Abstract
Background
Snakebite envenoming represents a significant and often neglected public health challenge, particularly in rural communities across tropical and subtropical regions. An estimated 1.2–5.5 million people are envenomed by snakebites annually. More than 125,000 of these bites are fatal, and 3–4 times as many results in disability/disfigurement. Despite its prevalence, collecting accurate epidemiological data on snakebite is challenging. This systematic review and meta-analysis collates global epidemiology data on snakebite morbidity and mortality.
Methods
Medline, Embase, Cochrane and CINAHL Plus databases were searched for articles published between 2001–2022. Pooled incidence and mortality were obtained using random effects modelling, heterogeneity (I2) was tested, and sensitivity analyses performed. Newcastle-Ottawa Scale assessed study quality.
Results
Out of the four databases, 5,312 articles were found. After removing duplicates, 3,953 articles were screened by title and abstract and 65 articles containing information on snakebite epidemiology, encompassing 663,460 snakebites, were selected for analysis. The people most at risk for snakebite were men (59%), engaged in agricultural labour (27.5%), and residing in rural areas (66.7%). More than half (57%) of the reported bites resulted in envenoming. Incidents occurred frequently in the summer season (38.5%), during daytime (56.7%), and bites were most often to the lower limb (56.4%). Envenoming severity was frequently mild (46.7%), treated in hospital (68.3%), and was treated with anti-venom (64.7%). The pooled global incidence and mortality was 69.4 /100,000 population (95%CI: 36.8 to 101.9) and 0.33/100,000 population (95%CI, 0.14 to 0.52) per year, respectively.
Stratified by continents, Asia had the highest incidence of 130.7/100,000 population (95%CI: 48.3 to 213.1) while Europe has the lowest with 0.7/100,000 population (95%CI: -0.2 to 1.5). The highest mortality was reported in Asia at 0.96/100,000 population (95% CI: 0.22 to 1.70), and Africa 0.44/100,000 population (95%CI: -0.03 to 0.84). Incidence was highest among inhabitants of lower-middle-income countries 132.7/100,000 population (95%CI: 55.4 to 209.9) while mortality was highest in low-income countries at 0.85/100,000 population (95% CI: -0.06 to 2.31).
Conclusion
Incidence and mortality rates noted here highlight the global impact of snakebite and underscore the critical need to address the burden of snakebite envenoming. It also reveals that while reported snakebite incidence was higher in lower-middle-income countries, the burden of mortality was greatest among inhabitants of low-income countries, again emphasising the need for greater efforts to tackle this neglected tropical disease.
Author summary
Snakebite envenoming poses a significant public health challenge, especially in rural tropical and subtropical areas. Annually, an estimated 1.2–5.5 million people are envenomed, with over 125,000 fatalities and many more suffering disabilities. Collecting accurate epidemiological data on snakebite is challenging. This systematic review and meta-analysis compile global data on snakebite morbidity and mortality.
After screening thousands of articles, 65 were selected, covering 663,460 snakebites. Men in agricultural areas were most at risk, with rural dwellers bearing the brunt. Most bites occurred in summer, during the day, and affected the lower limb. Envenoming severity was typically mild, often treated in hospitals with anti-venom.
Globally, the pooled incidence was 69.4/100,000 population per year and mortality was 0.33/100,000 population per year. Asia reported the highest incidence and mortality rates. Incidence was highest in lower-middle-income countries, while mortality was greatest in low-income countries.
These findings underscore the urgent need to address the burden of snakebite envenoming, particularly in resource-limited settings. Efforts must focus on prevention, treatment, and strengthening healthcare systems to mitigate the impact of this neglected tropical disease.
Introduction
Snakebite envenoming affects millions of people worldwide and is a significant source of mortality [1], primarily in rural and agricultural communities of tropical and subtropical countries [1]. In 2019, the World Health Organization (WHO) set a target to halve the number of deaths and cases of snakebite envenoming by 2030 [2]. According to the WHO, there are approximately 5.4 million snakebites and 1.8–2.7 million cases of envenomation globally each year, including 81,410–137,880 deaths and around three times as many individuals suffering from permanent disfigurement and/or disabilities, including limb amputations [1]. Regions such as Sub-Saharan Africa, Southeast Asia, and South Asia experience the highest incidence of snakebite, with up to 200,000 cases of envenoming estimated in Asia and 435,000 to 580,000 cases estimated to occur across Africa annually [1]. In South Asia, India experiences the highest mortality rate attributable to snakebite envenomation with approximately 45,900 deaths reported each year [3].
Several factors contribute to the risk of snakebite, including occupations that result in frequent exposure to snakes, such as agricultural work, farming, and herding, living in rural areas with close proximity to snake habitats, and insufficient knowledge about snakebite prevention. The lack of identification of venomous snakes and appropriate first aid measures, initial management by traditional healers, delay in reaching hospital and limited access to healthcare, including antivenoms, in rural areas further exacerbate the consequences of snakebite envenoming [4,5].
Despite the scale of snakebite across the world, reliable incidence and mortality data remain largely unavailable across snakebite endemic areas across rural equatorial regions; reliable data are instead mostly limited to a few developed countries where bites are relatively rare. Having information on the number of snakebites, envenomings, deaths, and long-term morbidity, is crucial for evaluating the impact of snakebite in these areas and for developing management guidelines, planning healthcare resources (especially antivenom availability), and providing appropriate training to healthcare professionals for effective snakebite treatment [6]. Furthermore, if we are to achieve the strategic goals set by the WHO to reduce snakebite-related deaths and disabilities by 50% by the year 2030 [1], comprehensive data on the incidence of snakebite and mortality rates are urgently needed. Therefore, this systematic review and metanalysis aims to assess and summarise the incidence of snakebite and resultant mortality available from published data globally.
Methodology
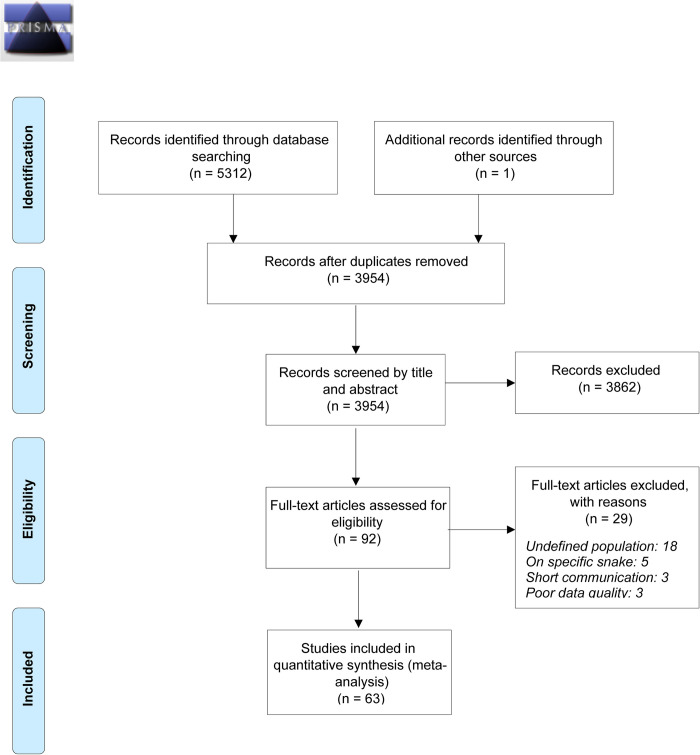
This systematic review and meta-analysis adhered to the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [7] and was registered with the International Prospective Register for Systematic Review (PROSPERO ID: CRD42022377613). The PRISMA flow diagram illustrates the identification and screening process of studies for inclusion in the meta-analysis (Fig 1). Two reviewers (BS and HAC) independently assessed all articles, extracted data, and completed the PRISMA checklist, which is included as supporting information (S1 PRISMA Checklist). As this review is based on published literature, no ethics approval was necessary.
Fig 1. PRISMA 2009 Flow Diagram.
Search strategy and study selection
Two authors (BNS and HAC) independently conducted a search for articles published between 01st January 2001 and 31st December 2022 on Medline, Embase, CINAHL Plus, and Cochrane. The authors used key terms recommended by the senior librarian at the University of Melbourne to conduct the search. The key search terms were the following: [Incidence OR prevalence OR epidemiology OR risk factors] AND [Snakebite OR Snake venom]. Searched articles were stored and managed using citation software EndNote X20. A detailed description of the search strategy has been provided as supplementary information (S1 Search strategy). The articles retrieved from the search were screened for eligibility based on their titles and abstracts by both BNS and HAC independently. Any articles that did not meet the criteria (outlined below) were excluded. Additionally, relevant publications’ reference lists were manually reviewed to identify any other studies. Inclusion and exclusion criteria were clearly defined for the study selection process. After a full read, BNS, HAC, and AA discussed and selected the final set of 65 articles. In case of any disagreement, the study lead, AA, made the final decision.
Selection criteria
To be eligible for inclusion, studies had to meet the following criteria: 1. Study of any design with published data, 2. Contained estimations of snakebite incidence and/or mortality due to snakebite envenoming in terms of actual number, incidence per 100,000 population or incidence per 100,000 population per year.
Studies were excluded if they met the following criteria: 1. Overlapping articles or duplicate data, editorials, reviews, short communication, case reports, preprints. 2. Meta-analyses or review articles. 3. Studies with undefined population or outcome. 4. Studies lacking sufficient methodological precision, 5. Focused on specific venomous snake or clinical complications.
For included studies, data containing name of first author, publication year, geographic location, study design and study period, all demographic details, number of snakebite cases and deaths due to snakebite were identified. Data were extracted and transferred to Microsoft Excel by two authors (BNS and HAC) for each eligible study.
Outcome measure
The primary outcome was the incidence of snakebite. All reported number were retrieved from the articles. Where the absolute annual number of snakebite envenoming cases reported by a given study was available, the incidence rate per 100,000 population was calculated using the country population or the catchment area population for the reporting year by referring to online sources.
The secondary outcome was mortality due to snakebites. Information on mortality was gathered by analysing the articles. Of the articles that reported total number of deaths were considered and Mortality rates (per 100,000 population) were calculated using the country populations of the reporting year as the denominator.
