Abstract
The present study investigated the potential role of different essential amino acids (AA) in striped catfish (Pangasius hypophthalmus). Fish (initial weight = 17.91±0.27 g, n = 260) were fed with eight isonitrogenous (30%), and isolipidic diets (6%) formulated to include different combinations of tryptophan (Trp), methionine (Met), and lysine (Lys) (T0: Zero AA, T1: Trp, T2: Lys, T3: Met, T4: Trp+Met, T5: Lys+Trp, T6: Met+Lys, T7: Lys+Trp+Met) for eight weeks. The dose of amino acid supplementation, whether individually or in combination, was 5g of each amino acid per kg of diet. The trial comprised eight treatments, with each treatment consisted of three replicates (n = 10/replicate). At the end of the growth experiment, the highest total body weight, crude protein, digestive enzymatic activity, immune response, and amino acids level were observed in treatments supplemented with amino acids compared to T0. After the growth experiment, fish in all treatments were exposed to Staphylococcus aureus (5×105 CFU/ml). For bacterial challenge trial, the T0 treatment was designated as positive (+ve T0) and negative control (-ve T0). Following the S. aureus challenge, fish fed with amino acids showed a better response to reactive oxygen species and lipid peroxidation, as indicated by the increased levels of catalase and superoxide dismutase. Conversely, the concentration of malondialdehyde gradually decreased in all treatments compared to the +ve T0 treatment. It is concluded that supplementation of amino acids improved the growth, protein content, and immunocompetency against S. aureus in striped catfish. The most favorable outcomes in striped catfish were shown by fish supplemented with T7 diet. These essential amino acids hold potential as efficient supplements for use in the intensive aquaculture for striped catfish.
1. Introduction
Striped catfish (Pangasius hypophthalmus), is an economically valuable fish and currently ranks among the most traded freshwater fish species worldwide [1]. The global production of this species was recorded as 2520.41 thousand tons in 2022 [2]. Enhancing catfish production can be achieved by emphasizing on high-quality feed [3], particularly by aligning the nutritional content of feed with specific dietary requirements [4, 5]. The demand for fishmeal and fish oil as feed components has increased dramatically to meet the rising needs of aquaculture production [6]. However, due to higher prices and limited supply of fishmeal, it has become necessary to search for alternative plant protein sources. It is important to consider that such alternative sources should have balanced dietary components and be capable of maintaining high growth rates [7]. High-quality feed is not limited to protein content alone but also encompasses essential amino acids to promote growth performance [8]. These amino acids greatly influence the growth patterns, reproductive performance, and development of a fish [9].
Among these essential amino acids, lysine is one of the most limiting amino acids in fish feed [10]. Lysine is found in significant proportions in fish muscle tissues such as rohu (Labeo rohita) (2.9%) [6], catla (Catla catla) (3.6g%) [11], juvenile black sea bream (Sparus aurata) (6.63%) [12], basa (Pangasius bocourti) (8.41%) [13]. It plays a pivotal role in growth [14], maintaining nitrogen balance [15], preventing excessive fat accumulation in the body [16], and maintaining osmotic pressure and acid-base balance in the body fluids [17]. It also known for inhibiting fin rotting of a fish, decreasing the mortality rates [18], enhancing protein deposition in the body and fillet content [19–21], increasing muscle growth in fish by rapidly increasing the size and length of muscle fibers through hyperplasia and hypertrophy [22], and being involved in the facilitation of ‘cross-linking’ protein, especially collagen [23]. The required range of lysine as a fish feed component varies between 2 to 4% of the total dietary protein for different fishes [24]. Furthermore, all finfish species require lysine as an essential dietary component, especially when alternative protein sources are used instead of fishmeal. In addition, fish fed with diets deficient in this essential amino acid showed reduced growth, and higher mortality rates [25].
After lysine, methionine is the second limiting amino acid in plant protein sources [5, 26]. These amino acids are involved in the synthesis of carnitine, which functions in the transportation of fatty acids to produce energy through oxidation [27], and increase protein retention [28]. Methionine supplementation can enhance protein synthesis in fish, as seen in blackhead seabream (17.25%) [29], improve growth performance in several fish species including common carp (Cyprinus carpio) [30], channel catfish (Ictalurus punctatus) [31], northern snakehead (Channa argus) [32], nile tilapia (Oreochromis niloticus) [33]. It also helps in maintaining antioxidant system [34], and enhance energy metabolism by synthesizing cysteine and glutathione through trans-sulphuration pathway [35]. On the other hand, studies have indicated that methionine deficiency leads to decreased survival rates, and reduced growth performance in Jian carp (Cyprinus carpio) [36], rainbow trout (Oncorhynchus mykiss) [37], juvenile red drum (Sciaenops ocellatus) [38]. Additionally, methionine deficiency can lead to the development of lenticular cataracts in seabream [39].
In contrast to methionine and lysine, tryptophan serves as the precursor for both serotonin (5-hydroxytryptamine, 5-HT) and melatonin (N-acetyl-5- methoxytryptamine). These compounds have significance in fortifying the immune response [40–42], improve growth performance [43], exert significant immunomodulatory influence, and function as potent scavengers of deleterious free radicals [44]. Numerous independent investigations have explored the effect of tryptophan, lysine, and methionine on growth performance and immune response of various fish species [45–50], including striped catfish [51], basa catfish [52]. To the best of our knowledge, there is no existing published data available on synergistic impact of these three amino acids on growth performance, disease resistance, and immunocompetency of striped catfish. Therefore, the purpose of the present investigation was to assess the potential influence of these essential amino acids on the growth, protein content, digestive enzymes, and immune response in striped catfish. The concentrations of these amino acids were selected based on the dietary requirement of catfish.
2. Materials and methods
2.1 Preparation of experimental diets
Isonitrogenous (30% crude protein) and isolipidic (6%) diets were formulated. Treatment diets were prepared by mixing the finely ground ingredients (grains were procured from local farmers in Pakistan while origin of soybean was USA; Table 1) with different combinations of methionine (Met), lysine (Lys), and tryptophan (Trp) (T0; zero supplementation, T1; Trp, T2; Lys; T3; Met: T4; Trp + Met, T5; Lys +Trp, T6; Met +Lys, T7; Lys + Trp +Met). L- lysine, L- methionine, and L- tryptophan were purchased (Sigma Aldrich, USA). A total of 0.50% of each amino acid was added to one kg of feed in each diet according to catfish dietary requirement [50]. All ingredients were thoroughly mixed and pellets (1mm) were made by using mechanical pellet machine (manufactured by PCSIR laboratories, Pakistan). The pellets were air-dried at room temperature and stored at 4°C. Ingredients and chemical composition of the treatment diets are given in Table 1. Amino acids profile of treatment diets is given in Table 2. Chemical composition and amino acid analysis of treatment diets were determined as mentioned in section 2.4.
Table 1. Ingredients and chemical composition of experimental diets.
| Ingredients (%) | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| Corn meal | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 |
| Rice polish | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| Wheat bran | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Canola meal | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Soybean meal | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 | 39.00 |
| Dicalcium phosphate | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Methionine | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 |
| Lysine | 1.58 | 1.58 | 1.58 | 1.58 | 1.58 | 1.58 | 1.58 | 1.58 |
| L-Threonine | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 |
| Trp (5g/Kg) | 0.00 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lys (5g/Kg) | 0.00 | 0.00 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Met (5g/Kg) | 0.00 | 0.00 | 0.00 | 0.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| Trp + Met (5g/Kg) | 0.00 | 0.00 | 0.00 | 0.00 | 0.50+0.50 | 0.00 | 0.00 | 0.00 |
| Lys + Trp (5g/Kg) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50+0.50 | 0.00 | 0.00 |
| Met + Lys (5g/Kg) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50+0.50 | 0.00 |
| Lys +Trp + Met (5g/Kg) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50+0.50+0.50 |
| Chemical Composition of Feed | ||||||||
| Moisture (%) | 10.02 | 10.12 | 10.20 | 10.03 | 10.21 | 10.01 | 10.02 | 10.13 |
| Crude Protein (%) | 29.99 | 30.10 | 30.10 | 30.03 | 30.12 | 30.11 | 30.09 | 30.12 |
| Crude Fat (%) | 6.16 | 6.16 | 6.18 | 6.19 | 6.17 | 6.19 | 6.18 | 6.17 |
| Crude Ash (%) | 8.72 | 8.81 | 8.61 | 8.90 | 8.93 | 8.82 | 8.74 | 8.76 |
(T0; C, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine). Tryptophan: (Trp), Methionine: (Met), Lysine: (Lys)
Table 2. Profile of amino acids (% of complete diet) in experimental diets.
| Amino Acids | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| Methionine | 0.11±0.02 | 0.19±0.12 | 0.21±0.02 | 0.65±0.22 | 0.65±0.01 | 0.11±0.21 | 0.65±0.22 | 0.65±0.02 |
| Tryptophan | 0.11±0.01 | 0.61±0.20 | 0.11±0.01 | 0.11±0.02 | 0.59±0.13 | 0.59±0.01 | 0.11±0.03 | 0.59±0.01 |
| Valine | 7.23±0.02 | 6.90±0.11 | 7.12±0.03 | 7.30±0.02 | 7.40±0.01 | 7.31±0.05 | 7.23±0.02 | 7.23±0.04 |
| Isoleucine | 1.80±0.04 | 1.81±0.21 | 1.81±0.12 | 1.80±0.22 | 1.82±0.12 | 1.81±0.02 | 1.83±0.14 | 1.82±0.15 |
| Leucine | 0.46±0.21 | 0.40±0.01 | 0.41±0.21 | 0.41±0.01 | 0.41±0.20 | 0.42±0.20 | 0.41±0.04 | 0.41±0.01 |
| Phenylalanine | 5.40±0.04 | 5.32±0.21 | 5.40±0.16 | 5.43±0.12 | 5.43±0.14 | 4.43±0.21 | 0.53±0.01 | 5.43±0.02 |
| Histidine | 0.07±0.04 | 0.07±0.01 | 0.07±0.03 | 0.08±0.13 | 0.08±0.12 | 0.08±0.10 | 0.08±0.04 | 0.09±0.01 |
| Lysine | 1.50±0.02 | 1.50±0.02 | 2.40±0.02 | 1.51±0.12 | 1.51±0.04 | 2.41±0.02 | 2.42±0.02 | 2.43±0.10 |
| Ornithine | 3.33±0.21 | 3.32±0.12 | 3.34±0.03 | 3.34±0.14 | 3.21±0.06 | 3.33±0.04 | 3.40±0.11 | 3.22±0.02 |
| Cysteine | 0.02±0.11 | 0.03±0.14 | 0.02±0.13 | 0.03±0.20 | 0.03±0.14 | 0.03±0.15 | 0.03±0.13 | 0.09±0.11 |
| Aspartic Acid | 0.22±0.04 | 0.23±0.22 | 0.22±0.02 | 0.23±0.14 | 0.23±0.01 | 0.24±0.02 | 0.23±0.04 | 0.23±0.02 |
| Asparagine | 1.55±0.22 | 1.56±0.32 | 1.56±0.03 | 1.57±0.15 | 1.57±0.03 | 1.58±0.02 | 1.58±0.03 | 1.53±0.01 |
| Serine | 0.21±0.01 | 0.21±0.12 | 0.21±0.13 | 0.21±0.05 | 0.22±0.13 | 0.23±0.15 | 0.21±0.16 | 0.22±0.17 |
| Glycine | 0.005±0.23 | 0.006±0.02 | 0.005±0.03 | 0.007±0.16 | 0.008±0.03 | 0.007±0.05 | 0.006±0.01 | 0.008±0.01 |
| Alanine | 1.36±0.11 | 1.36±0.02 | 1.36±0.13 | 1.37±0.14 | 1.35±0.13 | 1.36±0.11 | 1.37±0.11 | 1.36±0.13 |
| Tyrosine | 0.04±0.01 | 0.05±0.12 | 0.05±0.03 | 0.05±0.05 | 0.06±0.04 | 0.04±0.02 | 0.05±0.05 | 0.05±0.02 |
| Threonine | 6.08±0.21 | 6.11±0.04 | 5.91±0.02 | 6.03±0.14 | 5.83±0.13 | 6.11±0.05 | 6.02±0.04 | 6.11±0.02 |
(T0; C, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine), Tryptophan (Trp), Methionine (Met), Lysine (Lys).
