Abstract
Alpha-fetoprotein (AFP) is a glycoprotein that plays an important role in immune regulation with critical involvement in early human development and maintaining the immune balance during pregnancy. Postfetal development, the regulatory mechanisms controlling AFP undergo a shift and AFP gene transcription is suppressed. Instead, these enhancers refocus their activity to maintain albumin gene transcription throughout adulthood. During the postnatal period, AFP expression can increase in the setting of hepatocyte injury, regeneration, and malignant transformation. It is the first oncoprotein discovered and is routinely used as part of a screening strategy for HCC. AFP has been shown to be a powerful prognostic biomarker, and multiple HCC prognosis models confirmed the independent prognostic utility of AFP. AFP is also a useful predictive biomarker for monitoring the treatment response of HCC. In addition to its role as a biomarker, AFP plays important roles in immune modulation to promote tumorigenesis and thus has been investigated as a therapeutic target in HCC. In this review article, we aim to provide an overview of AFP, encompassing the discovery, biological role, and utility as an HCC biomarker in combination with other biomarkers and how it impacts clinical practice and future direction.
INTRODUCTION
Alpha-fetoprotein (AFP) is a glycoprotein with an immunoregulatory role during fetal development. It is a member of the albumin-like protein family, which also encompasses other proteins like human serum albumin and vitamin D–binding protein. 1 In adults, although expressed in minimal levels, elevated levels of AFP can be indicative of certain pathological conditions involving active liver regeneration, hepatitis, or cancer. AFP is secreted by HCC and other aggressive tumor phenotypes such as germ cell tumors and hepatoblastoma. AFP synthesis within tumors is more common among tumors characterized by large size, poor differentiation, and vascular invasion. Consequently, AFP levels convey essential prognostic information among patients with HCC. In this review, we describe the physiological and pathological functions of AFP and its diagnostic, prognostic, and predictive role in HCC. We also review its utility as an HCC biomarker in combination with other biomarkers and future directions.
BIOCHEMICAL STRUCTURE OF AFP
The initial isolation of AFP was made possible through immunochemical methods, a milestone achieved by Nishi. 2 With a molecular mass of ~70 kDa, its structure is composed of a single polypeptide chain that consists of 590 amino acid residues and is stabilized by a total of 15 disulfide (S-S) bridges. 3 This molecular architecture confers a high degree of conformational stability to AFP. Specifically, it retains its rigid native structure under a wide range of environmental conditions, such as a pH range between 4.5 and 10.5, temperatures below 70°C, urea concentrations below 7.5 M, and guanidinium hydrochloride concentrations below 2.0 M.4,5,6,7 It is noteworthy that this stability is particularly advantageous in clinical settings, where samples may be exposed to varying conditions during transport or storage, thus preserving the integrity of AFP as a biomarker.
The amino acid sequence of AFP is divided into 3 distinct domains, referred to as domains I, II, and III. Each of these domains is comprised of around 195 amino acid residues. Domains I, II, and III have 4, 5, and 6 putative S-S bonds, respectively. These domains are highly conserved in humans, rats, mice, and bovines, implying a preserved functional role of AFP across the different species.3,8 The sequence alignment has revealed about 28% homology between the domains of human AFP and serum albumin. Despite this similarity, significant differences exist between domains I, II, and III of AFP and serum albumin, suggesting that they possess unique functionalities, such as the fatty acid–binding capacity specific to AFP. It has been hypothesized that these structural differences could be key to unlocking further diagnostic or therapeutic uses for AFP, particularly if these distinct domains interact differently with other molecules in biological systems. 9
AFP exists in 3 major isoforms, which differ in their affinity for the lectin Lens culinaris agglutinin. These isoforms are known as AFP-L1, AFP-L2, and AFP-L3, and they are present in varying amounts under different pathological conditions. 10 Emerging research also indicates that these isoforms could provide additional diagnostic and prognostic information in HCC.11,12 The ratio of these isoforms has been found to be a predictor for malignancy, thereby adding another layer of clinical utility to AFP as a diagnostic biomarker in HCC.13,14
AFP DURING DEVELOPMENT AND PREGNANCY
AFP was first identified in the serum of human fetuses <5 months old, reflecting its critical involvement in early human development.15,16 Subsequent research using immunohistochemical and immunofluorescence techniques pinpointed the liver parenchyma as the exclusive site for AFP synthesis in the fetus. 17 Specifically, around the 12th week of gestation, as the yolk sack degenerates, the fetal liver takes over as the primary source of AFP production.
Research from the late 1980s has shown that both the absolute size of the fetus and its gestational age significantly influence AFP concentrations in both maternal and fetal circulations. Moreover, a strong correlation was observed between maternal AFP levels and those found in the cord arterial and venous blood, emphasizing the interrelatedness of these measures during pregnancy. 18 Maternal AFP levels can serve as key markers of fetal abnormalities, including neural tube defects and Down syndrome. 19 Currently, AFP is used as part of prenatal screening tests.
AFP has a compelling role in immunomodulation, specifically in maintaining the immune balance during pregnancy. Its primary function was initially understood to prevent fetal rejection by suppressing maternal immune responses. The first indirect evidence supporting AFP’s immunosuppressive characteristics came from studies where anti-AFP antibodies were infused into pregnant rabbits, leading to fetal rejection. 20 Further supporting this idea, experiments demonstrated that serum from pregnant mice inhibited antibody synthesis in vitro, an effect that was dependent on native AFP (nAFP). 21 However, it is important to note that nAFP may not be the sole contributor to suppressed immunity in pregnancy. For instance, studies have shown that pregnant mice exhibit diminished immune responses to vaccinations, but this phenomenon is not solely attributable to nAFP.
Following birth, the regulatory mechanisms controlling AFP undergo a shift. AFP enhancers, which promote AFP gene transcription during the fetal stage, are typically inhibited from the gene promoter postnatally. Instead, these enhancers refocus their activity to maintain albumin gene transcription throughout adulthood. 22 Subsequent studies also highlighted the potential role of AFP in stem cell research and regenerative medicine, given its early presence and role in developmental stages. In a model of partial hepatectomy, AFP was overexpressed acutely for 5 days in proliferated hepatocytes. 23 AFP overexpression was also noted in hepatocytes undergoing mitosis following galactosamine-induced live injury. 23
ROLE OF AFP IN IMMUNE MODULATION, TUMORIGENESIS, AND THERAPEUTIC TARGET
Role of AFP in antitumoral immunomodulation
In the field of oncology, tumor-derived AFP (tAFP) has broad immunosuppressive effects on multiple cell types, including natural killer cells, T cells, and dendritic cells (DCs), significantly contributing to the pathogenesis of HCC by impacting various immune cells. 24 A landmark study showed that while the nAFP and tAFP isoforms share nearly identical structures except for a single carbohydrate group, tAFP at physiological concentrations had a notable inhibitory effect on DC differentiation. This is not observed when using nAFP at physiological concentrations. 25 The authors showed that tAFP’s ability to inhibit DC differentiation and function depends on low molecular mass substances.
