Abstract
Background:
The addition of immune checkpoint inhibitors to neoadjuvant chemotherapy in operable advanced gastric or gastroesophageal junction (G/GEJ) cancer aroused wide interest. This study was designed to assess the efficacy and safety of neoadjuvant sintilimab, a programmed cell death protein-1 (PD-1) inhibitor, in combination with fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy for HER2-negative locally advanced G/GEJ cancer.
Methods:
Eligible patients with clinical stage cT4 and/or cN+M0 G/GEJ cancer were enroled in this phase II study. Patients received neoadjuvant sintilimab (200 mg every 3 weeks) for three cycles plus FLOT (50 mg/m2 docetaxel, 80 mg/m2 oxaliplatin, 200 mg/m2 calcium levofolinate, 2600 mg/m2 5-fluorouracil every 2 weeks) for four cycles before surgery, followed by four cycles of adjuvant FLOT with same dosages after resection. The primary endpoint was the pathological complete response (pCR) rate.
Results:
Thirty-two patients were enroled between August 2019 and September 2021, with a median follow-up of 34.8 (95% CI, 32.8–42.9) months. Thirty-two (100%) patients received neoadjuvant therapy, and 29 underwent surgery with an R0 resection rate of 93.1%. The pCR (TRG0) was achieved in 5 (17.2%; 95% CI, 5.8–35.8%) patients, and the major pathological response was 55.2%. Twenty-three (79.3%) patients had T downstaging, 21 (72.4%) had N downstaging, and 19 (65.5%) had overall TNM downstaging. Six (20.7%) patients experienced recurrence. Patients achieving pCR showed better event-free survival (EFS), disease-free survival (DFS), and overall survival (OS) than non-pCR. The estimated 3-year EFS rate, 3-year DFS rate, and 3-year OS rate were 71.4% (95% CI, 57.2–89.2%), 78.8% (95% CI, 65.1–95.5%), and 70.9% (95% CI, 54.8–91.6%), respectively. The objective response rate and disease control rate were 84.4% (95% CI, 68.3–93.1%) and 96.9% (95% CI, 84.3–99.5%), respectively. Twenty-five (86.2%) received adjuvant therapy. The main grade ≥3 treatment-related adverse events (TRAEs) were lymphopenia (34.4%), neutropenia (28.1%), and leukopenia (15.6%). no patients died from TRAE. The LDH level exhibited a better predictive value to pathological responses than PD-L1 and MSI status.
Conclusions:
The study demonstrated an encouraging efficacy and manageable safety profile of neoadjuvant sintilimab plus FLOT in HER2-negative locally advanced G/GEJ cancer, which suggested a potential therapeutic option for this population.
Keywords: sintilimab, FLOT, G/GEJ cancer, gastrointestinal neoplasms, neoadjuvant therapy
Introduction
Highlights
First study evaluating neoadjuvant sintilimab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel in resectable gastric or gastroesophageal junction cancer.
Favourable pathologic response and survival and safety profiles with this combination.
Patients achieving pathological complete response had better event-free survival, disease-free survival, and overall survival.
Lactate dehydrogenase level may be a predictor of pathologic response for resectable gastric or gastroesophageal junction cancer.
Gastric cancer (GC), including gastroesophageal junction (GEJ) cancer, is the fourth deadly leading cancer worldwide with almost 770 000 deaths annually and the highest mortality and incidence rates reported in Eastern Asia.1 Although the consensus on surgical resection has resulted in the improvement of curative effect during the past decades, most patients relapsed after surgery.2,3 Neoadjuvant therapy, as a well-established practice, has improved overall survival (OS) and tumour response beyond surgery alone in gastric (G) or GEJ (G/GEJ) cancer with the advantages of reducing tumour burden and assessing tumour response before surgery.4,5 Especially, accumulating randomized clinical trials have demonstrated the survival benefits of the addition of perioperative chemotherapy to surgery alone.6–9 Thus, this strategy was established as one of the standard treatments for resectable G/GEJ cancers. Nonetheless, overall improvements following the neoadjuvant chemotherapy were poor and disappointing, with pathological complete response (pCR) of 2–16%8,10,11 and a 5-year survival rate of 36–45%.6–8 Therefore, therapeutic breakthroughs in the neoadjuvant setting are still emphasized to improve pathological regression and long-term survival for patients with locally advanced G/GEJ cancer.
At present, based on the encouraging efficacy of immune checkpoint inhibitors (ICIs) in advanced G/GEJ cancer,12,13 the addition of ICIs to neoadjuvant chemotherapy has also raised great interest in treating patients with locally advanced, resectable G/GEJ cancer. Several trials have shown that the neoadjuvant therapy applied pre-surgically of ICIs plus chemotherapy played a pivotal role in radical treatment for this population.14–18 Despite the promising margin-free (R0) resection rates of 97.0–100.0% and pCR rates of 10.0–33.0%,14–18 the preliminary outcomes of these reports were few and limited to early-phase trials. Furthermore, numerous questions including the optimal choice of ICI and chemotherapy backbone and predictive roles of potential reliable biomarkers for response and survival remained unanswered. Therefore, while the addition of ICIs to neoadjuvant chemotherapy shows great potential in the treatment of locally advanced, resectable G/GEJ cancer, further research is needed to address the remaining uncertainties and optimize treatment strategies.
Sintilimab is a humanized IgG4 monoclonal antibody against programmed cell death protein-1 (PD-1),19 with higher binding affinity to human PD-1 than well-studied and approved PD-1 inhibitors like nivolumab and pembrolizumab.20 Sintilimab has shown promising anti-tumour efficacy and manageable safety profiles in various solid tumours.19,21 Notably, early results of a phase 2 study demonstrated an encouraging efficacy profile of sintilimab in combination with capecitabine and oxaliplatin (CapeOx) as neoadjuvant therapy for locally advanced, resectable G/GEJ cancer, with acceptable safety outcomes.18 Therefore, the combination of sintilimab and chemotherapy could be considered a potential neoadjuvant therapy for locally advanced G/GEJ cancer, warranting further investigation. Of note, fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) as a combination chemotherapy regimen was recommended in our study rather than the commonly used SOX regimen (S-1 plus oxaliplatin) in East Asia. A phase II randomized clinical trial in Chinese patients with locally advanced GC has demonstrated favourable safety and no significant differences in clinical downstaging, pathological response, and radiological response between neoadjuvant FLOT and SOX regimen.22 However, there was no data regarding FLOT plus ICI in the neoadjuvant setting for patients with locally advanced G/GEJ cancer. Taken together, this phase II study was conducted to assess the efficacy and safety of neoadjuvant sintilimab plus FLOT in patients with locally advanced G/GEJ cancer. We also investigated the relationships between potential biomarkers, tumour genetic profiles, and pCR.
Methods
Study design
This study was an investigator-initiated, single-arm, open-label, phase 2 study. It was approved by the Ethics Committee of the Hospital. The primary goal of this study was to evaluate the efficacy and safety of neoadjuvant sintilimab plus FLOT chemotherapy in patients with HER2-negative locally advanced G/GEJ cancer. The study was conducted in accordance with the International Conference on Harmonization of Good Clinical Practice guidelines, the Declaration of Helsinki, and applicable local laws and regulations. The patients were informed of the investigational nature of the study and provided written informed consent before enrolment. The work has been reported in line with the STROCSS criteria.23 Supplemental Digital Content 1, http://links.lww.com/JS9/B804.
Patient population
Eligible patients were previously untreated and aged between 18 and 75 years with histologically confirmed G/GEJ cancer. Patients had clinical stage cT4 and/or cN+M0 by endoscopic ultrasound or enhanced computed tomography (CT)/MRI according to the eighth edition of the American Joint Committee on Cancer TNM staging system.24 The tumour must be resectable before neoadjuvant therapy as evaluated by surgeons. Further inclusion criteria were Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, adequate hematopoietic, hepatic, and renal functions, availability to provide a tumour sample to evaluate PD-1 and its ligand (PD-L1) and microsatellite instability (MSI) status.
