Abstract
The Epstein-Barr virus (EBV) gH-gL complex includes a third glycoprotein, gp42. gp42 binds to HLA class II on the surfaces of B lymphocytes, and this interaction is essential for infection of the B cell. We report here that, in contrast, gp42 is dispensable for infection of epithelial cell line SVKCR2. A soluble form of gp42, gp42.Fc, can, however, inhibit infection of both cell types. Soluble gp42 can interact with EBV gH and gL and can rescue the ability of virus lacking gp42 to transform B cells, suggesting that a gH-gL-gp42.Fc complex can be formed by extrinsic addition of the soluble protein. Truncated forms of gp42.Fc that retain the ability to bind HLA class II but that cannot interact with gH and gL still inhibit B-cell infection by wild-type virus but cannot inhibit infection of SVKCR2 cells or rescue the ability of recombinant gp42-negative virus to transform B cells. An analysis of wild-type virions indicates the presence of more gH and gL than gp42. To explain these results, we describe a model in which wild-type EBV virions are proposed to contain two types of gH-gL complexes, one that includes gp42 and one that does not. We further propose that these two forms of the complex have mutually exclusive abilities to mediate the infection of B cells and epithelial cells. Conversion of one to the other concurrently alters the ability of virus to infect each cell type. The model also suggests that epithelial cells may express a molecule that serves the same cofactor function for this cell type as HLA class II does for B cells and that the gH-gL complex interacts directly with this putative epithelial cofactor.
All herpesviruses examined to date encode a complex of two glycoproteins, gH and gL, that appear to be necessary, if not sufficient, for virus penetration. Glycoprotein gH is generally thought to be the major player in virus cell fusion (5, 6, 8, 14, 20, 25, 26), while the role of gL is to serve as a chaperone, essential for folding and transport of functional gH (3, 11, 13, 20, 21, 28, 29). The Epstein-Barr virus (EBV) gH-gL complex follows this pattern. Glycoprotein gp85, the gH homolog, is retained in the endoplasmic reticulum in the absence of gp25, the EBV gL (38), and virosomes made from EBV proteins depleted of the gH-gL complex bind to cells but fail to fuse (9). The EBV gH-gL complex, however, includes a third glycoprotein, gp42, which is the product of the BZLF2 open reading frame (ORF) (18). This third component has also proven to be essential for penetration of the major target cell of EBV, the B lymphocyte. Several lines of evidence indicate that gp42 is a ligand for HLA class II and, further, that HLA class II functions as a cell surface cofactor for EBV entry into this cell type. Glycoprotein gp42 interacts with the β1 domain of HLA class II protein HLA-DR (30), and a monoclonal antibody (MAb) to gp42 called F-2-1 interferes with this interaction (17). MAb F-2-1 has no effect on EBV attachment via glycoprotein gp350/220 to its primary receptor, complement receptor type 2 (CR2; CD21) but inhibits the fusion of the virus with the B-cell membrane (22). Similarly, a MAb to HLA-DR or a soluble form of gp42 blocks B-cell transformation. Finally, B-cell lines which lack expression of HLA class II are not susceptible to superinfection with EBV unless expression of class II is restored (17). Most recently, we derived a recombinant virus with gp42 expression deleted and confirmed that loss of the glycoprotein resulted in a virus that attached to the B-cell surface but that failed to penetrate unless it was treated with the fusogenic agent polyethylene glycol (36).
Although most is known about the early interactions of EBV with B lymphocytes in vitro since these cells are readily available and easy to culture, infection is not restricted to this cell type in vivo. During our initial analysis of the biology of gp42 we had therefore examined its potential role in infection of a then newly derived model epithelial cell line, SVKCR2. SVKCR2 cells are transformed with simian virus 40 and stably transfected with B-cell receptor CR2 (19). They are poorly infectable with many strains of EBV, but in excess of 30% of the cells can be infected with the Akata strain of virus as judged by the expression of EBV latent protein EBNA 1 (18, 19). We found that MAb F-2-1 had no effect on the infection of SVKCR2 cells. At the same time, a second MAb, E1D1, which reacts with an epitope that can be formed by the coexpression of gH and gL in the absence of gp42, neutralized infection of SVKCR2 cells, but had no effect on the infection of lymphocytes. These data strongly suggested that the involvement of the gH-gL complex in the internalization of virus into the two cell types was different. We hypothesized that just as EBV has evolved a glycoprotein, gp350/220, which is uniquely adapted for attachment to B lymphocytes, so it has evolved a second glycoprotein, gp42, uniquely adapted for penetration into the same cell type (18). The implication was that gp42 might be dispensable for infection of epithelial cells.
Since we made our initial observations with SVKCR2 cells, several novel reagents, including the Akata strain virus with the expression of gp42 deleted, have become available. The recent insights into the role of HLA class II in B-cell infection also provided new impetus to reexamine the involvement of the gH-gL complex in epithelial cell infection. We report here that gp42 is not required for infection of SVKCR2 cells despite the fact that the soluble form of the protein that inhibits B-cell infection can also neutralize infection of SVKCR2 cells. To explain these apparently anomalous results, we describe a model which proposes that wild-type EBV virions contain two types of gH-gL complexes, one that includes gp42 and one that does not. We further propose that the tripartite “B-cell complexes” are not functional for infection of epithelial cells, just as the bipartite “epithelial cell complexes” are unable to mediate infection of the B lymphocyte.
MATERIALS AND METHODS
Cells.
Akata cells (33) (a gift from John Sixbey, St. Jude Children’s Research Hospital, Memphis, Tenn.) and P3HR1-Cl13 cells (10) (a gift from George Miller, Yale University, New Haven, Conn.) were grown in RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% heat-inactivated fetal bovine serum (Gibco/BRL Life Technologies, Grand Island, N.Y.). SVKCR2 cells (19) (a gift from A. B. Rickinson, University of Birmingham, Birmingham, England) were grown in Joklik’s modified Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, Utah) and 10 ng of cholera toxin (Sigma) per ml. Human leukocytes were obtained from heparinized adult peripheral blood or from cord blood by flotation on lymphocyte separation medium and were depleted of T cells by a double cycle of rosetting with sheep erythrocytes as previously described (18).
