Abstract
Background
Limited evidence exists on the population attributable fraction (PAF) of cancer cases and deaths in Latin America. In Peru several studies have been published regarding the PAF of various risk factors and their associated diseases. The objective of this study was to estimate the fraction of cancer cases and deaths attributable to potentially modifiable risk factors in Peru in 2018, before the COVID-19 pandemic in the population of 15 years old and older.
Methods
An ecological study was conducted using the prevalence of exposure of the Peruvian population to modifiable risk factors for cancer, the relative risk associated with each factor, and the number of cancer cases and deaths in 2018 as inputs. We used the Parkin formula with a Montecarlo statistical simulation model to calculate the PAF and confidence intervals. The number of new cancer cases and deaths attributed to each risk factor was determined by multiplying the number of cases and deaths in each gender by the PAF of each risk factor.
Findings
In Peru, 38.5% of new cases (34.5% in men and 42% in women) and 43.4% of cancer-related deaths (43.4% in men and 43.4% in women) were attributable to modifiable risk factors. The number of cancers attributable was 25,308 (10,439 in men and 14,869 in women) and the number of deaths attributable to cancer was 14,839 (6,953 in men and 7,886 in women). The predominant modifiable risk factors contributing to the highest number of cases and deaths were HPV infection (4,563 cases, 2,409 deaths), current tobacco use (3,348 cases, 2,180 deaths), and helicobacter pylori infection (2,677 cases, 1,873 deaths). Among the risk factors, oncogenic infections constituted the group with the highest PAF (16.6% for cases, 19.2% for deaths) followed by other unhealthy lifestyle factors (14.2% for cases, 16.7% for deaths), tobacco (7.2% for cases, 7.2% for deaths) and ultraviolet radiation (0.5% for cases, 0.3% for deaths).
Conclusions
Prior to the COVID-19 pandemic, 38.5% of cancer cases and 43.4% of cancer-related deaths in Peru were linked to modifiable risk factors in the population of 15 years old and older. Most preventable cancer cases and deaths were related to oncogenic infections, primarily caused by HPV and helicobacter pylori, followed by tobacco and obesity.
Keywords: Cancer, Population attributable fraction, Modifiable risk factors, Lifestyle, Preventive medicine, Peru
Introduction
Cancer constitutes a significant public health challenge in Peru due to the uncontrolled high prevalence of risk factors and disparities in accessing oncological services. This leads to delayed diagnoses and unequal treatment, ultimately increasing the risk of premature deaths among Peruvians [1–4]. In recent decades, transitions and social determinants in demographics, epidemiology, commercial diets, and nutrition, have significantly shaped Peru’s health profile, resulting in the dominance of non-communicable diseases such as cancer [5]. In addition, socio-economic factors such as poverty, education, gender, urbanization/rurality, ethnicity, race, environmental factors, and other health determinants influence Peruvians’ exposure to risk factors and access to healthcare [6]. Despite this, the country’s morbidity, and mortality continue to be affected by communicable diseases [1, 5–7].
According to the estimates from the International Agency for Research on Cancer (IARC) published by the Global Cancer Observatory, Peru registered 66,669 new cancer cases and 34,570 cancer-related deaths in 2018 (prior to the COVID-19 pandemic) [2, 3].
Potentially modifiable risk factors significantly contribute to numerous cancers, and the current estimation of this proportion within a population, known as the PAF, constitutes a valuable tool for prioritizing cancer prevention and control programs and interventions [8] and, in turn, allows us to estimate the percentage of cases that could have been avoided if exposure to associated risk factors had been minimized compared to the reference level [9]. There has been PAF studies assessing modifiable cancer risk factors in various regions such as North America (United States and Canada) [10, 11], Europe (England) [12], Asia (China, Japan) [13, 14] and the Middle East [15]. These studies have reported PAFs ranging from 23 to 45% for cancer cases and from 41 to 51% for cancer-related deaths. However, most cancer studies are focused on PAF for cancer cases, with only a few considering PAF for cancer-related deaths [10, 13, 14, 16].
0n the contrary, there are very little evidence of PAF for cases and deaths for cancer in Latin-American countries and do not exits PAF’s studies in Peru that consider all the risk factors and allow the estimation of the total number of cancer cases and deaths attributable to said risk factors. However, do exits PAF studies of some risk factors and derivate diseases (as can be observe on the tobacco consumption [17, 18] mortality tendencies for different cancer’s type, as gastric [19], kidney [20], cervical cancer [21], mesothelioma [22]). The sole Latin-American’s country that have one study of PAF for cancer is Brazil [16]; on the other hand, Chile has one PAF study that considers only lifestyle risk factors, not including oncogenic infections [23].
The objective of this study is to estimate the fraction of cancer cases and deaths in Peru attributable to potentially modifiable risk factors in 2018, prior to the COVID-19 pandemic.
Methods
An ecological study was conducted using data on the Peruvian population’s prevalence of exposure to modifiable risk factors for cancer, alongside cancer incidence and mortality preceding the COVID-19 pandemic in the population of 15 years old and older.
The modifiable risk factors reported in this study (Table 1), were obtained from a systematic review of the global burden of cancer attributable to risk factors 2010–2019 [24], in addition to primary published PAF studies [12–17, 23].
Table 1.
