Abstract
Background:
The liver is the most common site of metastasis from gastrointestinal stromal tumors (GISTs). The authors aimed to evaluate imatinib (IM) combined with hepatic resection (HR) or other local treatments such as radiofrequency ablation (RFA) and transarterial chemoembolization (TACE), compared to IM monotherapy in long-term survival benefits in patients suffering from GIST liver metastases.
Methods:
Our research encompassed 238 patients diagnosed with liver metastases of GISTs from January 2002 to April 2022 at the First Affiliated Hospital of Sun Yat-Sen University. The oncological outcomes of concern included overall survival (OS), progression-free survival (PFS), and liver-specific PFS.
Results:
Of all 238 patients, 126 were treated with IM alone (IM group), 81 with IM combined with HR (IM+HR group), and 31 with IM combined with RFA/TACE (IM+RFA/TACE group). The median follow-up time was 44.83 months. The median OS in the IM group was 132.60 months and was not reached in either the IM+HR group or the IM+RFA/TACE group. The 10-year OS rate in the IM+HR group was significantly superior to the IM group and the IM+RFA/TACE group (91.9% vs. 61.1% vs. 55.2%, respectively, P=0.015), and the liver-specific PFS (P=0.642) and PFS (P=0.369) in the three groups showed a beneficial trend in the combined treatment group. Multivariate analyses showed that age less than or equal to 60 years (HR 0.280, P<0.001) and IM+HR (HR 0.361, P=0.047) were independently associated with better OS. Achieving no evidence of disease through surgical intervention was independently correlated with enhanced OS (HR 0.099, P=0.034), liver-specific PFS (HR 0.388, P=0.014), and PFS (HR 0.402, P=0.004).
Conclusions:
In patients with GIST liver metastases, IM combined with HR might improve OS in selected patients compared with IM alone and IM combined with RFA/TACE. Achieving no evidence of disease status with surgical treatment of patients results in significant prolonging of OS, liver-specific PFS, and PFS.
Keywords: gastrointestinal stromal tumor, imatinib, liver metastases, surgery, survival
Introduction
Highlights
In patients with gastrointestinal stromal tumors liver metastases, imatinib (IM) combined with hepatic resection might improve overall survival compared with IM alone and IM combined with radiofrequency ablation or transarterial chemoembolization in selected patients.
Achieving no evidence of disease after surgical treatment is independently related to better overall survival, progression-free survival, and liver-specific progression-free survival in patients with gastrointestinal stromal tumors liver metastases.
Gastrointestinal stromal tumors (GISTs) represent the most prevalent type of mesenchymal tumor within the gastrointestinal tract, potentially originating from mutations in precursor cells typically responsible for the development of Cajal mesenchymal cells1,2. Approximately 15–50% of GISTs are diagnosed with metastatic disease, predominantly in the liver, accounting for 65% of all metastatic cases3,4. Treatment with imatinib (IM), a tyrosine kinase inhibitor (TKI) of KIT and PDGFRA, has significantly enhanced clinical outcomes for patients with advanced disease. Since its approval in February 2002 for treating metastatic or unresectable GISTs, 38% of patients have shown a partial response (PR), while 13.6% have experienced disease progression within 1–3 months of IM administration. Over half of the patients with metastases experienced disease progression within 2 years of IM treatment, attributed to secondary drug resistance5. However, the effectiveness of the second-line TKI, Sunitinib, following IM resistance is limited, yielding a median progression-free survival (PFS) of merely 5.6 months6.
Reducing tumor burden may prolong the duration of response to IM by reducing secondary drug resistance. Doctors in many centers have therefore started to combine surgical treatment [such as hepatic resection (HR) and locoregional therapies, such as radiofrequency ablation (RFA) and transarterial chemoembolization (TACE)] with IM in patients with GIST liver metastases7–10. However, to our knowledge, the choice of surgical therapy for GIST liver metastases is still controversial. Several retrospective studies have demonstrated that hepatectomy prolongs overall survival (OS) compared to TKI therapy alone8,11,12. Small sample sizes of retrospective studies have reported that RFA provides OS comparable to HR13. Another study found that TACE is a safe and effective treatment for GIST patients with liver metastases following TKI failure10. However, there are few studies comparing the survival benefits of different surgical modalities. Moreover, the timing of surgical therapy during IM therapy remains unclear; some scholars have proposed that patients with limited progression or stable disease treated with TKIs could benefit from surgical therapy14, while others have found that even patients with progressive disease could benefit from debulking therapy when a complete resection is performed15.
As far as we know, there is no study comparing the efficacy of IM combined with HR or RFA/TACE and IM alone in patients with GIST liver metastasis. Therefore, in our study, we aimed to assess the long-term survival benefits of a combination of different surgical treatments with IM versus IM alone in a large and relatively homogeneous cohort of patients with liver metastases from GISTs.
Materials and methods
Study design and treatment
This was a retrospective analysis, utilizing data gathered from the First Affiliated Hospital of Sun Yat-Sen University over a span of two decades, from January 2002 to April 2022.
The inclusion criteria were as follows: (1) pathological evidence of GIST of primary tumors; (2) liver metastases evidenced by biopsy or radiological findings; and (3) sufficient liver, hematologic, and renal function, coupled with an Eastern Cooperative Oncology Group performance status score ranging from 0 to 1. The exclusion criteria comprised: (1) IM was not used during treatment; (2) liver metastases appeared on second-line or later subsequent lines of TKI; (3) combined with other malignant tumors; and (4) failure to follow-up. The resolution to integrate surgical therapy was reached by a multidisciplinary consortium, encompassing surgeons, pathologists, medical oncologists, radiologists, nuclear medicine specialists and gastroenterologists.
The study protocol received approval from the ethical committees of the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China on 13 July 2023 [approval number: (2023) 352], adhering to the standards set forth in the 1975 Declaration of Helsinki. This study has been registered at clinicaltrials.gov (unique identification number: NCT06038552) and strictly followed Strengthening the reporting of cohort studies in surgery (STROCSS) criteria16 (Supplemental Digital Content 1, http://links.lww.com/JS9/B699).
Data collection
Comprehensive demographic and clinicopathological data were collected. Liver metastasis was demarcated as synchronous (identified concomitantly or within 6 months postprimary tumor diagnosis) or metachronous (post 6 months from primary tumor diagnosis)17. The efficacy of the IM regimen prior to surgical intervention was gauged using the Response Evaluation Criteria in Solid Tumors (RECIST)18. Limited progression was defined as progression limited to a single organ and within two lesions, and extensive progression was defined as the progression of more than two lesions in a single organ or of lesions in more than one organ. ‘Not applicable (NA)’ was used in reference to IM response evaluation not being applicable in cases where surgical treatment was performed before IM application. The purposes of surgical treatment were classified as no evidence of disease (NED) and non-NED. NED was defined as the resection or ablation of all macroscopic hepatic lesions and extrahepatic lesions. Since genetic mutation testing was not available until 2010, 38.66% (92/238) of patients in this study lacked genetic mutation information.
