Abstract
A transplantable human Epstein-Barr virus-associated gastric carcinoma (EBVaGC), designated KT, was propagated in severe combined immunodeficiency (SCID) mice for 12 passages. Mucin and cytokeratin expression and the Alu sequence in tumor DNA confirmed that the KT tumor was derived from human epithelial tissue. The identity of clonal EBV in the original and KT tumors was demonstrated by terminal repeat analysis of EBV DNA. The pattern of latency gene expression of EBV was the same in both tumors. EBER1 was presented similarly in tumor cell nuclei by in situ hybridization. Reverse transcription-PCR analysis also demonstrated Q-promoter-driven EBNA1 expression but not BZLF1, EBNA2, or LMP1 expression. Thus, the transplantable human EBVaGC KT retains the original EBV with the same latency gene expression and can serve as a model for this unique type of gastric carcinoma.
Epstein-Barr virus (EBV) has been shown to play a causative role in endemic Burkitt lymphoma (35) and nasopharyngeal carcinoma (NPC) (20), and the EBV genome has been identified in a wide variety of malignancies, including gastric carcinomas (10–12, 27, 32, 33). Although the proportion of EBV-associated gastric carcinoma (EBVaGC) varies from 6 to 16% (9, 14, 28, 31), the presence of EBVaGC has been reported worldwide. EBV is closely related to the genesis of EBVaGC (9). EBV-encoded small RNA is present in nearly all of the carcinoma cells in the intramucosal stage, and EBV in EBVaGC is monoclonal by Southern blot hybridization analysis with probes adjacent to the unique terminal repeat of EBV DNA. Furthermore, EBVaGC has unique morphologic, genetic, and phenotypic features. EBVaGC is frequently accompanied by dense infiltration of lymphocytes (27). The genetic pathway of EBVaGC differs considerably from that of EBV-negative gastric carcinoma [EBV(−)GC] (8). Variant forms of CD44, a cell-surface glycoprotein that acts as an adhesion molecule, are specifically expressed in EBVaGC (7).
In vitro cell culture and in vivo transplantation of neoplastic cells that retain the characteristics of the original tumor are indispensable tools for exploring the cell biology and molecular biology of a specific tumor. In NPC, the transplantation of tumors into nude mice (15, 17, 18) has been used for this purpose (4), since a stable cell line that carries the EBV genome in its nuclei has not yet been established. In gastric carcinoma, although a recent report showed successful EBV infection of human gastric carcinoma cells (34), this model may not reflect the natural condition of EBVaGC. Thus, to develop a model system for studying this unique type of gastric adenocarcinoma, we implanted EBVaGC tissue into severe combined immunodeficiency (SCID) mice instead of establishing cell lines.
Transplantation of EBVaGC into SCID mice.
To identify cases with EBVaGC before surgery, biopsied specimens of gastric carcinoma, obtained at Tokyo Metropolitan Komagome Hospital, were examined from 1994 to 1995 by in situ hybridization (ISH) of EBV-encoded small RNA as described previously. Fresh tissues were aseptically obtained from the tumor just after the stomach was resected to treat patients with EBVaGC. None of the patients had received chemotherapy before surgery. Informed consent was always obtained before the surgery.
Several pieces of tumor tissues were frozen in dry ice-hexane and stored at −80°C until used for DNA and RNA analyses. Then the tumor tissues were washed in sterile physiologic saline solution containing disinfectant, trimmed into pieces that were approximately 5 mm3, and inoculated subcutaneously into the backs of three SCID mice 5 to 10 weeks of age (Clea Japan, Tokyo, Japan). In one of three trials of implantation, EBVaGC tissues that had been engrafted to SCID mice grew to form a subcutaneous tumor in 12 weeks. Otherwise, implantation was considered to be unsuccessful, since tumor formation was not observed within 3 months after engraftment. The SCID tumor was designated KT, after the patient. The KT tumor was grown successfully in SCID mice for 12 passages. In the first passage, KT tumor cells doubled in size in 8.2 days.
