Abstract
In patients with impaired cell-mediated immune responses (e.g., lung transplant recipients and AIDS patients), cytomegalovirus (CMV) infection causes severe disease such as pneumonitis. However, although immunocompetency in the host can protect from CMV disease, the virus persists by evading the host immune defenses. A model of CMV infection of the endothelium has been developed in which inflammatory stimuli, such as the CC chemokine RANTES, bind to the endothelial cell surface, stimulating calcium flux during late times of CMV infection. At 96 h postinfection, CMV-infected cells express mRNA of the CMV-encoded CC chemokine receptor US28 but do not express mRNA of other CC chemokine receptors that bind RANTES (CCR1, CCR4, CCR5). Cloning and stable expression of the receptor CMV US28 in human kidney epithelial cells (293 cells) with and without the heterotrimeric G protein α16 indicated that CMV US28 couples to both Gαi and Gα16 proteins to activate calcium flux in response to the chemokines RANTES and MCP-3. Furthermore, cells that coexpress US28 and Gα16 responded to RANTES stimulation with activation of extracellular signal-regulated kinase, which could be attributed, in part, to specific Gα16 coupling. Thus, through expression of the CC chemokine receptor US28, CMV may utilize resident G proteins of the infected cell to manipulate cellular responses stimulated by chemokines.
Cytomegalovirus (CMV) infection is associated with a variety of human syndromes including pneumonitis, obliterative bronchiolitis, and vascular injury-associated atherosclerosis (6, 13, 19, 27). The relationship between infection and these responses of the host tissue remain unclear but, in part, may involve the expression of virally encoded genes and subsequent modification of the infected cell phenotype. After CMV infection, the host cell demonstrates activation manifested by increased effector responses such as inositol lipid hydrolysis, cellular levels of cyclic AMP and cyclic GMP, arachidonic acid metabolism, mobilization of intracellular calcium, and transcription of the c-jun, c-fos, and c-myc oncogenes (3, 8, 31, 41). A second phase of CMV-induced cell activation occurs at late times of infection (72 to 96 h) and is characterized by increased intracellular calcium concentrations (31). These data suggest that virus replication within the host cell is accompanied by activation that is characteristic of growth factor-induced cell activation (2, 4), perhaps enhanced by virus-encoded genes, to facilitate replication and infection.
Human CMV encodes a G-protein-coupled receptor homolog, US28 (11), that has binding affinity for CC chemokines (16, 23, 28). Such serpentine receptors for chemoattractants associate with heterotrimeric G proteins to initiate intracellular signaling that lead to downstream cell activation responses (9, 17, 45). Since the virally encoded receptor US28 has structural homology to the chemokine receptor subfamily of chemoattractant receptors, we have postulated that US28 may also couple with specific G proteins following stimulation, to activate cellular responses (17, 20, 45). The ligands for US28, CC chemokines RANTES, monocyte chemotactic protein 3 (MCP-3), and macrophage inflammatory protein 1α (MIP-1α), have chemoattractant properties for several cell populations, for example CD4+ T cells, monocytes, basophils, eosinophils, and, in the case of MCP-3, neutrophils (5, 33, 36, 38, 40, 42, 46); however, the effect of these chemokines when binding to US28 is unknown. For instance, expression of US28 during infection may function as a decoy receptor that binds CC chemokines and prevents their action on host cells rather than functioning with a principal role of transmitting a signal. Alternatively, we propose here that during CMV infection of the host cell, the virally encoded US28 is expressed on the surface of the infected cell as a functionally active receptor that mediates downstream effector functions in the infected cell following chemokine stimulation.
In this study, we demonstrated that RANTES binds to CMV-infected but not un-infected primary endothelial cells and stimulates intracellular calcium flux late in infection. We have ascribed RANTES-mediated signaling to the CMV-encoded G-protein-coupled chemokine receptor US28. During CMV infection of endothelial cells, mRNA of CMV US28 is expressed but mRNA of the other known CC chemokine receptors is not expressed. To study function, we cloned US28 from strain 4010 in human umbilical vein endothelial cells (HUVECs) and stably expressed the receptor with and without Gα16 in human kidney epithelial cells (293 cells). Using this expression system, we were able to study the mobilization of intracellular Ca2+ and activation of mitogen-activated protein (MAP) kinase pathways in response to CC chemokine stimulation. The data provide the first report that virally derived genes lead to activation of MAP kinases (ERK2).
MATERIALS AND METHODS
Materials.
RANTES, MCP-3, MIP-1α, and interleukin-8 (IL-8) were purchased from R&D Systems, Minneapolis, Minn. Materials for buffers and medium were purchased from Sigma Chemical Co (St. Louis, Mo.) unless stated otherwise. Pertussis toxin (PTX) was purchased from List Biological Laboratories, Inc. (Campbell, Calif.).
Cells.
HUVECs were harvested from umbilical veins by procedures described elsewhere (18). The cells were cultured in endothelial basal medium (Clonetics, Walkersville, Md.) supplemented with 5% fetal calf serum, 1 μg of hydrocortisone per ml, 10 ng of epithelial growth factor per ml, and penicillin-streptomycin-l-glutamine (HUVEC complete medium). Human kidney epithelial cells transformed with adenovirus (293 cells and 293 cells that stably express Gα16 [10]) were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine calf serum (HyClone, Logan, Utah) and penicillin-streptomycin-l-glutamine (complete medium).
Virus.
Human CMV strain 4010, isolated from the urine of an AIDS patient, was passaged in fibroblasts twice before being passaged in HUVECs. Subconfluent HUVEC monolayers were incubated at 0.1 PFU/cell with freshly harvested infected cells for 1 h, inoculum was removed, and cells were supplemented with fresh medium. The cells were maintained for 5 to 7 days in HUVEC complete medium at 37°C under 5% CO2. Mock infection involved incubation of subconfluent HUVEC monolayers with appropriate dilution of HUVEC equivalents and culture in parallel with CMV-infected cultures. Cell-free virus was prepared from supernatants of infected HUVEC cultures. The supernatant was subjected to low-speed centrifugation to remove cells and debris followed by ultracentrifugation at 45,000 × g at 4°C in an SW28 rotor (Beckman Instruments L5-50 ultracentrifuge). The pellet containing concentrated virus particles was resuspended in HUVEC complete medium and stored at −80°C.
Binding of 125I-RANTES.
Assays to determine the specific binding of RANTES to CMV-infected and uninfected HUVECs were performed by the binding methods described by Kuhn et al. (23). Briefly, cells were washed with ice-cold phosphate-buffered saline (PBS) and HEPES binding buffer (HBB) (50 mM HEPES [pH 7.2], 1 mM CaCl2, 5 mM MgCl2, 5% bovine serum albumin). Different concentrations of 125I-RANTES (specific activity, 2,200 Ci/mmol; (Dupont/New England Nuclear Life Science Products, Boston, Mass.) in the presence or absence of excess (10 μM) competing cold MCP-3 (R&D Systems, Minneapolis, Minn.) were added to the cells for 2 h at 4°C. Unbound RANTES was removed, and the cells were rinsed once with HBB and twice with PBS. The cells were dissolved in 0.5 N NaOH and counted on a Beckman gamma counter. Binding assays to determine the binding of RANTES to CMV-infected HUVECs at different times and to US28-expressing 293 cells involved preincubation with HBB for 60 min at 37°C; then 10 pM 125I-RANTES in increasing concentrations of unlabeled RANTES (10 pM to 100 nM) in HBB was incubated with the cells at 4°C for 60 min. The cells were rinsed with ice-cold PBS, lysed in 0.5 N NaOH, and counted.