Treatment protocol
Patients with GIST liver metastases were administered an initial IM dose of 400 mg daily, while those with KIT exon nine mutations received 600–800 mg daily. Patient surveillance, involving abdominal contrast-enhanced computed tomography (CT) scans or MRI, was undertaken every 3–12 months. All patients continued IM indefinitely unless progression or intolerable adverse reactions. The patients scheduled to receive HR discontinued the use of IM 2–14 days before elective surgery and continued after recovery from surgery.
Surgical treatment
HR
HR was carried out under the administration of general anesthesia and performed by a surgeon with 10 years of experience in gastrointestinal surgery. The determination of the surgical modality was established subsequent to comprehensive consultations tailored to each patient within the Department of Liver Surgery. The selection between anatomic and nonanatomic resection was guided by an assessment of the patient’s tumor burden and hepatic function. The surgical strategy was established in consideration of factors such as the residual liver volume, tumor positioning, and the surgeon’s personal preference. Intraoperative US was routinely used to aid in operative evaluation.
RFA
RFA procedures were conducted by an accomplished interventional radiologist boasting no less than a decade of specialized experience. RFA was executed utilizing the commercially accessible Cool-tip RFA system (Valleylab) or the RF 2000 system (Radio-Therapeutics Mountain View). The electrode was percutaneously inserted through a guide needle under real-time ultrasound guidance. The primary objective of the RFA procedures encompassed the complete elimination of the tumor entity, with a prescribed ablative margin of 0.5 cm meticulously factored in. For tumors larger than 3 cm, a series of systematically overlapping ablations were performed. Subsequent to the RFA, the needle track was coagulated to mitigate potential bleeding and inadvertent tumor dissemination.
TACE
TACE was meticulously conducted by seasoned interventional radiologists. Under a comprehensive assessment of the hepatic arterial blood supply, a selective catheter was introduced into the segmental or subsegmental arteries that supplied the tumor. The regimen of hepatic arterial infusion chemotherapy included an infusion of carboplatin 300 mg (Bristol-Myers Squibb), a mixture of 50 mg of epirubicin (Pharmourubicin, Pfizer) and 8 mg of mitomycin C (Zhejiang HI sun Pharmaceutical Co. Ltd.), intimately blended with 5 ml of lipiodol (Lipiodol Ultra-Fluid; Andre’ Guerbet Laboratories). Embolization was finally performed with either absorbable gelatin sponge particles (Geofoam; Hanzhou Alc Ltd, 1–2 mm in diameter) or polyvinyl alcohol particles (Alicen Pharm SCT&TEC CO., LTD., 350–560 μm in diameter). Following embolization, an angiographic assessment was performed to evaluate of the extent of vascular occlusion and the status of blood flow within other arterial vessels.
Definitions
OS was the span between the GIST liver metastases diagnosis and the date of death.
Liver-specific PFS within the IM group was the span between GIST liver metastases diagnosis and intrahepatic lesion progression, while in the combined therapy group, it spanned surgical intervention to intrahepatic lesion progression. PFS in the IM group was the span between GIST liver metastases diagnosis and disease progression, whereas in the combined therapy group, it spanned surgical intervention to disease progression.
Statistical analysis
Continuous variables were delineated as median values, assessed via the Mann–Whitney U test. Categorical variables, depicted as numerals and percentages, were compared using the χ² test. Survival curves were charted using the Kaplan–Meier paradigm and appraised with the log-rank test. Cox proportional hazard regression models were used to evaluate the prognostic relevance of potential survival predictors. The multivariable analysis included variables with P-values less than 0.10 on univariable analysis.
Propensity score matching (PSM) was applied in this study to diminish the selection bias. Participants were harmonized based on a logistic regression framework, matched in a 1:1:1 ratio utilizing a 0.1 caliper19. The matching variables included sex, age, primary site, metastatic phase, extrahepatic metastases, number of liver metastases and maximum tumor diameter.
A two-sided P value less than 0.05 indicated statistical significance. Statistical computations were facilitated using R (version 4.1.1) and SPSS 24.0.
Results
Patient characteristics
From January 2002 to April 2022, 330 consecutive patients were diagnosed with GIST liver metastases. Finally, a total of 238 patients were enrolled in our study (Fig. 1). Table 1 encapsulates the baseline characteristics of the patients, segregated into different treatment categories: IM therapy (n=126), IM combined with HR (n=81) and IM combined with RFA/TACE (n=31). There were 158 males (66.39%) and 80 females (33.61%). Seventy-four patients (31.09%) were over 60 years old. The primary tumor sites were distributed as follows: stomach (n=91, 38.24%), small intestine (n=134, 56.30%), colorectum (n=5, 2.10%), and other sites (n=8, 3.36%). There were 98 (41.18%) patients with synchronous liver metastases. The number of liver metastases was more than 3 in 133 (55.88%) patients, and the proportion was significantly lower in the combination therapy group (P<0.001). One hundred sixteen (48.74%) patients had liver metastases with a maximum diameter larger than 3 cm. Eighty-one (34.03%) patients had extrahepatic metastases. The most common driver mutation was KIT (90.41%, 132/146), and 92 (38.66%) patients did not have an information mutation. The evaluation of IM response before surgical treatment showed PR in nine patients (8.04%), stable disease in 34 patients (30.36%), extensive progress in six patients (5.36%), and limited progress in 27 patients (24.11%); 36 patients (32.14%) were not eligible for evaluation because surgical treatment was performed before the IM application. Of the 112 patients who received combination therapy, 60 (53.57%) achieved NED status after surgical therapy, and 52 (46.43%) did not. When contrasting the IM+RFA/TACE group with the IM+HR group, a significantly higher percentage of NED was observed in the latter (30.48 vs. 60.49%, P=0.018).
Figure 1.

Flowchart of the patients included in this study. GIST, gastrointestinal stromal tumors; HR, hepatic resection; IM, imatinib; RFA, radiofrequency ablation; TKI, tyrosine kinase inhibitor; TACE, transarterial chemoembolization.
Table 1.
