Abstract
An approach utilizing N-heterocyclic carbene for nitrile formation and desymmetrization reaction is developed. The process involves kinetic resolution, with the axially chiral aryl monoaldehydes obtained in moderate yields with excellent optical purities. These axially chiral aryl monoaldehydes can be conveniently transformed into functionalized molecules, showing great potential as catalysts in organic chemistry.
Introduction
Aryl aldehydes bearing chiral axis have received increasing attention in recent years [1–5]. In particular, these molecules have been found to be excellent chiral catalysts for enantioselective reactions, as demonstrated by Zhao et al. [6–10], Guo et al. [11–14], and others [15,16]. In a broader picture, aldehyde moieties have versatile applications in chemical synthesis [17–21] and exhibit various functions in natural products and bioactive molecules [22–25] (Fig. 1A). A number of synthetic approaches are now available to prepare axially chiral aryl aldehydes via enzymatic catalysis [26], transition metal catalysis [17,19–21,27–29], or organic catalysis [18,30,31]. Elegant examples include metal-catalyzed C-H activation or cross-coupling reactions, as demonstrated by Liao et al. [27], Chen et al. [17], Yang et al. [20], and others [19,21,28,29]. In the area of organic catalysis, Sparr et al. [18,30] reported an innovative approach to prepare axially chiral aryl aldehydes via an amine-catalyzed cascade reaction to set up new aryl cores and the chiral axis. Wu et al. [32] disclosed N-heterocyclic carbene (NHC)-catalyzed aldehyde-to-ester transformation with formal desymmetrization to prepare axially chiral aryl aldehyde.
Fig. 1.

Bioactive molecules and catalysts containing aryl aldehyde moiety and NHC-catalyzed atropoenantioselective nitrile formation and desymmetrization reaction. (A) Bioactive molecules and catalysts containing aryl aldehyde moieties. (B) This work: atroposelective synthesis of axially chiral biaryl aldehydes.
We are interested in employing NHC organic catalysts for unconventional transformations and functional molecules [33–46]. Research from Chen et al. [47], our laboratory [48], and Sun et al. [49] have shown that under the influence of NHC catalysts, aldehydes can be converted to nitriles (via the corresponding imine intermediates) under mild conditions. Fier and Maloney [50] developed this nitrile formation process as a protocol for deamination of primary sulfonamides. We recently converted monoaldehydes to the corresponding nitriles with axial chirality controlled [51].
Here, we disclose that through NHC-catalyzed nitrile formation, a desymmetrization/dynamic kinetic resolution process can be achieved and transfers symmetric dicarbaldehydes to axially chiral aryl monoaldehydes (Fig. 1B). Products from our approach containing cyanide and aldehyde groups are potential catalysts in organocatalysis. In contrast to earlier more conventional aldehyde conversions (such as ester formation), we hope our study encourages further exploration in innovating the NHC-catalyzed aldehyde-to-nitrile process for new applications.
Results
We initially employed biaryl dialdehyde (1a) and readily available p-toluenesulfinate (TsNH2, 2) as model substrates to screen reaction conditions for this transformation (Table). Subsequently, we performed a screening of various indanol-derived NHC pre-catalysts in the presence of NHEt2 in dichloroethane (DCE) solvent (Table, entries 1 to 3). Notably, the utilization of NHC pre-catalyst A [52] featuring the N-Mes group in this process could only generate a trace amount of the axially chiral aryl monoaldehyde 3a (entry 1). However, the product 3a could be given in moderate yields while utilizing NHC pre-catalysts B and C [53,54] (entries 2 and 3). Additionally, we observed the formation of a by-product biaryl dicarbonitrile 4 during this transformation process. Considering the impact of bases on nitrile formation during NHC catalysis, we conducted additional screening of various bases. However, when NHC pre-catalyst B was employed, the use of alternative bases resulted in a notable decrease on the product enantiomeric ratio (er) values (entries 4 to 7). Regrettably, our attempts to improve the results by screening various nonpolar and polar organic solvents did not yield positive outcomes (entries 8 to 11). However, it is worth noting that increasing the quantity of substrate 2 (TsNH2) resulted in a slight increase in yields and importantly improved the er values of product 3a. Moreover, in these cases, a nonchiral dicarbonitrile by-product 4 was obtained, with yields ranging from 18% to 21% (entries 12 and 13). Finally, when a 4-Å molecular sieve (MS) was used as the additive in the 1.0 mL of DCE, the target axially chiral product 3a was obtained in 63% yield and >99:1 er value (entry 14).