Potentially modifiable risk factors considered for each type of cancer
| TOPOGRAPHY | MODIFIABLE RISK FACTORS |
|---|---|
| Mouth, pharynx (C00-C14) | HPV infection, tobacco use, alcohol use, low fruit and vegetable consumption |
| Esophagus (C15) | Current tobacco use, obesity, sedentary behavior, alcohol use |
| Stomach (C16) | Helicobacter pylori infection, current tobacco use, obesity, red meat consumption, processed meat consumption |
| Colorectal (C18-C21) | Current tobacco use, sedentary behavior, alcohol use, overweight, obesity, red meat consumption, processed meat consumption, low fruit and vegetable consumption. |
| Liver (C22) | Hepatitis B virus, Hepatitis C virus, current tobacco use, obesity, overweight |
| Gallbladder (C23) | Obesity, overweight |
| Pancreas (C25) | Tobacco use, alcohol use, obesity |
| Larynx (C32) | HPV infection, current tobacco use, low fruit and vegetable consumption |
| Lung (C34) | Current tobacco use, passive exposure to tobacco smoke, sedentary behavior, low fruit and vegetable consumption |
| Melanoma of the skin (C43) | Ultraviolet radiation |
| Kaposi Sarcoma (C46) | HHV-8 infection, HIV |
| Breast (C50) | Tobacco use, passive exposure to tobacco smoke, obesity, overweight, sedentary behavior |
| Cervix (C53) | HPV infection |
| Endometrium (C54.1) | Obesity, overweight, sedentary behavior |
| Ovaries (C56) | Obesity, overweight |
| Penis (C60) | HPV infection |
| Kidney (C64) | Tobacco use, overweight, obesity |
| Bladder (C67) | Current tobacco use, overweight, obesity |
| Hodgkin’s Lymphoma (C81) | Epstein Barr virus, HIV, current tobacco use |
| Non-Hodgkin’s Lymphoma (C82-C85, C96.3) | Epstein Barr virus, HIV, Hepatitis C virus |
| Leukemias (C91-C95) | Tobacco use, obesity, overweight |
Prevalence of modifiable risk factors, cancer cases, and deaths
The prevalence of the Peruvian population’s exposure to modifiable risk factors for cancer was obtained from the following data sources:
Population surveys: The Demographic and Family Health Survey (ENDES 2018) provided data on cigarette and alcohol use, fruit and vegetable consumption, overweight and obesity among individuals aged 15 and above.
Research articles: These articles yielded information on the prevalence of oncogenic infections, sedentary behavior, and other relevant factors.
University thesis repositories: In some cases, specific information from both undergraduate and postgraduate thesis was obtained, mainly focusing on rare risk factors or those with limited studies in the Peruvian population, such as the prevalence of red meat and processed meat consumption (However, there are no thesis citations within the article).
Relative risk (RR)
The relative risk associated with each potentially modifiable risk factor was determined with a systematic search across databases including PUBMED, SCOPUS, EMBASE, COCHRANE, and SCIELO, prioritizing metanalysis, cohort and case-control studies, favoring recent studies covering American countries and controlling for cofounding variables through a multivariate statistical analysis. In cases where the systematic search did not yield studies with RR data for the potentially modifiable risk factors, research presenting odds ratios (OR) was used as a statistical approximation to RR.
Estimate of the PAF
The calculation of the PAF was performed using the formula described by Parkin et al. [25]:
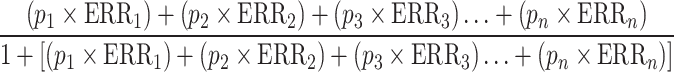 |
p1 represents the proportion of the population at exposure level 1 and subsequent levels, while ERR1 is the excess relative risk (relative risk − 1) at exposure level 1 and subsequent levels. The researchers calculated the PAF for the absence or decrease of risk factors. ERR was determined as the natural logarithm of the reciprocal of the relative risk, expressed by the formula:
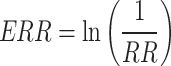 |
Cancer cases and deaths data in Peru were obtained from the Global Cancer Observatory (GLOBOCAN-Cancer today) which publishes the IARC estimates based on population cancer registry data from the year 2018 [3].
The calculation of the number of cancer cases and deaths attributed to each risk factor, categorized by gender, involved multiplying the number of cancer cases and deaths per gender by the PAF.
In case of cervical cancer and Kaposi sarcoma, a PAF of 100% was assumed, directly associated with VPH and VIH infections [26]. For skin melanoma, the PAF estimated by the IARC for the Peruvian population was derived from their “Cancers attributable to UV radiation” statistics available on the World Cancer Observatory website [27].
From an ethical point of view, this study did not imply risks as it utilized aggregate prevalence data from population surveys, relative risks from metanalysis and scientific journal articles, and the IARC cancer incidence and mortality estimates for Peru. Therefore, it did not require an informed consent. Additionally, the study received approval from the Research Ethics Committee from the Medical School of Ricardo Palma University (Expedited Review: PI-019-2023).
Results
In 2018, before to the COVID-19 pandemic, approximately 38.5% of new cancer cases in Peru were attributed to potentially modifiable risk factors. Cancers with the highest PAF, apart from cervical and Kaposi sarcoma, included larynx (85.6%), stomach (82.6%), liver (82.3%), lung (80.7%) and penis (75.0%). In contrast, cancers with lower PAF were ovarian cancer (8.4%), leukemia (12.8%), pancreatic cancer (21.8%), skin melanoma (25.0%) and bladder cancer (27.9%) (Table 2).
Table 2.
