Abstract
Background and Aims:
Trientine (TRI) and D-penicillamine (PEN) are used to treat copper overload in Wilson disease. Their main mode of action is thought to be through the facilitation of urinary copper excretion. In a recent study, TRI was noninferior to PEN despite lower 24-hour urinary copper excretion than PEN. We tested whether TRI and/or PEN also inhibit intestinal copper absorption.
Approach and Results:
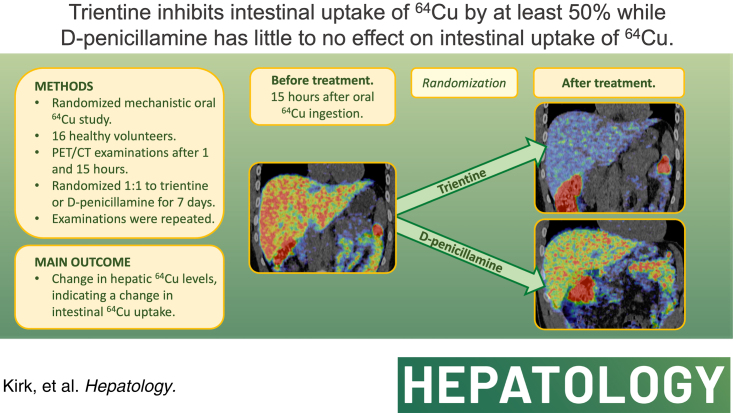
Sixteen healthy volunteers were examined with positron emission tomography (PET)/CT 1 and 15 hours after an oral Copper-64 (64Cu) dose. They then received 7 days of either PEN or TRI (trientine tetrahydrochloride), after which the 64Cu PET/CT scans were repeated. Venous blood samples were also collected. Pretreatment to posttreatment changes of the hepatic 64Cu uptake reflect the effect of drugs on intestinal absorption. 64Cu activity was normalized to dose and body weight and expressed as the mean standard uptake value. TRI (n=8) reduced hepatic 64Cu activity 1 hour after 64Cu dose from 6.17 (4.73) to 1.47 (2.97) standard uptake value, p<0.02, and after 15 hours from 14.24 (3.09) to 6.19 (3.43), p<0.02, indicating strong inhibition of intestinal 64Cu absorption. PEN (n=8) slightly reduced hepatic standard uptake value at 15 hours, from 16.30 (5.63) to 12.17 (1.44), p<0.04.
Conclusions:
In this mechanistic study, we show that TRI inhibits intestinal copper absorption, in addition to its cupriuretic effect. In contrast, PEN has modest effects on the intestinal copper absorption. This may explain why TRI and PEN are equally effective although urinary copper excretion is lower with TRI. The study questions whether the same therapeutic targets for 24-hour urinary excretion apply to both drugs.
INTRODUCTION
Wilson disease (WD) is a rare inherited autosomal recessive disorder that affects copper metabolism. Mutations in the ATP7B gene can lead to dysfunction of the gene product, the ATP7B protein. This protein is most abundantly expressed in the liver and mediates the incorporation of copper into ceruloplasmin in the trans-Golgi network and the biliary excretion of excess copper. When the ATP7B protein is dysfunctional, copper accumulates in the body with organ damage, especially in the liver and brain.1,2
According to current practice guidelines, the first-line treatment of WD is copper-chelating drugs such as D-penicillamine (PEN) and trientine (TRI).2,3 Their main mode of action is believed to be through copper chelation in blood and the promotion of urinary copper excretion.2,4–7 The cupriuretic effect of TRI is lower than that of PEN when compared at a mole-for-mole basis5,8 and therefore it has been assumed that higher doses of TRI were necessary to achieve comparable copper control.2,3 Still, it has been difficult to achieve similar cupriuretic effects with TRI compared to PEN in the initial phase of the treatment.9 Recently, a study with patients with clinically stable WD on PEN were randomized to continue PEN or switch to TRI in equal doses.10 The study demonstrated that TRI was noninferior to PEN in controlling nonceruloplasmin-bound copper, the primary endpoint. However, the 24-hour urinary copper excretion in the TRI group was only about half of that observed in the PEN group during the initial 24 weeks of the study. This raises the question of how TRI can be equally effective as PEN, despite exhibiting lower urinary copper excretion rates. Earlier studies suggested that TRI also inhibits intestinal copper uptake, although it was unclear if it was quantitatively important10,11 and whether it was in fact different from PEN.
Copper-64 (64Cu) positron emission tomography(PET)/CT scans can be used to quantify copper uptake in the intestines and liver of both patients with WD and healthy individuals.12–14 We have previously shown that a dose of orally administered 64Cu can be followed by 64Cu PET/CT showing quantitative hepatic uptake and subsequent biliary excretion.12,15 We have also demonstrated how the effect of zinc on intestinal copper uptake could be quantified using PET/CT to measure how the treatment reduced hepatic 64Cu levels after an oral dose of 64Cu.15
The present study aimed to examine and quantify the potential inhibition of intestinal copper uptake by TRI and PEN using 64Cu PET/CT. The measurements were performed before and after 7 days of treatment with either PEN or TRI. The study was performed in healthy human volunteers since the ATP7B protein is not involved in the transport of copper from the intestinal lumen to blood. Consequently, the findings are also applicable to the WD population.