Histopathology.
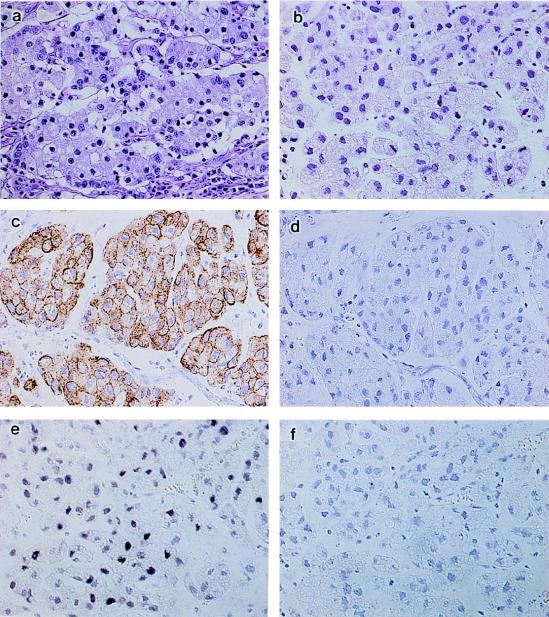
The original EBVaGC of KT was a 9.0 by 7.5 cm carcinoma on the anterior wall of the gastric body of a 58-year-old Japanese woman. It was an ulcerating lesion sharply demarcated by an elevated wall, and the carcinoma infiltrated to the subserosa. Histologically, the tumor was a poorly differentiated adenocarcinoma with solid nests, accompanied by lymphoid infiltration in the stroma (Fig. 1a). In a small fraction of the tumor, the nests consisted of carcinoma cells with prominent mucin in the cytoplasm, where lymphoid infiltration was minimal. The histology of the tumors in SCID mice (2nd, 4th, and 10th passage) reflected that of the original tumor but had some notable differences. First, the area composed of mucin-producing cells increased to occupy half of the tumor. Second, the area of poorly differentiated adenocarcinoma with solid nests, which was the predominant portion of the original tumor, was not accompanied by lymphoid infiltration (Fig. 1b).
FIG. 1.
Morphological evaluation of a human EBV-associated gastric carcinoma (EBVaGC) transplanted to SCID mice. (a) The original EBVaGC is a poorly differentiated adenocarcinoma with solid nests and lymphoid infiltration. (b) The transplanted tumor in SCID mice, designated the KT tumor, lacks lymphoid infiltration, and half of the tumor consists of mucus-producing cells, which were a minor component of the original tumor. Like the original tumor, the KT tumor shows cytokeratin immunoreactivity in the cytoplasm (c) but no positive staining when the cytokeratin antibody was replaced with mouse immunoglobulin (d). The KT tumor also shows a positive signal in the nuclei with EBER1 ISH (e) but no signal when the antisense oligoprobe was replaced with a sense probe (f). Magnification, ×80.
Confirmation that the SCID tumor was derived from a human epithelial malignancy.
In addition to histology and mucin production, immunohistochemistry disclosed that the tumor possessed an epithelial character; almost all of the neoplastic cells of the KT tumor were positively stained with anticytokeratin monoclonal antibody (Fig. 1c).