Risk of bias and quality assessment in individual studies
The terms risk of bias and quality assessment are commonly used interchangeably. Researchers use various tools to evaluate safeguards for internal validity and explore the potential reliability of evidence generated within a study [8,9]. In this review, using the Newcastle-Ottawa Scale (NOS) tool, BNS and HAC evaluated the risk of bias and quality for each eligible study [10]. The NOS is based on an eight-item score divided into three domains and is currently the most frequently utilised tool to assess study quality and risk of bias. It also offers flexibility for modification according to specific subjects [8]. This tool utilises a "star system" that assesses a study based on three main perspectives: the selection of study groups, the comparability of those groups, and the ascertainment of exposure of interest (outcome) for cohort, observational or cross-sectional studies. The overall quality of the study is rated as “good,” “fair,” or “poor,” based on the reviewing authors’ judgments about the study quality and risk of bias item for each included study; details can be found as supporting information (S1 Quality assessment). Minor discrepancies were resolved by lead author, AA.
Data analysis
The lead author (AA) analysed the data extracted from each study using Stata V.17 (StataCorp., College Station, Texas, United States of America). The senior author (AW) cross-checked the analysis, and any discrepancies were resolved through discussion. The overall incidence and mortality estimates, along with corresponding 95% confidence intervals, were calculated using random-effects models of restricted maximum-likelihood method [11] (i.e., an open model in which effects are not constant). In the presence of heterogeneity (as expected and observed), random-effect models have superior properties and are more conservative than fixed-effect models [12]. The fixed-effect model assumes that differences in observed effects result from sampling error, whereas the random-effects model suggests that the true effect might vary among studies due to inherent differences (heterogeneity) among studies. This approach makes it possible to estimate the variables by accounting for the heterogeneity of results and the weight of each study according to the number and type of population under review [13]. The χ2-test on Cochran’s Q statistic was used to test for between-study heterogeneity. The H and I2 indices were used to calculate this statistic, with I2 representing the percentage of total heterogeneity across studies based on true between-study differences rather than on chance. Conventionally, I2 values of 0–25% indicate low heterogeneity, 26–75% indicate moderate heterogeneity, and 76–100% indicate substantial heterogeneity [14]. To identify the possible sources of substantial/considerable heterogeneity, subgroup analysis was carried out for the following covariates: continent, economic classification [15], study design (Observational, Cross sectional and Cohort) and study setting (Community based, Database and Hospital based).
A sensitivity analysis was conducted using the ‘leave-one-out’ method to ascertain the influence of any single study on the overall result [16]. Publication bias was assessed by visual inspection of Begg’s funnel plots, Begg’s and Egger’s regression test [17,18]. The publication bias was declared in situations where the p-values from both Begg’s and Egger’s regression test were significant. All p-value <0.5 was considered as statistical significance.
Results
A comprehensive search of databases including Medline (2246), Embase (2837), CINAHL Plus (229), and Cochrane (0) added a total of 5,312 published articles from 2001 to 2022. After removing duplicates (1359), the remaining 3,954 articles were screened based on titles and abstracts and 3862 were excluded, as represented in Fig 1. From the remaining 92 articles, a thorough evaluation was conducted on a selection of articles to determine their eligibility for inclusion. As a result, 26 articles were excluded, leaving a final set of 63 [5,19–80] studies from 29 countries for this study. Table 1 provides a description of the included studies.
Table 1. Descriptions of the included studied.
| Study ID | Country | Study period (years) | Study design | Study population | Snake bite cases | Outcome included (I, M) | First author | Publication year |
|---|---|---|---|---|---|---|---|---|
| 1 | Brazil | 9 | Cohort | 8843000 | 1063 | I, M | Albuquerque PL et al [19] | 2013 |
| 2 | Cameroon | 1 | Cross sectional | 9924 | 66 | I, M | Alcoba G et al [20] | 2020 |
| 3 | Nepal | 1 | Cross sectional | 63454 | 166 | I, M | Alcoba G et al [21] | 2022 |
| 4 | Morocco | 16 | Cohort | 28550000 | 1423 | I, M | Arfaoui et al [22] | 2009 |
| 5 | India | 5 | Cohort | 27300000 | 6555 | I, M | Bhargava S et al [23] | 2018 |
| 6 | South Africa | 2.5 | Observational | 300000 | 333 | I, M | Blaylock R [24] | 2003 |
| 7 | USA | 5 | Cohort | 4440000 | 674 | I, M | Buchanan et al [25] | 2021 |
| 8 | Ghana | 5 | Cohort | 2457792 | 2973 | I | Ceesay B et al [26] | 2021 |
| 9 | Brazil | 11 | Cohort | 2449024 | 5568 | I, M | Ceron K et al [27] | 2021 |
| 10 | Turkey | 10 | Cohort | 63240194 | 550 | I | Cesaretli et al [28] | 2010 |
| 11 | Morocco | 5 | Cohort | 32223000 | 873 | I, M | Chafiq F et al [29] | 2016 |
| 12 | Taiwan | 5 | Cohort | 22952400 | 4647 | I, M | Chen CK et al [30] | 2015 |
| 13 | Brazil | 12 | Cohort | 189512052 | 326481 | I, M | Chippaux JP [31] | 2015 |
| 14 | Brazil | 10 | Cohort | 3168027 | 3909 | I, M | Costa M et al [32] | 2019 |
| 15 | Bosnia and Herzegovina | 23 | Cohort | 4,384,662 | 341 | I, M | Curic I et al [33] | 2009 |
| 16 | Australia | 8.6 | Cohort | 140000 | 216 | I | Currie BJ et al [34] | 2004 |
| 17 | Iran | 7 | Cohort | 293996 | 50 | I, M | Dehghani et al [35] | 2012 |
| 18 | Iran | 1 | Observational | 75373855 | 5172 | I, M | Dehghani R et al [36] | 2014 |
| 19 | Iran | 10 | Cohort | 79960000 | 53787 | I, M | Dehghani R et al [37] | 2014 |
| 20 | Iran | 1 | Cohort | 79960000 | 4917 | I, M | Dehghani R et al [37] | 2014 |
| 21 | Iran | 5 | Cohort | 66000 | 195 | I | Ebrahimi V et al [38] | 2018 |
| 22 | Sri Lanka | 1 | Cross sectional | 165665 | 677 | I | Ediriweera et al [39] | 2020 |
| 23 | Mozambique | 20 | Cohort | 7544 | 297 | I, M | Farooq et al [40] | 2022 |
| 24 | Kenya | 1 | Observational | 3613429 | 176 | I, M | Francis Okumu Ochola et al [41] | 2018 |
| 25 | India | 1 | Cross sectional | 402095 | 145 | I, M | Gajbhiye R et al [42] | 2019 |
| 26 | Burkina Faso | 5 | Cohort | 17051002 | 114126 | I, M | Gampini S et al [43] | 2016 |
| 27 | Ecuador | 10 | Cohort | 13000000 | 14720 | I, M | Gonzalez-Andrade F and Chippaux JP [44] | 2010 |
| 28 | India | 5 | Cohort | 580320 | 497 | I, M | Gupt A et al [45] | 2015 |
| 29 | Sudan | 5 | Observational | 39446096 | 63160 | I, M | H. Khalid and R. S. Azrag et al [46] | 2021 |
| 30 | Nicaragua | 5 | Cohort | 5900000 | 3286 | I, M | Hansson et al [47] | 2010 |
| 31 | Morocco | 15 | Cohort | 30896566 | 2053 | I, M | Hattimy et al [48] | 2018 |
| 32 | Bangladesh | 1 | Cross sectional | 819429 | 90 | I, M | Hossain J et al [49] | 2016 |
| 33 | Bulgaria | 9 | Cohort | 622867 | 68 | I, M | Iliev YT et al [50] | 2014 |
| 34 | Pakistan | 4 | Observational | 1136044 | 695 | I, M | Jamali et al [51] | 2022 |
| 35 | Sweden | 10 | Cohort | 25630000 | 1548 | I, M | Johnston CI et al [52] | 2017 |
| 36 | Nepal | 4 | Cohort | 5560000 | 265 | I | Karki et al [53] | 2019 |
| 37 | Iran | 5 | Observational | 133099 | 102 | I | Kassiri H et al [54] | 2019 |
| 38 | India | 1 | Observational | 263426 | 245 | I, M | Kharat R and Kedare R [55] | 2020 |
| 39 | Brazil | 4 | Cohort | 185235 | 351 | I, M | Leite Rde S et al [56] | 2013 |
| 40 | Croatia | 21 | Cohort | 496395 | 542 | I, M | Lucsic B et al [57] | 2006 |
| 41 | Brazil | 6 | Cohort | 15772000 | 2431 | I, M | Machado C et al [58] | 2012 |
| 42 | Nepal | 3 | Observational | 2356820 | 6993 | I, M | Magar CT et al [59] | 2013 |
| 43 | Myanmar | 1 | Cross sectional | 19877 | 24 | I | Mahmood MA et al [60] | 2018 |
| 44 | India | 2 | Cross sectional | 1952546 | 4871 | I, M | Majumder et al [61] | 2014 |
| 45 | Brazil | 6 | Cohort | 213159 | 304 | I | Oliveira HFA et al [62] | 2013 |
| 46 | Brazil | 1 | Observational | 407319 | 118 | I | Oliveira LP et al [63] | 2020 |
| 47 | Nepal | 1 | Observational | 249735 | 274 | I, M | Pandey DP [64] | 2018 |
| 48 | Nepal | 0.67 | Observational | 2500000 | 476 | I, M | Pandey et al [65] | 2022 |
| 49 | Nepal | 1 | Cross sectional | 1372 | 32 | I | Parajuli et al [66] | 2022 |
| 50 | Ecuador | 5 | Cohort | 103697 | 133 | I | Patino RSP et al [67] | 2022 |
| 51 | Panama | 2 | Cohort | 236489 | 390 | I | Pecchio M et al [68] | 2018 |
| 52 | Bangladesh | 1 | Cross sectional | 18857 | 98 | I, M | Rahman R et al [69] | 2010 |
| 53 | India | 8 | Cohort | 610577 | 409 | I | Rai A et al [70] | 2021 |
| 54 | Brazil | 2 | Cohort | 1562409 | 92 | I | Roriz et al [71] | 2017 |
| 55 | USA | 3 | Observational | 287201314 | 450 | I | Ruha AM et al [5] | 2017 |
| 56 | India | 5 | Cohort | 2145572 | 1633 | I, M | Sarkhel S et al [72] | 2017 |
| 57 | Costa Rica | 2 | Observational | 4764064 | 475 | I, M | Sasa M and Segura Cano SE [73] | 2020 |
| 58 | Brazil | 1 | Observational | 190755799 | 28716 | I, M | Schneider et al [74] | 2021 |
| 59 | South Korea | 6 | Cohort | 50326620 | 1335 | I, M | Senek MZF et al [75] | 2019 |
| 60 | Brazil | 1 | Observational | 137722 | 133 | I, M | Silva et al [76] | 2019 |
| 61 | Brazil | 8 | Cohort | 3168027 | 3019 | I, M | Tavares AV et al [77] | 2017 |
| 62 | Cameroon | 1 | Observational | 1409348 | 516 | I, M | Tchoffo et al [78] | 2019 |
| 63 | Lao PDR (Laos) | 1 | Cross sectional | 9856 | 35 | I | Vongphoumy I et al [79] | 2015 |
| 64 | Lao PDR (Laos) | 1 | Cross sectional | 7150 | 79 | I | Vongphoumy I et al [79] | 2015 |
| 65 | South Africa | 5 | Cohort | 3000000 | 879 | I | Wood et al [80] | 2016 |
From the selected 63 [5,19–80] articles two of the studies had consecutive information that was treated as separate studies and thus we have reported 65 studies in the overall analysis. As detailed in Table 2, a total of 663,460 snakebite cases were identified. Among these cases, approximately 58.9% were reported as male based on information from 57/65 (87.69%) studies. The age range of the affected individuals spanned from 0 to 92 years, as reported by 23/65 (35.38%) studies. Additionally, based on data from 19/65 (29.23%) and 27/65 (41.54%) studies, respectively, individuals in farming or agriculture professions (27.5%) and those living in rural areas (66.7%) were identified as being more vulnerable to snakebite.