Another potential mechanism of the immunosuppressive effect lies in the modulation of the regulatory suppressor cells. An early study documented AFP’s capacity to suppress certain T-cell–dependent immune reactions in both mouse and human in vitro settings. 26 A subsequent study showed AFP did not directly act on T cells but monocytes by suppressing their inflammatory processes. 27 The increase in production of prostaglandin E2, a potent immunoregulatory agent and the reduced secretion of inflammatory cytokines such as TNFα and IL-1β had led to a shift in CD4+ T-cell differentiation toward suppressive regulatory T cells with impaired T-cell–stimulatory capacity.28,29 In addition, AFP derived from hepatoma cells was shown to trigger polarization of M0 macrophage into M2-like phenotype through the PI3K/Akt pathway and therefore prevent phagocytosis. 30 AFP also hampers the proliferation of NK cells and T lymphocytes in addition to fostering the polarization of macrophages toward an M2-like phenotype, aiding in liver cancer immune escape.30,31 In addition, the interaction of AFP with lipid elements, such as polyunsaturated fatty acids, which are known to alter cellular metabolism,32,33 can influence the characteristics, functionality, and metabolic pathway engagement of DCs and natural killer cells. 34
Single-cell RNA sequencing combined with multiomics analysis has revealed that AFP-positive HCC is associated with a more suppressive microenvironment, likely mediated by the SPP1-CD44 axis. 35 A positive correlation has been observed between serum AFP levels and the percentage of CD4+CD25High+FOXP3+ regulatory T cells in patients with HCC. 36
Functional role of AFP in HCC tumorigenesis
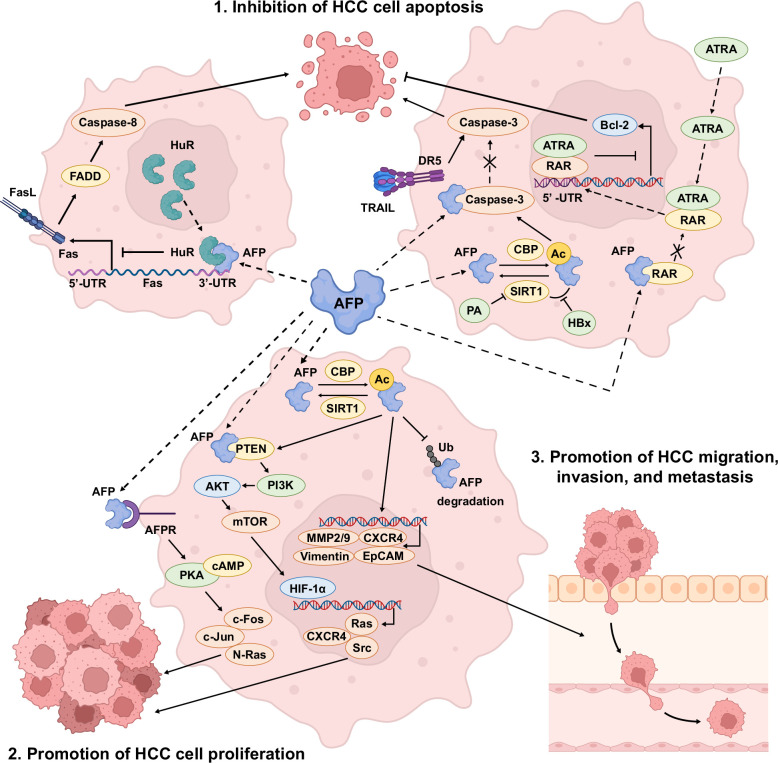
AFP has been reported to influence the ability of cancer cells to maintain and propagate their undifferentiated state. Liver tissue derived from HBV-related HCC was found to show higher levels of reprogramming proteins and stemness markers including pAKT(Ser473), Oct4, Klf4, Sox2, and c-myc compared to noncancerous liver tissues or non-HBV–related HCC that were negative for AFP. 37 In addition, several studies have shown the roles of AFP in tumor progression through inhibition of apoptosis, promotion of tumor proliferation, and promotion of tumor migration, invasion, and metastasis (Figure 1). The caspase-3 signal cascade is one of the pathways that AFP involves inhibiting HCC apoptosis. Lin et al 38 reported that AFP directly interacts with the caspase-3 active site to prevent its activation by tumor necrosis factor–related apoptosis-inducing ligand. Another study also demonstrated that AFP may regulate the p53/Bax/cytochrome c/caspase-3 pathway to inhibit cell apoptosis. 39 Recently, AFP has been identified to suppress the human antigen R–mediated Fas/Fas-associated death domain extrinsic apoptotic pathway. Interaction between AFP and human antigen R leads to the relocation of human antigen R from the nucleus to the cytoplasm, which inhibits Fas translation and subsequent suppression of Fas/Fas-associated death domain–mediated cell apoptosis. 40 Lastly, AFP was found to interfere with the all-trans retinoic acid–retinoic acid receptor (RAR) signaling pathway.41,42 Through binding to RAR, AFP competitively decreases the possibility of all-trans retinoic acid binding to RAR and inhibits its entrance into the nucleus. As such, less RAR binds to the 5ʹ-untranslated region of the Bcl-2 gene, thereby increasing the expression of Bcl-2, the antiapoptotic protein. 42
FIGURE 1.
Biological pathways of AFP involvement in the inhibition of HCC cell apoptosis, promotion of HCC cell proliferation, and promotion of HCC migration, invasion, and metastasis. Abbreviations: Ac, acetylation; AFP, alpha-fetoprotein; AFPR, AFP receptor; ATRA, all-trans retinoic acid; CBP, CREB binding protein; CXCR4, CXC chemokine receptor 4; DR5, death receptor 5; FADD, Fas-associated death domain; FasL, Fas ligand; HBx, hepatitis B virus X protein; HIF-1α, hypoxia-inducible factor-1α; HuR, human antigen R; MMP, matrix metalloproteinase; PA, palmitic acid; RAR, retinoic acid receptor; SIRT1, sirtuin type 1; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand; Ub, ubiquitin; UTR, untranslated region.
The stimulatory effect of AFP on HCC proliferation is achieved by activating the cAMP/PKA and PI3K/AKT/mTOR signaling pathways, respectively. Extracellular AFP binds to the AFP receptor on the cell membrane, resulting in cAMP accumulation and increased PKA activity. This triggers the overexpression of oncogenes such as c-Fos, c-Jun, and N-Ras, and mutant p53 and p21 proteins.43,44 On the other hand, intracellular AFP binding to PTEN activates the PI3K/AKT/mTOR pathway and upregulates the hypoxia-inducible factor-1α transcription.45,46 Hypoxia-inducible factor-1α subsequently binds to the promoters of Src, Ras, and CXCR4 in the nucleus to induce HCC cell proliferation. AFP also plays a crucial role in HCC invasion and metastasis, with serum levels correlating with distant metastasis, especially in small HCCs (diameter ≤5 cm). 47 Evidence from in vitro and in vivo studies showed that AFP promotes the level of metastasis-related proteins, including matrix metalloproteinase 2/9, CXC chemokine receptor 4, Vimentin, EpCAM, keratin 19, and integrin β1 through the PI3K/AKT pathway,48,49,50 and downregulates the expression of E-cadherin. 49
More recently, Xue et al 49 reported that the acetylation of AFP inhibits apoptosis by interacting with the aforementioned caspase-3 cascade, increases cell proliferation through enhancing the PTEN/PI3K/AKT pathway, and promotes migration and invasion of HCC. The acetylation status of AFP is regulated by acetyltransferase and deacetylase, CREB binding protein, and sirtuin type 1. 49 The authors also demonstrated that hepatitis B virus X protein and palmitic acid can promote AFP acetylation by inhibiting sirtuin type 1–mediated deacetylation, and lead to HCC progression. 49
AFP as a tumor antigen for immunotherapy of HCC
AFP’s unique expression in HCC cells and its minimal presence in normal adult tissues make it an ideal target for immunotherapy approaches.51,52 The journey of exploiting AFP for therapeutic purposes began with the development of AFP-based vaccines. Butterfield et al 53 identified 4 HLA-A*0201–restricted AFP epitopes and developed AFP peptide–based vaccinations for HCC. The study demonstrated that these vaccines were capable of inducing strong T-cell responses specifically targeted at AFP-expressing tumor cells, leading to a marked reduction in tumor growth and enhanced survival in treated mice. Subsequently, the initial phase I/II clinical trial focused on the immunizing efficiency of AFP peptides pulsed onto DC in patients with HCC. Moderate AFP-specific T-cell responses were detected to at least one of the peptides after vaccination. 54 However, no significant clinical benefits were observed in these patients, suggesting that additional approaches may be needed to enhance this antitumor response. To improve the efficacy of AFP-based HCC vaccines, further studies showed the efficacy of epitope-optimized AFP genetic vaccines in preventing carcinogen-induced murine autochthonous HCC. 51
In addition, T-cell–based therapies also exhibited potential in treating HCC. Comprehensive analyses of AFP-specific CD8+ T-cell responses in patients with HCC provided insights into the mechanisms of tumor immunity and the potential for T-cell–mediated therapy. 55 In this study, CD8+ T cells specific for the self-antigen AFP were found in the normal T-cell repertoire and were not centrally or peripherally depleted. High-avidity T-cell receptors, induced by AFP-derived peptides, were associated with significant antitumor effects in 15 patients with HCC, with a complete response in 1 and stabilized tumor growth in 8 patients. 56
More recent studies have explored the combination of AFP vaccines with immune checkpoint inhibitors. This approach has shown promise in slowing HCC progression in preclinical models. 57 The authors revealed that the combination therapy not only slowed tumor progression but also improved the overall immune response. This suggested a potential for overcoming the immunosuppressive environment often seen in HCC. Taken together, the synergistic effect of enhancing the immune response to AFP while simultaneously inhibiting immune checkpoints offers a multifaceted attack on tumor cells.
ETIOLOGY OF ELEVATED AFP
Table 1 summarizes the diverse etiologies for the elevation of AFP, categorized into hepatic and nonhepatic causes.58,59,60 Under hepatic neoplastic conditions, HCC is the primary malignancy known for increasing AFP levels, although it can be seen rarely in intrahepatic cholangiocarcinoma, and liver metastasis. These conditions underscore the protein’s significance as a biomarker in the detection and monitoring of hepatic malignancies. Table 1 also delineates non-neoplastic hepatic disorders such as liver cirrhosis, viral hepatitis (particularly those with active viral replication), and other liver diseases, which can contribute to elevated AFP, thus signaling liver regeneration, distress, or damage.
TABLE 1.
Etiology of elevated alpha-fetoprotein level a
| Hepatic causes of AFP elevation | Nonhepatic causes of AFP elevation | ||
|---|---|---|---|
| Neoplastic | Non-neoplastic | Neoplastic | Non-neoplastic |
| Hepatocellular carcinoma | Cirrhosis | Germ cell tumors (testicular and ovarian) | Normal pregnancy/infancy |
| Intrahepatic cholangiocarcinoma | Fulminant acute hepatitis | Gastric cancer | Colitis |
| Liver metastasis | Viral hepatitis | Sepsis | |
| Metabolic dysfunction–associated steatotic liver disease | Fetal disorders (gastroschisis, neural tube defect) |
||
| Biliary obstruction (intrahepatic and extrahepatic) |
Hereditary tyrosinemia type 1 | ||
| Drug-induced liver injury | Hereditary AFP persistence | ||
| Alcohol liver disease | Beckwith-Wiedemann syndrome | ||
| Hepatic inflammatory pseudotumor | Systemic lupus erythematosus | ||
| Neonatal hepatitis | Hirschsprung disease | ||
| Massive hepatic necrosis | Ataxia telangiectasia | ||
| Autoimmune hepatitis | |||
| Wilson disease | |||
| Hemochromatosis | |||
Modified from Hanif et al. 58
Abbreviation: AFP, alpha-fetoprotein.