Baseline clinical characteristics of all patients.
| Parameters | Total (n=238) | IM (n=126) | IM+HR (n=81) | IM+RFA/ TACE (n=31) | P | SMD |
|---|---|---|---|---|---|---|
| Age (years) | 0.297 | 0.150 | ||||
| ≤60 | 164 (68.91) | 82 (65.08) | 61 (75.31) | 21 (67.74) | ||
| >60 | 74 (31.09) | 44 (34.92) | 20 (24.69) | 10 (32.26) | ||
| Sex | 0.799 | 0.092 | ||||
| Female | 80 (33.61) | 42 (33.33) | 26 (32.10) | 12 (38.71) | ||
| Male | 158 (66.39) | 84 (66.67) | 55 (67.90) | 19 (61.29) | ||
| Primary sites | 0.496 | 0.239 | ||||
| Stomach | 91 (38.24) | 43 (34.13) | 32 (39.51) | 16 (51.61) | ||
| Small intestine | 134 (56.30) | 76 (60.32) | 45 (55.56) | 13 (41.94) | ||
| Colorectum | 5 (2.10) | 3 (2.38) | 2 (2.47) | 0 (0.00) | ||
| Others | 8 (3.36) | 4 (3.17) | 2 (2.47) | 2 (6.45) | ||
| Metastatic phase | 0.064 | 0.209 | ||||
| Synchronous | 98 (41.18) | 43 (34.13) | 40 (49.38) | 15 (48.39) | ||
| Metachronous | 140 (58.82) | 83 (65.87) | 41 (50.62) | 16 (51.61) | ||
| Number of metastases | <0.001 | 0.518 | ||||
| ≤3 | 105 (44.12) | 35 (27.78) | 52 (64.20) | 18 (58.06) | ||
| >3 | 133 (55.88) | 91 (72.22) | 29 (35.80) | 13 (41.94) | ||
| Largest diameter of metastases (cm) | 0.313 | 0.211 | ||||
| ≤3 | 122 (51.26) | 66 (52.38) | 37 (45.68) | 19 (61.29) | ||
| >3 | 116 (48.74) | 60 (47.62) | 44 (54.32) | 12 (38.71) | ||
| Extrahepatic metastases | 0.687 | 0.080 | ||||
| No | 157 (65.97) | 80 (63.49) | 56 (69.14) | 21 (67.74) | ||
| Yes | 81 (34.03) | 46 (36.51) | 25 (30.86) | 10 (32.26) | ||
| Gene mutation | 0.806 | 0.131 | ||||
| KIT_exon 9 | 27 (11.34) | 14 (11.11) | 8 (9.88) | 5 (16.13) | ||
| KIT_exon 11 | 105 (44.12) | 51 (40.48) | 40 (49.38) | 14 (45.16) | ||
| Wild type | 10 (4.20) | 6 (4.76) | 4 (4.94) | 0 (0.00) | ||
| Others | 4 (1.68) | 2 (1.59) | 1 (1.23) | 1 (3.23) | ||
| Unknown | 92 (38.66) | 53 (42.06) | 28 (34.57) | 11 (35.48) | ||
| IM response before surgical treatmenta | 0.615 | 0.180 | ||||
| PR | 9 (8.04) | — | 8 (9.88) | 1 (3.23) | ||
| SD | 34 (30.36) | — | 23 (28.40) | 11 (35.48) | ||
| Extensive progression | 6 (5.36) | — | 4 (4.94) | 2 (6.45) | ||
| Limited progression | 27 (24.11) | — | 18 (22.22) | 9 (29.03) | ||
| NA | 36 (32.14) | — | 28 (34.57) | 8 (25.81) | ||
| Status after surgical treatmenta | 0.018 | 0.517 | ||||
| NED | 60 (53.57) | — | 49 (60.49) | 11 (35.48) | ||
| non-NED | 52 (46.43) | — | 32 (39.51) | 20 (64.52) | ||
| Combined with other local therapy | — | — | ||||
| No | — | — | 63 (77.78) | — | ||
| RFA | — | — | 15 (18.52) | — | ||
| TACE | — | — | 2 (2.47) | — | ||
| RFA+TACE | — | — | 1 (1.23) | — |
Values are presented as n (%).
P-values were calculated using a two-sided χ2 test.
Suitable for surgical treatment group (n=112).
HR, hepatic resection; IM, imatinib; NA, not applicable; NED, no evidence of disease; PR, partial response; RFA, radiofrequency ablation; SD, stable disease; SMD, standard mean difference; TACE, transarterial chemoembolization.
Survival analysis
The median duration of follow-up was 44.83 months, ranging from 2.40 to 187.63 months. At the time of last follow-up, 24 patients (19.05%) in the IM group, five patients (6.17%) in the IM combined with HR group, and six patients (19.35%) in the IM combined with RFA/TACE group died from tumor progression. The 10-year OS rate was higher in the IM combined with HR group than in the IM group and the IM combined with RFA/TACE group (91.9% vs. 61.1% vs. 55.2%, respectively, P=0.015, Fig. 2A), while there was no significant difference between the IM group and the IM combined with RFA/TACE group (P=0.784, Fig. 2A). The liver-specific PFS (P=0.642) and PFS (P=0.369) in the three groups showed a beneficial trend in the combined treatment group (Fig. 2B, C). In the surgical treatment group, 36 patients received IM therapy after surgical treatment, and 76 patients received surgical treatment after IM application. However, no significant differences were observed in terms of OS (P=0.988), PFS (P=0.158), and liver-specific PFS (P=0.278) between these two subgroups (Fig. 3A–C).
Figure 2.