Patients who experienced active autoimmune diseases or had other primary malignancies at screening were ineligible for this study. Patients with active hepatitis B or C, chronic enteritis, prior allergy or intolerance to study drugs or their excipients, or human immunodeficiency virus (HIV)-positive were excluded from the study. Pregnant or lactating patients, as well as patients with childbearing potential who did not use contraception if sexually active, were also excluded. Detailed eligibility criteria were listed in Additional File 1, Supplemental Digital Content 2, http://links.lww.com/JS9/B805.
Procedures
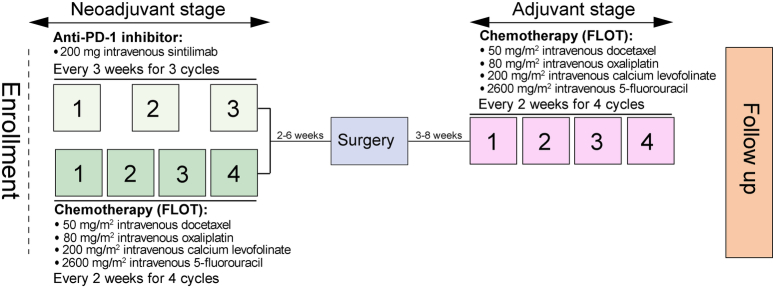
Neoadjuvant sintilimab plus FLOT were administered before surgery. Eligible patients received 200 mg intravenous sintilimab on day 1 every 3 weeks for 3 cycles. The investigator’s choice of FLOT chemotherapy consisted of 1-h 50 mg/m2 docetaxel, 2-h 80 mg/m2 oxaliplatin, and 200 mg/m2 calcium levofolinate and 24-h 2600 mg/m2 5-fluorouracil on day 1. All chemotherapy was administered intravenously and repeated every 2 weeks for 4 cycles. An overview of the therapeutic procedures was shown in Fig. 1.
Figure 1.
Treatment schedule. The treatment procedures for patients with locally advanced resectable G/GEJ cancer. FLOT, =fluorouracil, leucovorin, oxaliplatin, and docetaxel; G/GEJ, =gastric or gastroesophageal junction; PD-1, programmed death 1.
The criteria of dose modification or interruption were applicable and specified in the protocol. The dose reduction of sintilimab was not considered. The first chemotherapy dose was allowed to fluctuate up to 10% of the recommended dosage depending on the patient’s general health status. A maximum of two dose reductions of chemotherapy (to 80% or even 60% of the initial dose and then discontinuation) was permitted when serious treatment-related adverse events (TRAEs) occurred. The decision to discontinue medication was based on patients’ choices and investigators’ judgments.
Surgery was scheduled within 2–6 weeks after completion of neoadjuvant therapy for patients without tumour progression by preoperative imaging assessment. Patients with resectable disease proceeded to gastrectomy with D2 lymph node dissection, and the scope of resection was determined by the location and extent of the primary tumour according to Japanese gastric cancer treatment guidelines (version 4).25 Subsequent treatment regimens were confirmed by the investigators based on the assessment of patients’ clinical conditions if disease progression or metastasis occurred during the neoadjuvant therapy. Adjuvant chemotherapy with the FLOT regimen at the same dosages was recommended for four cycles at 3–8 weeks after surgery.
Assessments
Laboratory tests (including complete blood counts, chemistry, urinalysis, thyroid function tests, and electrocardiograms), physical examination, ECOG PS, gastroscopy with biopsy, and pathological evaluation were performed at baseline and before the start of each treatment cycle. Safety was assessed by TRAEs; TRAEs since the initiation of neoadjuvant therapy until the last dose of adjuvant therapy were graded according to the National Cancer Institute’s Common Toxicity Criteria for Adverse Events (NCI-CTCAE), version 5.0.26 Perioperative morbidity and mortality were recorded. Patients were followed up every 2–3 months after the completion of postoperative treatment until death. Postoperative surgical complications were graded according to the Clavien–Dindo classification.27
Tumour responses were assessed by two independent pathologists using Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST v1.1)28 with MRI or CT at baseline and every 2 cycles. Imaging studies were also performed before surgical resection to confirm resectability and response and before the first cycle of postoperative treatment for routine examination. Tumour staging was performed at baseline (clinical TNM Classification of Malignant Tumours: cTNM) and after surgery (post-neoadjuvant pathologic TNM Classification of Malignant Tumours: ypTNM) according to the 8th edition of the American Joint Committee on Cancer (AJCC) classification. Pathological regression in the primary tumour and lymph nodes after surgery was graded according to the Ryan criteria for tumour regression grading (TRG):29 TRG0 (no viable cancer cells), which was equivalent to a pCR; TRG1 (single cells or small groups of cancer cells, moderate response); TRG2 (residual cancer outgrown by fibrosis, minimal response); and TRG3 (minimal or no tumour cells killed, poor response).
Biomarker analysis
Tumour specimens from gastroscopy biopsy and surgical resection were collected for biomarker analysis. PD-L1 expression was assessed in formalin-fixed, paraffin-embedded (FFPE) tumour samples with the PD-L1 immunohistochemistry (IHC) 22C3 pharmDx assay (Dako, Glostrup, Denmark). A combined positive score (CPS) was used to characterize the PD-L1 expression. PD-L1 positivity was defined as CPS greater than or equal to 1, calculated as the number of all PD-L1-positive cells (tumour cells, lymphocytes, and macrophages) divided by the number of all tumour cells ×100.
The MSI/mismatch repair (MMR) status was assessed by next-generation sequencing (NGS) or IHC for MLH1, MSH2, MSH6, and PMS2.
Multiplex immunofluorescence (MIF) staining was used to evaluate the expression of CD8, FoxP3, CD3, CD19, and CD68 on tumour and/or tumour-infiltrating immune cells. Details regarding NGS, IHC, and MIF staining were provided in Additional Files 2–4, Supplemental Digital Content 2, http://links.lww.com/JS9/B805.
Venous blood samples were taken at baseline, preoperatively, and on day 7, one month, three months, and six months postoperatively and centrifuged to obtain the supernatant. The lactate dehydrogenase (LDH) activity, one feature of venous blood composition, was detected using a BECKMAN AU680 automatic biochemical analyzer (BECKMAN), according to manufacturers’ declarations.
Nanostring-based multigene assay
The NanoString nCounter platform was developed to measure the expression of 750 immune-related human genes from the FFPE-derived tumour tissues. The detailed method was presented in Additional File 5, Supplemental Digital Content 2, http://links.lww.com/JS9/B805.
Endpoints
The primary endpoint was the pCR rate, which was defined as the proportion of patients with grade 0 regression (TRG0). The secondary endpoints were objective response rate [ORR, calculated as the proportion of patients achieving complete response (CR) and partial response (PR)], disease control rate [DCR, referred to the proportion of patients with CR, PR, and stable disease (SD)], OS (defined as the period from enrolment to all-caused death), disease-free survival (DFS, defined as the time from surgery to disease progression, relapse, or death from any cause), event-free survival (EFS, defined as the time from enrolment to the disease progression that precluded surgery, postoperative disease progression or recurrence, or death from any cause), major pathological response (MPR) rate (defined as the proportion of patients with TRG0 and TRG1), R0 resection rate, downstaging, and safety. The exploratory endpoints were the pre-treatment and post-treatment dynamics of alterations in the tumour immune microenvironment or biomarkers and their correlation with the pathological response to the study treatment.
Statistical analysis
The sample size of 32 patients was determined based on the exploratory nature of this study.
Analyses of the primary endpoint and secondary endpoints (including DFS, MPR rate, R0 resection rate, downstaging, rate of each TRG after surgery, and surgical complications) were performed in the surgery set, which was defined as all eligible patients who received at least one dose of the study drug and underwent surgery based on an intention-to-treat (ITT) basis. EFS, OS, ORR, and DCR were analyzed in the full analysis set (FAS), which was defined as all patients who had received at least one dose of the study drug, regardless of whether they underwent surgery. Safety analysis was performed in the safety analysis set (SAS), which was defined as all patients who received the study drug medication at least once and had safety records. The biomarker population was defined as patients with at least one evaluable tumour sample.