2.2 Growth experiment
Trial was started after ethical approval from animal ethics committee (Ref. No.: Zoo/LCWU/932). Fish were collected from a local hatchery (Lahore, Pakistan) and transported to the aquaculture facility at Lahore College for Women University. Fish were acclimatized in three 600L tanks for a week and fed with prepared feed without any supplementation (diet in T0). After acclimatization, fish (initial weight = 17.91±0.27g, total no. = 260) were stocked in 24 (150L) glass aquaria (stocking density: 1.19kg/m3) for eight weeks. Each of the eight treatments had three replicates, while each replicate had ten fish. Remaining twenty fish were fed with a diet without amino acids supplementation to be used as the negative control in the bacterial challenge trial (section 2.3). These fish were reared in two separate glass aquaria, (10 fish each) under the same husbandry conditions. Daily ration was calculated based upon 2% of the biomass weight and each treatment was fed three times a day. A total of 10% water was exchanged on daily basis to maintain water quality at adequate level for the fish. Fish were reared at ambient temperature and photoperiod. The water quality parameters including dissolved oxygen (DO) (7.51±0.21mg/L), pH (7.21±0.41), and water temperature (29.00±1.00°C) were monitored on a daily basis. Ammonia, nitrite and nitrate in water were tested once a week and their values were noted to be lower than detection limit of water quality testing kits (API freshwater test kit, USA; LE144RS). Fish behavior was monitored on daily basis.
2.3 Bacterial challenge
2.3.1 Isolation of Staphylococcus aureus
S. aureus was obtained from diseased Labeo rohita fish originating from the University diagnostic laboratory, Department of Microbiology, University of Veterinary and Animal Sciences, Lahore Pakistan. A 10-gram portion of the diseased fish muscle sample was blended with 90 ml of sterile peptone water, generating a 1:10 dilution, to facilitate the enrichment of the target bacterial species. Subsequently, this mixture was incubated at 37°C for 6 hours following Akbar and Anal [53]. From dilutions, 0.5 ml was inoculated on to Mannitol Salt Agar (MSA) and incubated at 37°C for 24 hours. The emergence of colonies exhibiting a yellow hue was indicative of S. aureus and was subsequently validated through gram staining and coagulase production test using a Pastorex Staph Plus kit (Bio-Rad, Hercules, CA). The purified subculture was duly preserved to facilitate subsequent analyses.
2.3.2 Challenge with S. aureus
After the growth experiment, fish were challenged with S. aureus for 15 days. The S. aureus culture was prepared in 10 ml volume of nutrient broth (HiMedia Ltd., Lahore, Pakistan). Subsequently, the culture was vortexed, and incubated in shaker incubator for 24 hours at 37°C. The culture was centrifuged (Micro Prime Centrifuge, Pocklington, UK) at 5000 rpm for 15min at 4°C to get the hard pellet at the bottom. The obtained pellet underwent several washings, employing sterile phosphate buffer saline (PBS). Following thorough washing, the pellet was re-suspended in PBS (pH 7.4). To ascertain the optical density of bacterial suspension, a UV spectrophotometer was utilized to obtain concentration of 5×105 CFU/ml. The control group was split into two distinct subgroups: positive control (+ve T0) and negative control (-ve T0). Fish in -ve T0 was given bath with PBS only, whereas the other groups (+ve T0, T1, T2, T3, T4, T5, T6, and T7) (n = 15 for each treatment) were exposed to S. aureus (5×105 CFU/ml). Fish were bathed for 2 hours and the bath was repeated after seven days. Throughout the challenge period, all fish in all treatments were fed with their relevant diets.
2.4 Sample collection
At the end of the experiment, fish were fasted for 24 hours and anesthetized using clove oil (6ml/L; Sigma Aldrich USA) for five minutes. Five fish were randomly collected from each replicate of each treatment after growth experiment. All remaining fish were sampled after bacterial challenge. Total body weight, total body length, specific growth rate (SGR), feed conversion ratio (FCR), condition factor, and weight of viscera, and liver were calculated by using following formulae:
Blood was collected from caudal vein and stored in pro-coagulation clot activator (Xiangyuan Medical, Germany) and EDTA coated tubes (Xinle, China), respectively. Clot activator tubes were used to collect serum, while EDTA coated tubes were utilized for analysis of hematology and blood biochemistry. Blood samples were centrifuged at 5000 rpm for 20 min to extract plasma. It was stored at -20°C until assessed. Muscle samples were collected and stored at -20°C to determine chemical composition, and amino acid profile. Whereas, intestine samples were collected, washed with autoclaved sterile water, centrifuged at 5000 rpm, pellet collected at the bottom of centrifuge tubes and stored at 4°C to determine digestive enzymes. Fish muscles, liver, kidney, gut, and gills were collected for histological analysis.
2.5 Chemical composition and amino acid analysis
The chemical composition of muscles was analyzed using the protocol outlined by the association of official analytical chemists [54]. Muscle samples were dried in an oven at 80°C until a constant dry weight was achieved. The dried samples were then ground for further chemical analysis. Crude protein was determined using the Kjeldahl apparatus (PCSIR Laboratories, Pakistan). Crude lipids were determined following Folch method [55] in the Soxhlet apparatus (PCSIR Laboratories, Pakistan). The ash content in the muscles were determined by using furnace (PCSIR Laboratories, Pakistan). An amino acid analyzer (Biochrome 30+, Biochrome limited, Cambridge, UK) was used to quantify the amino acid contents of fish muscles and the analytical protocols followed by Ahmad et al. [56].
2.6 Digestive enzymes assay
Protease, amylase, and lipase enzymatic extracts from intestine samples were prepared following a method by Ding et al. [57]. Intestine samples were rinsed with sterile water and homogenized in phosphate buffer saline (PBS, 1g/10ml; pH = 7.5), and centrifuged at 5000 rpm for 20 minutes. The supernatant was collected and preserved at 4°C. Protease activity of intestine samples was determined using Folin-phenol reagent, according to Jin [58]. Quantification of amylase enzymes activity was carried out by utilizing iodine to detect the unhydrolyzed starch in samples, followed by Jiang [59]. Lipase activity was assessed by measuring the fatty acids released through the enzymatic breakdown of triglycerides, as described by Borlongan [60]. The enzymatic activities are expressed as intestine content units per liter (U/L).
2.7 Hematology, blood biochemistry and assays of antioxidant biomarkers
The levels of haemoglobin (g/dl), white blood cells (μL), red blood cells (μL), mean corpuscle volume (MCV; fL), haematocrit (HCT; %), platelets (μL), mean corpuscular haemoglobin (MCH; %), neutrophils (μL), lymphocytes (μL), monocytes (μL), and eosinophils (μL), were measured by using clinical hematology analyzer (Sysmex, China; KX-21N). Blood glucose level (mg/dl) was measured by using laboratory blood glucose analyzer (MegMedius, Karachi Pakistan; GLM-78). Triglycerides (mg/dl) were measured by using ELISA (Biocompare, USA) as per manufacturer’s protocol. Alanine aminotransferase (ALT; Unit/L), and aspartate aminotransferase (AST; Unit/L) were analyzed using analytical kits (Thermo Fisher Scientific, USA; Catalog # A7528-150, Catalog# A7561-150) on a clinical chemistry analyzer (Thermo Fisher Scientific, USA).
The SOD activity [enzyme commission number: EC.1.15.1.1], was assessed utilizing the SOD ELISA Kit (Pars Biochem; catalog# PRS-02005 hu). SOD activity was determined by measuring the auto-oxidation rates in the presence and absence of the sample, and results were expressed as μmol/L. The activity of catalase (enzyme commission number: EC.1.11.1.6) were determined spectrophotometrically (560nm) using catalase colorimetric activity kit (Thermo fisher scientific, USA; catalog# EIACATC), as per manufacturer instruction. Malondialdehyde (MDA; enzyme commission number: EC. 202-974-4) concentration was determined using ELISA Kit (catalog# PRS - 00991hu). MDA level was measured within the range of 0.3nmol/ml- 7nmol/ml at 450nm.
2.8 Histological analysis
At the end of the bacterial challenge, the intestine, gills, liver, muscles, and kidney were collected from each treatment (n = 5 of each organ), and placed in sterilized tubes containing 3ml of Bouin’s solution (Solarbio, Beijing, China). Following the samples were dehydrated and embedded in paraffin. Sections with a thickness of 5μm were then sliced from each sample and subjected to staining with hematoxylin and eosin according to Humason, [61].
2.9 Statistical analysis
Results were presented as mean ± standard error (S.E). Statistical analysis of the data was performed using one-way analysis of variance (ANOVA) with a significance level set at P< 0.05 to determine significant differences among groups. Based on the normality (Kolmogorov–Smirnov test) and Levene test of homogeneity of variances, variance between means were further analyzed using Duncan Multiple Range Test (DMRT). The parameters which showed significant variance after DMRT test have been mentioned with superscripts. All the Analyses were performed using SPSS version 20 (Armonk, NY: IBM Corp).
