Abstract
The SARS-CoV-2 subvariant BA.2.86 ‘Pirola’, first identified in Denmark in August 2023, has manifested with a significantly mutated spike protein profile, suggesting a heightened ability to evade vaccine-induced and infection-induced antibodies. This article outlines the epidemiological spread, immune response implications, and global responses to BA.2.86. Preliminary observations indicate community transmissions of the subvariant, even among those previously infected or vaccinated. Notably, the BA.2.86 infection has shown a potential to amplify antibody responses. The variant’s emergence has evoked memories of the Omicron variant’s rise in late 2021, though global immunity levels might modulate the impact of BA.2.86 impact differently. Continuous genomic surveillance, coupled with integrated diagnostic and epidemiological strategies, proves crucial in early detection and management. The emergence of BA.2.86 reaffirms the unpredictable nature of the COVID-19 pandemic, emphasizing the need for ongoing research, adaptability, and global collaboration.
Keywords: BA.2.86, community transmission, COVID-19, epidemiological spread, immune response, omicron, Pirola, SARS-CoV-2, spike protein, vaccine evasion
Introduction
The COVID-19 pandemic is caused by a virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the pandemic began in 2019, the virus has undergone numerous changes, resulting in the emergence of new variants and their subvariants. Each variant carries unique genetic signatures, which can potentially affect its transmissibility, virulence, and potential to circumvent the body’s immune system1–3.
The new Omicron subvariant BA.2.86 ‘Pirola’, emerging in Denmark with a heavily mutated spike protein, has swiftly drawn the global scientific community’s focus, particularly in terms of its potential resistance to the vaccines and treatments currently in use, and the broader implications for global public health tactics4. This article aims to offer an overview of BA.2.86, examining its characteristics, epidemiological spread, and implications for future pandemic management.
Origin and characteristics
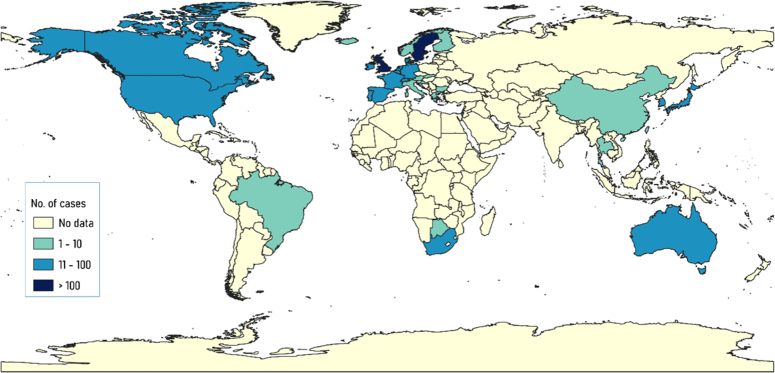
First reported in Denmark in August, the BA.2.86 subvariant rapidly expanded to eight countries, with a total of 24 cases identified by the end of the month5(Fig. 1). The BA.2.86 subvariant, characterized by a mutated spike protein with 29 substitutions, 4 deletions, and 1 insertion compared to its ancestor, BA.2, indicates a potential increased ability to evade antibodies induced by vaccines and prior infection. Out of the initial cases, none have been severely ill, though the data remains in its early stages6,7.
Figure 1.
Illustrates the global distribution of SARS-CoV-2 Omicron subvariant BA.2.86 as of 26 October 2023, reported by GISAID (Global Initiative on Sharing All Influenza Data). The figure was generated using QGIS (Quantum Geographic Information System).
The emergence of BA.2.86 has also drawn comparisons to the appearance of the Omicron variant in late 2021. However, given the broader global immunity resulting from previous infection waves and vaccine rollouts, the impact of BA.2.86 may differ from that of Omicron8.
Evolution of SARS-CoV-2 Omicron
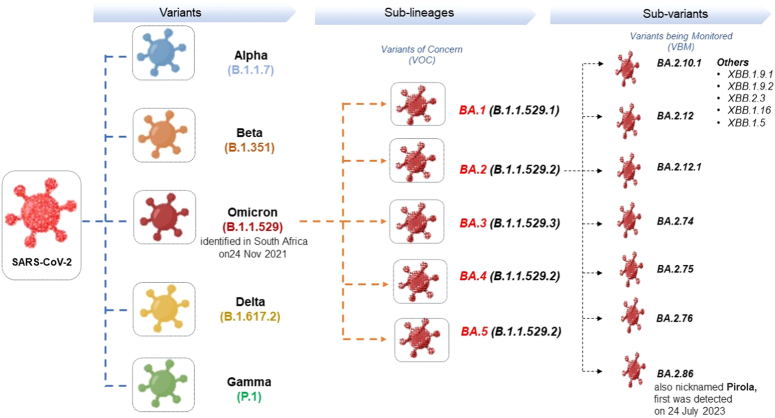
Figure 2 depicts the evolution of the SARS-CoV-2 virus, responsible for the COVID-19 pandemic, which has undergone significant genetic diversification since its emergence9. Among its many mutations, a noteworthy development is the evolution of the Omicron variant. The new subvariant, colloquially named ‘Pirola’, represents a continued lineage of the Omicron variant, which is characterized by a series of mutations that distinguish it from its predecessors. While the original Omicron variant was identified in South Africa on 24 November 2021, the Pirola subvariant was first detected several months later, on 24 July 2023. Its emergence underlines the virus’s capacity for mutation and the importance of vigilant genomic surveillance to monitor the evolution of the virus and potentially adjust public health responses accordingly (Fig. 2).