Confocal microscopy of intracellular calcium flux.
CMV-infected and mock-infected HUVECs in 24-well Corning trays were preincubated with Krebs-Ringer phosphate buffer (pH 7.2) plus 0.2% dextrose (5% dextrose in 0.2% NaCl, injectable [Abbott Laboratories, North Chicago, Ill.]), and 3 mM probenecid (KRPD/p) and then loaded with 2 μM Fluo-3 (Molecular Probes, Eugene, Oreg.) in KRPD/p for 45 min at 37°C in the dark. The cells were rinsed with KRPD/p and stimulated with 100 nM RANTES, MCP-3, or IL-8, with 10 μM ATP as a positive control, and were visualized with an inverted Nikon Diaphot epifluorescence microscope. The microscope was equipped for computer-controlled laser-scanning confocal microscopy (MRC-500; Bio-Rad Microscience, Cambridge, Mass.). Each field of cells, selected at random, was stimulated first with chemokine and then with ATP as a positive control. Images were collected at 15-s intervals for 225 s. The images were analyzed for relative fluorescence using NIH Image, v 5.6, software. The fluorescence of each image was calculated, and the difference from the total fluorescence was plotted over time.
Expression analysis of US28.
RNA was isolated from cells with Trizol reagent (Gibco-BRL Life Sciences, Gaithersburg, Md.) as described by the manufacturer. cDNA was obtained from the RNA precipitates by reverse transcription-PCR and amplified with specific primers to US28 as well as with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control. PCR products were analyzed by ethidium bromide or Vistra Green (Amersham Life Science, Arlington Heights, Ill.) staining of a 3.0% agarose gel and phosphorimaging.
Cloning of CMV US28.
Human CMV US28 was amplified by PCR from a cell lysate of the clinical isolate of CMV strain 4010 that had been propagated in HUVECs for approximately 12 passages. Amplification of US28 required specific primers that included nucleotides 219183 to 220289 of the CMV genome. The resultant product of approximately 1,100 bp was cloned directly into the EcoRI sites of the pCRII vector with the TA cloning kit (Invitrogen, San Diego, Calif.), and two separate clones were sequenced completely.
Creation of stably transfected cell lines.
The open reading frame of US28 was removed from the pCRII vector and cloned into the BamHI and XhoI sites of the hygromycin-selectable, stable episomal vector pCEP4 (Invitrogen, San Diego, Calif.). At 80% confluence in 30-mm dishes, 293 and 293/Gα16 cells were transfected with 2 μg of plasmid DNA by using Lipofectamine (Gibco-BRL Life Sciences) in serum-free DMEM medium. The cells were incubated in complete medium for 72 h and then split into complete medium with 300 μg of hygromycin B per ml. Colonies were allowed to form for 7 days, and individual clones were picked for propagation.
Spectrofluorometric intracellular [Ca2+] measurements.
Adherent 293 cells were removed from the plastic with 450 μM EDTA and then allowed to return to equilibrium for 15 min in Hanks balanced salt solution (HBSS) with Ca2+ and Mg2+ supplemented with 10 mM HEPES (pH 7.4) and 3 mM probenecid (HHBSS/p). The cells (5 × 106 cells/ml) were loaded with 1.0 μM Fura-2 AM (Molecular Probes) in HHBSS/p for 30 min at 37°C in the dark. They were then washed twice in HHBSS/p and resuspended at 2 × 106 cells/ml. Volumes of 1.5 ml of cell suspension were placed in a continuously stirred cuvette at 37°C in an 8000C Spectrofluorometer (SLM Instruments, Inc., Urbana, Ill.), and fluorescence was monitored at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. The data presented are the relative ratio of fluorescence excited at 340 and 380 nm.
MAP kinase assays.
Extracellular signal-regulated kinase (ERK2) activity was assayed by modified methods described previously (4a). 293.Gα16 and 293.Gα16/US28 cells were seeded at 2 × 105 cells/well (35 mm2) and incubated in DMEM–10% bovine calf serum for 48 h at 37°C. The cells were rinsed and incubated with serum-free DMEM for 4 h before stimulation. They were stimulated with 150 nM RANTES or MCP-3 in serum-free medium for 5 min at 37°C. After stimulation, they were rinsed twice with ice-cold PBS, lysed in RIPA buffer containing 2 μg of aprotonin per ml, and centrifuged to remove nuclei. Aliquots of the cell lysates were assayed for protein content. The cell lysates were immunoprecipitated with a 1:50 dilution of rabbit antiserum recognizing ERK2 (Santa Cruz Biotechnology, Santa Cruz, Calif.) and protein A-Sepharose (Sigma Chemical Co.) for 2 h at 4°C, and ERK2 kinase activity was assayed by phosphorylation of epidermal growth factor receptor662-681 peptide (provided by Bio Resource Center, Denver, Colo.) in the presence of [32P]ATP (Amersham Life Science) for 30 min at 30°C. The kinase mix was spotted onto P81 filter discs (Whatman, Clifton, N.J.) and rinsed three times with 200 mM phosphoric acid and once with acetone. The dried discs were counted with a Beckman scintillation counter.
RESULTS
Specific binding of RANTES to CMV-infected HUVECs.
Productive CMV infection of primary endothelial cells (HUVECs) was established with a clinical isolate of CMV, strain 4010, originally isolated from the urine of an AIDS patient and passaged only twice in fibroblasts. Productive infection was verified by immunoblotting of infected-cell lysates with monoclonal antibody to CMV IE1, development of the typical cytopathology of CMV plaques on HUVECs (data not shown), and titer determination assay of PFU as a measure of infectivity (Fig. 1A, inset). The growth curve of strain 4010 in HUVECs indicated that infectious virus was not produced until 72 h postinfection; the long replication of strain 4010 in HUVECs is characteristic of the growth curve of the laboratory strains AD169 and Towne in permissive fibroblasts (14, 15).
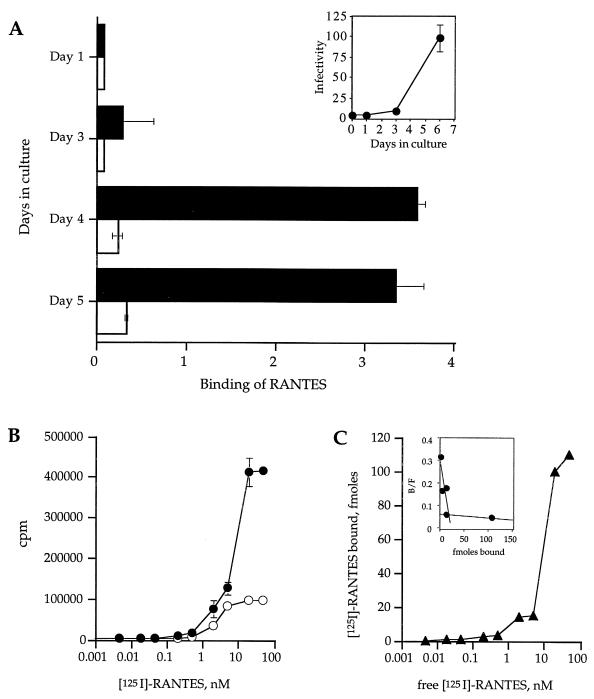
FIG. 1.
