Abstract
Objective
The aim of this study is to investigate the clinical value and potential prognostic significance of lung function assessment and Testin expression in non-small cell lung cancer (NSCLC) patients.
Methods
The NSCLC patients were classified into three groups according to lung function: group of normal lung function, group of PRISm (preserved ratio impaired spirometry) (FEV1, forced expiratory volume during the first second < 80% predicted and FEV1/FVC (forced vital capacity) ≥ 70%) and group of COPD (chronic obstructive pulmonary disease) (FEV1/FVC < 70%). The pre-operational clinicopathological characteristics of these patients were recorded and the markers of systemic inflammatory response, including neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and eosinophils (EOS), were compared between three groups. The expression of Testin in NSCLC samples was detected by IHC and we further explored the correlation between Testin expression and clinicopathological characteristics and prognosis of NSCLC patients. Finally, Cox regression analysis was conducted to study the prognostic factors of NSCLC patients.
Results
Of the 158 NSCLC patients, percentages of normal lung function, PRISm and COPD were 41.4%, 22.8% and 36.1%, respectively. Patients with tumor in the left lung were more likely to have pulmonary dysfunction (PRISm and COPD) than the right lung. The markers of systemic inflammatory response showed differences to various degree in the three groups and NSCLC patients with PRISm or COPD presented more unfavorable prognosis than patients with normal function. The expression of Testin correlated with lymph node metastasis, TNM stage and tumor invasion of NSCLC patients. Moreover, patients with low Testin expression exhibited poorer disease-free survival and overall survival than those with high Testin expression. In Cox regression analysis, we found that PRISm, COPD and Testin expression served as prognostic factors in NSCLC patients.
Conclusions
The presence of COPD or PRISm influenced systemic inflammatory response and prognosis of NSCLC patients. Testin expression correlated with clinicopathological features and could be potentially used as a prognostic marker in NSCLC.
Keywords: NSCLC, Lung function, Testin, Systemic inflammatory response, Prognosis
Introduction
Lung cancer is the dominant cause of cancer-related death worldwide, with NSCLC accounting for nearly 85% [1]. Smoking, airway inflammation and air pollution are the common pathogenic factors of NSCLC [2]. Airway obstruction such as chronic inflammation and even airflow restriction can promote the occurrence and development of NSCLC and affect the response of NSCLC to various treatment [3]. COPD is a major global health problem, which is defined as a FEV1 to FVC ratio smaller than 0.7 [4]. Similar to COPD, PRISm has unique impairment of lung function and do not meet the diagnostic criteria of COPD. As an “non-specific” spirometric pattern, PRISm is defined as a post-bronchodilator FEV1% predicted below 80% with a FEV1/FVC ratio ≥ 0.7 [5]. Both COPD and PRISm have been reported to be associated with prognosis of lung cancer patients [6, 7], but the relationship and molecular links between NSCLC and COPD/PRISm remains not fully elucidated.
Chronic inflammatory and immune responses play vital roles in the development and progression of COPD and PRISm. Serum inflammatory indicators, including neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR) and platelet to lymphocyte ratio (PLR) have been reported to predict treatment outcomes and help to identify patients most likely to benefit from treatment especially in immunotherapy [8, 9]. Moreover, previous studies have found a role of blood eosinophils (EOS) in the early response to PD-1 inhibitors in NSCLC patients [10]. In NSCLC patients treated with immune checkpoint inhibitors therapy, eosinophil measurements during treatment might be useful for predicting prolonged treatment failure [11]. However, there is no report on the difference and clinical value of pre-operative peripheral blood NLR/PLR/LMR/EOS in patients with NSCLC under normal lung function compared with PRISm or COPD.
Testin protein (encoded by TES) is expressed in almost all normal human tissues, while low or lack of Testin expression has been found in prostate cancer, endometrial carcinoma, ovarian, breast, acute lymphoblastic leukemia and nasopharyngeal carcinoma [12–17]. As a kind of tumor suppressor, Testin has been widely studied in gastrointestinal tract, breast, head and neck and intracranial tumors, but its research in lung tumors, especially in NSCLC, is less [18]. Our research team previously found that Testin was lowly expressed in NSCLC tissues compared to normal human lung tissues, and ectopic expression of the Testin gene inhibited cancer cell proliferation, invasion and colony formation in NSCLC cells [19]. Up to now, the nature of Testin expression in NSCLC tissues and its clinical significance has not been well clarified.
Herein, we compared the differences of serum inflammatory indicators, prognostic survival and Testin expression in NSCLC patients with PRISm or COPD. Our results indicated that the coexistence of COPD or PRISm influenced systemic inflammatory response and prognosis of NSCLC patients. And Testin expression correlated with clinicopathological characteristics and survival of NSCLC.