3. Results
3.1 Growth
A significant difference (P<0.05) was observed in all growth parameters among the eight treatments (Table 3). These parameters gradually increased in treatments with combined amino acids, compared to treatments supplemented with individual amino acids. All treatments showed a significant increase in all growth parameters compared to the T0 treatment, except for SGR. The SGR between T0, T1, and T2 showed insignificant differences, whereas all other treatments showed significant difference. The T0 treatment showed the lowest total body weight (38.82±0.28 g), and SGR (0.56±0.005%/day). The HSI between T0, T1, T2, and T3 showed an insignificant difference (P>0.05), whereas, T4, T5, T6, and T7 showed a significant increase (P<0.05). The T7 treatment showed the highest value of total body weight (58.21±1.25 g), SGR (1.07±0.005%/day), K (1.37±0.01%), HSI (2.68±0.09%), and VSI (4.85±0.23%). Similarly, the best FCR (0.71±0.004%) was also recorded in fish fed with T7 treatment.
Table 3. Summary of growth parameters in eight treatments at the end of the growth experiment.
Different superscripts across the rows represent the variance between treatments were calculated by Duncan multirange test of one-way ANOVA at P < 0.05.
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| TBW (g) | 38.82±0.28a | 39.52±0.36b | 38.51±0.42c | 38.82±0.27d | 45.27±0.31e | 43.58±0.68f | 45.56±0.31g | 58.21±1.25h |
| TBL (cm) | 12.00±0.12a | 12.78±0.42c | 12.08±0.27b | 12.00±0.22a | 14.31±0.28d | 14.54±0.21e | 14.31±0.33d | 16.21±0.41f |
| SGR (%/day) | 0.56±0.005a | 0.56±0.005a | 0.56±0.005a | 0.61±0.008b | 0.78±0.005d | 0.97±0.005e | 0.71±0.003c | 1.07±0.005f |
| K (%) | 2.25±0.005f | 2.25±0.005f | 2.17±0.008e | 1.89±0.01d | 1.55±0.005c | 1.54±0.005c | 1.42±0.008b | 1.37±0.005a |
| HSI (%) | 2.05±0.02a | 2.05±0.06a | 2.05±0.02a | 2.05±0.06a | 2.53±0.06c | 2.53±0.06c | 2.41±0.11b | 2.68±0.09d |
| VSI (%) | 2.48±0.04b | 2.48±0.35b | 2.48±0.04b | 2.41±0.06a | 4.01±0.14d | 4.01±0.14d | 3.65±0.07c | 4.85±0.23e |
| FCR | 1.31±0.005g | 1.31±0.005g | 1.23±0.008e | 1.29±0.008f | 0.99±0.005c | 1.03±0.005d | 0.95±0.01b | 0.71±0.004a |
T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine). TBW- total body weight, TBL- total body length, SGR- specific growth rate, K- condition factor, HSI-hepatosomatic index, VSI- viscerosomatic index, FCR- feed conversion ratio
3.2 Chemical composition and amino acid profile of muscles
Chemical composition (moisture content, crude protein, crude fat and crude ash) showed a significant difference (P<0.05) among all treatments at the end of the growth experiment (Table 4). The crude fat was significantly decreased among treatments supplemented with amino acids (P<0.05). The highest moisture content was observed in the T0 treatment compared to other treatments. The lowest crude ash was observed in T2 and T7 treatments compared to the other treatments. The crude protein in T0 treatment was the lowest (16.76±0.19), compared with other treatments. The highest concentration of crude protein (22.75±0.01%) was observed in T7 treatment. Results showed a significant difference (P<0.05) between essential amino acids (EAA) especially lysine, tryptophan, and methionine mostly among all treatments (Table 5). Treatments without amino acids (T0) had a significantly (P<0.05) lower concentration of essential amino acids (EAAs) and non-essential amino acids (NEAA) concentrations compared to other treatments (Table 5). The methionine (0.66±0.04%), lysine (2.46±0.08%), and tryptophan (0.53±0.04%) in the T0 treatment were significantly lower (P<0.05) compared to the other treatments. The significantly highest amino acids concentrations, methionine (0.99±0.02%), lysine (4.14±0.72%), and tryptophan (0.98±0.02%) were observed in T7 treatment. The amino acids arginine, alanine, and carnitine showed insignificant (P>0.05) differences between the T0, T1, and T2 treatment compared with others (Table 5).
Table 4. Chemical composition of muscles in eight treatments at the end of the growth experiment.
Different superscripts across the rows represent the variance between treatments were calculated by Duncan multirange test of one-way ANOVA at P < 0.05.
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| Moisture (%) | 70.21±0.93g | 68.21±0.57e | 69.00±0.98c | 64.32±0.93b | 67.21±0.57c | 66.16±0.83b | 63.01±0.73a | 69.01±0.98f |
| Crude Protein (%) | 16.76±0.19a | 17.45±0.15b | 17.45±0.15b | 18.37±0.03c | 19.25±0.01d | 20.12±0.08e | 21.86±0.02f | 22.75±0.01g |
| Crude Fat (%) | 8.26±0.01d | 9.45±0.11f | 8.51±0.14e | 8.52±0.14e | 7.47±0.07b | 7.12±0.34a | 7.75±0.007c | 7.75±0.007c |
| Crude Ash (%) | 4.43±0.16c | 4.65±0.02d | 4.21±0.02a | 5.57±0.15f | 5.96±0.01h | 5.86±0.01g | 4.92±0.01e | 4.35±0.02b |
(T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine).
Table 5. Essential amino acids (EAA) and non-essential amino acids (NEAA) contents from fish muscles from eight treatments at the end of the growth experiment; expressed as %.
Different superscripts across the rows represent the variance between treatments were calculated by Duncan multirange test of one-way ANOVA at P < 0.05.
| EAA | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| Methionine | 0.34±0.04a | 0.42±0.04 c | 0.41±0.04b | 0.97±0.04f | 0.99±0.02f | 0.69±0.02d | 0.78±0.05e | 0.99±0.02g |
| Tryptophan | 0.53±0.04a | 0.68±0.04d | 0.58±0.01b | 0.59±0.02bc | 0.79± 0.01f | 0.85± 0.03g | 0.59±0.01c | 0.98±0.02h |
| Valine | 1.97±1.06a | 2.20± 0.48b | 2.36±0.07c | 2.50±0.11e | 2.40±0.81d | 2.71±0.27e | 2.81±0.69f | 3.20±0.58g |
| Isoleucine | 3.60±0.05a | 3.93± 0.05b | 4.92±0.08c | 5.20±0.67d | 5.70±0.38f | 5.90±0.03g | 5.50±0.01e | 9.80±3.97h |
| Leucine | 0.36±0.05a | 0.65±0.07e | 0.57±0.01d | 0.65±0.06e | 0.78±0.04f | 0.42±0.01b | 0.52±0.02c | 1.16±0.04f |
| Phenylalanine | 1.12±0.04a | 1.43±0.04b | 1.65±0.05c | 1.46±0.03b | 1.88±0.04d | 2.26±0.01f | 2.02±0.05e | 2.33±0.06g |
| Histidine | 0.19±0.05c | 0.12±0.05a | 0.13±0.02b | 0.13±0.01b | 0.25±0.05d | 0.26±0.04e | 0.28±0.04f | 0.45±0.04g |
| Lysine | 2.46±0.08a | 2.47±0.01b | 3.48±0.51d | 2.62±0.75c | 2.72±0.41d | 3.74±0.55e | 3.99±0.02f | 4.14±0.72g |
| Arginine | 0.97±0.04bc | 0.99±0.01b | 0.99±0.02b | 1.03±0.04c | 1.03±0.04c | 1.12±0.37d | 1.16±0.04e | 1.19±0.04f |
| Ornithine | 1.22±0.71a | 1.32±0.71b | 2.70±0.58d | 1.52±0.67c | 2.80±0.67e | 3.30±0.97h | 3.21±0.61g | 2.92±1.85f |
| TEAA | 13.08 | 14.47 | 18.05 | 16.67 | 19.34 | 18.25 | 17.97 | 27.16 |
| NEAA | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| Cysteine | 0.09±0.01a | 0.13±0.01b | 0.17±0.01d | 0.39±0.03e | 0.15±0.01c | 0.17±0.04d | 0.47±0.03f | 0.59±0.02g |
| Aspartic Acid | 4.60±0.58a | 4.80±0.05b | 5.21±0.03e | 5.01±0.03d | 5.42±0.02f | 5.60±0.08g | 4.90±0.19c | 5.72±0.04h |
| Asparagine | 7.60±0.03a | 8.90±0.01c | 9.22±0.07d | 9.22±0.12d | 8.77±0.04b | 9.62±0.04c | 9.92±0.05d | 10.02±0.06e |
| Serine | 4.23±0.39a | 4.90±0.59d | 4.60±0.05c | 4.42±0.03b | 5.12±0.04e | 5.30±0.04f | 5.82±0.03g | 6.40±0.02h |
| Glutamine | 2.80±0.59a | 3.10±0.01b | 2.92±0.59ab | 4.52±0.81c | 5.16±0.61d | 5.60±0.81de | 6.63±0.58e | 8.42±0.64f |
| Glycine | 5.62±0.01a | 6.87±0.01b | 7.33±0.62d | 7.19±0.64c | 7.56±0.03e | 7.35±0.07d | 7.48±0.04 de | 8.62±1.01f |
| Alanine | 2.80±0.21a | 2.85±0.03a | 2.97±0.03b | 3.20±0.03c | 6.30±0.05e | 6.22±0.05e | 6.95±0.03d | 7.80±0.05f |
| Proline | 6.92±0.01a | 7.07±0.04b | 7.24±0.03cd | 7.15±0.05bc | 7.35±0.05d | 7.41±0.57d | 7.40±0.58d | 7.64±0.39e |
| Tyrosine | 0.25±0.07b | 0.30±0.09c | 0.69±0.03a | 0.29±0.01c | 0.35±0.04d | 0.23±0.01e | 0.44±0.05f | 0.57±0.06g |
| Ornithine | 1.22±0.71a | 1.21±0.58a | 1.62±0.67b | 1.90±0.67d | 1.90±0.9d | 1.60±0.41b | 1.82±1.85c | 2.01±0.61e |
| TNEAA | 36.13 | 39.04 | 41.97 | 43.29 | 48.08 | 49.1 | 51.83 | 57.79 |
(T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine). TEAA: total essential amino acids, TNEAA: total non-essential amino acids
3.3 Digestive enzymes assay
Dietary supplementation of amino acids significantly (P<0.05) increased the levels of amylase, lipase, and protease in the intestine (Table 6). The T0 treatment showed the lowest protease (35.01±0.57 unit/L), lipase (136.00± 0.57 unit/L), and amylase (20242.01±0.88 unit/L) at the end of the growth experiment. The protease, lipase, and amylase level were significantly (P<0.05) higher in T4, T5, and T6 treatments compared to T1, T2, and T3 treatments. The highest level of digestive enzymes was observed in T7 treatment. The T7 treatment showed the highest protease (55.21±0.57 unit/L), lipase (182.12±1.15 unit/L), and amylase (40411.03±0.66 unit/L) at the end of growth experiment.
