Abstract
BACKGROUND:
There are many FDA-approved drugs for advanced prostate cancer (PC), yet public interest in these drugs is not well understood. We compared public interest and state-level predictors of interest in five common oral adjunctive hormonal therapies.
METHODS:
Google Trends™ was queried for: “Enzalutamide”, “Abiraterone Acetate”, “Bicalutamide”, “Apalutamide”, and “Darolutamide” in the United States from January 2004 to November 2022. Data are presented as relative search index (RSI) by month. RSI ranges from 0 to 100 with 100 being peak popularity, 50 being half of the peak popularity, and 0 representing insufficient data to be determined.
RESULTS:
Several drugs abruptly increased in popularity following FDA approval including abiraterone, enzalutamide, and apalutamide. All drugs decreased in popularity from January 2020 to July 2020, corresponding with the COVID-19 pandemic. In the most recent 5 years, enzalutamide and abiraterone were the most common searched drugs, with bicalutamide a close 3rd place. States that did not expand Medicaid were significantly more likely to have bicalutamide as the top search drug vs. states that expanded Medicaid (p = 0.012). Across all states with data (n = 39), higher bicalutamide RSIs were significantly associated with lower household income (r = 0.385, p = 0.02) and greater percent of uninsured adults (r = 0.426, p = 0.007). This is the first study using Google Trends to compare advanced PC drugs by search popularity.
CONCLUSIONS:
Despite the emergence of more effective medications, bicalutamide remains relatively popular, particularly in states with lower household income, more uninsured adults, or those that did not expand Medicaid, possibly due to its lower cost.
INTRODUCTION
Advanced prostate cancer (PC) is often treated by inhibiting androgen activity [1]. Over the last ~10 years, several novel hormonal therapies (NHTs) received FDA-approval to treat metastatic and castration resistant PC in combination with androgen deprivation therapy (ADT). These NHTs include apalutamide, darolutamide, enzalutamide, and abiraterone acetate. While highly effective, they have more reported side effects and higher costs than older drugs [2]. Conversely, bicalutamide, a first-generation androgen-receptor antagonist with more than 2 decades on the market, has sustained widespread use despite being less effective than NHTs [3–5]. In a randomized clinical trial where men with chemotherapy naïve metastatic castration resistant PC received enzalutamide or bicalutamide, progression-free survival was significantly prolonged by enzalutamide compared to bicalutamide treatment [6]. Similarly, improved outcomes were observed with enzalutamide vs. bicalutamide when combined with ADT in men with metastatic hormone sensitive PC [3].
Reflective of the above data, NHTs are National Comprehensive Cancer Network category 1 recommendations for metastatic PC unlike bicalutamide. According to the 2020 American Urology Association guidelines, NHTs (abiraterone acetate with prednisone and enzalutamide) in combination with continued ADT should be offered to patients with newly diagnosed metastatic castration-resistant PC due to their survival benefits. In contrast, bicalutamide is not mentioned as first-line therapy for any stage of advanced PC [7, 8]. Furthermore, since 2014, the American Society of Clinical Oncology guidelines for treating metastatic castration-resistant PC state that although bicalutamide and other antiandrogens may be offered as treatment, this recommendation should include a discussion about their limited clinical benefit compared to abiraterone acetate and enzalutamide [9]. Ultimately, bicalutamide has not been as widely recommended as NHTs for the last decade due its lower effectiveness at treating PC than newer treatments.
Efficacy, safety, and tolerability are some of the drug characteristics considered when choosing a treatment. However, price and insurance coverage may play a crucial role in this decision as well. Generally, cost can be a significant barrier for patients, making it harder to access medications and increases the risk of nonadherence. According to Medicaid reimbursement data, the average expense for NHTs are thousands of dollars per claim, with apalutamide being the most expensive averaging $12,494.67 per claim in 2021. The expense of bicalutamide, in contrast, averages $16.44 per claim. How and whether this cost-saving factors into public interest in these drugs is unknown. Herein, to better understand public search interest of these advanced PC drugs, we studied the online search interest of NHTs and bicalutamide in the United States using Google Trends to examine time trends and explore factors driving public search interest. We hypothesized bicalutamide interest remains high even in the NHT era and is higher in states with lower median household income and with higher poverty.