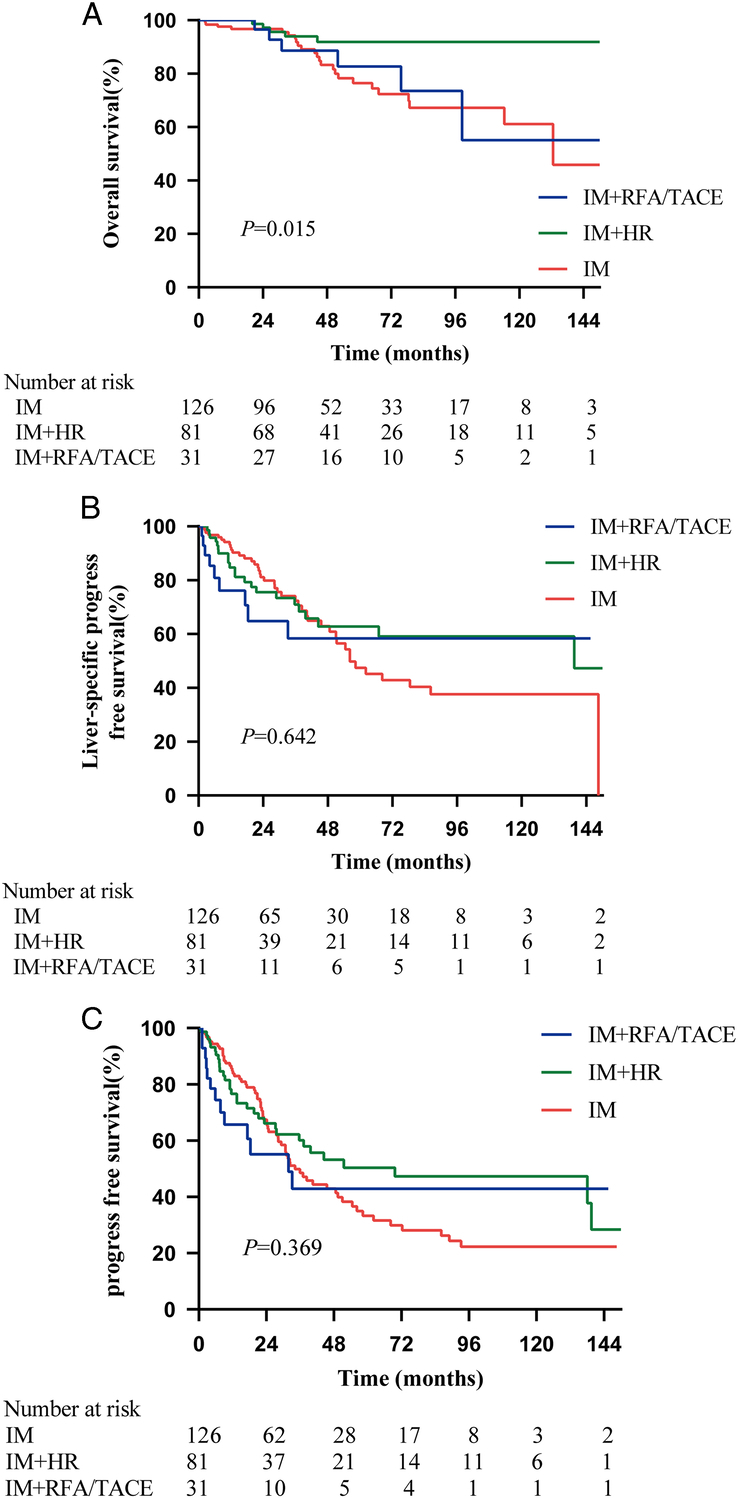
Kaplan–Meier survival curves comparing OS, liver-specific PFS and PFS in the IM, IM+HR, and IM+RFA/TACE groups, (A) OS, IM+HR vs. IM (HR (95% CI): 0.269 (0.129–0.560), P=0.004); IM+HR vs. IM+RFA/TACE (HR (95% CI): 0.310 (0.082–1.170), P=0.040); IM vs. IM+RFA/TACE (HR (95% CI): 1.133 (0.478–2,685), P=0.784); (B) liver-specific PFS, IM+HR vs. IM (HR (95% CI): 0.816 (0.497–1.339), P=0.422); IM+HR vs. IM+RFA/TACE (HR (95% CI): 0.736 (0.321–1.691), P=0.432); IM vs. IM+RFA/TACE (HR (95% CI): 0.880 (0.415–1.866), P=0.727); (C) PFS, IM+HR vs. IM (HR (95% CI): 0.755 (0.504–1.132), P=0.186); IM+HR vs. IM+RFA/TACE (HR (95% CT): 0.713 (0.354–1.436), P=0.300); IM vs. IM+RFA/TACE (HR (95% CI): 0.911 (0.493–1.682), P=0.757). HR, hepatic resection; HR, hazard ratio; IM, imatinib; OS, overall survival; PFS, progression-free survival; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Figure 3.

Kaplan–Meier survival curves comparing OS, liver-specific PFS and PFS in the surgical treatment before IM therapy group and surgical treatment after IM therapy group, (A) OS, HR (95% CI): 1.009 (0.295–3.453), P=0.988; (B) liver-specific PFS, HR (95% CI): 0.684 (0.342–1.370), P=0.278; (C) PFS, HR (95% CI): 0.652 (0.363–1.172), P=0.158. HR, hazard ratio; IM, imatinib; OS, overall survival; PFS, progression-free survival.
On multivariable analysis in the three groups, age (≤60 years, HR 0.280, P<0.001) and HR combined with IM (HR 0.361, P=0.047) emerged as significant prognostic factors for OS (Table 2). Age (≤60 years, HR 0.516, P=0.007) and the number of liver metastases (≤3, HR 0.530, P=0.017) were independently associated with better liver-specific PFS (Table S1, Supplemental Digital Content 2, http://links.lww.com/JS9/B700). Age (≤60 years, HR 0.572, P=0.006), the number of liver metastases (≤3, HR 0.466, P=0.001) and the absence of extrahepatic metastases (HR 0.522, P=0.001) were all independent factors associated with better PFS (Table S2, Supplemental Digital Content 2, http://links.lww.com/JS9/B700).
Table 2.
Prognostic factors for overall survival in the three groups.
| Variables | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|
| Sex (male vs. female) | 1.587 (0.743–3.390) | 0.233 | ||
| Age (≤60 vs. >60 years) | 0.275 (0.140–0.541) | <0.001 | 0.280 (0.142–0.555) | <0.001 |
| Primary sites (stomach vs. others) | 0.984 (0.489–1.983) | 0.965 | ||
| Metastatic phase (metachronous vs. synchronous) | 0.684 (0.352–1.328) | 0.262 | ||
| Number of metastases (≤3 vs. >3) | 0.431 (0.202–0.920) | 0.030 | 0.509 (0.229–1.131) | 0.098 |
| Largest diameter of metastases (≤3 vs. >3 cm) | 1.263 (0.649–2.458) | 0.492 | ||
| Extrahepatic metastases (no vs. yes) | 0.661 (0.332–1.319) | 0.240 | ||
| Therapy | ||||
| IM alone | Reference | – | Reference | – |
| IM+HR | 0.264 (0.100–0.696) | 0.007 | 0.361 (0.132–0.985) | 0.047 |
| IM+RFA/TACE | 0.887 (0.362–2.173) | 0.794 | 1.016 (0.404–2.551) | 0.974 |
HR, hazard ratio.
HR, hepatic resection; IM, imatinib; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Comparison of the IM+HR and IM+RFA/TACE groups
We would like to further investigate the effect of IM response before surgical treatment and the purpose of surgical treatment on prognosis. Multivariable analysis was performed in the IM+HR group and IM+RFA group and showed that NED was related to better OS (HR 0.099, P=0.034), liver-specific PFS (HR 0.388, P=0.014), and PFS (HR 0.402, P=0.004) (Tables 3, S3–4). IM response before surgical treatment was not an independent prognostic factor of OS, liver-specific PFS, or PFS (Tables 3, S3–4). Moreover, a number of liver metastases less than or equal to 3 was identified as a significant prognostic factor for both liver-specific PFS (HR 0.347, P=0.004) and PFS (HR 0.318, P<0.001) (Tables S3–4, Supplemental Digital Content 2, http://links.lww.com/JS9/B700). The absence of extrahepatic metastases was independent factors associated with better PFS (HR 0.479, P=0.018) (Table S4, Supplemental Digital Content 2, http://links.lww.com/JS9/B700). Patients treated with IM+HR had significantly better OS than those treated with IM+RFA/TACE (HR 0.310, P=0.040), but there was no significant difference in liver-specific PFS (HR 0.736, P=0.432) or PFS (HR 0.713, P=0.300) (Fig. 2A–C).