Virus.
Akata strain virus was obtained by induction of Akata cells with goat anti-human immunoglobulin (Ig) as described previously (18) and was harvested from unconcentrated clarified culture medium that had been passed through a 0.8-μm-pore-size filter. Akata strain virus lacking gp42 was obtained by a similar induction of Akata cells containing virus episomes that had undergone homologous recombination with a fragment of Akata virus DNA in which a neomycin resistance cassette had been inserted into the BZLF2 ORF 182 bp from the initiation codon as previously described (36). P3HR1 strain virus was obtained by induction of P3HR1-Cl13 cells with 30 ng of 12-o-tetradecanoylphorbol-13-acetate per ml, concentrated 100-fold by high-speed centrifugation, and resuspended in fresh medium as described previously (9). Virus was inactivated by irradiation of 1 ml of virus for 30 min in a 60-mm-diameter petri dish 8 cm from a short-wave germicidal lamp (GTE Sylvania Inc.).
Antibodies.
Five MAbs were used: F-2-1 against gp42 (18, 32); E1D1 (24), which reacts with the EBV gH-gL complex (18); ALVA 42, against the β chain of HLA-DR (7, 30); and two American Type Culture Collection antibodies, HB55 (also known as L423) (16) and HB180 (35) against the αβ heterodimer of HLA-DR. In addition an antibody to a synthetic peptide corresponding to residues 125 to 137 of gL was made (38). All antibodies were purified by chromatography on protein A (Sigma) coupled to Affigel-15 (Bio-Rad, Richmond, Calif.).
Fc constructs.
The soluble form of gp42, gp42.Fc, previously designated BZLF2.Fc (30), and the deletion mutants of BZLF2.Fc, ΔN58, ΔN90, and ΔN122, which included amino acids 59 to 223, 91 to 223, and 123 to 223 of the BZLF2 sequence, respectively, were made as described previously (30). All the mutant proteins and gp42.Fc bound to HLA class II (30, 31). An unrelated heterologous recombinant Fc chimeric protein, Cont.Fc, was constructed in a manner similar to that for gp42.Fc by fusing amino acids 19 to 258 of a vaccinia virus ORF (Copenhagen strain, p35) to the Fc portion of human IgG1 (37). All proteins were purified to homogeneity by affinity chromatography on protein A-agarose (4). Purity was confirmed by gel electrophoresis and silver staining of the gel.
Slot blots.
The amount of EBV DNA in virions was measured by hybridization with the BamHI W fragment of EBV DNA labeled with 32P by means of a random-primed DNA labeling kit (Boehringer Mannheim, Indianapolis, Ind.). Samples consisting of 150 μl of culture supernatant containing virus were filtered through a 0.8-μm-pore-size filter to remove cells and were digested for 10 min at room temperature with DNase I. Five microliters of 0.5 M EDTA was added, and virus particles were sedimented for 1 h at 16,000 × g. Sedimented virus was digested overnight at 56°C with proteinase K (12 mg per ml in 0.2 M EDTA), and serial dilutions were made in phosphate-buffered saline. The DNA in each sample was denatured by the addition of 1/10 volume of 5 N NaOH, neutralized with 2 M ammonium acetate, and applied to a nylon membrane and cross-linked by UV irradiation. Hybridizations were carried out as described previously (12) and quantified by scanning with a Molecular Dynamics Storm PhosphorImager.
B-cell transformation.
One hundred and twenty microliters of stock Akata virus was preincubated for 1 h at 4°C with 180 μl of medium containing Fc constructs or MAbs and then was added to 6 × 105 T-cell-depleted leukocytes for 60 min. Then, 540 μl of RPMI medium containing 10% heat-inactivated fetal calf serum was added, and 140 μl containing 105 cells was plated into each of 5 wells of a 96-well plate and incubated at 37°C. Cells were fed by replacing 50% of the medium with fresh medium at weekly intervals, and transformation was judged by outgrowth of cells over 5 weeks of culture.
Superinfection of Raji cells.
To test for superinfection and inhibition of superinfection of Raji cells, 1.5 × 106 cells were preincubated with Fc constructs or MAbs in 260 μl of phosphate-buffered saline or with medium alone for 1 h at 37°C. Stock P3HR1 virus (500 μl) in RPMI was added for an additional 2 h of incubation. Four milliliters of RPMI supplemented with serum was added, and cells were incubated for 3 days before being harvested for analysis by Western blotting.
Infection of SVKCR2 cells.
Six hundred to 1,000 microliters of virus was incubated with 200 μl of Fc constructs or MAbs for 1 h at 37°C, and then the solution was added to cells grown to 80 to 90% confluency in six-well tissue culture plates. Four hours later 4 ml of Joklik’s medium was added, and cells were reincubated for 4 days. Cells were removed from dishes by trypsinization, allowed to recover in medium with serum for 30 min at 37°C, washed in phosphate-buffered saline, and processed for Western blotting.
Western blots.
Cells were lysed in immunoprecipitation buffer, electrophoresed in polyacrylamide, and then electrically transferred onto nitrocellulose membranes (0.45-μm pore size; Schleicher and Schuell, Inc, Keene, N.H.) at 126 mA for 6 h. The transferred sheets were treated for at least 3 h with blocking buffer (10 mM Tris-HCl [pH 7.2], 0.15 M NaCl, 5% skim milk, 0.05% sodium azide) and reacted for at least 3 h with blocking buffer containing a 1/500 dilution of EBNA 1-positive human serum (SVKCR2 cells) or a 1/100 dilution of virus early-antigen (EA-D) MAbs (Capricorn Products, Inc., Scarborough, Maine) (superinfected Raji cells). They were then washed five times with wash buffer (10 mM Tris-HCl [pH 7.2], 0.15% NaCl, 0.3% Tween 20) for 10 min each. The washed sheets were reacted with alkaline phosphatase-conjugated goat anti-human antibodies or rabbit anti-mouse antibodies (Hyclone) for 3 h, and the bound anti-human or anti-mouse antibodies were detected by reacting them with substrate 5-bromo-4-chloro-indophosphate and Nitro Blue Tetrazolium (Sigma).
