Abstract
Background:
Sepsis syndromes are a major burden in the ICU with very high mortality. Vasopressin and copeptin are released in response to hypovolemia and have shown potential significance in diagnosing sepsis.
Objective:
To investigate the levels of copeptin in patients with sepsis syndromes and evaluate its relation with patient prognosis and mortality.
Methods:
Four databases were searched for literature published from inception to the 8th of November 2022. Original research articles where copeptin was measured in sepsis patients and compared with controls were included. Data extraction and synthesis: study characteristics, levels of copeptin in the participants, and copeptin assay description were extracted. Levels of copeptin in patients were pooled and compared with controls in terms of the standard mean difference (SMD) generated using a random-effects model.
Results:
Fifteen studies met the selection criteria. Copeptin levels were significantly higher in patients with sepsis, severe sepsis, and septic shock as compared to controls [(SMD: 1.49, 95% CI: 0.81–2.16, P<0.0001), (SMD: 1.94, 95% CI: 0.34–3.54, P=0.02), and (SMD: 2.17, 95% CI: 0.68–3.66, P=0.004), respectively]. The highest copeptin levels were noted in septic shock patients. The admission copeptin levels were significantly lower in survivors as compared to nonsurvivors (SMD: −1.73; 95% CI: −2.41 to −1.06, P<0.001).
Conclusion and Relevance:
Copeptin was significantly elevated in sepsis, severe sepsis, and septic shock. Survivors had a significantly lower copeptin during admission. Copeptin offered an excellent predictability to predict 1-month mortality. Measuring the copeptin in sepsis patients can aid treating physicians to foresee patients’ prognosis.
Keywords: copeptin, meta-analysis, sepsis, systematic review
Introduction
Highlights
Copeptin have shown potential significance in diagnosing sepsis.
Copeptin levels were significantly higher in patients with sepsis, severe sepsis, and septic shock as compared to controls.
Copeptin was significantly elevated in sepsis, severe sepsis, and septic shock. Survivors had a significantly lower copeptin during admission.
Sepsis syndromes are a common syndromic response to infections and are associated with physiological, biological, and biochemical abnormalities1. They result in life-threatening organ dysfunction caused by a dysregulated host response to infection2. The global incidence and death toll of sepsis is estimated to be 48.9 million and 11 million, respectively. Sepsis accounts for 1 in 5 global deaths, with half of the cases occurring in children3. Low-income and middle-income countries have a disproportionately higher burden of sepsis-related mortality due to the paucity of data and lack of resources for critical care4. Immediate response for septic shock management at the emergency department is a formidable challenge that can be hindered due to difficulties in establishing a diagnosis quickly5. With years of practice and treatment experiences, the criteria for sepsis syndromes categorization have changed over time. The updated definition establishes sepsis as a suspected infection without the requirement of a proven infection6. Previously, as per Sepsis-1, they were categorized into systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock. However, according to recent criteria (Sepsis-3), since SIRS could be the result of several noninfectious etiologies such as pancreatitis and myocardial infarction, and additionally, symptomatic overlap between sepsis and severe sepsis, only sepsis and septic shock remain in the current criteria. Sepsis-3 defines sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection2. Treatment initiatives at an early phase with prompt diagnosis can improve the prognosis in these patients7–10.
The alarming statistics of mortality associated with sepsis, especially in children, urge the clinicians for a rapid response in sepsis management by monitoring the trajectories of the disease. In the pathophysiology of sepsis, due to persistent hypovolemia, neuroendocrine compensation through vasopressin is observed for fluid and osmotic balance, regulation of blood pressure, and endocrine stress response11,12. Synthetic vasopressin serves as a potent vasoconstrictor, eventually aiding in organ perfusion, and for this reason, it is used to enhance perfusion in patients in the state of shock. Endogenous vasopressin is synthesized from the vasopressin gene and secreted from the posterior pituitary. Initially, a preprohormone is formed from the vasopressin gene that later undergoes post-translational modification producing arginine vasopressin13,14. Copeptin is a post-translational modification product from the same gene and is synthesized in stoichiometric equivalence to vasopressin, similar as insulin and C-peptide15,16. Despite elevations, the utility of analyzing plasma vasopressin concentration for sepsis monitoring in practice is restrained owing to its limitations of a shorter half-life, instability in vitro, rapid clearance at low temperatures, adherence to platelets, and most importantly, normalization to the normal range within a short duration13,14. In contrast, copeptin is stable with a longer half-life, remains elevated for several days following sepsis, and assays with short turn-around times are available. For this reason, copeptin has been extensively studied for its possible utility in sepsis diagnosis. In normal individuals, copeptin level ranges between 1.70 and 11.25 pmol/l and is increased in a variety of inflammatory conditions17–19. Apart from sepsis syndromes, copeptin concentration is also increased in acute disorders such as stroke and acute myocardial infarction, therefore rendering it as a nonspecific biomarker20. In recent research, copeptin has been proposed as a potential biomarker for sepsis syndromes21. Hence, this is the first meta-analysis which obtained the pooled estimate of the copeptin levels in sepsis patients and aimed to provide an exhaustive assessment of the diagnostic and prognostic value of copeptin as a biomarker in sepsis syndromes.
Materials and methods
Protocol and registration
The protocol for this systematic review and meta-analysis was prospectively registered in the international prospective register of systematic reviews. This systematic review was performed with respect to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA, Supplemental Digital Content 1, http://links.lww.com/JS9/B671, Supplemental Digital Content 2, http://links.lww.com/JS9/B672) updated guidelines 2020 and Assessing the methodological quality of systematic reviews (AMSTAR, Supplemental Digital Content 3, http://links.lww.com/JS9/B673) guidelines22,23.
Search strategy and study selection
We systematically searched the databases PubMed, Scopus, Web of Science, and Embase for relevant studies published from the inception of these databases to the 8th of November 2022. The keywords used were ‘copeptin’, ‘sepsis’, ‘severe sepsis’, and ‘septic shock’. These keywords were connected using the Boolean operators ‘AND’ and ‘OR’ for a systematic search.