Binding of 125I-RANTES to CMV-infected and uninfected HUVECs. (A) The inset shows the growth curve of CMV strain 4010 in primary endothelial cells (HUVECs). Subconfluent monolayers of HUVECs were infected with CMV strain 4010 stock at a multiplicity of infection of 0.1 and then harvested on days 1, 3, and 6 after infection. CMV-infected cells were subjected to titer determination on fresh HUVEC monolayers and assayed for infectivity. Infectivity is represented as 103 PFU per milliliter. Panel A shows binding of 10 pM 125I-RANTES in 100 pM unlabeled RANTES to CMV-infected (▪) or uninfected (□) monolayers. Binding is expressed as total bound labeled RANTES minus nonspecific binding of labeled RANTES relative to total free labeled RANTES and normalized to 20,000 cells. (B) Total binding of 125I-RANTES (•) and nonspecific binding (in the presence of 10 μM MCP-3) (○). (C) Specific binding was calculated by subtracting the nonspecific binding from the total binding and relating the resultant value to femtomoles (inset) by calculating the concentration from the specific activity of the 125I-RANTES.
The time course of expression of RANTES-specific binding on CMV-infected HUVECs was assessed by analysis of the binding of 125I-RANTES on days 1, 3, 4, and 5 of infection (Fig. 1A). No RANTES-specific binding was demonstrated on the infected cells on day 1 or 3 of infection, but on days 4 and 5 there was a significant increase in the specific binding of RANTES; the pattern mirrored the growth curve of CMV strain 4010 during culture in HUVECs (Fig. 1A).
Specific binding of RANTES was analyzed on day 4 of CMV infection of HUVECs (Fig. 1B and C). Increasing concentrations of labeled 125I-RANTES were added in the presence and absence of a 100-fold excess of competing cold MCP-3 to determine the total and nonspecific binding of RANTES (Fig. 1B). As shown in Fig. 1C, specific binding for RANTES is demonstrated with a Kd of approximately 10 nM, which compares to the reported Kd of 3.4 nM in US28-expressing K562 cells (16).
RANTES stimulates calcium flux in CMV-infected HUVECs.
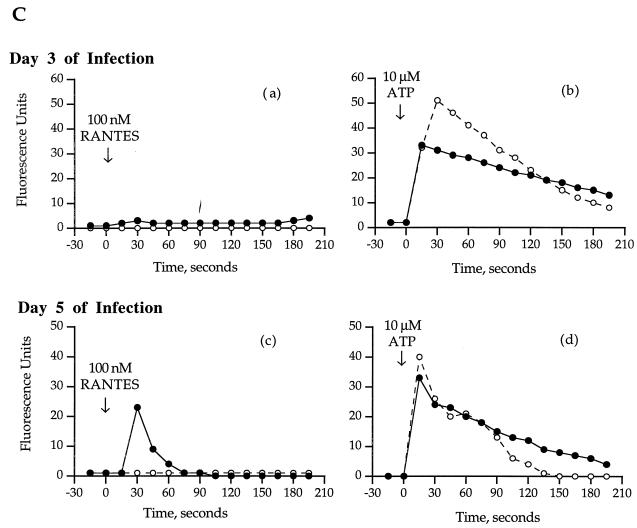
The consequences of RANTES binding to the infected cells were determined by induction of a cellular calcium flux by RANTES (Fig. 2). HUVEC monolayers were inoculated with CMV-infected HUVECs or mock-infected HUVECs and cultured at 37°C for 5 days. On days 1, 3, and 5 postinoculation, CMV-infected and mock-infected HUVEC monolayers were loaded with Fluo-3, rinsed, and assessed by confocal microscopy for a flux of intracellular calcium following stimulation. The positive control for intracellular calcium flux included stimulation with 10 μM ATP. The confocal images of stimulation of calcium flux in cells infected with CMV on day 5 of infection are shown in Fig. 2A and B, and the complete time courses of relative fluorescence following stimulation on days 3 and 5 of infection are presented in Fig. 2C. There was an increase in fluorescence in the CMV-infected cells following RANTES stimulation on day 5 of infection (Fig. 2B and C) that was not demonstrated on day 3 of infection (Fig. 2C). The RANTES-stimulated increase in fluorescence in the CMV-infected cells was intense in 20% of the cells per field (Fig. 2A) but transient compared with ATP. The mock-infected cells did not respond to RANTES stimulation at any time but did respond to ATP stimulation (Fig. 2C). It appears, therefore, that during CMV infection, a receptor for RANTES-specific signaling is expressed on infected endothelial cells at late times of infection.
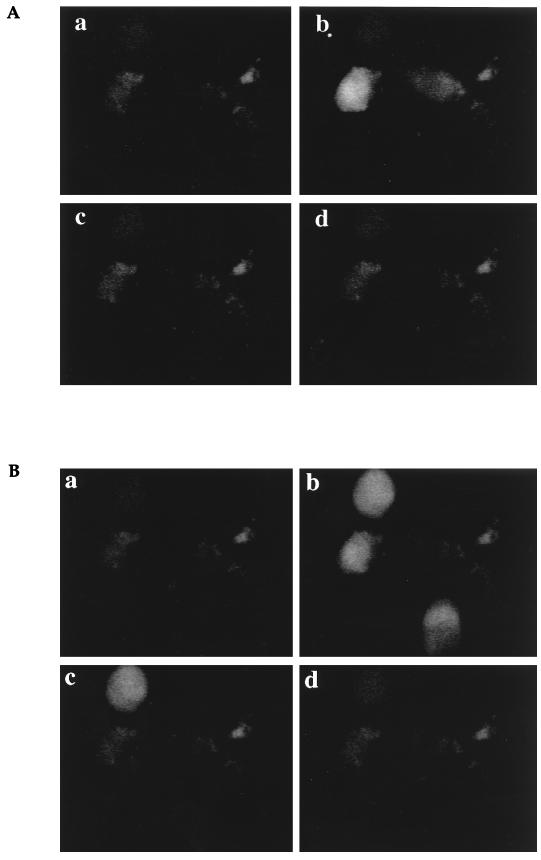
FIG. 2.
Calcium flux in CMV-infected HUVECs. (A and B) Monolayers of CMV-infected HUVECs on day 5 of culture were loaded with Fluo-3, and a single field was analyzed by confocal microscopy for calcium flux after stimulation with 100 nM RANTES (A) or 10 μM ATP (B). Images were captured after stimulation at time zero (a), 30 s (b), 60 s (c), and 90 s (d). (C) Time course of the calcium flux in CMV-infected and mock-infected HUVECs. The relative fluorescence from the images on day 3 (a and b) and day 5 (c and d) of infection was estimated with National Institutes of Health Image software. •, CMV-infected HUVECs; ○, uninfected HUVECs.