Categorical variables were summarized as frequencies [percentage (%)], and continuous variables were presented as medians with interquartile range (IQR) or range. The 95% CI of the pCR, MPR rate, R0 resection rate, ORR, and DCR were calculated using the Clopper-Pearson method. DFS, EFS, and OS were calculated using the Kaplan–Meier method with a 95% CI. The log-rank test determined survival differences across subgroups. Median follow-up was calculated using reverse Kaplan–Meier method. Patients without documented evidence of an event were censored at the date of last follow-up. Wilcoxon matched-pairs signed-rank test was used to assess the significant difference in serum LDH levels between the two groups. The diagnostic accuracy was determined using receiver operating characteristic curves (ROC) and its corresponding area under the ROC curve (AUC) and the optimal cut-off point with its corresponding sensitivity and specificity. Statistical comparisons of baseline characteristics between TRG0 and TRG1-3 groups according to clinicopathological variables were performed using Fisher’s exact test. Statistics of density in immune cells between TRG0 and TRG3 groups were analyzed using the two-tailed student’s t-tests. In order to identify differentially expressed genes related to the TRG, we performed the Mann–Whitney U test. All statistical tests were two-sided, with significance set at P less than 0.05. All analyses were conducted using R Statistics software version 4.1.0.
Results
Patient characteristics
Between August 2019 and September 2021, 35 patients were screened and 3 were excluded from the enrolment due to the use of banned treatment defined in this study. A total of 32 patients were enroled and received study treatment and were thus included in the FAS and SAS (Fig. 2). Besides, 3 patients did not proceed with surgery because of disease progression (n=1) and refusal of surgery (n=2); thus, 29 patients were included in the surgery set.
Figure 2.
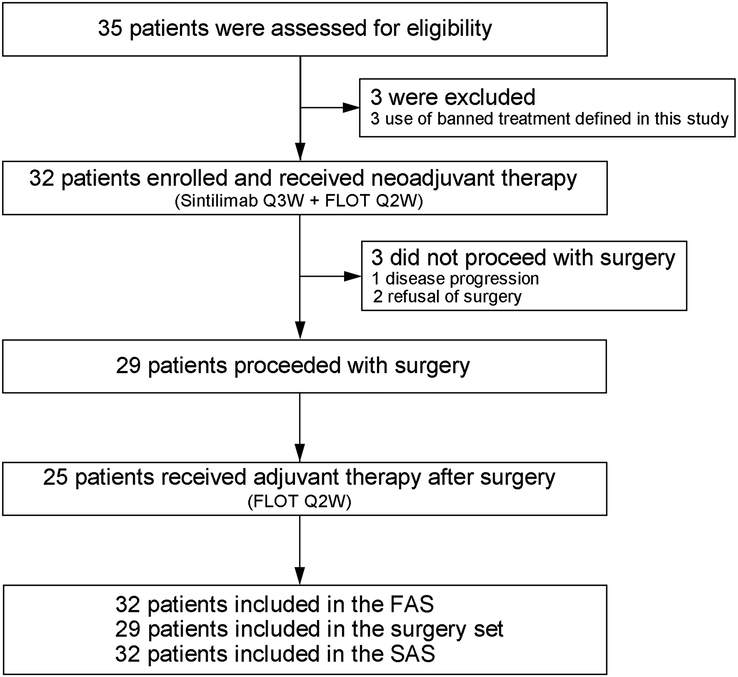
Consort diagram. FAS, full analysis set; FLOT, fluorouracil, leucovorin, oxaliplatin, and docetaxel; Q2W, every 2 weeks; Q3W, every 3 weeks; SAS, safety analysis set.
The baseline characteristics of 32 patients were shown in Table 1. The median age was 58 (range, 43–70) years and the majority of the patients were male (25/32, 78.1%). Most of the primary tumours were located in the G (26/32, 81.3%) and all cases had clinical stage III disease. The baseline clinical tumour stage of cT4a and the clinical node stage of N+ were observed in 19 (59.4%) and 32 (100.0%) patients, respectively. Twenty-nine (90.6%) patients were assessed for PD-L1 expression; of whom 13 (44.8%) had a CPS of greater than or equal to 1. All patients were evaluated for MSI status and most tumours (26/32, 81.3%) were MSI-low/microsatellite stability (MSS). There were no significant differences in clinical characteristics between the TRG0 and TRG1-3 groups (Additional File 6, Supplemental Digital Content 2, http://links.lww.com/JS9/B805).
Table 1.
Baseline characteristics.
| Characteristics | All patients (n=32) |
|---|---|
| Age, years-median (range) | 58 (43–70) |
| Sex, n (%) | |
| Male | 25 (78.1) |
| Female | 7 (21.9) |
| Primary tumour location, n (%) | |
| Gastric | 26 (81.3) |
| Gastric-oesophageal junction | 6 (18.7) |
| Histologic grade, n (%) | |
| Moderately differentiated | 7 (21.9) |
| Poorly differentiated | 19 (59.4) |
| Not evaluable | 6 (18.7) |
| Clinical T-stage, n (%) | |
| cT3 | 13 (40.6) |
| cT4a | 19 (59.4) |
| Clinical N-stage, n (%) | |
| cN0 | 0 |
| cN+ | 32 (100.0) |
| MSI status, n (%) | |
| MSI-H | 2 (6.3) |
| MSS | 26 (81.3) |
| Not reported or invalid | 4 (12.5) |
| PD-L1 status | 29 (90.6) |
| CPS <1a | 16 (55.2) |
| CPS ≥1a | 13 (44.8) |
| Clinical tumour, node, metastases (TNM) stage, n (%) | |
| III | 32 (100.0) |
| ECOG PS, n (%) | |
| 0 | 8 (25.0) |
| 1 | 24 (75.0) |
Data are presented as the median (IQR) or n (%).
CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group Performance Score; MSI, microsatellite instability; MSI-H, MSI-high; MSS, MSI-low/microsatellite stability; PD-L1, programmed cell death protein-1 and its ligand.
The proportion was calculated in the 29 patients with evaluable PD-L1 status.
Treatment
Of patients with neoadjuvant therapy, one requested surgery following three cycles of neoadjuvant therapy and one received only one cycle of sintilimab and three cycles of FLOT due to grade 3 increased alanine aminotransferase (ALT).
Totally 29 patients proceeded with gastrectomy with D2 lymph node dissection, with a mean time between the last neoadjuvant therapy and surgery of 26.8 (range, 19–73) days. Two (6.9%) patients underwent R1 resection with positive surgical margins; the R0 resection rate was 93.1% (95% CI, 77.2–99.2%). The mean number of lymph nodes resected was 26 (range, 14–53). The mean operative time was 219.1 (range, 166.0–272.2) min. The mean intraoperative blood loss was 210 (range, 14–406) ml. The mean length of postoperative hospital stay was 12 (range, 7–32; Additional File 7, Supplemental Digital Content 2, http://links.lww.com/JS9/B805) days.
Postoperative adjuvant therapy was initiated in 25 (86.2%) patients with 4 discontinuations due to the COVID-19 pandemic. Among them, 12 (41.4%) completed 4 cycles; while 5 (17.2%) received two, and 8 (27.6%) received three cycles of adjuvant therapy.
Pathological responses and survival outcomes
Based on the pathological evaluation of 29 patients in the surgery set, pCR (TRG0) was achieved in 5 (17.2%; 95% CI, 5.8–35.8%) patients. TRG1, TRG2, and TRG3 were observed in 11 (37.9%), 10 (34.5%), and 3 (10.3%) patients, respectively (Table 2 and Fig. 3). The MPR (TRG0/1) rate was 55.2% (95% CI, 35.7–73.6%). Compared with the clinical stage before treatment, 23 (79.3%) patients had T downstaging, 21 (72.4%) had N downstaging, and 19 (65.5%) had overall TNM downstaging.
