Abstract
A key set of connections necessary for the most complex brain functions are the long association cortico-cortical fiber tracts. These pathways have been described by the Dejerines and others using post mortem histological or brain dissection techniques. Given methodological limitations, these fiber connections have not been delineated completely in humans. Although the stem portions of fiber tracts have been identified in humans, their precise origins and terminations remain to be determined. By contrast, the origins and terminations as well as the stems of long cortico-cortical association fiber pathways in monkeys have been detailed in the macaque monkey brain using experimental tract tracing methods. Deepak Pandya made major contributions to the delineation of fiber tracts in the monkey brain. In the early 1990s, he compared his observations in monkeys with the original descriptions in humans by the Dejerines. With the advent of diffusion-weighted imaging, Dr. Pandya extended this line of investigation to the human brain with Dr. Nikos Makris. In this translational analysis of long association cortico-cortical fiber tracts, they applied a principle of extrapolation from monkey to human. In the present study, we addressed the reasoning and the complex methodology in translating brain structural connectivity from monkey to human in one cortico-cortical fiber tract, namely the superior fronto-occipital fascicle, which was delineated in both species by Dr. Pandya and colleagues. Furthermore, we represented this information in the form of connectional matrices in the context of the HOA2.0-ComPaRe framework, a homological monkey-to-human translational system used in neuroimaging.
Keywords: dMRI, DTI, fiber bundle, superior fronto-occipital fascicle, Harvard-Oxford Atlas, tractography
Graphical Abstract
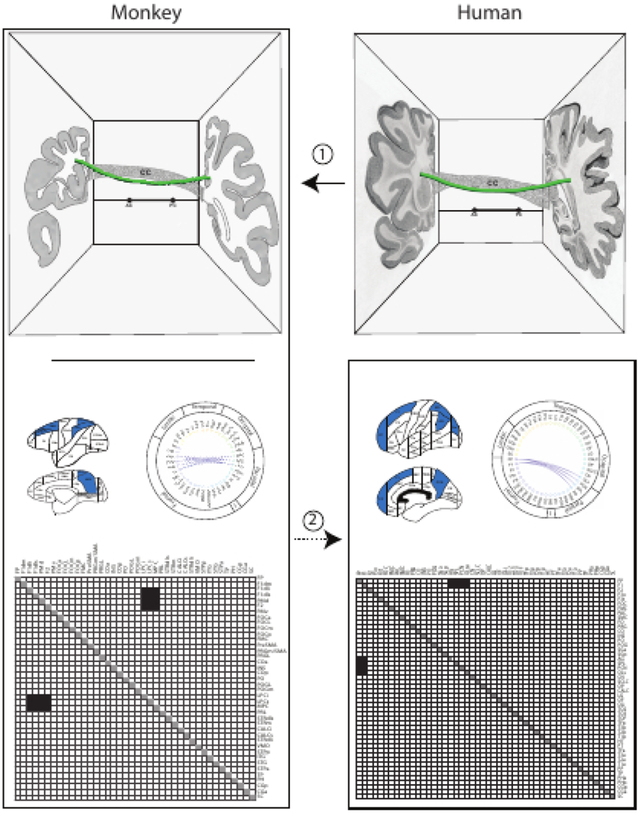
Direct information about origins and terminations of cortico-cortical long association fiber pathways in humans is extremely limited. The topographic location and trajectory of cortico-cortical association pathway stems in humans and non-human primates is similar (1), but only in non-human primates is there complete information on origins and terminations as well as stems. Thus, an extrapolation approach, from non-human primate to human (2), is applied in the present report to infer connectivity patterns for the superior fronto-occipital fascicle in the human brain.
1. Introduction
The scientific thinking of Deepak Pandya emphasized the importance of monkey neuroanatomical tract tracing data for understanding human brain connectivity in a homologically informed and conservative context ((Makris, 1999) – PhD thesis – Pandya 1st reader). As Dr. Pandya stated:
“In the monkey brain, it is known how different pathways correlate with radioactively labeled material, which has allowed their origins and terminations to be delineated precisely. However, this has not been done yet in humans. It should also be pointed out that structural brain connections are precise and architectonic. Because the stems of the principal pathways are similar in the monkey and the human brain, one can extrapolate the origins and terminations of the observed pathways to the human to correlate specific fiber pathways with cortical architectonic fields. Thus, given that this information is available in experimental animals but not yet in humans, structural brain connectivity in the nonhuman primate can guide the complete delineation of prominent human fiber tracts by means of extrapolation. Drawing these inferences, we can formulate in principle for each major human brain fiber bundle a similar map that characterizes the stem, origin and termination of that tract and, therefore, serves as a blueprint in terms of its connections.”
Dr. Pandya’s thinking underpinned a line of research starting in the early 1990s that he termed the “human project.” This project entailed extrapolating his observations of long cortico-cortical association fiber pathways in the macaque brain to the human brain. Dr. Pandya had observed that the white matter stems of association fiber pathways in the monkey brain were similar to those described by the Dejerines in the human brain (Dejerine and Dejerine-Klumpke, 1895, 1901). Stems are the compact portions of white matter pathways in which the axons converge as they course between their sites of origin and termination (Makris et al., 1997). Classic studies in the human brain delineated the shape, size and course of white matter pathway stems (e.g., (Dejerine and Dejerine-Klumpke, 1895, 1901; Ludwig and Klingler, 1956; Crosby et al., 1962)). Dr. Pandya observed this homological similarity very clearly based on his classic studies, and those based on the Dejerines. This experience provided the foundation for the thesis of Dr. Nikos Makris (Makris, 1999) under the mentorship of Dr. Pandya as primary advisor, and for subsequent papers by Dr. Makris and colleagues (Makris et al., 1997, 1999, 2002, 2005a, 2007, 2009; Makris and Pandya, 2009) that, for the first time, used anatomically-informed stem locations in the monkey and human brain to identify and validate stems of long association fiber tracts in human diffusion-weighted imaging data using the diffusion tensor imaging (DTI) model (Makris et al., 1997).
Delineation of the pathway stems was an important starting point in the determination of fiber pathways in humans. However, classic studies in the human brain (e.g., (Dejerine and Dejerine-Klumpke, 1895; Ludwig and Klingler, 1956)) as well as more modern studies using diffusion MRI-based tractography (e.g., (Mori et al., 1999; Mori and van Zijl, 2002; Tuch et al., 2002)) are unable to definitively show the origins and terminations of these pathways (see, e.g., (Schmahmann and Pandya, 2006; Mesulam, 2009; Pandya et al., 2015; Rushmore et al., 2020b)). By contrast, experimental tract tracing studies in the monkey allow for the precise definition of the three essential components of a white matter pathway, namely, origins and terminations as well as stems. Dr. Pandya emphasized that methods in the human brain could not provide a gold standard for the origins and terminations of cortico-cortical association fiber tracts. Thus, experimental pathway tracing data from the macaque, in addition to guiding and validating the localization of pathway stems in the human brain, could also be used to extrapolate the origins and terminations of long cortico-cortical association fiber tracts in humans using neuroimaging (e.g., (Mufson and Pandya, 1984; Seltzer and Pandya, 1986, 1989; Makris et al., 1997, 1999, 2002, 2005a, 2009; Petrides and Pandya, 1999, 2002, 2006, 2007; Tuch et al., 2002; Schmahmann and Pandya, 2006; Schmahmann et al., 2007; Makris and Pandya, 2009; Yeterian and Pandya, 2010; Pandya et al., 2015)). This approach has been applied to several major fiber tracts, such as the superior longitudinal fascicle (Makris et al., 2005a; Petrides and Pandya, 2006, 2007), the fronto-occipital fascicle (Makris et al., 2007), the middle longitudinal fascicle (Makris et al., 2009), and the extreme capsule (Makris and Pandya, 2009).