Materials and methods
Patient selection
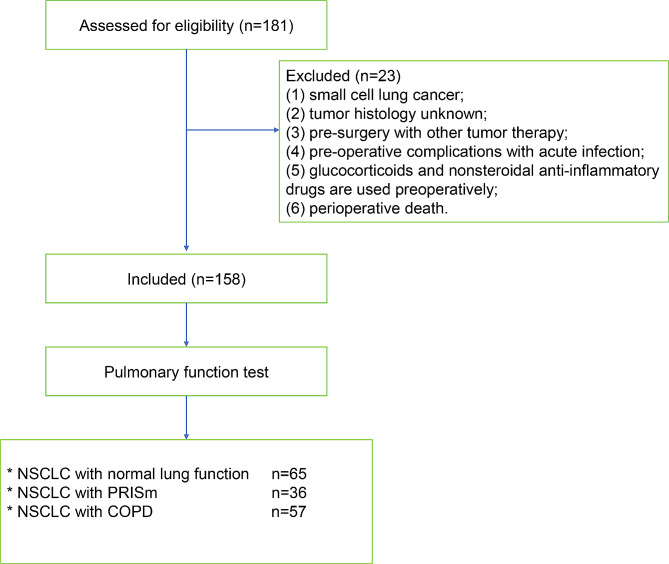
Lung cancer patients (stages I to IIIA), diagnosed with NSCLC from January 2010 to December 2018 at the Second Affiliated Hospital of Xu Zhou Medical University were consecutively enrolled in the study cohort. Inclusion criteria was: (1) NSCLC was diagnosed pathologically after surgery; (2) patients had undergone a baseline pulmonary function test; (3) complete medical records and follow-up information are available. Exclusion criteria was: (1) small cell lung cancer; (2) tumor histology unknown; (3) pre-surgery with other tumor therapy; (4) pre-operative complications with acute infection; (5) glucocorticoids and nonsteroidal anti-inflammatory drugs are used preoperatively; (6) perioperative death. Totally 158 patients were included in our study. Based on the results of lung function tests, participants were classified into three groups: the normal lung function group included patients with FEV1 ≥ 80% of predicted and FEV1/FVC ≥ 0.7; the PRISm group included patients with FEV1 < 80% of predicted value and FEV1/FVC ≥ 0.7; the COPD group included patients with FEV1/FVC < 0.7 (Fig. 1).
Fig. 1.
Screening and inclusion process for patients
Data acquisition
By consulting the electronic medical record system, demographic data included age, gender, smoking history, maximum tumor diameter, histological type, lymph node metastasis status, location, invasion, TNM stage (according to the eighth of TNM) classification), lung function and serum inflammatory indicators (NLR, PLR, LMR and EOS). The prognosis data of overall survival (OS) and disease-free survival (DFS) was obtained by phone follow-up and survival intervals were dated at December 31, 2020. In addition, we collected archived formalin-fixed and paraffin-embedded tumor tissues from 158 NSCLC patients. Ki-67 antigen is a protein associated with intracellular division and proliferation, which is expressed in S, G1, G2, and M phases of the cell cycle, and is often used as a reliable marker of tumor cell proliferation activity [20]. The expression of Testin and Ki-67 were analyzed by immunohistochemistry (IHC). Written informed consent was obtained from all patients, and protocols for this study were approved by the Ethics Committee of Second Affiliated Hospital of Xu Zhou Medical University.
Immunohistochemistry
Tumor Sect. (3-µm) were cut from formalin-fixed paraffin-embedded blocks and mounted on positive-charged slides. The primary antibody was goat anti-Testin and anti-Ki-67 polyclonal antibody (Santa Cruz Biotechnology). The paraffin sections were placed in a xylene bath for 10 min to remove paraffin, and repeated again and then placed in an ethanol gradient for rehydration. Antigen retrieval was performed with EDTA (pH 8.0) repair solution in a microwave, cooled to room temperature, treated with 3% H2O2 for 10 min for inactivation of endogenous peroxidase, rinsed with 1× PBST (0.1% Tween), incubated with 5% rabbit serum at room temperature for 15 min, and then incubated with primary antibody (1:100) at 4˚C overnight. The sections were then rinsed and incubated with biotin-labeled secondary antibody (SP KIT; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at 37˚C for 15 min, rinsed in 1× PBST (0.1% Tween) and then incubated with horseradish peroxidase (SP KIT; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) at 37˚C for 15 min. The sections were then treated with DAB for 10 min and the reaction was terminated. All sections were observed in at least five areas at a magnification of 400 by at least two investigators in a blinded manner. Cytoplasm and nuclei were counterstained with hematoxylin solution. Two pathologists who were blinded to patients’ clinical data examined all slides separately. The method of assessing the density of Testin in tumor was as described by our previous studies [19]. In brief, numbers of Testin in tumor were identified according to the immunohistochemical staining and were counted as follows: the total number of cells and positive cells were counted and the staining was scored as the percentages of positive cells: 0 (no staining) for specimens with positive cells ≤ 5%; 1 (weak staining) for specimens with positive cells > 5% and ≤ 25%; 2 (moderate staining) for specimens with positive cells > 25% and ≤ 50%; 3 (strong staining) for specimens with positive cells > 50%. Specimens with scores of ≤ 1 were regarded as negative and specimens with scores of > 1 were regarded as positive.
Statistical analysis
Continuous variables were presented as medians and inter-quartile ranges (IQRs) and were compared using the Kruskal–Wallis test. Categorical variables, expressed as the numbers and percentages of participants, were compared using the Chi-square test. The survival rate was estimated by using the Kaplan–Meier method and compared using the log-rank (Mantel–Cox) test. Risk factors for survival were analyzed using Cox proportional hazards regression model. Variables with a p-value < 0.20 in the univariate analysis were included in the multivariate analysis. In this risk-adjusted analysis, backward stepwise methods were applied to determine the independent factors that were associated with survival. Statistical significance was set at p < 0.05. All statistical analyses were performed using the SAS 9.13 software package (version 9.13; SAS Institute, Cary, NC USA) and Prism 8.0.