Table 2.
Radiological response in the FAS and pathological response in the surgery set.
| Best responses | |
|---|---|
| Radiological evaluation, n (%) | Patients (n=32) |
| CR | 4 (12.5) |
| PR | 23 (71.9) |
| SD | 4 (12.5) |
| PD | 1 (3.1) |
| ORR | 27 (84.4, 68.3–93.1%) |
| DCR | 31 (96.9, 84.3–99.5%) |
| Pathological evaluation, n (%) | Patients (n=29) |
| Pathology T-stage | |
| ypT0 | 5 (17.2) |
| ypT1 | 5 (17.2) |
| ypT2 | 4 (13.8) |
| ypT3 | 10 (34.5) |
| ypT4 | 3 (10.3) |
| ypTx | 2 (6.9) |
| Downing T | 23 (79.3) |
| Pathology N-stage | |
| ypN0 | 16 (55.2) |
| ypN1 | 7 (24.1) |
| ypN2 | 4 (13.8) |
| ypN3 | 2 (6.9) |
| Downing N | 21 (72.4) |
| Nerve invasion | |
| 0 | 17 (58.6) |
| 1 | 8 (27.6) |
| Unknown | 4 (13.8) |
| Vessel invasion | |
| 0 | 14 (48.3) |
| 1 | 11 (37.9) |
| Unknown | 4 (13.8) |
| Pathological regression (as per the Mandard criteria) | |
| TRG0 (pCR rate) | 5 (17.2) |
| TRG1 | 11 (37.9) |
| TRG2 | 10 (34.5) |
| TRG3 | 3 (10.3) |
| MPR rate | 16 (55.2) |
| TNM stage after operation | |
| ypT0N0 | 5 (17.2) |
| Stage I | 8 (27.6) |
| Stage II | 11 (37.9) |
| Stage III | 5 (17.2) |
Data are presented as n (%) or n (%, 95% CI).
CR, complete response; DCR, disease control rate; FAS, full analysis set; MPR, major pathological response; ORR, overall response rate; pCR, pathological complete response; PD, progressive disease; PR, partial response; SD, stable disease; TNM, tumour/node/metastases; TRG, tumour regression grading.
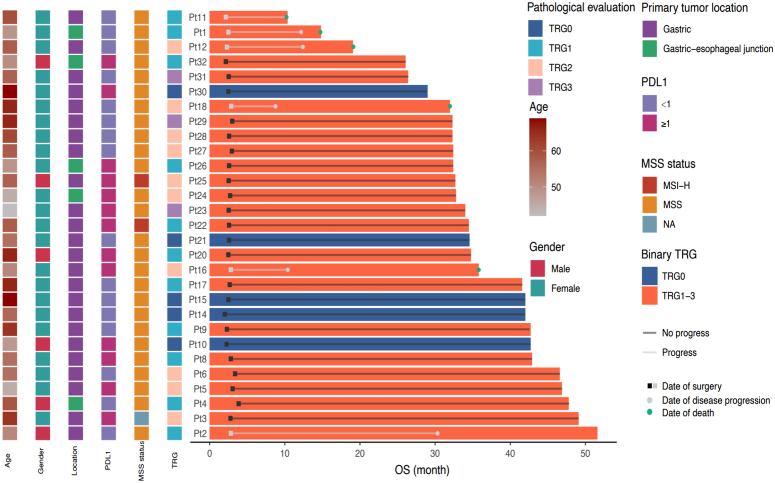
Figure 3.
Pathological response in the surgery set. The pathological response of each patient was assessed according to the Ryan criteria (n=29). MSI-H, high microsatellite instability; MSS, MSI-low/microsatellite stability; OS, overall survival; PD-L1, programmed death 1 and its ligand; Pt, patient; TRG, tumour regression grading.
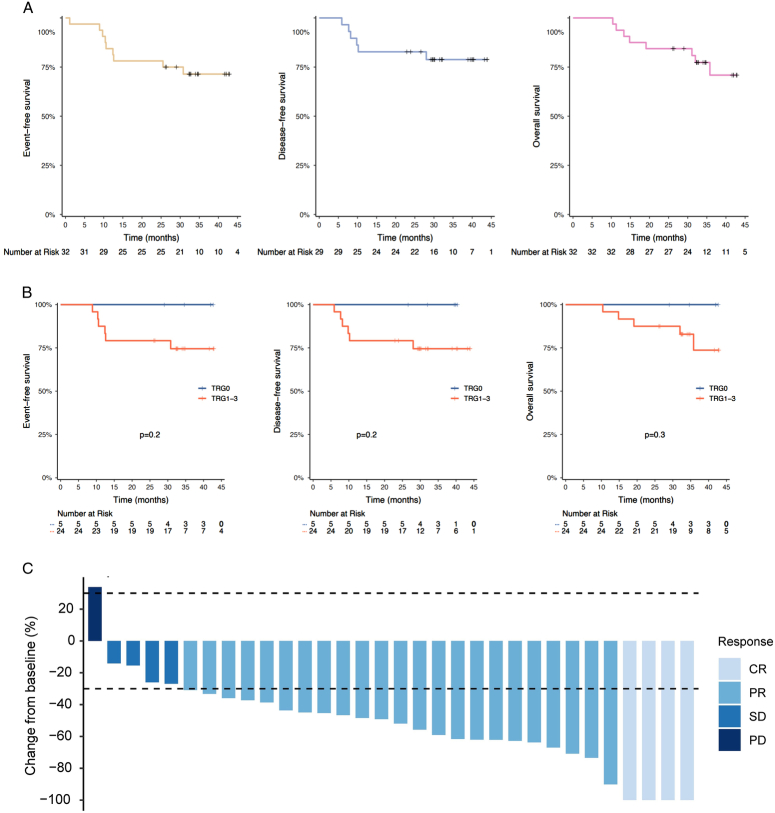
At the cut-off date of 1 November 2023, the median follow-up for surviving patients was 34.8 (95% CI, 32.8–42.9) months and 8 (25%) of 32 patients in the FAS population had died and 6 (20.7%) of 29 patients in the surgical setting had relapsed. Totally 11 (34.4%) patients were follow-up for over 3 years. Median DFS, median EFS, and median OS were not reached. The EFS, DFS, OS rates at 2 years were respectively estimated as 75% (95% CI, 61.4–91.6%), 82.8% (95% CI, 70.1–97.7%), and 84.4% (95% CI, 72.7–97.9%). The estimated 3-year EFS rate, 3-year DFS rate, and 3-year OS rate were 71.4% (95% CI, 57.2–89.2%), 78.8% (95% CI, 65.1–95.5%), and 70.9% (95% CI, 54.8–91.6%), respectively (Fig. 4A). Patients who achieved pCR (TRG0) showed numerically better EFS, DFS, and OS than patients with non-pCR (TRG1-3) (Fig. 4B). Of all 32 patients with preoperative imaging assessment, ORR was observed in 27 patients (84.4%; 95% CI, 68.3–93.1%), including 4 (12.5%) CR and 23 (71.9%) PR (Table 2). Four (12.5%) had SD per RECIST v1.1; thus, the DCR was 96.9% (95% CI, 84.3–99.5%). Only 1 (3.1%) had progressive disease (PD) before surgery. A total of 31 (96.9%) patients had tumour shrank and the best change in target lesion diameter from baseline was shown in Fig. 4C.
Figure 4.
Survival outcomes and tumour radiological response. (A) Kaplan–Meier curves for event-free survival and overall survival in the FAS population (n=32), and disease-free survival in the surgery set (n=29). (B) Kaplan–Meier curves for event-free survival, overall survival and disease-free survival in the surgery set (n=29) under the stratification factor of TRG status. (C) Waterfall plot of tumour size change from baseline to maximum percentage in FAS as per RECIST version 1.1 (n=32). CR, complete response; FAS, full analysis set; PD, progressive disease; PR, partial response; RECIST, response evaluation criteria in solid tumours; SD, stable disease; TRG, tumour regression grading.
