflective of an inverse correlation (and perhaps top-down inhibition) between activity in these regions (76).
IMPACT OF STRESS ON ADOLESCENT DEVELOPMENT OF MOTIVATED RESPONDING
As with earlier developmental stages, the extensive changes in neural circuitry occurring during adolescence leave the brain susceptible to environmental stressors that may alter normative developmental trajectories. In particular, because of the social restructuring that characterizes the transition to adolescence, in which there is a shift in focus from relationships with family members to relationships with peers (91, 92), exposure to social stressors can profoundly affect the normative developmental trajectory of the brain (5, 93–95). Notably, the effects of stress appear to be most robust when the stress occurs during adolescence (5, 96, 97) and can include a reduced ability to regulate distress (95, 98–100) and a marked increase in susceptibility to psychopathology (101). Exposure to social stress is just one example of the impacts that the environment can have on brain and behavioral development. Additional factors, such as diet, exercise, and drug use, among many others, have also been shown to dramatically influence brain development and contribute to the prevalence of psychiatric disorders.
NEURODEVELOPMENTAL STUDIES IN PSYCHIATRIC POPULATIONS
In addition to preclinical studies and neuroimaging studies of normative human brain circuit development, there have recently been parallel efforts to identify brain circuit alterations in psychiatric disorders that frequently emerge in developing populations, based on the assumption that there are discrete neural circuit alterations that correspond to the early signs and symptoms characterizing these disorders. The articles by Tromp et al. (102) and Jalbrzikowski et al. (103) in this issue of the Journal highlight these latest efforts.
With regard to anxiety disorders, which are among the most common psychiatric illnesses in adolescents, affecting as many as 1 in 10 (1, 104), there have been extensive investigations, using a variety of neuroimaging modalities, including task-based functional MRI (fMRI) studies, diffusion tensor imaging (DTI), and resting-state fMRI (rsfMRI) analyses. A general consensus among the task-based fMRI studies has been that there is marked elevation of amygdala activity in patients with anxiety disorders (105). In addition, decreased resting-state connectivity has been identified in patients with anxiety disorders compared with control subjects between the amygdala nuclei and prefrontal cortical structures, including the anterior cingulate cortex, medial PFC, and orbitofrontal cortex (106–108). Structural connectivity studies using DTI in patients with anxiety disorders have also identified decreased functional anisotropy, a measure of white matter microstructure, in the uncinate fasciculus, a white matter tract connecting cortical and hippocampal regions with subcortical structures, including the amygdala (109, 110). However, most of these studies were performed in adult patients with anxiety disorders. With regard to hyperactivation of the amygdala, the few studies in children and adolescents with anxiety disorders are consistent with studies in adult patients (111, 112). In addition, studies in young rhesus monkeys with increased anxiety-related behavioral temperament have reported decreased functional connectivity between the dorsolateral PFC and central amygdala as well as heightened metabolism in the amygdala (108, 113).
These findings should be considered in the context of the normative developmental changes in PFC-amygdala connectivity. As outlined above, multiple studies in typically developing populations have demonstrated an overall reduction in structural and functional connectivity between PFC and amygdala from childhood to adulthood (114, 115). This normative reduction in connectivity in this circuit likely represents a potential neurobiological basis for the improvements in emotion regulation observed over the course of development. However, during the adolescence time frame, there have been divergent reports in human neuroimaging studies of possible transient increases in amygdala-PFC functional connectivity (90, 116). These studies highlight the need for additional research assessing the transitions that occur during adolescence, when dynamic reorganization of multiple neural circuitry is taking place (75, 114).