In contrast, nonhepatic causes of AFP elevation are diverse, encompassing neoplastic conditions like germ cell tumors and gastric cancer, as well as a range of non-neoplastic conditions including normal pregnancy, various fetal disorders, and autoimmune diseases. Hepatic adenoma can undergo malignant transformation.61,62 While elevated AFP levels could be helpful in indicating malignant transformation in adenoma,63,64 contemporary guidelines do not recommend the use of AFP in monitoring the progression of hepatic adenoma into HCC given insufficient evidence of diagnostic utility. 65 This array of potential sources for AFP elevation highlights the biomarker’s broad clinical implications beyond liver pathology and necessitates a careful differential diagnosis when elevated levels are detected. Notably, the inclusion of liver metastasis as a neoplastic hepatic cause of AFP elevation expands the diagnostic context wherein AFP may serve as an indicator of secondary hepatic involvement by extrinsic malignancies.
AFP AS A DETECTION/SCREENING BIOMARKER FOR HCC
Performance of AFP for early-stage HCC detection
AFP holds the distinction of being the first recognized oncofetal biomarker. Its utility in liver cancer diagnosis dates back to initial discoveries in mouse hepatoma and was later confirmed in the serum of patients with HCC.66,67 Even as early as the 1960s, AFP was reported as a biomarker, not only for HCC but also to distinguish between primary and metastatic liver tumors.67,68,69 Since then, serial biomarker studies have been conducted to investigate the use of AFP as a biomarker for HCC detection/screening. Table 2 summarizes the performance of AFP and AFP-integrated biomarkers/panels for detecting early-stage HCC from at-risk patients with various phases of biomarker studies.70,91
TABLE 2.
Performance of AFP, AFP-L3%, and AFP-integrated biomarkers/panels for detecting early-stage HCC from at-risk patients
| Biomarker (cutoff) | Biomarker development phase a | Variables | Study type | Major etiology | No. subjects b | Definition of early-stage HCC | Sensitivity (%) | Specificity (%) | AUROC | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Blood-based protein marker | ||||||||||
| AFP | 2–4 | AFP | Meta-analysis | HBV, HCV, alcohol, MASLD | NA | BCLC 0/A or within Milan | 49 | 88 | NA | 70 |
| AFP (20 ng/mL) | 2 | AFP | Meta-analysis | HBV, HCV | 1722 | Resectable | 65 | 80 | NA | 69 |
| AFP | 3 | AFP | Meta-analysis | HBV, HCV, alcohol, MASLD | NA | BCLC 0/A or within Milan | 38 | 90 | NA | 70 |
| AFP | 4 | AFP | Meta-analysis | HCV, HBV, alcohol, MASLD | NA | BCLC 0/A or within Milan | 55 | 90 | NA | 70 |
| AFP-L3% | 2–3 | AFP-L3% | Meta-analysis | HBV, HCV, alcohol | 497 :1950 | BCLC 0/A or AJCC I | 34 | 92 | 0.76 | 71 |
| AFP-L3% (10%) | 3 | AFP-L3% | Cohort | HCV, alcohol, MASLD | 355; 484 | BCLC 0/A or single, ≤5 cm | 27–74 | 83–95 | 0.64 | 72,73 |
| AFP + AFP-L3% | 3 | AFP, AFP-L3% | Cohort | HBV | 42 : 168 c | NA | 66 d | 85 d | 0.78 d | 74 |
| AFP + DCP | 3 | AFP, DCP | Cohort | HBV, HCV | 42 : 168; 36 : 108; 39 : 77 c | NA | 46–86 d | 69–82 d | 0.61–0.88 d | 74,75,76 |
| AFP + AFP-L3% + DCP | 3 | AFP, AFP-L3%, DCP | Cohort | HCV, alcohol, MASLD | 484; 42 : 168 c | NA | 31–77 d | 66–91 d | 0.69 d | 73,74 |
| AFP-integrated clinical score | ||||||||||
| GALAD score (−0.63) | 2 | Gender, age, AFP, AFP-L3%, DCP | Meta-analysis | HBV, HCV, alcohol, MASLD | 1183 : 2838 | BCLC 0-A, AJCC I/II, or within Milan | 69 | 91 | 0.83 | 68 |
| GALAD score (−0.63) | 3 | Gender, age, AFP, AFP-L3%, DCP | Meta-analysis | HCV, alcohol, MASLD | 849 | BCLC 0/A or single ≤5 cm | 58 | 83 | 0.73 | 68 |
| HES algorithm | 3 | AFP, ΔAFP over the last year, age, platelets, ALT, interaction terms | Cohort | HCV, alcohol, MASLD | 355; 484 | BCLC 0/A or single ≤5 cm | 27–42 | 91–95 | 0.76 | 72,73 |
| Doylestown algorithm | 2 | Age, gender, logAFP, alkaline phosphatase, ALT | Case-control | HBV, HCV, others | 101 : 195 + 225 : 438 + 113 : 586 + 140 : 804 | BCLC 0/A | 35–58 | 90–95 | 0.77–0.89 | 77,78 |
| Doylestown Plus algorithm | 3 | Age, logAFP, PEG-precipitated IgG, fucosylated kininogen | Cohort | HCV, alcohol, MASLD | 17 : 58 | BCLC 0/A | 80 | 90 | NA | 79 |
| GALAD-C model | 2 | Gender, age, AFP, AFP-L3%, DCP | Case-control | HBV, HCV | 242 : 283 + 169 : 139 ; 395 : 846 c | NA | 85 d | 92 d | NA | 80,81 |
| GAAP model | 2 | Gender, age, AFP, DCP | Case-control | HBV, HCV | 242 : 283 + 169 : 139 ; 395 : 846; 199 : 508 c | NA | 75–88 d | 80–91 d | 0.92 d | 80,81,82 |
| ASAP model | 2 | Gender, age, AFP, DCP | Case-control | HBV | 318 : 603 + 286 : 211; 199 : 508 | BCLC 0/A | 73–74 | 88–90 | NA | 82,83,84 |
| AALP model | 2 | Age, AFP, AFP-L3%, DCP | Case-control | HBV, HCV | 395 : 846 | NA | 85 d | 92 d | 0.94 d | 81 |
| Plasma cfDNA+biomarkers | ||||||||||
| Multitarget HCC blood test (mt-HBT) | 2 | 3 cfDNA methylation markers (HOXA1, TSPYL5, B3GALT6), sex, AFP | Case-control | HCV, alcohol, MASLD, HBV | 81 : 404 + 78 : 245 | BCLC 0/A | 82 | 87 | 0.92 | 85 |
| HelioLiver test | 2 | 28 methylation markers, age, sex, AFP, AFP-L3%, DCP | Case-control | HBV, others | 46 : 236 + 37 : 125 | AJCC I/II | 76 | 91 | 0.92 | 86 |
| HCCscreen | 3 | Mutations in TP53, CTNNB1, AXIN1, TERT promoter, HBV integration breakpoint, AFP, DCP | Cohort | HBV | 331 | BCLC 0/A | 100 | 94 | NA | 87 |
| Ultrasound+AFP/GALAD score | ||||||||||
| Ultrasound+AFP | 2–4 | Ultrasound, AFP | Meta-analysis | HBV, HCV, alcohol, MASLD | 7140 | BCLC 0/A or within Milan | 63–74 | 84 | NA | 70,88 |
| Ultrasound+AFP (20 ng/mL) | 5 | Ultrasound, AFP | RCT | HBV | Screening : control=9373 : 9443 | NA | NA | NA | 37% reduction in HCC mortality | 89 |
| GALADUS score | 2 | Ultrasound, GALAD score | Case-control | HCV, MASLD, alcohol, HBV | 60 : 180 | BCLC 0/A | 88 | 94 | 0.97 | 90 |
Phase 1 biomarker study focuses on identifying potential biomarkers for early detection of HCC; phase 2 study is a case-control study to evaluate the biomarkers’ performance for distinguishing early-stage HCC from at-risk patients; phase 3 study adopts a PRoBE (prospective specimen collection and retrospective blinded evaluation) design, aiming to assess the time between HCC development and biomarker measurement; phase 4 study is a prospective cohort study to measure the biomarkers’ performance in a timely manner; and lastly, phase 5 biomarker study is a randomized study to evaluate whether the standard of case combined with the new biomarkers will reduce HCC mortality.
Numbers of subjects for case-control studies or nested case-control studies are shown as “early-stage HCC: at-risk control.” Numbers of subjects of cohort studies are represented as the entire study cohort. Numbers of subjects for training and validation sets are separately shown with “+” in between. Numbers of subjects of different studies are separately shown with “;” in between.
Indicates that the number is any-stage HCC instead of early-stage HCC.
Indicates that the sensitivity, specificity, and AUROC are for detecting any-stage HCC instead of early-stage HCC.
Abbreviations: AFP, alpha-fetoprotein; AJCC, American Joint Committee on Cancer; BCLC, Barcelona clinic liver cancer; cfDNA, cell-free DNA; DCP, des-gamma-carboxy prothrombin; GALAD, Gender, Age, AFP-L3%, AFP, and DCP; HES, Hepatocellular Carcinoma Early Detection Screening; IgG, immunoglobulin G; MASLD, metabolic dysfunction–associated steatotic liver disease; mt-HBT, multitarget HCC blood test; NA, not applicable; PEG, polyethylene glycol.