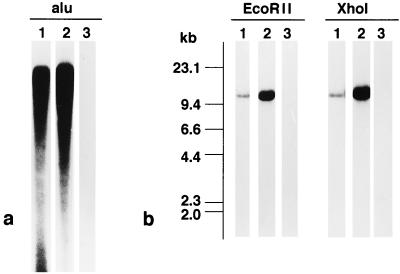
To further confirm that the tumors were derived from human tissue, the human Alu sequence was used as a probe for Southern blot analysis (18, 29). High-molecular-weight DNA was extracted from the original and SCID tumors (2nd, 4th, and 10th passage) using sodium dodecyl sulfate. Five micrograms of DNA was cleaved with BamHI and electrophoresed on a 0.5% agarose gel, and DNA fragments were transferred to a nylon membrane (Nytran; Schleicher & Schuell, Keene, N.H.). The blots were hybridized at 65°C for 48 h to 32P-labeled DNA probes and then washed twice at room temperature in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate. Band patterns were visualized by autoradiography. The probe, Alu (a gift from M. Miyaki, Tokyo Metropolitan Institute of Medical Science), was labeled with [32P]dCTP by the random primer labeling method (Prime-a-Gene labeling system; Promega, Madison, Wis.) and purified through a Nuctrap push column (Stratagene, La Jolla, Calif.). Thus, by Southern blot analysis it was found that DNAs prepared from both the original and KT tumors contained numerous human repetitive Alu sequences, whereas DNA from mouse tissues did not (Fig. 2a). This result confirms that the KT tumor is derived from human tissue.
FIG. 2.
Southern blot hybridization analysis of human EBVaGC in SCID mice. (a) When BamHI digests of DNA were subjected to Southern blot hybridization analysis, both the original and transplanted (KT) tumors showed repetitive bands of the human Alu sequence. (b) With EcoRII and XhoI fragments of EBV DNA, a single band of the same size was observed in the original EBVaGC and KT tumors. Lanes 1, original EBVaGC; lanes 2; KT tumor; lanes 3, mouse kidney.
Identity of clonal EBV.
The clonality of EBV in tumor tissues was evaluated by Southern blot analysis with BamHI-digested DNA probed with EcoRII or XhoI fragments that represent unique sequences at the left or right end, respectively, of the EBV genome (Fig. 2b). Single fragments of EBV terminal repeats of the same size were demonstrated in both the original and KT tumors. Furthermore, the fragments in the original and KT tumors were identical to both the left and right probes, indicating that EBV is present in an episomal form in both tumors. Thus, these facts indicate that the KT tumor retains the same EBV as the original tumor in the same condition, i.e., monoclonal and episomal forms, and that EBV in the KT tumors did not contain the linear form of EBV of the replicative phase.
Absence of BZLF1 gene expression.
Since the immediate-early BZLF1 protein disrupts viral latency through transactivation of early EBV genes, the strictness of EBV latency was assessed by evaluation of the expression of BZLF1 protein.
Total RNA was extracted from the original and KT tumors (10th passage) by the acid guanidium thiocyanate-phenol-chloroform extraction method as reported previously (6). To generate cDNA, 1 μl of oligo(dT)12–18 (500 μg/ml) and 12 μl of sterile, distilled water were added to 2 μg of total RNA, and the mixture was then heated at 70°C for 10 min and rapidly chilled on ice. Reagents were added to the mixture to give a final concentration of 50 mM Tris-HCl (pH 8.3), 75 mM potassium chloride, 3 mM magnesium chloride, 10 mM dithiothreitol, and 0.5 mM each deoxynucleoside triphosphate, and the mixture was incubated at 42°C for 2 min. After 200 U of SUPERSCRIPT II (Gibco BRL, Gaithersburg, Md.) was added, the mixture was incubated for 50 min at 42°C and then heated at 70°C for 15 min to stop the reaction. Details of the sequences and genome coordinates of primers and probes used to detect EBV transcripts are given in Table 1. The quality of RNA preparations was checked by 35 cycles of amplification of β-actin mRNA with the sense primer (5′-CCTTCCTGGGCATGGAGTCCT-3′), the antisense primer (5′-GGAGCAATGATCTTGATCTTC-3′), and the probe (CTGTGTTGGCGTACAGGTCTTTGCGGATGT) (5). The PCR mixture consisted of 1.25 U of AmpliTaq Gold (Perkin-Elmer, Foster City, Calif.), 20 mM Tris-HCl (pH 8.4), 50 mM potassium chloride, 1.5 mM magnesium chloride, 0.01% gelatin, 200 μM (each) deoxynucleoside triphosphate, 0.5 μM (each) primer, and synthesized cDNA, in a total volume of 50 μl. After heating at 95°C for 9 min, 40 cycles of amplification were performed with a thermal cycler model 480 (Perkin-Elmer); the thermal cycle profile was 1 min at 94°C, 2 min at 55°C, and 3 min at 72°C, with additional heating at 72°C for 10 min. Five microliters of each PCR product was then separated in 3% agarose gels, stained with ethidium bromide, and photographed under a UV transilluminator. Products were then subjected to Southern blot transfer onto nitrocellulose membrane (Protran; Schleicher & Schuell, Dassel, Germany). The filters were hybridized with 32P-end-labeled probes. The exposure time for autoradiography was 4 to 24 h for viral transcripts and 3 h for β-actin mRNA.
