Abstract
Avian leukosis virus (ALV) infection induces bursal lymphomas in chickens after proviral integration within the c-myc proto-oncogene and induces erythroblastosis after integration within the c-erbB proto-oncogene. A nested PCR assay was used to analyze the appearance of these integrations at an early stage of tumor induction after infection of embryos. Five to eight distinct proviral c-myc integration events were amplified from bursas of infected 35-day-old birds, in good agreement with the number of transformed bursal follicles arising with these integrations. Cells containing these integrations are remarkably common, with an estimated 1 in 350 bursal cells having proviral c-myc integrations. These integrations were clustered within the 3′ half of c-myc intron 1, in a pattern similar to that observed in bursal lymphomas. Bone marrow and spleen showed a similar number and pattern of integrations clustered within 3′ c-myc intron 1, indicating that this region is a common integration target whether or not that tissue undergoes tumor induction. While all tissues showed equivalent levels of viral infection, cells with c-myc integrations were much more abundant in the bursa than in other tissues, indicating that cells with proviral c-myc integrations are preferentially expanded within the bursal environment. Proviral integration within the c-erbB gene was also analyzed, to detect clustered c-erbB intron 14 integrations associated with erythroblastosis. Proviral c-erbB integrations were equally abundant in the bone marrow, spleen, and bursa. These integrations were randomly situated upstream of c-erbB exon 15, indicating that cells carrying 3′ intron 14 integrations must be selected during induction of erythroblastosis.
Analysis of avian leukosis virus (ALV) tumor induction allowed the identification of the molecular basis of oncogenesis by slowly transforming retroviruses (reviewed in reference 48). Proviral integration next to cellular proto-oncogenes and subsequent long terminal repeat (LTR)-driven oncogene expression can result in the development of monoclonal tumors. This particular retrovirus generates two distinct types of tumors depending on the cell type and proto-oncogene involved. The most common result of ALV infection is a bursal lymphoma involving proviral integration next to the c-myc proto-oncogene, in 50 to 100% of lymphoma-susceptible chickens infected as embryos or 1-day-old chicken (7, 15). Integration within the c-erbB proto-oncogene in erythrocyte precursors results in erythroblastosis with a more variable incidence of 5 to 80%, depending on the particular chicken strain analyzed (14, 16).
The c-myc gene encodes a transcription factor that functions in the regulation of cell growth and death, and deregulated c-myc expression is associated with a number of human and animal cancers (reviewed in reference 18). ALV integration within the c-myc gene (17) in immature B cells can result in the induction of metastatic bursal lymphomas in chickens (reviewed in reference 32). Examination of integrations from these lymphomas shows that nearly all proviruses have undergone deletion of the 5′ LTR (24, 25), so that the 3′ LTR can drive high levels of c-myc gene transcription (reviewed by Kung et al. [23]). The bursa is composed of roughly 10,000 follicles (34), each of which is derived from about two stem cells that establish follicles during embryonic hematopoeisis (49). Transformed or hyperproliferating follicles featuring clonal proviral c-myc integrations can be identified based on their enlarged size and differential staining with methyl green pyronin (10), beginning 4 weeks after ALV infection. Although 5 to 20 transformed follicles arise (12), only one or a few of these hyperproliferating follicles progress to form a metastatic lymphoma after 3 months of age, presumably after additional mutations and/or proviral integration next to proto-oncogenes such as c-bic (9, 47).
The c-erbB proto-oncogene encodes the epidermal growth factor receptor, a protein kinase which is involved in growth signalling in many cell types (33, 35). Proviral integration within this gene can result in erythroblastosis tumors involving erythroid precursors in the bone marrow and spleen, which arise 1 to 3 months after ALV infection (7). Interestingly, most of the proviral integrations from tumors map within the 3′ region of c-erbB intron 14 (16, 28, 40), so that a truncated gag-env-erbB fusion protein is produced which is thought to have constitutive kinase activity (33). The complete provirus is generally retained in c-erbB integrations, so that these fusion products are generated by transcription readthrough past the 3′ LTR followed by alternative splicing (28, 33). These readthrough transcripts are often transduced to generate recombinant viruses containing v-erbB sequences, which can be observed in about half of the erythroblastosis tumors that arise (28).
Examination of proviral integration sites within the c-myc or c-erbB genes of tumors reveals a nonrandom pattern of proviral integration. The majority of bursal lymphomas show integration within the 3′ region of c-myc intron 1, while occasional integrations are observed 5′ or 3′ of the gene (41, 44). The c-myc protein-coding sequence begins in exon 2, so that introduction of the LTR enhancer and promoter within intron 1 increases the production of wild-type c-myc mRNA and protein roughly 50-fold (29). It is not known why the 3′ region of intron 1 is the most common integration site observed in lymphomas. However, this region does contain unusual features, including DNase I-hypersensitive sites and AT-rich sequences (41, 43, 44), which could target this region for proviral integration. It is also possible that integration within this region of c-myc intron 1 is somehow selected during lymphomagenesis. The c-erbB gene integrations observed in erythroblastosis are also nonrandom, so that the majority of tumors show integrations clustered within a 300-bp region of intron 14. These intron sequences could be intrinsically susceptible to proviral integration, or integration within this region could be selected by its ability to generate an in-frame gag-env-erbB fusion protein (8).
We developed a PCR assay to amplify and characterize ALV proviral integrations within the c-myc and c-erbB genes at early stages of tumor induction. Cells carrying proviral c-myc or c-erbB integrations were detected in several tissues, indicating that the tissue specificity of lymphomagenesis or erythroblastosis is regulated at a stage after the initial integration event. While the 3′ region of c-myc intron 1 was a preferred integration site in infected tissues and in tumors, the 3′ region of c-erbB intron 14 was not a common integration site in infected tissues, indicating that those c-erbB integration sites are selected during the development of erythroblastosis tumors.
MATERIALS AND METHODS
Cell culture and virus infection of chicken embryos.
The S13, BK25, 293S, 1104HI, and BK3A cell lines were derived from bursal lymphomas of RAV-1-infected chickens (20, 24) and were cultured as previously described (42). S13 cells were used as a source of virus for infection of embryos. Virus was harvested from log-phase culture supernatants and was purified by low-speed centrifugation and 0.45-μm-pore-size cellulose-acetate filtration of the supernatant.
Line 15I5 × 71 fertilized eggs were provided by the USDA Avian Oncology Laboratory (Lansing, Mich.). Embryos 10 days old were infected by injection of 100 μl of S13 supernatant into a chorioallantoic vein, as described by Pink et al. (38). Hatched birds were sacrificed at 35 days of age by CO2 euthanasia; then the tissues were dissected, rinsed in saline, and stored at −70°C.
Genomic DNA preparation.
Frozen tissues were ground to a powder in a frozen mortar and pestle, and aliquots were removed for genomic DNA preparation. DNA was isolated from tissue samples or cell lines by proteinase K digestion, phenol-chloroform extraction, RNase A digestion, phenol-chloroform extraction, and ethanol precipitation (1). High-molecular-weight DNA was sheared by passage through a 21-gauge needle 10 times, and the DNA concentration was measured at 260 nm.
PCR amplification.