In a Cochrane systematic review and meta-analysis of phase 2 biomarker studies, at an AFP cutoff of 20 ng/mL, the sensitivity and specificity were 60% and 84%, respectively, for detecting HCC. 92 However, the sensitivity of AFP is compromised to 49% with a specificity of 88% among patients with early-stage HCC (Barcelona clinic liver cancer [BCLC] stage 0-A or within Milan criteria). 93 As a large proportion of patients with early-stage HCC do not exhibit elevated AFP levels,88,89 and AFP levels tend to be lower in HCC with nonviral etiologies, the sensitivity of AFP can diminish in a contemporary cohort of nonviral HCC. 94 Given that the limitation of AFP as a standalone biomarker for HCC is evident, Singal and colleagues performed landmark meta-analyses including phase 2–4 biomarker studies to investigate the performance of ultrasound with or without AFP in the setting of HCC screening. They reported that the addition of AFP to ultrasound improved sensitivity for early-stage HCC detection from 45%–52% to 63%–74%.93,95 In addition, AFP is the only biomarker that has been evaluated in a phase 5 biomarker study with ultrasound.96,97 In a large randomized controlled trial from China with 9373 patients in the screening arm or 9443 patients in the control arm, the authors showed that AFP combined with ultrasound resulted in a 37% reduction in HCC mortality compared to those without HCC screening. 96 Furthermore, with the rising incidence of nonviral HCC, it is imperative to assess the diagnostic accuracy of AFP in this specific subpopulation. However, cases of nonviral HCC, particularly those associated with NAFLD/metabolic dysfunction–associated fatty liver disease, have shown a lower likelihood of exhibiting high AFP levels (≥20 ng/mL). In a large cohort with 1.4k patients with HCC, the proportion of a high AFP was 64.9%, whereas it was 52.5% in those with NAFLD/metabolic dysfunction–associated fatty liver disease–related HCC. 98 Consistently, another cohort study indicated that patients with metabolic dysfunction–associated fatty liver disease–associated HCC typically had a lower elevation of AFP levels than those with HCV-related HCC. 99 As such, AFP is currently recommended for HCC surveillance with ultrasound by both the American Association for the Study of Liver Diseases (AASLD) 100 and the Asian Pacific Association for the Study of the Liver (APASL), 74 although the use of AFP is not endorsed by the European Association for the Study of the Liver (EASL). 101
Several studies investigated the longitudinal trend of AFP for HCC detection. 102 A recent large-scale phase 3 biomarker study with 2776 patients undergoing serial AFP monitoring in Taiwan demonstrated that a serial AFP increase of ≥10% was associated with a 12.1-fold increased risk of HCC in 6 months. 103 The risk increased 13-to-60-fold in patients with cirrhosis, hepatitis B or C, receiving antiviral therapy, or with AFP levels <20 ng/mL. Combining a serial AFP increase of ≥10% with AFP levels ≥20 ng/mL at 6 months prediagnosis significantly raised the HCC risk by 41.7 fold. 103 Despite these promising results, other studies reported limited sensitivity and specificity of serial AFP measurement for early-stage HCC detection. 102 Therefore, further studies are warranted to externally validate the findings, especially among patients with early-stage HCC and other races/ethnicities.
Fucosylated fraction of AFP (AFP-L3)
Alpha-fetoprotein L3 (AFP-L3), a variant of AFP that specifically binds to the lectin Lens culinaris agglutinin, emerges predominantly in patients with HCC. 71 It is often associated with advanced tumor characteristics, including larger tumor size, portal vein invasion, tumor stage, and higher grade.72,104
The percentage of AFP-L3 to total AFP, named AFP-L3%, has been studied as a biomarker for detecting HCC (Table 2).71,73,105 The sensitivity of measuring the percentage of AFP-L3 in AFP is inversely affected by the AFP level using the conventional detecting method, liquid-phase binding assay on an auto-analyzer (LiBASys).75,106 The introduction of new methodologies, including microfluidics-based separation and immunochips, has enabled not only the accurate measurement of AFP-L3% at very low AFP concentrations but also the use of smaller specimen volumes, particularly in cases where AFP levels are <20 ng/mL.76,107 Compared to AFP, while the specificity of AFP-L3% for early-stage HCC is excellent at 92%, its sensitivity is relatively low at 34% in phase 2 and 3 biomarker studies. 105 Recently, several phase 3 biomarker studies in Japan, South Korea, and the United States demonstrated that AFP-L3% has AUROCs of 0.60–0.80 a year before any-stage HCC diagnosis, with sensitivity ranging from 30% to 67% for detecting HCC.90,108,109,110 In these studies, the AUROC of AFP-L3% for the detection of BCLC stage 0-A HCC is similar between 0.76 and 0.89; however, its sensitivity varies widely from 27% to 74%.109,110 As such, larger phase 3 and phase 4-5 studies are required to evaluate its performance for clinical application.
Combination of AFP, AFP-L3%, and other biomarkers/panels for early-stage HCC detection
In the evolving landscape of HCC diagnostics, the search for novel biomarkers to enhance the accuracy of AFP is paramount. 91 The amalgamation of AFP with additional biomarkers, such as AFP-L3%, Des-Gamma-Carboxy Prothrombin (DCP, also known as prothrombin induced by vitamin K absence-II), Glypican-3, GP73, Heat Shock Protein 90 alpha, Midkine, and Osteopontin not only elevates the AUROC for early-stage HCC detection but also significantly outperforms AFP alone.77,78,111,112 For example, in a phase 3 study including 42 patients with HCC and 168 matched controls, Choi and colleagues compared the 3 most commonly used biomarkers, AFP, AFP-L3%, DCP as well as their combinations. They found AFP+AFP-L3% has the highest AUROCs of 0.78 and 0.71, respectively, at 6 and 12 months before HCC diagnosis. 90 In another phase 3 study containing patients without HBV, the combined use of AFP, AFP-L3%, and DCP resulted in a sensitivity of 47% and a specificity of 91% for early-stage HCC detection. 110 The performances of the AFP-integrated biomarkers/panels for early-stage HCC detection are also summarized in Table 2.
GALAD score, which incorporates Gender, Age, AFP-L3%, AFP, and DCP is a practical and accurate model for early-stage HCC detection and has been validated in various populations.79,91 The performance of the GALAD score for early-stage HCC detection remained stable across different etiologies and races/ethnicities, and a recent meta-analysis showed that the pooled AUROC of the GALAD score was 0.83 and 0.73 in phase 2 and phase 3 biomarker studies, respectively. 91 The sensitivity (69% vs. 58%) and specificity (91% vs. 85%) of the GALAD score at the commonly applied cutoff of −0.63 also decreased in the phase 3 studies, highlighting the need for further validation of novel biomarkers in cohort studies to avoid overestimation of their performance. 91 Recently, one of these phase 3 studies demonstrated better sensitivity for early-stage HCC detection with longitudinal measurement of the GALAD score than single time point measurement (69% vs. 54%). 109 Another study that combined ultrasound with the GALAD score to detect BCLC stage 0/A HCC augmented the AUROC to 0.98 and a remarkable sensitivity (95%) and specificity (91%), and remained accurate in the external validation cohort (AUROC: 0.97). 80
Similar to the GALAD score, the Hepatocellular Carcinoma Early Detection Screening (HES) Algorithm is another AFP-based algorithm that has been serially validated in multiple phase 2 studies81,82,83,84,113 and phase 3 studies.109,110 The HES algorithm incorporates the data of AFP, rate of AFP change within the last year, age, alanine aminotransferase (ALT), platelets, HCC etiology, and interaction terms (AFP and alanine aminotransferase, and AFP and platelets).81,82 In phase 3 studies, the sensitivity within 6 months before early-stage HCC diagnosis was comparable between the HES algorithm (39%–42%) and the GALAD score (31%–74%) at a specificity of 90%.109,110 However, the comparison of performance between the HES Algorithm, the GALAD score, AFP, AFP-L3%, and DCP remains controversial in these 2 studies109,110 and larger studies are warranted.