Radiolabeling and immunoprecipitation.
EBV proteins were labeled biosynthetically with [3H]glucosamine (20 Ci/mmol; Amersham Corp., Arlington Heights, Ill.) for 20 h starting 6 h after induction with anti-human IgG as previously described (38). Labeled cells were solubilized in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.2], 0.15 M NaCl, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.1 mM phenylmethylsulfonyl fluoride, 100 U of aprotinin per ml) and immunoprecipitated with antibody or precipitated with Fc constructs and protein A-Sepharose CL4B (Sigma). Precipitated proteins were washed, dissociated by being boiled in sample buffer containing 2-mercaptoethanol, and analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in acrylamide cross-linked with 0.28% N,N′-diallyltartardiamide, followed by fluorography. One milligram of gp42.Fc was extrinsically labeled with 1 mCi of 125I (Amersham) by the use of Iodobeads (Pierce, Rockford, Ill.), and free iodine was removed on a P-6 DG desalting column (Bio-Rad).
Binding of gp42.Fc to SVKCR2 cells.
SVKCR2 cells were treated for 2 days with 15 ng of recombinant human gamma interferon (Genzyme, Cambridge, Mass.) per ml to induce expression of HLA-DR. Induced and uninduced cells were washed twice in phosphate-buffered saline, removed from tissue culture dishes by trypsinization, and allowed to recover in medium at 37°C for 30 min. Expression of HLA-DR on induced cells was confirmed by immunofluorescence with antibody HB55. One million induced and uninduced cells were incubated in duplicate for 1 h on ice with approximately 10 μg of iodinated gp42.Fc in medium alone or with 10 μg of iodinated gp42.Fc in medium containing 100 μg of unlabeled gp42.Fc, 100 μg of unlabeled Cont.Fc, or 100 μg of MAb F-2-1. Cells were washed four times in phosphate-buffered saline, and the amount of radioactivity that remained associated with the cells was counted. The binding of iodinated gp42.Fc in the presence of unlabeled gp42.Fc, Cont.Fc, or MAb F-2-1 was expressed as the percent change in the amount of radioactivity that bound to uninduced SVKCR2 in the absence of unlabeled proteins.
Sucrose gradient sedimentation of virion proteins.
Forty million Akata cells were induced and labeled for 20 h with [3H]glucosamine. The cells were removed by low-speed centrifugation and filtration of the culture medium through a 1.2-μm-pore-size filter. Virus was concentrated by high-speed centrifugation and purified by centrifugation on a discontinuous dextran gradient (2, 23). Purified virus was lysed in RIPA buffer and centrifuged at 16,000 × g for 15 min in an Eppendorf centrifuge, and the supernatant was layered on a 9-ml continuous gradient of 5 to 25% sucrose in RIPA buffer poured onto a cushion of 0.75 ml of 60% sucrose. Gradients were centrifuged at 38,000 rpm for 18 h at 20°C in an SW41 rotor. One-milliliter fractions were collected (the top fraction being fraction 1 and the bottom being fraction 8), and each fraction was divided in two for immunoprecipitation.
RESULTS
Soluble gp42 but not antibodies to HLA class II can inhibit infection of SVKCR2 cells.
We have previously shown that MAb F-2-1, which blocks the interaction of gp42 and HLA class II, has no effect on the infection of SVKCR2 cells by EBV (18), and we and others (15) have been unable to detect any constitutive expression of HLA class II on SVKCR2 cells by using antibodies to HLA-DR. This strongly suggested that the infection of SVKCR2 cells, unlike the infection of B cells, did not involve an interaction between gp42 and HLA class II. To confirm this point, two types of experiments were performed. First, the effects of the antibodies to HLA class II that interfered with infection of the B lymphocyte were examined. As expected, none of the antibodies to HLA-DR, at the concentration which inhibited B-cell transformation (30 μg for 106 million cells; data not shown) had any affect on the induction of EBNA 1, although MAb E1D1 to gH-gL effectively inhibited infection (Fig. 1). Second, the effects of soluble gp42 were determined. To our surprise, soluble gp42 inhibited infection in a dose-dependent manner (Fig. 2). Previous workers have demonstrated the presence of HLA class II in the virion (15), so it was possible that gp42 was inhibiting infection by binding to virion-associated HLA class II. To eliminate this possibility, infections were repeated with the P3HR1 strain of virus. This virus strain is derived from cells that express only HLA-DQ, to which gp42 does not bind (31), and although P3HR1 virus does not infect SVKCR2 cells with great efficiency (19), we were able to detect the induction of EBNA 1 with a high-titer stock virus. Twenty micrograms of soluble gp42 also inhibited the infection of SVKCR2 cells by P3HR1 virus (Fig. 3).
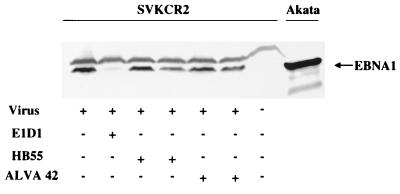
FIG. 1.
Effect of MAbs to HLA class II (HB55 and ALVA 42) or to gH-gL (E1D1) on the infection of SVKCR2 cells by Akata strain virus. Cells were harvested 4 days after infection, and equal numbers were analyzed by Western blotting for expression of EBNA 1. Blots were reacted with human serum containing antibody to EBNA 1 and with goat anti-human Ig conjugated to alkaline phosphatase. Uninduced Akata cells were included on the far right to demonstrate the electrophoretic mobility of EBNA 1 from this strain of virus. +, present; −, absent.
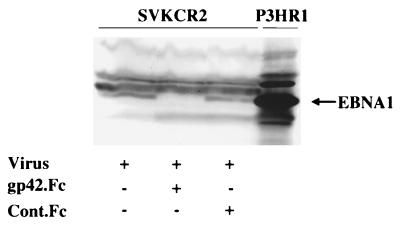
FIG. 2.