Table 6. Determination of digestive enzymes in fish intestine samples from all eight treatments at the end of the growth experiment.
Different superscripts across the rows represent the variance between treatments were calculated by Duncan multirange test of one-way ANOVA at P < 0.05.
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
|---|---|---|---|---|---|---|---|---|
| Amylase (Unit/L) | 20242.01±0.88b | 20241.23±0.57a | 20251.51±0.57c | 20261.11±0.57d | 37961.00±0.57g | 37952±0.57f | 36972.41±0.57e | 40411.03±0.66h |
| Lipase (Unit/L) | 136.00± 0.57a | 142.04±1.15c | 142.05±1.15c | 142.00±0.88b | 161.21± 0.57e | 146.13±1.15d | 172.12± 0.57f | 182.12±1.15g |
| Protease (Unit/L) | 35.01±0.57a | 39.34±0.57c | 45.41±0.57d | 37.23±0.57b | 53.03±0.57g | 49.06±0.57f | 47.01± 0.57e | 55.21±0.57h |
(T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine).
3.4 Hematology, blood biochemistry and antioxidant enzymes assay
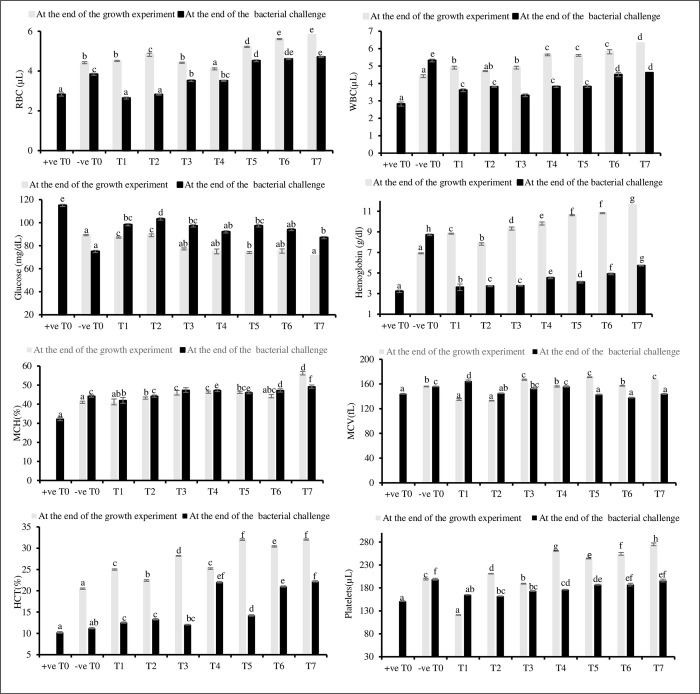
Most of the hematological parameters showed a significant difference (P<0.05) between the eight treatments both at the end of the growth experiment and after the bacterial challenge. Hematological parameters exhibited a similar pattern between treatments except that glucose gradually decreased and showed a significant difference (P<0.05) among T5, T6 and T7 treatments whereas, T0, T1, and T2 showed insignificant difference (P>0.05) (Fig 1). At the end of growth experiment, the hemoglobin (6.91±0.08 g/dl), white blood cells (4.42±0.12 μL), and hematocrit (20.51±0.08%) in theT0 treatment were observed to be the lowest compared to other treatments. All the hematological parameters were significantly (P<0.05) higher in the T7 treatment, except for glucose (71.23±1.85 mg/dL), at the end of the growth experiment. The treatments T4, T5, and T6, which were supplemented with synergistic amino acids, showed significantly higher hematological parameters compared to single or non-amino acid supplemented treatments (T1, T2, T3, and T0 treatment).
Fig 1. Analysis of hematological parameters in eight treatments at the end of the growth experiment and after bacterial challenge.
Different superscripts represent the variance between treatments were calculated by Duncan multirange test of one-way ANOVA at P < 0.05. different treatments are as follows: T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine. Hemoglobin (Hb), white blood cells (WBC), red blood cells (RBC), mean corpuscle volume (MCV), haematocrit (HCT), mean corpuscular hemoglobin (MCH), μL–microliter, fL- femtoliter.
The glucose level after bacterial challenge was observed to be significantly (P<0.05) highest in the +ve T0 treatment. The glucose level of the -ve T0 treatment after the bacterial challenge was observed to be significantly lowest (75.00±0.88 mg/dL), compared to other treatments. All the hematological parameters in the +ve T0 after bacterial challenge were observed to be significantly lowest. The T4, T5, and T6 treatments showed a significantly better response to bacteria compared to the T1, T2, and T3 treatments. All the hematological parameters in T7 treatment were significantly (P<0.05) higher after bacterial challenge compared to other treatments (Fig 1).
The blood biochemistry parameters in treatments fed combined amino acids demonstrated a notable increase, exhibiting a statistically significant difference (P<0.05) compared to treatments fed with individual and zero amino acids. The levels of triglycerides, ALT, and AST significantly lowered in all treatments compared to +ve T0 (Table 7). At the end of the growth experiment, the neutrophil count (35.21±0.57 μ/L), lymphocytes (65.21±2.61 μ/L), monocytes (3.31±0.05), and eosinophils (2.62±0.05 μ/L) were observed to be significantly lower (P<0.05) in the T0 treatment, compared to the other treatments. The levels of triglycerides (281.00±1.33 mg/dl), ALT (39.21±0.57 unit/L), and AST (30.41±0.57 unit/L) were significantly higher in the T0 treatment compared with other treatments at the end of the growth experiment. The highest levels of lymphocytes (79.02±0.57), monocytes (5.32±0.05), and eosinophils (4.54±0.05) were observed high in T7 treatment at the end of the growth experiment.
Table 7. Analysis of biochemical parameters of blood in eight treatments eight treatments at the end of the growth experiment and after the bacterial challenge.
Different superscripts across the rows represent the variance between treatments were calculated by Duncan multirange test of one-way ANOVA at P < 0.05.
| At the end of the growth experiment | |||||||||
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
| Neutrophils (μ/L) | 35.21±0.57a | 35.21±0.57b | 37.23±0.57c | 40.02± 0.57d | 46.00±0.57f | 45.02±0.57e | 46.00±0.57f | 47.00±0.57g | |
| Lymphocytes (μ/L) | 65.21±2.61a | 70.21±0.88c | 67.21±0.57b | 72.00±1.51e | 72.23±0.88f | 71.21±0.66d | 75.21±0.57g | 79.02±0.57h | |
| Monocytes (μ/L) | 3.31±0.05a | 3.32±0.33b | 3.52±0.33e | 3.32±0.33b | 4.81±0.05d | 5.32±0.05g | 5.21±0.05f | 5.32±0.05g | |
| Eosinophils (μ/L) | 2.62±0.05a | 2.82±0.05e | 3.12±0.57f | 2.63±0.05b | 2.72±0.33d | 2.71±0.05c | 3.52±0.05g | 4.54±0.05h | |
| Triglycerides(mg/dl) | 281.00±1.33f | 278.00±0.57e | 255.03±0.57d | 199.01±0.57b | 220.21±0.57c | 219.21±0.57c | 189.00±0.57a | 188.21±0.57a | |
| ALT (Unit/L) | 39.21±0.57g | 38.32±0.57f | 34.41±0.57e | 34.41±0.57e | 30.03±0.5bd | 29.32±0.57c | 29.02±0.57b | 24.03±0.57a | |
| AST (Unit/L) | 30.41±0.57f | 31.03±0.57g | 30.03±0.57 e | 23.04±0.88d | 21.32±0.66c | 23.02±0.57d | 20.32±0.57b | 18.54±0.57a | |
| Total Protein (g/dl) | 6.31±0.15b | 6.31±0.15b | 6.41±0.05c | 6.42±0.05c | 6.10±0.08a | 6.31±0.08b | 6.61±0.05d | 7.41±0.08e | |
| At the end of the bacterial challenge | |||||||||
| Parameters | -ve T0 | +ve T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| Neutrophils (μ/L) | 27.02±0.57i | 19.10±0.57a | 21.21±0.57d | 20.23±0.57b | 21.00±0.57 c | 25.03±0.57h | 24.21±0.57g | 23.23±0.57e | 24.00±0.57f |
| Lymphocytes (μ/L) | 68.32±0.33e | 52.21±1.45a | 69.01±0.57f | 66.23±0.57 c | 63.23±0.57b | 67.23±1.22d | 69.00±0.57f | 66.42±0.57c | 69.23±0.88g |
| Monocytes (μ/L) | 3.31±0.05f | 1.63±0.08a | 1.81±0.05b | 1.81±0.08 b | 1.95±0.03c | 2.31±0.33b | 2.11±0.05ad | 2.11±0.11d | 2.21±0.05e |
| Eosinophils (μ/L) | 2.33±0.33f | 1.82±0.05c | 1.62±0.05b | 1.52±0.08a | 1.83±0.05abc | 2.23±0.05bc | 2.34±0.08e | 2.21±0.05d | 2.43±0.05g |
| Triglycerides(mg/dl) | 303.00±1.52a | 385.00±1.85h | 372.00±1.21f | 374.02±0.57g | 323.03±2.84c | 330.32±0.57d | 340.00±0.57e | 338.00±0.88e | 315.00±2.88b |
| ALT (Unit/L) | 37.32±0.57a | 55.00±0.57g | 42.21±0.88e | 44.34±0.57f | 45.03±0.57d | 38.34±0.57b | 40.21±1.21d | 40.23±1.21d | 40.04±0.57c |
| AST (Unit/L) | 30.05±0.88a | 47.21±0.88i | 40.34±1.21d | 46.34±0.57h | 45.42±0.88g | 41.21±0.57f | 41.02±0.57e | 35.21±0.57b | 37.30±1.21c |
| Total Protein (g/dl) | 5.42±0.08h | 3.21±0.03a | 3.42±0.15b | 4.92±0.03e | 4.42±0.25c | 4.70±0.05d | 5.41±0.08d | 5.14±0.11f | 5.41±0.08g |
(T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine). alanine aminotransferase (ALT), aspartate aminotransferase (AST), μ/L- micro liter
At the end of the bacterial challenge, the +ve T0 treatment showed the highest levels of triglycerides, ALT, and AST. The total protein level of the -ve T0 treatment after the bacterial challenge was observed to be significantly highest (5.42±0.08 g/dL), compared to other treatments. The triglycerides, ALT, AST in the +ve T0 after the bacterial challenge were also observed to be significantly highest (P<0.05). The T4, T5, and T6 treatments exhibited a significantly better response to bacteria compared to the T1, T2, and T3 treatments. Eosinophils, monocytes, lymphocytes, and total protein in the T7 treatment were significantly (P<0.05) higher after bacterial challenge compared to other treatments (Table 7).