The Doylestown algorithm is also an algorithm well-validated in several phase 2 biomarker studies for HCC screening.114,115 On top of its original variables including age, gender, logAFP, alkaline phosphatase, and alanine aminotransferase, recently an updated version known as the Doylestown Plus algorithm incorporates the values of polyethylene glycol-precipitated immunoglobulin G and fucosylated kininogen. 85 The Doylestown Plus algorithm yielded promising results, with AUROC of 0.92 for detecting BCLC stage 0/A HCC any time before diagnosis, in a phase 3 study. 85
In addition to the aforementioned GALAD score and the algorithms, there are several AFP-integrated protein-based panels still under phase 2 biomarker validation (Table 2). For example, using the same variables included in the GALAD score, GALAD-C,86,87 GAAP,86,87,116 ASAP,116,117,118 and AALP 87 models were tailored and validated for HCC detection in Chinese populations. Another approach includes the incorporation of machine-learning models, such as gradient boosting that optimize the combination of AFP, AFP-L3%, and DCP to augment HCC detection. 119 This integration of computational prowess with clinical biomarkers is poised to improve the use of AFP and its combination for HCC screening. Lastly, the addition of AFP measurement to liquid biopsy techniques, such as circulating cell-free DNA and other extracellular markers (eg, cell-free microRNA, extracellular vesicles, or tumor-educated platelets), is evidencing notable potential.91,120,121,122,123,124 Currently, large phase 2 and phase 3 biomarker clinical trials are ongoing for validating a couple of promising AFP-embedded cell-free DNA–based panels including multitarget HCC blood test algorithm, 122 HelioLiver Test, 123 and HCCscreen. 124
Looking ahead, while more research is necessary to refine AFP’s utility with other biomarkers/panels across various HCC etiologies and to establish standardized cutoff values for broader applications, the focus is shifting toward the development of new predictive models. The challenge lies in effectively integrating statistical and machine-learning tools to tailor personalized HCC screening, enhancing the efficacy through novel blood-based biomarkers for AFP-negative HCC, and increasing provider awareness of these emerging diagnostic technologies to enhance early detection. 125
AFP AS A PROGNOSTIC BIOMARKER FOR HCC
Prognostic value of AFP
The prognostic significance of AFP levels before hepatectomy in HCC has been well-documented, with elevated AFP levels signaling a worse prognosis. Specifically, AFP levels exceeding 9000 ng/mL have been linked to shorter disease-free survival, while levels above 14,000 ng/mL are associated with decreased overall survival (OS), suggesting that high AFP levels could necessitate an upstaging of the disease. 126 In a large cohort study utilizing data from 78,743 patients with HCC within the Surveillance, Epidemiology, and End Results database, the level of AFP at the time of diagnosis was identified as a predictor of pathological grade, disease progression, and survival, even after adjusting for various confounding factors. This study highlighted that a positive AFP status carries a higher HR compared to other factors such as sex, race, marital status, and fibrosis severity. 127
Multiple prognostic scoring systems including MESH (model to estimate survival for HCC) system (tumor size, the presence of vascular invasion or metastasis, Child-Turcotte-Pugh score, performance status, AFP, and alkaline phosphatase), 128 the Taipei Integrated Scoring System (total tumor volume, Child-Turcotte-Pugh score, and AFP),129,130 and Cancer of the Liver Italian Program (CLIP) score (AFP, Child-Turcotte-Pugh score, invasion of portal vein, and tumor morphology) 130 incorporated AFP along with other predictors to predict the OS of patients with HCC.
AFP and liver transplantation—patient selection
The evolving role of AFP (combined with other biomarkers) in assessing eligibility for liver transplantation presents promising avenues for enhancing patient outcomes. Drawing on data from 1164 patients within the United Network for Organ Sharing database, it has been established that pretransplant AFP levels are independently predictive of survival rates in patients experiencing HCC recurrence. Notably, individuals exhibiting pretransplant AFP levels equal to or exceeding 500 ng/mL were found to have a 1.6-fold increased mortality risk compared to those with AFP levels at or below 20 ng/mL, a statistically significant finding (p<0.001). 131 Furthermore, the introduction of the New York/California (NYCA) score, which employs a dynamic AFP rate—defined as the difference between the maximum and final pre-liver transplant AFP levels—has shown significant potential in refining the selection process for candidates with HCC undergoing liver transplant. This dynamic assessment offers a more nuanced evaluation compared to static AFP measurements. The findings suggest that AFP-based models have a superior prognostic accuracy compared to the traditional Milan criteria, providing a more tailored approach to patient selection for transplantation. This implies that integrating AFP and similar biomarkers into the selection criteria could significantly improve the stratification of transplant candidates, potentially leading to better posttransplantation outcomes and a more equitable distribution of resources in the field of hepatology.
AFP and liver transplantation—posttransplant outcome
Metroticket 2.0 Model, which integrates AFP levels, further refines the prognostication of HCC-related death following liver transplantation, exemplifying the continued evolution of predictive modeling in HCC. 132 Similarly, the MORAL score, a prognostic model for HCC recurrence after liver transplant, incorporates preoperative information including tumor size, maximum AFP levels, neutrophil-lymphocyte ratio, and postoperative factors such as tumor grade and vascular invasion, offering a comprehensive view of patient prognosis. 133 A nomogram was developed based on both pretransplant characteristics, including AFP, cholesterol, neutrophil-lymphocyte ratio, and tumor size, and explant pathological features to predict posttransplant HCC recurrence. 134 The Shanghai criteria, involving 1078 patients with HCC who underwent liver transplantation, used AFP alongside other markers to predict OS or disease-free survival, highlighting the value of multifaceted diagnostic approaches. 135 Another scoring system, RETREAT, is derived from a multicenter cohort with external validation. 136 The score featured a high accuracy despite having 3 variables only (microvascular invasion, pretransplant AFP, and tumor size-related data). In addition, the R3-AFP predictive model, established using a large and international cohort of patients transplanted for HCC, includes various parameters like the number of nodules, the size of the largest nodule, the presence of microvascular invasion, nuclear grade, and the last pre-liver transplantation AFP value, demonstrating its utility in predicting HCC recurrence after transplantation. 137 Finally, a recent scoring system, RELAPSE, showed an accurate 2- and 5-year recurrence risk discrimination and was consistent with external validation. 138 Notably, the variables, which included AFP were identified through Fine and Gray competing risk analysis and machine-learning algorithms.
Moreover, the use of a dual biomarker model incorporating AFP-L3 and DCP has demonstrated high predictive power for posttransplant recurrence. An AFP-L3 level of 15% or higher, coupled with a DCP level of at least 7.5 ng/mL, was a strong indicator of the risk of early HCC recurrence in a prospective study of 285 patients who underwent liver transplantation. 139 This evidence supports the utility of these biomarkers not only in diagnosis but also in the strategic planning of postoperative care and surveillance.
Predictive and prognostic value of AFP in patients with HCC receiving systemic treatment
AFP plays a crucial prognostic role for immunotherapy-treated patients with HCC. In patients treated with atezolizumab and bevacizumab (Atezo-Bev), a combination of early AFP response (defined as a reduction in AFP ≥20% at 3 wk) and the albumin-bilirubin (ALBI) grade has been shown to correlate with the radiological response (using mRECIST criteria) and OS. 125 This finding illustrates the growing importance of AFP as a dynamic biomarker that can be used not only in diagnostic and prognostic settings but also in monitoring treatment efficacy and patient outcomes in the era of advanced therapeutic interventions. A multicenter study combined AFP and C-reactive protein as a predictive model for patients with HCC put on PD-(L)1-based immunotherapy. 140 The authors demonstrated an effective and simple stratification model using serum AFP (100 ng/mL) and C-reactive protein (1 mg/dL) to stratify patients’ OS, which was validated in another study with 2 cohorts: lenvatinib-immunotherapy combination and lenvatinib only. 141 However, given a low c-statistic of 0.62 in both training and validation sets, further modification of the score is warranted. AFP was also found to be a predictive biomarker for HCC. A randomized, double-blind, placebo-controlled, phase 3 trial with advanced HCC and AFP concentrations of 400 ng/mL or greater (REACH-2) showed improved OS in ramucirumab treatment arm. 142 This trial was a biomarker-enriched trial as the trial was designed based on the subgroup analysis of the prior negative REACH trial showing the survival benefit of ramicirumab among patients with HCC with AFP of 400 ng/mL or greater. 142
CONCLUSIONS
AFP is the first recognized oncofetal biomarker in HCC and remains a useful biomarker for early detection of cancer, especially when it is being used with imaging surveillance tests or another biomarker panel. Furthermore, it showed promising potential as an excellent prognostic and predictive biomarker and therapeutic target in HCC. Future studies should attempt to leverage machine-learning algorithms to refine the diagnostic, predictive, prognostic, and therapeutic capacity of AFP, which will likely further enhance the role of AFP in personalized patient care of HCC. 143
ACKNOWLEDGMENT
The authors thank BioRender.com for creating Figure 1.
Footnotes
Abbreviations: AASLD, American Association for the Study of Liver Diseases; AFP, alpha-fetoprotein; AFP-L3, alpha-fetoprotein L3; APASL, Asian Pacific Association for the Study of the Liver; BCLC, Barcelona clinic liver cancer; CLIP, Cancer of the Liver Italian Program; DC, dendritic cells; DCP, Des-Gamma-Carboxy Prothrombin; EASL, European Association for the Study of the Liver; GALAD, Gender, Age, AFP-L3%, AFP, and DCP; HES, Hepatocellular Carcinoma Early Detection Screening; nAFP, native AFP; OS, overall survival; RAR, retinoic acid receptor; tAFP, tumor-derived AFP.
Contributor Information
Yee Hui Yeo, Email: yeehui.yeo@cshs.org.
Yi-Te Lee, Email: Yi-Te.Lee@cshs.org.
Hsian-Rong Tseng, Email: HRTseng@mednet.ucla.edu.
Yazhen Zhu, Email: yazhenzhu@mednet.ucla.edu.
Sungyong You, Email: sungyong.you@cshs.org.
Vatche G. Agopian, Email: VAgopian@mednet.ucla.edu.
Ju Dong Yang, Email: judong.yang@cshs.org.
FUNDING INFORMATION
Hsian-Rong Tseng’s research is supported by the National Institutes of Health (R01CA253651 and R01CA253651-04S1). Yazhen Zhu’s research is supported by the National Institutes of Health (R01CA277530 and R01CA255727). Vatche G. Agopian’s research is supported by the National Institutes of Health (R01CA246304). Ju Dong Yang’s research is supported by the National Institutes of Health (K08CA259534 and R21 CA280444-01).
CONFLICTS OF INTEREST
Ju Dong Yang consults for AstraZeneca, Eisai, Exact, Exelixis, Fujifilm Medical Sciences, and Merck. Vatche G. Agopian consults for Early Diagnostics and Eximius Diagnostics. Yazhen Zhu owns stock in Eximius Diagnostics. The remaining authors have no conflicts to report.
