Abstract
Study objective:
Emergency department (ED)–initiated buprenorphine improves outcomes in patients with opioid use disorder; however, adoption varies widely. To reduce variability, we implemented a nurse-driven triage screening question in the electronic health record to identify patients with opioid use disorder, followed by targeted electronic health record prompts to measure withdrawal and guide next steps in management, including initiation of treatment. Our objective was to assess the impact of screening implementation in 3 urban, academic EDs.
Methods:
We conducted a quasiexperimental study of opioid use disorder–related ED visits using electronic health record data from January 2020 to June 2022. The triage protocol was implemented in 3 EDs between March and July 2021, and 2 other EDs in the health system served as controls. We evaluated changes in treatment measures over time and used a difference-in-differences analysis to compare outcomes in the 3 intervention EDs with those in the 2 controls.
Results:
There were 2,462 visits in the intervention hospitals (1,258 in the preperiod and 1,204 in the postperiod) and 731 in the control hospitals (459 in the preperiod and 272 in the postperiod). Patient characteristics within the intervention and control EDs were similar across the time periods. Compared with the control hospitals, the triage protocol was associated with a 17% greater increase in withdrawal assessment, using the Clinical Opioid Withdrawal Scale (COWS) (95% CI 7 to 27). Buprenorphine prescriptions at discharge also increased by 5% (95% CI 0% to 10%), and naloxone prescriptions increased by 12% points (95% CI 1% to 22%) in the intervention EDs relative to controls.
Conclusion:
An ED triage screening and treatment protocol led to increased assessment and treatment of opioid use disorder. Protocols designed to make screening and treatment the default practice have promise in increasing the implementation of evidence-based treatment ED opioid use disorder care.
INTRODUCTION
Deaths due to overdose continue to rise in the United States, driven by fentanyl,1 with over 90,000 deaths due to overdose last year.2 Medications for opioid use disorder, such as buprenorphine and methadone, reduce all-cause and overdose mortality by more than 50% and improve a host of other outcomes.3 Initiation of buprenorphine in the emergency department (ED) more than doubles treatment engagement at 30 days compared with referral alone4,5 and is cost effective.6 Offering buprenorphine to patients with opioid use disorder in EDs has become the standard of care in emergency medicine7 and is now easier with the recent policy changes that have eliminated the requirement for a DATA 2000 waiver (“X-waiver”) to prescribe buprenorphine.8
Despite the strength of the evidence, the adoption of ED-initiated buprenorphine is inconsistent. Among commercially insured patients presenting with nonfatal opioid overdose to US EDs, less than 20% received treatment in the subsequent 90 days, and Black patients were half as likely to undergo follow-up as White patients.9 The barriers to screening and treatment include limited clinician knowledge and experience, lack of streamlined workflows, competing demands, limited postdischarge care coordination resources, and continued stigma.10–13 Prior strategies to overcome these barriers have yielded mixed results. A recent multicenter, randomized controlled trial of user-initiated embedded decision support in the electronic health record to support the treatment of opioid use disorder did not increase the overall rate of ED-initiated buprenorphine.14 A multicomponent strategy to increase buprenorphine initiation in EDs, described previously by our group, was associated with increased use of buprenorphine overall.4 However, even in this highly engaged setting, adoption of buprenorphine prescribing varied substantially across providers.15 This is consistent with findings in other settings that showed low adoption of buprenorphine treatment even among trained prescribers.16
One implementation strategy that has the potential to increase adoption and reduce variability is leveraging insights from behavioral economics to modify the environment in which choices are made to “nudge,” but not mandate, individuals to behave in beneficial ways.17 Manipulation of default options takes advantage of status quo bias: the tendency to stick with the status quo because choosing differently is perceived as a loss or requires effort.18,19 Changing default options from “opt-in” to “opt-out” can increase the uptake of target clinical behaviors and has been shown to work in many contexts, from opioid prescription to end-of-life care.20,21 Given the known racial disparities in receipt of medications for opioid use disorder,9,22 it is also important to consider how default strategies influence treatment equity. Structuring choices to make evidence-based pathways the default practice may be a mechanism to reduce disparities by promoting appropriate care; however, there is also concern that disparities might widen if automated processes are not designed to promote equity.23
To reduce variability in OUD care among our EDs, we designed and implemented a strategy intended to make identification of opioid use disorder and prompting treatment the default practice. In collaboration with stakeholders, we implemented a nurse-driven triage screening protocol for opioid use disorder at Penn Medicine.24 A positive screening result triggers a cascade of targeted electronic health record prompts and clinical decision support to assess for withdrawal and initiate buprenorphine treatment. Our aim was to assess the impact of screening implementation on clinical outcomes including the identification of patients with opioid use disorder, assessment of withdrawal, and treatment with medications for opioid use disorder as well as equity in 3 urban, academic EDs using 2 other health system EDs as controls. We hypothesized that implementation of universal screening with automated clinical decision support would increase the identification and treatment of patients with opioid use disorder in the ED by streamlining care and making the treatment of opioid use disorder the default practice.
MATERIALS AND METHODS
Study Design and Setting
We conducted a quasiexperimental study using electronic health record data for patients with opioid use disorder–related ED visits at Penn Medicine between January 2020 and June 2022. We used a difference-in-differences design to estimate the effect of the triage screening and treatment protocol comparing 3 intervention EDs with 2 control EDs in the same health system.25,26 The study was approved by the University of Pennsylvania Institutional Review Board and follows the Transparent Reporting of Evaluations with Nonrandomized Designs reporting guideline.27
Penn Medicine is a large academic health system in the Philadelphia region, which has one of the highest rates of death due to overdose among large US urban areas.28 Our study included 5 hospitals: an urban tertiary referral hospital, an urban level I trauma center, an urban hospital with an associated psychiatric crisis center, and 2 suburban community hospitals. The annual number of ED visits at each hospital range from 45,000 to 72,000. In a prior study, our group demonstrated increases in the use of buprenorphine in the 3 urban EDs following implementation of a bundled strategy to support evidence-based initiation of treatment for opioid use disorder.15 However, substantial provider-level variability in buprenorphine prescriptions remained. Despite more than 90% of prescribers having an X-waiver, clinicians wrote buprenorphine prescriptions in only 11% of opioid use disorder–related encounters, and the individual prescription rates ranged from 0% to 61% of opioid use disorder–related encounters.15
Participant Selection
The primary analysis was conducted at the visit level. We included adult patients with opioid use disorder–related visits to EDs from January 2020 to June 2022. We identified opioid use disorder–related encounters using International Classification of Diseases, Tenth Revision (ICD-10), codes for opioid use disorder and overdose (Appendix E1, available at http://www.annemergmed.com).29 We included all patients regardless of the use of medications for opioid use disorder prior to the index visit. For the description of triage screening outcomes in intervention EDs, we included screening outcomes from all ED encounters following intervention implementation.
Intervention
The triage protocol was implemented in 3 health system EDs between March and July 2021, and the remaining 2 EDs served as controls. All 5 EDs use the same version of the electronic health record with the same order sets. For this quality improvement study, we selected the 3 intervention EDs based on feasibility; the intervention EDs are part of the same faculty group, with attending and resident physician crossover among the sites, and are in close proximity. The control hospitals have separate physician groups, and there is no clinician crossover between the hospitals.
Details of the design and implementation of the intervention have been described elsewhere24 and are shown in Figure 1. Briefly, we implemented a nurse-driven protocol for screening for opioid use disorder at ED triage coupled with automated prompts to clinicians to assess and treat opioid use disorder. The goal of the process was to make assessment and treatment of opioid use disorder the default process in the ED. The process was designed iteratively by our multidisciplinary research team with input from ED leaders, physicians, nurses, peers in recovery, outpatient providers, and operational experts in clinical decision support.24 All EDs had previously implemented initiatives to increase clinician readiness to treat opioid use disorder, including X-waiver training, development and dissemination of clinical pathways and order sets, training on Clinical Opioid Withdrawal Scale (COWS) measurement, and peer recovery specialist support.15 Prior to implementation, a series of brief nurse huddles provided training on the triage screening question, and information about the screening protocol was disseminated among faculty and resident physicians. Otherwise, the intervention was largely automated and applied to all ED clinicians, including trainees and rotating providers.
Figure 1.
