Abstract
Private sector engagement in health reform has been suggested to help reduce healthcare inequities in sub-Saharan Africa, where populations with the most need seek the least care. We study the effects of African Health Markets for Equity (AHME), a cluster randomized controlled trial carried out in Kenya from 2012 to 2020 at 199 private health clinics. AHME included four clinic-level interventions: social health insurance, social franchising, SafeCare quality-of-care certification programme and business support. This paper evaluates whether AHME increased the capacity of private health clinics to serve poor clients while maintaining or enhancing the quality of care provided. At endline, clinics that received AHME were 14.5 percentage points (pp) more likely to be empanelled with the National Health Insurance Fund (NHIF), served 51% more NHIF clients and served more clients from the middle three quintiles of the wealth distribution compared to control clinics. Comparing individuals living in households near AHME treatment and control clinics (N = 8241), AHME led to a 6.7-pp increase in the probability of holding any health insurance on average. We did not find any additional effect of AHME on insurance holding among poor households. We measured quality of care using a standardized patient (SP) experiment (N = 596 SP–provider interactions) where recruited and trained SPs were randomized to present as either ‘not poor’, and able to afford all services provided, or ‘poor’ by telling the provider they could only afford ∼300 Kenyan Shillings (US$3) in fees. We found that poor SPs received lower levels of both correct and unnecessary services, and AHME did not affect this. More work must be done to ensure that clients of all wealth levels receive high-quality care.
Keywords: Equity, healthcare utilization, public/private
Key messages.
We investigate whether a large-scale management intervention for private health clinics in Kenya can enable them to reach lower-wealth clients in their catchment areas.
The intervention increased clinics’ scale, allowing them to lower prices, which, in turn, led to more clients and more poor clients seeking care at these private health clinics.
However, standardized patients who presented with limited ability to afford services received a lower quality of care than non-poor clients at both treated and control clinics.
More work must be done to ensure that clients of all wealth levels not only have access to quality health care, but actually receive high-quality care at private clinics.
Introduction
Achieving equity in health and health care has long been at the forefront of the policy agenda in many African countries, including Kenya (Jehu-Appiah et al., 2011; Reich et al., 2016; McCollum et al., 2018; Paul et al., 2019; Banerjee et al., 2021; WHO, 2021). Nationally representative statistics from Kenya show that individuals from the lowest-wealth quintile are more likely to rate their health as very poor, but less likely to seek both public and private healthcare compared to individuals from wealthier quintiles (Ilinca et al., 2019). Barriers to accessing care are complex and include visit and travel fees, travel time and lost wages, all of which disproportionately affect individuals with low incomes (Bright et al., 2017; Montagu et al., 2020).
Expanding access to and improving the quality of private sector health care have been proposed as a potential solution to mitigate the inequities in access, particularly as private health sectors are substantial and growing sources of care in low- and middle-income countries (LMICs) (Hanson and Berman, 1998; Patouillard et al., 2007; Yoong et al., 2010; Chakraborty et al., 2019; Khetrapal et al., 2019). Private clinics offer various advantages to clients including extended operating hours and shorter wait times compared to public clinics.
However, there are significant knowledge gaps in how to ensure that private health markets function effectively for low-income individuals. Multiple barriers must be simultaneously addressed, including the cost of care, alignment between the insurance held by low-income individuals and the acceptance of such insurance by private clinics and the quality of care received by individuals with a limited budget. Recent qualitative research conducted in Kenya found that although poor individuals preferred to seek care at private providers, they often opt for public and faith-based providers due to their lower prices (Chakraborty et al., 2019). Relatively higher prices at private clinics can be exclusionary to lower-income households (Morgan et al., 2016). Additionally, investigating the alignment between the insurance coverage held by low-income individuals and the acceptance of such insurance by private clinics can provide insights into improving access. Last, it remains unclear whether poor clients, who may face financial constraints, receive the same quality of care and/or the same prices at private clinics as other clients.
This paper studies the ability of private health clinics to deliver affordable and high-quality care to poor clients as well as wealthier clients. Our analysis uses data from the African Health Markets for Equity (AHME) evaluation, a cluster randomized controlled trial carried out in Kenya from late 2012 through early 2020. AHME aimed to jointly address the supply side, demand side and policy environment through a coordinated package of interventions and networks of franchised healthcare clinics that delivered specified health services under a common brand. As part of AHME, clinics were encouraged to adopt social health insurance, and clinics received business and management training along with quality-of-care improvement plans. This study specifically examines the effects of AHME on the acceptance of and enrolment in public insurance, utilization patterns and the wealth of clients at private clinics and the quality of care provided. By analysing these factors, we aim to shed light on the impact of AHME on improving access, equity and the overall healthcare experience for individuals seeking services from private clinics in Kenya.
This study was conducted in Kenya, which is a good study setting because the private, for-profit healthcare sector is an important player in healthcare delivery. Specifically, private clinics are highly competitive and make up 42% of the 12 574 operational health facilities in the country (Center for Human Rights and Global Justice, 2021; Kenya Ministry of Health, 2021). These private clinics account for a substantial amount of total care volume: 12.4% and 29.4% of all visits to health facilities in urban and rural areas, respectively (Kenya Ministry of Health, 2014). Despite these volumes, research suggests that these private clinics may also be underused especially by poor clients, which provides opportunity for increasing their scale (Das et al., 2018). Finally, achieving universal health coverage through the expansion of the National Health Insurance Fund (NHIF) was a priority policy area during the study period, and it may have implications for the utilization of private healthcare services.
The AHME programme and its evaluation offer a unique opportunity to jointly examine a comprehensive intervention that includes both the supply and demand sides focused on healthcare equity, enabling a complete picture of private healthcare markets, and thus filling a gap in the literature on achieving healthcare equity. We also describe the results of a novel standardized patient (SP) survey that enables us to estimate the causal impact of a provider perceiving a client as poor on the quality and quantity of care services received. The impact of AHME on management and care delivery is forthcoming (Contreras-Loya et al., 2022). AHME has also been evaluated using qualitative methods (Suchman et al., 2019; Montagu et al., 2020), and the SP data from the impact evaluation have been used to understand the impact of clients demanding different medications (Kwan et al., 2022). Here, we focus on the effects of AHME on healthcare equity.
AHME programme framework and research questions
AHME was a cluster randomized controlled trial that investigated whether the combination of social health insurance and management training could improve access to and quality of private health clinics in Kenya. This paper aims to evaluate the effect of AHME on the expansion of private health clinics’ capacity to serve poor clients while maintaining or enhancing the quality of care provided.