One significant question is whether the neural circuit alterations observed in patients preceded the emergence of the disorder or rather reflect the chronicity or other sequelae of the disorder, such as effects of medications. In this issue of the Journal, the article by Tromp et al. (102) addresses this key question by performing detailed DTI studies in unmedicated preadolescent children (ages 8–12) with anxiety disorders and in age-matched control subjects. Based on tract-based DTI analyses, the authors found selectively decreased fractional anisotropy in the uncinate fasciculus in children with anxiety disorders. Interestingly, these neuroanatomical alterations were observed only in boys with anxiety disorders. While previous studies in adults, adolescents, and children with anxiety disorders have reported alterations in the uncinate fasciculus, no sex differences have been reported previously. This study is significant, as it is one of the first to suggest that children with anxiety disorders have an a priori alteration in PFC structural connectivity that cannot be explained by treatment with medications. Moreover, the finding that it is male specific suggests that an increased focus on assessing sex differences is crucial to comprehensively investigating the neural correlates underlying anxiety disorders.
In addition to anxiety disorders, altered cortico-limbic connectivity has been seen in other disorders, such as bipolar disorder (117) and schizophrenia (118). As with anxiety disorders, the developmental onset of these neural circuitry changes has yet to be established. The article by Jalbrzikowski et al. in this issue of the Journal (103) addresses the emergence of neural alterations in patients with psychotic spectrum disorders. A notable strength of their rsfMRI study is the large number of age-matched control subjects, for a total of 1,062 participants. These rsfMRI data sets were used to construct normative developmental trajectories of amygdala connectivity across late childhood through young adulthood (ages 10–25). The authors were able first to replicate their previous finding (115) that in normative subjects, there was an overall decrease in centromedial amygdala connectivity with multiple brain regions, including the ventrolateral PFC, dorsolateral PFC, caudate, and thalamus. These findings are consistent with an overall decrease in connectivity shown more broadly between cortical and subcortical structures across development. Having mapped this “growth chart,” the authors then compared centromedial amygdala connectivity with various brain regions in youths with psychotic spectrum disorders. Interestingly, they found that these youths failed to show a developmental decrease in functional connectivity between centromedial amygdala and striatum, thalamus, ventrolateral PFC, and occipital cortex. In fact, at the earliest age (10 years), connectivity between the centromedial amygdala and these other brain regions already appears to be significantly reduced in youths with psychotic spectrum disorders, and there is subsequently little or no additional reduction in connectivity—and in some patients an actual increase in connectivity occurs across the transition to young adulthood. One interpretation of these findings is that in late childhood, patients with psychotic spectrum disorders have undergone a general accelerated developmental decrease in amygdala connectivity, similar to the precocious development of amygdala-centric circuitry observed in individuals who have undergone early-life stress or deprivation and in preclinical rodent models of early-life stress (29, 30, 41, 42). Conversely, later in development, by young adulthood, patients with psychotic spectrum disorders may have an altered trajectory characterized by increased amygdala connectivity to the lateral PFC, caudate, and occipital cortex, indicating continued alterations of these circuits during the transition into adulthood.
CONCLUSIONS
As highlighted in both these articles in this issue of the Journal as well as previous human neuroimaging and pre-clinical studies, the maturation of neural connectivity patterns in typically developing subjects, as well as in patients with psychiatric disorders, is a dynamic, nonlinear process that does not occur uniformly in all brain regions. The Tromp et al. (102) study suggests that in children with anxiety disorders, a key white matter tract, the uncinate fasciculus, displays decreased microstructural integrity, highlighting the possibility that decreased PFC-limbic connectivity may be a key early neuroanatomical hallmark of these disorders. It is particularly interesting that this effect is apparent in boys and not girls. Conversely, in the Jalbrzikowski et al. study (103), youths in this same late childhood age range who have psychotic spectrum disorders, compared with healthy youths, display reduced amygdala-PFC connectivity that then does not decrease across later development, suggesting a fundamentally different maturation process that may underlie the affective dysregulation that often precedes and predicts increased psychotic symptoms.
