Abstract
Background
Iron deficiency is a significant cause of deferral in people wishing to donate blood. If iron removed from the body through blood donation is not replaced, then donors may become iron deficient. All donors are screened at each visit for low haemoglobin (Hb) levels. However, some deferred blood donors do not return to donate. Deferred first‐time donors are even less likely to return. Interventions that reduce the risk of provoking iron deficiency and anaemia in blood donors will therefore increase the number of blood donations. Currently, iron supplementation for blood donors is not a standard of care in many blood services. A systematic review is required to answer specific questions regarding the efficacy and safety of iron supplementation in blood donors.
Objectives
To assess the efficacy and safety of iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors.
Search methods
We ran the search on 18 November 2013. We searched Cochrane Injuries Group Specialised Register, CENTRAL, PubMed, MEDLINE (OvidSP), EMBASE (OvidSP), CINAHL (EBSCO Host) and six other databases. We also searched clinical trials registers and screened guidelines reference lists.
Selection criteria
Randomised controlled trials (RCTs) comparing iron supplementation versus placebo or control, oral versus parenteral iron supplementation, iron supplementation versus iron‐rich food supplements, and different doses, treatment durations and preparations of iron supplementation in healthy blood donors. Autologous blood donors were excluded.
Data collection and analysis
We combined data using random‐effects meta‐analyses. We evaluated heterogeneity using the I2 statistic; we explored considerable heterogeneity (I2 > 75%) in subgroup analyses. We carried out sensitivity analyses to assess the impact of trial quality on the results.
Main results
Thirty RCTs (4704 participants) met the eligibility criteria, including 19 comparisons of iron supplementation and placebo or control; one comparison of oral and parenteral iron supplementation; four comparisons of different doses of iron supplementation; one comparison of different treatment durations of iron supplementation; and 12 comparisons of different iron supplementation preparations.
Many studies were of low or uncertain methodological quality and therefore at high or uncertain risk of bias. We therefore rated the quality of the evidence for our outcomes as moderate. There was a statistically significant reduction in deferral due to low haemoglobin in donors who received iron supplementation compared with donors who received no iron supplementation, both at the first donation visit after commencement of iron supplementation (risk ratio (RR) 0.34; 95% confidence interval (CI) 0.21 to 0.55; four studies; 1194 participants; P value < 0.0001) and at subsequent donations (RR 0.25; 95% CI 0.15 to 0.41; three studies; 793 participants; P value < 0.00001). Supplementation also resulted in significantly higher haemoglobin levels (mean difference (MD) 2.36 g/L; 95% CI 0.06 to 4.66; eight studies; 847 participants, P value =0.04), and iron stores, including serum ferritin (MD 13.98 ng/mL; 95% CI 8.92 to 19.03; five studies; 640 participants; P value < 0.00001) and transferrin saturation (MD 3.91%; 95% CI 2.02 to 5.80; four studies; 344 participants; P value < 0.0001) prior to further donation. The differences were maintained after subsequent donation(s).
Adverse effects were widely reported and were more frequent in donors who received iron supplementation (RR 1.60; 95% CI 1.23 to 2.07; four studies; 1748 participants; P value = 0.0005). Adverse effects included constipation, diarrhoea, nausea, vomiting and taste disturbances, and some participants stopped treatment due to side effects.
Authors' conclusions
There is moderate quality evidence that rates of donor deferral due to low haemoglobin are considerably less in those taking iron supplements compared with those without iron supplementation, both at the first donation visit and at subsequent donation. Iron‐supplemented donors also show elevated haemoglobin and iron stores. These beneficial effects are balanced by more frequent adverse events in donors who receive iron supplementation than in those who do not; this is likely to limit acceptability and compliance. The long‐term effects of iron supplementation without measurement of iron stores are unknown. These considerations are likely to preclude widespread use of iron supplementation by tablets. Blood services may consider targeted use of supplementation in those at greatest risk of iron deficiency, personalised donation intervals and providing dietary advice.
Keywords: Female; Humans; Male; Iron Deficiencies; Anemia, Iron‐Deficiency; Anemia, Iron‐Deficiency/blood; Anemia, Iron‐Deficiency/etiology; Anemia, Iron‐Deficiency/prevention & control; Blood Donors; Blood Donors/statistics & numerical data; Constipation; Constipation/etiology; Ferritins; Ferritins/blood; Hemoglobin A; Hemoglobin A/analysis; Iron; Iron/blood; Iron, Dietary; Iron, Dietary/administration & dosage; Iron, Dietary/adverse effects; Randomized Controlled Trials as Topic; Sex Factors
Plain language summary
The effects of iron supplementation on iron deficiency and deferral in blood donors
Iron deficiency can cause symptoms of tiredness. The interval between blood donations is set by independent regulators to minimise iron deficiency in donors. Potential blood donors are screened each time they visit to give blood to see if they have iron deficiency. Donors who do not pass this screening test and so cannot give blood are deferred and asked to delay giving blood, but many of these donors do not return. If blood donors take iron tablets then the risk of becoming iron deficient may be reduced. However, the balance between the benefits of giving iron and the possible side effects is not clear. We have reviewed all the randomised trials testing the benefits of giving blood donors iron. The evidence is current up to November 2013.
We found 30 randomised trials of iron supplementation in blood donors with a total of 4704 participants. We found that some of the studies did not report details of their design very well and people in some of the studies left the study early and did not contribute data. Combining the results from four studies, we have shown that around 3% of donors who were given iron supplements were unable to give blood when they next came to donate because the levels of iron in their blood were too low, compared with 10% of donors who did not take iron. More than this, 4% of iron‐supplemented donors were unable to give blood at any future donation due to low iron levels, compared with around 20% of donors not given iron supplementation.
However, 29% of donors who took iron tablets experienced side effects compared with 17% of donors who were given dummy tablets. Combined data from two studies showed that the iron‐supplemented donors had nearly five times the chance of stomach upsets and changes in their taste compared to donors who did not take these tablets.
Due to the issues around how reliable the studies were, the quality of evidence is moderate and these results could change with more research.
Donors can benefit from iron tablets but the rate of side effects is high, which means in practice that giving all donors iron tablets is unlikely to be acceptable and we do not know whether giving iron causes extra problems over a long period of time. Blood services may target iron supplementation at groups or individuals who are at risk of iron deficiency or may try to reduce deferral by adjusting donation intervals to suit the donor's ability to give blood without becoming iron deficient or to give specific dietary advice to donors.
Summary of findings
Summary of findings for the main comparison. Iron supplementation for iron deficiency and/or anaemia in blood donors.
| Iron supplementation for iron deficiency and/or anaemia in blood donors | ||||||
| Patient or population: patients with iron deficiency and/or anaemia in blood donors Settings: national blood services Intervention: iron supplementation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Iron supplementation | |||||
| Low Hb deferral ‐ at first donation visit after commencement of treatment | Study population1 | RR 0.34 (0.21 to 0.55) | 1194 (4 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 105 per 1000 | 36 per 1000 (22 to 58) | |||||
| Low1 | ||||||
| 28 per 1000 | 10 per 1000 (6 to 15) | |||||
| High1 | ||||||
| 237 per 1000 | 81 per 1000 (50 to 130) | |||||
| Low Hb deferral ‐ after multiple donation visits | Study population1 | RR 0.25 (0.15 to 0.41) | 793 (3 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 199 per 1000 | 50 per 1000 (30 to 81) | |||||
| Low1 | ||||||
| 50 per 1000 | 12 per 1000 (8 to 20) | |||||
| High1 | ||||||
| 456 per 1000 | 114 per 1000 (68 to 187) | |||||
| Hb (g/L) ‐ before further donation Scale from: 123 to 152 | The mean Hb (g/L) ‐ before further donation in the control groups was 135.2 g/L | The mean Hb (g/L) ‐ before further donation in the intervention groups was 2.36 higher (0.06 to 4.66 higher)3 | 847 (8 studies) | ⊕⊕⊕⊝ moderate4 | ||
| Hb (g/L) ‐ after subsequent donation(s) Scale from: 127.3 to 129 | The mean Hb (g/L) ‐ after subsequent donation(s) in the control groups was 127.8 g/L | The mean Hb (g/L) ‐ after subsequent donation(s) in the intervention groups was 6.37 higher (2.36 to 10.39 higher)3 | 406 (3 studies) | ⊕⊕⊕⊝ moderate5 | ||
| Serum ferritin (ng/mL) ‐ before further donation Scale from: 12.9 to 57.8 | The mean serum ferritin (ng/mL) ‐ before further donation in the control groups was 21.1 ng/mL | The mean serum ferritin (ng/mL) ‐ before further donation in the intervention groups was 13.98 higher (8.92 to 19.03 higher)3 | 640 (5 studies) | ⊕⊕⊕⊝ moderate6 | ||
| Serum ferritin (ng/mL) ‐ after subsequent donation(s) Scale from: 18 to 19 | The mean serum ferritin (ng/mL) ‐ after subsequent donation(s) in the control groups was 18.6 ng/mL | The mean serum ferritin (ng/mL) ‐ after subsequent donation(s) in the intervention groups was 9.01 higher (5.76 to 12.25 higher) | 619 (3 studies) | ⊕⊕⊕⊝ moderate7 | ||
| Adverse effects (any) | 171 per 1000 | 274 per 1000 (210 to 354) | RR 1.6 (1.23 to 2.07) | 1748 (4 studies) | ⊕⊕⊕⊝ moderate8 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; Hb: haemoglobin; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Control risks will depend on study‐specific low haemoglobin deferral thresholds. Low and high control risks correspond to the minimum and maximum control risks in the included studies. 2Most of the information is from studies with an unclear risk of bias. All but one study had a high risk of attrition bias and two studies were partially commercially funded. Potential limitations are likely to lower confidence in the estimate of the effect. 3The range of scores is based on the lowest and highest estimate of the scores in the control groups in individual trials. 4Most of the information is from studies with an unclear risk of bias. Four studies had a high risk of attrition bias, one study received assistance with data analysis from suppliers of the iron supplementation and one study did not blind participants. Potential limitations are likely to lower confidence in the estimate of the effect. 5Most of the information is from studies with an unclear risk of bias. All studies had a high risk of attrition bias. Potential limitations are likely to lower confidence in the estimate of the effect. 6Most of the information is from studies with an unclear risk of bias. Two studies had a high risk of attrition bias and one study was partially commercially funded. Potential limitations are likely to lower confidence in the estimate of the effect. 7Most of the information is from studies with an unclear risk of bias. All studies had a high risk of attrition bias and one study was partially commercially funded. Potential limitations are likely to lower confidence in the estimate of the effect. 8Most of the information is from studies with an unclear risk of bias. Two studies had a high risk of attrition bias. Potential limitations are likely to lower confidence in the estimate of the effect.
Background
Description of the condition
Red blood cells are donated, tested and administered on an industrial scale. In the United Kingdom, in England and North Wales, NHS Blood and Transplant (NHSBT) collects around 1.9 million red cell donations per annum (NHSBT 2013). Meeting the blood requirements of health services is becoming increasingly difficult because of changes in attitudes in society to donation. Difficulties in meeting demands are compounded by the short supply of particularly useful blood groups such as O rhesus negative, found in 7% of the population, as well as the need to provide blood of specific blood group types in patients who have developed multiple or widely reactive antibodies. This current trend of declining rates of blood donation, combined with an increase in the requirement for blood components, is likely to continue in coming years (Seifried 2011).
Healthy blood donors can donate whole blood (a standard single red cell unit ‐ approximately 450 mL ‐ collected manually), a double‐dose of red cells (two red cell units collected by machine in a process termed apheresis) and platelets (referred to as plateletpheresis). To be able to donate, blood donors have to answer a number of questions regarding their lifestyle, health, risk of infection and travel prior to donation (donor health check ‐ DHC), as well as exceed minimum physical requirements such as weight and haemoglobin (Hb) level. Donors with major illness, recent cold, fever, infection, gastrointestinal upset or who are generally feeling unwell are deferred from donating. Iron deficiency remains a significant cause of morbidity in the general population and in blood donors (Baart 2011). Up to 5% of new donors cannot be accepted because of low haemoglobin levels and iron deficiency may be responsible for the deferral of a similar percentage of regular donors.
Blood donation removes 250 mg of iron from the body and there is considerable individual variation in body stores of iron, in dietary iron and in the intrinsic capacity to absorb and utilise dietary iron. If this iron is not replaced, then donors may become iron deficient or develop frank iron‐deficiency anaemia. NHSBT and other blood services have a duty of care for blood donors, to ensure that harmful effects of donations are avoided. The period since the last donation is inversely associated with the risk of deferral due to low haemoglobin (Baart 2011). Lower limits for donation intervals are set to minimise iron deficiency in repeat blood donors. Furthermore, all donors are screened for low haemoglobin levels. Failing to pass the haemoglobin screening test from a fingerprick sample of blood corresponding to an haemoglobin level of 135 g/L for males or 125 g/L for females (in the United Kingdom) leads to 'deferral' (i.e. at least temporary rejection from blood donation) (Blood Safety and Quality Regulations 2005). A further appointment to donate is arranged after the normal inert‐donation interval, currently 16 weeks for women and 12 weeks for men in England, to allow donors to replenish their iron stores.
Although these safeguards to protect donors are essential, they can have an adverse effect on future donor behaviour. We know that deferral is associated with donor non‐return, especially among first‐time donors (Custer 2007). Moreover, increasingly rigorous donor selection criteria, combined with demographic changes, have reduced the numbers of first‐time donors. It is therefore essential not only to minimise unnecessary deferral, but also to reduce the risk of provoking iron deficiency or anaemia in order to optimise the number of blood donors and donations.
A number of markers of iron deficiency have been studied in existing Cochrane reviews, both of iron stores (ferritin) and circulating iron and iron available for erythropoiesis (serum iron, total iron binding concentration (TIBC) and % transferrin saturation). Haemoglobin levels and measures of iron deficiency fall in regular blood donors, particularly in pre‐menopausal women (Alvarez‐Ossorio 2000; Finch 1977; Milman 1996; Skikne 1984; Worwood 1993). Currently, iron supplementation for blood donors is not a standard of care in NHSBT. Interestingly, there is some evidence from existing randomised controlled trials (RCTs) of improvement of exercise tolerance, mood disturbance and restless legs syndrome after treatment of iron‐deficient but non‐anaemic adults (Earley 2009; Grote 2009). However, there are at present no formal systematic reviews of the benefits of iron supplementation interventions in terms of improved haemoglobin, iron status, subjective symptoms of fatigue or mood disturbance or cognitive function in blood donors or, of crucial interest to the blood services, of their deferral for low haemoglobin at the next attendance at donor clinics, nor of the adverse effects and costs of these supplementation strategies.
In spite of medical, logistic and even ethical problems that may be faced in implementing a programme of iron supplementation for blood donors, a pragmatic review of the benefits and costs for the donor and the blood service is essential to inform policy. A systematic review has been undertaken to answer the specific questions of the efficacy and safety of iron supplementation in blood donors in preventing a fall in haemoglobin, improving iron stores and reducing systemic, neurological or cognitive symptoms in donors.
Description of the intervention
Iron supplementation interventions aim to increase iron stores in blood donors. Iron stores are regulated through absorption of iron and so interventions either directly or indirectly increase iron available for absorption. Interventions may be in the form of dietary advice to increase the amount of iron‐rich food or in the form of oral iron supplementation, such as iron salts. There are a variety of formulations with different side effect profiles in different people. It is also possible to give parenteral (intravenous or intramuscular) iron if iron is poorly absorbed. However, this is unlikely to be an intervention used for blood donors due to a variety of reasons.
How the intervention might work
Iron supplementation interventions aim to increase iron stores in blood donors by increasing iron available for absorption.
Why it is important to do this review
This review is important as maintaining the supply and health of blood donors is imperative for health services. The role of iron supplementation in maintaining the health of donors and their ability to donate is poorly understood and the available evidence has not been synthesised. A systematic review of the current evidence of the efficacy and safety of iron supplementation in blood donors is crucial to inform future trials and policy of iron supplementation in donors.
Objectives
To assess the efficacy and safety of iron supplementation to reduce deferral, iron deficiency and/or anaemia in blood donors.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Healthy prospective first‐time or repeat blood donors: whole blood donors (standard one unit collection), double‐dose red cell donors and platelet donors (referred to as plateletpheresis). Autologous blood donors (donation of blood for the donor's own use) were excluded.
Types of interventions
Iron supplementation versus placebo/control.
Iron supplementation: oral versus parenteral iron supplementation.
Iron supplementation versus iron‐rich food supplements (fortified foods with a quantifiable amount of iron).
Iron supplementation, dose A versus iron supplementation, dose B.
Iron supplementation, treatment duration A versus iron supplementation, treatment duration B.
Iron supplementation, preparation A versus iron supplementation, preparation B.
Types of outcome measures
Primary outcomes
Risk ratio of deferral of blood donors (number of prospective blood donors who are at least temporarily rejected from blood donation) due to low haemoglobin.
The low haemoglobin deferral threshold differs across studies according to the population and sex of the donor studied (see Characteristics of included studies).
Secondary outcomes
Mean levels of haemoglobin (Hb), mean cell volume (MCV), other blood indices and iron stores before further donations.
Mean levels of Hb, mean cell volume (MCV), other blood indices and iron stores after subsequent donations.
Health‐related quality of life, especially changes in cognitive function, 'mood' disturbances, aerobic power, fatigue score, physical activity.
Adverse effects from interventions received.
Compliance.
Analysis of blood indices were restricted to Hb, MCV, serum ferritin, serum or plasma iron, total iron binding capacity (TIBC) and transferrin saturation. We noted other reported blood indices and described these in the Characteristics of included studies tables.
Search methods for identification of studies
The SRI's Information Specialist (CD) formulated the search strategies in collaboration with the Cochrane Injuries Group.
In order to reduce publication and retrieval bias we did not restrict our search by language, date or publication status.
Electronic searches
We searched the following for RCTs and systematic reviews:
Cochrane Injuries Group Specialised Register (December 2013) (Appendix 1);
CENTRAL (Cochrane Central Register of Controlled Trials (2013, Issue 10) (Appendix 1);
PubMed (epublications only) (Appendix 2);
MEDLINE (OvidSP) (1948 to 18 November 2013) (Appendix 3);
EMBASE (OvidSP) (1974 to 18 November 2013) (Appendix 4);
CINAHL (EBSCO Host) (1982 to 18 November 2013) (Appendix 5);
British Nursing Index and Archive (1985 to 18 November 2013) (Appendix 6);
Transfusion Evidence Library (1980 to 18 November 2013) (Appendix 7);
LILACS (1982 to 18 November 2013) (Appendix 8);
IndMed (1985 to 18 November 2013) (Appendix 9);
KoreaMed (1997 to 18 November 2013) (Appendix 10);
PakMediNet (1995 to 18 November 2013) (Appendix 11);
Web of Science Conference Proceedings Citation Index‐Science (CPCI‐S) (1990 to 18 November 2013) (Appendix 12).
We searched the following clinical trials registers:
ClinicalTrials.gov (www.clinicaltrials.gov) (18 November 2013);
ISRCTN Register (18 November 2013);
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/) (18 November 2013);
UMIN‐CTR Japanese Clinical Trials Registry (http://www.umin.ac.jp/ctr/) (18 November 2013);
Hong Kong Clinical Trials Registry (http://www.hkclinicaltrials.com/) (18 November 2013).
Search strategies used to search the registers are listed in Appendix 13.
In MEDLINE we combined the search strategy with the Cochrane highly sensitive filter for identifying RCTs, as detailed in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We combined searches in EMBASE and CINAHL with adaptations of the relevant SIGN RCT filters (http://www.sign.ac.uk/methodology/filters.html).
Two of the ongoing trial databases listed in the protocol (the Chinese Clinical Trials Registry and the Sri Lanka Clinical Trials Registry) are now included within the WHO ICTRP database.
Searching other resources
Handsearching of reference lists
We checked references of all identified trials, relevant review articles and current treatment guidelines for further literature. These searches were limited to the 'first‐generation' reference lists.
Data collection and analysis
Selection of studies
One review author, Carolyn Doree (CD), initially screened all search hits for relevance against the eligibility criteria and discarded all those that were clearly irrelevant. Thereafter two other authors (GS and SF) independently screened all the remaining hits (titles, abstracts and full text) for relevance against the full eligibility criteria. We retrieved full‐text papers for all those references for which a decision of eligibility could not be made from title and abstract alone. Where possible, we sought further information from the authors where articles contained insufficient data to make a decision about eligibility. We resolved differences of opinion through discussion and consensus, where necessary with reference to a third author, David Roberts (DR). We have detailed studies which did not meet our eligibility criteria in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (GS and SF) independently extracted data onto customised forms. We piloted these forms with two included RCTs; we made subsequent changes to the data extraction form where appropriate and agreed by both authors. Throughout the data extraction process we resolved any disagreements by consensus. If agreement could not be met, we consulted a third author (DR). The review authors were not blinded to names of authors, institutions, journals or the outcomes of the trials. We translated papers requiring translation into English prior to data extraction.
Where data were reported graphically and we considered graphs to be of sufficient quality, two authors (GAS, SAF) estimated values from the graph and used the average value across both estimates.
Several studies reported outcomes separately for males and females. In these studies, we combined data for males and females to enable comparisons with other studies.
Assessment of risk of bias in included studies
Two review authors (GS and SF) independently assessed all included studies for possible risk of bias and made explicit judgements about whether studies were at risk of bias according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the design, conduct and analysis of the trial using a three‐point scale: low risk of bias, high risk of bias or unclear. To assess risk of bias, the authors included the following questions in the 'Risk of bias' table for each included study:
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated intervention adequately prevented (i.e. blinded) throughout the study?
Were incomplete outcome data adequately addressed for every outcome?
Were reports of the study free of selective outcome reporting?
Was the study apparently free of other problems that could put it at risk of bias?
We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
In many of the included studies, reporting of randomisation and blinding methods used was poor. Several studies reported only that the trial was "double‐blind". We interpreted "double‐blind" in the context of iron supplementation trials as an indication that the participants (but not necessarily the outcome assessors) were blinded, and have classified such studies as having a low risk of performance bias. Use of a placebo was not considered sufficient alone to indicate blinding of participants.
Measures of treatment effect
Dichotomous outcomes are presented as risk ratios (RR) with 95% confidence intervals (CI). For continuous outcomes, we recorded the mean and standard deviation. For continuous outcomes measured using the same scale, the effect measure is the mean difference (MD) with 95% CI.
Unit of analysis issues
Within each comparison of interventions of this review, for studies with more than two treatment arms, we avoided multiple pairwise comparisons of treatment groups by pooling treatment groups as appropriate. For dichotomous variables, we summed count data across groups and for continuous variables, we calculated the mean and standard deviation of the combined group from the mean and standard deviations of each subgroup.
Thus, for the comparison of iron supplementation versus placebo, we combined multiple iron supplementation trial arms for an overall comparison with the control or placebo arm. Similarly, in the comparison of different iron preparations, we combined different doses of an identical preparation for comparison with an alternative iron preparation.
For studies in which results were reported separately for males and females, we combined these data for the main analyses. We converted standard errors, P values and confidence intervals to standard deviations where necessary. We excluded studies in which continuous variables were reported as medians or geometric means without a measure of variation from the analysis.
We undertook conversion of units of total iron from µmol/L to µg/dL using 1 µg/dL = 0.179 µmol/L where necessary to allow meta‐analysis across studies reporting outcome values using different units.
Dealing with missing data
In view of the time that had elapsed since publication of the majority of studies, we made no attempt to contact individual study authors or institutions regarding missing data. We recorded the number of patients lost to follow‐up for each trial as unexplained or analysed undocumented differences between the number of patients randomised and the number of patients, and incorporated this into the assessment of risk of bias. Our preferred analysis was intention‐to‐treat (ITT), but where insufficient data were presented in the included studies, we used per‐protocol analysis. Studies which performed ITT analyses are shown in the Characteristics of included studies tables.
Assessment of heterogeneity
We assessed statistical heterogeneity of treatment effects between trials using Chi2 tests with a significant level at P < 0.1. We used the I2 statistic to quantify the amount of possible heterogeneity (where I2 > 30% denotes moderate heterogeneity and I2 > 75% denotes considerable heterogeneity). We assessed uncertainty in I2 values with 95% confidence intervals calculated using the test based method (Higgins 2002). We explored potential causes of heterogeneity by sensitivity and subgroup analyses.
We assessed clinical heterogeneity based on individual study characteristics (e.g. by examining differences in study quality, in the donation history and donor characteristics, and in the definition or measurement of outcomes of each study).
Assessment of reporting biases
We made every effort to identify unpublished studies through searching of conference abstracts and ongoing trial databases as described in the Search methods for identification of studies. We intended to assess publication bias using funnel plots but the number of included studies was lower than the minimum suggested for evaluation of funnel plot asymmetry for all outcomes (Higgins 2011), therefore formal assessment of publication bias was not possible.
Data synthesis
We performed meta‐analyses using Review Manager software (RevMan 2012). We had intended to carry out meta‐analyses using fixed‐effect models initially. However, in view of the differences in study participants (first‐time donors, repeat donors, deferred donors) in the included studies and the likely heterogeneity between these groups, we used random‐effects models for all meta‐analyses.
We assessed the dichotomous outcome of rate of low Hb deferral at the first post‐treatment donation visit as well as after multiple post‐donation visits (i.e. the final visit over study period) and cumulatively over all donation visits during the study period.
Few studies reported continuous outcomes as mean change from baseline values and therefore we compared endpoint (follow‐up) values for all comparisons, with the exception of one study (Borch‐Iohnsen 1993), in which no endpoint values were reported. Data from this study were reported graphically with no measures of variation and therefore were not analysed. We assessed continuous outcomes at the first post‐donation visit prior to donation, and after post‐treatment donation or donations. We excluded one study in which measurements were taken at the first post‐treatment visit as it was unclear whether the measurement was taken prior to or after donation (Blot 1980).
As well as the quantitative synthesis described above, we made an overall interpretation of the data based on a qualitative summary of the included studies.
We produced a 'Summary of findings' table using the GRADE profiler, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011).
Subgroup analysis and investigation of heterogeneity
We investigated heterogeneity by visual inspection of forest plots and by formal subgroup analyses by sex for the comparison of iron supplementation versus placebo by comparing outcomes between male‐ and female‐specific studies as well as sex‐specific results reported within individual studies. We included one study of predominantly (98.8%) male participants as a male‐specific study in subgroup analyses of sex (Radtke 2004b). The number of studies for all other comparisons precluded subgroup analysis.
Sensitivity analysis
We assessed the robustness of findings for the primary outcome, risk ratio of low Hb deferral and for Hb and serum ferritin levels using sensitivity analysis, including only those trials at low risk of performance bias, and including only those trials in which 25% or less of randomised participants were lost to follow‐up.
Results
Description of studies
Results of the search
Searches of electronic databases carried out in April 2011 and updated in May 2013 and November 2013 identified a total of 1951 references. Removal of duplicates resulted in 1032 references, which two review authors (GAS, SAF) screened independently in duplicate. We resolved discrepancies through discussion with a third review author (DR). Initial screening of these 1032 references for eligibility against the inclusion criteria excluded a further 964 references. Of the remaining 68 references, we excluded 21 after closer inspection of the full text showed that they did not fully meet the eligibility criteria (as described in the Characteristics of excluded studies). Eight additional references describing seven independent trials met the inclusion criteria but did not report sufficient data for inclusion; details are given in Studies awaiting classification). One other reference described a trial protocol (see Characteristics of ongoing studies). Searches of ongoing trial databases resulted in 33 ongoing trials for screening, six of which were unpublished trials relevant to this review and are included as ongoing studies. Study classification is summarised by a PRISMA flow diagram (Figure 1).
1.
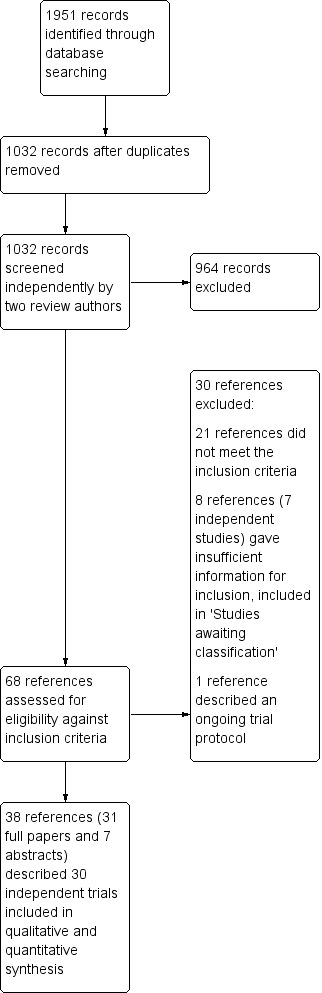
PRISMA study flow diagram.
Included studies
A total of 38 references (31 full papers and seven conference abstracts) describing 30 independent trials met the inclusion criteria. In one study, participants were stratified into two subgroups according to serum ferritin levels and each of the two treatments was randomised within both subgroups. For the purposes of this review, we treated these two independent participant subgroups as two separate trials (Mackintosh 1988_HSF; Mackintosh 1988_LSF).
Studies were carried out worldwide, including seven studies from the United States (Brittenham 1996; Cable 1988; Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b; Gordeuk 1990; Simon 1984), six from Sweden (Birgegard 2010; Ehn 1968; Frykman 1994; Lieden 1975; Lindholm 1981; Rybo 1971), four from South Africa (Jacobs 1993; Jacobs 2000; Mackintosh 1988_HSF; Mackintosh 1988_LSF), three from Germany (Busch 1972; Radtke 2004a; Radtke 2004b), three from Switzerland (Bucher 1973; Buzi 1980; Waldvogel 2012), two from Iran (Maghsudlu 2008; Mirrezaie 2008), two from Norway (Borch‐Iohnsen 1993; Rosvik 2010) and one each from France (Blot 1980), Italy (Landucci 1987) and Thailand (Linpisarn 1986).
Seven references describing six independent studies required translation into the English language (Blot 1980; Bucher 1973; Busch 1972; Buzi 1980; Ehn 1968; Lindholm 1981).
Participants
Six studies were of male donors only (Ehn 1968; Lieden 1975; Lindholm 1981; Linpisarn 1986; Mackintosh 1988_HSF; Mackintosh 1988_LSF), and in a further study 98.8% of participants were male (Radtke 2004b). Eleven studies included only females (Borch‐Iohnsen 1993; Brittenham 1996; Cable 1988; Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b; Gordeuk 1990; Maghsudlu 2008; Mirrezaie 2008; Simon 1984; Waldvogel 2012); eight of these were studies of women who were menstruating or of child‐bearing age (Borch‐Iohnsen 1993; Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b; Gordeuk 1990; Maghsudlu 2008; Mirrezaie 2008; Waldvogel 2012). Of the remaining 12 studies, five reported results separately for male and female donors (Birgegard 2010; Frykman 1994; Radtke 2004a; Rosvik 2010; Rybo 1971), six reported results pooled across male and female donors (Blot 1980; Bucher 1973; Busch 1972; Buzi 1980; Jacobs 1993; Landucci 1987), and one study did not specify the sex of the participants (Jacobs 2000). In studies of male and female donors, the percentage of male participants ranged from 3.1% to 71.2%.