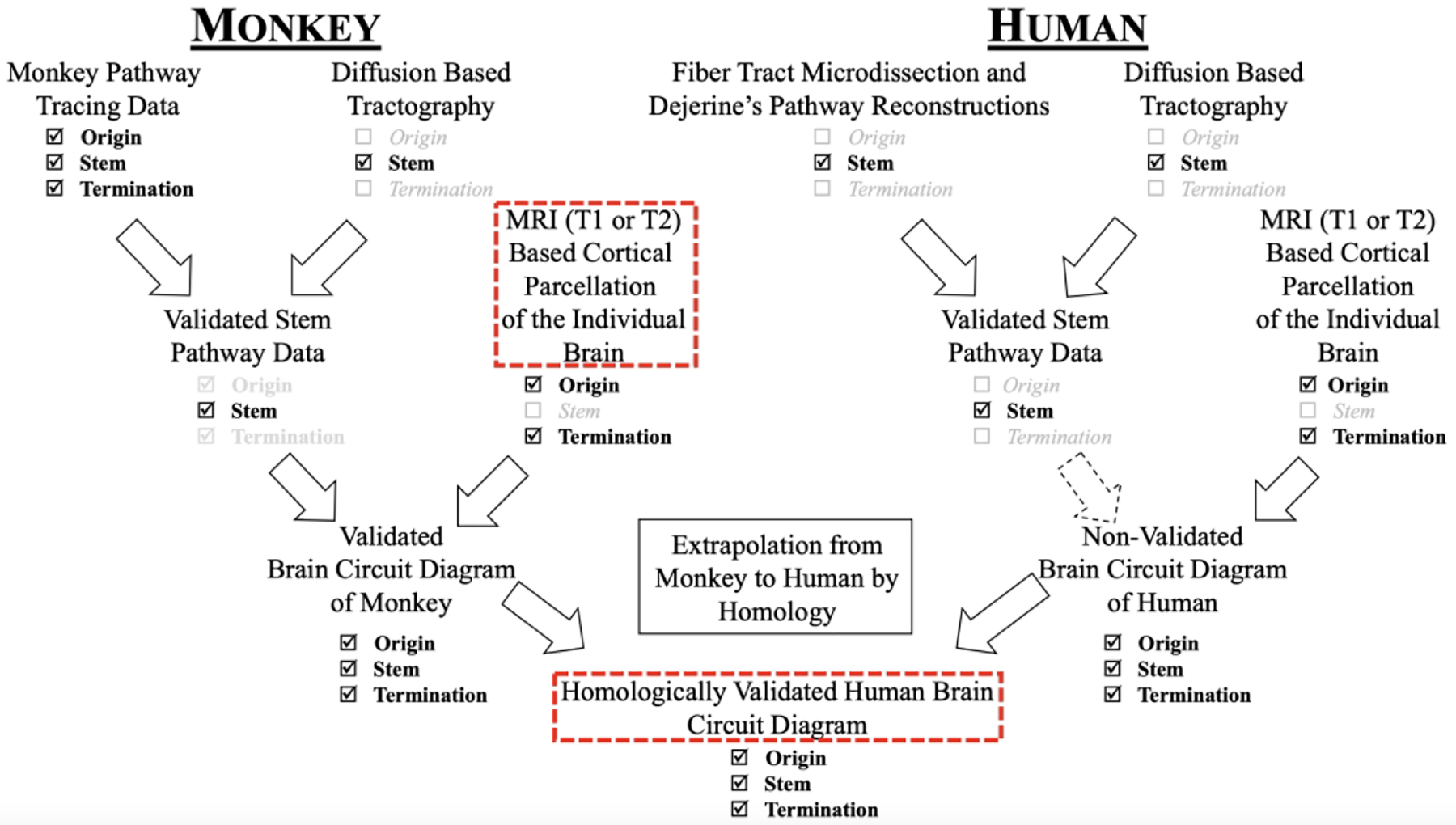
As indicated above, extrapolation at the level of neuroimaging has been performed for stems, but not yet for the origins and terminations of fiber pathways. To accomplish this goal, a systematic homological framework in the neuroimaging domain is required, as our group and others have recently proposed (e.g., (Mars et al., 2016; Van Essen and Glasser, 2018; Van Essen et al., 2019; Rushmore et al., 2020a, b, 2022)). The biggest difficulty in accomplishing this task lies in the fact that both a common neuroanatomical framework and an ad hoc technology platform are needed. These hurdles have been overcome by using an integrated comparative approach, which is presented and elaborated upon in the recently revised HOA2.0-ComPaRe system (Rushmore et al., 2022). The ontological and comparative nature of the HOA2.0-ComPaRe system allows for the origins and terminations of specific long association fiber tracts to be inferred in the human brain based on a process of extrapolation from monkey to human. This concept is depicted in detail in Figure 1 (from (Rushmore et al., 2020a)), and has been exemplified in studies of several long association fiber tracts by Deepak Pandya and colleagues (e.g., (Mufson and Pandya, 1984; Seltzer and Pandya, 1986, 1989; Makris et al., 1997, 1999, 2002, 2005a, 2009; Petrides and Pandya, 1999, 2002, 2006, 2007; Tuch et al., 2002; Schmahmann and Pandya, 2006; Schmahmann et al., 2007; Makris and Pandya, 2009; Yeterian and Pandya, 2010; Pandya et al., 2015)).
Figure 1:
Strategy for the validation of diffusion MRI tractography in human brains using monkey tract tracing data, fiber pathway stem location in monkey and human white matter, and cortical parcellation in humans and monkey brains. The dashed arrow denotes the lack of validated origins and terminations of human brain fiber pathways. Figure modified from (Rushmore et al., 2020a).
In this report, we present the first homologically-validated circuit diagram for an exemplar fiber tract in the human brain, the superior fronto-occipital fascicle (sFOF), in the context of the HOA2.0-ComPaRe framework (Rushmore et al., 2022). Specifically, we first present the rationale of the extrapolation methodology that Deepak Pandya originally proposed and used to map long association cortico-cortical fiber tracts across species in monkeys and humans. Second, we have collated monkey data on the location and connectivity of the sFOF from the autoradiographic tract tracing method of the Pandya group. Furthermore, we have included the limited validated human connectivity data on the stem location of the sFOF from diffusion MRI (dMRI; Makris et al., 1997), dMRI-based tractography (e.g., Catani et al., 2002; Makris et al., 2007) and histological methods (e.g., Bürgel et al., 2006; Dejerine & Dejerine-Klumpke, 1895, 1901). From these data, we have constructed, for the first time, the nominal matrix (i.e., a description of the origins and terminations of a fiber tract) and the topographical matrix (namely, the location of the stem of the fiber tract) for the sFOF in the human and monkey brain in an ontologically-comparable neuroimaging framework, i.e., HOA2.0-ComPaRe. We expect this formulation of structural brain connectivity in the form of comparative matrices to be extended to other fiber tracts and to be applied in basic and clinical neuroscience focused on structural connectivity as well as neuroimaging.
2. Materials and Methods
Rationale of Extrapolation Methodology
The rationale of extrapolation from non-human primate to human with respect to origins and terminations of long association cortico-cortical fiber tracts came from the historical approach that Deepak Pandya implemented in tracing fiber tracts originally in the rhesus macaque brain and then in the human. The principal method applied by Dr. Pandya and colleagues in the non-human primate was the autoradiographic pathway tracing technique (Cowan et al., 1972) in the period between the mid 1970s and late 1990s. The advent of dMRI-based tractography in the late 1990s and early 2000s (Mori et al., 1999; Tuch et al., 2002) allowed Dr. Pandya and colleagues to address important issues in structural connectivity in humans as well as monkeys. Given the impossibility of applying autoradiography or other invasive experimental tract tracing approaches in the human brain, Dr. Pandya and colleagues extrapolated knowledge acquired from experimental methods in monkeys to diffusion imaging-based tractography results in humans (Makris et al., 2002, 2005a, 2007, 2009; Makris and Pandya, 2009). Furthermore, they compared experimental autoradiographic data on the principal long association cortico-cortical fiber tracts in monkeys with diffusion imaging tractography (i.e., high angular resolution diffusion imaging, HARDI) results in the macaque brain (Schmahmann et al., 2007). This approach allowed a comparison of the long association cortico-cortical fiber tracts between the two species using similar diffusion imaging technology. It should be noted that in the Schmahmann et al. (2007) study in monkeys, the comparison of archival experimental data and dMRI-based tractography results was not performed in the same subjects. The extrapolation approach from monkey experimental autoradiography data to human dMRI-based tractography data was applied in all fiber pathway tractography projects in the human brain that Dr. Pandya worked on (Makris et al., 1997, 1999, 2002, 2005a, 2007, 2009; Makris and Pandya, 2009). This allowed a conservative identification of the origins and terminations of the fiber tracts in humans that shared a similar stem topography with monkey.
The end goal is that the combination of experimental tract tracing methods and dMRI-based tractography in monkeys can be used to infer aspects of connectional neuroanatomy in humans such as origins and terminations of pathways, which are not yet validated. This critical issue regarding lack of validation of these aspects of structural connectivity in the human brain has been elaborated upon in detail by Rushmore and colleagues (Rushmore et al., 2020b). Furthermore, a systematic approach for addressing this limitation has been proposed in two subsequent papers (Rushmore et al., 2020a, 2022). The present report provides in humans a first approximation of the complete connectional map of the sFOF following a conservative extrapolation approach that can be applied to other white matter pathways as well.
Comparative Data Collation
Mapping the connections of a white matter fiber pathway stem provided a novel insight in approaching the cartography of origins and terminations of a given fiber pathway in the brain. In the mid 1990s this seemed the most promising strategy to address the non-validated structural connectivity of human long cortico-cortical association fibers. This approach, under the guidance of Deepak Pandya, was first used for neuroimaging by the Center for Morphometric Analysis (Makris et al., 1999; Meyer et al., 1999). Accordingly, the neuroanatomy of the stem portion of fiber tracts represents critical information because it is the comparative nature of a fiber pathway stem that allows extrapolation or origins and terminations for that particular stem from the monkey to the human brain. Thus, a comparative data collation would be valid based on the concept of ontological similarity of stems between species and the extrapolation process that uses known information from one species (i.e., monkey) to infer unknown origins and terminations in a second species (i.e., human).