While enormous progress has been made in recent years in parsing out the neurobiological and behavioral patterns characteristic of developmental stages from infancy through adolescence, additional work in this area is necessary. In particular, the field is still limited in its understanding of how additional factors such as sex, genetic differences, early-life adversities, and other environmental factors influence the developmental landscape of learning and memory (14). Research elucidating typical and atypical brain development patterns will be crucial for identifying early-life risk factors that may underlie the emergence of mental illness and provide the key to treating susceptible individuals. Such an effort will depend in large part on optimizing clinical interventions specifically to treat symptoms as they manifest during childhood and adolescence, rather than relying on existing therapies that have largely been optimized for adults. The articles included in this issue of the Journal provide promising steps in this direction.
Acknowledgments
The authors acknowledge support from NIH grants NS052819 (Dr. Lee), 1TL1TR0002386-01 (Dr. Meyer), the Sackler Institute (Drs. Lee and Meyer), the Pritzker Neuropsychiatric Disorders Research Consortium (Dr. Lee), the New York–Presbyterian Youth Anxiety Center (Dr. Lee), and the DeWitt-Wallace Fund of the New York Community Trust (Drs. Lee and Meyer).
Footnotes
The authors report no financial relationships with commercial interests.
REFERENCES
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602 [DOI] [PubMed] [Google Scholar]
- 2.Paus T, Keshavan M, Giedd JN: Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 2008; 9:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Wang PS: The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health 2008; 29:115–129 [DOI] [PubMed] [Google Scholar]
- 4.Welsh JW, Knight JR, Hou SS-Y, et al. Association between substance use diagnoses and psychiatric disorders in an adolescent and young adult clinic-based population. J Adolesc Health 2017; 60:648–652 [DOI] [PubMed] [Google Scholar]
- 5.Andersen SL, Teicher MH: Stress, sensitive periods, and maturational events in adolescent depression. Trends Neurosci 2008; 31:183–191 [DOI] [PubMed] [Google Scholar]
- 6.Woodward LJ, Fergusson DM: Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry 2001; 40:1086–1093 [DOI] [PubMed] [Google Scholar]
- 7.Callaghan B, Meyer H, Opendak M, et al. Using a developmental ecology framework to align fear neurobiology across species. Annu Rev Clin Psychol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gee DG, Bath KG, Johnson CM, et al. Neurocognitive development of motivated behavior: dynamic changes across childhood and adolescence. J Neurosci 2018; 38:9433–9445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camp LL, Rudy JW: Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev Psychobiol 1988; 21:25–42 [DOI] [PubMed] [Google Scholar]
- 10.Graham AM, Fisher PA, Pfeifer JH: What sleeping babies hear: a functional MRI study of interparental conflict and infants’ emotion processing. Psychol Sci 2013; 24:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan RM, Landers M, Yeaman B, et al. Good memories of bad events in infancy. Nature 2000; 407:38–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alberini CM, Travaglia A: Infantile amnesia: a critical period of learning to learn and remember. J Neurosci 2017; 37:5783–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaghan BL, Li S, Richardson R: The elusive engram: what can infantile amnesia tell us about memory? Trends Neurosci 2014; 37:47–53 [DOI] [PubMed] [Google Scholar]
- 14.Pattwell SS, Bath KG: Emotional learning, stress, and development: an ever-changing landscape shaped by early-life experience. Neurobiol Learn Mem 2017; 143:36–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell BA, Spear NE: Ontogeny of memory. Psychol Rev 1972; 79:215–236 [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Raine A, Venables PH, et al. The development of skin conductance fear conditioning in children from ages 3 to 8 years. Dev Sci 2010; 13:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn CR, Klein DN, Lissek S, et al. The development of fear learning and generalization in 8–13 year-olds. Dev Psychobiol 2012; 54:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlund MW, Siegle GJ, Ladouceur CD, et al. Nothing to fear? Neural systems supporting avoidance behavior in healthy youths. Neuroimage 2010; 52:710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Hamlin AS, Richardson R: Fear extinction across development: the involvement of the medial prefrontal cortex as assessed by temporary inactivation and immunohistochemistry. J Neurosci 2009; 29:10802–10808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yap CSL, Richardson R: Extinction in the developing rat: an examination of renewal effects. Dev Psychobiol 2007; 49:565–575 [DOI] [PubMed] [Google Scholar]
- 21.Bouton ME: Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 2002; 52:976–986 [DOI] [PubMed] [Google Scholar]