Table.
Optimized conditions. Unless otherwise specified, the reactions were carried out using 1a (0.12 mmol), 2 (0.10 mmol), NHC (0.02 mmol), base (0.12 mmol), 4 Å MS (50 mg), and solvent (2.0 ml) at 40 °C under N2 for 24 h.

| ||||||
|---|---|---|---|---|---|---|
| Entry | NHC | Base | Solvent | 3a [%]a | Erb | 4a [%] |
| 1 | A | NHEt2 | DCE | Trace | - | - |
| 2 | B | NHEt2 | DCE | 50 | 96:4 | 16 |
| 3 | C | NHEt2 | DCE | 45 | 65:35 | 22 |
| 4 | B | Cs2CO3 | DCE | 43 | 63:37 | 15 |
| 5 | B | NaHCO3 | DCE | Trace | - | - |
| 6 | B | DBU | DCE | 44 | 79:21 | 19 |
| 7 | B | NEt3 | DCE | 55 | 82:18 | 21 |
| 8 | B | NHEt2 | DCM | 48 | 93:7 | 16 |
| 9 | B | NHEt2 | PhCH3 | 42 | 90:10 | 16 |
| 10 | B | NHEt2 | CHCl3 | 56 | 92:8 | 15 |
| 11 | B | NHEt2 | THF | 40 | 93:7 | 11 |
| 12c | B | NHEt2 | DCE | 54 | 98:2 | 18 |
| 13d | B | NHEt2 | DCE | 57 | 99:1 | 21 |
| 14d,e | B | NHEt2 | DCE | 63 | >99:1 | 15 |
DCE, 1,2-dichloroethane; DCM, dichloromethane.
a Isolated yield of 3a. b The er values of 3a were determined via HPLC on chiral stationary phase. c 1a (0.10 mmol), 2 (0.20 mmol). d 1a (0.10 mmol), 2 (0.30 mmol). e 1a (0.10 mmol), 2 (0.30 mmol), 4 Å MS (50 mg), and solvent (1.0 ml).
When the optimized reaction condition was obtained (Table, entry 14), we further explored the substrate scope of the atropoenantioselective nitrile formation process using a variety of biaryl dialdehydes 1 (Fig. 2). Whether one or two methyl groups are installed in ring A, with the corresponding aryl monoaldehydes obtained in improved yields with no erosion of the product er values (3b to 3e). However, the electron-donating group 4′-OCH3 introduced in ring A led to a lower enantioselectivity (3f). The halogen (F, Cl) could be attached to ring A of the benzaldehydes 1 to give corresponding products in moderate yields with retaining high optical purities (3g to 3j). Notably, the presence of 5′-Cl of ring A in the biaryl dialdehyde resulted in a lower er value of aryl aldehyde product 3k, albeit with a moderate yield. The 2′-methyl group on ring A played an important role in restricting axial rotation. For instance, when ethyl, -SMe, or alkenyl groups were incorporated at the 2′-position of ring A, the product yields of the aryl monoaldehydes were slightly changed (3l to 3n). Moreover, an obvious reduction in both product yields and er values could be observed when the 2′-halogen or 2′-phenyl group was introduced to ring A (3o to 3q). Furthermore, the NHC-catalyzed atropoenantioselective reaction exhibited promising performance even in the presence of aromatic fused rings in the dialdehydes, and all the corresponding aryl monoaldehydes could be obtained with excellent optical purities (3r to 3t). However, a slight decrease in the product er value occurred when 2′-CH3O-naphthyl was used to replace ring A (3u). In addition, we also tested substrate scope on the dialdehyde aromatic ring (ring B). Electron-donating (OMe) and halogen (F) groups were tolerated well and given corresponding aryl monoaldehyde products 3v and 3w in moderate yields and excellent er values.
Fig. 2.
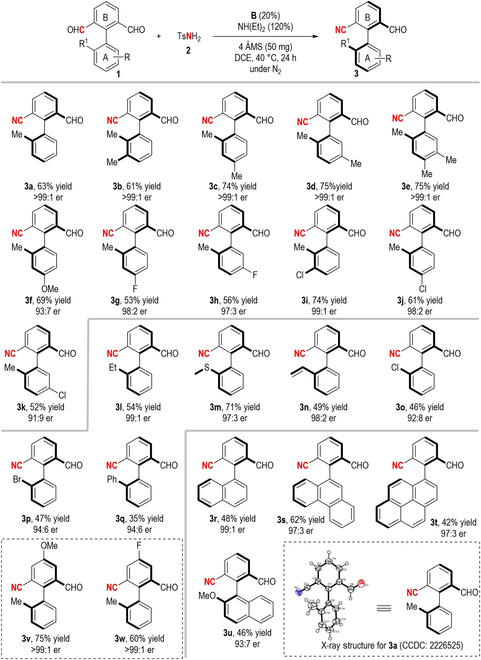
Scope of dialdehydes 1.
