Abstract
Vaccination with DNA and recombinant vaccinia viruses (rec.VV) has been studied with the coxsackievirus B3 (CVB3) model system. Plasmids encoding all structural proteins of CVB3, when injected intramuscularly, induced only low levels of virus-specific antibodies. However, DNA vaccination with the major structural protein VP1 protected 72.2% of mice from lethal challenge, whereas VP1 expressed by rec.VV was much less efficient.
Coxsackievirus B3 (CVB3), a member of the picornavirus group, is an important human pathogen. This virus, along with other enteroviruses, is involved in at least 50% of acute myocarditis cases and approximately 25% of dilated cardiomyopathy cases (2, 8). Despite the immense accumulation of molecular data and the observations that commonly useful vaccination procedures are applicable in murine models (4), so far there are no virus-specific preventive or therapeutic procedures available that protect humans against coxsackievirus-induced heart diseases. Immunization with DNA or recombinant vaccinia viruses (rec.VV) affords the opportunity to establish new preventive procedures against lethal CVB3 infections. In this study, we show that DNA vaccines can protect mice against CVB3-induced diseases and a comparison between immunization with DNA or rec.VV demonstrates that the efficiency of the induced protection was dependent on (i) the type of vaccine used and (ii) the CVB3 protein expressed.
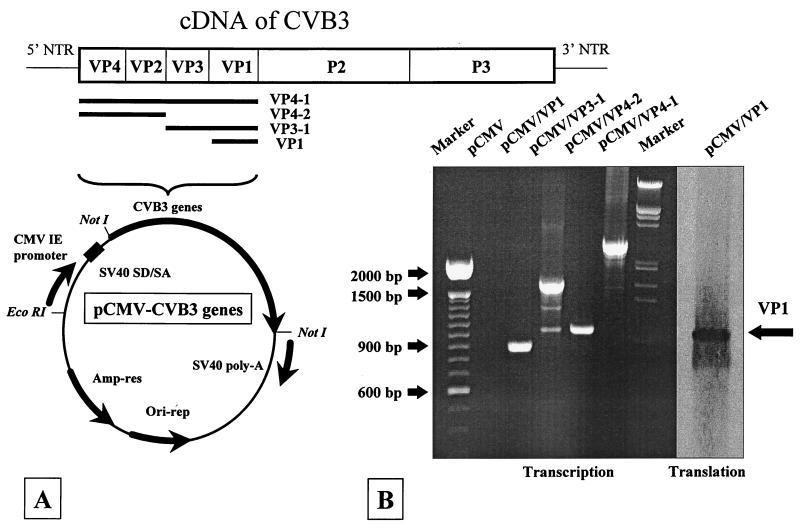
VP1 is the major capsid protein of CVB3, and several B- and T-cell epitopes are located within this protein (6). Therefore, after removing the reporter gene β-galactosidase from the parental vector pCMV-β (Clontech, Palo Alto, Calif.), the coding sequence specific for VP1 (851 bp) was amplified by PCR from the CVB3 cDNA (11), cloned into the plasmid pCMV, and named pCMV/VP1. In order to analyze the possibility that additional immunogenic epitopes may increase the immune reaction in vivo, we constructed the plasmids pCMV/VP4-2, pCMV/VP3-1, and pCMV/VP4-1, which encode overlapping sequences of all capsid proteins of CVB3 (Fig. 1A): VP4 and VP2 (995 bp), VP3 and VP1 (1,556 bp), and VP4 through VP1 (2,561 bp). Expression from these plasmids was confirmed in vitro by transient transfection of HeLa cells. After RNA isolation, DNase digestion, and reverse transcriptase reaction, the transcriptional activity of all plasmids was confirmed by PCR (Fig. 1B, Transcription). In addition, the translation of VP1 in pCMV/VP1-transfected HeLa cells was confirmed by Western blotting (Fig. 1B, Translation). Proteins VP4 through VP1, VP3 and VP1, and VP4 and VP2 are processed into single proteins during normal viral infection and were not recognized by the polyclonal antiserum; therefore, we could not confirm protein expression from these plasmids.
FIG. 1.