MATERIALS AND METHODS
We used Google Trends™ (http://trends.google.com) to query search popularity of five advanced PC medications. Google Trends analyzes the popularity of top search queries in Google Search worldwide. We examined data in the United States for search terms related to FDA-approved oral hormonal therapy drugs used in advanced PC. The search terms queried for these medications were the following: “Enzalutamide”, “Abiraterone Acetate”, “Bicalutamide”, “Apalutamide” and “Darolutamide”. These five medications were selected because of their similar clinical characteristics. They are taken orally, used specifically as an adjunctive medication in conjunction with standard ADT in advanced PC, and are not routinely taken concurrently with each other.
Search interest was measured as relative search index (RSI) by month. RSI ranges from 0 to 100 with 100 being peak popularity, 50 being half of the peak popularity, and 0 representing insufficient data to determine RSI. These drugs were searched as “Medications” in Google Trends so that the resulting RSI for each drug encompasses both the generic and brand name. We analyzed search popularity of medications in two times frames: “2004-present” and the 5 most recent years (11/1/17 to 11/1/22). The former represents the entirety of Google Trends available data, which best shows how search popularity of each drug changed over time. The latter was chosen to give a more contemporary view of search popularity.
To assess factors associated with search popularity, we compared statewide RSI to Medicaid expansion (yes/no) using a Chi-Square test set. We used Spearman correlation test to analyze statewide bicalutamide RSI to poverty rate, percent uninsured adults, unemployment rate, and median household income. Data on Medicaid expansion, statewide poverty, percent uninsured adults, unemployment rate, and median household income were reported from the Kaiser Family Foundation [10]. Statewide poverty was defined as the percentage of adults <65 years with income at or below 100% of the federal poverty level. All statistical tests were two-sided, and were performed using STATA, version 17.0 (StataCorp LLC). Statistical significance was defined as P < 0.05. Ethics clearance was not necessary as this study did not involve study participants and all data analyzed were available from publicly accessible databases. In lieu of a formal ethics committee, the principles of the Helsinki Declaration were followed.
RESULTS
In recent years, the three most searched medications were abiraterone, enzalutamide, and bicalutamide (Fig. 1). While rankings varied on a month-by-month basis, overall, enzalutamide was most searched followed by abiraterone and then bicalutamide during this time. Darolutamide was the least searched drug, although also is the most recently FDA-approved. Several drugs abruptly increased in RSI following important press announcements. Abiraterone search popularity spiked in July 2008 likely due to the results of its Phase I/II trial which were reported in May 2008 [11]. Several drugs increased in search popularity following FDA approval including abiraterone, enzalutamide, and apalutamide [12–14]. Search popularity decreased for all drugs from January 2020 to July 2020 and from September 2021 to November 2021, corresponding with the emergence of COVID-19 pandemic in Spring 2020 and a spike in COVID cases/deaths in Fall 2021, respectively [15].
Fig. 1.
Relative search interest of metastatic prostate cancer drugs.