Results
Clinicopathological characteristics of patients
The baseline clinicopathological characteristics of the patients were presented in Table 1. A total of 158 patients were included in our study, among which 91(57.6%) were younger than 65 years old and 123 (77.8%) were male. These patients were divided into NSCLC only (n = 65, 41.1%), NSCLC with PRISm (n = 36, 22.8%) and NSCLC with COPD (n = 57, 36.1%) groups based on pulmonary function. Clinicopathological characteristics of the patients were compared, and results indicated that there were no statistically significant differences in age, gender, smoking history, maximum tumor diameter, histological type, lymph node metastasis status, invasion, Testin expression, Ki-67 expression among the three groups (p > 0.05). Whereas the three groups differed in tumor location. Patients with tumor in the left lung were more likely to develop pulmonary dysfunction (PRISm and COPD) than the right lung (p = 0.027).
Table 1.
Clinicopathological parameters of included patients
| Characteristics | Overall | NSCLS only | NSCLC with PRISm | NSCLC with COPD | P value |
|---|---|---|---|---|---|
| n = 158 | n = 65(41.1%) | n = 36(22.8%) | n = 57(36.1%) | ||
| Age(years) | 0.428 | ||||
| ≤ 65 | 91(57.6%) | 41(63.1%) | 18(50%) | 32(56.1%) | |
| >65 | 67(42.4%) | 24(36.9%) | 18(50%) | 25(43.9%) | |
| Gender | 0.237 | ||||
| Male | 123(77.8%) | 54(83.1%) | 28(77.8%) | 40(70.2%) | |
| Female | 35(22.2%) | 11(16.9%) | 8(22.2%) | 17(29.8%) | |
| Smoking status(pack/year) | 0.464 | ||||
| <30 | 69(43.7%) | 28(43.1%) | 13(36.1%) | 28(49.1%) | |
| ≥ 30 | 89(56.3%) | 37(56.9% | 23(63.9%) | 29(50.9%) | |
| Maximum tumor diameter (cm) | 0.672 | ||||
| ≤ 3 | 60(37.95%) | 27(41.5%) | 12(33.3%) | 21(36.8%) | |
| 3< d ≤ 5 | 60(37.95%) | 21(32.3%) | 14(38.9%) | 25(43.9%) | |
| 5< d ≤ 7 | 20(12.7%) | 7(10.8%) | 6(16.7%) | 7(12.3%) | |
| >7 | 18(11.4%) | 10(15.4%) | 4(11.1%) | 4(7%) | |
| Histological type | 0.421 | ||||
| Squamous | 77(48.7%) | 30(46.2%) | 21(58.3%) | 26(45.6%) | |
| Adenocarcinoma | 66(41.8%) | 26(40%) | 13(36.1%) | 27(47.4%) | |
| Others | 15(9.5%) | 9(13.8% | 2(5.6%) | 4(7%) | |
| pN status | 0.228 | ||||
| N0 | 89(56.3%) | 32(49.2%) | 26(72.2%) | 31(54.4%) | |
| N1 | 34(21.5%) | 15(23.1%) | 6(16.7%) | 13(22.8%) | |
| N2-N3 | 35(22.2%) | 18(27.7%) | 4(11.1%) | 13(22.8%) | |
| Location | 0.027 | ||||
| Right lung | 76(48.1%) | 39(60%) | 12(33.3%) | 25(43.9%) | |
| Left lung | 82(51.9%) | 26(40%) | 24(66.7%) | 32(56.1%) | |
| Invasion | 0.455 | ||||
| Yes | 71(44.9%) | 30(46.2%) | 13(36.1%) | 28(49.1%) | |
| NO | 87(55.1%) | 35(53.8%) | 23(63.9%) | 29(50.9%) | |
| Textin expression | 0.943 | ||||
| High | 86(54.4%) | 36(55.4%) | 20(55.6%) | 30(52.6%) | |
| Low | 72(45.6%) | 29(44.6%) | 16(44.4%) | 27(47.4%) | |
| Ki-67 | 0.415 | ||||
| ≤ 30 | 63(39.9%) | 27(41.5%) | 11(30.6%) | 25(43.9%) | |
| >30 | 95(60.1%) | 38(58.5%) | 25(69.4%) | 32(56.1%) |
Serum inflammatory indicators of the three groups
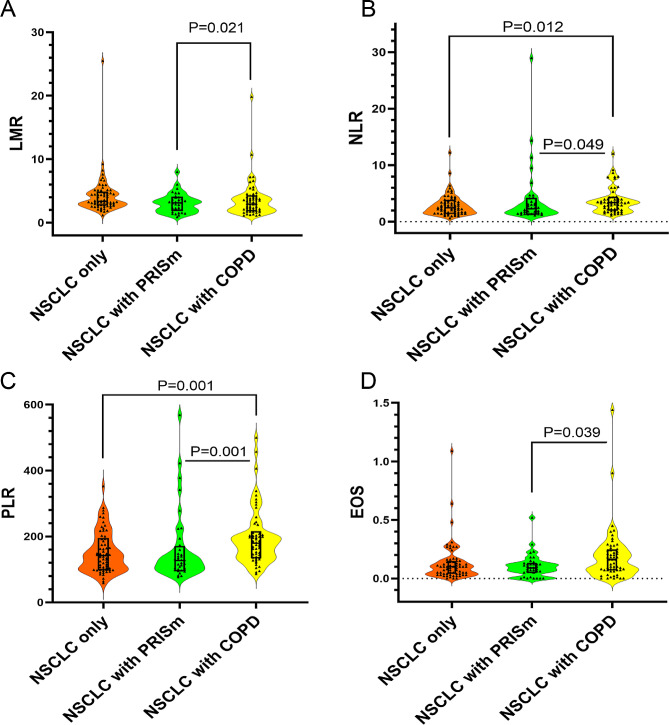
Lymphocyte to monocyte ratio (LMR) of COPD group was higher than that of PRISm group (p = 0.021) (Fig. 2A). For neutrophil to lymphocyte ratio (NLR), it was higher in COPD group compared with NSCLC only group (p = 0.012) or PRISm group (p = 0.049) (Fig. 2B). Similarly, platelet to lymphocyte ratio (PLR) was higher in COPD group compared with NSCLC only group (p = 0.001) or PRISm group (p = 0.001) (Fig. 2C). As for eosinophils (EOS), we found that it was higher in COPD group compared with PRISm group (Fig. 2D).