Expression of plasmid-encoded RNAs in tissue culture. (A) The β-galactosidase gene of the parental vector was replaced by sequences specific for the capsid proteins VP1 (851 bp), VP3 and VP1 (1,565 bp), VP4 and VP2 (995 bp), and VP4 to VP1 (2,561 bp) of CVB3. (B) Transcriptional activities of the plasmids pCMV/VP1, pCMV/VP3-1, pCMV/VP4-2, and pCMV/VP4-1 were analyzed by transient transfection of HeLa cells followed by RNA isolation, DNase treatment, cDNA synthesis, and PCR using the original primer pairs. No PCR product was detectable in pCMV-transfected cultures, demonstrating the specificity of the PCR conditions. In addition, the presence of VP1 in a protein extract of pCMV/VP1-transfected HeLa cells was analyzed (last lane) by Western blot analysis.
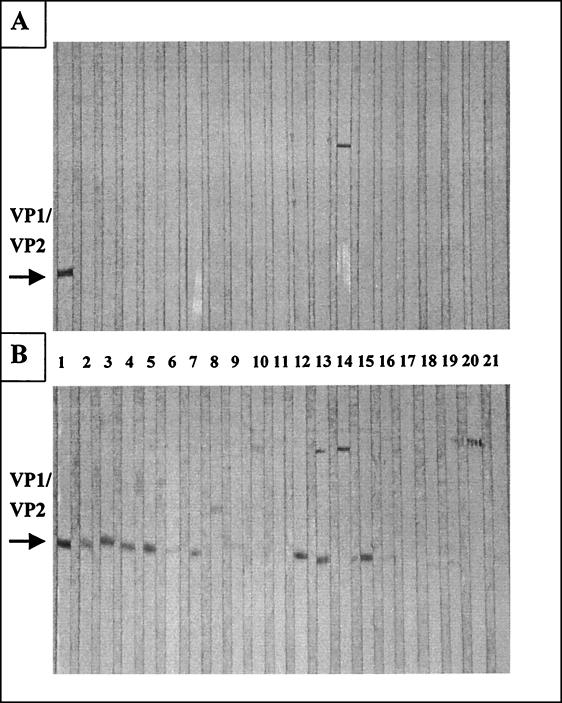
After the expression from the DNA vaccines was analyzed in vitro, BALB/c mice were inoculated intramuscularly (i.m.) twice in each quadriceps muscle separately with 100 μg of plasmid DNA at 4-week intervals. One group of mice remained untreated. All sera obtained prior to immunization were negative for CVB3 antibodies (data not shown). Four weeks after every injection, sera were analyzed for the presence of CVB3-specific antibodies by Western blotting and enzyme-linked immunosorbent assay (ELISA) (Fig. 2 and Table 1). Four weeks after the first plasmid inoculation, no virus-specific antibodies were detectable by Western blot analysis (Fig. 2A). However, 4 weeks after the second immunization, antibodies which were present in sera of pCMV/VP1- (lanes 2 to 6) as well as pCMV/VP4-2 (lanes 12 to 16)-immunized mice were able to bind virus-specific proteins with the molecular weight of capsid protein VP1 or VP2 (lane 1) of CVB3 (Fig. 2B). No or only a very few virus-specific antibodies were detectable in sera of mice treated with pCMV/VP3-1 (lanes 7 to 11) or pCMV/VP4-1 (lanes 17 to 21), using this method. In addition, levels of anti-CVB3 immunoglobulin M (IgM)- or IgG-specific antibodies were also assessed by ELISA, using purified CVB3 as a target antigen. pCMV-injected mice were used as negative controls. No increase of IgM titers in sera of all immunized mice was detectable in comparison to antibody concentrations in control mice (Table 1). This result may reflect the relatively late time point employed, when the IgM response may have already been converted to the production of IgG antibodies. However, it has been shown before that, by using the cytomegalovirus promoter system to express the influenza virus hemagglutinin glycoprotein, i.m. administration of plasmid DNA induced only low to undetectable IgM titers of ELISA activity in mice (5). In contrast, detectable IgG antibody levels were present after the second DNA inoculation, especially in sera of mice inoculated with the pCMV/VP1 and pCMV/VP4-2 constructs. This result confirms the data obtained by Western blot analysis suggesting that modest boosting has occurred (Table 1). Levels of IgG-specific antibodies raised by our DNA vaccines after the second immunization were low in comparison to antibody titers induced during acute viral infections, but low levels of IgG-specific or total antibody concentrations were also found in sera of mice inoculated with DNA vaccines against influenza virus or against lymphocytic choriomeningitis virus, respectively (5, 15, 16). These results may be due to the fact that (i) proteins encoded by our DNA vaccines may not be processed efficiently, (ii) using DNA vaccines, the expression of viral proteins is usually noncytopathic, and viral proteins are not released from transfected cells, or (iii) CVB3 is a cytoplasmic virus, and expression of CVB3-specific RNA sequences in the nucleus exposes them to unusual transcriptional and posttranscriptional pathways which could be responsible for low levels of protein concentrations in transfected target cells.
