Abstract
Background and Aims:
Pediatric acute liver failure (PALF) is a life-threatening condition. In Europe, the main causes are viral infections (12%–16%) and inherited metabolic diseases (14%–28%). Yet, in up to 50% of cases the underlying etiology remains elusive, challenging clinical management, including liver transplantation. We systematically studied indeterminate PALF cases referred for genetic evaluation by whole-exome sequencing (WES), and analyzed phenotypic and biochemical markers, and the diagnostic yield of WES in this condition.
Approach and Results:
With this international, multicenter observational study, patients (0–18 y) with indeterminate PALF were analyzed by WES. Data on the clinical and biochemical phenotype were retrieved and systematically analyzed.
Results:
In total, 260 indeterminate PALF patients from 19 countries were recruited between 2011 and 2022, of whom 59 had recurrent PALF. WES established a genetic diagnosis in 37% of cases (97/260). Diagnostic yield was highest in children with PALF in the first year of life (41%), and in children with recurrent acute liver failure (64%). Thirty-six distinct disease genes were identified. Defects in NBAS (n=20), MPV17 (n=8), and DGUOK (n=7) were the most frequent findings. When categorizing, the most frequent were mitochondrial diseases (45%), disorders of vesicular trafficking (28%), and cytosolic aminoacyl-tRNA synthetase deficiencies (10%). One-third of patients had a fatal outcome. Fifty-six patients received liver transplantation.
Conclusions:
This study elucidates a large contribution of genetic causes in PALF of indeterminate origin with an increasing spectrum of disease entities. The high proportion of diagnosed cases and potential treatment implications argue for exome or in future rapid genome sequencing in PALF diagnostics.
INTRODUCTION
Pediatric acute liver failure (PALF) is a rare, life-threatening clinical condition mainly affecting children in their first year of life.1 In the United States, PALF is mainly caused by paracetamol intoxication (13%), metabolic disorders (10%), and viral infections (8%).2 In Europe, a systematic collection of data on PALF etiologies is lacking. Single-center report inherited metabolic diseases (14%–28%) and viral infections (12%–16%) as the main causes of PALF in Europe.3,4 In US and European cohorts, the underlying etiology remained unclear in about half of cases, hampering clinical management including disease-specific therapies, particularly decision-making regarding liver transplantation.1–4 This uncertainty is critical for survival with a high burden for physicians, affected individuals, and their families. Therefore, establishing a causal diagnosis is central to PALF. Although standardized approaches regarding biochemical testing and targeted Sanger sequencing in children with PALF contributed to a higher diagnostic rate and helped to elucidate some PALF etiologies, a large fraction of PALF cases remained unclassified.5 Access to next-generation sequencing techniques unraveled novel or uncovered genetic causes of hitherto unsolved cases of PALF. An important milestone boosting whole-exome sequencing (WES) in acute liver failure (ALF) was the identification of biallelic variants in NBAS as a cause of recurrent acute liver failure (RALF) with onset in infancy (MIM: #616483).5,6 More recently, numerous NBAS cases7 but also reports on other rare genetic diseases associated with PALF were published, such as infantile liver failure syndrome type 1 due to variants in LARS1 (Mendelian Inheritance in Man (MIM): #615438),8,9 infantile liver failure syndrome type 3 due to variants in RINT1 (MIM: #618641),10 and transient infantile liver failure syndrome due to variants in TRMU (MIM: #613070).11 In the context of the rapidly growing knowledge on genetic causes of PALF, a single-center retrospective analysis of 148 PALF cases (between 2001 and 2011) identified the underlying genetic cause of formerly indeterminate cases in 27% using custom next-generation sequencing panel, indicating the high proportion of genetic causes among unsolved cases before the era of next-generation sequencing.12 However, a systematic WES study has not been performed to date in individuals with PALF of unknown etiology.
Therefore, in the present international, multicenter study, we aimed to study the proportion, type, and biochemical and clinical presentation of genetic diseases identified by WES in a cohort of individuals with PALF of indeterminate etiology.
METHODS
Study design and recruitment of patients
The study was carried out as an international, multicenter study including both prospective and retrospective cases. The inclusion criterion was the clinical diagnosis of PALF of unknown etiology in children aged 0–18 years. The clinical diagnosis of PALF was made according to local criteria, including patient phenotype and laboratory parameters such as plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, bilirubin and INR (international normalized ratio). Individuals were eligible if diagnostic workup did not establish the etiology of PALF; workup was broadly consistent within the collaborating expert centers for pediatric hepatology (for details see Supplemental Table S1, http://links.lww.com/HEP/I114). When there was more than one episode of PALF, cases were characterized as RALF. Data on country of origin, sex, clinical signs, and symptoms using the human phenotype ontology (HPO)13 as well as laboratory and histology data were retrieved by means of a specific case report form. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul and the ethical standards of the responsible committee on human experimentation. Informed consent to participate in the study was obtained from all patients and/or from their parents in case of minor patients. The study was approved by the ethcial committees of the Technical University Munich and the Medical Faculty Heidelberg (study number: S-035_2014).