Table 2. Summary of the included studies by demography, seasonal and clinical features.
| Variables | Categories | N | Number of studies reported | n | % |
|---|---|---|---|---|---|
| Total | 663460 | 65 | |||
| Gender | 167274 | 57 | |||
| Male | 98477 | 58.9 | |||
| Female | 57118 | 34.1 | |||
| Unknown | 11679 | 7.0 | |||
| Age | 23019 | 23 | |||
| Occupation | 12617 | 19 | |||
| Farming/agriculture | 3466 | 27.5 | |||
| Laboure | 545 | 4.3 | |||
| Housewife | 650 | 5.2 | |||
| Service | 314 | 2.5 | |||
| Unemployed | 151 | 1.2 | |||
| Student | 1042 | 8.3 | |||
| Others | 1575 | 12.5 | |||
| Unknown | 4874 | 38.6 | |||
| Area of residence | 27016 | 27 | |||
| Rural | 18020 | 66.7 | |||
| Peri-urban | 6976 | 25.8 | |||
| Urban | 508 | 1.9 | |||
| Unknown | 1512 | 5.6 | |||
| Snake type | 49920 | 34 | |||
| Venomous | 28607 | 57.3 | |||
| Non-venomous | 10310 | 20.7 | |||
| Unknown | 11003 | 22.0 | |||
| Location/place of bites | 9185 | 12 | |||
| Agriculture field | 1574 | 17.1 | |||
| Road or path | 1215 | 13.2 | |||
| Home | 2027 | 22.1 | |||
| Outdoor working area | 152 | 1.7 | |||
| Fishing | 1138 | 12.4 | |||
| Others | 1648 | 17.9 | |||
| Unknown | 1431 | 15.6 | |||
| Season | 13457 | 18 | |||
| Summer | 5175 | 38.5 | |||
| Monsoon | 2852 | 21.2 | |||
| Spring | 2253 | 16.7 | |||
| Autumn | 1053 | 7.8 | |||
| Winter | 490 | 3.6 | |||
| Unknown | 1634 | 12.1 | |||
| Bite time | 7380 | 15 | |||
| Day | 4181 | 56.7 | |||
| Night | 2568 | 34.8 | |||
| Unknown | 631 | 8.6 | |||
| Location of bite | 54254 | 39 | |||
| Lower limb | 30575 | 56.4 | |||
| Upper limb | 12940 | 23.9 | |||
| Others | 3261 | 6.0 | |||
| Unknown | 7478 | 13.8 | |||
| Severity of envenomation | 42864 | 27 | |||
| Mild | 20000 | 46.7 | |||
| Moderate | 12100 | 28.2 | |||
| Severe | 4232 | 9.9 | |||
| No envenomation | 205 | 0.5 | |||
| Unknown | 6327 | 14.8 | |||
| First-aid received | 11148 | 10 | |||
| Yes | 3158 | 28.3 | |||
| No | 7974 | 71.5 | |||
| Unknown | 16 | 0.1 | |||
| Treatment type | 3328 | 13 | |||
| Traditional | 759 | 22.81 | |||
| Formal treatment (in hospital) | 2272 | 68.3 | |||
| No treatment | 280 | 8.4 | |||
| Others | 17 | 0.5 | |||
| Use of anti-venom | 54483 | 33 | |||
| Yes | 35266 | 64.7 | |||
| No | 9923 | 18.2 | |||
| Unknown | 9294 | 17.1 |
Of the 49,920 cases that were examined across 34/65 (52.31%) studies, 57.3% of individuals were bitten by a venomous snake. According to 12/65 (18.46%) studies, 22.1% of these cases occurred at home, while 17.1% occurred in fields, and 13.2% occurred on roads or paths.
In terms of seasonal trends, 38.5% of snakebites were reported during summer, followed by 21.2% during monsoon season and 16.7% during spring, as reported by 18/65 (27.69%) studies. Additionally, as stated by 15/65 (23.08%) and 39/65 (60.00%) studies respectively, over half of all snakebites (56.7%) occurred during the daytime and 56.4% affected the lower limbs. In terms of severity, approximately 46.7% of cases as reported in 27/65 (41.54%) studies were considered mild, while 28.2% were classified as moderate and 9.9% were reported as severe.
Considering treatment details, 10/65 (15.38%) studies reported on first-aid treatment, 13/65 (20.00%) studies covered the type of treatment, and 33/65 (50.77%) studies reported on use of antivenom. Of the reported studies, more than two-thirds (71.5%) of the cases did not receive first-aid, 68.3% were treated in a hospital setting, and 64.7% received antivenom. Supplementary information containing detailed study-specific findings can be accessed for further insights (S1 Study-wise description).
Meta-analysis of incidence and mortality
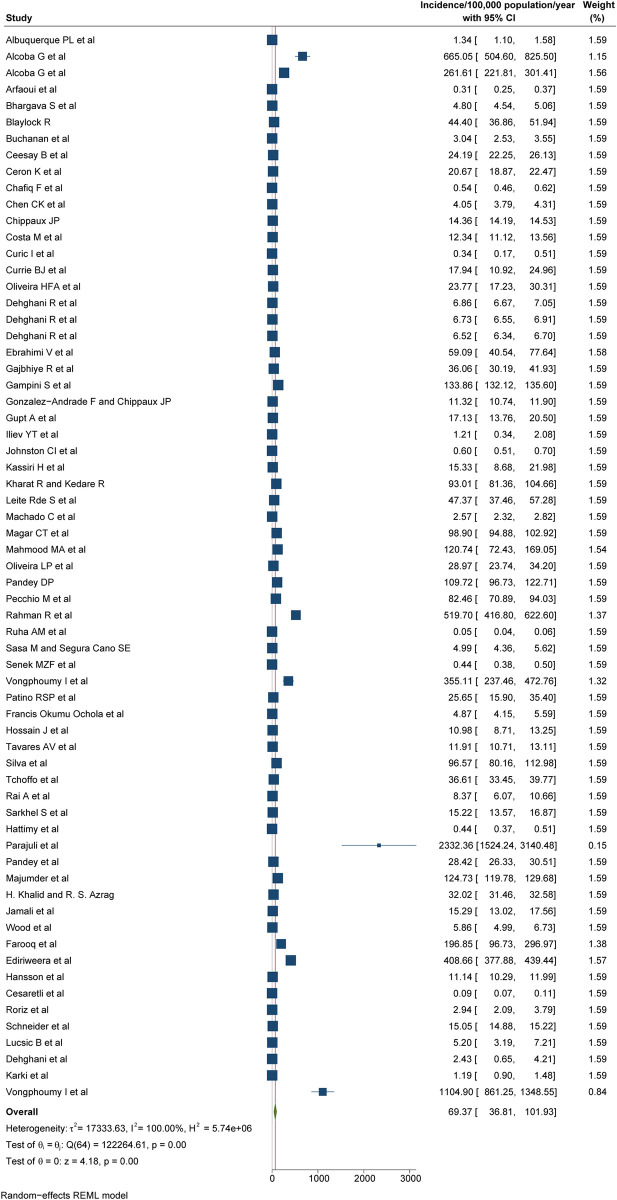
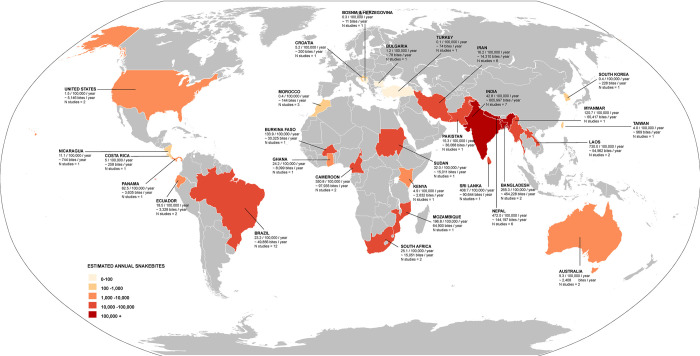
According to our most conservative estimates from 65 studies, the pooled global incidence of snakebite was 69.4/100,000 population (95% CI: 36.8 to 101.9; Fig 2), also mapped on Fig 3. Stratified by continents (Table 3), Asia has the highest incidence of 130.7/100,000 population (95% CI: 48.3 to 213.1), followed by Africa 84.2/100,000 population (95% CI: -6.0 to 174.5), South America 21.7/100,000 population (95% CI: 9.8 to 33.7), North America 19.9/100,000 population (95% CI: -10.2 to 50.1), Oceania 7.1/100,000 population (95% CI: -2.3 to 17.1) and Europe 0.7/100,000 population (95% CI: -0.2 to 1.5). The incidence was highest among inhabitants of lower-middle income countries at 132.6/100,000 population (95% CI: 55.4 to 209.9), followed by low income countries 72.5/100,000 population (95% CI: -47.8 to 192.8), middle countries 22.4/100,000 population (95% CI: 8.4 to 36.5), upper-middle income countries 15.8/100,000 population (95% CI: 2.5 to 29.2) and the lowest in high-income countries 12.4/100,000 population (95% CI: -4.5 to 29.2; Table 3). The pooled incidence of the studies that scored good, fair, or poor in our quality assessment was 183.7/100,000 population (95% CI: 19.9 to 347.5), 76.3/100,000 population (95% CI: 13.2 to 139.5), and 24.3/100,000 population (95% CI: 12.3 to 36.3), respectively (Table 3). High heterogeneity was observed to the reported incidences of snakebites (I2 >75%), with the absence of publication bias considering both Beggs test and Egger’s regression test (p<0.05). None of the following stratification helped identify the studies primarily responsible for the high heterogeneity: geographical location, economical classification, study quality, study setting and study design.