Aligned with AHME’s goals, we developed the framework depicted in Figure 1 to conceptualize one way to achieve high-quality, equitable health care. Through coverage and quality inputs, this can be achieved through (1) increasing access and acceptance of insurance among the population, (2) increasing access to and utilization of health care by the poor, (3) reducing the cost of care and ensuring private clinics’ business viability and (4) improving the quality of care provided by private healthcare clinics. The theory of change behind AHME was that by simultaneously addressing these four components—which we investigate one-by-one in this paper—healthcare equity could be improved among populations residing near AHME clinics in Kenya. Figure 1 also illustrates the mapping of each study goal to the corresponding surveys used to measure them.
Figure 1.
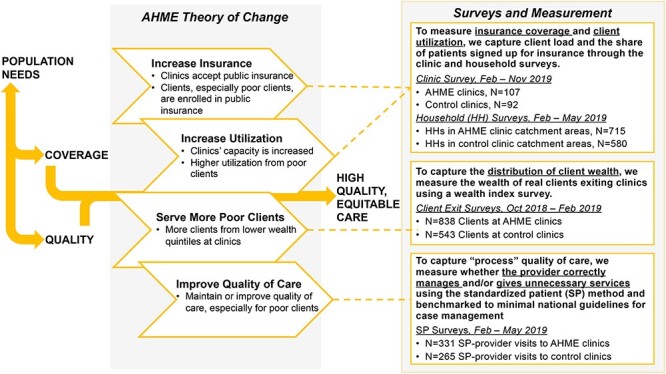
Conceptual framework and surveys
Note: This figure presents a conceptual framework of one way to achieve high-quality, equitable health care. Through coverage and quality inputs, high-quality, equitable care can be achieved by (1) increasing access and acceptance of insurance among the population, (2) increasing access to and utilization of health care by the poor, (3) reducing the cost of care and ensuring private clinics’ business viability and (4) improving the quality of care provided by private healthcare clinics.
The AHME programme comprised four clinic-level interventions: social health insurance, social franchising, SafeCare quality of care certification programme and business support. First, in terms of increasing social insurance, the AHME programme focused on providing clinics with NHIF empanelment support, which providers previously described as bureaucratic and time-consuming (Suchman et al., 2019). NHIF empanelment allowed clinics to treat insured clients and accept NHIF insurance payments for those services. To achieve this, clinics had to obtain accreditation from the Government of Kenya, indicating their eligibility to accept NHIF, and visibly display their acceptance of NHIF membership. By enrolling clients in NHIF and facilitating payment for their care, accreditation had the potential to expand the client base of clinics, particularly among those whose services were covered by membership premiums and the Government of Kenya subsidy.
With the goal of simultaneously increasing utilization and quality of care, the social franchising intervention bound the clinics under a common brand. Social franchising was tightly linked to quality-of-care certifications, including SafeCare, which focused on improving the clinical environment of AHME clinics to facilitate the delivery of high-quality services and has been rigorously studied as a stand-alone intervention in Tanzania (King et al., 2021). AHME implementing partners regularly visited clinics to check whether significant progress had been made on their quality improvement plan outlined at the beginning of the intervention.
AHME clinics also received business support, equipping clinic owners with the necessary skills to effectively manage their private health clinics as profitable and well-functioning businesses. Each clinic’s business structures and systems were assessed for quality, and a Business Improvement Plan was developed to address any identified gaps.
Materials and methods
Experimental design and compliance
Before the AHME programme began in 2012, we mapped all clinics in the evaluation study areas (with coverage in 35 of Kenya’s 47 counties) with the goal of randomizing clinics eligible for the programme into treatment and control groups, following the gold standard research design to identify causal effects due to the programme. Clinics were eligible if they were for-profit and privately run, they were licenced and all staff were licenced. Clinics were ineligible if they were faith-based, were already franchised or were physically close to an already franchised clinic. See the Clinic sample and survey section for more details.
The AHME intervention was randomized at the health clinic level, where clinics randomized to the treatment group received the full AHME programme and clinics randomized to the control group clinics did not. Within the treatment group, clinics received the AHME intervention in a randomized order and the control arm was randomly ordered to be consistent with the treatment arm process and facilitate subsequent honing of clinics in both arms This experimental design of the AHME impact evaluation allows us to conduct intent-to-treat (ITT) analyses to examine the effects of the programme on various dimensions of healthcare quality and equity for clinics assigned to the treatment group compared to those assigned to the control group. For more information about sampling and tracking of clinics, see the Clinic sample and survey section and Figure A1. We conducted two rounds of data collection: baseline to capture measures before clinics were exposed to the programme and endline to capture measures after the treatment group received 5–6 years of exposure to the programme. At baseline (2013–14), 123 clinics were randomized to the treatment group, and 109 to the control group control (N = 232 clinics). At endline (2019), we were unable to resurvey 33 clinics: 11 had closed, two were excluded because they were in conflict areas, 13 withdrew from the study and seven were excluded for other reasons such as they became faith-based clinics or were no longer for-profit for other reasons or were acquired by a large health system (see Figure A1 for more details). Our final evaluation analytic sample includes 199 clinics: 107 treated clinics and 92 control clinics.
To confirm our ability to conduct ITT analyses, we examined the extent of programme compliance and assessed if control group contamination occurred. We tracked which treatment and control group clinics received AHME’s four clinic-level interventions. Overall, compliance was high, especially among clinics that remained in AHME through the end of the study period. However, approximately one quarter of clinics disenrolled from AHME during the study period. Contamination—control group clinics receiving AHME programmes—was low. Because we present ITT analyses, we do not exclude control clinics if they received some AHME interventions. For more information on compliance and contamination, see the Compliance with the AHME intervention section.
We analysed data from the following surveys that we implemented to measure the impact of AHME on both clinic and household outcomes:
Baseline clinic surveys were collected from July 2013 to January 2014.
Baseline household surveys were collected from May to December 2015.
Endline clinic surveys were collected from February to November 2019.
Endline client exit surveys were conducted from October 2018 to February 2019.
Endline household surveys were collected from February to May 2019.
Endline SPs surveys were collected from February to May 2019.
These surveys spanned several years, and each is described in further detail later (more details in Appendix Figure A3).