With the exception of those studies of women of child‐bearing age, only two studies reported an age restriction on participants, which was from 18 to 56 years (Landucci 1987), and from 18 to 25 years (Lieden 1975). Participants in a third study were exclusively military service recruits (Ehn 1968).
Studies included both regular/repeat and first‐time donors. Thirteen studies recruited regular donors, defined as having donated at least five donations in the previous two years (Birgegard 2010), at least four donations (Mackintosh 1988_HSF; Mackintosh 1988_LSF), or two donations (Mirrezaie 2008) in the past year, with a median of three (range 0 to 21) previous donations over their lifetime (Maghsudlu 2008), or with an undefined donation history (Blot 1980; Borch‐Iohnsen 1993; Brittenham 1996; Frykman 1994; Radtke 2004a; Radtke 2004b; Rybo 1971; Simon 1984). Donors in six studies had made at least one previous donation (Gordeuk 1987a; Gordeuk 1990; Landucci 1987Lindholm 1981; Linpisarn 1986; Rosvik 2010), or included a majority (89%) of repeat donors (Waldvogel 2012). Only two studies recruited participants with no previous history of donation (Ehn 1968; Lieden 1975); the donation history was unknown in two studies (Bucher 1973; Busch 1972). Five studies were of deferred donors with Hb < 130 g/L (Buzi 1980), haematocrit < 35% (Devasthali 1991), low haematocrit (Gordeuk 1987b), or failing a copper sulphate test at enrolment (Jacobs 1993; Jacobs 2000), or donors deferred at their previous visit (Cable 1988).
Interventions
Nineteen studies included two trial arms, which included comparisons of iron supplementation versus placebo (Cable 1988; Gordeuk 1990; Linpisarn 1986; Maghsudlu 2008; Mirrezaie 2008; Radtke 2004b; Waldvogel 2012), or no iron supplementation (Brittenham 1996; Blot 1980; Rosvik 2010), oral versus parenteral iron supplementation (Birgegard 2010), different doses of the same iron preparation (Lieden 1975), and different preparations of iron supplementation (Borch‐Iohnsen 1993; Buzi 1980; Devasthali 1991; Frykman 1994; Gordeuk 1987b; Landucci 1987; Lindholm 1981).
In one report, participants were stratified according to serum ferritin levels and two independent trials were carried out, comparing iron supplementation with placebo (Mackintosh 1988_HSF; Mackintosh 1988_LSF).
Seven studies involved three trial arms, of two different iron preparations versus a placebo (Busch 1972; Gordeuk 1987a; Rybo 1971), two different doses of iron supplementation versus placebo (Ehn 1968; Radtke 2004a), iron supplementation and/or vitamin C (Simon 1984), and iron supplementation versus two doses of an alternative iron preparation (Jacobs 1993).
One four‐arm study compared different durations of iron supplementation or placebo, administered in vials, with iron supplementation administered in sachets at the full dose or replaced with placebo for the latter part of the trial (Bucher 1973); a second four‐arm study compared iron supplementation with two different levels of glycerophosphate and with an alternative iron preparation (Jacobs 2000).
From these studies, we were able to include 19 comparisons of iron supplementation and placebo or control; one comparison of oral and parenteral iron supplementation; four comparisons of different doses of iron supplementation; one comparison of different durations of iron supplementation; and 12 comparisons of different iron preparations.
Iron preparations included carbonyl or elemental iron, ferrous compounds (ferrous sulphate, ferrous carbonate, ferrous gluconate, ferrous glycine, ferrous fumarate, ferrous sulphate heptahydrate) and ferric compounds (ferric polymaltose, ferric protein succinylate, ferric sucrose, ferric glycerophosphate). The dose and duration of iron supplementation varied greatly across studies; from 50 mg ferrous sulphate three times daily for seven days, to 100 mg ferrous carbonate daily for one year (Lieden 1975). Four studies described iron preparations which included vitamin C (Blot 1980; Borch‐Iohnsen 1993; Busch 1972; Simon 1984). Full details of the interventions in each trial are given in Table 2.
1. Summary of study characteristics.
|
Studya |
Interventionb (elemental iron dose) | Reported outcomesc | Follow‐up time pointsd | Description of study participants |
| Birgegard 2010 | Fe2+SO4 (Duraferon) (100 mg daily for 20 days) | Hb, SeFe, RLS, AE | Week 4 and week 8 (non‐donation); donation 2 to 4 (♀) or 2 to 5 (♂); last donation is ≥ 1 year post‐1st donation | Experienced donors having given at least 5 donations in last 1 to 2 years |
| Fe3+sucrose (Venofer) (1 x 200 mg given intravenously) | ||||
| Blot 1980* | Fe2+SO4 + Vit C (Ferro‐Grad Abbott) (105 mg (+ 500 mg Vit C) daily for "following months") | Hb, MCV, SeFe, TIBC, AE, SI, Sat |
At second donation | Regular donors |
| Control (no placebo) | ||||
| Borch‐Iohnsen 1993 | Fe2+ fumarate + Vit C (Collett Iron) (20 mg (+ 120 mg Vit C) daily, treatment duration unclear) | Hb, SeFe, transferrin | 5 months after baseline measures | Female blood donors with depleted iron stores (serum ferritin < 20 μg/L and haemoglobin > 120 g/L) |
| Fe2+ fumarate (Vitalia Hemojern) (16 mg (+ 2 mg heme iron from porcine blood) daily, treatment duration unclear) | ||||
| Brittenham 1996 | Carbonyl iron (100 mg daily for 56 days) with scheduled visits | Mean no. donations per year | After 30 months | Females pledged to donate four times each year. |
| Control (scheduled visits only) | ||||
| Bucher 1973* | Fe2+SO4 (Resoferon) (37 mg 3 times daily for 28 days (one vial)) | Low Hb deferral, Hb, Hct, MCHC, transferrin, AE, PI | Day 14 and day 28 post‐donation | Healthy blood donors blood group B; haemoglobin 125 to 135 g/L |
| Fe2+SO4 (Resoferon) (37 mg 3 times daily for 28 days (28 sachets)) | ||||
| Fe2+SO4 (Resoferon) (37 mg 3 times daily for 4 days (4 sachets) followed by placebo for 24 days (24 sachets)) | ||||
| Placebo (3 times daily for 28 days (1 vial)) | ||||
| Busch 1972* | Fe2+SO4 (Eryfer) (50 mg (+ 222 mg Vit C + 84 mg NaHCO3) twice daily for 30 days) | AE | After 30 days of treatment | Blood donors |
| Fe2+SO4 (alternative) (50 mg (+ 222 mg Vit C) twice daily for 30 days) | ||||
| Placebo (273.8 mg maize starch + 1.2 mg Aerosil twice daily for 30 days) | ||||
| Buzi 1980* | Fe2+SO4 (Tardyferon) (80 mg (+ 80 mg muco‐protein) daily for 30 days) | Hb, Hct, TIBC, AE, SI | Day 2 after end of treatment | Deferred donors with Hb < 130 g/L (Hct < 37%) and no history of medical pathology for anaemia |
| Fe2+ fumarate (66 mg twice daily for 18 days) | ||||
| Cable 1988 | Fe2+ gluconate (Fergon) (37.5 mg twice daily for trial duration) | Low Hb deferral, Hb, SeFe, transferrin, ZP | ≥ 8 weeks since previous donation or 4 weeks since deferral, for 5 visits including initial visit |
Female donors failing previous Hb screen (some were eligible to donate at start of study) |
| Placebo (calcium phosphate twice daily) for trial duration | ||||
| Devasthali 1991 | Carbonyl iron (100 mg daily for 84 days) | Hb, MCV, SeFe, transferrin, TIBC, AE, SI | Weeks 0, 1, 3, 6, 12, 16 (none were donation visits) | Menstruating, non‐pregnant women 18 to 40 years old recently deferred from donation (Hct < 35%) with an absence of known medical disorders and no iron supplementation since deferral from blood donation and a MCV < 85 fL and ferritin < 12 μg/L |
| Fe2+SO4 (100 mg daily for 84 days) | ||||
|
Ehn 1968* (Adolfsson 1968*; Lieden 1975) |
Fe2+ succinate (Ferromyn S) (74 mg (+220 mg succinic acid) twice daily for 2 weeks) | Hb, Hct, TIBC, PA, SI | 2 months after 6 subsequent donations (inter‐donation interval 2 months) | Young, healthy, male conscripts with no past history of haematological, gastrointestinal or renal disorder. None had previous haemorrhage or had served as blood donors |
| Fe2+ succinate (Ferromyn S) (34 mg (+110 mg succinic acid) twice daily for 2 weeks) | ||||
| Placebo (twice daily for 2 weeks) | ||||
| Frykman 1994 | Fe2+ fumarate (Hemofer) (8 mg (+1.2 mg heme iron from porcine blood) twice daily for first month then second or third month) | Hb, SeFe, AE | After 3 months | Regular blood donors |
| Fe2+ fumarate (Erco‐Fer) (60 mg daily for first month then second or third month) | ||||
|
Gordeuk 1987a |
Carbonyl iron (600 mg 3 times daily for 7 days) | Hb, MCV, SeFe, TIBC, AE, SI, Sat, FEP | Day 56 after successful donation | Previous (at least once) female donors of child‐bearing age who were not pregnant and came to donate blood |
| Fe2+SO4 (60 mg 3 times daily for 7 days) | ||||
| Placebo (3 times daily for 7 days) | ||||
| Gordeuk 1987b | Carbonyl iron (600 mg 3 times daily for 21 days) | Hb, MCV, SeFe, TIBC, AE, SI, Sat, FEP | Weeks 1, 3, 6, 12, 16 | Female blood donors of child‐bearing age who were not pregnant recently deferred from repeat donation due to low Hct |
| Fe2+SO4 (60 mg 3 times daily for 21 days) | ||||
|
Gordeuk 1990 |
Carbonyl iron (100 mg daily for 56 days) | Low Hb deferral, Hb, MCV, SeFe, transferrin, TIBC, AE, SI | Day 56 after successful donation | Repeat female donors of child‐bearing age who were not pregnant and came to donate blood |
| Placebo (daily for 56 days) | ||||
| Jacobs 1993 | Fe2+SO4 (60 mg twice daily for 84 days) | Low Hb deferral, Hb, SeFe, NIA, AE, SI, Sat | Weeks 1, 2, 4, 8, 12. Not donation visits | Donors failing CuSO4 Hb screening test, i.e. deferred donors |
| Fe3+ polymaltose (100 mg daily for 84 days) | ||||
| Fe3+ polymaltose (100 mg twice daily for 84 days) | ||||
| Jacobs 2000 | Fe3+ polymaltose (100 mg (+3.6 mMol/L GlyP) twice daily for 84 days) | Hb, SeFe, transferrin, AE, SI, RCF | Weeks 4, 8 and 12. Not donation visits | Regular donors failing CuSO4 Hb screening test |
| Fe3+ polymaltose (100 mg (+1.9 mMol/L GlyP) twice daily for 84 days) | ||||
| Fe3+ polymaltose (100 mg twice daily for 84 days) | ||||
| Fe2+SO4 ("equivalent dose" twice daily for 84 days) | ||||
|
Landucci 1987 |
Fe3+ protein succinylate (Legofer) (80 mg daily for 30 days) | Hb, Hct, MCV, MCH, MCHC, SeFe, transferrin, AE, SI | End of trial: mean 30 +/‐ 2.2 days (range 23 to 33) | Blood donors aged 18 to 56 with low levels of stored iron (serum ferritin < 30 ng/100 mL) |
| Fe2+SO4 (105 mg daily for 30 days) | ||||
| Lieden 1975 | Fe3+ carbonate (100 mg daily for 1 year) | Low Hb deferral, TIBC, NIA, AE, SI, PCV | After 4th and 6th donations | Young, male, first‐time donor conscripts with no history of bleeding |
| Fe3+carbonate (20 mg daily for 1 year) | ||||
| Lindholm 1981* | Fe2+SO4 (ACO) (100 mg daily for 30 days) | Low Hb deferral, Hb, TIBC, AE, SI | After 1st, 2nd and 3rd donations | Previous donors (all except 14/500) without iron deficiency anaemia during the most recent years, could tolerate different iron preparations and intended to continue to give blood |
| Fe2+ fumarate (Erco‐Fer) (60 mg daily for 30 days) | ||||
|
Linpisarn 1986 |
"Elemental"” iron (56 mg daily for 90 days) | Hb, Hct, SeFe, transferrin | After ˜3 months (assumed no donations) | Male volunteer and paid blood donors who had previously donated |
| Placebo (daily for 90 days) | ||||
| Mackintosh 1988_LSF | Fe3+ polymaltose (Ferrimed DS) (100 mg twice daily for 56 days) | Hb, SeFe, AE | After 56 days of treatment (not donation visit) | Regular donors (at least 4 donations in previous year) passing the Hb test and with low serum ferritin (less than 20 μg/L) |
| Placebo (twice daily for 56 days) | ||||
| Mackintosh 1988_HSF | Fe3+ polymaltose (Ferrimed DS) (100 mg twice daily for 56 days) | Hb, SeFe, AE | After 56 days of treatment (not donation visit) | Regular donors (at least 4 donations in previous year) passing the Hb test and with high serum ferritin (between 50 and 150 μg/L) |
| Placebo (twice daily for 56 days) | ||||
|
Maghsudlu 2008 |
Fe2+SO4 (150 mg 3 times daily for 7 days) | Low Hb deferral, Hb, Hct, SeFe, TIBC, AE, SI, Sat | Visits 1 (4 months), 2 (8 months) and 3 (12 months) | Female, successful blood donors < 45 years who were not pregnant |
| Placebo (3 times daily for 7 days) | ||||
| Mirrezaie 2008 | Fe2+SO4 (50 mg daily for 56 days) | SeFe, AE | Day 7, 28 and 56. Not donation visits | Regular (at least 2 donations in past year) healthy female donors of childbearing age. 72% had previously been taking iron supplements |
| Placebo (daily for 56 days) | ||||
| Radtke 2004a | Fe2+ gluconate (20 mg (+ 400 mg Vit C) twice daily for 6 months) | Low Hb deferral, SeFe, transferrin, AE | ♂ = 2/4/6 months; ♀ = 3/6 months. All were donation visits | Regular, healthy donors |
| Fe2+ gluconate (+ 400 mg Vit C) (10 mg twice daily for 6 months) | ||||
| Placebo (+400 mg Vit C) (twice daily for 6 months) | ||||
| Radtke 2004b | Fe2+ Glycine SO4 (ferro sanol duodenal) (100 mg daily for 8 to 10 weeks) | Low Hb deferral | Before donation visits 1, 2, 3 Inter‐donation interval 8 to 10 weeks | Regular, healthy donors with a minimum body weight of 68 kg and Hb of 145 g/L giving 2‐unit RBC by apheresis |
| Placebo (daily for 8 to 10 weeks) | ||||
|
Rosvik 2010 |
Fe2+ Glycine SO4 (Niferex©) (100 mg daily for 8 days) | Hb, SeFe, transferrin | Day 8 (+/‐ 2) after initial donation | Donors with at least 1 prior donation |
| Control (no placebo) | ||||
|
Rybo 1971 |
Fe2+SO4 (100 mg twice daily for 14 days) | AE | Day 14 post‐donation | Regular blood donors |
| Fe2+SO4 heptahydrate (100 mg twice daily for 14 days) | ||||
| Placebo (twice daily for 14 days) | ||||
|
Simon 1984 |
Fe2+SO4 (37 mg daily for 56 days) | Hb, SeFe, TIBC | Donation visits 2, 3, 4, 5 etc. (inter‐donation interval 8 to 12 weeks, mean 9.5 weeks) with at least 4 donation visits | Regular, female blood donors committing to donate blood every 8 weeks for 1 year |
| Fe2+SO4 (37 mg (+75 mg Vit C) daily for 56 days) | ||||
| (100 mg Vit C daily for 56 days) | ||||
| Waldvogel 2012 | Fe2+SO4 (Tardyferon) (80 mg daily for 28 days) | Hb, SeFe, Cog, PA, AE | 1 week after donation (randomisation) and 4 weeks post‐randomisation | Successful female blood donors (non‐anaemic but iron‐deficient after donation) |
| Placebo (daily for 28 days) |
a* = translated.
bFe2+ SO4 = iron (II) sulphate; Fe3+sucrose = iron (III) sucrose; Fe2+ = ferrous (II) salt; Fe3+ = ferric (III) salt; NaHCO3 = sodium bicarbonate; Vit C = vitamin C; GlyP = glycerophosphate. All treatments were administered orally with the exception of ferric sucrose, given intravenously in Birgegard 2010.
cAE = adverse effects; Cog = cognitive function; FEP = free erythrocyte protoporphyrin; Hb = haemoglobin; Hct = haematocrit; MCH = mean cell Hb; MCHC = MCH concentration; MCV = mean cell volume; NIA = net iron absorption; PA = physical activity; PI = plasma iron; RLS = restless legs syndrome; Sat = percentage saturation; SeFe =serum ferritin; SI = serum iron; TIBC = total iron binding concentration; ZP = zinc protoporphyrin
d♂ = males, ♀ = females.
Outcomes
The duration of follow‐up varied greatly between studies. Ten studies reported outcomes after up to five donations subsequent to the administration of iron supplementation (Birgegard 2010; Brittenham 1996; Cable 1988; Ehn 1968; Lieden 1975; Lindholm 1981; Maghsudlu 2008; Radtke 2004a; Radtke 2004b; Simon 1984). In two studies, it was unclear whether any donations following treatment had taken place at the time of follow‐up (Blot 1980; Frykman 1994). Eighteen studies measured outcomes before any further donations. Time points ranged from a mean of eight days (Rosvik 2010) to 16 weeks (Devasthali 1991; Gordeuk 1987b), with a median follow‐up time before further donation of 56 days.
The primary outcome of this review, risk ratio of low Hb deferral, was reported in only eight studies (Bucher 1973; Cable 1988; Gordeuk 1990; Lieden 1975; Lindholm 1981; Maghsudlu 2008; Radtke 2004a; Radtke 2004b). Haemoglobin and serum ferritin levels were widely reported; other reported blood indices included mean cell/corpuscular volume (MCV), serum or plasma iron, total iron binding capacity (TIBC) and transferrin saturation. Health‐related quality of life measures were poorly reported; only two studies included measures of physical activity and fatigue (Ehn 1968; Waldvogel 2012), and no studies reported measures of mood disturbance or cognitive function. The majority of studies described adverse effects. Reported outcomes (including those not considered in this review) and endpoints in individual studies are shown in Table 2.
Excluded studies
We excluded 20 studies described in 21 references from the review following full‐text assessment against the eligibility criteria (see Characteristics of excluded studies). In summary, three studies included a single treatment arm, two studies allocated treatment without randomisation, in two studies randomisation of treatment could not be confirmed, three studies were of short‐term iron absorption levels, one study randomised vitamin C dose with all participants receiving identical iron supplementation, two observational studies included no iron supplementation, two studies were of non‐donors, one study was of plasmapheresis donors, one study reported results for both blood donors and non‐donors combined, one study was a commentary on iron deficiency in blood donors, one study administered erythropoietin to autologous blood donors and one study was no longer available in print.
Risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were restricted to where one review author deemed risk of bias as unclear rather than high or low and were mainly concerned with the interpretation of "double‐blind" (see Assessment of risk of bias in included studies). We resolved all disagreements by discussion and with further reference to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Overall, the risk of bias varied from low to high, with the majority of studies being unclear as to their quality (see Characteristics of included studies). This was mainly due to the age of the studies, with more recent studies using more rigorous methodologies. A summary of the risk of bias is shown in Figure 2.
2.
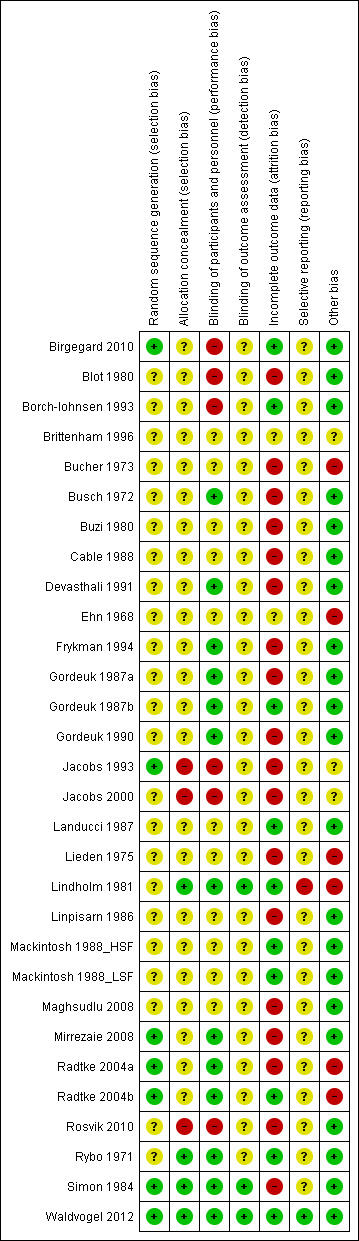
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 30 included studies, we assessed only seven as having a low likelihood of selection bias (Birgegard 2010; Jacobs 1993; Mirrezaie 2008; Radtke 2004a; Radtke 2004b; Simon 1984; Waldvogel 2012). In these studies, treatment allocation was randomised using web‐based systems, computer‐generated charts (n = 2), random block design (n = 3) and participant choice of randomised cards in envelopes. The risk of selection bias in 23 studies was unclear as none reported their method of randomisation.
No method for concealment of allocation was reported in 23 studies. In seven studies, two did not conceal allocation (Jacobs 2000: Rosvik 2010), and one used computer‐generated charts (Jacobs 1993); we assessed these as having a high risk of selection bias. We assessed four studies as being of low risk due to the use of code‐marked prescriptions (Lindholm 1981; Rybo 1971; Simon 1984; Waldvogel 2012).
Blinding
There was no blinding of participants in six of the studies so we considered these to have a high risk of performance bias (Birgegard 2010; Blot 1980; Borch‐Iohnsen 1993; Jacobs 1993; Jacobs 2000; Rosvik 2010). Eleven studies gave no indication of participant blinding so we recorded them as having an unclear risk. Of the 13 studies assessed as low risk, nine stated the studies as being double‐blind, either with the use of a placebo (Busch 1972; Gordeuk 1987a; Gordeuk 1987b; Gordeuk 1990; Mirrezaie 2008), or assumed to include participant blinding without explicitly stating so (Devasthali 1991; Frykman 1994; Radtke 2004a; Radtke 2004b); four used coded bottles for the prescriptions (Lindholm 1981; Rybo 1971; Simon 1984; Waldvogel 2012).
Only three of the 30 studies described any blinding of the outcome assessment and we rated them as low risk (Lindholm 1981: Simon 1984; Waldvogel 2012); we classified the remainder as unclear risk.
Incomplete outcome data
We investigated the completeness of data and have described the reasons for attrition or exclusion where reported for each included study and whether missing data were balanced across groups. We rated 10 studies as being low risk (Birgegard 2010; Borch‐Iohnsen 1993; Gordeuk 1987b; Landucci 1987; Lindholm 1981; Mackintosh 1988_HSF; Mackintosh 1988_LSF; Radtke 2004b; Rybo 1971; Waldvogel 2012), two were of unclear risk (Brittenham 1996; Ehn 1968), and the remainder had high risk of attrition bias, with a difference in missing data of greater than 5% between treatment arms, a high rate of loss to follow‐up or, in one case, because the number of participants randomised to each arm was not reported (Bucher 1973).
Selective reporting
All of the pre‐specified outcomes published in the protocol for the Waldvogel 2012 study were reported, and we deemed this study to have a low risk of bias. A high risk of bias was associated with one study, in which the authors failed to report three pre‐specified outcomes of interest (Lindholm 1981). In all 29 remaining studies, where all outcomes listed in the manuscript were reported but no study protocol was available to determine the full list of pre‐specified outcomes, the risk of reporting bias was unclear. No unpublished data were received, so there is currently no additional evidence of reporting bias.
Other potential sources of bias
We assessed each included study for other factors that might contribute to additional risk of bias. We have noted any concerns we had about other possible sources of bias and rated them thus:
high risk of further bias ‐ where the manufacturer has provided support, in terms of a grant (Radtke 2004a; Radtke 2004b), and additional help (Bucher 1973: Ehn 1968: Lieden 1975), or where the manufacturer supplied some co‐authors (Lindholm 1981);
unclear ‐ where the risk of further bias is uncertain, most commonly where a study has been supplied with iron supplements and/or placebo by a particular manufacturer (Borch‐Iohnsen 1993; Jacobs 1993; Jacobs 2000), or there were limited data available from a conference abstract (Brittenham 1996);
low risk of further bias ‐ where there was explicit declaration of independence from study sponsors (Birgegard 2010; Waldvogel 2012), or where no other sources of bias could be identified (18 studies).
Effects of interventions
See: Table 1
A. Iron supplementation versus placebo/control
Nineteen studies compared iron supplementation with placebo or control (see Table 2). Haemoglobin, serum ferritin and total iron binding capacity (TIBC) were reported graphically in one study (Simon 1984); we estimated data for this study from the graphs as described in the Methods section.
(1) Risk ratio of low Hb deferral (primary outcome)
Four studies reported low haemoglobin deferral rates (Gordeuk 1990; Maghsudlu 2008; Radtke 2004a; Radtke 2004b). One other study reported the mean number of donations per donor per year (Brittenham 1996). At the first donation visit after commencement of treatment, all four studies reported a lower rate of Hb deferral in donors who received iron supplementation than in those who had not, with three studies reporting a significant difference between treatment arms (Gordeuk 1990; Radtke 2004a; Radtke 2004b). Combined evidence from all four studies showed a significantly reduced risk of deferral due to low Hb at the first donation visit after treatment in donors who received iron supplementation (risk ratio (RR) 0.34; 95% confidence interval (CI) 0.21 to 0.55; four studies; 1194 participants; P value < 0.0001) (Analysis 1.1). There was no evidence of heterogeneity between studies (I2 = 0%; 95% CI 0% to 79.3%). This low Hb deferral risk reduction was maintained after multiple donation visits reported in three studies (RR 0.25; 95% CI 0.15 to 0.41; three studies; 793 participants; P value < 0.00001) (Maghsudlu 2008; Radtke 2004a; Radtke 2004b), and over cumulative donation visits (RR 0.31; 95% CI 0.18 to 0.52; four studies; 2740 participants; P value < 0.00001) (Analysis 1.1).
1.1. Analysis.
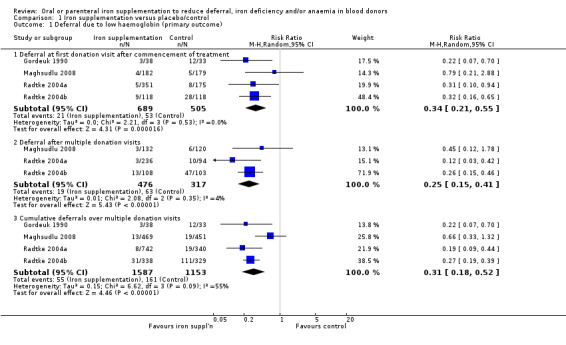
Comparison 1 Iron supplementation versus placebo/control, Outcome 1 Deferral due to low haemoglobin (primary outcome).
Subgroup analyses revealed no significant differences between male and female donors in low Hb deferral rates at first donation (P value = 0.90) (Analysis 10.1), after multiple donation visits (P value = 0.81) (Analysis 10.2) or over cumulative donation visits (P value = 0.85) (Analysis 10.3), although the number of studies provided low power to detect a difference between subgroups. Results were robust to performance bias (Analysis 11.1) and attrition bias (Analysis 12.1).
10.1. Analysis.
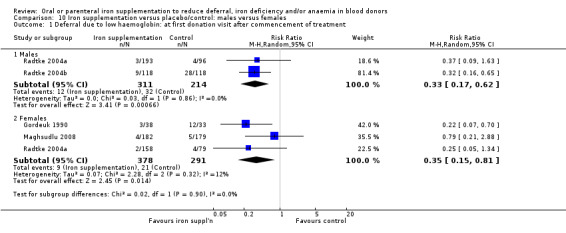
Comparison 10 Iron supplementation versus placebo/control: males versus females, Outcome 1 Deferral due to low haemoglobin: at first donation visit after commencement of treatment.
10.2. Analysis.
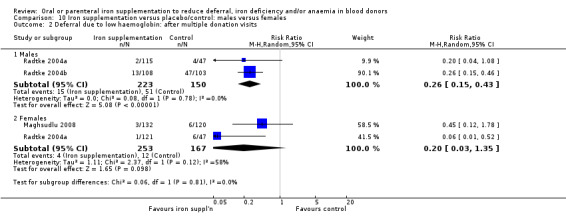
Comparison 10 Iron supplementation versus placebo/control: males versus females, Outcome 2 Deferral due to low haemoglobin: after multiple donation visits.
10.3. Analysis.
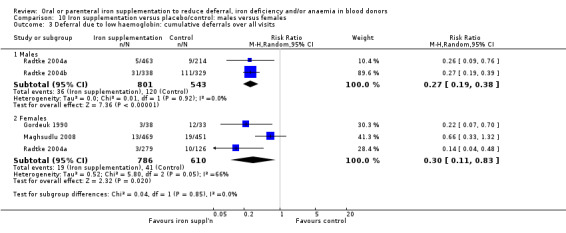
Comparison 10 Iron supplementation versus placebo/control: males versus females, Outcome 3 Deferral due to low haemoglobin: cumulative deferrals over all visits.
11.1. Analysis.
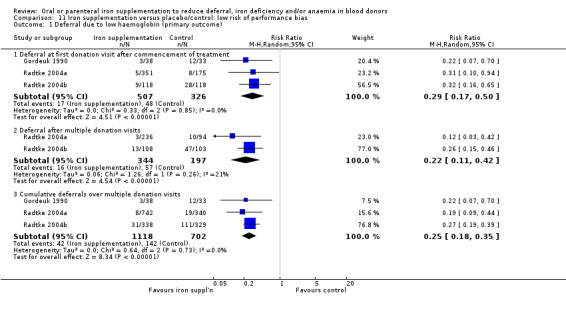
Comparison 11 Iron supplementation versus placebo/control: low risk of performance bias, Outcome 1 Deferral due to low haemoglobin (primary outcome).
12.1. Analysis.
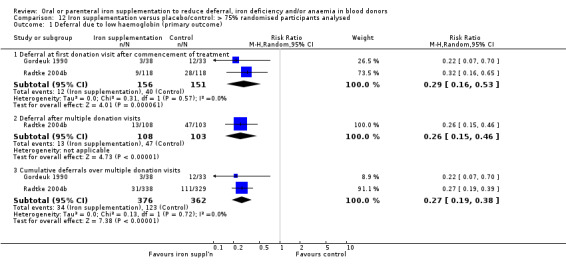
Comparison 12 Iron supplementation versus placebo/control: > 75% randomised participants analysed, Outcome 1 Deferral due to low haemoglobin (primary outcome).
