Abstract
Recent findings position the eukaryotic translation initiation factor eIF4E as a novel modulator of mRNA splicing, a process that impacts the form and function of resultant proteins. eIF4E physically interacts with the spliceosome and with some intron‐containing transcripts implying a direct role in some splicing events. Moreover, eIF4E drives the production of key components of the splicing machinery underpinning larger scale impacts on splicing. These drive eIF4E‐dependent reprogramming of the splicing signature. This work completes a series of studies demonstrating eIF4E acts in all the major mRNA maturation steps whereby eIF4E drives production of the RNA processing machinery and escorts some transcripts through various maturation steps. In this way, eIF4E couples the mRNA processing‐export‐translation axis linking nuclear mRNA processing to cytoplasmic translation. eIF4E elevation is linked to worse outcomes in acute myeloid leukemia patients where these activities are dysregulated. Understanding these effects provides new insight into post‐transcriptional control and eIF4E‐driven cancers.
Keywords: cancer, eIF4E, mRNA export, RNA maturation, RNA metabolism, splicing, translation
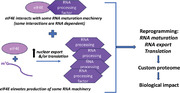
The eukaryotic translation initiation factor eIF4E reprograms splicing by elevating splice factor production and also, via physical interactions with the splicing machinery and substrate RNAs. This becomes dysregulated in high‐eIF4E acute myeloid leukemia. In combination with other studies, eIF4E is positioned to couple the major nuclear mRNA maturation steps with cytoplasmic translation.
INTRODUCTION
Essential to all cells is the capacity to synthesize proteins which constitute the physical basis for much of their molecular architecture and functions. Implicit to the generation of a proteome tailored to the cells’ requirements is the capacity to generate mRNAs that are correctly processed in the nucleus, exported through the nuclear pore complex to the cytoplasm, and subsequently associated with ribosomes to produce functional protein actors. The major steps in mRNA processing include the addition of the methyl‐7‐guanosine (m7G) cap on its 5′end known as capping, the excision of introns during splicing, and the addition of the polyA tail during cleavage and polyadenylation (CPA).[ 1 , 2 , 3 ] These steps are often physically tied to transcription allowing coordination between transcription and processing (Figure 1A).[ 2 , 3 ] However, transcription and RNA processing can be spatially decoupled; for example, splicing and CPA can also occur distal to transcription sites[ 4 , 5 ] (Figure 1A). In these cases, the basis for coordination of processing with transcription are less well understood. Moreover, dysregulation of RNA processing steps and/or a lack of coordination with nuclear mRNA export and/or translation impact the production of the requisite protein forms. Splicing serves as a prime example of this whereby aberrant splicing has varied impacts on mRNAs including the production of proteins with different functionalities, reducing protein levels due to mRNA decay and so forth.[ 6 , 7 , 8 , 9 , 10 , 11 ] About 20% of splicing occurs post‐transcriptionally at nuclear speckles.[ 4 ] In terms of biomedical relevance, splicing signatures are reprogrammed in many diseases including cancer.[ 6 , 11 , 12 ]
FIGURE 1.
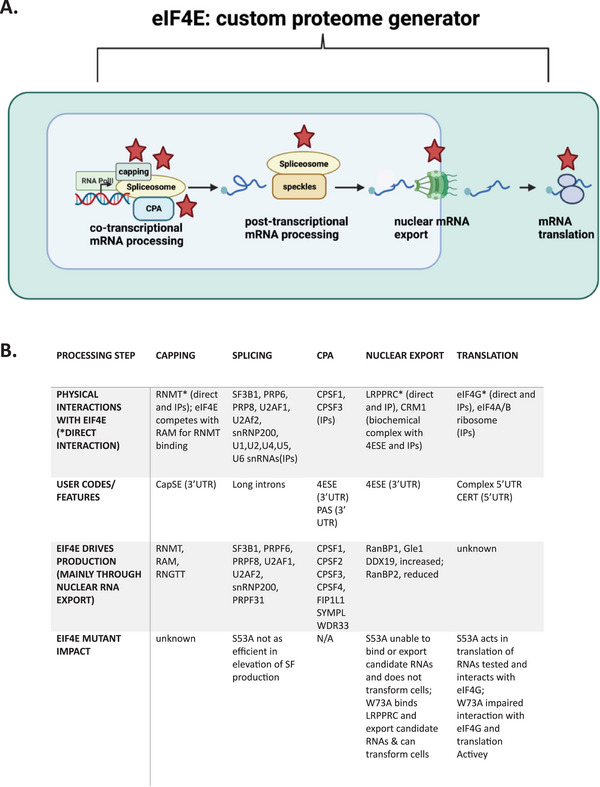
(A) eIF4E influences the major mRNA processing steps, RNA export and translation making it a master regulator of proteome generation. The major steps are shown with a focus on splicing. Note that capping and CPA can also occur post‐transcriptionally but are not shown for simplicity. Red stars indicate steps where eIF4E acts. In all cases, eIF4E interacts with the shown machinery and in some cases eIF4E also influences production of the components of said machines. Note that whether eIF4E influences co‐ or post‐transcriptional processing is unknown; here, we postulate its involved in both. Green barrel indicates the nuclear pore complex, blue is the nucleus and light green the cytoplasm. This figure was generated in Biorender. (B) Summary of factors that bind to and are regulated by eIF4E at each processing step. IPs indicate endogenous immunoprecipitation; direct indicates purified eIF4E and purified factor of interest directly binding; CERT, cytosine enriched regulator of translation identified in [ 29 ]; UTR, untranslated region; USER code, untranslated sequence for regulation. See text for details.
In this review, new findings are discussed whereby the eukaryotic translation initiation factor eIF4E employs a novel and pervasive strategy to reprogram splicing which is dysregulated in acute myeloid leukemia (AML). The finding that eIF4E influences splicing completes a series of studies which demonstrate roles for eIF4E in the major nuclear mRNA processing steps as well as in mRNA export and translation (Figure 1A,B).[ 13 , 14 , 15 , 16 , 17 , 18 ] Indeed, one potential means to coordinate mRNA processing with translation is to have a factor such as eIF4E provide connectivity in the system by coupling these events. In this way, connectivity and coordination could be maintained even when activities occur in spatially disparate locations such as the nucleus and cytoplasm. Additionally, it is postulated that cancer cells through hijacking eIF4E coordinately subvert the major steps in proteome generation (Figure 1A,B). Through this subversion, cancer cells can literally re‐write the proteome. In all, it is proposed that eIF4E can be viewed as a system‐wide coupler of the mRNA processing‐export‐translation axis.[ 13 , 14 , 15 , 17 , 19 ]
THE BIOCHEMICAL FUNCTIONS OF EIF4E AND ITS ROLES IN MRNA METABOLISM
eIF4E is a 25 kDa protein found in eukaryotes ranging from plants to animals.[ 20 , 21 , 22 ] Its primary sequence and three‐dimensional structure are highly conserved.[ 21 , 22 , 23 ] eIF4E is found in the nucleus and cytoplasm in a variety of organisms including yeast, mouse and human[ 24 , 25 , 26 , 27 , 28 ] (Figure 2). This factor is required for cell survival where its knockout in yeast or mice is lethal.[ 29 , 30 ] Its main biochemical function is to bind the methyl‐7‐guanosine (m7G) “cap” on the 5′ end of mRNAs and this activity is required for both its nuclear and cytoplasmic functions.[ 1 , 31 ] In the cytoplasm, eIF4E is found associated with ribosomes where it functions in cap‐dependent translation initiation of specific mRNAs.[ 19 ] It is also present in cytoplasmic structures such as P‐bodies (in the absence of ribosomes) where it plays roles in sequestering some transcripts from the translation machinery and/or protecting these from degradation.[ 1 , 32 , 33 ] In the nucleus, eIF4E is found diffusely throughout the nucleoplasm as well as in structures referred to as eIF4E nuclear bodies where it is absent from the nucleolus.[ 25 , 34 , 35 , 36 , 37 ] A subset of eIF4E nuclear bodies colocalize with promyelocytic leukemia (PML) nuclear bodies where PML potently reduces the affinity of eIF4E for the m7G cap while other eIF4E nuclear bodies colocalize with nuclear mRNA targets of eIF4E in the absence of PML.[ 25 , 34 , 36 , 37 , 38 , 39 ] eIF4E may associate with nuclear speckles which are sites of post‐transcriptional splicing.[ 28 ] In all, eIF4E appears to be in functionally distinct compartments within the nucleus.
FIGURE 2.
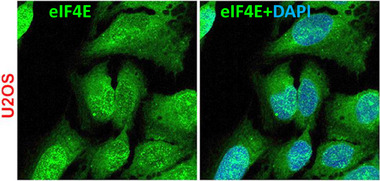
eIF4E is present in both the nucleus and cytoplasm of multiple cell types, U2Os cells are shown here. Immunofluorescence and confocal microscopy were used to monitor the localization of endogenous eIF4E from Ref.[13] Single section through the plane of cells is shown. Reprinted from Figure 1B of Ref.[13]
An important concept when considering post‐transcriptional control by eIF4E or any RNA binding protein (RBP) is delineating mechanisms of RNA specificity. For example, why are some RNAs sensitive to eIF4E at a given level while others are not? The RNA regulon provides a conceptual context to understand selectivity and coordination with regard to nuclear mRNA processing and translation.[ 40 , 41 , 42 ] Here, mRNAs with specific cis‐acting elements denoted USER codes (untranslated sequence elements for regulation) recruit RBPs that in turn recruit processing or translation machinery. Thus, the USER codes imbue selectivity for a given processing step in a specific context. Coordinated regulation of the RBPs involved is another means to co‐regulate these events. For groups of RNAs to be co‐regulated across different processing steps, they would contain the correct combination of USER codes.