PAF of cancer according to topography and potentially modifiable risk factors
| TOPOGRAPHY | PAF (%) | C.I. 95% | % TOTAL PAF |
|---|---|---|---|
| Mouth, pharynx (C00-C14) | |||
| HPV Infection | 8.1 | 6.5–9.8 | |
| Current tobacco use | 20.5 | 11.8–30.8 | 65.7 |
| Alcohol use | 36.1 | 23.2–48.2 | |
| Low fruit and vegetable consumption | 1.1 | 0.1–2.2 | |
| Esophagus (C15) | |||
| Current tobacco use | 11 | 6.9–15.9 | |
| Obesity | 12.3 | 3.9–36.3 | 60.8 |
| Sedentary behavior | 21.9 | 2.2–37.5 | |
| Alcohol use | 15.6 | 13.8–17.6 | |
| Stomach (C16) | |||
| Helicobacter pylori infection | 46.4 | 18.6–65.9 | |
| Current tobacco use | 8.3 | 3.8–14.4 | |
| Obesity | 2.8 | 0.7–5.1 | 82.6 |
| Red meat consumption | 18.9 | 10.2–18.2 | |
| Processed meat consumption | 6.2 | 1.0–11.5 | |
| Colorectal (C18-C21) | |||
| Current tobacco use | 2.1 | 1.1–3.1 | |
| Sedentary behavior | 18.5 | 8.3–27.1 | |
| Alcohol use | 4.1 | 2.1–6.3 | |
| Obesity | 4.1 | 2.4–6.1 | 53.6 |
| Overweight | 7.4 | 0.1–15.1 | |
| Red meat consumption | 5.9 | 1.5–9.8 | |
| Processed meat consumption as | 5 | 2.4–7.8 | |
| Low fruit and vegetable consumption | 6.6 | 0.9–12.4 | |
| Liver (C22) | |||
| Hepatitis B virus | 32.4 | 30.2–34.6 | |
| Hepatitis C virus | 21.5 | 20.1–23.1 | 82.3 |
| Current tobacco use | 5.7 | 3.8–6.6 | |
| Obesity | 16.6 | 10.2–23.3 | |
| Overweight | 6 | 0.8–11.4 | |
| Gallbladder (C23) | |||
| Obesity | 12.6 | 5.3–20.8 | 18.6 |
| Overweight | 6 | 2.6–9.6 | |
| Pancreas (C25) | |||
| Current tobacco use | 7.3 | 6.1–8.4 | |
| Alcohol use | 7.2 | 4.7–9.6 | 21.8 |
| Obesity | 7.2 | 3.1–11.7 | |
| Larynx (C32) | |||
| HPV infection | 22.1 | 10.9–37.0 | 85.6 |
| Current tobacco use | 38.8 | 18.5–60.6 | |
| Low fruit and vegetable consumption | 24.7 | 14.4–33.2 | |
| Lung (C34) | |||
| Current tobacco use | 44.3 | 17.5–70.0 | |
| Secondhand smoke | 7.7 | 5.0–10.2 | 80.7 |
| Sedentary behavior | 17 | 4.4–28.3 | |
| Low fruit and vegetable consumption | 11.7 | 4.3–19.3 | |
| Skin melanoma (C43) | |||
| Ultraviolet radiation | 25 | 20.7–29.4 | 25 |
| Kaposi sarcoma (C46) | |||
| HHV-8 infection | 100 | 96.4–100.0 | 100 |
| Breast (C50) | |||
| Current tobacco use | 9.2 | 1.2–25.8 | |
| Secondhand smoke | 5.6 | 2.1–9.0 | |
| Obesity | 8.5 | 6.3–10.8 | 41.8 |
| Overweight | 6 | 3.6–8.5 | |
| Sedentary behavior | 12.5 | 2.5–21.7 | |
| Cervix (C53) | |||
| HPV infection | 100 | 96.4–100.0 | 100 |
| Endometrium (C54.1) | |||
| Obesity | 28.4 | 22.3–34.7 | 58 |
| Overweight | 10.6 | 5.6–15.6 | |
| Sedentary behavior | 19 | 6.3–30 − 8 | |
| Ovary (C56) | |||
| Obesity | 5.9 | 3.5–8.4 | 8.4 |
| Overweight | 2.5 | 5.6–13.2 | |
| Penis (C60) | |||
| HPV infection | 75,0 | 45,6–88,5 | 75,0 |
| Kidney (C64) | |||
| Current tobacco use | 4.6 | 2.7–6.5 | 36.2 |
| Obesity | 16.9 | 13.3–20.4 | |
| Overweight | 14.8 | 11.4–18.0 | |
| Bladder (C67) | |||
| Current tobacco use | 20.8 | 18.0–23.6 | 27.9 |
| Obesity | 16.9 | 13.3–20.4 | |
| Overweight | 14.8 | 11.4–18.0 | |
| Hodgkin’s Lymphoma (C81) | |||
| Epstein Barr virus | 59.7 | 34.1–79.1 | 63.8 |
| HIV | 0.7 | 0.2–1.9 | |
| Current tobacco use | 3.4 | 1.3–5.7 | |
| Non-Hodgkin’s Lymphoma (C82-C85, C96.3) | |||
| Epstein Barr virus | 68 | 39.1–84.5 | 69.6 |
| HIV | 1 | 0.7–1.39 | |
| hepatitis C virus | 0.6 | 0.4–0.9 | |
| Leukemias (C91-C95) | |||
| Current tobacco use | 4.1 | 2.3–6.0 | 12.8 |
| Obesity | 5.5 | 3.7–7.6 | |
| Overweight | 3.3 | 1.5–5.0 |
The estimated fraction of cancer cases attributable to potentially modifiable risk factors was 34.5% in men and 42% in women, potentially preventing 25,308 cases of cancer (10,439 in men and 14,869 in women). In men, cancers with the highest PAF were Kaposi sarcoma, larynx (98.5%), lung (97.8%), liver (95.7%), mouth/pharynx (87.8%) and stomach (75.8%). In women, cancers with the highest PAF were cervix, Kaposi sarcoma, liver (73.7%), non-Hodgkin’s lymphoma (69.2%), stomach (66.3%), larynx (65.5%) and Hodgkin’s lymphoma (61.4%) (Table 3). The cancers with the highest number of preventable cases in men were stomach (2,526 cases), colorectal (1632 cases), lung (1,256 cases), non-Hodgkin’s lymphoma (1,134 cases) and liver (964 cases) and in women were cervix (4,270 cases), breast (2,868 cases), stomach (1,968 cases), colorectal (1,296 cases) and non-Hodgkin’s lymphoma (1,050 cases) (Table 3).
Table 3.