TABLE 1.
Sequences of primers and probes used in RT-PCR and Southern blot hybridization
| Transcript | Product size (bp) | Sequence (5′–3′) and type of primer |
|---|---|---|
| EBNA1a | 273 | GATGAGCGTTTGGGAGAGCTGATTCTGCA (Sense) |
| TCCTCGTCCATGGTTATCAC (Antisense) | ||
| AGACCTGGGAGCAGATTCAC (Probe) | ||
| EBNA2a | 339 | GCTGCTACGCATTAGAGACC (Sense) |
| TCCTGGTAGGGATTCGAGGG (Antisense) | ||
| CAGCACTGGCGTGTGACGTGGTGTAAAGTT (Probe) | ||
| Qp initiateda | 339 | AGGCGCGGGATAGCGTGCGCTACCGGA (Sense) |
| TCCTCGTCCATGGTTATCAC (Antisense) | ||
| AGACCTGGGAGCAGATTCAC (Probe) | ||
| Cp initiateda | 297 | CACTACAAGACCTACGCCTCTCCATCCATC (Sense) |
| TCTCCCCTAGGCCCTGAAGGTGAACCGCTT (Antisense) | ||
| GCGACCGGTGCCTTCTTAGGAGCTGTCCGA (Probe) | ||
| Wp initiateda | 235 | TCAGAGCGCCAGGAGTCCACACAAAT (Sense) |
| TCTCCCCTAGGCCCTGAAGGTGAACCGCTT (Antisense) | ||
| GCGACCGGTGCCTTCTTAGGAGCTGTCCGA (Probe) | ||
| LMP1a | 490 | TCCTCCTCTTGGCGCTACTG (Sense) |
| TCATCACTGTGTCGTTGTCC (Antisense) | ||
| GAAAGCACAATTCCAAGGAACAATGCCTG (Probe) | ||
| LMP2Ab | 280 | ATGACTCATCTCAACACATA (Sense) |
| CATGTTAGGCAAATTGCAAA (Antisense) | ||
| ATCCAGTATGCCTGCCTGTA (Probe) | ||
| LMP2Bb | 325 | CAGTGTAATCTGCACAAAGA (Sense) |
| CATGTTAGGCAAATTGCAAA (Antisense) | ||
| ATCCAGTATGCCTGCCTGTA (Probe) | ||
| BZLF1a | 453 | CATGTTTCAACCGCTCCGACTGG (Sense) |
| GCGCAGCCTGTCATTTTCAGATG (Antisense) | ||
| GCACGACGCACACGGAAACCACAACAGCCA (Probe) |
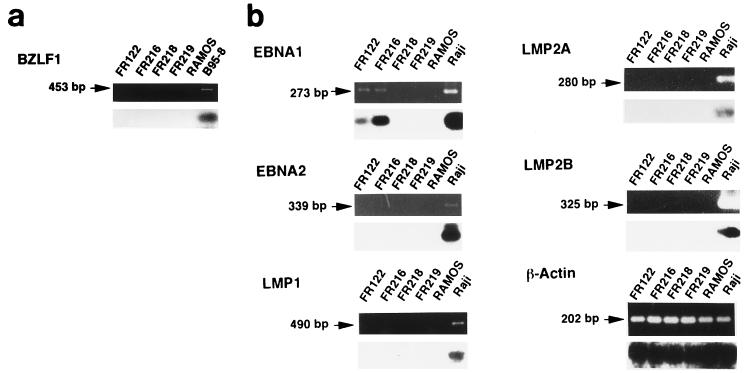
Reverse transcription (RT)-PCR analysis revealed that the original and KT tumors showed no evidence of the expression of BZLF1 mRNA (Fig. 3a). The sensitivity of this analysis was confirmed by detecting BZLF1 mRNA expression in B95-8 cells, an EBV-producing cell line. Thus, the regulation of EBV latency is relatively strict, even in a tumor transplanted into SCID mice.
