This is one of the many vivid memories shared by Mark, an intensive care unit (ICU) delirium survivor:
I vividly remember the feeling of a bullet burning through my brain before I woke up back in my torture; how had I survived? They had healing nanobots in me, so I could not die.
His story begins on the last day he remembers of 2015, December 26. Mark struggled to breathe at home, so his parents took him to the emergency department, where multiple teams evaluated him. Initially, he was admitted to a general care ward, where he was agitated and aggressive and subsequently intubated and transferred to the ICU within hours. This is where his delirium began.
Mark was sedated and in a medically induced coma for 3 weeks. What follows is the memory of what he experienced during his delirium; it is not pleasant for him to recall or share, but it needs to be told.
I was captured by a government agency and was tortured to find out the location of my family. I was waterboarded (probably due to my ARDS [acute respiratory distress syndrome] and lungs filling up with fluid), had my throat cut open, and was left to bleed out before being sutured up (placing of [central venous catheter]), and had my wrists cut open to bleed. Probes were inserted to shock me (placing of [arterial catheters]), and I was not allowed to sleep by being woken up constantly and bombarded by white noise (ICU machines and lights being turned on and off). These events were [interspersed] by my attempts to escape, all ending in recapture. This led me to find a gun in my delirium (not reality), putting it to the side of my head, and pulling the trigger. I vividly remember the feeling of a bullet burning through my brain before I woke up back in my torture; how had I survived? They had healing nanobots in me, so I could not die. All the staff in the ICU were my torturers.
Except for one staff member, Nurse Q. Mark recalls how he could hear and recognize Nurse Q’s voice through his delirium. Even when he was still receiving mechanical ventilation and not aware enough to interact, he recalls Nurse Q speaking and calming him.
Nurse Q spoke to me about what they were doing, and why, and was very protective in not allowing things to happen that they thought would harm me (I could hear them talking).
Mark was extremely weak after he woke up from sedation, having lost about 60 pounds. This translated into a long physical recovery. He was able to walk short distances (100 yards) when he was discharged from the hospital approximately 4 months later, but it took about 2 years to fully recover. The scale of his physical recovery, however, paled in comparison with that of his mental and emotional recovery. After his ICU stay, he suffered from depression in the hospital and anxiety and posttraumatic stress disorder after his stay. He says, “Seven years later, I still have issues with anxiety and low mood and have not yet fully recovered from my stay in ICU.”
Mark’s story allows us to recognize the importance of preventing this too-common experience. Effective evidence-based strategies for preventing and reducing delirium for future patients are available and reviewed in this article. The Table provides an overview of the existing evidence to apply the integrated approach of addressing patient needs, which involves (1) critical thinking by the clinician and (2) acting using the ABCDEF bundle to proactively prevent or reduce delirium in critically ill patients.
Table:
An Overview of Existing Evidence to Prevent or Reduce Delirium in Critically III Patients
| Intervention | Description and Evidence |
|---|---|
| Critical Thinking to Address Patient Needs | |
| Discomfort | Anxiety, depression, isolation, not being understood, sleep deprivation, thirst (internal sources), bed quality, endotracheal tubes, excess noise, immobility caused by equipment, lighting perfusion catheters (external sources) all can contribute to a patient’s discomfort.1 |
|
|
|
| Anxiety | > 50% of intensive care unit patients report experiencing some degree of anxiety. Music listening (either self-initiated or prescribed) is an effective, evidence-based strategy to reduce anxiety and reduce sedative exposure.2 |
|
|
|
| Communication | Several tools exist to support communication with nonverbal patients including an electronic tablet, a small dry-erase board, picture-letter boards, and mobile health applications such as VidaTalk.3 |
|
| |
| ABCDEF Bundle to Prevent and Reduce Delirium | |
| A: Assess Pain | Pain can precipitate and perpetuate delirium and agitation. Pain (at rest and during procedures) should be readily assessed, prevented when possible, and managed using validated tools (patient-report or observational based).4 Sedation does not treat pain. Sedation can mask signs of pain. Validated and reliable tools for nonverbal patients (PADIS guidelines4): • Critical Care Pain Observation Tool (CPOT, 4 items) • Behavioral Pain Scale (BPS, 3 items) |