REFERENCES
- 1.Deutsch HF. Chemistry and biology of alpha-fetoprotein. Adv Cancer Res. 1991;56:253–312. [DOI] [PubMed] [Google Scholar]
- 2.Nishi S. Isolation and characterization of a human fetal-alpha-globulin from the sera of fetuses and a hepatoma patient. Cancer Res. 1970;30:2507–2513. [PubMed] [Google Scholar]
- 3.Morinaga T, Sakai M, Wegmann TG, Tamaoki T. Primary structures of human alpha-fetoprotein and its mRNA. Proc Natl Acad Sci USA. 1983;80:4604–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zizkovsky V, Strop P, Korcakova J, Havranová M, Mikes F. Fluorescence spectroscopy, fluorescence polarization, and circular dichroism in studies on pH-dependent changes in the alpha-fetoprotein molecule. Ann NY Acad Sci. 1983;417:49–56. [DOI] [PubMed] [Google Scholar]
- 5.Kirkitadze MD, Narizhieva NV, Tomashevskiĭ A, Potekhin SA, Uverskiyiĭ VN. Stabilization of the alpha-fetoprotein structure with sucrose. Bioorg Khim. 1996;22:408–414. [PubMed] [Google Scholar]
- 6.Uverski VN, Kirkitadze MD, Narizhneva NV, Potekhin SA, Tomashevski AYU. Structural properties of alpha-fetoprotein from human cord serum: The protein molecule at low pH possesses all the properties of the molten globule. FEBS Lett. 1995;364:165–167. [DOI] [PubMed] [Google Scholar]
- 7.Uversky VN, Narizhneva NV, Ivanova TV, Tomashevski AY. Rigidity of human alpha-fetoprotein tertiary structure is under ligand control. Biochemistry. 1997;36:13638–13645. [DOI] [PubMed] [Google Scholar]
- 8.Baker ME. Evolution of alpha-fetoprotein: Sequence comparisons among AFP species and with albumin species. Tumour Biol. 1988;9:123–136. [DOI] [PubMed] [Google Scholar]
- 9.Terentiev AA, Moldogazieva NT. Structural and functional mapping of alpha-fetoprotein. Biochemistry (Mosc). 2006;71:120–132. [DOI] [PubMed] [Google Scholar]
- 10.AlSalloom AA. An update of biochemical markers of hepatocellular carcinoma. Int J Health Sci (Qassim). 2016;10:121–136. [PMC free article] [PubMed] [Google Scholar]
- 11.Yi X, Yu S, Bao Y. Alpha-fetoprotein-L3 in hepatocellular carcinoma: A meta-analysis. Clin Chim Acta. 2013;425:212–220. [DOI] [PubMed] [Google Scholar]
- 12.Force M, Park G, Chalikonda D, Roth C, Cohen M, Halegoua-DeMarzio D, et al. Alpha-fetoprotein (AFP) and AFP-L3 is most useful in detection of recurrence of hepatocellular carcinoma in patients after tumor ablation and with low AFP level. Viruses. 2022;14:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei T, Zhang W, Tan Q, Cui X, Dai Z. Electrochemical assay of the alpha fetoprotein-L3 isoform ratio to improve the diagnostic accuracy of hepatocellular carcinoma. Anal Chem. 2018;90:13051–13058. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Liu Q, Qin Y, Cao Y, Zhao J, Zhang K, et al. Ordered labeling-facilitated electrochemical assay of alpha-fetoprotein-L3 ratio for diagnosing hepatocellular carcinoma. ACS Appl Mater Interfaces. 2023;15:6411–6419. [DOI] [PubMed] [Google Scholar]
- 15.Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. [DOI] [PubMed] [Google Scholar]
- 16.Halbrecht I, Klibanski C. Identification of a new normal embryonic haemoglobin. Nature. 1956;178:794–795. [DOI] [PubMed] [Google Scholar]
- 17.Engelhardt NV, Goussev AI, Shipova LJ, Abelev GI. Immunofluorescent study of alpha-foetoprotein (alpha-fp) in liver and liver liver tumours. I. Technique of alpha-fp localization in tissue sections. Int J Cancer. 1971;7:198–206. [DOI] [PubMed] [Google Scholar]
- 18.Obiekwe BC, Malek N, Kitau MJ, Chard T. Maternal and fetal alphafetoprotein (AFP) levels at term. Relation to sex, weight and gestation of the infant. Acta Obstet Gynecol Scand. 1985;64:251–253. [DOI] [PubMed] [Google Scholar]
- 19.Racusin D, Villarreal S, Antony K, Harris R, Mastrobattista J, Lee W, et al. Role of maternal serum alpha-fetoprotein and ultrasonography in contemporary detection of Spina Bifida. Am J Perinatol. 2015;32:1287–1291. [DOI] [PubMed] [Google Scholar]
- 20.Slade B. Antibodies to alpha-foetoprotein cause foetal mortality in rabbits. Nature. 1973;246:493. [DOI] [PubMed] [Google Scholar]
- 21.Murgita RA. The immunosuppressive role of alpha-fetoprotein during pregnancy. Scand J Immunol. 1976;5:1003–1014. [DOI] [PubMed] [Google Scholar]
- 22.Camper SA, Tilghman SM. Postnatal repression of the alpha-fetoprotein gene is enhancer independent. Genes Dev. 1989;3:537–546. [DOI] [PubMed] [Google Scholar]
- 23.Sell S. Heterogeneity of alpha-fetoprotein (AFP) and albumin containing cells in normal and pathological permissive states for AFP production: AFP containing cells induced in adult rats recapitulate the appearance of AFP containing hepatocytes in fetal rats. Oncodev Biol Med. 1980;1:93–105. [PubMed] [Google Scholar]
- 24.Munson PV, Adamik J, Butterfield LH. Immunomodulatory impact of alpha-fetoprotein. Trends Immunol. 2022;43:438–448. [DOI] [PubMed] [Google Scholar]
- 25.Pardee AD, Shi J, Butterfield LH. Tumor-derived alpha-fetoprotein impairs the differentiation and T cell stimulatory activity of human dendritic cells. J Immunol. 2014;193:5723–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murgita RA, Goidl EA, Kontiainen S, Wigzell H. alpha-Fetoprotein induces suppressor T cells in vitro. Nature. 1977;267:257–259. [DOI] [PubMed] [Google Scholar]
- 27.Peck AB, Murgita RA, Wigzell H. Cellular and genetic restrictions in the immunoregulatory activity of alpha-fetoprotein. III. Role of the MLC-stimulating cell population in alpha-fetoprotein-induced suppression of T cell-mediated cytotoxicity. J Immunol. 1982;128:1134–1140. [PubMed] [Google Scholar]
- 28.Ritter M, Ali MY, Grimm CF, Weth R, Mohr L, Bocher WO, et al. Immunoregulation of dendritic and T cells by alpha-fetoprotein in patients with hepatocellular carcinoma. J Hepatol. 2004;41:999–1007. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto M, Tatsumi T, Miyagi T, Tsunematsu H, Aketa H, Hosui A, et al. α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells. Clin Exp Immunol. 2011;165:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Liu K, Zhang Q, Xu J, Liu J, Lin H, et al. Alpha fetoprotein promotes polarization of macrophages towards M2-like phenotype and inhibits macrophages to phagocytize hepatoma cells. Front Immunol. 2023;14:1081572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li M, Liu X, Zhou S, Li P, Li G. Effects of alpha fetoprotein on escape of Bel 7402 cells from attack of lymphocytes. BMC Cancer. 2005;5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlsson JA, Wold AE, Sandberg AS, Östman SM. The polyunsaturated fatty acids arachidonic acid and docosahexaenoic acid induce mouse dendritic cells maturation but reduce T-cell responses in vitro. PLoS One. 2015;10:e0143741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeyda M, Säemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, et al. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280:14293–14301. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita N, Yokoyama A, Hamazaki T, Yano S. Inhibition of natural killer cell activity of human lymphocytes by eicosapentaenoic acid. Biochem Biophys Res Commun. 1986;138:1058–1067. [DOI] [PubMed] [Google Scholar]
- 35.He H, Chen S, Fan Z, Dong Y, Wang Y, Li S, et al. Multi-dimensional single-cell characterization revealed suppressive immune microenvironment in AFP-positive hepatocellular carcinoma. Cell Discov. 2023;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahran AM, Nafady-Hego H, Mansor SG, Abbas WA, Abdel-Malek MO, Mekky MA, et al. Increased frequency and FOXP3 expression of human CD8+CD25High+ T lymphocytes and its relation to CD4 regulatory T cells in patients with hepatocellular carcinoma. Hum Immunol. 2019;80:510–516. [DOI] [PubMed] [Google Scholar]
- 37.Zhu M, Li W, Lu Y, Dong X, Lin B, Chen Y, et al. HBx drives alpha fetoprotein expression to promote initiation of liver cancer stem cells through activating PI3K/AKT signal pathway. Int J Cancer. 2017;140:1346–1355. [DOI] [PubMed] [Google Scholar]
- 38.Lin B, Zhu M, Wang W, Li W, Dong X, Chen Y, et al. Structural basis for alpha fetoprotein-mediated inhibition of caspase-3 activity in hepatocellular carcinoma cells. Int J Cancer. 2017;141:1413–1421. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Zhang Y, Zhang L, Zhang L, Mao J. Silencing alpha-fetoprotein expression induces growth arrest and apoptosis in human hepatocellular cancer cell. Cancer Lett. 2008;271:281–293. [DOI] [PubMed] [Google Scholar]