The stereochemical stability of the axially chiral biaryl aldehyde product 3a was then evaluated through the previous experimental approach [55–57] (Fig. 3). The procedure involved stirring the compound in mesitylene at 120 °C for 7 h. Consequently, a nearly racemic mixture of the targeted product 3a was obtained. By analyzing the rate of change in product enantiomeric excess (ee) values at 120 °C, it was possible to ascertain the rotational barrier to the axially chiral compound 3a. On the basis of the changing rate of product ee values at 120 °C, the rotational barrier of the axially chiral compound 3a was determined (ΔG# = 30.95 kcal/mol). Additionally, based on this barrier energy, a half-life to racemization at 120 °C was calculated and the half-life of product 3a is 1.92 h.
Fig. 3.

Evaluation of the stereochemical stability of 3a.
It is worth noting that this transformation can be put into practice at 5-mmol scales, resulting in the aryl aldehyde product 3a in 65% yield and >99:1 er value. The enantiomerically enriched axially chiral product 3a, containing both aldehyde and nitrile units, can serve as a potential catalyst in the aldehyde-catalyzed asymmetric reaction and could also be easily derived into a variety of functionalized molecules through simple protocols (Fig. 4). Aryl aldehyde product 3a could react with Wittig reagent to generate the axially chiral alkene product 5. Subsequently, the CN group of compound 5 can be reduced to an aldehyde group in 86% yield and >99:1 er value. Moreover, the optically enriched 3a could be efficiently reduced by NaBH4 to give alcohol 7 in quantitative yield with no erosion of optical purities. Then, the OH group of 7 can be substituted by the chloro group to form an unexpected product 8 in 70% yield with excellent enantioselectivity in this transformation. Furthermore, in the presence of potassium permanganate, compound 3a undergoes oxidation process to give axially chiral carboxylic acid 9 in good yield while maintaining its excellent optical purity. This axially chiral carboxylic acid 9 can be effectively employed as an organic catalyst for acid-catalyzed asymmetric reactions [58–64].
Fig. 4.

Large-scale synthesis and synthetic transformations of 3a.
It is worth noting that the formation of the intermediate (±)-10a is a reversible process from TsNH2 with aldehydes [65]. The formation of by-product 4a could affect the target product er values in this process (Table, entries 2, 12, and 13). Subsequently, we performed a control experiment using (rac)-3a as the starting material under standard conditions. In this control experiment (Fig. 5A), (S)-3a was obtained with a yield of 51% and an 82:18 er value. It is worth noting that by-product 4a was also generated in 45% yield. This result demonstrated that the kinetic resolution is critical for the product optical purity. Based on the experimental observation together with the reported literature [32,66–71], a proposed reaction pathway is depicted in Fig. 5B. First of all, the condensation of biaryl dialdehyde 1a with TsNH2 is generated to afford a racemic mixture of imines (±)-10a, then (S)-10a undergoes a faster desulfonylation reaction to give the corresponding atropisomeric benzaldehyde product (S)-3a in excellent enantioselectivity enabled by a chiral NHC catalyst. On the other hand, (R)-10a was dynamically hydrolyzed at a slower reaction rate than the starting material 1a. This process is desymmetrization/dynamic kinetic resolution, leading to the formation of the by-product 4a and improved enantioselectivity of product 3a, accompanied by the consumption of (R)-3a.
Fig. 5.