Effect of gp42.Fc, Cont.Fc, and MAb E1D1 on the infection of SVKCR2 cells by Akata strain virus. Cells were harvested 4 days after infection, and equal numbers were analyzed by Western blotting for expression of EBNA 1. Blots were reacted with human serum containing antibody to EBNA 1 and with goat anti-human Ig conjugated to alkaline phosphatase. Uninduced Akata cells were included on the far right to demonstrate the electrophoretic mobility of EBNA 1 for this strain of virus. The amounts of soluble proteins added (in micrograms) are indicated. +, present; −, absent.
FIG. 3.
Effect of gp42.Fc and Cont.Fc on infection of SVKCR2 cells by P3HR1 strain virus. Cells were harvested 4 days after infection, and equal numbers were analyzed by Western blotting for expression of EBNA 1. Blots were reacted with human serum containing antibody to EBNA 1 and with goat anti-human Ig conjugated to alkaline phosphatase. Uninduced P3HR1 cells were included on the far right to demonstrate the electrophoretic mobility of EBNA 1 for this strain of virus. +, present; −, absent.
Glycoprotein gp42 is not required for infection of epithelial cells.
The ability of soluble gp42 to inhibit the infection of SVKCR2 cells suggested that gp42 might not, after all, be dispensable for infection of this cell type. To determine if gp42 might be interacting with a cell surface molecule other than HLA class II, we iodinated gp42.Fc and examined its ability to bind specifically to SVKCR2 cells that lacked HLA class II or to SVKCR2 cells in which HLA class II expression had been induced by treatment with gamma interferon. The expression of HLA class II was confirmed by indirect immunofluorescence with antibody HB55 (data not shown). Although radioactivity associated with the cells, iodinated gp42.Fc did not bind specifically to those that lacked expression of HLA class II, as the amount of radioactivity bound did not change significantly in the presence of a 10-fold excess of unlabeled gp42.Fc, Cont.Fc, or MAb F-2-1. In contrast almost twice as much radioactivity bound to cells expressing HLA class II, and this increased binding could be significantly inhibited by unlabeled gp42.Fc and MAb F-2-1, but not by Cont.Fc. (Fig. 4). Although these results indicated that under the experimental conditions there was no evidence for an interaction between gp42.Fc and the epithelial cell surface like that between gp42.Fc and HLA class II, they did not completely rule out the possibility that a very weak interaction might contribute to the infectious process. To confirm that no interaction between gp42 and epithelial cells was required for infection, we therefore examined the behavior of a recombinant virus that lacked gp42. We have previously shown that this virus can bind to B cells but cannot infect them unless cells and bound virus are treated with polyethylene glycol to induce fusion. Equal amounts of wild-type and recombinant virus, as judged by a slot blot assay, were used for infection of SVKCR2 cells. Both viruses were equally capable of inducing EBNA 1 in SVKCR2 cells (Fig. 5), indicating that gp42 was not needed for infection. Soluble gp42 could still inhibit the infection of SVKCR2 cells by virus lacking gp42, but at least twice as much protein was required to inhibit the recombinant virus as was required to inhibit the wild-type virus (data not shown).
FIG. 4.
Binding of iodinated gp42.Fc to SVKCR2 cells cannot be competitively inhibited by excess unlabeled protein and shown to be specific unless cells are induced with gamma interferon to express HLA class II. The left side of the figure compares the amount of iodinated gp42.Fc bound to HLA class II-negative (HLA class II-ve) cells in the absence of any other protein (arbitrarily set at zero; bar to the extreme left) with the amount bound in the presence of a 10-fold excess of unlabeled gp42.Fc or Cont.Fc or 100 μg of MAb F-2-1. Since the data represent the averages of three experiments done with different batches of iodinated protein, they are expressed as percent changes in binding rather than as counts per minute. The right side of the figure shows the relative increase in binding of gp42.Fc to cells expressing HLA class II (HLA class II +ve) (almost 100 percent more, or twice the amount bound to HLA class II-negative cells) and shows that the addition of 100 μg of F-2-1 or a 10-fold excess of soluble gp42.Fc, but not of soluble Cont.Fc, significantly inhibited this binding. Vertical lines indicate the standard deviations of the averages of the three experiments.
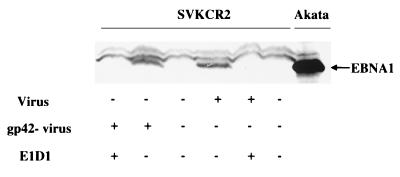
FIG. 5.
Comparison of the abilities of wild-type Akata virus and recombinant virus lacking gp42 to infect SVKCR2 cells in the presence or absence of MAb E1D1. Cells were harvested 4 days after infection, and equal numbers were analyzed by Western blotting for expression of EBNA 1. Blots were reacted with human serum containing antibody to EBNA 1 and with goat anti-human Ig conjugated to alkaline phosphatase. Uninduced Akata cells were included on the far right to demonstrate the electrophoretic mobility of EBNA 1 for this strain of virus. +, present; −, absent.
Soluble gp42 can complex with gH and gL and rescue the ability of virus that lacks gp42 to transform B cells.
