Abstract

Of the three Food and Drug Administration-approved melanocortin peptide drugs, two possess a cyclic scaffold, demonstrating that cyclized melanocortin peptides have therapeutic relevance. An extracyclic Arg residue, critical for pharmacological activity in the approved melanocortin cyclic drug setmelanotide, has also been demonstrated to increase the signal when fluorescently labeled cell-penetrating cyclic peptides are incubated with HeLa cells, with the maximal signal observed with three extracyclic Arg amino acids. Herein, a branching Lys residue was substituted into two macrocyclic melanocortin peptide agonists to incorporate 0–3 extracyclic Arg amino acids. Incorporation of the Arg residues resulted in equipotent or increased agonist potency at the mouse melanocortin receptors in vitro, suggesting that these substitutions were tolerated in the macrocyclic scaffolds. Further in vivo evaluation of one parent ligand (c[Pro-His-DPhe-Arg-Trp-Dap-Ala-Pro]) and the three Arg derivative (c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg-Arg-Arg)-Pro)] demonstrated that the three Arg derivative further decreased food intake compared to the parent macrocycle when the compounds were administered either via intrathecal injection or subcutaneous dosing. This suggests that three extracyclic Arg amino acids may be beneficial in the design of cyclic melanocortin ligands and that in vitro pharmacological profiling may not predict the in vivo efficacy of melanocortin ligands.
Keywords: melanocortin ligand, macrocycles, extracyclic Arg
The melanocortin receptors (MCRs) are a family of five known G protein-coupled receptors (GPCRs),1−8 agonists derived from the proopiomelanocortin (POMC) gene transcript9 including α-MSH, β-MSH, γ-MSH, and the adrenocorticotropic hormone (ACTH), and the two endogenous antagonists agouti10,11 and agouti-related protein (AGRP).12−14 This GPCR family is known to have many different biological activities attributed to the different receptor subtypes, including skin and hair pigmentation (MC1R),2,7 steroidogenesis (MC2R),7 energy homeostasis and appetite (MC3R and MC4R),15−19 sexual function,20−23 and exocrine gland function in rodents (MC5R).24 Three melanocortin peptide agonist ligands have been approved by the United States Food and Drug Administration (FDA). Afamelanotide (NDP-MSH, Scenesse), first reported by Hruby et al.,25 is used in the treatment of photosensitivity for individuals with erythropoietic protoporphyria.26 Bremelanotide (PT-141, Vylessi), a derivative of the cyclic peptide MTII27,28 with the C-terminal converted from a carboxyamide to a carboxylic acid, is used to treat hypoactive sexual desire disorder in premenopausal individuals.29 Setmelanotide (RM-493, Imcivree), first disclosed by Ipsen,30 is used for weight management in obese individuals with confirmed polymorphisms in rare genetic conditions.31 Due to the lack of selectivity of these approved peptide drugs for specific MCRs, off-target effects may occur. Central administration of NDP-MSH, approved for increasing skin pigmentation (presumably through the MC1R), decreased food intake (presumably through the MC3R/MC4R) when centrally administered in rats32 and rhesus monkeys.33 Effects on pigmentation/hair coloration (presumably through the MC1R) have been reported following administration of both bremelanotide (hypoactive sexual desire disorder, presumably through the MC4R)34 and setmelanotide (body weight regulation in genetic obesity, presumably through the MC4R).35,36 These data demonstrate that peptide melanocortin ligands possess clinically relevant therapeutic effects and that identifying novel scaffolds and compounds with unique pharmacological profiles at the MCRs may bypass the reported off-target effects of the approved melanocortin drugs.
While many melanocortin agonist peptides are derived from the naturally occurring melanocortin agonists from the POMC gene transcript, the endogenous antagonists may also serve as starting scaffolds to generate melanocortin ligands. The active forms of agouti and AGRP are large (approximately 110 residues in agouti37−39 and 50 amino acids in AGRP40). Both antagonists possess hypothesized hexapeptide active loops containing an Arg-Phe-Phe tripeptide motif that is critical for ligand binding and activity.41,42 Previous work incorporating the active loop sequence of AGRP into a DPro-Pro macrocyclic octapeptide, yielding c[Pro-Arg-Phe-Phe-Asn-Ala-Phe-DPro], resulted in a peptide with sub-micromolar antagonist potency at the mouse (m)MC4R.43 Further structure–activity relationship (SAR) studies on this scaffold demonstrated that replacing the Asn with a diaminopropionic acid (Dap) residue resulted in a peptide that was as potent as AGRP at the mMC4R, although it is shorter in length (8 vs 50 residues).43,44 Replacing the Arg-Phe-Phe antagonist motif with the known His-DPhe-Arg-Trp melanocortin agonist tetrapeptide sequence and truncation of residues within the macrocyclic core resulted in two agonist macrocycles (c[Pro-His-DPhe-Arg-Trp-Dap-Ala-DPro] and c[Pro-His-DPhe-Arg-Trp-Asn-Ala-Phe-DPro]) that possessed nanomolar agonist potency at the mMC4R.45 Further evaluation of one of the compounds in vivo (c[Pro-His-DPhe-Arg-Trp-Dap-Ala-DPro]) indicated that intrathecal (IT) or intracerebroventricular (ICV) administration of this compound resulted in a dose-dependent decrease in food intake,46 demonstrating the pharmacological efficacy of this compound scaffold. As these chimeric agonist compounds are derived from the endogenous melanocortin antagonists, they may possess a unique pharmacological profile that bypasses some of the off-target effects of the approved melanocortin therapeutics.
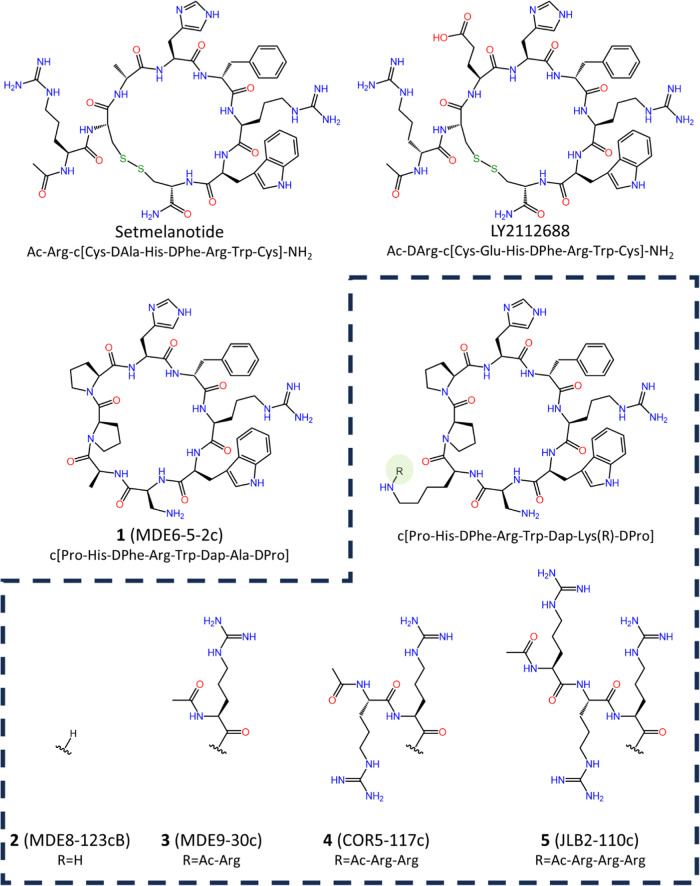
All three of the FDA-approved melanocortin peptides possess the known melanocortin agonist tetrapeptide sequence His-DPhe-Arg-Trp. Two of these compounds, bremelanotide and setmelanotide, are cyclized via a lactam and disulfide bridge, respectively. Setmelanotide (sequence Ac-Arg-c[Cys-DAla-His-DPhe-Arg-Trp-Cys]-NH2; Figure 1) possesses an extracyclic Arg, a basic residue unlike the extracyclic neutral Nle amino acid in bremelanotide (sequence Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-OH). A similar octapeptide to setmelanotide was reported by Eli Lilly.47 Derived following truncation, cyclization, and stereochemical inversion of the Phe residue in the linear β-MSH, an extracyclic Arg residue (sequence Ac-Arg-c[Cys-Glu-His-DPhe-Arg-Trp-Cys]-NH2) resulted in 0.44 nM potent affinity for the MC4R.47 Stereochemical inversion of the extracyclic Arg to DArg (sequence Ac-DArg-c[Cys-Glu-His-DPhe-Arg-Trp-Cys]-NH2, LY2112688, Figure 1) resulted in similar affinity for the MC4R (0.55 nM) and increased selectivity for the MC4R over the MC3R and MC1R.47 Removal of the extracyclic basic residue (DArg or Arg) decreased affinity fourfold at the MC4R in this series,47 suggesting that the basic charge was important for affinity. Although the development of LY2112688 was suspended after it was reported to increased blood pressure,48 the presence of the extracyclic basic residue in two different melanocortin peptide ligands, including the approved setmelanotide drug that reportedly does not affect blood pressure in certain patient populations,35 indicates that extracyclic basic motifs may be beneficial in cyclic melanocortin compounds.