- 22.Rudy JW: Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci 1993; 107:887–891 [DOI] [PubMed] [Google Scholar]
- 23.Pattwell SS, Bath KG, Casey BJ, et al. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc Natl Acad Sci USA 2011; 108:1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jablonski SA, Schiffino FL, Stanton ME: Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Dev Psychobiol 2012;54:714–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffino FL, Murawski NJ, Rosen JB, et al. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem 2011; 95:190–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ: Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 2011; 36:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, et al. Micro-stimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem 2006;13:728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson A, Brooks DC, Bouton ME: The role of the rat hippocampal system in several effects of context in extinction. Behav Neurosci 1995; 109:828–836 [DOI] [PubMed] [Google Scholar]
- 29.Tottenham N, Sheridan MA: A review of adversity, the amygdala, and the hippocampus: a consideration of developmental timing. Front Hum Neurosci 2010; 3:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 2013; 110:15638–15643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Harmelen AL, van Tol MJ, Dalgleish T, et al. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 2014; 9:2026–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson JL, Albert D, Iselin AM, et al. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc Cogn Affect Neurosci 2016; 11:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 2015; 77:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriceau S, Sullivan RM: Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci 2006; 9:1004–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunnar MR, Donzella B: Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 2002; 27:199–220 [DOI] [PubMed] [Google Scholar]
- 36.Gee DG, Gabard-Durnam L, Telzer EH, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci 2014; 25:2067–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gunnar MR, Hostinar CE, Sanchez MM, et al. Parental buffering of fear and stress neurobiology: reviewing parallels across rodent, monkey, and human models. Soc Neurosci 2015; 10:474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham AM, Buss C, Rasmussen JM, et al. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci 2016; 18:12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu A, Anh TT, Li Y, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 2015; 5:e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harlow HF, Mc Kinney WT Jr: Nonhuman primates and psychoses. J Autism Child Schizophr 1971; 1:368–375 [DOI] [PubMed] [Google Scholar]
- 41.Bath KG, Manzano-Nieves G, Goodwill H: Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm Behav 2016; 82:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thijssen S, Muetzel RL, Bakermans-Kranenburg MJ, et al. Insensitive parenting may accelerate the development of the amygdala-medial prefrontal cortex circuit. Dev Psychopathol 2017; 29:505–518 [DOI] [PubMed] [Google Scholar]
- 43.Callaghan BL, Richardson R: Early experiences and the development of emotional learning systems in rats. Biol Mood Anxiety Disord 2013; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callaghan BL, Tottenham N: The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci 2016; 7:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 2010; 67:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bick J, Fox N, Zeanah C, et al. Early deprivation, atypical brain development, and internalizing symptoms in late childhood. Neuroscience 2017; 342:140–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen SL: Stress, sensitive periods, and substance abuse. Neurobiol Stress 2018; 10:100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blakemore SJ, Choudhury S: Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry 2006; 47:296–312 [DOI] [PubMed] [Google Scholar]
- 49.Hwang K, Velanova K, Luna B: Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci 2010; 30:15535–15545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens MC, Skudlarski P, Pearlson GD, et al. Age-related cognitive gains are mediated by the effects of white matter development on brain network integration. Neuroimage 2009; 48:738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fair DA, Cohen AL, Power JD, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol 2009; 5:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durston S, Davidson MC, Tottenham N, et al. A shift from diffuse to focal cortical activity with development. Dev Sci 2006; 9:1–8 [DOI] [PubMed] [Google Scholar]