Matrix Formulation
We constructed a symmetrical matrix denoting the origins and terminations of the sFOF in terms of parcellation units, a form we term the nominal structural connectivity matrix (NSCM) (Figure 2 and Figure 3). We constructed these matrices for the human brain separately for connectional information acquired prior to the advent of diffusion imaging (pre-dMRI era), and for data obtained with dMRI-based tractography (dMRI era). The historical aspects of this topic have been elaborated upon in a separate publication (Makris et al., 2023).
Figure 2:
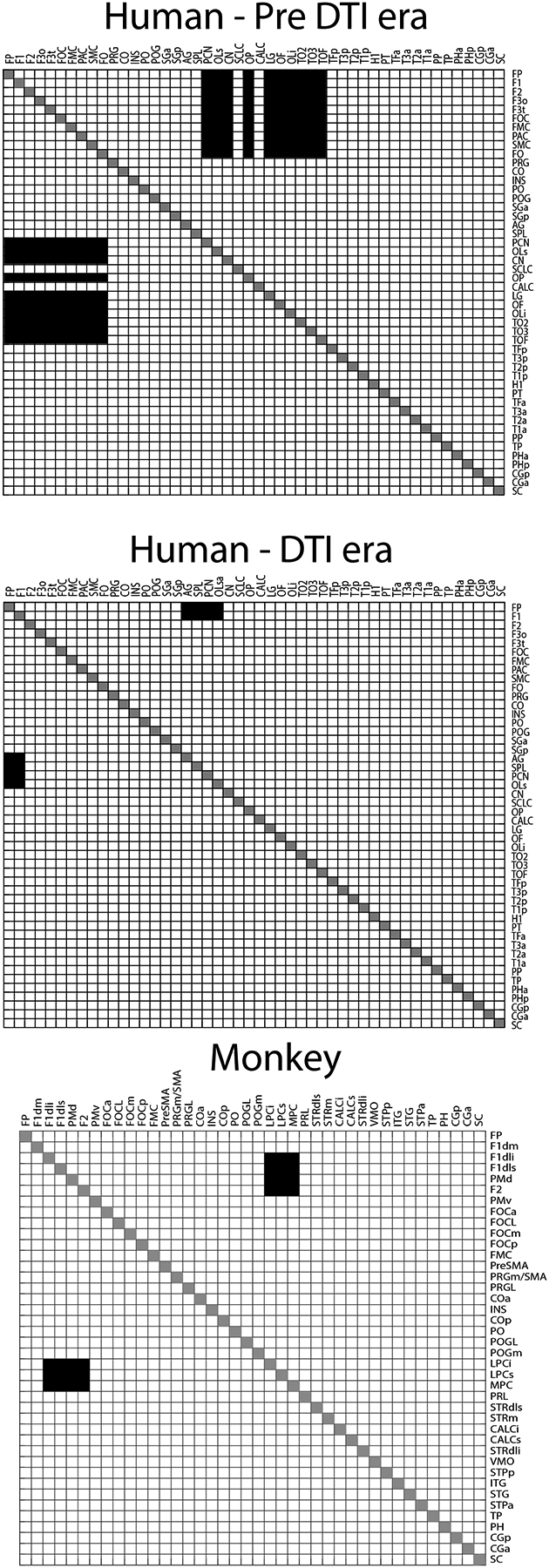
The nominal structural connectivity matrix (NSCM) of the sFOF for humans and monkeys represented as symmetric adjacency matrices. Upper: Connectional data obtained prior to the advent of diffusion tensor imaging (Pre-dMRI era). Middle: Connectional information derived from dMRI-based tractography of the human brain (dMRI era). Lower: Connectional data for the sFOF from experimental tract tracing methods in the monkey brain. See Table 1 for abbreviations.
Figure 3:
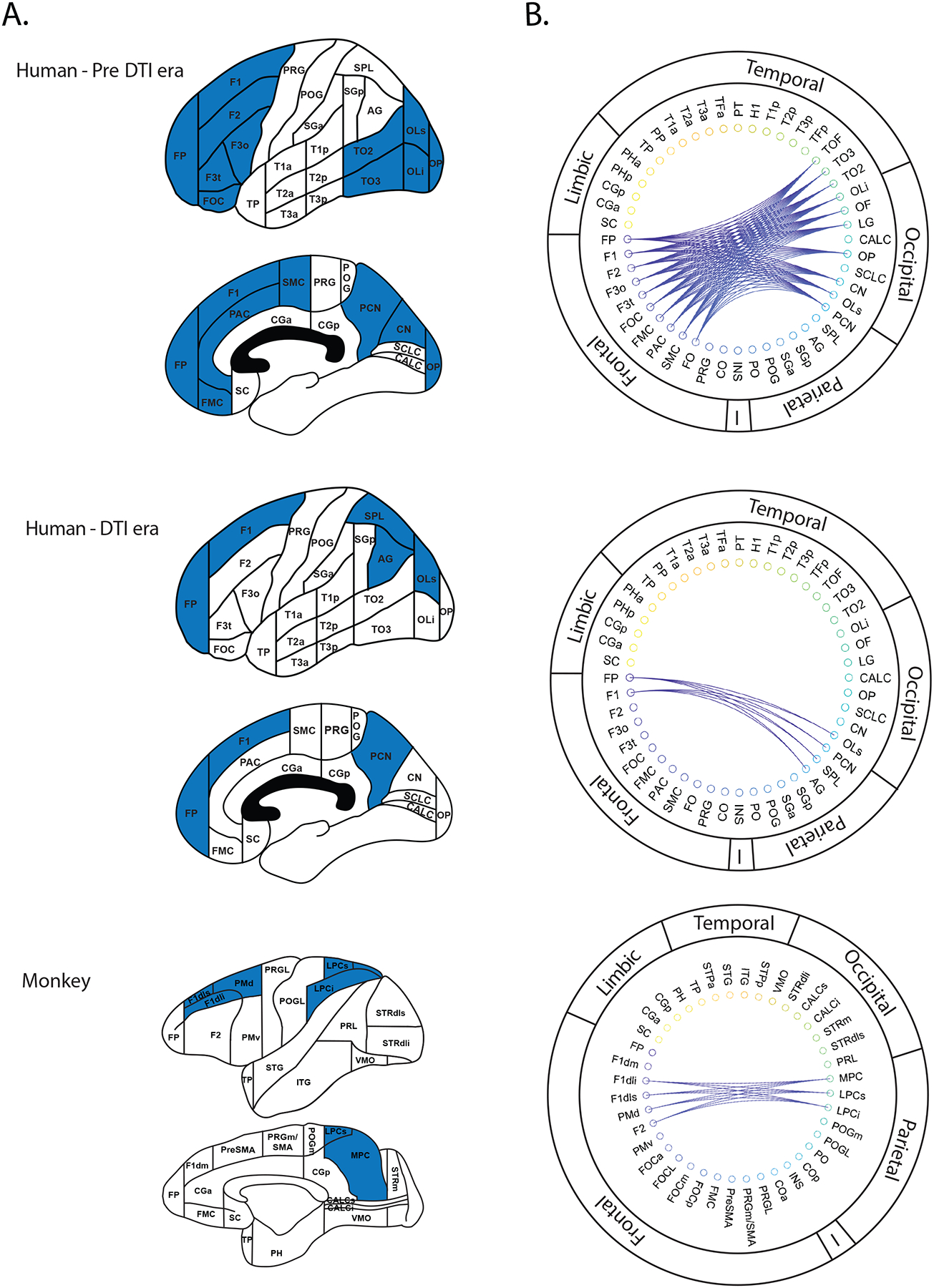
The nominal structural connectivity matrix (NSCM) of the sFOF represented with respect to cortical parcellation units for lateral and medial brain surfaces (A) and corresponding circular network diagrams (B). Upper: Connectional data in the human brain obtained prior to the advent of diffusion tensor imaging (Pre-dMRI era). Parcellation units (Caviness et al., 1996) connected by the sFOF are denoted in blue (A) and by connecting lines (B). Middle: Connectional data for the sFOF from dMRI-based tractography studies. Lower: Parcellation units connected by the sFOF in the monkey (Rushmore et al., 2022). See Table 1 for abbreviations.
The linkage between parcellation units and cortical regions was made through the use of the HOA2.0-ComPaRe system, and was done for both humans and monkeys. This provided a gray-matter centric approach to the connectivity of the sFOF, which interrelates origins and terminations for each cortical region of interest connected by the sFOF. Furthermore, we adopted an approach from the perspective of the white matter organization, i.e., a white-matter centric approach. This was based on the stem location of the sFOF, specifying the course of the stem in the cerebral cortical white matter. To accomplish this, we constructed a topographic structural connectivity matrix (TSCM) for the sFOF stem by aligning parcellation units with white matter sectors (Makris et al., 1999). This matrix associates the cortical parcellation units with the location of the white matter stem within the HOA2.0-ComPaRe framework.