METHODS
Study design
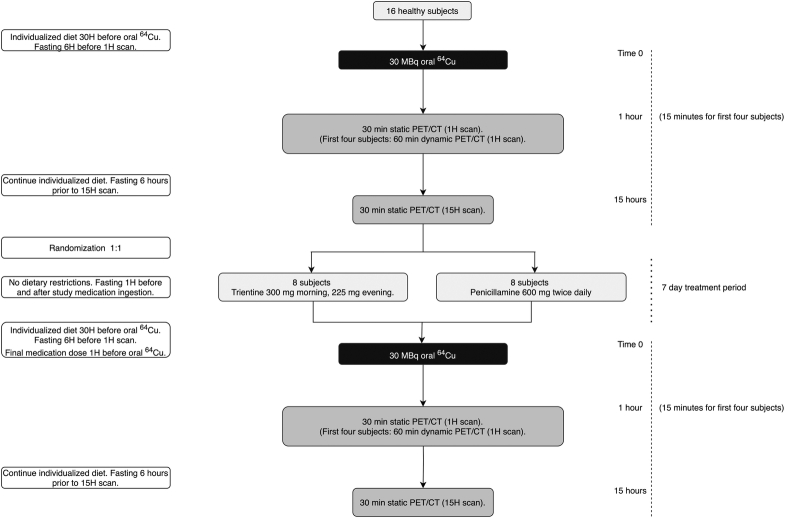
The study design of this mechanistic PET study is illustrated in Figure 1. Sixteen healthy volunteers (HVs) were initially examined by PET/CT one hour (1H) and fifteen hours (15H) after an oral 30 MBq dose of 64Cu. HVs were randomized 1:1 to the 2 treatments to increase the validity and quality of the mechanistic endpoint of the study. HVs either received trientine tetrahydrochloride (“Cuprior,” Orphalan) 300 mg morning and 225 mg evening or PEN (“Metalcaptase,” Heyl) 600 mg twice daily for 7 days before repeating the examinations. Since the study was conducted in healthy subjects, dosages were chosen to be low in the maintenance range typically used for treating WD. For the TRI dose, we used 800 mg trientine dihydrochloride as a starting point and a conversion factor of 0.64 as provided by Orphalan leading to a dose of 525 mg trientine tetrahydrochloride.
FIGURE 1.
Study design. Abbreviations: 1H, 1 hour; 15H, 15 hours; 30H, 30 hours; 64Cu, Copper-64; PET, positron emission tomography.
The HVs were instructed to fast 1 hour before and after the intake of study medicine and adherence was recorded by the participants using a printed form. Participants were further instructed to fast (water allowed) for 6 hours before each scan and were required to adhere to a controlled diet for 30 hours before the 1H scan lasting until the 15H scan. To achieve the latter, each subject recorded food intake in this timeframe before the first PET/CT scan and then repeated the same diet for the posttreatment examinations. The final dose of study medication was taken 1 hour before the oral dose of 64Cu and the first scan was 1H after the dose.
All authors had access to the study data and reviewed and approved the final manuscript.
Participants
The HVs were recruited through advertisements in local newspapers and social media. The inclusion criteria were age >18 years, BMI <30, and fertile women were required to use contraception and supply a negative pregnancy test on each day of 64Cu ingestion. Exclusion criteria were breastfeeding, pregnancy, claustrophobia, participation in a medical trial including a PET scan within the last year, known hypersensitivity to ingredients in the 64Cu tracer or study medications, and ineligibility as assessed by a medical doctor based on blood samples collected at baseline. Routine blood samples were drawn to rule out unknown liver or kidney disease. A selection of baseline parameters are presented in Table 1.
TABLE 1.
Baseline characteristics and select blood parameters collected at inclusion
| Trientine (n=8) | Penicillamine (n=8) | |
|---|---|---|
| Age (y) | 24 (13) | 21.5 (2.5) |
| Sex (male) | 4 | 4 |
| Ethnicity | Caucasian (7) South Asian (1) |
Caucasian (8) |
| BMI | 23.13 (3.32) | 23.01 (4.49) |
| ALT (U/L) | 22.50 (10.00) | 24.00 (12.50) |
| Bilirubin (μmol/L) | 11.00 (11.00) | 13.00 (3.50) |
| Albumin (g/L) | 41.50 (4.00) | 43.50 (6.50) |
| Creatinine (μmol/L) | 64.00 (9.50) | 74.50 (16.50) |
| Total copper (μmol/L) | 17.50 (7.80) | 16.05 (10.55) |
Note: Data presented as median (interquartile range).
Abbreviation: ALT, alanine aminotransferase.
Fertile women were required to use contraception for the duration of the study and 1 month following participation.
Sex was self-reported; an effort was made to balance the inclusion of sexes in this study.
Randomization
At the time of inclusion, each participant received an inclusion number and after the first scan, a randomization number with subsequent treatment modality was provided by the Hospital Pharmacy at Aarhus University Hospital. The Hospital Pharmacy created the randomization list using https://sealedenvelope.com. The medication was handed out following the baseline PET/CT scans.
64Cu PET/CT imaging
The PET/CT examinations were performed at the Department of Nuclear Medicine and PET-center, Aarhus University Hospital, Aarhus, Denmark. The 64Cu radioisotope was delivered from Hevesy Laboratory, DTU Nutech, Risø, Roskilde, Denmark. The preparation and quality control of the 64Cu tracer solution (a sterile acetate-buffered solution of 64CuCl2) has been detailed.12
Following oral 64Cu ingestion, venous blood samples were collected immediately (time=0), after 30 minutes and after 15 hours to measure 64Cu radioactivity. In the first 4 subjects, continuous PET/CT was performed from 15 to 75 minutes after the 64Cu dose which confirmed that there was no biliary 64Cu excretion taking place yet at this time (data not shown). Data from the final 30 minutes of these scans were used in the analysis as the 1H scan.