WES and variant prioritization
WES of cases and unaffected parents was performed at the Helmholtz Centre Munich (Munich, Germany) for research cases and the Institute of Human Genetics, Klinikum rechts der Isar, Technical University Munich (TUM, Munich, Germany) for diagnostic cases. WES data analysis was performed using an in-house pipeline of TUM.14 WES was performed on genomic DNA extracted from blood.15 Sequencing reads were aligned to human genome-build GRCh37/hg19 (UCSC Genome Browser) using the Burrows-Wheeler Aligner (v.0.7.5a).16 Single-nucleotide variants and small insertions and deletions were detected using the Genome Analysis Toolkit.17 Copy number variants were detected with ExomeDepth.18 Mitochondrial DNA (mtDNA) variants were assessed from exome data as described.19
A special focus was given to genes previously reported to be associated with ALF. This gene list was manually created and extended through searching the OMIM database for all entries matching the keyword “liver failure.” This resulted in a set of 243 distinct candidate genes (Supplemental Table S2, http://links.lww.com/HEP/I115). Variants were classified according to the guidelines of the American College of Medical Genetics and Genomics20 using the Python package “InterVar” and the ClinVar annotation.21
Phenotypic analysis
The following clinical variables were collected and analyzed: sex, weight, height, age at the time of the episode(s) of ALF/first symptoms of liver disease and at last assessment, patient survival, need for liver transplantation, and cause of death. Age ranges were defined as follows: neonatal period, age of onset at birth until day 28; infancy, age of onset before the second year of life; early childhood, age of onset between the second year of life and fifth year of life; childhood, age of onset between the fifth and 12th year of life; adolescence, age of onset between the 12th and 18th year of life. Laboratory data during the episode of liver failure were collected using the IU system (SI). Additionally, the most prominent hepatic and extrahepatic clinical features were provided as HPO terms by the referring clinicians.13 Based on the provided HPO terms, ancestral HPO terms were derived from the ontology using the R package “OntologyX.”22
Statistical analyses
Statistical analyses were performed using R version 4.0.4. For conducting survival analyses in R, the “Survival” and “Survminer” packages were used. The log-rank test was used to compare survival between different categorical variables.
Results
Study cohort
A total of 260 individuals were enrolled in this study between 2011 and 2022, with 111 individuals being prospectively enrolled and 149 patients enrolled retrospectively. Forty had been reported in publications on single disease gene discoveries or phenotypic spectrum studies.5–7,9–11,23–28 WES data were analyzed at the Technical University of Munich, of which 175 were examined as singletons and 85 by trio WES.
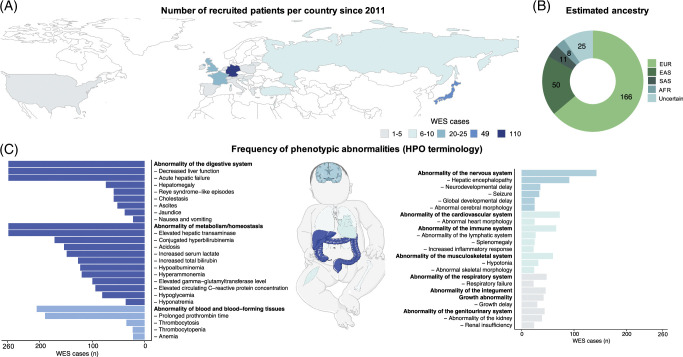
One hundred eighteen (118/260) patients were female (45%). Patients originated from centers in 19 countries in Europe, Asia, or North America (Figure 1A). The majority of patients (70%) were enrolled in Germany, Japan, and the United Kingdom, which is reflected in the estimated ethnic background based on the WES data (Figure 1B). Fifty-nine individuals (59/260) presented with RALF. On average, patients presented the second episode of ALF within 0.9 years (mean: 0.9 ± 0.9 y SD), whereas time intervals between ALF were significantly longer for further episodes (mean: 2.2 ± 4.8 y) (Supplemental Figure S1, http://links.lww.com/HEP/I108).
FIGURE 1.
Geographical origin, clinical phenotypes, and affected organ systems. (A) Geographic origin: the number of patients recruited within the different countries is indicated by color. (B) Ancestry prediction based on WES data. (C) Frequency of phenotypic abnormalities within the whole cohort using the HPO. HPO terms with a frequency of 20 and higher are displayed. The exact INR values were not available for all cases, however, for all patients with available INR information, INR levels were abnormally elevated. All frequencies are shown in absolute numbers. Missing data may have led to low numbers in some of the items (eg, cholestasis, conjugated hyperbilirubinemia). Abbreviations: AFR, African; EAS, east Asian; EUR, European; HPO, Human Phenotype Ontology; SAS, south Asian; WES, whole-exome sequencing.
Three hundred seventy-eight (378) distinct, nonredundant HPO terms were reported, resulting in a median of 12 HPO terms per patient (range 2–29) spanning a median of 5 organ systems (Figure 1B, Supplemental Table S3, http://links.lww.com/HEP/I116).
With respect to clinical liver involvement, 74 patients presented with hepatomegaly, 59 had cholestasis, and 52 ascites. Jaundice was present in 38 individuals. Besides the liver, main phenotypic presentations included abnormalities of metabolism (100%), the blood and blood-forming tissues (79%), the nervous system (55%), the cardiovascular system (28%), and the immune system (25%) (Figure 1C).
All patients had abnormal blood homeostasis or abnormalities of metabolism (according to the HPO terminology), including elevated aminotransferases, hyperbilirubinemia, acidosis, increased serum lactate, hypoalbuminemia, hyperammonemia, and hypoglycemia. Concerning blood and blood-forming tissue, the most frequent clinical features besides prolonged prothrombin time were thrombocytosis, thrombocytopenia, and anemia. Frequently reported neurological abnormalities were HE, neurodevelopmental delay, and seizures. Organ involvement stratified by clinical phenotype revealed only few differences between ALF and RALF cases, but abnormalities of the head and neck, nervous and musculoskeletal system were more common in the RALF than in the ALF group (Fisher Exact test, nominal p-value<0.05; Supplemental Figure S2, http://links.lww.com/HEP/I108).