The NSCM and TSCM together constitute the sine qua non condition for the determination of a fiber tract
The creation of both a nominal and a topographic matrix is necessary to characterize a given fiber tract. Namely, two different fiber tracts may have a similar nominal matrix, but follow different topographic courses in the white matter. Neither a TSCM nor a NSCM alone is sufficient to completely define a fiber tract. For example, SLF II and the sFOF both connect parietal areas with dorsolateral prefrontal areas, which would result in a similar NSCM. However, their stems have different trajectories within the cerebral cortical white matter, and therefore have different TSCMs. Thus, both matrices are necessary to provide sufficient information for the complete specification of a given fiber tract, that is, what it connects and also its location in the brain. Together, these matrices constitute the sine qua non condition for the accurate reconstruction of streamlines in dMRI-based tractography. The use of these two matrices will effect a reduction of false positive and false negative interpretations of dMRI-based tractography results. It should be noted that the absence of the TSCM in dMRI tractography has generated controversies (e.g., Forkel et al., 2014) in the identification and differentiation of fiber tracts such as SLF II and the sFOF. Moreover, the application of two distinct matrices takes advantage of validated data sets of different types: the nominal matrix leverages comparative connectivity data from the monkey that is not possible to achieve in the human (e.g., Rushmore et al., 2020b), while the topographic matrix takes advantage of validated stem locations in the human brain (Makris et al., 1997, 1999).
3. Results
We reviewed data on structural connectivity of the sFOF (i.e., origins, terminations, stems) in the published literature from the Pandya group using experimental tract tracing techniques in the macaque monkey, and dMRI-based tractography in both monkey and human. The literature directly relevant to the present study is listed in Table 2.
Table 2:
Superior Fronto-occipital Fascicle Articles
| Author | Year | Species | Name | Method | Supports sFOF existence? |
|---|---|---|---|---|---|
| Dejerine & Dejerine-Klumpke | 1895 | Human | OFF | Histology (potassium bichromate method) | Yes |
| Makris et al. | 1997 | Human | OF | dMRI | Yes |
| Ture et al. | 1997 | Human | sFOF | Dissection | No |
| Makris et al. | 1999 | Human | OF | dMRI | Yes |
| Catani et al. | 2002 | Human | sFOF | dMRI | Yes |
| Mori | 2002 | Human | sFOF | dMRI | No |
| Wakana et al. | 2004 | Human | sFOF | dMRI | Yes |
| Bürgel et al. | 2006 | Human | sOF | Histology (myelin staining) |
Yes |
| Petrides & Pandya | 2006 | Monkey | FOF | Anterograde Tracing | Yes |
| Schmahmann & Pandya | 2006 | Monkey | OFF / FOF | Anterograde Tracing | Yes |
| Makris et al. | 2007 | Human | OFF | dMRI | Yes |
| Yeterian & Pandya | 2010 | Monkey | OFF | Anterograde Tracing | Yes |
| Forkel et al. | 2014 | Human | sFOF | dMRI | No |
| Meola et al. | 2015 | Human | sFOF | dMRI Dissection |
No No |
| Bao et al. | 2017 | Human | sFOF | dMRI Dissection |
Yes No |
| Decramer et al. | 2018 | Monkey | sFOF | Dissection | Yes |
| Liu et al. | 2020 | Human | sFOF | dMRI Dissection |
No No |
Based on studies that describe the sFOF stem in a location consistent with that delineated by the Dejerines ((Dejerine and Dejerine-Klumpke, 1895), we produced connectivity matrices in the HOA2.0-ComPaRe framework, i.e., in the monkey and human, in three different forms. The first type of matrix is referred to as the nominal structural connectivity matrix (NSCM) and has been represented in three different ways. In the first form (Figure 2), the connections were visualized as an adjacency matrix with parcellation units forming the nodes of the graph. In the second form (Figure 3), the parcellation units connected by the sFOF were displayed on the human HOA brain template (Caviness et al., 1996). In the third form, the adjacency matrix was transformed to a circular connectivity diagram (Figure 3). The sFOF is also represented in terms of topographic location within the white matter HOA framework, referred to as the topographic structural connectivity matrix (TSCM) (Makris et al., 1999) (Figure 4). Finally, we represented the stem of the sFOF in the monkey and human brain to depict its similarity in both topography and morphology in both species (Figure 5). In both species, the sFOF stem is longitudinally oriented and elongated in the anterior-posterior dimension, and is located medial to the corona radiata, lateral or superior-lateral to the lateral ventricle and the body of the corpus callosum, and superior-lateral to the head and body of the caudate nucleus.
Figure 4:
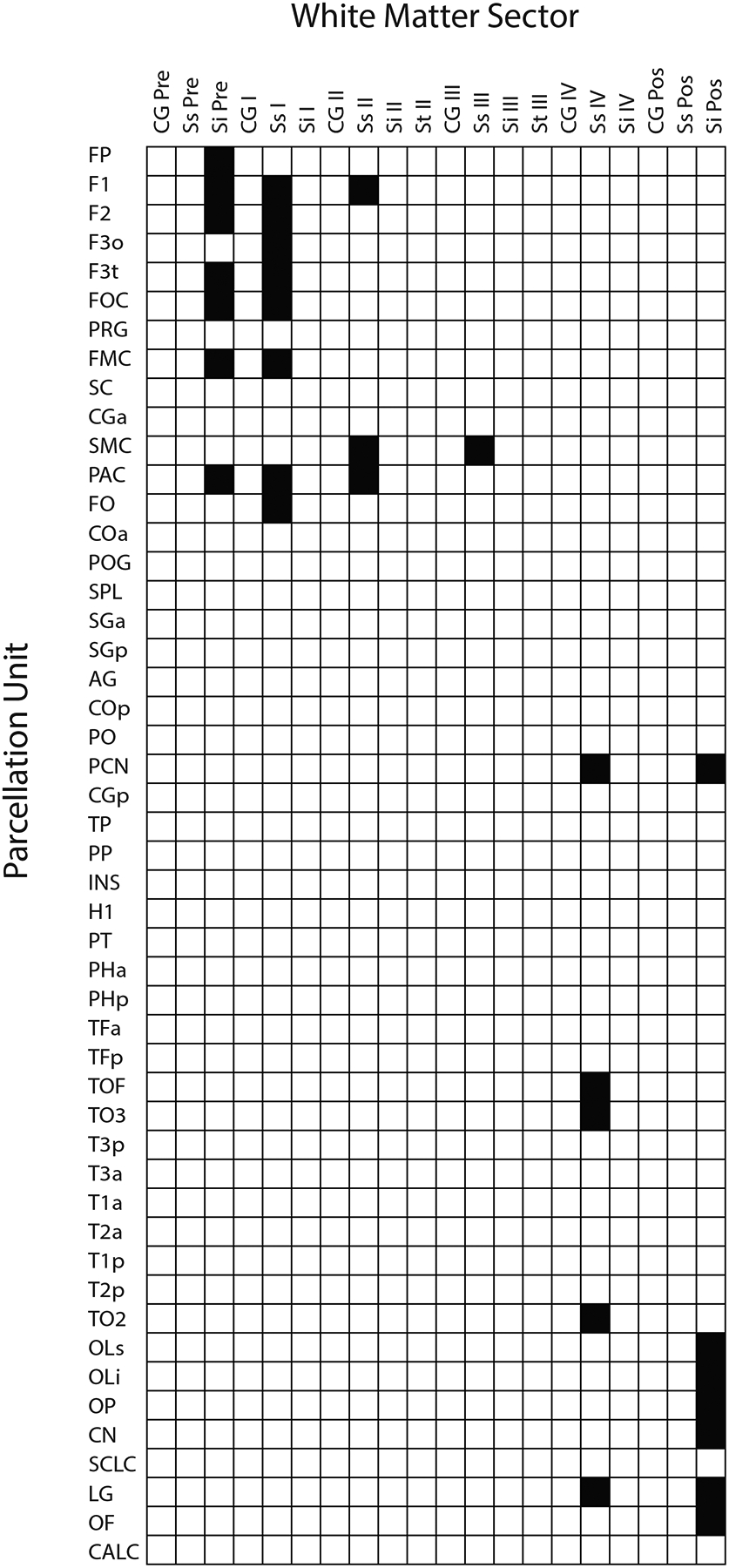
The topographic structural connectivity matrix (TSCM) of the sFOF in which the location of the sFOF stem is specified according to its location in hemispheric white matter sectors (columns) and originating from parcellation units (rows). White matter sectors and gray matter parcellation units are based on the HOA white and gray matter parcellation system for the human brain (Caviness et al., 1996; Makris et al., 1999; Meyer et al., 1999). It should be noted that for a complete definition of a fiber tract, two matrices are required: the topographic matrix, as described here, and the nominal matrix (e.g., Figures 2 and 3). The topographic matrix denotes the precise topography of the stem of the fiber tract in the hemispheric white matter, whereas the nominal matrix specifies its origins and terminations.