Catalase (CAT) showed an insignificant (P>0.05) difference between T0, and T1, whereas, T2, T3, T4, T5, T6, and T7 treatments showed a significant difference (P<0.05). The levels of catalase after the bacterial challenge were significantly different among all treatments. Superoxide dismutase (SOD), and malondialdehyde (MDA) were significantly different (P<0.05) among most of the treatment groups after the growth experiment and bacterial challenge (Table 8). The levels of CAT and SOD increased in response to the bacterial challenge in treatment groups. The highest level of CAT (4.58±0.02 μmol/L) and SOD (1.25±0.01μmol/L) were observed in the T7 group. On the other hand, the concentration of MDA gradually decreased in individual or combined amino acids supplemented groups (+ve T0>-ve T0> T1> T2>T3>T4>T5>T6>T7).
Table 8. Analysis of catalase (CAT), superoxide dismutase (SOD), and malondialdehyde (MDA) from fish blood serum of eight treatments at the end of the growth experiment and after bacterial challenge.
Different superscripts across the rows represent the variance between treatments were applied as a result of one way (Duncan multirange test) at P < 0.05.
| At the end of the growth experiment | |||||||||
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | |
| Catalase (μmol/L) | 0.25±0.01a | 0.25±0.01a | 0.27±0.01c | 0.26±0.01b | 0.29±0.01d | 0.66±0.02e | 1.36±0.01f | 2.78±0.03g | |
| SOD (μmol/L) | 0.13±0.01a | 0.14±0.01b | 0.18±0.01d | 0.17±0.01c | 0.26±0.01e | 0.27±0.01f | 0.38±0.02g | 1.18±0.01h | |
| MDA (μmol/L) | 1.25±0.02g | 0.25±0.01e | 0.72±0.23f | 0.23±0.01d | 0.22±0.01c | 0.15±0.01a | 0.17±0.01b | 0.14±0.01a | |
| At the end of the bacterial challenge | |||||||||
| Parameters | -ve T0 | +ve T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 |
| Catalase (μmol/L) | 0.58±0.01b | 0.15±0.01a | 1.25±0.01c | 2.11±0.01d | 2.13±0.01d | 3.23±0.01g | 2.30±0.01e | 2.54±0.01f | 4.58±0.02h |
| SOD (μmol/L) | 0.14±0.01a | 0.19±0.01b | 0.33±0.01d | 0.31±0.01c | 0.15±0.02a | 0.35±0.02e | 0.43±0.01f | 0.45±0.01g | 1.25±0.01h |
| MDA (μmol/L) | 0.14±0.01a | 1.54±0.01e | 0.32±0.01d | 0.25±0.02c | 0.33±0.01d | 0.24±0.01b | 0.23±0.02b | 0.24±0.01b | 0.14±0.01a |
(T0; control, T1; Tryptophan, T2; Lysine; T3; Methionine: T4; Tryptophan + Methionine, T5; Lysine +Tryptophan, T6; Methionine +Lysine, T7; Lysine + Tryptophan +Methionine), μmol/L: micro moles per liter.
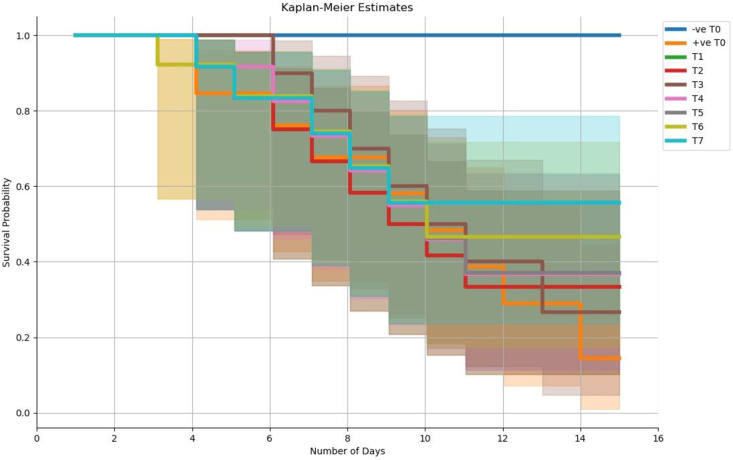
Survival rate after the bacterial challenge was presented through the Kaplan Meier survival curve. The best survival rate was observed in the T7 treatment compared to other treatments. The +ve T0 treatment showed less survival rate and high mortality (Fig 2).
Fig 2. Kaplan-Meier survival curves of striped catfish following infection by immersion challenge with S.
aureus. The results correspond to the survival percentage during 15 days post-infection of three replicates (n = 15/treatment). Kaplan-Meier survival data was analyzed by OriginPro software.
3.5 Histological analysis
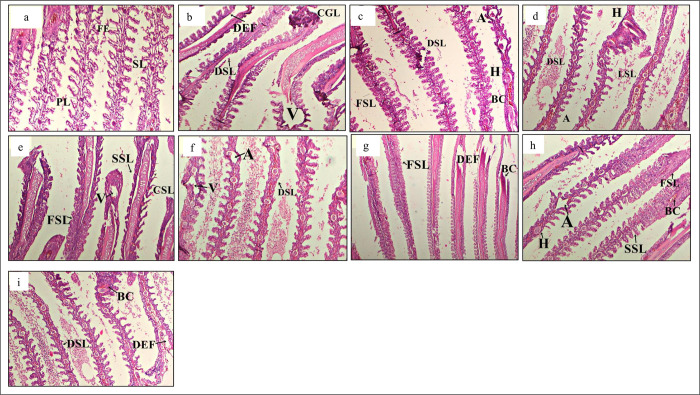
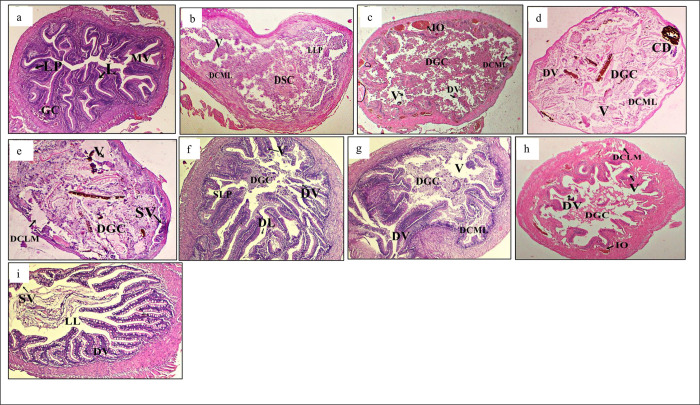
Several histopathological alterations were observed in the gill structure in all treatments (Fig 3A–3I). The histology of gills in the -ve T0 group exhibited the typical epithelial cell lining of lamellae (Fig 3A). In contrast, the groups exposed to S. aureus showed various structural changes, such as hemorrhage, intracellular oedema, disruption of gills with notable hypertrophy, loss of horizontal shaft with mucous membrane cellular proliferation (Fig 3B–3I). The +ve T0 treatment (Fig 3B) showed the highest anomalies and mortality. The gut structure of different treatments showed several pathologies (Fig 4A–4I). Histopathological analysis of the -ve T0 treatment showed a normal or less alterations in goblet cells, villi, and nucleus (Fig 4A). Meanwhile, the other treatments revealed structural anomalies such as excessive hypertrophy, the villi tended to fuse (FV), and the mucosal lining sloughed off, eventually leading to the large lumen (LL) (Fig 4B–4I).
Fig 3. Histological changes in gills.
Light micrographs of a paraffin section stained with eosin (40x). a; gills in -ve T0, b; gills t in +ve T0, c; gills T1, d; gills in T2, e; gills in T3, f; gills in T4, g; gills in T5, h; gills in T6, I; gills in T7. Primary lamellae, SL; Secondary lamellae, FSL; Fusion of secondary lamellae, DSL; Degeneration of secondary lamellae, HT; Hypertrophy, DPL; Degeneration of primary lamellae, TD; Tissue debris, A; Aneurism, BC; Blood congestion, V; vacuolation.
Fig 4. Histological changes in gut.
Light micrographs of a paraffin section stained with eosin (40x). a; gut in -ve T0, b; gut in +ve T0, c; gut in T1, d; gut in T2, e; gut in T3, f; gut in T4, g; gut in T5, h; gut in T6, I; gut in T7. GC; Goblet cells, LP; Laminar propria, N; Nucleus, L; Lumen, CE; Columnar epithelium, FV; Fusion of villi, LL; Large lumen, FLV; Flattened villi, DCML; Damaged circular muscle layer, DL; Distended lumen, DLML; Damaged longitudinal muscle layer, VF; Vacuole formation, SLP; Swelling of lamina propria, CCA; Cracked clay appearance of the tissues, SLML; Swelling of longitudinal muscle layer, DGC; Damaged goblet cells, DMM; Disarrangement of muscularis mucosa.
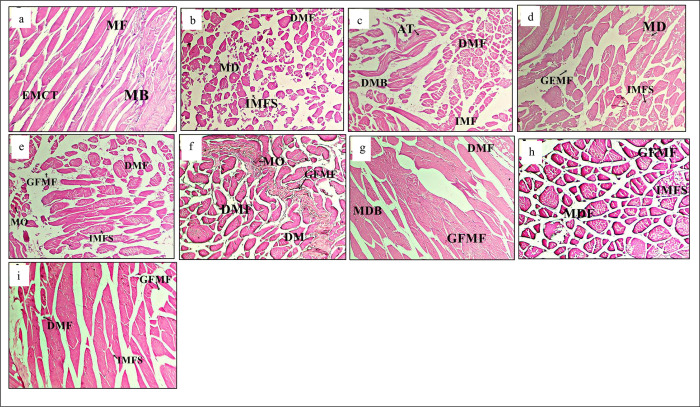
Several anomalies were observed in muscles structures of the eight treatments after the bacterial challenge (Fig 5A–5I). Muscle structures of the -ve T0 treatment showed less or no abnormalities (Fig 5A) as compared to other treatments. Whereas, different treatments showed structural changes like, muscle fibers degeneration, vacuole destabilization in muscle bundles and the increased inter myofibrillar space (IMFS) (Fig 5B–5I). The highest pathological alterations were observed in the muscles of +ve T0 treatment (Fig 5B). Liver in different treatments showed significant abnormalities (Fig 6A–6I). Treatment bathed with phosphate buffer saline and fed with zero amino acid supplement (-ve T0) showed normal hepatocytes, endothelium, and serous membrane that contained blood vessels (Fig 6A). On the other hand, different treatments showed pathologies such as necrosis, multinucleated nucleolus, oedema, hemosiderin, hematoma, intravenous tissue necrosis, edematous fluid intrusions (Fig 6B–6I).
Fig 5.