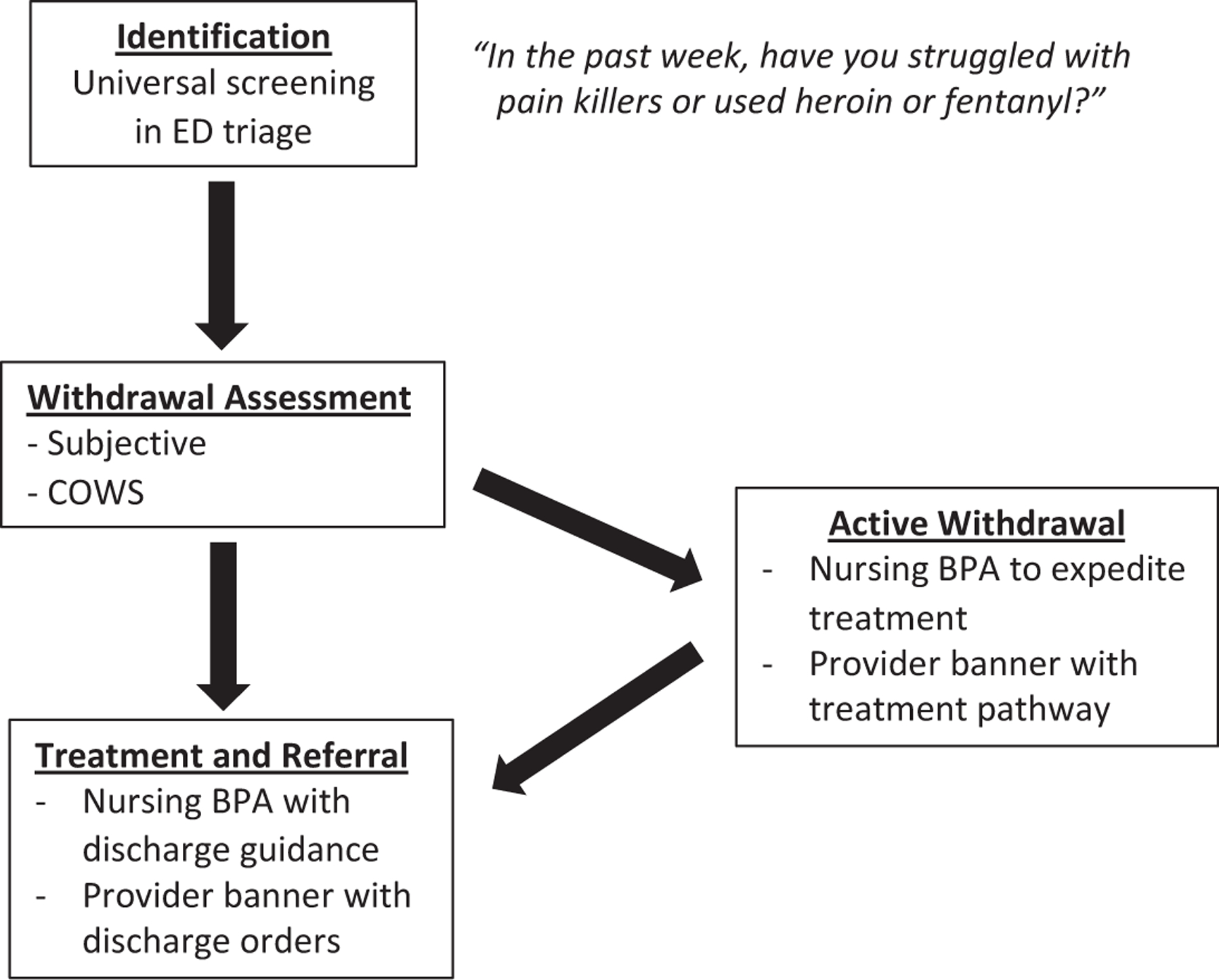
Emergency department triage screening and electronic health record prompts. The implementation strategy consisted of universal screening in ED triage, assessment of withdrawal, and tailored prompts to clinicians.23 Nurses received best practice alerts with clinical guidance, whereas physicians and advanced practice providers saw a noninterruptive banner linking to order sets with embedded decision support. Patients in active withdrawal also triggered prompts to clinicians and nurses to expedite treatment with appropriate clinical guidance. All EDs had previously implemented initiatives to increase ED clinicians’ readiness to treat opioid use disorder, including physician X-waiver training, development and dissemination of clinical pathways and order sets, training on COWS measurement, and peer recovery specialist support. Prior to implementation, nurses received training on the use of the screening question at triage, and information about the screening protocol was disseminated among faculty and resident physicians. Otherwise, the intervention was largely automated and applied to all ED clinicians, including trainees and rotating providers. BPA, best practice alert; COWS, Clinical Opioid Withdrawal Scale; ED, emergency department.
In the intervention EDs, all patients were queried about recent struggles with opioids using a single screening question (Figure 1). Answering yes to the question or presentation with overdose was considered a positive screen result, which then prompted the nurse to assess for withdrawal using COWS, a 11-item scale that quantitatively assesses signs and symptoms of opioid withdrawal.30 Patients in active withdrawal triggered a nursing best practice alert and a noninterruptive banner for providers. The best practice alert advised nurses to expedite care for patients in withdrawal, including guidance to discuss withdrawal management with medications for opioid use disorder with the physicians and provide take-home naloxone to patients.24 For patients in active withdrawal, treatment could be initiated either by a provider in triage before patients were roomed or by adjusting triage prioritization. Originally, positive screen results also triggered an automated notification to peer recovery specialists. The automated step was deimplemented shortly after protocol rollout because increased scope of responsibilities for peers outside the ED made it challenging to respond to every alert. However, ED clinicians retained the ability to consult peers as needed. All positive screen results also generated guidance on discharge orders and referrals.
Data Source
We used data from the Epic electronic health record via the Clarity reporting database (Hyperspace 2017; Epic Systems Corporation). The rates of missingness for our primary and secondary outcomes were 0%, and no patients were excluded because of missing data.
Outcomes
We evaluated clinical care measures over time, including COWS assessment, treatment with medications for opioid use disorder, and naloxone prescriptions at discharge for overdose prevention. The prespecified primary outcome was COWS assessment because completion of COWS was the most proximal step in the treatment pathway, indicating that screening was completed, that there was recognition of the need for treatment, and that an assessment was conducted. COWS was also used to guide specific treatment steps based on whether a patient was in withdrawal. Secondary outcomes included the use of medications for opioid use disorder and prescriptions for take-home naloxone. We measured the total use of buprenorphine per opioid-related ED encounter, a composite metric that included buprenorphine administration in the ED and/or buprenorphine prescription at discharge. We also measured ED administration of methadone, including either continuation of outpatient methadone treatment after confirmation with an opioid treatment program or a one-time, low dose of methadone for withdrawal management.31 Although the decision support guided providers toward buprenorphine as the first-line treatment, the choice of medications for opioid use disorder was left to clinicians. For patients preferring methadone, our institution has internal guidance on initial methadone dosing, with psychiatric consultation required for admitted patients in whom methadone maintenance is started. To capture the use of methadone or buprenorphine, both of which are evidence-based treatments, we included a composite measure of total medications used for opioid use disorder, which included any buprenorphine during or after the ED visit or methadone administration in the ED. We also measured the proportion of patients with naloxone prescriptions at discharge and follow-up visits in our health system for outpatient treatment of opioid use disorder within 14 days. Finally, to evaluate the provider-level variability in prescriptions, we measured provider-level buprenorphine prescriptions at discharge per opioid use disorder–related encounter. For discharge prescriptions and follow-up, we evaluated outcomes only in those patients who were discharged from the ED.
Other Variables
We extracted screening responses and patient characteristics from the electronic health record, including age, sex, race/ethnicity, and insurance status. We also extracted comorbid mental health and substance use disorders using ICD-10 codes, calculated Charlson Comorbidity Index scores using previously coded diagnoses in patient records, and extracted prior ED and hospital visits within the study health system for our cohort.
Analysis
We used descriptive statistics to characterize the sample and triage screening outcomes, and we compared patient and visit characteristics between the preperiod and postperiod among the treatment and control hospitals using difference in proportions for independent samples, reporting mean deltas and 95% confidence intervals.
The screening protocol had staggered implementation, with 2 EDs implementing in March 2021 and a third in July 2021. To visualize outcomes over time, we plotted each outcome in the treatment hospitals using the implementation date as time 0 and displayed outcomes from 9 months prior to implementation through June 2022 (11 to 13 months following implementation depending on the implementation date). We also plotted outcomes in the control hospitals, with time 0 being the midpoint between implementation dates in the treatment hospitals.