In mRNA translation, eIF4E‐capped mRNA complexes directly bind the eIF4G platform protein.[ 19 , 43 ] This complex is recruited to the ribosome through interactions of eIF4G with eIF3 which in turn interacts with the ribosome. eIF4E overexpression increased translation efficiency (more ribosomes per transcript) for selected mRNAs which in turn causes inappropriate elevation of oncogenic proteins in the absence of increased transcription. eIF4E does not stimulate the translation efficiency of all capped mRNAs. Specificity arises from the presence of highly structured elements within the 5′ untranslated regions (UTR) in target mRNAs which act as USER codes for translation.[ 19 ] Some more defined 5′ UTR elements have been suggested such as the cytosine enriched regulator of translation (CERT).[ 29 ] Examples of specificity include observations that eIF4E stimulates ODC translation efficiency but not that of housekeeping transcripts like GAPDH.[ 24 , 25 , 26 ] It is noteworthy that cap‐dependent translation can occur independently of eIF4E utilizing other cap‐binding factors such as eIF3d, eIF4E2, the cap‐binding complex (CBC), and PARN.[ 19 ] Both cellular context and USER codes within the transcripts themselves underlie which cap‐binding protein is employed.
In the nucleus, eIF4E's best‐described role is the promotion of the nuclear export of a subset of mRNAs which increases their availability to the ribosomes and thus amplifies protein production without altering transcription or a priori translation efficiency.[ 24 , 25 , 44 , 45 ] eIF4E overexpression increased export of CCND1 and ODC but not GAPDH transcripts.[ 34 , 45 , 46 ] It was since discovered that eIF4E physically associated with the nuclear export machinery as well as ∼3000 nuclear mRNAs including transcripts that act in pathways driving malignant transformation and RNA metabolism.[ 18 , 47 ] Targets mRNAs contain a ∼50‐nucleotide element in their 3′UTR denoted the eIF4E sensitivity element (4ESE) which is comprised of a stem‐paired loop originally identified in CCDN1 and PIM1 (Figure 1B).[ 17 , 34 , 35 , 46 ] This USER code for mRNA export is necessary and sufficient for eIF4E‐dependent mRNA export. Specifically, eIF4E associates with the m7G cap of RNAs containing a 4ESE USER code which directly binds LRPPRC, which in turn binds directly to both eIF4E and CRM1/XPO1.[ 17 ] In this way, eIF4E engages the CRM1/XPO1 export receptor, rather than the bulk mRNA export receptor NXF1, to transit through the nuclear pore.[ 17 , 34 , 35 , 46 ] Mutations which disrupt the 4ESE structure, as observed by biophysical methods, disrupt formation of the export complex and the export process itself.[ 17 ] When elevated, eIF4E also remodels the nuclear pore complex presumably to aid in recycling and release of mRNA cargoes on the cytoplasmic side of the nuclear pore.[ 48 ] Independent of nuclear export, eIF4E elevation leads to increased capping efficiency of hundreds of transcripts whereby nuclear eIF4E physically interacts with the capping enzyme RNMT as well as drives production of the capping machinery through stimulating the export and translation of their corresponding transcripts[ 14 , 49 ] (Figure 1B). The USER code for capping differs from the 4ESE. The Cap‐sensitivity element (CapSE) increased the capping of model mRNAs and recruited RNMT. Similarly, with regard to cleavage and polyadenylation (CPA), eIF4E physically associates with the CPA machinery, drives production of some of this machinery through its RNA export function, and impacts on cleavage efficiency for a few examined endogenous RNAs[ 15 ] (Figure 1B). Model mRNAs that contained the 4ESE element had increased eIF4E‐dependent PAS cleavage efficiency relative to control mRNAs suggestive of a common USER code between eIF4E‐dependent CPA and mRNA export at least for some targets. A unifying feature for its impacts on these RNA processing steps is the capacity of eIF4E to act as an m7G‐cap chaperone, escorting RNAs through multiple processing steps as well as modulating levels of the pertinent machinery[ 1 , 50 ] (Figure 1A,B). The physical interactions with selected processing machinery appears to be made specific, at least in part, through the USER codes and their capacity to recruit pertinent factors.
EIF4E IN CANCER
Both the nuclear and cytoplasmic activities of eIF4E can be dysregulated in malignancy and contribute to its oncogenic potential.[ 16 , 18 , 36 , 44 , 51 , 52 , 53 , 54 , 55 , 56 ] Elevation of eIF4E activity and/or levels in cancer can occur through a broad variety of mechanisms, for example, dysregulated mTOR signaling, Myc or NFkB induced transcription, gene amplification and others.[ 44 , 57 , 58 , 59 ] eIF4E elevation promotes loss of contact inhibition, growth in soft agar, apoptotic rescue, enhanced motility, and so forth.[ 44 , 59 , 60 , 61 ] In xenograft mouse models, eIF4E elevation corresponds to increased tumor numbers, invasion, and metastases.[ 56 , 59 , 63 ] In transgenic models, mice overexpressing eIF4E develop a variety of cancers.[ 64 ] eIF4E‐mediated transformation relies both on its capacity to increase the nuclear mRNA export and translation efficiency of oncogenic mRNAs.[ 14 , 18 , 25 , 34 , 46 , 48 , 53 , 65 ] eIF4E levels are increased in many cancers including in a subset of AML patients with its elevation generally correlating with poor prognosis.[ 36 , 44 , 51 , 52 , 53 , 66 ] Nuclear enrichment of eIF4E in high‐eIF4E AML specimens correlated with elevated capping and eIF4E‐dependent RNA export relative to healthy volunteers or normal‐eIF4E AML patients.[ 36 , 52 , 53 , 66 ] Survival analysis of a ∼370 AML patient cohort indicated that high‐eIF4E AML patients had substantially reduced survival.[ 13 ] Thus, eIF4E elevation alone is a significant predictor of poor prognosis in AML.
It is important to note that in cells with normal levels of eIF4E, its genetic reduction or pharmacological targeting impair its RNA processing impacts relative to untreated controls, supporting a role for eIF4E in these events even when it is not elevated.[ 61 , 62 ]
EIF4E DYSREGULATION REPROGRAMS THE SPLICING SIGNATURE
Splicing is the removal of introns and joining of flanking exons in pre‐messenger RNAs and some non‐coding RNAs.[ 67 ] Nearly all splicing in mammalian cells is catalyzed by the major spliceosome, an intricate assembly of >150 proteins and five uridine‐rich small nuclear UsnRNAs (U1, U2, U4, U5, and U6 snRNAs).[ 67 ] The resulting protein‐UsnRNA complexes are referred to as snRNPs and act in a series of stepwise complexes to select the excision points in the transcript followed by intron removal and re‐ligation.[ 67 ] Alternative splicing (AS) generates greater diversity in the proteome by producing multiple mRNAs from the same precursor.[ 10 ] About 95% of multi‐exonic genes undergo AS.[ 68 ] AS events include altered selection of the 5′ or 3′ splice sites, skipped exons (SE), inclusion of mutually exclusive exons (MXE) or intron retention (IR).[ 6 , 7 , 8 , 9 , 10 , 68 ] For about 60% of AS events, splicing alters protein levels or generates proteins which manifest new activities or loss‐of‐function.[ 6 , 7 , 8 , 9 , 10 , 68 ] Such proteins can possess opposing functions to their constitutive counterparts, lead to mis‐localization, generate transcripts that rapidly degrade contributing to protein loss and/or other effects.[ 6 , 7 , 8 , 9 , 10 , 11 , 12 ]
eIF4E represents a prototypical driver of aberrant splicing whereby it elicits effects through physical association with components of the spliceosome and via stimulation of SF protein production[ 13 ] (Figure 1B). For example, eIF4E dysregulation alone is sufficient to modulate ∼1000 splicing events in ∼800 transcripts in eIF4E‐overexpressing U2Os cells relative to vector controls. Importantly, eIF4E overexpression did not induce known SF mutations. Similarly, segregation of AML patients without known SF mutations into high‐eIF4E and normal‐eIF4E AML cohorts revealed ∼4600 transcripts underwent differential splicing based on eIF4E levels. Differential splicing and eIF4E status corresponded to substantially reduced overall survival with 136 days for high‐eIF4E patients versus 1396 days for normal‐eIF4E patients.[ 13 ] In both AML and U2Os cells, SE and MXE were the predominant events with these equally distributed in terms of their stimulation or repression. In this way, eIF4E did not stimulate all splicing but repressed or promoted splicing of selected events. Targeted events typically involved long, structured introns and occurred in transcripts that contained more exons. A comparison of splice targets between AML and U2Os cells revealed ∼450 transcripts in common indicative of a core group of targets that undergo reprogramming by eIF4E. Many of these transcripts are involved in pathways that support the oncogenic phenotypes associated with eIF4E including Myc activation, DNA repair, and the adaptive immune response.[ 13 ] eIF4E overexpression was not linked to substantial alteration in transcript levels.[ 13 ]
The impact of eIF4E on splicing does not recapitulate that observed for other reported incidents of splicing dysregulation.[ 13 ] For example, a comparison of splicing signatures derived from secondary AML (sAML) specimens with elevated PRPF6 and SF3B1 protein levels but no SF mutations[ 71 ] revealed that only 363/4670 of eIF4E AS targets (363/963 for sAML targets) were in common. Thus, the elevation of PRPF6 and SF3B1 alone were insufficient to recapitulate the eIF4E‐dependent splicing profile. Moreover, splicing patterns arising from eIF4E overexpression were not a recapitulation of those observed in AML cells with SF mutations. For instance, a comparison of splicing events arising in high‐eIF4E AML relative to AML cells with SF3B1 mutation[ 6 ] showed only a modest overlap (47/4670) of eIF4E targets but included a substantial fraction of SF3B1 targets (47/83 of SF3B1).[ 13 ] Moreover, these signatures differ in event type influenced, for example, SF3B1 mutation altered 3′ splice site usage while eIF4E impacts mainly SE or MXE events.[ 13 , 72 ] Myc dysregulation is also associated with modified splicing[ 11 ] where it elicits increased transcription necessitating a dependency on the spliceosome to process these new transcripts,[ 70 ] while eIF4E elevation did not lead to substantial alterations to transcript levels. Interestingly, Myc is an mRNA export and translation target of eIF4E and thus eIF4E elevation leads to increased Myc protein levels.[ 16 ] Conversely, eIF4E is a Myc transcriptional target.[ 57 ] However, there were only ∼150 common target transcripts between these groups.[ 13 , 73 ] In all, eIF4E‐dependent splicing reprogramming is not a recapitulation of any single downstream target. This highlights the extensive reprogramming arising from its combinatorial impacts on SF expression and its physical interactions with the spliceosome discussed below.