- 40.Chen T, Dai X, Dai J, Ding C, Zhang Z, Lin Z, et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Wang S, Jiang W, Li H, Liu Z, Zhang C, et al. Impact of intracellular alpha fetoprotein on retinoic acid receptors-mediated expression of GADD153 in human hepatoma cell lines. Int J Cancer. 2012;130:754–764. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Zhang J, Wang J, Yan Y, Zhang C. Alpha-fetoprotein accelerates the progression of hepatocellular carcinoma by promoting Bcl-2 gene expression through an RA-RAR signalling pathway. J Cell Mol Med. 2020;24:13804–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li MS, Li PF, Chen Q, Du GG, Li G. Alpha-fetoprotein stimulated the expression of some oncogenes in human hepatocellular carcinoma Bel 7402 cells. World J Gastroenterol. 2004;10:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenkova LN, Dudich EI, Dudich IV. Induction of apoptosis in human hepatoma cells by alpha-fetoprotein. Tumour Biol. 1997;18:261–273. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Xiao L, Zhao M, Zhou J, Zhang Q, Wang H, et al. Molecular analysis of AFP and HSA interactions with PTEN protein. BioMed Res Int. 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu M, Guo J, Li W, Lu Y, Fu S, Xie X, et al. Hepatitis B virus X protein induces expression of alpha-fetoprotein and activates PI3K/mTOR signaling pathway in liver cells. Oncotarget. 2015;6:12196–12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y-Q, Wang A-J, Zhang T-T, Chen SH. Association of alpha-fetoprotein and metastasis for small hepatocellular carcinoma: A propensity-matched analysis. Sci Rep. 2022;12:15676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Y, Zhu M, Li W, Lin B, Dong X, Chen Y, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med. 2016;20:549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue J, Cao Z, Cheng Y, Wang J, Liu Y, Yang R, et al. Acetylation of alpha-fetoprotein promotes hepatocellular carcinoma progression. Cancer Lett. 2020;471:12–26. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Xu L, Zhu X, Wang P, Chi H, Meng Z. Activation of phosphatidylinositol 3-kinase/Akt signaling mediates sorafenib-induced invasion and metastasis in hepatocellular carcinoma. Oncol Rep. 2014;32:1465–1472. [DOI] [PubMed] [Google Scholar]
- 51.Hong Y, Peng Y, Guo ZS, Guevara-Patino J, Pang J, Butterfield LH, et al. Epitope-optimized alpha-fetoprotein genetic vaccines prevent carcinogen-induced murine autochthonous hepatocellular carcinoma. Hepatology. 2014;59:1448–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y, Hong Y, Mizejewski GJ. Engineering alpha-fetoprotein-based gene vaccines to prevent and treat hepatocellular carcinoma: Review and future prospects. Immunotherapy. 2014;6:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butterfield LH, Koh A, Meng W, Vollmer CM, Ribas A, Dissette V, et al. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59:3134–3142. [PubMed] [Google Scholar]
- 54.Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–2825. [DOI] [PubMed] [Google Scholar]
- 55.Thimme R, Neagu M, Boettler T, Neumann-Haefelin C, Kersting N, Geissler M, et al. Comprehensive analysis of the alpha-fetoprotein-specific CD8+ T cell responses in patients with hepatocellular carcinoma. Hepatology. 2008;48:1821–1833. [DOI] [PubMed] [Google Scholar]
- 56.Nakagawa H, Mizukoshi E, Kobayashi E, Tamai T, Hamana H, Ozawa T, et al. Association between high-avidity T-cell receptors, induced by alpha-fetoprotein-derived peptides, and anti-tumor effects in patients with hepatocellular carcinoma. Gastroenterology. 2017;152:1395–1406 e10. [DOI] [PubMed] [Google Scholar]
- 57.Lu X, Deng S, Xu J, Green BL, Zhang H, Cui G, et al. Combination of AFP vaccine and immune checkpoint inhibitors slows hepatocellular carcinoma progression in preclinical models. J Clin Invest. 2023;133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, et al. Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol. 2022;28:216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kew M. Alpha-fetoprotein in primary liver cancer and other diseases. Gut. 1974;15:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zawadzki ZA, Kraj MA. Alpha fetoprotein in hepatocellular disease and neoplastic disorders. Am J Gastroenterol. 1974;61:45–52. [PubMed] [Google Scholar]
- 61.Liau SS, Qureshi MS, Praseedom R, Huguet E. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J Gastrointest Surg. 2013;17:1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoot JHMB, Coelen RJS, De Jong MC, Dejong CHC. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: A systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010;12:509–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson KA, Weber SM, Fong Y, Blumgart LH. Malignant transformation of hepatic adenoma with recurrence after resection. HPB (Oxford). 2002;4:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vijay A, Elaffandi A, Khalaf H. Hepatocellular adenoma: An update. World J Hepatol. 2015;7:2603–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.European Association for the Study of the L . EASL Clinical Practice Guidelines on the management of benign liver tumours. J Hepatol. 2016;65:386–398. [DOI] [PubMed] [Google Scholar]
- 66.Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963;1:174–180. [DOI] [PubMed] [Google Scholar]
- 67.IuS T. [Detection of embryo-specific alpha-globulin in the blood serum of a patient with primary liver cancer]. Vopr Med Khim. 1964;10:90–91. [PubMed] [Google Scholar]
- 68.Abelev GI, Assecritova IV, Kraevsky NA, Perova SD, Perevodchikova NI. Embryonal serum alpha-globulin in cancer patients: diagnostic value. Int J Cancer. 1967;2:551–558. [DOI] [PubMed] [Google Scholar]
- 69.Tatarinov Iu S. [Content of the embryo-specific alpha-globulin in the serum of the fetus, newborn infant and adult man with primary liver cancer]. Vopr Med Khim. 1965;11:20–24. [PubMed] [Google Scholar]
- 70.Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, et al. International Liver Cancer Association (ILCA) White Paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160:2572–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, et al. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419–5423. [PubMed] [Google Scholar]
- 72.Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, et al. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–1383. [DOI] [PubMed] [Google Scholar]
- 73.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamagata Y, Katoh H, Nakamura K, Tanaka T, Satomura S, Matsuura S. Determination of alpha-fetoprotein concentration based on liquid-phase binding assay using anion exchange chromatography and sulfated peptide introduced antibody. J Immunol Methods. 1998;212:161–168. [DOI] [PubMed] [Google Scholar]
- 76.Kagebayashi C, Yamaguchi I, Akinaga A, Kitano H, Yokoyama K, Satomura M, et al. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal Biochem. 2009;388:306–311. [DOI] [PubMed] [Google Scholar]
- 77.Yu R, Xiang X, Tan Z, Zhou Y, Wang H, Deng G. Efficacy of PIVKA-II in prediction and early detection of hepatocellular carcinoma: A nested case-control study in Chinese patients. Sci Rep. 2016;6:35050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–153. [DOI] [PubMed] [Google Scholar]
- 80.Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, et al. GALAD score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol Biomarkers Prev. 2019;28:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146:1249–55 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White DL, Richardson P, Tayoub N, Davila JA, Kanwal F, El-Serag HB. The updated model: An adjusted serum alpha-fetoprotein-based algorithm for hepatocellular carcinoma detection with hepatitis C virus-related cirrhosis. Gastroenterology. 2015;149:1986–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tayob N, Richardson P, White DL, Yu X, Davila JA, Kanwal F, et al. Evaluating screening approaches for hepatocellular carcinoma in a cohort of HCV related cirrhosis patients from the Veteran’s Affairs Health Care System. BMC Med Res Methodol. 2018;18:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tayob N, Christie I, Richardson P, Feng Z, White DL, Davila J, et al. Validation of the hepatocellular carcinoma early detection screening (HES) algorithm in a cohort of veterans with cirrhosis. Clin Gastroenterol Hepatol. 2019;17:1886–1893.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singal AG, Tayob N, Mehta A, Marrero JA, Jin Q, Lau J, et al. Doylestown Plus and GALAD demonstrate high sensitivity for HCC detection in patients with cirrhosis. Clin Gastroenterol Hepatol. 2022;20:953–955 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu M, Wu R, Liu X, Xu H, Chi X, Wang X, et al. Validation of the GALAD model and establishment of GAAP model for diagnosis of hepatocellular carcinoma in Chinese patients. J Hepatocell Carcinoma. 2020;7:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren T, Hou X, Zhang X, Chen D, Li J, Zhu Y, et al. Validation of combined AFP, AFP-L3, and PIVKA II for diagnosis and monitoring of hepatocellular carcinoma in Chinese patients. Heliyon. 2023;9:e21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. [DOI] [PubMed] [Google Scholar]
- 89.Breborowicz J. Microheterogeneity of human alphafetoprotein. Tumour Biol. 1988;9:3–14. [DOI] [PubMed] [Google Scholar]
- 90.Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69:1983–1994. [DOI] [PubMed] [Google Scholar]
- 91.Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision HCC screening. Hepatology. 2023;78:319–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colli A, Nadarevic T, Miletic D, Giljaca V, Fraquelli M, Štimac D, et al. Abdominal ultrasound and alpha-foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev. 2021;4:CD013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singal AG, Haaland B, Parikh ND, Ozbay AB, Kirshner C, Chakankar S, et al. Comparison of a multitarget blood test to ultrasound and alpha-fetoprotein for hepatocellular carcinoma surveillance: Results of a network meta-analysis. Hepatol Commun. 2022;6:2925–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gopal P, Yopp AC, Waljee AK, Chiang J, Nehra M, Kandunoori P, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12:870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology. 2018;154:1706–1718 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. [DOI] [PubMed] [Google Scholar]