FIG. 2.
Detection of CVB3-specific antibody levels induced by DNA vaccine. BALB/c mice were injected twice i.m. with plasmid DNA at 28-day intervals. Four weeks after every injection, sera were obtained and analyzed for the presence of CVB3-specific antibodies by Western blot analysis. (A) After the first immunization, no virus-specific antibodies were detectable. (B) After the second immunization, antibodies which were present in sera of pCMV/VP1 (lanes 2 to 6)- and pCMV/VP4-2 (lanes 12 to 16)-immunized mice were able to bind virus-specific proteins with the same molecular weight as the capsid protein VP1 or VP2 of CVB3 (lane 1, as a positive control). Only a few or no virus-specific antibodies were present in samples of pCMV/VP3-1 (lanes 7 to 11)- and pCMV/VP4-1 (lanes 17 to 21)-treated mice. The serum dilution was 1:25. The results are representative of three different experiments.
TABLE 1.
Detection of CVB3-specific antibody levels induced by DNA vaccines by ELISAa
| Plasmid DNA | Dilution of 1:25 (OD490)
|
Dilution of 1:50 (OD490)
|
||
|---|---|---|---|---|
| Immunization 1 | Immunization 2 | Immunization 1 | Immunization 2 | |
| IgM-specific antibodies | ||||
| pCMV | 0.25 ± 0.04 | 0.30 ± 0.06 | 0.18 ± 0.01 | 0.24 ± 0.04 |
| pCMV/VP1 | 0.29 ± 0.08 | 0.26 ± 0.04 | 0.24 ± 0.06 | 0.21 ± 0.04 |
| pCMV/VP3-1 | 0.22 ± 0.03 | 0.32 ± 0.08 | 0.20 ± 0.04 | 0.26 ± 0.05 |
| pCMV/VP4-2 | 0.22 ± 0.06 | 0.36 ± 0.07 | 0.18 ± 0.06 | 0.29 ± 0.05 |
| pCMV/VP4-1 | 0.19 ± 0.04 | 0.39 ± 0.13 | 0.16 ± 0.02 | 0.31 ± 0.09 |
| IgG-specific antibodies | ||||
| pCMV | 0.17 ± 0.02 | 0.17 ± 0.04b | 0.15 ± 0.02 | 0.15 ± 0.03 |
| pCMV/VP1 | 0.19 ± 0.03 | 0.30 ± 0.08 | 0.15 ± 0.01 | 0.21 ± 0.05 |
| pCMV/VP3-1 | 0.16 ± 0.01 | 0.22 ± 0.04 | 0.14 ± 0.01 | 0.18 ± 0.03 |
| pCMV/VP4-2 | 0.17 ± 0.02 | 0.33 ± 0.13 | 0.14 ± 0.01 | 0.25 ± 0.07 |
| pCMV/VP4-1 | 0.15 ± 0.01 | 0.27 ± 0.07 | 0.13 ± 0.01 | 0.21 ± 0.03 |
After the first and the second immunizations, the presence of IgM- or IgG-specific CVB3 antibodies was characterized by ELISA, demonstrated as the optical density at 490 nm (OD490). Four mice in each group were used in each experiment. The data shown are the mean values ± standard deviation. The results are representative of three different experiments.
The statistical comparisons were carried out with Microsoft Excel by using Student’s t test (pCMV/VP1 > pCMV [P < 0.008], pCMV/VP3-1 > pCMV [P < 0.3], pCMV/VP4-2 > pCMV [P < 0.003], and pCMV/VP4-1 > pCMV [P < 0.03]). Significant differences are only detectable in sera of pCMV/VP1-, pCMV/VP4-1-, and pCMV/VP4-2-vaccinated mice.