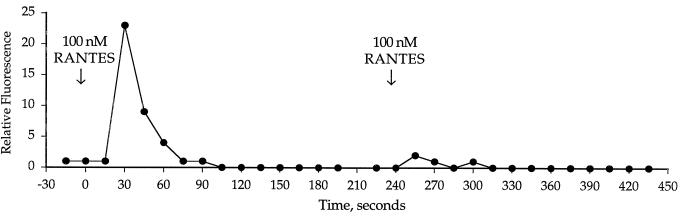
Desensitization of a signal by a second exposure to stimulus reflects receptor-mediated events. The CMV-infected cells were desensitized to a second stimulation of RANTES, thereby suggesting that a receptor was responsible for the RANTES- mediated signaling (Fig. 3). There was no response in the infected cells to the CXC chemokine IL-8, and there was an insignificant response to 500 nM MCP-3 (data not shown).
FIG. 3.
Desensitization of the intracellular calcium flux in CMV-infected HUVECs in response to a second stimulation of RANTES.
Human CC chemokine receptor mRNA is not expressed.
To determine whether an endogenous CC chemokine receptor was upregulated during CMV infection, we determined whether mRNA of the known CC chemokine receptors were present in uninfected and CMV-infected HUVECs. The RNA from uninfected and CMV-infected HUVECs was isolated along with the RNA from peripheral blood lymphocytes as a control for CC chemokine receptor expression and analyzed by an RNase protection assay with a commercially available template probe that would identify all six of the known human CC chemokine receptors (CCR1, CCR2a and CCR2b, CCR3, CCR4, and CCR5) (Pharmingen, San Diego, Calif.). Peripheral blood lymphocytes contained RNA of all six of the expected CC chemokine receptors; however, uninfected and CMV-infected HUVECs did not express mRNA for the known CC chemokine receptors (data not shown). mRNAs of the housekeeping genes, GAPDH and L32, were detected in comparable amounts in each of the cell types. Thus, neither uninfected nor CMV-infected HUVECs express mRNA for any of the six well-characterized CC chemokine receptors; these data suggest that acquisition of RANTES binding may be due to expression of the virally encoded CC chemokine receptor US28.
Expression of US28 mRNA during CMV infection of HUVECs.
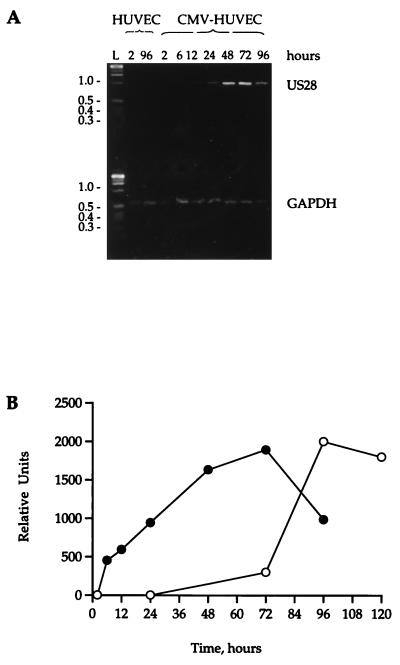
To determine whether the CMV-encoded CC chemokine receptor US28 was expressed during CMV infection of HUVECs, subconfluent HUVEC monolayers were infected with cell-free CMV at 0.01 PFU/cell and, at specific times after infection, were harvested for mRNA. By using reverse transcription-PCR techniques with US28- and GAPDH-specific primers, US28 mRNA could be detected as early as 6 h postinfection; the level of US28 mRNA reached a plateau by 48 to 72 h postinfection before declining at 96 h (Fig. 4A). There was no US28 mRNA present in the mock-infected cells at any time, and the levels of GAPDH in the infected and mock-infected cells remained constant.
FIG. 4.
Expression of US28 mRNA during CMV infection. (A) RNA was isolated from mock-infected or CMV-infected HUVECs at specific times (2, 6, 12, 24, 48, 72, and 96 h) after infection. RNA was transcribed to cDNA with Moloney murine leukemia virus reverse transcriptase, and specific fragments of US28 and GAPDH were identified by PCR. Samples were run on a 3.0% agarose gel and stained with Vistra Green. (B) Appearance of US28 mRNA and RANTES binding during CMV infection. The intensities of the bands in panel A were quantified by fluorescence imaging on a Storm phosphorimager. The arbitrary units of mRNA concentration are plotted against the time course of RANTES binding during infection.
Comparison of the time course of appearance of US28 mRNA with binding of the chemokine RANTES to the cells during CMV infection indicated that there was a lag of approximately 48 h between the first appearance of mRNA and the appearance of a functional RANTES receptor on the cell surface (Fig. 4B).
Expression of US28 in 293 cells and 293.Gα16 cells.
To further study the function of the virally encoded CC chemokine receptor, we isolated a clone of US28 from the CMV clinical strain 4010 that had been propagated exclusively through HUVECs. Comparison of US28(4010) to previously cloned US28 sequences indicated that our clone of US28 differed, with five amino acid dissimilarities, from the US28 sequence of strain VHL, which had also been propagated exclusively in HUVECs (44). There was remarkable identity between the predicted amino acid sequence of US28(4010) and the sequences of US28 of the laboratory strains AD169 and Towne (16, 28), such that the sequence of US28(4010) differed only at position 19, where a nonpolar alanine (4010) is replaced by a positively charged aspartic acid (AD169). While this substitution may herald alterations in the structure or function of US28, the implications of this amino acid change have not been pursued in this study.
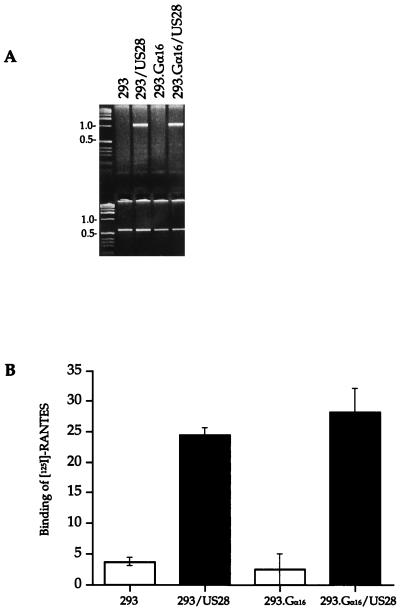
To confirm that US28 was expressed in the 293 and 293.Gα16 cells, US28 mRNA (lanes 2 and 4) was identified in the transfected cell cultures, whereas the parent cells did not express US28 mRNA (lanes 1 and 3) (Fig. 5A). Specific binding of 125I-RANTES to US28-expressing and nonexpressing 293 and 293.Gα16 cells indicated that a RANTES receptor was expressed on the cell surface in the US28-expressing cells but not in the nonexpressing cells (Fig. 5B).
FIG. 5.