THE TWO‐PRONG IMPACT OF EIF4E ON SPLICING: ELEVATION OF SFS AND PHYSICAL INTERACTIONS WITH THE SPLICING MACHINERY
Interestingly, eIF4E elevation is sufficient to drive the production of multiple SFs simultaneously laying the foundation for its wide‐scale reprogramming[ 13 ] (Figure 1B). Indeed, nuclear eIF4E associated with ∼100 RNAs encoding splicing‐related factors with more than 50 components of the spliceosome including constituents of each of the major snRNPs, for example, SF3B1, U2AF1, U2AF2, PRPF6, PRPF8, PRPF19, PRPF31, SNRNP200. These mRNAs were nuclear export targets of eIF4E and for PRPF8, SF3B1, and U2AF2 also eIF4E‐dependent translation targets. Correspondingly, these SF protein levels were elevated upon eIF4E overexpression and decreased by genetic reduction of eIF4E. Importantly, modulation of eIF4E did not influence the steady‐state transcription or RNA stability of SF transcripts tested. Moreover, eIF4E did not affect UsnRNA levels, and thus likely did not alter the number of spliceosomes. eIF4E modulated the splicing of 5 SFs in U2OS and ∼20 SFs in AML indicating that aberrant splicing of SFs could also contribute to the splicing signature. Morevoer, these findings could suggest that eIF4E plays a role in the production of specialized spliceosomes. Finally, high‐eIF4E AML patients exhibited substantial SF elevation relative to normal‐eIF4E AML patients highlighting the clinical relevance of SF elevation to the observed splicing signatures.
Moreover, eIF4E physically interacted with many of the SFs for which it controls production (Figure 1B).[ 13 ] Specifically, endogenous eIF4E immunoprecipitated with constituents of the major snRNPs including PRPF6, PRPF8, PRPF19, PRPF31, SF3B1, U2AF1, U2AF2, and SNRNP200 as well as the corresponding UsnRNAs. The subset of protein‐eIF4E interactions examined were RNA‐ and cap‐dependent suggesting eIF4E's association with the splicing machinery relied on capped, substrate pre‐mRNAs. This observation brings up the possibility that eIF4E acts as a cap‐chaperone escorting some unspliced mRNAs to the spliceosome. For some transcripts, nuclear eIF4E interacts with both intron‐containing pre‐mRNAs and mature mRNA products. A comparison of mRNAs found in nuclear RIPs with those identified as eIF4E‐dependent splicing targets indicated ∼1300 transcripts were physically associated with eIF4E out of ∼4600 that underwent eIF4E‐dependent splicing in AML patients.[ 13 ] In all, these studies reveal a two‐tier system whereby some eIF4E‐dependent splicing events arise solely due to the altered splicing landscape produced by eIF4E while others are also modulated directly through eIF4E's association with the m7G cap on target RNAs (Figures 1 and 3).
FIGURE 3.
Summary of molecular events that underpin splicing dysregulation. Importantly, different drivers of splicing reprogramming generate disparate splicing signatures. Only a few examples were provided for simplicity. See text for more information.
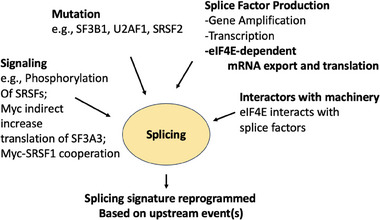
Some earlier studies intimated a connection between eIF4E and splicing. For example, eIF4E altered splicing of SXL in Drosophila where it also physically interacted with components of the U2 snRNP.[ 74 ] Another group suggested that eIF4E may co‐localize with SRSF2 (formerly known as Sc35), a marker of splicing speckles.[ 28 ] An interesting counterpoint is that the splicing co‐factor SRSF1 (formerly known as SF2/ASF) plays a role in selected eIF4E‐dependent, cap‐dependent translation[ 75 ] potentially implicating it as another coupler of splicing and translation similar to eIF4E. Another potential link arises from studies demonstrating that SRSF1 cooperates with MYC for its oncogenic activity, and it was suggested that MYC activation of eIF4E and subsequent enhanced eIF4E‐phosphorylation were indirectly responsible, but this was not directly tested.[ 76 ] Other studies noted that SFs can be regulated at the translation level, but these may or may not be driven by eIF4E. For example, SF3B1 transcripts have their translation regulated through N6 adenosine methylation which appears to be eIF4E‐dependent.[ 77 ] Independently, Myc hyperactivation stimulates protein synthesis of splicing factor SF3A3 in an eIF3d‐dependent manner, leading to metabolic reprogramming that amplifies its oncogenic potential.[ 78 ]
ABERRANT SPLICING IN MALIGNANCY
Splicing can be dysregulated through inappropriate elevation of SF production, signaling and mutation (Figure 3).[ 11 , 79 ] For instance, in a diverse set of solid tumors, SRSF1 and SRSF6 levels are upregulated.[ 10 ] The reasons underpinning elevation can be context dependent, for example, SRSF1 is elevated in some cancers via gene amplification and in others by Myc‐induced transcription.[ 11 ] In sAML specimens without SF mutations, PRPF6 and SF3B1 are elevated relative to healthy volunteers by unknown mechanisms.[ 71 ] Importantly, these alterations drive selected splicing reprogramming. Clearly understanding how SF production becomes dysregulated in cancer is critical for the development of future therapeutic strategies.
The most frequently mutated SFs in the heme‐malignancies known as myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN) are SF3B1, SRSF2, and U2AF1 with these associated with progression to AML, accounting for ∼5%–10% of AML patients.[ 6 , 7 , 10 , 72 , 80 , 81 , 82 ] Patients harbor only one SF mutation since they appear to be synthetically lethal. eIF4E increases production and binds both SF3B1 and U2AF1 suggesting that these SFs are more widely dysregulated in AML than predicted from the frequency of mutations alone.[ 13 ] High‐eIF4E AML patients in our splicing analysis did not harbor SF mutations[ 13 ]; however, in the database there were high‐eIF4E AML patients with SF mutations and thus elevated eIF4E and SF mutation were not mutually exclusive (Leucegene.ca). Unlike eIF4E elevation which is linked to poor outcomes in patients,[ 13 ] SF3B1 mutation can be linked to good or poor prognosis in MDS depending on co‐occurring conditions.[ 72 , 83 , 84 ] Additionally, mutation of other AML‐associated proteins such as RUNX1, while rare, are associated with altered splicing in MDS and AML.[ 85 ] Importantly, AML is characterized by disrupted splicing far more frequently than SF or RUNX mutation occur in patients.[ 12 ]
CLINICAL TARGETING OF ABERRANT SPLICING
Compounds are available to target aberrant splicing in cancer, most of which focus on SF3B1[ 69 , 81 ] (Figure 4). An obvious concern for targeting an essential activity such as splicing is the tolerability at both the cellular and organismal levels. Relevant to the development of therapeutic interventions, modulation of the core components of the spliceosome do not necessarily elicit global impairment of splicing but rather induce reprogramming.[ 86 ] For example, while SF3B1 is a core component of the spliceosome, its pharmacological inhibition only alters ∼10% of splicing events.[ 69 , 80 , 81 ] SF‐targeting compounds generally affect tumor cells more so than normal controls providing a therapeutic window.[ 80 , 87 , 88 ] SF3B1‐targeting compounds include Pladienolide B (PB), Sudemycin and Spliceostatin A (SSA) in the laboratory and E‐1707 and H3B‐8800 in clinical trials (Figure 4). These compounds bind to wildtype or mutant SF3B1 with the exception of R1074H mutant which no longer binds PB leading to resistance.[ 72 ] By contrast, cells harboring other SF3B1 mutations are more sensitive to SSA and E‐1707 indicating that the compound binding‐site together with the mutation influence outcome. Mutation‐based enhanced sensitivity likely arises because cells no longer survive further impairment of splicing and importantly, these compounds also bind wildtype SF3B1 which is present given mutations are heterozygotic. In early phase clinical trials, SF3B1 was targeted in epithelial malignancies with E‐1707 which was generally well tolerated but halted due to partial vision loss in two patients.[ 89 , 90 ] Results with the next generation inhibitor H3B‐8800 were recently reported in patients with AML or related hematological malignancies.[ 91 ] Patients were enrolled regardless of SF3B1 mutation status which was tracked post facto and mutation status did not generally correspond to outcomes. There were no objective clinical responses observed although 15% had reduced transfusion dependency. Molecular correlates suggested that SF3B1 activity was inhibited importantly demonstrating that H3B‐8800 targeted splicing in humans and that targeting splicing was associated with acceptable toxicities.[ 91 ]
FIGURE 4.