(2) Hb levels, mean cell volume (MCV), other blood indices and iron stores
i. Haemoglobin (Hb)
Mean Hb levels were reported in 12 studies (Bucher 1973; Cable 1988; Ehn 1968; Gordeuk 1987a; Gordeuk 1990; Linpisarn 1986; Mackintosh 1988_HSF; Mackintosh 1988_LSF; Maghsudlu 2008; Rosvik 2010; Simon 1984; Waldvogel 2012), although in one study results were reported graphically and data extraction could not be undertaken (Ehn 1968); this study reported no significant differences between treatment groups.
In eight studies which reported Hb levels at follow‐up prior to further donation (Bucher 1973; Gordeuk 1987a; Gordeuk 1990; Linpisarn 1986; Mackintosh 1988_HSF; Mackintosh 1988_LSF; Rosvik 2010; Waldvogel 2012), meta‐analysis showed that iron supplementation resulted in significantly higher levels of Hb at follow‐up (mean difference (MD) 2.36 g/L; 95% CI 0.06 to 4.66; eight studies; 847 participants, P value = 0.04), showing a beneficial effect of iron supplementation. A moderate level of heterogeneity was observed between studies (I2 = 69%; 95% CI 34.3% to 85.1%) (Analysis 1.2).
1.2. Analysis.
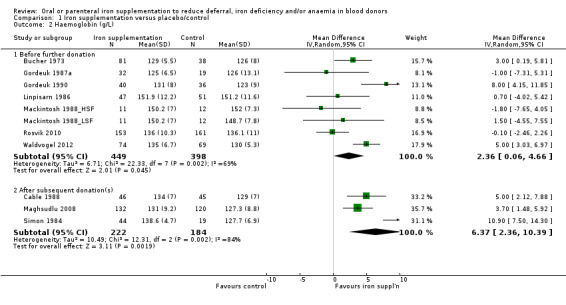
Comparison 1 Iron supplementation versus placebo/control, Outcome 2 Haemoglobin (g/L).
Sensitivity analyses showed that the effect of iron supplementation on Hb levels before further donation remained significant when five studies with a high or unclear risk of performance bias were excluded (MD 4.76 g/L; 95% CI 1.07 to 8.45; three studies; 270 participants; P value = 0.01) (Analysis 11.2), and when studies with less than 75% of randomised participants included in the analysis were excluded (MD 2.90 g/L; 95% CI 0.23 to 5.57; six studies; 698 participants; P value = 0.03) (Analysis 12.2).
11.2. Analysis.
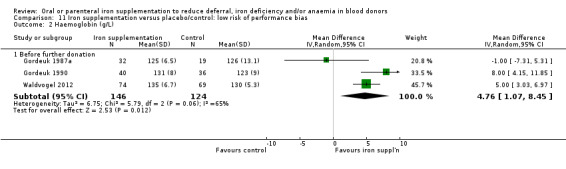
Comparison 11 Iron supplementation versus placebo/control: low risk of performance bias, Outcome 2 Haemoglobin (g/L).
12.2. Analysis.
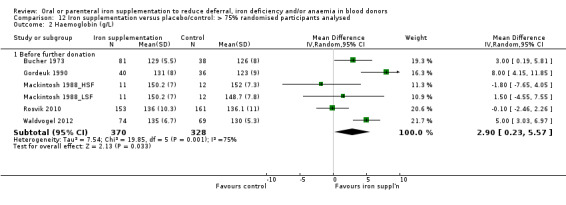
Comparison 12 Iron supplementation versus placebo/control: > 75% randomised participants analysed, Outcome 2 Haemoglobin (g/L).
Subgroup analysis by sex revealed that the difference in Hb level between treatment arms was found in female donors (MD 3.56 g/L; 95% CI 0.21 to 6.92; four studies; 431 participants; P value = 0.04), but not male donors (MD 0.08 g/L; 95% CI ‐1.90 to 2.05; four studies; 297 participants; P value = 0.94). A test for subgroup differences was not significant (P value = 0.08) (Analysis 10.4), although the number of studies provided low power to detect a difference between subgroups. We observed no heterogeneity across four studies reporting Hb levels in males (I2 = 0%; 95% CI 0% to 32.8%); however, four studies reporting Hb levels in females showed high heterogeneity (I2 = 80%; 95% CI 48.3% to 92.6%), with no clear differences apparent between these studies.
10.4. Analysis.
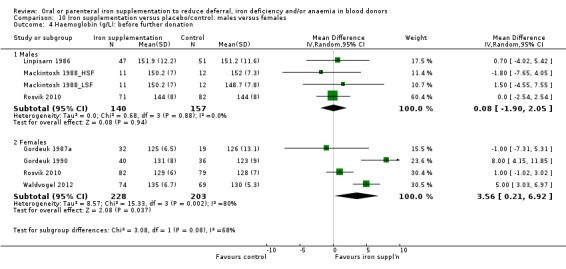
Comparison 10 Iron supplementation versus placebo/control: males versus females, Outcome 4 Haemoglobin (g/L): before further donation.
Hb after subsequent donation(s) was reported in three studies (Cable 1988; Maghsudlu 2008; Simon 1984). We found a significant difference in Hb levels between treatment arms in favour of iron supplementation after donation (MD 6.37 g/L; 95% CI 2.36 to 10.39; three studies; 406 participants; P value = 0.002) (Analysis 1.2), with high heterogeneity across studies (I2 = 84%; 95% CI 50.9% to 94.6%). Visual inspection of the forest plot showed a particularly strong effect from one study of menstruating female blood donors with an inter‐donation interval of between eight and 12 weeks (Simon 1984). There was no residual evidence for heterogeneity when this study was excluded (I2 = 0%).
ii. Mean corpuscular volume (MCV)
Two studies reported MCV before further donation; both studies reported higher mean MCV levels at follow‐up in donors who received iron supplementation compared with those who did not (Gordeuk 1987a; Gordeuk 1990). However, meta‐analyses of these two studies did not provide evidence for a difference in MCV between treatment arms (MD 1.37 fL; 95% CI ‐0.17 to 2.92; two studies; 127 participants; P value = 0.08) (Analysis 1.3).
1.3. Analysis.
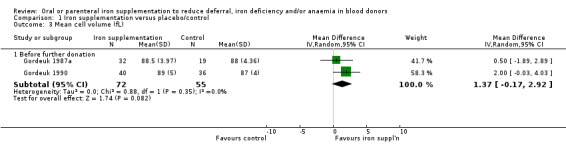
Comparison 1 Iron supplementation versus placebo/control, Outcome 3 Mean cell volume (fL).
iii. Serum ferritin
Serum ferritin (ng/mL) before further donation was reported as an outcome in nine studies (Gordeuk 1987a; Gordeuk 1990; Linpisarn 1986; Mackintosh 1988_HSF; Mackintosh 1988_LSF; Mirrezaie 2008; Radtke 2004a; Rosvik 2010; Waldvogel 2012). However, three studies reported serum ferritin as geometric mean (Gordeuk 1987a; Gordeuk 1990; Rosvik 2010), and a fourth study reported median serum ferritin values, with no suitable measure of variation for inclusion of the data from these studies in a quantitative synthesis (Linpisarn 1986). In these four studies, one reported a significant difference in serum ferritin between treatment arms in favour of iron supplementation (Rosvik 2010); two reported significant increases in serum ferritin from baseline in the iron supplementation group but not the placebo/control group (Gordeuk 1987a; Gordeuk 1990), and one reported no difference in serum ferritin between treatment groups (Gordeuk 1987a).
In five studies, we found significantly higher mean serum ferritin levels at follow‐up in donors who received iron supplementation compared with those who did not in all but one study (Mackintosh 1988_HSF), in which donors were pre‐selected for high serum ferritin (between 50 and 150 ng/mL). Meta‐analysis of all five studies showed that iron supplementation resulted in significantly higher levels of serum ferritin (MD 13.98 ng/mL; 95% CI 8.92 to 19.03; five studies; 640 participants; P value < 0.00001). We found a moderate level of heterogeneity (I2 = 68%; 95% CI 16.0% to 87.5%) between studies (Analysis 1.4). The effect remained significant when we excluded the study with high baseline serum ferritin levels (MD 13.67 ng/mL; 95% CI 8.39 to 18.95; four studies; 617 participants; P value < 0.00001).
1.4. Analysis.
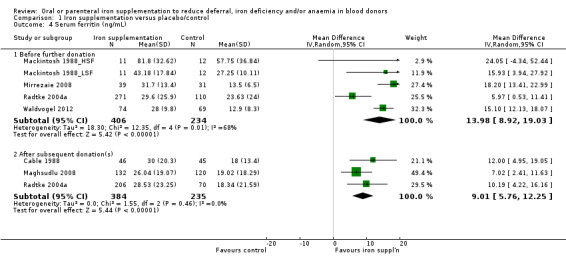
Comparison 1 Iron supplementation versus placebo/control, Outcome 4 Serum ferritin (ng/mL).
Subgroup analysis showed that the significant improvement in serum ferritin associated with iron supplementation was found in both male and female donors (males: MD 10.94 ng/mL; 95% CI 1.00 to 20.88; three studies; 265 participants; P value = 0.03; females: MD 14.39 ng/mL; 95% CI 9.90 to 18.88; three studies; 375 participants; P value < 0.00001; test for subgroup differences: P value = 0.53) (Analysis 10.5).
10.5. Analysis.
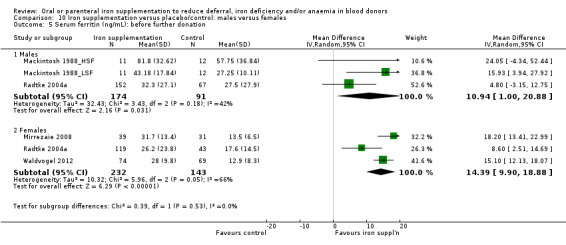
Comparison 10 Iron supplementation versus placebo/control: males versus females, Outcome 5 Serum ferritin (ng/mL): before further donation.
Sensitivity analyses showed that the increase in serum ferritin levels associated with iron supplementation before further donation remained significant when we excluded two studies with an unclear risk of performance bias in a sensitivity analysis (MD 13.31 ng/mL; 95% CI 7.22 to 19.40; three studies; 594 participants; P value < 0.0001) (Analysis 11.3) and when studies with less than 75% of randomised participants included in the analysis were excluded (MD 15.24 ng/mL; 95% CI 12.37 to 18.11; three studies; 189 participants; P value < 0.00001) (Analysis 12.3).
11.3. Analysis.
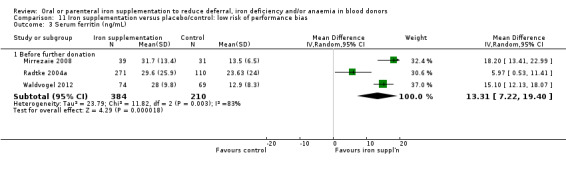
Comparison 11 Iron supplementation versus placebo/control: low risk of performance bias, Outcome 3 Serum ferritin (ng/mL).
12.3. Analysis.
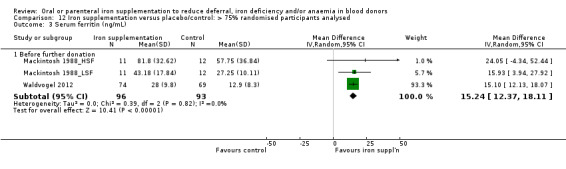
Comparison 12 Iron supplementation versus placebo/control: > 75% randomised participants analysed, Outcome 3 Serum ferritin (ng/mL).
Mean serum ferritin levels after subsequent donation(s) were reported in three trials (Cable 1988; Maghsudlu 2008; Radtke 2004a). One other study reported serum ferritin levels graphically as geometric mean values (Simon 1984). Meta‐analysis of the three trials reporting mean values showed that the significant difference in mean serum ferritin in favour of iron supplementation was maintained after subsequent donation(s) (MD 9.01 ng/mL; 95% CI 5.76 to 12.25; three studies; 619 participants; P value < 0.00001), with no evidence for heterogeneity across studies (I2 = 0%; 95% CI 0% to 86.7%) (Analysis 1.4).
iv. Serum or plasma iron
Serum or plasma iron concentration was reported in five studies (Bucher 1973; Ehn 1968; Gordeuk 1987a; Gordeuk 1990; Maghsudlu 2008), although in one study results were reported graphically and data extraction could not be undertaken (Ehn 1968); no significant differences between treatment groups were reported in this study. In three studies which reported serum or plasma iron concentration before further donation (Bucher 1973; Gordeuk 1987a; Gordeuk 1990), meta‐analysis showed no evidence for a difference between treatment arms (MD 11.76 μg/dL; 95% CI ‐1.67 to 25.20; three studies; 246 participants; P value = 0.09), with moderate heterogeneity across studies (I2 = 59%; 95% CI 0% to 88.4%). Only one study reported serum or plasma iron concentration after post‐treatment donation(s); this study found significantly higher levels of serum iron in donors who received iron supplementation (MD 7.89 μg/dL; 95% CI 1.12 to 14.66; 252 participants; P value = 0.02) (Maghsudlu 2008) (Analysis 1.5).
1.5. Analysis.
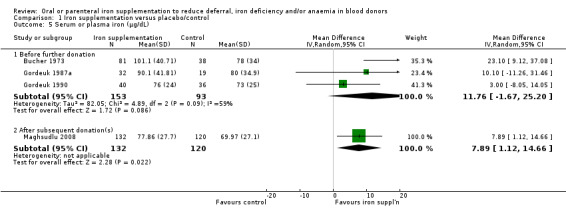
Comparison 1 Iron supplementation versus placebo/control, Outcome 5 Serum or plasma iron (μg/dL).
v. Total iron binding capacity (TIBC)
TIBC was reported in five studies (Ehn 1968; Gordeuk 1987a; Gordeuk 1990; Maghsudlu 2008; Simon 1984), although in one study results were reported graphically and data extraction could not be undertaken (Ehn 1968); this study reported no significant differences between treatment groups. Two studies reported TIBC before further donation (Gordeuk 1987a; Gordeuk 1990), and two studies reported values after subsequent donation(s) (Maghsudlu 2008; Simon 1984). Iron supplementation resulted in significantly lower levels of TIBC consistent with a beneficial effect of iron supplementation both before further donation (MD ‐32.05 μg/dL; 95% CI ‐61.45 to ‐2.65; two studies; 127 participants; P value = 0.03) and after subsequent donations (MD ‐42.64 μg/dL; 95% CI ‐56.99 to ‐28.28; two studies; 315 participants; P value < 0.00001), with low to moderate heterogeneity across studies (I2 = 43%; I2 = 29% respectively) (Analysis 1.6).
1.6. Analysis.
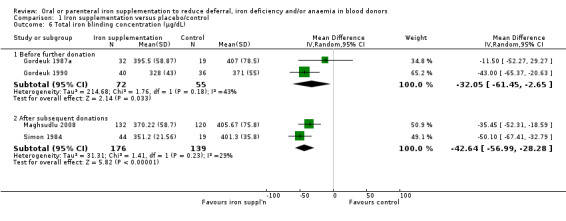
Comparison 1 Iron supplementation versus placebo/control, Outcome 6 Total iron blinding concentration (μg/dL).
vi. Transferrin saturation (%)
Transferrin saturation (also described as saturation of TIBC) was reported in four studies (Bucher 1973; Cable 1988; Gordeuk 1990; Linpisarn 1986). Meta‐analysis of these four studies showed that iron supplementation resulted in significantly higher transferrin saturation levels (MD 3.91%; 95% CI 2.02 to 5.80; four studies; 344 participants; P value < 0.0001), with no evidence for heterogeneity across studies (I2 = 0%; 95% CI 0% to 60.9%) (Analysis 1.7). Evidence from two studies showed that an increase in transferrin saturation in iron‐supplemented donors compared with placebo was maintained after subsequent donations (MD 4.84%; 95% CI 2.78 to 6.90; two studies; 343 participants; P value < 0.00001) (Cable 1988; Maghsudlu 2008) (Analysis 1.7).
1.7. Analysis.
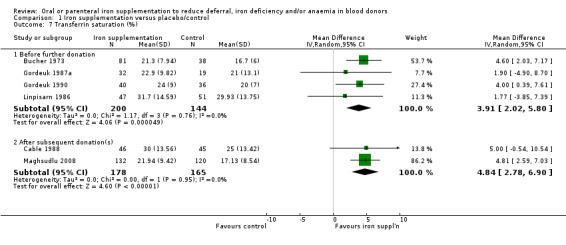
Comparison 1 Iron supplementation versus placebo/control, Outcome 7 Transferrin saturation (%).
(3) Health‐related quality of life and physical activity
One study reported health‐related quality of life as an outcome, which was assessed by fatigue (level of fatigue on a visual analogue scale and a subjective fatigue severity scale), quality of life (SF‐12V2 self questionnaire: vitality, physical and mental condition) and aerobic capacity (Chester step test) (Waldvogel 2012). In this study, there were no differences in health‐related quality of life measures after four weeks of treatment in any of the measures used, with the exception of physical condition (MD 2.40; 95% CI 0.93 to 3.87; one study; 133 participants; P value = 0.001) (Analysis 1.8).
1.8. Analysis.
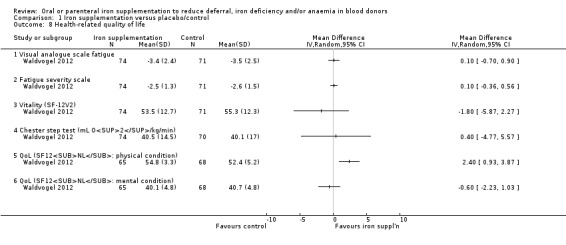
Comparison 1 Iron supplementation versus placebo/control, Outcome 8 Health‐related quality of life.
In one other study which reported physical capacity using a bike test, no standard deviations were provided and therefore a formal assessment of the results from this study was not possible (Ehn 1968). No significant differences between treatment groups were reported.
(4) Adverse effects
Fourteen studies reported adverse effects as an outcome although two studies reported adverse effects in the treatment group only (Blot 1980; Rosvik 2010), and five studies did not report adverse effects separately for each treatment arm (Bucher 1973; Mackintosh 1988_HSF; Mackintosh 1988_LSF; Radtke 2004a; Radtke 2004b). Reported adverse effects included constipation, diarrhoea, nausea, vomiting, gastric discomfort, abdominal cramps, headache, dizziness and taste disturbances. Four studies reported the occurrence of cumulative adverse events (Gordeuk 1987a; Maghsudlu 2008; Rybo 1971; Waldvogel 2012). Meta‐analysis of these four studies showed a significant increased risk of adverse effects associated with iron supplementation (RR 1.60; 95% CI 1.23 to 2.07; four studies; 1748 participants; P value = 0.0005) (Analysis 1.9).
1.9. Analysis.
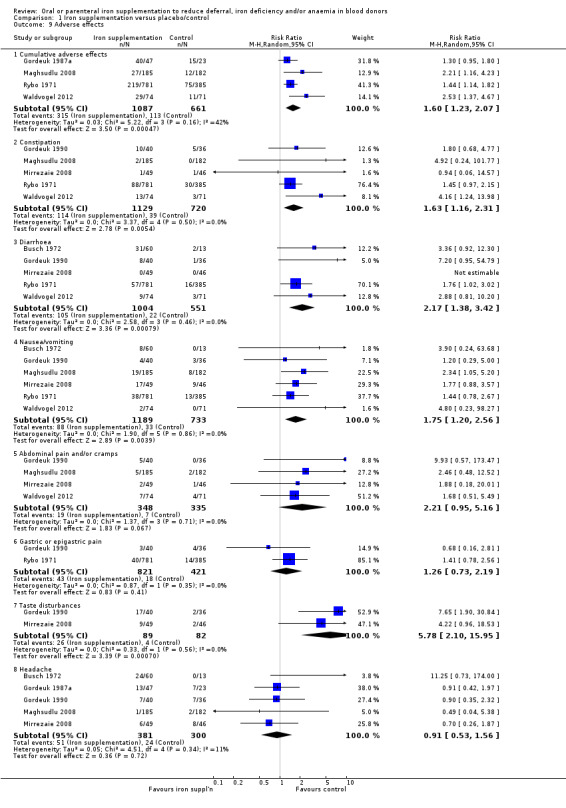
Comparison 1 Iron supplementation versus placebo/control, Outcome 9 Adverse effects.
Meta‐analysis of studies reporting specific adverse effects showed that iron supplementation was associated with an increased risk of constipation (RR 1.63; 95% CI 1.16 to 2.31; five studies; 1849 participants; P value = 0.005), diarrhoea (RR 2.17; 95% CI 1.38 to 3.42; five studies; 1555 participants; P value = 0.0008), nausea/vomiting (RR 1.75; 95% CI 1.20 to 2.56; six studies; 1922 participants; P value = 0.004) and taste disturbances (RR 5.78; 95% CI 2.10 to 15.95; two studies; 171 participants; P value = 0.0007), whilst there was insufficient evidence for an increased risk of abdominal pain and/or cramps (RR 2.21; 95% CI 0.95 to 5.16; four studies; 683 participants; P value = 0.07), gastric/epigastric pain (RR 1.26; 95% CI 0.73 to 2.19; two studies; 1242 participants; P value = 0.41) or headache (RR 0.91; 95% CI 0.53 to 1.56; five studies; 681 participants; P value = 0.72) (Analysis 1.9).
(5) Compliance
Three studies reported compliance as continuation of treatment (iron supplementation or placebo) (Busch 1972; Gordeuk 1987a; Rybo 1971). Meta‐analysis showed a high risk of discontinuation of treatment in participants who received iron supplementation compared with those who received placebo, although this difference failed to meet statistical significance (RR 0.83; 95% CI 0.68 to 1.01; three studies; 1336 participants; P value = 0.06) (Analysis 1.10).
1.10. Analysis.
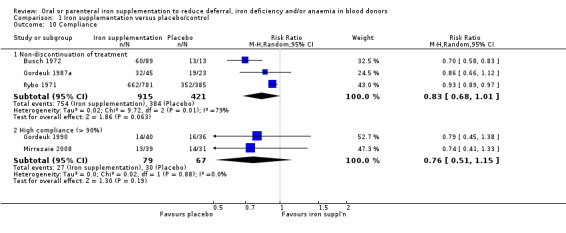
Comparison 1 Iron supplementation versus placebo/control, Outcome 10 Compliance.
Five studies reported the number of individuals who achieved a high compliance rate, defined as 100% compliance (Gordeuk 1990; Rosvik 2010), or over 90% compliance (Mirrezaie 2008; Radtke 2004a; Radtke 2004b); however only two trials reported compliance rates separately for both treatment groups (Gordeuk 1990; Mirrezaie 2008). Meta‐analysis of these two trials showed no evidence for a difference in compliance rates between treatment groups (RR 0.76; 95% CI 0.51 to 1.15; two studies; 146 participants; P value = 0.19) (Analysis 1.10). Compliance (ingestion of over 90% of tablets) was poor in one‐third of men and one‐quarter of women in one study (Radtke 2004a); a second study by the same authors reported that compliance was "largely similar in both groups" (Radtke 2004b). One study reported that full compliance was achieved in 92.8% of participants who received iron supplementation (Rosvik 2010). In the Blot 1980 study, iron supplementation was "well adhered to" in 81.8% of patients although no definition of compliance was given.
One study reported the number of days over a total treatment period of 28 days on which tablets were taken (Bucher 1973); tablets were taken for between 87.7% and 93.4% of total treatment days in participants who received iron supplementation compared with 88.2% in the placebo group. One study reported a mean of 1.6 (standard deviation (SD) 0.4) tablets per day were taken in the iron supplementation group compared with 1.5 (SD 0.7) in the placebo group (Cable 1988). A compliance rate of 96% was reported in the Waldvogel 2012 study, with similar adherence in both groups. In the Ehn 1968 study, no more than 10 tablets were forgotten by any participant over the entire study period.
Seven studies identified reasons for non‐compliance or discontinuation of treatment associated with adverse effects (Blot 1980; Busch 1972; Cable 1988; Gordeuk 1987a; Gordeuk 1990; Rybo 1971; Waldvogel 2012).
B. Iron supplementation: oral versus parenteral iron supplementation
One study compared oral and parenteral iron supplementation (Birgegard 2010).
(1) Risk ratio of low Hb deferral (primary outcome)
Low Hb deferral was not reported in this study.
(2) Hb levels, MCV, other blood indices and iron stores
i. Haemoglobin (Hb)
Hb was reported as an outcome in this study, although results were given descriptively, whereby "no significant differences in Hb between the treatment groups were seen".
ii. Mean corpuscular volume (MCV)
MCV was not reported in this study.
iii. Serum ferritin
Mean serum ferritin was reported both before further donation and after four (women) or five (men) subsequent donations. There was no difference in post‐treatment serum ferritin levels at follow‐up prior to further donation between treatment arms (MD 2.10 ng/mL; 95% CI ‐5.91 to 10.11; 120 participants; P value = 0.61) (Analysis 2.1). However, after further multiple donations, the mean serum ferritin level was significantly higher in donors who received iron supplementation intravenously in a single dose after each donation, compared with those who were administered oral iron supplements (MD 7.65 ng/mL; 95% CI 0.36 to 14.94; 120 participants; P value = 0.04) (Analysis 2.1).
2.1. Analysis.
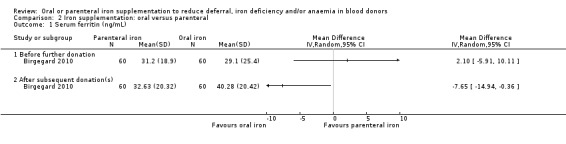
Comparison 2 Iron supplementation: oral versus parenteral, Outcome 1 Serum ferritin (ng/mL).
iv. Serum or plasma iron
Serum or plasma iron levels were not reported in this study.
v. Total iron binding capacity (TIBC)
TIBC was not reported in this study.
vi. Transferrin saturation
Transferrin saturation was not reported in this study.
(3) Health‐related quality of life and physical activity
No measures of health‐related quality of life or physical activity were used in this study.
(4) Adverse effects
No serious adverse effects occurred during the trial. In donors who received oral iron supplementation, two cases of constipation and two cases of diarrhoea were reported. In addition, six donors who received oral iron supplementation reported gastric discomfort. A non‐severe headache in one donor was the only adverse event reported in the intravenous iron group.
This study also compared the frequencies of restless leg syndrome, measured by the International Restless Legs Syndrome Study Group Severity Scale (IRLS) in each treatment group. A significant difference in IRLS score between treatment groups in favour of intravenous administration of iron was reported; however, no standard deviations were reported to enable an estimation of the effect size.
(5) Compliance
Treatment compliance (defined as 100% of medication taken) between visit four and seven ranged from 88% to 91.7% for oral iron supplementation compared with 88% to 100% for iron administered intravenously.
C. Iron supplementation versus iron‐rich food supplements
No studies comparing iron supplementation versus iron‐rich food supplements were identified.
D. Iron supplementation, dose A versus iron supplementation, dose B
Four studies compared different doses of iron supplementation (Ehn 1968; Jacobs 1993; Lieden 1975; Radtke 2004a). Details of the doses and duration of treatment in individual studies are given in Table 2.
(1) Risk ratio of low Hb deferral (primary outcome)
Two studies reported low Hb deferral at donation although in the first of these, discrepancies in the paper prevented reliable extraction of the data (Jacobs 1993; Radtke 2004a). In the second study, there was no evidence for a difference in the rate of low Hb deferral between treatment groups either at the first donation visit after commencement of iron supplementation (RR 0.66; 95% CI 0.11 to 3.92; 351 participants; P value = 0.65), after subsequent donation visits (RR 1.87; 95% CI 0.17 to 20.33; 236 participants; P value = 0.61) or over cumulative donation visits (RR 0.98; 95% CI 0.25 to 3.90; 742 participants; P value = 0.98) (Radtke 2004a) (Analysis 3.1).
3.1. Analysis.
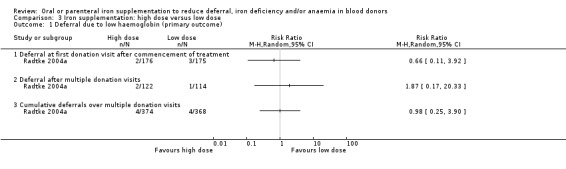
Comparison 3 Iron supplementation: high dose versus low dose, Outcome 1 Deferral due to low haemoglobin (primary outcome).
(2) Hb levels, MCV, other blood indices and iron stores
i. Haemoglobin (Hb)
Hb levels before further donation were reported in one study (Jacobs 1993), in which there was no evidence for a difference in haemoglobin levels at follow‐up between treatment dosage groups (MD 5.00 g/L; 95% CI ‐0.47 to 10.47; 85 participants; P value = 0.07) (Analysis 3.2). One study reported Hb levels after subsequent donation(s) (Ehn 1968); however, this study reported results graphically and data extraction could not be undertaken; no significant differences between groups were reported in this study.
3.2. Analysis.
Comparison 3 Iron supplementation: high dose versus low dose, Outcome 2 Haemoglobin (g/L).
ii. Mean corpuscular volume (MCV)
No studies reported MCV as an outcome.
iii. Serum ferritin
Two studies reported serum ferritin levels at follow‐up before further donation (Jacobs 1993; Radtke 2004a). Meta‐analysis of these two studies showed no evidence for a difference in serum ferritin between dosage groups (MD 2.89 ng/mL; 95% CI ‐1.83 to 7.60; two studies; 356 participants; P value = 0.23). However, in one study which reported serum ferritin level after two (female) or three (male) subsequent donations, there was a significant difference in serum ferritin in favour of a higher dose (20 mg twice daily compared with 10 mg twice daily) of iron supplementation (MD 7.96 ng/mL; 95% CI 1.68 to 14.24; 206 participants; P value = 0.01) (Radtke 2004a) (Analysis 3.3).
3.3. Analysis.
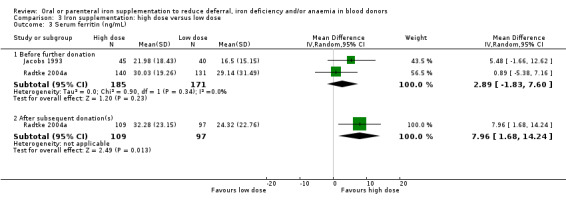
Comparison 3 Iron supplementation: high dose versus low dose, Outcome 3 Serum ferritin (ng/mL).
iv. Serum or plasma iron
One study reported serum iron levels after subsequent donations (Lieden 1975). There was no evidence for a difference in serum iron levels between treatment groups in this study (MD 21.00 μg/dL; 95% CI ‐7.70 to 49.70; 17 participants; P value = 0.15) (Analysis 3.4). A further study reported results graphically and data extraction could not be undertaken; no significant differences between groups were reported (Ehn 1968).
3.4. Analysis.
Comparison 3 Iron supplementation: high dose versus low dose, Outcome 4 Serum or plasma iron (μg/dL).
v. Total iron binding capacity (TIBC)
Two studies reported TIBC after subsequent donations (Ehn 1968; Lieden 1975), although in the first of these results were reported graphically and no data extraction could be undertaken; this study reported no significant differences between treatment groups. In the second study, there was no evidence for a difference in TIBC between treatment groups (MD ‐27.00 μg/dL; 95% CI ‐78.22 to 24.22; 17 participants; P value = 0.30) (Analysis 3.5).
