Abstract
We have used a strategy for colocalization of Psi (Ψ)-tethered ribozymes and targets to demonstrate that Ψ sequences are capable of specific interaction in the cytoplasm of both packaging and nonpackaging cells. These results indicate that current in vitro dimerization models may have in vivo counterparts. The methodology used may be applied to further genetic analyses on Ψ domain interactions in vivo.
All retroviruses require packaging of two genomic RNAs for infectivity. The viral sequences that direct correct encapsidation, known as the psi (Ψ) element, are located in the 5′ end of the genomic RNA beginning downstream from the primer binding site and extending into the 5′ gag coding region of the viral RNA (1, 4, 16, 23). Extracted retroviral genomic RNAs of various origins have been shown by electron microscopy to be linked close to their 5′ ends in a parallel orientation, forming dimers (5, 15, 18); the dimerized region maps within the Ψ element. Sequences within the Ψ element forming a series of conserved stem-loops have been shown to be capable of dimer formation under appropriate in vitro conditions. Deletion or modification of these sequences in the context of the virus have effects on encapsidation or infectivity, depending on the exact mutation. Consequently, dimerization is thought to be required in vivo both in selective encapsidation of unspliced viral genomic-length RNAs and in the early steps of replication occurring immediately postinfection.
In Moloney murine leukemia virus (MoMuLV), in vitro analyses have demonstrated that dimerization can occur in the absence of virally encoded proteins under appropriate salt and temperature conditions (11, 19, 22). Kinetic studies suggest that dimerization is a multistep process involving a series of intermolecular associations between structured elements in the Ψ domains. The addition of the MoMuLV nucleocapsid protein 10 (NCp10), derived from the gag precursor in vivo, stimulates the in vitro formation of dimers (7). Thus far, stimulation of dimer formation by NCp10 is confined to subgenomic RNAs, and no in vitro dimerization of full-length viral genomic RNAs has yet been observed. Based upon the in vitro observations, it is believed that formation of stable dimers of genomic viral RNAs in vivo requires virally encoded gag protein(s).
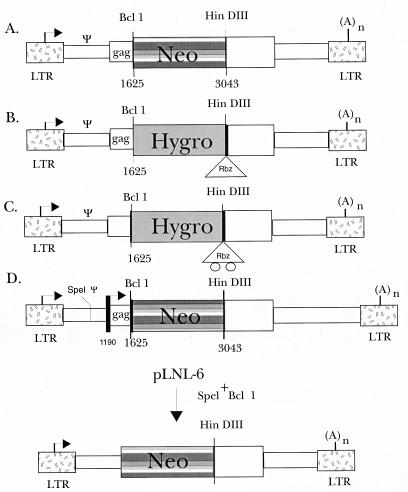
In order to investigate the in vivo mechanics of the dimerization reaction, we have devised a ribozyme strategy which takes advantage of the demonstrated virion colocalization of Ψ-tethered ribozyme and targets (21). We wished to determine whether or not the Ψ element would allow ribozyme-target colocalization in the cytoplasm of the infected cell independent of packaging, and if so, whether viral proteins were required for this process. The experimental design therefore is dependent upon the formation of heterodimers between the ribozyme-encoding RNAs and the target RNAs. Many factors could bias the formation of homodimers over heterodimers, including the nature of the Ψ element, the levels of transcripts, and the coexpression of transcripts. To address this problem, we have designed target and ribozyme retroviral vectors derived from the MoMuLV vector pLNL-6 (1) (Fig. 1A) which utilize antibiotic resistance markers to monitor transfection efficiency. All of the constructs are deficient in the coding regions for pol, env, and the major part of the gag gene but retain the sequences known to be required for dimerization and packaging (the Ψ region) (Fig. 1A). Each contains the MoMuLV long terminal repeats (LTRs) which direct transcription of only a single RNA initiating in the 5′ LTR and terminating in the 3′ LTR (Fig. 1A). For clarity, pLNL-6 is referred to as pΨneo. An additional pair of constructs derived from pLNL-6 have the neo gene replaced by the hygro gene, maintain the MoMuLV Ψ sequences, and have either a functional anti-neo ribozyme (pΨhygRbz) or a ribozyme crippled by a double point mutation (pΨhyg*Rbz) (Fig. 1B and C and Fig. 2) appended immediately after the hygro coding region.
FIG. 1.