Fig. 2.
Characteristics of serum inflammatory indicators in three groups. (A) Lymphocyte to monocyte ratio (LMR) in three groups. (B) Neutrophil to lymphocyte ratio (NLR) in three groups. (C) Platelet to lymphocyte ratio (PLR) in three groups. (D) Eosinophils (EOS) in three groups
Prognosis of the three groups
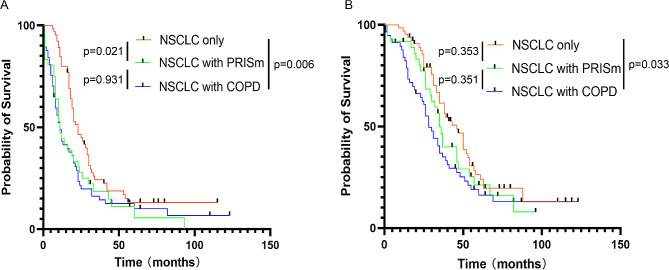
There were significant differences of disease-free survival (DFS) in PRISm (p = 0.021) and COPD (p = 0.006) group compared with NSCLC only group, while there was no difference between PRISm and COPD group (p = 0.931) (Fig. 3A). For overall survival (OS), there was significant difference between NSCLC only group and COPD group (p = 0.033). Nevertheless, no statistic difference of overall survival was found between other groups (NSCLC only group vs. PRISm group, p = 0.353; PRISm group vs. COPD group, p = 0.351) (Fig. 3B).
Fig. 3.
Prognosis of the three groups. (A) Disease-free survival in three groups. (B) Overall survival in three groups
Testin expression with clinicopathological characteristics of NSCLC patients
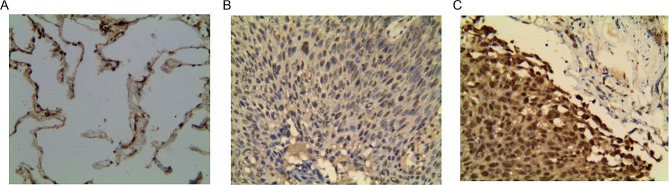
The expression of Testin protein in 158 NSCLC patients was detected by IHC (Figure 4A-C). These patients were divided into group of Testin low expression and group of Testin high expression according to the IHC staining of Testin. Combined with the clinicopathological characteristics of patients, we found that Testin expression correlated with pN status (p = 0.006), TNM stage (p = 0.0012) and tumor invasion (p = 0.0055). Patients of Testin low expression exhibited more advanced characteristics in lymph node metastasis, TNM stage and tumor invasion (Table 2).
Fig. 4.
Examples of Testin in NSCLC. (A) No Testin expression. (B) Testin low expression. (C) Testin high expression
Table 2.
Clinicopathological characteristics of patients grouped by Testin expression
| Clinicopathological features | Testin | Total | P Value | |
|---|---|---|---|---|
| Low (n = 72) | High(n = 86) | |||
| Age (years) | 0.425 | |||
| ≤ 65 | 39(54.2%) | 52(60.5%) | 91(57.6%) | |
| >65 | 33(45.8%) | 34(39.5%) | 67((42.4%) | |
| Gender | 0.129 | |||
| Male | 60(83.3%) | 63(73.3%) | 123(77.8%) | |
| Female | 12(16.7%0 | 23(26.7%) | 35(22.2%) | |
| Maximum tumor diameter (cm) | 0.494 | |||
| ≤ 3 | 25(34.7%) | 35(40.7%) | 60(37.95%) | |
| 3 < d ≤ 5 | 26(36.1%) | 34(39.5%) | 60(37.95%) | |
| 5 < d ≤ 7 | 10(13.9%) | 10(11.6%) | 20(12.7%) | |
| > 7 | 11(15.3%) | 7(8.2%) | 18(11.4%) | |
| Histological type | 0.580 | |||
| Squamous | 32(44.4%) | 45(52.3%) | 77(48.7%) | |
| Adenocarcinoma | 32(44.4%) | 34(39.5%) | 66(41.8%) | |
| others | 8(11.2%) | 7(8.2%) | 15(9.5%) | |
| Tumor differentiation | 0.642 | |||
| Low-medium | 42(58.3%) | 47(54.7%) | 89(56.3%) | |
| Medium-high | 30(41.7%) | 39(45.3%) | 69(43.7%) | |
| pN status | 0.006 | |||
| N0 | 29(40.3%) | 60(69.8%) | 89(56.3%) | |
| N1 | 19(26.4%) | 15(17.4%) | 34((21.5%) | |
| N2-N3 | 24(33.3%) | 11(12.8%) | 35(22.2%) | |
| Ki-67 | 0.162 | |||
| ≤30 | 33(45.8%) | 30(34.9%) | 63(39.9%) | |
| >30 | 39(54.2%) | 56(65.1%) | 95(60.1%) | |
| TNM stage | 0.0012 | |||
| I | 8(9.4%) | 24(32.9%) | 32(20.3%) | |
| II | 36(42.4%) | 22(30.1%) | 58(36.7%) | |
| III | 41(48.2%) | 27(37.0%) | 68(43.0%) | |
| Invasion | 0.0055 | |||
| Yes | 41(56.9) | 30(34.9) | 71(44.9%) | |
| NO | 31(43.1) | 56(65.1) | 87(55.1%) | |
| Group | 0.472 | |||
| NSCLC only | 26(36.1%) | 39(45.3%) | 65(41.1%) | |
| PRISm | 17(23.6%) | 19(22.1%) | 36(22.8%) | |
| COPD | 29(40.3%) | 28(32.6%) | 57(36.1%) | |
Testin expression with prognosis of NSCLC patients