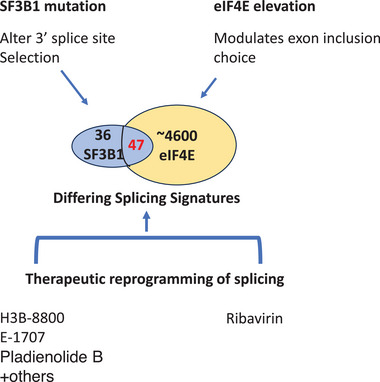
Therapeutic implications of splicing dysregulation. Overlap of 47 transcripts that are targeted in both elevated eIF4E and SF3B1 mutation, splicing events in the same transcripts can differ leading to distinct outcomes. Data are derived from analysis of AML patients.[ 13 ] Below, pharmacological agents that can reprogram splicing. Note that most SF3B1 targeting compounds bind both the mutant and wildtype forms of SF3B1 and thus influence splicing in either case. Ribavirin, H3B‐8800 and E‐1707 are the only of these compounds tried in human clinical trials published to date.[ 89 , 90 , 91 ]
As discussed above, high‐eIF4E AML was characterized by dysregulated splicing arising from the multi‐level impact of eIF4E on many SFs including SF3B1[ 13 ] (Figure 1B). eIF4E has been targeted pharmacologically and such inhibition could also correct eIF4E‐dependent splicing in high‐eIF4E AML patients.[ 51 , 52 , 53 , 92 , 93 ] The first eIF4E inhibitor discovered was the antiviral drug ribavirin which acts as a m7G‐cap competitor by directly binding eIF4E in its cap‐binding site as shown by NMR and other biophysical techniques.[ 94 , 95 , 96 , 97 ] Ribavirin inhibits eIF4E's activities in splicing, CPA, mRNA export, translation, and oncogenic transformation[ 55 , 66 , 94 , 95 , 97 ] (Davis and Borden, unpublished observation) while capping remains to be tested. Genetic reduction of eIF4E decreases ribavirin activity indicating it acts via eIF4E.[ 56 , 98 ] There were three clinical trials using ribavirin in high‐eIF4E AML patients which resulted in objective clinical responses including remissions.[ 51 , 52 , 53 ] The first study was the only trial reported to assess the impact of targeting eIF4E with ribavirin in the absence of any other drugs enabling an analysis of the impact of impairing eIF4E function alone in patients.[ 53 ] In this study, patients who had molecular targeting of eIF4E corresponded to those who achieved objective clinical responses including complete remissions. Ribavirin disrupts eIF4E‐m7G‐capped mRNA interactions and blocks eIF4E's association with its nuclear importer Importin 8 leading to cytoplasmic retention of eIF4E and clinical responses.[ 52 , 53 , 65 ] In turn, ribavirin impairs eIF4E‐dependent splicing, RNA export and translation to a similar extent as genetic reduction of eIF4E, for example.[ 13 , 18 , 54 , 55 , 56 , 61 , 95 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 ] In this way, ribavirin likely acts as a corrector of eIF4E‐dependent splicing as well as its other RNA processing activities. Further studies in ribavirin treated patients are warranted to directly assess this possibility. Ribavirin relapse in patients coincided with impaired drug import into the cell and/or chemical deactivation of ribavirin.[ 51 , 52 , 94 ] Both contribute to nuclear re‐entry of eIF4E and subsequent increased eIF4E‐dependent mRNA export.[ 53 , 65 , 94 ] Presumably this also leads to re‐emergence of eIF4E‐dependent splicing signatures. Clinical trials with ribavirin in combination with other drugs have also been completed in castration‐resistant prostate cancer and head & neck cancer trials where objective clinical responses were observed.[ 92 , 93 ] Given high‐eIF4E AML patients can also harbor SF mutations, it would be interesting to examine if combining splicing inhibitors with ribavirin leads to better outcomes.
OPEN QUESTIONS AND FUTURE DIRECTIONS
The newly discovered role for eIF4E in splicing completes a series of studies which demonstrate that this factor can modulate the major nuclear mRNA processing steps. eIF4E elevates the leves of some of the machinery and regulates the processing of selected transcripts via its role as a m7G cap chaperone and its capacity to interact with components of the capping, splicing, CPA, mRNA export and translation machineries[ 13 , 14 , 15 , 17 , 19 , 25 , 34 , 46 , 105 , 106 ] (Figure 1A,B). Specific transcripts can be modulated by eIF4E at different steps based on their complement of USER codes.[ 1 , 16 , 40 , 41 , 42 ] RNAs with the right combination of USER codes are sensitive to eIF4E at multiple levels of processing, for example, mRNAs with both a 4ESE in their 3′UTR and a long structured 5′ UTR will be sensitive to export and translation respectively[ 1 , 16 ] (Figure 1B).
A key question remains: Where in the nucleus does eIF4E interact with this splicing and other processing machinery? Generally speaking, capping, splicing, and CPA occur close to sites of transcription[ 1 , 2 , 3 ]; however, these events can also occur post‐transcriptionally (Figure 1A,B).[ 4 , 5 ] If eIF4E associated with sites of transcription it would provide a clear model by which eIF4E modulated multiple processes synchronously. Indeed, our preliminary data show an eIF4E‐RNA polymerase II interaction (Mars and Borden, unpublished observation). Transcription rates can influence the propensity to skip exons, the most prevalent splicing event influenced by eIF4E. Thus, studies into whether eIF4E directly or indirectly modulates these rates are important. Interestingly, splicing of long introns, such as those found in eIF4E‐sensitive transcripts, is considered to occur post‐transcriptionally at splicing speckles[ 4 , 107 ] and eIF4E may associate with nuclear speckles.[ 28 ] This could be further investigated using a combination of fluorescence in situ hybridization to detect target intron‐containing mRNAs, immunofluorescence for proteins and confocal microscopy. It is also important to establish whether eIF4E interacts with SF subcomplexes and/or catalytically active spliceosomes using biochemical methods. Studies using PARCLIP experiments will aide in the identification of eIF4E‐specific splicing USER codes that can be leveraged to dissect their functionalities. Additionally, eIF4E influences the production of the splicing, capping, and CPA machinery through its mRNA export and in some cases also translation activities.[ 13 , 14 , 15 , 25 , 36 , 37 , 105 ] Given the evolutionary conservation from plants to human of both eIF4E and these mRNA processing steps, we postulate that eIF4E could, coordinate these processes in a broad array of organisms, but this remains to be directly tested.
We postulate that eIF4E acts as a prototypical coupler linking the major mRNA processing steps with translation to elicit selective re‐programming which enables regulation of the RNA processing‐export‐translation axis. If eIF4E were to impact the system of post‐transcriptional processing, one would predict that modulating eIF4E should have a multi‐level impact simultaneously on the entire system. For example, elevation of eIF4E protein levels in cells or patients coordinately promotes specific capping, splicing, nuclear export, and translation events stemming in part from the elevation of specific components of these machineries.[ 13 , 14 , 15 , 25 , 36 , 37 , 105 ] Conversely, genetic reduction of eIF4E levels decreased the levels of specific constituents of the machineries and associated activities.[ 13 ] Modulation of splicing, RNA export and translation steps are all m7G cap‐dependent and inhibited by the addition of cap analogues or cap competitors such as ribavirin, and/or mutation of the cap‐binding site.[ 13 , 14 , 18 , 25 , 34 , 46 , 48 , 61 , 65 ] Indeed, ribavirin treatment impairs eIF4E‐dependent splicing, RNA export, CPA and translation providing a pharmacological example of coordinated impairment of eIF4E‐dependent processes[ 13 , 18 , 65 ] (Davis and Borden, unpublished observations). Some of these eIF4E functions can be genetically separated[ 39 , 48 ] (Figure 1A,B). The S53A eIF4E mutant does not associate with the nuclear mRNAs examined and is thus unable to promote their RNA export and in turn, has impaired capacity to elevate SF levels and a reduced capacity to transform cells.[ 13 , 48 ] However, the S53A mutant still binds the m7G cap in vitro and to capped mRNAs in the cytoplasm, and promotes their translation efficiency as readily as wildtype eIF4E.[ 108 , 109 ] By contrast, the W73A eIF4E mutant impairs translation through reduced binding of eIF4G but still binds the m7G cap with the same affinity as wildtype eIF4E, promotes eIF4E‐dependent mRNA export since it still binds the LRPPRC export co‐factor and retains its oncogenic potential.[ 17 , 25 , 35 ]
There are specific features related to nuclear and cytoplasmic eIF4E that could influence its role as a coupler of the mRNA processing‐export‐translation axis that require further dissection. One means to control eIF4E and its related functions is through modifying its nuclear‐cytoplasmic compartmentalization. Ribavirin or m7G cap analogue treatment led to cytoplasmic retention of eIF4E through abrogating the eIF4E‐Importin 8 interaction.[ 65 ] Protein factors can similarly impact cellular compartmentalization, for example, overexpression of the proline rich homeodomain PRH, also known at the haematopoietically expressed homeodomain protein Hex, or reduction in Importin 8 levels suppressed eIF4E‐dependent RNA export through cytoplasmic retention of eIF4E.[ 65 , 105 , 110 ] Indeed, ribavirin treatment, PRH/Hex overexpression or Importin 8 reduction decrease both nuclear levels of eIF4E and its oncogenic potential.[ 65 , 105 ] Ribavirin impairs eIF4E‐dependent export and translation; however, the impact of Importin 8 has not yet been examined on translation. Cytoplasmic retention of eIF4E would be anticipated to impact splicing and its other nuclear activities, this needs to be examined. Similarly, phosphorylation of eIF4E is linked to its oncogenic potential and its capacity to export selected RNAs[ 111 ]; and more controversially, to increased translational efficiency for specific RNAs.[ 112 , 113 , 114 ] It will be interesting to study if phosphorylation correlated with eIF4E‐dependent splicing as this would be an excellent way to modulate the eIF4E‐dependent mRNA processing‐export‐translation axis.
The closest comparator to eIF4E in terms of impacts on gene expression is the cap binding complex (CBC) which is comprised of the NCBP1‐NCBP2 heterodimer, the latter subunit directly binds the m7G cap on transcripts and CBC immunoprecipitates with transcription, splicing, CPA, and export machinery. Moreover, it recruits some transcripts to the translation apparatus during the pioneer round of translation or specific stresses.[ 1 , 115 , 116 , 117 , 118 ] Preliminary data suggest an interaction between eIF4E and RNA Polymerase II (Mars and Borden, unpublished observation), which could further extend the parallel between these factors. eIF4E influences the production of factors involved in capping, CPA, and splicing allowing it to globally effect these events combinatorically; however, CBC does not appear to impact on the production of these. It is not clear whether CBC and eIF4E escorts the same RNA species through each step in nuclear processing and translation or if there is cap‐chaperone switching whereby mRNAs may associate with eIF4E or CBC depending on the processing step.[ 1 ] In this way, eIF4E mediated coupling does not necessarily (but can) involve the chaperoning of specific RNAs through each step, but moreover engages coordinated, simultaneous reprogramming of the RNA processing machinery.
Coupling was reported between some nuclear mRNA processing factors and translation previously. The bulk RNA export factor, NXF1/TAP, facilitates the export and translation of a viral derived intron‐retained transcript. Indeed, NXF1/TAP interacted with polysomes and increased translation efficiency of the viral RNA.[ 119 ] The splicing co‐factor SRSF1 increased translation efficiency of ∼1500 transcripts and for candidate RNAs tested both splicing and translation was enhanced; however, whether SRSF1 interacted with polysomes is not yet known.[ 120 , 121 ] SRSF1 also activated the mTOR kinase cascade deactivating the eIF4E inhibitor 4EBP1 and thereby activating eIF4E‐dependent translation.[ 75 ] SRSF1 appears to employ the same USER code, the SRSF1‐binding site, for splicing and translation.[ 121 ] By contrast, the USER codes for eIF4E differ depending on the step, for example, the 4ESE sensitizes RNAs to eIF4E‐dependent export, while the complex, structured 5′ UTR sensitizes RNAs at the translation level.[ 17 , 19 , 34 , 35 ] Each USER code recruits distinct partners to the eIF4E‐mRNA complexes (Figure 1B). Another difference is that SRSF1 has no ascribed function in mRNA export or CPA, in contrast to eIF4E. The mutual link with mTOR suggests intersectionality between SRSF1 and eIF4E‐dependent mRNA processing programs.