The search results were exported into Mendeley Desktop version 1.19.8 and duplicates were removed. Studies were then screened by title and abstract followed by full-text screening based on the selection criteria. Those studies that met the selection criteria were included in the review. The citations of the included studies were also checked for any eligible studies. Any discrepancies during the study selection were solved via discussion between the authors.
Selection criteria
All studies that fulfilled the following selection criteria were included: Full-text available in English language; Original research where copeptin was measured in human participants with sepsis syndromes; Control population was defined and not assigned to patients with SIRS.
The studies clarified if patients with hypothalamic-pituitary- adrenal axis disorders were excluded. The copeptin assay description was elaborated.
We excluded all studies that did not include a control population. Likewise, case reports, review articles, research letters, correspondences, commentaries, and expert perspectives were excluded.
Risk of bias assessment
Considering the nonrandomized design of the included studies, the quality assessment was performed using the ROBINS-I (Risk of bias in nonrandomized studies-I) bias assessment tool24. The tool assesses the potential risk of bias in studies under six domains: (1) selection of comparison groups; (2) bias due to confounding; (3) ascertainment of exposure; (4) measurement of outcomes; (5) missing data; and (6) reporting of results. The risk of bias of a study was considered to be low if at least five domains possessed low risk, moderate if any two domains possessed moderate risk, serious if at least one domain possessed serious risk, and critical if at least one of the domains possessed critical risk of bias. Two reviewers (R.T. and S.S.) independently performed a blinded risk of bias assessment. A third reviewer (S.B.) created a PDF document of the included studies with author identity and affiliations removed.
Outcomes of interest
Our primary outcomes of interest included evaluation the levels of copeptin in patients with sepsis syndromes as compared to controls devoid of any infective symptoms and to compare the copeptin levels between survivors and nonsurvivors. Secondary outcomes of interest include comparing the copeptin levels in different severities of sepsis and evaluation of copeptin levels for predicting mortality in patients during follow-up.
Data extraction
Two reviewers (A.B. and R.T.) extracted data to a prespecified Excel sheet. The following data items were extracted: (1) descriptive characteristics of studies included (author, year, sepsis syndromes studied, number of patients and controls, age, sex, and overall study findings); (2) patient characteristics (sepsis diagnostic criteria, health and organ status scores, sepsis origin, and undergoing treatment); (3) assay description (methodology, sampling, sample handling, and assay sensitivities variability); and (4) predictability (area under curve (AUC) values, cut-off values, sensitivity, and specificity).
Data synthesis
Ratios and percentages were used to express discrete variables. Continuous variables were expressed as mean and SD, or median and interquartile range or range. Since meta-analysis from continuous data requires data in terms of mean and SD, the data from studies reporting copeptin levels in terms of median and interquartile range were converted into mean and SD following the conversion formula proposed by Hozo et al.25 for variables with an unknown distribution.
Statistical analysis
Six distinct analyses were performed: comparison of copeptin levels between (1) sepsis and controls, (2) severe sepsis and controls, (3) septic shock and controls, (4) sepsis and severe sepsis, (5) severe sepsis and septic shock, and (6) survivors and nonsurvivors. Since the diagnostic criteria for sepsis varied and consequently the copeptin levels could differ based on each criteria’s patient selection, subgroup analysis was performed where studies utilizing Sepsis-1, Sepsis-2, and Sepsis-3 definitions were placed into distinct subgroups. The objective of the results from our meta-analysis was determined to be more generalizable, and therefore, with reference to a relevant research, standardized mean difference (SMD) was used to express the pooled difference in the copeptin levels between the aforementioned comparison groups26. SMD was generated using the inverse-variance method and expressed along with a 95% CI. Statistical significance was set for a P-value <0.05. The heterogeneity was estimated using Cochran’s Q and I 2 statistics. A low, moderate, high, and substantial heterogeneity was considered for I 2 <40%, I 2=40–69%, I 2=70–90%, and I 2 >90%, respectively. Considering the variation in the population demographics and moderate to substantial heterogeneity, a random-effects model was employed to conduct a meta-analysis using Review Manager (RevMan) version 5.4.1. To test for the influence of the findings of individual studies on the pooled effect, sensitivity analyses were performed by omitting each study at a time from the analysis. The publication bias among the included studies was assessed by constructing funnel plots and using Egger’s and Begg’s tests to check for funnel plot asymmetry. A P-value <0.05 indicated that publication bias is present.
Results
Study search and study selection
The database search retrieved a total of 1650 studies. After removing duplicates and screening by title and abstract, 117 studies were subjected for full-text screening in accordance to the selection criteria. Finally, 15 studies that met our selection criteria were included for qualitative and quantitative synthesis of this systematic review (Fig. 1).
Figure 1.
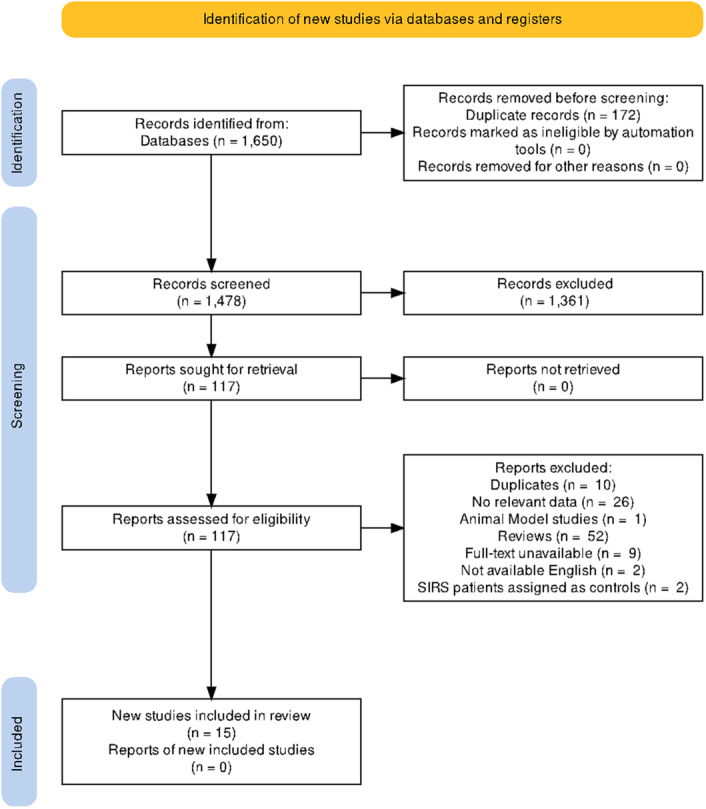
PRISMA guidelines.