Clinic sample and surveys
Baseline data were collected for 232 clinics in the AHME treatment and control groups, and 199 clinics were included in the final analytic sample and completed endline surveys (treatment clinics: N = 107; control clinics: N = 92; for more details, see the Clinic sample and survey section).
To understand the effect of AHME on clinics’ enrolment in NHIF and other management outcomes such as capacity, we collected data in the clinics’ last full operational month at the time of the survey. We reviewed clinics’ financial records and also administered a survey to the clinic’s owner, manager or in-charge to collect information on the types of services the clinic offered, information on NHIF empanelment and the number of clients in the last month. Clinic survey questionnaires included data extraction forms for capturing quantitative information, particularly for the service utilization indicators. Other data sources included Ministry of Health reporting forms (such as MoH 707), electronic databases, electronic records, electronic or written medical records, logbooks, registers, receipts, stock cards, clinic accounting records, payroll records and performance reports.
Household sample and surveys
To understand if AHME, which was delivered at the clinic level, impacted demand-side outcomes, we surveyed local households living in clinics’ catchment areas. Households themselves were not randomized, but were assigned to the AHME treatment or control group based on whether they were in a catchment area of a clinic that received AHME or was in the control group. To identify these households, at baseline, we surveyed individuals exiting AHME treatment and control clinics, and if they met our eligibility criteria and consented, we subsequently completed a household survey at their home. Further details are provided in the Household sample and survey section and Figure A2.
This survey measured household member composition, demographics and health insurance, in addition to the inputs to the wealth index. At endline, 1295 household surveys were completed representing 8241 individuals living in households associated with 199 clinics. See the Household sample and survey section and Figure A2 for details on household attrition.
For households at baseline, we constructed an asset wealth index using data from the baseline household survey. This survey collected the same list of assets and used the same measurement approach as the 2015 Demographic and Health Surveys (DHS) fielded in the Kenya Malaria Indicator Survey, henceforth referred to as the DHS (Kenya Ministry of Health, 2021), which includes nationally representative wealth quintiles. Because the AHME household baseline survey collected the same set of asset questions (assets in the DHS wealth survey), in the same time frame (2015) and in the same place (Kenya), we were able to apply the 2015 DHS asset weights to our data. The resulting baseline wealth index is valid for comparing the wealth of AHME households to national wealth quintiles for Kenya in 2015.
Client exit sample and survey
To understand whether AHME achieved its goal of expanding affordable health care for the poor, we conducted exit interviews with 1381 clients as they were leaving 182 AHME clinics at endline (treatment N = 102, control N = 80). This sample was designed to be representative of the client population that each clinic served after the AHME intervention took place and thus will capture any shift in patient characteristics that took place because of AHME.
During endline exit interviews (October 2018 to February 2019), respondents were asked to provide information on infrastructure and asset ownership within their household. From these data, we used principal components analysis to construct an asset wealth index to calculate internal wealth quintiles. This wealth index is valid for comparing the relative wealth of clients exiting AHME treatment and control clinics, but is not valid for comparing to nationally representative samples. The nationally representative DHS weights could not be applied here because there was no 2018 or 2019 DHS (see the Measuring household wealth using two wealth indexes and Client exit sample and survey sections for more details). All results calculated using the client exit survey were then reweighted using the probability of an NHIF client being sampled at that clinic, whether the exit interview respondent reported having NHIF coverage and whether the clinic was NHIF empanelled.
SP sample, survey and experiment
To determine whether AHME had an effect on whether providers gave different levels of correct or unnecessary care to clients living above vs at or below the lower-middle-income class poverty line, we implemented an experiment using the SP method. SPs are healthy individuals recruited and trained to portray pre-designed health scenarios at sampled clinics. The SP method is the state-of-the-art method for assessing provider practice during a one-time interaction with a patient or a client (Kwan et al., 2019). We designed the SP surveys to capture healthcare process and outcome measures related to adult curative services, one of the main outpatient services covered by the AHME intervention. Our SP data reflect quality of health care received by clients seeking walk-in, outpatient at AHME treatment and control clinics. Specifically, in January 2019, 40 individuals were locally recruited, trained and hired as SPs portraying a walk-in client. The SP visits were conducted between February and May 2019. To ensure accurate and comprehensive recall, within 1–3 h after each SP visit, SPs completed an exit questionnaire administered by a fieldwork supervisor. For further details, see the SPs section.
We measure three quality-of-care dimensions: correct case management, whether any unnecessary laboratory tests were prescribed and whether any non-efficacious medicines were prescribed. To define correct case management vs otherwise, necessary vs unnecessary laboratory tests and efficacious vs non-efficacious medicines, we worked with a Technical Advisory Group of four local Kenyan clinicians and benchmarked classifications of care quality to the Kenya Ministry of Health guidelines for correct case management for conditions presented by the SPs. Definitions of these variables are presented in Table A3. Importantly, these definitions are not mutually exclusive. For example, if a provider ordered a correct test for malaria and prescribed an additional medicine that is not efficacious for malaria, that visit would be coded as correct case management and as unnecessary non-efficacious medicine prescribed.
SPs were randomly assigned to portray an adult client with either an acute case of malaria or asthma, case presentations that we pool in this paper to improve statistical power. To specifically identify the causal effect of whether AHME changed how providers managed clients above vs below the lower-middle-income class poverty line, we implemented an experimental study within the SP surveys by varying a single characteristic in the SP case presentation in the case scenario and randomly assigning the experimental variant to the AHME evaluation clinic sample. Using statistical software, half of the SPs portraying asthma were randomly assigned to tell their provider that they could only afford Kenya shillings (KSH) 300 in fees, corresponding to an approximate benchmark for the World Bank lower-middle-income class poverty line value of US$3.20, adjusted for purchasing power parity. For malaria, clinics received two SP visits—one assigned to fully afford all services and one that could only afford KSH 300 of services. For asthma, half of the sample clinics were randomly assigned to receive an SP who could fully afford all services, and the other half, one who could only afford KSH 300. All SP visits were unannounced, and all SPs were blinded to AHME treatment status at the clinic level, to our outcomes and to the definitions of our outcomes. SPs visited each of the N = 199 clinics included in the clinic analytical sample. In analyses using SP data, we include an indicator variable for the type of case (asthma vs malaria). For more details on SP randomization, see the SPs section.