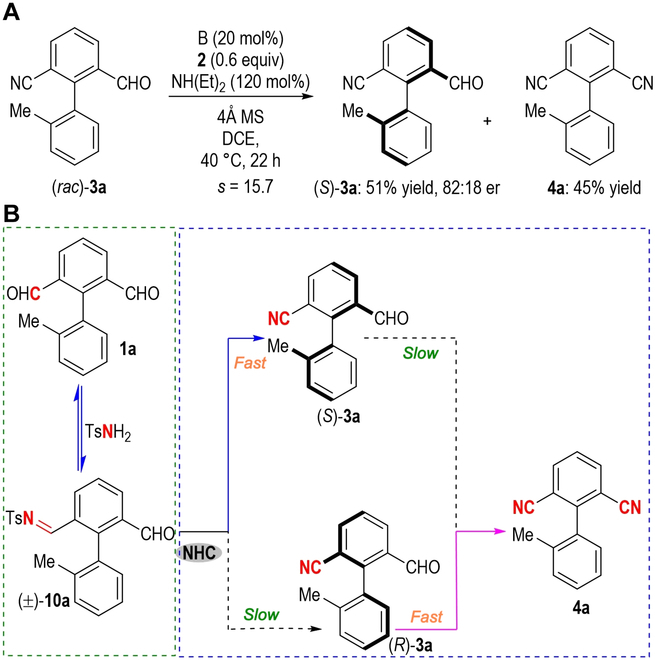
Mechanistic study. (A) Control experiment. (B) Proposed reaction mechanism.
Discussion
We have developed an atropoenantioselective NHC-catalyzed nitrile formation and desymmetrization reaction. Various substituents can be tolerated well, with the corresponding aryl monoaldehydes afforded in moderate to good yields and good to excellent er values. The stereochemical stability of aryl aldehyde has been assessed using thermal dynamic methods. Notably, this synthetic protocol is well-suited for large-scale synthesis, and the resulting products, containing both aldehyde and nitrile functional groups, can be conveniently transformed into diverse functional molecules without marked loss of optical purities. As we continue our research, we aim to explore further synthetic applications for these atropoisomerically enriched aryl monoaldehydes.
Methods
In a glove box, dicarbaldehyde 1 (0.10 mmol), NHC pre-catalyst B (0.02 mmol, 7.5 mg), TsNH2 2 (0.30 mmol), and 4 Å MS (50 mg) were added into a 4.0-ml vial containing a magnetic stir bar. Subsequently, anhydrous DCE (1.0 ml) and N,N-diethylamine (NHEt2) (0.12 mmol, 12.4 μl) were added into the vial using a syringe. The resulting reaction mixture was stirred at 40 °C for 24 h. The mixture was directly purified by column chromatography (silica gel, eluent: petroleum ether/ethyl acetate = 20:1) to obtain the target product 3.
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program of China (2022YFD1700300); the National Natural Science Foundation of China (22071036); the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023) at Guizhou University; Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province [Qianjiaohe KY (2020)004]; the Guizhou Province First-Class Disciplines Project [(Yiliu Xueke Jianshe Xiangmu)-GNYL(2017)008]; Guizhou University (China); Singapore National Research Foundation under its NRF Investigatorship (NRF-NRFI2016-06) and Competitive Research Program (NRF-CRP22-2019-0002); Ministry of Education, Singapore, under its MOE AcRF Tier 1 Award (RG7/20, RG70/21), MOE AcRF Tier 2 (MOE2019-T2-2-117), and MOE AcRF Tier 3 Award (MOE2018-T3-1-003); a Chair Professorship Grant; and Nanyang Technological University.
Author contributions: Y.L. and T.L. conceived the idea and designed the experiments. Z.J., Y.R.C., and T.L. supervised the work. Y.C. conducted most of the experiments. T.L., Z.J., and Y.R.C. drafted the manuscript with assistance from all co-authors. All authors partly contributed to the experiments and discussions.