Risk of bias assessment
The result of the blinded risk of bias assessment is displayed in Supplementary Table 1 (Supplemental Digital Content 4, http://links.lww.com/JS9/B674). Briefly, four studies possessed a moderate overall risk of bias while the rest possessed a low overall risk of bias.
Descriptive characteristics of the included studies
The detailed descriptive characteristics of the included studies are shown in Table 1. Briefly, 15 studies were included in this meta-analysis21,27–40. The studies were conducted in different geographical locations: North America, Europe, and Asia. Five studies reported the copeptin levels in all three sepsis syndromes, sepsis, severe sepsis, and septic shock, whereas others reported in at least one of them. The number of patients with sepsis syndromes ranged from 15 to 186. The patients were adults except for two studies that studied the pediatric population33,37. The number of controls ranged from 15 to 155 individuals. In most of the studies, healthy individuals were assigned as controls. Battista et al. assigned the patients with gastrointestinal bleeding as controls and Lee et al. assigned the controls to hospitalized patients with nonsepsis cases. Overall, a majority of studies found significantly elevated copeptin levels in patients with sepsis syndromes as compared to controls. All studies relating copeptin levels with survival outcomes found lower levels in survivors as compared to nonsurvivors. The diagnostic criteria for sepsis syndromes; however, differed. A majority of studies used the 2001 Sepsis-2 (SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference) definition to select and classify patients with sepsis syndromes. A few studies reported the Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores of the patients. The source of sepsis infection was respiratory infections in the majority of studies. Two studies reported that the patients were being treated with fluid resuscitation and a vasoactive drug, norepinephrine (Supplementary Table 2, Supplemental Digital Content 4, http://links.lww.com/JS9/B674).
Table 1.
Descriptive characteristics of the included studies.
| S.N. | References | Study country | Sepsis definition | Patients with sepsis syndromes (Cases) | Controls | Age of patients | Sex of patients | Overall findings |
|---|---|---|---|---|---|---|---|---|
| 1. | Ameen et al. 28 | Saudi Arabia | Sepsis-2 | 39 | NR | 61.2 (5) | 21:18 | Significantly lower copeptin in survivors (P=0.001). |
| 2. | Assaad et al. 29 | Egypt | Sepsis-2 | S=20 SS=20 SPS=20 |
20 Healthy individuals | S =49.5 (19.28), SS= 56.8 (13.16)SPS= 58.7 (12.1) | S= 13:7 SS= 9:11 SPS= 11:9 |
Significantly elevated copeptin in patients with sepsis syndromes as compared to controls (P<0.001) |
| 3. | Battista et al. 30 | Italy | Sepsis-2 | S=24 SS=25 SPS=15 |
15 GI bleeding= 15 | S= 67.1 (20-85) SS= 68.8 (50-83) SPS= 77.5 (66-93) |
S= 13:11 SS= 9: 16 SPS= 4:11 |
Copeptin levels differed in sepsis, severe sepsis, and septic shock as compared to controls (P<0.05). |
| 4. | Jiang et al. 31 | China | Sepsis-2 | 41 | NR | 62.07 (11.89) | 25:16 | Significantly lower admission copeptin in sepsis survivors (P<0.05). |
| 5. | Jochberger et al. 21 | Austria | Sepsis-2 | 25 | 70 Healthy individuals | 60 (12) | 16:9 | Significantly elevated copeptin in patients with sepsis as compared to controls (P<0.001) |
| 6. | Koch et al. 32 | Germany | Sepsis-3 | 145 | 66 Healthy individuals | 64 (18-90) | 133:85 | Significantly elevated copeptin in patients with sepsis as compared to controls; however, no significant differences in the copeptin levels between sepsis and nonsepsis patients. |
| 7. | Kloter et al. 33 | Switzerland, France, USA | Sepsis-3 | 645 | NR | 61 (20.3) | 56: 44 | Copeptin significantly associated with mortality (P=0.002) |
| 8. | Lee et al. 26 | Singapore | Sepsis-2 | S= 53 SPS= 13 |
70 Nonsepsis patients (previously well children admitted for GI endoscopy, circumcision and chronic orthopedic conditions) | S= 52.6 (42.6) SPS= 100 (58.4) months |
S= 27: 26 SPS= 6:7 |
No difference in the copeptin levels between sepsis, septic shock, and controls. |
| 9. | Lesur et al. 34 | Canada | Sepsis-2 | 37 | 14 Healthy individuals | 59.8 (17) | 22:15 | Significantly higher copeptin in sepsis patients as compared to non-septic and healthy participants. |
| 10. | Morgenthaler et al. 35 | Switzerland | Sepsis-1 | S=22 SS= 15 SPS= 16 |
84 Healthy individuals | 57 (15) | 1.2:1 | Copeptin levels significantly elevated in sepsis syndromes. |
| 11. | Palmiere et al. 36 | Switzerland | Sepsis-1 | 28 | 28 | NR | NR | Copeptin levels significantly elevated in sepsis patients as compared to controls (P<0.001). |
| 12. | Schlapbach et al. 27 | Switzerland | NR | 30 | 155 | 31.5 (29–34) gestational weeks | 38 (32–40) | No significant difference in the copeptin levels in sepsis and controls (P=0.30). |
| 13. | Sobhy et al. 37 | Egypt | Sepsis-2 | S=20 SS=20 SPS=20 |
10 Healthy individuals | S= 60.8 (13.5) SS= 59.8 (11.6) SPS= 50.3 (15.5) |
S= 2:3 SS= 9:11 SPS= 11:9 |
Copeptin levels significantly elevated in sepsis syndromes as compared to controls (P<0.001). |
| 14. | Struck et al. 38 | Germany | Sepsis-1 | 35 | 50 | NR | NR | Significantly elevated copeptin levels in septic shock as compared to controls (P<0.0001). |
| 15. | Zhang et al. 39 | China | Sepsis-2 | S= 186 SS= 97 SPS= 95 |
50 Healthy individuals | S= 71 (59–78) SS= 73 (60–78) SPS= 73 (65–78) |
S= 61:39 SS= 63: 37 SPS= 58:42 |
Significantly elevated copeptin levels in sepsis syndromes as compared to controls (P<0.001). |
Age is expressed as mean (SD) or median (IQR), Sex is expressed as ratio; GI, gastrointestinal; NR, not reported.