FIG. 3.
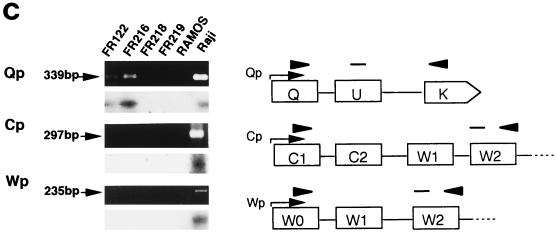
RT-PCR analysis of BZLF1 gene and EBV latency gene mRNAs in human EBVaGC in SCID mice. Transcripts of the BZLF1 gene (a) and the EBNA1, EBNA2, LMP1, LMP2A, and LMP2B genes (b) of EBV were amplified by RT-PCR, electrophoresed, and visualized with ethidium bromide staining (upper panel), and each blot was then hybridized with a specific internal probe (lower panel). (a) Neither the original EBVaGC (FR122) nor the KT tumor (FR216) show BZLF1 transcript, whereas B95-8, an EBV-producing cell line, does. (b) Among the EBV latency genes, both the original EBVaGC (FR122) and the KT tumor (FR216) show EBNA1 transcript but not EBNA2, LMP1, LMP2A, or LMP2B transcript. All of the transcripts of the EBV latency genes examined were seen in an EBV-containing lymphoma cell line (Raji), whereas none were observed in a lymphoma cell line that is negative for EBV (Ramos) or in mouse tissues (FR218, liver; and FR219, kidney). β-Actin mRNA was readily amplified from all of the tissues tested. (c) Promoter usage of EBNA1 mRNA in human EBVaGC in SCID mice. Three promoters of EBV DNA, Cp, Wp, and Qp, are mutually exclusive for the expression of EBNA1. Each promoter pair and the corresponding exon was amplified by RT-PCR, as illustrated. Both the original EBVaGC (FR122) and the KT tumor (FR216) show Qp-driven EBNA mRNA but not Cp- or Wp-driven transcript. The latter two EBNA1 transcripts are demonstrated in an EBV-containing lymphoma cell line (Raji). None of the transcripts are amplified in a lymphoma cell line that is negative for EBV (Ramos) or in mouse tissues.
Pattern of latency gene expression of EBV.
EBV-associated malignancy is classified according to expression of the latency gene (2, 3). Latency I is observed in Burkitt lymphoma, which is characterized as EBER(+), EBNA1(+), EBNA2(−), and LMP1(−). Latency III is observed in lymphoblastoid cell lines, opportunistic lymphoma in immunodeficiency, and pyothorax-associated lymphoma, which are EBER(+), EBNA1(+), EBNA2(+), and LMP1(+). NPC is characteristic of latency II, with EBER(+), EBNA1(+), EBNA2(−), and LMP1(+) between latencies I and III.
By EBER1 ISH, we confirmed that nearly 100% of the neoplastic cells in the original tumor showed signals positive for EBER1. The neoplastic cells of the KT tumor (2nd, 4th, and 10th passage) also showed signals positive for EBER1 (Fig. 1e), although the intensity of the staining was relatively weak.