Empirical strategy
For all sets of outcomes, we estimate ITT models using linear regression to compare outcomes among units randomly assigned to receive the AHME intervention compared to those in the control group. The primary independent variable is an indicator, 1 if the clinic was assigned to the AHME treatment group at baseline and 0, otherwise. Individuals’ and households’ AHME treatment status is determined by the AHME treatment status of the clinic catchment area from which they were recruited at baseline. Dependent variables were constructed from the data collected using the household, clinic, client exit and SP surveys. In all models, we cluster standard errors at the clinic level. For more details on variable construction, see Table A3.
To evaluate the impact of the AHME programme on poor individuals in the household survey sample, we interact an indicator for if their household wealth score is in the lowest two quintiles with the indicator for AHME, and include a main term for poor household. When the unit of analysis is the client, which includes both clinic exit interviews and SP analyses, AHME treatment status is determined by the treatment status of the clinic visited by the client or SP. SP analyses also include SP individual and SP case fixed effects.
Data collection and analysis
The research team in conjunction with local enumerators applied rigorous quality assurance standards during data collection. Questionnaires were developed by the research team with input from Kenyan researcher and enumerator teams to ensure culturally appropriate phrasing and accurate translation into local languages. Data collection quality was safeguarded through extensive training of field staff and piloting, as well as having backchecks (two different enumerators administered the same survey for a subset of respondents to ensure accuracy), spot checks (supervisors monitored surveying during unannounced visits) and high-frequency checks (analysis on incoming data to flag any anomalies in the data). All data were collected using SurveyCTO software, and all analyses were conducted using Stata 16 (StataCorp LLC, College Station, TX).
Results
Balance at baseline
We evaluated the balance of demographic, socioeconomic and infrastructure indicators collected at baseline for the clinic and household samples and found that overall randomization was successful. Table 1 compares characteristics of individuals and clinics assigned to the AHME treatment and control groups. For both individual and clinic characteristics, we cannot reject the null hypothesis that the joint differences in characteristics of the AHME treatment and control groups are zero (F stat = 1.35 and 1.51, respectively).
Table 1.
Baseline balance of household and clinic characteristics
| AHME treatment | Control | Difference | |||
|---|---|---|---|---|---|
| Mean | Standard error | Mean | Standard error | t-test | |
| Panel A: individuals from household surveys | |||||
| Female | 0.52 | 0.01 | 0.52 | 0.01 | 0.00 |
| Age | 19.91 | 0.31 | 19.52 | 0.31 | 0.39 |
| Head of household is female | 0.02 | 0.00 | 0.02 | 0.00 | 0.00 |
| Head of household age | 42.24 | 0.64 | 41.92 | 0.63 | 0.32 |
| Number of household members | 7.19 | 0.17 | 7.54 | 0.18 | −0.34 |
| Any insurance in household | 0.39 | 0.03 | 0.33 | 0.03 | 0.06 |
| Insured household members | 0.29 | 0.03 | 0.24 | 0.02 | 0.05 |
| Wealth quintile 1 (poorest) | 0.08 | 0.02 | 0.14 | 0.03 | −0.06** |
| Wealth quintile 2 | 0.21 | 0.02 | 0.24 | 0.02 | −0.02 |
| Wealth quintile 3 | 0.22 | 0.02 | 0.22 | 0.02 | 0.00 |
| Wealth quintile 4 | 0.25 | 0.02 | 0.18 | 0.02 | 0.07*** |
| Wealth quintile 5 (wealthiest) | 0.24 | 0.03 | 0.23 | 0.03 | 0.02 |
| Number of individuals | 4485 | 3756 | |||
| F-test of joint significance | 1.35 | ||||
| Panel B: clinics | |||||
| Number of clients | 87.04 | 7.10 | 95.56 | 9.13 | 8.52 |
| Years ownership | 10.64 | 0.59 | 9.86 | 0.68 | −0.78 |
| Antenatal care | 0.67 | 0.05 | 0.67 | 0.05 | 0.00 |
| Labour and delivery services | 0.4 | 0.05 | 0.41 | 0.05 | 0.01 |
| Postnatal care | 0.64 | 0.05 | 0.62 | 0.05 | −0.02 |
| Child immunization | 0.3 | 0.04 | 0.46 | 0.05 | 0.16** |
| Well-baby check-ups | 0.62 | 0.05 | 0.73 | 0.05 | 0.12* |
| TB treatment for adults | 0.1 | 0.03 | 0.06 | 0.02 | −0.04 |
| Provides inpatient services | 0.18 | 0.04 | 0.24 | 0.04 | 0.06 |
| Family planning clients | 0.16 | 0.01 | 0.17 | 0.01 | 0.01 |
| Unit cost per client | 13.64 | 1.26 | 13.92 | 1.48 | 0.28 |
| Profit margin | 0.39 | 0.02 | 0.37 | 0.03 | −0.02 |
| NHIF empanelled | 0.14 | 0.03 | 0.08 | 0.03 | −0.06 |
| Provides health insurance to businesses | 0.2 | 0.04 | 0.15 | 0.04 | −0.05 |
| Sells medicines to the public | 0.51 | 0.05 | 0.48 | 0.05 | −0.03 |
| Number of clinics | 107 | 92 | |||
| F-test of joint significance | 1.50 | ||||
Note: Robust standard errors are given clustered at the clinic level. The last column contains t-tests for the difference in means by treatment group. All missing values were imputed with the mean. The last row of each panel includes the F-statistic for a test of joint significance, conducted separately for the individual sample and the clinic sample. The number of clients was measured in previous week. Profit margin is an average calculated over previous 6 months.
*, ** and *** significance at the 1, 5 and 10% critical level.
Abbreviation: TB, tuberculosis.
Effects on household wealth and enrolment in health insurance
We first describe the demand-side effects of the AHME intervention with results from the household survey. Table 1 describes characteristics of 8241 individuals recruited at baseline from treatment and control clinics’ catchment areas. The average household contained seven members and reported a male head of household who was 42 years old.
Household wealth, measured using the Kenya DHS asset wealth index methodology, was grouped into five quintiles poorest (Quintile 1) to wealthiest (Quintile 5) (Kenya Ministry of Health, 2014). If the households included in this study were perfectly representative of all Kenyan households, we would observe 20% of our sample in each quintile. Instead, we find that households in AHME clinics’ baseline catchment areas were wealthier on average than both the control group and the national distribution: 8% of AHME households were in the lowest-wealth quintile and 24% and 25% were in the highest two wealth quintiles, respectively (Table 1). The control group more closely resembles the national distribution; however, the poorest quintile is still under-represented. These results likely reflect the fact that private health clinics, a key eligibility criterion for the clinics in the AHME intervention, are not often located in the poorest areas and instead are in regions where middle- and upper-income households reside.