Figure 1.
Structures of setmelanotide, LY2112688, 1 (MDE6-5-2c), 2 (MDE8-123cB), 3 (MDE9-30c), 4 (COR5-117c), and 5 (JLB2-110c).
Basic residues have also been demonstrated to enhance cellular uptake of various compounds, as reported with basic histones and homopolymers enhancing the uptake of radiolabeled albumin in sarcoma-180 cells.49 Cell-penetrating peptides, including sequences derived from the HIV TAT peptide,50,51 contain multiple basic residues. These amino acids are critical for cellular uptake. HeLa cells incubated with fluorescently labeled peptides containing the TAT(48–57) basic region (Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg; basic residues underlined) fluoresce, whereas incubation with labeled peptides containing truncated basic regions [TAT(37–53), missing three Arg residues] did not result in a fluorescence signal.52 Fluorescence is also observed following incubation of Jurkat cells with labeled homopolymers of basic amino acids, with the highest signal observed for Arg9 compared to Lys9, His9, or Orn9.53 Further examination indicated that a minimum of six Arg residues were required for a measurable fluorescence signal with increasing signal observed by increasing the chain length up to nine Arg residues, the fluorescence signal was similar for chains of l- or d-Arg isomers, and the maximal signal was observed for a Arg15 peptide compared to Arg7, Arg9, Arg20, Arg25, and Arg30 chains.53 Similar results were observed when cyclic FITC-labeled poly-Arg peptides were incubated with HeLa cells.54 In a scaffold with four to nine cyclized Arg residues (FITC-βA-c[Lys-Argx-Glu]-NH2), the highest fluorescence signal was observed with nine Arg.54 It was also observed that shifting some of the Arg residues outside of the cyclic portion of the peptide increased the fluorescence. Within the scaffold FITC-βA-Argy-c[Lys-Argz-Glu]-NH2 (total of seven Arg within each compound, where y = 0, 1, 2, 3, and 4 and z = 7, 6, 5, 4, and 3), compounds with extracyclic Arg residues were found to result in a higher fluorescence signal compared to the no extracyclic Arg amino acid control, with the highest fluorescence observed following incubation of the compound with three extracyclic Arg residues (FITC-βA-Arg3-c[Lys-Arg4-Glu]-NH2).54 These data indicate that incorporation of Arg residues can result in higher cellular uptake of a peptide ligand and that within a cyclic peptide scaffold, three Arg residues may promote the highest uptake.
Due to the off-target effects of approved peptide melanocortin agonist compounds, the observation that an extracyclic Arg residue increases MC4R agonist affinity, and that extracyclic Arg residues increase the fluorescence signal of cells incubated with cyclic cell-penetrating peptides, herein, 0–3 Arg amino acids were incorporated into two melanocortin agonist scaffolds to determine the functional effects of incorporating extracyclic Arg amino acids. These compounds were assayed for in vitro agonist activity at HEK293 cells stably expressing the mMC1R, mMC3R, mMC4R, and mMC5R. Due to the observed subnanomolar mMC4R agonist potency of these compounds herein, two compounds (one possessing three extracyclic Arg residues, one possessing zero extracyclic Arg residues) were administered in vivo to measure the functional efficacy of these compounds in mice. As initial proof-of-principle studies, these compounds were both directly injected into the central nervous system via intrathecal (IT) administration and systemically dosed via subcutaneous (SQ) injection to probe the potential effects of the extracyclic Arg residues in macrocyclic melanocortin ligands on feeding and routes of administration.
Results and Discussion
Peptides were synthesized manually using microwave irradiation using standard fluorenylmethoxycarbonyl (Fmoc) chemical techniques.55,56 Disulfide cyclization of the LY2112688 and setmelanotide ligands was performed on resin with iodine using a previously described protocol.57,58 The head-to-tail cyclization of the remaining macrocyclic peptides through formation of the Pro-His amide bond has previously been described.45 For branched macrocycles possessing 1–3 Arg residues, a Dde-Lys(Fmoc)-OH residue was incorporated into the linear peptide resin. Due to the presumed importance of the His-DPhe-Arg-Trp tetrapeptide agonist pharmacophore sequence, the DPro-Pro dipeptide cyclization motif, and the observed activity differential between the Dap/Asn position in agonist and antagonist ligands within this compound series, the Ala position was selected for substitution with the Dde-Lys(Fmoc)-OH amino acid. Following incorporation of this residue, the Fmoc group was removed from the ε-amine using 2% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in DMF, followed by incorporation of the extracyclic Arg residue(s). The terminal Arg amino acid was attached as an Ac-Arg(Pmc)-OH residue, followed by removal of the Dde protecting group on the α-amine of the branching Lys using 2% hydrazine in DMF, and the protected linear chain was synthesized before subsequent cleavage and macrocyclization. All peptides were purified using semipreparative reverse-phase high-pressure liquid chromatography (RP-HPLC). Peptide purity was assessed by analytical RP-HPLC in two different solvent systems, and the correct molecular mass was determined by ESI-MS (University of Minnesota Mass Spectrometry Laboratory; Table 1).
Table 1. Characterization of Peptides Synthesized in This Studya.
| peptide | compound ID | sequence | k’ (MeCN) | k’ (MeOH) | M (cal) | M (obs) | purity |
|---|---|---|---|---|---|---|---|
| LY2112688 | Ac-DArg-c[Cys-Glu-His-DPhe-Arg-Trp-Cys]-NH2 | 4.1 | 7.0 | 1174.5 | 1174.6 | >95% | |
| setmelanotide | Ac-Arg-c[Cys-DAla-His-DPhe-Arg-Trp-Cys]-NH2 | 4.3 | 7.1 | 1116.5 | 1116.5 | >98% | |
| 1 | MDE6-5-2c | c[Pro-His-DPhe-Arg-Trp-Dap-Ala-DPro] | 3.8 | 7.0 | 977.5 | 977.1 | >98% |
| 2 | MDE8-123cB | c[Pro-His-DPhe-Arg-Trp-Dap-Lys-DPro] | 3.6 | 7.3 | 1034.6 | 1034.8 | >99% |
| 3 | MDE9-30c | c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg)-DPro] | 4.0 | 7.8 | 1232.7 | 1232.8 | >96% |
| 4 | COR5-117c | c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg-Arg)-DPro] | 3.7 | 5.8 | 1388.8 | 1388.8 | >85% |
| 5 | JLB2-110c | c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg-Arg-Arg)-DPro] | 4.3 | 7.3 | 1544.9 | 1544.9 | >99% |
| 6 | MDE5-149-2c | c[Pro-His-DPhe-Arg-Trp-Asn-Ala-Phe-DPro] | 7.0 | 11.2 | 1152.6 | 1152.5 | >95% |
| 7 | MDE8-128c | c[Pro-His-DPhe-Arg-Trp-Asn-Lys-Phe-DPro] | 6.4 | 10.4 | 1209.6 | 1209.9 | >96% |
| 8 | MDE9-39cB | c[Pro-His-DPhe-Arg-Trp-Asn-Lys(Ac-Arg)-Phe-DPro] | 5.7 | 9.4 | 1407.7 | 1407.7 | >97% |
| 9 | COR2-138c | c[Pro-His-DPhe-Arg-Trp-Asn-Lys(Ac-Arg-Arg)-Phe-DPro] | 5.4 | 8.8 | 1563.8 | 1564.1 | >99% |
| 10 | COR2-78c | c[Pro-His-DPhe-Arg-Trp-Asn-Lys(Ac-Arg-Arg-Arg)-Phe-DPro] | 5.3 | 9.1 | 1719.9 | 1720.3 | >95% |
HPLC k’ = [(peptide retention time – solvent retention time)/solvent retention time] in acetonitrile (MeCN; 10% acetonitrile in 0.1% trifluoroacetic acid/water and a gradient to 90% acetonitrile over 35 min) or methanol (MeOH; 10% methanol in 0.1% trifluoroacetic acid/water and a gradient to 90% methanol over 35 min). An analytical Vydac C18 column (Vydac 218TP104) was used with a flow rate of 1.5 mL/min. The peptide purity was determined by HPLC at a wavelength of 214 nm.
The peptides were assayed for agonist activity in HEK293 cells stably expressing the mMC1R, mMC3R, mMC4R, and mMC5R using the AlphaScreen cAMP assay according to the manufacturer’s instructions and as previously reported.45,59 As the mMC2R is only activated by ACTH,60 it was excluded from this study. The tool compounds Ac-His-DPhe-Arg-Trp-NH2 and LY211268847,48 and approved melanocortin therapeutics NDP-MSH26 and setmelanotide31 were included as positive controls. Since the AlphaScreen cAMP assay is a competition assay resulting in a lower signal at higher concentrations of the agonist compound, the concentration–activity curves were normalized to NDP-MSH as previously described.59,61,62 Due to the inherent error of the assay, compounds that were within a threefold potency range were considered equipotent herein.