3.5. Analysis.
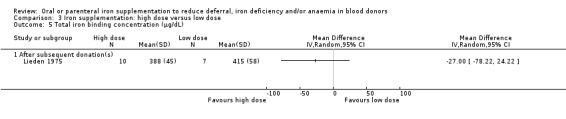
Comparison 3 Iron supplementation: high dose versus low dose, Outcome 5 Total iron binding concentration (μg/dL).
vi. Transferrin saturation (%)
Transferrin saturation before further donation was reported in one study (Jacobs 1993), in which there was no evidence for a difference in transferrin saturation (MD 5.10%; 95% CI ‐0.46 to 10.66; 85 participants; P value = 0.07) (Analysis 3.6). No studies reported transferrin saturation after subsequent donation(s).
3.6. Analysis.
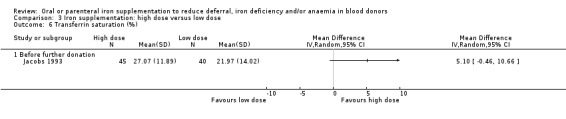
Comparison 3 Iron supplementation: high dose versus low dose, Outcome 6 Transferrin saturation (%).
(3) Health‐related quality of life and physical activity
Physical capacity using a bike test was reported in one study (Ehn 1968); however, no standard deviations were provided and therefore a formal assessment of these results was not possible. No significant differences between treatment groups were reported.
(4) Adverse effects
Adverse effects were reported descriptively in two studies (Jacobs 1993; Radtke 2004a), in which no significant differences in the frequency of adverse effects between treatment groups were found. Specific adverse effects were not reported.
(5) Compliance
Compliance was measured in all four studies although was not reported in one study (Jacobs 1993), and no study reported compliance separately per treatment group. Two studies reported compliance as no more than 10 tablets forgotten during the whole period (Ehn 1968; Lieden 1975), which occurred in all and 75% of participants respectively. In the third study compliance, defined as the ingestion of at least 90% of prescribed capsules, was poor in one‐third of male and one‐quarter of female participants (Radtke 2004a).
E. Iron supplementation, treatment duration A versus iron supplementation, treatment duration B
One study compared different durations of iron supplementation between groups (3108 mg over 28 days versus 444 mg over four days) (Bucher 1973).
(1) Risk ratio of low Hb deferral (primary outcome)
Low Hb deferral was not reported in this study.
(2) Hb levels, MCV, other blood indices and iron stores
i. Haemoglobin (Hb)
Post‐treatment Hb levels before further donation visits were reported in this study; there was no evidence of a difference in Hb levels between treatment arms (MD 1.00 g/L; 95% CI ‐0.93 to 2.93; 123 participants; P value = 0.31) (Analysis 4.1).
4.1. Analysis.

Comparison 4 Iron supplementation: long versus short treatment duration, Outcome 1 Haemoglobin (g/L).
ii. Mean corpuscular volume (MCV)
MCV was not reported in this study.
iii. Serum ferritin
Serum ferritin was not reported in this study.
iv. Serum or plasma iron
Significantly higher plasma iron levels before further donation visits were found in donors who received iron supplementation for the longer treatment duration of 28 days (MD 24.12 μg/dL; 95% CI 9.36 to 38.88; 123 participants; P value = 0.001) (Analysis 4.2).
4.2. Analysis.

Comparison 4 Iron supplementation: long versus short treatment duration, Outcome 2 Serum or plasma iron (μg/dL).
v. Total iron binding capacity (TIBC)
TIBC was not reported in this study.
vi. Transferrin saturation (%)
Significantly higher transferrin saturation levels before further donation visits between treatment arms were found in donors who received a longer treatment duration of iron supplementation (MD 4.81%; 95% CI 1.93 to 7.69; 123 participants; P value = 0.001) (Analysis 4.3).
4.3. Analysis.

Comparison 4 Iron supplementation: long versus short treatment duration, Outcome 3 Transferrin saturation (%).
(3) Health‐related quality of life and physical activity
No measures of health‐related quality of life or physical activity were used in this study.
(4) Adverse effects
Mainly minor side effects were reported in 19% to 29% of donors; this study did not provide results separately for each treatment group.
(5) Compliance
This study reported treatment compliance only in participants who received treatment for the longer period of 28 days; in these participants, tablets were taken on between 87.7% and 93.4% of total treatment days.
F. Iron supplementation, preparation A versus iron supplementation, preparation B
Twelve studies compared different preparations of iron supplementation, which included a comparison of ferrous sulphate versus ferrous fumarate (Buzi 1980; Lindholm 1981), ferrous sulphate versus carbonyl iron (Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b), ferrous sulphate versus two or three different doses of ferric polymaltose (Jacobs 1993; Jacobs 2000), ferrous sulphate versus ferric protein succinylate (Landucci 1987), two different preparations of ferrous sulphate (Busch 1972; Rybo 1971), and two different preparations of ferrous fumarate: heme iron versus non‐heme iron (Frykman 1994), or non‐heme iron versus a lower dose of non‐heme iron but supplemented with heme iron (Borch‐Iohnsen 1993).
Three studies reported Hb levels and other blood indices graphically (Borch‐Iohnsen 1993; Devasthali 1991; Gordeuk 1987b). In the first study, we estimated data from the graphs as described in the Methods section (Devasthali 1991). However, in two studies, graphs were of insufficient quality to allow reliable data extraction (Borch‐Iohnsen 1993; Gordeuk 1987b), and in one case (Borch‐Iohnsen 1993), did not include standard deviations.
(1) Risk ratio of low Hb deferral (primary outcome)
One study comparing ferrous sulphate with ferric polymaltose reported low Hb deferral rates (Jacobs 1993); however these data could not be extracted due to data discrepancies in the study report.
In the comparison of ferrous sulphate with ferrous fumarate, there was no evidence from one study of a difference in the rate of low Hb deferral after multiple donation visits (RR 0.51; 95% CI 0.05 to 5.60; 419 participants; P value = 0.58) (Lindholm 1981) (Analysis 5.1).
5.1. Analysis.
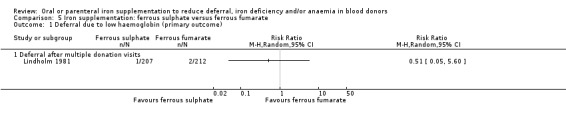
Comparison 5 Iron supplementation: ferrous sulphate versus ferrous fumarate, Outcome 1 Deferral due to low haemoglobin (primary outcome).
(2) Hb levels, MCV, other blood indices and iron stores
i. Haemoglobin (Hb)
Two studies comparing ferrous sulphate and ferrous fumarate reported Hb levels (Buzi 1980; Lindholm 1981); there was no evidence for a difference in Hb levels between iron preparations either before further donation (MD 2.00; 95% CI ‐2.85 to 6.85; one study; 61 participants; P value = 0.42) (Buzi 1980), or after two subsequent donations (MD 1.00 g/L; 95% CI ‐0.79 to 2.79; one study; 346 participants; P value = 0.27) (Lindholm 1981) (Analysis 5.2).
5.2. Analysis.
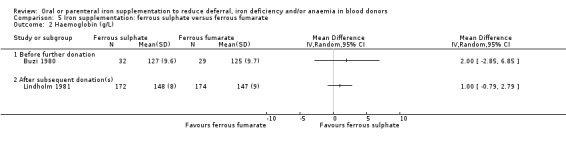
Comparison 5 Iron supplementation: ferrous sulphate versus ferrous fumarate, Outcome 2 Haemoglobin (g/L).
In the comparison of ferrous sulphate with carbonyl iron, three studies reported Hb levels before further donation (Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b), although in one study data were reported graphically and could not be extracted (Gordeuk 1987b). Combined evidence from two studies showed no evidence for a difference in Hb levels between treatment arms (MD 0.76 g/L; 95% CI ‐2.98 to 4.49; two studies; 79 participants; P value = 0.69) (Analysis 6.1).
6.1. Analysis.
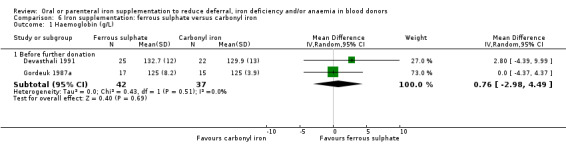
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 1 Haemoglobin (g/L).
Similarly, there was no evidence for a difference in Hb levels before further donation from three trials (Jacobs 1993; Jacobs 2000; Landucci 1987), which compared ferrous sulphate with ferric compounds as iron preparations (MD 2.36 g/L; 95% CI ‐0.63 to 5.34; three studies; 261 participants; P value = 0.12) (Analysis 7.1).
7.1. Analysis.
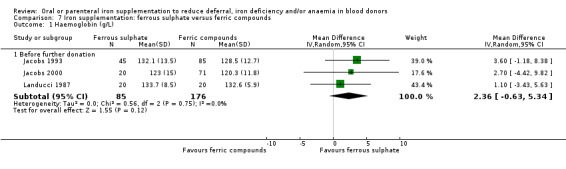
Comparison 7 Iron supplementation: ferrous sulphate versus ferric compounds, Outcome 1 Haemoglobin (g/L).
In the comparison of two preparations of ferrous fumarate (20 mg compared with 16 mg plus 2 mg heme iron from porcine blood), there was no evidence for a difference in mean change from baseline Hb levels (MD ‐20.0 g/L; 95% CI ‐80.59 to 40.59; one study; 34 participants; P value = 0.52) (Analysis 9.1). In another study (Frykman 1994), which compared two different preparations of iron fumarate, Hb levels were reported as median values and therefore no formal assessment of the effect size could be undertaken.
9.1. Analysis.
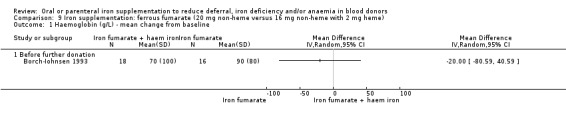
Comparison 9 Iron supplementation: ferrous fumarate (20 mg non‐heme versus 16 mg non‐heme with 2 mg heme), Outcome 1 Haemoglobin (g/L) ‐ mean change from baseline.
ii. Mean corpuscular volume (MCV)
Three studies which compared ferrous sulphate with carbonyl iron reported MCV before further donation (Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b), although graphical data reporting in one study precluded data extraction (Gordeuk 1987b). Combined evidence from two studies showed no difference in MCV after treatment (MD 0.62 fL; 95% CI ‐ 1.49 to 2.74; two studies; 79 participants; P value = 0.56) (Analysis 6.2).
6.2. Analysis.
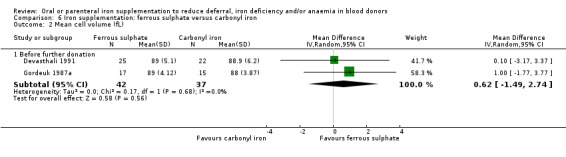
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 2 Mean cell volume (fL).
There was also no difference in MCV before further donation in a study of ferrous sulphate compared with ferric protein succinylate (MD 1.00 fL; 95% CI ‐1.09 to 3.09; 40 participants; P value = 0.35) (Landucci 1987) (Analysis 7.2).
7.2. Analysis.
Comparison 7 Iron supplementation: ferrous sulphate versus ferric compounds, Outcome 2 Mean cell volume (fL).
iii. Serum ferritin
Serum ferritin levels before further donation were reported in three studies (Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b), which compared ferrous sulphate with carbonyl iron, although data from two studies could not be extracted (Devasthali 1991; Gordeuk 1987b), and one study reported geometric mean values (Gordeuk 1987a).
There was also no evidence for a difference in serum ferritin levels before further donation from three studies (Jacobs 1993; Jacobs 2000; Landucci 1987), which compared ferrous sulphate with ferrous compounds (MD 8.07 ng/mL; 95% CI ‐1.50 to 17.63; three studies; 261 participants; P value = 0.10) (Analysis 7.3).
7.3. Analysis.
Comparison 7 Iron supplementation: ferrous sulphate versus ferric compounds, Outcome 3 Serum ferritin (ng/mL).
In the comparison of two preparations of ferrous fumarate (with and without heme iron), there was no evidence for a difference in mean change from baseline serum ferritin levels (MD ‐4.00 ng/mL; 95% CI ‐12.44 to 4.44; one study; 34 participants; P value = 0.35) (Analysis 9.2). Another study which compared different preparations of iron fumarate reported serum ferritin as median levels and therefore no formal assessment of the effect size could be undertaken (Frykman 1994).
9.2. Analysis.
Comparison 9 Iron supplementation: ferrous fumarate (20 mg non‐heme versus 16 mg non‐heme with 2 mg heme), Outcome 2 Serum ferritin (ng/mL) ‐ mean change from baseline.
iv. Serum or plasma iron
In two studies which compared serum iron levels between ferrous sulphate and ferrous fumarate preparations, there was no evidence for a difference in serum iron levels between treatment arms either before further donation (MD 7.00 μg/dL; 95% CI ‐7.14 to 21.14; one study; 61 participants; P value = 0.33) (Buzi 1980), or after subsequent donations (MD 0.00 μg/dL; 95% CI ‐7.06 to 7.06; one study; 346 participants; P value = 1.00) (Lindholm 1981) (Analysis 5.3).
5.3. Analysis.
Comparison 5 Iron supplementation: ferrous sulphate versus ferrous fumarate, Outcome 3 Serum or plasma iron (μg/dL).
Combined evidence from two studies (Devasthali 1991; Gordeuk 1987a), which compared ferrous sulphate and carbonyl iron, also showed no difference in serum iron levels before further donation (MD ‐1.76 μg/dL; 95% CI ‐26.49 to 22.97; two studies; 79 participants; P value = 0.89) (Analysis 6.3). In a third study, graphical data were of insufficient quality to allow extraction (Gordeuk 1987b).
6.3. Analysis.
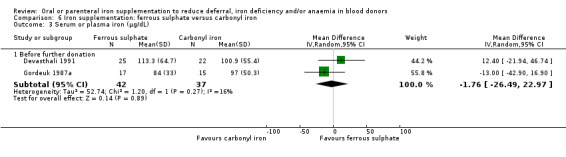
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 3 Serum or plasma iron (μg/dL).
Similarly, there was no evidence for a difference in serum iron levels between ferrous sulphate and ferric compound iron preparations before further donations (Jacobs 2000; Landucci 1987) (MD 0.88; 95% CI ‐3.25 to 5.00; two studies; 131 participants; P value = 0.68) (Analysis 7.4).
7.4. Analysis.
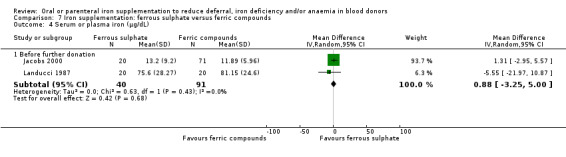
Comparison 7 Iron supplementation: ferrous sulphate versus ferric compounds, Outcome 4 Serum or plasma iron (μg/dL).
v. Total iron binding capacity (TIBC)
There was no evidence for a difference in TIBC either before further donation (MD 0.00 μg/dL; 95% CI ‐3.76 to 3.76; one study; 61 participants; P value = 1.00) (Buzi 1980), or after subsequent donations (MD 5.59 μg/dL; 95% CI ‐2.65 to 13.83; one study; 346 participants; P value = 0.18) (Lindholm 1981) (Analysis 5.4), in studies which compared ferrous sulphate with ferrous fumarate.
5.4. Analysis.
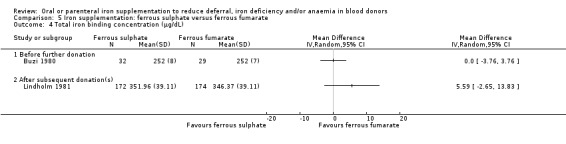
Comparison 5 Iron supplementation: ferrous sulphate versus ferrous fumarate, Outcome 4 Total iron binding concentration (μg/dL).
There was also no evidence from two studies of a difference in TIBC before further donation between ferrous sulphate and carbonyl iron (MD ‐9.75 μg/dL; 95% CI ‐52.65 to 33.16; two studies; 79 participants; P value = 0.66) (Devasthali 1991; Gordeuk 1987a) (Analysis 6.4).
6.4. Analysis.
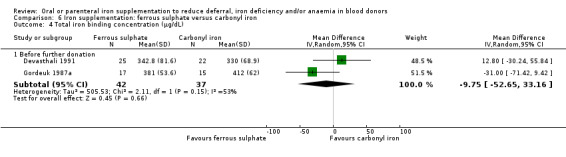
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 4 Total iron binding concentration (μg/dL).
In the comparison of two preparations of ferrous fumarate (with and without heme iron), there was no evidence for a difference in mean change from baseline TIBC levels (MD 0.60 μg/dL; 95% CI ‐2.77 to 3.97; one study; 34 participants; P value = 0.73) (Analysis 9.3).
9.3. Analysis.

Comparison 9 Iron supplementation: ferrous fumarate (20 mg non‐heme versus 16 mg non‐heme with 2 mg heme), Outcome 3 Total iron binding concentration (μg/dL) ‐ mean change from baseline.
vi. Transferrin saturation
Three studies which compared ferrous sulphate with carbonyl iron reported transferrin saturation (Devasthali 1991; Gordeuk 1987a; Gordeuk 1987b), although two of these reported data graphically and the quality of the graphs in the latter study precluded data extraction (Devasthali 1991; Gordeuk 1987b). Meta‐analysis of two studies showed no evidence for a difference in transferrin saturation between treatment arms (MD 2.45%; 95% CI ‐3.37 to 8.26; two studies; 79 studies; P value = 0.41) (Analysis 6.5).
6.5. Analysis.
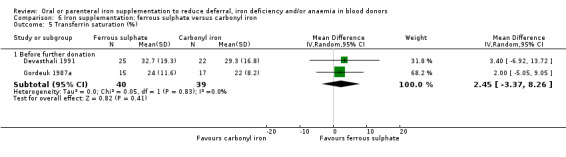
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 5 Transferrin saturation (%).
However, combined evidence from two studies, which compared ferrous sulphate with ferric polymaltose, showed significantly higher levels of transferrin saturation before further donation in donors who received ferrous sulphate (MD 5.33%; 95% CI 1.61 to 9.05 to; two studies; 221 participants; P value = 0.005), demonstrating a beneficial effect of ferrous sulphate over ferric polymaltose (Jacobs 1993; Jacobs 2000) (Analysis 7.5).
7.5. Analysis.
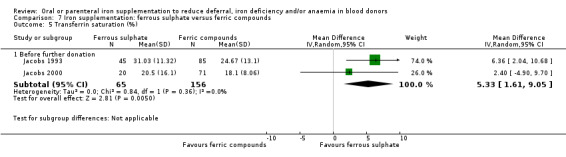
Comparison 7 Iron supplementation: ferrous sulphate versus ferric compounds, Outcome 5 Transferrin saturation (%).
(3) Health‐related quality of life and physical activity
No measures of health‐related quality of life or physical activity were used in studies which compared different iron preparations.
(4) Adverse effects
In the comparison of ferrous sulphate with ferrous fumarate, one study showed a significant increase in overall adverse effects associated with ferrous sulphate (RR 1.40; 95% CI 1.04 to 1.88; 131 participants; P value = 0.03) (Lindholm 1981) (Analysis 5.5). However, there were no significant differences observed in individual studies (Buzi 1980; Lindholm 1981), or from meta‐analysis of the frequencies of specific adverse effects, which included constipation, diarrhoea, nausea/vomiting and abdominal pain and/or cramps.
5.5. Analysis.
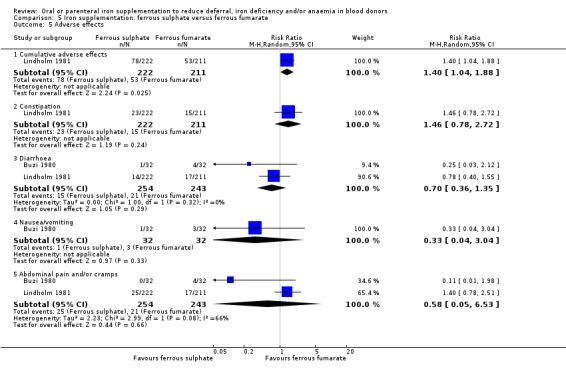
Comparison 5 Iron supplementation: ferrous sulphate versus ferrous fumarate, Outcome 5 Adverse effects.
There was no evidence for a difference in the overall frequency of adverse effects between ferrous sulphate and carbonyl iron from a meta‐analysis of two studies (Devasthali 1991; Gordeuk 1987a) (RR 0.89; 95% CI 0.75 to 1.06; two studies; 96 participants; P value = 0.18) (Analysis 6.6), or in the frequency of specific adverse effects which included constipation, diarrhoea, nausea/vomiting, abdominal pain and/or cramps, gastric/epigastric pain and headache. However, carbonyl iron was associated with an increased risk of taste disturbances compared with ferrous sulphate (RR 0.43; 95% CI 0.25 to 0.74; two studies; 96 participants; P value = 0.002) (Analysis 6.6).
6.6. Analysis.
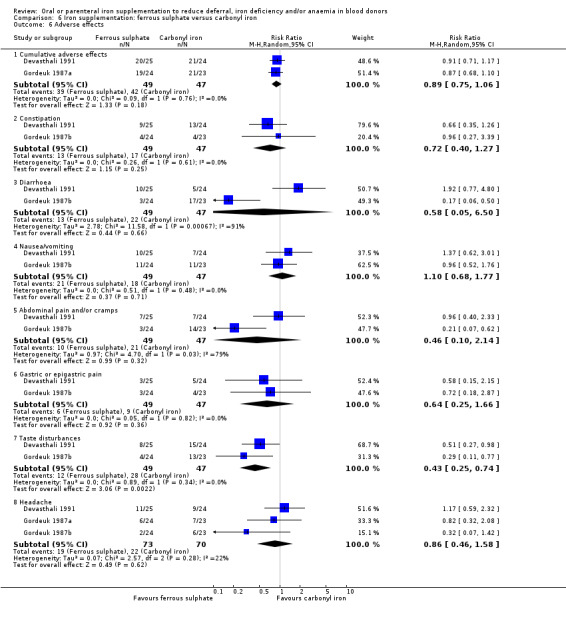
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 6 Adverse effects.
Adverse effects were not reported in any of the studies which compared ferrous sulphate with ferric compound iron preparations.
In the comparison of heme iron with non‐heme iron, one study reported a higher frequency of cumulative adverse effects for non‐heme iron than heme iron (25% versus 14%) (Frykman 1994), although the absence of actual numbers of participants prevented a statistical evaluation of this difference. In this study, participants who received non‐heme iron also experienced a higher rate of gastric pain (19% versus 6%), obstipation (35% versus 14%) and diarrhoea (37% versus 26%).
In one study, which compared EryferⓇ with an alternative ferrous sulphate preparation (Busch 1972), a lower frequency of cumulative adverse effects was observed in participants who received EryferⓇ than in those who received the alternative preparation in both the morning (11.2% versus 4.7%) and the evening (10.0% versus 2.9%), although actual numbers were not reported and therefore no formal statistical assessment of these differences could be undertaken. In this study, adverse effects were predominantly gastrointestinal complaints, which included loss of appetite, indigestion and diarrhoea. In a second study, which compared ferrous sulphate with an alternative sustained release iron preparation (Rybo 1971), there were no significant differences in the frequency of cumulative adverse effects (RR 1.14; 95% CI 0.91 to 1.43; 781 participants; P value = 0.26) or specific adverse effects including constipation, diarrhoea, nausea/vomiting and epigastric pain (Analysis 8.1).
8.1. Analysis.
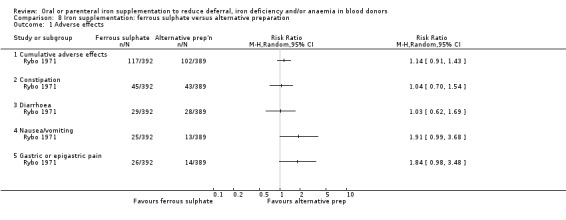
Comparison 8 Iron supplementation: ferrous sulphate versus alternative preparation, Outcome 1 Adverse effects.
(5) Compliance
In the comparison of ferrous sulphate with ferrous fumarate, there was no difference in the number of participants who were 100% compliant over the treatment period (77.5% versus 82.5% respectively) (Lindholm 1981).
In a meta‐analysis of two studies comparing ferrous sulphate with carbonyl iron (Gordeuk 1987a; Gordeuk 1987b), there was no difference in compliance between treatment arms (RR 0.95; 95% CI 0.84 to 1.09; two studies; 95 participants; P value = 0.48) (Analysis 6.7). A third study reported full compliance in both treatment arms (Devasthali 1991).
6.7. Analysis.
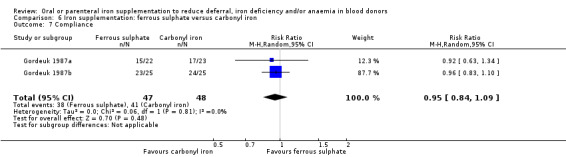
Comparison 6 Iron supplementation: ferrous sulphate versus carbonyl iron, Outcome 7 Compliance.
Compliance was reported descriptively in two studies (Jacobs 1993; Jacobs 2000), which compared ferrous sulphate with ferric compound iron preparations; the first of these reported only that some patients stopped treatment due to adverse effects (Jacobs 1993). In the second study, tolerance was reported to be "much better with the complex exceeding 80% but this was only 60% with the ferrous sulphate" (Jacobs 2000).
The number of study participants who discontinued treatment was similar for ferrous sulphate and an alternative sustained‐release iron preparation (85.1% versus 86.6% respectively) (Rybo 1971). However, in the study of EryferⓇ, compared with an alternative ferrous sulphate preparation (Busch 1972), compliance was significantly higher in participants who received EryferⓇ than in those who received the alternative preparation (RR 1.80; 95% CI 1.24 to 2.61; one study; 89 participants; P value = 0.002) (Analysis 8.2). We did not carry out meta‐analysis due to the differences in alternative iron preparation between the two studies.
8.2. Analysis.
Comparison 8 Iron supplementation: ferrous sulphate versus alternative preparation, Outcome 2 Compliance.
Discussion
Iron deficiency is a significant cause of deferral in people wishing to donate blood. Donation intervals are set to minimise iron deficiency in repeat blood donors and all donors are screened pre‐donation at each repeat visit for low haemoglobin levels. Deferral of donation for a period of six months through failure to pass the haemoglobin threshold is associated with failure of donors to return to give blood. Avoiding iron deficiency is therefore essential not only to minimise symptoms and morbidity in donors and hence increase retention of donors in the long term, but also to maintain the efficiency of a donor session, where deferral is costly and disruptive. Iron supplementation for blood donors has been considered and in some settings has been implemented for certain groups of 'at risk' donors. Rigorous evidence for the cost and benefits of iron supplementation is essential to guide policy.
Summary of main results
The relative and absolute benefits of iron supplementation
Thirty randomised controlled trials (RCTs) met the eligibility criteria, including comparisons of iron supplementation with placebo, as well as different methods of administration, doses, duration and preparations of iron supplementation. Meta‐analysis of four studies showed a significantly reduced risk of deferral due to low haemoglobin in donors who received iron supplementation compared with donors who received no iron supplementation, both at the first donation visit (risk ratio (RR) 0.34; 95% confidence interval (CI) 0.21 to 0.55; four studies; 1194 participants; P value < 0.0001) and at subsequent donations (RR 0.25; 95% CI 0.15 to 0.41; three studies; 793 participants; P value < 0.00001). There is also a clear benefit of iron supplementation on markers of iron stores but the effect of iron on haemoglobin level, although significant, is low. Detailed comparison of the effect of iron supplementation on iron stores is hampered by different assay methods and lack of standardisation.
Based on data from the four studies, the absolute risk of deferral due to low Hb at the first donation visit after receiving iron supplementation is 3.6% in iron‐supplemented donors compared with 10.5% in controls. The corresponding absolute risks of deferral after multiple donation visits are 5.0% and 19.9% respectively, based on data from three studies.
Evidence from a single study of parenteral versus oral iron suggests that parenteral iron is significantly more effective than oral iron in increasing serum ferritin levels. There may also be fewer minor side effects in donors given parenteral compared with oral iron, but widespread use of parenteral iron would not be practical in this population. However, this review has identified four ongoing randomised trials and one study awaiting classification which include iron administered intravenously to blood donors. Nevertheless, it seems unlikely that parenteral iron would be accepted on a mass scale, particularly given recent evidence from a systematic review that use of parenteral iron is associated with an increased risk of infection (Litton 2013).
Side effects of iron supplementation
The benefits of iron supplementation with tablets are substantial but the rate of significant side effects is high, which is likely to limit acceptability and compliance. However, adverse effects were widespread and were more frequent in donors who received iron supplementation than in those who did not (RR 1.60; 95% CI 1.23 to 2.07; four studies; 1748 participants; P value = 0.0005), with a significantly increased risk of gastrointestinal upset and taste disturbances.
Due to the adverse effects associated with iron supplementation, treatment compliance is an issue. The absolute risk of adverse effects is 29% in iron‐supplemented donors compared with an absolute risk of 17% in controls. The impact of these side effects on compliance is uncertain. Although seven studies identified reasons for non‐compliance or discontinuation of treatment associated with adverse effects (Blot 1980; Busch 1972; Cable 1988; Gordeuk 1987a; Gordeuk 1990; Rybo 1971; Waldvogel 2012), only two trials reported compliance rates separately for both treatment groups (Gordeuk 1990; Mirrezaie 2008) and, taken together, showed no evidence for a difference in compliance rates between treatment groups (RR 0.76; 95% CI 0.51 to 1.15; two studies; 146 participants; P value = 0.19). Compliance (as measured by ingestion of over 90% of tablets) was poorly documented in many of the studies but variable when reported (Radtke 2004a; Radtke 2004b). There are unlikely to be significant cost issues associated with iron supplements but compliance may be a more important issue if iron supplementation is targeted at large numbers of donors in routine operational practice.
The long‐term effects of iron supplementation without measurement of iron stores are unknown and in other contexts iron given indiscriminately has had deleterious consequences in some populations (Oppenheimer 2001; Sazawal 2006). These considerations are likely to preclude widespread use of iron supplementation by tablets.
Overall completeness and applicability of evidence
There are very few studies of the effects of iron supplementation on physical capacity and quality of life in blood donors.
Differences between studies in terms of type of participants, the preparation, dose and duration of treatment and the time at which outcomes were measured, as well as inter‐donation interval, inevitably limited investigation of the effect of iron supplementation on physical capacity and quality of life. There is very limited evidence in non‐blood donors that iron supplementation of iron‐deficient non‐anaemic adults improves some aspects of cognitive function (Falkingham 2010). Further evidence of the effect of iron stores on cognitive function and physical activity or capacity in donors from adequately powered RCTs would be crucial to inform future policies.