To estimate the impact of the triage protocol, we used a difference-in-differences analysis to assess changes in our primary outcome as well as other treatment and process measures in the 3 intervention EDs compared with those in the 2 controls. Because the use of medications for opioid use disorder has increased over time,32 a simple pre-post comparison may inappropriately estimate the effect of the interventions. The difference-in-difference approach allows for comparison between the intervention hospitals and control hospitals within the same health system to account for secular trends.33 In order to use this approach, we tested for parallel trends in the preperiod and assumed that these trends would have continued had there been no exposure.33
We fit a linear regression model to examine the effect of the intervention on both the absolute proportion of patients receiving each outcome and the rate of change in outcomes between the sites. We chose a linear model because it has been shown that linear models provide consistent causal estimates for association for binary outcomes34 and that nonlinear models for difference-in-differences analysis can be problematic.35 Our model included a binary variable that indicated implementation of the screening intervention (“treatment”), calendar time (ED visit date), and an indicator for the time period (preimplantation or postimplementation), with time 0 representing the implementation date or the midpoint between dates for the control sites. The model also included a difference-in-differences estimator—the interaction term between time period and treatment group—which indicates whether the rates of change in outcomes differed based on exposure to the intervention. We also accounted for clustering by hospital in our model using robust standard errors. We also performed several sensitivity analyses to assess the robustness of our results.25,26 These included limiting our sample to the first visit only for any given patient to account for the correlation within person due to repeated visits and adjusting for covariates that differed significantly between the sites at baseline (race, insurance status, and Charlson Comorbidity Index score). In addition, we assessed whether the intervention had differential effects by sex or race by stratifying outcomes by race and sex. All analyses were conducted using R statistical software.36
Finally, for buprenorphine prescriptions, we included a provider-level analysis of the rate of buprenorphine prescriptions at discharge per opioid use disorder–related encounter before and after the interventions after restricting reporting to providers with 10 or more opioid use disorder–related encounters. We reported descriptive statistics for the intervention and control hospitals by prescriber quartile.
RESULTS
Screening Outcomes
During the study period, triage screening was completed for 96.8% of visits (153,149 encounters, Table 1). Of those screened, 1.6% (2,477 encounters) yielded a positive result, including 1.5% responding “Yes” to the screening question and 0.1% presenting with suspected overdose. The mean total time in triage was 3.5 minutes overall: 4.0 minutes for patients screening positive and 0.9 minutes for patients with suspected overdose. This amounted to approximately 2 to 3 positive screen results per day in each intervention ED, adding approximately 1 to 2 minutes daily to ED throughput times.
Table 1.
Triage screening outcomes.
| Screening Completion, n (%) | n=158,087 |
|---|---|
| Screening complete | 153,149 (96.8) |
| Screening incomplete | 4,938 (3.2) |
| Screening outcomes * | |
| Yes | 2,289 (1.5) |
| Overdose | 188 (0.1) |
| Unable to assess | 2,578 (1.7) |
| No | 142,165 (93.4) |
| Proportion of positive screen result † | 2,477 (1.6) |
| Triage time in minutes, mean (SD) | |
| Overall | 3.5 (16.8) |
| Yes | 4.0 (27.1) |
| Overdose | 0.91 (60.1) |
| No | 3.3 (18.5) |
| Unable to assess | 4.4 (24.1) |
| Missing (screening not completed) | 3.7 (14.2) |
Outcomes reported from triage screening process among those who underwent screening.
Yes or overdose.
Patient and Visit Characteristics
Over the study period, there were a total of 3,193 opioid use disorder–related visits to the study EDs based on opioid use disorder–related ICD-10 codes: 2,462 in the intervention hospitals and 731 in the control hospitals. The patient characteristics are shown in Table 2, broken down by preperiod and postperiod. The majority of patients were men and middle aged, with greater numbers of Black and publicly insured patients in the intervention hospitals than in the controls. The characteristics were similar within the hospitals across time, with the exception of slightly fewer men in the postperiod in the control hospitals. The proportion of patients admitted to the hospital decreased in both the sites over time. The control sites had more White, commercially insured patients with lower illness severity and ED utilization than the intervention sites.
Table 2.
Characteristics of patients seen for opioid use disorder–related visits in study emergency departments.
| Intervention EDs |
Control EDs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
Preperiod |
Postperiod |
Overall |
Preperiod |
Postperiod |
|||||
| Patient Characteristics | N=2,462 | n=1,258 | n=1,204 | Delta | 95% CI | N=731 | n=459 | n=272 | Delta | 95% CI |
| Age, mean (SD) | 41.4 (14.1) | 41.3 (13.4) | 41.4 (14.8) | −0.2 | −1.3 to 0.9 | 41.0 (14.1) | 41.4 (14.2) | 40.3 (13.9) | 1.1 | −1 to 3.2 |
| Male sex, n (%) | 1,632 (66.3) | 823 (65.4) | 809 (67.2) | 1.8 | − 2 to 5.6 | 470 (64.3) | 304 (66.2) | 166 (61.0) | 16.6 | 8.9–24.2 |
| Race, n (%) | ||||||||||
| Asian/Pacific Islander | 17 (0.7) | 11 (0.9) | 6 (0.5) | −0.4 | −1.1 to 0.4 | 12 (1.6) | 9 (2.0) | 3 (1.1) | −0.9 | −2.9 to 1.2 |
| Black | 943 (38.3) | 458 (36.4) | 485 (40.3) | 3.9 | 0–7.8 | 108 (14.8) | 69 (15.0) | 39 (14.3) | −0.7 | −6.3 to 4.9 |
| Other race/missing data | 96 (3.9) | 49 (3.9) | 47 (3.9) | 0 | −1.5 to 1.5 | 25 (3.4) | 14 (3.1) | 11 (4.0) | 1.0 | −2.1 to 4.1 |
| White | 1,406 (57.1) | 740 (58.8) | 666 (55.3) | −3.5 | −7.5 to 0.5 | 586 (80.2) | 367 (80.0) | 219 (80.5) | 0.6 | −5.7 to 6.8 |
| Hispanic ethnicity, n (%) | 172 (7.0) | 93 (7.4) | 79 (6.6) | −0.8 | −2.9 to 1.3 | 61 (8.3) | 46 (10.0) | 15 (5.5) | −4.5 | −8.7 to 0.4 |
| Insurance, n (%) | ||||||||||
| Commercial | 410 (16.7) | 212 (16.9) | 198 (16.4) | −0.4 | −3.4 to 2.6 | 358 (49.0) | 223 (48.6) | 135 (49.6) | 1.0 | −6.7 to 8.8 |
| Medicaid | 1,651 (67.1) | 824 (65.5) | 827 (68.7) | 3.2 | −0.6 to 7 | 195 (26.7) | 118 (25.7) | 77 (28.3) | 2.6 | −4.4 to 9.6 |
| Medicare | 293 (11.9) | 166 (13.2) | 127 (10.5) | −2.6 | −5.3 to 0 | 115 (15.7) | 80 (17.4) | 35 (12.9) | −4.6 | −10.1 to 1 |
| Uninsured/unknown | 108 (4.4) | 56 (4.5) | 52 (4.3) | −0.1 | −1.8 to 1.6 | 63 (8.6) | 38 (8.3) | 25 (9.2) | 0.9 | −3.6 to 5.5 |
| Charlson Comorbidity Index score, n (%) | 1.00 (2.0) | 1.1 (2.1) | 0.9 (1.9) | 0.1 | 0–0.3 | 0.5 (1.3) | 0.6 (1.4) | 0.4 (1.1) | 0.2 | 0–0.4 |
| ED visits in past 12 months, mean (SD) | 3.56 (7.9) | 3.4 (8.0) | 3.7 (7.9) | −0.3 | −0.9 to 0.4 | 1.2 (2.6) | 1.3 (3.9) | 1.0 (1.6) | 0.3 | 0–0.6 |
| Hospital admissions in past 12 months, mean (SD) | 0.65 (1.9) | 0.7 (2.2) | 0.6 (1.7) | 0.1 | 0–0.3 | 0.3 (0.9) | 0.3 (1.0) | 0.2 (0.7) | 0.1 | 0–0.2 |
| Disposition, n (%) | ||||||||||
| Admitted to hospital | 512 (20.8) | 354 (28.1) | 158 (13.1) | −15.8 | −19 to −12.7 | 115 (15.7) | 88 (19.2) | 27 (9.9) | −9.2 | −14.6 to −3.9 |
| Discharged from ED | 1,548 (62.8) | 683 (54.3) | 865 (71.8) | 17.8 | 14.1–21.6 | 342 (46.8) | 179 (39.0) | 163 (59.9) | 22.7 | 15.1–30.2 |
| Other disposition* | 402 (16.3) | 221 (17.6) | 181 (15.0) | −2.5 | −5.5 to 0.5 | 274 (37.5) | 192 (41.8) | 82 (30.1) | 11.7 | −19.1 to −4.3 |
CI, Confidence interval.