RT-PCR analyses (Fig. 3b) demonstrated the presence of EBNA1 mRNA (BamHI U and K exons) in both the original and KT tumors. On the other hand, neither of these tumors expressed detectable EBNA2, LMP1, or LMP2 mRNA. The RNA preparation from an EBV-containing lymphoma cell line, Raji, yielded strong signals for all of the latency genes, whereas Ramos (a Burkitt lymphoma cell line that is negative for EBV) and mouse tissues showed mRNA only for β-actin.
Promotor usage of EBNA1.
The expression of EBNA mRNA depends on the activities of three mutually exclusive EBNA promoters: BamHI C, W, and Q (Cp, Wp, and Qp, respectively) (17, 21, 23, 24). Cp and Wp each initiate large primary transcripts which are differentially spliced into all six EBNA mRNAs as observed in EBV-immortalized B lymphoblastoid cell lines. The third EBNA promoter in latency I or II, which initiates the selective expression of EBNA1 mRNA by bypassing the coding regions of other EBNA genes, was once reported to be the BamHI-F promoter (Fp) (23, 26) but is now known to be Qp (13, 17, 24, 25).
As shown in Fig. 3c, EBNA1 mRNA in the latent phase consists of specific exons for Cp, Wp, or Qp and common exons, such as BamHI-U and -K. By RT-PCR analysis, Qp-driven EBNA mRNA was clearly detected in both tumors, while Cp- or Wp-driven EBNA mRNA was not detected at all. On the other hand, Cp- and Wp-driven EBNA1 exons were demonstrated in Raji but not in any of the transcripts in Ramos or mouse tissues by RT-PCR. These results indicate that the original and KT tumors are EBV latency I. This is consistent with previous studies (30) and demonstrates that the pattern of latency gene expression is faithfully reflected in KT tumors.
KT tumor as a model for EBVaGC.
A stable cell line of NPC that carries the EBV genome in its nuclei has not yet been established (15), suggesting that an in vitro environment excludes the EBV genome from epithelial cells. Since EBVaGC is also an epithelial malignancy, we first tried to establish a transplantable tumor of EBVaGC in nude mice. However, several unsuccessful attempts led us to adopt SCID mice as a host for human EBVaGC. While the reason for the unsuccessful transplantation in nude mice is not clear, the implantation of EBVaGC might depend on a subtle balance between carcinoma and infiltrated lymphocytes within the tumor.
Interestingly, the proportion of mucin-producing carcinoma in KT tumors was greater than that in the original tumor, in which solid-type poorly differentiated adenocarcinoma was accompanied by a dense infiltration of lymphocytes. Since mucin production and lymphocytic infiltration were inversely correlated in the original tumor, lymphocytes may block the full differentiation of carcinoma cells. Alternatively, the carcinoma cells may themselves produce certain cytokines that induce the migration of lymphocytes. KT tumors enable researchers to study the cytokine expression of carcinoma cells, since the contribution of infiltrating lymphocytes can be neglected in the assay of cytokines in this system.
EBVaGC is considered to develop from a single EBV-infected epithelial cell (9), but the precise role of EBV in the development and maintenance of EBVaGC has not been clarified. Previously, we demonstrated that EBVaGC and EBV(−)GC may have different genetic pathways: deletion of 5q and/or 17p and microsatellite instability are extremely rare in EBVaGC (8). In Burkitt lymphoma, which is also a latency I EBV-associated malignancy (22), translocation of c-myc to the immunoglobulin gene is primarily responsible for the genesis of lymphoma (1, 16, 19). We can now investigate such a mechanism in EBVaGC with KT tumors. Furthermore, we believe that KT tumors will be a useful in vivo model for various investigations related to gene therapy or immunotherapy of EBVaGC.
Acknowledgments
Y. Iwasaki and J.-M. Chong contributed equally to this work.
This work was supported by a Grant-in Aid for Scientific Research on Priority Areas (09253103) from the Ministry of Education, Science, Sports, and Culture of Japan and by the Tokyo metropolitan government.