Expression of CMV US28 in 293 cells. (A) Identification of mRNA transcripts of US28 in 293 and 293.Gα16 cells expressing US28. cDNA of mRNA collected from cell lysates was amplified with specific primers to HCMV US28 and GAPDH. (B) Binding of 10 pM 125I-RANTES to 293 and 293.Gα16 cells without US28 (□) or expressing US28 (▪). RANTES binding for each cell type was calculated relative to the total cpm and normalized to 5 × 105 cells.
Activation of intracellular calcium flux in response to CC chemokine ligation to US28.
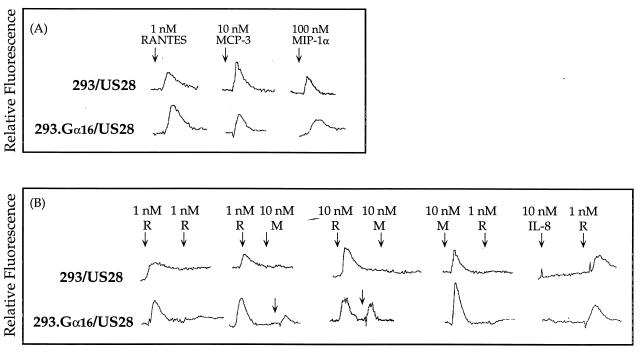
Previous studies have shown that stimulation of CMV US28 by the CC chemokines RANTES and MIP-1α activates the mobilization of intracellular Ca2+ concentrations (16, 23). Our results confirm these findings and also show that cells expressing CMV US28 can undergo an intracellular calcium flux in response to stimulation by MCP-3 (Fig. 6). The US28-mediated response was 10-fold lower following stimulation with MCP-3 (10 nM) than with RANTES (1 nM) and was 100-fold less following stimulation with MIP- 1α (100 nM) (Fig. 6A). There was no difference in the stimulation of intracellular Ca2+ flux in response to the chemokines with respect to expression of the Gα16 subunit (Fig. 6A). 293 and 293.Gα16 cells that do not express US28 did not show intracellular calcium flux following stimulation with high concentrations of RANTES, MCP-3, MCP-1, or MIP-1α.
FIG. 6.
Tracings of intracellular Ca2+ flux in cells expressing US28 after stimulation with chemokines. Each tracing is representative of three separate runs. (A) Single stimulation of 293 and 293.Gα16 cells expressing US28 with RANTES, MCP-3, and MIP-1α. (B) Sequential stimulation of 293 and 293.Gα16 cells expressing US28 with either 1 or 10 nM RANTES (R) or 10 nM MCP-3 (M).
Desensitization of US28-mediated intracellular calcium flux following stimulation with CC chemokines.
The chemokines RANTES and MCP-3 were added sequentially to test the cross-desensitization of the US28 response (Fig. 6B). Stimulation of cells expressing US28 with and without Gα16 with RANTES at the higher dose of 10 nM significantly desensitized the receptor to a second stimulation with 10 nM MCP-3. Likewise, prestimulation with MCP-3 (10 nM) completely desensitized receptor activity to stimulation with RANTES (1 nM). Coexpression of Gα16 enhanced the response by US28 following stimulation with RANTES (1 nM); the cells that coexpress US28 and Gα16 protein were not completely desensitized to secondary stimulation by MCP-3. Stimulation of cells with the CXC chemokine IL-8 did not activate a flux of intracellular calcium and did not desensitize the receptor to a second stimulation with 1 nM RANTES.
US28 activates intracellular Ca2+ mobilization through specific G proteins.
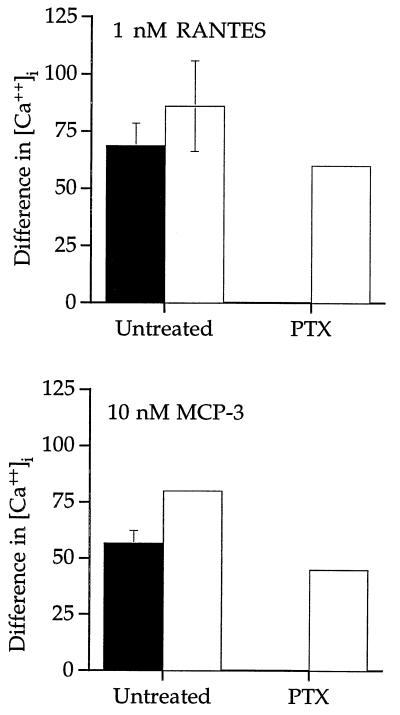
Treatment of the cells with the Gαi class inhibitor PTX completely inhibited RANTES and MCP-3 stimulation of intracellular Ca2+ flux in 293 cells expressing US28 (Fig. 7). In 293 cells that coexpress US28 with Gα16 (not a substrate for PTX), PTX did not inhibit RANTES and MCP-3 stimulation of intracellular calcium mobilization. Hence, US28 couples to Gαi proteins as well as PTX-insensitive Gα16 proteins to activate intracellular calcium flux following chemokine stimulation.
FIG. 7.
HCMV-US28 signaling of intracellular Ca2+ flux in the presence of 100 μg of PTX per ml. ▪, 293 cells expressing US28; □, 293.Gα16 cells expressing US28. The difference in intracellular calcium concentration is represented for each stimulation. Data are the mean of three experiments.
Activation of MAP kinases in 293 cells expressing CMV US28 with or without Gα16 by specific CC chemokines.
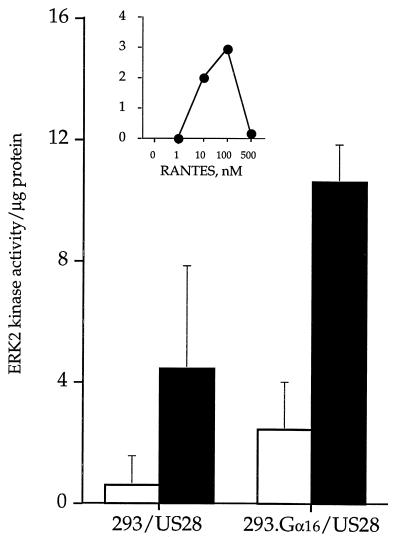
Several reports indicate that CMV infection of the host cell initiates membrane-associated cellular responses that mimic growth factor-induced cell activation (1–3, 7); however, the associated signaling cascades that link the membrane events and effector response have not been defined. Since chemoattractant G-protein-coupled receptors activate pathways typical of those attributed to growth factor stimulation (4a, 9, 21, 30, 45), we investigated CMV-encoded US28 activation of ERK2 MAP kinase in terms of phosphorylation of the epidermal growth factor receptor peptide in response to chemokine stimulation (Fig. 8). A dose-response curve of RANTES stimulation of 293.Gα16/US28 cells following a 5-min incubation indicated that reactivity peaks at 100 nM RANTES (Fig. 8, inset).
FIG. 8.
Activation of MAP kinase pathways by RANTES stimulation. Activation of ERK2 MAP kinase in 293 cells, and 293 cells expressing US28, 293.Gα16 cells, and 293.Gα16 cells expressing US28. MAP (ERK2) kinase was immunoprecipitated from unstimulated cells (□) and cells stimulated with 150 nM RANTES (▪). The data are the mean of three experiments. The inset represents a RANTES dose-response curve of ERK2 activation in 293.Gα16 cells expressing US28.