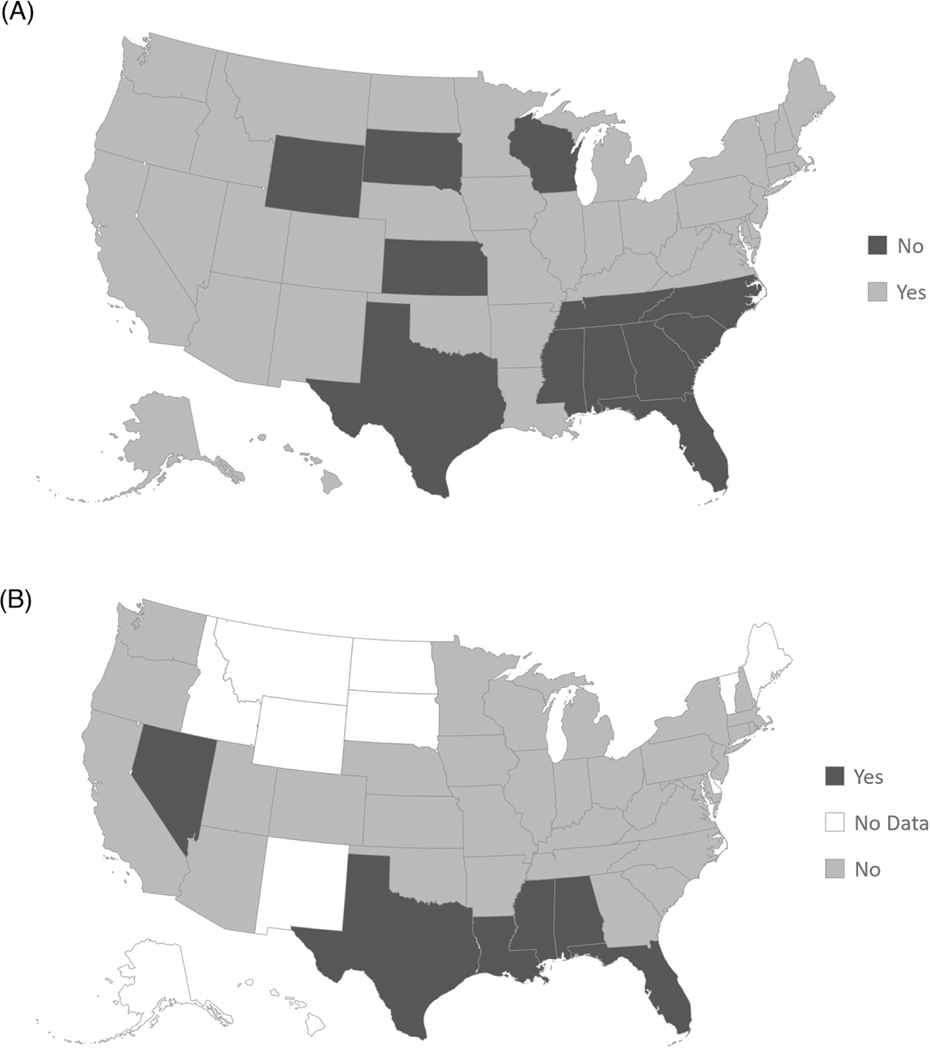
From November 1st, 2017, to November 1st, 2022, search data were available for 39 states (including the District of Columbia). The 12 States without search data were some of the least populated states: Alaska, Delaware, Hawaii, Idaho, West Virginia, New Mexico, Wyoming, Montana, North Dakota, South Dakota, Vermont, and Maine. Of the 39 states with data, bicalutamide was the most popular in 6 states, 4 of which did not expand Medicaid (Fig. 2) [16]. These four states included Texas, Mississippi, Alabama, and Florida. The remaining two states, Nevada and Louisiana, expanded Medicaid in 2014 and 2016, respectively [16]. Of the 39 states with search popularity data, States that did not expand Medicaid were significantly more likely to have bicalutamide as the top searched drug vs. states that did expand Medicaid where abiraterone or enzalutamide were more likely to be the top search (p = 0.012).
Fig. 2. Comparison of Medicaid Expansion to Bicalutamide Search Popularity.
A States that Expanded Medicaid as of 11–1-2022, B States where Bicalutamide is the Most Searched.