Competing interests: The authors declare that they have no competing interests.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Materials
Figs. S1 and S2
Tables S1 to S8
References
- 1.Li S, Chen X-Y, Enders D. Aldehyde catalysis: New options for asymmetric organocatalytic reactions. Chem. 2018;4(9):2026–2028. [Google Scholar]
- 2.Wang Q, Gu Q, You S-L. Enantioselective carbonyl catalysis enabled by chiral aldehydes. Angew Chem Int Ed. 2019;58(21):6818–6825. [DOI] [PubMed] [Google Scholar]
- 3.Liao G, Zhang T, Lin Z-K, Shi B-F. Transition metal-catalyzed enantioselective C-H functionalization via chiral transient directing group strategies. Angew Chem Int Ed. 2020;59(45):19773–19786. [DOI] [PubMed] [Google Scholar]
- 4.Wen W, Guo Q-X. Recent advances in chiral aldehyde catalysis for asymmetric functionalization of amines. Synthesis. 2022;55(05):719–732. [Google Scholar]
- 5.Xiao X, Zhao B. Vitamin B(6)-based biomimetic asymmetric catalysis. Acc Chem Res. 2023;56(9):1097–1117. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Gong X, Li J, Li Y, Ma J, Hou C, Zhao G, Yuan W, Zhao B. Carbonyl catalysis enables a biomimetic asymmetric Mannich reaction. Science. 2018;360(6396):1438–1442. [DOI] [PubMed] [Google Scholar]
- 7.Cheng A, Zhang L, Zhou Q, Liu T, Cao J, Zhao G, Zhang K, Song G, Zhao B. Efficient asymmetric biomimetic aldol reaction of glycinates and trifluoromethyl ketones by carbonyl catalysis. Angew Chem Int Ed. 2021;60(37):20166–20172. [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Zhou Q, Song G, Song Y, Zhao G, Ding K, Zhao B. Enantioselective synthesis of pyroglutamic acid esters from glycinate via carbonyl catalysis. Angew Chem Int Ed. 2021;60(19):10588–10592. [DOI] [PubMed] [Google Scholar]
- 9.Hou C, Peng B, Ye S, Yin Z, Cao J, Xiao X, Zhao B. Catalytic asymmetric α C(sp3)–H addition of benzylamines to aldehydes. Nat. Catal. 2022;5(11):1061–1068. [Google Scholar]
- 10.Ji P, Liu X, Xu J, Zhang X, Guo J, Chen W-W, Zhao B. Direct asymmetric α-C–H addition of N-unprotected propargylic amines to trifluoromethyl ketones by carbonyl catalysis. Angew Chem Int Ed. 2022;61(48): Article e202206111. [DOI] [PubMed] [Google Scholar]
- 11.Wen W, Chen L, Luo M-J, Zhang Y, Chen Y-C, Ouyang Q, Guo Q-X. Chiral aldehyde catalysis for the catalytic asymmetric activation of glycine esters. J Am Chem Soc. 2018;140(30):9774–9780. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Luo M-J, Zhu F, Wen W, Guo Q-X. Combining chiral aldehyde catalysis and transition-metal catalysis for enantioselective α-allylic alkylation of amino acid esters. J Am Chem Soc. 2019;141(13):5159–5163. [DOI] [PubMed] [Google Scholar]
- 13.Shen H-R, Li C-X, Jiang X, Lin Y, Liu J-H, Zhu F, Wu Z-L, Cai T, Wen W, He R-X, et al. Chiral aldehyde catalysis enables direct asymmetric α-substitution reaction of N-unprotected amino acids with halohydrocarbons. Chem Sci. 2023;14(21):5665–5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q-W, Wen W, Guo Q-X. Chiral aldehyde–palladium catalysis enables asymmetric synthesis of α-alkyl tryptophans via cascade Heck-alkylation reaction. Org Lett. 2023;25(17):3163–3167. [DOI] [PubMed] [Google Scholar]
- 15.Guimond N, MacDonald MJ, Lemieux V, Beauchemin AM, Beauchemin AM. Catalysis through temporary intramolecularity: Mechanistic investigations on aldehyde-catalyzed cope-type hydroamination lead to the discovery of a more efficient tethering catalyst. J Am Chem Soc. 2012;134(40):16571–16577. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald MJ, Hesp CR, Schipper DJ, Pesant M, Beauchemin AM. Highly enantioselective intermolecular hydroamination of allylic amines with chiral aldehydes as tethering catalysts. Chem Eur J. 2013;19(8):2597–2601. [DOI] [PubMed] [Google Scholar]
- 17.Chen W-W, Zhao Q, Xu M-H, Lin G-Q. Nickel-catalyzed asymmetric Ullmann coupling for the synthesis of axially chiral tetra-ortho-substituted biaryl dials. Org Lett. 2010;12(5):1072–1075. [DOI] [PubMed] [Google Scholar]