|
|
|
| B: Breathing | Invasive ventilation is a risk factor for delirium. It may also increase anxiety, discomfort, agitation, and restlessness.5 Both spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs) should occur at least once daily (SAT occurs if patient is sedated). Richmond Agitation and Sedation Scale (RASS) is a widely validated and reliable tool for assessing level of awareness. |
|
|
|
| C: Choice of Sedation | Evaluate the need for sedation. Interprofessional discussion should occur with health care team (including patient and family). Perform a risk vs benefit analysis with unified understanding of high risks for prolonged sedation. PADIS guidelines outline best practice recommendations for sedative type.4 Benzodiazepines are not recommended, as they increase the risk of delirium.4 |
|
|
|
| D: Delirium | Detect delirium using validated and reliable tools at least once per shift or with any acute change. • CAM-ICU (4 items, < 2 min to administer and document) • ICDSC (8 items, <2 minutes to administer and document) • Training on the use of these two tools is well-established and available online through the Critical Illness, Brain Dysfunction, and Survivorship (CIBS) Center Incorporate interventions to reduce or prevent delirium throughout shift. • Hourly reorientation • Cognitive stimulation • Activity/Mobility • Sleep hygiene • Environmental modifications • Family involvement |
|
|
|
| E: Early Mobility | “Walk when wild, walk when sluggish.”6—Dr Bill Beninati Use early mobility assessments and protocols to assess the patient’s ability. A recent study reported a 95% decrease in the odds of delirium with daily mobilization.7 Refer to the PADIS guidelines and discussion in this article for a full review of evidence for this bundle element. |
|
|
|
| F: Family | The presence and involvement of family in routine care reduces the risk of delirium by almost 40%.5 This is either in person or virtual. Family can assist with SAT, reorientation, cognitive stimulation, and many other tasks such as oral care, suctioning, bathing, pushing the wheelchair or intravenous poles during ambulation, and many other patient care needs.8 |
Abbreviations: CAM-ICU, Confusion Assessment Method for the ICU; ICDSC, Intensive Care Delirium Screening Checklist; PADIS guidelines, 2018 Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU.
Delirium is an acute physiologic disruption of the brain leading to a sharp change and fluctuation of consciousness and cognition and suggests the existence of an underlying acute encephalopathy.9,10 Upwards of 50% to 75% of critically ill patients will experience delirium in the ICU, with significant consequence.11 Critically ill patients with delirium are twice as likely to die compared with the average patient in the ICU (according to a meta-analysis; odds ratio [OR], 7.09; 95% CI, 3.60-14.0), to develop dementia (OR, 2.30; 95%CI, 1.85-2.86), to stay an additional 1.9 days in the ICU, and to be discharged to an institution instead of returning home (relative risk, 2.17).11,12
Delirium is incredibly distressing to the patient, family, and clinicians. Survivors of delirium often have vivid memories of hallucinations, delusions, and fear that they experienced during delirium.13 Approximately 50% of ICU survivors will experience post–intensive care syndrome, a consequence of delirium, leading to an inability to return to work, reduced quality of life for patients and their families, and continued deficits in cognitive, physical, and mental health abilities.14
Delirium is a health care emergency. This article presents available evidence to prevent and reduce delirium in the critical care setting. Implications for advanced practice registered nurses (APRNs) and nursing leadership at the patient care unit and system levels are discussed. We recognize that some readers may be familiar with the ABCDEF bundle, or the care concepts represented within the bundle. We also recognize how the COVID-19 pandemic changed the critical care environment and that many clinicians may not be as familiar with the bundle or how to integrate or use it in their daily patient care routines. Therefore, we define the bundle elements, tools, and assessments in the Table and provide an example to demonstrate how bundle integration can be accomplished within a shift.
Overview and Integration of Existing Evidence
Delirium care must use an integrated approach—symptom assessment combined with critical thinking—to put tools and strategies into action to prevent and reduce delirium. This integrated approach is crucial because delirium is a complex, multifactorial syndrome that requires anticipation of patient needs, assessment of symptoms, and continued action to effectively prevent or reduce the occurrence, duration, and severity.