Biochemical characterization of the whole cohort showed broad ranges for plasma ALT activity with a median of 907 U/L (range: 20–19,200 U/L) and AST activity with a median of 1939 (range: 36–31,800 U/L) (Figure 2A, B). When analyzed separately, patients with RALF had a significantly higher ALT activity with a median of 3,900 U/L (range: 167–19,200 U/L) (p-value 2.3×10−11, Wilcoxon test) and AST activity with a median of 4804 (range: 74–31,800 U/L) (p-value 5.5×10-8, Wilcoxon test) compared to the activity levels in the ALF group with ALT activity with a median of 582 U/L (range: 20–15,528 U/L) and AST activity with a median of 1395 (range: 36–21,227 U/L). Median maximal INR was 3 (range: 1.21–15.7), and median maximal total bilirubin was 140 µmol/L (range: 4.62–1219.2 µmol/L). (Figure 2C–E). Sixteen patients in the ALF cohort showed a bilirubin level below 17.1 µmol/L (1 mg/dL) and hence a low bilirubin phenotype. Five patients were reported to have a conjugated bilirubin below 5.1 µmol/L (0.3 mg/dL). Bilirubin and INR levels did not differ significantly between patients with ALF and RALF.
FIGURE 2.
Biochemical characterization of the cohort. Laboratory characterization of ALF and RALF including aspartate aminotransferase, alanine aminotransferase, liver function (INR), total bilirubin as well as direct bilirubin using violin plots. Bold dots indicate the median, bars indicate the 25th to 75th percentile; green dots represent values within the reference range for normal values according to a consensus of Deutsche Gesellschaft für Klinische Chemie und Laboratoriumsmedizin and Verband der Diagnostika- und Diagnostikageräte-Hersteller.29 *p≤0.05 **p≤0.01 ***p≤0.001 ****p≤0.0001 by Wilcoxon test. Abbreviations: ALF, acute liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; RALF, recurrent acute liver failure.
Liver biopsy data were available for 104 patients, with steatosis (36/104) and fibrosis (34/104) being the most commonly reported histologic findings. Steatosis was found significantly more frequent in patients with RALF than in patients with ALF, whereas other histologic findings exhibited no differences between RALF and ALF (nominal p-value<0.05; Supplemental Figure S3, http://links.lww.com/HEP/I108).
At the time point of data analysis, 181 patients were alive (134 with native liver survival, 47 with liver transplantation), while 79 children had died, 9 of those after liver transplantation (Figure 3A). Age of onset of ALF (first episode in patients with a RALF phenotype) ranged from neonatal to juvenile, with the majority of patients presenting within the first year of life (data available for 209 patients) (Figure 3B). The majority of deaths due to ALF (72%) occurred within the first year of life (Figure 3C). Comparison of the overall survival rate in the two clinical subgroups revealed a significantly higher native liver survival rate in the RALF subgroup as compared to the ALF subgroup (Figure 3D). When stratified by age of ALF onset, decreased native liver survival probability was associated with a younger age of onset (Figure 3E). A trigger for ALF was reported in 82 cases, with infections being the most prominent one (77 cases).
FIGURE 3.
Outcome of patients with pediatric acute liver failure. (A) Patient and native liver survival. (B) Timeline for age at first ALF in years. (C) Timeline for age of death after the first ALF in years. (D) Kaplan-Meier plot for the native liver survival after the first ALF stratified by the clinical phenotype in months. p-value calculation by log-rank test. (E) Kaplan-Meier plot for the native liver survival after the first ALF stratified by the age of onset. Abbreviations: ALF, acute liver failure; RALF, recurrent acute liver failure.
Genetics
WES analysis established a genetic diagnosis in 97/260 of so far genetically unsolved cases (37%). Nineteen patients remained unsolved with variants of uncertain significance in OMIM disease genes or candidate disease genes. In the remaining 144/260 patients (55%), no variant could be prioritized. Pathogenic and likely pathogenic variants were detected in 36 distinct ALF disease-associated genes, of which more than half (21/36, 58%) were reported in single cases only (Figure 4A). Defects in NBAS (21%), MPV17 (8%), and DGUOK (7%) occurred most frequently. In 92/97 cases, the causative genetic defect was inherited in an autosomal recessive fashion; among these, 53/92 were homozygous for disease-causing variants (Supplemental Table S4, http://links.lww.com/HEP/I117). In total, 108 distinct variants classified as “likely pathogenic” or “pathogenic” according to ACMG criteria within the 36 genes were found. Of the 108 variants, 56 (52%) were already reported as “likely pathogenic” or “pathogenic” in ClinVar at the time point of WES analysis. Missense variants formed the major proportion of all disease-causing variants. Out of the 36 cases in this study who had consanguineous parents, a genetic diagnosis was established in 22 (61%). Additionally, 20 out of 22 genetically diagnosed cases were found to be homozygous for the disease-causing variant.
FIGURE 4.
Molecular etiology of pediatric acute liver failure. (A) Genetic spectrum of pediatric acute liver failure; (B) diagnostic yield, stratified by ALF vs. RALF; (C) age of onset stratified by disease groups; and (D) native liver survival after first ALF stratified by the 2 most frequent disease groups. Abbreviations: ALF, acute liver failure; RALF, recurrent acute liver failure; WES, whole-exome sequencing.
When categorizing the disease genes into functional groups, most of the cases are associated with mitochondrial diseases (45%), followed by disorders of vesicular trafficking (28%) and cytosolic aminoacyl-tRNA synthetase deficiencies (10%) (Figure 4A). Diagnostic yield was higher in patients with ALF within the first 5 years of life (40%; n=187) compared to children 6 years of age or above (14%; n=21) (Supplemental Figure S4, http://links.lww.com/HEP/I108). Highest yield was achieved in infancy (41%; n=123) and in children with RALF (64%; n=59) (Figure 4B). The majority of patients with disorders of vesicular trafficking developed RALF. NBAS deficiency was the most frequent diagnosis in this group. In the majority of mitochondrial diseases (68%), ALF occurred within the first 6 months of life (median 2.5 months), whereas disorders of vesicular trafficking and cytosolic aminoacyl-tRNA synthetases were characterized by an age of onset of half a year and older (Figure 4C).