It is possible that effects of iron supplementation on physical function may take place quickly and be found in trials of short‐term iron supplementation. However, immediately after donation these effects are confounded by fluctuations in haemoglobin. Furthermore, a reduction in physical or mental function may only be seen in those who are iron deficient over a longer period of time. While power calculations are difficult without more preliminary data, studies may indeed need larger sample sizes and longer follow‐up periods to see the effects of iron supplementation on wider measures of physical and mental function.
One potentially very important question for further study is whether low and/or intermittent iron supplementation may have a similar effect in reducing anaemia and low haemoglobin deferral to a higher dose or continuous iron supplementation, but with reduced side effects. Only one study directly addressed different durations of iron supplementation in donors and compared iron supplementation of 3108 mg over 28 days versus 444 mg over four days (Bucher 1973). Unfortunately, deferral due to low haemoglobin was not reported and adverse events were not discussed by study group. Our review can therefore only search for differences in outcomes among different trials of iron supplementation where iron was given for different durations.
The combined evidence from all four studies where deferral rates were reported showed a significantly reduced risk of deferral due to low Hb at the first donation visit after treatment in donors who received iron supplementation (RR 0.34; 95% CI 0.21 to 0.55; four studies; 1194 participants; P value < 0.0001) and this low Hb deferral risk reduction was maintained after multiple and/or cumulative donation visits, with no evidence of heterogeneity between studies (I2 = 0%; 95% CI 0% to 79.3%) (Gordeuk 1990; Maghsudlu 2008; Radtke 2004a; Radtke 2004b). Furthermore, there were no significant differences between male and female donors in low Hb deferral rates at first donation, after multiple donation visits or over cumulative donation visits (Analysis 10.1; Analysis 10.2; Analysis 10.3). Looking at the effect of different iron dosing schedules on iron stores, meta‐analysis of two studies which reported serum ferritin levels at follow‐up before further donation showed no evidence for a difference in serum ferritin between dosage groups (Jacobs 1993; Radtke 2004a). However, in one study which reported serum ferritin levels after two (female) or three (male) subsequent donations, there was a significant difference in serum ferritin in favour of a higher dose (20 mg twice daily compared with 10 mg twice daily) of iron supplementation (Radtke 2004a (Analysis 3.3). These differences did not translate into a significant difference in deferral rates.
The question of the effect of duration or intensity of iron supplementation and outcome has been addressed in RCTs of iron supplementation in pregnancy and a recent Cochrane review of intermittent versus daily iron supplementation concluded that there was a reduced incidence of mild to moderate anaemia in women taking daily compared to intermittent iron supplements, but no differences in the incidence of adverse outcomes of the pregnancy or in the neonate, as far as could be ascertained. Nevertheless, there was a significantly reduced incidence of side effects in those women receiving intermittent compared to daily iron supplementation (RR 0.57; 95% CI 0.34 to 0.87) (Peña‐Rosas 2012).
One physiological explanation for the broadly similar effects of lower versus higher doses of iron is that absorption of iron is greater when iron stores are low and the proportion of iron absorbed is reduced as iron stores rise, reducing the benefit and possibly increasing the side effects as the dose and duration of iron supplementation is increased. Examining the benefits and adverse events of lower versus higher‐dose regimes of iron supplementation would quite clearly be a priority for further work.
No trials were reported from lower‐middle‐income or low‐income counties. In many parts of the world recruitment of donors and blood safety has been the main focus of concern and research, while low deferral rates in repeat donors have been less of a priority. Nevertheless, in many parts of the world iron deficiency is very common (Lim 2012; Miller 2013), and high deferral rates in first‐time donors who fail to meet the haemoglobin threshold are certainly observed. Iron replacement in areas where malaria and other protozoa or community‐acquired bacterial infections are prevalent may predispose to infection (Drakesmith 2012), and it is likely that the question of iron replacement in donors will become an important topic of research in middle‐income and low‐income countries as the donor base increases and a higher proportion of donors are repeat donors. The safety of iron supplementation with regard to infection will require careful scrutiny in well‐designed trials, although it may be more straightforward to predict the likelihood of deferral to stratify donation intervals to reduce deferral in groups of donors at greater risk of iron deficiency.
This review highlights the limited amount of data available for iron supplementation in the context of blood donation. The number of studies is limited for each comparison and they usually involve a small number of participants. Small study effects (bias) should be considered when interpreting the results. The majority of the data are from studies comparing iron supplementation versus placebo, but even there numbers are small. For example, only a few events are recorded in each study when looking at the effect of iron supplementation on low Hb deferral.
Quality of the evidence
Thirty RCTs including a total of 4704 participants met the eligibility criteria, including 19 comparisons of iron supplementation and placebo or control; one comparison of oral and parenteral iron supplementation; four comparisons of different doses of iron supplementation; one comparison of different treatment durations of iron supplementation; and 12 comparisons of different iron supplementation preparations. However, the number of studies included in meta‐analyses was limited by differences in methods of outcome reporting, treatment duration and length of follow‐up between studies. The reduction in deferral due to low haemoglobin shows a large effect, but due to the risk of bias in the included studies we have downgraded the quality of the evidence to moderate (see 'Summary of findings' table).
Heterogeneity confidence intervals for I2 were generally wide due to the low number of studies included in each analysis. Visual inspection of forest plots revealed no obvious heterogeneity due to date of publication or study size.
Potential biases in the review process
The risk of bias was high or unclear in many studies, probably due to poor methods of reporting in studies published before 1990, including five that were published before 1980. Precise definition of the quality of studies is difficult as judgement is based on limited evidence, but there is no definitive evidence of substantial bias nor of systematic error through poor quality studies.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no other reviews in this area for comparison of our findings with other analyses.
Authors' conclusions
Implications for practice.
Overall, there is moderate quality evidence that iron supplementation has a substantial effect on reducing the risk of low haemoglobin deferral, but a significant proportion of donors taking iron suffer side effects. Blood services seeking a reduction in the levels of deferral due to low haemoglobin would wish to consider any reasonable methods to prevent iron deficiency, weighing cost against benefits and feasibility. With this in mind, possible courses for future action by blood services would be the targeted use of supplementation at groups or individuals at greater risk of iron deficiency, stratified or personalised donation intervals and/or dietary advice.
Implications for research.
The effect of dose and preparation of iron on both efficacy and the frequency of side effects is unclear from the existing studies. Crucially, the studies do not allow any definition of the relationship between dose and duration of iron supplementation and benefits or side effects. These questions would have to be explored, in large‐scale randomised controlled trials (RCTs) or pilot studies, before widespread use of iron supplementation in donors could be considered. Potential differences in methods used to assess biomarkers could be important when interpreting the absolute change in biomarker values (such as ferritin). There is very limited evidence that dietary advice to improve iron store is efficacious and there are no trials of dietary advice in donors. Further work in this area should include RCTs of a range of interventions to determine efficacy precisely. Finally, there are few existing randomised trials of the effects of iron supplementation on the physical capacity and quality of life of blood donors; future studies should include an assessment of these measures in iron‐supplemented donors.
Acknowledgements
We thank Susan Brunskill for her advice during the development of the study protocol. We are grateful to Emma Sydenham and Deirdre Beecher for their help and advice during the preparation of this review.
This research was supported by the NHSBT and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme (SF, CD) and the NIHR under its Programme Grant Scheme (NIHR‐RP‐PG‐0310‐1004, SF). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Appendices
Appendix 1. Search strategies for CENTRAL and the Cochrane Injuries Group Specialised Register
Cochrane Injuries Group Specialised Register
#1 (((blood donor* OR blood donat*) AND (iron OR anaemi* OR anemi* OR ferritin OR ferrous OR haemoglobin* OR hemoglobin* OR Hb OR ferric OR ferropaeni* or ferropeni*))) AND ( INREGISTER) [REFERENCE] [STANDARD]
Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library)
#1 MeSH descriptor: [Blood Donors] this term only #2 MeSH descriptor: [Cytapheresis] explode all trees #3 (red blood cell* or RBC* or blood or platelet* or apheresis or plateletpheresis) near/6 (donor* or donat*) #4 (donor* or donat* or interdonat*) near/10 (defer* or delay* or exclu* or reject* or "turn* away" or interval*) #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Anemia] explode all trees #7 MeSH descriptor: [Ferritins] this term only #8 MeSH descriptor: [Hemoglobins] this term only #9 MeSH descriptor: [Iron] this term only #10 MeSH descriptor: [Iron Compounds] explode all trees #11 (iron or anaemi* or anemi* or ferritin or ferrous):ti #12 iron near/3 (rich or enrich* or food* or diet* or absorp* or store* or storing or status or deficien* or deplet* or oral* or supplement* or salt* or complex or inject* or infusion* or intravenous* or replace* or product* or tablet* or pill* or capsule* or sulphate or sulfate or therap*) #13 ((ferritin or iron or haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) near/3 (level* or low* or below or concentration* or cutoff or rais* or increas*)) #14 ferrous next (sulfate or sulphate or fumarate or fumerate) #15 (ferropaeni* or ferropeni* or Albafort* or Fchem‐Sol* or Fe‐Max or Femiron or CapletFeostat* or Feosol or Fer Iron or Fer‐Gen‐Sol or Fer‐in‐Sol or Ferate* or TRFer* or Feratab or FeroSul or Fergon* or Ferra* or TDFerretts* or Ferro‐Sequels* or Ferro‐Time* or Ferrospace* or Fumasorb* or Hemocyte* or Ironmar* or Mol‐Iron* or Nephro* or FernNephro* or Siderol* or IronTandem* or Yiero‐Gota* or Yieronia* or (Ferra near/2 Caps) or Ferro‐Bob or Slow Fe or Slow Release Iron or Infed* or Dexferrum* or Ferrlecit* or Venofer*) #16 parenteral iron or iron dextran* or iron sucrose or ferric gluconate or heme iron or non‐heme iron #17 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 #18 #5 and #17
Appendix 2. PubMed search strategy
(blood donor* OR blood donat*) AND (iron OR anaemi* OR anemi* OR ferritin OR ferrous OR haemoglobin* OR hemoglobin* OR Hb OR ferric OR ferropaeni* or ferropeni*) AND (random* OR blind* OR trial* OR study OR groups OR control OR controlled) AND (publisher[sb] NOT pubstatusnihms)
Appendix 3. MEDLINE (OvidSP) search strategy
1. BLOOD DONORS/ 2. exp CYTAPHERESIS/ 3. ((red blood cell* or red cell* or RBC* or blood or platelet* or apheresis or plateletpheres*) adj6 (donat* or donor*)).tw. 4. ((donor* or donat* or interdonat*) adj10 (defer* or delay* or exclu* or reject* or turn* away or interval*)).tw. 5. or/1‐4 6. exp ANEMIA/ 7. FERRITINS/ 8. HEMOGLOBINS/ 9. IRON/ 10. exp IRON COMPOUNDS/ 11. (iron or anaemi* or anemi* or ferritin or ferrous).ti. 12. (iron adj3 (rich or enrich* or food* or diet* or absorp* or store* or storing or status or deficien* or deplet* or oral* or supplement* or salt* or complex or inject* or infusion* or intravenous* or replace* or product* or tablet* or pill* or capsule* or sulphate or sulfate or therap*)).ab. 13. ((ferritin or iron or haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) adj3 (level* or low* or below or concentration* or cutoff or cut off or rais* or increas*)).tw. 14. (ferrous adj (sulfate or sulphate or fumarate or fumerate)).ab. 15. (ferropaeni* or ferropeni* or Albafort* or Fchem‐Sol* or Fe‐Max or Femiron or CapletFeostat* or Feosol or Fer Iron or Fer‐Gen‐Sol or Fer‐in‐Sol or Ferate* or TRFer* or Feratab or FeroSul or Fergon* or Ferra* or TDFerretts* or Ferro‐Sequels* or Ferro‐Time* or Ferrospace* or Fumasorb* or Hemocyte* or Ironmar* or Mol‐Iron* or Nephro* or FernNephro* or Siderol* or IronTandem* or Yiero‐Gota* or Yieronia* or (Ferra adj2 Caps) or Ferro‐Bob or Slow Fe or Slow Release Iron or Infed* or Dexferrum* or Ferrlecit* or Venofer*).tw. 16. (parenteral iron or iron dextran* or iron sucrose or ferric gluconate or heme iron or non‐heme iron).ab. 17. or/6‐16 18. 5 and 17
Appendix 4. EMBASE (OvidSP) search strategy
1. BLOOD DONOR/ 2. APHERESIS/ 3. THROMBOCYTOPHERESIS/ 4. ((red blood cell* or red cell* or RBC* or blood or platelet* or apheresis or plateletpheres*) adj6 (donat* or donor*)).tw. 5. ((donor* or donat* or interdonat*) adj10 (defer* or delay* or exclu* or reject* or turn* away or interval*)).tw. 6. or/1‐5 7. exp IRON DEFICIENCY ANEMIA/ 8. IRON DEFICIENCY/ 9. HEMOGLOBIN BLOOD LEVEL/ 10. IRON DEPLETION/ 11. *IRON/12. IRON THERAPY/ 13. exp ANTIANEMIC AGENT/ 14. (iron or anaemi* or anemi* or ferritin or ferrous).ti. 15. (iron adj3 (rich or enrich* or food* or diet* or absorp* or store* or storing or status or deficien* or deplet* or oral* or supplement* or salt* or complex or inject* or infusion* or intravenous* or replace* or product* or tablet* or pill* or capsule* or sulphate or sulfate or therap*)).ab. 16. ((ferritin or iron or haemoglobin or hemoglobin or Hb or haematocrit or hematocrit or Hct) adj3 (level* or low* or below or concentration* or cutoff or cut off or rais* or increas*)).tw. 17. (ferrous adj (sulfate or sulphate or fumarate or fumerate)).ab. 18. (ferropaeni* or ferropeni* or Albafort* or Fchem‐Sol* or Fe‐Max or Femiron or CapletFeostat* or Feosol or Fer Iron or Fer‐Gen‐Sol or Fer‐in‐Sol or Ferate* or TRFer* or Feratab or FeroSul or Fergon* or Ferra* or TDFerretts* or Ferro‐Sequels* or Ferro‐Time* or Ferrospace* or Fumasorb* or Hemocyte* or Ironmar* or Mol‐Iron* or Nephro* or FernNephro* or Siderol* or IronTandem* or Yiero‐Gota* or Yieronia* or (Ferra adj2 Caps) or Ferro‐Bob or Slow Fe or Slow Release Iron or Infed* or Dexferrum* or Ferrlecit* or Venofer*).tw. 19. (parenteral iron or iron dextran* or iron sucrose or ferric gluconate or heme iron or non‐heme iron).ab. 20. or/7‐19 21. 6 and 20
Appendix 5. CINAHL (EBSCOhost) search strategy
1. (MH "Blood Donors") 2. (MH "Cytapheresis+") 3. TI ( (("red blood cell*" OR RBC* OR blood OR platelet* OR plateletpheresis OR apheresis) AND (donat* OR donor*)) ) OR AB ( (("red blood cell*" OR RBC* OR blood OR platelet* OR plateletpheresis OR apheresis) AND (donat* OR donor*)) ) 4. TI ( ((donor* OR donat* OR interdonat*) AND (defer* OR delay* OR exclu* OR reject* OR turn* AND away OR interval*)) ) OR AB ( ((donor* OR donat* OR interdonat*) AND (defer* OR delay* OR exclu* OR reject* OR turn* AND away OR interval*)) ) 5. S1 OR S2 OR S3 OR S4 6. (MH "Anemia, Iron Deficiency") 7. (MH "Ferritin") 8. (MH "Transferrin") 9. (MH "Iron") 10. (MH "Iron Compounds+") 11. TI (iron OR anaemi* OR anemi* OR ferritin OR ferrous) 12. AB (ferrous AND (sulfate OR sulphate OR fumarate OR fumerate)) 13. AB (iron AND (rich OR enrich* OR food* OR diet* OR absorp* OR store* OR storing OR status OR deficien* OR deplet* OR oral* OR supplement* OR salt* OR complex OR inject* OR infusion* OR intravenous* OR replace* OR product* OR tablet* OR pill* OR capsule* OR sulphate OR sulfate OR therap*)) 14. AB ("parenteral iron" OR "iron dextran*" OR "iron sucrose" OR "ferric gluconate" OR "heme iron" OR "non‐heme iron") 15. TI ( (ferropaeni* OR ferropeni* OR Albafort* OR Fchem‐Sol* OR Fe‐Max OR Femiron OR CapletFeostat* OR Feosol OR "Fer Iron" OR Fer‐Gen‐Sol OR Fer‐in‐Sol OR Ferate* OR TRFer* OR Feratab OR FeroSul OR Fergon* OR Ferra* OR TDFerretts* OR Ferro‐Sequels* OR Ferro‐Time* OR Ferrospace* OR Fumasorb* OR Hemocyte* OR Ironmar* OR Mol‐Iron* OR Nephro* OR FernNephro* OR Siderol* OR IronTandem* OR Yiero‐Gota* OR Yieronia* OR "Ferra Caps" OR Ferro‐Bob OR "Slow Fe" OR "Slow Release Iron" OR Infed* OR Dexferrum* OR Ferrlecit* OR Venofer*) ) OR AB ( (ferropaeni* OR ferropeni* OR Albafort* OR Fchem‐Sol* OR Fe‐Max OR Femiron OR CapletFeostat* OR Feosol OR "Fer Iron" OR Fer‐Gen‐Sol OR Fer‐in‐Sol OR Ferate* OR TRFer* OR Feratab OR FeroSul OR Fergon* OR Ferra* OR TDFerretts* OR Ferro‐Sequels* OR Ferro‐Time* OR Ferrospace* OR Fumasorb* OR Hemocyte* OR Ironmar* OR Mol‐Iron* OR Nephro* OR FernNephro* OR Siderol* OR IronTandem* OR Yiero‐Gota* OR Yieronia* OR "Ferra Caps" OR Ferro‐Bob OR "Slow Fe" OR "Slow Release Iron" OR Infed* OR Dexferrum* OR Ferrlecit* OR Venofer*) ) 16. TI ( ((ferritin OR iron OR haemoglobin OR hemoglobin OR Hb OR haematocrit OR hematocrit OR Hct) AND (level* OR low* OR below OR concentration* OR cutoff OR "cut off" OR rais* OR increas*)) ) OR AB ( ((ferritin OR iron OR haemoglobin OR hemoglobin OR Hb OR haematocrit OR hematocrit OR Hct) AND (level* OR low* OR below OR concentration* OR cutoff OR "cut off" OR rais* OR increas*)) ) 17. S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 18. S6 AND S17
Appendix 6. BNI (NHS Evidence) search strategy
1 (("red blood cell*" OR RBC* OR blood OR platelet* OR plateletpheresis OR aphersis) AND (donat* OR donor*)).ti,ab 2 ((donor* OR donat* OR interdonat*) AND (defer* OR delay* OR exclu* OR reject* OR "turn* away" OR interval*)).ti,ab 3. 1 OR 2 4. (iron OR anaemi* OR anemi* OR ferritin OR ferrous).ti 5. (ferrous AND (sulfate OR sulphate OR fumarate OR fumerate)).ab 6. (iron AND (rich OR enrich* OR food* OR diet* OR absorp* OR store* OR storing OR status OR deficien* OR deplet* OR oral* OR supplement* OR salt* OR complex OR inject* OR infusion* OR intravenous* OR replace* OR product* OR tablet* OR pill* OR capsule* OR sulphate OR sulfate OR therap*)).ab 7. ("parenteral iron" OR "iron dextran*" OR "iron sucrose" OR "ferric gluconate" OR "heme iron" OR "non‐heme iron").ab 8. (ferropaeni* OR ferropeni* OR Albafort* OR Fchem‐Sol* OR Fe‐Max OR Femiron OR CapletFeostat* OR Feosol OR "Fer Iron" OR Fer‐Gen‐Sol OR Fer‐in‐Sol OR Ferate* OR TRFer* OR Feratab OR FeroSul OR Fergon* OR Ferra* OR TDFerretts* OR Ferro‐Sequels* OR Ferro‐Time* OR Ferrospace* OR Fumasorb* OR Hemocyte* OR Ironmar* OR Mol‐Iron* OR Nephro* OR FernNephro* OR Siderol* OR IronTandem* OR Yiero‐Gota* OR Yieronia* OR "Ferra Caps" OR Ferro‐Bob OR "Slow Fe" OR "Slow Release Iron" OR Infed* OR Dexferrum* OR Ferrlecit* OR Venofer*).ti,ab 9. ((ferritin OR iron OR haemoglobin OR hemoglobin OR Hb OR haematocrit OR hematocrit OR Hct) AND (level* OR low* OR below OR concentration* OR cutoff OR "cut off" OR rais* OR increas*)).ti,ab 10. 4 OR 5 OR 6 OR 7 OR 8 OR 9 11. 3 AND 10
Appendix 7. Transfusion Evidence Library search strategy
(keywords:"blood donors" OR title:(donor OR donors OR donation)) AND title:(iron OR anaemic OR anemic OR anaemia OR anemia OR ferritin OR ferrous OR haemoglobin OR hemoglobin OR Hb OR ferric OR ferropaenia or ferropaenic OR ferropenia OR ferropenic)
Appendix 8. LILACS search strategy
db:("LILACS") AND type_of_study:("clinical_trials") AND (donor* OR donat*) AND (iron OR anaemi* OR anemi* OR ferritin OR ferrous OR haemoglobin* OR hemoglobin* OR hb OR ferric OR ferropaeni* OR ferropeni*)
Appendix 9. IndMed search strategy
(donor$ or donat$) AND (iron or anaemi$ or anemi$ or ferritin or ferrous or haemoglobin$ or hemoglobin$ or hb or ferric or ferropaeni$ or ferropeni$) AND (random$ or blind$ or trial or control$ or group$)
Appendix 10. KoreaMed search strategy
"Blood Donors" [MH] AND "Randomized Controlled Trial" [PT] OR "blood donor" [ALL] AND "Randomized Controlled Trial" [PT] OR "blood donors"[ALL] AND "Randomized Controlled Trial" [PT] OR "blood donation" [ALL] AND "Randomized Controlled Trial" [PT]
Appendix 11. PakMediNet search strategy
(donor OR donors OR donation) AND (randomized OR randomized OR randomly OR trial)
Appendix 12. Web of Science ‐ CPCI‐S search strategy
#1 TI=(red blood cell* or red cell* or RBC* or blood or platelet* or apheresis or plateletpheres*) #2 TI=(defer* or delay* or exclu* or reject* or turn* away or interval*) #3 #1 OR #2 #4=TI=(donor* or donat* or interdonat*) #5 #3 AND #4 #6 TI=(iron OR anaemi* OR anemi* OR ferritin OR ferrous) #7 TI=(ferropaeni* OR ferropeni* OR Albafort* OR Fchem‐Sol* OR Fe‐Max OR Femiron OR CapletFeostat* OR Feosol OR "Fer Iron" OR Fer‐Gen‐Sol OR Fer‐in‐Sol OR Ferate* OR TRFer* OR Feratab OR FeroSul OR Fergon* OR Ferra* OR TDFerretts* OR Ferro‐Sequels* OR Ferro‐Time* OR Ferrospace* OR Fumasorb* OR Hemocyte* OR Ironmar* OR Mol‐Iron* OR Nephro* OR FernNephro* OR Siderol* OR IronTandem* OR Yiero‐Gota* OR Yieronia* OR "Ferra Caps" OR Ferro‐Bob OR "Slow Fe" OR "Slow Release Iron" OR Infed* OR Dexferrum* OR Ferrlecit* OR Venofer*) #8 TI=((haemoglobin OR hemoglobin OR Hb OR haematocrit OR hematocrit OR Hct) AND (level* OR low* OR below OR concentration* OR cutoff OR "cut off" OR rais* OR increas*)) #9 #6 OR #7 OR #8 #10 TS=(randomized OR randomised OR trial) #11 #5 AND #9 AND #10
Appendix 13. Clinical trials registries search strategies
Clinicaltrials.gov
(Search Terms: blood donors OR blood donation) AND (Intervention: iron OR ferritin OR ferrous OR ferric) OR (Condition: iron deficiency OR haemoglobin OR hemoglobin OR Hb OR ferropaenic or ferropenic)
WHO ICTRP
(Title: donor OR donors OR donate OR donated OR donation OR donations OR donating) AND (Intervention: iron OR ferritin OR ferrous OR ferric) OR (Condition: iron deficiency OR haemoglobin OR hemoglobin OR Hb OR ferropaenic or ferropenic)
ISRCTN
“blood donor" OR "blood donors" OR "blood donation" OR "blood donations" OR "platelet donors" OR "platelet donation" OR "platelet donations"
UMIN‐CTR & Hong Kong Clinical Trials Registry
donor OR donors OR donation
Data and analyses
Comparison 1. Iron supplementation versus placebo/control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deferral due to low haemoglobin (primary outcome) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Deferral at first donation visit after commencement of treatment | 4 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.21, 0.55] |
| 1.2 Deferral after multiple donation visits | 3 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.15, 0.41] |
| 1.3 Cumulative deferrals over multiple donation visits | 4 | 2740 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.18, 0.52] |
| 2 Haemoglobin (g/L) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Before further donation | 8 | 847 | Mean Difference (IV, Random, 95% CI) | 2.36 [0.06, 4.66] |
| 2.2 After subsequent donation(s) | 3 | 406 | Mean Difference (IV, Random, 95% CI) | 6.37 [2.36, 10.39] |
| 3 Mean cell volume (fL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before further donation | 2 | 127 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐0.17, 2.92] |
| 4 Serum ferritin (ng/mL) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Before further donation | 5 | 640 | Mean Difference (IV, Random, 95% CI) | 13.98 [8.92, 19.03] |
| 4.2 After subsequent donation(s) | 3 | 619 | Mean Difference (IV, Random, 95% CI) | 9.01 [5.76, 12.25] |
| 5 Serum or plasma iron (μg/dL) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Before further donation | 3 | 246 | Mean Difference (IV, Random, 95% CI) | 11.76 [‐1.67, 25.20] |
| 5.2 After subsequent donation(s) | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 7.89 [1.12, 14.66] |
| 6 Total iron blinding concentration (μg/dL) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Before further donation | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐32.05 [‐61.45, ‐2.65] |
| 6.2 After subsequent donations | 2 | 315 | Mean Difference (IV, Random, 95% CI) | ‐42.64 [‐56.99, ‐28.28] |
| 7 Transferrin saturation (%) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Before further donation | 4 | 344 | Mean Difference (IV, Random, 95% CI) | 3.91 [2.02, 5.80] |
| 7.2 After subsequent donation(s) | 2 | 343 | Mean Difference (IV, Random, 95% CI) | 4.84 [2.78, 6.90] |
| 8 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Visual analogue scale fatigue | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Fatigue severity scale | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Vitality (SF‐12V2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Chester step test (mL O2/kg/min) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 QoL (SF12NL: physical condition) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 QoL (SF12NL: mental condition) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse effects | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Cumulative adverse effects | 4 | 1748 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.23, 2.07] |
| 9.2 Constipation | 5 | 1849 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.16, 2.31] |
| 9.3 Diarrhoea | 5 | 1555 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [1.38, 3.42] |
| 9.4 Nausea/vomiting | 6 | 1922 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.20, 2.56] |
| 9.5 Abdominal pain and/or cramps | 4 | 683 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [0.95, 5.16] |
| 9.6 Gastric or epigastric pain | 2 | 1242 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.73, 2.19] |
| 9.7 Taste disturbances | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 5.78 [2.10, 15.95] |
| 9.8 Headache | 5 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.53, 1.56] |
| 10 Compliance | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Non‐discontinuation of treatment | 3 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.68, 1.01] |
| 10.2 High compliance (> 90%) | 2 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.51, 1.15] |
Comparison 2. Iron supplementation: oral versus parenteral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum ferritin (ng/mL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After subsequent donation(s) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Iron supplementation: high dose versus low dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deferral due to low haemoglobin (primary outcome) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Deferral at first donation visit after commencement of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Deferral after multiple donation visits | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Cumulative deferrals over multiple donation visits | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Haemoglobin (g/L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serum ferritin (ng/mL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before further donation | 2 | 356 | Mean Difference (IV, Random, 95% CI) | 2.89 [‐1.83, 7.60] |
| 3.2 After subsequent donation(s) | 1 | 206 | Mean Difference (IV, Random, 95% CI) | 7.96 [1.68, 14.24] |
| 4 Serum or plasma iron (μg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 After subsequent donation(s) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Total iron binding concentration (μg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 After subsequent donation(s) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Transferrin saturation (%) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Iron supplementation: long versus short treatment duration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin (g/L) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Serum or plasma iron (μg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Transferrin saturation (%) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Iron supplementation: ferrous sulphate versus ferrous fumarate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deferral due to low haemoglobin (primary outcome) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Deferral after multiple donation visits | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Haemoglobin (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 After subsequent donation(s) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serum or plasma iron (μg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 After subsequent donation(s) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total iron binding concentration (μg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 After subsequent donation(s) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Cumulative adverse effects | 1 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.04, 1.88] |
| 5.2 Constipation | 1 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.78, 2.72] |
| 5.3 Diarrhoea | 2 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.36, 1.35] |
| 5.4 Nausea/vomiting | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 3.04] |
| 5.5 Abdominal pain and/or cramps | 2 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 6.53] |
Comparison 6. Iron supplementation: ferrous sulphate versus carbonyl iron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin (g/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Before further donation | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.76 [‐2.98, 4.49] |
| 2 Mean cell volume (fL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Before further donation | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐1.49, 2.74] |
| 3 Serum or plasma iron (μg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before further donation | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐1.76 [‐26.49, 22.97] |
| 4 Total iron binding concentration (μg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Before further donation | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐9.75 [‐52.65, 33.16] |
| 5 Transferrin saturation (%) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Before further donation | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 2.45 [‐3.37, 8.26] |
| 6 Adverse effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Cumulative adverse effects | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.75, 1.06] |
| 6.2 Constipation | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.40, 1.27] |
| 6.3 Diarrhoea | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.05, 6.50] |
| 6.4 Nausea/vomiting | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.68, 1.77] |
| 6.5 Abdominal pain and/or cramps | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.10, 2.14] |
| 6.6 Gastric or epigastric pain | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.25, 1.66] |
| 6.7 Taste disturbances | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.25, 0.74] |
| 6.8 Headache | 3 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.46, 1.58] |
| 7 Compliance | 2 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.09] |
Comparison 7. Iron supplementation: ferrous sulphate versus ferric compounds.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin (g/L) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Before further donation | 3 | 261 | Mean Difference (IV, Random, 95% CI) | 2.36 [‐0.63, 5.34] |
| 2 Mean cell volume (fL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Serum ferritin (ng/mL) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before further donation | 3 | 261 | Mean Difference (IV, Random, 95% CI) | 8.07 [‐1.50, 17.63] |
| 4 Serum or plasma iron (μg/dL) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Before further donation | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 0.88 [‐3.25, 5.00] |
| 5 Transferrin saturation (%) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Before further donation | 2 | 221 | Mean Difference (IV, Random, 95% CI) | 5.33 [1.61, 9.05] |
Comparison 8. Iron supplementation: ferrous sulphate versus alternative preparation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Cumulative adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Constipation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Diarrhoea | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Nausea/vomiting | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Gastric or epigastric pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Compliance | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Non‐discontinuation of treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Iron supplementation: ferrous fumarate (20 mg non‐heme versus 16 mg non‐heme with 2 mg heme).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin (g/L) ‐ mean change from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Serum ferritin (ng/mL) ‐ mean change from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total iron binding concentration (μg/dL) ‐ mean change from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Before further donation | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Iron supplementation versus placebo/control: males versus females.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deferral due to low haemoglobin: at first donation visit after commencement of treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Males | 2 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.17, 0.62] |
| 1.2 Females | 3 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.81] |
| 2 Deferral due to low haemoglobin: after multiple donation visits | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Males | 2 | 373 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.15, 0.43] |
| 2.2 Females | 2 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.03, 1.35] |
| 3 Deferral due to low haemoglobin: cumulative deferrals over all visits | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Males | 2 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.19, 0.38] |
| 3.2 Females | 3 | 1396 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.83] |
| 4 Haemoglobin (g/L): before further donation | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Males | 4 | 297 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐1.90, 2.05] |
| 4.2 Females | 4 | 431 | Mean Difference (IV, Random, 95% CI) | 3.56 [0.21, 6.92] |
| 5 Serum ferritin (ng/mL): before further donation | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Males | 3 | 265 | Mean Difference (IV, Random, 95% CI) | 10.94 [1.00, 20.88] |
| 5.2 Females | 3 | 375 | Mean Difference (IV, Random, 95% CI) | 14.39 [9.90, 18.88] |
| 6 Serum ferritin (ng/mL): after subsequent donation(s) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Males | 1 | 142 | Mean Difference (IV, Random, 95% CI) | 7.70 [‐2.36, 17.76] |
| 6.2 Females | 3 | 477 | Mean Difference (IV, Random, 95% CI) | 10.00 [6.13, 13.87] |
10.6. Analysis.
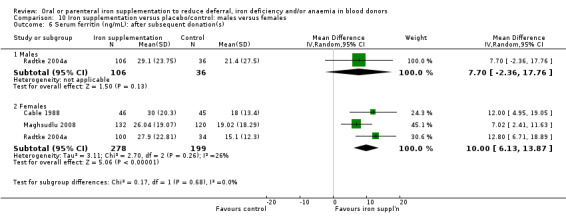
Comparison 10 Iron supplementation versus placebo/control: males versus females, Outcome 6 Serum ferritin (ng/mL): after subsequent donation(s).