In yeast, the RNA polymerase II subunit Rpb4/7 interacts with some transcripts co‐transcriptionally and potentiates their CPA, nuclear export under stress conditions, as well as translation through physical interactions with eIF3.[ 123 , 124 , 125 ] Interestingly, loss of Rpb4 or Rpb7 substantially reduced the number of polysomes[ 123 ]; by contrast genetic eIF4E reduction in mice does not alter polysome profiles or global translation relative to wildtype animals.[ 29 ] The number of transcripts impacted at the translation level by Rpb4/7, the extent of overlap of transcripts with its CPA or translation targets or if Rbp4/7 alters production of the above RNA processing machinery is not yet determined. There is no evident homologue of Rpb4/7 in metazoans, and impacts on splicing, a rare event in yeast, are unknown. In all, these observations suggest that mechanisms coupling RNA processing and translation between yeast and metazoans may share similarities but otherwise be quite distinct.
CONCLUSIONS
In summary, eIF4E is a new actor in the regulation of splicing. Its dysregulation leads to widescale changes to the splicing signature which in AML correlate with poor outcomes. Moreover, the identification of the mechanisms by which splicing become dysregulated will unravel new mechanisms for reprogramming splicing particularly in the absence of SF mutations. Moreover, a model is presented whereby eIF4E co‐regulates and interacts with factors involved in the mRNA processing‐export‐translation axis uniquely positioning it to act in coupling or coordinating these processes. eIF4E appears unique in both elevating production of the RNA processing machinery and physically interacting with these. Diseases of dysregulated eIF4E, such as in high‐eIF4E AML, are maladies of generalized disruption of the mRNA processing‐export‐translation axis leading to widescale reprogramming of the proteome. This model could provide the basis for the development of new therapeutic directions.
CONFLICT OF INTEREST STATEMENT
The author declares no conflicts of interest.
ACKNOWLEDGEMENTS
Katherine L. B. Borden is grateful for critical reading of the manuscript by Drs Jean‐Clement Mars and Biljana Culjkovic‐Kraljacic. Confocal micrographs were kindly provided by Dr Culjkovic‐Kraljacic. Katherine L. B. Borden is supported by grants to the National Institutes of Health (RO1 CA98571 and CA80728), Canadian Institutes for Health Research (PJT 159785) and holds a Canada Research Chair in Molecular Biology of the Cell Nucleus.
Borden, K. L. B. (2024). The eukaryotic translation initiation factor eIF4E unexpectedly acts in splicing thereby coupling mRNA processing with translation. BioEssays, 46, e2300145. 10.1002/bies.202300145
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Mars, J.‐C. , Ghram, M. , Culjkovic‐Kraljacic, B. , & Borden, K. L. B. (2021). The cap‐binding complex CBC and the eukaryotic translation factor eIF4E: Co‐conspirators in cap‐dependent RNA maturation and translation. Cancers (Basel), 13, 6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maniatis, T. , & Reed, R. (2002). An extensive network of coupling among gene expression machines. Nature, 416, 499–506. [DOI] [PubMed] [Google Scholar]
- 3. Neugebauer, K. M. (2002). On the importance of being co‐transcriptional. Journal of Cell Science, 115, 3865–3871. [DOI] [PubMed] [Google Scholar]
- 4. Girard, C. , Will, C. L. , Peng, J. , Makarov, E. M. , Kastner, B. , Lemm, I. , Urlaub, H. , Hartmuth, K. , & Lührmann, R. (2012). Post‐transcriptional spliceosomes are retained in nuclear speckles until splicing completion. Nature Communications, 3, 994. [DOI] [PubMed] [Google Scholar]
- 5. Prasanth, K. V. , Prasanth, S. G. , Xuan, Z. , Hearn, S. , Freier, S. M. , Bennett, C. F. , Zhang, M. Q. , & Spector, D. L. (2005). Regulating gene expression through RNA nuclear retention. Cell, 123, 249–263. [DOI] [PubMed] [Google Scholar]
- 6. Hershberger, C. E. , Moyer, D. C. , Adema, V. , Kerr, C. M. , Walter, W. , Hutter, S. , Meggendorfer, M. , Baer, C. , Kern, W. , Nadarajah, N. , Twardziok, S. , Sekeres, M. A. , Haferlach, C. , Haferlach, T. , Maciejewski, J. P. , & Padgett, R. A. (2021). Complex landscape of alternative splicing in myeloid neoplasms. Leukemia, 35, 1108–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gurnari, C. , Pagliuca, S. , & Visconte, V. (2021). Alternative splicing in myeloid malignancies. Biomedicines, 9, 1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang, E. , & Aifantis, I. (2020). RNA splicing and cancer. Trends in Cancer, 6, 631–644. [DOI] [PubMed] [Google Scholar]
- 9. Bonnal, S. C. , López‐Oreja, I. , & Valcárcel, J. (2020). Roles and mechanisms of alternative splicing in cancer – Implications for care. Nature reviews Clinical oncology, 17, 457–474. [DOI] [PubMed] [Google Scholar]
- 10. Urbanski, L. M. , Leclair, N. , & Anczuków, O. (2018). Alternative‐splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdisciplinary Reviews: RNA, 9, e1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anczuków, O. , & Krainer, A. R. (2016). Splicing‐factor alterations in cancers. RNA, 22, 1285–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rivera, O. D. , Mallory, M. J. , Quesnel‐Vallières, M. , Chatrikhi, R. , Schultz, D. C. , Carroll, M. , Barash, Y. , Cherry, S. , & Lynch, K. W. (2021). Alternative splicing redefines landscape of commonly mutated genes in acute myeloid leukemia. PNAS, 118, e2014967118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghram, M. , Morris, G. , Culjkovic‐Kraljacic, B. , Mars, J.‐C. , Gendron, P. , Skrabanek, L. , Revuelta, M. V. , Cerchietti, L. , Guzman, M. L. , & Borden, K. L. B. (2023). The eukaryotic translation initiation factor eIF4E reprograms alternative splicing. Embo Journal, 42, e110496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Culjkovic‐Kraljacic, B. , Skrabanek, L. , Revuelta, M. V. , Gasiorek, J. , Cowling, V. H. , Cerchietti, L. , & Borden, K. L. B. (2020). The eukaryotic translation initiation factor eIF4E elevates steady‐state m(7)G capping of coding and noncoding transcripts. PNAS, 117, 26773–26783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davis, M. R. , Delaleau, M. , & Borden, K. L. B. (2019). Nuclear eIF4E stimulates 3'‐end cleavage of target RNAs. Cell Reports, 27, 1397–1408.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Culjkovic‐Kraljacic, B. , & Borden, K. L. B. (2018). The impact of post‐transcriptional control: Better living through RNA regulons. Frontiers in Genetics, 9, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Volpon, L. , Culjkovic‐Kraljacic, B. , Sohn, H. S. , Blanchet‐Cohen, A. , Osborne, M. J. , & Borden, K. L. B. (2017). A biochemical framework for eIF4E‐dependent mRNA export and nuclear recycling of the export machinery. RNA, 23, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Culjkovic‐Kraljacic, B. , Fernando, T. M. , Marullo, R. , Calvo‐Vidal, N. , Verma, A. , Yang, S. , Tabbò, F. , Gaudiano, M. , Zahreddine, H. , Goldstein, R. L. , Patel, J. , Taldone, T. , Chiosis, G. , Ladetto, M. , Ghione, P. , Machiorlatti, R. , Elemento, O. , Inghirami, G. , Melnick, A. , … Cerchietti, L. (2016). Combinatorial targeting of nuclear export and translation of RNA inhibits aggressive B‐cell lymphomas. Blood, 127, 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borden, K. L. B. , & Volpon, L. (2020). The diversity, plasticity, and adaptability of cap‐dependent translation initiation and the associated machinery. RNA Biology, 17, 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coutinho De Oliveira, L. , Volpon, L. , Rahardjo, A. K. , Osborne, M. J. , Culjkovic‐Kraljacic, B. , Trahan, C. , Oeffinger, M. , Kwok, B. H. , & Borden, K. L. B. (2019). Structural studies of the eIF4E‐VPg complex reveal a direct competition for capped RNA: Implications for translation. PNAS, 116, 24056–24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volpon, L. , Osborne, M. J. , & Borden, K. L. B. (2019). Biochemical and structural insights into the eukaryotic translation initiation factor eIF4E. Current Protein & Peptide Science, 20, 525–535. [DOI] [PubMed] [Google Scholar]
- 22. Joshi, B. , Lee, K. , Maeder, D. L. , & Jagus, R. (2005). Phylogenetic analysis of eIF4E‐family members. BMC Evolutionary Biology, 5, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volpon, L. , Osborne, M. J. , Topisirovic, I. , Siddiqui, N. , & Borden, K. L. (2006). Cap‐free structure of eIF4E suggests a basis for conformational regulation by its ligands. Embo Journal, 25, 5138–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lejbkowicz, F. , Goyer, C. , Darveau, A. , Neron, S. , Lemieux, R. , & Sonenberg, N. (1992). A fraction of the mRNA 5' cap‐binding protein, eukaryotic initiation factor 4E, localizes to the nucleus. PNAS, 89, 9612–9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen, N. (2001). PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. Embo Journal, 20, 4547–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iborra, F. J. , Jackson, D. A. , & Cook, P. R. (2001). Coupled transcription and translation within nuclei of mammalian cells. Science, 293, 1139–1142. [DOI] [PubMed] [Google Scholar]