PAF, cancer cases and deaths attributable to potentially modifiable risk factors in Peruvian men and women
| TOPOGRAPHY | MEN | WOMEN | ||||||
|---|---|---|---|---|---|---|---|---|
| PAF (%) | C.I. 95% | Cases | Deaths | PAF (%) | C.I. 95% | Cases | Deaths | |
| Mouth, pharynx | 87.8 | 64.7–100.0 | 464 | 178 | 29.2 | 15.7–45.1 | 155 | 58 |
| Esophagus | 69.1 | 33.6–100.0 | 186 | 174 | 40.2 | 9.3–83.6 | 32 | 29 |
| Stomach | 75.8 | 33.6–100.0 | 2,526 | 2,018 | 66.3 | 25.8–100.0 | 1,968 | 1,532 |
| Colorectal | 72.5 | 32.2–100.0 | 1,632 | 845 | 54.3 | 23.0–81.8 | 1,296 | 652 |
| Liver | 95.7 | 75.6–100.0 | 964 | 967 | 73.7 | 57.0–91.4 | 797 | 787 |
| Gallbladder | 8.1 | 3.1–13.7 | 20 | 13 | 25.6 | 9.8–42.2 | 199 | 116 |
| Pancreas | 26.7 | 16.8–37.6 | 191 | 184 | 19.4 | 13.4–25.1 | 173 | 165 |
| Larynx | 98.5 | 53.0–100.0 | 165 | 95 | 65.5 | 32.9–100.0 | 43 | 24 |
| Lung | 97.8 | 46.7–100.0 | 1,388 | 1,256 | 41.6 | 13.2–100.0 | 611 | 542 |
| Skin melanoma | 46.9 | 43.8–50.0 | 301 | 97 | 1.2 | 0.9–2.7 | 8 | 2 |
| Kaposi Sarcoma | 100.0 | 96.4–100.0 | 297 | 56 | 100.0 | 96.4–100.0 | 53 | 19 |
| Breast | NA | NA | NA | NA | 41.8 | 15.6–75.8 | 2,868 | 762 |
| Cervix | NA | NA | NA | NA | 100.0 | 96.4–100.0 | 4,270 | 2,288 |
| Endometrium | NA | NA | NA | NA | 58.0 | 34.1–81.1 | 725 | 180 |
| Ovary | NA | NA | NA | NA | 8.4 | 9.0–21.6 | 106 | 66 |
| Penis | 75.0 | 45.6–88.5 | 214 | 60 | NA | NA | NA | NA |
| Kidney | 33.4 | 23.3–44.0 | 394 | 167 | 34.3 | 27.3–41.5 | 265 | 102 |
| Bladder | 36.7 | 28.9–44.7 | 267 | 89 | 15.1 | 9.1–21.2 | 57 | 23 |
| Hodgkin’s Lymphoma | 66.4 | 36.8–91.4 | 127 | 52 | 61.4 | 35.0–82.6 | 107 | 34 |
| Non-Hodgkin’s Lymphoma | 70.2 | 40.5–87.7 | 1,134 | 568 | 69.2 | 39.9–86.4 | 1,050 | 437 |
| Leukemias | 15.8 | 6.4–26.9 | 169 | 132 | 10.2 | 1.9–14.8 | 85 | 68 |
| TOTAL | 10,439 | 6,951 | 14,868 | 7,886 | ||||
NA: Not applicable
Oncogenic infections constitute the primary group of modifiable attributable factors; potentially preventing up to 10,883 cancer cases annually (PAF: 16.6%). Controlling HPV infection could prevent up to 4,563 cases annually. The second most important group of modifiable factors in preventing cancer compromises other lifestyle factors (PAF: 14.2%), potentially preventing up to 9,362 cases annually; obesity is associated with the highest number of annual cancer cases, potentially preventing up to 2,087 cases if controlled. The third most important group of modifiable factors for cancer in Peru is tobacco exposure (PAF: 7.2%), contributing to 4,754 annual cases. Avoiding direct tobacco consumption could potentially prevent treating 3,348 cases of cancer annually. Finally, exposure to ultraviolet radiation stands as the least attributable modifiable factor (PAF: 0.5%) accountable for 309 cancers annually (Tables 4 and 5).
Table 4.
Number of cancer cases and deaths attributable to potentially modifiable risk factors in Peru distributed by genders
| FACTOR CLUSTERING | MODIFIABLE RISK FACTORS |
MEN | WOMEN | BOTH GENDERS | |||
|---|---|---|---|---|---|---|---|
| CASES | DEATHS | CASES | DEATHS | CASES | DEATHS | ||
|
Tobacco (Lifestyle) |
Current tobacco use | 2,157 | 1,572 | 1,191 | 608 | 3,348 | 2,180 |
| Secondhand smoke | 106 | 92 | 1,300 | 206 | 1,406 | 298 | |
| Other unhealthy lifestyle factors (Lifestyle) | Sedentary behavior | 778 | 494 | 1,270 | 723 | 2,048 | 1,217 |
| Overweight | 420 | 301 | 1,221 | 409 | 1,641 | 710 | |
| Obesity | 868 | 630 | 1,219 | 768 | 2,087 | 1,398 | |
| Red meat consumption | 761 | 573 | 702 | 507 | 1,463 | 1,080 | |
| Processed meat consumption | 319 | 226 | 304 | 204 | 623 | 430 | |
| Low fruit and vegetable consumption | 486 | 320 | 145 | 125 | 631 | 445 | |
| Alcohol use | 593 | 325 | 276 | 98 | 869 | 423 | |
| UV Radiation (Environment-lifestyle). | UV Radiation | 301 | 97 | 8 | 2 | 309 | 99 |
|
Infections (Lifestyle) |
Helicobacter pylori | 1,213 | 971 | 1,464 | 902 | 2,677 | 1,873 |
| HBV | 376 | 369 | 233 | 302 | 609 | 671 | |
| HCV | 227 | 218 | 67 | 234 | 294 | 452 | |
| HPV | 293 | 98 | 4,270 | 2,311 | 4,563 | 2,409 | |
| HHV8 | 297 | 56 | 53 | 19 | 350 | 75 | |
| HIV | 29 | 14 | 11 | 5 | 40 | 19 | |
| EBV | 1,215 | 597 | 1,135 | 463 | 2,350 | 1,060 | |
| TOTAL | 10,439 | 6,953 | 14,869 | 7,886 | 25,308 | 14,839 | |
Table 5.