The Table outlines strategies to support the assessment of patient needs. This includes, but is not limited to, the ability of the patient to communicate needs and discomfort, the ability to know or understand what is happening to them and in their room, and the assessment of symptoms such as anxiety, nausea, discomfort, thirst, and fear that may hinder their ability to fully participate in their care.1–3 To achieve these abilities, the ABCDEF bundle is operationalized to minimize sedation, treat pain, optimize breathing, address delirium, increase mobility, and integrate family involvement.4,11
The Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) guidelines and the ABCDEF bundle are operational tools to prevent and treat delirium. The 2018 PADIS guidelines outline the evidence and provide a framework for the implementation of best practices to optimize patient health and recovery during their ICU stay.4 The ABCDEF bundle is a toolkit used to apply the PADIS guidelines to each patient.4,15 A study of 15 226 adult ICU patients reported significantly reduced rates of delirium, coma, mortality, and institutionalization with ABCDEF bundle use16 (Figure). In this study, there was an observed 25% to 50% decrease in delirium despite variations in adherence to all components of the ABCDEF bundle. The more components applied, the less delirium occurred.11,16 This evidence provides a starting point for practice. Depending on the clinical situation, completing each ABCDEF bundle component every shift may be difficult. However, achieving even 1 or 2 components will aid in reducing delirium.
Figure:
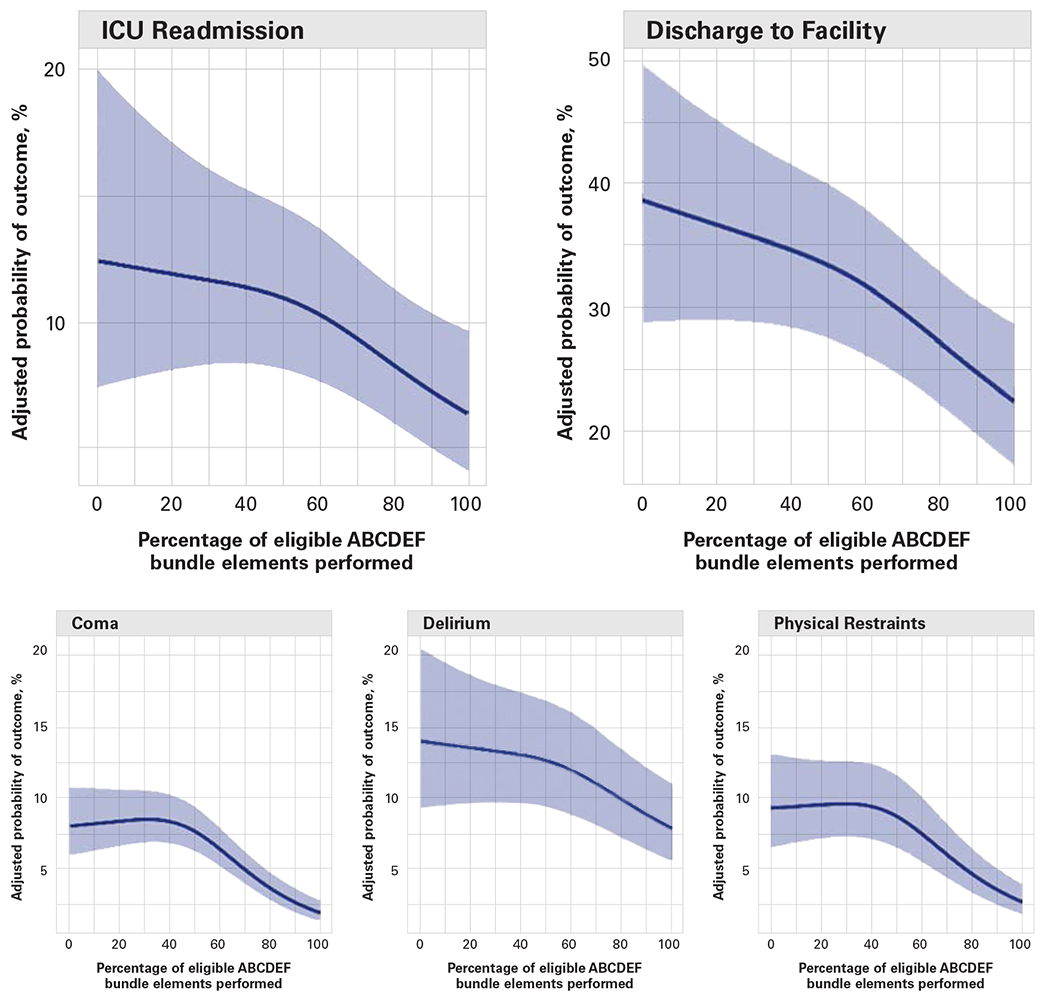
The outcomes of mechanical ventilation, significant pain, coma, delirium, physical restraints, ICU readmission, and discharge to a facility and their association with the percentage of ABCDEF bundle elements performed.17 ICU indicates intensive care unit.