Subsequent PET/CT scans were performed at 1H and 15H after 64Cu ingestion.
The PET/CT procedure, image analysis, blood analysis, and radiotracer preparation have been detailed.12,16
Following an initial CT topogram ensuring that the liver, bile ducts, and proximal gut were within the planned field of view, a low-dose CT scan of the thorax, abdomen, and pelvis was performed for attenuation correction of the PET data. TrueX + Time of Flight image reconstruction was used with 4 iterations and 5 subsets, Gaussian filter FWHM 2.0 mm, and a 440×440 matrix. PET images from the clavicula to the upper femur were acquired through continuous bed motion with a scan speed of 0.4 mm/second allowing for good count statistics.
PET/CT scans were analyzed using PMOD (version 4.0, PMOD Technologies LLC). Five spherical volumes of interest (VOIs) were placed in the right liver lobe, each 4 mL in volume for a total of ~1.5% of the liver volume. This approach has been previously detailed and eliminates signal spillout from other organs such as the colon, large blood vessels within the liver, and the effect of respiratory motion.15 Three VOIs were placed in the luminal center of the transverse colon, each 0.5 mL in volume. Due to the temporospatial movement of intestinal contents, colonic 64Cu activity at 1H was likely zero, and any present signal likely spilled out from nearby segments of the small intestines. A hand-drawn VOI was created to include only the kidney parenchyma without a renal pelvis or calyces—only the left kidney was analyzed to avoid spillout from the liver. A single VOI was placed centrally within the bladder at 0.5 mL in volume, to estimate urinary 64Cu excretion.
Outcome
To quantify the inhibition of intestinal 64Cu uptake by the 2 treatments, hepatic 64Cu levels were measured before and after treatment due to the first-pass extraction of copper from the portal circulation into the liver. This method was previously used to estimate the similar effects of zinc.15 The activity was expressed as mean standard uptake value (SUV) which is the mean activity per mL organ normalized to a dose of 64Cu and body weight after correction for radioactivity decay:
where A is activity concentration in Bq/mL; BW is patient weight in g; and D is the ingested dose in Bq.
The inhibition of intestinal uptake was estimated as the posttreatment hepatic SUV reduction at the scan 1H or 15H after the oral 64Cu dose as has been detailed.15
Secondary endpoints included the 64Cu activity in venous samples and other organs of interest including the colon, kidney, and bladder. Venous 64Cu levels, given as kBq/mL were corrected for decay, body weight, and ingested dose.
Safety as measured by biochemical change from baseline was assessed by medical doctors involved in the study (authors Thomas D. Sandahl and Peter Ott) who also assessed any reported adverse events.
Statistical analysis
REDCap was used for data management to store baseline information, contact information, and more on HVs. PET data were extracted from the PMOD program.
The normality of data was tested using histograms and QQ plots as well as the Shapiro-Wilk test.
As data were generally not normally distributed, data were analyzed using the Mann-Whitney U test or Wilcoxon signed-rank test where appropriate. A p value <0.05 was considered statistically significant. Data are presented using the median (interquartile range) unless otherwise specified.
STATA version 17.0 (StataCorp LP) was used to perform statistical analysis, and Graphpad Prism version 9.5.1 (Graphpad Software) was used to generate figures.
Sample size
The target sample size was determined based on experience from a previous study by our team.15 In that study, which was very similar to the present study, we included 10 participants per group and were able to quantitate drug-induced changes in intestinal copper uptake. For this present study, we made improvements to the methodology using dynamic PET/CT to select the best starting time point for PET scans and introduced individualized controlled diets. Given the binding affinity and mode of action of both TRI and PEN, we estimated that we should be able to detect clinically relevant changes using 8 subjects per group for the primary outcome.
Ethics and approvals
The study was conducted in accordance with both the Declarations of Helsinki and Istanbul.
The protocol and all amendments were approved by the Danish Medicines Agency (2021061093) and the regional Ethics Committee (1-10-72-343-20). The study is registered with the European Union Drug Regulating Authorities Clinical Trials Database (EudraCT) (2020-005832-31). The Good Clinical Practice unit at Aarhus and Aalborg University Hospitals monitored the study. All participants gave informed written consent before inclusion in the study.
RESULTS
Participants
Of 45 screened individuals, 16 HVs were eligible for and included in the study from June 8, 2021, to April 25, 2022. All 16 HVs completed the trial. All 16 HVs were evaluated for the primary endpoint. Baseline characteristics and select blood parameters are available (Table 1). Compliance in relation to the study drug was 100% in the PEN group and 97% in the TRI group. In both groups, compliance for fasting in relation to the administration of study medication, before each scan and before 64Cu ingestion, was 100%.
Hepatic and gallbladder 64Cu content
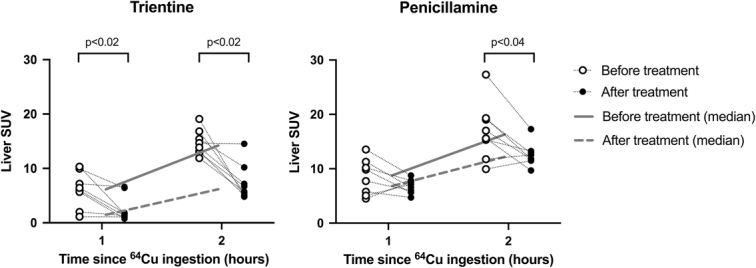
Before the treatment, hepatic 64Cu activity expressed as mean SUV was similar in the 2 groups 1 and 15 hours after the oral 64Cu dose, as expected (Table 2 and Figure 2).