- 18.Witzig RM, Fäseke VC, Häussinger D, Sparr C. Atroposelective synthesis of tetra-ortho-substituted biaryls by catalyst-controlled non-canonical polyketide cyclizations. Nat Catal. 2019;2(10):925–930. [Google Scholar]
- 19.Dhawa U, Tian C, Wdowik T, Oliveira JCA, Hao J, Ackermann L. Enantioselective pallada-electrocatalyzed C-H activation by transient directing groups: Expedient access to helicenes. Angew Chem Int Ed. 2020;59(32):13451–13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Sun J, Gu W, Tang W. Enantioselective cross-coupling for axially chiral tetra-ortho-substituted biaryls and asymmetric synthesis of gossypol. J Am Chem Soc. 2020;142(17):8036–8043. [DOI] [PubMed] [Google Scholar]
- 21.Perveen S, Zhang S, Wang L, Song P, Ouyang Y, Jiao J, Duan X-H, Li P. Synthesis of axially chiral biaryls via enantioselective Ullmann coupling of ortho-chlorinated aryl aldehydes enabled by a chiral 2,2′-bipyridine ligand. Angew Chem Int Ed. 2022;61(47): Article e202212108. [DOI] [PubMed] [Google Scholar]
- 22.Ran H, Li S-M. Fungal benzene carbaldehydes: Occurrence, structural diversity, activities and biosynthesis. Nat Prod Rep. 2021;38(1):240–263. [DOI] [PubMed] [Google Scholar]
- 23.Scazzocchio F, Cometa MF, Tomassini L, Palmery M. Antibacterial activity of Hydrastis canadensis extract and its major isolated alkaloids. Planta Med. 2001;67(6):561–564. [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Cai G, Guo Y, Gao C, Huang W, Zhang Z, Lu H, Liu K, Chen J, Xiong X, et al. Exploring marine-derived ascochlorins as novel human dihydroorotate dehydrogenase inhibitors for treatment of triple-negative breast cancer. J Med Chem. 2021;64(18):13918–13932. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J-Q, Fan S-R, Wei X, Zhang C-X, Zhang D-M, Chen M-F, He X. Synthesis and biological evaluation of marine natural product, cryptoechinuline D derivatives as novel antiangiogenic agents. Bioorg Med Chem Lett. 2022;65: Article 128717. [DOI] [PubMed] [Google Scholar]
- 26.Staniland S, Yuan B, Gimenez-Agullo N, Marcelli T, Willies SC, Grainger DM, Turner NJ, Clayden J. Enzymatic desymmetrising redox reactions for the asymmetric synthesis of biaryl atropisomers. Chem Eur J. 2014;20(41):13084–13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao G, Chen H-M, Xia Y-N, Li B, Yao Q-J, Shi B-F. Synthesis of chiral aldehyde catalysts by Pd-catalyzed atroposelective C-H naphthylation. Angew Chem Int Ed. 2019;58(33):11464–11468. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Xu Q, Wu J, Fan J, Xie M. Construction of N-C axial chirality through atroposelective C-H olefination of N-arylindoles by palladium/amino acid cooperative catalysis. Org Lett. 2019;21(16):6361–6365. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Xu Q, Fan J, Zhou L, Liu N, Zhu L, Wu J, Xie M. Pd(II)-catalyzed enantioconvergent twofold C–H annulation to access atropisomeric aldehydes: A platform for diversity-oriented-synthesis. Org Chem Front. 2021;8(13):3404–3412. [Google Scholar]
- 30.Witzig RM, Lotter D, Fäseke VC, Sparr C. Stereoselective arene-forming aldol condensation: Catalyst-controlled synthesis of axially chiral compounds. Chem Eur J. 2017;23(53):12960–12966. [DOI] [PubMed] [Google Scholar]
- 31.Lotter D, Castrogiovanni A, Neuburger M, Sparr C. Catalyst-controlled stereodivergent synthesis of atropisomeric multiaxis systems. ACS Cent Sci. 2018;4(5):656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Li M, Sun J, Zheng G, Zhang Q. Synthesis of axially chiral aldehydes by N-heterocyclic-carbene-catalyzed desymmetrization followed by kinetic resolution. Angew Chem Int Ed. 2022;61(14): Article e202117340. [DOI] [PubMed] [Google Scholar]
- 33.Enders D, Niemeier O, Henseler A. Organocatalysis by N-heterocyclic carbenes. Chem Rev. 2007;107(12):5606–5655. [DOI] [PubMed] [Google Scholar]
- 34.Bugaut X, Glorius F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem Soc Rev. 2012;41(9):3511–3522. [DOI] [PubMed] [Google Scholar]