Fraction of cancer cases attributable to potentially modifiable risk factors in Peru distributed by gender
| FACTOR CLUSTERING | MODIFIABLE RISK FACTORS |
MEN | WOMEN | BOTH GENDERS | |||
|---|---|---|---|---|---|---|---|
| PAF CASES | PAF CLUSTERED | PAF CASES | PAF CLUSTERED | PAF CASES | PAF CLUSTERED | ||
|
Tobacco (Lifestyle) |
Current tobacco use | 7.1 | 7.5 | 3.4 | 7.0 | 5.1 | 7.2 |
| Secondhand smoke | 0.3 | 3.7 | 2.1 | ||||
| Other unhealthy lifestyle factors (Lifestyle) | Sedentary behavior | 2.6 | 13.9 | 3.6 | 14.5 | 3.1 | 14.2 |
| Overweight | 1.4 | 3.4 | 2.5 | ||||
| Obesity | 2.9 | 3.4 | 3.2 | ||||
| Red meat consumption | 2.5 | 2.0 | 2.2 | ||||
| Processed meat consumption | 1.1 | 0.9 | 0.9 | ||||
| Low fruit and vegetable consumption | 1.6 | 0.4 | 1.0 | ||||
| Alcohol use | 2.0 | 0.8 | 1.3 | ||||
| UV Radiation (Environment-lifestyle). | UV Radiation | 1.0 | 1.0 | 0.0 | 0.0 | 0.5 | 0.5 |
|
Infections (Lifestyle) |
Helicobacter pylori | 4.0 | 12.0 | 4.1 | 20.4 | 4.1 | 16.6 |
| HBV | 1.2 | 0.7 | 0.9 | ||||
| HCV | 0.7 | 0.2 | 0.4 | ||||
| HPV | 1.0 | 12.1 | 6.9 | ||||
| HHV8 | 1.0 | 0.1 | 0.5 | ||||
| HIV | 0.1 | 0.0 | 0.1 | ||||
| EBV | 4.0 | 3.2 | 3.6 | ||||
| TOTAL | All risk factors | 34.5 | 34.4 | 42.0 | 41.9 | 38.5 | 38.5 |
In 2018, 43.4% of cancer deaths in Peru were attributed to potentially modifiable risk factors, with percentages of 43.4% in men and 43.4% in women, resulting in 14,839 deaths (6,953 in men and 7,886 in women). Preventable cancer deaths by controlling modifiable factors include gastric cancer (2,018), lung (1,256), liver (967), colorectal (845) and non-Hodgkin lymphoma (568) in men; while in women, preventable deaths include cervical cancer (2,288), gastric cancer (1,532), liver cancer (787), breast cancer (762) and lung cancer (542) (Table 3).
In the present study, oncogenic infections are the main group of modifiable factors that determine cancer mortality in Peru (PAF: 19.2%) responsible for 6,559 deaths. Controlling HPV infection, could potentially prevent 2,409 deaths annually. The second group of modifiable factors comprises other unhealthy lifestyle factors (PAF: 16.7%) associated with 5,703 deaths annually. Addressing obesity could prevent 1,398 cancer deaths. The third factor impacting cancer mortality in Peru in 2018 was tobacco exposure (PAF: 7.2%), contributing to 2,478 deaths annually, with direct tobacco consumption associated to 2,180 cancer deaths annually. Lastly, exposure to ultraviolet radiation had a PAF of 0.3% and was responsible for 99 deaths (Tables 4 and 6).
Table 6.
Fraction of cancer deaths attributable to potentially modifiable risk factors in Peru distributed by gender
| FACTOR CLUSTERING | MODIFIABLE RISK FACTORS | MEN | WOMEN | BOTH GENDERS | |||
|---|---|---|---|---|---|---|---|
| PAF DEATHS | PAF CLUSTERED | PAF DEATHS | PAF CLUSTERED | PAF DEATHS | PAF CLUSTERED | ||
|
Tobacco (Lifestyle) |
Current tobacco use | 9.8 | 10.4 | 3.3 | 4.5 | 6.4 | 7.2 |
| Secondhand smoke | 0.6 | 1.1 | 0.9 | ||||
| Other unhealthy lifestyle factors (Lifestyle) | Sedentary behavior | 3.0 | 17.9 | 4.0 | 15.6 | 3.6 | 16.7 |
| Overweight | 1.9 | 2.2 | 2.1 | ||||
| Obesity | 3.9 | 4.2 | 4.1 | ||||
| Red meat consumption | 3.6 | 2.8 | 3.2 | ||||
| Processed meat consumption | 1.4 | 1.1 | 1.3 | ||||
| Low fruit and vegetable consumption | 2.0 | 0.7 | 1.3 | ||||
| Alcohol use | 2.0 | 0.5 | 1.2 | ||||
| UV Radiation (Environment-lifestyle). | UV Radiation | 0.6 | 0.6 | 0.0 | 0.0 | 0.3 | 0.3 |
|
Infections (Lifestyle) |
Helicobacter pylori | 6.1 | 14.5 | 5.0 | 23.3 | 5.5 | 19.2 |
| HBV | 2.3 | 1.7 | 2.0 | ||||
| HCV | 1.4 | 1.3 | 1.3 | ||||
| HPV | 0.6 | 12.7 | 7.0 | ||||
| HHV8 | 0.3 | 0.1 | 0.2 | ||||
| HIV | 0.1 | 0.0 | 0.1 | ||||
| EBV | 3.7 | 2.5 | 3.1 | ||||
| TOTAL | All risk factors | 43.4 | 43.4 | 43.4 | 43.4 | 43.4 | 43.4 |
Discussion
This study, conducted in 2018, prior to the COVID-19 pandemic in the population of 15 years old and older, revealed that 38.5% of new cancer cases and 43.4% of cancer-related deaths in Peru were attributable to potentially modifiable risk factors. Oncogenic infections, alongside unhealthy lifestyles, accounted for one-third of cancer cases and nearly two-fifths of cancer deaths. Notably, HPV infection, current tobacco use and helicobacter pylori infection emerged as the primary risk factors contributing to a higher number that cause greater number of cancer cases and deaths.
The fraction of cancer cases attributable to potentially modifiable risk factors in Peru lies between intermediate ranges compared to other countries. It is higher than countries such as Australia [28] (32.0%), Eastern Mediterranean countries [15] (33.4%), Japan [14] (35,9%) and Canada [29] (33–37%). However, it is lower than the US [9] (42.0%) and similar to the United Kingdom [12] (37.7%). Regarding cancer mortality, Peru’s fraction attributable to modifiable factors is intermediate compared to Japan [14], (41%) and lower than the US [10] (45.1%) and China [30] (45.2%). Brazil [16] stands out as the only country of Latin America with a PAF including oncogenic infections and unhealthy lifestyles among cancer risk factors. In terms of fraction attributable to cases, Peru exhibited a higher PAF compared to Brazil (38.4% versus 34.2%), while demonstrating a similar PAF for attributable deaths (43.4% Peru versus 42% in Brazil).