Patients with disorders of vesicular trafficking rarely died, while patients with mitochondrial disease showed a high mortality. For the other disease groups, patient numbers were too low for analyses of survival (Figure 4D).
The 3 most frequent disease groups in this cohort presented a broad phenotypic spectrum with multiple organs involved (Supplemental Figure S5, http://links.lww.com/HEP/I108).
Liver biopsy data were available for 44 genetically solved cases (Supplemental Figure S6, http://links.lww.com/HEP/I108). While fibrosis was most common in patients with mitochondrial disorders, cirrhosis was found most frequently in patients with cytosolic tRNA synthetase deficiencies. Steatosis was the commonest finding in all three distinct disease groups, and necrosis was only rarely reported in any of the distinct disease groups. No significant differences in necrosis or inflammation incidence could be identified between the groups.
Subcohort fulfilling PALF study group inclusion criteria
The inclusion criteria of our cohort differed from those of the US PALF study group (PALFSG,1). We investigated whether applying the more stringent PALFSG inclusion criteria to our cohort would yield different results. A total of 145/201 patients with ALF in our study fulfilled the PALFSG inclusion criteria. Comparing this subgroup with the overall cohort, there were no differences apart from INR (and the associated HPO umbrella term blood and blood-forming tissue), which was significantly higher in the subgroup fulfilling PALFSG criteria (for details see Supplemental Table S5, http://links.lww.com/HEP/I118).
DISCUSSION
PALF is a life-threatening event with very heterogenous etiologies. Despite ongoing efforts of standardizing diagnostic approaches, in one-third to half of cases no causal diagnosis is achieved.30 In 2015, variants in NBAS were identified as a novel cause of PALF,5 the number of individuals diagnosed with NBAS deficiency rose rapidly6,7 and motivated to further explore the genetic landscape of PALF of unknown etiology. Hence, the pediatric acute liver failure exome sequencing (PALFES) study was started including up to now 260 individuals with PALF of unknown etiology from three different continents. This represents by far the largest cohort of patients with unresolved PALF studied by WES reported in the literature to date. A genetic diagnosis was established in 37% of patients with unresolved PALF; in patients with RALF the diagnostic yield was even higher (64%).
With the elucidation of about 40% of hitherto unexplained PALF cases, genetic diseases likely represent the largest group of PALF in terms of etiology. Nevertheless, it is noteworthy that 55% of patients in this series had no evidence of an underlying genetic disorder. Transcriptome or proteome analyses may further increase the diagnostic rate of genetic diseases by providing functional evidence on inconclusive candidate variants detected or by discovery of functional relevant variants missed by the genetic analysis of our cohort. Even disregarding this, a substantial part of indeterminate PALF is likely of nongenetic origin.31 According to Squires et al,32 the fraction of PALF cases with unknown etiology is particularly high in the first 3 years of life, which is the age range in which our study demonstrates the highest molecular diagnostic yield by WES.
Three main groups of genetic diseases underlying indeterminate PALF have been identified: mitochondrial disorders, disorders of vesicular trafficking, and cytosolic aminoacyl-tRNA synthetase deficiencies (Figure 5). Mitochondrial genetic diagnoses were noticeably more frequent within our study than had previously been reported.32 Still, in our cohort, one of the early reported genetic causes of PALF, mutations in mitochondrial polymerase gamma (POLG) associated with Alpers syndrome, was rarely observed. Because it is a known cause of PALF, POLG-suspected cases may have undergone targeted genetic testing and are therefore not included in this cohort. However, we cannot exclude the possibility of enrollment bias in the PALFES cohort, as some of the participating centers were studying a cohort of pediatric patients with mitochondrial diseases in parallel with the PALFES study, and thus mitochondrial-related ALF cases may have been assigned more frequently.33 In addition, the mere fact that a genetic study was conducted may have led to a higher inclusion rate of patients with suspected genetic causes. Specifically, publications on new genetic causes of RALF during the study led to an increased sending of RALF cases. Therefore, the unbiased diagnostic yield should likely be lower, with a potentially lower rate of mitochondrial disease and genetic causes of RALF. This suggestion is supported by the high proportion of RALF cases in our cohort, for which a genetic cause is more likely to be expected.
FIGURE 5.
(A) Mitochondrial disease genes causing pediatric acute liver failure. (B) Disorders of vesicular trafficking and aminoacyl-tRNA synthetase deficiency (box) leading to pediatric acute liver failure. Genes with pathogenic variants that have been identified in cases within this study are shown in bold.
A potential limitation of our study was the lack of an internationally standardized definition of PALF, which is also reflected in our cohort. Most of the individuals included in our cohort (201/260 including also RALF cases) met the stringent inclusion criteria of the longitudinal studies of PALF conducted by the “PALF Study Group” in the United States.1 Although the PALF Study Group inclusion criteria do not represent definition criteria of PALF, they are frequently used as such. Our analyses show that these more stringent criteria would not lead to different results, including diagnostic yield, age at onset, histology, survival, organ involvement, histology, and disease genes.
In general, PALF cases with a clear biochemical fingerprint (such as tyrosinemia type I, with elevated succinylacetone in urine or dried blood spots) are typically diagnosed based on metabolic investigations, and genetically confirmed in the further workup. The 3 disease groups identified in this study share the feature that no specific laboratory biomarker or metabolic fingerprint by itself can establish the molecular diagnosis or point specifically to a single disease gene. Accordingly, we could not identify a clear biochemical or clinical profile in the cases now diagnosed genetically by WES using already established investigations. More comprehensive metabolomic studies would be needed to extend the search for diagnostic biomarkers. Consequently, with our current knowledge, we advocate performing genetic analyses using WES or whole-genome sequencing in parallel to metabolic analyses as the timely decision on further appropriate treatment options such as transplantation and/or specific drug therapy or dietary management in case of metabolic diseases will depend on the underlying disorder.