Copeptin assay performance and predictability
The majority of studies performed copeptin measurement using enzyme-linked immunosorbent assay (ELISA). Three studies utilized chemiluminescence immunoassay, two studies utilized fluorescent immunoassay, one utilized high-performance liquid chromatography, and another utilized Time-resolved Amplified Cryptate Emission (TRACE). In most studies, venous blood sampling was performed on an ethylene-diamine tetra acetic acid (EDTA) anticoagulated vial. For neonates, umbilical cord blood was sampled. The blood samples obtained ranged from immediate admission to at most 72 h after admission. A few studies reported that the sampling was performed prior to the initiation of therapy. Serum or plasma was generated by centrifugation and cryopreserved at temperatures of −4°C to −80°C prior to analysis. Numerous studies also reported the assay detection limit and variability. The lowest detection limit observed was 0.24 pmol/l using ELISA in the study of Lee et al. with an inter-assay variability of <15%. The lowest interassay variability noted was <12% using TRACE in the study of Battista et al. with a detection limit of 0.9 pmol/l. No significant differences were observed in the assay performance with sample storage temperature (Table 2).
Table 2.
Copeptin performance and predictability.
| Copeptin performance | ||||||
|---|---|---|---|---|---|---|
| Author | Copeptin assay | Clinical sample | Sampling point | Sample storage | Detection limit | Variability |
| Ameen et al. 28 | ELISA | Venous blood, EDTA | <24 h of admission | −20°C | NR | NR |
| Assaad et al. 29 | ELISA | Blood | <48 h of admission | −20°C | NR | NR |
| Battista et al. 30 | TRACE | Plasma | Up to 72 h of admission | −70°C | 0.9 pmol/l | Inter-assay variation <12% |
| Jiang et al. 31 | ELISA | Venous blood | Up to 72 h of admission | −4°C | NR | NR |
| Jochberger et al. 21 | CLIA | EDTA plasma | 24 h of admission | −80°C | 1.7 pmol/l | Inter-assay variation <20% |
| Koch et al. 32 | Fluorescent immunoassay | EDTA Plasma | At admission | −80°C | NR | NR |
| Kloter et al. 33 | CLIA | Blood | NR | −20°C | 0.4 pmol/l | NR |
| Lee et al. 26 | ELISA | Arterial/ venous blood | <24 h of admission | −20°C | 0.24 pmol/l | Inter-assay variation <15% |
| Lesur et al. 34 | ELISA | Blood | NR | NR | Upper limit 248 pmol/l | NR |
| Morgenthaler et al. 35 | CLIA | Arterial/ venous blood | On admission | −70°C | 1.7 pmol/l | Inter-assay variation <20% |
| Palmiere et al. 36 | ELISA | Venous blood | Prior to autopsy | −70°C | 3.9 pmol/l | Inter-assay variation <20% |
| Schlapbach et al. 27 | Fluorescent immunoassay | Umbilical blood | As soon as birth | −80°C | 4.8 pmol/l | NR |
| Sobhy et al. 37 | ELISA | Serum | On admission | −80°C | NR | NR |
| Struck et al. 38 | HPLC | Serum/plasma | NR | −20°C | NR | NR |
| Zhang et al. 39 | NR | |||||
| Copeptin predictability | ||||||
| Author | Outcome | Cut-off | AUC | AUC 95% CI | Sensitivity | Specificity |
| Ameen et al. 28 | Mortality within 28th day | NR | 0.845 | 0.724–0.966 | NR | NR |
| Assaad et al. 29 | Mortality within 10th day | >11 pmol/l | 0.660 | NR | 57.89% | 76.19% |
| Battista et al. 30 | Mortality within 30th day | 23.2 pmol/l | 0.845 | NR | 74% | 87% |
| Jiang et al. 31 | Mortality within 28th day | NR | 0.951 | NR | NR | NR |
| Jochberger et al. 21 | NR | |||||
| Koch et al. 32 | NR | |||||
| Kloter et al. 33 | Mortality within 30th day | 0.820 | 0.76-0.89 | NR | NR | |
| Lee et al. 26 | NR | |||||
| Lesur et al. 34 | NR | |||||
| Morgenthaler et al. 35 | Not defined | 96 pmol/l | 0.750 | 0.610–0.860 | 61.5% | 83.8% |
| Palmiere et al. 36 | NR | |||||
| Schlapbach et al. 27 | NR | |||||
| Sobhy et al. 37 | Mortality | 58.1 pmol/l | 0.880 | NR | 96.6% | 61.3% |
| Struck et al. 38 | NR | |||||
| Zhang et al. 39 | Mortality within 28th day | 21.5 pmol/l | 0.826 | 0.780–0.871 | 85.3% | 59.8% |
We further investigated the predictability of admission copeptin levels with mortality outcomes via the AUC values of the receiver operating characteristics analysis performed by the studies that we included (Table 2). An excellent AUC value of 0.951 was observed in the study of Jiang et al. in relation to predicting mortality up to 28 days of admission. Similar AUCs were observed for studies investigating copeptin predictability for 1-month mortality (AUC= 0.826–0.880). However, the AUC was only satisfactory in the case of Assaad et al.’s study, which analyzed the predictability of copeptin for mortality within 10 days of admission (AUC=0.660). Thus, copeptin levels measurement in patients with sepsis syndromes at admission was found to predict 1-month mortality well. For mortality risk assessment, different cut-offs were established. Sobhy et al. found that a copeptin level cut-off of 58.1 pmol/l at admission predicted mortality with 96.6% sensitivity and 61.3% specificity. A lower cut-off of 11 pmol/l offered a lower sensitivity of 57.89%. The highest specificity (87%) was observed at a cut-off of 23.2 pmol/l.