Linear maps of retroviral vectors used in this study. The MoMuLV-based retroviral vector, pLNL-6 (pΨneo) was kindly provided by A. Dusty Miller of the Fred Hutchinson Cancer Research Center, Seattle, Wash. (1), and served as the basic vector for all of the constructs in this study. Target plasmids pΨneo (A) and pΔΨneo (D) contain the neomycin phosphotransferase (neo) gene, allowing selection of transfected cells with G418. Retroviral vectors expressing a ribozyme (pΨhygRbz) (B) or a mutant ribozyme (pΨhyg*Rbz) (C) were derived from pLNL-6 by replacement of the neo gene with hygromycin phosphotransferase (hygro)-Rbz or hygro-*Rbz constructs. Specifically, a 1.1-kb BamHI fragment containing the hygro gene was subcloned upstream of either a functional or a mutant ribozyme gene in pBluescript. The hygro-ribozyme segments were inserted into the BclI and HindIII sites of pLNL-6 (B and C). This functionally destroyed both the BclI and BamHI sites in the LTR-hygromycin-LTR (LHL) vectors.
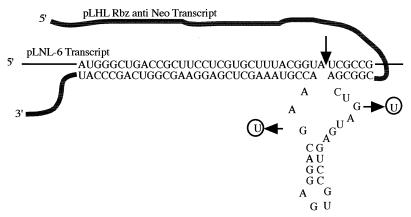
FIG. 2.
Pairing of the anti-neo ribozyme with its target in LNL-6. The ribozyme cleavage site in the neo transcript is between nucleotides 2391 and 2392 in the pLNL-6 sequence and is approximately 1,800 nucleotides downstream of the start point of transcription. Since the ribozyme cloning site in the pΨhygRbz vector was constrained to a site just downstream of the hygro coding region, the neo target site was chosen to position it at the same relative distance from the messenger cap site as the ribozyme, to enhance the probability of the ribozyme pairing arms interacting with the target sequence in the dimerized RNAs. The ribozyme has asymmetric base pairing, with 6 bp in stem I and 32 bp in stem III. The long stem III facilitates the ribozyme and target association, whereas once cleavage has taken place, the short stem I facilitates rapid product dissociation and possibly hastens the ultimate degradation of the cleaved RNA (16). To control for ribozyme versus antisense activity, we generated a mutant, inactive version of the anti-neo ribozyme and inserted it in place of the functional ribozyme in pΨhyg*Rbz (Fig. 1C).
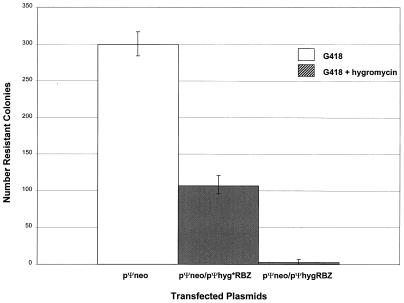
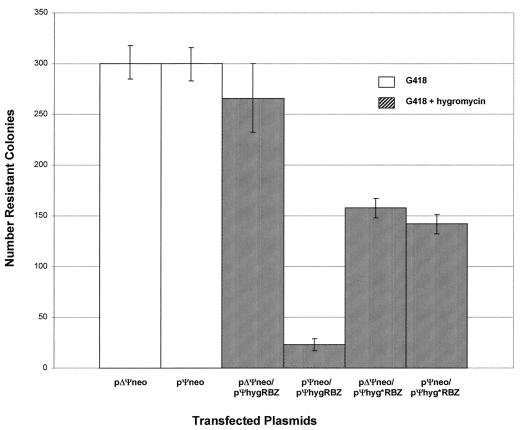
Initially, we wished to ascertain whether or not the ribozyme targeting would give a readily discernible phenotype. The ribozyme and target retroviral vectors were transfected and/or cotransfected into the murine retroviral PA317 (ATCC) packaging cell line to take advantage of constitutively produced viral proteins that enhance the dimerization of the viral RNAs in vitro (8, 9). The PA317 cell line is a derivative of NIH 3T3 mouse embryo fibroblasts carrying truncated mouse retroviral DNA (lacking the Ψ element, the 3′ LTR, and part of the 5′ LTR) and the herpes simplex thymidine kinase (TK) gene (both in pBR322). To ensure maintenance of the packaging phenotype, PA317 cells were reselected with HAT (hypoxanthine, aminopterin, and thymidine). The PA317 cells were maintained in Dulbecco-modified minimum essential medium (DMEM)-high glucose (Irvine Scientific) supplemented with 10% fetal bovine serum. PA317 cells (105 cells) were plated into 60-mm tissue culture dishes (Falcon) 24 h prior to transfection, and the medium was changed 5 to 6 h before transfection. Cells were transfected according to the manufacturer’s protocol for Cell Phect (Pharmacia Biochem) by using the following plasmid combinations: (i) pΨneo alone, (ii) pΨneo plus pΨhygRbz, and (iii) pΨneo plus pΨhyg*Rbz (2:1 ratio of Rbz to target vectors; 9 μg of total plasmid DNA). Cells were subjected to a glycerol shock after 16 h. Selection was begun 24 h later in medium containing 500 μg of G418 (Mediatech)/ml plus 50 μg of hygromycin/ml after trypsinization, dilution, and replating. Selection was continued until well-isolated colonies appeared (3 to 5 weeks). The results from transfections carried out in triplicate are presented in Fig. 3. The number of stably transfected G418-resistant colonies was reduced approximately 100-fold in the presence of the functional ribozyme and 2-fold in the presence of the crippled ribozyme relative to colonies arising after transfection with pΨneo alone. These results are consistent with a specific ribozyme effect.