This integrated approach is iterative and cyclical. The use of feedback loops (ie, reassessment of symptoms) helps to inform the next step in care. The ABCDEF bundle can be used as a checklist; however, each step is interconnected as shown through the example. The ability to address patient symptoms and provide interventions to reduce delirium (cognitive stimulation, mobility, etc) is related to the patient’s ability to communicate, which is dependent on the level of sedation. If the patient’s condition requires sedation, continual action is required to address signs of discomfort, anxiety, pain, and delirium; clinicians should also continually reassess the need for sedation. The least amount of sedation needed should be used to maximize patient communication of needs and ABCDEF bundle actions.4,11,15 The ABCDEF bundle is A: assess, prevent, and manage pain (and anxiety, fear, thirst, etc); B: both spontaneous awakening trials (SATs) and spontaneous breathing trials (SBTs); C: choice of analgesia and sedation; D: delirium—assess, prevent, and manage; E: exercise and early mobility; and F: family involvement (see Table).
Delirium Care in Action: ICU Liberation With the Awake-and-Walking ICU
The ICU Liberation Campaign is an initiative through the Society of Critical Care Medicine that provides evidence-based strategies to use the PADIS guidelines and the ABCDEF bundle in practice.17 We provide examples of the ICU Liberation Campaign by describing a day in the awake-and-walking ICU in the next section. In an awake-and-walking ICU, an interprofessional team masters critical thinking and the tools in the PADIS guidelines and the ABCDEF bundle to ensure that all patients are awake and actively mobile unless specific contraindications are present. Although the Table presents the ABCDEF bundle in a prescribed order, in clinical care, the bundle elements are integrated within patient care and are often not sequential. One of the first awake-and-walking ICUs was founded by 2 APRNs and a physician in the late 1990s at Latter Day Saints Hospital in Salt Lake City, Utah.18 Their 2007 study demonstrated it was safe and feasible to walk intubated patients in acute respiratory failure who had higher illness severity.19 Five randomized controlled trials and 8 observational studies have substantiated this finding as reported by the 2018 PADIS guidelines.4 The awake-and-walking ICU team approach is supported by the PADIS guidelines and the results from the ICU Liberation Collaborative.4,17
A Day in the Awake-and-Walking ICU
Upon admission, and at the start of every shift, the patient’s needs are evaluated from a symptom perspective, and the necessity of sedation (if intubated) is reviewed. Patient comfort is addressed upfront to reduce any symptoms of pain (A: assess pain), anxiety, fear, discomfort, thirst, and other patient needs (critical thinking) that may prohibit the patient’s ability to participate in their care.
Mechanical ventilation is not an automatic indication for sedation.11,20 When sedation is necessary—such as in cases of status epilepticus, intracranial hypertension, or difficulty with oxygen consumption—PADIS guidelines are followed to choose the least harmful sedative (C: choice of sedation). Upon resolution of the underlying medical condition, SATs and SBTs (B: breathing) should be resumed with the goal of sedation cessation. The lowest dose to achieve a Richmond Agitation and Sedation Score (RASS) of −2 to +1 (light sedation) should be used during sedation except for severe cases, such as those listed above.4
Critical care sedation practices have advanced over the past 2 decades. Although the present-day ICU sedation practice recommendations are light sedation and the avoidance of benzodiazepines, deep sedation remains common practice in some ICUs, especially after the COVID-19 pandemic.21 A common misconception is that deeper sedation is necessary for any intubated patient on mechanical ventilation, and that it will improve safety, comfort, and survival.21,22 Deep sedation leads to multiple downstream consequences including unassessed symptoms, and thus, untreated anxiety, discomfort, and pain; increased delirium risk; and impaired mobility and communication.11,21 Deep sedation is a barrier to early mobility and family engagement because deep sedation prevents patient interaction. Re-engaging with the PADIS guidelines and the ABCDEF bundle are critical to lead with best practice recommendations.21
An SAT and SBT should occur at least once per shift when a patient is sedated (B: breathing). Critical thinking regarding patient needs (ie, symptoms) and how to use the ABCDEF bundle tools are essential to the success of SATs and SBTs. Agitation and restlessness during awakening trials can be a barrier to success. Clinicians need to evaluate and treat root causes of agitation such as fear, inability to communicate, anxiety, delirium, pain, and physical or emotional needs prior to an SAT.1,3,4 Explaining the steps in the SAT and SBT to the patient and family prior to initiation prepares both parties for how the SAT and SBT will feel and sets expectations. Successful SBTs and SATs involve a team approach with the nurse, patient, family, physician, respiratory therapist, and occupational, physical, and speech therapists (F: family). As the patient becomes more aware, signs of agitation and underlying delirium may occur. Family and clinician presence, reassurance, and reorientation help to mitigate these signs and coordinate breathing efforts (D: delirium). This is where the saying “Walk when wild, walk when sluggish”7 by Dr Bill Beninati comes into play (E: early mobility). Activity and mobility are excellent team-oriented tools to apply and activate the patient’s brain, increasing awareness, and therefore preventing or reducing delirium; occupational, physical, and speech therapy are key partners in activity and mobility interventions.