- 35.Hopkinson MN, Richter C, Schedler M, Glorius F. An overview of N-heterocyclic carbenes. Nature. 2014;510(7506):485–496. [DOI] [PubMed] [Google Scholar]
- 36.Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem Rev. 2015;115(17):9307–9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Wang H, Jin Z, Chi YR. N-heterocyclic carbene organocatalysis: Activation modes and typical reactive intermediates. Chin J Chem. 2020;38(10):1167–1202. [Google Scholar]
- 38.Chen K-Q, Sheng H, Liu Q, Shao P-L, Chen X-Y. N-heterocyclic carbene-catalyzed radical reactions. Sci China Chem. 2020;64(1):7–16. [Google Scholar]
- 39.Dai L, Ye S. Recent advances in N-heterocyclic carbene-catalyzed radical reactions. Chin Chem Lett. 2021;32(2):660–667. [Google Scholar]
- 40.Li T, Jin Z, Chi YR. N-heterocyclic carbene-catalyzed arene formation reactions. Sci China Chem. 2021;65(2):210–223. [Google Scholar]
- 41.Zhao C, Blaszczyk SA, Wang J. Asymmetric reactions of N-heterocyclic carbene (NHC)-based chiral acyl azoliums and azolium enolates. Green Synth Catal. 2021;2(2):198–215. [Google Scholar]
- 42.Zhang B, Wang J. Assembly of versatile fluorine-containing structures via N-heterocyclic carbene organocatalysis. Sci China Chem. 2022;65(9):1691–1703. [Google Scholar]
- 43.Lv J, Xu J, Pan X, Jin Z, Chi YR. Carbene-catalyzed activation of cyclopropylcarbaldehydes for Mannich reaction and δ-lactam formation: Remote enantioselectivity control and dynamic kinetic asymmetric transformation. Sci China Chem. 2021;64(6):985–990. [Google Scholar]
- 44.Wang G, Zhang M, Guan Y, Zhang Y, Hong X, Wei C, Zheng P, Wei D, Fu Z, Chi YR, et al. Desymmetrization of cyclic 1,3-diketones under N-heterocyclic carbene organocatalysis: Access to organofluorines with multiple stereogenic centers. Research. 2021;2021:9867915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Wang H, Jin Z, Chi YR. Development of green and low-cost chiral oxidants for asymmetric catalytic hydroxylation of enals. Green Synth Catal. 2021;2(3):295–298. [Google Scholar]
- 46.Lv J, Nong Y, Chen K, Wang Q, Jin J, Li T, Jin Z, Chi YR. N-heterocyclic carbene catalyzed C-acylation reaction for access to linear aminoenones. Chin Chem Lett. 2023;34(1): Article 107570. [Google Scholar]
- 47.Chen D-D, Hou X-L, Dai L-X. Unexpected transfer of tosyl group of ArCH=NTs-catalyzed by N-heterocyclic carbene. J Org Chem. 2008;73(14):5578–5581. [DOI] [PubMed] [Google Scholar]
- 48.Jin Z, Xu J, Yang S, Song B-A, Chi YR. Enantioselective sulfonation of enones with sulfonyl imines by cooperative N-heterocyclic-carbene/thiourea/tertiary-amine multicatalysis. Angew Chem Int Ed. 2013;52(47):12354–12358. [DOI] [PubMed] [Google Scholar]
- 49.Sun F, Yin T, Wang Y, Feng A, Yang L, Wu W, Yu C, Li T, Wei D, Yao C. A combined experimental and computational study of NHC-promoted desulfonylation of tosylated aldimines. Org Chem Front. 2020;7(3):578–583. [Google Scholar]
- 50.Fier PS, Maloney KM. NHC-catalyzed deamination of primary sulfonamides: A platform for late-stage functionalization. J Am Chem Soc. 2019;141(4):1441–1445. [DOI] [PubMed] [Google Scholar]
- 51.Lv Y, Luo G, Liu Q, Jin Z, Zhang X, Chi YR. Catalytic atroposelective synthesis of axially chiral benzonitriles via chirality control during bond dissociation and CN group formation. Nat Commun. 2022;13(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He M, Strube JR, Bode JW. Highly enantioselective azadiene Diels-Alder reactions catalyzed by chiral N-heterocyclic carbenes. J Am Chem Soc. 2006;128(26):8418–8420. [DOI] [PubMed] [Google Scholar]
- 53.Kerr MS, de Alaniz JR, Rovis T. A highly enantioselective catalytic intramolecular Stetter reaction. J Am Chem Soc. 2002;124(35):10298–10299. [DOI] [PubMed] [Google Scholar]
- 54.Kerr MS, Rovis T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric Stetter reaction. J Am Chem Soc. 2004;126:8876–8877. [DOI] [PubMed] [Google Scholar]