Table 3.
Prognostic factors for overall survival in the imatinib combined with hepatic resection group (IM+HR) and imatinib combined with radiofrequency ablation or transarterial chemoembolization group (IM+RFA/TACE).
| Variables | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|
| Sex (male vs. female) | 1.235 (0.327–4.658) | 0.756 | ||
| Age (≤60 vs. >60 years) | 0.234 (0.070–0.783) | 0.018 | 0.395 (0.107–1.451) | 0.162 |
| Primary Sites (stomach vs. others) | 2.077 (0.631–6.838) | 0.239 | ||
| Metastatic phase (metachronous vs. synchronous) | 0.778 (0.237–2.552) | 0.678 | ||
| Number of Metastases (≤3 vs. >3) | 0.511 (0.156–1.674) | 0.267 | ||
| Largest diameter of metastases (≤3 vs. >3 cm) | 1.305 (0.398–4.281) | 0.661 | ||
| Extrahepatic metastases (no vs. yes) | 0.358 (0.109–1.176) | 0.090 | 0.538 (0.148–1.955) | 0.346 |
| IM response before surgical treatment (PR+SD+NA vs. PD) | 0.540 (0.164–1.772) | 0.310 | ||
| Status after surgical treatment (NED vs. non-NED) | 0.065 (0.008–0.511) | 0.009 | 0.099 (0.012–0.835) | 0.034 |
| Therapy (IM+HR vs. IM+RFA/TACE) | 0.308 (0.094–1.012) | 0.052 | 0.625 (0.184–2.119) | 0.450 |
HR, hazard ratio.
NA, not applicable; NED, no evidence of disease; PD, progressive disease; PR, partial response; SD, stable disease.
Survival analysis after PSM
To minimize the impact of selection bias, sex, age, primary site, metastatic phase, extrahepatic metastases, number of liver metastases and maximum tumor diameter were evaluated at a 1:1:1 ratio. Following PSM, the baseline characteristics of the 81 patients in the three treatment groups were summarized in Table 4. The 10-year OS rate exhibited an upward trend but did not reach statistical significance in the IM+HR group compared to the IM group and IM+RFA/TACE group (93.3% vs 63.0% vs 55.2%, P=0.187, Fig. 4A). The liver-specific PFS (P=0.497) and PFS (P=0.174) in the three groups were not significantly different (Fig. 4B, C). On multivariable analysis in the three groups, age (≤60 years, HR 0.155, P=0.004) emerged as a significant prognostic factor for OS (Table 5). Age (≤60 years, HR 0.360, P=0.012) and the number of liver metastases (≤3, HR 0.376, P=0.015) were independently associated with better PFS but not liver-specific PFS (Tables S5–6, Supplemental Digital Content 2, http://links.lww.com/JS9/B700). In comparing the IM+HR group with the IM+RFA/TACE group after PSM, multivariate analysis indicated that NED (HR 0.264, P=0.034) was an independent factor for liver-specific PFS but not for OS or PFS. The surgical treatment strategy and IM response before surgical treatment were not significant prognostic factors (Tables 6, S7–8).
Table 4.
Baseline clinical characteristics of the three groups after propensity score matching.
| Parameters | Total (n=81) | IM (n=27) | IM+HR (n=27) | IM+RFA/ TACE (n=27) | P | SMD |
|---|---|---|---|---|---|---|
| Age (years) | 0.934 | 0.058 | ||||
| ≤60 | 62 (76.54) | 20 (74.07) | 21 (77.78) | 21 (77.78) | ||
| >60 | 19 (23.46) | 7 (25.93) | 6 (22.22) | 6 (22.22) | ||
| Sex | 0.354 | 0.262 | ||||
| Female | 28 (34.57) | 7 (25.93) | 9 (33.33) | 12 (44.44) | ||
| Male | 53 (65.43) | 20 (74.07) | 18 (66.67) | 15 (55.56) | ||
| Primary sites | 0.663 | 0.050 | ||||
| Stomach | 37 (45.68) | 13 (48.15) | 12 (44.44) | 12 (44.44) | ||
| Small intestine | 40 (49.38) | 13 (48.15) | 14 (51.85) | 13 (48.15) | ||
| Colorectum | 1 (1.23) | 0 (0.00) | 1 (3.70) | 0 (0.00) | ||
| Others | 3 (3.70) | 1 (3.70) | 0 (0.00) | 2 (7.41) | ||
| Metastatic phase | 1.000 | <0.001 | ||||
| Synchronous | 45 (55.56) | 15 (55.56) | 15 (55.56) | 15 (55.56) | ||
| Metachronous | 36 (44.44) | 12 (44.44) | 12 (44.44) | 12 (44.44) | ||
| Number of metastases | 1.000 | <0.001 | ||||
| ≤3 | 42 (51.85) | 14 (51.85) | 14 (51.85) | 14 (51.85) | ||
| >3 | 39 (48.15) | 13 (48.15) | 13 (48.15) | 13 (48.15) | ||
| Largest diameter of metastases (cm) | 0.951 | 0.050 | ||||
| ≤3 | 44 (54.32) | 14 (51.85) | 15 (55.56) | 15 (55.56) | ||
| >3 | 37 (45.68) | 13 (48.15) | 12 (44.44) | 12 (44.44) | ||
| Extrahepatic metastases | 0.479 | 0.186 | ||||
| No | 66 (81.48) | 23 (85.19) | 23 (85.19) | 20 (74.07) | ||
| Yes | 15 (18.52) | 4 (14.81) | 4 (14.81) | 7 (25.93) | ||
| Gene mutation | 0.351 | 0.100 | ||||
| KIT_exon 9 | 12 (14.81) | 4 (14.81) | 3 (11.11) | 5 (18.52) | ||
| KIT_exon 11 | 33 (40.74) | 11 (40.74) | 11 (40.74) | 11 (40.74) | ||
| Wild type | 3 (3.70) | 0 (0.00) | 3 (11.11) | 0 (0.00) | ||
| Others | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Unknown | 33 (40.74) | 12 (44.44) | 10 (37.04) | 11 (40.74) | ||
| IM response before surgical treatmenta | 0.511 | 0.083 | ||||
| PR | 5 (9.26) | — | 4 (14.81) | 1 (3.70) | ||
| SD | 20 (37.04) | — | 9 (33.33) | 11 (40.74) | ||
| Extensive progression | 1 (1.85) | — | 1 (3.70) | 0 (0.00) | ||
| Limited progression | 14 (27.45) | — | 6 (22.22) | 8 (29.63) | ||
| NA | 14 (27.45) | — | 7 (25.93) | 7 (25.93) | ||
| Status after surgical treatmenta | 0.056 | 0.538 | ||||
| NED | 29 (53.70) | — | 18 (66.67) | 11 (40.74) | ||
| non-NED | 25 (46.30) | — | 9 (33.33) | 16 (59.26) | ||
| Combined with other local therapy | — | — | ||||
| No | — | — | 19 (70.37) | — | ||
| RFA | — | — | 5 (18.52) | — | ||
| TACE | — | — | 2 (7.41) | — | ||
| RFA+TACE | — | — | 1 (3.70) | — |
Values are presented as n (%).