Other disposition includes transfer to other facilities and deaths.
Impact on COWS Measurement and Treatment Outcomes
Next, we assessed the rate of COWS measurement, our primary outcome, over time across the intervention and control hospitals (Table 3, Figure 2). In the intervention EDs, the mean proportion with COWS measurement increased by 21.5%, from 26% in the preperiod to 48% in the postperiod (95% CI 17.7 to 25.3), whereas there was no significant change in the control hospitals. The increased rate of COWS measurement in the intervention hospitals was maintained throughout the postperiod (Figure 2). Among patients who reported subjective symptoms of withdrawal (n=810), the COWS score was measured in 91.6%.
Table 3.
Outcomes of opioid use disorder–related visits in intervention and control emergency departments.
| Intervention EDs |
Control EDs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Outcomes | Overall | Preperiod | Postperiod | Delta | 95% CI | Overall | Preperiod | Postperiod | Delta | 95% CI |
| All patients | n=2,462 | n=1,258 | n=1,204 | n=731 | n=459 | n=272 | ||||
| COWS measured, n (%) | 909 (36.9) | 332 (26.4) | 577 (47.9) | 21.5 | 17.7–25.3 | 83 (11.4) | 44 (9.6) | 39 (14.3) | 4.8 | −0.5 to 10 |
| Any medication for opioid use disorder, n (%)* | 751 (30.5) | 334 (26.6) | 417 (34.6) | 8.1 | 4.4–11.8 | 54 (7.4) | 32 (7.0) | 22 (8.1) | 1.1 | −3.2 to 5.4 |
| Any buprenorphine, n (%) | 588 (23.9) | 256 (20.3) | 332 (27.6) | 7.2 | 3.8–10.7 | 37 (5.1) | 21 (4.6) | 16 (5.9) | 1.3 | −2.4 to 5 |
| Buprenorphine in ED, n (%) | 416 (16.9) | 183 (14.5) | 233 (19.4) | 4.8 | 1.8–7.8 | 27 (3.7) | 15 (3.3) | 12 (4.4) | 1.1 | −2.1 to 4.4 |
| Methadone in ED, n (%) | 168 (6.8) | 81 (6.4) | 87 (7.2) | 0.8 | 1.3 to 2.9 | 17 (2.3) | 11 (2.4) | 6 (2.2) | −0.2 | −2.6 to 2.2 |
| Discharged from ED | n=1,548 | n=683 | n=865 | n=342 | n=179 | n=163 | ||||
| Buprenorphine at discharge, n (%) | 337(21.8) | 131 (19.2) | 206 (23.8) | 4.6 | 0.4–8.9 | 5 (1.5) | 3 (1.7) | 2 (1.2) | −0.4 | −3.4 to 2.5 |
| Naloxone at discharge, n (%) | 715 (46.2) | 246 (36.0) | 469 (54.2) | 18.2 | 13.2–23.2 | 72 (21.1) | 31 (17.3) | 41 (25.2) | 7.8 | −1.4 to 17.1 |
| Follow-up visit at 14 days, n (%) | 400 (25.8) | 160 (23.4) | 240 (27.7) | 4.3 | 0.2 to 8.8 | 40 (11.7) | 28 (15.6) | 12 (7.4) | −8.3 | −15.5 to −1 |
Any medication for opioid use disorder includes administration of buprenorphine or methadone in the ED and/or prescription of buprenorphine at discharge.
Figure 2.
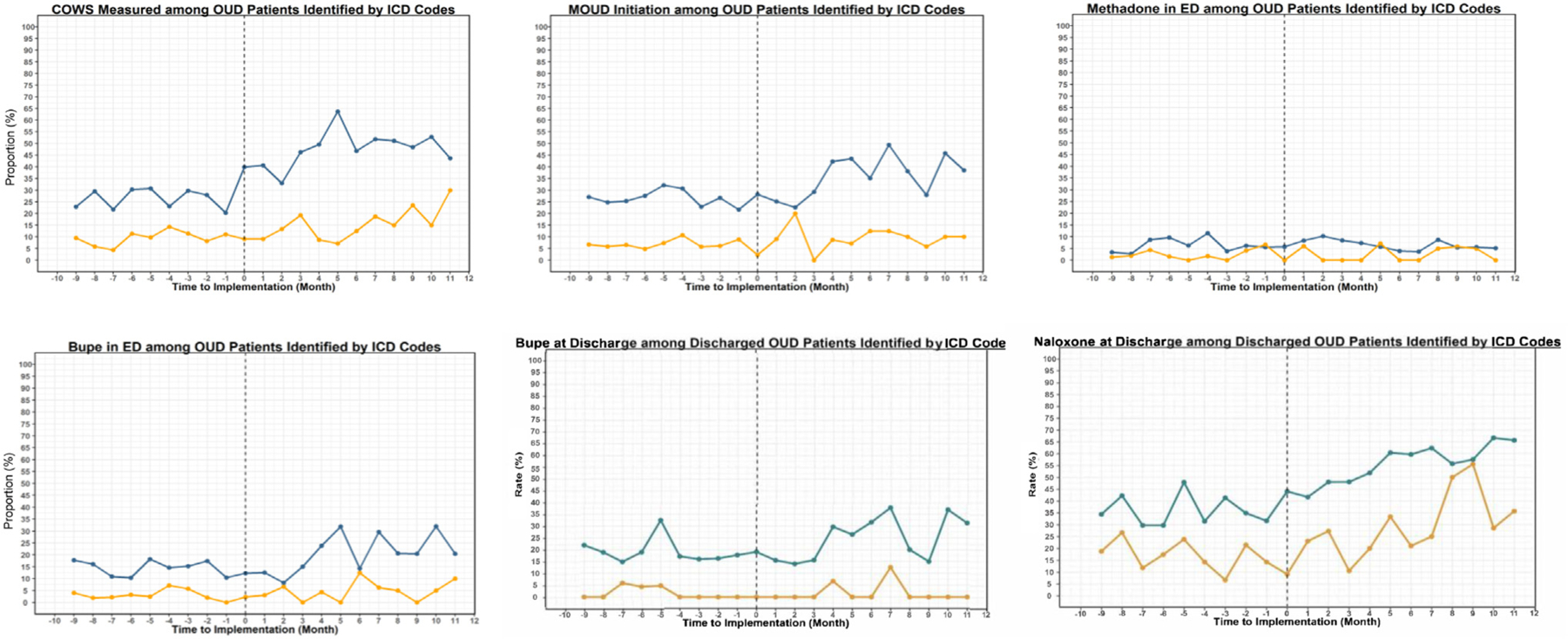
Treatment outcomes before and after the implementation of emergency department intervention in intervention and control EDs. Blue represents the intervention EDs, and yellow represents the control EDs. Bupe, buprenorphine; ICD, International Classification of Diseases; MOUD, medication for opioid use disorder; OUD, opioid use disorder.
We also assessed changes in the secondary outcomes over time. All buprenorphine-related measures and naloxone prescriptions at discharge—the other outcomes most directly targeted by the screening pathway—increased significantly over time in the intervention hospitals but not in the controls (Table 3, Figure 2). There were no changes in ED methadone administration in either group. The intervention hospitals showed no change in 14-day follow-up in the health system, although this did not account for referrals outside the system, whereas 14-day follow-up declined in the control hospitals.
Next, we performed a difference-in-differences analysis to evaluate whether exposure to screening and the treatment intervention was associated with greater changes in the outcomes in the intervention versus control hospitals (Table 4). In our model, the total rate of COWS measurement increased by 17% more in the intervention hospitals compared with that in the control hospitals (95% CI 7% to 27%). In addition, the prescriptions for buprenorphine at discharge increased by 5% more in the intervention hospitals relative to that in the controls (95% CI 0% to 10%), and naloxone prescriptions at discharge increased by 12% more in the intervention sites (95% CI 1% to 22%). The total use of medication for opioid use disorder, methadone administration, and ED buprenorphine administration did not increase significantly in the intervention EDs relative to those in the control sites.