Safety and tolerability
In the SAS population, 32 (100.0%) patients experienced at least one TRAE during neoadjuvant therapy (Table 3). Common TRAEs of any grade were nausea (100.0%), decreased appetite (100.0%), hypodynamia (100.0%), lymphopenia (96.9%), anaemia (84.4%), leukopenia (75.0%), and neutropenia (65.6%). The majority of TRAEs were grade 1–2. Grade 3 or higher TRAEs occurred in 19 (59.4%) patients and mainly included lymphopenia (34.4%), neutropenia (28.1%), leukopenia (15.6%), and anaemia (12.5%). Of note, 6 (18.8%) patients had hypothyroidism, which was considered a potential immunologic cause. No grade 5 TRAEs were reported.
Table 3.
Treatment-related adverse events (TRAEs) in the SAS population.
| Safety population (n=32), n (%) | ||||
|---|---|---|---|---|
| Any grade | Grade 1–2 | Grade 3 | Grade 4 | |
| Nausea | 32 (100.0) | 31 (96.9) | 1 (3.1) | 0 |
| Decreased appetite | 32 (100.0) | 32 (100.0) | 0 | 0 |
| Hypodynamia | 32 (100.0) | 29 (90.6) | 3 (9.4) | 0 |
| Lymphopenia | 31 (96.9) | 20 (62.5) | 7 (21.9) | 4 (12.5) |
| Anaemia | 27 (84.4) | 23 (71.9) | 4 (12.5) | 0 |
| Leukopenia | 24 (75.0) | 19 (59.4) | 3 (9.4) | 2 (6.3) |
| Neutropenia | 21 (65.6) | 12 (37.5) | 5 (15.6) | 4 (12.5) |
| Alopecia | 17 (53.1) | 17 (53.1) | 0 | 0 |
| Increased alanine aminotransferase | 17 (53.1) | 16 (50.0) | 1 (3.1) | 0 |
| Increased aspartate aminotransferase | 17 (53.1) | 17 (53.1) | 0 | 0 |
| Vomiting | 14 (43.8) | 13 (40.6) | 1 (3.1) | 0 |
| Decreased platelet count | 13 (40.6) | 12 (37.5) | 1 (3.1) | 0 |
| Diarrhoea | 12 (37.5) | 10 (31.2) | 2 (6.3) | 0 |
| Hypokalemia | 7 (21.9) | 7 (21.9) | 0 | 0 |
| Hypothyroidism | 6 (18.8) | 6 (18.8) | 0 | 0 |
| Hyperbilirubinemia | 5 (15.6) | 5 (15.6) | 0 | 0 |
| Fever | 5 (15.6) | 5 (15.6) | 0 | 0 |
| Peripheral neuropathy | 4 (12.5) | 4 (12.5) | 0 | 0 |
| Subclinical hypothyroidism | 3 (9.4) | 3 (9.4) | 0 | 0 |
| Hypoproteinemia | 3 (9.4) | 3 (9.4) | 0 | 0 |
| Abdominal pain | 3 (9.4) | 2 (6.3) | 1 (3.1) | 0 |
| Febrile neutropenia | 3 (9.4) | 0 | 3 (9.4) | 0 |
| Hyperthyroidism | 2 (6.3) | 2 (6.3) | 0 | 0 |
| OEdema | 2 (6.3) | 2 (6.3) | 0 | 0 |
| Proteinuria | 2 (6.3) | 2 (6.3) | 0 | 0 |
| Dyspnoea | 1 (3.1) | 1 (3.1) | 0 | 0 |
Data are n (%).
SAS, safety analysis set.
Among 29 patients in the surgery set, 15 (51.7%) patients experienced grade 1–2 surgical complications, mainly including anaemia (44.8%), fever (13.8%), weight loss (10.3%), and intestinal obstruction (10.3%) (Table 4). No grade 3 or higher surgical complications and postoperative mortality were observed. No surgical complications leading to readmission within 30 days, emergency reoperation, or intensive care occurred.
Table 4.
Surgical complications in the surgery set.
| Patients (n=29), n (%) | |||
|---|---|---|---|
| Surgical complication | Any grade | Grade 1–2 | Grade 3 or higher |
| Anaemia | 13 (44.8) | 13 (44.8) | 0 |
| Fever | 4 (13.8) | 4 (13.8) | 0 |
| Weight loss | 3 (10.3) | 3 (10.3) | 0 |
| Intestinal obstruction | 3 (10.3) | 3 (10.3) | 0 |
| Intra-abdominal infections | 2 (6.9) | 2 (6.9) | 0 |
| Pneumonia | 2 (6.9) | 2 (6.9) | 0 |
| Bloodstream infection | 2 (6.9) | 2 (6.9) | 0 |
| Anastomotic leak | 2 (6.9) | 2 (6.9) | 0 |
| Hypoproteinemia | 1 (3.4) | 1 (3.4) | 0 |
| Pancreatic fistula | 1 (3.4) | 1 (3.4) | 0 |
| Incision infection | 1 (3.4) | 1 (3.4) | 0 |
Data are n (%).
Biomarker analysis
Regarding MSI status, only 2 patients were identified as MSI-H with high PD-L1 expression (CPS ≥1) and did not achieve the pCR (Fig. 3). Therefore, we were unable to provide information on the relationship between MSI-H and tumour response in this study.
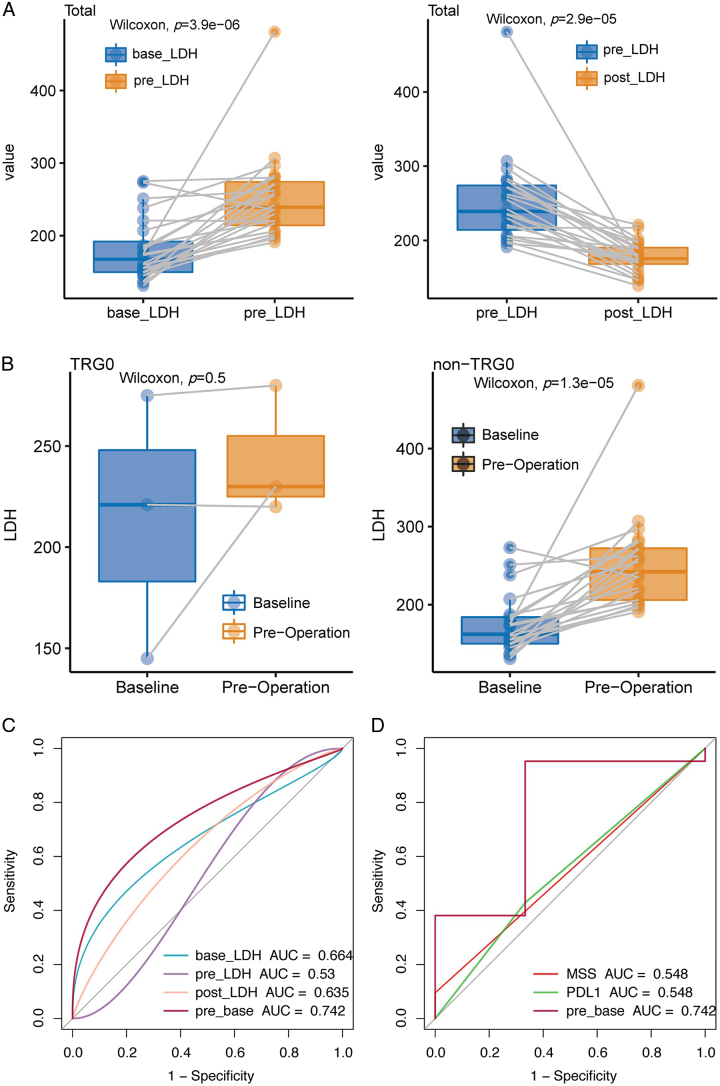
The LDH activity was detected in 24 of 29 surgical patients. Elevated LDH levels following the neoadjuvant therapy and decreased LDH after surgery were observed (Fig. 5A). In addition, we divided the patients into two groups according to pathological responses: the TGR0 group representing marked tumour regression, and the TRG1-3 group showing mild or no regression. It was found that the TRG1-3 group had a higher LDH level after neoadjuvant therapy, while there was no significant difference in the TRG0 group (Fig. 5B). We defined baseline LDH level as the base-LDH group, preoperative as the pre-LDH group, the difference between pre-LDH and base-LDH as the pre-base group, and postoperative as the post-LDH group. ROC analysis showed that LDH levels in the pre-base group exhibited a better predictive value for pathological response than LDH contents in base-LDH, pre-LDH, and post-LDH groups (Fig. 5C). Moreover, compared with PD-L1 expression and MSS/MSI-H status, LDH levels in the pre-base group could significantly differentiate patients with different pathological responses (Fig. 5D). AUC, optimal cut-off value, sensitivity, and specificity for the diagnosis of pathological response were shown in Additional File 8, Supplemental Digital Content 2, http://links.lww.com/JS9/B805.