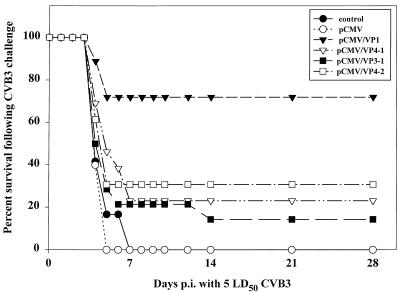
Four weeks after the second vaccination, mice were subjected to intraperitoneal (i.p.) challenge with a normally lethal dose of CVB3 (Nancy), a cardiopathogenic strain (designated H3) of the original stock of CVB3 obtained from J. F. Woodruff and isolated by S. A. Huber. The virus was propagated and purified as described previously (7). After the challenge, the number of surviving animals was monitored up to 28 days postinfection (p.i.). No excess mortality was noted beyond this time point. As shown in Fig. 3, the most effective vaccine in our study was the pCMV/VP1 construct, conferring protection on 72.2% of mice (13 of 18). i.m. inoculation of the plasmids pCMV/VP4-1, pCMV/VP3-1, and pCMV/VP4-2 was less effective, inducing incomplete protection of between 30.8% (4 of 13 with pCMV/VP4-2), 23.1% (3 of 13 with pCMV/VP4-1), and 14.3% (2 of 14 with pCMV/VP3-1). Because VP1 is expressed as a fusion protein in pCMV/VP3-1 and pCMV/VP4-1, the immune response to this molecule may be less able to recognize the native VP1 molecule. A similar argument may apply to the failure of the pCMV/VP4-2 construct, since previous work (1) shows that VP2 contains important epitopes. The expression of VP2, VP3, and VP4 as single proteins should clarify this issue. However, the low level of anti-CVB3-specific antibody is unlikely to explain the good protection we induced by administration of the pCMV/VP1 construct. It is likely that immune responses other than antibodies play a role in the pCMV/VP1-induced protection. Therefore, the cellular immune response in vaccinated mice is under investigation now.
FIG. 3.
Protection against normally lethal CVB3 challenge induced by DNA vaccination. BALB/c mice were immunized with two injections of plasmid DNA encoding either CVB3 VP1, VP4 and VP2, VP3 and VP1, or VP4 through VP1; with two injections of a control plasmid, pCMV; or with PBS alone. Four weeks after the second inoculation, mice were challenged with 5 LD50s of CVB3 i.p. The percentage of animals surviving is shown over a period of 28 days. The results presented summarize data from three independent experiments, using at least three to four mice in each group.
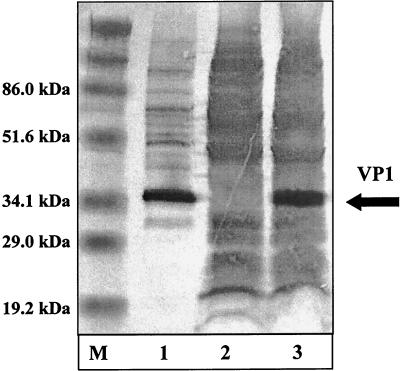
In order to analyze a second immunization procedure, using rec.VV, the PCR-derived subgenomic CVB3 fragment of VP1 was cloned into the StuI-SpeI site of the transfer plasmid pSC11 (3). After homologous recombination with the wild-type virus, rec.VV VV-VP1 was isolated by using standard methods (12, 14). Protein expression of VP1 was analyzed by Western blotting (Fig. 4). Protein bands, specific for VP1 of CVB3, were easily detectable in CVB3- and VV-VP1-infected HeLa cell extracts (lanes 1 and 3) when probed with a murine antiserum raised against the VP1 protein of CVB3. No virus-specific band was detectable in lysates from cells infected with parental VV expressing the vector-derived β-galactosidase (VVSC11) only (lane 2).
FIG. 4.
Expression of the coxsackievirus protein VP1 from rec.VV. Detection of protein VP1 by Western blot analysis. After electrophoresis, samples containing 30 μg of protein from CVB3-infected (lane 1), VVSC11-infected (lane 2), and VV-VP1-infected (lane 3) HeLa cells were blotted on a nitrocellulose membrane. A primary antibody (diluted 1:2,500) was applied to detect the VP1 protein. A prestained protein marker was used as a size standard (lane M).