Oncogenic infections accounted for the highest PAF in Peru, contributing to 16.6% of cancer cases and 19.2% of deaths. This PAF is notably higher than those reported in other countries such as the USA [10], Canada [29], Australia [28] and the UK [12], where infections explain 3.3%, 3.7%, 2.9% and 3.6% of cancer cases, respectively. In Japan [14], the reported fraction is slightly lower at 16.6%. In Latin America, the study performed in Brazil [16] places oncogenic infections in second place, following tobacco, however, the exact PAF value wasn´t provided. The burden of modifiable risk factors for cancer in Peru is attributed to the “double burden of disease”, a common phenomenon in low- to middle-income countries facing many disparities. These countries must confront the increasing burden of non-communicable diseases, and unresolved communicable diseases rooted in structural health determinants [31–34].
This study underscores Peru’s struggle to control communicable diseases as a contributing factor to cancer despite existing policies, plans and strategies aimed at prevention, vaccination, screening and early detection of many associated neoplasms [1, 2].
The second highest PAF in Peru was attributed to unhealthy lifestyle (excluding tobacco consumption), similar to findings obtained by Islami in the US [9] for cancer cases (14.2% Peru versus 13.9% US) and slightly higher for cancer deaths (16.7% versus 14.9%). When including tobacco as a part of unhealthy lifestyle factors, Peru’s PAF was 21.4%, slightly lower than that reported by neighboring countries like Chile (30%) and Brazil (26.5%). These disparities found in the Region are attributed to the various levels of epidemiological transition among countries. Typically, countries with higher incomes and aging population tend to have a greater prevalence of unhealthy lifestyles [1, 5, 6, 35]. Currently, Chile boasts the highest aging rate in the Andean region, reaching 58%, placing it at stage 9 of transition according to the categorization proposed by CEPAL [36]. Meanwhile, Peru stands at stage 6 in this framework.
In Peru, the fraction attributable to modifiable factors such as tobacco and ultraviolet radiation for the occurrence of cancer cases and deaths is notably lower compared to most studies reported in other countries [10, 12, 14, 28, 29], including neighboring countries like Chile [23] and Brazil [16]. These findings are explained by successful tobacco control policies that have reduced consumption trends over the past two decades. However, poor access to diagnosis and under-reporting of lung cancer is possible, especially in rural remote regions away from the capital [2, 37]. On the other hand, UV radiation holds minimal relevance in Peru, as most skin melanomas are unrelated to UV radiation (acral melanomas) [38].
Our data underscores the imperative need to strengthen cancer prevention and control policies in Peru, emphasizing interventions proven to offer high cost/effectiveness. Prioritizing optimal vaccination coverage against oncogenic infections such as HPV and HBV [39, 40]. Simultaneously, implementing effective strategies to promote healthy lifestyles in the population, including reducing tobacco consumption, increasing physical activity, and fostering healthy dietary habits is essential [8, 37]. On the other hand, evidence from various researches, such as the analysis of mortality trends related to gastric cancer, demonstrates that improving access to safe water can significantly reduce the burden of gastric cancer attributed to Helicobacter pylori [34, 40–43].
This research significantly contributes to understanding cancer causality within the socio-health context, serving as valuable input for researchers, academics and decision-makers involved in the formulation of policies, plans, strategies and cost-effective interventions. We reaffirm the importance of adopting a territorial approach to address the most vulnerable regions, aiming to reduce cancer incidence and mortality. This approach optimizes the collective efforts of both the State and society.
One limitation of the PAF research model was the bias from the use of secondary information sources, that could result in some level of underreporting. To mitigate this, we relied on estimates of cases and deaths from the IARC, based on data from population-based cancer registries in Peru, which could have been affected in a smaller extent by the underreporting compared to vital records for deaths.
It should also be taken into account that PAF studies consider the effect of each risk’s factor in an independent way; it means, they do not consider possible interactions or confounding effects between factors, which constitutes a limitation of this methodology.
Another limitation was the scarcity of studies that reported the PAF for modifiable risk factors specifically for cancer deaths, published in the past decade. Most studies focused on PAF for new cancer cases, affecting the comparability of our results. However, leveraging previous studies conducted in Latin America and other regions allowed contextualization of the outcomes obtained for Peru.
There are some differences in the considered risk factors, compared to studies such as those by Islami (10 for the USA or Poirier [28] for Canada, which do not include Epstein Barr virus infection as a risk factor for Hodgkin’s and non-Hodgkin lymphoma, unlike this study that did include EBV. Additionally, prostate cancer was not included in our study due to insufficient evidence of modifiable risk factors. The fraction attributable to ionizing radiation could not be obtained due to a lack of exposed population data in Peru. Lastly, although firewood is used as cooking fuel in a fifth of Peruvian households, this study did not include exposure to firewood as a risk factor for lung cancer, as most PAF studies for this cancer did not consider it.
Interpreting the results obtained in this study requires caution, considering the existing methodological particularities between studies, which might not have been conducted within the same timeframe, age groups or included the same cancer locations.
Conclusion
Oncogenic infections accounted for the highest PAF, contributing to 16.6% of cancer cases and 19.1% of deaths. The burden of modifiable risk factors for cancer in Peru is attributed to the “double burden of disease”. Despite existing policies, plans and strategies for prevention, vaccination, screening, and early detection of various related neoplasms, this study underscores the lack of control of communicable diseases as a cause of cancer in Peru.
In 2018, prior to the COVID-19 pandemic, 38.5% of new cases and 43.4% of cancer deaths in Peru were attributable to potentially modifiable risk factors in the population of 15 years old and older. Oncogenic infections, combined with unhealthy lifestyle choices, accounted for one-third of cancer cases and almost two-fifths of cancer deaths. HPV infection, current tobacco use, and Helicobacter pylori infection were identified as the primary risk factors contributing to a higher number of cancer cases and deaths.
Implications of all the available evidence
Our findings underscore the urgency to strengthen cancer prevention and control policies in Peru. This involves prioritizing cost-effective interventions, such as achieving optimal vaccination coverage against oncogenic infections such as HPV and HBV. Simultaneously, implementing effective strategies to promote healthy lifestyles in the population, including reducing tobacco consumption, increasing physical activity, and fostering healthy dietary habits is essential. On the other hand, the strategy of improving access to safe water can significantly reduce the burden of stomach cancer attributable to helicobacter pylori.