Figure 5.
The predictive value of the LDH levels/PD-L1 expression/MSI status to pathological response. (A) Boxplots of the LDH levels between the baseline group and the pre-operation group and between the pre-operation group and the post-operation group. (B) The correlation between variations in the LDH levels after neoadjuvant therapy and pathological response (non-TRG0 and TRG0). (C) The receiver operating characteristic curves of predictive value regarding serum LDH levels at baseline (base), pre-operation (pre), post-operation (post), or between baseline and pre-operation (pre-base) to the pathological response. (D) The receiver operating characteristic curves of predictive value regarding serum LDH levels at the pre-base group, PD-L1 expression, and MSI status to the pathological response. AUC, area under the receiver operating characteristic curve; LDH, lactate dehydrogenase; MSI, microsatellite instability; MSS, MSI-low/microsatellite stability; PD-L1, programmed death 1 and its ligand; TRG, tumour regression grading.
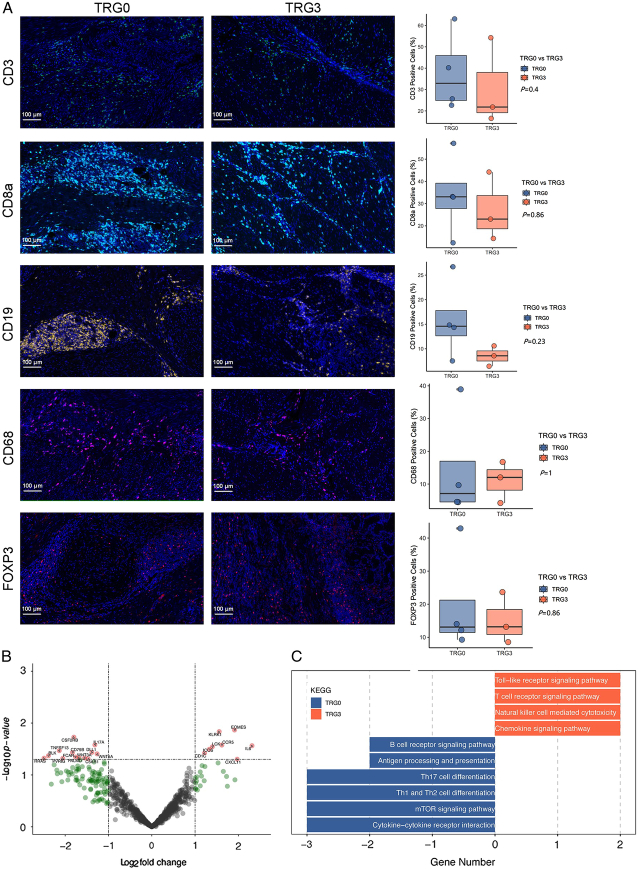
Since partial responders (TRG1 and 2) did not show a distinct immune profile, the opposite ends of the histopathological response spectrum were compared [TRG0, complete responders (CRs), n=5 vs. TRG3, non-responders (NRs), n=3]. MIF staining showed that despite no statistically significant (P>0.05), the increase of CD3+ T cells, CD8+ T cells, and CD19+ B cells, and the decrease in CD68+ tumour-associated macrophages (TAM) was observed in the TRG0 group (Fig. 6A). In addition, FoxP3+ regulatory T cells (Tregs) were not significantly different between the TRG0 and TRG3 groups. To identify transcriptional differences in the tumour immune microenvironment in patients with differential pathological responses to this combination, NanoString analysis was performed to measure the expression of 770 immune-related genes. A differential gene expression analysis comparing CRs and NRs revealed a total of 25 significantly differentially expressed genes (DEGs); therefore, 9 were upregulated and 16 were downregulated (Fig. 6B). IL6 and HRAS genes were amongst the most highly and least expressed, respectively. KEGG enrichment analysis demonstrated that the Toll-like receptor, T-cell receptor signalling pathway, natural killer cell-mediated cytotoxicity, and chemokine signalling pathway were prominent in the TRG3 group. In contrast, the mTOR signalling pathway, antigen processing and presentation, and Th17 cell differentiation significantly enriched in the TRG0 group (Fig. 6C). Collectively, these data suggested the dynamic complexity and diverse behaviour of the tumour microenvironment in patients with different pathological responses.
Figure 6.
Dynamic complexity and diverse behaviour of tumour microenvironment in patients with different pathological responses. (A) Immune cell infiltration in tumour parenchyma by multiple immunofluorescence staining. Densities of the following cells are compared between patients with TRG0 (n=5) and TRG3 (n=3): infiltrating CD3+ cells, infiltrating CD8+ cells, infiltrating CD19+ cells, infiltrating CD68+ cells, infiltrating FOXP3 cells. (B) Volcano plot displaying significantly differentially expressed immune-related genes between TRG0 and TRG3 groups. (C) Immune pathways with a significantly different expression between TRG0 and TRG3 groups per gene set enrichment analysis. TRG, tumour regression grading.
Discussion
Neoadjuvant chemotherapy has been widely utilized to enhance R0 resection rates and improve survival time for patients with locally advanced GC, given its low survival rate. The role of immunotherapy combined with chemotherapy in the perioperative treatment of G/GEJ cancers is unknown. To our knowledge, this was the first long-term follow-up study to investigate the feasibility of neoadjuvant sintilimab plus FLOT chemotherapy in patients with resectable G/GEJ cancer, which demonstrated encouraging efficacy with a pCR (ypT0N0) rate of 17.2%, an MPR rate of 55.2%, and manageable safety profiles. The estimated 3-year EFS rate, 3-year DFS rate, and 3-year OS rate were 71.4% (95% CI, 57.2–89.2%), 78.8% (95% CI, 65.1–95.5%), and 70.9% (95% CI, 54.8–91.6%), respectively. These data may provide potential evidence for the neoadjuvant PD-1 blockade plus chemotherapy for this population.
Regarding perioperative standard chemotherapy for G/GEJ cancer, the neoadjuvant FLOT regimen has been shown to achieve higher rates of pCR at 16.0% and MPR at 37.0% compared to the SOX and CapeOx regimens.8 However, there is still some controversy regarding whether neoadjuvant FLOT is equally beneficial compared to the popular SOX or XELOX regimens in East Asian patients.22 A phase II randomized trial conducted in China demonstrated that only 2% of patients in the FLOT group achieved a pathological complete regression, whereas none of the patients in the SOX group achieved pCR.22 In contrast, our study reported even more promising results, with a numerically higher rate of pCR at 17.2% and MPR at 55.2%. Additionally, the R0 resection rate of 93.1% might be slightly underestimated due to two patients refusing surgery after radiological assessment indicating CR and PR. These encouraging outcomes can be attributed to the additional therapeutic benefits of neoadjuvant ICIs in combination with chemotherapy, compared to chemotherapy alone, for advanced G/GEJ cancer.