At endline, individuals in the AHME treatment group were 6.7 percentage points (pp) more likely to be enrolled in health insurance (Table 2, Column 1, P-value < 0.05), a 23.9% increase relative to the control group mean of 28%. This result is largely attributable to an increase in enrolment in both public (including NHIF) insurance (18.3%) and private insurance plans (53.2%). While AHME increased enrolment in insurance on average, the programme did not differentially impact poor households (Table 2, Columns 2, 4 and 6, interaction between AHME and poor household P-value > 0.1).
Table 2.
Effect of AHME on individuals’ health insurance from household surveys
| (1) | (2) | (3) | (4) | (5) | (6) | |
|---|---|---|---|---|---|---|
| Individual has health insurance | ||||||
| Any | Public insurance | Private insurance | ||||
| AHME treatment | ||||||
| Coefficient | 0.067 | 0.054 | 0.046 | 0.041 | 0.033 | 0.025 |
| Standard error | 0.031 | 0.034 | 0.029 | 0.031 | 0.019 | 0.021 |
| P-value | 0.015 | 0.055 | 0.052 | 0.096 | 0.038 | 0.126 |
| Poor household | ||||||
| Coefficient | −0.193 | −0.193 | −0.044 | |||
| Standard error | 0.031 | 0.028 | 0.018 | |||
| P-value | 0.000 | 0.000 | 0.008 | |||
| AHME × poor household | ||||||
| Coefficient | −0.009 | −0.035 | 0.018 | |||
| Standard error | 0.046 | 0.039 | 0.030 | |||
| P-value | 0.578 | 0.811 | 0.276 | |||
| Mean control group | 0.280 | 0.280 | 0.251 | 0.251 | 0.062 | 0.062 |
| Observations | 8241 | 8241 | 8241 | 8241 | 8241 | 8241 |
| Clinics | 199 | 199 | 199 | 199 | 199 | 199 |
Note: Robust standard errors clustered at the index clinic level. One-sided P-values are given. Dependent variable in Models 1–3 is an indicator for access to health insurance at endline. Public Insurance includes NHIF civil servants, NHIF HISP, NHIF Supa Cover, NHIF student NEMIS, Linda Mama, universal health coverage and county health coverage. Private insurance includes employer-sponsored insurance, employer pays healthcare costs directly and all other non-public insurance providers. Poor household is an indicator for if the household’s wealth score at baseline was in the lowest two quintiles. AHME × poor household is an interaction term between the AHME treatment indicator, and the indicator for if the household is in the lowest two wealth quintiles.
Effects on clinic insurance enrolment and scale
We next describe the supply-side effects of the AHME intervention measured using the clinic survey. Table 1 presents characteristics of clinics in the AHME treatment and control groups at baseline. At baseline, AHME clinics saw 87 clients per week on average compared to 96 in the control group. Fourteen percent of AHME clinics were NHIF empanelled compared to 10% of control clinics. We find that AHME was successful at increasing clinic enrolment in NHIF: AHME clinics were 14.5pp more likely to be empanelled at endline (Table 3, P = 0.015). AHME was also successful at increasing clinics’ client load: treated clinics served 26.0% more clients (P = 0.056) and 51.3% more NHIF clients (p = 0.051), each measured in the clinic’s last operational month (Table 3).
Table 3.
Effect of AHME on clinic-level outcomes from clinic surveys
| (1) | (2) | (3) | |
|---|---|---|---|
| Clinic is NHIF empanelled | Number of clients (log) | Number of NHIF clients (asinh) | |
| AHME treatment | |||
| Coefficient | 0.145 | 0.260 | 0.513 |
| Standard error | 0.066 | 0.162 | 0.312 |
| P-value | 0.015 | 0.056 | 0.051 |
| Mean control group | 0.214 | 385 | 25 |
| Clinics | 187 | 187 | 187 |
Note: Robust standard errors clustered at the clinic level and one-sided P-values (H0: beta ≤ 0) are given. Dependent variable in Column 1 is an indicator for NHIF empanelment, and that in Column 2 is the log number of clients. The dependent variable in Column 3 was transformed using the inverse hyperbolic arc sine (asinh) because the outcome contains zeros. Both the number of clients and NHIF clients were measured in the last full operational month.
Effects on client wealth distribution
To reiterate, we found that AHME increased the scale of clinics and the likelihood clinics were able to accept NHIF insurance, both necessary but not sufficient factors for increasing healthcare access by poor patients. To understand if clinics served a larger number of poor clients, we use the client exit survey at endline, which was conducted after the AHME intervention was delivered. This survey measured the household wealth of clients exiting AHME treatment and control clinics. Here, we measured wealth using an internally comparable asset wealth score, grouped into quintiles (Appendix Table A5). Indeed, we find that AHME clinics were more likely to serve clients from lower-wealth quintiles. Figure 2 shows that the distribution of client wealth at AHME clinics is shifted to the left of that of control clinics, and the Kolmogorov–Smirnov test suggests non-equivalent distributions (Figure 2, P-value < 0.01). We find that AHME clinics served fewer clients from the top wealth quintile and more from the middle three quintiles (Figure 2 and Appendix Table A5).
Figure 2.
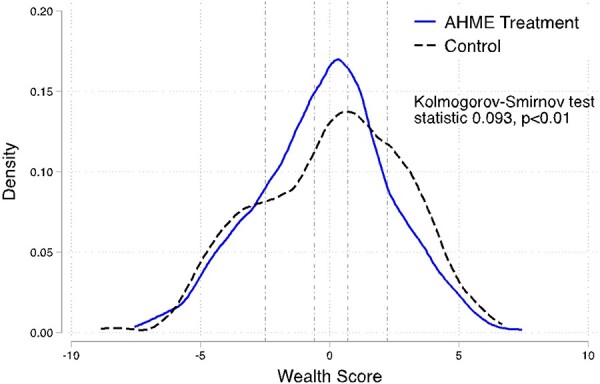
Distribution of wealth score among clients at AHME treatment and control clinics at endline
Note: Distribution of the AHME internal asset wealth index shown. All results are reweighted based on the share of NHIF clients seen at that clinic the month prior to the clinic survey to account for survey sampling. Test statistic and P-value for the Kolmogorov–Smirnov test for equality of distribution functions shown indicate that we reject the null hypothesis of equal distributions. Vertical dashed lines divide the distributions into wealth quintiles from quintile 1 (poorest) to 5 (wealthiest) moving left to right.