FIG. 3.
Ribozyme-mediated inhibition of G418-resistant transfectants in cultured PA317 Cells. The columns show the average numbers of transformed colonies obtained after G418 plus hygromycin selection from three separate experiments; the error bars indicate the data range.
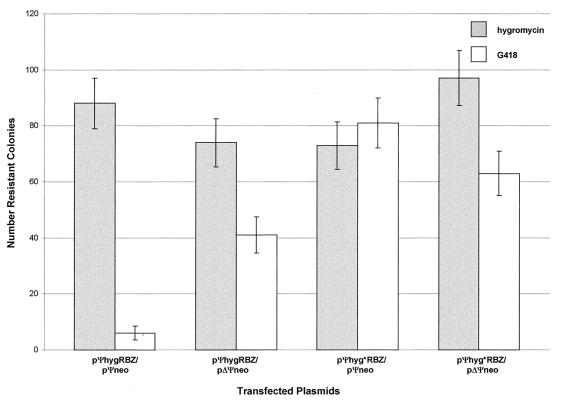
In order to verify that the reduction in G418-resistant clones was a consequence of ribozyme-mediated destruction of the neo transcript and was not due to nonspecific toxicities, we carried out an experiment using the same constructs but scoring separately for G418 and hygromycin resistance (Fig. 4). To assay for colocalization, one ribozyme and one target construct were cotransfected into target cells in all four combinations as above (for ΔΨ construct, see below). After transfection, the cells were divided equally, and one half was subjected to G418 and the other half to hygromycin B (50 μg/ml; Sigma) selection. Since only the ribozyme and mutant ribozyme constructs carry the hygro gene, the number of hygromycin-resistant colonies serves to normalize the transfection efficiencies. In all cases, the numbers of hygromycin-resistant clones were similar, regardless of the combination of vectors used in the transfections (Fig. 4). The number of G418-resistant colonies monitored neo gene expression from the target vectors. As observed in the first set of experiments, the functional ribozyme severely inhibited the formation of G418-resistant colonies, whereas the mutant ribozyme had little effect. Consequently, nonspecific effects of dimerization, such as interference with translation, cannot be solely responsible for the reduction in G418-resistant colony formation; otherwise, significantly fewer hygromycin-resistant colonies would arise in the pΨneo-pΨhygRbz than in the pΔΨneo-pΨhygRbz combination. Likewise, the modest reduction in G418-resistant colonies in the mutant ribozyme transfections supports this view.
FIG. 4.
Ψ-mediated colocalization of ribozyme and target RNAs in PA317 packaging cells. Each column shows the average number of colonies for duplicate transfections obtained under the indicated selection conditions; the error bars show the data range.
In order to examine the role of the Ψ region in ribozyme targeting and efficacy, a 752-base segment of pLNL-6 encompassing sequences known to be required for dimerization and packaging of the viral RNAs was deleted by cleaving the unique SpeI and BclI sites in the vector, filling in the ends, and blunt-end ligating them together. We refer to the construct with Ψ deleted as pΔΨneo (Fig. 1D).