- 27. Lang, V. , Zanchin, N. I. , Lünsdorf, H. , Tuite, M. , & Mccarthy, J. E. (1994). Initiation factor eIF‐4E of Saccharomyces cerevisiae. Distribution within the cell, binding to mRNA, and consequences of its overproduction. Journal of Biological Chemistry, 269, 6117–6123. [PubMed] [Google Scholar]
- 28. Dostie, J. , Lejbkowicz, F. , & Sonenberg, N. (2000). Nuclear eukaryotic initiation factor 4E (eIF4E) colocalizes with splicing factors in speckles. Journal of Cell Biology, 148, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Truitt, M. L. , Conn, C. S. , Shi, Z. , Pang, X. , Tokuyasu, T. , Coady, A. M. , Seo, Y. , Barna, M. , & Ruggero, D. (2015). Differential requirements for eIF4E dose in normal development and cancer. Cell, 162, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Altmann, M. , Müller, P. P. , Pelletier, J. , Sonenberg, N. , & Trachsel, H. (1989). A mammalian translation initiation factor can substitute for its yeast homologue in vivo. Journal of Biological Chemistry, 264, 12145–12147. [PubMed] [Google Scholar]
- 31. Osborne, M. J. , & Borden, K. L. B. (2015). The eukaryotic translation initiation factor eIF4E in the nucleus: Taking the road less traveled. Immunological Reviews, 263, 210–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferraiuolo, M. A. , Basak, S. , Dostie, J. , Murray, E. L. , Schoenberg, D. R. , & Sonenberg, N. (2005). A role for the eIF4E‐binding protein 4E‐T in P‐body formation and mRNA decay. Journal of Cell Biology, 170, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrei, M. A. , Ingelfinger, D. , Heintzmann, R. , Achsel, T. , Rivera‐Pomar, R. , & Lührmann, R. (2005). A role for eIF4E and eIF4E‐transporter in targeting mRNPs to mammalian processing bodies. RNA, 11, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Culjkovic, B. , Topisirovic, I. , Skrabanek, L. , Ruiz‐Gutierrez, M. , & Borden, K. L. B. (2005). eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3'UTR. Journal of Cell Biology, 169, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Topisirovic, I. , Siddiqui, N. , Lapointe, V. L. , Trost, M. , Thibault, P. , Bangeranye, C. , Piñol‐Roma, S. , & Borden, K. L. B. (2009). Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export‐competent RNP. Embo Journal, 28, 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Topisirovic, I. , Guzman, M. L. , Mcconnell, M. J. , Licht, J. D. , Culjkovic, B. , Neering, S. J. , Jordan, C. T. , & Borden, K. L. B. (2003). Aberrant eukaryotic translation initiation factor 4E‐dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Molecular and Cellular Biology, 23, 8992–9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Topisirovic, I. , Capili, A. D. , & Borden, K. L. B. (2002). Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E‐dependent mRNA transport of cyclin D1 in a PML‐dependent manner. Molecular and Cellular Biology, 22, 6183–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Volpon, L. , Osborne, M. J. , Capul, A. A. , De La Torre, J. C. , & Borden, K. L. B. (2010). Structural characterization of the Z RING‐eIF4E complex reveals a distinct mode of control for eIF4E. PNAS, 107, 5441–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kentsis, A. , Dwyer, E. C. , Perez, J. M. , Sharma, M. , Chen, A. , Pan, Z. Q. , & Borden, K. L. B. (2001). The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. Journal of Molecular Biology, 312, 609–623. [DOI] [PubMed] [Google Scholar]
- 40. Blackinton, J. G. , & Keene, J. D. (2014). Post‐transcriptional RNA regulons affecting cell cycle and proliferation. Seminars in Cell & Developmental Biology, 34, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Keene, J. D. , & Lager, P. J. (2005). Post‐transcriptional operons and regulons co‐ordinating gene expression. Chromosome Research, 13, 327–337. [DOI] [PubMed] [Google Scholar]
- 42. Keene, J. D. , & Tenenbaum, S. A. (2002). Eukaryotic mRNPs may represent posttranscriptional operons. Molecular Cell, 9, 1161–1167. [DOI] [PubMed] [Google Scholar]
- 43. Sonenberg, N. , & Gingras, A.‐C. (1998). The mRNA 5' cap‐binding protein eIF4E and control of cell growth. Current Opinion in Cell Biology, 10, 268–275. [DOI] [PubMed] [Google Scholar]
- 44. Culjkovic, B. , & Borden, K. L. (2009). Understanding and targeting the eukaryotic translation initiation factor eIF4E in head and neck cancer. Journal of Oncology, 2009, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rousseau, D. , Kaspar, R. , Rosenwald, I. , Gehrke, L. , & Sonenberg, N. (1996). Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. PNAS, 93, 1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Culjkovic, B. , Topisirovic, I. , Skrabanek, L. , Ruiz‐Gutierrez, M. , & Borden, K. L. B. (2006). eIF4E is a central node of an RNA regulon that governs cellular proliferation. Journal of Cell Biology, 175, 415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Culjkovic, B. , Topisirovic, I. , & Borden, K. L. B. (2007). Controlling gene expression through RNA regulons: The role of the eukaryotic translation initiation factor eIF4E. Cell Cycle, 6, 65–69. [DOI] [PubMed] [Google Scholar]
- 48. Culjkovic‐Kraljacic, B. , Baguet, A. , Volpon, L. , Amri, A. , & Borden, K. L. B. (2012). The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Reports, 2, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Osborne, M. J. , Volpon, L. , Memarpoor‐Yazdi, M. , Pillay, S. , Thambipillai, A. , Czarnota, S. , Culjkovic‐Kraljacic, B. , Trahan, C. , Oeffinger, M. , Cowling, V. H. , & Borden, K. L. B. (2022). Identification and characterization of the interaction between the methyl‐7‐guanosine cap maturation enzyme RNMT and the cap‐binding protein eIF4E. Journal of Molecular Biology, 434, 167451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borden, K. L. (2016). The eukaryotic translation initiation factor eIF4E wears a “cap” for many occasions. Translation (Austin), 4, e1220899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Assouline, S. , Gasiorek, J. , Bergeron, J. , Lambert, C. , Culjkovic‐Kraljacic, B. , Cocolakis, E. , Zakaria, C. , Szlachtycz, D. , Yee, K. , & Borden, K. L. B. (2023). Molecular targeting of the UDP‐glucuronosyltransferase enzymes in high‐eukaryotic translation initiation factor 4E refractory/relapsed acute myeloid leukemia patients: a randomized phase II trial of vismodegib, ribavirin with or without decitabine. Haematologica, 10.3324/haematol.2023.282791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Assouline, S. , Culjkovic‐Kraljacic, B. , Bergeron, J. , Caplan, S. , Cocolakis, E. , Lambert, C. , Lau, C. J. , Zahreddine, H. A. , Miller, W. H. , & Borden, K. L. B. (2015). A phase I trial of ribavirin and low‐dose cytarabine for the treatment of relapsed and refractory acute myeloid leukemia with elevated eIF4E. Haematologica, 100, e7–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Assouline, S. , Culjkovic, B. , Cocolakis, E. , Rousseau, C. , Beslu, N. , Amri, A. , Caplan, S. , Leber, B. , Roy, D.‐C. , Miller, W. H. , & Borden, K. L. B. (2009). Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): A proof‐of‐principle clinical trial with ribavirin. Blood, 114, 257–260. [DOI] [PubMed] [Google Scholar]
- 54. Urtishak, K. A. , Wang, L.‐S. , Culjkovic‐Kraljacic, B. , Davenport, J. W. , Porazzi, P. , Vincent, T. L. , Teachey, D. T. , Tasian, S. K. , Moore, J. S. , Seif, A. E. , Jin, S. , Barrett, J. S. , Robinson, B. W. , Chen, I.‐M. L. , Harvey, R. C. , Carroll, M. P. , Carroll, A. J. , Heerema, N. A. , Devidas, M. , … Felix, C. A. (2019). Targeting EIF4E signaling with ribavirin in infant acute lymphoblastic leukemia. Oncogene, 38, 2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bollmann, F. , Fechir, K. , Nowag, S. , Koch, K. , Art, J. , Kleinert, H. , & Pautz, A. (2013). Human inducible nitric oxide synthase (iNOS) expression depends on chromosome region maintenance 1 (CRM1)‐ and eukaryotic translation initiation factor 4E (elF4E)‐mediated nucleocytoplasmic mRNA transport. Nitric Oxide, 30, 49–59. [DOI] [PubMed] [Google Scholar]
- 56. Pettersson, F. , Yau, C. , Dobocan, M. C. , Culjkovic‐Kraljacic, B. , Retrouvay, H. , Puckett, R. , Flores, L. M. , Krop, I. E. , Rousseau, C. , Cocolakis, E. , Borden, K. L. B. , Benz, C. C. , & Miller, W. H. (2011). Ribavirin treatment effects on breast cancers overexpressing eIF4E, a biomarker with prognostic specificity for luminal B‐type breast cancer. Clinical Cancer Research, 17, 2874–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schmidt, E. V. (2004). The role of c‐myc in regulation of translation initiation. Oncogene, 23, 3217–3221. [DOI] [PubMed] [Google Scholar]
- 58. Hariri, F. , Arguello, M. , Volpon, L. , Culjkovic‐Kraljacic, B. , Nielsen, T. H. , Hiscott, J. , Mann, K. K. , & Borden, K. L. B. (2013). The eukaryotic translation initiation factor eIF4E is a direct transcriptional target of NF‐kappaB and is aberrantly regulated in acute myeloid leukemia. Leukemia, 27, 2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. De Benedetti, A. , & Harris, A. L. (1999). eIF4E expression in tumors: its possible role in progression and malignacies. The International Journal of Biochemicstry and Cell Biology, 31, 59–72. [DOI] [PubMed] [Google Scholar]