P-values were calculated using a two-sided χ2 test.
Suitable for surgical treatment group (n=112).
HR, hepatic resection; IM, imatinib; NA, not applicable; NED, no evidence of disease; PR, partial response; RFA, radiofrequency ablation; SD, stable disease; SMD, standard mean difference; TACE, transarterial chemoembolization.
Figure 4.
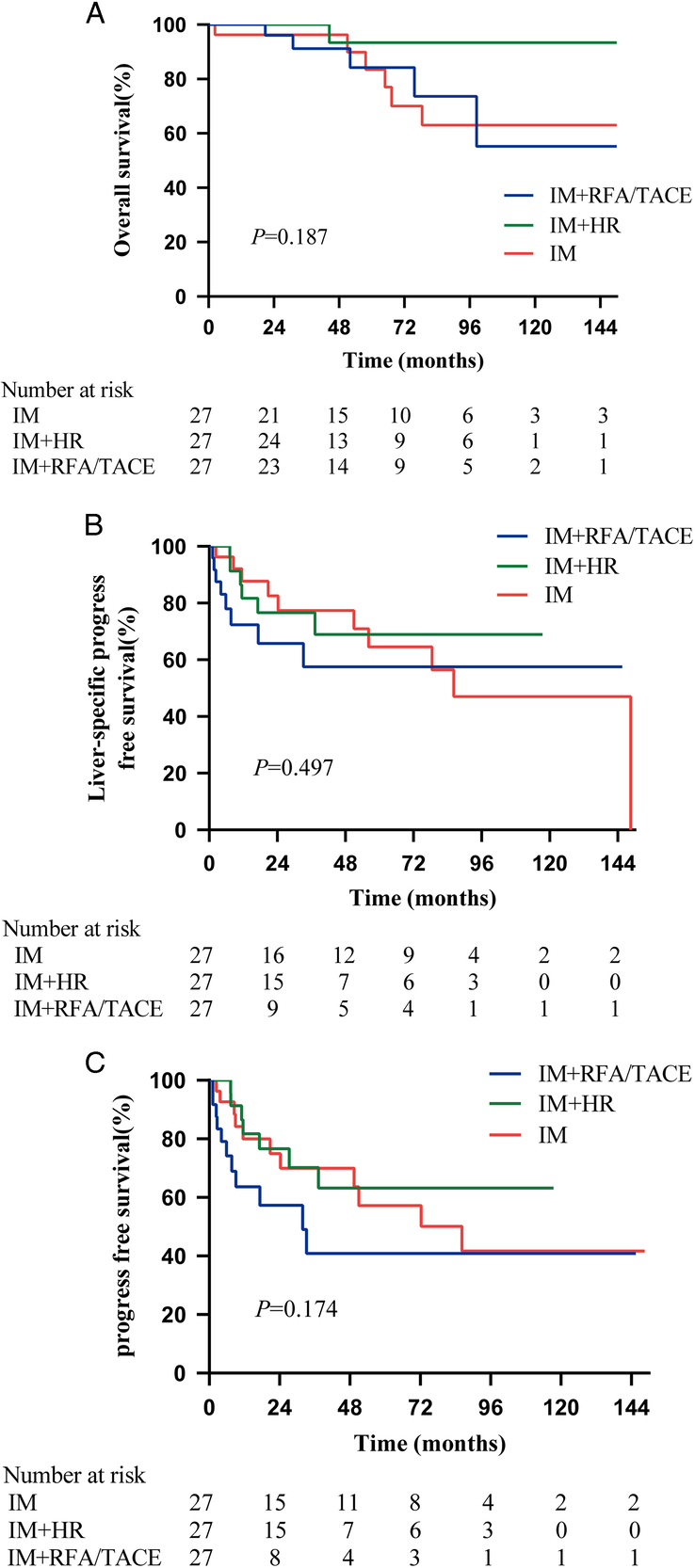
Kaplan–Meier survival curves comparing OS, liver-specific PFS and PFS in the IM, IM+HR and IM+RFA/TACE groups after PSM. (A) OS, IM+HR vs. IM (HR (95% CI): 0.190 (0.043–0.838), P=0.085); IM+HR vs. IM+RFA/TACE (HR (95% CI): 0.183 (0.037–0.906), P=0.080); IM vs. IM+RFA/TACE (HR (95% CI): 1.041 (0.318–3.404), P=0.947); (B) liver-specific PFS, IM+HR vs. IM (HR (95% CI): 0.812 (0.301–2.188), P=0.677); IM+HR vs. IM+RFA/TACE (HR (95% CI): 0.556 (0.193–1.603), P=0.269); IM vs. IM+RFA/TACE (HR (95% CI): 0.707 (0.269–1.844), P=0.447); (C) PFS, IM+HR vs. IM (HR (95% CI): 0.717 (0.284–1.810), P=0.489); IM+HR vs. IM+RFA/TACE (HR (95% CI): 0441 (0.172–1.128), P=0.080); IM vs. IM+RFA/TACE (HR (95% CI): 0.611 (0.258–1.445), P=0.238). HR, hazard ratio; HR, hepatic resection; IM, imatinib; OS, overall survival; PFS, progression-free survival; PSM, propensity score matching; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Table 5.
Prognostic factors for overall survival in the three groups after propensity score matching.
| Variables | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|
| Sex (male vs. female) | 1.947 (0.526–7.208) | 0.319 | ||
| Age (≤60 vs. >60 years) | 0.118 (0.034–0.409) | 0.001 | 0.155 (0.044–0.551) | 0.004 |
| Primary Sites (stomach vs. others) | 1.186 (0.375–3.752) | 0.772 | ||
| Metastatic phase (metachronous vs. synchronous) | 0.360 (0.096–1.347) | 0.129 | ||
| Number of Metastases (≤3 vs. >3) | 0.353 (0.095–1.306) | 0.119 | ||
| Largest diameter of metastases (≤3 vs. >3 cm) | 0.695 (0.220–2.191) | 0.534 | ||
| Extrahepatic metastases (no vs. yes) | 0.272 (0.086–0.860) | 0.027 | 0.442 (0.130–1.511) | 0.193 |
| Therapy | ||||
| IM alone | Reference | – | Reference | – |
| IM+HR | 0.180 (0.022–1.495) | 0.112 | 0.264 (0.030–2.328) | 0.230 |
| IM+RFA/TACE | 0.965 (0.294–3.173) | 0.953 | 1.022 (0.302–3.460) | 0.972 |
HR, hazard ratio.