- 53.Larsen B, Luna B: Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 2018; 94:179–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson C, Wilbrecht L: Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev Cogn Neurosci 2011; 1:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer HC, Bucci DJ: Age differences in appetitive Pavlovian conditioning and extinction in rats. Physiol Behav 2016; 167:354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simon NW, Gregory TA, Wood J, et al. Differences in response initiation and behavioral flexibility between adolescent and adult rats. Behav Neurosci 2013; 127:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hare TA, Tottenham N, Galvan A, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry 2008; 63:927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer HC, Bucci DJ: Setting the occasion for adolescent inhibitory control. Neurobiol Learn Mem 2017; 143:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spear LP: The Behavioral Neuroscience of Adolescence. New York, Norton, 2010 [Google Scholar]
- 60.Somerville LH, Hare T, Casey BJ: Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci 2011; 23:2123–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galván A: The teenage brain: sensitivity to rewards. Curr Dir Psychol Sci 2013; 22:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rovee CK, Rovee DT: Conjugate reinforcement of infant exploratory behavior. J Exp Child Psychol 1969; 8:33–39 [DOI] [PubMed] [Google Scholar]
- 63.Fareri DS, Martin LN, Delgado MR: Reward-related processing in the human brain: developmental considerations. Dev Psychopathol 2008; 20:1191–1211 [DOI] [PubMed] [Google Scholar]
- 64.Casey BJ, Jones RM, Hare TA: The adolescent brain. Ann N Y Acad Sci 2008; 1124:111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hefner K, Holmes A: Ontogeny of fear-, anxiety-, and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res 2007; 176:210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrow MC, Boring FW, Keough TE, et al. Differential GSR conditioning as a function of age. Dev Psychol 1969; 1:299–302 [Google Scholar]
- 67.Pattwell SS, Duhoux S, Hartley CA, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci USA 2012; 109:16318–16323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCallum J, Kim JH, Richardson R: Impaired extinction retention in adolescent rats: effects of d-cycloserine. Neuropsychopharmacology 2010; 35:2134–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker KD, Bisby MA, Richardson R: Impaired fear extinction in adolescent rodents: behavioural and neural analyses. Neurosci Biobehav Rev 2016; 70:59–73 [DOI] [PubMed] [Google Scholar]
- 70.Johnson DC, Casey BJ: Easy to remember, difficult to forget: the development of fear regulation. Dev Cogn Neurosci 2015; 11:42–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim JH, Li S, Richardson R: Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb Cortex 2011; 21:530–538 [DOI] [PubMed] [Google Scholar]
- 72.Chambers RA, Taylor JR, Potenza MN: Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 2003; 160:1041–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spear LP: The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24:417–463 [DOI] [PubMed] [Google Scholar]
- 74.Luna B, Marek S, Larsen B, et al. An integrative model of the maturation of cognitive control. Annu Rev Neurosci 2015; 38:151–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pattwell SS, Liston C, Jing D, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun 2016; 7:11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 2013; 33:4584–4593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gabard-Durnam LJ, Gee DG, Goff B, et al. Stimulus-elicited connectivity influences resting-state connectivity years later in human development: a prospective study. J Neurosci 2016; 36:4771–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Casey BJ, Tottenham N, Liston C, et al. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 2005; 9:104–110 [DOI] [PubMed] [Google Scholar]
- 79.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 1999; 2:861–863 [DOI] [PubMed] [Google Scholar]
- 80.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 2004; 101:8174–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meyer HC, Bucci DJ: Imbalanced activity in the orbitofrontal cortex and nucleus accumbens impairs behavioral inhibition. Curr Biol 2016; 26:2834–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Somerville LH: Systems neuroscience: the balancing act of behavioral regulation. Curr Biol 2016; 26:R925–R926 [DOI] [PubMed] [Google Scholar]
- 83.Arruda-Carvalho M, Wu W-C, Cummings KA, et al. Optogenetic examination of prefrontal-amygdala synaptic development. J Neurosci 2017; 37:2976–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cunningham MG, Bhattacharyya S, Benes FM: Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol 2002; 453:116–130 [DOI] [PubMed] [Google Scholar]
- 85.Cunningham MG, Bhattacharyya S, Benes FM: Increasing interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex 2008; 18:1529–1535 [DOI] [PubMed] [Google Scholar]
- 86.Chan T, Kyere K, Davis BR, et al. The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J Neurosci 2011; 31:4991–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sotres-Bayon F, Sierra-Mercado D,Pardilla-Delgado E, et al. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 2012; 76:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quirk GJ, Beer JS: Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 2006; 16:723–727 [DOI] [PubMed] [Google Scholar]