- 60. Carroll, M. , & Borden, K. L. B. (2013). The oncogene eIF4E: using biochemical insights to target cancer. Journal of Interferon & Cytokine Research, 33, 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zahreddine, H. A. , Culjkovic‐Kraljacic, B. , Emond, A. , Pettersson, F. , Midura, R. , Lauer, M. , Del Rincon, S. , Cali, V. , Assouline, S. , Miller, W. H. , Hascall, V. , & Borden, K. L. (2017). The eukaryotic translation initiation factor eIF4E harnesses hyaluronan production to drive its malignant activity. Elife, 6, e29830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Culjkovic, B. , Tan, K. , Orolicki, S. , Amri, A. , Meloche, S. , & Borden, K. L. B. (2008). The eIF4E RNA regulon promotes the Akt signaling pathway. Journal of Cell Biology, 181, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pettersson, F. , Del Rincon, S. V. , Emond, A. , Huor, B. , Ngan, E. , Ng, J. , Dobocan, M. C. , Siegel, P. M. , & Miller, W. H. (2015). Genetic and pharmacolgic inhibition of eIF4E reduces breast cancer cell migration, invasion and metastasis. Cancer Research, 75(6), 1102–1112. [DOI] [PubMed] [Google Scholar]
- 64. Ruggero, D. , Montanaro, L. , Ma, L. , Xu, W. , Londei, P. , Cordon‐Cardo, C. , & Pandolfi, P. P. (2004). The translation factor eIF‐4E promotes tumor formation and cooperates with c‐Myc in lymphomagenesis. Nature Medicine, 10, 484–486. [DOI] [PubMed] [Google Scholar]
- 65. Volpon, L. , Culjkovic‐Kraljacic, B. , Osborne, M. J. , Ramteke, A. , Sun, Q. , Niesman, A. , Chook, Y. M. , & Borden, K. L. B. (2016). Importin 8 mediates m7G cap‐sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. PNAS, 113, 5263–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kraljacic, B. C. , Arguello, M. , Amri, A. , Cormack, G. , & Borden, K. (2011). Inhibition of eIF4E with ribavirin cooperates with common chemotherapies in primary acute myeloid leukemia specimens. Leukemia, 25, 1197–1200. [DOI] [PubMed] [Google Scholar]
- 67. Wahl, M. C. , Will, C. L. , & Lührmann, R. (2009). The spliceosome: Design principles of a dynamic RNP machine. Cell, 136, 701–718. [DOI] [PubMed] [Google Scholar]
- 68. Pan, Q. , Shai, O. , Lee, L. J. , Frey, B. J. , & Blencowe, B. J. (2008). Deep surveying of alternative splicing complexity in the human transcriptome by high‐throughput sequencing. Nature Genetics, 40, 1413–1415. [DOI] [PubMed] [Google Scholar]
- 69. Effenberger, K. A. , Urabe, V. K. , & Jurica, M. S. (2017). Modulating splicing with small molecular inhibitors of the spliceosome. Wiley Interdisciplinary Reviews: RNA, 8, 10.1002/wrna.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hsu, T. Y.‐T. , Simon, L. M. , Neill, N. J. , Marcotte, R. , Sayad, A. , Bland, C. S. , Echeverria, G. V. , Sun, T. , Kurley, S. J. , Tyagi, S. , Karlin, K. L. , Dominguez‐Vidaña, R. , Hartman, J. D. , Renwick, A. , Scorsone, K. , Bernardi, R. J. , Skinner, S. O. , Jain, A. , Orellana, M. , … Westbrook, T. F. (2015). The spliceosome is a therapeutic vulnerability in MYC‐driven cancer. Nature, 525, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Crews, L. A. , Balaian, L. , Delos Santos, N. P. , Leu, H. S. , Court, A. C. , Lazzari, E. , Sadarangani, A. , Zipeto, M. A. , La Clair, J. J. , Villa, R. , Kulidjian, A. , Storb, R. , Morris, S. R. , Ball, E. D. , Burkart, M. D. , & Jamieson, C. H. M. (2016). RNA splicing modulation selectively impairs leukemia stem cell maintenance in secondary human AML. Cell Stem Cell, 19, 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhou, Z. , Gong, Q. , Wang, Y. , Li, M. , Wang, L. , Ding, H. , & Li, P. (2020). The biological function and clinical significance of SF3B1 mutations in cancer. Biomarker Research, 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Phillips, J. W. , Pan, Y. , Tsai, B. L. , Xie, Z. , Demirdjian, L. , Xiao, W. , Yang, H. T. , Zhang, Y. , Lin, C. H. , Cheng, D. , Hu, Q. , Liu, S. , Black, D. L. , Witte, O. N. , & Xing, Y. (2020). Pathway‐guided analysis identifies Myc‐dependent alternative pre‐mRNA splicing in aggressive prostate cancers. PNAS, 117, 5269–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Graham, P. L. , Yanowitz, J. L. , Penn, J. K. M. , Deshpande, G. , & Schedl, P. (2011). The translation initiation factor eIF4E regulates the sex‐specific expression of the master switch gene Sxl in Drosophila melanogaster. Plos Genetics, 7, e1002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Michlewski, G. , Sanford, J. R. , & Cáceres, J. F. (2008). The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E‐BP1. Molecular Cell, 30, 179–189. [DOI] [PubMed] [Google Scholar]
- 76. Anczuków, O. , Rosenberg, A. Z. , Akerman, M. , Das, S. , Zhan, L. , Karni, R. , Muthuswamy, S. K. , & Krainer, A. R. (2012). The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nature Structural & Molecular Biology, 19, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cieśla, M. , Ngoc, P. C. T. , Muthukumar, S. , Todisco, G. , Madej, M. , Fritz, H. , Dimitriou, M. , Incarnato, D. , Hellström‐Lindberg, E. , & Bellodi, C. (2023). m(6)A‐driven SF3B1 translation control steers splicing to direct genome integrity and leukemogenesis. Molecular Cell, 83, 1165–1179.e11. [DOI] [PubMed] [Google Scholar]
- 78. Cieśla, M. , Ngoc, P. C. T. , Cordero, E. , Martinez, Á. S. , Morsing, M. , Muthukumar, S. , Beneventi, G. , Madej, M. , Munita, R. , Jönsson, T. , Lövgren, K. , Ebbesson, A. , Nodin, B. , Hedenfalk, I. , Jirström, K. , Vallon‐Christersson, J. , Honeth, G. , Staaf, J. , Incarnato, D. , … Bellodi, C. (2021). Oncogenic translation directs spliceosome dynamics revealing an integral role for SF3A3 in breast cancer. Molecular Cell, 81, 1453–1468.e12. [DOI] [PubMed] [Google Scholar]
- 79. Bradley, R. K. , & Anczuków, O. (2023). RNA splicing dysregulation and the hallmarks of cancer. Nature Reviews Cancer, 23, 135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Saez, B. , Walter, M. J. , & Graubert, T. A. (2017). Splicing factor gene mutations in hematologic malignancies. Blood, 129, 1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Taylor, J. , & Lee, S. C. (2019). Mutations in spliceosome genes and therapeutic opportunities in myeloid malignancies. Genes, Chromosomes & Cancer, 58, 889–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Visconte, V. , O Nakashima, M. , & J Rogers, H. (2019). Mutations in splicing factor genes in myeloid malignancies: Significance and impact on clinical features. Cancers (Basel), 11, 1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huber, S. , Haferlach, T. , Meggendorfer, M. , Hutter, S. , Hoermann, G. , Baer, C. , Kern, W. , & Haferlach, C. (2022). SF3B1 mutations in AML are strongly associated with MECOM rearrangements and may be indicative of an MDS pre‐phase. Leukemia, 36, 2927–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mangaonkar, A. A. , Lasho, T. L. , Finke, C. , Ketterling, R. P. , Reichard, K. K. , Mccullough, K. , Gangat, N. , Al‐Kali, A. , Begna, K. H. , Hogan, W. H. , Litzow, M. R. , Alkhateeb, H. , Shah, M. , Pardanani, A. , Tefferi, A. , Al Ali, N. H. , Talati, C. , Sallman, D. , Padron, E. , … Patnaik, M. M. (2022). SF3B1‐mutant myelodysplastic syndrome/myeloproliferative neoplasms: A unique molecular and prognostic entity. Haematologica, 107, 1189–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Huang, Y.‐J. , Chen, J.‐Y. , Yan, M. , Davis, A. G. , Miyauchi, S. , Chen, L. , Hao, Y. , Katz, S. , Bejar, R. , Abdel‐Wahab, O. , Fu, X.‐D. , & Zhang, D.‐E. (2022). RUNX1 deficiency cooperates with SRSF2 mutation to induce multilineage hematopoietic defects characteristic of MDS. Blood Advances, 6, 6078–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Papasaikas, P. , Tejedor, J. R. , Vigevani, L. , & Valcárcel, J. (2015). Functional splicing network reveals extensive regulatory potential of the core spliceosomal machinery. Molecular Cell, 57, 7–22. [DOI] [PubMed] [Google Scholar]
- 87. Kaida, D. , Motoyoshi, H. , Tashiro, E. , Nojima, T. , Hagiwara, M. , Ishigami, K. , Watanabe, H. , Kitahara, T. , Yoshida, T. , Nakajima, H. , Tani, T. , Horinouchi, S. , & Yoshida, M. (2007). Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre‐mRNA. Nature Chemical Biology, 3, 576–583. [DOI] [PubMed] [Google Scholar]
- 88. Fan, L. , Lagisetti, C. , Edwards, C. C. , Webb, T. R. , & Potter, P. M. (2011). Sudemycins, novel small molecule analogues of FR901464, induce alternative gene splicing. Acs Chemical Biology, 6, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eskens, F. A. L. M. , Ramos, F. J. , Burger, H. , O'brien, J. P. , Piera, A. , De Jonge, M. J. A. , Mizui, Y. , Wiemer, E. A. C. , Carreras, M. J. , Baselga, J. , & Tabernero, J. (2013). Phase I pharmacokinetic and pharmacodynamic study of the first‐in‐class spliceosome inhibitor E7107 in patients with advanced solid tumors. Clinical Cancer Research, 19, 6296–6304. [DOI] [PubMed] [Google Scholar]
- 90. Hong, D. S. , Kurzrock, R. , Naing, A. , Wheler, J. J. , Falchook, G. S. , Schiffman, J. S. , Faulkner, N. , Pilat, M. J. , O'brien, J. , & Lorusso, P. (2014). A phase I, open‐label, single‐arm, dose‐escalation study of E7107, a precursor messenger ribonucleic acid (pre‐mRNA) splicesome inhibitor administered intravenously on days 1 and 8 every 21 days to patients with solid tumors. Investigational New Drugs, 32, 436–444. [DOI] [PubMed] [Google Scholar]
- 91. Steensma Dea . (2021). Results of a clinical trial of H3B‐8800, a splicing modulator, in patients with myelodysplatic syndromes (MDS), acute myeloid leukemia (AML) or chronic myelomonocytic leukemia (CMML). Leukemia, 35(12), 3542–3550.34172893 [Google Scholar]