Comparison 11. Iron supplementation versus placebo/control: low risk of performance bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deferral due to low haemoglobin (primary outcome) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Deferral at first donation visit after commencement of treatment | 3 | 833 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.50] |
| 1.2 Deferral after multiple donation visits | 2 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.11, 0.42] |
| 1.3 Cumulative deferrals over multiple donation visits | 3 | 1820 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.18, 0.35] |
| 2 Haemoglobin (g/L) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Before further donation | 3 | 270 | Mean Difference (IV, Random, 95% CI) | 4.76 [1.07, 8.45] |
| 3 Serum ferritin (ng/mL) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before further donation | 3 | 594 | Mean Difference (IV, Random, 95% CI) | 13.31 [7.22, 19.40] |
Comparison 12. Iron supplementation versus placebo/control: > 75% randomised participants analysed.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Deferral due to low haemoglobin (primary outcome) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Deferral at first donation visit after commencement of treatment | 2 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.16, 0.53] |
| 1.2 Deferral after multiple donation visits | 1 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.15, 0.46] |
| 1.3 Cumulative deferrals over multiple donation visits | 2 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.19, 0.38] |
| 2 Haemoglobin (g/L) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Before further donation | 6 | 698 | Mean Difference (IV, Random, 95% CI) | 2.90 [0.23, 5.57] |
| 3 Serum ferritin (ng/mL) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Before further donation | 3 | 189 | Mean Difference (IV, Random, 95% CI) | 15.24 [12.37, 18.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Birgegard 2010.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Sweden Study setting: single blood donation unit Number of participants randomised: Treatment arm 1: 60 Treatment arm 2: 60 Number of participants analysed (at 12 months): Treatment arm 1: 57 Treatment arm 2: 55 Follow‐up time points: 3 (female) or 4 (male) donations subsequent to baseline donation. Final donation is at least 1 year after first donation Hb threshold for deferral from donation: not reported Source of funding: supported by an unrestricted grant from Renapharma AB |
|
| Participants | Regular donors with at least 5 previous donations within the past 1 to 2 years Mean age (years): Treatment arm 1: 50.3 (SD 8.0) years Treatment arm 2: 50.3 (SD 8.0) years Sex (male/female): 41.7%/58.3% |
|
| Interventions | Treatment arm 1: oral iron (Fe2+) sulphate corresponding to Fe2+ 100 mg (Duraferon): one 100 mg tablet taken daily for 20 days after each blood donation. Total dose: 200 mg after each donation Treatment arm 2: intravenous iron (III) sucrose (Venofer); 200 mg (10 mL of 20 mg/mL iron (III) as iron sucrose, corresponding to 200 mg iron (III), given after each blood donation. Total dose: 200 mg after each donation |
|
| Outcomes | Iron status at end of study (primary outcome); iron status at other time points as well as in men versus women and younger versus older women; RLS frequency and severity | |
| Notes | This study performed intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central randomisation was performed via a web‐based system using the minimisation method to ensure baseline balance for age and blood B‐Hb" |
| Allocation concealment (selection bias) | Unclear risk | "Central randomisation was performed via a web‐based system using the minimisation method to ensure baseline balance for age and blood B‐Hb" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of randomised participants with missing data was similar in each group (3/60 versus 5/60 after 12 months) with less than 5% difference in attrition rate between treatment arms) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | "The study was supported by an unrestricted grant from Renapharma AB" |
Blot 1980.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: France Study setting: single transfusion centre Number of participants randomised: Treatment arm 1: 91 Treatment arm 2: 80 Number of participants analysed: Treatment arm 1: 73 (42 male, 31 female) Treatment arm 2: 70 (43 male, 27 female) Follow‐up time points: first donation after baseline donation Hb threshold for deferral from donation: not reported Source of funding: not reported |
|
| Participants | Regular donors (at least 1 prior donation) Mean age (years): combined treatment arms: 36.4 (SD 9.5, range 20 to 59) (male); 34.1 (SD 10.8, range 18 to 56) (female) Sex (male/female): 57.3%/62.7% |
|
| Interventions | Treatment arm 1: oral iron and ascorbic acid (Fero‐Grad Abbott); 105 mg iron and 500 mg ascorbic acid taken daily in the morning Total dose: 3150 mg per month Treatment arm 2: control (no iron supplementation or placebo) |
|
| Outcomes | Haemoglobin; MCV; total serum iron; total iron binding capacity; serum ferritin | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (18/91 versus 10/80) and 10.5% of randomised participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Borch‐Iohnsen 1993.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial (with an additional 2 randomised arms of non‐donors) Country of study: Norway Study setting: not reported Number of participants randomised: Treatment arm 1: 18 Treatment arm 2: 16 Number of participants analysed: Treatment arm 1: 18 Treatment arm 2: 16 Follow‐up time points: 5 months after baseline measures Hb threshold for deferral from donation: not reported Source of funding: not reported. Oral iron supplements were supplied by Collett‐Marwell Hauge A/S and Cederroth AB |
|
| Participants | Female blood donors with depleted iron stores (serum ferritin < 20 μg/L and haemoglobin > 120 g/L) Mean age (years): not reported (range 30 to 50) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: iron fumarate 20 mg + 120 mg ascorbic acid taken daily; treatment duration unclear Treatment arm 2: heme iron from porcine blood 2 mg plus iron fumarate 16 mg taken daily; treatment duration unclear |
|
| Outcomes | Haemoglobin; serum ferritin; transferrin. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Brittenham 1996.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: USA Study setting: not reported Number of participants randomised: not reported Number of participants analysed: not reported Follow‐up time points: 30 months Hb threshold for deferral from donation: not reported Source of funding: not reported |
|
| Participants | Female donors who pledged to donate at least 4 units of blood each year Mean age (years): not reported Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral carbonyl iron; 100 mg taken daily for 56 days after each scheduled blood donation. Total dose: 5600 mg after each donation Treatment arm 2: unscheduled control (no iron supplementation or placebo; unscheduled visits) Treatment arm 3: scheduled control (no iron supplementation but donations scheduled as per carbonyl iron group) |
|
| Outcomes | Mean annual rate of donation; haemoglobin; serum ferritin | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants randomised to each treatment arm was not reported |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified study outcomes were not reported in this published conference abstract |
| Other bias | Unclear risk | This published conference abstract provided limited data to establish potential sources of bias |
Bucher 1973.
| Methods |
Type of study: parallel, 4‐arm randomised controlled trial Country of study: Switzerland Study setting: not reported Number of participants randomised: not reported Number of participants analysed: Treatment arm 1: 43 Treatment arm 2: 38 Treatment arm 3: 38 Treatment arm 4: 42 Follow‐up time points: day 14 and day 28 after baseline Hb threshold for deferral from donation: 135 g/L Source of funding: supported by Ciba‐Geigy who supplied Resoferon and placebos and contributed to the planning and statistical analysis |
|
| Participants | Healthy blood donors with blood group B and haemoglobin levels 125 to 135 g/L Mean age (years): Treatment arm 1: 44 Treatment arm 2: 40 Treatment arm 3: 41 Treatment arm 4: 42 Sex (male/female): 22.5%/77.5% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate (Resoferon, Ciba‐Geigy); 37 mg provided as 3 tablets in a vial, taken 3 times daily for 28 days. Total dose: 3108 mg Treatment arm 2: oral placebo provided as 3 pills in a vial taken 3 times daily for 28 days Treatment arm 3: oral ferrous sulphate (Resoferon; Ciba‐Geigy); 37 mg tablets provided as 3 pills in sachets, taken 3 times daily for 28 days. Total dose: 3108 mg Treatment arm 4: oral ferrous sulphate (Resoferon, Ciba‐Geigy); 37 mg tablets provided as 3 pills in sachets, taken 3 times daily for 4 days, followed by oral placebo provided as 3 pills in sachets, taken 3 times daily for the remaining 24 days. Total dose: 444 mg |
|
| Outcomes | Haemoglobin; haematocrit; plasma iron; total iron binding capacity; mean cell haemoglobin concentration; transferrin saturation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some withdrawals were described but the number of participants randomised to each treatment arm was not reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | High risk | Ciba‐Geigy supplied the iron supplement and placebo, and provided help in planning and statistical analysis |
Busch 1972.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: Germany Study setting: not reported Number of participants randomised: not reported Number of participants analysed: (completing intake): Treatment arm 1: 43 Treatment arm 2: 17 Treatment arm 3: 13 Follow‐up time points: after 30 days of treatment Hb threshold for deferral from donation: not reported Source of funding: not reported |
|
| Participants | Blood donors Mean age (years): not reported Sex (male/female): Treatment arm 1: 71.2%/28.8% Treatment arm 2: 64.9%/35.1% Treatment arm 3: 69.2%/30.8% |
|
| Interventions | Treatment arm 1: oral iron sulphate (Fe2+) (Eryfer, Farbweke Hoechst AG); 152 mg (50 mg Fe2+) + 222 mg ascorbic acid + 84 mg Na Bicarb, taken twice daily for 30 days. Total dose: 9120 mg (3000 mg Fe2+) Treatment arm 2: oral iron sulphate (alternative commercial preparation); 152 mg (50 mg Fe2+) + 222 mg ascorbic acid taken twice daily for 30 days. Total dose: 9120 mg (3000 mg Fe2+) Treatment arm 3: oral placebo (maize starch 273.8 mg + Airosil 1.2 mg) taken twice daily for 28 days |
|
| Outcomes | Side effects (primary outcome) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" and a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some withdrawals were described but the number of participants randomised to each treatment arm was not reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Buzi 1980.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Switzerland Study setting: single centre blood transfusion service Number of participants randomised: Treatment arm 1: 32 Treatment arm 2: 32 Number of participants analysed: Treatment arm 1: 32 Treatment arm 2: 32 Follow‐up time points: 2 days after end of treatment Hb threshold for deferral from donation: 130 g/L Source of funding: not reported |
|
| Participants | Deferred donors with Hb < 130 g/L (Hct < 37%) Mean age (years): Treatment arm 1: 42.9 Treatment arm 2: 44.6 Sex (male/female): 3.1%/96.9% |
|
| Interventions | Treatment arm 1: oral elementary iron (Tardyferon, Robapharm SA, Basel); 80 mg + 80 mg of mucoprotein, with slow release of iron sulphate ions over at least 6 hours, taken daily for 30 days. Total dose: 2400 mg Fe2+ Treatment arm 2: oral iron comparator, 2 capsules containing 66 mg iron, liberated by iron fumarate, taken twice daily for 18 days Total dose: 2376 mg Fe2+ |
|
| Outcomes | Haemoglobin; haematocrit; serum iron; total iron binding capacity; side effects | |
| Notes | 3 individuals switched treatment; it is unclear whether they were included or withdrawn from the study) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 individuals stopped the comparator treatment and converted to the alternative treatment (Tardyferon), but it is unclear whether they were withdrawn from the study or analysed in the comparator arm |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Cable 1988.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: USA Study setting: multiple American Red Cross blood mobiles Number of participants randomised: Treatment arm 1: 100 Treatment arm 2: 100 Number of participants analysed: (2 or more visits): Treatment arm 1: 57 Treatment arm 2: 64 Follow‐up time points: 4 visits subsequent to baseline visit with minimum 8 weeks since previous donation or 4 weeks since deferral Hb threshold for deferral from donation: 125 g/L (Hct 38%) Source of funding: supported by American Red Cross funds |
|
| Participants | Female donors failing a previous Hb screen (Hct 33% to 41%) prior to start of study Mean age (years): Treatment arm 1: 34 (SD 10) Treatment arm 2: 34 (SD 9) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral ferrous gluconate (Fergon, Winthrop‐Breon Laboratories, New York); 75 mg elemental iron taken twice daily. Duration of treatment not reported Treatment arm 2: oral calcium phosphate placebo taken twice daily. Duration of treatment not reported |
|
| Outcomes | Venous haemoglobin; red cell zinc protoporphyrin; serum ferritin; iron and iron‐binding capacity; transferrin saturation; blood donations over initial and 4 subsequent visits | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (43/100 versus 36/100 after 2 or more visits) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Devasthali 1991.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: USA Study setting: Northern Ohio Red Cross Number of participants randomised: Treatment arm 1: 24 Treatment arm 2: 25 Number of participants analysed (at week 16): Treatment arm 1: 21 Treatment arm 2: 25 Follow‐up time points: weeks 1, 3, 6, 12, 16 after baseline (none were donation visits) Hb threshold for deferral from donation: not reported (Hct 35%) Source of funding: not reported |
|
| Participants | Menstruating, non‐pregnant female donors recently deferred from donation (Hct < 35%); MCV < 85 fL and ferritin < 12 μg/L at start of study Mean age (years): both treatment groups combined: range 18 to 40 Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral carbonyl iron, 100 mg taken daily at bedtime, at least 2 hours after last meal for 84 days. Total dose: 8400 mg Treatment arm 2: oral ferrous sulphate, 500 mg (100 mg Fe2+) taken daily at bedtime, at least 2 hours after last meal for 84 days. Total dose: 8400 mg Fe2+ |
|
| Outcomes | Haemoglobin; mean corpuscular volume; reticulocyte count; platelet count; erythrocyte protoporphyrin; ferritin; serum iron; total iron binding capacity; transferrin saturation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 withdrawals at week 16 were reported, all from same treatment arm (carbonyl iron) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Ehn 1968.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: Sweden Study setting: single centre Number of participants randomised: not reported Number of participants analysed: (after 4 donations): Treatment arm 1: 12 Treatment arm 2: 12 Treatment arm 3: 18 Follow‐up time points: 2 months after 6 donations subsequent to baseline donation; inter‐donation interval of 2 months Hb threshold for deferral from donation: not reported Source of funding: supported by AB Hassle, Molndal Medical Research Fund, County of Ostergotland |
|
| Participants | Young male first‐time donor conscripts Mean age (years): both arms combined (subset of 58 subjects): mean 20, range 18 to 23 Sex (male/female): 100%/0% |
|
| Interventions | Treatment arm 1: oral iron (Ferromyn S, AB Hassle); 37 mg as ferrous succinate + 0.11 g succinic acid taken as 2 tablets twice daily for 2 weeks. Total dose: 2000 mg Treatment arm 2: oral iron (Ferromyn S, AB Hassle); 37 mg as ferrous succinate + 0.11 g succinic acid taken twice daily for 2 weeks. Total dose: 1000 mg Treatment arm 3: oral placebo taken twice daily |
|
| Outcomes | Haemoglobin; serum iron, total iron binding capacity; stainable bone marrow iron; sideroblasts; desferrioxamine test | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some withdrawals were described but the number of participants randomised to each treatment arm was not reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | High risk | The study was supported by AB Hassle, manufacturers of iron supplements |
Frykman 1994.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Sweden Study setting: single blood donor centre Number of participants randomised: Treatment arm 1: 50 Treatment arm 2: 50 Number of participants analysed: Treatment arm 1: 48 Treatment arm 2: 44 Follow‐up time points: 3 months from baseline Hb threshold for deferral from donation: not reported Source of funding: not reported |
|
| Participants | Regular blood donors Mean age (years): Treatment arm 1: median age 45 (male), 44.5 (female) Treatment arm 2: median age 41 (male), 45 (female) Sex (male/female): Treatment arm 1: 46.9%/53.1% Treatment arm 2: 47.9%/52.1% |
|
| Interventions | Treatment arm 1: oral heme iron combination (Hemofer, Aktiva Pharmaceuticals, Sweden); 1.2 mg heme iron from porcine blood + 8 mg Fe2+ as iron fumarate, taken twice daily over 3 months, with a placebo replacement for the second or third month of treatment. Total dose: 144 mg Heme + 960 mg Fe2+ Treatment arm 2: oral non‐heme iron (Erco‐fer Orion Pharmaceutica, Finland); 60 mg Fe2+ as iron fumarate, taken once daily over 3 months, with a placebo replacement for the second or third month of treatment. Total dose: 3600 mg Fe2+ |
|
| Outcomes | Tolerance of Hemofer (primary outcome); serum ferritin; haemoglobin | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (2/50 versus 6/50) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Gordeuk 1987a.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: USA Study setting: multi‐centre: 3 fixed site collection facilities Number of participants randomised: Treatment arm 1: 24 Treatment arm 2: 26 Treatment arm 3: 25 Number of participants analysed: Treatment arm 1: 15 Treatment arm 2: 17 Treatment arm 3: 19 Follow‐up time points: day 56 after baseline donation Hb threshold for deferral from donation: not reported (Hct 38%) Source of funding: sponsored in part by Food and Drug Administration Orphan Drugs Development Grant |
|
| Participants | Female donors of child‐bearing age with Hct ≥ 38% who had donated blood at least once previously Mean age (years): not reported Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral carbonyl iron, 600 mg taken 3 times daily for 7 days. Total dose: 12,600 mg Treatment arm 2: oral ferrous sulphate, 300 mg (60 mg Fe2+) taken 3 times daily for 7 days. Total dose: 1260 mg Fe2+ Treatment arm 3: oral placebo, taken 3 times daily for 7 days |
|
| Outcomes | Haemoglobin; mean corpuscular volume; free erythrocyte protoporphyrin; serum ferritin; serum iron; total iron binding capacity; transferrin saturation; adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" and identical capsules were used for both treatment and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (9/26 versus 6/25) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Gordeuk 1987b.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: USA Study setting: blood donors to the Northern Ohio Red Cross Number of participants randomised: Treatment arm 1: 25 Treatment arm 2: 25 Number of participants analysed: Treatment arm 1: 18 Treatment arm 2: 18 Follow‐up time points: weeks 1, 3, 6, 12, 16 after baseline Hb threshold for deferral from donation: not reported (Hct 38%) Source of funding: not reported |
|
| Participants | Menstruating, non‐pregnant female donors with recent deferral from repeat blood donation due to haematocrit levels (Hct < 38%) Mean age (years): not reported Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral carbonyl iron, 600 mg taken 3 times daily for 21 days. Total dose: 3780 mg Fe2+ Treatment arm 2: oral ferrous sulphate iron, 300 mg (60 mg Fe2+) taken 3 times daily for 21 days. Total dose: 3780 mg Fe2+ |
|
| Outcomes | Free erythrocyte protoporphyrin; serum ferritin; serum iron; total iron binding capacity; transferrin saturation; haemoglobin; mean corpuscular volume; white blood cell and platelet count; occult blood; side effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" and identical capsules were used for both treatment arms |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of withdrawals was the same in each treatment group (7/25 versus 7/25) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Gordeuk 1990.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: USA Study setting: multiple fixed site blood donor centres Number of participants randomised: Treatment arm 1: 50 Treatment arm 2: 49 Number of participants analysed: Treatment arm 1: 40 Treatment arm 2: 36 Follow‐up time points: day 56 after baseline donation Hb threshold for deferral from donation: 125 g/L (Hct 38%) Source of funding: supported in part by Food and Drug Administration Orphan Drugs Development Grant and by National Heart, Lung and Blood Institute of the National Institutes of Health |
|
| Participants | Female repeat donors with Hb ≥ 125 g/L Mean age (years): not reported (range 18 to 40) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral carbonyl iron (100 mg elemental iron) taken daily for 56 days. Total dose: 5600 mg Treatment arm 2: oral placebo, taken daily for 56 days |
|
| Outcomes | Haemoglobin; haematocrit; MCV; serum ferritin; free red cell protoporphyrin; serum iron; total iron binding capacity; transferrin saturation; net iron absorption; adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" and identical capsules were used for both treatment arms |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants lost to follow‐up was high (30%) and differed between treatment arms (10/50 versus 13/49) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Jacobs 1993.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: South Africa Study setting: single hospital donor centre Number of participants randomised: Treatment arm 1: 51 Treatment arm 2: 53 Treatment arm 3: 55 Number of participants analysed: Treatment arm 1: 45 Treatment arm 2: 40 Treatment arm 3: 45 Follow‐up time points: non‐donation visits at weeks 1, 2, 4, 8, 12 after baseline Hb threshold for deferral from donation: 135 g/L (male) or 125 g/L (female) Source of funding: supported by the University of Cape Town Leukaemia Centre and Staff Research (Cancer, Becker and Foote) Fund, the Gwendoline Moore Trust, the National Cancer Association, the Medical Research Council and the Michael Chanani, Kaliski and MA Richardson bequests |
|
| Participants | Deferred donors who failed CuSO4 haemoglobin screening test Mean age (years): combined groups: 32 (range 17 to 64) Sex (male/female): Treatment arm 1: 9.8%/90.2% Treatment arm 2: 9.4%/90.6% Treatment arm 3: 21.8%/78.2% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate (Hausman Laboratories) 60 mg taken twice daily in the fasting state for 12 weeks. Total dose: 10,080 mg Treatment arm 2: oral iron (Hausman Laboratories); 100 mg chewable ferric polymaltose taken daily with breakfast for 12 weeks. Total dose: 8400 mg Treatment arm 3: oral iron (Hausman Laboratories); 100 mg chewable ferric polymaltose taken twice daily with breakfast and supper for 12 weeks. Total dose: 16,800 mg |
|
| Outcomes | Full blood count; serum iron; total iron binding capacity; serum ferritin; adverse effects; compliance | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Individuals were randomly assigned, using a computer‐generated chart" |
| Allocation concealment (selection bias) | High risk | "Individuals were randomly assigned, using a computer‐generated chart" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (6/51 versus 13/53 versus 10/55) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Unclear risk | Iron supplements were donated by manufacturer |
Jacobs 2000.
| Methods |
Type of study: parallel, 4‐arm randomised controlled trial Country of study: South Africa Study setting: single hospital donor centre Number of participants randomised: Treatment arm 1: 40 Treatment arm 2: 35 Treatment arm 3: 45 Treatment arm 4: 47 Number of participants analysed: Treatment arm 1: 24 Treatment arm 2: 23 Treatment arm 3: 24 Treatment arm 4: 20 Follow‐up time points: non‐donation visits at weeks 4, 8, 12 after baseline Hb threshold for deferral from donation: not reported Source of funding: trial material supplied by Vifor International; data management and statistical analysis carried out by Clindata International |
|
| Participants | Regular donors, deferred by CuSO4 haemoglobin test Mean age (years): not reported Sex (male/female): not reported |
|
| Interventions | Treatment arm 1: oral iron polymaltose complex (IPC) (Vifor International); 100 mg + 1.8 mol/L glycerophosphate (GlyP) taken twice daily for 12 weeks. Total dose: 16,800 mg IPC + 302.4 mol/L GlyP Treatment arm 2: oral iron polymaltose complex (IPC) (Vifor International); 100 mg + 0.9 mol/L glycerophosphate (GlyP) taken twice daily for 12 weeks. Total dose: 16,800 mg IPC + 151.2 mol/L GlyP Treatment arm 3: oral iron polymaltose complex (IPC) (Vifor International); 100 mg taken twice daily for 12 weeks. Total dose: 16,800 mg IPC Treatment arm 4: oral ferrous sulphate taken twice daily at an "equivalent dose". Total dose: not reported |
|
| Outcomes | Haemoglobin; serum iron; transferrin saturation; serum ferritin; red cell ferritin | |
| Notes | This study performed intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Treatment allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants lost to follow‐up was high (14%) and differed between treatment arms (4/40 versus 3/35 versus 3/45 versus 14/47) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Unclear risk | Iron supplements were donated by the manufacturer (Vifor International) |
Landucci 1987.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Italy Study setting: not reported Number of participants randomised: Treatment arm 1: 20 Treatment arm 2: 20 Number of participants analysed: Treatment arm 1: 20 Treatment arm 2: 20 Follow‐up time points: mean 30 (SD 2.2, range 23 to 33) days after baseline Hb threshold for deferral from donation: not reported Source of funding: not reported |
|
| Participants | Iron‐depleted blood donors (serum ferritin < 30 ng/100 mL) Mean age (years): both groups combined: mean 36.2 (SD 7.9, range 18 to 56) Sex (male/female): 27.5%/72.5% |
|
| Interventions | Treatment arm 1: oral iron protein succinylate (80 mg Fe3+) (Legofer, Farmades SpA, Rome), taken daily for a mean duration across both treatment groups of 30 (SD 2.2) days. Total dose: 2400 mg Fe3+ Treatment arm 2: oral iron sulphate (105 mg Fe2+) taken daily for a mean duration across both treatment groups of 30 (SD 2.2) days. Total dose: 3150 mg Fe2+ |
|
| Outcomes | Red blood cell count; haemoglobin; haematocrit; mean corpuscular volume, mean corpuscular haemoglobin content; mean corpuscular haemoglobin concentration; serum iron; serum transferrin; serum ferritin | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Lieden 1975.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Sweden Study setting: single Number of participants randomised: Treatment arm 1: 10 Treatment arm 2: 10 Number of participants analysed: Treatment arm 1: 10 Treatment arm 2: 7 Follow‐up time points: mean 36.4 (20 mg) or 24.5 (100 mg) days after third donation after baseline; mean 20.4 (20 mg) or 18.6 (100 mg) days after fifth donation after baseline. Mean interval 7.5 weeks between donations Hb threshold for deferral from donation: not reported Source of funding: supported by a grant from AB Astra, Sodertalje |
|
| Participants | Young, male, first‐time donor conscripts Mean age (years): not reported Sex (male/female): 100%/0% |
|
| Interventions | Treatment arm 1: oral ferrous carbonate (AB Astra), 100 mg stabilised with sugar in pellet form, taken daily for 1 year. Total dose: 36,500 mg Treatment arm 2: oral ferrous carbonate (AB Astra), 20 mg stabilised with sugar in pellet form, taken daily for 1 year. Total dose: 7300 mg |
|
| Outcomes | Packed cell volume; serum iron; total iron binding capacity; stainable bone marrow iron; iron absorption; faecal iron | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 randomised participants withdrew from the study; all were in the same treatment group (20 mg ferrous carbonate) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | High risk | Supported by AB Astra, manufacturer of ferrous carbonate |
Lindholm 1981.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Sweden Study setting: single hospital blood donor centre Number of participants randomised: Treatment arm 1: 250 Treatment arm 2: 250 Number of participants analysed (at test 2): Treatment arm 1: 219 Treatment arm 2: 210 Follow‐up time points: after first, second and third donations. Median inter‐donation interval of between 51 and 75 days Hb threshold for deferral from donation: 125 g/L Source of funding: not reported |
|
| Participants | Previous donors (97.2%) with no iron deficiency anaemia during recent years Mean age (years): not reported Sex (male/female): 100%/0% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate (Ferrosulfat ACO, Orion Pharmaceutica, Finland); 100 mg taken daily for 30 days; repeated as second donation. Total dose: 3000 mg per donation Treatment arm 2: oral iron (Erco‐Fer, Orion Pharmaceutica, Finland); 60 mg taken daily for 30 days; repeated as second donation. Total dose: 1800 mg per donation |
|
| Outcomes | Side effects and haemoglobin concentration (primary outcome) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | Treatment was allocated by "code‐marked bags of prescriptions" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants were blinded: code‐marked bags of prescriptions which said "1 tablet at bedtime for 30 days" were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The study was conducted with the investigator blinded to the procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of randomised participants with missing data was similar in each group (31/250 versus 40/250 after the second test) with less than 5% difference in attrition rate between treatment arms |
| Selective reporting (reporting bias) | High risk | Pre‐specified outcomes of serum ferritin, total iron binding capacity and compliance were not reported |
| Other bias | High risk | 2 of the 3 co‐authors were with the manufacturer of an iron supplement used in the study |
Linpisarn 1986.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Thailand Study setting: single hospital blood transfusion unit Number of participants randomised: Treatment arm 1: 72 Treatment arm 2: 69 Number of participants analysed: Treatment arm 1: 47 Treatment arm 2: 51 Follow‐up time points: approximately 3 months after baseline; mean 97.1 (SD 10.8) days (iron supplementation group); mean 95.5 (SD 9.1) days (placebo group) Hb threshold for deferral from donation: 120 g/L Source of funding: supported by a grant from the Royal Thai Government given to the Research Institute for Health Sciences |
|
| Participants | Male volunteer and paid blood donors who had previously given blood consecutively 4 times or more, each with an interval of 6 months or less. Body weight > 50 kg; haemoglobin > 12 gm% from CuSO4 test Mean age (years): not reported Sex (male/female): 100%/0% |
|
| Interventions | Treatment arm 1: oral elemental iron, 56 mg taken daily for 90 days. Total dose: 5040 mg Treatment arm 2: oral placebo taken daily for 90 days |
|
| Outcomes | Haemoglobin; serum iron; total iron binding capacity; serum ferritin | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants lost to follow‐up was high (30%) and differed between treatment arms (25/72 versus 18/69) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Mackintosh 1988_HSF.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: South Africa Study setting: Western Province Blood Transfusion Service Number of participants randomised: Treatment arm 1: 11 Treatment arm 2: 12 Number of participants analysed: Treatment arm 1: 11 Treatment arm 2: 12 Follow‐up time points: after 56 days of treatment Hb threshold for deferral from donation: 135 g/L Source of funding: supported by the University of Cape Town Leukaemia Centre and Staff Research (Cancer) Fund, the National Cancer Institute and the Medical Research Council. Iron polymaltose donated by Hausman Laboratories |
|
| Participants | Regular donors with a minimum of 4 units in the preceding 12 months; haemoglobin ≥ 135 g/L and serum ferritin between 50 and 150 μg/L Mean age (years): not reported Sex (male/female): 100%/0% |
|
| Interventions | Treatment arm 1: oral elemental iron (Ferrimed DS, Hausman Laboratories, Switzerland); 100 mg as chewable tablets taken twice daily for 56 days. Total dose: 11,200 mg Treatment arm 2: oral placebo taken twice daily for 56 days. Total dose: 11,200 mg |
|
| Outcomes | Serum ferritin; haemoglobin; adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | |
Mackintosh 1988_LSF.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: South Africa Study setting: Western Province Blood Transfusion Service Number of participants randomised: Treatment arm 1: 11 Treatment arm 2: 12 Number of participants analysed: Treatment arm 1: 11 Treatment arm 2: 12 Follow‐up time points: after 56 days of treatment Hb threshold for deferral from donation: 135 g/L Source of funding: supported by the University of Cape Town Leukaemia Centre and Staff Research (Cancer) Fund, the National Cancer Institute and the Medical Research Council. Iron polymaltose donated by Hausman Laboratories |
|
| Participants | Regular donors with a minimum of 4 units in the preceding 12 months; haemoglobin ≥ 135 g/L and serum ferritin b < 20 μg/L Mean age (years): not reported Sex (male/female): 100%/0% |
|
| Interventions | Treatment arm 1: oral elemental iron (Ferrimed DS, Hausman Laboratories, Switzerland); 100 mg as chewable tablets taken twice daily for 56 days. Total dose: 11,200 mg Treatment arm 2: oral placebo taken twice daily for 56 days. Total dose: 11,200 mg |
|
| Outcomes | Serum ferritin; haemoglobin; adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | |
Maghsudlu 2008.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Iran Study setting: multi‐centre: 2 blood transfusion centres Number of participants randomised: Treatment arm 1: 207 Treatment arm 2: 205 Number of participants analysed: Treatment arm 1: 132 Treatment arm 2: 120 Follow‐up time points: visits at 4, 8, 12 months after baseline Hb threshold for deferral from donation: 125 g/L Source of funding: supported by the Research Centre of the Iranian Blood Transfusion Organisation |
|
| Participants | Successful female, non‐pregnant donors of childbearing age Mean age (years): both groups pooled: mean 28.7 (SD 7.2, maximum age 45) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate, 150 mg (50 mg elemental iron) taken 3 times daily for 7 days; repeated after each of 3 donations. Total dose: 3150 mg for each donation Treatment arm 2: oral placebo taken 3 times daily for 7 days; repeated after each of 3 donations. Total dose: 3150 mg for each donation |
|
| Outcomes | Compliance; adverse effects; haemoglobin; haematocrit; serum ferritin; serum iron; total iron binding capacity; transferrin saturation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of study participants was not reported although a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants lost to follow‐up was high (39%) and differed between treatment arms (75/207 versus 85/205) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Mirrezaie 2008.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Iran Study setting: multi‐centre: 2 blood transfusion centres Number of participants randomised: Treatment arm 1: 49 Treatment arm 2: 46 Number of participants analysed: Treatment arm 1: 39 Treatment arm 2: 31 Follow‐up time points: non‐donation visits on day 7 (adverse effects only); day 28; day 56 after baseline donation Hb threshold for deferral from donation: 120 g/L Source of funding: supported under a co‐operative agreement from Shiraz University of Medical Sciences and Shiraz Blood Transfusion Organization |
|
| Participants | Regular female donors of childbearing age with more than one blood donation in the last year; haemoglobin ≥ 120 g/L Mean age (years): Treatment arm 1: mean 34.2 (SD 9.3, range 18 to 49) Treatment arm 2: mean 34.0 (SD 8.0, range 18 to 49) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate equivalent to 50 mg elemental iron, taken daily for 8 weeks. Total dose: 2800 mg Treatment arm 2: oral placebo taken daily for 56 days. |
|
| Outcomes | Serum ferritin; compliance; adverse effects | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment randomly by a "random block design" |
| Allocation concealment (selection bias) | Unclear risk | Participants were assigned to treatment randomly by a "random block design" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" and a placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (10/49 versus 15/46) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Radtke 2004a.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: Germany Study setting: single blood transfusion centre Number of participants randomised: Treatment arm 1: 176 Treatment arm 2: 175 Treatment arm 3: 175 Number of participants analysed: (at visit 3 (males) or visit 2 (females)): Treatment arm 1: 54 males, 57 females Treatment arm 2: 54 males, 44 females Treatment arm 3: 40 males, 40 females Follow‐up time points: donation visits at 2, 4, 6 months (male) or 3, 6 months (female) Hb threshold for deferral from donation: 135 g/L (male) or 125 g/L (female) Source of funding: supported in part by a grant from Phyt‐Immun GmbH, Homburg, Germany |
|
| Participants | Regular donors with haemoglobin ≥ 135 g/L (males) or ≥ 125 g/L (females) Mean age (years): mean not reported, all groups combined: range 19 to 67 (males), 19 to 65 (females) Sex (male/female): Treatment arm 1: 55.1%/44.9% Treatment arm 2: 54.9%/45.1% Treatment arm 3: 54.9%/45.1% |
|
| Interventions | Treatment arm 1: oral ferrous gluconate (Phyt‐Immun GmbH, Homburg, Germany); 40 mg + 400 mg ascorbic acid taken daily for 6 months. Total dose: 7520 mg Treatment arm 2: oral ferrous gluconate (Phyt‐Immun GmbH, Homburg, Germany); 20 mg + 400 mg ascorbic acid taken daily for 6 months with 400 mg ascorbic acid. Total dose: 3760 mg Treatment arm 3: oral placebo + 400 mg ascorbic acid taken daily for 6 months |
|
| Outcomes | Storage iron (logarithm of the ratio of transferrin receptor to ferritin concentration) (primary outcome); donations and deferrals; serum ferritin; % with depleted iron stores; adverse effects; compliance | |
| Notes | This study performed intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to treatment by "block randomization with variable block length" |
| Allocation concealment (selection bias) | Unclear risk | Patients were assigned to treatment by "block randomization with variable block length" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (63/173 versus 72/175 versus 88/175 at the second visit) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | High risk | This study performed intention‐to‐treat analysis. The study was supported in part by a grant from Phyt‐Immun GmbH, Homburg, Germany |
Radtke 2004b.