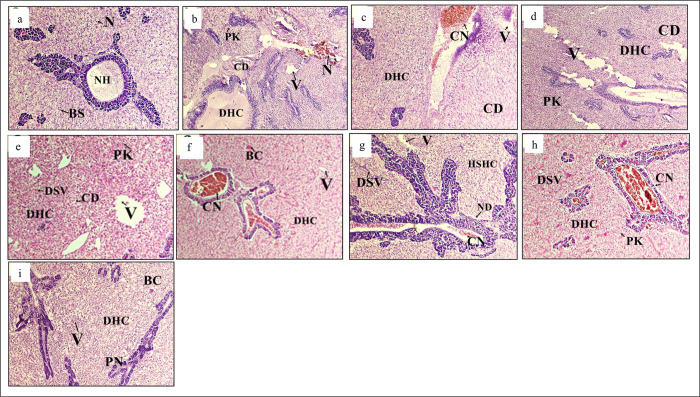
Fig 6. Histological changes in liver.
Light micrographs of a paraffin section stained with eosin (40x). a; liver in -ve T0, b; liver in +ve T0, c; liver in T1, d; liver in T2, e; liver in T3, f; liver in T4, g; liver in T5, h; liver in T6, I; liver in T7. NH; Normal hepatocytes, GC; Granular cytoplasm, BC; Blood congestion HD; Hepatocyte generation, CSN; Central spheroidal hepatocyte nucleus, N; Cell necrosis, PN; Pyknotic nuclei, CD; Cytoplasmic degeneration, IEF; Infiltration of oedematous fluid, rCV; Rupturing of the central vein, V; Vacuolization of hepatocytes. DHC; degeneration of hepatocytes.
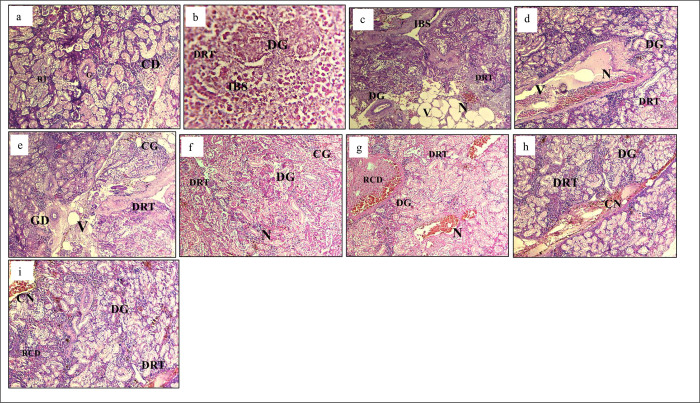
Kidney structure of eight treatments exhibited anomalies. Less or no structural abnormalities were observed in kidney structure of -ve T0 treatment (Fig 7A). However, the +ve T0 treatment displayed the highest structural abnormalities among all other treatments (Fig 7B). Severe structural changes among treatments were observed (T1>T2>T3>T4>T5>T6>T7) (Fig 7C–7I).
Fig 7. Histological changes in kidney.
Light micrographs of a paraffin section stained with eosin (40x). a; kidney in -ve T0, b; kidney in +ve T0, c; kidney in T1, d; kidney in T2, e; kidney in T3, f; kidney in T4, g; kidney in T5, h; kidney in T6, I; kidney in T7. G; Glomerulus, CD; Collecting duct, DG; Degenerative glomerulus, IBS; Increased bowman space, FRT; Fusion of renal tubule, DRT; Degenerative renal tubule, CG; Congestion of glomerulus, N; Necrosis, H; Hemorrhage, A; Atrophy.
4. Discussion
The present study showed that striped catfish fed a diet without methionine, lysine, and tryptophan supplementation exhibited poor growth. Total body weight, hepatosomatic index, and specific growth rate were lower in the T0 treatment. However, these parameters gradually increased in dietary treatments with combined amino acids as compared to individual amino acid supplementation. The highest growth rate probably accounts for better feed utilization efficiency, a faster absorption rate as well as greater availability of free methionine, lysine, and tryptophan in tissues to be utilized [62]. Present findings were similar to the experimental results in Chinese sucker (Myxocyprinus asiaticus) [63], yellowtail (Seriola quinqueradiata) [64], common carp [65], large yellow croaker (Pseudosciaena crocea) [66], rohu [67], Atlantic salmon (Salmo salar) [68], juvenile hybrid striped bass (Morone saxatilis) [69], and juvenile Jian carp (Cyprinus carpio) [70].
Fish growth is positively associated with the accretion of protein, fat, and other nutrients [5]. The present study demonstrates a significant decrease (P<0.05) in crude lipid, and an increase in crude protein in most treatments compared with those fed with the T0 diet. The highest protein level was observed in fish fed with the T7 diet (22.75±0.01%), which may be due to high activation of the target of rapamycin (TOR) signaling pathway. The TOR signaling pathway is involved in improving protein synthesis and growth in fish [71]. These results coincide with other fish species, including silver perch [72], yellow crocker [73], and silver pompano [74]. The growth of fish is also intricately linked to their digestive and absorptive capabilities, which, in turn, depends upon the performance of digestive and brush border enzymes [75]. At the end of the present study, the activities of intestinal amylase, trypsin, and lipase exhibited a decline in fish fed a diet without methionine supplementation (T0), while they displayed an increase in fish that were fed diets containing optimal levels of methionine, lysine, and tryptophan.
In the present research, the comprehensive hematological profile following the growth experiment and the bacterial challenge underwent significant modification as a result of either tryptophan, or methionine or lysine supplementation. Furthermore, a notably higher number of white blood cells (WBC) was noted in the all treatments compared with T0. This augmentation in WBC count aligns with previous findings in European seabass [76], where the provision of varying levels of methionine hydroxy analogue led to increased survival rates and enhanced humoral and cellular responses after injection of Aeromonas hydrophila [77]. Tryptophan and methionine have been substantiated to play pivotal roles in bolstering the immune response, while concurrently demonstrating the ability to modulate metabolic pathways implicated in enhancing the efficacy of the immune system [78].
In fish, intestinal health has been correlated with the intestinal physical barrier, which mainly consists of intestinal epithelial cells and tight junction proteins (such as claudins, occludin and ZO-1) [79]. Intestinal inflammation is accompanied by the excessive production of reactive oxygen species (ROS), which causes lipid peroxidation and protein oxidation damage in intestinal epithelial cells [70]. Lipid peroxidation and protein oxidation in tissues can be reflected by the MDA [80]. In this study, dietary supplementation of amino acids, either individual or combined, showed a significant decrease in the serum MDA contents of striped catfish, suggesting that an appropriate amino acid level might have inhibited lipid peroxidation and protein oxidation in the fish intestine [81]. The possible inhibition of oxidative damage is considered closely related to the improvement in non-enzymatic and enzymatic antioxidant capacities in fish [82]. Previous studies indicated that nutrients, such as histidine [83, 84], and lysine, increased the glutathione (GSH) content in the intestine of fish. Additionally, SOD and GPx are major antioxidant enzymes in fish [85]. In present study, the activities of SOD and CAT in the serum were the highest in the appropriately supplemented diets, suggesting that amino acids could improve the enzymatic antioxidant capacity in fish.
The histological alterations during the bacterial challenge test correlated with haemato-biochemical and antioxidant enzyme data [86]. This study elucidates notable variations in various tissues, including muscles, gills, kidneys, liver, and gut. The greatest tissue damage was observed in the +ve T0 treatment. The gills are particularly susceptible to waterborne pathogens due to their perpetual exposure to the external environment [87, 88]. In +ve T0 treatment, the gills displayed a significant prevalence of histological abnormalities when compared to other treatments. This result in erythrocytes congestion within the marginal channel [89]. In contrast, the liver histology of amino acid treated groups showed characteristics reminiscent of those found in negative control group (-ve T0). The liver’s impaired ability to efficiently remove foreign particles results in the degeneration of hepatocytes and congestion within sinusoid’s [90]. The presence of extracellular toxin generated by S. aureus might be the underlying factor responsible for the formation of lipid vacuoles and the occurrence of necrosis in the liver. Comparable hepatic irregularities, including the infiltration of lymphocytes, focal necrosis and the presence of cytoplasmic fat vacuoles, have been similarly observed in carp [91].
Fish exposed to S. aureus, kidney tissues displayed severe necrosis and observable changes in the glomeruli. Notably, the glomerular epithelium in the kidney of catfish afflicted by S. aureus exhibited noticeable histological alterations [92]. A pronounced elevation in the height of intestinal villi and reduction in adverse effects of S. aureus within the dietary groups might be due to action of amino acids inhabiting the intestine, causing consequent reduction in pH and inhibiting fermenting indigestible carbohydrates. Histopathological results support and confirm our examined hematological parameters and consistent with previous findings of pathological examination of S. aureus.
5. Conclusion
The present results demonstrated that dietary supplementation of methionine, lysine, and tryptophan in striped catfish significantly improved growth performance and enhanced digestive and absorptive function. Additionally, amino acid supplementation provided protection to the hepatopancreas and intestine from lipid peroxidation and protein oxidation by enhancing enzymatic antioxidant capacity (SOD, Catalase and MDA activities). Based on observed weight gain, intestinal trypsin and hepatopancreatic anti-hydroxy radical activities, the dietary requirement for methionine, lysine, and tryptophan in catfish is suggested to be 5g/kg. The most favorable outcomes in striped catfish were observed in fish supplemented with the combined three amino acids in the diet (T7). Further studies are needed to elucidate the specific molecular mechanisms through which these amino acids mediate antioxidant defense in fish.