Our study demonstrates that the lack of biomarker specificity also holds true for lactate in serum, as lactate concentration could not differentiate between mitochondrial disorders from other causes of PALF, indicating that lactic acidemia is also a secondary finding in severe liver dysfunction such as PALF. Extremely high levels of AST and ALT have been associated with NBAS deficiency,6 often presenting as RALF. This reflects the significantly higher AST/ALT levels in this group; however, diagnosis cannot be ascertained based on this finding. Age of onset can direct the clinical suspicion toward possible genetic disorders underlying PALF, with mitochondrial disorder most frequently occurring in the neonatal period. Another frequent cause of neonatal ALF is gestational alloimmune liver disease presenting typically with low AST and ALT levels. Such cases have not been included in our study. In our cohort, only 5 out of 50 neonates showed ALT<100 U/L. Of note, in 2 of the 5 cases with low ALT, pathogenic variants in DGUOK were found to be responsible for the phenotype. There is a substantial overlap among the different disease groups with respect to age of onset.
An unexpected histological finding in the PALFES cohort is the high percentage (30%) of progressive liver remodeling. This indicates that a subgroup has a preceding subclinical disease, with PALF as the first clinical presentation.
The most commonly affected extrahepatic organ system was the nervous system, with HE and neurodevelopmental delay reported most often. In analogy to biochemical parameters, also the clinical parameters in our study did not discriminate between cases with and without a genetic diagnosis. This again emphasizes the relevance and necessity of genome-wide genetic analyses in PALF to determine the underlying cause.
Establishing a (genetic) diagnosis is crucial for clinical management and outcome of children with PALF. While causative treatments are not available for most of the genetic disorders detected in our cohort, there are specific management approaches for several diseases. In patients with TRMU deficiency, it has been shown that cysteine supplementation improved survival significantly compared to patients without cysteine supplementation.11 Forced antipyretic management in ILFS1, ILFS2, and ILFS3 may help to avoid fever-triggered RALF.6,7,9,10 Additionally, the decision on transplantation is dependent on the underlying disorder and the expected outcome in relation to both transplant liver survival and extrahepatic features and finally, the risk of recurrence of PALF in another child of the family can only be predicted with a genetic diagnosis. Our study demonstrates that outcome differs depending on the underlying disease etiology; native liver survival is significantly higher in individuals with disorders of vesicular trafficking compared to mitochondrial diseases. The role of liver transplantation for individuals with PALF due to genetic diseases is discussed controversially in the literature especially for mitochondrial disorders, due to potentially unfavorable neurological, cardiac, or neuromuscular involvement and poor outcome, arguing against liver transplantation.34 However, there are mitochondrial disorders such as TRMU or hepatic DLD deficiency where extrahepatic involvement is scarce with a favorable neurological prognosis.11,35 Moreover, multisystemic mitochondrial disorders are not contradicting liver transplant in general, as there are reports of patients, for example, with DGUOK deficiency, with minor neurological involvement who received a liver transplantation and had a satisfactory post-transplant course.11,36,37
In order to help decision-making, diagnosis of PALF and the identification of the etiology needs to be established within a short period of time as the clinical situation typically is critical and decisions are time-sensitive. Turnaround time in our study was not assessed systematically, and individuals have also been enrolled retrospectively where turnaround time was not essential for clinical care. However, it is impractical to wait for a long time to receive WES results in critically ill patients. Hence, from a technical point of view, genome sequencing would be faster and more accurate than WES, with reported turnaround time as low as 24 hours, being a promising option to achieve genetic diagnoses especially in critically ill children.38,39 Higher investment costs and demanding computational power hampers availability of this technology in most hospitals, but will likely be available in the near future in many countries. Again, rapid establishment of a (genetic) diagnosis has the potential to both save lives and reduce costs.38,39
In conclusion, this study demonstrates that a relevant number of indeterminate PALF cases can be solved by WES. This shifts PALF in the range of other rare disorders for which WES/whole-genome sequencing is now a well-established diagnostic procedure. Major identified groups are mitochondrial disorders, disorders of vesicular trafficking, and cytosolic aminoacyl-tRNA synthetase deficiencies. An ascertained diagnosis helps physicians make treatment decisions and can save lives and costs. For these reasons, we assert that WES, or in the near future whole-genome sequencing, should be within a first-line diagnostic approach for every child presenting with PALF.
Supplementary Material
ACKNOWLEDGMENTS
First and foremost, the authors thank affected patients and families. Furthermore, the authors thank all clinicians involved in the care of the recruited patients who contributed to this collection of data. The authors also thank the funding agencies.
Footnotes
Abbreviations: ALF, acute liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HPO, human phenotype ontology; INR, international normalized ratio; PALF, pediatric acute liver failure; PALFES, pediatric acute liver failure exome sequencing; PALFSG, PALF study group; POLG, polymerase gamma; RALF, recurrent acute liver failure; SI, IU system; TUM, Technical University Munich; WES, whole-exome sequencing.
Dominic Lenz, Lea D. Schlieben and Masaru Shimura share first authorship and contributed equally to the paper. Kei Muramaya, Christian Staufner and Holger Prokisch are shared last authors and contributed equally to the paper.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepjournal.com.
Contributor Information
Birgit Knoppke, Email: Birgit.Knoppke@klinik.uni-regensburg.de.
Martina Kohl, Email: Martina.Kohl-Sobania@uksh.de.
Heike Kölbel, Email: heike.koelbel@uk-essen.de.
Stefan Kölker, Email: Stefan.Koelker@med.uni-heidelberg.de.
Vassiliki Konstantopoulou, Email: vassiliki.konstantopoulou@meduniwien.ac.at.
Tatiana Krylova, Email: krylova@med-gen.ru.
Zarife Kuloğlu, Email: zarifekuloglu@yahoo.com.