The failure to detect any specific binding of gp42.Fc to SVKCR2 cells and the dispensability of gp42 for infection of this cell type implied that the soluble protein had to be inhibiting infection by binding to virus. We had already ruled out the possibility that inhibition could be attributed solely to binding to HLA class II in the virion. The next most likely possibility seemed to be that it was binding to gH or gL. To test this possibility, we determined whether soluble gp42.Fc in conjunction with protein A-agarose beads, which bind to the Fc portion of the protein, could precipitate gH and gL from lysates of Akata cells that had been induced to express proteins either of wild-type virus or of recombinant virus that lacked gp42. An anti-peptide antibody to gL immunoprecipitated gH, gL, and gp42 from the wild-type virus and the bipartite complex of gH and gL from the recombinant virus (Fig. 6A). Antibody ALVA 42 to HLA- DR immunoprecipitated the α and β chains of HLA class II from both. gp42.Fc precipitated glycoproteins that comigrated with HLA class II, with gH, and with gL. These results further suggested that gp42.Fc was able to reform a tripartite complex from one that contained only gH and gL. If this were indeed the case, it would indicate that gp42.Fc might also be able to rescue the ability of the recombinant virus that lacked gp42 to infect normal B cells. To test this possibility, we infected T-cell-depleted peripheral blood leukocytes with virus that lacked gp42 in the presence of a range of concentrations of gp42.Fc. Foci of transformation became apparent in the presence, but not the absence, of gp42.Fc (Table 1), and when the cells grew out further, the presence of recombinant virus was confirmed by Southern blotting with a probe for the neomycin resistance gene (36). The rescue of virus over a limited concentration range was consistent with high concentrations containing enough of the soluble protein both to saturate gH-gL complexes and compete for binding to HLA class II on the cell surface and with low concentrations not providing a sufficient reconstitution of gH-gL-gp42.Fc.
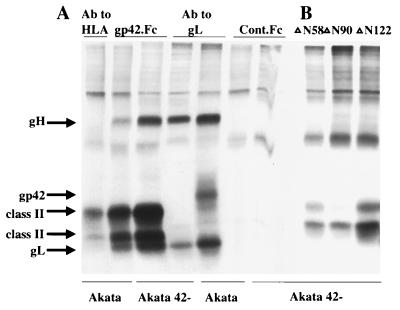
FIG. 6.
SDS-PAGE analysis of proteins precipitated from Akata cells harboring wild-type (Akata) or recombinant (Akata gp42-) episomes. Cells were induced with anti-human Ig and labeled with [3H]glucosamine. Proteins were immunoprecipitated by MAb ALVA 42 to HLA class II or anti-peptide antibody to gL, or by gp42.Fc or Cont.Fc (A) or were precipitated by truncated constructs ΔN58, ΔN90, and ΔN122 (B).
TABLE 1.
Ability of gp42.Fc to rescue the ability of Akata virus lacking gp42 to transform T-cell-depleted leukocytes
| Amt of soluble construct (ng/well) | Transformationa by Akata virus lacking gp42 at a dilution of:
|
||
|---|---|---|---|
| 1/2 in the presence of gp42.Fc | 1/6 in the presence of:
|
||
| gp42.Fc | Cont.Fc | ||
| 10,000 | 1/6 | 2/6 | NDb |
| 3,000 | 1/6 | 3/6 | ND |
| 1,000 | 4/6 | 2/6 | 0/6 |
| 300 | 1/6 | 4/6 | 0/6 |
| 100 | 3/6 | 3/6 | 0/6 |
| 30 | 5/6 | 3/6 | 0/6 |
| 10 | 6/6 | 5/6 | 0/6 |
| 3 | 6/6 | 6/6 | 0/6 |
| 1 | 3/6 | 5/6 | 0/6 |
| 0.3 | 0/6 | 1/6 | 0/6 |
| 0.1 | 0/6 | 0/6 | ND |
| 0.03 | 0/6 | 0/6 | ND |
| 0 | 0/6 | 0/6 | 0/6 |
Number of transformed wells/total number of wells infected. Wild-type Akata virus at the same two dilutions transformed six of six wells in the absence of any soluble construct.
ND, not done.
Rescue of B-cell infection by virus lacking gp42 is not a result of cell signalling via HLA class II.
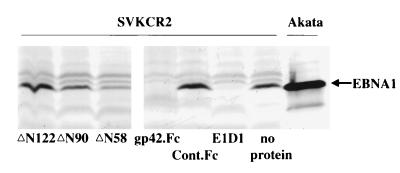
The ability of gp42.Fc to rescue the ability of recombinant virus to transform B cells could also be explained if the soluble protein, rather than promoting a physical interaction between the gH-gL complex and HLA class II, were instead providing a signal via HLA class II that was essential to render the B cell permissive for infection. Two types of experiments were done to examine this possibility. First, mixtures of recombinant virus and wild-type virus that had been inactivated by UV irradiation were tested for the ability to transform B cells. No transformation was seen over a range of dilutions of wild-type virus despite the fact that the irradiated wild-type virus should still have been able to provide a coincident signal via HLA class II (Table 2). Second, three forms of recombinant gp42.Fc from which increasing amounts of amino terminal sequence had been deleted were examined for their abilities to rescue transformation by recombinant virus. The deletion of up to 122 amino acids from the BZLF2-derived sequences at the amino terminus of gp42.Fc still resulted in proteins, ΔN122, ΔN90, and ΔN58, that could bind to HLA class II (30, 31). Precipitation analyses, however, showed that although each of the proteins could precipitate HLA class II, none of them could precipitate gH or gL (Fig. 6B). In keeping with this phenotype, i.e., binding to HLA class II but not interacting with gH-gL, neither ΔN122 nor ΔN90 was able to inhibit the infection of SVKCR2 cells (Fig. 7). The protein from which the smallest amount was deleted, ΔN58, and which may have retained a reduced ability to interact with gH and gL that we could detect in our precipitation analyses, gave a slightly reduced EBNA 1 signal. In addition, it was found that none of the proteins was able to rescue the ability of virus lacking gp42 to infect B cells in an assay run in parallel with that shown in Table 1. However, since each was still able to bind to HLA class II, each was still able to inhibit superinfection of the B-cell line Raji with P3HR1 strain virus with an ability that decreased with the extent of the deletion (Fig. 8).
TABLE 2.
Effect of UV irradiation on the ability of Akata virus lacking gp42 to transform T-cell-depleted leukocytes
| Akata virus mixture (wild type, UV irradiated, gp42 negative)a | Transformationb |
|---|---|
| None, none, none | 0/6 |
| 1/200, none, none | 6/6 |
| None, 1/5, none | 0/6 |
| None, none, 1/1 | 0/6 |
| None, 1/2, 1/2 | 0/6 |
| None, 1/5, 1/2 | 0/6 |
| None, 1/10, 1/2 | 0/6 |
| None, 1/20, 1/2 | 0/6 |
| None, 1/5, 1/5 | 0/6 |
| None, 1/20, 1/5 | 0/6 |
Fractions are dilutions of virus used in the mixture.