- 89.Perlman SB, Pelphrey KA: Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol 2011; 108:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qin S, Young CB, Supekar K, et al. Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci USA 2012; 109:7941–7946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nelson EE, Leibenluft E, McClure EB, et al. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med 2005; 35:163–174 [DOI] [PubMed] [Google Scholar]
- 92.Doremus-Fitzwater TL, Varlinskaya EI, Spear LP: Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn 2010; 72:114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Adams T, Rosenkranz JA: Social isolation during postweaning development causes hypoactivity of neurons in the medial nucleus of the male rat amygdala. Neuropsychopharmacology 2016; 41:1929–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gan JO, Bowline E, Lourenco FS, et al. Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice. Neuroscience 2014; 258:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sebastian CL, Tan GC, Roiser JP, et al. Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage 2011; 57:686–694 [DOI] [PubMed] [Google Scholar]
- 96.Jankord R, Solomon MB, Albertz J, et al. Stress vulnerability during adolescent development in rats. Endocrinology 2011; 152:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Einon DF, Morgan MJ: A critical period for social isolation in the rat. Dev Psychobiol 1977; 10:123–132 [DOI] [PubMed] [Google Scholar]
- 98.Arseneault L, Bowes L, Shakoor S: Bullying victimization in youths and mental health problems: “much ado about nothing”? Psychol Med 2010; 40:717–729 [DOI] [PubMed] [Google Scholar]
- 99.Skelly MJ, Chappell AE, Carter E, et al. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: possible role of disrupted noradrenergic signaling. Neuropharmacology 2015; 97:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukkes JL, Mokin MV, Scholl JL, et al. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav 2009; 55:248–256 [DOI] [PubMed] [Google Scholar]
- 101.Spear LP: Heightened stress responsivity and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol 2009; 21:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tromp DPM, Williams LE, Fox AS, et al. Altered uncinate fasciculus microstructure in childhood anxiety disorders in boys but not girls. Am J Psychiatry 2019; 176:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jalbrzikowski M, Murty VP, Tervo-Clemmens B, et al. Age-associated deviations of amygdala functional connectivity in youths with psychosis spectrum disorders: relevance to psychotic symptoms. Am J Psychiatry 2019; 176:196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010; 49:980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Etkin A, Wager TD: Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Etkin A, Prater KE, Hoeft F, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 2010; 167:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 2011; 56:881–889 [DOI] [PubMed] [Google Scholar]
- 108.Birn RM, Shackman AJ, Oler JA, et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry 2014; 19:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phan KL, Orlichenko A, Boyd E, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry 2009; 66:691–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tromp DP, Grupe DW, Oathes DJ, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry 2012; 69:925–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Williams LE, Oler JA, Fox AS, et al. Fear of the unknown: uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology 2015; 40:1428–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liao M, Yang F, Zhang Y, et al. White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry 2014; 14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox AS, Oler JA, Shackman AJ, et al. Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci USA 2015; 112:9118–9122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Casey BJ, Heller AS, Gee DG, et al. Development of the emotional brain. Neurosci Lett 2017; S0304-3940(17)30964-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jalbrzikowski M, Larsen B, Hallquist MN, et al. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry 2017; 82:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabard-Durnam LJ, Flannery J, Goff B, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage 2014; 95:193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh MK, Kelley RG, Chang KD, et al. Intrinsic amygdala functional connectivity in youth with bipolar I disorder. J Am Acad Child Adolesc Psychiatry 2015; 54:763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anticevic A, Tang Y, Cho YT, et al. Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr Bull 2014; 40:1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]