Fig 2. Pooled incidence of snakebite, REML-Restricted Maximum Likelihood.
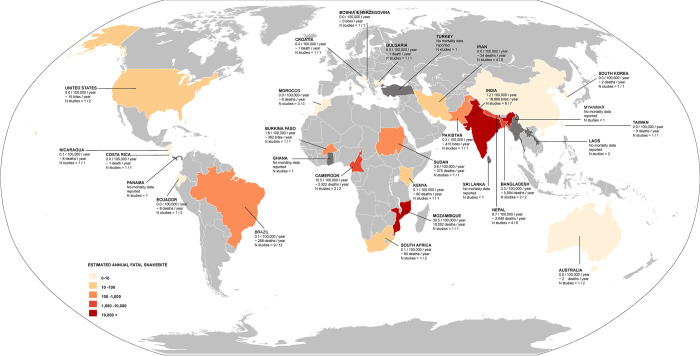
Fig 3. World map with incidence of snakebites per 100,000 population per year across the globe.
Study number pre county have been provided. For countries with multiple studies, average incidence per 100,000 population per year have been provided and noted. (The direct link to the base layer of the map: https://commons.wikimedia.org/wiki/File:BlankMap-World.svg).
Table 3. Sub-group analysis for the overall pooled incidence of the included studies.
| Variable | No of studies | Incidence/100,000 population/ year (95% CI*), p-value | I2 | Egger test (p-value) | Beggs test (p-value) |
|---|---|---|---|---|---|
| Continent | |||||
| Africa | 12 | 84.2 (-6.0 to 174.5), <0.01 | 100.0 | 0.001 | 0.4507 |
| Asia | 29 | 130.7 (48.3 to 213.1), <0.01 | 100.0 | 0.001 | 0.1486 |
| Europe | 2 | 0.7 (-0.2 to 1.5), 0.05 | 73.25 | - | |
| North America | 5 | 19.9 (-10.2 to 50.1), <0.01 | 99.99 | 0.026 | 0.2207 |
| Oceania | 3 | 7.1 (-2.3 to 17.1), <0.01 | 98.61 | 0.001 | 0.2963 |
| South America | 14 | 21.7 (9.8 to 33.7), <0.01 | 99.99 | 0.001 | 0.2284 |
| Economy | |||||
| Low-income countries | 2 | 72.5 (-47.8 to 192.8), <0.01 | 99.99 | ||
| Lower-middle income countries | 34 | 132.6 (55.4 to 209.9), <0.01 | 100.0 | 0.001 | 0.4767 |
| Middle-income countries | 12 | 22.4 (8.4 to 36.5), <0.01 | 99.99 | 0.001 | 0.3037 |
| Upper-middle countries | 8 | 15.8 (2.5 to 29.2), <0.01 | 99.99 | 0.001 | 0.9015 |
| High-income countries | 9 | 12.4 (-4.5 to 29.2), <0.01 | 100.0 | 0.001 | 0.9170 |
| Study Quality | |||||
| Good | 12 | 183.7 (19.9 to 347.5), <0.01 | 100.0 | 0.001 | 0.9453 |
| Fair | 21 | 76.3 (13.2 to 139.5), <0.01 | 100.0 | 0.001 | 0.2639 |
| Poor | 32 | 24.3 (12.3 to 36.3), <0.01 | 100.0 | 0.001 | 0.8840 |
| Study setting | |||||
| Registry/database | 14 | 306.2 (79.0 to 533.4), 0.01 | 100.0 | 0.001 | 0.7426 |
| Community based | 26 | 42.4 (7.8 to 76.9), 0.01 | 100.0 | 0.001 | 0.3780 |
| Hospital based | 25 | 24.4 (13.3 to 35.1), 0.01 | 100.0 | 0.001 | 0.6913 |
| Study design | |||||
| Observational | 16 | 35.4 (17.7 to 53.1), <0.01 | 100.0 | 0.001 | 0.7526 |
| Cross sectional | 12 | 422.5 (147.2 to 697.9), <0.01 | 100.0 | 0.001 | 0.3727 |
| Cohort | 37 | 18.9 (9.3 to 28.5), 0.01 | 100.0 | 0.001 | 1.0000 |
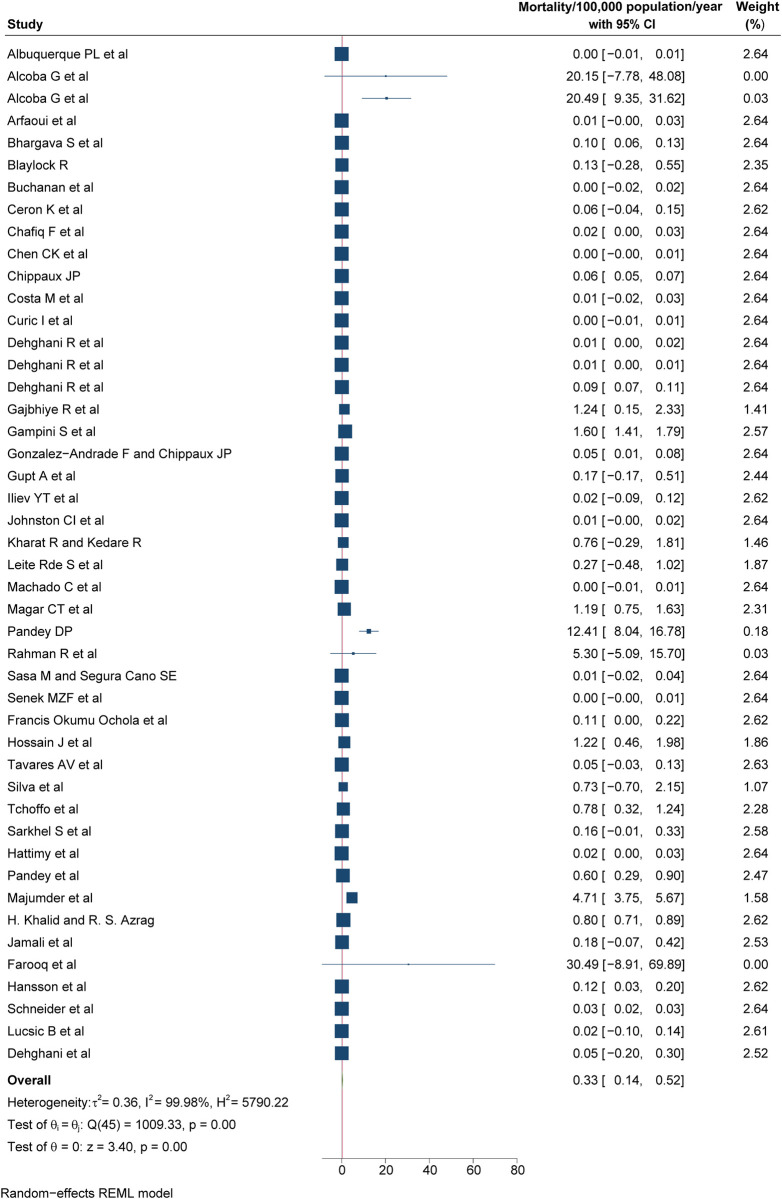
Based on the 46/65 (69.23%) studies that provided mortality data, the overall global mortality due to snakebites was estimated at 0.33/100,000 population (95% CI: 0.14 to 0.52; Fig 4) and mapped on world map (Fig 5). When stratified by continents (Table 4), Asia had the highest mortality at 0.96/100,000 population (95% CI: 0.22 to 1.7), followed by Africa at 0.44/100,000 population (95% CI: -0.03 to 0.84), North America at 0.03/100,000 population (95% CI: -0.02 to 0.08) and South America at 0.03/100,000 (95% CI: 0.01 to 0.05) had a similar mortality rate, whereas Europe and Oceania had the similar at 0.01/100,000 population (95% CI: -0.01 to 0.02) and 0.01/100,000 population (95% CI: -0.00 to 0.02), respectively. Among different income categories (Table 4), the mortality was highest among inhabitants of low-income countries at 0.85/100,000 population (95% CI: -0.60 to 2.31), followed by lower-middle income countries at 0.74/100,000 population (95% CI: 0.25 to 1.23), middle-income countries at 0.02/100,000 population (95% CI: 0.01 to 0.04), upper-middle income countries at 0.01/100,000 population (95% CI: -0.01 to 0.04), and the lowest rate was observed in high-income countries at 0.00/100,000 population (95% CI: -0.00 to 0.01). The pooled mortality rate was lowest for studies scored as poor with 0.03/100,000 population (95% CI: 0.01 to 0.05), followed by studies scored as fair 0.44/100,000 population (95% CI: 0.17 to 0.70), and was 1.21/100,000 population (95%CI: —0.49 to 2.92) for studies scored as good in the quality assessment (Table 4). The heterogeneity of the mortality studies was high (I2 >75%), and did not reduce by geographical location, economical classification, study quality, study setting and study design stratification, with mostly the absence of publication bias considering both Beggs test and Egger’s regression test (p<0.05).