We thank S. Imai for technical suggestions and T. Sakurai and K. Hidano for helping to prepare the manuscript.
REFERENCES
- 1.Battey J, Moulding C, Taub R, Murphy W, Stewart T, Potter H, Lenoir G, Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983;34:779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- 2.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks L A, Lear A L, Young L S, Rickinson A B. Transcripts from the Epstein-Barr virus BamHI A fragment are detectable in all three forms of virus latency. J Virol. 1993;67:3182–3190. doi: 10.1128/jvi.67.6.3182-3190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Busson P, Ganem G, Flores P, Mugneret F, Clausse B, Caillou B, Braham K, Wakasugi H, Lipinski M, Tursz T. Establishment and characterization of three transplantable EBV-containing nasopharyngeal carcinomas. Int J Cancer. 1988;42:599–606. doi: 10.1002/ijc.2910420422. [DOI] [PubMed] [Google Scholar]
- 5.Busson P, McCoy R, Sadler R, Gilligan K, Tursz T, Raab-Traub N. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992;66:3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 7.Chong J-M, Fukayama M, Hayashi Y, Funata N, Takizawa T, Koike M, Muraoka M, Kikuchi-Yanoshita R, Miyaki M, Mizuno S. Expression of CD44 variants in gastric carcinoma with or without Epstein-Barr virus. Int J Cancer. 1997;74:450–454. doi: 10.1002/(sici)1097-0215(19970822)74:4<450::aid-ijc16>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Chong J-M, Fukayama M, Hayashi Y, Takizawa T, Koike M, Konishi M, Kikuchi-Yanoshita R, Miyaki M. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 1994;54:4595–4597. [PubMed] [Google Scholar]
- 9.Fukayama M, Hayashi Y, Iwasaki Y, Chong J-M, Ooba T, Takizawa T, Koike M, Mizutani S, Miyaki M, Hirai K. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994;71:73–81. [PubMed] [Google Scholar]
- 10.Fukayama M, Hayashi Y, Ooba T, Funata N, Ibuka T, Koike M, Hebisawa A, Kurasawa A, Fukayama M, Nakahiro K, Kudoh S. Pyothorax-associated lymphoma: development of Epstein-Barr virus-associated lymphoma within the inflammatory cavity. Pathol Int. 1995;45:825–831. doi: 10.1111/j.1440-1827.1995.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukayama M, Ibuka T, Hayashi Y, Ooba T, Koike M, Mizutani S. Epstein-Barr virus in pyothorax-associated pleural lymphoma. Am J Pathol. 1993;143:1044–1049. [PMC free article] [PubMed] [Google Scholar]
- 12.Harabuchi Y, Yamanaka N, Kataura A, Imai S, Kinoshita T, Mizuno F, Osato T. Epstein-Barr virus in nasal T-cell lymphomas in patients with lethal midline granuloma. Lancet. 1991;335:128–130. doi: 10.1016/0140-6736(90)90002-m. [DOI] [PubMed] [Google Scholar]
- 13.Lear A L, Rowe M, Kurilla M G, Lee S, Henderson S, Kieff E, Rickinson A B. The Epstein-Barr virus (EBV) nuclear antigen 1 BamHI F promoter is activated on entry of EBV-transformed B cells into the lytic cycle. J Virol. 1992;66:7461–7468. doi: 10.1128/jvi.66.12.7461-7468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leoncini L, Vindigni C, Megha T, Funtó I, Pacenti L, Musaró M, Renieri A, Seri M, Anagnostopoulos J, Tosi P. Epstein-Barr virus and gastric cancer: data and unanswered questions. Int J Cancer. 1993;53:898–901. doi: 10.1002/ijc.2910530605. [DOI] [PubMed] [Google Scholar]
- 15.Lin C-T, Dee A N, Chen W, Chan W-Y. Association of Epstein-Barr virus, human papilloma virus, and cytomegalovirus with nine nasopharyngeal carcinoma cell lines. Lab Invest. 1994;71:731–736. [PubMed] [Google Scholar]