Copeptin levels between sepsis patients and controls
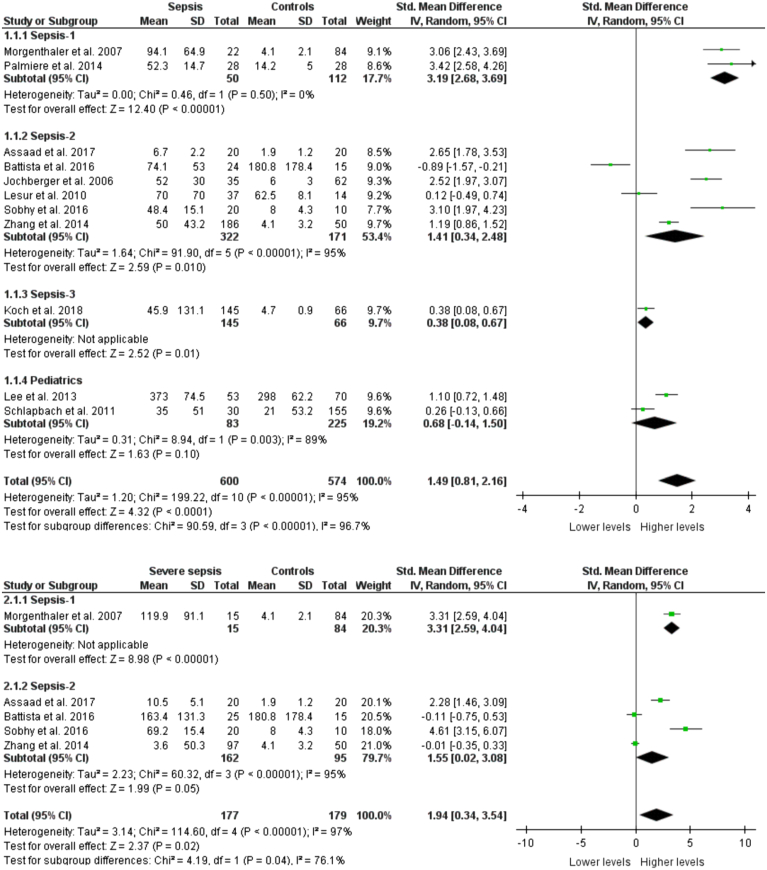
Eleven studies reported the copeptin levels in sepsis patients and controls. There were altogether 1164 participants with 590 being sepsis patients and 574 being controls. The pooled copeptin levels were significantly higher in sepsis patients as compared to controls (SMD: 1.49, 95% CI: 0.81–2.16, P<0.0001) as shown in Figure 2. No significant publication bias was detected [Egger’s test (P=0.0844), Begg’s test (P=0.3115)]. In the sensitivity analysis, no significant changes in the direction of pooled effect were noted on the omission of each of the 11 studies from the analysis, one at a time, thus indicating the robustness of the finding. Upon the omission of the pediatric subgroup from the overall analysis, the results did not change. These findings did not vary based on the different sepsis definitions used in the included studies. Interestingly, we specifically found in the pediatric population that copeptin levels did not significantly differ between cases and controls (SMD: 0.68, 95% CI: −0.14–1.50, P=0.10). Only adult sepsis patients diagnosed based on Sepsis-1, Sepsis-2, and Sepsis-3 had significant differences.
Figure 2.
Copeptin levels between sepsis, severe sepsis patients, and controls stratified with sepsis definition.
Copeptin levels between severe sepsis patients and controls
Five studies with 177 patients with severe sepsis and 179 controls measured copeptin levels. Severe sepsis patients had significantly elevated pooled copeptin levels as compared to controls (SMD: 1.94, 95% CI: 0.34–3.54, P=0.02) as shown in Figure 2. There was no significant publication bias [Egger’s test (P=0.0780), Begg’s test (P=0.1416)]. The pooled effect was similar in studies based on Sepsis-1 and Sepsis-2 criteria. In the sensitivity analysis, the pooled effect became insignificant when the study of Sobhy et al. was excluded.