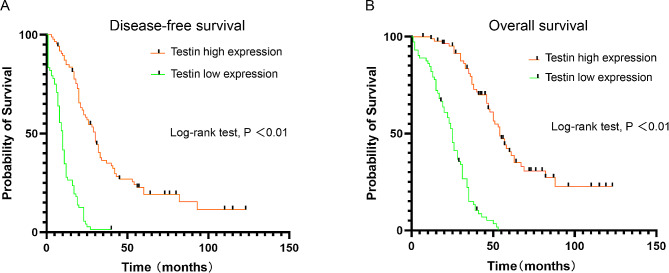
Patients with Testin low expression had poorer disease-free survival(10.0 ± 10.0 months)than those with Testin high expression(27.5 ± 26.0 months) (p<0.01) (Fig. 5A). Likewise, patients with Testin low expression had more unfavorable overall survival time(25.0 ± 16.75 months) than those with Testin high expression(47.0 ± 27.5 months)(p<0.01) (Fig. 5B).
Fig. 5.
The relationship between Testin expression and prognosis. (A) Testin expression with disease-free survival; (B) Testin expression with overall survival
Prognostic analysis of NSCLC patients
Univariate Cox regression was performed to analyze the prognostic factors of NSCLC. Results showed that old age (p = 0.0294), stage III (p = 0.0079), Testin low expression (p < 0.0001), N1-N3 (p = 0.0053), low-medium differentiation (p = 0.0438), invasion (p < 0.0001), group of PRISm or COPD (p = 0.0082) were associated with poor prognosis for disease-free survival (DFS). While old age (p = 0.0002), stage III (p = 0.0002), Testin low expression (p < 0.0001), T3-T4 (p = 0.0042), N1-N3 (p = 0.001), invasion (p = 0.0055), group of PRISm or COPD (p = 0.0264) were associated with poor prognosis for overall survival (OS) (Table 3). Furthermore, multivariate Cox regression analysis revealed that age (HR = 1.041; 95%CI = 1.021–1.064; p < 0.0001), Testin expression (HR = 0.156; 95%CI = 0.101–0.241; p < 0.0001), Ki-67 expression (HR = 1.624; 95%CI = 1.120–2.354; p = 0.0106), invasion (HR = 1.866; 95%CI = 1.281–2.720; p = 0.0012) and group of PRISm or COPD (HR = 1.291; 95%CI = 1.056–1.577; p = 0.0126) were independent prognostic factors for disease free survival (DFS) (Table 4). And for overall survival (OS), age (HR = 1.067; 95%CI = 1.043–1.092; p < 0.0001), stage (HR = 1.584; 95%CI = 1.075–2.334; p = 0.0200), Testin expression (HR = 0.123; 95%CI = 0.076–0.198; p < 0.0001) and group of PRISm or COPD (HR = 1.299; 95%CI = 1.048–1.610; p = 0.017) were independent prognostic factors (Table 5).
Table 3.
Univariate Cox regression
| Variables | Disease free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| Hazard radio | 95% CI | p | Hazard radio | 95% CI | p | |
| Gender | 0.382 | 0.4896 | ||||
| Male | 1 | 1 | ||||
| Female | 1.194 | 0.803–1.775 | 1.165 | 0.757–1.795 | ||
| Age | 0.0294 | 0.0002 | ||||
| ≤ 60 | 1 | 1 | ||||
| > 60 | 1.022 | 1.002–1.043 | 1.042 | 1.020–1.066 | ||
| Stage | 0.0079 | 0.0002 | ||||
| I-II | 1 | 1 | ||||
| III | 1.59 | 1.129–2.239 | 2.026 | 1.404–2.923 | ||
| Testin expression | <0.0001 | <0.0001 | ||||
| Low | 1 | 1 | ||||
| High | 0.193 | 0.130–0.287 | 0.141 | 0.090–0.219 | ||
| Ki-67 | 0.2764 | 0.8062 | ||||
| ≤ 30 | 1 | 1 | ||||
| > 30 | 1.209 | 0.859-1.700 | 1.047 | 0.725–1.513 | ||
| pT status | 0.0548 | 0.0042 | ||||
| T1-T2 | 1 | 1 | ||||
| T3-T4 | 1.4 | 0.993–1.975 | 1.707 | 1.183–2.464 | ||
| pN status | 0.0053 | 0.001 | ||||
| N0 | 1 | 1 | ||||
| N1-N3 | 1.62 | 1.154–2.272 | 1.844 | 1.280–2.658 | ||
| Pathology | 0.3625 | 0.0559 | ||||
| S | 1 | 1 | ||||
| A-AS Mixed | 1.169 | 0.835–1.636 | 1.428 | 0.991–2.058 | ||
| Differentiation | 0.0438 | 0.0953 | ||||
| Low-medium | 1 | 1 | ||||
| Medium-high | 0.707 | 0.505–0.990 | 0.734 | 0.511–1.056 | ||
| Invasion | <0.0001 | 0.0055 | ||||
| None | 1 | 1 | ||||
| Yes | 2.022 | 1.421–2.877 | 1.714 | 1.171–2.507 | ||
| Tumor size (cm) | 0.2377 | 0.2328 | ||||
| ≤ 5 | 1 | 1 | ||||
| >5 | 1.267 | 0.855–1.876 | 1.288 | 0.850–1.950 | ||
| Group | 0.0082 | 0.0264 | ||||
| NSCLC only | 1 | 1 | ||||
| PRISm or COPD | 1.29 | 1.068–1.558 | 1.262 | 1.028–1.551 | ||
Table 4.