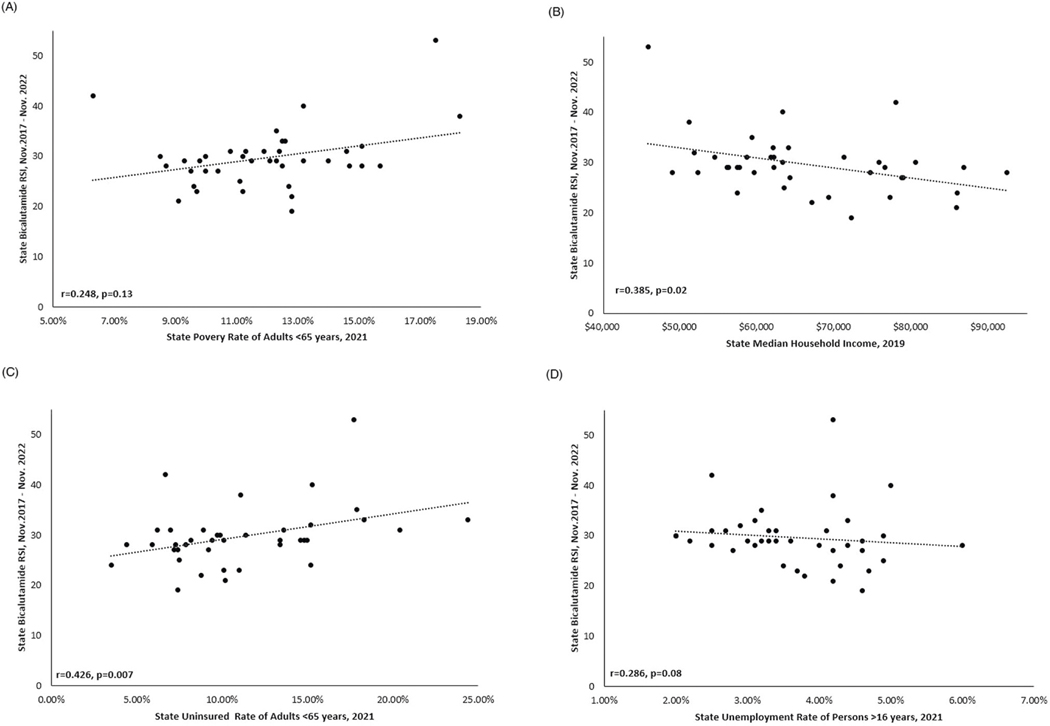
We compared RSI of bicalutamide from November 2017 to November 2022 to statewide poverty, household income, and percent of uninsured adults aged <65 years (Fig. 3). Bicalutamide was most popular in Mississippi with an RSI of 53. Mississippi also had the lowest median household income of $45,792, and the third- highest poverty level at 17.5%. Across all 39 states with data, higher bicalutamide RSIs were associated with higher state level poverty (r = 0.248, p = 0.13), lower household income (r = 0.385, p = 0.02), greater percent of uninsured adults (r = 0.426, p = 0.007), and higher unemployment (r = 0.286, p = 0.08), though associations for poverty and unemployment did not reach statistical significance.
Fig. 3. Association between Statewide BIcalutamide and Socioeconomic Factors.
A Poverty Rate, B Median Household Income, C Percent Uninsured, and D Unemployment Rate.
DISCUSSION
NHTs have emerged over the past decade as highly effective medications used to treat advanced PC. Yet, bicalutamide, an older less effective medication, has sustained widespread use. This discrepancy may be indicative of some public perception of these drugs, which is not well understood. To address this, we used Google Trends to analyze search popularity of NHTs and bicalutamide in the United States to examine time trends and explore factors driving public search interest. We found that despite newer and more effective agents available, public search interest in bicalutamide remained high. Moreover, searches for bicalutamide were significantly more common in states that did not expand Medicaid, had higher uninsured adults, and lower household income. These data suggests that the cost-savings of bicalutamide may be driving its continued search popularity even in the most recent few years despite the availability of newer and more effective medications [3, 5].