There was an increase of ERK2 MAP kinase activity in 293 and 293.Gα16 cells expressing US28 after stimulation by RANTES, and although these cells coexpressing Gα16 inconsistently demonstrated a high level of background ERK2 MAP kinase activity, there was reproducible stimulation of the cells in response to RANTES (Fig. 8). US28 receptor in the absence of Gα16 protein appeared less responsive to RANTES stimulation in terms of ERK2 MAP kinase activation. Cells without US28 did not significantly respond to RANTES stimulation, although the backgrounds were elevated (data not shown).
CMV US28 couples to Gαi and Gα16 proteins to activate MAP kinase after stimulation with RANTES.
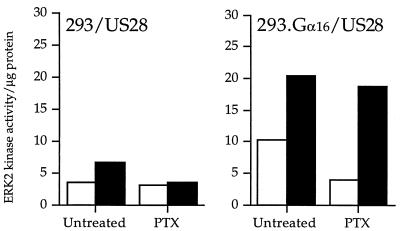
Pretreatment of US28-expressing 293 cells with PTX did not significantly inhibit RANTES-stimulated ERK2 MAP kinase activation in the cells that coexpress Gα16 and US28 cells (Fig. 9). However, as expected, the small increase in ERK2 MAP kinase activity in the US28 cells without Gα16 was sensitive to PTX inhibition, confirming that US28 couples weakly to Gαi class proteins to stimulate ERK2 MAP kinase activity. This experiment suggests that pretreatment with PTX may inhibit Gαi-protein-mediated MAP kinase signaling but does not affect specific coupling of US28 to Gα16 proteins to activate the MAP kinase pathway in response to chemokine stimulation.
FIG. 9.
CMV US28 signaling of ERK2 MAP kinase activity in the presence of PTX. □, unstimulated cells; ▪, cells stimulated with 150 nM RANTES. The data represent a single experiment representative of three experiments.
DISCUSSION
In this study, we demonstrated that the CC chemokine RANTES binds to CMV-infected endothelial cells and stimulates intracellular calcium flux through a putative RANTES receptor on the infected cell. Pursuant to these studies, we have ascribed at least some of these RANTES-mediated activities to the CMV-encoded RANTES receptor US28 such that during CMV infection, none of the human CC chemokine receptors (CCR1, CCR2a or CCR2b, CCR3, CCR4, and CCR5) are expressed in primary endothelial cells. In addition, US28 couples to specific G proteins and, through these associations, activates intracellular calcium flux and the MAP kinase pathway in response to stimulation by CC chemokines. This study suggests that cell activation mechanisms during CMV infection may involve the CMV protein US28.
We have developed a model of CMV infection of the endothelium by propagation of a pathogenic clinical isolate of CMV, strain 4010, through primary endothelial cells (HUVECs). Although endothelial cells cultured in vitro are not susceptible to productive CMV infection with the laboratory strains AD169 and Towne (15, 39, 44), the clinical isolate strain 4010 infects HUVECs to produce infectious virus particles in a growth pattern similar to that of the laboratory strain AD169 in permissive fibroblasts (15). In addition, at late times of infection, specific proteins that bind the CC chemokine RANTES are expressed on the surface of infected HUVECs; RANTES- specific binding was not detected in uninfected HUVECs. In functional studies of Fluo-3-loaded CMV-infected HUVECs, RANTES stimulation induced intracellular calcium flux at late times of infection, thereby indicating that during infection a functional RANTES-specific receptor was expressed on the infected-cell surface.
In support of our hypothesis that a virally encoded CC chemokine receptor is expressed during CMV infection of endothelial cells and activates specific intracellular pathways in response to CC chemokine stimulation, we confirmed that endogenous human CC chemokine receptors were not expressed during CMV infection. RNA from CMV-infected and mock-infected HUVECs was analyzed by an RNase protection assay with a radiolabeled multiprobe specific for the six well-characterized human chemokine receptors. None of these receptors were expressed in the HUVECs, whereas all the receptors were expressed in peripheral blood lymphocytes. In addition, CMV infection of HUVECs did not upregulate the expression of previously undetectable receptors. These data do not preclude the possibility that an unknown endogenous CC chemokine receptor exists. It should be noted that the Duffy antigen receptor, which is expressed in endothelial cells from such organs as the brain and kidneys, also binds CC chemokines (24, 34) and may be upregulated during CMV infection. However, chemokine binding to the Duffy antigen receptor does not activate intracellular signaling pathways (29).
During infection, US28 mRNA was expressed as early as 6 h and its level peaked at 48 to 72 h (Fig. 5A). It is interesting, however, that there appeared to be a lag time between the first appearance of US28 mRNA in the infected cell and the acquisition of specific RANTES binding. The delayed expression of the functional receptor on the cell surface may be due to prolonged posttranslational modifications of the seven transmembrane-spanning proteins.
It is likely that CMV-activated cellular responses during infection are preceded by CMV-regulated intracellular signaling pathways. CMV-induced activation of the transcriptional machinery and arachidonic acid release by CMV infection (1, 8) suggested to us the possibility that MAP kinase pathways activated through signaling receptors expressed on the cell surface were involved (32). Cell activation in CMV-infected cells may be due in part to membrane expression of de novo-synthesized CMV late proteins, and we propose that US28 is a candidate for this CMV-encoded gene. Our evidence that US28 may be a functional chemokine receptor includes the observations that during CMV infection of HUVECs, (i) RANTES specifically binds to the infected-cell surface and stimulates the intracellular calcium flux and (ii) the infected cells express CMV-encoded US28, a CC chemokine receptor, but do not express any known human CC chemokine receptors. Thus, since our infection model involves a clinical isolate passaged through primary endothelial cells, we cloned US28 from the 4010-infected HUVECs and stably expressed it in human epithelial kidney cells (293 cells) to determine whether US28 from a clinical isolate may have specific functions that contribute to virulence. In addition, we were interested in the ability of US28 to couple with specific G proteins, and so we coexpressed US28 with the Gα16 protein in 293 cells.
Heterotrimeric G proteins are expressed in a tissue-specific fashion, and thus expression of receptors in cells with different repertoire of G proteins might exert different effects. CMV infects many different cell types and therefore may be able to manipulate specific populations of cells through coupling of the virally encoded US28 with host G proteins (e.g., Gα16 subunits are specifically expressed in hematopoietic cells) to activate intracellular signaling pathways. In this study, US28 couples with Gα16 and Gαi class proteins to activate the intracellular calcium flux after stimulation with a spectrum of CC chemokines but not CXC chemokines such as IL-8. The US28-expressing cells were responsive to stimulation by RANTES but were less responsive to similar concentrations of the other CC chemokines, MCP-3 and MIP-1α, thereby confirming previous reports that RANTES may be the primary ligand for US28 activation (16, 23). In 293 cells expressing US28, RANTES and MCP-3 cross-desensitize, suggesting a common activation pathway of intracellular calcium signaling (Fig. 6B). However, in 293 cells that coexpress US28 and Gα16, the desensitization to MCP-3 by RANTES was diminished (Fig. 6B). The ability of US28 to depend on specific G proteins was confirmed by pretreatment of the cells with the Gαi inhibitor PTX. PTX completely inhibited US28-mediated function in the US28-expressing 293 cells but did not inhibit the US28-mediated calcium flux in the 293 cells that coexpressed US28 and the PTX-resistant Gα16, thereby indicating that US28 couples to G as well as Gαi proteins (Fig. 7). The lack of response by MCP-3 (500 nM) in the CMV-infected cells compared to the US28-transfected 293 cells may indicate that the appropriate repertoire of cellular signaling proteins essential for US28 function in response to MCP-3 stimulation was not present in the HUVECs, but MCP-3 is 10-fold less active than RANTES as a stimulant in the US28-expressing 293 cells, and this may also affect the ability of the infected endothelial cells to respond.