Fig 4. Pooled mortality of snakebite, REML-Restricted Maximum Likelihood.
Fig 5. World map with mortality of snakebites per 100,000 population per year across the globe.
Study number pre county have been provided. For countries with multiple studies, average mortality per 100,000 population per year have been provided and noted. (The direct link to the base layer of the map: https://commons.wikimedia.org/wiki/File:BlankMap-World.svg).
Table 4. Sub-group analysis for the overall pooled mortality of the included studies.
| Variable | No of studies | Mortality/100,000 population/ year (95% CI*), p-value | I2 | Egger test (p-value) | Beggs test (p-value) |
|---|---|---|---|---|---|
| Continent | |||||
| Africa | 10 | 0.44 (-0.03 to 0.84), <0.01 | 99.93 | 0.027 | 0.2105 |
| Asia | 19 | 0.96 (0.22 to 1.70), <0.01 | 100.0 | <0.001 | 0.0501 |
| Europe | 2 | 0.01 (-0.01 to 0.02), 0.91 | 0.00 | ||
| North America | 3 | 0.03 (-0.02 to 0.08) | 84.44 | 0.017 | 0.2963 |
| Oceania | 2 | 0.01 (-0.00 to 0.02), 0.87 | 0.01 | 1.000 | |
| South America | 10 | 0.03 (0.01 to 0.05), <0.01 | 89.58 | 0.149 | 0.8580 |
| Economy | |||||
| Low-income countries | 2 | 0.85 (-0.60 to 2.31), <0.01 | 99.48 | 1.000 | 0.0059 |
| Lower-middle income countries | 25 | 0.74 (0.25 to 1.23), <0.01 | 99.99 | <0.001 | 0.0500 |
| Middle-income countries | 9 | 0.02 (0.01 to 0.04), <0.01 | 90.87 | 0.179 | 0.6022 |
| Upper-middle countries | 4 | 0.01 (-0.01 to 0.04), <0.01 | 50.45 | 0.015 | 0.2207 |
| High-income countries | 6 | 0.00 (-0.00 to 0.01), <0.01 | 0.08 | 0.484 | 0.4524 |
| Study Quality | |||||
| Good | 7 | 1.21 (-0.49 to 2.92), <0.01 | 99.99 | <0.001 | 0.0715 |
| Fair | 17 | 0.44 (0.17 to 0.70), <0.01 | 99.97 | 0.004 | 0.5366 |
| Poor | 22 | 0.03 (0.01 to 0.05), <0.01 | 94.26 | <0.001 | 0.0068 |
| Study setting | |||||
| Registry/database | 9 | 2.61 (-0.01 to 5.32), <0.01 | 100.0 | 0.0007 | 0.7545 |
| Community based | 20 | 0.23 (0.02 to 0.44), <0.01 | 99.97 | 0.0001 | 0.0125 |
| Hospital based | 17 | 0.06 (0.02 to 0.09), <0.01 | 95.45 | 0.0001 | 0.1494 |
| Study design | |||||
| Observational | 12 | 0.28 (0.07 to 0.50), <0.01 | 99.92 | 0.0001 | 0.2437 |
| Cross sectional | 7 | 3.38 (0.16 to 6.60), <0.01 | 98.31 | 0.0029 | 1.0000 |
| Cohort | 27 | 0.15 (0.02 to 0.29), <0.01 | 99.94 | 0.0001 | 0.0059 |
Sensitivity analysis
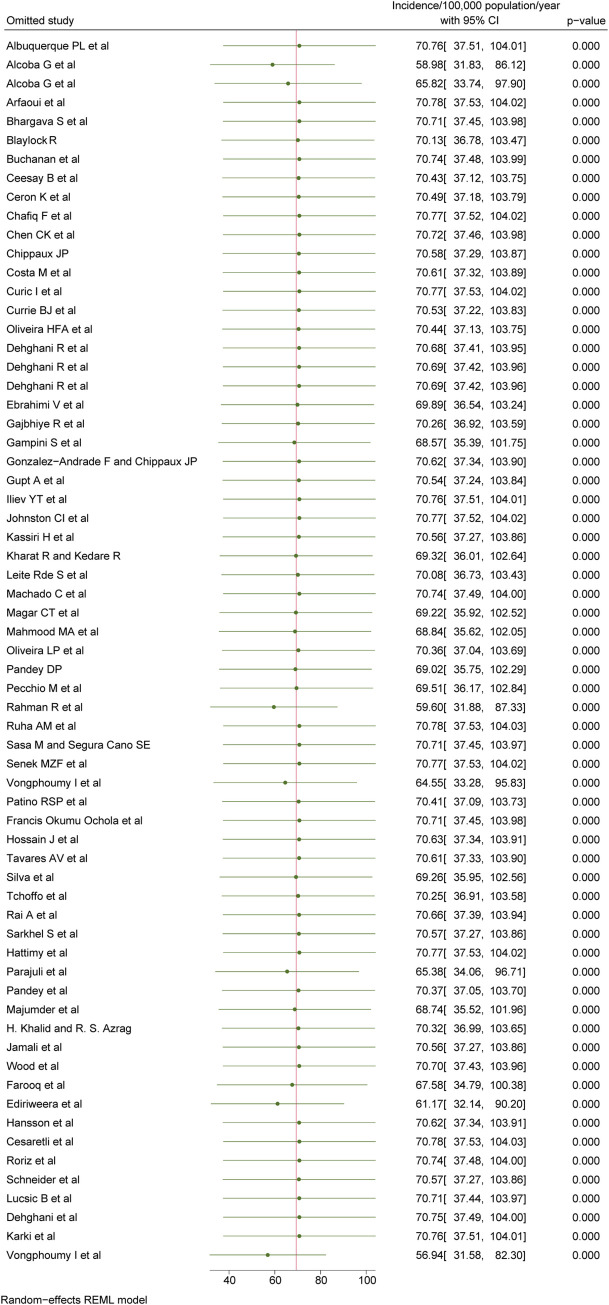
Sensitivity analysis indicated that the pooled incidence estimation was relatively robust to the exclusion of any one study from the overall meta-analysis and did not change by more than 10% (Fig 6) except when leaving out the following four individual studies: Alcoba G et al [pooled incidence: 58.9 (95% CI: 31.8 to 86.1)] [20], Rahman R et al [pooled incidence: 59.6 (95% CI: 31.9 to 87.3)] [69], Ediriweera et al [pooled incidence: 61.2 (95% CI: 32.3 to 103.9)] [39] and Vongphoumy et al [pooled incidence: 56.9 (95% CI: 31.6 to 82.3)] [79]. The overall heterogeneity was unaffected (I2 = 100.0%).
Fig 6. Sensitivity analysis for the studies reporting incidence.
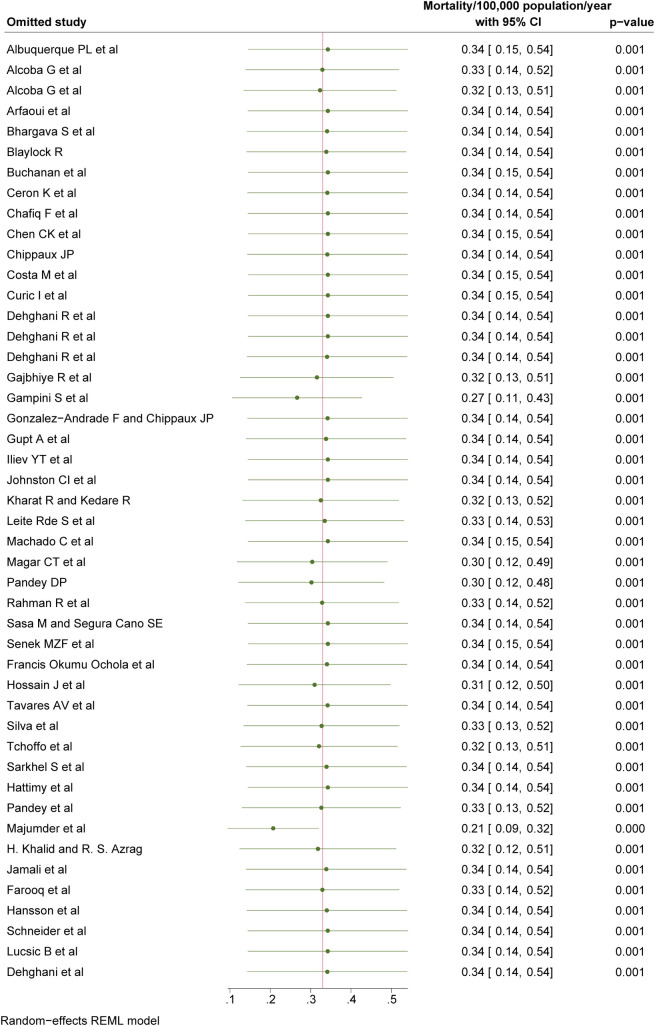
Sensitivity analysis for the mortality status showed that the pooled mortality did not significantly change after excluding studies one by one, and the change was within 10% with the exception of two studies: Gampini et al. [pooled mortality: 0.27 (95% CI: 0.11 to 0.43)] [43], and Majumder et al. [pooled mortality: 0.21 (95% CI: 0.09 to 0.32)] [61] (Fig 7). However, the overall heterogeneity was unaffected with the removal of these two studies (I2 = 100.0%). This indicates insensitivity of the overall pooled-Incidence rate.
Fig 7. Sensitivity analysis for the studies reporting mortality.