The primers used for nested PCR amplification of proviral c-myc integrations were from the U5 LTR region (L1, 5′-TGATGGCCGGACCGTTGATTCCCTGACGACTA-3′; L2, 5′-TACGAGCACATACATGAAGCAGAAG-3′) at bp +67 to +92 relative to the viral transcription start site (4) and from c-myc exon 2 (M1, 5′-TGGCGAGCTTCTCCGACACCACCTTCTC-3′; M2, 5′-TGCCCCGCTGCTGCGCCGCCAGGTAGAAGT-3′) at bp +5 to +432 relative to the exon 2 5′ border (44). The LTR primer sequences chosen are not conserved in endogenous virus LTRs (21, 45), so that they do not misprime from endogenous viral sequences. Proviral integrations were amplified from genomic DNA in ThermoPol buffer (New England Biolabs) [10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, 0.1% Triton X-100, 20 mM Tris-HCl (pH 8.8)] with 400 μM dATP, 400 μM dTTP, 400 μM dCTP, 320 μM dGTP, 80 μM 7-deaza dGTP (Boehringer Mannheim), and 1 μM each L1 and M1 primers, in a total volume of 50 μl. After denaturation at 98°C for 10 min, 4 U of Taq polymerase (Perkin Elmer-Cetus) and 0.08 U of Vent polymerase (New England Biolabs) were added at 90°C. Thermal cycling was performed for two cycles at 96°C for 2 min and 61.5°C for 5 min and then for 23 cycles at 95°C for 1 min and 61.5°C for 5 min, followed by 1 cycle of 72°C for 10 min. A second round of PCR was carried out under the same conditions with 2 μM each L2 and M2 primers, using 6 μl of a 1:500 dilution of the first-round PCR products.
Primers used for amplification of viral LTR sequences were sense L3 (5′-CGCGGTACCCAGGATATAGTATTTCGC-3′) at bp −295 and antisense L4 (5′-GCGAAGCTTATTGAAGCCTTCTGCTTC-3′); at bp +99 relative to the transcription start site (4). Genomic DNA (0.15 μg) was amplified in Taq buffer (50 mM KCl, 100 μg of gelatin per ml, 10 mM Tris-HCl [pH 8.0]) with 3 mM MgCl2, 80 μM each deoxynucleoside triphosphate, and 1.65 μM each L3 and L4 primers. DNA was denatured at 94°C for 5 min, and then 1 U of Taq was added at 90°C. The PCR profile was 94°C for 1 min, 55°C for 45 s, and 72°C for 45 s for 25 cycles, followed by 72°C for 10 min for 1 cycle.
Proviral c-erbB integrations were amplified with the nested L1 and L2 LTR primers and c-erbB exon 15 primers E1 (5′-CGTGTACAGTTTGGATGGCAGAGC-3′) and E2 (5′-GGCAAACAGCATTGGCATCTGC-3′) from bp +108 to +155 within erb exon 15 relative to the exon 15 5′ border (16, 19). Genomic DNA (2.5 μg) was amplified first with 1 μM each L1 and E1 primers and 400 μM each deoxynucleoside triphosphate in Taq buffer. The mixture was denatured at 94°C 4 min, 4 U of Taq was added, and thermal cycling was carried out at 94°C for 20 s, 60°C for 20 s, and 72°C for 2 min for 25 cycles, followed by 72°C for 10 min for 1 cycle. The reaction mixture was diluted 50-fold, and 2 μl was added for a second round of PCR with the L2 and E2 primers, under the same amplification conditions. In some PCRs, the E3 primer (5′-CAGACCAGGGTATCATTGTC-3′; located at bp +81 was used instead of the E2 primer.
Southern blot hybridization.
The c-myc intron 1 probe used for Southern blot hybridization was prepared by PCR amplification of 0.25 μg of genomic DNA from an uninfected bursa, with 1 μM each M3 (5′-TGTACTAGTTCTCCGTGCTCTCGGCTTG-3′) and M4 (5′-GGCAAGCTTCCTCGAAGTAGAAGTAGGG-3′) primers located from bp −663 to +93 relative to the exon 2 5′ border (44). Nested PCR conditions were the same as for the amplification of proviral c-myc integrations (described above), except that the annealing and extension temperatures were both 62°C and cycling was carried out for 20 cycles. The resulting 772-bp PCR product was purified by agarose gel electrophoresis and glass bead treatment (Qiagen). The 410-bp ALV LTR probe was obtained by KpnI and HindIII restriction of pALV-LUC, a plasmid containing a complete strain RAV-1 LTR (6), followed by gel purification. The 384-bp c-erbB probe was prepared with c-erbB primers E3 and E4 (5′-CTTGACCATCAGGCAGAGG-3′, located at bp −303 relative to the exon 15 5′ border) to amplify 0.5 μg of DNA from uninfected bursa.
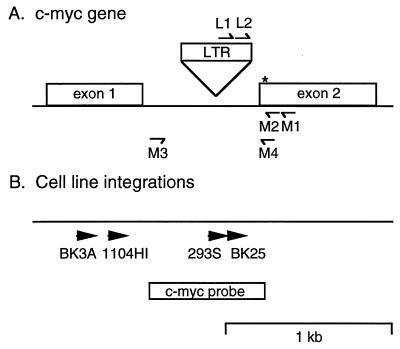
PCR products (5 μl) were separated on 2% agarose gels in Tris-borate buffer and were transferred to nitrocellulose. The probes were labelled by random priming with [32P]dCTP (Boehringer Mannheim) and were hybridized to blots. For PhosphorImager analysis, the blots were scanned and quantitated with ImageQuant software (Molecular Dynamics). The mixtures of cell line DNA carrying defined numbers of proviral c-myc integrations were used as internal standards, assuming that one cell contains about 2.5 pg of genomic DNA (5). These cell lines contain amplified proviral c-myc genes, as determined by Southern blot hybridization. The BK3A cell line contains one copy, 1104HI has two copies, and 293S and BK25 have four copies of proviral c-myc integrations. These factors were taken into account when calculating the number of copies of each integration per microgram of genomic DNA.
DNA sequencing of proviral c-myc integrations.
Amplified proviral c-myc integrations were separated by agarose gel electrophoresis, and the bands migrating at about 400 bp were excised and purified by treatment with glass beads (Qiagen). DNA was made blunt ended by Pfu polymerase treatment and cloned into the PCR Script plasmid, followed by propagation in Epicurean Coli XL1 Blue (Stratagene). Plasmids were purified by cesium chloride gradient ultracentrifugation and sequenced by the dideoxy method (Amersham U.S. Biochemical) with M13 reverse (5′-GGAAACAGCTATGACCATG-3′) and T7 (5′-TAATACGACTCACTATAGGG-3′) primers.
RESULTS
Identification of proviral c-myc gene integrations by PCR amplification.
Integration of proviral sequences within the c-myc gene occurs most often upstream of exon 2 in ALV-induced bursal lymphomas (41, 44). These integrations can be specifically detected by PCR amplification with sense LTR and antisense c-myc exon 2 primers, as illustrated in the map of a typical c-myc gene proviral integration shown in Fig. 1A. This should allow analysis of cells with proviral c-myc integrations at early stages of tumor induction, before they can be detected by Southern blot hybridization (15). U5 LTR sequences and c-myc exon 2 sequences were used to design the L1 and M1 PCR primers for amplification of these proviral c-myc integrations from ALV-infected birds. Nested LTR L2 and c-myc M2 primers were used in a second round of PCR to increase the specificity of amplification. Bursal lymphoma cell lines carrying clonal proviral c-myc integrations were used as test templates to develop specific PCR amplification of mixtures of integrations. The BK3A, 1104HI, 293S, and BK25 cell lines each have sense orientation proviral integrations ranging from 160 to 1,050 bp upstream of c-myc exon 2 (Fig. 1B), as determined by Southern blot analysis (24, 43). The conditions developed for specific amplification of these integrations included the use of mixture of Taq and Vent polymerases, as well as the use of 7-deaza-dGTP in the pool of nucleotide precursors (26), obtain extension through GC-rich regions in c-myc intron 1. These modifications of standard PCR conditions were required to efficiently amplify proviral c-myc integrations (data not shown).