Table 4.
Linear regression models for treatment outcomes*.
| Outcome | Model Term | Estimate | 95% CI |
|---|---|---|---|
| All patients | |||
| COWS measurement rate | Calendar time | 0.01 | 0.00–0.02 |
| Time period | −0.06 | −0.15 to 0.04 | |
| Intervention | 0.18 | 0.05–0.31 | |
| Time period#Intervention ED | 0.17 | 0.07–0.27 | |
| Total medications for opioid use disorder | Calendar time | 0.01 | 0.00–0.01 |
| Time period | −0.05 | −0.15 to 0.06 | |
| Intervention | 0.19 | 0.05–0.33 | |
| Time period#Intervention ED | 0.05 | −0.01 to 0.11 | |
| Methadone administered in the ED | Calendar time | 0.00 | 0.00–0.01 |
| Time period | −0.02 | −0.05 to 0.01 | |
| Intervention | 0.04 | 0.01–0.07 | |
| Time period#Intervention ED | 0.00 | −0.01 to 0.01 | |
| Buprenorphine administered in the ED | Calendar time | 0.01 | 0.00–0.01 |
| Time period | −0.04 | −0.11 to 0.02 | |
| Intervention | 0.11 | 0.02–0.21 | |
| Time period#Intervention ED | 0.04 | −0.02 to 0.10 | |
| Discharged from ED | |||
| Buprenorphine prescription at discharge | Calendar time | 0.00 | 0.00–0.01 |
| Time period | −0.05 | −0.11 to 0.01 | |
| Intervention | 0.16 | 0.03–0.30 | |
| Time period#Intervention ED | 0.05 | 0.00–0.10 | |
| Naloxone prescribed at discharge | Calendar time | 0.01 | 0.00–0.02 |
| Time period | −0.06 | −0.13 to 0.02 | |
| Intervention | 0.17 | −0.04 to 0.40 | |
| Time period#Intervention ED | 0.12 | 0.01–0.22 |
All models are reported at the patient visit level and adjusted for calendar time. The difference-in-differences estimator is the interaction term between the hospital (intervention vs control) and time period (preperiod vs postperiod), indicated as time period#intervention. For context, an estimate of 0.17 of periods#intervention indicates a 17%-point increase in the outcome relative to the control EDs.
We performed several sensitivity analyses to test the robustness of our results. First, we limited our models to the first visit for each patient in our cohort (1,817 unique patients in intervention EDs and 618 in the controls). These results were similar to the primary analysis (Tables E1 and E2, available at http://www.annemergmed.com). We also included a sensitivity analysis adjusting for covariates at the hospital site level that differed significantly between the sites at baseline (race, insurance status, and Charlson Comorbidity Index score). Again, the results were similar to those of the primary analysis (Table E3, available at http://www.annemergmed.com).
We also assessed the changes in the outcomes in the intervention and control EDs stratified by race (Table 5). Overall, we saw similar increases in our COWS measurement across racial groups in the intervention EDs. Buprenorphine use was higher in Black patients than in White patients in the intervention EDs across time, whereas methadone administration was higher in White patients in both the time periods, with no significant change in either group over time. We also assessed the outcomes by sex in the preperiod and postperiod and observed mixed results, although increases in buprenorphine were greater in men than in women in the intervention EDs (Table E4, available at http://www.annemergmed.com).
Table 5.
Outcomes of opioid use disorder–related visits in intervention and control emergency departments by race.
| Intervention EDs |
Control EDs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes by Race | Overall | Before | After | Delta | 95% CI | Overall | Before | After | Delta | 95% CI |
| All patients White | n=1,406 | n=740 | n=666 | n=586 | n=367 | n=219 | ||||
| All patients Black | n=1,056 | n=518 | n=538 | n=145 | n=92 | n=53 | ||||
| COWS measured, n (%) | ||||||||||
| Black | 340 (36.1) | 119 (26.0) | 221 (45.6) | 19.6 | 13.4–25.8 | 12 (11.1) | 6 (8.7) | 6 (15.4) | 6.7 | −8.4 to 21.8 |
| White | 522 (37.1) | 195 (26.4) | 327 (49.1) | 22.7 | 17.7–27.8 | 64 (10.9) | 33 (9.0) | 31 (14.2) | 5.2 | −0.7 to 11 |
| Any medication for opioid use disorder, n (%) | ||||||||||
| Black | 307 (32.6) | 130 (28.4) | 177 (36.5) | 8.1 | 1.9–14.3 | 9 (8.3) | 4 (5.8) | 5 (12.8) | 7 | −6.8 to 20.9 |
| White | 414 (29.4) | 187 (25.3) | 227 (34.1) | 8.8 | 3.9–13.7 | 42 (7.2) | 26 (7.1) | 16 (7.3) | 0.2 | −4.3 to 4.8 |
| Any buprenorphine, n (%) | ||||||||||
| Black | 281 (29.8) | 118 (25.8) | 163 (33.6) | 7.8 | 1.8–13.9 | 6 (5.6) | 1 (1.4) | 5 (12.8) | 11.4 | −1.5 to 24.2 |
| White | 284 (20.2) | 126 (17.0) | 158 (23.7) | 6.7 | 2.3–11.1 | 28 (4.8) | 18 (4.9) | 10 (4.6) | −0.3 | −4.2 to 3.5 |
| Buprenorphine in ED, n (%) | ||||||||||
| Black | 181 (19.2) | 72 (15.7) | 109 (22.5) | 6.8 | 1.6–12 | 5 (4.6) | 1 (1.4) | 4 (10.3) | 8.8 | −3.1 to 20.7 |
| White | 215 (15.3) | 99 (13.4) | 116 (17.4) | 4 | 0.1–8 | 21 (3.6) | 13 (3.5) | 8 (3.7) | 0.1 | −3.1 to 3.3 |
| Methadone in ED, n (%) | ||||||||||
| Black | 28 (3.0) | 12 (2.6) | 16 (3.3) | 0.7 | −1.7 to 3.1 | 3 (2.8) | 3 (4.3) | 0 (0.0) | −4.3 | −11.2 to 2.5 |
| White | 133 (9.5) | 64 (8.6) | 69 (10.4) | 1.7 | −1.5 to 4.9 | 14 (2.4) | 8 (2.2) | 6 (2.7) | 0.6 | −2.4 to 3.6 |
| Discharged patients Black | n=632 | n=275 | n=357 | n=46 | n=20 | n=26 | ||||
| Discharged patients White | n=852 | n=377 | n=475 | n=276 | n=149 | n=127 | ||||
| Buprenorphine at discharge, n (%) | ||||||||||
| Black | 171 (27.1) | 73 (26.5) | 98 (27.5) | 0.9 | −6.4 to 8.2 | 2 (4.3) | 1 (5.0) | 1 (3.8) | −1.2 | −14.4 to 12.1 |
| White | 154 (18.1) | 55 (14.6) | 99 (20.8) | 6.3 | 0.9–11.6 | 2 (0.7) | 1 (0.7) | 1 (0.8) | 0.1 | −2 to 2.3 |
| Naloxone at discharge, n (%) | ||||||||||
| Black | 313 (49.5) | 118 (42.9) | 195 (54.6) | 11.7 | 3.6–19.8 | 8 (17.4) | 3 (15.0) | 5 (19.2) | 4.2 | −21.8 to 30.2 |
| White | 374 (43.9) | 119 (31.6) | 255 (53.7) | 22.1 | 15.4–28.8 | 57 (20.7) | 25 (16.8) | 32 (25.2) | 8.4 | −2 to 18.8 |
| Follow-up visit at 14 days, n (%) | ||||||||||
| Black | 149 (23.6) | 62 (22.5) | 87 (24.4) | 1.8 | −5.1 to 8.8 | 8 (17.4) | 4 (20.0) | 4 (15.4) | −4.6 | −31.4 to 22.2 |
| White | 239 (28.1) | 93 (24.7) | 146 (30.7) | 6.1 | −0.2 to 12.3 | 31 (11.2) | 23 (15.4) | 8 (6.3) | −9.1 | −17 to −1.2 |
Provider-Level Variation
A goal of the universal screening protocol was to reduce provider-level variation in the adoption of buprenorphine prescribing. To assess whether this goal was met, we performed a provider-level analysis of buprenorphine prescriptions per opioid use disorder–related encounter broken into prescribing quartiles for the intervention EDs and halves for the control EDs because of a small sample size (Figure 3).