Number of transformed wells/total number of wells infected.
FIG. 7.
Effect of truncated constructs ΔN58, ΔN90, and ΔN122 and gp42.Fc, Cont.Fc, and MAb E1D1 on infection of SVKCR2 cells with Akata strain virus. Cells were harvested 4 days after infection, and equal numbers were analyzed by Western blotting for expression of EBNA 1. Blots were reacted with human serum containing antibody to EBNA 1 and with goat anti-human Ig conjugated to alkaline phosphatase. Uninduced Akata cells were included on the far right to demonstrate the electrophoretic mobility of EBNA 1 for this strain of virus.
FIG. 8.
Effect of truncated constructs ΔN58, ΔN90, and ΔN122 and Cont.Fc on superinfection of Raji cells by P3HR1 strain virus. Cells were harvested 3 days after infection, and equal numbers were analyzed by Western blotting with a MAb for expression of EA-D.
Akata virus contains more gH and gL in the virion than gp42.
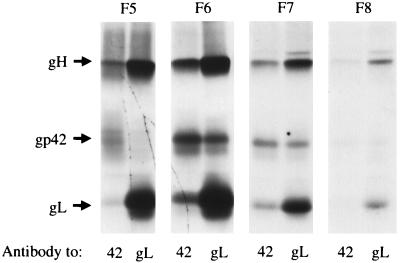
The ability of recombinant virus to infect SVKCR2 cells, despite its failure to transform B cells, indicated that although gp42 was essential for B-cell infection, a complex that included only gH and gL was sufficient for infection of SVKCR2 cells. To examine whether it was possible that such a bipartite complex might exist in wild-type virus, virus was purified from the supernatant medium of Akata cells that had been labeled with [3H]glucosamine, lysed, and separated by velocity sedimentation through a 5 to 20% sucrose gradient. Each fraction was divided into two for immunoprecipitation with anti-peptide antibody to gL or MAb F-2-1. The sequences of gp42, gL, and gH include 4, 3, and 5 potential N-linked glycosylation sites, respectively, and the apparent mass of each protein changes by approximately 10 kDa after digestion with N-glycanase (18). The autoradiogram, however, clearly indicated that, as previously seen in virus-producing cells (18), more labeled gH and gL was present in virus than gp42, and whereas complexes immunoprecipitated by the antibody to gp42 appeared to contain similar amounts of each labeled protein, those immunoprecipitated with the antibody to gL appeared to contain relatively more gH and gL (Fig. 9). This result was consistent with the existence of complexes of gH and gL that lacked the third component, gp42.
FIG. 9.
SDS-PAGE analysis of proteins immunoprecipitated by MAb F-2-1 to gp42 or anti-peptide antibody to gL from virus harvested from the supernatant medium of Akata cells labeled with [3H]glucosamine and purified by centrifugation on a discontinuous dextran gradient. Prior to immunoprecipitation solubilized virus proteins were sedimented through a gradient of 5 to 25% sucrose and separated into 10 fractions. Each fraction was divided in two for immunoprecipitated. The fraction immunoprecipitated in each pair of lanes is indicated at the top (F5 [fifth from the top of the gradient] to F8 [toward the bottom of the gradient]).
DISCUSSION
The entry of EBV into lymphocytes has been the subject of considerable research effort. The attachment process involving the interaction of gp350 and CR2 has been well studied, and the involvement of the gH-gL-gp42 complex with HLA class II leading to fusion of the virus envelope and the cell membrane is being explored. Much less is known about entry into the epithelial cell. Although there is currently much discussion concerning the importance of epithelial cell infection in the establishment and maintenance of EBV in the healthy individual (34), the virus clearly accesses epithelial cells in immunocompromised individuals who develop oral hairy leukoplakia and in individuals who suffer from nasopharyngeal carcinomas (27), so from a biomedical perspective the process is very important. Until relatively recently the major impediment to investigating early events in epithelial cell infection was the lack of a permissive, culturable cell. The SVKCR2 cell line was developed to fill this need (19), and while it does so by making use of the B-cell receptor CR2, which may not be the attachment protein used by the virus to infect the epithelium in vivo, there is no compelling reason to suppose that subsequent penetration events are not authentic. In this context we conclude that entry into B cells and epithelial cells involves very different usage of the EBV gH-gL-gp42 complex.
The experiments with a recombinant virus in which the BZLF2 ORF is disrupted by a neomycin resistance cassette unequivocally demonstrated that gp42, the glycoprotein that mediates an essential interaction with HLA class II on the B-cell surface, plays no role in infection of SVKCR2 cells. This was entirely compatible with the observation that expression of HLA class II on the epithelial cells is not relevant to infection but raised some interesting questions as to why a soluble exogenous form of gp42 was able to interfere with the process. The aggregate of biochemical and biologic evidence provided here and previously (17, 18) and summarized in Table 3 indicates that gp42.Fc binds to gH-gL complexes in virus particles that are missing the native full-length protein and suggests that it is the conversion of bipartite gH-gL complexes to tripartite gH-gL-gp42.Fc complexes that is responsible for the inhibitory effects of gp42.Fc on epithelial cell infection.
TABLE 3.
Summary of the effects of antibody and soluble forms of gp42 on the ability of wild-type virus and virus lacking gp42 to infect B cells and the epithelial line SVKCR2
| MAb or protein | Protein(s) reacting with MAb or protein | Infection in presence of MAb or proteina of:
|
|||
|---|---|---|---|---|---|
| B cells by:
|
Epithelial cells by:
|
||||
| Wild-type virus | gp42− virus | Wild-type virus | gp42− virus | ||
| None | None | + | − | + | + |
| E1D1 | gH-gL | + | ND | − | − |
| F-2-1 | gp42 | − | ND | + | ND |
| ALVA 42 | HLA- DR | − | ND | + | ND |
| gp42.Fc | HLA- DR and gH-gL | − | + | − | − |
| ΔN58 | HLA- DR | − | − | + | ND |
| ΔN90 | HLA- DR | − | − | + | ND |
| ΔN122 | HLA- DR | ± | − | + | ND |
+, infection present; −, infection absent; ±, low-level infection; ND, not done; gp42−, gp42 negative.