- 16.Neri A, Barriga F, Knowles D M, Magrath I T, Dalla-Favera R. Different regions of the immunoglobulin heavy-chain locus are involved in chromosomal translocations in distinct pathogenetic forms of Burkitt lymphoma. Proc Natl Acad Sci USA. 1988;85:2748–2752. doi: 10.1073/pnas.85.8.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996;70:623–627. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochiya T, Fujiyama A, Fukushige S, Hatada I, Matsubara K. Molecular cloning of an oncogene from a human hepatocellular carcinoma. Proc Natl Acad Sci USA. 1986;83:4993–4997. doi: 10.1073/pnas.83.14.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelicci P-G, Knowles II D M, Magrath I, Dalla-Favera R. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci USA. 1986;83:2984–2988. doi: 10.1073/pnas.83.9.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raab-Traub N, Flynn K, Pearson G, Huang A, Levine P, Lanier A, Pagano J. The differentiated form of nasopharyngeal carcinoma contains Epstein-Barr virus DNA. Int J Cancer. 1987;39:25–29. doi: 10.1002/ijc.2910390106. [DOI] [PubMed] [Google Scholar]
- 21.Rogers R P, Woisetschlaeger M, Speck S H. Alternative splicing dictates translational start in Epstein-Barr virus transcripts. EMBO J. 1990;9:2273–2277. doi: 10.1002/j.1460-2075.1990.tb07398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe M, Lear A L, Croom-Carter D, Davies A H, Rickinson A B. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol. 1992;66:122–131. doi: 10.1128/jvi.66.1.122-131.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sample J, Brooks L, Sample C, Young L, Rowe M, Gregory C, Rickinson A, Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci USA. 1991;88:6343–6347. doi: 10.1073/pnas.88.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaefer B C, Strominger J L, Speck S H. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer B C, Strominger J L, Speck S H. The Epstein-Barr virus BamHI F promoter is an early lytic promoter: lack of correlation with EBNA 1 gene transcription in group 1 Burkitt’s lymphoma cell lines. J Virol. 1995;69:5039–5047. doi: 10.1128/jvi.69.8.5039-5047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer B C, Woisetschlaeger M, Strominger J L, Speck S H. Exclusive expression of Epstein-Barr virus nuclear antigen 1 in Burkitt lympnoma arises from a third promoter, distinct from the promoters used in latently infected lymphocytes. Proc Natl Acad Sci USA. 1991;88:6550–6554. doi: 10.1073/pnas.88.15.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss L M. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Am J Pathol. 1991;139:469–474. [PMC free article] [PubMed] [Google Scholar]
- 28.Shibata D, Weiss L M. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol. 1992;140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 29.Shih C, Weinberg R A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982;29:161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura M, Imai S, Tokunaga M, Koizumi S, Uchizawa M, Okamoto K, Osato T. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer. 1996;74:625–631. doi: 10.1038/bjc.1996.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokunaga M, Land C E, Uemura Y, Tokudome T, Tanaka S, Sato E. Epstein-Barr virus in gastric carcinoma. Am J Pathol. 1993;143:1250–1254. [PMC free article] [PubMed] [Google Scholar]
- 32.Tokunaga M, Uemura Y, Tokudome T, Ishidate T, Masuda H, Okazaki E, Kaneko K, Naoe S, Ito M, Okamura A, Shimada A, Sato E, Land C E. Epstein-Barr virus-related gastric cancer in Japan: a molecular patho-epidemiological study. Acta Pathol Jpn. 1993;43:574–581. doi: 10.1111/j.1440-1827.1993.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 33.Weiss L M, Movahed L A, Warnke R A, Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med. 1989;320:502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997;71:5688–5691. doi: 10.1128/jvi.71.7.5688-5691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.zur Hausen H, Schulte-Holthausen H, Klein G, Henle W, Henle G, Clifford P, Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970;228:1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]