TABLE 2.
64Cu levels in the liver, colon, kidney, and bladder before and after treatment, by treatment group
| Trientine (n=8) | Penicillamine (n=8) | |||
|---|---|---|---|---|
| Time post-64Cu dose (h) | 1H | 15H | 1H | 15H |
| Liver SUV, pretreatment | 6.17 (4.73) | 14.24 (3.09) | 8.78 (5.30) | 16.30 (5.63) |
| Liver SUV, posttreatment | 1.47 (2.97) | 6.19 (3.43) | 6.85 (1.90) | 12.17 (1.44) |
| Pre- vs. Posttreatment | p<0.02 | p<0.02 | NS | p<0.04 |
| Colon SUV, pretreatment | 0.60 (0.51) | 48.59 (20.16) | 1.38 (1.12) | 26.48 (22.06) |
| Colon SUV, posttreatment | 1.09 (1.48) | 60.18 (24.69) | 0.80 (0.72) | 29.33 (37.77) |
| Pre- vs. Posttreatment | NS | p<0.03 | NS | NS |
| Kidney SUV, pretreatment | 0.88 (0.98) | 1.59 (0.36) | 0.98 (0.72) | 1.54 (0.58) |
| Kidney SUV, posttreatment | 0.21 (0.16) | 0.79 (0.49) | 1.10 (0.62) | 1.52 (0.66) |
| Pre- vs. Posttreatment | p<0.02 | p<0.03 | NS | NS |
| Bladder SUV, pretreatment | 0.05 (0.11) | 0.17 (0.19) | 0.03 (0.02) | 0.17 (0.18) |
| Bladder SUV, posttreatment | 0.24 (0.31) | 0.34 (0.52) | 0.43 (0.60) | 1.34 (1.94) |
| Pre- vs. Posttreatment | NS | NS | p<0.02 | NS (p<0.07) |
Note: Data presented as median (interquartile range). p values calculated using Wilcoxon Rank Sum. N=16 (8 in the TRI and 8 in the PEN group).
Values in bold are statistically significant (p < 0.05).
Abbreviations: 1H, 1 hour; 15H, 15 hours; 64Cu, Copper-64; NS, not significant; SUV, standard uptake value, a measure of 64Cu concentration in the organ.
FIGURE 2.
64Cu levels in the liver 1 and 15 hours after 64Cu dose before and after treatment with either trientine or penicillamine. Median line. N=16 (8 in the trientine and 8 in the penicillamine group) (p values calculated using Wilcoxon Rank Sum). Abbreviations: 64Cu, Copper-64; SUV, standard uptake value, a measure of 64Cu concentration in the organ.
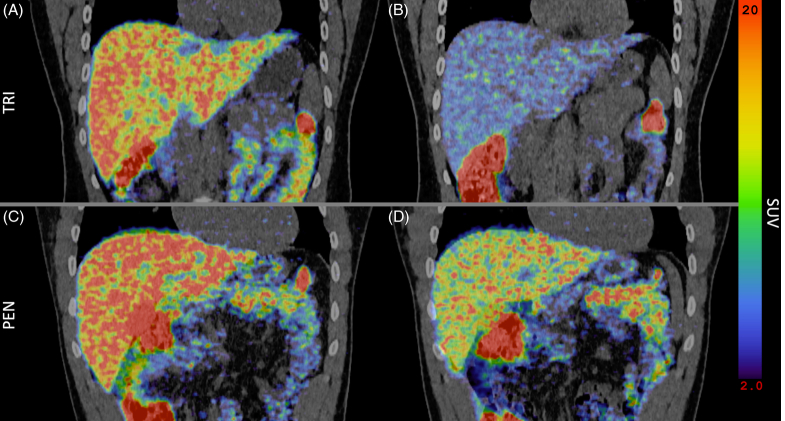
After 7 days of treatment with TRI, hepatic SUV was statistically significantly reduced compared to the baseline measurements on both scans (Table 2 and Figure 3). This indicated a reduced hepatic exposure to 64Cu due to decreased intestinal 64Cu uptake. In the PEN-treated group, liver SUV was only slightly lower than the pretreatment levels, and only after 15 hours (Table 2).
FIGURE 3.
Fused coronal abdominal PET/CT scan showing the liver 15 hours after an oral 64Cu dose. (A, C) Before treatment. (B) After treatment with TRI. (D) After treatment with PEN. Abbreviations: 64Cu, Copper-64; PEN, D-penicillamine; PET, positron emission tomography; SUV, standard uptake value, a measure of 64Cu concentration in the organ; TRI, trientine.
Venous 64Cu levels
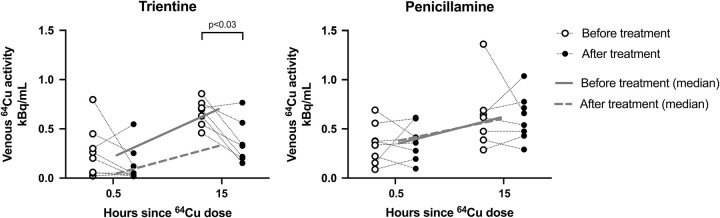
As seen in Figure 4, TRI reduced venous 64Cu both 30 minutes and 15 hours after the 64Cu dose, as expected if TRI inhibited intestinal copper uptake. The same was not observed with PEN. 15H data were missing in 2 cases (1 TRI, 1 PEN).