| Methods |
Type of study: cross‐over, 2‐arm randomised controlled trial Country of study: Germany Study setting: not reported Number of participants randomised: Treatment arm 1: 131 Treatment arm 2: 129 Number of participants analysed (at visit 3): Treatment arm 1: 108 Treatment arm 2: 103 Follow‐up time points: 3 donation visits subsequent to baseline donation; inter‐donation interval 8 to 10 weeks Hb threshold for deferral from donation: 145 g/L (before first donation); 140 g/L (before subsequent donations) Source of funding: supported in part by a grant from Sanol/Schwarz Pharma, Monheim, Germany |
|
| Participants | Regular donors with minimum body weight 68 kg; meeting the Hb threshold Mean age (years): not reported; all groups combined: range 18 to 62 Sex (male/female): 98.8%/1.2% |
|
| Interventions | Treatment arm 1: oral iron (iron(II)‐glycine‐sulphate‐complex (Sanol/Schwartz Pharma, Monheim, Germany), 100 mg Fe2+ taken daily between baseline and 3 subsequent visits (between 56 and 70 days). Total dose: 16,800 mg to 21,000 mg Treatment arm 2: oral placebo (Sanol/Schwartz Pharma, Monheim, Germany); taken daily between baseline and 3 subsequent visits (between 56 and 70 days) |
|
| Outcomes | Donations and deferrals; haemoglobin; serum ferritin; adverse effects; compliance | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to treatment by "block randomization with variable block length" |
| Allocation concealment (selection bias) | Unclear risk | Patients were assigned to treatment by "block randomization with variable block length" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of study participants was not reported although the study was described as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of randomised participants with missing data was similar in each group (23/131 versus 26/129 at the third visit) with less than 5% difference in attrition rate between treatment arms |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | High risk | The study was supported in part by a grant from Phyt‐Immun GmbH, Homburg, Germany |
Rosvik 2010.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Norway Study setting: hospital blood bank Number of participants randomised: Treatment arm 1: 198 Treatment arm 2: 201 Number of participants analysed: Treatment arm 1: 153 (71 male, 82 female) Treatment arm 2: 161 (82 male, 79 female) Follow‐up time points: mean 8 (SD 2) days after initial donation Hb threshold for deferral from donation: not reported Source of funding: supported by a grant from Western Norway Regional Health Authority |
|
| Participants | Donors with at least 1 previous donation; Hb ≥ 135 g/L (males) or ≥ 125 g/L (females); serum ferritin > 20 μg/L; willingness to return 8 (+/‐ 2) days after donation for follow‐up Mean age (years): both treatment groups pooled: mean 42.8 (SD 11.4, range 18 to 69) (male), 43.2 (SD 12.1, range 20 to 70) (female) Sex (male/female): Treatment arm 1: 50%/50% Treatment arm 2: 50.7%/49.3% |
|
| Interventions | Treatment arm 1: oral ferroglycin sulphate complex (Niferex©); 100 mg taken daily for 8 days. Total dose: 800 mg Treatment arm 2: control (no iron supplementation or placebo) |
|
| Outcomes | Short‐term change in iron status (primary outcome); effects of iron supplementation in donors with variants in the classical HFE‐gene of hereditary haemochromatosis compared to a HFE wildtype group | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Treatment allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of participants lost to follow‐up was high (20%) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Rybo 1971.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: Sweden Study setting: hospital blood bank Number of participants randomised: Treatment arm 1: 460 (261 male, 199 female) Treatment arm 2: 460 (260 male, 200 female) Treatment arm 3: 456 (257 male, 199 female) Number of participants analysed: Treatment arm 1: 392 (212 male, 180 female) Treatment arm 2: 389 (214 male, 175 female) Treatment arm 3: 385 (205 male, 180 female) Follow‐up time points: 14 days post‐donation Hb threshold for deferral from donation: not reported Source of funding: not reported |
|
| Participants | Regular blood donors Mean age (years): not reported Sex (male/female): Treatment arm 1: 56.7%/63.3% Treatment arm 2: 56.5%/63.5% Treatment arm 3: 56.4%/63.6% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate (AB Hassle, Goteborg); 100 mg taken twice daily for 14 days. Total dose: 2800 mg Treatment arm 2: oral iron (AB Hassle, Goteborg) as 100 mg sustained‐release tablets (40% iron release within 1 hour and 100% within 6 hours) taken twice daily for 14 days. Total dose: 2800 mg Treatment arm 3: oral placebo (AB Hassle, Goteborg) taken twice daily for 14 days. Total dose: 2800 mg |
|
| Outcomes | Adverse effects; compliance | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Low risk | "Subjects received tablets in a coded bottle labelled 'iron tablets to blood donors'" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Subjects received tablets in a coded bottle labelled 'iron tablets to blood donors'" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of randomised participants with missing data was similar in each group (68/460 versus 61/460) with less than 5% difference in attrition rate between treatment arms) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | No other sources of bias identified |
Simon 1984.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial followed by non‐randomised cross‐over period Country of study: USA Study setting: multiple fixed or hospital donation sites and monthly blood drives Number of participants randomised: Treatment arm 1: 55 Treatment arm 2: 49 Treatment arm 3: 57 Number of participants analysed: Treatment arm 1: 22 Treatment arm 2: 22 Treatment arm 3: 19 Follow‐up time points: at each of at least 3 donation visits subsequent to baseline donation; inter‐donation interval 8 to 12 weeks; mean 9.5 weeks. After 4 subsequent visits, median number of days since baseline = 266, range 225 to 403 days Hb threshold for deferral from donation: not reported (Hct 38%) Source of funding: supported by a grant from Blood Systems Research Foundation, Scottsdale, Arizona. Iron supplements supplied by Schering Corporation of Kenilworth, New Jersey |
|
| Participants | Regular, actively menstruating, female donors who could commit to donate blood close to every 8 weeks for 1 year Mean age (years): Treatment arm 1: mean 28 (SD 5, range 18 to 40) Treatment arm 2: mean 26 (SD 6, range 20 to 42) Treatment arm 3: mean 30 (SD 7, range 19 to 40) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate (Schering Corporation, Kenilworth, New Jersey); 39 mg elemental iron taken daily for 56 days. Total dose: 2184 mg Fe2+ Treatment arm 2: oral ferrous sulphate (Schering Corporation, Kenilworth, New Jersey); 39 mg elemental iron + 75 mg vitamin C taken daily for 56 days. Total dose: 2184 mg Fe2+ Treatment arm 3: oral placebo (Schering Corporation, Kenilworth, New Jersey) containing 75 mg vitamin C only, taken daily for 56 days |
|
| Outcomes | Haemoglobin; haematocrit; total iron binding capacity; serum ferritin | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Donors were "randomly assigned to one of three groups by pulling a card with an A, B, or C on it from an envelope in which the cards had been placed in random order" |
| Allocation concealment (selection bias) | Low risk | Donors were "given a bottle of medication marked either A, B, or C according to the card pulled. The bottles were filled at the pharmacy according to their code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants were "given a bottle of medication marked either A, B, or C according to the card pulled. The bottles were filled at the pharmacy according to their code" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The randomisation code was broken only after all participants had been in the study for at least 1 year |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals differed between treatment arms (33/55 versus 27/49 versus 39/57) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes listed in the manuscript were reported, but no study protocol was available to determine the full list of pre‐specified outcomes |
| Other bias | Low risk | |
Waldvogel 2012.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Switzerland Study setting: Lausanne Blood Transfusion Centre Number of participants randomised: Treatment arm 1: 78 Treatment arm 2: 76 Number of participants analysed: Treatment arm 1: 74 Treatment arm 2: 71 Follow‐up time points: 4 weeks after randomisation (5 weeks after initial donation) Hb threshold for deferral from donation: 120 g/L Source of funding: supported by Pierre Fabre Medicament, Boulogne, France |
|
| Participants | Successful female donors, non‐anaemic but iron‐deficient after donation (Hb ≥ 120 g/L and ferritin ≤ 30 ng/mL 1 week after donation) Mean age (years): Treatment arm 1: mean 32.9 (SD 8.4) Treatment arm 2: mean 30.7 (SD 8.8) Sex (male/female): 0%/100% |
|
| Interventions | Treatment arm 1: oral ferrous sulphate (Tardyferon, Robapharm, Boulogne, France); 80 mg taken daily for 28 days. Total dose: 2240 mg Treatment arm 2: oral placebo taken daily for 28 days |
|
| Outcomes | Level of fatigue perceived by donors on a 10‐point visual analogue scale; subjective fatigue evaluated by the Fatigue Severity Scale (primary outcome); change in aerobic capacity; depression and quality of life; ferritin; haemoglobin | |
| Notes | This study performed intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A simple random allocation sequence without restriction was generated by an independent pharmacy according to a pre‐established computer generated list" |
| Allocation concealment (selection bias) | Low risk | "Each drug package was identified with a unique number according to the randomisation schedule and given to the nurse in charge of the participant. The code was held by the pharmacist and remained unbroken until the end of the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Each drug package was identified with a unique number according to the randomisation schedule and given to the nurse in charge of the participant. The code was held by the pharmacist and remained unbroken until the end of the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All investigators and the statistician remained blinded until the end of the statistical analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants with missing data was similar in each group (4/78 versus 4/75) with less than 5% difference in attrition rate between treatment arms) |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified in the published protocol were reported |
| Other bias | Low risk | The study was supported by Pierre Fabre Medicament, Boulogne, France, owners of Robapharm who manufacture Tardyferon. However, the study was accorded "total independence in study design, data analysis and interpretation, and in the writing of the manuscript" |
CuSO4 = copper sulphate
Fe2+ = ferrous compound
Fe3+ = ferric compound
GlyP = glycerophosphate Hb = haemoglobin Hct = haematocrit IPC = iron polymaltose complex MCV = mean cell volume RLS = restless legs syndrome SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aramburu 1991 | This study is no longer available |
| Bier‐Ulrich 2003 | A randomised controlled trial of iron supplementation versus placebo in donors undergoing plasmapheresis |
| Blagoevska 2005 | This randomised trial of both blood donors and non‐donors was published in abstract form in 2005. No further publications have been identified and attempts to contact the authors have been unsuccessful |
| Borch‐Iohnsen 1989 | A study of women (non‐donors) of child‐bearing age with low serum ferritin levels, randomised to receive one of 3 iron supplementation preparations |
| Brittenham 2011 | A commentary on iron deficiency in blood donors |
| Brugnara 1993 | A cohort study of erythropoietin in male autologous blood donors |
| Bryant 2012 | A non‐randomised, comparative study of ferrous sulphate or ferrous gluconate in deferred versus non‐deferred blood donors |
| Chiamchanya 2013 | A randomised study of different doses of vitamin C with separate identical doses of iron supplementation |
| Gordeuk 1986 | A single‐arm safety study of incremental doses of elemental carbonyl iron in blood donors |
| Hoppe 2006 | A randomised study of hourly iron absorption rates after receiving 2 types of iron‐fortified rolls in regular blood donors. Each donor received a number of different iron preparations over 5 days |
| Kaltwasser 1982 | This trial was published in abstract form in 2005. It is unclear whether treatment was randomised. No further publications have been identified |
| Lieden 1975b | A non‐randomised study of ferrous carbonate versus placebo in first‐time donors (male conscripts) |
| Lopes 1999 | A randomised trial of daily versus weekly doses of ferrous sulphate in women (non‐donors) or child‐bearing age |
| Magnussen 2008 | A non‐randomised trial comparing 2 iron preparations in blood donors, where treatment allocation was made on the basis of adverse effects |
| Nielsen 1973 | A study of the iron absorption from slow‐release and rapid‐release iron preparations in blood donors, where the 2 types of treatment were given on alternate days for 10 days |
| Nielsen 1976 | A study of the iron absorption from slow‐release and rapid‐release iron preparations in blood donors, where the 2 types of treatment were given on alternate days for 10 days |
| Radtke 2005 | A single‐arm trial of elemental iron in blood donors |
| Remy 1956 | A retrospective commentary on the individual serum iron levels in 40 repeat blood donors |
| Tobias 1981 | A prospective study of blood donors who received either ferrous fumarate or a trace of elemental iron. It was unclear whether treatment was randomised |
| Zanella 1989 | A retrospective/prospective study of the iron status of regular blood donors. No iron supplementation was administered |
Characteristics of studies awaiting assessment [ordered by study ID]
Dara 2013.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: India Study setting: not reported Number of participants randomised: Treatment arm 1: 98 Treatment arm 2: 102 Number of participants analysed: Treatment arm 1: 64 Treatment arm 2: 62 Follow‐up time points: 1 month after whole blood donation and at the time of next blood donation (3 to 6 months) Source of funding: not reported |
| Participants | Regular blood donors who had donated at least twice in the previous year Mean age (years): not reported (range 20 to 59) Sex (male/female): not reported |
| Interventions | Treatment arm 1: 98.6 mg oral ferrous fumarate taken daily for 21 days Treatment arm 2: placebo (glucose capsules) taken daily for 21 days |
| Outcomes | Haemoglobin, haematocrit, serum ferritin, red cell indices (MCV, MCH, MCHC, RBC count) and red cell distribution at 1 month and at next donation |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Ekermo 2013.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Sweden Study setting: not reported Number of participants randomised: 210 (numbers in each treatment arm not reported) Number of participants analysed: not reported Follow‐up time points: 4 donations (females) or 5 donations (males) after baseline Source of funding: not reported |
| Participants | Regular blood donors who had donated at least 5 previous whole blood donations Mean age (years): not reported Sex (male/female): not reported |
| Interventions | Treatment arm 1: infusion of dextriferron 1000 mg after first donation in the study and then 200 mg after each donation Treatment arm 2: 100 mg Fe2+ (20 tablets) after each donation |
| Outcomes | Change in haemoglobin from baseline at second visit (primary outcome); change in haemoglobin from baseline at subsequent visits; iron status, health status (SF‐36), International RLS Study Group Rating Scale score and fatigue status at each blood donation; incidence and severity of adverse events, tolerance and compliance |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Grosz 2011.
| Methods |
Type of study: parallel, 4‐arm randomised controlled trial Country of study: Norway Study setting: Oslo Blood Bank Number of participants randomised: 621 (numbers in each treatment arm not reported) Number of participants analysed: not reported Follow‐up time points: 30 days after whole blood donation Source of funding: not reported |
| Participants | Active blood donors who provided written informed consent Mean age (years): not reported Sex (male/female): not reported |
| Interventions | Treatment arm 1: standard 20‐day iron supplementation of one tablet (Niferex©) 100 mg Fe2+ per day Treatment arm 2: standard 20‐day iron supplementation combined with a daily high‐dose vitamin C in the form of 1 tablet of 750 mg ascorbic acid (Pharma Nord©) daily for 30 days Treatment arm 3: 30‐day high‐dose vitamin C supplementation only Treatment arm 4: no form of supplementation |
| Outcomes | Serum ferritin; haemoglobin, serum iron at 30 days |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Kiss 2013.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: USA Study setting: not reported Number of participants randomised: 214 (numbers in each treatment arm not reported) Number of participants analysed: Treatment arm 1: 106 Treatment arm 2: 101 Follow‐up time points: day 56 after baseline donation Source of funding: not reported |
| Participants | Repeat donors (more than 1 donation in past year) Mean age (years): not reported Sex (male/female): 37%/63% |
| Interventions | Treatment arm 1: oral iron 39 mg taken daily for 24 weeks. Total dose: 6552 mg Treatment arm 2: oral placebo, taken daily for 24 weeks |
| Outcomes | Haemoglobin; serum ferritin; time to recovery of 80% of post‐donation decrease in haemoglobin; % donors with venous Hb < 125 g/L at day 56 post‐donation; reticulocyte counts (in a subset of donors) |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Marks 2011.
| Methods |
Type of study: parallel, 2‐arm, double‐blind, randomised controlled trial Country of study: Australia Study setting: Australian Red Cross Blood Service Number of participants randomised: 282 (numbers in each treatment arm not reported) Number of participants analysed: not reported Follow‐up time points: 12 weeks Source of funding: not reported |
| Participants | Returning female whole blood donors aged 18 to 45 years Mean age (years): not reported Sex (male/female): 0%/100% |
| Interventions | Treatment arm 1: carbonyl iron (Feosol), 45 mg daily for 8 weeks Treatment arm 2: placebo |
| Outcomes | Total body iron (calculated from serum ferritin, serum transferrin receptor) and prevalence of iron deficiency (primary outcomes); eligibility to donate at week 12, incidence of gastrointestinal complaints, venous Hb |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Stotzer 2013.
| Methods |
Type of study: parallel, 3‐arm randomised controlled trial Country of study: Germany Study setting: not reported Number of participants randomised: The number of participants randomised in each group is unclear Number of participants analysed: Treatment arm 1: 50 Treatment arm 2: 46 Treatment arm 3: 46 Follow‐up time points: blood parameters were observed for a period of at least 18 months but unclear how many donations were sought/given during this period Source of funding: not reported |
| Participants | Repeat blood donors Mean age (years): not reported Sex (male/female): not reported |
| Interventions | Treatment arm 1: 50 mg elemental iron given daily for 24 days. Total iron: 1200 mg Treatment arm 2: 20 mg elemental iron (+ vitamins) given daily for 60 days as food supplement. Total iron: 1200 mg Treatment arm 3: 100 mg ascorbic acid over an observation period of 2 years |
| Outcomes | Low Hb deferrals; laboratory parameters; venous Hb; mean corpuscular volume; ferritin; zinc protoporphyrin; safety and tolerance parameters |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Wong 2012.
| Methods |
Type of study: parallel, 2‐arm randomised controlled trial Country of study: Hong Kong Study setting: not reported Number of participants randomised: Treatment arm 1: 112 Treatment arm 2: 111 Number of participants analysed: Treatment arm 1: 102 Treatment arm 2: 101 Follow‐up time points: next scheduled donation visit (men: 3 months; women: 4 months) Source of funding: not reported |
| Participants | Repeat blood donors Mean age (years): not reported Sex (male/female): 50%/50% |
| Interventions | Treatment arm 1: 300 mg iron fumarate (Fortifer) equivalent to 100 mg of elemental iron, once daily for 2 weeks Treatment arm 2: no iron supplementation |
| Outcomes | Low Hb deferral; serum ferritin levels; adverse effects; compliance |
| Notes | Insufficient information was included in this conference abstract for inclusion in the review. No further publications of this study have been identified |
Fe2+ = ferrous compound
Hb = haemoglobin MCH = mean cell Hb MCV = mean cell volume MCHC = MCH concentration RBC = red blood cell RLS = restless legs syndrome
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000911897.
| Trial name or title | Monitoring of iron status and clinical evaluation of interventions intended to maintain clinically adequate iron status in women who are new blood donors |
| Methods | A phase 3, randomised, open‐label, parallel study |
| Participants | New female blood donors: Accepted as a whole blood donor at the time of the first and second whole blood donations and attending with the intention of giving a third donation within 12 months of the previous donation |
| Interventions | Treatment arm 1: 30‐day course of a nutritional iron supplement (18 mg carbonyl iron tablets) at a dose of 1 tablet daily over a 30‐day period, or 1 tablet daily until finished if some days are omitted for any reason Treatment arm 2: verbal and written dietary advice to enhance iron intake by increasing awareness of food choices that may increase their intake of available food iron |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | September 2012 |
| Contact information | Dr James M Faed, Blood Bank Dunedin Hospital Private Bag 6133 Dunedin 9016, New Zealand. Email: jim.faed@nzblood.co.nz |
| Notes | — |
Bryant ongoing.
| Trial name or title | Strategies to reduce iron deficiency (STRIDE) study |
| Methods | A multi‐centre, randomised, double‐blind, parallel, efficacy study |
| Participants | Frequent blood donors: 3 or more (men) or 2 or more (women) donations in the prior 12 months |
| Interventions | Treatment arm 1: donors given a letter thanking them for donating Treatment arm 2: donors given a letter informing them of their plasma ferritin level and recommending action to prevent iron deficiency, such as taking iron supplements or delaying donation Treatment arm 3: donors given 60 placebo pills following each donation Treatment arm 4: donors given 60 pills with 19 mg elemental iron (the amount in a typical multiple vitamin with iron) following each donation Treatment arm 5: donors given 60 pills with 38 mg elemental iron following each donation |
| Outcomes | Efficacy (specific outcomes not reported) |
| Starting date | Not reported |
| Contact information | B J Bryant, Blood Bank, University of Texas Medical Branch, Galveston, Texas, United States. Email: bbryant@utmb.edu |
| Notes | Enrolment of 660 donors complete; study now entering the 2‐year longitudinal follow‐up phase |
EUCTR2009‐010623‐64‐SE.
| Trial name or title | A clinical open, randomised study of oral iron (Duroferon®) vs. intravenous iron (Ferinject®) for iron substitution in blood donors |
| Methods | A phase 4, randomised, open‐label, parallel safety/efficacy study |
| Participants | Blood donors:
|
| Interventions | Treatment arm 1: intravenous ferric carboxymaltose ‐ Ferinject (Vifor, France) Treatment arm 2: oral ferrous sulphate ‐ Duroferon, 100 mg |
| Outcomes |
Primary outcome: Change in blood Hb analysed at the second blood donation Secondary outcomes:
|
| Starting date | Not specified |
| Contact information | — |
| Notes | Sponsored by Renapharma AB |
EUCTR2010‐023790‐19‐DK.
| Trial name or title | A randomised, prospective, double‐blind, comparative, placebo‐controlled trial of intravenous administration of iron isomaltoside 1000 (Monofer®) via infusions of blood donors with iron deficiency |
| Methods | A phase 4, randomised, double‐blind, parallel, safety/efficacy study |
| Participants | Female, first‐time donors
|
| Interventions | Treatment arm 1: intravenous iron oligosaccharide complex: Monofer® Treatment arm 2: intravenous placebo infusion |
| Outcomes |
Primary outcome: Change in Hb levels from baseline (before the first donation) to just before the third donation Secondary outcomes:
|
| Starting date | August 2011 |
| Contact information | Lars Lykke Thomsen, Pharmacosmos CRO, Rørvangsvej 30, Holbæk, 4300, Denmark. Email: llt@pharmacosmos.com |
| Notes | Sponsored by Pharmacosmos A/S. Trial ended prematurely in March 2012 |
EUCTR2012‐001529‐28‐DK.
| Trial name or title | A randomized, prospective, double‐blind, comparative placebo‐controlled study of intravenous iron isomaltoside 1000 (Monofer®) administered by infusions to iron‐deficient blood donors |
| Methods | A phase 3, randomised, double‐blind, parallel, safety/efficacy study |
| Participants | Female blood donors:
|
| Interventions | Treatment arm 1: intravenous iron oligosaccharide complex: Monofer® Treatment arm 2: intravenous placebo infusion |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | June 2012 |
| Contact information | Clinical R&D, Pharmacosmos CRO, Rørvangsvej 30, Holbæk, 4300, Denmark. Email: llt@pharmacosmos.com |
| Notes | Sponsored by Pharmacosmos A/S. This trial is ongoing |
NCT01519830.
| Trial name or title | Iron Substitution in Blood Donors (ISUB) |
| Methods | A phase 4, randomised, double‐blind (subject, investigator, outcomes assessor), parallel, efficacy study |
| Participants | Healthy regular blood donors:
|
| Interventions | Treatment arm 1: intravenous iron ‐ iron carboxymaltose (Ferinject) Treatment arm 2: placebo ‐ 0.9% NaCl solution |
| Outcomes |
Primary outcome: Difference in fatigue on a 10‐point numeric scale after intravenous substitution of iron or placebo at 6 weeks (measured by questionnaire) Secondary outcomes:
|
| Starting date | January 2012 |
| Contact information | Peter Keller, MD, Universitätklinik für Hämatologie, Inselspital Bern, CH‐3010 Bern |
| Notes | This study was completed in January 2013 but no publications have been identified |
NCT01787526.
| Trial name or title | Intravenous high dose iron in blood donors (IronWoMan) |
| Methods | A phase 3, randomised, open‐label, parallel, safety/efficacy study |
| Participants | Blood donors:
|
| Interventions | Treatment arm 1: oral iron in a corresponding dose of 10 g (assuming an absorption of 10%, 100 capsules a 100 mg iron each) taken over 8 to 12 weeks Treatment arm 2: high‐dose intravenous iron (ferric carboxymaltose, 1000 mg) |
| Outcomes |
Primary outcome: Transferrin saturation (%) at visit 1 Secondary outcomes:
|
| Starting date | September 2013 |
| Contact information | Karin Amrein, MD (Principal Investigator), Medical University Graz, Austria 8036. Email: karin.amrein@medunigraz.at |
| Notes | Estimated completion date: August 2015 |
Hb = haemoglobin
NaCl = sodium chloride RLS = restless legs syndrome
Differences between protocol and review
We have redefined the comparison of iron supplementation schedule A versus schedule B as a comparison of treatment duration A versus treatment duration B for clarity and to avoid any ambiguity in definition. In addition, we have renamed the comparison of iron supplementation manufacturer A versus manufacturer B as iron preparation A versus iron preparation B to describe more accurately the different iron compounds.