In the present study, NDP-MSH possessed subnanomolar agonist activity at the assayed MCRs, with EC50 values of 0.009, 0.08, 0.39, and 0.17 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively (Table 2 and Figure 2). These values are similar to the previously reported 0.015, 0.09, 0.39, and 0.11 nM values for these receptors.45 The tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 also possessed agonist pharmacology (4.2, 27, 3.9, and 1.9 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively; Figure 2) similar to previously published results (14, 60, 14, and 10 nM).45
Table 2. Peptide Agonist Pharmacology at the Mouse MCRsa.
| EC50 (nM) | ||||||
|---|---|---|---|---|---|---|
| peptide | compound ID | sequence | mMC1R | mMC3R | mMC4R | mMC5R |
| NDP-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.009 ± 0.002 | 0.08 ± 0.01 | 0.39 ± 0.07 | 0.17 ± 0.02 | |
| Ac-His-DPhe-Arg-Trp-NH2 | Ac-His-DPhe-Arg-Trp-NH2 | 4.2 ± 0.8 | 27 ± 7 | 3.9 ± 0.7 | 1.9 ± 0.2 | |
| LY2112688 | Ac-DArg-c[Cys-Glu-His-DPhe-Arg-Trp-Cys]-NH2 | 0.6 ± 0.2 | 0.16 ± 0.05 | 0.048 ± 0.009 | 0.05 ± 0.01 | |
| Setmelanotide | Ac-Arg-c[Cys-DAla-His-DPhe-Arg-Trp-Cys]-NH2 | 0.5 ± 0.2 | 0.08 ± 0.03 | 0.036 ± 0.004 | 0.08 ± 0.02 | |
| 1 | MDE6-5-2c | c[Pro-His-DPhe-Arg-Trp-Dap-Ala-DPro] | 0.24 ± 0.09 | 8 ± 2 | 0.5 ± 0.1 | 0.183 ± 0.009 |
| 2 | MDE8-123cB | c[Pro-His-DPhe-Arg-Trp-Dap-Lys-DPro] | 0.4 ± 0.1 | 7 ± 2 | 0.34 ± 0.08 | 0.21 ± 0.07 |
| 3 | MDE9-30c | c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg)-DPro] | 0.2 ± 0.1 | 5.3 ± 0.2 | 0.31 ± 0.06 | 0.18 ± 0.02 |
| 4 | COR5-117c | c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg-Arg)-DPro] | 0.16 ± 0.04 | 5 ± 1 | 0.26 ± 0.06 | 0.19 ± 0.01 |
| 5 | JLB2-110c | c[Pro-His-DPhe-Arg-Trp-Dap-Lys(Ac-Arg-Arg-Arg)-DPro] | 0.27 ± 0.05 | 8 ± 2 | 0.34 ± 0.09 | 0.29 ± 0.02 |
| 6 | MDE5-149-2c | c[Pro-His-DPhe-Arg-Trp-Asn-Ala-Phe-DPro] | 0.13 ± 0.04 | 22 ± 6 | 1.0 ± 0.2 | 0.32 ± 0.02 |
| 7 | MDE8-128c | c[Pro-His-DPhe-Arg-Trp-Asn-Lys-Phe-DPro] | 0.07 ± 0.01 | 11 ± 4 | 0.29 ± 0.05 | 0.17 ± 0.03 |
| 8 | MDE9-39cB | c[Pro-His-DPhe-Arg-Trp-Asn-Lys(Ac-Arg)-Phe-DPro] | 0.03 ± 0.01 | 4.0 ± 0.4 | 0.27 ± 0.07 | 0.25 ± 0.03 |
| 9 | COR2-138c | c[Pro-His-DPhe-Arg-Trp-Asn-Lys(Ac-Arg-Arg)-Phe-DPro] | 0.026 ± 0.004 | 2.1 ± 0.3 | 0.13 ± 0.03 | 0.28 ± 0.02 |
| 10 | COR2-78c | c[Pro-His-DPhe-Arg-Trp-Asn-Lys(Ac-Arg-Arg-Arg)-Phe-DPro] | 0.04 ± 0.01 | 2.3 ± 0.7 | 0.20 ± 0.05 | 0.37 ± 0.04 |
The indicated errors represent the standard error of the mean from at least three independent experiments performed in duplicate.
Figure 2.

Illustration of the agonist pharmacology of NDP-MSH, Ac-His-DPhe-Arg-Trp-NH2, setmelanotide, LY2112688, 1 (MDE6-5-2c), and 5 (JLB2-110c) at the mMC1R, mMC3R, mMC4R, and mMC5R.
The LY2112688 compound possessed subnanomolar agonist potencies at the MCRs assayed (0.6, 0.16, 0.048, and 0.05 at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively; Table 2 and Figure 2). At the human MCRs, LY2112688 was reported to possess the highest affinity for the hMC4R (0.5 nM),47 with 30-fold decreased affinity for the hMC1R (17 nM),47 100-fold decreased affinity for the hMC3R (57 nM),47 and 3000-fold decreased affinity for the hMC5R (1700 nM).48 This cyclic peptide was reported to possess subnanomolar functional activity at the hMC4R (0.28 nM)47 and was described to possess “approximately sixfold greater potency for MC4R- vs MC3R-mediated functional response”.48 Thus, the threefold difference in functional response between the mMC3R and mMC4R is similar to the described sixfold difference at the corresponding human receptors (and more similar than the 100-fold difference in binding affinity for the human receptors). Differences between binding affinity and functional activity may also explain the differences observed in functional potency at the mMC4R and mMC5R (equipotent) and 3000-fold difference in binding affinity at the hMC4R and hMC5R. The large difference may also be explained by species difference at the MC5R. The linear peptide γ2-MSH was reported to possess micromolar agonist potency at the hMC5R (2700 nM) and nanomolar potency at the mMC5R (42 nM),63 demonstrating that profiling the pharmacological activity of the same ligand at different species MCRs may lead to differing functional potencies. Species differences were also reported for the melanocortin compound TCMCB07, which was a more potent agonist at the dog MC5R (EC50 = 0.0012 nM) compared to the human MC5R (EC50 = 1.42 nM) and rat MC5R (EC50 = 5.82 nM).64
The approved melanocortin therapeutic setmelanotide was found to be a subnanomolar agonist (Table 2 and Figure 2) at the mMC1R (0.5 nM), mMC3R (0.08 nM), mMC4R (0.036 nM), and mMC5R (0.08 nM). Subnanomolar potency was also observed at the rat MC4R (0.28 nM).65 Additional characterization at other murine melanocortin receptors was not reported. The same group reported similar functional potency at the hMC4R (0.27 nM), 20-fold selectivity for the hMC4R over the hMC3R (5.3 nM) and hMC1R (5.8 nM), and 5900-fold selectivity for the hMC4R over the hMC5R (1600 nM).65 Another group has reported setmelanotide to possess EC50 values of 0.15 nM at the hMC3R and 0.39 nM or less than 0.05 nM at the hMC4R, depending on the expression level of the hMC4R.66 These data along with the present study support that setmelanotide possesses subnanomolar agonist potency at the MC4R across multiple species. The selectivity of setmelanotide appears to be species-dependent. The present study suggests that setmelanotide is not functionally selective for the mMC4R over the mMC3R or mMC5R, while this ligand may be selective for the hMC4R over the hMC3R (depending on hMC4R receptor expression levels) and is selective for the hMC4R over the hMC5R.
The macrocyclic octapeptide lead ligand 1 (MDE6-5-2c; Figure 1) was observed to possess agonist activity at the assayed MCRs, with EC50 values of 0.24, 8, 0.5, and 0.18 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively (Table 2 and Figure 2). This activity profile at the mouse MCRs is similar to a prior report of this compound, with agonist potencies listed as 1.1, 40, 1.6, and 0.3 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively.45 Replacing the Ala within the macrocycle with Lys, 2 (MDE8-123cB), resulted in a ligand with equipotent activity at the mMC1R (0.4 nM), mMC3R (7 nM), mMC4R (0.34 nM), and mMC5R (0.21 nM) compared to the lead 1 (MDE6-5-2c), suggesting that substitution and introduction of a positive charge at this position did not affect the observed pharmacological activity. Addition of 1 (3, MDE9-30c), 2 (4, COR5-117c), and 3 (5, JLB2-110c) extracyclic Arg residues at the ε-amine of the Lys side chain (Figure 1) did not alter the agonist pharmacological profile or agonist potencies of the resulting ligands compared to the lead 1 (MDE6-5-2c), as all compounds were within a twofold potency range at the assayed receptors (Table 2).