FIGURE 4.
Venous 64Cu levels following an oral 64Cu dose, before and after treatment with either trientine or penicillamine. Corrected for decay, body weight, and ingested dose. Median line. N=16 (8 in the trientine and 8 in the penicillamine group). One missing value in each group at 15 hours after treatment (p values calculated using Wilcoxon Rank Sum). Abbreviation: 64Cu, Copper-64.
64Cu content in the colon lumen
Changes in SUV in the colon lumen may reflect increased biliary excretion of 64Cu or reduced intestinal uptake of 64Cu. On TRI, SUV in the colon lumen at 15H increased from 48.59 (20.16) before treatment to 60.18 (24.69), p<0.03, as was expected if TRI were to inhibit intestinal uptake of the orally ingested 64Cu (Table 2). No significant change was seen for PEN, 26.48 (22.06) versus 29.33 (37.77).
However, care should be taken in interpreting changes in colon lumen SUV due to peristaltic movements of the intestines, interindividual differences in intestinal transit times, and other factors.
64Cu content in kidneys and urinary bladder
TRI significantly reduced 64Cu in the kidneys at both 1H and 15H as would be expected if less 64Cu was available for renal uptake from the lower venous concentrations (Table 2). In contrast, PEN did not alter 64Cu levels in the kidneys.
Measurements of bladder SUV were disturbed by variability in bladder volume between scans leading to uncertainty of the measurements. There was a trend toward an increase in bladder 64Cu for both treatments as would be expected given the cupriuretic effect of the drugs. The changes, however, only reached statistical significance for PEN (Table 2).
Safety measurements and adverse events
Standard biochemistry values measured at the end of the trial were not significantly different from baseline, except for a modest increase in creatinine in the TRI group, 64.00 (9.50) μmol/L versus 68.00 (11.50), and an increase in potassium, 3.65 (0.30) mmol/L versus 4.05 (0.15) in the PEN group. None of these changes were considered clinically significant (Table 1).
Within the TRI group, total copper decreased from 17.50 (7.80) μmol/L to 14.85 (10.85), p < 0.05, while total copper was unchanged after PEN, 16.05 (10.55) μmol/L versus 15.50 (13.45).
All blood samples before and after treatment are reported in supplementary materials (Supplemental Table S1, http://links.lww.com/HEP/I151).
Eighteen treatment-emergent adverse events were reported by 11 subjects, 3 in the PEN and 8 in the TRI group; none of these were considered severe (Supplemental Table S2, http://links.lww.com/HEP/I151). Of these, 6 were considered possibly related to the study treatment, all in the TRI group.
None of the reported treatment-emergent adverse events persisted after the trial conclusion and no further treatment-emergent adverse events were reported when subjects were contacted 2–3 weeks after the trial conclusion.
DISCUSSION
In this randomized mechanistic PET study, intestinal absorption of 64Cu and the effects of PEN and TRI on this process were investigated using PET/CT. The reduction of hepatic 64Cu content was used as a primary endpoint and was indicative of intestinal 64Cu uptake. Sixteen HV were examined by PET/CT 1 (1H) and 15 hours (15H) after oral 64Cu administration before and after 7 days of treatment with either trientine tetrahydrochloride or PEN. The main finding was that treatment with TRI reduced hepatic 64Cu content by ≈75% at 1H and by ≈55% at 15H. Accordingly, venous 64Cu activity was reduced by ≈ 80% at 30 minutes and by ≈50% at 15H. The same was not observed after PEN treatment, strongly suggesting that TRI and not PEN reduced intestinal uptake of 64Cu by at least 50%. This conclusion is further supported by findings that TRI reduced renal 64Cu activity and increased 64Cu in colonic contents at 15H.
These results help to explain why in several previous reports TRI was equally efficacious to PEN in controlling nonceruloplasmin-bound copper in WD despite it being less effective at inducing urinary copper excretion.7,9,10,17
In a recent comparable 64Cu PET/CT study in HV, zinc treatment resulted in a reduction in intestinal 64Cu uptake of ~50% as measured 13H after an oral 64Cu dose, similar to the effect seen here with TRI.15 The effect of zinc is most likely facilitated by induction of intestinal metallothioneins, and it is unknown if the effect of TRI is similar or related to the chelation of copper in the intestinal lumen.18,19 However, an increase in bladder 64Cu with treatment with TRI was observed, and this would not be expected to occur following zinc treatment.