FIG. 1.
Map of proviral c-myc gene integrations. (A) The U5 LTR L1 and L2 and c-myc exon 2 M1 and M2 primers used for nested PCR amplification of proviral c-myc gene integrations are depicted. The asterisk represents the start site of c-myc translation. (B) The sites of proviral c-myc integration in the BK3A, 1104HI, 293S, and BK25 bursal lymphoma cell lines are depicted. The positions of the c-myc probe sequences (amplified with the M3 and M4 primers) used for Southern blot hybridization are also shown.
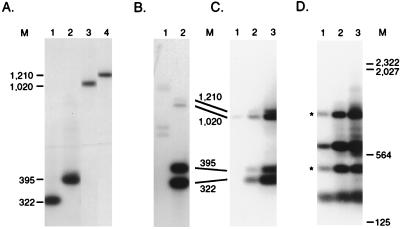
Nested PCR amplification of the BK3A, 1104HI, 293S, and BK25 cell lines each yielded nested PCR products from 322 to 1,210 bp in size (Fig. 2A), in good agreement with the size expected from Southern blot mapping of their integration sites (24, 43). The BK3A and 1104HI cell lines having larger amplification products were analyzed at 500 copies of each integration, while the smaller 293S and BK25 products were tested at 160 copies each, to partially correct for the less efficient amplification of larger targets. These products were detected with a c-myc intron 1 probe that is 5′ of the c-myc PCR primers (Fig. 1B). These integrations were also faithfully amplified from mixtures of the BK3A, 1104HI, 293, and BK25 cell line DNAs (Fig. 2B). The first-round PCR products from the cell line mixture were 327 bp larger than the nested second-round PCR products as expected (Fig. 2B), indicating that each proviral integration is specifically amplified by both of these primer sets. This amplification method is quantitative, since increasing the amount of cell line DNA analyzed (in a constant background of 3 μg of genomic DNA from uninfected bursa) increases the yield of PCR products (Fig. 2C). Titer determinations indicate that these integrations can be detected at low copy numbers in these mixtures (Fig. 2C and data not shown). Taken together, these findings indicate that mixtures of proviral c-myc integrations can be specifically amplified by this PCR assay.
FIG. 2.
PCR amplification of proviral c-myc gene integrations. (A) Genomic DNA from bursal lymphoma cell lines was amplified in a nested PCR assay with sense LTR and antisense c-myc primers and then subjected to Southern blot hybridization with the c-myc intron 1 probe. The BK25 (lane 1) and 293S (lane 2) cell lines were assayed at 160 copies of each proviral c-myc integration, while the 1104 HI (lane 3) and BK3A (lane 4) cell lines were analyzed at 500 copies each. The size of each PCR product is indicated in base pairs. (B) Mixtures of 160 copies or 500 copies of genomic DNA from each of the four cell lines were amplified as for panel A, except that 1 μg of DNA from uninfected chicken bursa was added to each reaction mixture. The first-round PCR products were analyzed in lane 1, and the second-round PCR products were analyzed in lane 2. (C) Mixtures of DNA from the four cell lines were assayed after nested PCR amplification of 1.6 and 5 copies (lane 1), 16 and 50 copies (lane 2), or 160 and 500 copies (lane 3). All the samples were amplified in a background of 3 μg of uninfected genomic DNA. (D) Bursal genomic DNA from an ALV-infected 35-day-old bursa (bird 23) was assayed at 0.5 μg (lane 1), 1 μg (lane 2), or 3 μg (lane 3). DNA from an uninfected bird was added so that the total DNA amount amplified was 3 μg in each lane. The asterisks identify integrations used for analysis of the abundance of cells with these integrations. The migration of HindIII-digested lambda DNA molecular weight marker (M) is indicated in base pairs.
The PCR assay was then used to determine whether proviral c-myc integrations can be detected in bursal DNA from ALV-infected birds. Day 10 line 15I5 × 71 embryos were infected by intravenous injection of RAV-1 strain ALV, a technique which efficiently induces lymphomas (38). Hatched birds were analyzed at 35 days of age, since this is the earliest stage at which the first signs of transformed follicle hyperplasia can be detected (2, 31). Analysis of amplified DNA from one typical ALV-infected 35-day-old bird showed multiple proviral c-myc integrations (Fig. 2D), while products were not observed in DNA from an uninfected 35-day-old sibling (data not shown). These PCR products ranged in size from 200 to 1,130 bp, corresponding to integration sites 38 to 968 bp upstream of c-myc exon 2. The four major PCR products were detected at all DNA concentrations tested, while less abundant products were detected only at higher levels of input DNA.
Phosphorimaging and comparison of the amplification signals from the bursal DNA samples with those of the cell line mixtures having known copy numbers allowed an estimation of the abundance of bursal cells with proviral c-myc integrations. The intensity of the 400- and 1,100-bp bursal integration products (Fig. 2D) was compared with the intensity of the corresponding cell mixture products of similar size (Fig. 2C). These products were estimated to represent about 250 or 350 integrations per μg of input DNA, respectively. Since each cell contains 2.5 pg genomic DNA (5), this would indicate a frequency of 1 proviral integration per 1,150 or 1,600 cells, for each integration event of average abundance. The bursa contains roughly 10,000 follicles (34), so that the expected abundance of cells with each particular integration is 1 in 10,000. The higher than expected detection of each integration event probably reflects hyperproliferation of these cells, since transformed follicles are two to four times larger than normal bursal follicles (10, 12). Overall, cells having proviral c-myc integrations must be abundant, since the four major integration events in this sample would represent roughly 1 of every 350 bursal cells.
Bursal proviral c-myc integrations cluster within c-myc intron 1.