HR, hepatic resection; IM, imatinib; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Table 6.
Prognostic factors for overall survival in the imatinib combined with hepatic resection group (IM+HR) and imatinib combined with radiofrequency ablation or transarterial chemoembolization group (IM+RFA/TACE) after propensity score matching.
| Variables | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|
| Sex (male vs. female) | 1.077 (0.197–5.902) | 0.932 | ||
| Age (≤60 vs. >60 years) | 0.156 (0.025–0.954) | 0.044 | 0.119 (0.017–0.856) | 0.035 |
| Primary Sites (stomach vs. others) | 1.032 (0.328–8.123) | 0.550 | ||
| Metastatic phase (metachronous vs. synchronous) | 0.466 (0.084–2.602) | 0.384 | ||
| Number of Metastases (≤3 vs. >3) | 0.199 (0.023–1.709) | 0.141 | ||
| Largest diameter of metastases (≤3 vs. >3 cm) | 0.802 (0.162–3.979) | 0.781 | ||
| Extrahepatic metastases (no vs. yes) | 0.377 (0.068–2.081) | 0.263 | ||
| IM response before surgical treatment (PR+SD+NA vs. PD) | 0.476 (0.095–2.388) | 0.367 | ||
| Status after surgical treatment (NED vs. non-NED) | 0.150 (0.017–1.295) | 0.084 | 0.122 (0.013–1.136) | 0.065 |
| Therapy (IM+HR vs. IM+RFA/TACE) | 0.182 (0.021–1.560) | 0.120 |
HR, hazard ratio.
NA, not applicable; NED, no evidence of disease; PD, progressive disease; PR, partial response; SD, stable disease.
Discussion
In our study, we investigated the efficacy of surgical treatments in GIST liver metastases and found that the OS of IM combined with HR was better than that of IM combined with RFA/TACE and IM alone. Age, HR, and NED status after surgical treatment were predictors of OS. After PSM, compared with IM and IM combined with RFA/TACE, IM combined with HR showed a better OS trend but no significant difference, which may have been caused by missing too many cases during the matching analysis process.
The extension of patient survival with IM in metastatic GIST is well established, while the problem of disease progression after drug resistance is almost inevitable with the increasing duration of targeted drug therapy20. Elimination or reduction of drug-resistant lesions may improve survival, and several retrospective studies in small samples have shown that resection combined with IM was associated with a good prognosis for patients with GIST metastatic disease, especially complete resection of residual metastases11. Xue et al.8 revealed that patients received TKI combined with hepatectomy had a higher not statistically different 5-year survival rate compared with TKI alone (91.5 vs. 78.3%, P=0.083). However, there was great heterogeneity in previous studies; for example, hepatectomy was not strictly limited to the duration of IM treatment, and information on lesion response to IM before surgery was lacking8,12. Unlike previous studies, we followed a standardized treatment protocol, involving IM therapy before or after HR, and evaluated the IM response before surgical treatment. Our study showed that a substantial 30.8% improvement in the 10-year OS rate with IM+HR compared to IM alone (91.9 vs. 61.1%, P=0.015). Age less than or equal to 60 years and NED status after surgical treatment were independently associated with OS. Therefore, we advocate that resectability of all metastatic lesions, including extrahepatic metastases, should be a necessary condition for surgical decision-making for GIST liver metastases.
In the present study, we could not conclude that there is a relationship between prognosis and the IM response before surgical treatment. This may be related to poor compliance and long IM withdrawal times before or after surgery in some patients. From previous literature reports, surgical procedures are recommended in cases of PR, stable, or limited progression of TKI11,14.
Clinicopathological data from 249 patients with advanced GIST was analyzed in a Korean retrospective study to determine whether surgical treatment prior to IM therapy improved outcomes. The results showed that patients with advanced GIST who received R0/1 resection and then received IM treatment regularly did not have any significant difference in PFS and OS compared with patients in whom IM was applied before surgery, although there was a trend of improvement in PFS and OS21. Our study similarly compared outcomes in GIST liver metastases patients who underwent surgery after IM therapy versus those who received IM after surgery. The results showed no significant differences in OS, PFS, and liver-specific PFS between the two groups.
RFA or TACE may be considered for some patients who have fewer lesions or small-diameter tumors. Prior small studies have suggested these approaches for drug-resistant or unresectable GIST liver metastases10,13,22. Chen et al.13 showed comparable efficacy of RFA and surgery (mOS: 47 months versus not reached, P=0.413). Another study by Cao et al.10 highlighted the survival benefit of TACE for GIST patients with liver metastases compared to TKI monotherapy (68.5 weeks vs. 25.7 weeks, P=0.0001). It is safe and effective, but its clinical efficacy has not been good compared with that of other treatments. In our study, the combination of HR was associated with improved OS compared with the combination of RFA or TACE and IM alone, while the combination of RFA or TACE did not improve prognosis compared with IM alone. Possible explanations for our results may be as follows: first, RFA is not a radical treatment for lesions larger than 3 cm23. TACE is the most commonly used treatment for unresectable hepatocellular carcinoma24,25, and only curative-intent hepatectomy provides a better survival benefit than TKI therapy alone in GIST liver metastasis patients, while resection of drug-resistant lesions does not11,26,27. Moreover, the inclusion of a small number of patients who received RFA or TACE made it difficult to show statistically significant differences in the comparison of OS.
In our study, it was not found that IM combined with surgical treatment improved liver-specific PFS or PFS compared to IM alone, but it improved OS. This was probably because in this retrospective study with a long time span, some patients had less regular intervals between CT reviews or reviewed in other hospitals, making it difficult to determine accurate times to progression. However, the survival outcome of OS was obtained relatively easily and accurately.
While our study offers valuable insights, it is not without limitations. First, the retrospective design introduces potential selection biases. In this study, candidates for HR were chosen case-by-case, considering anatomic difficulties, number of lesions, and comorbidities, etc. Furthermore, patients with a lower tumor burden and could achieve NED after surgical treatment tended to perform HR. After PSM, there was no clear statistically significant difference in the OS outcome after HR or RFA/TACE therapy, which partially because of the loss of too many cases during the matching process. Therefore, we tended to believe that HR might have a better long-term prognosis than RFA/TACE in selected patients. Second, the relatively small number of patients in the RFA/TACE group limited the robustness of our analysis. Third, some patients did not receive regular radiological tests, resulting in a possible delayed recording of disease progression. Moreover, the time span of this study was too long to obtain information on the complications and side effects of various treatments. Multicenter real-world studies or RCTs would be necessary for obtaining more evidence in balance between the risk and benefits of HR.