The implementation of SAT and SBT protocols must use a coordinated, team approach to identify the appropriate timing and needed safety screens.23 The timing of these interventions is important. If an SAT and SBT are approached without all team members present, the patient, nurse, and family may not have the necessary support to successfully achieve the desired outcomes (ie, extubation, no sedation). An approach with an incomplete team increases the risk of a failed attempt and the resumption of sedation.
Integrated in the awake-and-walking ICU approach is routine delirium assessment (at least once per shift and with every acute change; D: delirium). This increases the rate of early detection, leading to faster mitigation of signs and behaviors associated with delirium. Interweaving reorientation, cognitive stimulation (conversation, music, games, etc), physical activity, sleep enhancement (bundle cares, ear plugs, and eye mask at night), and family presence into every shift actively works to prevent and mitigate delirium. Family members can assist with many interventions (F: family).9 Awake-and-walking ICUs have open doors and accept families as a part of the ICU team in the preservation of patients’ lives.
Acute encephalopathy is the medical term for delirium (formally called acute brain failure), and it is as essential to screen for it as it is to assess for things such as acute renal failure. When clinicians understand that delirium is potentially fatal or can have life-long repercussions, they will have a sense of urgency to prevent, detect, and intervene. Avoiding or minimizing sedation allows use of delirium prevention and treatment tools such as mobility, cognitive stimulation, sleep enhancement, and family presence.
At the end of every shift, celebrate the small wins as a team (patient, family, health care members) that occurred to maintain the patient’s comfort (ie, symptom management), and the patient’s cognitive (ie, delirium negative) and functional state (ie, maintaining baseline functional ability). This appreciation for small wins creates the positive feedback necessary to make incremental gains.
APRN’s Leader Role: Next Steps
The APRN in an awake-and-walking ICU plays a key role in assisting patients to navigate the complex maze of critical illness and achieve the ultimate goal, to survive and thrive. APRNs particularly provide continuity of care and the big picture perspective that guides critical thinking and decision-making that can determine the trajectory of a patient’s life in the ICU and beyond. An APRN, particularly a clinical nurse specialist (CNS), can lead the interdisciplinary team in collaboration and facilitate the use of each discipline’s expertise to meet the nuanced needs of each patient. APRNs stand with the health care team including physicians, nurses, therapists, pharmacists, and families, to protect and liberate patients from delirium through thoughtful implementation of the ABCDEF bundle.
The CNS is integral to implementing change at the unit and system level. A compliance focus has driven the adoption of the PADIS guidelines and the ABCDEF bundle, leading to clinicians not understanding the “why” behind these tools. Integrating a CNS into the implementation and compliance team can help clinicians learn and use these best practices to optimize patient outcomes.