Figure 5:
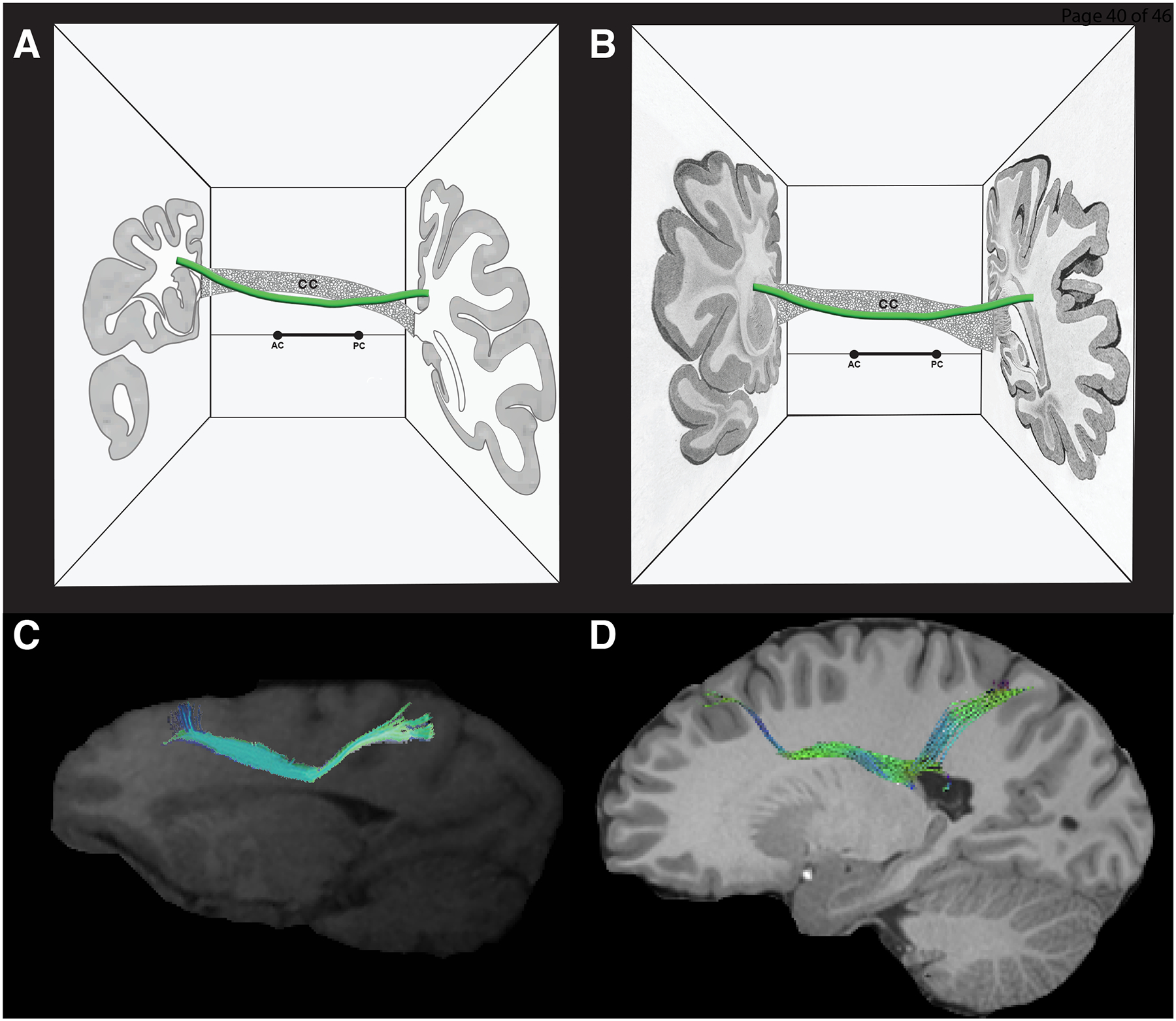
In this figure, the morphologic and topographic similarity of the sFOF stem between monkey (A, C) and human (B, D) is depicted. Specifically, the stem of the sFOF has a longitudinal orientation and elongated shape in the anterior-posterior dimension and is located medial to the corona radiata, lateral or superior-lateral to the lateral ventricle and the body of the corpus callosum, and superior-lateral to the head and body of the caudate nucleus. A, B: Schematic figures illustrating the location of the stem of the sFOF (green) in the monkey brain (A) and in the human brain (B). The topological relationship of the sFOF stem in both figures is shown with respect to the corpus callosum (CC), the lateral ventricle and the caudate nucleus. The two coronal sections in A and B have been modified, respectively, from the monkey brain atlas of Schmahmann and Pandya (2006) and the human brain atlas of Dejerine and Dejerine-Klumpke (Dejerine and Dejerine-Klumpke, 1895). C, D: The stem of the sFOF is shown in the context of parasagittal MRI images modified from Schmahmann et al. (2007) in the monkey brain and Makris et al. (2007) in the human brain. Abbreviations: AC – anterior commissure; CC – corpus callosum; PC – posterior commissure.
4. Discussion
Here for the first time we have compiled connectional matrices based on human and monkey brain source data for an exemplar fiber tract, the superior fronto-occipital fascicle (sFOF). Two matrices were constructed from human source material, one from data collected before the advent of diffusion imaging (pre-dMRI era), and the other from the results of dMRI-based tractographic methods (dMRI era). The pre-dMRI era matrix shows that the sFOF connected widespread regions of the frontal, parietal, occipital and temporal lobes. This pattern of connectivity is a result of the methods used before the development of dMRI-based tractography, reflecting a difficulty in elaborating the gray matter origins and terminations of the pathway. However, the methods used by the Dejerines and others revealed basic features of the stem of the sFOF in terms of its general trajectory, shape, width (size, half centimeter) and its topographic relationship with neighboring structures, specifically the lateral ventricle, caudate nucleus, corpus callosum, cingulum, superior longitudinal fasciculus and the corona radiata ((Dejerine and Dejerine-Klumpke, 1895), vol. 1, pp. 861–2). The overall conclusions of the Dejerines were as follows: “The occipito-frontal fasciculus therefore constitutes a long association pathway, which connects the temporo-occipital lobe to the frontal lobe, to the convexity of the hemisphere and to the insula (by the fibers going to the external capsule). Like all the long association fascicles, it is made up of fibers of unequal length belonging only in part of their path to the occipito-frontal fasciculus: this is distinguished from the long association bundles only by its location, which is deep and subependymal, it is in fact located inward [medial] to the projection system, while the other long association bundles occupy an eccentric [outward] position with respect to this system.” ((Dejerine and Dejerine-Klumpke, 1895), vol. 1, pp. 763).
In the present study, information from the pre-dMRI era was used as a basis for including only those dMRI era studies that agreed on the location of the stem of the sFOF in delineating this pathway. The resulting connectional matrix of the sFOF shows a substantially restricted subset of regions compared to those described in the pre-dMRI era (Figure 3). Namely, connections of the sFOF were between frontal, parietal, and occipital regions. This connectional matrix is very similar to that derived from experimental tract tracing methods of the sFOF in the monkey (Petrides and Pandya, 2006; Schmahmann and Pandya, 2006; Schmahmann et al., 2007; Yeterian and Pandya, 2010). Given the expansion of the cortical association areas in humans with respect to monkeys, the results of human and monkey studies show that the sFOF has more extensive connections in the occipital cortex in humans. More specifically, Makris, Pandya and colleagues (Makris et al., 2007)) have shown that the sFOF (designated as occipitofrontal fascicle, OFF, in their paper) extends to the lateral superior occipital region of interest, whereas in monkey studies it extends to the occipitoparietal region (area PO or V6, classically considered Brodmann’s area 19 (e.g., (Von Bonin and Bailey, 1947; Colby et al., 1988; Galletti et al., 1996; Brodmann, 1999; Luppino et al., 2005)).
Since monkey experimental tracing studies reveal origins and terminations of pathways, they are considered to provide a gold standard for connectional neuroanatomy of pathways in the human brain. We conclude that data on human fiber pathways from dMRI-based methods can be refined using knowledge of the stem topography, and should be interpreted in the context of experimental findings from monkey tract tracing studies that determine pathway origins and terminations with greater accuracy.
A comparison of connectional matrices between monkey and human brains is enabled by the HOA2.0-ComPaRe framework of the cerebral cortex (Rushmore et al., 2022), which is designed to interrelate cerebral cortical parcellation units between the two species. This approach relies on a comparative architectonic context. With respect to white matter pathway analysis, such a system is an important step toward extrapolating connectional results such as matrices from the monkey to the human brain, with the ultimate goal of validating white matter pathways in the human brain with regard to origins and terminations in addition to pathway stems.
An additional approach in refining dMRI-based connections is to include the location of the white matter pathway as a guide for tractography algorithms. Unlike origins and terminations, information on stem locations of white matter pathways is available in the human brain, and has been validated (Makris et al., 1997, 1999). In the present study, we have specified the stem location of the sFOF in the human brain per se (Makris et al., 1997, 1999). Incorporating the stem location of the sFOF into a dMRI-based tractographic analysis facilitated the accuracy of the results. However, many dMRI-based studies of pathways such as the sFOF do not explicitly consider the stem location. As a result, the reconstructed pathway may not align with the anatomical stem location, resulting in a misinterpretation of the course of the pathway and introducing questions regarding the existence of the pathway per se.