Alice Kuster, Email: alice.kuster@chu-nantes.fr.
Martin W. Laass, Email: Martin.Laass@uniklinikum-dresden.de.
Elke Lainka, Email: elke.lainka@uk-essen.de.
Eberhard Lurz, Email: Eberhard.Lurz@med.uni-muenchen.de.
Hanna Mandel, Email: hmandel2637@gmail.com.
Katharina Mayerhanser, Email: Katharina.Mayerhanser@mri.tum.de.
Johannes A. Mayr, Email: H.Mayr@salk.at.
Patrick McKiernan, Email: pat.mckiernan@nhs.net.
Patricia McClean, Email: pmcclean@doctors.org.uk.
Valerie McLin, Email: valerie.mclin@hcuge.ch.
Karine Mention, Email: karine.mention@chru-lille.fr.
Hanna Müller, Email: hamuell@med.uni-marburg.de.
Laurent Pasquier, Email: laurent.pasquier@chu-rennes.fr.
Martin Pavlov, Email: martin.pavlov@helmholtz-munich.de.
Natalia Pechatnikova, Email: funny85@bk.ru.
Bianca Peters, Email: bianca.peters@med.uni-heidelberg.de.
Danijela Petković Ramadža, Email: dramadza@gmail.com.
Dorota Piekutowska-Abramczuk, Email: d.abramczuk@ipczd.pl.
Denisa Pilic, Email: denisa.pilic@uk-essen.de.
Sanjay Rajwal, Email: sanjay.rajwal@nhs.net.
Nathalie Rock, Email: Nathalie.Rock@hcuge.ch.
René Santer, Email: r.santer@uke.de.
Wilfried Schenk, Email: wilfried.schenk@klinikum-augsburg.de.
Natalia Semenova, Email: semenova@med-gen.ru.
Christiane Sokollik, Email: Christiane.sokollik@insel.ch.
Ekkehard Sturm, Email: ekkehard.sturm@med.uni-tuebingen.de.
Robert W. Taylor, Email: Robert.Taylor@newcastle.ac.uk.
Eva Tschiedel, Email: eva.tschiedel@uk-essen.de.
Vaidotas Urbonas, Email: vaidotas.urbonas@vuvl.lt.
Roser Urreizti, Email: roserurreizti@gmail.com.
Jan Vermehren, Email: gastro-elki@gesundheitnord.de.
Jerry Vockley, Email: vockleyg@upmc.edu.
Georg-Friedrich Vogel, Email: Georg.Vogel@i-med.ac.at.
Matias Wagner, Email: matias.wagner@mri.tum.de.
Wendy van der Woerd, Email: W.vanderWoerd@umcutrecht.nl.
Saskia B. Wortmann, Email: s.wortmann@salk.at.
Ekaterina Zakharova, Email: doctor.zakharova@gmail.com.
Georg F. Hoffmann, Email: georg.hoffmann@med.uni-heidelberg.de.
Thomas Meitinger, Email: thomas.meitinger@mri.tum.de.
Kei Murayama, Email: kmuraya@mri.biglobe.ne.jp.
Christian Staufner, Email: christian.staufner@med.uni-heidelberg.de.
Holger Prokisch, Email: prokisch@helmholtz-muenchen.de.
AUTHOR CONTRIBUTIONS
Dominic Lenz, Lea D. Schlieben, Masaru Shimura, Kei Murayama, Christian Staufner, and Holger Prokisch were responsible for the conception and design of the study. Data acquisition was performed by Dominic Lenz, Lea D. Schlieben, Masaru Shimura, Alyssa Bianzano, Riccardo Berutti, Rüdiger Adam, Denise Aldrian, Ivo Baric, Ulrich Baumann, Neslihan E. Bozbulut, Philip Bufler, Birutė Burnytė, Pier L. Calvo, Ellen Crushell, Buket Dalgiç, Anibh M. Das, Antal Dezsőfi, Felix Distelmaier, Alexander Fichtner, Peter Freisinger, Harald Gaspar, Louise Goujon, Nedim Hadzic, Steffen Hartleif, Sven F. Garbade, Bianca Hegen, Maja Hempel, Stephan Henning, Andre Hoerning, Roderick Houwen, Joanne Hughes, Raffaele Iorio, Katarzyna Iwanicka-Pronicka, Martin Jankofsky, Norman Junge, Ino Kanavaki, Aydan Kansu, Sonja Kaspar, Simone Kathemann, Deidre Kelly, Ceyda T. Kirsaçlioğlu, Birgit Knoppke, Martina Kohl, Heike Kölbel, Vassiliki Konstantopoulou, Tatiana Krylova, Zarife Kuloğlu, Alice Kuster, Martin W. Laass, Elke Lainka, Eberhard Lurz, Hanna Mandel, Johannes A. Mayr, Patrick McKiernan, Patricia McClean, Valerie McLin, Karine Mention, Hanna Müller, Laurent Pasquier, Natalia Pechatnikova, Bianca Peters, Danijela Petković Ramadža, Dorota Piekutowska-Abramczuk, Denisa Pilic, Sanjay Rajwal, Nathalie Rock, Agnès Roetig, René Santer, Wilfried Schenk, Natalia Semenov, Christiane Sokollik, Ekkehard Sturm, Robert W. Taylor, Eva Tschiedel, Vaidotas Urbonas, Roser Urreizti, Jan Vermehren, Jerry Vockley, Georg-Friedrich Vogel, Wendy van der Woerd, Saskia B. Wortmann, and Ekaterina Zakharova.