Discussion
Snakebites occur when humans and snakes come into contact as a consequence of their intersecting behavioural ecologies. As both human and snake ecologies differ seasonally and geographically, the number of interactions and their outcomes vary widely over the course of the year and across the planet. This review focused on the morbidity and mortality of snakebites based on available published data globally. We estimate the global incidence of snakebite as 69.4/100,000 population (95% CI: 36.8 to 101.9), and mortality as 0.33/100,000 population (95% CI: 0.14 to 0.52). The highest estimated morbidity rates of snakebite were observed in Asia and Africa, with the lowest incidence rates observed in Europe. Similarly, Asia had the highest mortality rate at 0.96/100,000 population (95% CI: 0.22 to 1.7), followed by Africa at 0.44/100,000 population (95% CI: -0.03 to 0.84). These findings were in line with those of Kasturiratne et al., who estimated the highest number of deaths due to snakebite to occur in South Asia, followed by sub-Saharan Africa, while the lowest numbers of deaths were estimated for Australasia, Southern Latin America, and Western Europe [6].
A study using regional data estimates, which were derived from country-specific data within a defined region, published global estimates of the incidence of venomous snakebite indicating that between the years 1990 and 2019, there were approximately 1,200,000 to 5,500,000 snakebite envenoming incidents, resulting in 63,400 deaths, worldwide. Our study examined the period from 2000 to 2022, focusing on all snakebite (venomous and non-venomous). During this period, the estimated number of snakebite incidents was 18,390,000 and mortality was 1,390,000. In this review, among the 49,920 cases from 34 studies, it was found that 57.3% of individuals were envenomed. Previous studies have suggested that snakebites resulting in envenoming ranged from 12% to 87% of the total number of snakebites [6].
Certain human activities and geographical locations significantly increase the risk of encountering snakes. Individuals residing in tropical regions and engaged in rural lifestyles and agricultural professions are at a higher risk of snakebite. In the 19th century, the bite incidence was very high among farmers [81], but agricultural mechanisation has undoubtedly reduced the risk of snakebites significantly. Especially in Europe, where agricultural activities are no longer a common association of snakebite [82]. This is reinforced by the results of this review, in which we found that 22.1% of cases occurred at home, while 17.1% occurred in agriculture fields. This has somewhat altered the circumstances surrounding snakebites, especially among populations susceptible to bites, particularly children. Seasonal patterns reveal a broader distribution of snakebite incidents during spring and summer [83]. This systematic review also found that 39.5% of the total number of snakebites occurred during summer, followed by 21.2% during the monsoon season and 16.7% during spring. It should be noted that while monsoon seasons occur in tropical regions, they do not occur in more temperate regions where snakebites are also endemic. Thus, the seasonality of snakebite should be considered on a regional basis.
According to research conducted by Ralph et al. in 2019, the mortality rate following a venomous snakebite increases if antivenom is not administered within six hours [84]. This review identified Asia as the region with the highest recorded mortality rates, followed by Africa. Many countries in South Asia are classified as lower-middle income countries and a combination of ecological factors, socioeconomic vulnerability, and limited capacity within their healthcare systems is likely to contribute to the burden of snakebite envenoming within these regions. For example, individuals may turn to traditional healers or visit clinics with inadequate knowledge on how to treat snakebite envenoming or which lack the necessary antivenom for life-saving treatment [84,85,86]. Countries in Sub-Saharan Africa have been reported to face similar challenges. The production of antivenom may also be insufficient for the incidence of snakebite in a region or may be disproportionately available in private clinics rendering them unaffordable to those most at risk of being envenomed. The presence of political conflict and humanitarian crises further exacerbate the situation [87,88]. In contrast, South America, and Europe exhibit lower mortality rates from snakebites. In Europe, where high-quality healthcare services are readily accessible and well-distributed, individuals are more inclined to seek prompt medical attention. Snake bite mortality in South America may be lower due to the presence of improved snakebite management systems, which include the development of locally effective antivenoms [89,90]. This proactive approach contributes to better outcomes in managing snakebite incidents. In this review, the pooled incidence and mortality for the hospital-based studies showed the lowest compared to community-based studies and studies with a data source from a database or registry support the community health seeking behaviour where people do not seek conventional medical care and are therefore missed in hospital record.
In terms of public health, gaining a comprehensive understanding of the disease burden is essential for effectively addressing its consequences. Previous evidence has highlighted the significant impact of snakebite envenoming, with an estimated 63,400 deaths (95% UI 38,900–78,600) and 2.9 million years of life lost (YLLs; 1.8 million–3.7 million) in 2019. These statistics establish snakebite envenoming as one of the deadliest neglected tropical diseases (NTDs) based on the Global Burden of Disease study in 2019 [91].
The consequences of snakebite envenoming include the need for antivenom, hospitalization, intensive care unit care, surgery, long-term sequelae (i.e., disability/disfigurement), and death. To estimate the total number of snakebites, we relied on data regarding the prevalence of snakebite from studies conducted in various regions worldwide. However, the estimated number of bites exhibited considerable variation amongst studies, likely due to methodological differences. This heterogeneity implies that our estimation of the total number of snakebites is only a rough approximation. The actual magnitude of the snakebite burden may not be accurately represented in recorded data, as a significant proportion of individuals with asymptomatic, mild, or even life-threatening bites may not seek medical treatment at hospitals and health clinics that collate data pertaining to these injuries. Furthermore, it is impossible to determine, particularly in community surveys, whether "all bites" encompasses bites from non-venomous snakes and/or dry bites from venomous snakes. While these non-envenoming bites may not contribute significantly to the overall disease burden, the opportunity cost of the bite can still have adverse effects on the victims and their households. Furthermore, data regarding the incidence of non-envenoming snakebite are potentially useful as an index of the prevalence of antagonistic encounters between humans and snakes (of which bites resulting in envenoming are a subset). These data are undoubtedly underreported, but we encourage researchers to collect them, including as much information as possible regarding the circumstances of snakebites, whether or not envenoming results. A deeper understanding of the ecology of snakebite may be one pathway towards reducing its prevalence.
The strength of this review is the fact that our analyses encompassed a comprehensive selection of 65 studies, shedding light on the global incidence and mortality of snakebites across all regions. In the study by Kasturiratne et al on the global burden of snakebite, several assumptions were used to ensure the representativeness of the data. In instances where no data were accessible for a specific country to calculate the incidence, the lowest incidence rate within a neighbouring country was used. Additionally, a country was considered as free of snakebites if there was no literature indicating occurrences since 1985. Furthermore, a country was considered to have no mortality due to snakebites, even if reports of snakebites existed, if no mortality statistics had been reported to the WHO mortality database from 1990 to the present date [6]. However, in this review, absolute annual number of snakebite envenoming cases and country population or the catchment area population for the reporting year reported by a given study was used to calculate the incidence rate per 100,000 population, online sources were used when country population or the catchment area population was not available in the study. However, this study retains certain limitations that need to be acknowledged. Firstly, there was a high level of heterogeneity observed in the evaluated outcomes. Incidence data vary widely across countries and among studies, with differences in study methodology contributing most notably to this variability along with geographical location, and the circumstances surrounding the snakebite incidents. It is important to note that most of the articles examined were not standardised epidemiological studies. However, this heterogeneity may serve as an indicator of variations among the studied populations, study design and settings, helping to identify the underlying causes for these differences. Secondly, the results of the sensitivity analysis highlighted the impact of including or excluding the study conducted by Alcoba G et al, Rahman R et al, Ediriweera et al and Vongphoumy et al. The inclusion of these studies led to an overall pooled incidence rate rage of 69.4/100,000 population, while their exclusion resulted in a lower pooled incidence rate in a range between 56.9/100,000 population and 61.2/100,000 population. Therefore, caution is needed when interpreting and generalising the findings. Thirdly, it is important to note that this study only included articles published in the English language. Consequently, there is a possibility that relevant studies published in other languages may have been excluded from analysis. Overall, these strengths and limitations should be considered when interpreting the findings of this review.