BALB/c mice were inoculated i.p. with 107 PFU of VV-VP1. Four weeks postvaccination, sera were analyzed for the presence of anti-CVB3 antibodies by ELISA, using purified CVB3 as a target antigen. VVSC11-infected and noninfected mice were used as negative controls. Low amounts of detectable IgG-specific antibody concentrations were present only in sera of mice inoculated with VV-VP1, similar to the findings of some other groups using rec.VV to express foreign proteins (9, 10). After being bled, mice were challenged with 5 50% lethal doses (LD50) of CVB3 and the number of surviving animals was monitored for up to 28 days p.i. No excess mortality was noted beyond this time point. As shown in Table 2, vaccination with VV-VP1 was not effective in BALB/c mice, in contrast to the protection induced by pCMV/VP1. Using VV-VP1, only 5.6% (1 of 18) of mice survived the lethal CVB3 challenge. However, there was a delay of death in VV-VP1-vaccinated mice, which died up to and during a period of 21 days postchallenge, whereas mice of all control groups were dead 7 days postchallenge. In contrast, using C57BL/6 mice in VV-VP1 immunization studies, we were able to induce up to 50% protection (data not shown). The question remains as to why the VP1 expression by rec.VV was ineffective in inducing a protective immune response in BALB/c mice in comparison to the VP1 expression by a DNA vaccine. One possible explanation for this result is that some mouse strains, like the BALB/c mice in the VV-VP1 studies, could be nonresponders when rec.VV is used for immunization studies, as demonstrated by Schirmbeck et al. (13). They found that DNA vaccination was able to induce an immune response against a hepatitis B virus surface antigen in a specific strain of mice which were nonresponders when rec.VV was used as an expression vehicle.
TABLE 2.
Efficiency of pCMV/VP1- or VV-VP1-induced protection against normally lethal CVB3 challenge
| Exptl groupa vaccinated with | IgG antibody levels after vaccinationb (ELISA, OD490) | % Survival following CVB3 challenge at indicated day p.i.c
|
|
|---|---|---|---|
| 7 | 28 | ||
| pCMV | 0.17 ± 0.04 | 0 | 0 |
| pCMV/VP1 | 0.30 ± 0.08 | 72.2 | 72.2 |
| VVSC11 | 0.14 ± 0.02 | 0 | 0 |
| VV-VP1 | 0.21 ± 0.02 | 28.6 | 5.6 |
| Nonvaccinated | 0.12 ± 0.02 | 0 | 0 |
BALB/c mice were immunized either with 2 × 200 μg of pCMV/VP1 or with a single dose of 107 PFU of VV-VP1. Control mice remained uninfected, were inoculated with 2 × 200 μg of pCMV, or were infected with 107 PFU of VVSC11.
Four weeks after the last injection, sera were obtained and analyzed for the presence of CVB3-specific antibodies by ELISA. The serum dilution was 1:25. The data are the mean values ± standard deviation. OD490, optical density at 490 nm.
Four weeks after immunization, mice were challenged with 5 LD50s of CVB3 i.p. The percentage of animals surviving is shown at days 7 and 28 after challenge. The results are representative of three different experiments.
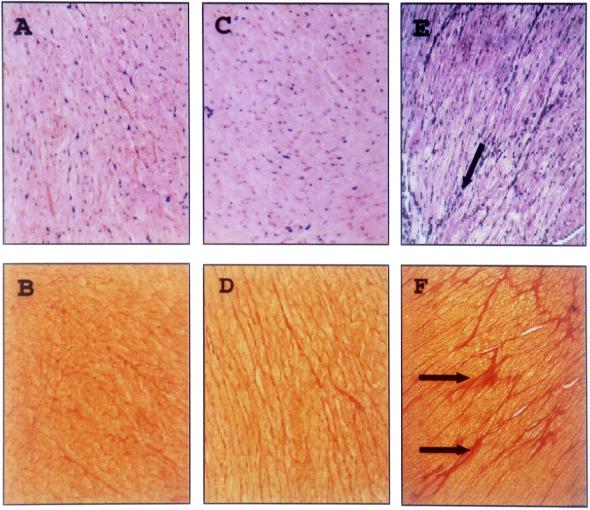
Furthermore, 45-day-p.i. paraffin sections of murine heart tissue were stained with hematoxylin-eosin or with Sirius red and analyzed microscopically for ongoing inflammation or fibrosis. In heart tissue of nonimmunized surviving BALB/c mice, infected with 1 LD50 of CVB3, histopathological changes were detectable. This was demonstrated by the presence of fibrotic tissue (Fig. 5E and F), indicating tissue destruction earlier during the infection. Using heart tissue of plasmid- or rec.VV-vaccinated mice, we could not detect any disorders in the myocardium of surviving animals, indicating that CVB3 was not able to cause tissue destruction, inflammation, or fibrosis in protected animals (Fig. 5A through D). Further experiments will be focused on the characterization of the viral load in the murine heart tissue of vaccinated mice after challenge and the characterization of the differences between both immunization procedures in causing protection.