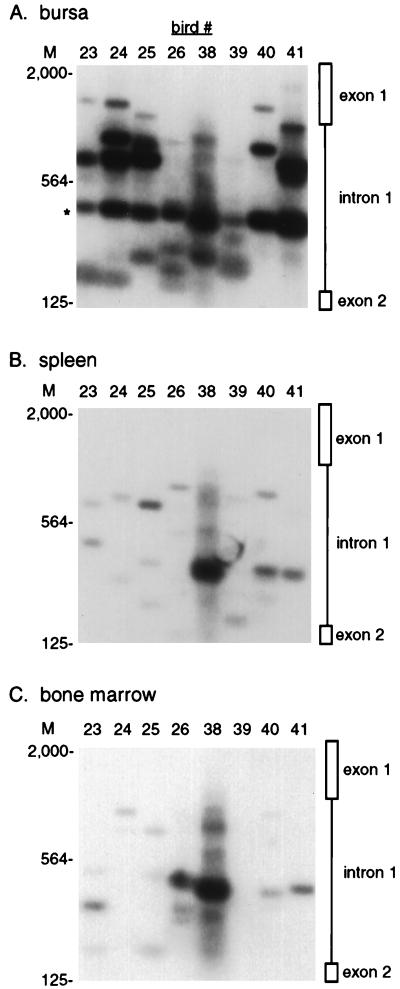
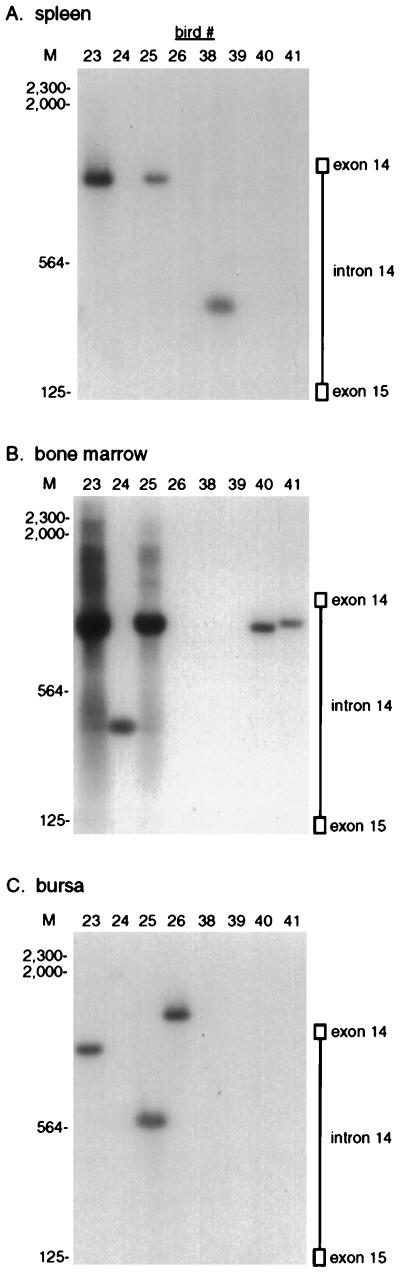
The nested PCR assay was used to analyze proviral c-myc integrations in eight 35-day-old ALV-infected birds, obtained from two separate infection experiments. A 1-μg portion of DNA was analyzed from each sample, since this amount detects the major integration events (Fig. 2D and data not shown). Bursal DNA from each bird shows a distinct pattern of five to eight proviral c-myc integrations (Fig. 3A). This number corresponds well to the number of transformed follicles counted by methyl green pyronine staining of serially sectioned bursal samples (12, 31) from infected siblings (data not shown). These integrations range in size from 200 to 1,500 bp, so that a few integrations lie within exon 1 while the majority lie within intron 1 (Fig. 3A).
FIG. 3.
Proviral c-myc integrations in different tissues from ALV-infected birds. A 1-μg sample of genomic DNA from 35-day-old ALV-infected birds was amplified in the nested PCR assay with LTR and c-myc primers and then subjected to Southern blot hybridization with the c-myc intron 1 probe. The migration of HindIII-digested lambda DNA molecular size marker (M) is indicated in base pairs. The positions of integrations within exon 1 and intron 1 are indicated. Blots were exposed to film for 6 h. (A) Bursal DNA. The asterisk marks the 400-bp integration products that were cloned and sequenced from birds 23 and 25. (B) Spleen. (C) Bone marrow.
Several of these integrations were cloned and sequenced, to determine whether the nested PCR assay amplifies authentic proviral c-myc integrations. The integrations chosen map to the clustered region of integration sites in 3′ intron 1. The products from birds 23 and 25 migrating at about 400 bp (Fig. 3A), were gel purified and cloned, and several of the resulting plasmids were sequenced from each bird. One integration event was identified in bird 23, and two distinct integrations were identified in bird 25. These clones all contained the correct proviral c-myc junction sequences, featuring loss of the terminal U5 LTR CA nucleotides at the proviral integration site (data not shown). The integration sites were situated 237, 235, and 225 bp 5′ of exon 2, as expected from their size on Southern blots (Fig. 3A). These findings confirm that the nested PCR assay specifically and accurately amplifies proviral c-myc integrations.
Cells with proviral c-myc integrations arise in nonbursal tissues.
The PCR assay was used to analyze bone marrow, spleen, and brain DNA samples from the same 35-day-old birds, to determine whether proviral c-myc integrations can arise in tissues that do not give rise to tumors. DNA from spleen (Fig. 3B), bone marrow (Fig. 3C), and brain (see Fig. 6A) were all analyzed in the same experiment with the bursal samples (Fig. 3A), and the same autoradiographic exposures are shown, so that these panels can be directly compared. Proviral c-myc integrations were observed in all of these tissues. The integrations in spleen and bone marrow covered the 1,000-bp region upstream of c-myc exon 2, similar to the range observed in bursal samples (Fig. 3). The average number of integrations detected in the spleen (five per bird) was smaller than that in the bursa (six per bird), while bone marrow contained only about three integrations per bird. Spleen cell integrations are five times less abundant on average than bursal integrations, and bone marrow integrations are 10 times less abundant, as judged by phosphorimaging analysis. A few of the spleen and bone marrow integrations, however, are as abundant as some of the bursal integrations. These findings indicate that proviral integration and deregulation of c-myc gene expression can promote proliferation in nonbursal tissues. Most of the integration sites detected in different tissues from individual birds were of different sizes, indicating that these events arose independently in each tissue. However, a few of these integrations were of similar size in some of the tissues (e.g., the 380-bp band in bursa and bone marrow of bird 38 [Fig. 3]), suggesting that these cells could have been derived from the same original integration event.
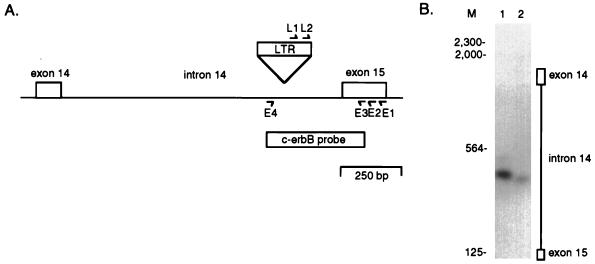
FIG. 6.
PCR amplification of proviral c-erbB gene integrations. (A) Map of the c-erbB gene depicting the U5 LTR and c-erbB exon 15 primers used for nested PCR amplification of proviral c-erbB integrations. The positions of the c-erbB probe sequences (amplified with E3 and E4 primers) used for Southern blot hybridization are shown. (B) Genomic DNA (1 μg) from bird 24 bone marrow was amplified in a nested PCR assay with sense LTR and antisense c-erbB primers and then subjected to Southern blot hybridization with the c-erbB probe. First-round PCR for both samples used primers L1 and E1. The migration of HindIII-digested lambda DNA molecular size marker (M) is indicated in base pairs. The positions of the integrations within intron 14 and exon 14 are indicated. Lanes: 1, second-round PCR with primers L2 and E2; 2, second-round PCR with primers L2 and E3.
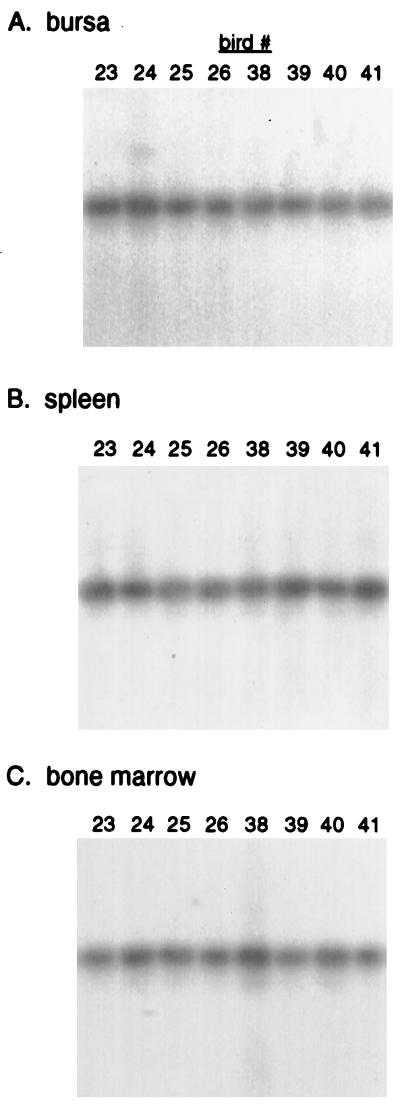
The lower levels of c-myc integrations observed in spleen and bone marrow are not due to differences in viral infection of PCR efficiency. PCR analysis with 5′ and 3′ LTR primers to detect proviral LTR sequences indicates that the bursa, spleen, and bone marrow are equally infected at this age (Fig. 4). Moreover, the glyceraldehyde-3-phosphate dehydrogenase cellular gene was amplified to the same extent from each DNA sample (data not shown), indicating that all samples contained equivalent amounts of amplifiable genomic DNA. These findings indicate that while all of the tissues show similar levels of viral infection, the absolute number and abundance of proviral c-myc integrations is greatest in bursal tissue.