Conclusion
The actual magnitude of snakebite burden may not be accurately represented in recorded data due to methodological differences among included studies. However, incidence and mortality rates mentioned in this report serve as a stark reminder of the worldwide significance of snakebites and emphasise the urgent necessity to address the burden they impose. These findings also shed light on a crucial disparity: although reported snakebite incidence was higher in upper-middle-income countries the highest mortality rates occurred among residents of low-income countries. This striking contrast further emphasizes the imperative for intensified investigation of interventions aimed at combatting this neglected tropical disease.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Acknowledgments
We acknowledge the intellectual support received from the Librarian of the University of Melbourne.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Health and Medical Research Council (nhmrc.gov.au: Grant ID 13/093/002 AVRU to AA, TNWJ and AW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gutiérrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, Warrell DA. Snakebite envenoming. Nature reviews Disease primers. 2017;3(1):1–21. [DOI] [PubMed] [Google Scholar]
- 2.Organization WH. Snakebite envenoming: a strategy for prevention and control. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. Snakebite mortality in India: a nationally representative mortality survey. PLoS neglected tropical diseases. 2011;5(4):e1018. doi: 10.1371/journal.pntd.0001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharat R, Kedare R. Epidemiological Profile of Snake Bites over 1-Year Period from Tertiary Care Centre in Maval Region of Maharashtra, India. Indian Journal of Forensic Medicine & Toxicology. 2020;14(1):44–9. [Google Scholar]
- 5.Ruha A-M, Kleinschmidt KC, Greene S, Spyres MB, Brent J, Wax P, et al. The epidemiology, clinical course, and management of snakebites in the North American Snakebite Registry. Journal of Medical Toxicology. 2017;13:309–20. doi: 10.1007/s13181-017-0633-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS medicine. 2008;5(11):e218. doi: 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W-65-W-94. [DOI] [PubMed] [Google Scholar]
- 8.Ma L-L, Wang Y-Y, Yang Z-H, Huang D, Weng H, Zeng X-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Medical Research. 2020;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuya-Kanamori L, Xu C, Hasan SS, Doi SA. Quality versus risk-of-bias assessment in clinical research. Journal of clinical epidemiology. 2021;129:172–5. doi: 10.1016/j.jclinepi.2020.09.044 [DOI] [PubMed] [Google Scholar]
- 10.Wells GAS B.; O’Connell D.; Peterson J.; Welch V.; Losos M.; Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinicalepidemiology/oxford.asp (accessed on 16 February 2023). [ [Google Scholar]
- 11.Mantel N. Statistical aspects of the analysis of data from retrospective studies of diseases. J Natl Cancer Inst. 1959;105:125–9. [PubMed] [Google Scholar]
- 12.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Archives of Public Health. 2014;72:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis John Wiley & Sons. Ltd, Chichester, UK. 2009. [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 15.Serajuddin U, Hamadeh N. New World Bank country classifications by income level: 2020–2021. World Bank Blogs. 2020;1. [Google Scholar]
- 16.Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta-analysis. Research synthesis methods. 2010;1(2):112–25. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Chu H, Murad MH, Hong C, Qu Z, Cole SR, et al. Empirical comparison of publication bias tests in meta-analysis. Journal of general internal medicine. 2018;33:1260–7. doi: 10.1007/s11606-018-4425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. Journal of clinical epidemiology. 2001;54(10):1046–55. doi: 10.1016/s0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 19.Albuquerque PL, Silva Junior GB, Jacinto CN, Lima CB, Lima JB, Veras MdSB, et al. Epidemiological profile of snakebite accidents in a metropolitan area of northeast Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 2013;55:347–51. doi: 10.1590/S0036-46652013000500009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcoba G, Chabloz M, Eyong J, Wanda F, Ochoa C, Comte E, et al. Snakebite epidemiology and health-seeking behavior in Akonolinga health district, Cameroon: cross-sectional study. PLoS neglected tropical diseases. 2020;14(6):e0008334. doi: 10.1371/journal.pntd.0008334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcoba G, Sharma SK, Bolon I, Ochoa C, Martins SB, Subedi M, et al. Snakebite epidemiology in humans and domestic animals across the Terai region in Nepal: a multicluster random survey. The Lancet Global Health. 2022;10(3):e398–e408. doi: 10.1016/S2214-109X(22)00028-6 [DOI] [PubMed] [Google Scholar]
- 22.Arfaoui A, Hmimou R, Ouammi L, Soulaymani A, Mokhtari A, Chafiq F, et al. Epidemiological profile of snakebites in Morocco. Journal of Venomous Animals and Toxins including Tropical Diseases. 2009;15:653–66. [Google Scholar]
- 23.Bhargava S, Kaur R, Singh R. Epidemiological profile of snake-bite cases from Haryana: A five year (2011–2015) retrospective study. Journal of forensic and legal medicine. 2018;54:9–13. doi: 10.1016/j.jflm.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 24.Blaylock R. Epidemiology of snakebite in Eshowe, KwaZulu-Natal, South Africa. Toxicon. 2004;43(2):159–66. [DOI] [PubMed] [Google Scholar]
- 25.Buchanan J, Thurman J, Hargis C, Kirkpatrick L, Huecker M. Snakebites reported to the Kentucky Regional Poison Control Centers for the years 2012–2016. Wilderness & Environmental Medicine. 2021;32(2):143–8. [DOI] [PubMed] [Google Scholar]
- 26.Ceesay B, Taal A, Kalisa M, Odikro MA, Agbope D, Kenu E. Analysis of snakebite data in Volta and Oti Regions, Ghana, 2019. Pan African Medical Journal. 2021;40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceron K, Vieira C, Carvalho PS, Carrillo JFC, Alonso J, Santana DJ. Epidemiology of snake envenomation from Mato Grosso do Sul, Brazil. PLoS Neglected Tropical Diseases. 2021;15(9):e0009737. doi: 10.1371/journal.pntd.0009737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cesaretli Y, Ozkan O. Snakebites in Turkey: epidemiological and clinical aspects between the years 1995 and 2004. Journal of Venomous Animals and Toxins Including Tropical Diseases. 2010;16:579–86. [DOI] [PubMed] [Google Scholar]
- 29.Chafiq F, Hattimy FE, Rhalem N, Chippaux J-P, Soulaymani A, Mokhtari A, et al. Snakebites notified to the poison control center of Morocco between 2009 and 2013. Journal of venomous animals and toxins including tropical diseases. 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C-K, Lin C-C, Shih F-Y, Chaou C-H, Lin JC-C, Lai T-I, et al. Population-based study of venomous snakebite in Taiwan. Journal of Acute Medicine. 2015;5(2):38–42. [Google Scholar]
- 31.Chippaux J-P. Epidemiology of envenomations by terrestrial venomous animals in Brazil based on case reporting: from obvious facts to contingencies. Journal of venomous animals and toxins including tropical diseases. 2015;21:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa MKBd Fonseca CSd, Navoni JA Freire EMX. Snakebite accidents in Rio Grande do Norte state, Brazil: Epidemiology, health management and influence of the environmental scenario. Tropical Medicine & International Health. 2019;24(4):432–41. [DOI] [PubMed] [Google Scholar]
- 33.Curić I, Curić S, Bradarić I, Bubalo P, Bebek-Ivanković H, Nikolić J, et al. Snakebites in Mostar region, Bosnia and Herzegovina. Collegium antropologicum. 2009;33(2):93–8. [PubMed] [Google Scholar]
- 34.Currie BJ. Snakebite in tropical Australia: a prospective study in the “Top End” of the Northern Territory. Medical Journal of Australia. 2004;181(11–12):693–7. doi: 10.5694/j.1326-5377.2004.tb06526.x [DOI] [PubMed] [Google Scholar]
- 35.Dehghani R, Rabani D, Shahi MP, Jazayeri M, Bidgoli MS. Incidence of snake bites in Kashan, Iran during an eight year period (2004–2011). Archives of trauma research. 2012;1(2):67. doi: 10.5812/atr.6445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dehghani R, Dadpour B, Mehrpour O. Epidemiological profile of snakebite in Iran, 2009–2010 based on information of Ministry of Health and Medical Education. International journal of medical toxicology and forensic medicine. 2014;4(2):33–41. [Google Scholar]
- 37.Dehghani R, Fathi B, Shahi MP, Jazayeri M. Ten years of snakebites in Iran. Toxicon. 2014;90:291–8. doi: 10.1016/j.toxicon.2014.08.063 [DOI] [PubMed] [Google Scholar]
- 38.Ebrahimi V, Hamdami E, Khademian M, Moemenbellah-Fard M, Vazirianzadeh B. Epidemiologic prediction of snake bites in tropical south Iran: Using seasonal time series methods. Clinical Epidemiology and Global Health. 2018;6(4):208–15. [Google Scholar]
- 39.Ediriweera DS, Kasthuriratne A, Pathmeswaran A, Gunawardene NK, Jayamanne SF, Murray K, et al. Evaluating spatiotemporal dynamics of snakebite in Sri Lanka: Monthly incidence mapping from a national representative survey sample. PLoS neglected tropical diseases. 2021;15(6):e0009447. doi: 10.1371/journal.pntd.0009447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farooq H, Bero C, Guilengue Y, Elias C, Massingue Y, Mucopote I, et al. Snakebite incidence in rural sub-Saharan Africa might be severely underestimated. Toxicon. 2022;219:106932. doi: 10.1016/j.toxicon.2022.106932 [DOI] [PubMed] [Google Scholar]
- 41.Ochola FO, Okumu MO, Muchemi GM, Mbaria JM, Gikunju JK. Epidemiology of snake bites in selected areas of Kenya. Pan African Medical Journal. 2018;29(1):1–14. doi: 10.11604/pamj.2018.29.217.15366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajbhiye R, Khan S, Kokate P, Mashal I, Kharat S, Bodade S, et al. Incidence & management practices of snakebite: A retrospective study at Sub-District Hospital, Dahanu, Maharashtra, India. The Indian Journal of Medical Research. 2019;150(4):412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gampini S, Nassouri S, Chippaux J-P, Semde R. Retrospective study on the incidence of envenomation and accessibility to antivenom in Burkina Faso. Journal of Venomous Animals and Toxins including Tropical Diseases. 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González-Andrade F, Chippaux J-P. Snake bite envenomation in Ecuador. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010;104(9):588–91. doi: 10.1016/j.trstmh.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 45.Gupt A, Bhatnagar T, Murthy B. Epidemiological profile and management of snakebite cases–A cross sectional study from Himachal Pradesh, India. Clinical Epidemiology and Global Health. 2015;3:S96–S100. [Google Scholar]