The interim results of the DANTE trial reported a pCR rate of 25% in patients treated with a combination of atezolizumab and FLOT chemotherapy.30 It was important to note that the pCR rate in our trial (17.2% vs. 25.0%) was moderately lower compared to the DANTE trial using the same chemotherapy backbone. There were a couple of reasons that could explain this difference. Firstly, there was some controversy regarding whether neoadjuvant FLOT chemotherapy is equally beneficial in East Asian patients compared to German patients.22 In addition, due to the lack of head-to-head comparisons, it was difficult to directly compare treatment outcomes and safety profiles between anti-PD-1 and anti-PD-L1 therapies. Secondly, it was worth noting that our study had poorer baseline characteristics. All of our patients (100.0%) had locally advanced tumours (cT3/T4) and lymph node involvement (cN+), whereas the proportions in the DANTE trial were 77.0% and 78.0%, respectively.30 Therefore, it was possible that our patients had inferior outcomes compared to those in the DANTE trial.
Regarding relapse and survival, our study estimated a 3-year DFS rate of 78.8% (95% CI, 65.1–95.5%) and a 3-year OS rate of 70.9% (95% CI, 54.8–91.6%). With a sufficient follow-up period, our findings indicated that patients who achieved a pCR had numerically better EFS, DFS, and OS compared to those who did not achieve a pCR. It was important to note that previous reports on neoadjuvant immunotherapy had short follow-up time and immature OS data. Therefore, our results were mainly compared with the FLOT4 trial and showed better outcomes than what has been reported in that trial (the 3-year DFS rate was 48%, and the 3-year OS rate was 58%).31–35
Furthermore, the promising 3-year survival rates observed in our study can be partly attributed to the fact that over half of the patients (55.2%) achieved nodal downstaging to ypN0 after treatment. Nodal downstaging has been recognized as an important indicator of the effectiveness of neoadjuvant therapy for G/GEJ cancer and has a positive impact on the survival of this patient population.36–38 Additionally, our study observed a remarkable downstaging of the overall TNM stage (65.5%), which was higher compared to previously reported rates (43.3–55.2%).39,40 This downstaging indicated that neoadjuvant therapy effectively reduced the tumour burden before surgery. Moreover, the evaluation of radiological response using RECIST criteria was important for assessing the effectiveness of neoadjuvant therapy for G/GEJ cancer.41 In our study, an ORR of 84.4% was observed, and tumour shrinkage was observed in 31 out of 32 patients (96.9%). These findings suggested that neoadjuvant therapy significantly reduced the tumour size, which may have contributed to the low recurrence rate (20.7%) observed in our study.
Although prior studies showed that patients with PD-L1-positive and MSI-H may achieve prolonged response duration,42–45 no compelling conclusions regarding correlations between both and pathological response were drawn due to the limited sample size in this study. Although preoperative LDH level was an unfavourable prognosticator for the prediction of survival (OS and DFS) in patients with D2-resected GC,46 limited data were available on the changes in LDH levels on the pathological response in G/GEJ cancer. In our study, a significantly increased LDH level from baseline was observed in the non-pCR (TRG1-3) group after neoadjuvant therapy, while no obvious LDH changes in the pCR (TRG0) group; it may indicate the potential of LDH as a biomarker for selecting patients who achieved better pCR and tumour regression from neoadjuvant anti-PD-1 plus chemotherapy. These results have prompted us to consider whether different tumour regression grades correspond to varying degrees of LDH level changes from baseline; regrettably, this would require further exploration in the future, as we did not refine the subgrouping in our current study. Of note, results with better pathologic response achieved in patients with preoperative three-/four/drug chemotherapy compared to those with two-drug regimen8,9 indicated that chemotherapy intensity may affect histologic tumour regression; we reasoned that the addition of higher-intensity chemotherapy to the PD-1 inhibitors may result in stronger tumour regression, reflected in smaller or even non-significant changes in LDH levels. Overall, LDH as a potential biomarker may monitor response to therapeutic interventions and help clinicians develop more personalized treatment regimens for patients with different prognosis, warranting further investigation for prognostic value. In addition, we also found that LDH in the pre-base group (AUC=0.742), as a potential cost-effective biomarker, may have a higher predictive value to pathological response than MSI/MSI-H (AUC=0.548) or PD-L1 (AUC=0.548). Despite the provision of some insights, the results should be interpreted with caution due to the limited sample size and urge the need for further large-scale multicenter randomized trials.
Increasing evidence suggested that elevated levels of CD19+ B cells, CD3+ T cells, CD8+ T cells, and FoxP3 Tregs, as well as decreased levels of CD68+ TAMs, were significantly correlated with a better prognosis in GC.47–49 Although our study did not show statistically significant differences in outcomes, we observed a similar trend in these tumour-infiltrating lymphocytes (TILs). Whether the infiltration density of TILs is considered as the predictive biomarkers for prognosis warrants additional observation and verification in studies with a larger sample size, which may allow further customization of treatments and prediction of individualized therapeutic responses.
The safety profiles of neoadjuvant sintilimab plus FLOT chemotherapy were manageable with the most frequent TRAEs being nausea, decreased appetite, and hypodynamia, which was similar to previous findings.18,22,50 No new safety signals were identified. The lower incidence of commonly reported grade 3–4 haematological toxicity including leucopenia (15.7% vs. 28.0%) and neutropenia (28.1% vs. 52.0%) was observed in our study over the FLOT4 trial.50 It may be due to the use of a perioperative granulocyte colony-stimulating factor in the study. Moreover, it demonstrated that the addition of sintilimab might not increase toxicity mainly caused by chemotherapy. Similar outcomes were seen in a study of neoadjuvant sintilimab plus CapeOx chemotherapy.18 Of note, compared with patients with chemotherapy backbone alone (1.0%),50 12.5% experienced grade 3-4 anaemia in this study. We recommended that the surveillance for anaemia should be strengthened in the application of this combination regimen. All surgical complications were mild (grade 1–2) in our study. On the whole, our data indicated that sintilimab plus FLOT might be safe but also reminded us to pay more attention to and improve the management of AEs during immunotherapy.
There were several limitations in this study. First, since there was no available prior data for anti-PD-1 plus chemotherapy in patients with G/GEJ cancer at the time of design, the sample size was not calculated. Therefore, the study was exploratory and empirically estimated the sample size. Second, the single-arm, single-centre phase 2 design might lead to potential selection bias. In this setting, a compelling assessment of the contribution of the combination regimen was difficult. Third, the biomarker correlative analyses were exploratory. Therefore, it was limited regarding the ability to identify new biological processes associated with treatment response. The clinical response observed in this study should be further accompanied and explained with biomarker and translational studies.
Conclusions
Neoadjuvant sintilimab plus FLOT regimen showed encouraging clinical benefits and acceptable safety, providing a feasible and promising treatment option for locally advanced, resectable G/GEJ cancer. Well-designed phase 3 randomized controlled trials are warranted soon to further confirm our findings. Moreover, predictive biomarkers for pathological response and survival should also be the focus of future studies while precision medicine is awaited.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University (No. 2019214). Written informed consent was obtained from each patient. All procedures in the present study conformed to the ethical standards of the institutional and/or national research committee, Good Clinical Practice guidelines, and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All patients provided informed consent for the publication of any associated data.
Consent
Not applicable.
Source of funding
The costs of publication will be funded by grants from the Joint Funds of the National Natural Science Foundation of China (U2004132, N.L.), Leading Talents in Health Science and Technology Innovation for Young and Middle-aged People in Henan Province (YXKC2021008, N.L.), the National Natural Science Fund Youth Fund of China (82003041, C.W.), and Key Scientific and Technological Projects in Henan Province (222102310324, C.W.).