Communication with the overall health care team, including the patient and family, is also integral to the success of the PADIS guidelines and the ABCDEF bundle. The patient must be an active participant regardless of ventilation status. If the patient is unable to participate, then corrective steps to improve awareness (minimizing sedation, mobilizing, reorientation) must be taken. Patients who are unable to communicate may experience heightened anxiety, distress, and helplessness. These symptoms may exacerbate delirium. Several methods to augment and support communication in nonverbal, or minimally verbal, patients exist and are available for use with patients in the ICU.3
System-level and unit-level changes assist in fully integrating this approach into ICU care. This integration should follow a team approach, including a patient and family representative and bedside clinicians. Details on staffing and unit culture within the awake-and-walking ICU are available.18,19 Additionally, the Society for Critical Care Medicine has in-depth resources on barriers and strategies to successful implementation, including a publication on the interpretation and implementation of the PADIS guidelines by Balas et al.15,23,24 A change management framework, such as Agile implementation, is important to guide the interprofessional team as protocols and policies are localized, tested, evaluated, and implemented.25
Initial and continuing education of the entire ICU team, including patients and families, is important for the optimal use of this approach. Nurse education specialists are key to supporting this initiative. Explaining the “why” behind the actions is essential to communicating the need for and the vision behind the ICU Liberation Campaign and a team approach as shown in the awake-and walking ICU examples. Understanding the reality of delirium and the role of sedation in delirium; receiving practical training on how to manage symptoms and behaviors related to anxiety, fear, agitation, delirium, or restlessness to meet patient needs; and learning how to collaboratively respond to delirium are all examples of educational needs. In addition to education, developing and implementing nudges, or reminders, for the teams will help sustain the education provided. Celebrating the small wins in the moment as a team is one example of a nudge that provides positive feedback and learnings that can be built on in the next shift.
Conclusion
As illustrated by Mark, delirium is a terrifying experience with significant downstream consequences. Evidence-based tools and approaches exist to prevent and reduce delirium. The awake-and-walking ICU approach provides a blueprint for other ICUs to follow, to fully integrate this approach into every shift at the bedside. Mark shares his conclusions about his experience of ICU delirium:
These are priorities going forward from my perspective. First is the need for [examining] sedation, as it increases delirium risk. The PADIS [guidelines] recommended sedatives should only be used for safety or clinical reasons. Second, sedentary positions and inactivity lead to weight loss and weakness. This can be combated by a less aggressive sedation plan, allowing the patient to safely work with therapists to stop muscle loss. Lastly, prevention is better than cure, it is better for everyone if the patient does not get weak, as it causes so many complications.
In this article, available evidence to prevent and reduce delirium in the ICU setting was presented and discussed. Implications for APRNs and nursing leadership at a patient care unit and system level were discussed and recommendations were made for next steps.
ACKNOWLEDGMENTS
The authors of this article would like to express their appreciation to Sikandar Khan, do, ms, David E. Dwyer, phd, rn, and Jesus Casida, phd, rn, apn-c, for their thoughtful review and feedback on the development of this article.
The authors declare no conflicts of interest. Heidi Lindroth is supported by a grant from the National Institute of Health, National Institute on Aging, 1K23AG07662-01.
Contributor Information
Kali Dayton, ICU Sedation and Mobility Consultant, Dayton ICU Consulting, Washington.
Mark Hudson, ICU survivor and patient advocate for improved ICU care; podcaster of the ICU Life and Recovery podcast; and a student at the School of Psychology and Counselling, The Open University, Milton Keynes, United Kingdom.
Heidi Lindroth, clinician-nurse scientist, Department of Nursing, Mayo Clinic, 200 1st St SW, Mayo Clinic, Rochester, MN, 55902; and an affiliate scientist, Center for Innovation and Implementation Science and the Center for Aging Research, Regenstrief Institute, School of Medicine, Indiana University, Indianapolis, Indiana.