To assay for colocalization, one ribozyme and one target construct were cotransfected into PA317 cells in all four combinations, along with single transfection controls. The assay is based on the premise that if in vivo Ψ-dependent colocalization occurs intracellularly, fewer G418-resistant colonies should form when the ribozyme vector is cotransfected with the Ψ-containing target than when it is cotransfected with the ΔΨ target, due to enhancement of ribozyme activity via Ψ-dependent colocalization with the target. The numbers of G418- and G418- and hygromycin-resistant colonies were scored (Fig. 5). Once again there was a marked reduction in the number of G418-resistant clones obtained when the pΨneo target was cotransfected with pΨhygRbz, compared to pΨhyg*Rbz. Most importantly, the dramatic reduction in G418-resistant colonies was lost following removal of the Ψ element from the target vector (pΔΨneo-pΨhygRbz). Removal of the Ψ sequence from the target did not affect the number of G418-resistant stable clones obtained, showing that the presence or absence of the Ψ sequence itself does not affect neo gene expression (Fig. 5). Consequently, colony formation cannot be due to viral spread or superinfection. Moreover, the variation in the number of hygromycin-resistant colonies generated by pΨhyg*RBZ and pΨhygRBZ is always less than twofold, regardless of the cotransfected target. The same is true whether hygromycin selection alone (Fig. 4) or hygromycin and G418 selection is used (Fig. 5; compare pΨneo-pΨhyg*Rbz, pΔΨneo-pΨhyg*Rbz, and pΔΨneo-pΨhygRbz). We conclude from these results that Ψ-directed association of retroviral RNAs occurs intracellularly prior to viral encapsidation.
FIG. 5.
Ribozyme activity assay of Ψ-mediated colocalization in PA317 packaging cells. In the negative control (no plasmid), all cells died within 1 week. Each column shows the average number of colonies for duplicate transfections obtained under the indicated selection conditions; the error bars show the data range.
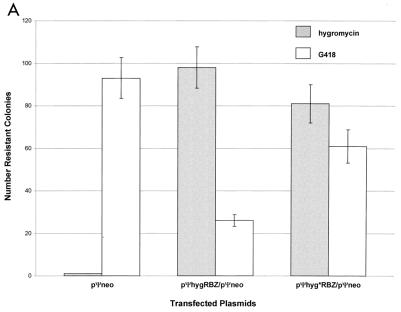
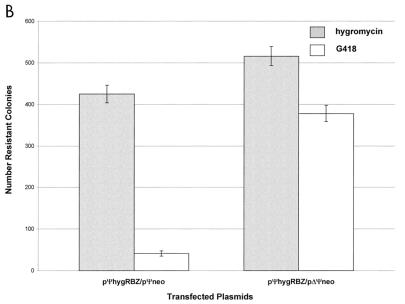
Dimerization of retroviral RNA can occur independently of nucleocapsid protein in vitro (14). In order to evaluate the role of nucleocapsid proteins in dimerization as measured by ribozyme-mediated inactivation of the neo transcript in vivo, we repeated the transfection assay using the human 293 nonpackaging cell line. The protocol was identical to that used with PA317 cells, except that cells were glycerol shocked 6 h posttransfection. The results obtained from these transfection experiments suggest that intracellular dimerization and consequent ribozyme-target colocalization can occur in the absence of the viral nucleocapsid proteins, albeit less efficiently than in their presence (Fig. 6A). The data from the 293 cell transfections confirm that the presence of the Ψ element is essential for this process (Fig. 6B).
FIG. 6.
Ψ-mediated colocalization of RNAs in 293 nonpackaging cells. Each column shows the average number of colonies for duplicate transfections obtained under the indicated selection conditions; the error bars show the data range. (A) Ribozyme-specific inhibition of target expression; (B) Ψ-dependent inhibition of target expression.
Sullenger and Cech (21) demonstrated Ψ-dependent colocalization of a retroviral vector RNA expressing a ribozyme with a Ψ-tethered lacZ target. Their results demonstrated that copackaging of these two RNAs into virions resulted in reduction of the titer of lacZ transduction by 90%, clearly a function of ribozyme destruction of the target RNA. They were unable to detect ribozyme-mediated reduction of LacZ activity in the cell cytoplasm and saw the ribozyme effect only after virion packaging and transduction of target cells. A difference between their experimental approach and ours was that their target RNA was expressed from a stably transformed cell line, and the ribozyme-encoding viral vector was transduced into these cells. In contrast, we cotransfected the two DNAs into the same cells, thereby allowing coordinated expression of the RNAs and increasing the likelihood that the Ψ elements from the two vectors could interact. In addition, we have shown that >90% of the transcripts generated by pLNL6 (pΨneo) and derivatives containing other ribozymes are unspliced and therefore contain the entire Ψ domain, which optimizes chances for colocalization in these experiments (24).