FIG. 4.
Analysis of viral infection in different tissues from ALV-infected birds. Genomic DNA (0.15 μg) from 35-day-old ALV-infected birds was amplified with LTR primers and then subjected to by Southern blot hybridization with LTR sequences. (A) Bursa. (B) Spleen. (C) Bone marrow.
Four brain samples were analyzed to determine whether proviral c-myc integrations can arise in nonhematopoietic tissue. Only one sample showed a single c-myc integration (Fig. 5A). However, the brain showed a high level of viral infection similar to that of the other tissues, as judged by PCR amplification of viral LTR sequences (Fig. 5B). In addition, cellular glyceraldehyde-3-phosphate dehydrogenase sequences were efficiently amplified from brain DNA samples (data not shown). These findings suggest that cells with proviral c-myc integrations arise at a lower frequency in the brain than in hematopoietic tissue or that they fail to proliferate after c-myc gene deregulation. Interestingly, the one proviral c-myc integration observed is nearly as abundant as those in hematopoietic tissues, suggesting that cells with these integrations can proliferate in the central nervous system (CNS). It is interesting that this brain integration is of the same size as the abundant integration observed in the bursa and bone marrow of bird 38. This could represent a target cell capable of proliferating in many tissues, unlike the majority of the integration events, which are restricted to only one tissue at this early stage of tumor induction.
FIG. 5.
Proviral c-myc integration in brain tissue. (A) A 1-μg sample of genomic brain DNA from each of the 35-day-old ALV-infected birds was amplified in the nested PCR assay with LTR and c-myc primers and then subjected to Southern blot hybridization with the c-myc intron 1 probe. The blot was exposed to film for 6 h. The migration of HindIII-digested lambda DNA molecular size marker (M) is indicated in base pairs. (B) Genomic brain DNA (0.15 μg) was amplified with LTR primers and then subjected to Southern blot hybridization with LTR sequences.
Detection of proviral c-erbB integrations.
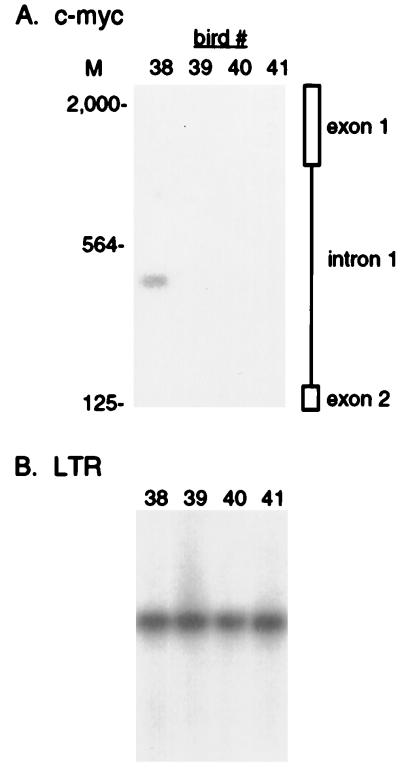
ALV also induces erythroblastosis after proviral integration within the c-erbB proto-oncogene in 5 to 10% of infected line 15I5 × 71 birds (16). The majority of the proviral integrations observed in erythroblastosis tumors map to intron 14 of the c-erbB gene (28, 40). This should allow the detection of proviral c-erbB integrations by nested PCR amplification with LTR L1 and L2 and c-erbB exon 15 E1 and E2 primers, as illustrated in Fig. 6A. Amplification of genomic DNA from the bone marrow of bird 24 shows one 408-bp proviral integration product (Fig. 6B), placing the proviral junction sequence within intron 14 of the c-erbB gene (Fig. 6A). This PCR product is specific, since it hybridizes to a c-erbB probe internal to the PCR primers. As a further control, the first-round PCR products were amplified with a more 5′ E3 primer for the second round of PCR. This produces a PCR product that is 27 bp smaller than the L2-E2 product (Fig. 6B), confirming the specificity of the PCR amplification.
This PCR assay was used to analyze the occurrence of proviral c-erbB integrations in different tissues for comparison with the pattern of proviral c-myc integration. The spleen and bone marrow are the target organs for erythroblastosis, while the bursa and brain are not involved. Three of the eight spleen samples analyzed showed proviral c-erbB integrations (Fig. 7A), and five of the eight bone marrow samples showed integrations (Fig. 7B). The samples containing proviral c-erbB integrations appear to be abundant in both tissues, as judged by the very short autoradiographic exposure required to detect these products. The samples that did not show integrations were negative even after very long autoradiographic exposures (data not shown). The frequency of erythroblastosis is low in this particular chicken strain (16), so that these negative samples may represent birds that are not undergoing erythroblastosis. Interestingly, cells carrying c-erbB integrations also were found in three of the eight bursal samples (Fig. 7C). The majority of the c-erbB integrations mapped to different sites in each tissue, indicating that these integrations arose independently within each compartment. Bursal cells with c-erbB integrations were as abundant as those of the spleen and bone marrow, even though the bursa is not involved in erythroblastosis. This indicates that the tissue-specific induction of erythroblastosis in bone marrow and spleen occurs at a step after the initial proliferation of cells carrying c-erbB integrations. Analysis of the four brain samples did not identify any c-erbB integrations (data not shown), suggesting either that c-erbB gene integrations do not arise in the brain or that abnormal c-erbB gene expression is negatively selected in this tissue.
FIG. 7.
Proviral c-erbB integrations in different tissues from ALV-infected birds. Genomic DNA (2.5 μg) from 35-day-old ALV-infected birds was amplified in the nested PCR assay with LTR L1 and L2 and c-erbB E1 and E2 primers and then subjected to Southern blot hybridization with the c-erbB intron 14 probe. The migration of molecular size markers (M) is shown in base pairs. The blots were exposed to film for 25 min. The migration of HindIII-digested lambda DNA molecular size marker (M) is indicated in base pairs. (A) Spleen. (B) Bone marrow. (C) Bursa.
In most of the positive samples, only one or two c-erbB integration events were observed in each tissue (Fig. 7). However, two of the bone marrow samples (from birds 23 and 25 [Fig. 7C]) showed one abundant integration of at about 1,000 bp in 5′ intron 14 and also multiple minor bands ranging from 100 to more than 3,000 bp in size, which would map from exon 12 through exon 15. These PCR products appear to be specific, since they were also amplified with the E3 primer (data not shown). These minor products could potentially arise from the transduction of c-erbB sequences within recombinant viruses, which arise in about half of erythroblastosis tumors (28). Retroviral recombination and further infection could generate proviral integrations with c-erbB exon 15 sequences at different positions relative to the 5′ LTR. It is possible that the tissue samples showing this phenomenon harbored initial c-erbB integrations that favor the production of readthrough proviral c-erbB transcripts, so that the frequency of c-erbB transduction and integration is increased.