Figure 2.
Evolutionary mapping of SARS-CoV-2 variants: focusing on the emergence of Omicron subvariant BA.2.86 ‘Pirola’.
Transmissibility, infectivity, and immune evasion
Due to its potential impact on transmissibility, infectivity, and immune response evasion, BA.2.86 is causing significant concern. ‘Transmissibility’ refers to how easily the variant spreads from person to person, with higher transmissibility potentially leading to rapid outbreaks and complicating containment efforts. ‘Infectivity’ relates to how effectively the virus can enter and multiply within human cells, which can influence the severity of the disease it causes. The most alarming aspect is ‘immune evasion’, which is the variant’s ability to overcome the defense mechanisms built by previous infections or vaccinations10. Variants with improved immune evasion can cause breakthrough infections even in populations that were previously considered protected. The BA.2.86 subvariant, characterized by its heavily mutated spike protein, could significantly influence three critical areas: transmissibility, infectivity, and immune evasion. The spike protein, which is directly linked to infectivity, is essential for the virus’s entry into human cells. Moreover, mutations in this protein may affect not only the virus’s ability to spread but also its capacity to avoid being neutralized by antibodies11. Therefore, thoroughly understanding these factors is crucial in evaluating the risks posed by any new variant and in devising effective public health strategies.
The potential impact on society and economy
The socio-economic impact of BA.2.86 can be substantial. Due to its faster spread compared to previous variants, it could escalate transmission rates, consequently overburdening healthcare systems and depleting resources. This could lead to more hospitalizations, affecting the workforce due to sickness and quarantine rules. Moreover, the emergence of new variants can negatively influence various sectors, leading to economic disruptions. Understanding the genetic makeup and consequences of these subvariants is vital for devising effective public health measures and mitigating their socio-economic impacts12.
Global response strategies
The rapid identification and tracking of new SARS-CoV-2 variants, like BA.2.86, can play a key role in future pandemic management. Collective immunity from vaccinations or natural infections, especially against the Omicron variants, is crucial in limiting the spread of BA.2.86 globally. Ongoing, detailed surveillance of BA.2.86’s evolution is critical to anticipate and respond to potentially more infectious or harmful sublineages13.
Denmark has exemplified effective pandemic response by integrating diagnostic and epidemiological strategies, significantly aiding in the early detection of BA.2.86. Wastewater surveillance has also been essential in gauging the variant’s prevalence4. The continuous evolution of the Omicron variant has led to a revaluation of treatment strategies to enhance immune protection. Recent studies underscore the role of hybrid immunity in effectively combating both the original SARS-CoV-2 strain and other variants of concern (VOCs). Adapting to the virus’s evolution necessitates a comprehensive approach that integrates vaccine development, antiviral treatments, and ongoing research.
The emergence of BA.2.86 underscores the continuous evolution of the virus, influenced by global immunity levels, vaccination campaigns, and treatment strategies. It is crucial to understand the variant’s potential effects on disease severity, vaccine efficacy, and transmission dynamics to ensure an effective pandemic response14.
Novel treatment strategies
The global health community is emphasizing the importance of continuous genomic surveillance to track the evolution and spread of this subvariant. This surveillance is critical in understanding the variant’s characteristics and its response to existing treatments and vaccines15. There is a focus on adapting current vaccine strategies. Given the potential for BA.2.86 to evade immunity from previous infections or vaccinations, researchers are exploring the modification of existing vaccines to enhance their efficacy against this and other emerging variants16. In addition to vaccines, the treatment regime for BA.2.86 includes the use of antiviral drugs17. Agents like molnupiravir and the combination of nirmatrelvir and ritonavir, which have shown effectiveness against Omicron and its subvariants, are being considered for treatment18,19. Ongoing research is essential to fully understand the efficacy of these drugs against BA.2.86 and to develop new therapeutic options. Moreover, the concept of hybrid immunity is gaining attention. This involves combining the immunity acquired from previous infections with the immunity provided by vaccination. Studies suggest that hybrid immunity might offer more robust protection against various variants, including BA.2.8620,21.
Conclusion
The detection of BA.2.86 highlights the ever-evolving challenge posed by SARS-CoV-2. While the long-term implications of this subvariant remain to be fully understood, its emergence underscores the importance of continuous genomic surveillance, global collaboration, and preparedness. The COVID-19 pandemic’s trajectory remains unpredictable, emphasizing the need for adaptability in response strategies and ongoing research efforts.
Ethical approval
No ethical approval is required.
Sources of funding
No funding was received.
Author contribution
All authors have equally contributed.