The Ψ elements of retroviruses are required for the production of functional virions by virtue of their intrinsic ability to dimerize. A combination of chemical probing data, sequence analysis of related viruses, and computer folding was used to predict a complex secondary structure for the MoMuLV domain (2, 22) containing 10 stem-loop structures. Based on the predicted structure and further in vitro data, Girard et al. (11, 12) proposed a model for a sequential series of intermolecular interactions between Ψ domains leading to dimerization. First, transient intermolecular pairing between complementary sequences in loops 1 and 4 and loops 2 and 5 promote weak association of genomic RNA monomers. This association is thought to bring the critical C278-to-G303 stem-loop into apposition and induce a conformational rearrangement where the stem-loop opens, facilitating loop-loop annealing by the internal autocomplementary sequence 5′-U288AGCUA293-3′ and formation of a more stable dimer. Kinetic analyses of Ψ-mediated dimer formation in the presence of NCp10 shows that the protein does not modify the steps in dimerization but rather lowers the energy barrier to the annealing step by destabilizing the critical C278-to-G303 stem-loop (11, 12).
In the present study, we observe Ψ-mediated association (as measured by Ψ-dependent ribozyme activity) in both packaging and nonpackaging cell lines. Since gag-derived proteins, including NCp10, are absent in the nonpackaging line, the observed ribozyme activity reflects gag-independent Ψ interactions. Therefore, our results are consistent with the existence in vivo of the weak interactions in the first step of dimerization proposed in the above model. One possibility is that this interaction occurs within the nucleus, perhaps transiently, facilitating ribozyme cleavage prior to nucleus-to-cytoplasm transport. A second possibility (the two are not mutually exclusive) is that the ribozyme-target association occurs in the cytoplasm in a manner that allows both translation of resistance proteins and ribozyme cleavage. Certainly, the Ψ association itself does not appear to be inhibitory to translation (since hygromycin resistance is unaffected by any of the constructs and the mutant ribozyme has only a weak inhibitory effect on G418 resistance). This would be consistent with the existence of a weak interaction, as it is difficult to imagine that translation could proceed effectively through the fully stabilized dimer structure. It is possible that a changeover from translation to packaging of the viral genomic sequences during the latter stages of the viral replication cycle might reflect a transition from the formation of loose dimers to tight (gag-dependent) dimers.
The results presented here demonstrate that Ψ-mediated ribozyme-target heterodimers can be formed quite efficiently and that such heterodimerization can occur independently of packaging. Thus, our Ψ-ribozyme system could serve as a model for examining critical components and steps of the dimerization and subsequent packaging reactions in vivo. Since all known retroviruses have dimerization domains, these analyses could be carried out on other retroviruses, including the lentiviruses, and could conceivably be used for the development of inhibitory trans-acting sequences which compete with normal dimerization. These studies may also prove to be a useful paradigm for studying other intracellular RNA dimerization, and consequently RNA-RNA annealing reactions (3, 10, 17, 20).
Acknowledgments
We thank R. Reinke, R. Thomas, L. Ieshu, and W. J. Fitzgerald for technical assistance and S. Westaway for critical reading of the manuscript.
This work was supported by grants AI29329, AI38592, and GM53933 from the National Institutes of Health.