The pattern of proviral c-erbB integration sites observed in infected tissues from 35-day-old birds is different from that observed in erythroblastosis tumors. The majority of erythroblastosis tumors from line 15I chickens show clustered integrations within a 300-bp 3′ region of intron 14, while 5′ intron 14 or exon 15 integrations are observed less commonly (28, 40). In contrast, the integrations from infected tissues of line 15I5 × 71 35-day-old birds show no apparent clustering within 3′ intron 14 (Fig. 7), since only 2 of the 11 integrations detected are 100 to 400 bp in size. Instead, the PCR products range in size from 300 to 2500 bp, mapping at different sites from exon 12 through intron 14 (8). These findings indicate that the 3′ region of c-erbB intron 14 is not a preferred site of proviral integration. Instead, cells having these integrations must be selected during the induction of erythroblastosis.
DISCUSSION
A nested PCR assay which specifically amplifies proviral c-myc integrations from ALV-infected birds at early stages of tumor induction, before these integrations can be detected by Southern blot hybridization, was developed. Amplification of bursal samples reveals five to eight proviral c-myc integrations per bird. This number corresponds well to the number of transformed follicles counted in infected siblings, indicating that this PCR assay detects the majority of distinct proviral c-myc integration events that result in transformation of bursal follicles. Comparison of bursal integrations with cell lines having known numbers of integrations allowed an estimation of the abundance of bursal cells with proviral c-myc integrations. The estimate of roughly 1 in 1,150 to 1 in 1,600 bursal cells carrying each distinct integration was surprisingly high relative to the expected frequency of 1 transformed follicle per 10,000 normal follicles. This suggests that cells with c-myc integrations hyperproliferate even faster than the expanding population of normal bursal cells, as supported by the increased size of transformed follicles (10, 12). Normal bursal follicles contain about 2 × 105 cells at this age (50), indicating that cells with c-myc integrations must expand rapidly within the weeks after infection.
Cells with proviral c-myc integrations are also common in tissues that do not support the development of lymphomas. This suggests that integration and c-myc gene deregulation can support proliferation without leading to tumor induction. However, spleen and bone marrow cells with c-myc integrations are much less abundant than in the bursa. These tissues also show a reduced number of distinct integration events. All of the tissues show equal levels of viral infection, indicating that cells with proviral c-myc integrations arise less often in nonbursal tissues and/or that these cells are less likely to proliferate after integration and c-myc gene deregulation. The bursal environment is required for induction of lymphoma, since surgical or chemical ablation of the bursa prevents ALV lymphomagenesis (37, 39). Growth factors within the bursa could support high levels of c-myc-induced proliferation or could prevent the apoptosis that can occur when c-myc is expressed at high levels (11, 51).
Proviral c-myc integration in the brain was much less common than in hematopoietic tissues. The single integration event observed was as abundant as some of the hematopoietic integrations, indicating that the cells harboring this integration can proliferate within the brain. ALV causes neurological dysfunction in 20% of infected birds, involving CNS inflammation and infiltration of lymphoid and myelomonocytic cells (13). These abnormalities could involve cells with proviral integrations next to c-myc or other proto-oncogenes that can activate abnormal cell growth. Interestingly, the CNS integration observed is a similar size to a major integration found in the bursa and bone marrow. This integration could have arisen in a cell type capable of migrating and proliferating in many tissues, unlike the majority of the integration events, which are restricted to only one tissue compartment at this early stage. This could also represent a cell in the initial stages of metastasis, although extrabursal tumors are not visibly detected until after 4 months of age (31).
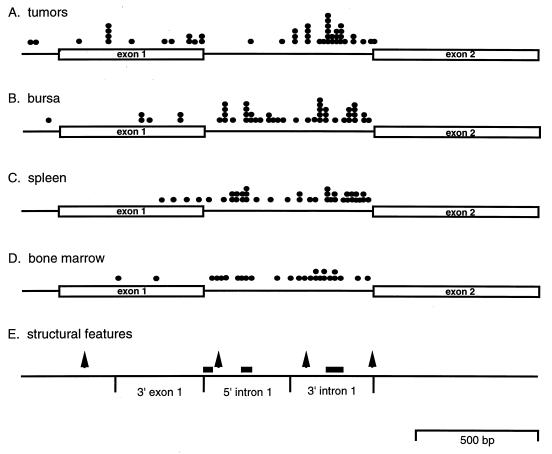
Shih et al. (44) and Robinson and Gagnon (41) previously analyzed the sites of proviral c-myc integration in a large number of ALV bursal lymphomas and metastases. While some tumor integrations map within noncoding exon 1, the majority of these integrations map within the 3′ half of c-myc intron 1, as illustrated in Fig. 8A. This clustering of integrations could reflect an intrinsic preference for proviral integration within this region, or cells with these integrations could be selected during tumor induction. These possibilities can be distinguished by comparing proviral c-myc integration sites in tumors with those we identified in infected tissues that are not undergoing tumor induction.
FIG. 8.
Map of proviral c-myc integration sites in tumors and in infected tissues. (A) The sites of proviral c-myc integration in ALV-induced tumors are summarized from Shih et al. (44) and Robinson and Gagnon (41). (B) Integration sites in infected bursa, mapped with data from Fig. 3A. (C) Integration sites in infected spleen tissues, with data from Fig. 3B. (D) Integration sites in infected bone marrow, with data from Fig. 3C. (E) Structural features of the c-myc gene. Arrows represent DNase I-hypersensitive sites. Bars represent AT-rich sequences. The 350-bp 3′ exon 1, 5′ intron 1, and 3′ intron 1 regions used to analyze integration frequencies in Table 1 are outlined.
The proviral integration sites mapped by PCR assays of infected bursa, spleen, and bone marrow were plotted according to their position within the c-myc gene (Fig. 8). These integrations lie within exon 1 and intron 1, with the majority of the sites clustering within 3′ intron 1. The distribution of these proviral c-myc integrations was further analyzed by tabulating the number of integrations found in 3′ c-myc exon 1, 5′ intron 1, or 3′ intron 1. Each region is approximately 350 bp long. Approximately half of the integrations from tumors or from tissues of infected birds cluster within 3′ intron 1 (Table 1). Interestingly, 35 to 40% of the integrations from infected tissues map to the 5′ region of intron 1, while these sites are involved in only 4% of tumors. Swift et al. (46) proposed that integration within the 5′ half of intron 1 may not be favorable for c-myc gene transcription or translation. However, cells with integrations in 5′ or 3′ intron 1 proliferate to a similar extent in infected tissues as judged by their abundance in PCR assays (Fig. 3), indicating that integrations in either region support the expansion of these cell populations. Thus, cells having 5′ intron 1 integrations appear to be selected against during tumor progression. These findings also indicate that the 3′ region of intron 1 is a preferential integration target whether or not that tissue supports tumor induction. This region is also targeted by reticuloendotheliosis virus, which can induce bursal lymphomas after integration within the c-myc gene (46).
TABLE 1.