Copeptin levels between septic shock patients and controls
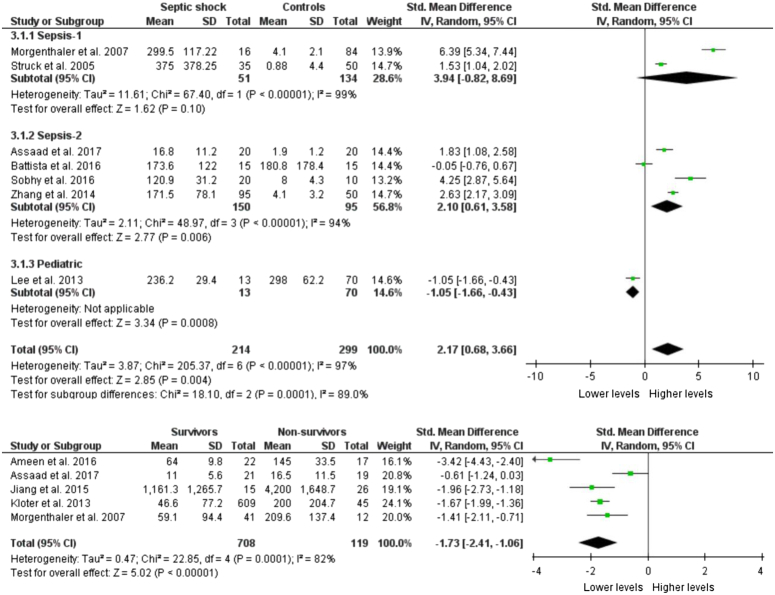
Seven studies reported the copeptin levels in altogether 214 septic shock patients and 299 controls. The pooled copeptin levels were significantly higher in septic shock patients as compared to controls (SMD: 2.17, 95% CI: 0.68–3.66, P=0.004) as shown in Figure 3. Specifically, in studies based on the Sepsis-1 definition, the results were not significant (P=0.10). In studies whose patient selection was based on Sepsis-2, the copeptin levels were significantly higher (P=0.006). In one study performed in the pediatric population, it was found that septic shock patients had significantly lower copeptin than controls (P=0.0008). No significant publication bias was detected [Egger’s test (P=0.4567), Begg’s test (P=0.4527)]. In the sensitivity analysis, no significant changes in the direction of the pooled effect were noted on the omission of each of the seven studies from the analysis, one at a time.
Figure 3.
Copeptin levels between septic shock patients and controls (up) and survivors and nonsurvivors (down).
Copeptin levels between survivors and nonsurvivors
Five studies studied the copeptin levels in accordance with survival. Out of 708 patients, 119 died while 827 survived. Copeptin levels were measured within 72 h of admission and follow-up was performed for a survival outcome to at most 30 days. The copeptin levels were significantly lower in survivors as compared to nonsurvivors (SMD: −1.73; 95% CI: −2.41 to −1.06, P<0.00001) as shown in Figure 3. No significant publication bias was observed [Egger’s test (P=0.6960), Begg’s test (P=0.1416)].
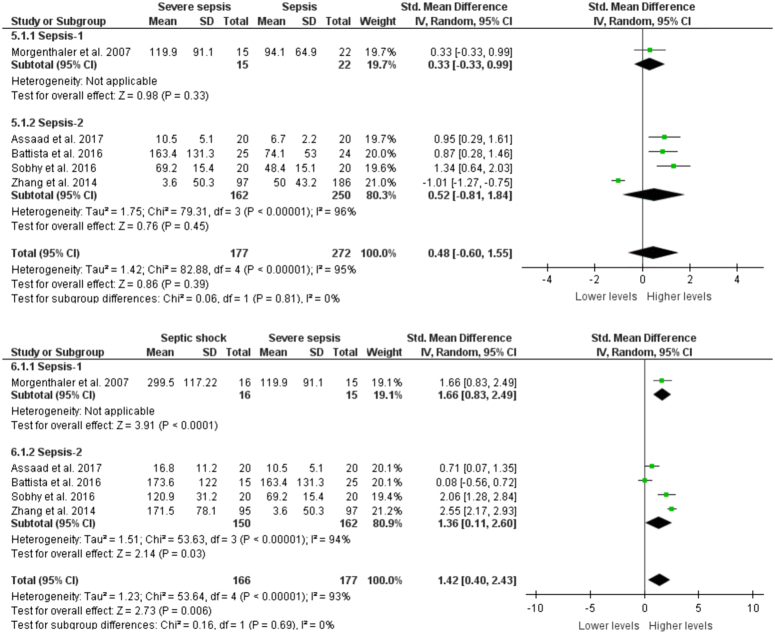
Comparison of copeptin levels with sepsis severity
There were no significant differences in the copeptin levels between sepsis and severe sepsis patients in studies based on both Sepsis-1 and Sepsis-2 definitions (SMD: 0.48, 95% CI: −0.60–1.55, P=0.39). In contrast, septic shock patients had significantly elevated copeptin levels as compared to severe sepsis patients in both the definitions (SMD: 1.42, 95% CI: 0.40–2.43, P=0.006) (Fig. 4).
Figure 4.
Copeptin levels between sepsis, severe sepsis, and septic shock.
Discussion
This meta-analysis aimed to analyze the copeptin levels in patients with sepsis syndromes. Patients categorized as sepsis, severe sepsis, and septic shock were evaluated and all presented with significantly elevated copeptin levels. The copeptin levels were the highest in patients with septic shock. We further found that the admission copeptin levels were significantly lower in survivors as compared to nonsurvivors. These findings were consistent throughout the studies and did not vary based on the sepsis diagnostic criteria. However, from two studies measuring copeptin in healthy and sepsis infants, no significant differences were detected, which implies the need for more studies on copeptin in newborns in order to diagnose and monitor neonatal sepsis.
Numerous studies28,29,35,39,40 have also found that the copeptin levels are significantly elevated during septicemia and increase proportionally with sepsis progression and severity. Likewise, studies have also reported lower admission copeptin levels in sepsis patients who survived27,30,35 and these findings are similar to the findings that this meta-analysis produced. In contrast, the findings of Battista et al. and Schlapbach et al. are conflicting. They found that the copeptin levels in sepsis did not differ significantly as compared to controls. Overall, our findings suggest that measuring copeptin at the admission of patients with sepsis syndromes significantly aids in monitoring severity, progression, and predicting the risk of mortality. Reports of higher copeptin levels in patients with multi-organ dysfunction syndrome (MODS) as compared to non-MODS patients further validate the association of copeptin with sepsis severity.