Of the four NHTs assessed, abiraterone acetate and enzalutamide were the most searched, each maintaining as RSI double that of apalutamide, the third most search NHT. This is expected since both abiraterone acetate and enzalutamide have been on the market the longest, have the widest indications, and are thus generally more well known. Further, abiraterone, enzalutamide, and apalutamide had large, expected spikes in search popularity in May 2011, September 2012, and February 2018, respectively, which all shortly followed each drug’s FDA Approval [12–14]. Once abiraterone acetate was FDA approved in 2011, it became more widely searched than bicalutamide, which was the only other approved drug on the market for advanced PC. Abiraterone acetate surpassing bicalutamide in search popularity is not surprising given its increased efficacy relative to bicalutamide [5]. Further, enzalutamide was FDA approved in August 2012, and has since been relatively tied with abiraterone as the most searched NHT. This is despite two meta-analyses of randomized control trials and observational studies both finding that enzalutamide was slightly superior to abiraterone in time to PSA progression, PSA response rate, and progression-free survival [17, 18]. In contrast, enzalutamide is more expensive than abiraterone through Medicaid, especially given the availability of generic abiraterone. The relative cost-savings of abiraterone may explain why its search popularity is on par with the slightly more effective enzalutamide, though this needs further study.
Bicalutamide had an RSI of 100 in 2004 and remained the most searched advanced PC medication until July 2008 at which point abiraterone acetate was briefly the most searched following results of its Phase I/II trial. Bicalutamide returned to being the most searched a few months later and remained the most searched until abiraterone acetate gained FDA approval in 2011. It was expected that bicalutamide was the most searched during these time periods as it was the only of five medications that was FDA-approved and available. Yet, even as more effective FDA-approved NHTs emerged, bicalutamide still remained highly searched. Given that bicalutamide is still often used to block testosterone surges from ADT, we would not expect interest to drop to zero. However, from the pre-NHT era to the early-NHT era where NHTs were only approved for mCRPC, to recent times where NHTs gained approvals in earlier stages of disease and thus the indications where only bicalutamide were used have declined, it is noteworthy that interest in bicalutamide remained relatively unchanged. Though bicalutamide has uses besides advanced PC that may contribute to its search popularity, including for alopecia and androgen-receptor positive triple negative breast cancer, it is much more inexpensive than NHTs [19]. The cost-savings of bicalutamide may be driving its popularity the most. Among the medications searched, bicalutamide is the least expensive with average Medicaid costs of $16.44 per claim in 2021 [20]. Enzalutamide and abiraterone, however, cost an average of $11,234.21 and $3,064.85 per claim through Medicaid [20]. This in part may explain why in 2020 total Medicaid claims for bicalutamide (n = 18,150) was more than enzalutamide (n = 8684) and abiraterone acetate (n = 7981) combined [20].
In the United States, it is well documented that financial barriers and inadequate insurance coverage can restrict patients’ ability to receive healthcare, including prescription drugs. Ali et al. found that barriers including federal poverty level, out-of-pocket medication cost, and unemployment were associated with poorer access to prescription medications [21]. And further, West et al. found that among psychiatric patients in Medicaid programs, the most common medication access problems were due to inability to access clinically indicated medication refills or new prescriptions because Medicaid would not approve or cover them [22]. It may be possible that bicalutamide remains relatively popular as patients may face similar financial and insurance barriers. Indeed, the link between high bicalutamide RSI and states without Medicaid expansion, lower household income, and higher percent uninsured supports this hypothesis. Ultimately, further studies are needed to confirm the link between lower socioeconomic status and continued high interest in bicalutamide.