Author contributions
Conceptualization: JADV, WR; data curation: JADV, WR, WCH, LECL; formal analysis: JADV, WR, WCH; funding acquisition: JADV; investigation: JADV, WR, WCH, LECL, NG, JALC, ITM; methodology: JADV, WR, WCH; project administration: JADV, LECL, JALC; resources: LECL, ITM; software: WCH, JALC; supervision: JADV, WR; validation: JADV, DV; visualization: JADV, NG, JALC, ITM; writing– original draft: JADV, WR; writing– review: JADV, WR, WCH, LECL, NG, JALC, ITM, DV; editing: JADV, WR, JALC, DV, ITM.
Funding
This project received funding from a research grant provided by the Ricardo Palma University (ACU N0 2619 − 2019).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
From an ethical standpoint, this study did not imply risks to individuals, as it used aggregated prevalence data from population surveys, relative risks from metanalysis and scientific journal articles, along with IARC cancer incidence and mortality estimates for Peru, thus not requiring an informed consent. Additionally, the study received approval from the Research Ethics Committee from the Medical School of Ricardo Palma University (Expedited Review: PI-019-2023).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Piñeros M, Ramos W, Antoni S, Abriata G, Medina LE, Miranda JJ, et al. Cancer patterns, trends, and transitions in Peru: a regional perspective. Lancet Oncol. 2017;18(10):e573–86. doi: 10.1016/S1470-2045(17)30377-7. [DOI] [PubMed] [Google Scholar]
- 2.Ramos W, Guerrero N, Medina J, Guerrero P. Análisis de la situación del Cáncer en el Perú, 2018. Lima: Ministerio de Salud del Perú; 2022. https://www.dge.gob.pe/epipublic/uploads/asis/asis_2020_27_120833.pdf
- 3.International Agency for Reasearch on Cancer. Cancer Today. Lyon, IARC; 2018. https://gco.iarc.fr/today/home. Accessed 20 Dec 2013.
- 4.Centro Nacional de Epidemiología, Prevención y Control de Enfermedades, Ministerio de Salud del Perú. Carga de Enfermedad en el Perú. Estimación de los años de vida saludable perdidos 2016. Lima: Ministerio de Salud del Perú. 2019. https://www.dge.gob.pe/portal/docs/tools/Cargaenfermedad2016.pdf. Accessed 20 Dec 2013.
- 5.Valdez W, Miranda J, Ramos W. State of the epidemiologic transition in Peru during 1990 and 2006. Rev Peru Epidemiol. 2011;15(3):1–5. [Google Scholar]
- 6.Ramos W, Venegas D, Honorio H, Pesantes J, Arrasco J, Yagui M. Non-communicable diseases: effect of major transitions and social determinants. Rev Peru Epidemiol. 2014;18(S1):e06. [Google Scholar]
- 7.Centro Nacional de Epidemiología . Prevención Y Control De Enfermedades, Ministerio De Salud Del Perú. Análisis De Situación De Salud Del Perú, 2021. Lima: CDC-MINSA; 2023. [Google Scholar]
- 8.De La Cruz-Vargas JA, Ramos W, Chanduví W, Espinoza R, Guerrero N, Loayza-Castro J, et al. Feasibility study to evaluate the proportion of cancer attributable to modificable risk factors in Peru and Latin America. Rev Fac Med Hum. 2020;20(1):114–22. doi: 10.25176/RFMH.v20i1.2657. [DOI] [Google Scholar]
- 9.Teh HS, Woon YL. Burden of cancers attributable to modifiable risk factors in Malaysia. BMC Public Health. 2021;21:1–10. doi: 10.1186/s12889-021-10412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DR, Friedenreich CM, Ruan Y, Poirier AE, Walter SD, King WD, et al. The burden of cancer attributable to modifiable risk factors in Canada: methods overview. Prev Med. 2019;122:3–8. doi: 10.1016/j.ypmed.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118(8):1130–41. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islami F, Chen W, Yu XQ, Lortet-Tieulent J, Zheng R, Flanders WD, et al. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol. 2017;28(10):2567–74. doi: 10.1093/annonc/mdx342. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, Hirabayashi M, Abe SK, Katanoda K, Sawada N, Lin Y, et al. Burden of cancer attributable to modifiable factors in Japan in 2015. Glob Health Med. 2022;4(1):26–36. doi: 10.35772/ghm.2021.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kulhánová I, Znaor A, Shield KD, Arnold M, Vignat J, Charafeddine M, et al. Proportion of cancers attributable to major lifestyle and environmental risk factors in the Eastern Mediterranean region. Int J Cancer. 2020;146(3):646–56. doi: 10.1002/ijc.32284. [DOI] [PubMed] [Google Scholar]
- 16.Azevedo e Silva G, de Moura L, Curado MP, Gomes FS, Otero U, Rezende LFM, et al. The fraction of Cancer Attributable to Ways of Life, infections, Occupation, and Environmental Agents in Brazil in 2020. PLoS ONE. 2016;11(2):e0148761. doi: 10.1371/journal.pone.0148761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichon-Riviere A, Bardach A, Rodríguez Cairoli F, Casarini A, Espinola N, Perelli L, et al. Health, economic and social burden of tobacco in Latin America and the expected gains of fully implementing taxes, plain packaging, advertising bans and smoke-free environments control measures: a modelling study. Tob Control. 2023:tc–2022–057618. 10.1136/tc-2022-057618 [DOI] [PMC free article] [PubMed]
- 18.Torres-Roman JS, Valcarcel B, Martinez-Herrera JF, Bazalar-Palacios J, La Vecchia C, Raez LE. Mortality trends for Lung Cancer and Smoking Prevalence in Peru. Asian Pac J cancer Prevention: APJCP. 2022;23(2):435. doi: 10.31557/APJCP.2022.23.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Roman JS, Julca-Marín D, Ticona-Tiña D, Quispe-Vicuña C, Bazalar-Palacios J, De La Cruz-Ku G, et al. Trends in gastric cancer mortality 2005–2020 in Peru and its geographical areas: a joinpoint regression analysis. Cancer Epidemiol. 2023;87:102485. doi: 10.1016/j.canep.2023.102485. [DOI] [PubMed] [Google Scholar]