Lea D. Schlieben, Dmitrii Smirnov, Robert Kopajtich, Theresa Brunet, Melanie Brugger, Matias Wagner, Katharina Mayerhanser, and Thomas Meitinger interpreted genetic results. Lea D. Schlieben was responsible for the statistical analysis and data visualization. Dominic Lenz and Lea D. Schlieben drafted the article. Masaru Shimura, Kei Murayama, Christian Staufner, Holger Prokisch, Georg F. Hoffmann, Thomas Meitinger, and Stefan Kölker critically revised the content. Christian Staufner and Holger Prokisch supervised the study.
FUNDING INFORMATION
This study was supported by the BMBF (German Federal Ministry of Education and Research) through the mitoNET German Network for Mitochondrial Diseases (grant numbers 01GM1906B), PerMiM Personalized Mitochondrial Medicine (grant number 01KU2016A), the EJP RD project GENOMIT [grant number 01GM1207 and I4695-B supported by the Austrian Science Funds, FWF (to Johannes A. Mayr)], the Dietmar Hopp Foundation, St. Leon-Rot, Germany (grant number 23011235) and the German Liver Foundation (grant number S163/10052/2018). Masaru Shimura acknowledges the research support from JSPS Overseas Research Fellowships. This work was supported in part by the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development, AMED (JP22ek0109468, JP22kk0305015).
Robert W. Taylor is funded by the Wellcome Centre for Mitochondrial Research (203105/Z/16/Z), the Mitochondrial Disease Patient Cohort (UK) (G0800674), the Medical Research Council International Centre for Genomic Medicine in Neuromuscular Disease (MR/S005021/1), the Medical Research Council (MR/W019027/1), the Lily Foundation, the Pathological Society, the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust and the UK NHS Highly Specialized Service for Rare Mitochondrial Disorders of Adults and Children.
CONFLICTS OF INTEREST
Ulrich Baumann consults, advises, is on the speakers’ bureau, and received grants from Albireo and Mirum. He advises and received grants from Alexion and Astellas. He consults for Vivet. Pier Luigi Calvo advises Albireo/Ipsen, Mirum, and Nestle. Felix Distelmaier received grants from Danone, DFG, Elterninitiative Kinderkrebsklinik E.V., Margarete-Breuer Stiftung, PTC Therapeutics, Santhera, and TANGO2 Research Foundation. Elke Lainka advises Albireo and Mirum. Eberhard Lurz consults, advises, and is on the speakers’ bureau for Albireo, Mirum, Nutricia, and Takeda. The remaining authors have no conflicts to report.
REFERENCES
- 1.Squires RH, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squires JE, Alonso EM. Response to: comment on the “NASPGHAN position paper on the diagnosis and management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2022;74:138–58. [DOI] [PubMed] [Google Scholar]
- 3.Kathemann S, Bechmann LP, Sowa J-P, Manka P, Dechêne A, Gerner P, et al. Etiology, outcome and prognostic factors of childhood acute liver failure in a German Single Center. Ann Hepatol. 2015;14:722–8. [PubMed] [Google Scholar]
- 4.Hegarty R, Hadzic N, Gissen P, Dhawan A. Inherited metabolic disorders presenting as acute liver failure in newborns and young children: King’s College Hospital experience. Eur J Pediatr. 2015;174:1387–92. [DOI] [PubMed] [Google Scholar]
- 5.Haack TB, Staufner C, Köpke MG, Straub BK, Kölker S, Thiel C, et al. Biallelic mutations in NBAS cause recurrent acute liver failure with onset in infancy. Am J Hum Genet. 2015;97:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staufner C, Haack TB, Köpke MG, Straub BK, Kölker S, Thiel C, et al. Recurrent acute liver failure due to NBAS deficiency: Phenotypic spectrum, disease mechanisms, and therapeutic concepts. J Inherit Metab Dis. 2016;39:3–16. [DOI] [PubMed] [Google Scholar]
- 7.Staufner C, Peters B, Wagner M, Alameer S, Barić I, Broué P, et al. Defining clinical subgroups and genotype–phenotype correlations in NBAS-associated disease across 110 patients. Genet Med. 2020;22:610–21. [DOI] [PubMed] [Google Scholar]
- 8.Casey JP, McGettigan P, Lynam-Lennon N, McDermott M, Regan R, Conroy J, et al. Identification of a mutation in LARS as a novel cause of infantile hepatopathy. Mol Genet Metab. 2012;106:351–8. [DOI] [PubMed] [Google Scholar]
- 9.Lenz D, Smith DEC, Crushell E, Husain RA, Salomons GS, Alhaddad B, et al. Genotypic diversity and phenotypic spectrum of infantile liver failure syndrome type 1 due to variants in LARS1. Genet Med. 2020;22:1863–73. [DOI] [PubMed] [Google Scholar]
- 10.Cousin MA, Conboy E, Wang J-S, Lenz D, Schwab TL, Williams M, et al. RINT1 Bi-allelic variations cause infantile-onset recurrent acute liver failure and skeletal abnormalities. Am J Hum Genet. 2019;105:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel GF, Mozer-Glassberg Y, Landau YE, Schlieben LD, Prokisch H, Feichtinger RG, et al. Genotypic and phenotypic spectrum of infantile liver failure due to pathogenic TRMU variants. Genet Med. 2022;25:100314. [DOI] [PubMed] [Google Scholar]
- 12.Hegarty R, Gibson P, Sambrotta M, Strautnieks S, Foskett P, Ellard S, et al. Study of acute liver failure in children using next generation sequencing technology. J Pediatr. 2021;236:124–30. [DOI] [PubMed] [Google Scholar]
- 13.Robinson PN, Köhler S, Bauer S, Seelow D, Horn D, Mundlos S. The human phenotype ontology: A tool for annotating and analyzing human hereditary disease. Am J Hum Genet. 2008;83:610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berutti R, Schwarzmayr T, Strom TM. Exome Variant Annotation Database 2022.