Effects on quality of care for poor clients
Finally, we report results comparing SP visits with healthcare providers at AHME treatment and control clinics. We test for whether quality of care was differential based on if a client presents as poor. We examine quality-of-care outcomes on three dimensions: an indicator for correct case management, an indicator for whether any unnecessary laboratory tests were prescribed, and an indicator for whether any non-efficacious medicines were prescribed (see the SPs section for details on correct and unnecessary/non-efficacious care). We find that correct case management was lower and the probability of an SP receiving any unnecessary laboratory tests was higher on average at AHME clinics compared to control clinics; however, these differences were not statistically significant (Table 4, Columns 1 and 2, respectively).
Table 4.
Effects of AHME and being poor on correct and unnecessary actions: SP regression models
| (1) | (2) | (3) | |
|---|---|---|---|
| Correct case management | Any unnecessary laboratory tests | Any non-efficacious medicines | |
| AHME treatment | |||
| Coefficient | −0.062 | 0.054 | −0.045 |
| Standard error | 0.048 | 0.041 | 0.049 |
| P-value | 0.200 | 0.187 | 0.361 |
| AHME treatment × poor | |||
| Coefficient | −0.048 | −0.085 | 0.059 |
| Standard error | 0.073 | 0.065 | 0.080 |
| P-value | 0.508 | 0.196 | 0.458 |
| Poor | |||
| Coefficient | −0.208 | −0.013 | −0.259 |
| Standard error | 0.063 | 0.057 | 0.069 |
| P-value | 0.001 | 0.816 | 0.000 |
| Mean AHME control group | 0.728 | 0.211 | 0.66 |
| Observations | 596 | 596 | 596 |
Note: The table shows multivariate regressions using SP data. All models contain robust standard errors clustered at the SP individual and clinic levels, SP fixed effects and covariates for the AHME treatment indicator, an indicator for the poor SP experiment and an interaction term for AHME treatment and poor. All models pool the asthma and malaria SP visits and control for the case scenario. In Model 1, correct case management is a 0–1 binary measure constructed specific to each scenario. For asthma, SP data were coded as correctly managed, 1 if the provider treated the case with an inhaler or bronchodilator and 0 otherwise. For malaria, SP data were coded as correctly managed, 1 if the provider ordered a malaria rapid diagnostic test or a malaria microscopy test and 0 otherwise. For Model 2, any unnecessary laboratory test is a 0–1 binary measure constructed specific to each scenario. For asthma, SP data were coded as any unnecessary laboratory tests, 1 if providers ordered any laboratory test and 0 otherwise. For malaria, SP data were coded as any unnecessary laboratory tests, 1 if providers ordered any laboratory test excluding malaria rapid diagnostic test, malaria microscopy, blood count and brucellosis test and 0 otherwise. For Model 3, any non-efficacious medicine is a 0–1 binary measure constructed specific to each scenario. For asthma, SP data were coded as any non-efficacious medicines, 1 if providers dispensed/prescribed any medicines excluding inhaler or bronchodilators and 0 otherwise. For malaria, SP data were coded as any non-efficacious medicines, 1 if providers dispensed/prescribed any medicines excluding artemether lumefantrine and paracetamol and 0 otherwise.
We find no evidence that the AHME programme worked to improve quality of care for poor clients; the interaction term between AHME and poor SP is not statistically significant in any of our quality-of-care models (Table 4, Columns 1–3). However, when controlling for the AHME intervention, SPs who presented as poor, that is, told the provider that they could only afford KSH 300 in fees, were 20.8pp less likely to receive correct case management than SPs who did not present as poor (Table 4, Column 1, P-value < 0.01). SPs who presented as poor were 25.9pp less likely to be prescribed any non-efficacious medicines (Table 4, Column 3, P-value < 0.01), indicating that poor SPs received a lower overall volume of services than non-poor SPs.
Discussion
This study documents the healthcare equity effects of AHME, a cluster randomized controlled trial that investigated whether the combination of social health insurance and management training could improve access to and quality of private health clinics in Kenya. AHME had substantial effects on clinics: clinics in the treatment group reported more clients—a 26.0% increase in total clients and a 51.3% increase in NHIF clients. AHME also increased the likelihood that individuals living in the catchment areas of treated households had any, public or private health insurance, with increases of 6.7pp, 4.6pp and 2.5pp, respectively. However, we do not find evidence that AHME achieved its goal of encouraging pro-poor private health care: we find that poor households were substantially less likely to have health insurance, and there was no additional effect of AHME for this group. Similarly, using SP method experiments, we found no evidence that AHME worked to improve the quality of care for poor clients; however, irrespective of the programme, SPs who presented as poor were 20.8pp less likely to receive correct case management. We also did not find that AHME clinics served more clients from the lowest-wealth quintile.
AHME focused on social health insurance as a mechanism through which low-income households can receive high-quality health care at private or public clinics. Specifically, the AHME intervention encouraged and assisted private clinics to empanel under the NHIF scheme. On the supply side, AHME was successful in removing frictions in insurance enrolment, which providers previously described as bureaucratic and time-consuming (Suchman et al., 2019) resulting both in large effects on clinic empanelment and large increases in the number of clients with public insurance served. At baseline, 8% of control group clinics were NHIF empanelled, and at endline, 21% of control group clinics were empanelled. This increase likely reflects the large nation-wide effort to increase access to and use of NHIF that occurred in the years between baseline and endline (Barasa et al., 2018). Despite this secular trend, at endline, AHME clinics were 14.5pp more likely to be NHIF empanelled than the counterfactual.
As AHME was largely a private sector clinic engagement and management programme, our finding that individuals in the AHME treatment group were 24% more likely to have any health insurance at endline compared to control clinics are welcome. While we cannot explicitly test these mechanisms, there are several ways through which increased clinic NHIF empanelment might increase demand-side insurance enrolment beyond the secular trend. Future research is needed to understand these mechanisms, and specifically whether clinics’ advertising that they accepted NHIF insurance—e.g. through signs—helped to encourage uptake of insurance in the AHME treatment group. Second, after clinics empanelled with NHIF, local clients had a higher incentive to enrol in NHIF and also to visit NHIF-accepting clinics. However, we found no additional benefit of AHME for those from poor households, who are much less likely to hold any health insurance than wealthier households in both the treatment and control groups. More work must be done to improve insurance enrolment of this population specifically.