REFERENCES
- 1.Adam M A, Miller A D. Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretroviral RNA into virions. J Virol. 1988;62:471–480. doi: 10.1128/jvi.62.10.3802-3806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alford R L, Honda S, Laurence C B, Belmont J W. RNA secondary structure analysis of the packaging signal for Moloney murine leukemia virus. Virology. 1991;183:611–619. doi: 10.1016/0042-6822(91)90990-s. [DOI] [PubMed] [Google Scholar]
- 3.Awang G, Sen D. Mode of dimerization of HIV-1 genomic RNA. Biochemistry. 1993;32:11453–11457. doi: 10.1021/bi00093a024. [DOI] [PubMed] [Google Scholar]
- 4.Bender M A, Palmer T D, Gelinas R E, Miller A D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987;61:1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender W, Davidson N. Mapping of poly(A) sequences in the electron microscope reveals unusual structure of type C oncornavirus RNA molecules. Cell. 1976;7:595–607. doi: 10.1016/0092-8674(76)90210-5. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand E, Rossi J J. Anti-HIV hammerhead ribozymes: targeting strategies and optimization of intracellular function. In: Eckstein F, Lilley D, editors. Nucleic acids and molecular biology. New York, N.Y: Springer-Verlag; 1996. pp. 301–313. [Google Scholar]
- 7.Bonnet-Mathonière B, Girard P-M, Muriaux D, Paoletti J. Nucleocapsid protein 10 activates dimerization of the RNA of Moloney murine leukemia virus in vitro. Eur J Biochem. 1996;238:129–135. doi: 10.1111/j.1432-1033.1996.0129q.x. [DOI] [PubMed] [Google Scholar]
- 8.de Rocquigny H, Gabus C, Vincent A, Fournié-Zaluski M-C, Roques B, Darlix J-L. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc Natl Acad Sci USA. 1992;89:6472–6476. doi: 10.1073/pnas.89.14.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng Y-X, Copeland T D, Henderson L E, Gorelick R J, Bosche W J, Levin J G, Rein A. HIV-1 nucleocapsid protein induces “maturation” of dimeric retroviral RNA in vitro. Proc Natl Acad Sci USA. 1996;93:7577–7581. doi: 10.1073/pnas.93.15.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu W, Rein A. Maturation of dimeric viral RNA of Moloney murine leukemia virus. J Virol. 1993;67:5443–5449. doi: 10.1128/jvi.67.9.5443-5449.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard P-M, Bonnet-Mathonière B, Muriaux D, Paoletti J. Auto complementary sequence in the 5′ leader region is responsible for dimerization of MoMuLV genomic DNA. Biochemistry. 1995;34:9785–9794. doi: 10.1021/bi00030a016. [DOI] [PubMed] [Google Scholar]
- 12.Girard P-M, de Rocquigny H, Roques B-P, Paoletti J. A model of PSI dimerization: destabilization of the C278-G303 stem-loop by the nucleocapsid protein (NCp10) of MoMuLV. Biochemistry. 1996;35:8705–8714. doi: 10.1021/bi952454s. [DOI] [PubMed] [Google Scholar]
- 13.Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983;25:179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- 14.Housset V, de Rocquigny H, Roques B P, Darlix J-L. Basic amino acids flanking the zinc finger of Moloney murine leukemia virus nucleocapsid protein NCp10 are critical for virus infectivity. J Virol. 1993;67:2537–2545. doi: 10.1128/jvi.67.5.2537-2545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung H J, Hu S, Bender W, Bailey J M, Davidson N, Nicolson M O, McAllister R M. RD-114, baboon, and wooly monkey viral RNAs compared in size and structure. Cell. 1976;7:609–620. doi: 10.1016/0092-8674(76)90211-7. [DOI] [PubMed] [Google Scholar]
- 16.Mann R, Mulligan R C, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- 17.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murti K G, Bondurant M, Tereba A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J Virol. 1981;37:411–419. doi: 10.1128/jvi.37.1.411-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy C, Tounekti N, Mougel M, Darlix J-L, Paoletti C, Ehresmann C, Ehresmann B, Paoletti J. An analytical study of the dimerization of in vitro generated RNA of Moloney murine leukemia virus (MoMuLV) Nucleic Acids Res. 1990;18:7287–7292. doi: 10.1093/nar/18.24.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakuragi J-I, Panganiban A T. Human immunodeficiency virus type 1 RNA outside the primary encapsidation and dimer linkage region affects RNA dimer stability in vivo. J Virol. 1997;71:3250–3254. doi: 10.1128/jvi.71.4.3250-3254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullenger B A, Cech T R. Tethering ribozymes to a retroviral packaging signal for destruction of viral RNA. Science. 1993;262:1566–1569. doi: 10.1126/science.8248806. [DOI] [PubMed] [Google Scholar]
- 22.Tounekti N, Mougel M, Roy C, Marquet R, Darlix J-L, Paoletti J-L, Ehresmann B, Ehresmann C. Effect of dimerization on the conformation of the encapsidation Ψ domain of Moloney murine leukemia virus RNA. J Mol Biol. 1992;223:205–220. doi: 10.1016/0022-2836(92)90726-z. [DOI] [PubMed] [Google Scholar]
- 23.Varmus H, Swanstorm R. Replication of retroviruses. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 369–512. [Google Scholar]
- 24.Zhou C, Bahner I C, Larson G P, Zaia J A, Rossi J J, Kohn D B. Inhibition of HIV-1 in human T-lymphocytes by retrovirally transduced anti-tat and rev hammerhead ribozymes. Gene. 1994;149:33–39. doi: 10.1016/0378-1119(94)90409-x. [DOI] [PubMed] [Google Scholar]