There are some differences in outcomes between the protocol and the review.
In the protocol for this review, the outcomes "Number of blood donors with a change in iron stores" and "Number of donors with a change in Hb levels, mean cell volume (MCV) and other blood indices before donations" are vague, since it is unclear how "a change" in these measures should be defined. Any level of change in these measures may be of clinical interest. Hence, we have replaced these outcomes with a new single outcome, which now includes iron stores as well as all other measures before donation as follows:
"Mean levels of Hb, MCV, other blood indices and iron stores before further donations".
Also, the outcome "Rate of change in Hb levels, MCV, other blood indices and iron stores between donations" included in the protocol for this review does not take into account the likely scenario of different numbers of donations at different time points between studies, and between demographic groups within studies (e.g. males versus females). Therefore we have redefined this outcome as measured after subsequent donations as follows:
"Mean levels of Hb, MCV, other blood indices and iron stores after subsequent donations".
We have removed the outcome "Total number of successful donations (per donor and per intervention)", defined in the protocol, since this measure is directly correlated with the primary outcome "Risk ratio of deferral of blood donors (number of prospective blood donors who are at least temporarily rejected from blood donation) due to low haemoglobin" and is therefore deemed uninformative.
Finally, treatment compliance is an important issue in oral iron supplementation and we have added this as a new outcome.
We had intended to analyse continuous outcomes as mean change from baseline; however, few studies reported continuous outcomes as mean change from baseline values and therefore we have compared endpoint (follow‐up) values for all comparisons.
In view of the differences in study participants (first‐time donors, repeat donors, deferred donors) in the included studies and the likely heterogeneity between these groups, we used random‐effects models rather than fixed‐effect models for all meta‐analyses.
We had intended to contact study authors in order to obtain information that was missing or unclear in the published report; however, this was not done due to the time that had elapsed since publication of the majority of studies.
We did not perform sensitivity analyses based on allocation concealment due to the high number of studies deemed to have a high risk of bias associated with allocation concealment. We had also intended to carry out subgroup analyses by donation history, menopausal status, Hb threshold for donation and trial setting, but this was not possible due to a paucity of studies reporting these factors. These will be addressed in future updates of this review if sufficient data are available.
Contributions of authors
Graham Smith is a content expert for this review (stem cells) and carried out the screening and selection of trials, data extraction and assessment of risk of bias, analysis of results and preparation of the protocol and final report.
Sheila Fisher is a methodological expert for this review, and carried out the screening and selection of trials, data extraction and assessment of risk of bias, analysis of results and preparation of the final report.
Carolyn Dorée is an information specialist, who developed and implemented the search strategies and contributed to the preparation of the protocol and final report.
Emanuele Di Angelantonio is a content expert for this review (blood donors and iron) and contributed to the preparation of the final report.
David Roberts is a content expert for this review (red blood cells and transfusion medicine), and assisted with eligibility screening and contributed to the preparation of the protocol and final report.
Sources of support
Internal sources
Research & Development, NHS Blood and Transplant, UK.
External sources
No sources of support supplied
Declarations of interest
Graham Smith: none known
Sheila Fisher: none known
Carolyn Doree: none known
Emanuele Di Angelantonio: reported serving as an honorary consultant for the National Health Service Blood Transfusion; receiving royalties from Elsevier (France) and that he has received grants from the NHS Blood and Transplant, British Heart Foundation and the Medical Research Council.
David Roberts: none known
New
References
References to studies included in this review
Birgegard 2010 {published data only}
- Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sanguinis 2010;99(4):354‐61. [PUBMED: 20598107] [DOI] [PubMed] [Google Scholar]
- Schneider K, Birgegard G, Ulfberg J. Healthy blood donors are iron deficient!. Transfusion. 59th Annual Meeting of the American Association of Blood Banks; 2006 Oct. 2006; Vol. 49 Suppl 9:83A; Abstract SP144.
Blot 1980 {published data only}
- Blot I, Chenayer M, Diakhate L, Leluc R, Gross E, Tchernia G. Iron reserves in blood donors. Should we prescribe systematic iron supplementation? [Etude des reserves en fer chez les donneurs de sang. Faut‐il prescrire une supplementation martiale systematique?]. Revue Francaise de Transfusion et Immuno‐Hematologie 1980;23(2):119‐29. [PUBMED: 7455480] [DOI] [PubMed] [Google Scholar]
Borch‐Iohnsen 1993 {published data only}
- Borch‐Iohnsen B, Halvorsen R, Stenberg V, Flesland O, Mowinckel P. The effect of daily low‐dose iron supplements in female blood donors with depleted iron stores: comparison with female non‐donors. Scandinavian Journal of Clinical and Laboratory Investigation 1993;53(8):789‐91. [DOI] [PubMed] [Google Scholar]
Brittenham 1996 {published data only}
- Brittenham GM, Gordeuk VR, Bravo JR, Hirschler NV, Piliavin JA, Goormastic M, et al. Carbonyl iron supplementation for female blood donors. Blood. 1996; Vol. 88; 10 Suppl Pt 2:89b; Abstract 3085.
Bucher 1973 {published data only}
- Bucher U, Baumann E, Keller T. Iron substitution in blood donors. II. Practical experience with iron substitution [Eisensubstitution bei Blutspendern. II. Praktische Erfahrungen mit der Eisensubstitution]. Schweizerische Medizinische Wochenschrift 1973;103(46):1634‐40. [PUBMED: 4751063] [PubMed] [Google Scholar]
Busch 1972 {published data only}
- Busch H, Gohrbandt G. Prevention of iron deficiency conditions in blood donors. Tolerance studies using the new iron preparation Eryfer [Zur Propylaxe von eisenmangelzustanden bei Blutspendern. Vertraglichkeitsstudie mit dem neuen eisenpraparat Eryfer]. Munchener Medizinische Wochenschrift (1950) 1972;114(36):1543‐6. [PUBMED: 4561493] [PubMed] [Google Scholar]
Buzi 1980 {published data only}
- Buzi E, Siegenthaler P. Iron substitution in blood donors. Two different iron preparations from the galenic viewpoint [Substitution de fer chez les donneurs de sang. Etudes portant sur deux preparations de fer differentes au point de vue galenique]. Schweizerische Rundschau fur Medizin Praxis = Revue Suisse de Medecine Praxis 1980;69(47):1744‐7. [PUBMED: 7255330] [PubMed] [Google Scholar]
Cable 1988 {published data only}
- Cable RG, Morse EE, Keltonic J, Kakaiya R, Kiraly T. Iron supplementation in female blood donors deferred by copper sulfate screening. Transfusion 1988;28(5):422‐6. [PUBMED: 3047920] [DOI] [PubMed] [Google Scholar]
Devasthali 1991 {published data only}
- Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double‐blind study. European Journal of Haematology 1991;46(5):272‐8. [PUBMED: 2044721] [DOI] [PubMed] [Google Scholar]
Ehn 1968 {published data only}
- Adolfsson L, Ehn L, Lieden G, Oldfelt CO. Blood donors' needs of iron (II) [Jarnbehov hos blodgivare (II)]. Lakartidningen 1968;65(51):5109‐14. [PubMed] [Google Scholar]
- Ehn L, Lieden G, Oldfelt CO. Need of iron in blood donors [Jarnbehov hos blodgivare]. Lakartidningen 1968;65(7):687‐97. [PUBMED: 5723566] [PubMed] [Google Scholar]
- Lieden G. Iron supplement to blood donors. I. Trials with intermittent iron supply. Acta Medica Scandinavica 1975;197(1‐2):31‐6. [PUBMED: 1092131] [DOI] [PubMed] [Google Scholar]
Frykman 1994 {published data only}
- Frykman E, Bystrom M, Jansson U, Edberg A, Hansen T. Side effects of iron supplements in blood donors: superior tolerance of heme iron. Journal of Laboratory and Clinical Medicine 1994;123(4):561‐4. [PUBMED: 8145004] [PubMed] [Google Scholar]
Gordeuk 1987a {published data only}
- Gordeuk VR, Brittenham GM, Hughes MA, Keating LJ. Carbonyl iron for short‐term supplementation in female blood donors. Transfusion 1987;27(1):80‐5. [PUBMED: 3810831] [DOI] [PubMed] [Google Scholar]
Gordeuk 1987b {published data only}
- Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High‐dose carbonyl iron for iron deficiency anemia: a randomized double‐blind trial. American Journal of Clinical Nutrition 1987;46(6):1029‐34. [PUBMED: 3318377] [DOI] [PubMed] [Google Scholar]
Gordeuk 1990 {published data only}
- Gordeuk VR, Brittenham GM, Bravo J, Hughes MA, Keating LJ. Prevention of iron deficiency with carbonyl iron in female blood donors. Transfusion 1990;30(3):239‐45. [PUBMED: 2180144] [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Brittenham GM, Bravo JA, Hughes MA, Keating LJ. Prevention of iron deficiency in female blood donors with short term carbonyl iron therapy. Blood. 29th Annual Meeting of the American Society of Hematology; 1987 Dec 4‐8; Washington (DC). 1987; Vol. 5 Suppl 1:329a; Abstract 1172.
Jacobs 1993 {published data only}
- Jacobs P, Fransman D, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron‐deficient blood donors. Journal of Clinical Apheresis 1993;8(2):89‐95. [PUBMED: 8226711] [DOI] [PubMed] [Google Scholar]
Jacobs 2000 {published data only}
- Jacobs P, Wood L, Bird AR. Erythrocytes: better tolerance of iron polymaltose complex compared with ferrous sulphate in the treatment of anaemia. Hematology (Amsterdam, Netherlands) 2000;5(1):77‐83. [PUBMED: 11399604] [DOI] [PubMed] [Google Scholar]
Landucci 1987 {published data only}
- Landucci G, Frontespezi S. Treatment of iron deficiency conditions in blood donors: controlled study of iron sulphate versus iron protein succinylate. Journal of International Medical Research 1987;15(6):379‐82. [PUBMED: 3325320] [DOI] [PubMed] [Google Scholar]
Lieden 1975 {published data only}
- Lieden G, Ehn L, Höglund S, Reizenstein P. Difficulties in preventing iron deficiency in blood donors. European Journal of Clinical Investigation 1973;3:250. [Google Scholar]
- Lieden G, Höglund S, Ehn L. Iron supplement to blood donors. II. Effect of continuous iron supply. Acta Medica Scandinavica 1975;197(1‐2):37‐41. [PUBMED: 1124659] [PubMed] [Google Scholar]
Lindholm 1981 {published data only}
- Lindholm A, Creutzer B, Skinhoj A. Iron supplement to blood donors: a comparative study of two iron preparations. Opuscula Medica 1981;26(4):134‐7. [Google Scholar]
Linpisarn 1986 {published data only}
- Linpisarn S, Kunachiwa W, Laokuldilok T, Laokuldilok J, Keawvichit R, Kulapongs P. Iron status and the effect of iron supplementation in Thai male blood donors in northern Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1986;17(2):177‐83. [PUBMED: 3787305] [PubMed] [Google Scholar]
Mackintosh 1988_HSF {published data only}
- Mackintosh W, Jacobs P. Response in serum ferritin and haemoglobin to iron therapy in blood donors. American Journal of Hematology 1988;27:17‐9. [DOI] [PubMed] [Google Scholar]
Mackintosh 1988_LSF {published data only}
- Mackintosh W, Jacobs P. Response in serum ferritin and haemoglobin to iron therapy in blood donors. American Journal of Hematology 1988;27:17‐9. [DOI] [PubMed] [Google Scholar]
Maghsudlu 2008 {published data only}
- Maghsudlu M, Nasizadeh S, Toogeh GR, Zandieh T, Parandoush S, Rezayani M. Short‐term ferrous sulfate supplementation in female blood donors. Transfusion 2008;48(6):1192‐7. [PUBMED: 18363581] [DOI] [PubMed] [Google Scholar]
- Maghsudlu M, Zandieh T, Togeh GH, Parandush S, Rezaiani M. Effect of short‐term ferrous sulfate on iron stores in female blood donors. Vox Sanguinis. XVth Regional Congress of the International Society of Blood Transfusion (ISBT); 2006 Sept; Madrid. 2007; Vol. 93 Suppl 1:69‐70; Abstract P‐044.
Mirrezaie 2008 {published data only}
- Mirrezaie SM, Parsi R, Torabgahromi SA, Askarian M. Low dose, short‐term iron supplementation in female blood donors of childbearing age: a randomized, double‐masked, placebo‐controlled study. Iranian Journal of Medical Sciences 2008;33(3):138‐43. [Google Scholar]
Radtke 2004a {published data only}
- Kiesewetter H, Tegtmeier J, Salama A, Radtke H. Comparison of iron loss in whole blood donors. Vox Sanguinis. XVth Regional Congress of the International Society of Blood Transfusion; 2005 July 2; Athens. 2005; Vol. 89 Suppl 1:38.
- Radtke H, Mayer B, Rocker L, Salama A, Kiesewetter H. Iron supplementation and 2‐unit red blood cell apheresis: a randomized, double‐blind, placebo‐controlled study. Transfusion 2004;44(10):1463‐7. [PUBMED: 15383019] [DOI] [PubMed] [Google Scholar]
Radtke 2004b {published data only}
- Radtke H, Tegtmeier J, Rocker L, Salama A, Kiesewetter H. Daily doses of 20 mg of elemental iron compensate for iron loss in regular blood donors: a randomized, double‐blind, placebo‐controlled study. Transfusion 2004;44(10):1427‐32. [PUBMED: 15383014] [DOI] [PubMed] [Google Scholar]
Rosvik 2010 {published data only}
- Rosvik AS, Hervig T, Wentzel‐Larsen T, Ulvik RJ. Effect of iron supplementation on iron status during the first week after blood donation. Vox Sanguinis 2010;98(3 Pt 1):e249‐56. [PUBMED: 19874572] [DOI] [PubMed] [Google Scholar]
Rybo 1971 {published data only}
- Rybo G, Solvell L. Side‐effect studies on a new sustained release iron preparation. Scandinavian Journal of Haematology 1971;8(4):257‐64. [PUBMED: 5134472] [DOI] [PubMed] [Google Scholar]
Simon 1984 {published data only}
- Simon TL, Garry PJ. Iron supplementation of menstruating female blood donors. Transfusion 1981;21(5):634. [DOI] [PubMed] [Google Scholar]
- Simon TL, Hunt WC, Garry PJ. Iron supplementation for menstruating female blood donors. Transfusion 1984;24(6):469‐72. [PUBMED: 6506175] [DOI] [PubMed] [Google Scholar]
Waldvogel 2012 {published data only}
- Pedrazzini B, Waldvogel S, Cornuz J, Vaucher P, Bize R, Tissot JD, et al. The impact of iron supplementation efficiency in female blood donors with a decreased ferritin level and no anaemia. Rationale and design of a randomised controlled trial: a study protocol. Trials 2009;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel S, Pedrazzini B, Vaucher P, Bize R, Cornuz J, Tissot JD, et al. Clinical evaluation of iron treatment efficiency among non‐anemic but iron‐deficient female blood donors: a randomized controlled trial. BMC Medicine 2012;10:8. [PUBMED: 22272750] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aramburu 1991 {published data only}
- Aramburu E, Arrieta A, Medarde A. Study of iron supplementation in female blood donors. Sangre 1991;36 Suppl 2:38. [Google Scholar]
Bier‐Ulrich 2003 {published data only}
- Bier‐Ulrich AM, Haubelt H, Anders C, Nagel D, Schneider S, Siegler KE, et al. The impact of intensive serial plasmapheresis and iron supplementation on iron metabolism and Hb concentration in menstruating women: a prospective randomized placebo‐controlled double‐blind study. Transfusion 2003;43(3):405‐10. [PUBMED: 12675729] [DOI] [PubMed] [Google Scholar]
Blagoevska 2005 {published data only}
- Blagoevska M, Grubovik R, Makarovska T, Samonikov J, Balamovski M, Ortakovska S. Assessment of therapeutic efficacy of iron in treatment of sideropenic anemia. ISBT Congress Abstract Book. XVI Regional Congress of the International Society of Blood Transfusion (ISBT); 2005 Nov; Bangkok. 2005.
Borch‐Iohnsen 1989 {published data only}
- Borch‐Iohnsen B, Meltzer HM, Trygg K, Stenberg V, Reinskou T. Women and iron deficiency‐‐a problem? Iron levels in a group of fertile Norwegian women and the bioavailability of 3 low‐dose iron supplements in women with low iron stores [Kvinner og jernmangel‐‐et problem? Jernstatus i en gruppe norske fertile kvinner og biotilgjengeligheten av tre lavdose jerntilskudd hos kvinner med lave jernlagre]. Tidsskrift for den Norske Laegeforening : Tidsskrift for Praktisk Medicin, ny Raekke 1989;109(19‐21):1990‐5. [PUBMED: 2665180] [PubMed] [Google Scholar]
Brittenham 2011 {published data only}
- Brittenham GM. Iron deficiency in whole blood donors. Transfusion 2011;51(3):458‐61. [PUBMED: 21388389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brugnara 1993 {published data only}
- Brugnara C, Chambers LA, Malynn E, Goldberg MA, Kruskall MS. Red blood cell regeneration induced by subcutaneous recombinant erythropoietin: iron‐deficient erythropoiesis in iron‐replete subjects. Blood 1993;81(4):956‐64. [PUBMED: 8428002] [PubMed] [Google Scholar]
Bryant 2012 {published data only}
- Bryant B, Yau YY, Arceo S, Hopkins J, Leitman S. Role of iron replacement therapy in the routine management of blood donors. American Journal of Hematology. 3rd Annual Sickle Cell Disease Research and Educational Symposium and Grant Writing Institute and Annual National Sickle Cell Disease Scientific Meeting; Fort Lauderdale (FL). 2009; Vol. 84(8):E359.
- Bryant BJ, Yau YY, Arceo SM, Daniel‐Johnson J, Hopkins JA, Leitman SF. Iron replacement therapy in the routine management of blood donors. Transfusion 2012;52(7):1566‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiamchanya 2013 {published data only}
- Chiamchanya N. Rapid recovery time of hemoglobin level in female regular blood donors with ferrous fumarate and high dose of ascorbic acid supplement. Journal of the Medical Association of Thailand 2013;96(2):165‐71. [PubMed] [Google Scholar]
Gordeuk 1986 {published data only}
- Gordeuk VR, Brittenham GM, McLaren CE, Hughes MA, Keating LJ. Carbonyl iron therapy for iron deficiency anemia. Blood 1986;67(3):745‐52. [PUBMED: 3947745] [PubMed] [Google Scholar]
Hoppe 2006 {published data only}
- Hoppe M, Hulthen L, Hallberg L. The relative bioavailability in humans of elemental iron powders for use in food fortification. European Journal of Nutrition 2006;45(1):37‐44. [PUBMED: 15864409] [DOI] [PubMed] [Google Scholar]
Kaltwasser 1982 {published data only}
- Kaltwasser JP, Schafer M, Seidl S. Iron prophylaxis in blood donors. Blut 1982;45:79‐80. [Google Scholar]
Lieden 1975b {published data only}
- Lieden G, Höglund S, Ehn L. Changes in certain iron metabolism variables after a single blood donation. Acta Medica Scandinavica 1975;197(1‐2):27‐30. [DOI] [PubMed] [Google Scholar]
Lopes 1999 {published data only}
- Lopes MC, Ferreira LO, Batista Filho M. Use of daily and weekly ferrous sulfate to treat anemic childbearing‐age women [Uso diario e semanal de sulfato ferroso no tratamento de anemia em mulheres no periodo reprodutivo.]. Cadernos de Saude Publica / Ministerio da Saude, Fundacao Oswaldo Cruz, Escola Nacional de Saude Publica 1999;15(4):799‐808. [PUBMED: 10633202] [DOI] [PubMed] [Google Scholar]
Magnussen 2008 {published data only}
- Magnussen K, Bork N, Asmussen L. The effect of a standardized protocol for iron supplementation to blood donors low in hemoglobin concentration. Transfusion 2008;48(4):749‐54. [PUBMED: 18194390] [DOI] [PubMed] [Google Scholar]
Nielsen 1973 {published data only}
- Nielsen JB, Ikkala E, Solvell L, Bjorn‐Rasmussen E, Ekenved G. Absorption of iron from sustained release and rapidly disintegrating tablets. Influence of daily numbers of administrations. Acta Medica Scandinavica 1973;1‐2(1):123‐7. [PUBMED: 4727245] [DOI] [PubMed] [Google Scholar]
Nielsen 1976 {published data only}
- Nielsen JB, Ikkala E, Solvell L, Bjorn‐Rasmussen E, Ekenved G. Absorption of iron from slow‐release and rapidly‐disintegrating tablets ‐ a comparative study in normal subjects, blood donors and subjects with iron deficiency anaemia. Scandinavian Journal of Haematology. Supplementum 1976;28:89‐97. [PUBMED: 1064905] [DOI] [PubMed] [Google Scholar]
Radtke 2005 {published data only}
- Radtke H, Tegtmeier J, Rocker L, Salama A, Kiesewetter H. Compensating for iron loss in regular blood donors using ferrous gluconate and ascorbic acid. Transfusion 2005;45(7):1236‐7. [PUBMED: 15987376] [DOI] [PubMed] [Google Scholar]
Remy 1956 {published data only}
- Remy D, Zockler H. Prophylactic iron therapy of habitual blood donors [Medikamentose Eisenprophylaxe bei Dauerblutspendern]. Blut 1956;2(1):32‐6. [PUBMED: 13315518] [DOI] [PubMed] [Google Scholar]
Tobias 1981 {published data only}
- Tobias KI, Camp FR Jr, Linville A, Dedich SN, Parrish J, White WD. A laboratory report on iron supplementation in blood donors. I. American Journal of Forensic Medicine and Pathology 1981;2(3):225‐30. [PUBMED: 7325132] [DOI] [PubMed] [Google Scholar]
Zanella 1989 {published data only}
- Zanella A, Milani S, Silvani C, Gridelli L, Berzuini A, Rossi F, et al. Monitoring hemoglobin and iron status in blood donors to prevent iron deficiency. Bibliotheca Nutritio et Dieta 1989;44:131‐43. [PUBMED: 2783107] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dara 2013 {published data only}
- Dara R, Khetan D, Marwaha N. Role of iron supplementation in prevention of iron deficiency in regular blood donors: randomised, placebo controlled study. 23rd Regional Congress of the International Society of Blood Transfusion, Amsterdam; 2‐5 June 2013. Vox Sanguinis. 2013; Vol. 105:122; Abstract P‐167.
Ekermo 2013 {published data only}
- Ekermo BE, Forsberg P, Schedvin G, Berlin G. An open, randomised, clinical study of oral versus intravenous iron for iron substitution in blood donors. Transfusion. 2013; Vol. 53 Suppl 2:36A; Abstract S48‐030G.
Grosz 2011 {published data only}
- Grosz D, Larsen TT, Svenningsen V, Wistner R, Heier HE. Combining high dose vitamin C with oral iron supplementation for a more effective rise in serum ferritin among blood donors. 21st Regional Congress of the ISBT, Europe, Lisbon, 18‐22 June 2011. Vox Sanguinis. 2011; Vol. 101:143.
Kiss 2013 {published data only}
- Kiss JE, Cable RG, Brambilla D, Simone G, Mast AE, Spencer BR, et al. Hemoglobin recovery after blood donation and the effects of iron supplementation: the Hemoglobin and Iron Recovery Study (HEIRS). Transfusion. 2013; Vol. 53 Suppl 2:14A; Abstract P5.
Marks 2011 {published data only}
- Marks DC, Speedy J, Robinson K, Capper HR, Mondy PJ, Brama T, et al. Post‐donation iron supplementation with 45 mg carbonyl iron is effective and well tolerated. Transfusion. 2011; Vol. 51:98A.
- Speedy J, Marks DC, Robinson KL, Capper HR, Mondy PJ, Brama T, et al. Iron management in blood donors: a trials of post‐donation iron replacement. Transfusion Medicine 2012;22(3):222‐3. [Google Scholar]
Stotzer 2013 {published data only}
- Stotzer F, Schneider S, Metzgeroth G, Brade J, Muller‐Steinhardt M, Kluter H. Influence of iron substitution with a standard drug vs a food supplement on blood donation in repeated blood donors. Transfusion Medicine and Hemotherapy. 2013; Vol. 40:6‐7; Abstract SPE‐V06.
Wong 2012 {published data only}
- Wong HK, Lee CK, Leung JNS, Lee IYM, Tsoi WC, Lin CK. Optimizing the iron status of regular Chinese blood donors. 32nd International Congress of the AMMTAC, 7‐12 July 2012, Cancun, Mexico. Vox Sanguinis. 2012:10385‐6.
References to ongoing studies
ACTRN12612000911897 {published data only}
- ACTRN12612000911897. Monitoring of iron status and clinical evaluation of interventions intended to maintain clinically adequate iron status in women who are new blood donors. http://apps.who.int/trialsearch/trial.aspx?trialid=ACTRN12612000911897 (accessed 28 May 2013).
Bryant ongoing {published data only}
- Bryant BJ, Schlumpf KS, Kiss JE, Cable RG, Spencer BR, Vij V, Mast AE. Enrollment data from the Strategies to Reduce Iron Deficiency (STRIDE) study. Transfusion. 2012; Vol. 52:30A‐1A.
EUCTR2009‐010623‐64‐SE {published data only}
- EUCTR2009‐010623‐64‐SE. A clinical open, randomised study of oral iron (Duroferon®) vs. intravenous iron (Ferinject®) for iron substitution in blood donors. http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2009‐010623‐64‐SE (accessed 28 May 2013).
EUCTR2010‐023790‐19‐DK {published data only}
- EUCTR2010‐023790‐19‐DK. A randomised, prospective, double‐blind, comparative, placebo‐controlled trial of intravenous administration of iron isomaltoside 1000 (Monofer®) via infusions of blood donors with iron deficiency. http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2010‐023790‐19‐DK (accessed 28 May 2013).
EUCTR2012‐001529‐28‐DK {published data only}
- EUCTR2012‐001529‐28‐DK. A randomized, prospective, double‐blind, comparative placebo‐controlled study of intravenous iron isomaltoside 1000 (Monofer®) administered by infusions to iron‐deficient blood donors. http://apps.who.int/trialsearch/trial.aspx?trialid=EUCTR2012‐001529‐28‐DK (accessed 28 May 2013).
NCT01519830 {published data only}
- NCT01519830. Iron Substitution in Blood Donors (ISUB). http://clinicaltrials.gov/show/NCT01519830 (accessed 28 May 2013).
NCT01787526 {published data only}
- NCT01787526. Intravenous High Dose Iron in Blood Donors (IronWoMan). http://clinicaltrials.gov/show/NCT01787526 (accessed 28 May 2013).
Additional references
Alvarez‐Ossorio 2000
- Alvarez‐Ossorio L, Kirchner H, Kluter H, Schlenke P. Low ferritin levels indicate the need for iron supplementation: strategy to minimize iron‐depletion in regular blood donors. Transfusion Medicine 2000;10(2):107‐12. [DOI] [PubMed] [Google Scholar]
Baart 2011
- Baart Am, Kort WL, Moons KG, Vergouwe Y. Prediction of low haemoglobin levels in whole blood donors. Vox Sanguinis 2001;100(2):204‐11. [DOI] [PubMed] [Google Scholar]
Blood Safety and Quality Regulations 2005
- http://www.legislation.gov.uk/uksi/2005/50/contents/made. 2005 (accessed 18 February 2011).
Custer 2007
- Custer B, Chinn A, Hirscler NV, Busch MP, Murphy EL. The consequence of temporary deferral on future whole blood donation. Transfusion 2007;47(8):1514‐23. [DOI] [PubMed] [Google Scholar]
Drakesmith 2012
- Drakesmith H, Prentice AM. Hepcidin and the iron‐infection axis. Science 2012;338(6108):768‐72. [DOI] [PubMed] [Google Scholar]
Earley 2009
- Earley CJ, Horska A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double‐blind, placebo‐controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Medicine 2009;10(2):206‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falkingham 2010
- Falkingham M, Abdelhamid A, Curtis P, Fairweather‐Tait S, Dye L, Hooper L. The effects of oral iron supplementation on cognition in older children and adults: a systematic review and meta‐analysis. Nutrition Journal 2010;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 1977
- Finch CA, Cook JD, Labbe RF, Culala M. Effect of blood donation on iron stores as evaluated by serum ferritin. Blood 1977;50(3):441‐7. [PubMed] [Google Scholar]
Grote 2009
- Grote KL, Leissner L, Hedner J, Ulfberg J. A randomized, double blind, placebo controlled, multi‐center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Movement Disorders 2009;24(10):1445‐52. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson S. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lim 2012
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2224‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litton 2013
- Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta‐analysis of randomised clinical trials. BMJ 2013;347:f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2013
- Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harbour Perspectives in Medicine 2013;3(7):a011866. [DOI: 10.1101/cshperspect.a011866] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milman 1996
- Milman N. Serrum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. International Journal of Haematology 1996;63(2):103‐35. [DOI] [PubMed] [Google Scholar]
NHSBT 2013
- NHSBT. Annual Report. http://www.nhsbt.nhs.uk/annualreview/ 2013.
Oppenheimer 2001
- Oppenheimer SJ. Iron and its relation to immunity and infectious disease. Journal of Nutrition 2001;131(2):616S‐35S. [DOI] [PubMed] [Google Scholar]
Peña‐Rosas 2012
- Peña‐Rosas JP, De‐Regil LM, Dowswell T, Viteri FE. Intermittent oral iron supplementation during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009997] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Sazawal 2006
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community‐based, randomised, placebo‐controlled trial. Lancet 2006;367(9505):133‐4. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Wiley ‐ Blackwell, 2008:Chapter 12.
Seifried 2011
- Seifried E, Klueter H, Weidmann C, Staudenmaier T, Schrezenmeier H, Henschler R, et al. How much blood is needed?. Vox Sanguinis 2011;100(1):10‐21. [DOI] [PubMed] [Google Scholar]
Skikne 1984
- Skikne B, Lynch S, Borek CD, Cook J. Iron and blood donation. Clinical Haematology 1984;13(1):271‐81. [PubMed] [Google Scholar]
Worwood 1993
- Worwood M, Darke C. Serum ferritin, blood donation, iron stores and haemochromatosis. Transfusion Medicine 1993;2(1):21‐8. [DOI] [PubMed] [Google Scholar]