Finally, we investigated the quality of care at AHME treatment and control clinics using a novel variation on an SP experiment. Research has found that low-income individuals receive lower-quality medical care (Haemmerli et al., 2021), and we aimed to examine whether AHME had an effect on levels of quality of care received by patients who could fully afford all services vs those who could only afford up to KSH 300 (US$3). By implementing an experiment within the SP method, SPs randomized to state that they could only afford KSH 300 (US$3) led to receive fewer health services, and the programme had no effect on this. Regardless of AHME treatment status, we found that when providers faced a budget constraint, there was a reduction in both minimally correct and unnecessary care, which is being further examined in greater detail in a forthcoming paper.
This paper provides a unique opportunity to simultaneously examine the supply and demand-side factors that must work together to achieve healthcare equity in LMICs (Balabanova et al., 2010). AHME focused on access to public health insurance, where the government is the payer, as a mechanism to increase use of affordable, high-quality private care for poor individuals. We find suggestive evidence that NHIF works in this way in Kenya, especially after supply-side frictions in enrolment were reduced for clinics. Efforts to reduce the cost of care must also ensure that the quality of care is maintained. We found that providers reduced the quantity of both necessary and unnecessary care when SPs presented as poor clients, leading to this population receiving a lower quality of care overall. Future research should use this SP method to study quality and quantity of care in public clinics compared to private clinics. More work must be done to ensure that providers do not reduce necessary care provided in response to clients’ budget constraints.
Limitations
This study has several limitations. First, because the AHME intervention included only private clinics and surrounding households, the AHME evaluation sample did not perfectly represent the distribution of wealth in Kenya and instead contained disproportionately more households from the top two wealth quintiles and fewer from the bottom quintile. While it is somewhat unsurprising that private clinics were located in relatively wealthier areas, it is worth understanding further how the spatial distribution of private health sectors in study settings may not provide equal access to all parts of the wealth distribution in a region or country and that they may skew to where wealthier populations reside. For this reason, our results may not generalize to all regions or populations in Kenya. Second, because nationally representative asset weights were not available at endline, our endline wealth index is only valid for comparisons between AHME treatment and control groups and not valid for comparisons to national wealth quintiles. Third, healthcare quality is a multidimensional and complex topic, and this study likely was not able to measure all aspects of healthcare quality. In this study, the SP method was a good fit as SPs require a one-time visit for services that (1) do not subject the client to invasive procedures; (2) can only assess tracer health conditions that have been validated for ethical research and (3) do not require established client services or follow-up visits, such as those related to chronic conditions or other ailments. However, in this study, we were not able to logistically implement the SP method in a manner that would allow us to identify the causal effects of the AHME programme on differences in quality of care received by clients who were insured compared to those uninsured, holding constant all other aspects.
Conclusion
The effects of AHME suggest that aligning the insurance type accepted by clinics with that held by individuals can effectively encourage lower-income clients to utilize private sector healthcare services. Additionally, private, for-profit clinics that received management training were able to serve a greater number of clients from the middle wealth distribution. These results indicate that management training combined with social health insurance and franchising is a viable solution for sustaining profitable private healthcare clinics and to encourage pro-poor health care in Kenya, instead of solely relying on them to provide charitable care. However, further research is necessary to comprehend how to maintain high-quality health care for the low-income clients who utilize clinics in the private sector.
Supplementary Material
Acknowledgements
The authors would like to thank Nicole Perales, David Contreras-Loya, Rita Cuckovich, Afke Jagar and Innovations for Poverty Action Kenya for their research and technical assistance; Jishnu Das for his support; our SP Technical Advisory Group (Catherine Wanjiru, John Wanangwe, Jacob Odipo, Daniel Waithaka) for their expertise; Andrew Muriithi, Pheliciah Mwachofi, Purity Kimuru, Rodgers Kedoge and Salome Omondi for their supervision and the SPs for their communication and hard work.
Contributor Information
Claire E Boone, Booth School of Business, University of Chicago, 5807 S. Woodlawn Ave, Chicago, IL 60637, USA.
Paul J Gertler, Haas School of Business, University of California Berkeley, 2220 Piedmont Ave, Berkeley, CA 94720, USA.
Grace Makana Barasa, Innovations for Poverty Action, Sandalwood Lane, Nairobi, Kenya.
Joshua Gruber, Center for Effective Global Action, University of California Berkeley, Giannini Hall, 251 Berkeley, CA 94720, USA.
Ada Kwan, Division of Pulmonary and Critical Care Medicine, University of California San Francisco, 1001 Potrero Avenue, San Francisco, CA 94110, USA.
Supplementary data
Supplementary data are available at Health Policy and Planning online.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This project was funded by a grant from the Bill and Melinda Gates Foundation (Opportunity ID: OPP1044138). The former UK Department of International Development provided funding for the grant. Claire Boone was supported by the NIH National Institute on Aging (NIA) under grant award T32 AG000243 (PI: David Meltzer, MD, PhD). The contents are solely the responsibility of the author and do not necessarily represent the official views of the NIA.
Author Contributions
C.E.B., A.K., P.J.G. and J.G. contributed to conception or design of the work. G.M.B., A.K. and J.G. contributed to data collection. C.E.B., A.K., J.G. and P.J.G. contributed to data analysis and interpretation. C.E.B., A.K., G.M.B., J.G. and P.J.G. contributed to drafting of the article. C.E.B., A.K., G.M.B., J.G. and P.J.G. contributed to critical revision of the article. Final approval of the version is to be submitted by C.E.B., A.K., G.M.B., J.G. and P.J.G.
Reflexivity statement
This research paper represents a collaborative effort from a diverse team of five coauthors, led by a female doctoral student. Our team is diverse along several dimensions: seniority (student to full professor), gender (females and males), race/ethnicity (three different groups), location (global south and global north) and academic discipline (health policy, medical school and business school).