Figure 3.
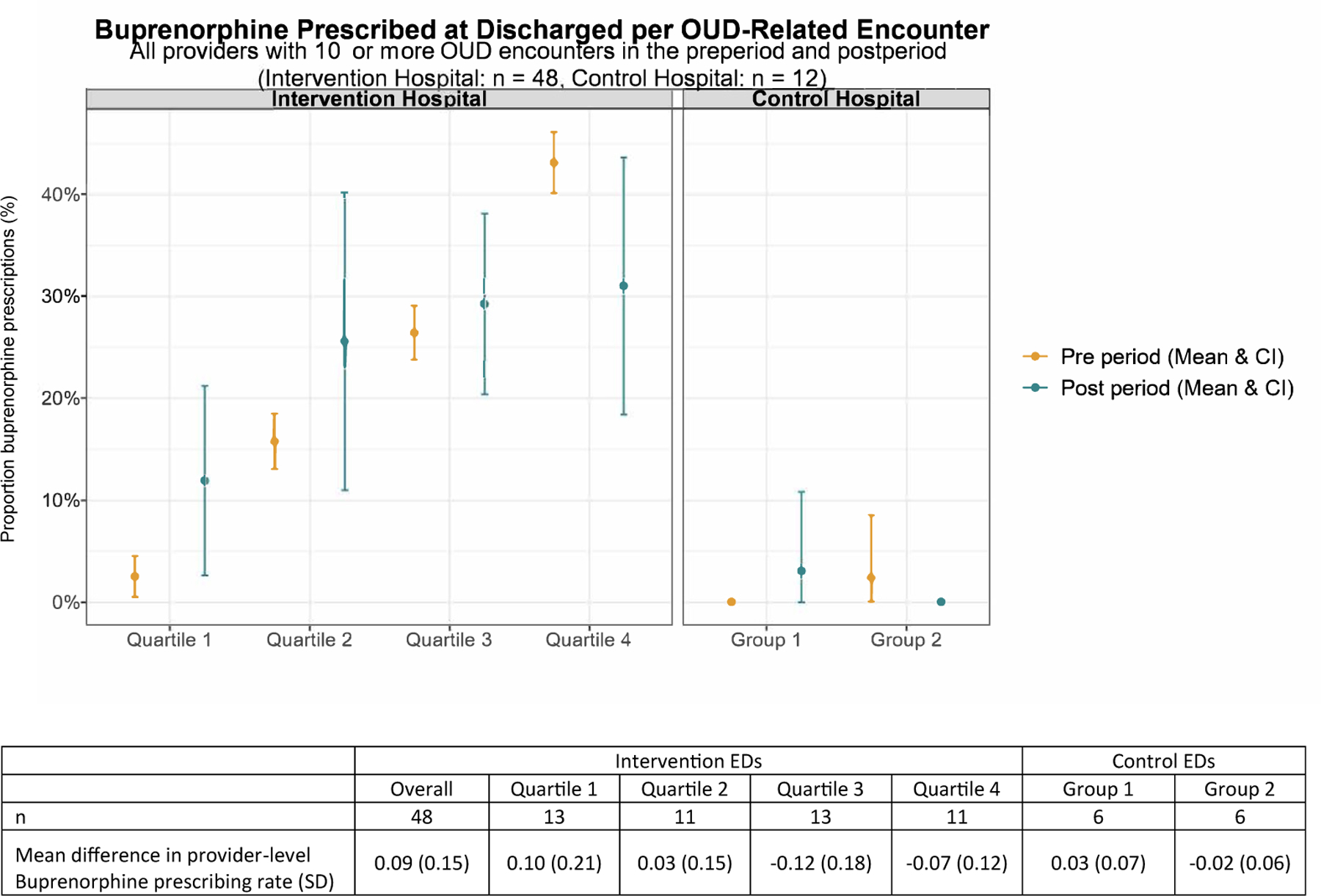
Provider-level buprenorphine prescription in the preperiod and postperiod. Mean buprenorphine prescription in the preperiod and postperiod by quartile (intervention hospitals) and half (control hospitals). Yellow represents the preperiod and blue represents the postperiod mean prescription rates.
Among attending physicians with 10 or more opioid use disorder–related encounters over the study period (48 in the intervention hospitals and 12 in the control hospitals), the mean provider-level buprenorphine prescription rate per opioid use disorder–related encounter went from 21% to 24% among discharged patients in the intervention hospitals and 1% to 2% among those in the control hospitals. When broken down by quartile (Figure 3), the change in the provider-level prescription rate was driven by increases in the lower quartiles, with the lowest quartile increasing by 10% and the second quartile increasing by 1%.
LIMITATIONS
Our study has several important limitations. First, we presented results from a single urban, academic health system in a city highly affected by the opioid crisis. The intervention EDs had also previously implemented treatment guidelines, and most physicians were X-waivered; so, results may not be generalizable to all settings. Second, we observed differences in the patient populations between the intervention and control ED settings. Although the quasiexperimental design accounts for these differences as well as secular and local trends, there could have been unmeasured time-dependent confounders that influenced the outcomes. There is also potential for misclassification of the use of electronic health record data that rely on ICD-10 diagnoses, a known limitation of electronic health records for identification of patients with opioid use disorder.37 However, misclassification error is unlikely to vary significantly across time periods and by hospital because the same coders were used and, therefore, is unlikely to substantially bias the results. Finally, all outcomes were reported as proportions; so, they may not account for increases in the rate of diagnosis of opioid use disorder that may have been observed after implementation of the screening protocols. We also lack complete data on long-term outcomes, including continued medication, treatment engagement outside of our health system, or mortality. Despite these limitations, our study provides an important look at the impact of universal ED screening on the identification, assessment, and treatment of opioid use disorder in a large academic health system.
DISCUSSION
In this implementation study of an ED triage and treatment protocol for opioid use disorder, we found that universal triage screening with automated clinical decision support was widely adopted, feasible, and associated with significant increases in the assessment and treatment of opioid use disorder. Patients in hospitals implementing the protocols were more likely to be assessed for withdrawal as well as receive buprenorphine and naloxone prescriptions at discharge, driven largely by increases in prescriptions among lower-adopting providers. There was a relative increase of 26% in buprenorphine prescriptions at discharge among the intervention EDs, with absolute treatment rates of 35% for any medications for opioid use disorder and 28% for any buprenorphine in the postperiod. Put another way, for every 20 patients with opioid use disorder exposed to this new pathway, one additional patient received a buprenorphine prescription at discharge. This occurred even in study EDs with high baseline rates of treatment compared with levels reported in other large studies (5% to 12%)9,14,38 and amid a period of growing concerns about challenges initiating buprenorphine for patients using fentanyl, the dominant opioid in Philadelphia.39,40 Finally, our intervention was implemented as part of usual care, required few additional resources, and involved all-comers to the EDs, making the findings generalizable and representing real-world evidence at scale. ED visits represent an important opportunity for engagement in treatment and harm reduction for patients with opioid use disorder, and recent policy changes with the removal of the X-waiver requirement means that many more ED clinicians can now prescribe buprenorphine nationally.8 Rapidly scalable strategies such as the one presented here are critical to address gaps in the treatment of opioid use disorder and reduce overdose deaths.
Our findings suggest that universal screening coupled with targeted electronic health record prompts is an effective implementation strategy to increase broader adoption of treatment of opioid use disorder in ED settings that can overcome the numerous barriers to initiation of buprenorphine.12,13,41 We leveraged behavioral insights—including the concept of “nudges”—to design clinician electronic health record workflows for the following: (1) make identification of opioid use disorder and active withdrawal the default practice, (2) activate tailored prompts to nurses and physicians in the existing workflow to signal the importance of timely care for opioid use disorder, and (3) navigate clinicians to clinical decision support to make evidence-based treatment easy. Our finding that the largest gains were among low-adopting physicians suggests that the intervention reduced variability by making it easier to deliver evidence-based care.