Our findings must be interpreted within the context of the study design. First, our study is limited to the United States of which data were available for 39 states. Second, search popularity does not necessarily correlate with medication usage. Third, it is both unknown who is searching for these medications and why they are searching for them. For example, bicalutamide is used off-label for treatment of early puberty in boys and as part of feminizing hormone therapy in transgender women [23, 24]. The non-PC treatment related searches of some these medications could not be excluded in our data and would contribute to a higher RSI versus the remaining medications with fewer non-PC treatment related searches. Finally, given the limits of Google Trends, we were only able to examine state-level data creating heterogeneity across searchers from the same state. Given noise tends to bias the results to the null, our results may have underestimated the strength of the link between socioeconomic status and bicalutamide search popularity, though future individual-level data are needed to confirm this.
CONCLUSIONS
This is the first study to compare advanced PC drug search popularity on Google. Search popularity seemed to increase the most following FDA approval. Despite the emergence of more effective medications, bicalutamide remains relatively popular, especially in states with lower household income, more uninsured adults or that did not expand Medicaid, possibly due to its lower cost.
ACKNOWLEDGEMENTS
SD was supported by the Patient-Centered Outcomes Research Training in Urologic and Gynecologic Cancers (PCORT UroGynCan: T32CA251072). JD was supported by NIH grants TL1 DK132768 and U2C DK 129496. GCG was supported by the Department of Defense Health Disparities Research Fellowship. NAF was supported by an NIH grant T32CA240172. JG was supported by an 2022 PCF Young Investigator Award, Department of Defense Health Disparity Research Award W81XWH-19-1-0748, and American Cancer Society Research Scholar Grant RSG-18-018-01-CPHPS.
Footnotes
COMPETING INTERESTS
SJF – consultant for Bayer, Astellas, Astra Zeneca, Janssen, Pfizer, Merck, Sanofi and speaker for Astra Zeneca and Sanofi. EP – receives research funding from Pfizer/Astellas, speaker for Bayer and Myovant, advisor for Bayer and Janssen. JG – Consultant for Janssen, Myovant, Astellas, Pfizer, Bayer, Elsevier, Exelixis, and Incyte. WA – Consultant for Bayer, Janssen, Pfizer, Astellas, Myovant. The other authors declare no competing interests.
Reprints and permission information is available at http://www.nature.com/reprints
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author, SD, upon reasonable request.
REFERENCES
- 1.Desai K, McManus JM, Sharifi N. Hormonal therapy for prostate cancer. Endocr Rev. 2021;42:354–73. 10.1210/endrev/bnab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori K, Mostafaei H, Pradere B, Motlagh RS, Quhal F, Laukhtina E, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25:1892–900. 10.1007/s10147-020-01777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaishampayan UN, Heilbrun LK, Monk P 3rd, Tejwani S, Sonpavde G, Hwang C, et al. Clinical efficacy of enzalutamide vs bicalutamide combined with androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: a randomized clinical trial. JAMA Netw Open. 2021;4:e2034633. 10.1001/jamanetworkopen.2020.34633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koukourakis MI, Kakouratos C, Kalamida D, Mitrakas A, Pouliliou S, Xanthopoulou E, et al. Comparison of the effect of the antiandrogen apalutamide (ARN-509) versus bicalutamide on the androgen receptor pathway in prostate cancer cell lines. Anticancer Drugs. 2018;29:323–33. 10.1097/cad.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 5.Ueda T, Shiraishi T, Ito S, Ohashi M, Matsugasumi T, Yamada Y, et al. Abiraterone acetate versus bicalutamide in combination with gonadotropin releasing hormone antagonist therapy for high risk metastatic hormone sensitive prostate cancer. Sci Rep. 2021;11:10094. 10.1038/s41598-021-89609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siemens DR, Klotz L, Heidenreich A, Chowdhury S, Villers A, Baron B, et al. Efficacy and safety of enzalutamide vs bicalutamide in younger and older patients with metastatic castration resistant prostate cancer in the TERRAIN trial. J Urol. 2018;199:147–54. 10.1016/j.juro.2017.08.080. [DOI] [PubMed] [Google Scholar]
- 7.Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART I. J Urol. 2021;205:14–21. 10.1097/ju.0000000000001375. [DOI] [PubMed] [Google Scholar]
- 8.Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART II. J Urol. 2021;205:22–9. 10.1097/ju.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 9.Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer:American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J Clin Oncol. 2014;32:3436–48. 10.1200/jco.2013.54.8404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KFF. 2022/04/21/T16:15:42+00:00. 2022. https://www.kff.org/statedata/.