In this study, the tetrahydrochloride formulation of TRI was used. TRI is currently available in 2 formulations, as either trientine dihydrochloride or tetrahydrochloride, and the demonstrated inhibition of intestinal 64Cu absorption is likely similar.20,21 In accordance, Siegemund et al11 reported that trientine dihydrochloride reduced 64Cu activity in the liver region by 80% 4 hours after an oral dose of 64Cu. Even though they did not report later time points and their method could not rule out the possibility of enhanced biliary excretion, their study suggests that also the trientine dihydrochloride formulation inhibited intestinal copper uptake. The mechanism by which TRI inhibits intestinal uptake is not clear, but we assume that a fraction of the TRI dose simply chelates copper in the intestinal lumen. In that case, there may be quantitative differences since the dihydrochloride formulation has a lower bioavailability.22
A critical question is whether the findings in this study can be extrapolated for HV to the WD population. Because WD is rare, it was not feasible to recruit enough patients with WD for this study at our center. However, the data are still likely relevant for patients with WD. Dietary copper is taken up by enterocytes in the small intestine by the copper transporter CTR1 and released to the portal venous system where the liver rapidly clears over 95% of copper from the systemic circulation, much of this through first-pass metabolism, also by CTR1.4,23–26 Thus, ATP7B dysfunction affects copper metabolism only after dietary copper reaches the liver and does not affect copper uptake from the small intestine or the portal venous system, as was demonstrated by normalization of copper balance following liver transplantation in patients with WD.25,27–29 Taken together, the results in HV should also be valid for patients with WD. Similar studies on WD treatments to reduce intestinal copper uptake were performed in HV, further strengthening the choice of methodology for this study.15,30
The scans were performed at time points 1H and 15H after the oral dose of 64Cu. The 1H time point was chosen because biliary excretion can be ruled out as a bias at that time point, even in the non-Wilsonian population.12 That was further confirmed in the present study where PET/CT recordings from 15 to 75 minutes after the 64Cu dose showed no signs of biliary 64Cu excretion in 4 participants. TRI dramatically reduced both hepatic and venous 64Cu activity 1H after dosing which strongly suggests inhibited intestinal uptake. To ensure that treatments did not only delay intestinal copper uptake, the final 15H scan was performed. At 15H, TRI reduced hepatic and venous 64Cu activity; however, at this time point, biliary excretion of 64Cu was present in the healthy subjects. We found no evidence that the reduced hepatic 64Cu activity at 15H in TRI-treated HV was the result of enhanced biliary excretion of 64Cu.
In contrast to TRI, PEN had comparatively little effect on intestinal absorption of orally administered 64Cu. In PEN-treated individuals, hepatic 64Cu was roughly unchanged at 1H and only slightly reduced at 15H relative to the reduction by TRI at the same time point. In addition, there was no change in venous 64Cu levels at any time, nor in renal or colonic activity. We propose that lower 15H hepatic 64Cu was secondary to increased urinary excretion induced by PEN, as also indicated by the increased bladder SUV on PEN. However, a minor inhibitory effect by PEN on intestinal 64Cu absorption could not be excluded.
The therapeutic effect of inhibiting intestinal uptake of copper by about 50% will in itself prevent copper accumulation and is thus comparable to the urinary excretion achieved by PEN during the initial phase of treatment.9 The results suggest that target ranges for on-treatment 24-hour urinary copper during TRI and PEN treatment in WD should be further developed. Because of TRI’s effect on intestinal uptake, the relationship between dose and 24-hour urinary excretion may be complex. A potential alternative may be to monitor the effect of treatment by use of the 24-hour urinary excretion after 48-hour treatment pause. However, previous studies focused only on PEN and there is no standardization for this methodology on TRI.2,9,31
Our data provide a possible explanation for the apparent paradoxical finding in the CHELATE trial that TRI was noninferior to PEN in controlling nonceruloplasmin-bound copper, while the average urinary copper excretion was lower with TRI (≈0.3 mg/24H) than with PEN (≈0.5 mg/24H).10 Normally, the intestinal absorption of copper is 50% of the ingested dose,32 and TRI would seem to reduce this further to 25%. If patients in the chelate study practiced a moderate copper-restricted diet of 1 mg/d, TRI would be expected to reduce intestinal uptake with ≈0.25 mg/24H resulting in a total decoppering effect of 0.55 mg/24H similar to that of PEN.
Our study has limitations. While the CHELATE trial used an mg-to-mg conversion of PEN to TRI, we used a 1200 mg PEN to 525 mg TRI comparison, but even with this lower TRI dose, the effect on intestinal uptake could be demonstrated. We did not collect stool for measurements of 64Cu activity because a 15H sampling may not represent full bowel content and because of interference with biliary 64Cu excretion. The study was performed with HVs and not in patients with WD, as would seem relevant. However, the rarity of WD, and concerns about placebo treatment33 would make that difficult, and as detailed above intestinal uptake of copper should be the same in healthy persons and patients with WD. While a large sample size would provide greater certainty, the chosen sample size is not unusual for studies employing complex and expensive methodologies like PET to demonstrate an effect on intestinal uptake of copper. The inhibitory effect of TRI on intestinal uptake was assessed 1 hour after drug intake and it is unclear how long this effect will last. In addition, further and larger studies are needed to explore how the dual mode of action by TRI translates into effects and the monitoring of treatments.
In conclusion, this study supports the mechanism of action whereby TRI reduces intestinal copper uptake in addition to its already-known cupriuretic effects. This may explain why, in a previous study, TRI treatment in WD was similarly effective to PEN to control nonceruloplasmin-bound copper even though the urinary copper excretion was not enhanced to the same extent.10 Specific target ranges for 24-hour urinary copper excretion during TRI need to be further developed. Since we know that the urinary copper excretion is dose dependent for this medication, whether there is a dose-dependence for its effect on intestinal copper absorption remains to be determined. Further studies may help demonstrate this action, and add to the understanding of the mechanism of action for this drug in treating WD.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the dedicated staff at the Department of Hepatology and Gastroenterology as well as the Department of Nuclear Medicine and PET-center, Aarhus University Hospital, for their competent assistance with the study. In particular, technologists Dorte Schmidt Jespersen, Mie Ringgaard Dollerup, and Rikke Birkegaard Bertelsen offered thorough and dedicated assistance to the project. The authors also wish to thank the participants for volunteering in this study; without them this study would not have been possible.