The macrocyclic nonapeptide 6 (MDE5-149-2c) scaffold possessed similar agonist potencies compared to 1 (MDE6-5-2c) at the assayed receptors, with EC50 values of 0.13, 22, 1.0, and 0.32 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively. These potencies are similar to the previously reported EC50 values of 0.35, 32, 1.4, and 0.45 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively.45 Replacement of the Ala residue in this macrocyclic scaffold with Lys resulted in the peptide 7 (MDE8-128c) that was equipotent at the mMC1R (0.07 nM), mMC3R (11 nM), mMC4R (0.29 nM), and mMC5R (0.17 nM) compared to the lead macrocycle 6 (MDE5-149-2c). Attaching 1 (8, MDE9-39cB), 2 (9, COR2-138c), or 3 (10, COR2-78c) Arg residues to the ε-amine of the Lys residue resulted in macrocyclic peptides with equipotent functional activity at the mMC5R compared to the lead nonapeptide macrocycle 6 (MDE5-149-2c). These peptides elongated by 1–3 Arg through the Lys residue were more potent at the mMC1R (3- to 5-fold), mMC3R (5- to 10-fold), and mMC4R (4- to 8-fold) compared to 6 (MDE5-149-2c).
Selectivity Profiles
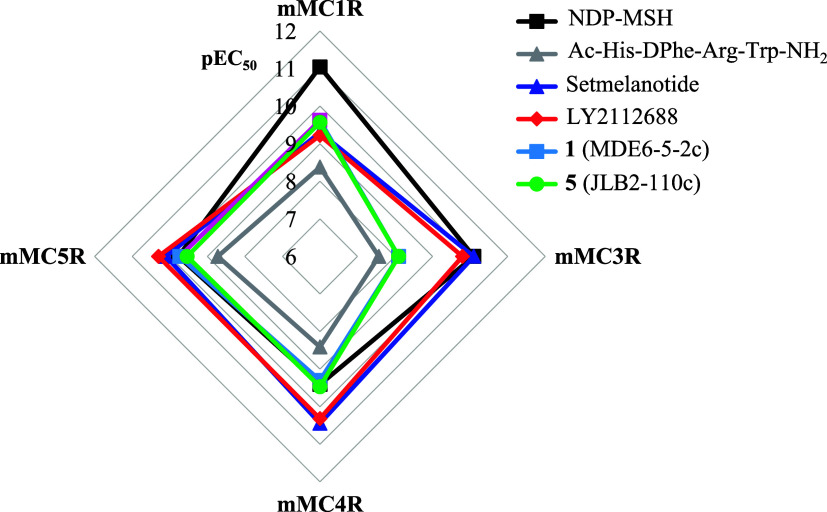
To visualize ligand selectivity at the different MCRs, the agonist EC50 values of select compounds were converted to pEC50 and incorporated into a radar plot (Figure 3). Each spoke represents a different MCR, and the distance from the center of the graph indicates the agonist potency at that receptor (more potent compounds graphed further from the center of the plot). The control peptide NDP-MSH is most selective for the mMC1R, with the corresponding plot showing NDP-MSH (black squares) as the furthest from the center in the MC1R spoke. The control tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 is equipotent at the mMC1R (4.2 nM), mMC4R (3.9 nM), and mMC5R (1.9 nM), with decreased potency at the mMC3R (27 nM). This can be visualized in the radar plot for Ac-His-DPhe-Arg-Trp-NH2 (gray triangle) as being between the 8 and 9 lines for pEC50 at the mMC1R, mMC4R, and mMC5R, while crossing between the 7 and 8 lines at the mMC3R spoke.
Figure 3.
Summary of the agonist pharmacology of NDP-MSH, Ac-His-DPhe-Arg-Trp-NH2, setmelanotide, LY2112688, 1 (MDE6-5-2c), and 5 (JLB2-110c) at the mMC1R, mMC3R, mMC4R, and mMC5R. Each spoke of the radar plot represents the indicated MCR. Data are plotted as pEC50 values ascending outward (the more potent the compound, the further it resides from the center of the graph).
Setmelanotide and LY2112688 have been reported as 20- to 30-fold selective for the MC4R using the human receptors.47,65 From the present study at the mouse MCRs, it is observed that these compounds are equipotent at the mMC3R, mMC4R, and mMC5R and 13- to 14-fold selective for the mMC4R over the mMC1R. In the radar plot, this is visualized by the inward deflection toward the center of the graph at the mMC1R relative to the remaining receptors for setmelanotide (blue triangle) and LY2112688 (red diamond; Figure 3). Thus, while these compounds possess picomolar agonist potency at the mMC4R, the relative lack of selectivity between the mMC3R, mMC4R, and mMC5R suggests that caution should be exercised when attributing in vivo pharmacological activity solely to the MC4R following administration of these compounds in mice.
As representative compounds for the macrocycles described herein, compounds 1 (MDE6-5-2c; blue square) and 5 (JLB2-110c; green circle) were plotted in the radar graph. The equipotency of these compounds at the different MCRs is visualized by overlapping plots at the different spokes on the graph (Figure 3). Although these compounds are about 10-fold less potent at the mMC4R compared to LY2112688 and setmelanotide, they are more selective for the mMC4R over the mMC3R, as evidenced by the shift toward the center of the plot on the mMC3R spoke. The 16- to 24-fold selectivity for the mMC4R over the mMC3R for these compounds may be useful in the development of future ligands that are more potent mMC4R agonist compounds and maintain selectivity over the mMC3R, to generate probe molecules that can selectively test the in vivo functions of the different receptors and as potential therapeutic lead compounds that bypass off-target effects due to activity at the different MCRs.
In Vivo Feeding Studies
The in vitro pharmacological activity profiles suggested that melanocortin macrocyclic peptides without extracyclic Arg residues possessed similar agonist potency to compounds with 0–3 extracyclic Arg amino acids (Table 2). As an initial examination on the effect of incorporating Arg residues to a macrocyclic melanocortin agonist in vivo, the octapeptide macrocyclic lead compound 1 (MDE6-5-2c) and corresponding three-Arg derivative 5 (JLB2-110c) were selected for administration in mice. These compounds were equipotent at the MCRs (Table 2, Figures 2 and 3). It was hypothesized that any observed differences following in vivo administration would presumably be due to intrinsic property variances of the compounds (enhanced uptake, different pharmacokinetic [PK] parameters, increased stability, solubility differences, etc. as examples) and not due to potency differences at the MCRs. Compounds were administered both directly to the CNS via intrathecal (IT) injection and systemically via subcutaneous (SQ) injection to examine possible effects via two different methods of compound administration.
It is well known that central administration of MC4R agonist ligands decreases (and antagonist compounds increases) food intake in mice.18,46,67 The octapeptide macrocyclic lead peptide 1 (MDE6-5-2c) was previously administered to mice via IT injection and resulted in a dose-dependent decrease in food intake in male mice following doses of 2 and 5 nmol/mouse, with a 10 nmol/mouse dose having a similar effect to the 5 nmol/mouse dose.46 These results guided the selection of a 3-point dose response profile for the three-Arg derivative 5 (JLB2-110c) following IT administration (Figure 4A). Food intake following doses of 5, 1, and 0.2 nmol/mouse was compared to that of the vehicle control. Both male and female mice were used in approximately equal amounts per treatment group in this study. Since no statistical differences were observed between sexes, male and female data were collapsed into the different treatment groups for data analysis. Compared to vehicle-treated mice, all doses of 5 (JLB2-110c) resulted in a statistically significant decrease in food intake at 2, 4, 6, and 8 h following compound administration (Figure 4A). A dose-dependent decrease in cumulative food intake was observed over this time period, where higher levels of compound administration resulted in a decreased food intake. Animals appeared healthy following dosing of all compounds, and no difference in ambulatory activity was observed, suggesting an on-target food intake effect by the compound.
Figure 4.

Cumulative food intake following single administration of 5 (JLB2-110c), 1 (MDE6-5-2c), or vehicle control directly into the spinal column (intrathecal, IT) in wild-type (C57BL6) mice using a crossover experimental design. Both male and female mice were examined in approximately equal amounts. No statistically significant differences between sexes were observed in cumulative food intake relative to the vehicle control, so sexes were collapsed into compound treatment groups (N = 13). (A) Dose-dependent decrease in cumulative food intake compared to the vehicle control was observed following single IT administrations of 5 (JLB2-110c) (0.2, 1, and 5 nmol/mouse). Significant decreases were observed at 2, 4, 6, and 8 h compared to the vehicle control for each dose. (B) Cumulative food intake was decreased following a 5 nmol IT dose of either 5 (JLB2-110c) or 1 (MDE6-5-2c) compared to the vehicle control. Significant decreases were observed at 2, 4, 6, and 8 h compared to the vehicle control for both compounds. At 4, 6, and 8 h postadministration, 5 (JLB2-110c) significantly decreased cumulative food intake compared to 1 (MDE6-5-2c). **p < 0.01 for 0.2 nmol 5 (JLB2-110c) compared to the vehicle. ***p < 0.001 for 0.2, 1, and 5 nmol 5 (JLB2-110c) and 5 nmol 1 (MDE6-5-2c) compared to the vehicle. ##p < 0.01, ###p < 0.001 for 5 nmol 5 (JLB2-110c) compared to 5 nmol 1 (MDE6-5-2c).