In addition, the positive findings in this study suggest that coupling screening with prompts and clinical decision support is needed to overcome the limitations of each of these strategies offered on their own. The EMergency department initiated BuprenorphinE for opioid use Disorder trial—a recent multisite, cluster, randomized trial of physician-facing, user-initiated clinical decision support—did not show increased rates of ED-initiated buprenorphine across 5 health systems.14 Although clinical decision support may address gaps in knowledge or readiness to initiate buprenorphine, the EMergency department initiated BuprenorphinE for opioid use Disorder results suggest that these strategies need to be paired with other interventions to change behavior.42 Similarly, although there are no formal recommendations on ED screening for drug use,43–45 screening for other unmet medical or social needs in ED settings is not always impactful, particularly when coupled with passive referral processes.46,47 In the current study, we coupled universal screening with tailored prompts and clinical decision support. These components emerged through a stakeholder-engaged design process, which helped to identify key levers for behavior change in our local context.24
The intervention was associated with changes in both process and treatment outcomes, particularly in the assessment of withdrawal and prescriptions for buprenorphine and naloxone at discharge. We saw the largest increases in our primary outcome, COWS assessment, which is the most proximal management step in initiating medications for opioid use disorder and the behavior most directly targeted by electronic health record prompts. Buprenorphine is typically administered in the ED once a patient experiences mild-to-moderate withdrawal symptoms (COWS >8 to 12); so, not all patients are ready for initiation of buprenorphine during their visit.48 However, patients can receive a prescription for buprenorphine to be initiated off-site, a procedure that is safe, effective, and the standard of care in many places.49,50 The increase seen in buprenorphine prescribing at discharge, which was targeted in the decision support pathway, suggests that the triage pathway helped prompt treatment initiation after discharge for patients without withdrawal in the ED who may have been missed if they did not present with withdrawal symptoms during their visit.51 We observed similar increases in naloxone prescriptions at discharge, an evidence-based but underutilized harm reduction intervention that should be provided for any patient with opioid use disorder or at risk of overdose.48 We did not observe significant increases in the 14-day follow-up rates in the intervention hospitals; however, many patients were seen for follow-up outside the health system; so, the significance of this finding is unclear.
Finally, we examined the outcomes of our intervention by race and sex. Over the past several years, there have been significant increases in deaths due to overdose among non-White populations both locally and nationally52,53; so, it is critical to understand whether default strategies are promoting equity. Our primary outcome—COWS assessment—increased across different racial groups and in men and women—with rates in the postperiod similar by race in the intervention EDs (49% in White and 46% in Black patients) and increased in women relative to those in men (27% vs 20%, respectively). The use of medication for opioid use disorder was similar or higher in Black patients in the intervention EDs in the postperiod, with any medications for opioid use disorder used in 34% of White patients and 37% of Black patients and any buprenorphine used in 24% of White patients and 34% of Black patients. Only methadone was administered more frequently in White patients in the intervention EDs during the postperiod (10% in White patients vs 3% in Black patients). This is in contrast to prior literature that demonstrated lower rates of buprenorphine treatment following an ED visit for nonfatal overdose in Black patients compared with those in White patients as well as racial disparities in buprenorphine prescriptions overall.9,22,54,55 Although there is still substantial work to be done to understand racial disparities in the treatment of opioid use disorder,56 it is critical to examine whether interventions have disproportionate effects to assess whether they reinforce versus reduce pre-existing disparities.
In conclusion, an ED triage screening and treatment protocol led to increases in the assessment of COWS and treatment with medications for opioid use disorder. Protocols designed to identify patients and prompt treatment—making treatment the default practice—have promise in increasing the implementation of evidence-based treatment care for opioid use disorder in EDs.
Supplementary Material
Editor’s Capsule Summary.
What is already known on this topic
Buprenorphine and naloxone aid risk reduction for those with an opioid use disorder (OUD).
What question this study addressed
Does an emergency department (ED) triage screening tool to detect opioid use disorder–related patient visits impact identification and initiation of medications to aid?
What this study adds to our knowledge
Studying 3,193 total visits in 5 EDs in one health system (3 serving as controls), the triage protocol for opioid use disorder increased detection of opioid withdrawal and the prescribing of buprenorphine and naloxone.
How this is relevant to clinical practice
Structured ED programs that identify and prompt action for patients with opioid use disorder can change care.
Funding and support:
By Annals’ policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). The authors have stated that no such relationships exist. This work was supported by the Penn Injury Science Center (CDC 19R49CE003083). Dr. Lowenstein was also supported by the National Institute on Drug Abuse (grant K23DA055087), and Dr. Delgado was also supported by the National Institute of Child Health and Human Development (grant K23HD090272001) and by a philanthropic grant from the Abramson Family Foundation.
Footnotes
Readers: click on the link to go directly to a survey in which you can provide feedback to Annals on this particular article. A podcast for this article is available at www.annemergmed.com.
Supervising editor: Donald M. Yealy, MD. Specific detailed information about possible conflict of interest for individual editors is available at https://www.annemergmed.com/editors.
All authors attest to meeting the four ICMJE.org authorship criteria:(1) Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND (2) Drafting the work or revising it critically for important intellectual content; AND (3) Final approval of the version to be published; AND (4) Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This work was presented as an oral abstract at the Society of Academic Emergency Medicine Annual Meeting in New Orleans, LA, on May 12, 2022 and as a poster at the Society of General Internal Medicine Annual Meeting in Orlando, FL, on April 7, 2022.
Contributor Information
Margaret Lowenstein, Division of General Internal Medicine, Perelman School of Medicine at the University of Pennsylvania, Center for Addiction Medicine and Policy, University of Pennsylvania Health System, Philadelphia, PA.
Jeanmarie Perrone, Department of Emergency Medicine, Perelman School of Medicine at the University of Pennsylvania, Center for Addiction Medicine and Policy, University of Pennsylvania Health System, Philadelphia, PA.
Rachel McFadden, Department of Emergency Medicine, Perelman School of Medicine at the University of Pennsylvania, Center for Addiction Medicine and Policy, University of Pennsylvania Health System, Philadelphia, PA.
Ruiying Aria Xiong, Division of General Internal Medicine, University of Pennsylvania, and Penn Medicine Nudge Unit, University of Pennsylvania Health System, Philadelphia, PA.
Zachary F. Meisel, Department of Emergency Medicine, University of Pennsylvania Health System, Philadelphia, PA.
Nicole O’Donnell, Department of Emergency Medicine, Perelman School of Medicine at the University of Pennsylvania, Center for Addiction Medicine and Policy, University of Pennsylvania Health System, Philadelphia, PA.
Dina Abdel-Rahman, Department of Emergency Medicine, University of Pennsylvania, and Penn Medicine Nudge Unit, University of Pennsylvania Health System, Philadelphia, PA.
Jeffrey Moon, Department of Emergency Medicine, University of Pennsylvania Health System, Philadelphia, PA.
Nandita Mitra, Department of Biostatistics and Epidemiology, University of Pennsylvania Health System, Philadelphia, PA.
Mucio Kit Delgado, Department of Emergency Medicine, University of Pennsylvania, and Penn Medicine Nudge Unit, University of Pennsylvania Health System, Philadelphia, PA.