Supporting information
(XLSX)
Acknowledgments
The authors acknowledge R.S.N Janjua (SoyPak, Pvt. Ltd.) for his support in provision of fish and technical support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Agriculture Organization of the United Nations. Fisheries Department. The State of World Fisheries and Aquaculture, 2000. Food & Agriculture Org.; 2000. [Google Scholar]
- 2.FAO. Food and Agriculture Organization. The State of World Fisheries and Aquaculture 2022. FAO, Rome. doi: 10.4060/cc0461en [DOI] [Google Scholar]
- 3.Singh AK, Lakra WS. Culture of Pangasianodon hypophthalmus into India: Impacts and present scenario. Pak J Biol Sci. 2012; 15(1): 19–26. doi: 10.3923/pjbs.2012 [DOI] [PubMed] [Google Scholar]
- 4.Mubarak AS, Tias DT, Sulmartiwi L. Pemberian dolomit pada kultur Daphnia spp. Sistem daily feeding pada populasi Daphnia spp. dan kestabilan kualitas air. J Ilm Peri dan Kel. 2009;1(1):67–72. doi: 10.20473/jipk.v1i1.11700 [DOI] [Google Scholar]
- 5.Aristasari E, Nur‘Aini RA, Nopita W, Lamid M, Al-Arif MA. The growth, protein content, and fatty acid of catfish meat (pangasius sp.) With the addition of different lysine doses in commercial feed. In IOP Conference Series: Eart Environ Sci. 2020; 441.1: 012018. doi: 10.1088/1755-1315/441/1/012018 [DOI] [Google Scholar]
- 6.Gatlin III DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, et al. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aqua res. 2007;38(6):551–79. [Google Scholar]
- 7.Tacon AG, Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aqua. 2008;285(1–4):146–58. [Google Scholar]
- 8.Arsad SC, Stavrakakis V, Turpin P, Rossa Y, Risjani LA, Sari FS, et al. IOP Conference Series: Eart Environ Sci. 2019; 236, 012044 [Google Scholar]
- 9.Wilson RP. Amino acid requirements of finfish and crustaceans. InAmino acids in animal nutrition 2003; 427–447. Wallingford UK: CABI Publishing. [Google Scholar]
- 10.Small BC, Soares JH. 2000. Quantitative dietary requirement of juvenile striped bass. Aquacult. Nutr 6:207–212. [Google Scholar]
- 11.Khan MA. Dietary l‐lysine requirement of fingerling stinging catfish, Heteropneustes fossilis (B loch) for optimizing growth, feed conversion, protein and lysine deposition. Aqua Res. 2013;44(4):523–33. [Google Scholar]
- 12.Mohanty B, Mahanty A, Ganguly S, Sankar TV, Chakraborty K, Rangasamy A, et al. Amino acid compositions of 27 food fishes and their importance in clinical nutrition. J amino acid. 2014. doi: 10.1155/2014/269797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain B, Mahboob S, Hassan M, Liaqat F, Sultana T, Tariq H. Comparative analysis of proximate composition of head from wild and farmed Catla catla. J Anim Plant Sci. 2011;21(2):207–10. [Google Scholar]
- 14.Lu J, Hua Y, Fu WZ, Zhou F, Yang BB, Xiao JX, et al. Effects of supplementation coated lysine and methionine in mixture protein diets on growth performance, digestibility and serum biochemical indices of juvenile black sea bream, Acanthopagrus schlegelii. Tur J Fish Aqua Sci. 2014;14(3):633–42. [Google Scholar]
- 15.Danuwat P, Rimruthai P, Phattanawan C, Peerarat D. Determination of essential amino acids in Pangasius bocourti. J Food Pro Tech. 2016;7(2). [Google Scholar]
- 16.Ozorio ROA, Verreth JAJ, Aragao CR, Vermeulen CJ, Schrama JW, Verstegen MWA, et al. Dietary carnitine supplements increased lipids metabolism and decreased protein oxidation in African catfish. J Aqua Trop.2003; 18:225–238. [Google Scholar]
- 17.Chiu YN, Austic LE, Rumsey GL. 1988. Effect of feeding level and dietary electrolyte on the arginine of rainbow trout. Aqua.1988; 69:79–91. [Google Scholar]
- 18.Li P, Mai K, Trushenski J, Wu G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aqua feeds. J Amino Acid. 2009; 37:43–53. [DOI] [PubMed] [Google Scholar]
- 19.Sveier H, Raae AJ, Lied E. Growth and protein turnover in Atlantic salmon (Salmo salar L.); the effect of dietary protein level and protein particle size. Aqua. 2000; 185:101–120. [Google Scholar]
- 20.Furuya WM, Furuya VRB. Nutritional innovations on amino acids supplementation in Nile tilapia diets. Revista Brasileira de Zootecnia. 2010; 39:88–94. [Google Scholar]
- 21.Hamid SNIN, Abdullah MF, Zakaria Z, Yusof SJHM, Abdullah R. Formulation of Fish Feed with Optimum Protein-bound Lysine for African Catfish (Clarias Gariepinus) Fingerlings, Proced Engineer. 2016; 148:361–369. [Google Scholar]
- 22.Valente LMP, Moutou KA, Conceição LEC, Engrola S, Fernandes JMO, Johnston IA. What determines growth potential and juvenile quality of farmed fish species. Rev Aqua. 2013; 5:168–193. [Google Scholar]
- 23.Huang D, Liang H, Ge X, Zhu J, Li S, Wang Y, et al. Effects of dietary lysine levels on growth performance and glycolipid metabolism via the AKT/FoxO1 pathway in juvenile largemouth bass, Micropterus salmoides. Aqua Nutr. 2022;1–5. [Google Scholar]
- 24.Wilson RP. Amino acid and protein, fish nutrition, 3rd ed. Academic press, London. 2002; 162–164. [Google Scholar]
- 25.Ketolea HG. Requirement for dietary lysine and arginine by fry of rainbow trout. J Ani Sci; 1983, 56:101–107. [DOI] [PubMed] [Google Scholar]
- 26.University of Maryland—UNM (2006) Lysine. Edu. Maryland. Available at: https://umd.edu/. Accessed on 25 July 2006. [Google Scholar]
- 27.Chu ZJ, Gong Y, Lin YC, Yuan YC, Cai WJ, Gong SY, et al. Optimal dietary methionine requirement of juvenile Chinese sucker, Myxocyprinus asiaticus. Aqua Nutr. 2014;20(3):253–64. [Google Scholar]
- 28.Nunes AJ, Sá MV, Browdy CL, Vazquez-Anon M. Practical supplementation of shrimp and fish feeds with crystalline amino acids. Aqua. 2014; 431:20–7. [Google Scholar]
- 29.Harpaz Sheenan. L-Carnitine and Its Attributed Functions in Fish Culture and Nutrition. Aqua. 2005;3–21. doi: 10.1016/j.aquaculture.2005.04.007 [DOI] [Google Scholar]
- 30.Alissianto YR, Sandriani ZA, Rahardja BS. The effect of amino acid lysine and methionine addition on feed toward the growth and retention on mud crab (Scylla serrata). In IOP Conference Series: Eart Environ Sci. 2018; 137(1): 012059. [Google Scholar]
- 31.Cai Y, Burtle GJ. Methionine requirement of channel catfish fed soybean meal-corn-based diets. J Ani Sci. 1996;74(3):514–21. doi: 10.2527/1996.743514x [DOI] [PubMed] [Google Scholar]
- 32.Miao S, Chang E, Han B, Zhang X, Liu X, Zhou Z, et al. Dietary tryptophan requirement of northern snakehead, Channa argus (Cantor, 1842). Aqua. 2021; 542:736904. doi: 10.1016/j.aquaculture.2021.736904 [DOI] [Google Scholar]
- 33.Prabu E, Felix N, Uma A, Ahilan B, Antony C. Metabolic responses of juvenile GIFT strain of Nile tilapia (Oreochromis niloticus) to dietary L‐tryptophan supplementation. Aqua Nutr. 2020;26(5):1713–23. doi: 10.1111/anu.13122 [DOI] [Google Scholar]
- 34.Elango R. Methionine nutrition and metabolism: insights from animal studies to inform human nutrition. 2020. J Nutr. 150; 2518–2523. doi: 10.1093/jn/nxaa155 [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Yang P, He C, Chi S, Li S, Mai K, et al. Effects of dietary methionine on growth performance and metabolism through modulating nutrient-related pathways in largemouth bass (Micropterus salmoides). Aqua Rep. 2021; 20:100642. [Google Scholar]
- 36.Tang L, Wang GX, Jiang J, Feng L, Yang L, Li SH, et al. Effect of methionine on intestinal enzymes activities, microflora and humoral immune of juvenile Jian carp (Cyprinus carpio var. Jian). Aqua Nutr. 2009;15(5):477–83. [Google Scholar]
- 37.Poston HA. Response of rainbow trout to source and level of supplemental dietary methionine. Comparative Biochemistry and Physiology—Part A. 1986; 83:739–744 [Google Scholar]
- 38.Goff JB, Gatlin III DM. Evaluation of different sulfur amino acid compounds in the diet of red drum, Sciaenops ocellatus, and sparing value of cystine for methionine. Aqua. 2004;241(1–4):465–77. [Google Scholar]
- 39.Takagi S, Shimeno S, Hosokawa H, Ukawa M. Effect of lysine and methionine supplementation to a soy protein concentrate diet for red sea bream Pagrus major. Fish Sci. 2001;67(6):1088–96. [Google Scholar]
- 40.Wen H, Feng L, Jiang W, Liu Y, Jiang J, Li S, et al. Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2014;40(1):275–87. [DOI] [PubMed] [Google Scholar]
- 41.Viola S, Lahav E. Effects of lysine supplementation in practical carp feeds on total protein sparing and reduction of pollution. Isra J Aqua. 1991;43(3):112–8. [Google Scholar]
- 42.Robinson EH, Li MH. Use of plant proteins in catfish feeds: replacement of soybean meal with cottonseed meal and replacement of fish meal with soybean meal and cottonseed meal. J World Aqua Soci. 1994;25(2):271–6. [Google Scholar]
- 43.Fu Y, Liang X, Li D, Gao H, Wang Y, Li W, et al. Effect of dietary tryptophan on growth, intestinal microbiota, and intestinal gene expression in an improved triploid crucian carp. Front Nutr. 2021; 8:676035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purwaningsih S. Aktivitas Antioksidan dan Komposisi Kimia Keong Matah Merah (Cerithidea obtusa) (Antioxidant Activity and Nutrient Composition of Matah Merah Snail (Cerithidea obtusa). Ilmu Kelautan: Indo J Mar Sci. 2012;17(1):39–48. doi: 10.1088/1755-1315/441/1/012018 [DOI] [Google Scholar]
- 45.Grimble RF, Grimble GK. Immunonutrition: role of sulfur amino acids, related amino acids, and polyamines. Nutr. 1998;14(7–8):605–10.doi: 10.1016/s0899-9007(98)80041-5 [DOI] [PubMed] [Google Scholar]
- 46.El-Husseiny O, El-Haroun ER, Goda A, Hassan I, Woodward B, et al. Effects of lysine and tryptophan supplementations in plant protein-based diets on the performance of Nile tilapia (Oreochromis niloticus). J Appl Aqua. 2017;29(3–4):266–76. [Google Scholar]
- 47.Zhou XQ, Zhao CR, Jiang J, Feng L, Liu Y. Dietary lysine requirement of juvenile Jian carp (Cyprinus carpio var. Jian). Aqua Nut. 2008;14(5):381–6. [Google Scholar]