To directly compare the compounds in vivo, a 5 nmol/mouse dose of 1 (MDE6-5-2c) was administered IT (Figure 4B). Similar to 5 (JLB2-110c) and as previously reported,46 this dose of 1 (MDE6-5-2c) significantly decreased food intake at 2, 4, 6, and 8 h compared to vehicle-treated mice. Comparing the two compounds, 5 (JLB2-110c) resulted in statistically significant decreased cumulative food intake compared to 1 (MDE6-5-2c) at 4, 6, and 8 h when both compounds were administered at 5 nmol/mouse (Figure 4B). These results demonstrate that although 1 (MDE6-5-2c) and 5 (JLB2-110c) possessed equipotent in vitro pharmacological activities at the mouse MCRs, 5 (JLB2-110c) resulted in a more robust food intake suppression response compared to 1 (MDE6-5-2c) in vivo.
To explore the potential differences between Arg-containing melanocortin macrocyclic ligands following systemic administration, both compounds were also injected subcutaneously (SQ) at a 7.5 mg/kg dose in male mice (Figure 5). This subcutaneous route of administration is used for the FDA-approved setmelanotide melanocortin peptide drug35,68 as well as insulins, GLP-1 peptides, and other antidiabetic drugs. Therefore, this route has been validated for clinically relevant peptide drugs that modulate appetite. Both 5 (JLB2-110c) and 1 (MDE6-5-2c) significantly decreased food intake at 2, 4, 6, and 8 h following SQ administration compared to the vehicle saline control (Figure 5). Similar to IT administration, dosing of 5 (JLB2-110c) resulted in statistically significant decreased food intake at 6 and 8 h (p < 0.05) relative to 1 (MDE6-5-2c), suggesting that incorporation of three Arg residues in a melanocortin macrocyclic agonist scaffold results in a more robust response in vivo following system administration. The reason for the enhanced response could include increased stability, enhanced permeability, different PK parameters, solubility differences, PD, etc.
Figure 5.
Cumulative food intake following single administration of 5 (JLB2-110c), 1 (MDE6-5-2c), or vehicle control subcutaneously (SQ) in wild-type (C57BL6) male mice (n = 7). Significant decreases were observed at 2, 4, 6, and 8 h compared to the vehicle control for both compounds. At 6 and 8 h, postadministration of 5 (JLB2-110c) significantly decreased cumulative food intake compared to 1 (MDE6-5-2c). *p < 0.05 for 7.5 mg/kg 1 (MDE6-5-2c) and 5 (JLB2-110c) compared to saline, **p < 0.01 for 7.5 mg/kg 1 (MDE6-5-2c) and 5 (JLB2-110c) compared to saline, ***p < 0.001 for 7.5 mg/kg 1 (MDE6-5-2c) and 5 (JLB2-110c) compared to saline, and #p < 0.05 for 7.5 nmol 5 (JLB2-110c) compared to 7.5 nmol 1 (MDE6-5-2c).
Conclusions
In the present study, the potential in vitro and in vivo pharmacological effects of extracyclic Arg residues were investigated on two melanocortin agonist macrocyclic scaffolds. The agonist potencies of macrocycles possessing 0–3 Arg residues, select melanocortin tool compounds, and two approved melanocortin peptide drugs were determined at the mouse MCRs. While NDP-MSH and Ac-His-DPhe-Arg-Trp-NH2 were similar to previously reported values, LY2112688 and setmelanotide were observed to be relatively equipotent at the mMC3R, mMC4R, and mMC5R, an unexpected result compared to reports at the human receptors. These differences may be due to species differences at the MCRs or past reliance on binding data and not functional analysis. Extracyclic Arg residues incorporated into an octapeptide scaffold did not alter in vitro agonist pharmacology and resulted in more potent agonist EC50 values in a nonapeptide scaffold at the mMC1R, mMC3R, and mMC4R. When tested in vivo following IT administration, the addition of three extracyclic Arg residues to an octapeptide macrocyclic scaffold dose-dependently decreased food intake and decreased food intake relative to the octapeptide macrocyclic starting scaffold. These data demonstrated that although addition of extracyclic Arg residues did not alter in vitro agonist pharmacology, a differential response in vivo was observed. A similar effect was observed following SQ administration, indicating that the addition of a three Arg extracyclic motif resulted in a more robust in vivo response following systemic delivery. This information may be used in the design of more potent, selective compounds that can be administered systemically.
Experimental Section
General Peptide Synthesis and Purification
Unless otherwise mentioned, amino acids, resins, and coupling reagents were purchased from Peptides International. The Fmoc-DArg(Pbf)-OH, Fmoc-His(Boc)-OH, Ac-Arg(Pmc)-OH, and Dde-Lys(Fmoc) amino acids were purchased from Bachem. Dichloromethane (DCM), methanol (MeOH), acetonitrile (MeCN), dimethylformamide (DMF), and anhydrous diethyl ether were purchased from Fisher (Fair Lawn, NJ). Trifluoroacetic acid (TFA), dimethyl sulfoxide (DMSO), piperidine, triisopropylsilane (TIS), thioanisole, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), hydrazine, and N,N-diisopropylethylamine (DIEA) were purchased from Sigma-Aldrich (St. Louis, MO). All reagents and chemicals were ACS grade or better and were used without further purification.
Peptides were synthesized using standard N-α-fluorenylmethoxycarbonyl (Fmoc) methodologies55,56 on a manual microwave synthesizer (Discover SPS; CEM, Matthews, NC) using iterative deprotection and amino acid coupling steps. The Fmoc group was removed by a two-stage deprotection strategy with 20% piperidine in DMF (1 × 2 min at room temperature, followed by 1 × 4 min using microwave irradiation to 75 °C and 30W). Microwave coupling reactions were carried out at 75 °C and 30W for 5 min (10 min for coupling Arg residues), except for coupling His, which utilized a lower temperature (50 °C for 5 min). Most coupling reactions utilized 3.1 equiv of the amino acid, 3 equiv of HBTU, and 5 equiv of DIEA. For coupling Arg and DArg, higher equivalents were employed (5.1 equiv of Arg, 5 equiv of HBTU, and 7.1 equiv of DIEA). In all cases, reaction progress was monitored using a ninhydrin69 or chloranil70 assay, and deprotection and coupling reactions were repeated if necessary.
The crude tetrapeptides were purified on a C18 RP-HPLC semipreparative column (Vydac 218TP1010, 1.0 cm × 25 cm) using a Shimadzu system equipped with a UV detector. Peptide purity (λ = 214 nm) was determined by analytical RP-HPLC (Vydac 218TP104, 0.46 cm × 25 cm) on a Shimadzu system equipped with a PDA detector in two solvent systems (MeCN and MeOH), and the correct exact mass was obtained by ESI-MS (Table 1; Bruker BioTOF II ESI/TOF-MS; LeClaire-Dow Instrumentation Facility, University of Minnesota).
Cyclized DPro-Pro Peptides
Peptides were synthesized using H-Pro-2-chlorotrityl resin, as described above. To generate a branch point to incorporate additional Arg residues outside of the macrocyclic backbone, a Dde-Lys(Fmoc)-OH-protected residue was added. The Fmoc-side chain protecting group was removed using 2% DBU in DMF (3 × for 3 min at room temperature), and Arg residues (1–3) were added. For the addition of the Arg residues, DBU was used to remove the Fmoc group. The terminal Arg was incorporated as an Ac-Arg(Pmc)–OH. Following the addition of the Arg residues, the Dde was removed from the Lys residue using 2% hydrazine in DMF solution (3 × for 3 min at room temperature), and additional amino acids were added as described in the general peptide section. Before cleavage, the terminal Fmoc group was removed.
After completion of the syntheses, peptides were cleaved with 99:1 DCM:TFA solution (2.5 mL for 1.5 min, 4 ×). The cleavage solutions were then concentrated, and side chain-protected peptides were precipitated using ice-cold ethyl ether. Peptides were cyclized to generate the Pro-His amide bond in DCM with BOP (3 equiv) and HOBt (3 equiv) overnight, and the DCM was removed under a vacuum. Without further purification, the cyclized peptides were side chain-deprotected using a 95:2.5:2.5 TFA/TIS/H2O solution for 2 h, the solution was then concentrated, and peptides were precipitated using ice-cold ethyl ether and purified as described above.
Disulfide Cyclization Protocol
Following the removal of the terminal Fmoc group, peptides were acetylated by adding a 3:1 acetic anhydride:pyridine solution and mixing for 30 min at room temperature. Following DMF washes, the peptide was cyclized on resin using 15 equiv of iodine dissolved in DMF in the dark while bubbling N2 gas for 2 h. The resin was washed 10 × with DMF, followed by 10 × washes with DCM. Cyclized peptides were cleaved from resin and side chain-deprotected using a 91:3:3:3 mixture of TFA/H2O/TIS/thioanisole for 2 h at room temperature and then precipitated using ice-cold diethyl ether. Crude peptides were pelleted on a Sorvall Legend XTR centrifuge (4000 rpm, 4 °C, 4 min), dried overnight in a vacuum desiccator, and purified as described above.
cAMP “AlphaScreen” Bioassay
The purified tetrapeptides were dissolved in DMSO at a stock concentration of 10–2 M (NDP-MSH in H2O at a stock concentration of 10–4 M) and assayed using HEK293 cells stably expressing the mouse MC1R, MC3R, MC4R, and MC5R and the “AlphaScreen” cAMP bioassay kit (PerkinElmer) according to the manufacturer’s instructions and as previously described.45,59
Before experimental compounds were assayed, a panel of known melanocortin ligands (α-MSH, NDP-MSH, MTII, γ2-MSH, Ac-His-DPhe-Arg-Trp-NH2, and SHU9119) was assayed on the different cell lines. The potencies, relative selectivity, and pharmacological activities (i.e., partial agonist and antagonist for SHU9119 at the mMC3R, antagonist without partial agonist activity for SHU9119 at the MC4R) of these compounds were evaluated and compared to historical values to validate the identity of the cell lines used.