The possibility that CMV infection modifies the activation of MAP kinase signal transduction represents a novel mechanism for recruitment of transcriptional activity in the infected cells. Our data suggest that CMV infection regulates the MAP kinase-signaling pathways of the host cell through the CMV-encoded G protein coupled receptor US28. Our analyses included activation of the ERK2 MAP kinase, a member of the Ras-dependent signaling cascade that is traditionally activated by growth factors (12, 25). Although both cell lines expressing US28 demonstrated activation of ERK2 in response to RANTES stimulation, the response was enhanced in cells that coexpressed US28 and Gα16. PTX pretreatment did not inhibit RANTES stimulation of ERK2 MAP kinase activity in the cells that coexpressed US28 with the PTX-resistant Gα16 proteins, whereas RANTES stimulation of ERK2 MAP kinase activity was completely inhibited in the US28-expressing cells that did not express Gα16, indicating that US28 couples, albeit weakly, to Gαi-class proteins. These results complement the work recently published by Epstein and colleagues, wherein CMV activation of several pathways including MAP kinase in smooth muscle cells, which presumably do not contain Gα16, was blocked by PTX treatment (34a).
This report of the intracellular signaling pathways activated by US28, a homolog of human CC chemokine receptors, may provide information on the signaling pathways activated by chemokine receptors expressed on responding immune cells. Exhaustive studies of chemoattractant receptors describe activation of the MAP kinase pathways through ligand stimulation, for example, fMLP and C5a (9, 45), and the CXC chemokine receptor for IL-8 (21). However, studies of CC chemokine receptor activation have been limited to reports of calcium mobilization and adenylate cyclase studies (26, 35, 37). As a representative of CC chemokine receptors, despite its viral origin, US28 has demonstrated a functional ability to activate the MAP kinase-signaling pathway through ERK2 MAP kinase in response to CC chemokine stimulation. The activation of ERK2 kinase by chemokine stimulation of US28 suggests that US28 may be able to mimic specific aspects of growth factor-regulated cell activation.
The expression of Gα16 protein in human cells of hematopoietic origin suggests that CMV may be able to manipulate these cells through US28. CMV has an enhanced tropism for infection of hematopoietic cells (particularly monocytes), and these cells have been implicated as a site of CMV latency (43). The coupling of CMV US28 with the Gα16 subunit may allow endogenous chemokines to activate intracellular signaling to promote monocyte proliferation. To date, we can only speculate that this leads to viral persistence. However the link between CMV and atherosclerosis (19, 22), where macrophage activation plays an important role, suggests that expression of G protein receptors by viruses may exert pleiotropic effects on host cellular responses. Future studies will determine whether CMV encodes proteins, such as US28, to enhance specific functions in cell types that may be important for viral persistence.
ACKNOWLEDGMENTS
We thank J. Nichol for advice about the cloning of CMV US28.
This work was supported by the National Institutes of Health: grants HL-34303 and GM-30324 and by Specialized Center for Research in ARDS (adult respiratory distress syndrome) grant HL-40784.
REFERENCES
- 1.AbuBaker S, Boldogh I, Albrecht T. HCMV, stimulation of [3H] release from [3H]-arachidonic acid prelabelled cells. Arch Virol. 1990;113:255–266. doi: 10.1007/BF01316678. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht T, Boldogh I, Fons M. Receptor-initiated activation of cells and their oncogenes by herpes-family viruses. J Invest Dermatol. 1992;98:29S–35S. doi: 10.1111/1523-1747.ep12462169. [DOI] [PubMed] [Google Scholar]
- 3.Albrecht T, Boldogh I, Fons M, AbuBakar S, Deng C. Cell activation signals and the pathogenesis of HCMV. Intervirology. 1990;31:68–75. doi: 10.1159/000150140. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht T, Boldogh I, Fons M, Lee C, AbuBakar S, Russell J, Au W. Cell-activation responses to CMV infection. Relationship to phasing of CMV replication and to the induction of cellular damage. Subcell Biochem. 1989;15:157–202. [PubMed] [Google Scholar]
- 4a.Avdi N, Winston B W, Russel M, Young S K, Johnson G L, Worthen G S. Activation of MEKK by FMLP in human neutrophils: mapping pathways for MAP kinase activation. J Biol Chem. 1996;271:33598–33606. doi: 10.1074/jbc.271.52.33598. [DOI] [PubMed] [Google Scholar]
- 5.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 6.Bando K, Paradis I, Similo S, Konishi H, Komatsu K, Zullo T, Yousem S, Close J, Zeevi A, Duquesnoy R, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110:4–13. doi: 10.1016/S0022-5223(05)80003-0. [DOI] [PubMed] [Google Scholar]
- 7.Boldogh I, AbuBakar S, Deng C, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991;65:1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boldogh I, Abubakar S, Fons M, Deng C, Albrecht T. Activation of cellular oncogenes by clinical isolates and laboratory strains of HCMV. J Med Virol. 1991;34:241–247. doi: 10.1002/jmv.1890340409. [DOI] [PubMed] [Google Scholar]
- 9.Buhl A, Avdi N, Worthen G, Johnson G. Mapping of the C5a receptor signal transduction network in human neutrophils. Proc Natl Acad Sci USA. 1994;91:9190–9194. doi: 10.1073/pnas.91.19.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buhl A, Eisfelder B, Worthen G, Johnson G, Russell M. Selective coupling of the human anaphylatoxin C5a receptor and α16 in human kidney 293 cells. FEBS Lett. 1993;323:132–134. doi: 10.1016/0014-5793(93)81464-b. [DOI] [PubMed] [Google Scholar]
- 11.Chee M, Satchwell S, Preddie E, Weston K, Barrell B. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature. 1990;344:774–777. doi: 10.1038/344774a0. [DOI] [PubMed] [Google Scholar]
- 12.Egan S, Weinberg R. The pathway to signal achievement. Nature. 1993;365:781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- 13.Ettinger N, Bailey T, Trulock E, Storch G, anderson D, Raab S, Spitznagel E, Dresler C, Cooper J. Cytomegalovirus infection and pneumonitis. Impact after isolated lung transplantation. Am Rev Respir Dis. 1993;147:1017–1023. doi: 10.1164/ajrccm/147.4.1017. [DOI] [PubMed] [Google Scholar]
- 14.Fish K, Depto A, Moses A, Britt W, Nelson J. Growth kinetics of HCMV are altered in monocyte-derived macrophages. J Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman H, Macarak E, MacGregor R, Wolfe J, Kefalides N. Virus infection of endothelial cells. J Infect Dis. 1981;143:266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- 16.Gao J-L, Murphy P. Human cytomegalovirus open reading frame US28 encodes a functional β-chemokine receptor. J Biol Chem. 1994;269:28539–28542. [PubMed] [Google Scholar]