- 48.Fatma Abidi S, Khan MA. Growth, protein retention, and body composition of fingerling Indian major carp, rohu, Labeo rohita (Hamilton), fed diets with various levels of lysine. J World Aqua Soci. 2010;41(5):791–9. [Google Scholar]
- 49.Marchão RS, Copatti CE, Ribeiro FB, Bomfim MA, de Lima MS, Batista VF, et al. Evaluation of dietary tryptophan requirement on growth, whole-body composition, and hematobiochemical parameters of tambaqui (Colossoma macropomum) in the fattening phase. Aqua Intern. 2023;1–20. [Google Scholar]
- 50.Jobling M. National Research Council (NRC): Nutrient requirements of fish and shrimp: The National Academies Press, Washington, DC, 2011, 376+ XVI pp, £ 128 (Hardback), ISBN: 978-0-309-16338-5. [Google Scholar]
- 51.Rachmawati D, Nurhayati D. Effect of dietary lysine on the growth performance of Pangasius hypophthalmus. Depik. 2022;11(2):111–6. [Google Scholar]
- 52.Yuangsoi B, Wongmaneeprateep S, Sangsue D. The optimal dietary DL-methionine on growth performance, body composition and amino acids profile of Pangasius catfish (Pangasius bocourti). Aqua Aquar Conser Legisl. 2016;9(2):369–78. [Google Scholar]
- 53.Akbar A, Anal AK. Occurrence of Staphylococcus aureus and evaluation of anti-staphylococcal activity of Lactococcus lactis subsp. lactis in ready-to-eat poultry meat. Annal Microbiol. 2014; 64 (1): 131–138. doi: 10.1007/s13213-013-0641-x [DOI] [Google Scholar]
- 54.AOAC. 2005. Official method of Analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24. [Google Scholar]
- 55.Folch J, Lees M, Sloane-Stanley GM. A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues. J Biol Chem. 1957; 226, 497–509. doi: 10.1083/jcb.1.2.173 [DOI] [PubMed] [Google Scholar]
- 56.Ahmad B, Ali A, Naz D, Raziq S, Khan A, Aziz A, et al. Biochemical composition of fish and changes during processing and storage. Biosci Res. 2020; 18(2),1903–1913. [Google Scholar]
- 57.Ding X, Li ZJ, Chen YQ, Lin HZ, Yang YY, Yang K. Effects of probiotics on growth and activities of digestive enzymes of Pennaus vannamei. J Fish Sci. 2004; 11, 580–584. doi: 10.1016/j.aquaculture.2007.05.035 [DOI] [Google Scholar]
- 58.Jin ZL. The Evaluation Principle and Method of Functional Food. Beijing Publishers, Beijing. 1995. doi: 10.1111/jwas.12615 [DOI] [Google Scholar]
- 59.Jiang CK. Manual of Enzyme Activity Measuring. Science and Technology Press, Shanghai. 1988. doi: 10.1038/s41598-022-11940-z [DOI] [Google Scholar]
- 60.Borlongan IG. Studies on the digestive lipases of milkfish, Chanos chanos. Aquac. 1990; 89, 315–325. doi: 10.1016/0044-8486(90)90135-A [DOI] [Google Scholar]
- 61.Humason GL. Animal Tissue Techniques. WH Freeman and Co, San Francisco. Open J Ani Sci. 1979; 5(2): 641. [Google Scholar]
- 62.El‐Saidy DM, Gaber MM. Complete replacement of fish meal by soybean meal with dietary L‐lysine supplementation for Nile tilapia Oreochromis niloticus (L.) fingerlings. J World Aqua Soci. 2002;33(3):297–306. [Google Scholar]
- 63.Yu DH, Gong SY, Yuan YC, Luo Z, Lin YC, Li Q. Effect of partial replacement of fish meal with soybean meal and feeding frequency on growth, feed utilization and body composition of juvenile Chinese sucker, Myxocyprinus asiaticus (Bleeker). Aqua Res. 2013;44(3):388–94. doi: 10.1111/j.1365-2109.201103043.x [DOI] [Google Scholar]
- 64.Ruchimat T, Masumoto T, Hosokawa H, Itoh Y, Shimeno S. Quantitative lysine requirement of yellowtail (Seriola quinqueradiata). Aqua. 1997; 158(3–4), 331–339. doi: 10.1016/s0044-8486(97)00215-9 [DOI] [Google Scholar]
- 65.Schwarz FJ, Kirchgessner M, Deuringer U. Studies on the methionine requirement of carp (Cyprinus carpio). Aqua. 1998; 161:121–9 [Google Scholar]
- 66.Mai K, Wan J, Ai Q, Xu W, Liufu Z, Zhang L, et al. Dietary methionine 835 requirement of large yellow croaker, Pseudosciaena crocea R. Aqua. 2006; 253:564–72. doi: 10.1186%2Fs40104-017-0194-0 [Google Scholar]
- 67.Abidi SF, Khan MA. Total sulphur amino acid requirement and cystine 688 replacement value for fingerling rohu, Labeo rohita: effects on growth, nutrient retention and body composition. Aqua Nutr. 2011; 17:583–94. 690 4. [Google Scholar]
- 68.Sveier H, Nordås H, Berge GE, Lied E. Dietary inclusion of crystalline D- and 838 L-methionine: effects on growth, feed and protein utilization, and digestibility in small and large Atlantic salmon (Salmon salar L.). Aqua Nutr. 2001; 7:169–81. [Google Scholar]
- 69.Li P, Burr GS, Wen Q, Goff JB, Murthy HS, Gatlin DM. Dietary sufficiency of sulfur amino acid compounds influences plasma ascorbic acid concentrations and liver peroxidation of juvenile hybrid striped bass (Morone chrysops×M. saxatilis). Aqua. 2009; 287:414–8. [Google Scholar]
- 70.Tang L, Wang GH, Jiang J, Feng L, Liu Y, Li SH. Effect of methionine on 684 intestinal enzymes activities, microflora and humoral immune of juvenile 685 Jian carp (Cyprinus carpio var. Jian). Aqua Nutr. 2009; 15:477–83. [Google Scholar]
- 71.Ahmad I, Dar NA. Dietary valine improved growth, immunity, enzymatic activities and expression of TOR signaling cascade genes in rainbow trout, Oncorhynchus mykiss fingerlings. Sci Rep. 2021; 11(1): 22089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang S, Liu F, Liou C. Assessment of dietary lysine requirement for silver perch (Bidyanus bidyanus) juveniles. Aqua. 2011; 312(1–4): 102–108. doi: 10.1016/j.aquaculture.2010.12.011 [DOI] [Google Scholar]
- 73.Xie F, Ai Q, Mai K, Xu W, Wang X. Dietary lysine requirement of large yellow croaker (Pseudosciaena crocea, Richardson 1846) larvae. Aqua Res. 2011; 43(6), 917–928. doi: 10.1111/j.1365-2109.2011.02906.x [DOI] [Google Scholar]
- 74.Ebeneezar S, Vijayagopal P, Srivastava PP, Gupta S, Varghese T, Prabu DL, et al. Dietary lysine requirement of juvenile silver pompano, Trachinotus blochii (Lacepede, 1801). Aqua. 2019; 511:734234. doi: 10.1016/j.aquaculture.2019.734234 [DOI] [Google Scholar]
- 75.Bakke AM, Glover C, Krogdahl Å. Feeding, digestion and absorption of nutrients. Fish Physiol. 2010; 30: 57–110. Academic Press. [Google Scholar]
- 76.Machado M, Azeredo R, Fontinha F, Fernández-Boo S, Conceição LE, et al. Dietary methionine improves the European seabass (Dicentrarchus labrax) immune status, inflammatory response, and disease resistance. Front Immunol. 2018; 9:2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Powell CD, Chowdhury MK, Bureau DP. Assessing the bioavailability of L‐methionine and a methionine hydroxy analogue (MHA‐Ca) compared to DL‐methionine in rainbow trout (Oncorhynchus mykiss). Aqua Res. 2017;48(1):332–46. [Google Scholar]
- 78.Niklasson L. Intestinal Mucosal Immunology of Salmonids. Response to Stress and Infection and Crosstalk with the Physical Barrier, University of Gothenburg. 2013. [Google Scholar]
- 79.Jiang WD, Wen HL, Liu Y, Jiang J, Wu P, Zhao J, et al. Enhanced muscle nutrient content and flesh quality, resulting from tryptophan, is associated with antioxidative damage referred to the Nrf2 and TOR signaling factors in young grass carp (Ctenopharyngodon idella): avoid tryptophan deficiency or excess. Food Chem. 2016; 199: 210–219. doi: 10.1016/j.foodchem.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 80.Kuang SY, Xiao WW, Feng L, Liu Y, Jiang J, Jiang WD. Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellf Immunol. 2012; 32: 629e36. doi: 10.1016/j.fsi.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 81.Bitzer-Quintero OK, D avalos-Marín AJ, Ortiz GG, ARdA Meza, TorresMendoza BM, Robles RG. Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Bio Pharmacol. 2010;64: 77e81. doi: 10.1016/j.biopha.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 82.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol. 2003; 201:28–36. doi: 10.1002/path.1409 [DOI] [PubMed] [Google Scholar]
- 83.Feng L, Zhao B, Chen GF, Jiang WD, Liu Y, Jiang J. Effects of dietary histidine on antioxidant capacity in juvenile Jian carp (Cyprinus carpio). Fish Physiology Biochemistry. 2013; 39:559e71. [DOI] [PubMed] [Google Scholar]
- 84.Trenzado CE, Morales AE, Higuera M. Physiological Effects of Crowding in Rainbow Trout, Oncorhynchus mykiss, Selected for Low and High Stress Responsiveness. Aqua. 2006; 258, 583–593. doi: 10.1016/j.aquaculture.2006.03.045 [DOI] [Google Scholar]
- 85.Fernandes MN, Mazon AF. Environmental pollution and fish gill morphology. J Environ Prot. 2003; 8: 203–231. [Google Scholar]
- 86.Özak AA, Demirkale İ, Yanar A. First record of two species of parasitic copepods on immigrant pufferfishes (Tetraodontiformes: Tetraodontidae) caught in the eastern Mediterranean Sea. Turk J Fish Aqua Sci. 2012; 12(3). [Google Scholar]
- 87.Camargo MMP, Martinez CBR. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neot Ichthyol. 2007; 5:327–336. [Google Scholar]
- 88.Palladino A, De Felice E, Attanasio C, Barone CM, Crasto A, D Angelo L, et al. A Morphological and Ultrastructural Study of the Anterior Digestive Tract of Adult Nile Tilapia Oreochromis niloticus. Anim. 2023; 13: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okwari OO, Ettarh RR, Akpogomeh BA, Eteng MU. Gastric anti-secretory and anti-ulcerogenic effects of Dombeya buettneri in rats. J Ethnopharmacol. 2000; 71: 315–319. doi: 10.1016/s0378-8741(99)00196-8 [DOI] [PubMed] [Google Scholar]
- 90.El-Barbary MI. Some clinical, microbiological and molecular characteristics of Aeromonas hydrophila isolated from various naturally infected fishes. Aqua Intern. 2010; 18: 943–954. [Google Scholar]
- 91.Miyazaki T, Kaige NA. histopathological study on motile aeromonad disease of Crucian carp. Fish Pathol. 1985; 21: 181–185 [Google Scholar]
- 92.Khafagy AR, El-Gamal RM, Hala FA, Samaa AM, Aly SM. Prevalence and histopathological changes of Staphylococcus aureus infection of catfish (Clarias garipeneaus). 1st International Conference (Centr Lab Aqua Res Coop World Fish). 2017; 1 20–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.