- 11.Danila DC, Rathkopf DE, Morris MJ, Slovin SF, Schwartz LH, Farmer K, et al. Abiraterone acetate and prednisone in patients (Pts) with progressive metastatic castration resistant prostate cancer (CRPC) after failure of docetaxel-based chemotherapy. J Clin Oncol. 2008;26:5019. 10.1200/jco.2008.26.15_suppl.5019. [DOI] [Google Scholar]
- 12.FDA approves abiraterone acetate in combination with prednisone for high-risk metastatic castration-sensitive prostate cancer. FDA. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abiraterone-acetate-combination-prednisone-high-risk-metastatic-castration-sensitive. [Google Scholar]
- 13.Drug Approval Package: Xtandi (enzalutamide) NDA # 203415. 2012. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203415Orig1s000Approv.pdf.
- 14.FDA approves apalutamide for non-metastatic castration-resistant prostate cancer. FDA. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-apalutamide-non-metastatic-castration-resistant-prostate-cancer. [Google Scholar]
- 15.United States - COVID-19 Overview - Johns Hopkins. Johns Hopkins Coronavirus Resource Center. 2022. https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 16.KFF. 2022/11/09/T14:44:01+00:00. 2022. https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map/.
- 17.Chopra A, Georgieva M, Lopes G, Yeo CM, Haaland B. Abiraterone or enzalutamide in advanced castration-resistant prostate cancer: an indirect comparison. Prostate. 2017;77:639–46. 10.1002/pros.23309. [DOI] [PubMed] [Google Scholar]
- 18.Kang M, Jeong CW, Kwak C, Ku JH, Kim HH. Comparing the clinical efficacy of abiraterone acetate, enzalutamide, and orteronel in patients with metastatic castration-resistant prostate cancer by performing a network meta-analysis of eight randomized controlled trials. Oncotarget. 2017;8:59690–7. 10.18632/oncotarget.17741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho RM, Santos LDN, Ramos PM, Machado CJ, Acioly P, Frattini SC, et al. Bicalutamide and the new perspectives for female pattern hair loss treatment: what dermatologists should know. J Cosmet Dermatol. 2022;21:4171–5. 10.1111/jocd.14773. [DOI] [PubMed] [Google Scholar]
- 20.Data.Medicaid.gov. State Drug Utilization Data 2021. Accessed August 21, 2023. https://data.medicaid.gov/dataset/eec7fbe6-c4c4-5915-b3d0-be5828ef4e9d.
- 21.Ali AM, Cobran EK, Young HN. Barriers associated with access to prescription medications in patients diagnosed with type 2 diabetes mellitus treated at federally qualified health centers. Pharmacy. 2022;10. 10.3390/pharmacy10040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West JC, Wilk JE, Rae DS, Muszynski IS, Stipec MR, Alter CL, et al. Medicaid prescription drug policies and medication access and continuity: findings from ten states. Psychiatr Serv. 2009;60:601–10. 10.1176/ps.2009.60.5.601. [DOI] [PubMed] [Google Scholar]
- 23.Anawalt BD. Chapter 140 - Gynecomastia. In: Jameson JL, De Groot LJ, de Kretser DM, Giudice LC, Grossman AB, Melmed S, et al. , editors. Endocrinology: adult and pediatric, 7th ed. Philadelphia: W.B. Saunders; 2016. p. 2421–30.e5. [Google Scholar]
- 24.Randolph JF Jr. Gender-affirming hormone therapy for transgender females. Clin Obstet Gynecol. 2018;61:705–21. 10.1097/grf.0000000000000396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, SD, upon reasonable request.