Conflicts of interest disclosure
There are no conflicts of interest.
Guarantor
Ahmad Neyazi.
Data availability statement
We have not collected any primary data for this research. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 11 January 2024
Contributor Information
Prakasini Satapathy, Email: prakasini.satapathy@gmail.com.
Pawan Kumar, Email: pawankumar705c@gmail.com.
Jeetendra K. Gupta, Email: jk.gupta@gla.ac.in.
Ali A. Rabaan, Email: arabaan@gmail.com.
Nawal A. Al Kaabi, Email: alkaabin971@gmail.com.
Dibyalochan Mohanty, Email: mohantypharmacy@anurag.edu.in.
Pathakala Naveen, Email: Naveenpharmacy@anurag.edu.in.
Mahalaqua Nazli Khatib, Email: nazli.786@rediffmail.com.
Shilpa Gaidhane, Email: drshilpagaidhane@gmail.com.
Quazi Syed Zahiruddin, Email: zahirquazi@gmail.com.
Ahmad Neyazi, Email: ahmadniazi000@gmail.com.
References
- 1.Samanta J, Mahapatra SJ, Kumar N, et al. Virus related acute pancreatitis and virus superinfection in the ‘Dual disease’ model of acute pancreatitis and SARS-Co-V2 infection: a multicentre prospective study. Pancreatology 2022;22:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee S, Bhattacharya M, Nag S, et al. A detailed overview of SARS-CoV-2 Omicron: its sub-variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses 2023;15:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu N, Kohli P, Mishra C, et al. To evaluate the effect of COVID-19 pandemic and national lockdown on patient care at a tertiary-care ophthalmology institute. Indian J Ophthalmol 2020;68:1540–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen M, Moller FT, Gunalan V, et al. First cases of SARS-CoV-2 BA.2.86 in Denmark, 2023. Euro Surveill 2023;28:2300460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris E. CDC assesses risk from BA.2.86, highly mutated COVID-19 variant. JAMA 2023;330:1029. [DOI] [PubMed] [Google Scholar]
- 6.Looi MK. Covid-19: scientists sound alarm over new BA.2.86 “Pirola” variant. BMJ 2023;382:1964. [DOI] [PubMed] [Google Scholar]
- 7.Mahase E. Covid-19: new “Pirola” variant BA.2.86 continues to spread in UK and US. BMJ 2023:382.2097. [DOI] [PubMed] [Google Scholar]
- 8.Qu P, Xu K, Faraone JN, et al. Immune evasion, infectivity, and fusogenicity of SARS-CoV-2 Omicron BA.2.86 and FLip variants. bioRxiv 2023;2023:557206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markov PV, Ghafari M, Beer M, et al. The evolution of SARS-CoV-2. Nature Reviews Microbiology 2023;21:361–379. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Yu Y, Jian F, et al. Antigenicity and infectivity characterisation of SARS-CoV-2 BA.2.86. Lancet Infect Dis 2023;23:e457–e459. [DOI] [PubMed] [Google Scholar]
- 11.Uriu K, Ito J, Kosugi Y, et al. Transmissibility, infectivity, and immune evasion of the SARS-CoV-2 BA.2.86 variant. Lancet Infect Dis 2023;23:e460–e461. [DOI] [PubMed] [Google Scholar]
- 12.da Silva SJR. The emergence of new SARS-CoV-2 omicron subvariants introduces uncertainty about the end of the COVID-19 pandemic. Front Med 2022;9:1010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Lu L, Jiang S. SARS-CoV-2 Omicron subvariant BA.2.86: limited potential for global spread. Signal Transduct Target Ther 2023;8:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuchipudi SV. How evasive and transmissible is the newest omicron offshoot, BA.2.86, that causes COVID-19? 4 questions answered. The Conversation 2023; 12 September 2023.
- 15.Zeghbib S, Kemenesi G, Jakab F. The importance of equally accessible genomic surveillance in the age of pandemics. Biol Futura 2023;74:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanelli P, Rezza G. COVID-19 vaccination strategies and their adaptation to the emergence of SARS-CoV-2 variants. Vaccines (Basel) 2022;10:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faiyazuddin M, Sophia A, Ashique S, et al. Virulence traits and novel drug delivery strategies for mucormycosis post-COVID-19: a comprehensive review. Front Immunol 2023;14:1264502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun M, Lai H, Zhang Z, et al. Molnupiravir for the treatment of non-severe COVID-19: a systematic review and meta-analysis of 14 randomized trials with 34 570 patients-authors’ response. J Antimicrob Chemother 2023;78:3009–3012. [DOI] [PubMed] [Google Scholar]
- 19.Schilling WHK, Jittamala P, Watson JA, et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. Lancet Infect Dis 2024;24:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection, vaccination, and hybrid immunity against symptomatic Alpha, Beta, and Delta SARS-CoV-2 infections: an observational study. EBioMedicine 2023;95:104734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasrado N, Barouch DH. SARS-CoV-2 hybrid immunity: the best of both worlds. J Infect Dis 2023;228:1311–1313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have not collected any primary data for this research. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.