Footnotes
Abbreviations: 1H, 1 hour; 15H, 15 hours; 64Cu, Copper-64; HV, healthy volunteer; PEN, D-Penicillamine; PET, positron emission tomography; SUV, standard uptake value; TRI, trientine; VOI, volume of interest; WD, Wilson disease.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Frederik Teicher Kirk, Email: frkirk@rm.dk.
Ditte Emilie Munk, Email: dittmu@rm.dk.
Eugene Scott Swenson, Email: eugene.swenson@alexion.com.
Adam Michael Quicquaro, Email: adam.quicquaro@alexion.com.
Mikkel Holm Vendelbo, Email: mikkvend@rm.dk.
Michael L. Schilsky, Email: michael.schilsky@yale.edu.
Peter Ott, Email: peterott@rm.dk.
Thomas Damgaard Sandahl, Email: thomsand@rm.dk.
AUTHOR CONTRIBUTIONS
Frederik Teicher Kirk, Ditte Emilie Munk, Eugene Scott Swenson, Adam Michael Quicquaro, Peter Ott, and Thomas Damgaard Sandahl conceptualized the study and designed the protocol. Frederik Teicher Kirk, Ditte Emilie Munk, and Mikkel Holm Vendelbo performed the experiments. Frederik Teicher Kirk performed the formal analysis. Frederik Teicher Kirk, Peter Ott, and Thomas Damgaard Sandahl did the original draft preparation. Ditte Emilie Munk, Eugene Scott Swenson, Adam Michael Quicquaro, Mikkel Holm Vendelbo, and Michael L. Schilsky reviewed and edited the manuscript. Frederik Teicher Kirk, Peter Ott, and Thomas Damgaard Sandahl acquired the funding. All authors have read and agreed to the final version of the manuscript. Frederik Teicher Kirk and Thomas Damgaard Sandahl verified the data.
FUNDING INFORMATION
The study was part of a study series on the effects of drugs in WD that were carried out at Aarhus University Hospital and financed by Alexion, AstraZeneca Rare Disease, Boston, MA, USA. Two authors, Eugene Scott Swenson and Adam Michael Quicquaro, are employees of the funding source. The study design was agreed upon by all authors, and Eugene Scott Swenson and Adam Michael Quicquaro participated in data interpretation. However, the investigators (Frederik Teicher Kirk, Ditte Emilie Munk, Mikkel Holm Vendelbo, Michael L. Schilsky, Peter Ott, and Thomas Damgaard Sandahl) maintained full control of the manuscript. The study was also supported by an unrestricted research grant from The Memorial Foundation of manufacturer Vilhelm Pedersen and Wife.
CONFLICTS OF INTEREST
Frederik Teicher Kirk received grants from Alexion and AstraZeneca. Eugene Scott Swenson is employed by, owns stock, and holds intellectual property rights with Alexion and AstraZeneca. Adam Michael Quicquaro is employed by, owns stock, and holds intellectual property rights with Alexion and AstraZeneca. Michael L. Schilsky advises Arbomed and DepyMed. He received grants from Orphalan, Vivet, and the Wilson Disease Association. Peter Ott consults for Orphalan. He advises Mexbrain, Vivex, and Yaqrit. He received grants from Alexion and Univar. Thomas Damgaard Sandahl consults for Arbormed, Univar, and Ultragenyx. He advises and received grants from Alexion. He is on the speakers’ bureau for Orphalan. He received grants from AstraZeneca. The remaining authors have no conflicts to report.
REFERENCES
- 1.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol. 2012;56:671–685. doi: 10.1016/j.jhep.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, et al. A multidisciplinary approach to the diagnosis and management of Wilson disease: Executive summary of the 2022 practice guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology. 2023;77:1428–1455. [DOI] [PubMed] [Google Scholar]
- 4.Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985;65:238–309. [DOI] [PubMed] [Google Scholar]
- 5.Walshe JM. Copper chelation in patients with Wilson’s disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q J Med. 1973;42:441–452. [PubMed] [Google Scholar]
- 6.Wiggelinkhuizen M, Tilanus MEC, Bollen CW, Houwen RHJ. Systematic review: Clinical efficacy of chelator agents and zinc in the initial treatment of Wilson disease. Aliment Pharmacol Ther. 2009;29:947–958. [DOI] [PubMed] [Google Scholar]
- 7.Weiss KH, Thurik F, Gotthardt DN, Schäfer M, Teufel U, Wiegand F, et al. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11:1028–35.e1-2. [DOI] [PubMed] [Google Scholar]
- 8.Walshe JM. Management of penicillamine nephropathy in Wilson’s disease: A new chelating agent. Lancet. 1969;294:1401–1402. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffenberger J, Lohse CM, Gotthardt D, Rupp C, Weiler M, Teufel U, et al. Long-term evaluation of urinary copper excretion and non-caeruloplasmin associated copper in Wilson disease patients under medical treatment. J Inherit Metab Dis. 2019;42:371–380. [DOI] [PubMed] [Google Scholar]
- 10.Schilsky ML, Czlonkowska A, Zuin M, Cassiman D, Twardowschy C, Poujois A, et al. Trientine tetrahydrochloride versus penicillamine for maintenance therapy in Wilson disease (CHELATE): A randomised, open-label, non-inferiority, phase 3 trial. Lancet Gastroenterol Hepatol. 2022;7:1092–1102. [DOI] [PubMed] [Google Scholar]