The two major pieces of evidence for the reconstitution of a tripartite complex of gH, gL, and gp42.Fc are (i) the ability of gp42.Fc to precipitate gH and gL from virus proteins solubilized in RIPA buffer and (ii) its ability to rescue the transforming ability of recombinant virus lacking the expression of gp42. The dose dependency of this rescue is also consistent with the inhibitory effects of gp42.Fc on wild-type virus. Once all available gH-gL complexes are converted to gH-gL-gp42.Fc complexes, gp42.Fc can presumably compete for binding to HLA class II on the cell surface. In addition, the truncated forms of gp42.Fc that can still bind HLA class II but that can no longer interact with gH and gL lose their ability to rescue recombinant virus, while they retain their ability to inhibit B-cell infection by wild-type virus. If gp42.Fc were supplying an essential signalling event by binding to HLA class II, rather than physically bridging gH-gL and HLA class II, the truncated molecules should be able to restore infection by the recombinant virus as effectively as the original construct. Instead, their failure to associate with gH-gL and reform a tripartite complex rendered them ineffective. It might also be expected that a mixture of inactivated wild-type virus and recombinant virus would result in B-cell transformation if a coincident signal were all that was required. This was not the case.
It was already clear from previous work that virus carrying only a bipartite complex of gH and gL is unable to infect B cells (36). These new data suggest the converse, namely, that virus carrying only a tripartite complex of gH, gL, and gp42.Fc is unable to infect epithelial cells. Such a model would predict that truncated forms of gp42.Fc which retain the ability to bind to HLA class II but which lose the ability to associate with gH and gL would still inhibit B-cell infection by wild-type virus but would fail to inhibit the infectivity of wild-type virus for epithelial cells. Both predictions were experimentally confirmed.
A model in which tripartite complexes of gH-gL-gp42.Fc, or by extension complexes of gH-gL-gp42, cannot infect epithelial cells then calls for an explanation. One possibility is that gp42 sterically inhibits some interaction of gH-gL with the epithelial cell surface. This in turn would suggest that there might be a “receptor” on epithelial cells, absent on B cells, used by the complex in a manner analogous to the usage of HLA class II by gH-gL-gp42 on B lymphocytes. It would be consistent with our previous findings with the two MAbs F-2-1 and E1D1 (18). F-2-1 inhibits the interaction of gp42 with HLA class II (17) and blocks the infection of B cells without having any effect on epithelial cell infection. E1D1, which reacts with the gH-gL complex in the absence of gp42, has no effect on B-cell infection but efficiently neutralizes the infection of SVKCR2 cells. Preliminary data obtained with a gastric carcinoma line recently shown to be infectable with EBV (39) indicate that this observation can be extended beyond the SVKCR2 cell line (1). It could be explained if the epitope recognized by E1D1 is involved in an interaction with the putative gH-gL receptor on epithelial cells.
From an evolutionary standpoint it might be argued that acquisition of gp42 is a late event in the divergence of EBV as a gamma herpesvirus with a host range expanded from epithelial cells to lymphocytes. The model proposed here would, however, necessitate keeping a proportion of gH-gL complexes free of gp42 if infection of epithelial cells were not to be compromised. The examination of the relative amounts of gH, gL, and gp42 in virions would suggest that this has in fact happened. Knox and Young (15) have proposed that the presence of HLA class II in virions contributes to the ease with which the Akata strain of EBV can infect the SVKCR2 cell line. We can detect no HLA class II associated with the gH-gL-gp42 complex in virus, which we interpret as suggesting that a conformational change occurs after virus binding to CR2 to allow gp42-HLA class II interaction. The relatively small amount of gp42 in the virion makes it difficult to determine if a portion might be associated with HLA class II alone (gels such as that shown in Fig. 9 have been exposed for 4 months). However, if HLA class II in virus were able to act as a sink for gp42 and increase the number of complexes that contained only gH and gL, a more efficient infection of SVKCR2 cells might be expected. The next challenge will be to search for an epithelial cell receptor with which the EBV gH-gL complex can interact.
ACKNOWLEDGMENTS
This research was supported by Public Health Service grant AI20662 from the National Institute of Allergy and Infectious Diseases.
We thank Susan M. Turk for excellent technical assistance, Immunex Research and Development Corporation for reagents, and Melanie K. Spriggs for helpful discussions.
REFERENCES
- 1.Borza, C., and L. M. Hutt-Fletcher. Unpublished data.