Traditional neuroanatomists such as the Dejerines (Dejerine and Dejerine-Klumpke, 1895), Zilles (Bürgel et al., 1997), and Pandya (Petrides and Pandya, 2006; Schmahmann and Pandya, 2006; Makris et al., 2007; Yeterian and Pandya, 2010) have endorsed the existence of the sFOF in the monkey and human brain without reservation. By contrast, fiber microdissection and dMRI-based tractographic studies have not consistently demonstrated this fiber tract in humans (Ludwig and Klingler, 1956; Türe et al., 1997; Catani et al., 2002; Mori, 2002; Wakana et al., 2004; Forkel et al., 2014; Meola et al., 2015; Bao et al., 2017; Decramer et al., 2018; Liu et al., 2020). It is clear that this remains an unresolved issue. In this regard it is important to note that differences in anatomical observations may be due to differences in methodology. Absence of evidence is not evidence of absence, and we should remain open to future studies with more advanced methodologies to address this issue. The novel aspect of this manuscript is a clear demonstration of the existence of the sFOF in humans and monkeys, with shared features regarding the stem portion in terms of location (medial vs. lateral), trajectory (i.e., between frontal and occipital parietal cortices), and topographic relationships with neighboring and similar brain structures (e.g., lateral ventricle, Muratoff’s bundle, internal capsule, caudate nucleus, corpus callosum).
Thus, one approach to the optimization of dMRI-based tractography results could include both validated information about pathway stem location in the human brain, and comparative data from monkey pathway tracing studies. Both types of information, when used in conjunction, are expected to reduce the number of false positive and false negative observations, thus improving the results of dMRI-based connectional studies in the human brain.
A Historical Context for the Pandya School of Structural Connectivity
Dr. Pandya’s approach to structural connectivity in the monkey brain was a touchstone for understanding the organization of the human brain. This thinking is aligned and continuous with Norman Geschwind’s school of thought in behavioral neurology, of which Deepak Pandya was the foundational neuroanatomist. It should be emphasized as well that the HOA approach in neuroimaging developed by Verne Caviness and David Kennedy was supported and co-founded by Albert Galaburda, as reflected in their foundational papers on MRI-based cortical parcellation (Rademacher et al., 1992, 1993) and in the later work of Jeremy Schmahmann on MRI-based cerebellar parcellation (Makris et al., 2003, 2005b). Furthermore, this same approach is continued in the seminal paper of 1997 on DTI-based morphometry of long cortico-cortical association fiber tracts in the human brain co-authored by Deepak Pandya, Edith Kaplan and Nikos Makris (Makris et al., 1997); in this paper, validation of DT-MRI was performed for the first time based on the original work of the Dejerines under the guidance of Dr. Pandya as the primary doctoral dissertation advisor of Dr. Makris (Makris, 1999). It should be noted that in 1992 Dr. Pandya explained to Dr. Makris the extraordinary similarity of the Dejerines’ anatomical descriptions of long association cortico-cortical fiber tracts in humans as compared to his own experimental findings in macaque brains. Dr. Pandya encouraged Dr. Makris to undertake a dissertation on this topic, which Dr. Pandya referred to as “the human brain project” specifically interrelating non-human primate and human histological data for long association cortico-cortical fiber tracts. In 1994 with the advent of diffusion tensor MRI (DTI), which was made available by Van Wedeen at Massachusetts General Hospital, Drs. Makris, Pandya and colleagues were able to validate, for the first time, the use of DTI for delineating and visualizing in color the long cortico-cortical association fiber tracts in humans (Makris et al., 1997). Subsequently, in 1999, Nikos Makris and Edward Yeterian were able to integrate all principal fiber tracts in the human brain in the original HOA framework (Makris et al., 1999). Thus, the Norman Geschwind school of thought established in Boston in the early to mid 1960s (e.g., (Geschwind, 1965a; b; Geschwind and Galaburda, 1985a; b; c)), which revived the European behavioral neurology tradition of Carl Wernicke and Jules Dejerine in late 1800’s, has been fully reflected in the approach of the Center for Morphometric Analysis and the HOA framework for both morphometric analysis of the brain as well as structural connectivity (e.g., (Rademacher et al., 1992; Caviness et al., 1996; Makris et al., 1997, 1999, 2005a; Meyer et al., 1999; Rushmore et al., 2022)).
The Importance of the Monkey in Interpreting Human Connectional Data
Deepak Pandya’s approach to the connectional neuroanatomy of the monkey cerebrum provides a blueprint for all cortico-cortical long association fiber tracts in the human brain. Such a blueprint comprises fiber tracts that are conserved across species. Each monkey fiber tract contains complete information regarding stem, origin and termination. This provides a tremendous advantage of monkey structural connectivity over human structural connectivity in which there is no definitive information with respect to precise origins and terminations, as opposed to stems, for any long association cortico-cortical fiber tract. We have elaborated in detail on this critical issue in a previous publication (Rushmore et al., 2020b). There is evidence supporting the conservation of long cortico-cortical association pathways between monkey and human (Makris et al., 1999), namely the stems of these pathways are comparable in terms of location and trajectory. Therefore, the assumption that an extrapolation from monkey to human with respect to pathway origins and terminations in the latter seems reasonable.
That said, caution should be used in any extrapolation to account for the possibility that there are fiber bundles in the human brain that are not documented in the monkey. For example, there is ample evidence for the existence of the cingulum in both the human and the monkey brain. By contrast, however, although the inferior fronto-occipital fascicle has been well documented in the human brain (Ludwig and Klingler, 1956), it remains uncertain in the monkey brain due to a lack of tract tracing data (Schmahmann and Pandya, 2007; Sarubbo and Petit, 2019). Furthermore, given the presence of additional gyri such as the middle frontal and middle temporal gyri as well as the considerable expansion of multimodal association areas in humans, it is expected that there is a significantly richer structural connectivity of the brain circuit diagram. This issue has been addressed in detail by our group and others (e.g., (Mesulam, 2000; Makris et al., 2005a; Schmahmann and Pandya, 2006; Rilling et al., 2008; Pandya et al., 2015)). Given the similarities but also the differences between the human and the monkey brain, caution is warranted in transferring knowledge from one species to the other as well as in understanding the specific methodologies used to achieve the observations in each species. The latter point regarding methodology is especially important given that dMRI-based tractography is a common methodology used across species, whereas this is not feasible with experimental tract tracing methods. Therefore, a thoroughly validated dMRI-based tractographic methodology in monkeys can be transferred to dMRI-based tractography in humans.
This process of transferring a common methodology that has been validated in one species to an identical but not validated methodology in another species would ensure a higher degree of validity in the interpretation of results in the second species. In the current state of the art in human brain structural connectivity methodology, validated dMRI-based tractography in monkeys in the same individual could generate optimal imaging algorithms that could be used in validating dMRI-based tractography studies in the human brain. Future studies should use validated dMRI acquisition protocols as well as validated dMRI-based tractography analysis algorithms in the human brain. Ultimately, novel tract tracing techniques need to be developed for safe use in human brains in vivo. In this regard there has been research in experimental animal neuroimaging using nanoparticles that has not yet met criteria for safe use in humans (Suffredini et al., 2014; Gharagouzloo et al., 2017).
Circuitry Formulation
The sFOF is one of the principal long association cortico-cortical fiber tracts connecting the frontal and parietal lobes in both humans (e.g., (Makris et al., 2007)) and monkeys (e.g., (Petrides and Pandya, 2006; Schmahmann and Pandya, 2006; Schmahmann et al., 2007; Yeterian and Pandya, 2010)). The dorsolateral prefrontal and parietal cortical regions can function as an integrated circuit for such cognitive processes as visuospatial analysis and attention by means of the sFOF in both species (e.g., (Makris et al., 2007; Yeterian and Pandya, 2010)).
Limitations and Future Studies
There is currently a debate regarding the superior fronto-occipital fascicle in monkeys and humans. Future studies should extensively address the structural connectivity of this fiber tract in both species, as well as its relative proximity to neighboring fiber tracts such as the Muratoff bundle, the corticospinal tract, the subdivisions of the superior longitudinal fascicle and the cingulum bundle in humans and monkeys (e.g., (Bürgel et al., 1997, 2006; Schmahmann and Pandya, 2007; Forkel et al., 2014; Meola et al., 2015; Bao et al., 2017)).