- 92. Dunn, L. A. , Fury, M. G. , Sherman, E. J. , Ho, A. A. , Katabi, N. , Haque, S. S. , & Pfister, D. G. (2018). Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus‐associated oropharyngeal squamous cell cancer. Head & Neck, 40, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kosaka, T. , Maeda, T. , Shinojima, T. , Nagata, H. , Mizuno, R. , & Oya, M. (2017). A clinical study to evaluate the efficacy and safety of docetaxal with ribavirin in patients with progressive castration resistant prostate cancer who have previously received docetaxol alone. Journal of Clinical Oncology, e14010–e14010. https://ascopubs.org/doi/abs/10.1200/JCO2017.35.15_suppl.e14010 [Google Scholar]
- 94. Zahreddine, H. A. , Culjkovic‐Kraljacic, B. , Assouline, S. , Gendron, P. , Romeo, A. A. , Morris, S. J. , Cormack, G. , Jaquith, J. B. , Cerchietti, L. , Cocolakis, E. , Amri, A. , Bergeron, J. , Leber, B. , Becker, M. W. , Pei, S. , Jordan, C. T. , Miller, W. H. , & Borden, K. L. B. (2014). The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature, 511, 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kentsis, A. , Topisirovic, I. , Culjkovic, B. , Shao, L. , & Borden, K. L. B. (2004). Ribavirin suppresses eIF4E‐mediated oncogenic transformation by physical mimicry of the 7‐methyl guanosine mRNA cap. PNAS, 101, 18105–18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Volpon, L. , Osborne, M. J. , Zahreddine, H. , Romeo, A. A. , & Borden, K. L. B. (2013). Conformational changes induced in the eukaryotic translation initiation factor eIF4E by a clinically relevant inhibitor, ribavirin triphosphate. Biochemical and Biophysical Research Communications, 434, 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kentsis, A. , Volpon, L. , Topisirovic, I. , Soll, C. E. , Culjkovic, B. , Shao, L. , & Borden, K. L. B. (2005). Further evidence that ribavirin interacts with eIF4E. Rna, 11, 1762–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pettersson, F. , Del Rincon, S. V. , Emond, A. , Huor, B. , Ngan, E. , Ng, J. , Dobocan, M. C. , Siegel, P. M. , & Miller, W. H. (2015). Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Research, 75, 1102–1112. [DOI] [PubMed] [Google Scholar]
- 99. Xi, C. , Wang, L. , Yu, J. , Ye, H. , Cao, L. , & Gong, Z. (2018). Inhibition of eukaryotic translation initiation factor 4E is effective against chemo‐resistance in colon and cervical cancer. Biochemical and Biophysical Research Communications, 503, 2286–2292. [DOI] [PubMed] [Google Scholar]
- 100. Wang, G. , Li, Z. , Li, Z. , Huang, Y. , Mao, X. , Xu, C. , & Cui, S. (2017). Targeting eIF4E inhibits growth, survival and angiogenesis in retinoblastoma and enhances efficacy of chemotherapy. Biomedicine & Pharmacotherapy, 96, 750–756. [DOI] [PubMed] [Google Scholar]
- 101. Dai, D. , Chen, H. , Tang, J. , & Tang, Y. (2017). Inhibition of mTOR/eIF4E by anti‐viral drug ribavirin effectively enhances the effects of paclitaxel in oral tongue squamous cell carcinoma. Biochemical and Biophysical Research Communications, 482, 1259–1264. [DOI] [PubMed] [Google Scholar]
- 102. Attar‐Schneider, O. , Drucker, L. , & Gottfried, M. (2016). Migration and epithelial‐to‐mesenchymal transition of lung cancer can be targeted via translation initiation factors eIF4E and eIF4GI. Laboratory Investigation, 96, 1004–1015. [DOI] [PubMed] [Google Scholar]
- 103. Shi, F. , Len, Y. , Gong, Y. , Shi, R. , Yang, X. , Naren, D. , & Yan, T. (2015). Ribavirin inhibits the activity of mTOR/eIF4E, ERK/Mnk1/eIF4E signaling pathway and synergizes with tyrosine kinase inhibitor imatinib to impair Bcr‐Abl mediated proliferation and apoptosis in Ph+ leukemia. PLoS ONE, 10, e0136746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hayman, T. J. , Williams, E. S. , Jamal, M. , Shankavaram, U. T. , Camphausen, K. , & Tofilon, P. J. (2012). Translation initiation factor eIF4E is a target for tumor cell radiosensitization. Cancer Research, 72, 2362–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Topisirovic, I. (2003). The proline‐rich homeodomain protein, PRH, is a tissue‐specific inhibitor of eIF4E‐dependent cyclin D1 mRNA transport and growth. Embo Journal, 22, 689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rosenwald, I. B. , Kaspar, R. , Rousseau, D. , Gehrke, L. , Leboulch, P. , Chen, J.‐J. , Schmidt, E. V. , Sonenberg, N. , & London, I. M. (1995). Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post‐transcriptional levels. Journal of Biological Chemistry, 270, 21176–21180. [DOI] [PubMed] [Google Scholar]
- 107. Boutz, P. L. , Bhutkar, A. , & Sharp, P. A. (2015). Detained introns are a novel, widespread class of post‐transcriptionally spliced introns. Genes & Development, 29, 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kaufman, R. J. , Murtha‐Riel, P. , Pittman, D. D. , & Davies, M. V. (1993). Characterization of wild‐type and Ser53 mutant eukaryotic initiation factor 4E overexpression in mammalian cells. Journal of Biological Chemistry, 268, 11902–11909. [PubMed] [Google Scholar]
- 109. Zhang, Y. , Klein, H. L. , & Schneider, R. J. (1995). Role of Ser‐53 phosphorylation in the activity of human translation initiation factor eIF‐4E in mammalian and yeast cells. Gene, 163, 283–288. [DOI] [PubMed] [Google Scholar]
- 110. Topisirovic, I. , & Borden, K. L. (2005). Homeodomain proteins and eukaryotic translation initiation factor 4E (eIF4E): An unexpected relationship. Histology and Histopathology, 20, 1275–1284. [DOI] [PubMed] [Google Scholar]
- 111. Topisirovic, I. , Ruiz‐Gutierrez, M. , & Borden, K. L. B. (2004). Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Research, 64, 8639–8642. [DOI] [PubMed] [Google Scholar]
- 112. Ueda, T. , Watanabe‐Fukunaga, R. , Fukuyama, H. , Nagata, S. , & Fukunaga, R. (2004). Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Molecular and Cellular Biology, 24, 6539–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Morley, S. J. , & Naegele, S. (2002). Phosphorylation of eukaryotic initiation factor (eIF) 4E is not required for de novo protein synthesis following recovery from hypertonic stress in human kidney cells. Journal of Biological Chemistry, 277, 32855–32859. [DOI] [PubMed] [Google Scholar]
- 114. Lachance, P. E. D. , Miron, M. , Raught, B. , Sonenberg, N. , & Lasko, P. (2002). Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Molecular and Cellular Biology, 22, 1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rambout, X. , & Maquat, L. E. (2020). The nuclear cap‐binding complex as choreographer of gene transcription and pre‐mRNA processing. Genes & Development, 34, 1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pabis, M. , Neufeld, N. , Steiner, M. C. , Bojic, T. , Shav‐Tal, Y. , & Neugebauer, K. M. (2013). The nuclear cap‐binding complex interacts with the U4/U6.U5 tri‐snRNP and promotes spliceosome assembly in mammalian cells. RNA, 19, 1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shi, M. , Zhang, H. , Wu, X. , He, Z. , Wang, L. , Yin, S. , Tian, B. , Li, G. , & Cheng, H. (2017). ALYREF mainly binds to the 5' and the 3' regions of the mRNA in vivo. Nucleic Acids Research, 45, 9640–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Choe, J. , Oh, N. , Park, S. , Lee, Y. K. , Song, O.‐K. , Locker, N. , Chi, S.‐G. , & Kim, Y. K. (2012). Translation initiation on mRNAs bound by nuclear cap‐binding protein complex CBP80/20 requires interaction between CBP80/20‐dependent translation initiation factor and eukaryotic translation initiation factor 3g. Journal of Biological Chemistry, 287, 18500–18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jin, L. , Guzik, B. W. , Bor, Y.‐C. , Rekosh, D. , & Hammarskjöld, M.‐L. (2003). Tap and NXT promote translation of unspliced mRNA. Genes & Development, 17, 3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Maslon, M. M. , Heras, S. R. , Bellora, N. , Eyras, E. , & Cáceres, J. F. (2014). The translational landscape of the splicing factor SRSF1 and its role in mitosis. Elife, 3, e02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sanford, J. R. , Coutinho, P. , Hackett, J. A. , Wang, X. , Ranahan, W. , & Caceres, J. F. (2008). Identification of nuclear and cytoplasmic mRNA targets for the shuttling protein SF2/ASF. PLoS ONE, 3, e3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Topisirovic, I. , Kentsis, A. , Perez, J. M. , Guzman, M. L. , Jordan, C. T. , & Borden, K. L. B. (2005). Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels. Molecular and Cellular Biology, 25, 1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Harel‐Sharvit, L. , Eldad, N. , Haimovich, G. , Barkai, O. , Duek, L. , & Choder, M. (2010). RNA polymerase II subunits link transcription and mRNA decay to translation. Cell, 143, 552–563. [DOI] [PubMed] [Google Scholar]
- 124. Farago, M. , Nahari, T. , Hammel, C. , Cole, C. N. , & Choder, M. (2003). Rpb4p, a subunit of RNA polymerase II, mediates mRNA export during stress. Molecular Biology of the Cell, 14, 2744–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fischer, J. , Song, Y. S. , Yosef, N. , Di Iulio, J. , Churchman, L. S. , & Choder, M. (2020). The yeast exoribonuclease Xrn1 and associated factors modulate RNA polymerase II processivity in 5' and 3' gene regions. Journal of Biological Chemistry, 295, 11435–11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