Ethical approval
This study involves human participants and was approved by Innovations for Poverty Action’s IRB board (Protocol No. 1085) and Kenya Medical Research Institute (Non-SSC Protocol No. 372). The study also received local research permission in Kenya National Commission for Science, Technology and Innovation Permit #NA-COSTI/P/19/5343/28310. Our study analyses data from the SP method, which involves recruiting, hiring and training individuals to portray tracer health conditions. Similar to other SP studies with similar designs and embedded in an intervention, we sought a waiver of provider informed consent to conduct the SP study. The request for a waiver was based on a recent study commissioned by the US Department of Health and Human Services to assess the ethics of simulated patient studies. Supported by a pilot study conducted in Nairobi that validated the SP method in the Kenyan context, both ethics committees approved the waiver request within the AHME evaluation study because (1) combining informed consent with the congregation of providers during trainings and the implementation of interventions during the study period posed threats to the scientific validity of the study objectives, as well as to the risk of SP detection, and (2) there is no more than minimal risk of participation to the SPs or providers, as reported in the Nairobi SP pilot and validation study (Daniels et al.). Ethics committee approvals with the waiver of informed consent were provided conditional on our agreement to return to all clinics visited by SPs to disclose the SP study to them and to provide them with an opportunity to ask questions and discuss any concerns. During 1–23 January 2020, we informed all clinics and providers that they received SPs. Finally, the study was registered with the American Economic Association Registry, trial number AEARCTR-0000217, and the Pan African Clinical Trial Registry, trial number PACTR201502000770329.
Conflict of interest statement
The authors declare no conflicts of interest.
References
- Balabanova D, McKee M, Mills A, Walt G, Haines A. 2010. What can global health institutions do to help strengthen health systems in low income countries? Health Research Policy and Systems 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Finkelstein A, Hanna R et al. 2021. The challenges of Universal Health Insurance in developing countries: experimental evidence from Indonesia’s National Health Insurance. American Economic Review 111: 3035–63. [Google Scholar]
- Barasa E, Rogo K, Mwaura N, Chuma J. 2018. Kenya National Hospital Insurance Fund Reforms. Implications and Lessons for Universal Health Coverage. Health Systems & Reform 4: 346–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright T, Felix L, Kuper H, Polack S. 2017. A systematic review of strategies to increase access to health services among children in low and middle income countries. BMC Health Services Research 17: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Human Rights and Global Justice . 2021. Wrong Prescription: The Impact of Privatizing Healthcare in Kenya. The Economic and Social Rights Centre-Hakijamii and the Center for Human Rights and Global Justice at New York University School of Law. New York: Center for Human Rights and Global Justice.
- Chakraborty NM, Montagu D, Wanderi J, Oduor C. 2019. Who serves the poor? An equity analysis of public and private providers of family planning and child health services in Kenya. Front Public Health 6: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Loya D, Gertler P, Kwan A. 2022. Managerial Practices and Altruism in Health Care Delivery.
- Das J, Woskie L, Rajbhandari R, Abbasi K, Jha A. 2018. Rethinking assumptions about delivery of healthcare: implications for universal health coverage. BMJ 361: k1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerli M, Powell-Jackson T, Goodman C, Thabrany H, Wiseman V. 2021. Poor quality for the poor? A study of inequalities in service readiness and provider knowledge in Indonesian primary health care facilities. International Journal for Equity in Health 20: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K, Berman P. 1998. Private health care provision in developing countries: a preliminary analysis of levels and composition. Health Policy and Planning 13: 195–211. [DOI] [PubMed] [Google Scholar]
- Ilinca S, Di Giorgio L, Salari P, Chuma J. 2019. Socio-economic inequality and inequity in use of health care services in Kenya: evidence from the fourth Kenya household health expenditure and utilization survey. International Journal for Equity in Health 18: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehu-Appiah C, Aryeetey G, Spaan E et al. 2011. Equity aspects of the National Health Insurance Scheme in Ghana: who is enrolling, who is not and why? Social Science & Medicine 72: 157–65. [DOI] [PubMed] [Google Scholar]
- Kenya Ministry of Health . 2014. Kenya household Health expenditure and utilisation survey.Kenya.
- Kenya Ministry of Health . 2021. Kenya Master Health Facility List [WWW Document]. http://kmhfl.health.go.ke/#/home, accessed 26 October 2021.
- Khetrapal S, Acharya A, Mills A. 2019. Assessment of the public-private-partnerships model of a national health insurance scheme in India. Social Science & Medicine 243: 112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JJC, Powell-Jackson T, Makungu C et al. 2021. Effect of a multifaceted intervention to improve clinical quality of care through stepwise certification (SafeCare) in health-care facilities in Tanzania: a cluster-randomised controlled trial. The Lancet Global Health 9: e1262–e1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A, Boone CE, Sulis G, Gertler PJ. 2022. Do private providers give patients what they demand, even if it is inappropriate? A randomised study using unannounced standardised patients in Kenya. BMJ Open 12: e058746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A, Daniels B, Bergkvist S et al. 2019. Use of standardised patients for healthcare quality research in low- and middle-income countries. BMJ Global Health 4: e001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum R, Theobald S, Otiso L et al. 2018. Priority setting for health in the context of devolution in Kenya: implications for health equity and community-based primary care. Health Policy and Planning 33: 729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu D, Suchman L, Seefeld CA. 2020. Equity lessons from a large scale private-sector healthcare intervention in Ghana and Kenya: results for a multi-year qualitative study. Gates Open Research 4: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R, Ensor T, Waters H. 2016. Performance of private sector health care: implications for universal health coverage. The Lancet 388: 606–12. [DOI] [PubMed] [Google Scholar]
- Patouillard E, Goodman CA, Hanson KG, Mills AJ. 2007. Can working with the private for-profit sector improve utilization of quality health services by the poor? A systematic review of the literature. International Journal for Equity in Health 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul E, Deville C, Bodson O et al. 2019. How is equity approached in universal health coverage? An analysis of global and country policy documents in Benin and Senegal. International Journal for Equity in Health 18: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich MR, Harris J, Ikegami N et al. 2016. Moving towards universal health coverage: lessons from 11 country studies. The Lancet 387: 811–6. [DOI] [PubMed] [Google Scholar]
- Suchman L, Montagu D, Seefeld CA. 2019. African Health Markets for Equity Qualitative Evaluation Final Report. UCSF Institute for Global Health Sciences. [Google Scholar]
- WHO . 2021. Universal health coverage (UHC) [WWW Document]. https://www.who.int/news-room/fact-sheets/detail/universal-health-coverage-(uhc), accessed 7 October 2021.
- Yoong J, Burger N, Spreng C, Sood N, Noor AM. 2010. Private sector participation and health system performance in sub-Saharan Africa. PLoS One 5: e13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