A significant issue underlying the debate on the existence of the sFOF pertains to the lack of complete and detailed descriptions of the imaging and analysis parameters across studies. These are factors that can have a significant impact on the results but are not always completely described, as seen in a comparison of two dMRI-based tractography studies of the human sFOF (Meola et al., 2015; Bao et al., 2017). Both studies used the same tractography analysis program, but only Bao et al. observed the existence of the sFOF. Importantly, Bao et al. reported the exact analysis parameters that were used, whereas Meola did not. Unless the parameters are explicitly presented, it is not possible to systematically compare observations from different studies. In this regard, it is important to recognize that many publicly available analysis tools in neuroimaging have default parameters and settings, although these can be changed by individual investigators. The use of different parameters can produce different anatomical results and thus make it difficult to interpret conflicting observations regarding any pathway, e.g., the sFOF. A more accurate interpretation of findings across multiple studies requires that future tractography studies using diffusion imaging report detailed and complete analysis parameters.
The Pandya comparative anatomical approach provides a conservative and complementary framework for interpreting conflicting data in human brain dMRI-based tractography. With respect to the existence of debatable cortico-cortical association fiber tracts in humans such as the sFOF, this extrapolation approach provides a solid comparative knowledge basis as a testbed for further investigations. Moreover, as MRI technology improves and allows for visualization of cytoarchitecture in the cerebral cortex, the parcellation frameworks in monkey and human will become more directly comparable (Rushmore et al., 2022).
Conclusion
We have presented the first homologically-validated circuit diagram for an exemplar fiber tract in the human brain, the superior fronto-occipital fascicle, in the context of the HOA2.0-ComPaRe framework (Rushmore et al., 2022)). We have used the extrapolation methodology originally proposed by Deepak Pandya to map the sFOF by collating monkey and human data. Furthermore, the Pandya comparative anatomical approach provides a conservative and complementary framework for interpreting conflicting data in human brain dMRI-based tractography. Following this approach, we have constructed nominal and topographical matrices for the sFOF in the human and monkey brain in the HOA2.0-ComPaRe anatomical framework used in neuroimaging. This formulation of structural brain connectivity in the form of comparative matrices can be extended to other fiber tracts and applied in the study of structural connectivity in basic and clinical neuroscience and neuroimaging.
Table 1:
Abbreviations
| AG | Angular gyrus |
| CALC | Intracalcarine cortex |
| CALCi | Calcarine cortex, inferior |
| CALCs | Calcarine cortex, superior |
| CGa | Cingulate gyrus, anterior division |
| CGp | Cingulate gyrus, posterior division |
| CN | Cuneal cortex |
| CO | Central opercular cortex |
| COa | Central opercular cortex – anterior |
| COp | Central opercular cortex – posterior |
| DTI | Diffusion tensor imaging |
| F1 | Superior frontal gyrus |
| F1dm | Superior frontal gyrus – dorsomedial |
| F1dli | Superior frontal gyrus – dorsolateral inferior |
| F1dls | Superior frontal gyrus – dorsolateral superior |
| F2 | Middle frontal gyrus |
| F3o | Inferior frontal gyrus, pars opercularis |
| F3t | Inferior frontal gyrus, pars triangularis |
| FMC | Frontal medial cortex |
| FO | Frontal opercular cortex |
| FOC | Frontal orbital cortex |
| FOCa | Anterior frontal orbital cortex |
| FOCL | Lateral frontal orbital cortex |
| FOCm | Medial frontal orbital cortex |
| FOCp | Posterior frontal orbital cortex |
| FP | Frontal pole |
| H1 | Heschl’s gyrus |
| HOA | Harvard-oxford atlas |
| INS | Insula cortex |
| ITG | Inferior temporal gyrus |
| LG | Lingual gyrus |
| LPCi | Inferior lateral parietal cortex |
| LPCs | Superior lateral parietal cortex |
| MPC | Medial parietal cortex |
| NSCM | Nominal structural connectivity matrix |
| OF | Occipital fusiform gyrus |
| OLi | Lateral occipital cortex, inferior |
| OLs | Lateral occipital cortex, superior |
| OP | Occipital pole |
| PAC | Paracingulate gyrus |
| PCN | Precuneal cortex |
| PH | Parahippocampal cortex |
| PHa | Parahippocampal gyrus, anterior |
| PHp | Parahippocampal gyrus, posterior |
| PMd | Dorsal premotor cortex |
| PMv | Ventral premotor cortex |
| PO | Parietal operculum |
| POG | Postcentral gyrus |
| POGL | Postcentral gyrus, lateral |
| PP | Planum polare |
| PreSMA | Pre-supplementary motor area |
| PRG | Precentral gyrus |
| PRGL | Precentral gyrus, lateral |
| PRGm/SMA | Precentral gyrus, medial/supplementary motor area |
| PRL | Prelunate gyrus |
| PT | Planum temporale |
| SC | Subcallosal cortex |
| SCLC | Supracalcarine cortex |
| sFOF | Superior fronto-occipital fascicle |
| SGa | Supramarginal gyrus, anterior |
| SGp | Supramarginal gyrus, posterior |
| SMC | Supplementary motor cortex |
| SPL | Superior parietal lobule |
| STG | Superior temporal gyrus |
| STPa | Supratemporal plane, anterior |
| STPp | Supratemporal plane, posterior |
| STRdli | Striate cortex, dorsolateral inferior |
| STRdls | Striate cortex, dorsolateral superior |
| STRm | Striate cortex, medial |
| T1a | Supra temporal plane, anterior |
| T1p | Superior temporal gyrus, posterior |
| T2a | Middle temporal gyrus, anterior |
| T2p | Middle temporal gyrus, posterior |
| T3a | Inferior temporal gyrus, anterior |
| T3p | Inferior temporal gyrus, posterior |
| TFa | Temporal frontal cortex, anterior |
| TFp | Temporal frontal cortex, posterior |
| TO2 | Middle temporal gyrus, temporo-occipital |
| TO3 | Inferior temporal gyrus, temporo-occipital |
| TOF | Temporal occipital fusiform cortex |
| TP | Temporal pole |
| TSCM | Topographic structural connectivity matrix |
| VMO | Ventromedial occipital |
Acknowledgments
This work was supported in part by Institute of Health (NIH) grants R01 MH112748 (to NM), R01 MH111917 (to NM), R01 NS125307 (to NM, RR), R21 DA042271 (to NM), K24 MH116366 (to NM), R01 AG042512 (to NM), R01MH125860 (NM).
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
Literature Cited
- Bao Y, Wang Y, Wang W, Wang Y. 2017. The Superior Fronto-Occipital Fasciculus in the Human Brain Revealed by Diffusion Spectrum Imaging Tractography: An Anatomical Reality or a Methodological Artifact? Front Neuroanat 11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K 1999. Brodmann’s “Localisation in the Cerebral Cortex.” Springer. [Google Scholar]
- Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K. 2006. White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29:1092–1105. [DOI] [PubMed] [Google Scholar]
- Bürgel U, Mecklenburg I, Blohm U, Zilles K. 1997. Histological visualization of long fiber tracts in the white matter of adult human brains. J Hirnforsch 38:397–404. [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. 2002. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94. [DOI] [PubMed] [Google Scholar]
- Caviness VS Jr, Meyer J, Makris N, Kennedy DN. 1996. MRI-based topographic parcellation of human neocortex: An anatomically specified method with estimate of reliability. J Cogn Neurosci 8:566–587. [DOI] [PubMed] [Google Scholar]
- Colby CL, Gattass R, Olson CR, Gross CG. 1988. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J Comp Neurol 269:392–413. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA. 1972. The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res 37:21–51. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphreys T, Lauer EW. 1962. Correlative Anatomy of the Nervous System. New York, NY: Macmillan. [Google Scholar]
- Decramer T, Swinnen S, van Loon J, Janssen P, Theys T. 2018. White matter tract anatomy in the rhesus monkey: a fiber dissection study. Brain Struct Funct 223:3681–3688. [DOI] [PubMed] [Google Scholar]
- Dejerine J, Dejerine-Klumpke A. 1895. Anatomie des Centres Nerveux: Méthodes générales d’étude-embryologie-histogénèse et histologie. Anatomie du cerveau. Paris, France: Rueff et Cie. [Google Scholar]
- Dejerine J, Dejerine-Klumpke A. 1901. Anatomie des Centres Nerveux. Paris, France: Rueff et Cie. [Google Scholar]
- Forkel SJ, Thiebaut de Schotten M, Kawadler JM, Dell’Acqua F, Danek A, Catani M. 2014. The anatomy of fronto-occipital connections from early blunt dissections to contemporary tractography. Cortex 56:73–84. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Battaglini PP, Shipp S, Zeki S. 1996. Functional Demarcation of a Border Between Areas V6 and V6A in the Superior Parietal Gyrus of the Macaque Monkey. Eur J Neurosci 8:30–52. [DOI] [PubMed] [Google Scholar]
- Geschwind N 1965a. Disconnexion syndromes in animals and man. II. Brain 88:585–644. [DOI] [PubMed] [Google Scholar]
- Geschwind N 1965b. The problem of language in relation to the phylogenetic development of the brain. Sist Nerv 17:411–419. [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. 1985a. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol 42:428–459. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. 1985b. Cerebral lateralization. Biological mechanisms, associations, and pathology: III. A hypothesis and a program for research. Arch Neurol 42:634–654. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. 1985c. Cerebral lateralization. Biological mechanisms, associations, and pathology: II. A hypothesis and a program for research. Arch Neurol 42:521–552. [DOI] [PubMed] [Google Scholar]