- 55.Ma C, Sheng F-T, Wang H-Q, Deng S, Zhang Y-C, Jiao Y, Tan W, Shi F. Atroposelective access to oxindole-based axially chiral styrenes via the strategy of catalytic kinetic resolution. J Am Chem Soc. 2020;142(37):15686–15696. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q, Zhang W-W, Song H, Wang J, Zheng C, Gu Q, You S-L. Rhodium-catalyzed atroposelective oxidative C–H/C–H cross-coupling reaction of 1-aryl isoquinoline derivatives with electron-rich heteroarenes. J Am Chem Soc. 2020;142(37):15678–15685. [DOI] [PubMed] [Google Scholar]
- 57.Jin J, Huang X, Xu J, Li T, Peng X, Zhu X, Zhang J, Jin Z, Chi YR. Carbene-catalyzed atroposelective annulation and desymmetrization of urazoles. Org Lett. 2021;23(10):3991–3996. [DOI] [PubMed] [Google Scholar]
- 58.Akiyama T, Mori K. Stronger bronsted acids: Recent progress. Chem Rev. 2015;115(17):9277–9306. [DOI] [PubMed] [Google Scholar]
- 59.Min C, Seidel D. Asymmetric Brønsted acid catalysis with chiral carboxylic acids. Chem Soc Rev. 2017;46(19):5889–5902. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto T, Hirose M, Maruoka K. Asymmetric imino aza-enamine reaction catalyzed by axially chiral dicarboxylic acid: Use of arylaldehyde N,N-dialkylhydrazones as acyl anion equivalent. J Am Chem Soc. 2008;130(24):7556–7557. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto T, Kimura H, Kawamata Y, Maruoka K. Generation and exploitation of acyclic azomethine imines in chiral Brønsted acid catalysis. Nat Chem. 2011;3(8):642–646. [DOI] [PubMed] [Google Scholar]
- 62.Zhu Z, Odagi M, Supantanapong N, Xu W, Saame J, Kirm H-U, Abboud KA, Leito I, Seidel D. Modular design of chiral conjugate-base-stabilized carboxylic acids: Catalytic enantioselective [4 + 2] cycloadditions of acetals. J Am Chem Soc. 2020;142(36):15252–15258. [DOI] [PubMed] [Google Scholar]
- 63.Zhou T, Qian P-F, Li J-Y, Zhou Y-B, Li H-C, Chen H-Y, Shi B-F. Efficient synthesis of sulfur-stereogenic sulfoximines via Ru(II)-catalyzed enantioselective C-H functionalization enabled by chiral carboxylic acid. J Am Chem Soc. 2021;143(18):6810–6816. [DOI] [PubMed] [Google Scholar]
- 64.Wu Z-Y, Yin P, Ju H-X, Chen Z-Q, Li C, Li S-C, Liang H-W, Zhu J-F, Yu S-H. Natural nanofibrous cellulose-derived solid acid catalysts. Research. 2019;2019:6262719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Layer RW. The chemistry of imines. Chem Rev. 2002;63(5):489–510. [Google Scholar]
- 66.Hayashi T, Niizuma S, Kamikawa T, Suzuki N, Uozumi Y. Catalytic asymmetric synthesis of axially chiral biaryls by palladium-catalyzed enantioposition-selective cross-coupling. J Am Chem Soc. 1995;117(35):9101–9102. [Google Scholar]
- 67.Mori K, Ichikawa Y, Kobayashi M, Shibata Y, Yamanaka M, Akiyama T. Enantioselective synthesis of multisubstituted biaryl skeleton by chiral phosphoric acid catalyzed desymmetrization/kinetic resolution sequence. J Am Chem Soc. 2013;135(10):3964–3970. [DOI] [PubMed] [Google Scholar]
- 68.Lu S, Poh SB, Rong Z-Q, Zhao Y. NHC-catalyzed atroposelective acylation of phenols: Access to enantiopure NOBIN analogs by desymmetrization. Org Lett. 2019;21(15):6169–6172. [DOI] [PubMed] [Google Scholar]
- 69.Yang G, Guo D, Meng D, Wang J. NHC-catalyzed atropoenantioselective synthesis of axially chiral biaryl amino alcohols via a cooperative strategy. Nat Commun. 2019;10(1):3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai L, Liu Y, Xu Q, Wang M, Zhu Q, Yu P, Zhong G, Zeng X. A dynamic kinetic resolution approach to axially chiral diaryl ethers by catalytic atroposelective transfer hydrogenation. Angew Chem Int Ed. 2023;62(7): Article e202216534. [DOI] [PubMed] [Google Scholar]
- 71.Zhu M, Jiang H-J, Sharanov I, Irran E, Oestreich M. Atroposelective silylation of 1,1′-biaryl-2,6-diols by a chiral counteranion directed desymmetrization enhanced by a subsequent kinetic resolution. Angew Chem Int Ed. 2023;62: Article e202304475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 and S2
Tables S1 to S8
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