Multivariate Cox regression of disease free survival
| Variables | Disease free survival | ||
|---|---|---|---|
| Hazard radio | 95% CI | p | |
| Age | 1.042 | 1.021–1.064 | <0.0001 |
| Testin | 0.156 | 0.101–0.241 | <0.0001 |
| Invasion | 1.866 | 1.281–2.720 | 0.0012 |
| Group of PRISm or COPD | 1.291 | 1.056–1.577 | 0.0126 |
Table 5.
Multivariate Cox regression of overall survival
| Variables | Overall survival | ||
|---|---|---|---|
| Hazard radio | 95% CI | p | |
| Age | 1.067 | 1.043–1.092 | <0.0001 |
| Stage | 1.584 | 1.075–2.334 | 0.02 |
| Testin | 0.123 | 0.076–0.198 | <0.0001 |
| Group of PRISm and COPD | 1.299 | 1.048–1.610 | 0.017 |
Discussion
Chronic airway inflammation plays an important role in the occurrence and development of chronic obstructive pulmonary disease and lung cancer [21]. Siemes et al. followed up a group of more than 7000 people without malignant tumors for about 10 years. The results showed that the possibility of cancer increased when CRP (C-reactive protein) > 3 mg/dl [22]. Danish researchers measured baseline CRP, fibrinogen and leukocyte levels in 8656 patients with COPD and followed them up for 5 years [23]. It was found that patients with elevated inflammatory indexes had a four-fold increased risk of lung cancer. PRISm was previously considered to be an unclassified and restricted lung function impairment disease [24]. Like COPD, it has the change of decreased FEV1 and chronic airway inflammation. Patients with PRISm are more likely to be male, smoker, and tend to have a higher basis of metabolic syndrome and cardiovascular and cerebrovascular diseases [25, 26].A recent study from South Korea demonstrated that NSCLC patients with PRISm had a worse survival prognosis than those with COPD or normal lung function [6]. And COPD was associated with postoperative development of respiratory failure after thoracic surgery [27]. Moreover, for patients with COPD, smoking cessation is useful to improve symptoms, respiratory function and metabolic parameters in the short term [28]. In our study, we found that patients with PRISm or COPD exhibited poorer disease-free survival (DFS) than the normal lung function group. When comparing the overall survival (OS) of NSCLC patients with different lung function states in the three groups, there was only significant difference between COPD group and normal lung function group. The reason why it is not completely consistent with previous studies may be related to the baseline level differences of the patients, such as the selected population, sample size, age and tumor stage.
In the occurrence, development and prognosis of chronic lung inflammation and lung tumors, systemic inflammatory indicators, such as NLR, LMR, PLR and EOS, can play a critical role in innate and adaptive immunity as effective markers [29, 30]. Neutrophils are constantly recruited and activated in COPD lungs, producing a large number of oxidants such as ROS, causing DNA oxidative damage and increasing the incidence of lung tumors. Neutrophil elastase secreted by neutrophils can directly activate TLR4 signaling pathway, thus promoting the expression of CXCL8 in bronchial epithelial cells and the production of mucin by activating EGFR [31, 32]. From the perspective of adaptive immunity, COPD pulmonary inflammation affects the ability of CD8+ cells to clear tumor cells, and impacts the balance of Th1/Th2, the proportion of regulatory T cells and Th17 cells, and the expression of programmed cell death protein 1 (PD-1) and its ligand (PD-L1) in CD8+ T cells, resulting in cell cycle arrest and T cell inactivity [33]. A study including 3,656 patients showed NLR to be potentially a useful biomarker to predict the poor prognosis for NSCLC [34]. The research of Lin et al. indicated that LMR was potentially used as an independent prognostic factor for survival in previously untreated metastatic NSCLC [35]. In a study comprising 3430 patients, Gu et al. found that PLR was associated with unfavorable survival in advanced NSCLC [36]. Similarly, peripheral EOS was reported to be connected with the effect of immune checkpoint inhibitor treatment in NSCLC patients [37]. In our study, we found that there were differences to various degree of serum inflammatory indicators in different lung function groups. The coexistence of PRISm or COPD influenced the systemic inflammatory response of NSCLC patients and these results provided a basis for the individualized treatment and management of NSCLC patients with pulmonary dysfunction.