Author contribution
N.L. and C.W. designed the study and drafted the paper. Z.L., Q.F., B.Z., J.Z., X.B.W., C.M.L., J.B.W., and W.Y.D. collected the patient data. J.L., Y.J.M. and L.Y.B. reviewed the radiological results. Q.X.X. independently reviewed the pathological results (TRG). M.Y.W. collected the patient data for adverse effects. S.X.L. edited the final paper. All authors read and approved the paper for publication.
Conflicts of interest disclosure
The authors declare that they have no competing interests.
Research registration unique identifying number (UIN)
ClinicalTrials.gov NCT04341857.
Availability of data and materials
The datasets supporting the results of the present study can be obtained from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
The authors thank the Department of Pathology, the Department of General Surgery, and the GCP office of the medical institution conducting clinical trials at Henan cancer hospital.
Supplementary Material
Footnotes
N.L., Z.L., Q.F. contributed equally to this work and should be considered co-first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 5 February 2024
Contributor Information
Zhi Li, Email: 1295718200@qq.com.
Qiang Fu, Email: mmqiangqiang@163.com.
Bin Zhang, Email: binzhang666@126.com.
Jian Zhang, Email: zhangjian1971258@sina.com.
Xiang-Bin Wan, Email: wxbzlyy@126.com.
Chao-Min Lu, Email: lcmzklyx888@126.com.
Jin-Bang Wang, Email: 13623833860@163.com.
Wen-Ying Deng, Email: psc1969@sohu.com.
Yi-Jie Ma, Email: mayijie1987@126.com.
Liang-Yu Bie, Email: bieliangyu@163.com.
Meng-Yu Wang, Email: mengyu15560112382@163.com.
Jing Li, Email: lijingqingqing@163.com.
Qing-Xin Xia, Email: 13838173710@139.com.
Chen Wei, Email: 384348513@qq.com.
Su-Xia Luo, Email: zlyyluosuxia0361@zzu.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Johnston FM, Beckman M. Updates on management of gastric cancer. Curr Oncol Rep 2019;21:67. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20:167–192. [DOI] [PubMed] [Google Scholar]
- 4.Aggelis V, Cunningham D, Lordick F, et al. Peri-operative therapy for operable gastroesophageal adenocarcinoma: past, present and future. Ann Oncol 2018;29:1377–1385. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad MU, Javadi C, Poultsides GA. Neoadjuvant treatment strategies for resectable proximal gastric, gastroesophageal junction and distal esophageal cancer. Cancers (Basel) 2022;14:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 7.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–1721. [DOI] [PubMed] [Google Scholar]
- 8.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–1957. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Liang H, Li Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 2021;22:1081–1092. [DOI] [PubMed] [Google Scholar]
- 10.Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki Y, Terashima M, Mizusawa J, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer 2021;24:492–502. [DOI] [PubMed] [Google Scholar]
- 12.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123–133. [DOI] [PubMed] [Google Scholar]
- 14.Ding X, Li B, Xue Q, et al. Perioperative sintilimab combination with SOX for resectable locally advanced gastric/gastroesophageal junction cancer(GC/GEJC): Initial findings of a single-arm phase II trial. J Clin Oncol 2022;40:294.34890242 [Google Scholar]
- 15.Liu N, Liu Z, Zhou Y, et al. Efficacy and safety of camrelizumab combined with FLOT versus FLOT alone as neoadjuvant therapy in patients with resectable locally advanced gastric and gastroesophageal junction adenocarcinoma who received D2 radical gastrectomy. J Clin Oncol 2021;39:e16020. [Google Scholar]
- 16.Liu Y, Han G, Li H, et al. Camrelizumab combined with FLOFOX as neoadjuvant therapy for resectable locally advanced gastric and gastroesophageal junction adenocarcinoma: Updated results of efficacy and safety. J Clin Oncol 2021;39:4036. [Google Scholar]
- 17.Li H, Deng J, Ge S, et al. Phase II study of perioperative toripalimab in combination with FLOT in patients with locally advanced resectable gastric/gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 2021;39:4050. [Google Scholar]
- 18.Jiang H, Yu X, Li N, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer 2022;10:e003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Mai W, Jiang W, et al. Sintilimab: a promising anti-tumor PD-1 antibody. Front Oncol 2020;10:594558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Fei K, Jing H, et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. MAbs 2019;11:1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J-M, Jia R, Wang Y, et al. A first-in-human phase 1a trial of sintilimab (IBI308), a monoclonal antibody targeting programmed death-1 (PD-1), in Chinese patients with advanced solid tumors. J Clin Oncol 2018;36:e15125. [Google Scholar]
- 22.Sah BK, Zhang B, Zhang H, et al. Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer. Nat Commun 2020;11:6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 24.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 25.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–92. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 29.Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005;47:141–146. [DOI] [PubMed] [Google Scholar]
- 30.Al-Batran S-E, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: Interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J Clin Oncol 2022;40 suppl: Abstract 4003. [Google Scholar]
- 31.Li JY, Huang XZ, Gao P, et al. Survival landscape of different tumor regression grades and pathologic complete response in rectal cancer after neoadjuvant therapy based on reconstructed individual patient data. BMC Cancer 2021;21:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 33.Hellmann MD, Chaft JE, William WN, Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934–939. [DOI] [PubMed] [Google Scholar]
- 35.Donohoe CL, O’Farrell NJ, Grant T, et al. Classification of pathologic response to neoadjuvant therapy in esophageal and junctional cancer: assessment of existing measures and proposal of a novel 3-point standard. Ann Surg 2013;258:784–792; discussion 792. [DOI] [PubMed] [Google Scholar]
- 36.Ikoma N, Estrella JS, Hofstetter W, et al. Nodal downstaging in gastric cancer patients: promising survival if ypN0 is achieved. Ann Surg Oncol 2018;25:2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu L, Xing Z, Huang M, et al. Nodal downstaging to ypN0 after neoadjuvant chemotherapy positively impacts on survival of cT4N+ GC/GEJ patients. J Surg Oncol 2022;126:1403–1412. [DOI] [PubMed] [Google Scholar]
- 38.Noble F, Nolan L, Bateman AC, et al. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J Gastroenterol 2013;19:9282–9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Ugo D, Persiani R, Rausei S, et al. Response to neoadjuvant chemotherapy and effects of tumor regression in gastric cancer. Eur J Surg Oncol 2006;32:1105–1109. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Yang X, Yan C, et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: a single-arm, open-label, phase II trial. Eur J Cancer 2020;130:12–19. [DOI] [PubMed] [Google Scholar]
- 41.Iwasa S, Kudo T, Takahari D, et al. Practical guidance for the evaluation of disease progression and the decision to change treatment in patients with advanced gastric cancer receiving chemotherapy. Int J Clin Oncol 2020;25:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto T, Kurokawa Y, Takahashi T, et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer 2019;22:785–792. [DOI] [PubMed] [Google Scholar]
- 43.Zhu L, Li Z, Wang Y, et al. Microsatellite instability and survival in gastric cancer: a systematic review and meta‑analysis. Mol Clin Oncol 2015;3:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salem ME, Puccini A, Grothey A, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res 2018;16:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang C, Wu HM, Yu WM, et al. Research status on immunotherapy trials of gastric cancer. World J Clin Cases 2021;9:5782–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang ZX, Yang LP, Qiu MZ, et al. Prognostic value of preoperative serum lactate dehydrogenase levels for resectable gastric cancer and prognostic nomograms. Oncotarget 2016;7:39945–39956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Yan Y, Yang Y, et al. High infiltration of tumor-associated macrophages influences poor prognosis in human gastric cancer patients, associates with the phenomenon of EMT. Medicine (Baltimore) 2016;95:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolcetti R, De Re V, Canzonieri V. Immunotherapy for gastric cancer: time for a personalized approach? Int J Mol Sci 2018;19:1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu QM, Yu CD, Ling ZQ. Elevated circulating CD19+ lymphocytes predict survival advantage in patients with gastric cancer. Asian Pac J Cancer Prev 2012;13:2219–2224. [DOI] [PubMed] [Google Scholar]
- 50.Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697–1708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the results of the present study can be obtained from the corresponding author upon reasonable request.