- Gharagouzloo CA, Timms L, Qiao J, Fang Z, Nneji J, Pandya A, Kulkarni P, van de Ven AL, Ferris C, Sridhar S. 2017. Quantitative vascular neuroimaging of the rat brain using superparamagnetic nanoparticles: New insights on vascular organization and brain function. Neuroimage 163:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kinoshita M, Shinohara H, Hori O, Ozaki N, Nakada M. 2020. Does the superior fronto-occipital fascicle exist in the human brain? Fiber dissection and brain functional mapping in 90 patients with gliomas. NeuroImage Clin 25:102192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig E, Klingler J. 1956. Atlas Cerebri Humani. Basel, Switzerland: Karger. [Google Scholar]
- Luppino G, Hamed SB, Gamberini M, Matelli M, Galletti C. 2005. Occipital (V6) and parietal (V6A) areas in the anterior wall of the parieto-occipital sulcus of the macaque: a cytoarchitectonic study. Eur J Neurosci 21:3056–3076. [DOI] [PubMed] [Google Scholar]
- Makris N 1999. Delineation of human association fiber pathways using histologic and magnetic resonance methodologies. PhD Thesis in Behavioral Neuroscience (DN Pandya, Advisor), Boston University School of Medicine. [Google Scholar]
- Makris N, Hodge SM, Haselgrove C, Kennedy DN, Dale A, Fischl B, Rosen BR, Harris G, Caviness VS Jr, Schmahmann JD. 2003. Human cerebellum: surface-assisted cortical parcellation and volumetry with magnetic resonance imaging. J Cogn Neurosci 15:584–599. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN. 2005a. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. 1999. MRI-based topographic parcellation of human cerebral white matter and nuclei: II. Rationale and applications with systematics of cerebral connectivity. Neuroimage 9:18–45. [DOI] [PubMed] [Google Scholar]
- Makris N, Pandya DN. 2009. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct 213:343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. 2009. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 19:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Sorg S, Kennedy DN, Caviness VS, Pandya DN. 2007. The occipitofrontal fascicle in humans: A quantitative, in vivo, DT-MRI study. Neuroimage 37:1100–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Worth AJ, Jenkins BG, Garrido L, Sorensen AG, Wedeen V, Tuch DS, Wu O, Cudkowicz ME, Caviness VS, Rosen BR, Kennedy DN. 2002. Diffusion tensor imaging. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editor. Neuropsychopharmacology: The Fifth Generation of Progress. Vol. 3. Philadelphia, PA: Lipincott. p 357–371. [Google Scholar]
- Makris N, Rushmore R, Kaiser J, Albaugh M, Kubicki M, Rathi Y, Zhang F, O’Donnell LJ, Yeterian E, Caviness VS, Kennedy DN. 2023. A Proposed Human Structural Brain Connectivity Matrix in the Center for Morphometric Analysis Harvard-Oxford Atlas Framework: A historical perspective and future direction for enhancing the precision of human structural connectivity with a novel neuroanatomical typology. Dev Neurosci 45:161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Schlerf JE, Hodge SM, Haselgrove C, Albaugh MD, Seidman LJ, Rauch SL, Harris G, Biederman J, Caviness VS Jr, Kennedy DN, Schmahmann JD. 2005b. MRI-based surface-assisted parcellation of human cerebellar cortex: an anatomically specified method with estimate of reliability. Neuroimage 25:1146–1160. [DOI] [PubMed] [Google Scholar]
- Makris N, Worth AJ, Sorensen AG, Papadimitriou GM, Wu O, Reese TG, Wedeen VJ, Davis TL, Stakes JW, Caviness VS, Kaplan E, Rosen BR, Pandya DN, Kennedy DN. 1997. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol 42:951–962. [DOI] [PubMed] [Google Scholar]
- Mars RB, Foxley S, Verhagen L, Jbabdi S, Sallet J, Noonan MP, Neubert F-X, Andersson JL, Croxson PL, Dunbar RIM, Khrapitchev AA, Sibson NR, Miller KL, Rushworth MFS. 2016. The extreme capsule fiber complex in humans and macaque monkeys: a comparative diffusion MRI tractography study. Brain Struct Funct 221:4059–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meola A, Comert A, Yeh F-C, Stefaneanu L, Fernandez-Miranda JC. 2015. The controversial existence of the human superior fronto-occipital fasciculus: Connectome-based tractographic study with microdissection validation. Hum Brain Mapp 36:4964–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M 2009. Defining neurocognitive networks in the BOLD new world of computed connectivity. Neuron 62:1–3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. 2000. Principles of Behavioral and Cognitive Neurology. Oxford University Press. [Google Scholar]
- Meyer JW, Makris N, Bates JF, Caviness VS, Kennedy DN. 1999. MRI-Based topographic parcellation of human cerebral white matter: I. Technical foundations. Neuroimage 9:1–17. [DOI] [PubMed] [Google Scholar]
- Mori S 2002. Two and three-dimensional analyses of brain white matter architecture using diffusion imaging. CNS Spectr 7:529–534. [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. 1999. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PCM. 2002. Fiber tracking: principles and strategies - a technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN. 1984. Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol 225:31–43. [DOI] [PubMed] [Google Scholar]
- Pandya D, Petrides M, Cipolloni PB. 2015. Cerebral Cortex: Architecture, Connections, and the Dual Origin Concept. New York, NY: Oxford University Press. [Google Scholar]
- Petrides M, Pandya DN. 1999. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns: Dorsolateral prefrontal cortex in human and monkey. Eur J Neurosci 11:1011–1036. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2002. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey: Ventrolateral prefrontal cortex in human and monkey. Eur J Neurosci 16:291–310. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2006. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol 498:227–251. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. 2007. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci 27:11573–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher J, Caviness VS Jr, Steinmetz H, Galaburda AM. 1993. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex 3:313–329. [DOI] [PubMed] [Google Scholar]
- Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS Jr. 1992. Human cerebral cortex: localization, parcellation, and morphometry with magnetic resonance imaging. J Cogn Neurosci 4:352–374. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TEJ. 2008. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11:426–428. [DOI] [PubMed] [Google Scholar]
- Rushmore RJ, Bouix S, Kubicki M, Rathi Y, Rosene DL, Yeterian EH, Makris N. 2020a. MRI-based parcellation and morphometry of the individual rhesus monkey brain: the macaque Harvard-Oxford Atlas (mHOA), a translational system referencing a standardized ontology. Brain Imaging Behav 15:1589–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore RJ, Bouix S, Kubicki M, Rathi Y, Yeterian E, Makris N. 2022. HOA2.0-ComPaRe: A next generation Harvard-Oxford Atlas comparative parcellation reasoning method for human and macaque individual brain parcellation and atlases of the cerebral cortex. Frontiers in Neuroanatomy 16:1035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore RJ, Bouix S, Kubicki M, Rathi Y, Yeterian EH, Makris N. 2020b. How human Is human connectional neuroanatomy? Front Neuroanat 14:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarubbo S, Petit L. 2019. Editorial: Organization of the white matter anatomy in the human brain. Front Neuroanat 13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya D. 2006. Fiber Pathways of the Brain. New York, NY: Oxford University Press. [Google Scholar]
- Schmahmann JD, Pandya DN. 2007. The complex history of the fronto-occipital fasciculus. J Hist Neurosci 16:362–377. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, Wedeen VJ. 2007. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1986. Posterior parietal projections to the intraparietal sulcus of the rhesus monkey. Exp Brain Res 62:459–469. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. 1989. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J Comp Neurol 281:97–113. [DOI] [PubMed] [Google Scholar]
- Suffredini G, East JE, Levy LM. 2014. New applications of nanotechnology for neuroimaging. AJNR Am J Neuroradiol 35:1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. 2002. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med 48:577–582. [DOI] [PubMed] [Google Scholar]
- Türe U, Yaşargil MG, Pait TG. 1997. Is there a superior occipitofrontal fasciculus? A microsurgical anatomic study. Neurosurgery 40:1226–1232. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Donahue CJ, Coalson TS, Kennedy H, Hayashi T, Glasser MF. 2019. Cerebral cortical folding, parcellation, and connectivity in humans, nonhuman primates, and mice. Proc Natl Acad Sci U S A 116:26173–26180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF. 2018. Parcellating Cerebral Cortex: How Invasive Animal Studies Inform Noninvasive Mapmaking in Humans. Neuron 99:640–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bonin G, Bailey P. 1947. The neocortex of Macaca mulatta. Champaign, IL, US: University of Illinois Press The neocortex of Macaca mulatta. (Illinois Monogr. med. Sci. [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. 2004. Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. 2010. Fiber pathways and cortical connections of preoccipital areas in rhesus monkeys. J Comp Neurol 518:3725–3751. [DOI] [PubMed] [Google Scholar]