The possible underlying mechanism of copeptin elevation underlies the fact that it is a surrogate marker for vasopressin. A lowered blood pressure and volume trigger the release of stress hormones including cortisol and vasopressin. This is accomplished by a closely regulated baroreceptive and osmoreceptive mechanisms. Diminishing blood pressure and volume depolarizes the supraoptic nuclei (SON) and/or paraventricular nuclei (PVN), which generates action potential through the hypothalamic-neurohypophyseal tract resulting in the release of vasopressin via exocytosis of neurosecretory granules, secreting vasopressin into the blood19. The longer half-life of copeptin has allowed easier production and harvest of anti-copeptin antibodies in rabbits for in vitro diagnostic use. A majority of the studies we included measured copeptin via ELISA. We detected no significant differences in the assay results in accordance to sample storage. Morgenthaler et al.35 studied the sandwich immunoassay to estimate copeptin and observed a precisely linear copeptin measurement within the range of 2.25–1215 pmol/l. Overall, the assays for copeptin demonstrated a good detection limit, a wide linearity range, and low inter-assay result variability, therefore, suggesting easier access to the testing in clinical practice. We further investigated the utility of copeptin in predicting mortality and found that the 1-month mortality risk prediction was excellent; however, the 10-day mortality was only satisfactory as found in Assaad et al.’s study. This implies the possible utility of measuring copeptin at the admission of the patients, which aids to foresee the patient’s prognosis and the risk of poor outcomes. In patients presenting to the emergency department with sepsis symptoms, a high copeptin level indicates the requirement of utmost attention, and appropriate therapy in order to secure survival. In contrast, the continuous monitoring of copeptin has not been shown to hold greater significance since copeptin levels fall inappropriately low in late sepsis with advanced vasodilatory shock. This is attributed to the diminished baroreceptor-induced vasopressin release. In such patients, the vasoconstrictors were found to produce a poor response due to the desensitization of vasoconstricting receptors19.
Numerous studies have concluded that analysis of copeptin in combination with health evaluation schemes including SOFA and APACHE II scores offered a greater sensitivity and specificity to predicting prognosis and mortality. Jiang et al.30 found a significant positive correlation between the serum copeptin levels and the APACHE II scores of sepsis patients. Supportively, Zhang et al 39. found the highest AUC value for copeptin combined with cortisol, mortality in emergency department sepsis (MEDS) score, and procalcitonin in predicting septic shock and mortality. Furthermore, the MODS score has been shown to vary significantly based on sepsis severity (42). These risk scores does add significant value in predicting prognosis alongside copeptin. Several other biomarkers have been emerging to diagnose and monitor sepsis prognosis, which include procalcitonin and presepsin. Procalcitonin is known to increase in the early stages of bacterial infection and higher concentrations have been shown in infection from gram-positive bacteria therefore aiding in antimicrobial stewardship 30. Similarly, presepsin is a soluble CD14 molecule that acts as a receptor of the lipopolysaccharide complex of bacterial endotoxins and are found to significantly elevate in sepsis. These biomarkers too have shown strong correlation with SOFA scores of Sepsis-341. Clinicians dealing with sepsis should therefore relate all diagnostic parameters including copeptin when predicting prognosis and assessing the risk of mortality.
Copeptin’s use as a diagnostic and prognostic tool has been practiced in a wide variety of diseases apart from sepsis syndromes. Copeptin has been found to elevate significantly in acute myocardial infarction, heart failure, lower respiratory tract infections, diabetes, renal disease, and central nervous system disorders. Copeptin has further been implicated in distinguishing between inappropriate and appropriate vasopressin secretion. When plasma copeptin is combined with urine sodium as a ratio, it has been shown that copeptin can differentiate hyponatremia from normovolemia and hyponatremia from hypovolemia. Therefore, when interpreting copeptin levels, clinicians should keep in mind that it is a nonspecific marker and elevations could result from aforementioned overlapping conditions and not solely due to prevailing sepsis.
Our study holds both strengths and limitations. This is the first meta-analysis investigating the potential biomarker role of copeptin in sepsis syndromes. We investigated copeptin levels in all sepsis categories and compared it in accordance with the severity and survival of the patient. Importantly, we stratified our analysis based on different sepsis diagnostic criteria. The distribution of male and female patients was similar in all studies, which diminished the bias that copeptin is slightly higher in female subjects. The health evaluation scores including SOFA and APACHE II were assessed. We further analyzed the predictability of copeptin to mortality outcome along with appropriate cut-off values and corresponding sensitivities and specificities. One limitation of this study is that the ongoing treatment of the patients could not be accessed due to unavailability. Similarly, most of the studies did not report the health evaluation score. Lastly, due to our stringent selection criteria to achieve homogeneity, the omission of studies with relevant data might have occurred.
Conclusions
Overall, patients with all categories of sepsis syndromes irrespective of different sepsis definitions had elevated copeptin levels. Survivors had a significantly low copeptin level during admission. Measuring the copeptin levels of patients suspected or diagnosed with sepsis can prove to be beneficial in foreseeing the patient’s prognosis and risk of mortality. However, more studies with a larger population size should be performed and the findings should be evaluated before incorporating the testing of copeptin in routine intensive care practice.
Ethical approval
Not required.
Consent
Not applicable.
Sources of funding
No funding was available for the study.
Author contribution
A.B. and S.S.: wrote the original manuscript, reviewed, and edited the original manuscript; A.B., S.S., S.B., R.T., S.B., S.T., E.T.T., S.P.A., and R.S.: reviewed and edited the original manuscript.
Conflicts of interest disclosure
Authors have no conflicts of interest to declare.
Research registration unique identifying number (UIN)
PROSPERO ID: CRD42022377471.
Guarantor
Sangam Shah.
Data availability statement
All required information are in manuscript itself.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 17 January 2024
Contributor Information
Abhinav Bhattarai, Email: abhinavbhattarai9@gmail.com.
Sangam Shah, Email: sangam.shah.1997@gmail.com.
Sujata Baidya, Email: sujatabaidya46@gmail.com.
Ranjana Thapa, Email: theranjana8848@gmail.com.
Suyog Bhandari, Email: suyogbhandari1@gmail.com.
Eans T. Tuladhar, Email: eanstara@gmail.com.
Subhash P. Acharya, Email: drsuvash@gmail.com.
Ranjit Sah, Email: ranjitsah@iom.edu.np.