Briefly, cells 70–90% confluent were dislodged with Versene (Gibco) at 37 °C and plated at 10 000 cells/well in a 384-well plate (Optiplate) with 10 μL of freshly prepared stimulation buffer (1× HBSS, 5 mM HEPES, 0.5 mM IBMX, 0.1% BSA, pH = 7.4) with 0.5 μg of anti-cAMP acceptor beads per well. The cells were stimulated with the addition of 5 μL of stimulation buffer containing peptide (concentrations from 10–4 to 10–13 M, determined by ligand potency) or forskolin (10–4 M) and incubated in the dark at room temperature for 2 h.
Following stimulation, streptavidin donor beads (0.5 μg) and biotinylated-cAMP (0.62 μmol) were added to the wells in a green light environment with 10 μL of lysis buffer (5 mM HEPES, 0.3% Tween-20, 0.1% BSA, pH = 7.4), and the plates were incubated in the dark at room temperature for an additional 2 h. Plates were read on an Enspire (PerkinElmer) α-plate reader using a prenormalized assay protocol (set by the manufacturer).
Data Analysis
The EC50 values represent the mean of at least three independent experiments performed in duplicate replicates. The EC50 values and associated standard errors (SEM) were determined by fitting the data to a nonlinear least-squares analysis using the PRISM program (version 4.0, GraphPad Inc.). The peptides were assayed as TFA salts and not corrected for the peptide content.
Animals
This study was conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota. Wild-type (WT) male and female mice with a mixed 129/Sv × C57BL/6J background derived from an in-house breeding colony were used throughout this experiment as previously reported in the literature.46,62,67,71 Mice were housed in a temperature-controlled room (23–25 °C) and maintained on a reversed 12 h light/dark cycle (lights off at 11:00 am). Mice had ad libitum access to normal chow (Harlan Teklad 2018 Diet: 18.6% crude protein, 6.2% crude fat, 3.5% crude fiber, with an energy density of 3.1 kcal/g) and water.
IT Study Design
All intrathecal (IT)46,67,72 administration experiments were a crossover design, and standard chow was provided ad libitum. Mice were housed in standard polycarbonate conventional cages provided by the University of Minnesota’s Research Animal Resources. Compounds were dissolved to a stock solution of 5 nmol/5 μL in saline. On the day of compound administration, an aliquot of stock solution was diluted with saline to concentrations of 1 or 0.2 nmol in 5 μL of saline if needed. The desired experimental dose (5 μL) or saline control (5 μL) was administered via IT injection 2 h before lights out (t = 0 h). Food intake was manually measured at T = 0, 2, 4, 6, and 8 hours postinjection using a standard laboratory-scale balance (Sartorius Quintix 512-1s, Bohemia, New York). Mice were given 6–7 days for recovery between treatments to re-establish pretreatment body weight and feeding patterns. Male and female mice were used in approximately equal numbers (n ≥ 5 for each sex). No statistical difference between sexes was observed for cumulative intake relative to the vehicle control, so the sexes were collapsed into compound treatment groups (n = 13 per treatment group). Data were analyzed using the PRISM program (v9.0; GraphPad Inc.) by a two-way ANOVA with a simple effects within rows multiple comparison relative to vehicle control-treated mice in order to compare individual doses to vehicle control administration at each time point. Statistical significance is considered if p < 0.05.
SQ Study Design
The subcutaneous (SQ) administration experiment was a crossover design, and standard chow was provided ad libitum. Male mice (N = 7) were housed in standard polycarbonate conventional cages provided by the University of Minnesota’s Research Animal Resources. Compounds were dissolved to a concentration of 7.5 mg/kg at 10 mL/kg in saline. The desired experimental dose (7.5 mg/kg) was administered via SQ injection 2 h before lights out (t = 0 h). Food intake was manually measured at T = 0, 2, 4, 6, and 8 h postinjection using a standard laboratory-scale balance (Sartorius Quintix 512-1s, Bohemia, New York). Mice were given 6–7 days for recovery between treatments to re-establish pretreatment body weight and feeding patterns. Data were analyzed using the PRISM program (v9.0; GraphPad Inc.) by a two-way ANOVA with a simple effects within rows multiple comparison relative to vehicle control-treated mice in order to compare individual doses to vehicle control administration at each time point. Statistical significance is considered if p < 0.05.
Acknowledgments
This work was supported by the NIH Grants R01DK124504 and R01DK091906. The TOC graphical abstract was created with BioRender.com software.
Glossary
Abbreviations Used
- ACTH
adrenocorticotropic hormone
- AGRP
agouti-related protein
- cAMP
3′,5′-cyclic adenosine monophosphate
- DIEA
N,N-diisopropylamine
- ESI-MS
electrospray ionization mass spectrometry
- FDA
Food and Drug Administration
- Fmoc
fluorenylmethoxycarbonyl
- GPCR
G protein-coupled receptor
- HBSS
Hanks’ balanced salt solution
- HBTU
2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- IBMX
isobutylmethylxanthine
- IT
intrathecal
- MBHA
methylbenzhydrylamine
- MC1R
melanocortin 1 receptor
- MC2R
melanocortin 2 receptor
- MC3R
melanocortin 3 receptor
- MC4R
melanocortin 4 receptor
- MC5R
melanocortin 5 receptor
- MCR
melanocortin receptor
- MeCN
acetonitrile
- MeOH
methanol
- MSH
melanocyte-stimulating hormone
- NDP-MSH
4-norleucine, 7-d-phenylalanine-α-melanocyte-stimulating hormone (melanotan I)
- PDA
photodiode array
- POMC
proopiomelanocortin
- RP-HPLC
reversed-phase high-pressure liquid chromatography
- SAR
structure–activity relationship
- SEM
standard error of the mean
- SQ
subcutaneous
- TIS
triisopropylsilane
The authors declare no competing financial interest.