- 97.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. [DOI] [PubMed] [Google Scholar]
- 98.Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Than NN, Ghazanfar A, Hodson J, Tehami N, Coldham C, Mergental H, et al. Comparing clinical presentations, treatments and outcomes of hepatocellular carcinoma due to hepatitis C and non-alcoholic fatty liver disease. QJM. 2017;110:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 102.Turshudzhyan A, Wu GY. Persistently rising alpha-fetoprotein in the diagnosis of hepatocellular carcinoma: A review. J Clin Transl Hepatol. 2022;10:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Su TH, Chang SH, Chen CL, Liao SH, Tseng TC, Hsu SJ, et al. Serial increase and high alpha-fetoprotein levels predict the development of hepatocellular carcinoma in 6 months. Hepatol Res. 2023;53:1021–1030. [DOI] [PubMed] [Google Scholar]
- 104.Wong RJ, Ahmed A, Gish RG. Elevated alpha-fetoprotein: Differential diagnosis—Hepatocellular carcinoma and other disorders. Clin Liver Dis. 2015;19:309–323. [DOI] [PubMed] [Google Scholar]
- 105.Zhou JM, Wang T, Zhang KH. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2021;100:e27673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katoh H, Nakamura K, Tanaka T, Satomura S, Matsuura S. Automatic and simultaneous analysis of Lens culinaris agglutinin-reactive alpha-fetoprotein ratio and total alpha-fetoprotein concentration. Anal Chem. 1998;70:2110–2114. [DOI] [PubMed] [Google Scholar]
- 107.Ma H, Sun X, Chen L, Cheng W, Han XX, Zhao B, et al. Multiplex immunochips for high-accuracy detection of AFP-L3% based on surface-enhanced Raman scattering: Implications for early liver cancer diagnosis. Anal Chem. 2017;89:8877–8883. [DOI] [PubMed] [Google Scholar]
- 108.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. High-sensitivity Lens culinaris agglutinin-reactive alpha-fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol. 2014;49:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singal AG, Tayob N, Mehta A, Marrero JA, El‐Serag H, Jin Q, et al. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology. 2022;75:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): A phase 3 biomarker study in the United States. Clin Gastroenterol Hepatol. 2023;21:415–423 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang T, Zhang KH. New blood biomarkers for the diagnosis of AFP-negative hepatocellular carcinoma. Front Oncol. 2020;10:1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pang B, Leng Y, Wang X, Wang Y, Jiang L. A meta-analysis and of clinical values of 11 blood biomarkers, such as AFP, DCP, and GP73 for diagnosis of hepatocellular carcinoma. Ann Med. 2023;55:42–61. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Tayob N, Corley DA, Christie I, Almers L, Rahal AK, Richardson P, et al. Validation of the updated hepatocellular carcinoma early detection screening algorithm in a community-based cohort of patients with cirrhosis of multiple etiologies. Clin Gastroenterol Hepatol. 2021;19:1443–1450 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang M, Devarajan K, Singal AG, Marrero JA, Dai J, Feng Z, et al. The Doylestown algorithm: A test to improve the performance of AFP in the detection of hepatocellular carcinoma. Cancer Prev Res (Phila). 2016;9:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang M, Sanda M, Comunale MA, Herrera H, Swindell C, Kono Y, et al. Changes in the glycosylation of Kininogen and the development of a Kininogen-based algorithm for the early detection of HCC. Cancer Epidemiol Biomarkers Prev. 2017;26:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Y, Yang X, Shao Y, Zhao H, Jiang J, Huang P, et al. Comparison of diagnostic performance of AFP, DCP and two diagnostic models in hepatocellular carcinoma: A retrospective study. Ann Hepatol. 2023;28:101099. [DOI] [PubMed] [Google Scholar]
- 117.Yang T, Xing H, Wang G, Wang N, Liu M, Yan C, et al. A novel online calculator based on serum biomarkers to detect hepatocellular carcinoma among patients with hepatitis B. Clin Chem. 2019;65:1543–1553. [DOI] [PubMed] [Google Scholar]
- 118.Liu SY, Li C, Sun LY, Guan MC, Gu LH, Yin DX, et al. ASAP score versus GALAD score for detection of hepatitis C-related hepatocellular carcinoma: A multicenter case-control analysis. Front Oncol. 2022;12:1018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sato M, Morimoto K, Kajihara S, Tateishi R, Shiina S, Koike K, et al. Machine-learning approach for the development of a novel predictive model for the diagnosis of hepatocellular carcinoma. Sci Rep. 2019;9:7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Von Felden J, Garcia-Lezana T, Dogra N, Gonzalez-Kozlova E, Ahsen ME, Craig A, et al. Unannotated small RNA clusters associated with circulating extracellular vesicles detect early stage liver cancer. Gut. 2021;71:1935–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun N, Zhang C, Lee YT, Tran BV, Wang J, Kim H, et al. HCC EV ECG score: An extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma. Hepatology. 2023;77:774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022;20:173–182 e7. [DOI] [PubMed] [Google Scholar]
- 123.Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, et al. A multi-analyte cell-free DNA-based blood test for early detection of hepatocellular carcinoma. Hepatol Commun. 2022;6:1753–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, et al. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci USA. 2019;116:6308–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campani C, Bamba‐Funck J, Campion B, Sidali S, Blaise L, Ganne‐Carrié N, et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab. Liver Int. 2023;43:708–717. [DOI] [PubMed] [Google Scholar]
- 126.Chan MY, She WH, Dai WC, Tsang SHY, Chok KSH, Chan ACY, et al. Prognostic value of preoperative alpha-fetoprotein (AFP) level in patients receiving curative hepatectomy—An analysis of 1,182 patients in Hong Kong. Transl Gastroenterol Hepatol. 2019;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7:12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Su CW, et al. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer. 2016;63:25–33. [DOI] [PubMed] [Google Scholar]
- 129.Hsu CY, Huang YH, Hsia CY, Su CW, Lin HC, Loong CC, et al. A new prognostic model for hepatocellular carcinoma based on total tumor volume: The Taipei Integrated Scoring System. J Hepatol. 2010;53:108–117. [DOI] [PubMed] [Google Scholar]
- 130.Daniele B, Annunziata M, Barletta E, Tinessa V, Di Maio M. Cancer of the Liver Italian Program (CLIP) score for staging hepatocellular carcinoma. Hepatol Res. 2007;37(suppl 2):S206–S209. [DOI] [PubMed] [Google Scholar]
- 131.Mahmud N, John B, Taddei TH, Goldberg DS. Pre-transplant alpha-fetoprotein is associated with post-transplant hepatocellular carcinoma recurrence mortality. Clin Transplant. 2019;33:e13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154:128–139. [DOI] [PubMed] [Google Scholar]
- 133.Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, et al. Recurrence after liver transplantation for hepatocellular carcinoma: A new MORAL to the story. Ann Surg. 2017;265:557–564. [DOI] [PubMed] [Google Scholar]
- 134.Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: Analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. [DOI] [PubMed] [Google Scholar]
- 135.Fan J, Yang GS, Fu ZR, Peng ZH, Xia Q, Peng CH, et al. Liver transplantation outcomes in 1,078 hepatocellular carcinoma patients: A multi-center experience in Shanghai, China. J Cancer Res Clin Oncol. 2009;135:1403–1412. [DOI] [PubMed] [Google Scholar]
- 136.Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Costentin C, Piñero F, Degroote H, Notarpaolo A, Boin IF, Boudjema K, et al. R3-AFP score is a new composite tool to refine prediction of hepatocellular carcinoma recurrence after liver transplantation. JHEP Rep. 2022;4:100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tran BV, Moris D, Markovic D, Zaribafzadeh H, Henao R, Lai Q, et al. Development and validation of a REcurrent Liver cAncer Prediction ScorE (RELAPSE) following liver transplantation in patients with hepatocellular carcinoma: Analysis of the US Multicenter HCC Transplant Consortium. Liver Transpl. 2023;29:683–697. [DOI] [PubMed] [Google Scholar]
- 139.Norman JS, Li PJ, Kotwani P, Shui AM, Yao F, Mehta N. AFP-L3 and DCP strongly predict early hepatocellular carcinoma recurrence after liver transplantation. J Hepatol. 2023;79:1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—Development and validation of the CRAFITY score. J Hepatol. 2022;76:353–363. [DOI] [PubMed] [Google Scholar]
- 141.Yang Y, Ouyang J, Zhou Y, Zhou J, Zhao H. The CRAFITY score: A promising prognostic predictor for patients with hepatocellular carcinoma treated with tyrosine kinase inhibitor and immunotherapy combinations. J Hepatol. 2022;77:574–576. [DOI] [PubMed] [Google Scholar]
- 142.Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–870. [DOI] [PubMed] [Google Scholar]
- 143.Sato M, Moriyama M, Fukumoto T, Yamada T, Wake T, Nakagomi R, et al. Development of a transformer model for predicting the prognosis of patients with hepatocellular carcinoma after radiofrequency ablation. Hepatol Int. 2024;18:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]