- 17.Gerard N, Gerard C. The chemoattractant receptor for human C5a anaphylatoxin. Nature. 1991;349:614–616. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 18.Gimbrone M, Jr, Shefton E, Cruise S. Isolation and primary culture of endothelial cells from human umbilical vessels. Tissue Cult Assoc Manual. 1978;4:813. [Google Scholar]
- 19.Grattan M, Moreno-Cabral C, Starnes V, Oyer P, Stinson E, Shumway N. CMV infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989;261:3561–3566. [PubMed] [Google Scholar]
- 20.Holmes W, Lee J, Kuang W, Rice G, Wood W. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:16275–16278. [PubMed] [Google Scholar]
- 21.Knall C, Young S, Nick J, Buhl A, Worthen G. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 22.Koskinen P, Nieminen M, Mattila S, Hayry P, Lautenschlager I. The correlation between symptomatic CMV infection and CMV antigenemia in heart allograft recipients. Transplantation. 1993;55:547–551. doi: 10.1097/00007890-199303000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn D, Beall C, Kolattukudy P. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun. 1995;211:325–330. doi: 10.1006/bbrc.1995.1814. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Wang Z, Horuk R, Hellelgesser J, Lou Y, Hadley T, Peiper S. The promiscuous chemokine binding profile of the Duffy antigen/receptor for chemokines is primarily localized to sequences in the amino-terminal domain. J Biol Chem. 1995;270:26239–26245. doi: 10.1074/jbc.270.44.26239. [DOI] [PubMed] [Google Scholar]
- 25.Marshall C. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 26.McColl S, Hachicha M, Levasseur S, Neote K, Schall T. Uncoupling of early signal transduction events from effector function in human peripheral blood neutrophils in response to recombinant MIP-1α and 1β. J Immunol. 1993;150:4550–4560. [PubMed] [Google Scholar]
- 27.Melnick J, Adam E, DeBakey M. Possible role of CMV in atherogenesis. JAMA. 1990;263:2204–2207. [PubMed] [Google Scholar]
- 28.Neote K, DiGregorio D, Mak J, Horuk R, Schall T. Molecular cloning, functional expression and signaling characteristics of a CC chemokine receptor. Cell. 1993;72:415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 29.Neote K, Mak J, Jr L K, Schall T. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood. 1994;84:44–52. [PubMed] [Google Scholar]
- 30.Nick J, Avdi N, Gerwins P, Johnson G, Worthen G. Activation of a p38 mitogen-activated protein kinase in human neutrophils by LPS. J Immunol. 1996;156:4867–4875. [PubMed] [Google Scholar]
- 31.Nokta M, Eaton D, Steinsland O, Albrecht T. Ca2+ responses in CMV-infected fibroblasts of human origin. Virology. 1987;157:259–267. doi: 10.1016/0042-6822(87)90268-6. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki K, Sagata N. The MOS/MAP kinase pathway stabilizes c-fox by phosphorylation and augments its transforming activity in NIH 3T3 cells. EMBO J. 1995;14:5048–5059. doi: 10.1002/j.1460-2075.1995.tb00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opdenakker G, Froyen G, Fiten P, Proost P, VanDamme J. Human monocyte chemotactic protein-3 (MCP-3): molecular cloning of the cDNA and comparison with other chemokines. Biochem Biophys Res Commun. 1993;191:535–542. doi: 10.1006/bbrc.1993.1251. [DOI] [PubMed] [Google Scholar]
- 34.Peiper S, Wang Z, Neote K, Martin A, Showell H, Conklyn M, Ogborne K, Hadley T, Lu Z, Hesselgesser J. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med. 1995;181:1311–1317. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Shibutani T, Johnson T M, Yu Z X, Ferrans V J, Moss J, Epstein S E. Pertussis toxin-sensitive G proteins as mediators of the signal transduction pathways activated by cytomegalovirus infection of smooth muscle cells. J Clin Invest. 1997;100:2054–2061. doi: 10.1172/JCI119738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sozzani S, Molino M, Locati M, Luini W, Cerletti C, Vecchi A, Mantovani A. Receptor-activated calcium influx in human monocytes exposed to monocyte chemotactic protein-1 and related cytokines. J Immunol. 1993;150:1544–1553. [PubMed] [Google Scholar]
- 36.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Damme J V, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a and a distinct set of chemokines. J Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 37.Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, Vita N, Damme J V, Mantovani A. Receptors and transduction pathways for monocyte chemotactic protein-2 and MCP-3. J Immunol. 1994;152:3615–3622. [PubMed] [Google Scholar]
- 38.Sozzani S, Zhou D, Locati M, Rieppi M, Proost P, Magazin M, Vita N, Damme J V, Mantovani A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3: similarities and differences with MCP-1. J Immunol. 1994;152:3615. [PubMed] [Google Scholar]
- 39.Span A, Dam-Mieras M V, Mullers W, Endert J, Muller A, Bruggeman C. The effect of virus infection on the adherence of leukocytes or platelets to endothelial cells. Eur J Clin Invest. 1991;21:331–338. doi: 10.1111/j.1365-2362.1991.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 40.Uguccioni M, D’Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1α, MIP-1β on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- 41.Valyi-Nagy T, Bandi Z, Boldogh I, Albrecht T. Hydrolysis of inositol lipids: an early signal of HCMV infection. Arch Virol. 1988;101:199–207. doi: 10.1007/BF01311001. [DOI] [PubMed] [Google Scholar]
- 42.VanDamme J, Proost P, Lenaerts J, Opedenakker G. Structural and functional identification of two human, tumor-derived monocyte chemotactic proteins (MCP-2 and MCP-3) belonging to the chemokine family. J Exp Med. 1992;176:59. doi: 10.1084/jem.176.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Laer D, Serr A, Meyer-Konig U, Kirste G, Hufert F, Haller O. Human cytomegalovirus immediate early and late transcripts are expressed in all major leukocyte populations in vivo. J Infect Dis. 1995;172:365–370. doi: 10.1093/infdis/172.2.365. [DOI] [PubMed] [Google Scholar]
- 44.Waldman W, Roberts W, Davis D, Williams M, Sedmak D, Stephens R. Preservation of natural endothelial cytopathogenicity of CMV by propagation in endothelial cells. Arch Virol. 1991;117:143–164. doi: 10.1007/BF01310761. [DOI] [PubMed] [Google Scholar]
- 45.Worthen G, Avdi N, Buhl A, Suzuki N, Johnson G. fMLP activates Ras and Raf in human neutrophils. J Clin Invest. 1994;94:815–823. doi: 10.1172/JCI117401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu L, McVica D, Ben-Baruch A, Kuhns D, Johnston J, Oppenheim J, Wang J. MCP-3 interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995;25:2612–2617. doi: 10.1002/eji.1830250931. [DOI] [PubMed] [Google Scholar]