Testin is a protein expressed in a wide range of normal human tissues and locates in the cytoplasm along stress fibers being recruited to focal adhesions [38]. Latest reports indicated that Testin served as a tumor suppressor in the carcinogenesis of multiple types of cancers, including colorectal cancer [39], childhood acute lymphoblastic leukaemia [40],nasopharyngeal carcinoma [17], breast cancer [41] and gastric cancer [42], however the mechanism of loss of Testin expression is still unknown. Testin participates in the processes of cell cycle, tumor growth, apoptosis, epithelial-mesenchymal transition, angiogenesis, and metastasis [18], suggesting its potential usage in the diagnosis and therapy of cancer. Our previous study identified that Testin expression was reduced in NSCLC cell lines and overexpression of Testin significantly inhibited tumor growth of NSCLC both in vitro and in vivo [19]. In the present study, our results revealed that although there was no difference in Testin expression among the three groups of different pulmonary function, Testin expression correlated with poor clinicopathological parameters of NSCLC patients, including pN status, TNM stage and tumor invasion. Furthermore, patients with relative higher expression of Testin survive longer than those with lower ones and Cox regression analysis indicated that Testin served as an independent risk factor of prognosis for NSCLC patients. Our study adds the understanding of Testin in NSCLC and provides potential markers for the diagnosis and treatment of NSCLC.
In conclusion, this study assessed the pre-operational clinicopathological characteristics of NSCLC patients with PRISm or COPD. NSCLC patients with different lung function exerted differences in systemic inflammatory response and prognosis. Testin was clinically relevant with NSCLC and served as a promising marker for predicting prognosis of operatable NSCLC patients.
Author contributions
Chunhua Ling and Jing Luo designed and supervised the study. Yanmin Zhang and Qian Zhang collected tissue samples and integrated medical information of the patients. Qian Wang performed IHC experiments and Gaoming Wang was responsible for statistical analysis. All authors read and approved the final version of the manuscript.
Funding
This research was supported by Xuzhou Applied Basic Research Science and Technology Project (No. KC19039), National Natural Science Foundation of China (No. 82273325), the Science and Technology Program of Xu Zhou (KC22166) and Science and Technology Project of Xuzhou Health Commission (XWKYHT20210551).
Data availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanmin Zhang, Gaoming Wang and Qian Zhang contributed equally to this work.
Contributor Information
Jing Luo, Email: luojing_2767983@163.com.
Chunhua Ling, Email: lingchunhua83@163.com.
References
- 1.Tan WL, et al. Asian Thoracic Oncology Research Group Expert Consensus Statement on Optimal Management of Stage III NSCLC. J Thorac Oncol. 2020;15(3):324–43. doi: 10.1016/j.jtho.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Houghton AM. Common mechanisms linking Chronic Obstructive Pulmonary Disease and Lung Cancer. Ann Am Thorac Soc. 2018;15(Suppl 4):S273–7. doi: 10.1513/AnnalsATS.201808-537MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim JU, et al. Impact of Combined Chronic Obstructive Pulmonary Disease Status and systemic inflammation on Outcome of Advanced NSCLC: Multicenter Retrospective Cohort Study. Int J Chron Obstruct Pulmon Dis. 2020;15:3323–34. doi: 10.2147/COPD.S274354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viniol C, Vogelmeier CF. Exacerbations COPD Eur Respir Rev, 2018. 27(147). [DOI] [PMC free article] [PubMed]
- 5.Wei X, et al. Imaging Features of Chronic Bronchitis with preserved ratio and impaired spirometry (PRISm) Lung. 2018;196(6):649–58. doi: 10.1007/s00408-018-0162-2. [DOI] [PubMed] [Google Scholar]
- 6.Heo IR, et al. Impact of coexistent preserved ratio impaired spirometry on the survival of patients with lung cancer: analysis of data from the Korean Association for Lung Cancer Registry. Thorac Cancer. 2021;12(18):2478–86. doi: 10.1111/1759-7714.14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caramori G, et al. Molecular links between COPD and lung cancer: new targets for drug discovery? Expert Opin Ther Targets. 2019;23(6):539–53. doi: 10.1080/14728222.2019.1615884. [DOI] [PubMed] [Google Scholar]
- 8.Mandaliya H, et al. Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung canc er inflammation index (ALI) Transl Lung Cancer Res. 2019;8(6):886–94. doi: 10.21037/tlcr.2019.11.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo A, et al. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with Nivolumab in Pretreated Non-small Cell Lung Cancer (NSCLC): a large Retrospective Multicenter Study. Adv Ther. 2020;37(3):1145–55. doi: 10.1007/s12325-020-01229-w. [DOI] [PubMed] [Google Scholar]
- 10.Sibille A, et al. Clinical benefit to programmed death-1 inhibition for non-small-cell lung cancer is associated with h igher blood eosinophil levels. Acta Oncol. 2020;59(3):257–9. doi: 10.1080/0284186X.2019.1695063. [DOI] [PubMed] [Google Scholar]
- 11.Osawa H et al. Association between time to treatment failure and peripheral eosinophils in patients with non-small c ell lung cancer treated with immune checkpoint inhibitors. Pol Arch Intern Med, 2021. 131(10). [DOI] [PubMed]
- 12.Gu Z, et al. TESTIN suppresses tumor growth and invasion via manipulating cell cycle progression in endometrial carcinoma. Med Sci Monit. 2014;20:980–7. doi: 10.12659/MSM.890544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H, et al. Frequent hypermethylation and loss of heterozygosity of the testis derived transcript gene in ovarian cancer. Cancer Sci. 2010;101(5):1255–60. doi: 10.1111/j.1349-7006.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, et al. Testin is a tumor suppressor and prognostic marker in breast cancer. Cancer Sci. 2012;103(12):2092–101. doi: 10.1111/cas.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weeks RJ, et al. TESTIN induces Rapid Death and suppresses proliferation in Childhood B Acute Lymphoblastic Leukaemia cells. PLoS ONE. 2016;11(3):e0151341. doi: 10.1371/journal.pone.0151341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong R, et al. TESTIN was commonly hypermethylated and involved in the epithelial-mesenchymal transition of endometrial cancer. APMIS. 2015;123(5):394–400. doi: 10.1111/apm.12361. [DOI] [PubMed] [Google Scholar]