- 20.Torres-Roman JS, De la Cruz-Ku G, Juárez-Leon V, Calderón-Solano D, Bazalar-Palacios J, Vecchia CL, et al. Mortality trends and geographic distribution of kidney cancer in Peru: a secondary analysis. BMC Urol. 2023;23(1):51. doi: 10.1186/s12894-023-01208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres-Roman JS, Ronceros-Cardenas L, Valcarcel B, Arce-Huamani MA, Bazalar-Palacios J, Ybaseta-Medina J, et al. Cervical cancer mortality in Peru: regional trend analysis from 2008–2017. BMC Public Health. 2021;21:1–10. doi: 10.1186/s12889-021-10274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Roman JS, Gomez-Rubio V, Sanchez-Trujillo L, Delgado-Rosas E, Puche-Vergara F, Sanz-Anquela JM, et al. Geographic study of mortality due to mesothelioma in Peru and its evolution. Cancer Epidemiol. 2020;68:101791. doi: 10.1016/j.canep.2020.101791. [DOI] [PubMed] [Google Scholar]
- 23.Rezende LFM, Murata E, Giannichi B, Tomita LY, Wagner GA, Sanchez ZM, et al. Cancer cases and deaths attributable to lifestyle risk factors in Chile. BMC Cancer. 2020;20(1):693. doi: 10.1186/s12885-020-07187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.GBD 2019 Cancer Risk Factors Collaborators The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2022;400(10352):563–91. doi: 10.1016/S0140-6736(22)01438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkin DM. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(S2):S2–5. doi: 10.1038/bjc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin A, Park S, Shin HR, Park EH, Park SK, Oh JK, et al. Population attributable fraction of infection-related cancers in Korea. Ann Oncol. 2011;22(6):1435–42. doi: 10.1093/annonc/mdq592. [DOI] [PubMed] [Google Scholar]
- 27.Arnold M, Lam F, Ervik M, Soerjomataram I. Cancers attributable to UV radiation. Lyon, France: International Agency for Research on Cancer; 2018. https://gco.iarc.fr/causes/uv
- 28.Whiteman DC, Webb PM, Green AC, Neale RE, Fritschi L, Bain CJ, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health. 2015;39(5):477–84. doi: 10.1111/1753-6405.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirier AE, Ruan Y, Volesky KD, King WD, O’Sullivan DE, Gogna P, et al. The current and future burden of cancer attributable to modifiable risk factors in Canada: Summary of results. Prev Med. 2019;122:140–7. doi: 10.1016/j.ypmed.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer statistics? Cancer Commun (Lond) 2019;39(1):22. doi: 10.1186/s40880-019-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bygbjerg IC. Double burden of noncommunicable and infectious diseases in developing countries. Science. 2012;337(6101):1499–501. doi: 10.1126/science.1223466. [DOI] [PubMed] [Google Scholar]
- 32.Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg. 2006;100(3):191–9. doi: 10.1016/j.trstmh.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31(1):100–10. doi: 10.1093/carcin/bgp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venegas-Ojeda D, Agüero-Palacios YD. Gastric cancer mortality rate trend in Peru: segmented regression model from 1995 to 2013. Rev Fac Med Hum. 2021;21(1):28–39. doi: 10.25176/RFMH.v21i1.3592. [DOI] [Google Scholar]
- 35.Szot Meza J. Demographic-epidemiologic transition in Chile, 1960–2001. Rev Esp Salud Publica. 2003;77(5):605–13. doi: 10.1590/S1135-57272003000500009. [DOI] [PubMed] [Google Scholar]
- 36.Turra C, Fernandes F. La transición demográfica: oportunidades y desafíos en la senda hacia el logro de los Objetivos de Desarrollo Sostenible en América Latina y el Caribe, Documentos de Proyectos (LC/TS.2020/105). Santiago, Comisión Económica para América Latina y el Caribe (CEPAL); 2021. https://www.cepal.org/es/publicaciones/46805-la-transicion-demografica-oportunidades-desafios-la-senda-logro-objetivos
- 37.Torres-Roman JS, Valcarcel B, Martinez-Herrera JF, Bazalar-Palacios J, La Vecchia C, Raez LE. Mortality trends for Lung Cancer and Smoking Prevalence in Peru. Asian Pac J Cancer Prev. 2022;23(2):435–43. doi: 10.31557/APJCP.2022.23.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basurto-Lozada P, Molina-Aguilar C, Castaneda-Garcia C, Vázquez-Cruz ME, Garcia-Salinas OI, Álvarez-Cano A, et al. Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res. 2021;34(1):59–71. doi: 10.1111/pcmr.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palladini A, Landuzzi L, Lollini PL, Nanni P. Cancer immunoprevention: from mice to early clinical trials. BMC Immunol. 2018;19(1):16. doi: 10.1186/s12865-018-0253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lollini PL, Nicoletti G, Landuzzi L, Cavallo F, Forni G, De Giovanni C, et al. Vaccines and other immunological approaches for cancer immunoprevention. Curr Drug Targets. 2011;12(13):1957–73. doi: 10.2174/138945011798184146. [DOI] [PubMed] [Google Scholar]
- 41.Castillo M, Bernabe L, Castaneda CA, Chavez I, Ruiz E, Barreda F, et al. Helicobacter Pylori detected in tap water of Peruvian patients with gastric Cancer. Asian Pac J Cancer Prev. 2019;20(11):3193–6. doi: 10.31557/APJCP.2019.20.11.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichelberger L, Murphy G, Etemadi A, Abnet CC, Islami F, Shakeri R, et al. Risk of gastric cancer by water source: evidence from the Golestan case-control study. PLoS ONE. 2015;10(5):e0128491. doi: 10.1371/journal.pone.0128491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein PD, Graham DY, Gaillour A, Opekun AR, Smith EO. Water source as risk factor for Helicobacter pylori infection in Peruvian children. Gastrointest Physiol Working Group Lancet. 1991;337(8756):1503–6. doi: 10.1016/0140-6736(91)93196-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