- 15.Zech M, Jech R, Boesch S, Škorvánek M, Weber S, Wagner M, et al. Monogenic variants in dystonia: An exome-wide sequencing study. Lancet Neurol. 2020;19:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25:1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, del Angel G, Levy-Moonshine A, et al. From FastQ data to high-confidence variant calls: The genome analysis toolkit best practices pipeline. Curr Protoc Bioinforma. 2013;43:11.10.1–11.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plagnol V, Curtis J, Epstein M, Mok KY, Stebbings E, Grigoriadou S, et al. A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics. 2012;28:2747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner M, Berutti R, Lorenz-Depiereux B, Graf E, Eckstein G, Mayr JA, et al. Mitochondrial DNA mutation analysis from exome sequencing—a more holistic approach in diagnostics of suspected mitochondrial disease. J Inherit Metab Dis. 2019;42:909–17. [DOI] [PubMed] [Google Scholar]
- 20.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Wang K. InterVar: Clinical interpretation of genetic variants by the 2015 ACMG-AMP Guidelines. Am J Hum Genet. 2017;100:267–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene D, Richardson S, Turro E. OntologyX: A suite of R packages for working with ontological data. Bioinformatics. 2017;33:1104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lonlay P, Valnot I, Barrientos A, Gorbatyuk M, Tzagoloff A, Taanman J-W, et al. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat Genet. 2001;29:57–60. [DOI] [PubMed] [Google Scholar]
- 24.Kleine-Eggebrecht N, Staufner C, Kathemann S, Elgizouli M, Kopajtich R, Prokisch H, et al. Mutation in ITCH gene can cause syndromic multisystem autoimmune disease with acute liver failure. Pediatrics. 2019;143:e20181554. [DOI] [PubMed] [Google Scholar]
- 25.Lenz D, Stahl M, Seidl E, Schöndorf D, Brennenstuhl H, Gesenhues F, et al. Rescue of respiratory failure in pulmonary alveolar proteinosis due to pathogenic MARS1 variants. Pediatr Pulmonol. 2020;55:3057–66. [DOI] [PubMed] [Google Scholar]
- 26.Calvo PL, Tandoi F, Haak TB, Brunati A, Pinon M, Olio DD, et al. NBAS mutations cause acute liver failure: When acetaminophen is not a culprit. Ital J Pediatr. 2017;43:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz D, McClean P, Kansu A, Bonnen PE, Ranucci G, Thiel C, et al. SCYL1 variants cause a syndrome with lowγ-glutamyl-transferase cholestasis, acute liver failure, and neurodegeneration (CALFAN. Genet Med. 2018;20:1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vavassori S, Chou J, Faletti LE, Haunerdinger V, Opitz L, Joset P, et al. Multisystem inflammation and susceptibility to viral infections in human ZNFX1 deficiency. J Allergy Clin Immunol. 2021;148:381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas L, Müller M, Schumann G, Weidemann G, Klein G, Lunau S, et al. Consensus of DGKL and VDGH for interim reference intervals on enzymes in serum Konsensus von DGKL und VDGH zu vorläufigen Referenzbereichen für Serumenzyme. J Lab Med. 2005;29:301–8. [Google Scholar]
- 30.Narkewicz MR, Horslen S, Hardison RM, Shneider BL, Rodriguez-Baez N, Alonso EM, et al. A learning collaborative approach increases specificity of diagnosis of acute liver failure in pediatric patients. Clin Gastroenterol Hepatol. 2018;16:1801–10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chapin CA, Burn T, Meijome T, Loomes KM, Melin-Aldana H, Kreiger PA, et al. Indeterminate pediatric acute liver failure is uniquely characterized by a CD103+CD8+ T-cell infiltrate. Hepatology. 2018;68:1087–100. [DOI] [PubMed] [Google Scholar]
- 32.Squires JE, Alonso EM, Ibrahim SH, Kasper V, Kehar M, Martinez M, et al. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper on the diagnosis and management of pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2022;74:138–58. [DOI] [PubMed] [Google Scholar]
- 33.Stenton SL, Shimura M, Piekutowska-Abramczuk D, Freisinger P, Distelmaier F, Mayr JA, et al. Diagnosing pediatric mitochondrial disease: lessons from 2,000 exomes. MedRxiv. 2021;6:21 21259171. [Google Scholar]
- 34.Ayers M, Horslen SP, Gómez AM, Squires JE. Mitochondrial hepatopathy. Pediatr Liver Dis. 2022;26:421–38. [DOI] [PubMed] [Google Scholar]
- 35.Brassier A, Ottolenghi C, Boutron A, Bertrand A-M, Valmary-Degano S, Cervoni J-P, et al. Dihydrolipoamide dehydrogenase deficiency: A still overlooked cause of recurrent acute liver failure and Reye-like syndrome. Mol Genet Metab. 2013;109:28–32. [DOI] [PubMed] [Google Scholar]
- 36.Jankowska I, Czubkowski P, Rokicki D, Lipiński P, Piekutowska-Abramczuk D, Ciara E, et al. Acute liver failure due to DGUOK deficiency–is liver transplantation justified. Clin Res Hepatol Gastroenterol. 2021;45:101408. [DOI] [PubMed] [Google Scholar]
- 37.Grabhorn E, Tsiakas K, Herden U, Fischer L, Freisinger P, Marquardt T, et al. Long-term outcomes after liver transplantation for deoxyguanosine kinase deficiency: A single-center experience and a review of the literature. Liver Transpl. 2014;20:464–72. [DOI] [PubMed] [Google Scholar]
- 38.Owen MJ, Lefebvre S, Hansen C, Kunard CM, Dimmock DP, Smith LD, et al. An automated 13.5 hour system for scalable diagnosis and acute management guidance for genetic diseases. Nat Commun. 2022;13:4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of Singleton and Trio, rapid genome and exome sequencing in ill infants. Am J Hum Genet. 2019;105:719–33. [DOI] [PMC free article] [PubMed] [Google Scholar]