- 17.Zhong Z, Zhang F, Yin SC. Effects of TESTIN gene expression on proliferation and migration of the 5-8F nasopharyngeal carcinoma cell line. Asian Pac J Cancer Prev. 2015;16(6):2555–9. doi: 10.7314/APJCP.2015.16.6.2555. [DOI] [PubMed] [Google Scholar]
- 18.Popiel A, Kobierzycki C, Dziegiel P. The role of Testin in Human cancers. Pathol Oncol Res. 2019;25(4):1279–84. doi: 10.1007/s12253-018-0488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, et al. Testin is a tumor suppressor in non-small cell lung cancer. Oncol Rep. 2017;37(2):1027–35. doi: 10.3892/or.2016.5316. [DOI] [PubMed] [Google Scholar]
- 20.Menon SS, et al. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45. doi: 10.1016/j.cca.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Ceelen JJ, Langen RC, Schols AM. Systemic inflammation in chronic obstructive pulmonary disease and lung cancer: common driver of pulm onary cachexia? Curr Opin Support Palliat Care. 2014;8(4):339–45. doi: 10.1097/SPC.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 22.Siemes C, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam S tudy. J Clin Oncol. 2006;24(33):5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 23.Thomsen M, et al. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(10):982–8. doi: 10.1164/rccm.201206-1113OC. [DOI] [PubMed] [Google Scholar]
- 24.Marruchella A. Preserved ratio impaired spirometry and interstitial lung abnormalities in smokers. Am J Respir Crit Care Med. 2019;199(10):1293. doi: 10.1164/rccm.201812-2305LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijnant SRA et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J, 2020. 55(1). [DOI] [PubMed]
- 26.Heo IR, Kim HC, Kim TH. Health-Related Quality of Life and related factors in persons with preserved ratio impaired spirometr y: data from the Korea National Health and Nutrition Examination Surve. Med (Kaunas), 2020. 57(1). [DOI] [PMC free article] [PubMed]
- 27.Pezzuto A, et al. Predictors of respiratory failure after thoracic surgery: a retrospective cohort study with comparison between lobar and sub-lobar resection. J Int Med Res. 2022;50(6):3000605221094531. doi: 10.1177/03000605221094531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pezzuto A, et al. Short-term benefits of Smoking Cessation improve respiratory function and metabolism in smokers. Int J Chron Obstruct Pulmon Dis. 2023;18:2861–5. doi: 10.2147/COPD.S423148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing X, et al. Systemic inflammatory response markers Associated with Infertility and Endometrioma or Uterine Leiomyoma in Endometriosis. Ther Clin Risk Manag. 2020;16:403–12. doi: 10.2147/TCRM.S232849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tataroglu C, et al. Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC) Lung Cancer. 2004;43(1):47–54. doi: 10.1016/j.lungcan.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Lantuejoul S, et al. Telomere maintenance and DNA damage responses during lung carcinogenesis. Clin Cancer Res. 2010;16(11):2979–88. doi: 10.1158/1078-0432.CCR-10-0142. [DOI] [PubMed] [Google Scholar]
- 32.Caramori G, et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66(6):521–7. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- 33.McKendry RT, et al. Dysregulation of antiviral function of CD8(+) T cells in the Chronic Obstructive Pulmonary Disease Lu Ng. Role of the PD-1-PD-L1 Axis. Am J Respir Crit Care Med. 2016;193(6):642–51. doi: 10.1164/rccm.201504-0782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu XB, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analy sis. Sci Rep. 2015;5:12493. doi: 10.1038/srep12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin GN, et al. Prognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated m etastatic non-small cell lung cancer patients receiving platinum-based doublet. Med Oncol. 2014;31(7):70. doi: 10.1007/s12032-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 36.Gu X, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3,430 p atients. Sci Rep. 2016;6:23893. doi: 10.1038/srep23893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osawa H, et al. Absolute increase in the number and proportion of Peripheral eosinophils Associated with Immune checkpoint inhibitor treatment in non-small cell Lung Cancer patients. Cancer Diagn Progn. 2021;1(5):485–90. doi: 10.21873/cdp.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papanikolaou M, Crump SM, Abbott GW. The focal adhesion protein Testin modulates KCNE2 potassium channel beta subunit activity. Channels (Austin) 2021;15(1):229–38. doi: 10.1080/19336950.2021.1874119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Qiao Q. The relationship between TESTIN expression and the prognosis of colorectal cancer. Pathol Res Pract. 2022;232:153744. doi: 10.1016/j.prp.2021.153744. [DOI] [PubMed] [Google Scholar]
- 40.Weeks RJ, et al. Silencing of TESTIN by dense biallelic promoter methylation is the most common molecular event in childhood acute lymphoblastic leukaemia. Mol Cancer. 2010;9:163. doi: 10.1186/1476-4598-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarti M, et al. Differential expression of testin and survivin in breast cancer subtypes. Oncol Rep. 2013;30(2):824–32. doi: 10.3892/or.2013.2502. [DOI] [PubMed] [Google Scholar]
- 42.Ma H, et al. Extensive analysis of D7S486 in primary gastric cancer supports TESTIN as a candidate tumor suppressor gene. Mol Cancer. 2010;9:190. doi: 10.1186/1476-4598-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.