- 46.Khalid H, Azrag RS. Retrospective hospital-based study on snakebite envenomation in Sudan. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2021;115(9):992–7. doi: 10.1093/trstmh/trab085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson E, Cuadra S, Oudin A, de Jong K, Stroh E, Torén K, et al. Mapping snakebite epidemiology in Nicaragua–pitfalls and possible solutions. PLoS neglected tropical diseases. 2010;4(11):e896. doi: 10.1371/journal.pntd.0000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chafiq F, Hami H, Mokhtari A, Soulaymani A, Rachida SB. Geographical distribution of health indicators related to snake bites and envenomation in Morocco between 1999 and 2013. Epidemiology and Health. 2018;40. doi: 10.4178/epih.e2018024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hossain J, Biswas A, Rahman F, Mashreky SR, Dalal K, Rahman A. Snakebite Epidemiology in Bangladesh: A national community based health and injury survey. Health. 2016;8:479–86. [Google Scholar]
- 50.Iliev YT, Tufkova SG, Zagorov MY, Nikolova SM. Snake venom poisoning in the Plovdiv region from 2004 to 2012. Folia medica. 2014;56(1). doi: 10.2478/folmed-2014-0005 [DOI] [PubMed] [Google Scholar]
- 51.Jamali A, Yousif M, Kareem S, Ali S, Salman B, Imtiaz S. Epidemiology and complications of snakebite in rural area of Badin and Thar Sind, Pakistan. JPMA The Journal of the Pakistan Medical Association. 2022;72(8):1591–7. doi: 10.47391/JPMA.4547 [DOI] [PubMed] [Google Scholar]
- 52.Johnston CI, Ryan NM, Page CB, Buckley NA, Brown SG, O’Leary MA, et al. The Australian snakebite project, 2005–2015 (ASP-20). Medical journal of Australia. 2017;207(3):119–25. doi: 10.5694/mja17.00094 [DOI] [PubMed] [Google Scholar]
- 53.Karki D, Sharma B, Koirala R, Nagila A. Epidemiology and Clinical Outcome of Snakebite in Western Nepal: A Retrospective Study. Journal of Gandaki Medical College-Nepal. 2019;12(1):53–7. [Google Scholar]
- 54.Kassiri H, Khodkar I, Kazemi S, Kasiri N, Lotfi M. Epidemiological analysis of snakebite victims in southwestern Iran. Journal of Acute Disease. 2019;8(6):260–4. [Google Scholar]
- 55.Kharat R, Kedare R. Epidemiological Profile of Snake Bites over 1-Year Period from Tertiary Care Centre in Maval Region of Maharashtra, India. Indian Journal of Forensic Medicine & Toxicology. 2020;14(1). [Google Scholar]
- 56.Leite RdS, Targino ITG, Lopes YACF, Barros RM, Vieira AA. Epidemiology of snakebite accidents in the municipalities of the state of Paraíba, Brazil. Ciência & Saúde Coletiva. 2013;18:1463–71. [DOI] [PubMed] [Google Scholar]
- 57.Lukšić B, Bradarić N, Prgomet S. Venomous snakebites in southern Croatia. Collegium antropologicum. 2006;30(1):191–7. [PubMed] [Google Scholar]
- 58.Machado C, Bochner R, Fiszon JT. Epidemiological profile of snakebites in Rio de Janeiro, Brazil, 2001–2006. Journal of Venomous Animals and Toxins including Tropical Diseases. 2012;18:217–24. [Google Scholar]
- 59.Magar CT, Devkota K, Gupta R, Shrestha RK, Sharma SK, Pandey DP. A hospital based epidemiological study of snakebite in Western Development Region, Nepal. Toxicon. 2013;69:98–102. doi: 10.1016/j.toxicon.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 60.Mahmood MA, Halliday D, Cumming R, Thwin K-T, Kyaw MMZ, White J, et al. Snakebite incidence in two townships in Mandalay Division, Myanmar. PLoS neglected tropical diseases. 2018;12(7):e0006643. doi: 10.1371/journal.pntd.0006643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Majumder D, Sinha A, Bhattacharya SK, Ram R, Dasgupta U, Ram A. Epidemiological profile of snake bite in south 24 Parganas district of West Bengal with focus on underreporting of snake bite deaths. Indian journal of public health. 2014;58(1):17–21. doi: 10.4103/0019-557X.128158 [DOI] [PubMed] [Google Scholar]
- 62.Oliveira HFAD Barros RM, Pasquino JA Peixoto LR, Sousa JA, Leite RdS. Snakebite cases in the municipalities of the State of Paraíba, Brazil. Revista da Sociedade Brasileira de Medicina Tropical. 2013;46:617–24. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira LPd Moreira JGdV, Sachett JdAG Monteiro WM, Meneguetti DUdO Bernarde PS. Snakebites in rio branco and surrounding region, Acre, Western Brazilian Amazon. Revista da Sociedade Brasileira de Medicina Tropical. 2020;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pandey DP. Epidemiology of snakebites based on field survey in Chitwan and Nawalparasi districts, Nepal. Journal of Medical toxicology. 2007;3:164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandey DP, Shrestha BR, Acharya KP, Shah KJ, Thapa-Magar C, Dhakal IP, et al. A prospective study of snakebite in a tertiary care hospital in south-western Nepal. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2023;117(6):435–43. doi: 10.1093/trstmh/trac127 [DOI] [PubMed] [Google Scholar]
- 66.Parajuli SB, Heera K, Luitel A, Shrestha M, Upadhyaya B. Snakebite Incidence, General Awareness and Belief of Snakebite Management at a Rural Municipality of Eastern Nepal. Journal of Nobel Medical College. 2022;11(2):67–73. [Google Scholar]
- 67.Patiño RS, Salazar-Valenzuela D, Robles-Loaiza AA, Santacruz-Ortega P, Almeida JR. A retrospective study of clinical and epidemiological characteristics of snakebite in Napo Province, Ecuadorian Amazon. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2023;117(2):118–27. doi: 10.1093/trstmh/trac071 [DOI] [PubMed] [Google Scholar]
- 68.Pecchio M, Suárez JA, Hesse S, Hersh AM, Gundacker ND. Descriptive epidemiology of snakebites in the Veraguas province of Panama, 2007–2008. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2018;112(10):463–6. doi: 10.1093/trstmh/try076 [DOI] [PubMed] [Google Scholar]
- 69.Rahman R, Faiz MA, Selim S, Rahman B, Basher A, Jones A, et al. Annual incidence of snake bite in rural Bangladesh. PLoS neglected tropical diseases. 2010;4(10):e860. doi: 10.1371/journal.pntd.0000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rai A, Chettri M, Dewan S, Khandelwal B, Chettri B. Epidemiological study of snakebite cases in Sikkim: Risk modeling with regard to the habitat suitability of common venomous snakes. PLoS neglected tropical diseases. 2021;15(11):e0009800. doi: 10.1371/journal.pntd.0009800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roriz KRPS Zaqueo KD, Setubal SS Katsuragawa TH, Silva RRd Fernandes CFC, et al. Epidemiological study of snakebite cases in Brazilian Western Amazonia. Revista da Sociedade Brasileira de Medicina Tropical. 2018;51:338–46. doi: 10.1590/0037-8682-0489-2017 [DOI] [PubMed] [Google Scholar]
- 72.Sarkhel S, Ghosh R, Mana K, Gantait K. A hospital based epidemiological study of snakebite in Paschim Medinipur district, West Bengal, India. Toxicology Reports. 2017;4:415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasa M, Cano SES. New insights into snakebite epidemiology in Costa Rica: A retrospective evaluation of medical records. Toxicon: X. 2020;7:100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider MC, Vuckovic M, Montebello L, Sarpy C, Huang Q, Galan DI, et al. Snakebites in rural areas of Brazil by race: indigenous the most exposed group. International journal of environmental research and public health. 2021;18(17):9365. doi: 10.3390/ijerph18179365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Senek MZF, Kong SY, Shin SD, Sun KM, Kim J, Ro YS. Epidemiological profile and outcomes of snakebite injuries treated in emergency departments in South Korea, 2011–2016: a descriptive study. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2019;113(10):590–8. doi: 10.1093/trstmh/trz050 [DOI] [PubMed] [Google Scholar]
- 76.Silva AMd, Colombini M, Moura-da-Silva AM, Souza RMd, Monteiro WM, Bernarde PS. Epidemiological and clinical aspects of snakebites in the upper Juruá River region, western Brazilian Amazonia. Acta Amazonica. 2019;50:90–9. [Google Scholar]
- 77.Tavares AV, Araújo KAMd, Marques MRdV, Vieira AA, Leite RdS. The epidemiology of snakebite in the Rio Grande do Norte state, Northeastern Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 2017;59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tchoffo D, Kamgno J, Kekeunou S, Yadufashije C, Nana Djeunga HC, Nkwescheu AS. High snakebite underreporting rate in the Centre Region of Cameroon: an observational study. BMC Public Health. 2019;19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vongphoumy I, Phongmany P, Sydala S, Prasith N, Reintjes R, Blessmann J. Snakebites in two rural districts in Lao PDR: community-based surveys disclose high incidence of an invisible public health problem. PLoS neglected tropical diseases. 2015;9(6):e0003887. doi: 10.1371/journal.pntd.0003887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wood D, Sartorius B, Hift R. Snakebite in north-eastern South Africa: clinical characteristics and risks for severity. South African Family Practice. 2016;58(2):62–7. [Google Scholar]
- 81.Viaud-Grand-Marais A. Etudes médicales sur les serpents de la Vendée et de la Loire inferieure: De l’impr. L. Toinon & ce; 1869. [Google Scholar]
- 82.Chippaux J-P. Epidemiology of snakebites in Europe: a systematic review of the literature. Toxicon. 2012;59(1):86–99. doi: 10.1016/j.toxicon.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 83.Stahel E, Wellauer R, Freyvogel T. Vergiftungen durch einheimische Vipern (Vipera berus und Vipera aspis). Eine retrospektive Studie an 113 Patienten. Schweiz Med Wochenschr. 1985;115(890):6. [PubMed] [Google Scholar]
- 84.Ralph R, Sharma SK, Faiz MA, Ribeiro I, Rijal S, Chappuis F, et al. The timing is right to end snakebite deaths in South Asia. bmj. 2019;364. doi: 10.1136/bmj.k5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in South Asia: a review. PLoS neglected tropical diseases. 2010;4(1):e603. doi: 10.1371/journal.pntd.0000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox S, Rathuwithana A, Kasturiratne A, Lalloo D, De Silva H. Underestimation of snakebite mortality by hospital statistics in the Monaragala District of Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(7):693–5. doi: 10.1016/j.trstmh.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 87.Alcoba G, Potet J, Vatrinet R, Singh S, Nanclares C, Kruse A, et al. Snakebite envenoming in humanitarian crises and migration: a scoping review and the Médecins Sans Frontières experience. Toxicon: X. 2022;13:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Habib AG, Musa BM, Iliyasu G, Hamza M, Kuznik A, Chippaux J-P. Challenges and prospects of snake antivenom supply in sub-Saharan Africa. PLoS Neglected Tropical Diseases. 2020;14(8):e0008374. doi: 10.1371/journal.pntd.0008374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gutiérrez JM, Fan HW. Improving the control of snakebite envenomation in Latin America and the Caribbean: a discussion on pending issues. Oxford University Press; 2018. p. 523–6. [DOI] [PubMed] [Google Scholar]
- 90.Gutiérrez JM, Higashi HG, Wen FH, Burnouf T. Strengthening antivenom production in Central and South American public laboratories: report of a workshop. Toxicon. 2007;49(1):30–5. doi: 10.1016/j.toxicon.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 91.Abbafati C, Machado D, Cislaghi B, Salman O, Karanikolos M, McKee M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.