References
- Chhajlani V.; Muceniece R.; Wikberg J. E. Molecular cloning of a novel human melanocortin receptor. Biochem. Biophys. Res. Commun. 1993, 195 (2), 866–873. 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- Chhajlani V.; Wikberg J. E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992, 309 (3), 417–420. 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- Gantz I.; Konda Y.; Tashiro T.; Shimoto Y.; Miwa H.; Munzert G.; Watson S. J.; DelValle J.; Yamada T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993, 268 (11), 8246–8250. 10.1016/S0021-9258(18)53088-X. [DOI] [PubMed] [Google Scholar]
- Gantz I.; Miwa H.; Konda Y.; Shimoto Y.; Tashiro T.; Watson S. J.; DelValle J.; Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993, 268 (20), 15174–15179. 10.1016/S0021-9258(18)82452-8. [DOI] [PubMed] [Google Scholar]
- Gantz I.; Shimoto Y.; Konda Y.; Miwa H.; Dickinson C. J.; Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200 (3), 1214–1220. 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- Griffon N.; Mignon V.; Facchinetti P.; Diaz J.; Schwartz J. C.; Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200 (2), 1007–1014. 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Cone R. D. The cloning of a family of genes that encode the melanocortin receptors. Science 1992, 257 (5074), 1248–1251. 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L.; Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Low M. J.; Tatro J. B.; Entwistle M. L.; Simerly R. B.; Cone R. D. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. U.S.A. 1993, 90 (19), 8856–8860. 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S.; Inoue A.; Kita T.; Inoue A.; Nakamura M.; Chang A. C.; Cohen S. N.; Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature 1979, 278 (5703), 423–427. 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Bultman S. J.; Michaud E. J.; Woychik R. P. Molecular characterization of the mouse agouti locus. Cell 1992, 71 (7), 1195–1204. 10.1016/S0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- Miller M. W.; Duhl D. M.; Vrieling H.; Cordes S. P.; Ollmann M. M.; Winkes B. M.; Barsh G. S. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 1993, 7 (3), 454–467. 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- Fong T. M.; Mao C.; MacNeil T.; Kalyani R.; Smith T.; Weinberg D.; Tota M. R.; Van der Ploeg L. H. T. ART (protein product of agouti-related transcript) as an antagonist of MC-3 and MC-4 receptors. Biochem. Biophys. Res. Commun. 1997, 237 (3), 629–631. 10.1006/bbrc.1997.7200. [DOI] [PubMed] [Google Scholar]
- Ollmann M. M.; Wilson B. D.; Yang Y. K.; Kerns J. A.; Chen Y. R.; Gantz I.; Barsh G. S. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997, 278 (5335), 135–138. 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Shutter J. R.; Graham M.; Kinsey A. C.; Scully S.; Luthy R.; Stark K. L. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997, 11 (5), 593–602. 10.1101/gad.11.5.593. [DOI] [PubMed] [Google Scholar]
- Butler A. A.; Kesterson R. A.; Khong K.; Cullen M. J.; Pelleymounter M. A.; Dekoning J.; Baetscher M.; Cone R. D. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 2000, 141 (9), 3518–3521. 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Chen A. S.; Marsh D. J.; Trumbauer M. E.; Frazier E. G.; Guan X. M.; Yu H.; Rosenblum C. I.; Vongs A.; Feng Y.; Cao L. H.; Metzger J. M.; Strack A. M.; Camacho R. E.; Mellin T. N.; Nunes C. N.; Min W.; Fisher J.; Gopal-Truter S.; MacIntyre D. E.; Chen H. Y.; Van der Ploeg L. H. T. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000, 26 (1), 97–102. 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Huszar D.; Lynch C. A.; Fairchild-Huntress V.; Dunmore J. H.; Fang Q.; Berkemeier L. R.; Gu W.; Kesterson R. A.; Boston B. A.; Cone R. D.; Smith F. J.; Campfield L. A.; Burn P.; Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997, 88 (1), 131–141. 10.1016/S0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Fan W.; Boston B. A.; Kesterson R. A.; Hruby V. J.; Cone R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 1997, 385 (6612), 165–168. 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Irani B. G.; Xiang Z. M.; Yarandi H. N.; Holder J. R.; Moore M. C.; Bauzo R. M.; Proneth B.; Shaw A. M.; Millard W. J.; Chambers J. B.; Benoit S. C.; Clegg D. J.; Haskell-Luevano C. Implication of the melanocortin-3 receptor in the regulation of food intake. Eur. J. Pharmacol. 2011, 660 (1), 80–87. 10.1016/j.ejphar.2010.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr R. T.; Lines R.; Levine N.; Brooks C.; Xiang L.; Hruby V. J.; Hadley M. E. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996, 58 (20), 1777–1784. 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Hadley M. E. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides 2005, 26 (10), 1687–1689. 10.1016/j.peptides.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H. T.; Martin W. J.; Howard A. D.; Nargund R. P.; Austin C. P.; Guan X. M.; Drisko J.; Cashen D.; Sebhat I.; Patchett A. A.; Figueroa D. J.; DiLella A. G.; Connolly B. M.; Weinberg D. H.; Tan C. P.; Palyha O. C.; Pong S. S.; MacNeil T.; Rosenblum C.; Vongs A.; Tang R.; Yu H.; Sailer A. W.; Fong T. M.; Huang C.; Tota M. R.; Chang R. S.; Stearns R.; Tamvakopoulos C.; Christ G.; Drazen D. L.; Spar B. D.; Nelson R. J.; MacIntyre D. E. A role for the melanocortin 4 receptor in sexual function. Proc. Natl. Acad. Sci. U.S.A. 2002, 99 (17), 11381–11386. 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells H.; Fuciarelli K.; Hansen J.; Hadley M. E.; Hruby V. J.; Dorr R.; Levine N. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. J. Urol. 1998, 160 (2), 389–393. 10.1016/S0022-5347(01)62903-3. [DOI] [PubMed] [Google Scholar]
- Chen W.; Kelly M. A.; Opitz-Araya X.; Thomas R. E.; Low M. J.; Cone R. D. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 1997, 91 (6), 789–798. 10.1016/S0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Sawyer T. K.; Sanfilippo P. J.; Hruby V. J.; Engel M. H.; Heward C. B.; Burnett J. B.; Hadley M. E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological-activity. Proc. Natl. Acad. Sci. U.S.A. 1980, 77 (10), 5754–5758. 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Approves First Treatment to Increase Pain-Free Light Exposure in Patients with a Rare Disorder. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. (accessed Oct 8, 2019).
- Al-Obeidi F.; Castrucci A. M. D.; Hadley M. E.; Hruby V. J. Potent and prolonged acting cyclic lactam analogs of α-melanotropin: Design based on molecular-dynamics. J. Med. Chem. 1989, 32 (12), 2555–2561. 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- Al-Obeidi F.; Hadley M. E.; Pettitt B. M.; Hruby V. J. Design of a new class of superpotent cyclic alpha-melanotropins based on quenched dynamic simulations. J. Am. Chem. Soc. 1989, 111 (9), 3413–3416. 10.1021/ja00191a044. [DOI] [Google Scholar]
- FDA Approves New Treatment for Hypoactive Sexual Desire Disorder in Premenopausal Women. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-hypoactive-sexual-desire-disorder-premenopausal-women. (accessed June 21, 2019).
- Dong Z. Xin.; Moreau J.-P.. Melanocortin Receptor Ligands. WO2007/008704. (18 Janurary 2007).
- FDA Approves First Treatment for Weight Management for People with Certain Rare Genetic Conditions. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-first-treatment-weight-management-people-certain-rare-genetic-conditions. (accessed Nov 27, 2020).
- Brown K. S.; Gentry R. M.; Rowland N. E. Central injection in rats of α-melanocyte-stimulating hormone analog: Effects on food intake and brain Fos. Regul. Pept. 1998, 78 (1–3), 89–94. 10.1016/S0167-0115(98)00127-X. [DOI] [PubMed] [Google Scholar]
- Koegler F. H.; Grove K. L.; Schiffmacher A.; Smith M. S.; Cameron J. L. Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 2001, 142 (6), 2586–2592. 10.1210/endo.142.6.8198. [DOI] [PubMed] [Google Scholar]
- Clayton A. H.; Kingsberg S. A.; Portman D.; Sadiq A.; Krop J.; Jordan R.; Lucas J.; Simon J. A. Safety profile of bremelanotide across the clinical development program. J. Women’s Health 2022, 31 (2), 171–182. 10.1089/jwh.2021.0191. [DOI] [PubMed] [Google Scholar]
- Kühnen P.; Clement K.; Wiegand S.; Blankenstein O.; Gottesdiener K.; Martini L. L.; Mai K.; Blume-Peytavi U.; Gruters A.; Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 2016, 375 (3), 240–246. 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- Kanti V.; Puder L.; Jahnke I.; Krabusch P. M.; Kottner J.; Vogt A.; Richter C.; Andruck A.; Lechner L.; Poitou C.; Krude H.; Gottesdiener K.; Clement K.; Farooqi I. S.; Wiegand S.; Kuhnen P.; Blume-Peytavi U. A melanocortin-4 receptor agonist induces skin and hair pigmentation in patients with monogenic mutations in the leptin-melanocortin pathway. Skin Pharmacol. Physiol. 2021, 34 (6), 307–316. 10.1159/000516282. [DOI] [PubMed] [Google Scholar]
- Ollmann M. M.; Barsh G. S. Down-regulation of melanocortin receptor signaling mediated by the amino terminus of agouti protein in Xenopus melanophores. J. Biol. Chem. 1999, 274 (22), 15837–15846. 10.1074/jbc.274.22.15837. [DOI] [PubMed] [Google Scholar]
- Ollmann M. M.; Lamoreux M. L.; Wilson B. D.; Barsh G. S. Interaction of agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998, 12 (3), 316–330. 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.; Gunn T. M.; Bouley D. M.; Lu X. Y.; Watson S. J.; Schlossman S. F.; Duke-Cohan J. S.; Barsh G. S. A biochemical function for attractin in agouti-induced pigmentation and obesity. Nat. Genet. 2001, 27 (1), 40–47. 10.1038/83741. [DOI] [PubMed] [Google Scholar]