REFERENCES
- 1.Increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. Centers for Disease Control and Prevention. Accessed March 27, 2023. https://emergency.cdc.gov/han/2020/han00438.asp [Google Scholar]
- 2.Provisional drug overdose death counts. Centers for Disease Control and Prevention. Accessed March 27, 2023. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm [Google Scholar]
- 3.Medications for opioid use disorder save lives. The National Academies Press; 2019. Accessed March 27, 2023. https://www.nap.edu/catalog/25310/medications-for-opioid-use-disorder-save-lives [PubMed] [Google Scholar]
- 4.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313:1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks D Initiating medication-assisted treatment for patients presenting with opioid withdrawal. Educ Manag. 2017;29:85–96. [Google Scholar]
- 6.Busch SH, Fiellin DA, Chawarski MC, et al. Cost-effectiveness of emergency department-initiated treatment for opioid dependence. Addiction. 2017;112:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.E-QUAL network opioid initiative. College of Emergency Physicians. Accessed March 27, 2023. https://www.acep.org/administration/quality/equal/emergency-quality-network-e-qual/e-qual-opioid-initiative/ [Google Scholar]
- 8.X-waiver no longer required to treat opioid use disorder. American College of Emergency Physicians. Accessed January 25, 2023. https://www.acep.org/news/acep-newsroom-articles/x-waiver-no-longer-required-to-treat-opioid-use-disorder/ [Google Scholar]
- 9.Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of treatment for opioid use disorder following nonfatal overdose in commercially insured patients. JAMA Netw Open. 2020;3:e205852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andraka-Christou B, Capone MJ. A qualitative study comparing physician-reported barriers to treating addiction using buprenorphine and extended-release naltrexone in U.S. office-based practices. Int J Drug Policy. 2018;54:9–17. [DOI] [PubMed] [Google Scholar]
- 11.Haffajee RL, Bohnert AS, Lagisetty PA. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med. 2018;54(Suppl 3):S230–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and facilitators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3:e204561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37:1787–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melnick ER, Nath B, Dziura JD, et al. User centered clinical decision support to implement initiation of buprenorphine for opioid use disorder in the emergency department: EMBED pragmatic cluster randomized controlled trial. BMJ (Clin Res Ed). 2022;377:e069271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowenstein M, Perrone J, Xiong RA, et al. Sustained implementation of a multicomponent strategy to increase emergency department-initiated interventions for opioid use disorder. Ann Emerg Med. 2022;79:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan A, Anderman J, Deseran T, et al. Monthly patient volumes of buprenorphine-waivered clinicians in the US. JAMA Netw Open. 2020;3:e2014045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. Yale University Press; 2008. [Google Scholar]
- 18.Samuelson W, Zeckhauser R. Status quo bias in decision making. J Risk Uncertainty. 1988;1:7–59. [Google Scholar]
- 19.Kahneman D, Knetsch JL, Thaler RH. Anomalies: the endowment effect, loss aversion, and status quo bias. J Econ Perspect. 1991;5:193–206. [Google Scholar]
- 20.Delgado MK, Shofer FS, Patel MS, et al. Association between electronic medical record implementation of default opioid prescription quantities and prescribing behavior in two emergency departments. J Gen Intern Med. 2018;33:409–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpern SD, Loewenstein G, Volpp KG, et al. Default options in advance directives influence how patients set goals for end-of-life care. Health Aff (Millwood). 2013;32:408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagisetty PA, Ross R, Bohnert A, et al. Buprenorphine treatment divide by race/ethnicity and payment. JAMA Psychiatry. 2019;76:979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mrkva K, Posner NA, Reeck C, et al. Do nudges reduce disparities? Choice architecture compensates for low consumer knowledge. J Mark. 2021;85:67–84. [Google Scholar]
- 24.Lowenstein M, McFadden R, Abdel-Rahman D, et al. Redesign of opioid use disorder screening and treatment in the ED. NEJM Catal Innov Care Deliv. 2022;3. 10.1056/CAT.21.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuart EA, Huskamp HA, Duckworth K, et al. Using propensity scores in difference-in-differences models to estimate the effects of a policy change. Health Serv Outcomes Res Methodol. 2014;14:166–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abadie A Semiparametric difference-in-differences estimators. Rev Econ Stud. 2005;72:1–19. [Google Scholar]
- 27.Des Jarlais DC, Lyles C, Crepaz N, et al. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Final report & recommendations. The Mayor’s Task Force to Combat the Opioid Epidemic in Philadelphia. Accessed March 27, 2023. http://dbhids.org/wp-content/uploads/2017/05/OTF_Report.pdf [Google Scholar]
- 29.2021 DSM-5 diagnoses and new ICD-10-CM codes as ordered in the ICD-10-CM classification. American Psychiatric Association. Accessed March 27, 2023. https://www.psychiatry.org/psychiatrists/practice/dsm/updates-to-dsm/coding-updates/2021-coding-updates#section_1 [Google Scholar]
- 30.Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS). J Psychoactive Drugs. 2003;35:253–259. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Electronic Code of Federal Regulations. Medication assisted treatment for opioid use disorders. In: Title 42: Public Health, Part 8 - Medication Assisted Treatment for Opioid Use Disorders. Accessed March 27, 2023. https://www.ecfr.gov/cgi-bin/text-idx?node=pt42.1.8&rgn=div5#se42.1.8_112 [Google Scholar]
- 32.Krawczyk N, Rivera BD, Jent V, et al. Has the treatment gap for opioid use disorder narrowed in the U.S.?: a yearly assessment from 2010 to 2019. Int J Drug Policy. 2022;110:103786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312:2401–2402. [DOI] [PubMed] [Google Scholar]
- 34.Gomila R Logistic or linear? Estimating causal effects of experimental treatments on binary outcomes using regression analysis. J Exp Psychol Gen. 2021;150:700–709. [DOI] [PubMed] [Google Scholar]
- 35.Lechner M The estimation of causal effects by difference-in-difference methods. Found Trends Econom. 2011;4:165–224. [Google Scholar]
- 36.R: A Language and Environment for Statistical Computing [computer program]. 2018; Vienna, Austria. [Google Scholar]
- 37.Howell BA, Abel EA, Park D, et al. Validity of incident opioid use disorder (OUD) diagnoses in administrative data: a chart verification study. J Gen Intern Med. 2021;36:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland WC, Nath B, Li F, et al. Interrupted time series of user-centered clinical decision support implementation for emergency department-initiated buprenorphine for opioid use disorder. Acad Emerg Med. 2020;27:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spadaro A, Sarker A, Hogg-Bremer W, et al. Reddit discussions about buprenorphine associated precipitated withdrawal in the era of fentanyl. Clin Toxicol (Phila). 2022;60:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varshneya NB, Thakrar AP, Hobelmann JG, et al. Evidence of buprenorphine-precipitated withdrawal in persons who use fentanyl. J Addict Med. 2022;16:e265–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart MT, Coulibaly N, Schwartz D, et al. Emergency department-based efforts to offer medication treatment for opioid use disorder: what can we learn from current approaches? J Subst Abuse Treat. 2021;129:108479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwan JL, Lo L, Ferguson J, et al. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ (Clin Res Ed). 2020;370:m3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawk K, D’Onofrio G. Emergency department screening and interventions for substance use disorders. Addict Sci Clin Pract. 2018;13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahota PK, Shastry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139–146. [DOI] [PubMed] [Google Scholar]
- 45.Wallace AS, Luther B, Guo JW, et al. Implementing a social determinants screening and referral infrastructure during routine emergency department visits, Utah, 2017–2018. Prev Chronic Dis. 2020;17:E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulie P, Steinmetz E, Johnson S, et al. A health-related social needs referral program for Medicaid beneficiaries treated in an emergency department. Am J Emerg Med. 2021;47:119–124. [DOI] [PubMed] [Google Scholar]
- 47.Wallace AS, Luther BL, Sisler SM, et al. Integrating social determinants of health screening and referral during routine emergency department care: evaluation of reach and implementation challenges. Implement Sci Commun. 2021;2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawk K, Hoppe J, Ketcham E, et al. Consensus recommendations on the treatment of opioid use disorder in the emergency department. Ann Emerg Med. 2021;78:434–442. [DOI] [PubMed] [Google Scholar]
- 49.Lee JD, Grossman E, DiRocco D, et al. Home buprenorphine/naloxone induction in primary care. J Gen Intern Med. 2009;24:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin SA, Chiodo LM, Bosse JD, et al. The next stage of buprenorphine care for opioid use disorder. Ann Intern Med. 2018;169:628–635. [DOI] [PubMed] [Google Scholar]
- 51.Faude S, Delgado MK, Perrone J, et al. Variability in opioid use disorder clinical presentations and treatment in the emergency department: a mixed-methods study. Am J Emerg Med. 2023;66:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khatri UG, Pizzicato LN, Viner K, et al. Racial/ethnic disparities in unintentional fatal and nonfatal emergency medical services-attended opioid overdoses during the COVID-19 pandemic in Philadelphia. JAMA Netw Open. 2021;4:e2034878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman J, Mann NC, Hansen H, et al. Racial/ethnic, social, and geographic trends in overdose-associated cardiac arrests observed by US emergency medical services during the COVID-19 pandemic. JAMA Psychiatry. 2021;78:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunphy CC, Zhang K, Xu L, et al. Racial‒ethnic disparities of buprenorphine and vivitrol receipt in Medicaid. Am J Prev Med. 2022;63:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holland WC, Li F, Nath B, et al. Racial and ethnic disparities in emergency department-initiated buprenorphine across five healthcare systems. Acad Emerg Med. Published online January 19, 2023. 10.1111/acem.14668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.James K, Jordan A. The opioid crisis in black communities. J Law Med Ethics. 2018;46:404–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