Proviral integration within different regions of the c-myc gene
| Source | % of integrations found ina:
|
||
|---|---|---|---|
| 3′ exon 1 | 5′ intron 1 | 3′ intron 1 | |
| Tumors | 17 | 4 | 62 |
| Bursa | 10 | 40 | 48 |
| Spleen | 10 | 36 | 54 |
| Bone marrow | 8 | 35 | 58 |
The 3′ region of c-myc intron 1 features sequence motifs that could be involved in targeting proviral integration to this region. These motifs include DNase I-hypersensitive sites (43) and an AT-rich region (Fig. 8E). The location of the DNase I-hypersensitive sites does not correlate particularly strongly with preferred integration sites in the 3′ half of intron 1. Additional hypersensitive sites found in 5′ intron 1 and exon 1 are also not preferred integration sites (Fig. 8), suggesting that DNase I-hypersensitive sites are probably not sufficient to target proviral integration to 3′ intron 1. A 67-bp AT-rich stretch occurs within the 3′ half of intron 1; however, shorter AT stretches that are not preferential integration sites occur upstream (Fig. 8E). It is possible that more than one of these motifs work cooperatively to attract proviral integration to the 3′ intron 1 region. This region could also attract integration due to other features such as matrix-associated regions, which appear to be common sites of proviral integration (22, 27).
The pattern of proviral c-erbB integration differs from that of c-myc integration in several ways. A smaller absolute number of c-erbB integration events are observed, suggesting that the c-erbB gene is a less common integration target or that cells that can support altered c-erbB gene expression are less common than cells that tolerate deregulated c-myc expression. Many of the tissues fail to show c-erbB integrations at all, in contrast to the ubiquitous appearance of c-myc integrations. This probably reflects the lower incidence of erythroblastosis (5 to 10%) relative to lymphomagenesis (70 to 100%) in this chicken strain (16). The tissue distribution of c-myc and c-erbB integrations is also quite different. Cells with c-erbB integrations occur with roughly equal abundance in bursa, bone marrow, and spleen, indicating that cells with c-erbB integrations can proliferate equally in tumor target and nontarget tissues. In contrast, cells with proviral c-myc integrations are preferentially expanded within the bursal environment.
The 3′ region of c-myc intron 1 is a preferred integration target whether or not that tissue supports tumor induction. However, c-erbB integrations are randomly distributed upstream of exon 15 in infected tissues, while tumors show a strong preference for integrations within the 3′ region of c-erbB intron 14, indicating that there must be selection for 3′ intron 14 c-erbB integrations during induction of erythroblastosis. This could reflect a requirement for correct mRNA splicing to produce an in-frame gag-erbB fusion protein with constitutive kinase activity, which can promote the transformation of erythroblasts within the spleen or bone marrow (33). It is also possible that the low incidence of erythroblastosis in line 15I5 × 71 chickens is due to an altered c-erbB intron 14 conformation that is somehow less susceptible to proviral integration than that of the parental line 15I strain, which shows a high incidence of erythroblastosis tumors with 3′ intron 14 integrations.
These studies reveal that cells with c-erbB and c-myc proto-oncogene integrations are abundant in many tissues at an early stage after ALV infection, whether or not these tissues are targets for tumor induction by either oncogene. Nason-Burchenal and Wolff (30) also found that proviral gag-myb transcripts arise in many tissues after murine leukemia virus infection of mice, even though the tissues containing proviral c-myb gene integrations do not necessarily give rise to tumors (3). There is no information available on the biological effects of these widespread integrations in tissues that do not give rise to tumors. However, it is possible that abnormal LTR-driven proto-oncogene expression in different tissues could contribute to the anemia, stunted growth, and other symptoms often observed in ALV-infected birds (reviewed in reference 36). Cells with proto-oncogene integrations in the CNS could also potentially contribute to the neurological dysfunction and inflammation observed after ALV infection (13). Similar phenomena are likely to occur in infections involving mammalian retroviruses and lentiviruses and could account for some of the systemic and neurologic sequelae of these infections.
ACKNOWLEDGMENTS
We thank Maxine Linial and Paul Neiman (Fred Hutchinson Cancer Research Center) for their helpful comments on the manuscript. We thank Larry Bacon, Ali Fadly, and Laura Parks (USDA Avian Disease and Oncology Laboratory, Lansing, Mich.) for providing eggs and advice, and we thank Sue Atwood, Lynn Bergmeyer, and Tom Cummins (Johnson & Johnson, Rochester, N.Y.) for their PCR advice.
This work was supported by NIH grant CA68328 to A. Ruddell.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Baba T W, Humphries E H. Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci USA. 1985;82:213–216. doi: 10.1073/pnas.82.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belli B, Wolff L, Nazarov V, Fan H. Proviral activation of the c-myc proto-oncogene is detectable in preleukemic mice infected neonatally with Moloney murine leukemia virus but not in resulting end-stage T lymphomas. J Virol. 1995;69:5138–5141. doi: 10.1128/jvi.69.8.5138-5141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizub D, Katz R A, Skalka A M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984;49:557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom S E, Delany M E, Muscarella D E. Constant and variable features of avian chromosomes. In: Etches R J, Gibbons A M V, editors. Manipulation of the avian genome. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 39–60. [Google Scholar]
- 6.Bowers W J, Baglia L A, Ruddell A. Regulation of avian leukosis virus long terminal repeat-enhanced transcription by C/EBP-Rel interactions. J Virol. 1996;70:3051–3059. doi: 10.1128/jvi.70.5.3051-3059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burmester B R, Fontes A K, Walter W G. Pathogenicity of a viral strain (RPL12) causing avian visceral lymphomatosis and related neoplasms. III. Influence of host age and route of inoculation. J Natl Cancer Inst. 1960;42:1423–1442. [PubMed] [Google Scholar]
- 8.Callaghan T, Antczak M, Flickinger T, Raines M, Myers M, Kung H J. A complete description of the EGF-receptor exon structure: implication in oncogenic activation and domain evolution. Oncogene. 1993;8:2939–2948. [PubMed] [Google Scholar]
- 9.Clurman B E, Hayward W S. Multiple proto-oncogene activations in avian leukosis virus-induced lymphomas: evidence for stage-specific events. Mol Cell Biol. 1989;9:2657–2664. doi: 10.1128/mcb.9.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper M D, Payne L N, Dent P B, Burmester B R, Good R A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968;41:373–378. [PubMed] [Google Scholar]
- 11.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 12.Ewert D L, deBoer G F. Avian lymphoid leukosis: mechanisms of lymphomagenesis. Adv Vet Sci Comp Med. 1988;32:37–55. doi: 10.1016/b978-0-12-039232-2.50006-2. [DOI] [PubMed] [Google Scholar]
- 13.Ewert D L, Steiner I, DuHadaway J. In ovo infection with the avian retrovirus RAV-1 leads to persistent infection of the central nervous system. Lab Invest. 1990;62:156–162. [PubMed] [Google Scholar]
- 14.Fadly A M, Witter R L. Resistance of line 6(3) chickens to reticuloendotheliosis-virus-induced bursa-associated lymphomas. Int J Cancer. 1986;38:139–43. doi: 10.1002/ijc.2910380122. [DOI] [PubMed] [Google Scholar]
- 15.Fung Y K, Fadly A M, Crittenden L B, Kung H J. Avian lymphoid leukosis virus infection and DNA integration in the preleukotic bursal tissues: a comparative study of susceptible and resistant lines. Virology. 1982;119:411–421. doi: 10.1016/0042-6822(82)90100-3. [DOI] [PubMed] [Google Scholar]