References
- 1.Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ 2007;335:879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito S, De Simone G, Boccia G, et al. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist 2017;10:204–212. [DOI] [PubMed] [Google Scholar]
- 3.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephen AH, Montoya RL, Aluisio AR. Sepsis and septic shock in low- and middle-income countries. Surg Infect (Larchmt) 2020;21:571–578. [DOI] [PubMed] [Google Scholar]
- 5.Gavelli F, Castello LM, Avanzi GC. Management of sepsis and septic shock in the emergency department. Intern Emerg Med 2021;16:1649–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 7.Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017;196:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017;376:2235–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA 2018;320:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 2018;44:925–928. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell HK, Lee HJ, Macbeth AH, et al. Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol 2008;84:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson GL. Antidiuretic hormone. Normal and disordered function. Endocrinol Metab Clin North Am 2001;30:671–vii. [DOI] [PubMed] [Google Scholar]
- 13.Robertson GL, Mahr EA, Athar S, et al. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 1973;52:2340–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preibisz JJ, Sealey JE, Laragh JH, et al. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 1983;5(2 Pt 2):I129–I138. [DOI] [PubMed] [Google Scholar]
- 15.Mu D, Ma C, Cheng J, et al. Copeptin in fluid disorders and stress. Clin Chim Acta 2022;529:46–60. [DOI] [PubMed] [Google Scholar]
- 16.Mu D, Cheng J, Qiu L, et al. Copeptin as a diagnostic and prognostic biomarker in cardiovascular diseases. Front Cardiovasc Med 2022;9:901990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenske WK, Schnyder I, Koch G, et al. Release and decay kinetics of copeptin vs AVP in response to osmotic alterations in healthy volunteers. J Clin Endocrinol Metab 2018;103:505–513. [DOI] [PubMed] [Google Scholar]
- 18.Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–119. [DOI] [PubMed] [Google Scholar]
- 19.Gomes DA, de Almeida Beltrão RL, de Oliveira Junior FM, et al. Vasopressin and copeptin release during sepsis and septic shock. Peptides 2021;136:170437. [DOI] [PubMed] [Google Scholar]
- 20.Möckel M, Searle J. Copeptin-marker of acute myocardial infarction. Curr Atheroscler Rep 2014;16:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jochberger S, Morgenthaler NG, Mayr VD, et al. Copeptin and arginine vasopressin concentrations in critically ill patients. J Clin Endocrinol Metab 2006;91:4381–4386. [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 23.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2 : a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both; 2017. [DOI] [PMC free article] [PubMed]
- 24.Chapter 25: Assessing risk of bias in a non-randomized study | Cochrane Training [Internet]. Accessed 22 July 2022. https://training.cochrane.org/handbook/current/chapter-25#_Ref396934606
- 25.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeshima N, Sozu T, Tajika A, et al. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol 2014;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ameen A, Abdel Rehim M, Shaaban YH. Endocrine And metabolic alterations may underlie mortality of severe sepsis and septic shock patients admitted to ICU. J Egypt Soc Parasitol 2016;46:109–116. [DOI] [PubMed] [Google Scholar]
- 28.Assaad SN, Salam MMA, El, et al. The potential of serum copeptin as a prognostic marker of mortality in patients with sepsis, severe sepsis, or septic shock. Egypt J Obesity, Diabetes Endocrinol 2016;2:131. [Google Scholar]
- 29.Battista S, Audisio U, Galluzzo C, et al. Assessment of diagnostic and prognostic role of copeptin in the clinical setting of sepsis. Biomed Res Int 2016;2016:3624730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Feng B, Gao D, et al. Plasma concentrations of copeptin, C-reactive protein and procalcitonin are positively correlated with APACHE II scores in patients with sepsis. J Int Med Res 2015;43:188–195. [DOI] [PubMed] [Google Scholar]
- 31.Koch A, Yagmur E, Hoss A, et al. Clinical relevance of copeptin plasma levels as a biomarker of disease severity and mortality in critically ill patients. J Clin Lab Anal 2018;32:e22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kloter M, Gregoriano C, Haag E, et al. Risk assessment of sepsis through measurement of proAVP (copeptin): a secondary analysis of the TRIAGE study. Endocr Connect 2021;10:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JH, Chan YH, Lai OF, et al. Vasopressin and copeptin levels in children with sepsis and septic shock. Intensive Care Med 2013;39:747–753. [DOI] [PubMed] [Google Scholar]
- 34.Lesur O, Roussy JF, Chagnon F, et al. Proven infection-related sepsis induces a differential stress response early after ICU admission. Crit Care 2010;14:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgenthaler NG, Müller B, Struck J, et al. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock 2007;28:219–226. [DOI] [PubMed] [Google Scholar]
- 36.Palmiere C, Augsburger M. Copeptin as a diagnostic biomarker for sepsis-related deaths. Peptides 2014;59:75–78. [DOI] [PubMed] [Google Scholar]
- 37.Schlapbach LJ, Frey S, Bigler S, et al. Copeptin concentration in cord blood in infants with early-onset sepsis, chorioamnionitis and perinatal asphyxia. BMC Pediatr 2011;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides 2005;26:2500–2504. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Dong G, Zhao X, et al. Prognostic significance of hypothalamic-pituitary-adrenal axis hormones in early sepsis: a study performed in the emergency department. Intensive Care Med 2014;40:1499–1508. [DOI] [PubMed] [Google Scholar]
- 40.Sobhy EM, Naguib MM, Hammad MG, et al. Prognostic value of the biomarker copeptin in critically ill patients with sepsis. Kasr Al Ainy Med J 2016;22:123–128. [Google Scholar]
- 41.Lee S, Song J, Park DW, et al. Diagnostic and prognostic value of presepsin and procalcitonin in non-infectious organ failure, sepsis, and septic shock: a prospective observational study according to the Sepsis-3 definitions. BMC Infect Dis 2022;22:8. doi: 10.1186/s12879-021-07012-8. PMID: 34983420; PMCID: PMC8725484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All required information are in manuscript itself.