- 11.Siegemund R, Lößner J, Günther K, Kühn HJ, Bachmann H. Mode of action of triethylenetetramine dihydrochloride on copper metabolism in Wilson’s disease. Acta Neurol Scand. 1991;83:364–366. [DOI] [PubMed] [Google Scholar]
- 12.Kjærgaard K, Sandahl TD, Frisch K, Vase KH, Keiding S, Vilstrup H, et al. Intravenous and oral copper kinetics, biodistribution and dosimetry in healthy humans studied by [(64)Cu]copper PET/CT. EJNMMI Radiopharm Chem. 2020;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandahl TD, Gormsen LC, Kjærgaard K, Vendelbo MH, Munk DE, Munk OL, et al. The pathophysiology of Wilson’s disease visualized: A human (64) Cu PET study. Hepatology. 2022;75:1461–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murillo O, Collantes M, Gazquez C, Moreno D, Hernandez-Alcoceba R, Barberia M, et al. High value of (64)Cu as a tool to evaluate the restoration of physiological copper excretion after gene therapy in Wilson’s disease. Mol Ther Methods Clin Dev. 2022;26:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munk DE, Lund Laursen T, Teicher Kirk F, Vilstrup H, Ala A, Gormsen LC, et al. Effect of oral zinc regimens on human hepatic copper content: a randomized intervention study. Sci Rep. 2022;12:14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emilie Munk D, Teicher Kirk F, Vendelbo M, Vase K, Munk O, Ott P, et al. Positron emission tomography using 64-copper as a tracer for the study of copper-related disorders. J Vis Exp. 2023. doi: 10.3791/65109. [DOI] [PubMed] [Google Scholar]
- 17.Weiss KH, Kruse C, Manolaki N, Zuin M, Ferenci P, van Scheppingen D, et al. Multicentre, retrospective study to assess long-term outcomes of chelator based treatment with trientine in Wilson disease patients withdrawn from therapy with d -penicillamine. Eur J Gastroenterol Hepatol. 2022;34:940–947. [DOI] [PubMed] [Google Scholar]
- 18.Sturniolo GC, Mestriner C, Irato P, Albergoni V, Longo G, D'Incà R. Zinc therapy increases duodenal concentrations of metallothionein and iron in Wilson’s disease patients. Am J Gastroenterol. 1999;94:334–338. [DOI] [PubMed] [Google Scholar]
- 19.Yuzbasiyan-Gurkan V, Grider A, Nostrant T, Cousins RJ, Brewer GJ. Treatment of Wilson’s disease with zinc: X. Intestinal metallothionein induction. J Lab Clin Med. 1992;120:380–386. [PubMed] [Google Scholar]
- 20.Woimant F, Debray D, Morvan E, Obadia MA, Poujois A. Efficacy and safety of two salts of trientine in the treatment of Wilson’s disease. J Clin Med. 2022;11:3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohr I, Bourhis H, Woimant F, Obadia MA, Morgil M, Morvan E, et al. Experience on switching trientine formulations in Wilson disease: Efficacy and safety after initiation of TETA 4HCl as substitute for TETA 2HCl. J Gastroenterol Hepatol. 2023;38:219–224. [DOI] [PubMed] [Google Scholar]
- 22.Weiss KH, Thompson C, Dogterom P, Chiou Y, Morley T, Jackson B, et al. Comparison of the pharmacokinetic profiles of trientine tetrahydrochloride and trientine dihydrochloride in healthy subjects. Eur J Drug Metab Pharmacokinet. 2021;46:665–675. [DOI] [PubMed] [Google Scholar]
- 23.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. [DOI] [PubMed] [Google Scholar]
- 24.Prohaska JR. Role of copper transporters in copper homeostasis. Am J Clin Nutr. 2008;88:826s–829ss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tao T. Hepatic copper metabolism: insights from genetic disease. Hepatology. 2003;37:1241–1247. [DOI] [PubMed] [Google Scholar]
- 26.Turnlund JR. Copper nutriture, bioavailability, and the influence of dietary factors. J Am Diet Assoc. 1988;88:303–308. [PubMed] [Google Scholar]
- 27.Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: Insights from biochemical and cell biological approaches. J Membr Biol. 2003;191:1–12. [DOI] [PubMed] [Google Scholar]
- 28.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–2111. [DOI] [PubMed] [Google Scholar]
- 29.Poujois A, Sobesky R, Meissner WG, Brunet AS, Broussolle E, Laurencin C, et al. Liver transplantation as a rescue therapy for severe neurologic forms of Wilson disease. Neurology. 2020;94:e2189–e2202. [DOI] [PubMed] [Google Scholar]
- 30.Henderson LM, Brewer GJ, Dressman JB, Swidan SZ, DuRoss DJ, Adair CH, et al. Use of zinc tolerance test and 24-hour urinary zinc content to assess oral zinc absorption. J Am Coll Nutr. 1996;15:79–83. [DOI] [PubMed] [Google Scholar]
- 31.Dziezyc K. Measurement of urinary copper excretion after 48-h d-penicillamine cessation as a compliance assessment in Wilson’s disease. Funct Neurol. 2015;30:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnlund JR, Keyes WR, Anderson HL, Acord LL. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am J Clin Nutr. 1989;49:870–878. [DOI] [PubMed] [Google Scholar]
- 33.Ott P, Ala A, Askari FK, Czlonkowska A, Hilgers RD, Poujois A, et al. Designing clinical trials in Wilson’s disease. Hepatology. 2021;74:3460–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]