- 2.Dolyniuk M, Pritchett R, Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976;17:935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duus K M, Hatfield C, Grose C. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology. 1995;210:429–440. doi: 10.1006/viro.1995.1359. [DOI] [PubMed] [Google Scholar]
- 4.Fanslow W C, Anderson D M, Grabstein K H, Clark E A, Cosman D, Armitage R J. Soluble forms of CD40 inhibit biologic responses of human B cells. J Immunol. 1992;149:655–660. [PubMed] [Google Scholar]
- 5.Forghani B, Ni L, Grose C. Neutralization epitope of the varicella-zoster virus gH:gL glycoprotein complex. Virology. 1994;199:458–462. doi: 10.1006/viro.1994.1145. [DOI] [PubMed] [Google Scholar]
- 6.Fuller A O, Santos R E, Spear P G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989;63:3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gayle M A, Sims J E, Dower S K, Slack J L. Monoclonal antibody 1994-01 (also known as ALVA 42) reported to recognize type II IL-1 receptor is specific for HLA- DR alpha and beta chains. Cytokine. 1994;6:83–86. doi: 10.1016/1043-4666(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 8.Gompels U A, Minson A. The properties and sequence of glycoprotein H of herpes simplex type 1. Virology. 1986;153:230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 9.Haddad R S, Hutt-Fletcher L M. Depletion of virosomes made with Epstein-Barr virus proteins abolishes their ability to fuse with virus receptor-bearing cells. J Virol. 1989;63:4998–5005. doi: 10.1128/jvi.63.12.4998-5005.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heston L, Rabson M, Brown N, Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982;295:160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson A C, Johnson D C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutt-Fletcher L M, Balachandran N, LeBlanc P A. Modification of Epstein-Barr virus replication by tunicamycin. J Virol. 1986;57:117–123. doi: 10.1128/jvi.57.1.117-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye J F, Gompels U A, Minson A C. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J Gen Virol. 1992;73:2693–2698. doi: 10.1099/0022-1317-73-10-2693. [DOI] [PubMed] [Google Scholar]
- 14.Keller P M, Davison A J, Lowe R S, Riemen M W, Ellis R W. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology. 1987;157:526–533. doi: 10.1016/0042-6822(87)90295-9. [DOI] [PubMed] [Google Scholar]
- 15.Knox P G, Young L S. Epstein-Barr virus infection of CR2-transfected epithelial cells reveals the presence of MHC class II on the virion. Virology. 1995;213:147–157. doi: 10.1006/viro.1995.1555. [DOI] [PubMed] [Google Scholar]
- 16.Lampson L A, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293–299. [PubMed] [Google Scholar]
- 17.Li Q X, Spriggs M K, Kovats S, Turk S M, Comeau M R, Nepom B, Hutt-Fletcher L M. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J Virol. 1997;71:4657–4662. doi: 10.1128/jvi.71.6.4657-4662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q X, Turk S M, Hutt-Fletcher L M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995;69:3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q X, Young L S, Niedobitek G, Dawson C W, Birkenbach M, Wang F, Rickinson A B. Epstein-Barr virus infection and replication in a human epithelial system. Nature. 1992;356:347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 20.Liu D X, Gompels U A, Foa-Tomasi L, Campadelli-Fiumi G. Human herpesvirus 6 glycoprotein H and L homologues are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology. 1993;197:12–22. doi: 10.1006/viro.1993.1562. [DOI] [PubMed] [Google Scholar]
- 21.Liu D X, Gompels U A, Nicholas J, Lelliott C. Identification and expression of the human herpesvirus 6 glycoprotein H and interaction with an accessory 40K glycoprotein. J Gen Virol. 1993;74:1847–1857. doi: 10.1099/0022-1317-74-9-1847. [DOI] [PubMed] [Google Scholar]
- 22.Miller N, Hutt-Fletcher L M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988;62:2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemerow G R, Cooper N R. Isolation of Epstein-Barr virus and studies of its neutralization by human IgG and complement. J Immunol. 1981;127:272–278. [PubMed] [Google Scholar]
- 24.Oba D E, Hutt-Fletcher L M. Induction of antibodies to the Epstein-Barr virus glycoprotein gp85 with a synthetic peptide corresponding to a sequence in the BXLF2 open reading frame. J Virol. 1988;62:1108–1114. doi: 10.1128/jvi.62.4.1108-1114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peeters B, Dewind N, Broer R, Gielkins A, Moormann R. Glycoprotein H of pseudorabies virus is essential for entry and cell-to-cell spread of the virus. J Virol. 1992;66:3888–3892. doi: 10.1128/jvi.66.6.3888-3892.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasmussen L, Resta S R, Merigan T C. Human cytomegalovirus glycoprotein-receptor interactions. Transplant Proc. 1991;23:60–63. [PubMed] [Google Scholar]
- 27.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. New York, N.Y: Raven Press; 1996. pp. 2397–2446. [Google Scholar]
- 28.Roop C, Hutchison L, Johnson D C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells and its particles lack glycoprotein H. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaete R R, Perot K, Scott P I, Nelson J A, Stinski M F, Pachl C. Co-expression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology. 1993;193:853–861. doi: 10.1006/viro.1993.1194. [DOI] [PubMed] [Google Scholar]
- 30.Spriggs M K, Armitage R J, Comeau M R, Strockbine L, Farrah T, MacDuff B, Ulrich D, Alderson M R, Müllberg J, Cohen J I. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA- DRβ chain and inhibits antigen presentation. J Virol. 1996;70:5557–5563. doi: 10.1128/jvi.70.8.5557-5563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spriggs, M. K., and M. R. Comeau. Unpublished data.
- 32.Strnad B C, Schuster T, Klein R, Hopkins III R F, Witmer T, Neubauer R H, Rabin H. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. J Virol. 1982;41:258–264. doi: 10.1128/jvi.41.1.258-264.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada K. Cross-linking of cell surface immunoglobulin induces Epstein-Barr virus in Burkitt lymphoma lines. Int J Cancer. 1984;33:27–32. doi: 10.1002/ijc.2910330106. [DOI] [PubMed] [Google Scholar]
- 34.Thorley-Lawson D A, Miyashita E M, Khan G. Epstein-Barr virus and the B cell: that’s all it takes. Trends Microbiol. 1996;4:204–208. doi: 10.1016/s0966-842x(96)90020-7. [DOI] [PubMed] [Google Scholar]
- 35.Van Vooris W C, Steinman R M, Hair L S, Luban J, Witmer M D, Koide S, Cohn Z. Specific anti-mononuclear antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983;158:126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Hutt-Fletcher L M. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J Virol. 1998;72:158–163. doi: 10.1128/jvi.72.1.158-163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Z, Fanslow W C, Seldin M F, Rousseau A M, Painter S L, Comeau M R, Cohen J I, Spriggs M K. Herpesvirus Saimiri encodes a new cytokine, IL17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 38.Yaswen L R, Stephens E B, Davenport L C, Hutt-Fletcher L M. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology. 1993;195:387–396. doi: 10.1006/viro.1993.1388. [DOI] [PubMed] [Google Scholar]
- 39.Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997;71:5688–5691. doi: 10.1128/jvi.71.7.5688-5691.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]