REFERENCES
- 1.Luckhardt EM, Gunnels MS, Chlan LL. Assessing discomfort in critically ill patients: a narrative review of the literature. Crit Care Nurse. 2022;42(4):47–54. doi: 10.4037/ccn2022280 [DOI] [PubMed] [Google Scholar]
- 2.Chlan LL. Engaging critically ill patients in symptom management: thinking outside the box! Am J Crit Care. 2016;25(4):293–300. doi: 10.4037/ajcc2016932 [DOI] [PubMed] [Google Scholar]
- 3.Happ MB. Giving voice: nurse-patient communication in the intensive care unit. Am J Crit Care. 2021;30(4):256–265. doi: 10.4037/ajcc2021666 [DOI] [PubMed] [Google Scholar]
- 4.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/ccm.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 5.Pun BT, Badenes R, Heras La Calle G, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayton K, Seitz Brenna. Walking Home from the ICU. Walking home from the ICU episode 131: occupational therapists as leaders in the ICU. May 23, 2023. https://day-tonicuconsulting.com/walking-home-from-the-icu-podcast/walking-home-from-the-icu-episode-131-occupational-therapists-as-leaders-in-the-icu
- 7.Bersaneti MDR, Whitaker IY. Association between nonpharmacological strategies and delirium in the intensive care unit. Nurs Crit Care. 2022;27(6):859–866. doi: 10.1111/nicc.12750 [DOI] [PubMed] [Google Scholar]
- 8.Hetland BD, McAndrew NS, Kupzyk KA, et al. Family caregiver preferences and contributions related to patient care in the ICU. West J Nurs Res. 2022;44(3):214–226. doi: 10.1177/01939459211062954 [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 10.Oldham MA. Delirium disorder: unity in diversity. Gen Hosp Psychiatry. 2022;74:32–38. doi: 10.1016/j.genhosppsych.2021.11.007 [DOI] [PubMed] [Google Scholar]
- 11.Stollings JL, Kotfis K, Chanques G, Pun BT, Pandharipande PP, Ely EW. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021;47(10):1089–1103. doi: 10.1007/s00134-021-06503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aung Thein MZ, Pereira JV, Nitchingham A, Caplan GA. A call to action for delirium research: meta-analysis and regression of delirium associated mortality. BMC Geriatr. 2020;20(1):325. doi: 10.1186/s12877-020-01723-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehm LM, Jones AC, Selim AA, et al. Delirium-related distress in the ICU: a qualitative meta-synthesis of patient and family perspectives and experiences. Int J Nurs Stud. 2021;122:104030. doi: 10.1016/j.ijnurstu.2021.104030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colbenson GA, Johnson A, Wilson ME. Post-intensive care syndrome: impact, prevention, and management. Breathe (Sheff). 2019;15(2):98–101. doi: 10.1183/20734735.0013-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balas MC, Weinhouse GL, Denehy L, et al. Interpreting and implementing the 2018 Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption Clinical Practice Guideline. Crit Care Med. 2018;46(9):1464–1470. doi: 10.1097/CCM.0000000000003307 [DOI] [PubMed] [Google Scholar]
- 16.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47(1):3–14. doi: 10.1097/ccm.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society of Critical Care Medicine. ICU liberation. Accessed July 13, 2023. https://www.sccm.org/iculiberation [Google Scholar]
- 18.Bailey PP, Miller RR 3rd, Clemmer TP. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10)(suppl):S429–S435. doi: 10.1097/CCM.0b013e3181b6e227 [DOI] [PubMed] [Google Scholar]
- 19.Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87 [DOI] [PubMed] [Google Scholar]
- 20.Strom T Sedation in the ICU. Dan Med J. 2012;59(5):B4458. [PubMed] [Google Scholar]
- 21.Stollings JL, Balas MC, Chanques G. Evoluation of sedation management in the intensive care unit (ICU). Intens Care Med. 2022;48(11):1625–1628. doi: 10.1007/s00134-022-06806-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttormson JL, Chlan L, Tracy MF, Hetland B, Mandrekar J. Nurses’ attitudes and practices related to sedation: a national survey. Am J Crit Care. 2019;28(4):255–263. doi: 10.4037/ajcc2019526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balas MC, Pun BT, Pasero C, et al. Common challenges to effective ABCDEF bundle implementation: the ICU liberation campaign experience. Crit Care Nurse. 2019;39(1):46–60. doi: 10.4037/ccn2019927 [DOI] [PubMed] [Google Scholar]
- 24.Stollings JL, Devlin JW, Pun BT, et al. Implementing the ABCDEF bundle: top 8 questions asked during the ICU liberation ABCDEF bundle improvement collaborative. Crit Care Nurse. 2019;39(1):36–45. doi: 10.4037/ccn2019981 [DOI] [PubMed] [Google Scholar]
- 25.Azar J, Kelley K, Dunscomb J, et al. Using the agile implementation model to reduce central line-associated bloodstream infections. Am J Infect Control. 2019;47(1):33–37. doi: 10.1016/j.ajic.2018.07.0085 [DOI] [PubMed] [Google Scholar]