- Creemers J. W. M.; Pritchard L. E.; Gyte A.; Le Rouzic P.; Meulemans S.; Wardlaw S. L.; Zhu X.; Steiner D. F.; Davies N.; Armstrong D.; Lawrence C. B.; Luckman S. M.; Schmitz C. A.; Davies R. A.; Brennand J. C.; White A. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83–132: Interaction between AGRP83–132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology 2006, 147 (4), 1621–1631. 10.1210/en.2005-1373. [DOI] [PubMed] [Google Scholar]
- Kiefer L. L.; Veal J. M.; Mountjoy K. G.; Wilkinson W. O. Melanocortin receptor binding determinants in the agouti protein. Biochemistry 1998, 37 (4), 991–997. 10.1021/bi971913h. [DOI] [PubMed] [Google Scholar]
- Tota M. R.; Smith T. S.; Mao C.; MacNeil T.; Mosley R. T.; Van der Ploeg L. H. T.; Fong T. M. Molecular interaction of agouti protein and agouti-related protein with human melanocortin receptors. Biochemistry 1999, 38 (3), 897–904. 10.1021/bi9815602. [DOI] [PubMed] [Google Scholar]
- Ericson M. D.; Wilczynski A.; Sorensen N. B.; Xiang Z. M.; Haskell-Luevano C. Discovery of a β-hairpin octapeptide, c[Pro-Arg-Phe-Phe-Dap-Ala-Phe-DPro], mimetic of agouti-related protein(87–132) [AGRP(87–132)] with equipotent mouse melanocortin-4 receptor (mMC4R) antagonist pharmacology. J. Med. Chem. 2015, 58 (11), 4638–4647. 10.1021/acs.jmedchem.5b00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. D.; Freeman K. T.; Schnell S. M.; Fleming K. A.; Haskell-Luevano C. Structure-activity relationship studies on a macrocyclic agouti-related protein (AGRP) scaffold reveal agouti signaling protein (ASP) residue substitutions maintain melanocortin-4 receptor antagonist potency and result in inverse agonist pharmacology at the melanocortin-5 receptor. J. Med. Chem. 2017, 60 (19), 8103–8114. 10.1021/acs.jmedchem.7b00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M. D.; Freeman K. T.; Schnell S. M.; Haskell-Luevano C. A macrocyclic agouti-related protein/[Nle4,DPhe7]α-melanocyte stimulating hormone chimeric scaffold produces subnanomolar melanocortin receptor ligands. J. Med. Chem. 2017, 60 (2), 805–813. 10.1021/acs.jmedchem.6b01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adank D. N.; Lunzer M. M.; Ericson M. D.; Koeperich Z. M.; Wilber S. L.; Fleming K. A.; Haskell-Luevano C. Comparative intracerebroventricular and intrathecal administration of a nanomolar macrocyclic melanocortin receptor agonist MDE6–5-2c (c[Pro-His-DPhe-Arg-Trp-Dap-Ala-DPro]) decreases food intake in mice. ACS Chem. Neurosci. 2020, 11 (19), 3051–3063. 10.1021/acschemneuro.0c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J. P.; Hsiung H. M.; Flora D. B.; Edwards P.; Smith D. P.; Zhang X. Y.; Gadski R. A.; Heiman M. L.; Hertel J. L.; Emmerson P. J.; Husain S.; O’Brien T. P.; Kahl S. D.; Smiley D. L.; Zhang L.; Dimarchi R. D.; Yan L. Z. Discovery of a β-MSH-derived MC-4R selective agonist. J. Med. Chem. 2005, 48 (9), 3095–3098. 10.1021/jm0501432. [DOI] [PubMed] [Google Scholar]
- Greenfield J. R.; Miller J. W.; Keogh J. M.; Henning E.; Satterwhite J. H.; Cameron G. S.; Astruc B.; Mayer J. P.; Brage S.; See T. C.; Lomas D. J.; O’Rahilly S.; Farooqi I. S. Modulation of blood pressure by central melanocortinergic pathways. New Engl. J. Med. 2009, 360 (1), 44–52. 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Ryser H.-P.; Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science 1965, 150 (3695), 501–503. 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- Frankel A. D.; Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55 (6), 1189–1193. 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Green M.; Loewenstein P. M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55 (6), 1179–1188. 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Vivès E.; Brodin P.; Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272 (25), 16010–16017. 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Mitchell D. J.; Kim D. T.; Steinman L.; Fathman C. G.; Rothbard J. B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56 (5), 318–325. 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Traboulsi H.; Larkin H.; Bonin M. A.; Volkov L.; Lavoie C. L.; Marsault E. Macrocyclic cell penetrating peptides: a study of structure-penetration properties. Bioconjugate Chem. 2015, 26 (3), 405–411. 10.1021/acs.bioconjchem.5b00023. [DOI] [PubMed] [Google Scholar]
- Carpino L. A.; Han G. Y. 9-Fluorenylmethoxycarbonyl function, a new base-sensitive amino-protecting group. J. Am. Chem. Soc. 1970, 92 (19), 5748–5749. 10.1021/ja00722a043. [DOI] [Google Scholar]
- Carpino L. A.; Han G. Y. 9-fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 1972, 37 (22), 3404–3409. 10.1021/jo00795a005. [DOI] [Google Scholar]
- Joseph C. G.; Bauzo R. M.; Xiang Z. M.; Shaw A. M.; Millard W. J.; Haskell-Luevano C. Elongation studies of the human agouti-related protein (AGRP) core decapeptide (Yc[CRFFNAFC]Y) results in antagonism at the mouse melanocortin-3 receptor. Peptides 2003, 24 (2), 263–270. 10.1016/S0196-9781(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Hargittai B.; Barany G. Controlled syntheses of natural and disulfide-mispaired regioisomers of alpha-conotoxin SI. J. Pept. Res. 1999, 54 (6), 468–479. 10.1034/j.1399-3011.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- Lensing C. J.; Freeman K. T.; Schnell S. M.; Adank D. N.; Speth R. C.; Haskell-Luevano C. An in vitro and in vivo investigation of bivalent ligands that display preferential binding and functional activity for different melanocortin receptor homodimers. J. Med. Chem. 2016, 59 (7), 3112–3128. 10.1021/acs.jmedchem.5b01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiöth H. B.; Chhajlani V.; Muceniece R.; Klusa V.; Wikberg J. E. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996, 59 (10), 797–801. 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- Elster L.; Elling C.; Heding A. Bioluminescence resonance energy transfer as a screening assay: Focus on partial and inverse agonism. J. Biomol. Screening 2007, 12 (1), 41–49. 10.1177/1087057106295895. [DOI] [PubMed] [Google Scholar]
- Lensing C. J.; Freeman K. T.; Schnell S. M.; Speth R. C.; Zarth A. T.; Haskell-Luevano C. Developing a biased unmatched bivalent ligand (BUmBL) design strategy to target the GPCR homodimer allosteric signaling (cAMP over β-arrestin 2 recruitment) within the melanocortin receptors. J. Med. Chem. 2019, 62 (1), 144–158. 10.1021/acs.jmedchem.8b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph C. G.; Yao H.; Scott J. W.; Sorensen N. B.; Marnane R. N.; Mountjoy K. G.; Haskell-Luevano C. γ2-Melanocyte stimulation hormone (γ2-MSH) truncation studies results in the cautionary note that γ2-MSH is not selective for the mouse MC3R over the mouse MC5R. Peptides 2010, 31 (12), 2304–2313. 10.1016/j.peptides.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber K. A.; Ji R.-L.; Gallazzi F.; Jiang S.; Van Doren S. R.; Tao Y.-X.; Newton Northup J. Development of a therapeutic peptide for cachexia suggests a platform approach for drug-like peptides. ACS Pharmacol. Transl. Sci. 2022, 5 (5), 344–361. 10.1021/acsptsci.1c00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K. G.; Sutton G. M.; Dong J. Z.; Roubert P.; Plas P.; Halem H. A.; Culler M. D.; Yang H.; Dixit V. D.; Butler A. A. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R-and MC4R-deficient C57BL/6J mice. Peptides 2009, 30 (10), 1892–1900. 10.1016/j.peptides.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durek T.; Kaas Q.; White A. M.; Weidmann J.; Fuaad A. A.; Cheneval O.; Schroeder C. I.; de Veer S. J.; Dellsen A.; Osterlund T.; Larsson N.; Knerr L.; Bauer U.; Plowright A. T.; Craik D. J. Melanocortin 1 receptor agonists based on a bivalent, bicyclic peptide framework. J. Med. Chem. 2021, 64 (14), 9906–9915. 10.1021/acs.jmedchem.1c00095. [DOI] [PubMed] [Google Scholar]
- Adank D. N.; Lunzer M. M.; Lensing C. J.; Wilber S. L.; Gancarz A. M.; Haskell-Luevano C. Comparative in vivo investigation of intrathecal and intracerebroventricular administration with melanocortin ligands MTII and AGRP into mice. ACS Chem. Neurosci. 2018, 9 (2), 320–327. 10.1021/acschemneuro.7b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément K.; Biebermann H.; Farooqi I. S.; Van der Ploeg L.; Wolters B.; Poitou C.; Puder L.; Fiedorek F.; Gottesdiener K.; Kleinau G.; Heyder N.; Scheerer P.; Blume-Peytavi U.; Jahnke I.; Sharma S.; Mokrosinski J.; Wiegand S.; Muller A.; Weiss K.; Mai K.; Spranger J.; Gruters A.; Blankenstein O.; Krude H.; Kuhnen P. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018, 24, 551–555. 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- Kaiser E.; Colescott R. L.; Bossinger C. D.; Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34 (2), 595–598. 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Christensen T. A Qualitative test for monitoring coupling completeness in solid-phase peptide-synthesis using chloranil. Acta Chem. Scand. B 1979, 33 (10), 763–766. 10.3891/acta.chem.scand.33b-0763. [DOI] [Google Scholar]
- Lensing C. J.; Adank D. N.; Doering S. R.; Wilber S. L.; Andreasen A.; Schaub J. W.; Xiang Z. M.; Haskell-Luevano C. Ac-Trp-DPhe(p-l)-Arg-Trp-NH2, a 250-fold selective melanocortin-4 receptor (MC4R) antagonist over the melanocortin-3 receptor (MC3R), affects energy homeostasis in male and female mice differently. ACS Chem. Neurosci. 2016, 7 (9), 1283–1291. 10.1021/acschemneuro.6b00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden J. L. K.; Wilcox G. L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980, 67 (2–3), 313–316. 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]