In conclusion, we provided a large and relatively homogeneous cohort of GIST liver metastases patients and found that IM combined with HR was superior to IM alone or IM combined with RFA/TACE in improving OS in selected patients. Achieving NED status with surgical treatment results in significantly improved OS, liver-specific PFS, and PFS.
Ethical approval
This study protocol received approval from the ethical committees of the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China on 13 July 2023 [approval number: (2023) 352].
Consent
This study is a retrospective study with little risk to the subjects, and the informed consent of the subjects is not required. the ethical committees of the First Affiliated Hospital of Sun Yat-sen University agreed to exempt the subject informed consent.
Sources of funding
None.
Author contribution
X.Z. and Z.C.: study concept and design; H.W., Y.H., and S.H.: drafting of the manuscript; H.W., Y.H., S.H., H.X., W.X., L.T., Y.D., X.L., Y.Y., and Z.S.: acquisition of data; H.W., Y.H., S.H., and H.X.: analysis and interpretation of data; X.Z., Z.C., and S.S.: critical revision of the manuscript; H.W. and Q.Z.: statistical analysis; X.Z. and Z.C.: study supervision. All authors read and approved the final manuscript.
Conflicts of interest disclosure
The authors report no conflicts of interest in this work.
Research registration unique identifying number (UIN)
This retrospective study was registered with clinicaltrials.gov (unique identification number: NCT06038552).
Guarantor
Xinhua Zhang.
Data availability statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Haoxiang Wen, Yihao Huang, and Shaoqing Huang contributed equally to this manuscript.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 17 January 2024
Contributor Information
Haoxiang Wen, Email: wenhx3@mail2.sysu.edu.cn.
Yihao Huang, Email: huangyh86@mail2.sysu.edu.cn.
Shaoqing Huang, Email: huangshq59@mail2.sysu.edu.cn.
Han Xiao, Email: xiaoh69@mail.sysu.edu.cn.
Wenxuan Xie, Email: xiewenx@mail2.sysu.edu.cn.
Qian Zhou, Email: zhouq49@mail.sysu.edu.cn.
Li Tan, Email: tanli5@mail2.sysu.edu.cn.
Yuqi Ding, Email: dingyq8@mail2.sysu.edu.cn.
Xiaofei Liu, Email: liuxf78@mail2.sysu.edu.cn.
Yang Yu, Email: yuyang63@mail2.sysu.edu.cn.
Zimin Song, Email: songzm@mail2.sysu.edu.cn.
Shunli Shen, Email: shenshli@mail.sysu.edu.cn.
Zebin Chen, Email: chenzb23@mail.sysu.edu.cn.
Xinhua Zhang, Email: zhangxinhua@mail.sysu.edu.cn.
References
- 1.Tryggvason G, Gíslason HG, Magnússon MK, et al. Gastrointestinal stromal tumors in Iceland, 1990-2003: the icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 2005;117:289–293. [DOI] [PubMed] [Google Scholar]
- 2.Chan KH, Chan CW, Chow WH, et al. Gastrointestinal stromal tumors in a cohort of Chinese patients in Hong Kong. World J Gastroenterol 2006;12:2223–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang DY, Wang X, Yuan WJ, et al. Metastatic pattern and prognosis of gastrointestinal stromal tumor (GIST): a SEER-based analysis. Clin Transl Oncol 2019;21:1654–1662. [DOI] [PubMed] [Google Scholar]
- 4.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472–480. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–1338. [DOI] [PubMed] [Google Scholar]
- 7.Turley RS, Peng PD, Reddy SK, et al. Hepatic resection for metastatic gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Cancer 2012;118:3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue A, Gao X, He Y, et al. Role of surgery in the management of liver metastases from gastrointestinal stromal tumors. Front Oncol 2022;12:903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon IS, Shin JH, Han K, et al. Ultrasound-guided intraoperative radiofrequency ablation and surgical resection for liver metastasis from malignant gastrointestinal stromal tumors. Korean J Radiol 2018;19:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao G, Li J, Shen L, et al. Transcatheter arterial chemoembolization for gastrointestinal stromal tumors with liver metastases. World J Gastroenterol 2012;18:6134–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton TL, Walker BS, Billingsley KG, et al. Hepatic metastases in gastrointestinal stromal tumors: oncologic outcomes with curative-intent hepatectomy, resection of treatment-resistant disease, and tyrosine kinase inhibitor therapy alone. HPB (Oxford) 2022;24:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao B, Peng J, Tang J, et al. Liver surgery prolongs the survival of patients with gastrointestinal stromal tumor liver metastasis: a retrospective study from a single center. Cancer Manag Res 2018;10:6121–6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Li C, Yang H, et al. Radiofrequency ablation versus resection for resectable liver metastases of gastrointestinal stromal tumours: results from three national centres in China. Clin Res Hepatol Gastroenterol 2019;43:317–323. [DOI] [PubMed] [Google Scholar]
- 14.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325–2331. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa J, Kanda T, Hirota S, et al. Surgical interventions for focal progression of advanced gastrointestinal stromal tumors during imatinib therapy. Int J Clin Oncol 2007;12:212–217. [DOI] [PubMed] [Google Scholar]
- 16.Agha R, Abdall-Razak A, Crossley E, et al. STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int J Surg 2019;72:156–165. [DOI] [PubMed] [Google Scholar]
- 17.Vassos N, Agaimy A, Hohenberger W, et al. Management of liver metastases of gastrointestinal stromal tumors (GIST). Ann Hepatol 2015;14:531–539. [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 19.Rassen JA, Shelat AA, Franklin JM, et al. Matching by propensity score in cohort studies with three treatment groups. Epidemiology 2013;24:401–409. [DOI] [PubMed] [Google Scholar]
- 20.Casali PG, Zalcberg J, Le Cesne A, et al. Ten-year progression-free and overall survival in patients with unresectable or metastatic gi stromal tumors: long-term analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J Clin Oncol 2017;35:1713–1720. [DOI] [PubMed] [Google Scholar]
- 21.An HJ, Ryu MH, Ryoo BY, et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol 2013;20:4212–4218. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Vauthey JN, Abdalla EK, et al. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg 2006;141:537–544. [DOI] [PubMed] [Google Scholar]
- 23.Cucchetti A, Piscaglia F, Cescon M, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300–307. [DOI] [PubMed] [Google Scholar]
- 24.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011;37:212–220. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–380. [DOI] [PubMed] [Google Scholar]
- 27.European Association for the Study of the Liver . European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma [published correction appears in J Hepatol. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.