FIG. 5.
Histology of myocardial tissue from pCMV/VP1- and VV-VP1-vaccinated mice after CVB3 challenge. Paraffin sections from heart tissue of pCMV/VP1 (A and B)- and VV-VP1 (C and D)-vaccinated and nonimmunized (E and F) BALB/c mice 45 days following challenge with 5 LD50 of CVB3 for the immunized mice and 1 LD50 of CVB3 for the nonimmunized mice were stained with hematoxylin-eosin (A, C, and E) or Sirius red (B, D, and F); connective tissue is stained red. No infiltrating immune cells or unusual high levels of connective tissue, indicating ongoing myocarditis or fibrosis, were detectable in immunized mice. In contrast, fibrotic tissue, indicating former virus-caused tissue destruction, was present in heart samples from nonimmunized mice (E and F, arrows). Magnification, ×125.
Acknowledgments
Kirk U. Knowlton is acknowledged for the CVB3 cDNA clone. We thank Katrin Klement for excellent technical assistance.
This work was supported by BMBF grant 01229602/01229104 ZP5 (to A.H.) and by NIH grant AI-42314 (to J.L.W.).
REFERENCES
- 1.Beatrice S T, Katze M G, Zajac B A, Crowell R L. Induction of neutralizing antibodies by the coxsackievirus B3 virion polypeptide VP2. Virology. 1980;104:426–438. doi: 10.1016/0042-6822(80)90345-1. [DOI] [PubMed] [Google Scholar]
- 2.Bowles N E, Olsen E G J, Richardson P J, Archard L C. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;i:1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabati S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening in recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fohlman J, Pauksen K, Morein B, Bjare U, Ilback N G, Friman G. High yield production of an inactivated coxsackie B3 adjuvant vaccine with protective effect against experimental myocarditis. Scand J Infect Dis Suppl. 1993;88:103–108. [PubMed] [Google Scholar]
- 5.Fynan E F, Webster R G, Fuller D H, Haynes J R, Satoro J C, Robinson L. DNA vaccines: protective immunization by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci USA. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haarmann C M, Schwimmbeck P L, Mertens T, Schultheiss H-P, Strauer B E. Identification of serotype-specific and nonserotype-specific B-cell epitopes of coxsackie B virus using synthetic peptides. Virology. 1994;200:381–389. doi: 10.1006/viro.1994.1202. [DOI] [PubMed] [Google Scholar]
- 7.Henke A, Huber S, Stelzner A, Whitton J L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandolf R, Hofschneider P H. Viral heart disease. Semin Immunopathol. 1989;11:1–13. doi: 10.1007/BF00197080. [DOI] [PubMed] [Google Scholar]
- 9.Klavinskis L S, Whitton J L, Oldstone M B A. Molecularly engineered vaccine which expresses an immunodominant T-cell epitope induces cytotoxic T lymphocytes that confer protection from lethal virus infection. J Virol. 1989;63:4311–4316. doi: 10.1128/jvi.63.10.4311-4316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klavinskis L S, Whitton J L, Joly E, Oldstone M B A. Vaccination and protection from a lethal viral infection: identification, incorporation, and use of a cytotoxic T lymphocyte glycoprotein epitope. Virology. 1990;178:393–400. doi: 10.1016/0042-6822(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 11.Knowlton K U, Jeon E-S, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70:7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackett M, Smith G L, Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984;49:857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schirmbeck R, Bohm W, Ando K, Chisari F V, Reimann J. Nucleic acid vaccination primes hepatitis B virus surface antigen-specific cytotoxic T lymphocytes in nonresponder mice. J Virol. 1995;69:5929–5934. doi: 10.1128/jvi.69.10.5929-5934.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitton J L, Gebhard J R, Lewicki H, Tishon A, Oldstone Michael B A. Molecular definition of a major cytotoxic T-lymphocyte epitope in the glycoprotein of lymphocytic choriomeningitis virus. J Virol. 1988;62:687–695. doi: 10.1128/jvi.62.3.687-695.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama M, Zhang J, Whitton J L. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama M, Hasset D E, Zhang J, Whitton J L. DNA immunization can stimulate florid local inflammation, and the antiviral immunity induced varies depending on injection site. Vaccine. 1997;15:553–560. doi: 10.1016/s0264-410x(97)00213-2. [DOI] [PubMed] [Google Scholar]