- 16.Fung Y K, Lewis W G, Crittenden L B, Kung H J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983;33:357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- 17.Hayward W S, Neel B G, Astrin S M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981;290:475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- 18.Henriksson M, Luscher B. Proteins of the myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:110–182. doi: 10.1016/s0065-230x(08)60353-x. [DOI] [PubMed] [Google Scholar]
- 19.Henry C, Coquillaud M, Saule S, Stehelin D, Debuire B. The four C-terminal amino acids of the v-erbA polypeptide are encoded by an intronic sequence of the v-erbB oncogene. Virology. 1985;140:179–182. doi: 10.1016/0042-6822(85)90457-x. [DOI] [PubMed] [Google Scholar]
- 20.Hihara H, Shimizu T, Yamamoto H. Establishment of tumor cell lines cultured from chickens with avian lymphoid leukosis. Natl Inst Anim Health Q. 1974;14:163–173. [PubMed] [Google Scholar]
- 21.Hughes S H. Sequence of the long terminal repeat and adjacent segments of the endogenous avian virus Rous-associated virus 0. J Virol. 1982;43:191–200. doi: 10.1128/jvi.43.1.191-200.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitamura Y, Lee Y M, Coffin J M. Nonrandom integration of retroviral DNA in vitro: effect of CpG methylation. Proc Natl Acad Sci USA. 1992;89:5532–5536. doi: 10.1073/pnas.89.12.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kung H J, Boerkel C, Carter T H. Retroviral mutagenesis of cellular oncogenes: a review with insights into the mechanisms of insertional activation. Curr Top Microbiol Immunol. 1991;171:1–25. doi: 10.1007/978-3-642-76524-7_1. [DOI] [PubMed] [Google Scholar]
- 24.Linial M, Groudine M. Transcription of three c-myc exons is enhanced in chicken bursal lymphoma cell lines. Proc Natl Acad Sci USA. 1985;82:53–57. doi: 10.1073/pnas.82.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linial M, Gunderson N, Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985;230:1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- 26.McConlogue L, Brow M A, Innis M A. Structure-independent DNA amplification by PCR using 7-deaza-2′-deoxyguanosine. Nucleic Acids Res. 1988;16:9869. doi: 10.1093/nar/16.20.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mielke C, Maass K, Tummler M, Bode J. Anatomy of highly expressing chromosomal sites targeted by retroviral vectors. Biochemistry. 1996;35:2239–2252. doi: 10.1021/bi952393y. [DOI] [PubMed] [Google Scholar]
- 28.Miles B D, Robinson H L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985;54:295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan J H, Parsons J T. Characterization of c-myc proteins from avian bursal lymphoma cell lines. Virology. 1986;150:178–186. doi: 10.1016/0042-6822(86)90277-1. [DOI] [PubMed] [Google Scholar]
- 30.Nason-Burchenal K, Wolff L. Activation of c-myb is an early bone-marrow event in a murine model for acute promonocytic leukemia. Proc Natl Acad Sci USA. 1993;90:1619–1623. doi: 10.1073/pnas.90.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neiman P E, Jordan L, Weiss R A, Payne L N. Malignant lymphomas of the bursa of fabricus: analysis of early transformation. In: Essex M, et al., editors. Viruses in naturally occurring cancers. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. pp. 519–528. [Google Scholar]
- 32.Neiman P E, Summers J, Thomas S J, Xuereb S, Loring G. Genetic instability and apoptotic cell death during neoplastic progression of v-myc-initiated B-cell lymphomas in the bursa of Fabricius. Cold Spring Harbor Symp Quant Biol. 1994;59:509–515. doi: 10.1101/sqb.1994.059.01.056. [DOI] [PubMed] [Google Scholar]
- 33.Nilsen T W, Maroney P A, Goodwin R G, Rottman F M, Crittenden L B, Raines M A, Kung H J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985;41:719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- 34.Olah I, Glick B. The number and size of the follicular epithelium (FE) and follicles in the bursa of Fabricius. Poult Sci. 1978;57:1445–50. doi: 10.3382/ps.0571445. [DOI] [PubMed] [Google Scholar]
- 35.Partanen A M. Epidermal growth factor and transforming growth factor-alpha in the development of epithelial-mesenchymal organs of the mouse. Curr Top Dev Biol. 1990;24:31–55. [PubMed] [Google Scholar]
- 36.Payne L N. Biology of avian retroviruses. In: Levy J, editor. The Retroviridae. Vol. 1. New York, N.Y: Plenum Press; 1992. pp. 299–404. [Google Scholar]
- 37.Peterson R D A, Burmester B R, Fredrickson T N, Purchase H G, Good R A. Relationships among visceral lymphomatosis, bursa of Fabricius, and bursa-dependent lymphoid tissue of the chicken. J Natl Cancer Inst. 1966;36:585–598. doi: 10.1093/jnci/36.4.585. [DOI] [PubMed] [Google Scholar]
- 38.Pink J R L, Jotereau F, Houssaint E, Weber W T. Avian embryos in immunology. In: Lefkovits I, Pernis B, editors. Immunological methods. III. Orlando, Fla: Harcourt Brace Jovanovich; 1985. pp. 385–402. [Google Scholar]
- 39.Purchase H G, Gilmour P G, Romero C H, Okazaki W. Post-infection genetic resistance to avian lymphoid leukosis resides in B target cells. Nature. 1979;270:61–62. doi: 10.1038/270061a0. [DOI] [PubMed] [Google Scholar]
- 40.Raines M A, Lewis W G, Crittenden L B, Kung H J. c-erbB activation in avian leukosis virus-induced erythroblastosis: clustered integration sites and the arrangement of provirus in the c-erbB alleles. Proc Natl Acad Sci USA. 1985;82:2287–2291. doi: 10.1073/pnas.82.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson H L, Gagnon G C. Patterns of proviral insertion and deletion in avian leukosis virus-induced lymphomas. J Virol. 1986;57:28–36. doi: 10.1128/jvi.57.1.28-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruddell A, Linial M, Schubach W, Groudine M. Lability of leukosis virus enhancer-binding proteins in avian hematopoietic cells. J Virol. 1988;62:2728–2735. doi: 10.1128/jvi.62.8.2728-2735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schubach W, Groudine M. Alteration of c-myc chromatin structure by avian leukosis virus integration. Nature. 1984;307:702–708. doi: 10.1038/307702a0. [DOI] [PubMed] [Google Scholar]
- 44.Shih C K, Linial M, Goodenow M M, Hayward W S. Nucleotide sequence 5′ of the chicken c-myc coding region: localization of a noncoding exon that is absent from myc transcripts in most avian leukosis virus-induced lymphomas. Proc Natl Acad Sci USA. 1984;81:4697–4701. doi: 10.1073/pnas.81.15.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith E J, Bizub D, Scholl D R, Skalka A M. Characterization of a solitary long terminal repeat of avian endogenous virus origin. Virology. 1984;134:493–496. doi: 10.1016/0042-6822(84)90319-2. [DOI] [PubMed] [Google Scholar]
- 46.Swift R A, Shaller E, Witter R L, Kung H J. Insertional activation of c-myc by reticuloendotheliosis virus in chicken B lymphoma: nonrandom distribution and orientation of the proviruses. J Virol. 1985;54:869–872. doi: 10.1128/jvi.54.3.869-872.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tam W, Ben-Yehuda D, Hayward W S. bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA. Mol Cell Biol. 1997;17:1490–1502. doi: 10.1128/mcb.17.3.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varmus H E. Form and function of retroviral proviruses. Science. 1982;216:812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- 49.Weill J-C, Reynaud C-A. The chicken B cell compartment. Science. 1987;238:1094–1098. doi: 10.1126/science.3317827. [DOI] [PubMed] [Google Scholar]
- 50.Weill J C, Reynaud C A, Lassila O, Pink J R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci USA. 1986;83:3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wurm F M, Gwinn K A, Kingston R E. Inducible overproduction of the mouse c-myc protein in mammalian cells. Proc Natl Acad Sci USA. 1986;83:5414–5418. doi: 10.1073/pnas.83.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]