Abstract
INTRODUCTION:
Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline (LatAm-FINGERS) is the first non-pharmacological multicenter randomized clinical trial (RCT) to prevent cognitive impairment in Latin America (LA). Our aim is to present the study design and discuss the strategies used for multicultural harmonization.
METHODS:
This 1-year RCT (working on a 1-year extension) investigates the feasibility of a multi-domain lifestyle intervention in LA and the efficacy of the intervention, primarily on cognitive function. An external harmonization process was carried out to follow the FINGER model, and an internal harmonization was performed to ensure this study was feasible and comparable across the 12 participating LA countries.
RESULTS:
Currently, 1549 participants have been screened, and 815 randomized. Participants are ethnically diverse (56% are Nestizo) and have high cardiovascular risk (39% have metabolic syndrome).
DISCUSSION:
LatAm-FINGERS overcame a significant challenge to combine the region’s diversity into a multi-domain risk reduction intervention feasible across LA while preserving the original FINGER design.
Keywords: Alzheimer’s disease, cognitive impairment, dementia lifestyle, multidomain intervention, prevention, randomized controlled trial, World-Wide FINGERS
1 ∣. BACKGROUND
Dementia is a major public health challenge. It currently affects > 50 million people worldwide, particularly in low- and middle-income countries (LMIC), where «two thirds of dementia patients live.1 At least 40% of dementia cases might be attributable to 12 potentially modifiable risk factors at different periods during the life course.2 There is growing evidence that the age-specific prevalence of dementia has decreased in many high-income countries during the past 2 decades, which is probably related to control of risk factors.2-4
Changes in modifiable risk factors for dementia prevalence could bring potential benefits for dementia prevention in Latin America (LA). Indeed, the impact of the modifiable risk factors is more significant in LA, where the proportion of dementia cases estimated to be preventable by eliminating nine risk factors is as high as 56%.3,5-7
Evidence for the efficacy of dementia prevention strategies is growing steadily. In recent years, several randomized clinical trials tested whether single interventions on lifestyle factors would improve cognitive function in older persons, but the results were mixed.4 In contrast, robust positive effects on cognitive function were found for multi-domain interventions, as in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER).5 FINGER included participants with high dementia risk and consisted of regular physical exercise, a Mediterranean-like diet (Healthy Nordic diet), cognitive and social stimulation, and control of cardiovascular risk factors.
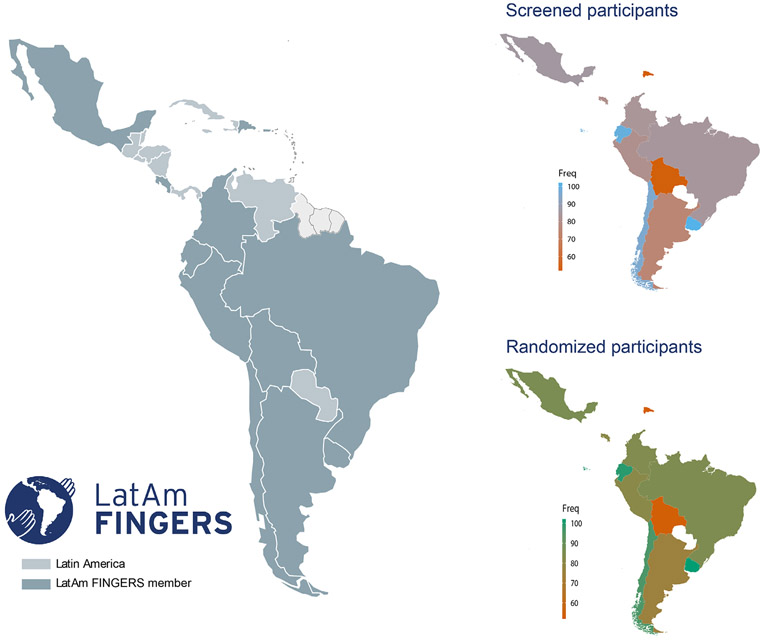
The positive results of the FINGER trial on cognition inspired other initiatives to confirm its findings using similar approaches.8 Worldwide initiatives include projects such as the Alzheimer’s Association U.S. Study to Protect Brain Health through Lifestyle Intervention to Reduce Risk (U.S. POINTER, United States), the Multimodal Interventions to Delay Dementia and Disability in Rural China (MIND-CHINA, China) study, the Australian Multidomain Approach to Reduce Dementia Risk by Protecting Brain Health with Lifestyle Intervention (AU-ARROW, Australia) study, among others. However, no multi-domain intervention has been tested yet in LA. The Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline (LatAm-FINGERS) is the first attempt to bring together LA countries in an intervention to reduce cognitive decline. It gathers 13 centers across 12 LA countries and is open to new incorporations (Figure 1).
FIGURE 1.
On the left, countries that are part of the LatAm-FINGERS trial. On the right, screened and randomized participants by country. LatAm-FINGERS, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline
LatAm-FINGERS offers two ideal conditions to test a multi-domain intervention to prevent cognitive decline. First, LA has a higher prevalence of modifiable risk factors than Finland, and we hypothesize a more significant benefit from this intervention in LA participants. Second, the LA population presents singular socio-cultural and ethnic characteristics that vastly differ from those in Finland. Data demonstrating the feasibility and efficacy of this multi-domain intervention in LA would confirm the generalizability of FINGER findings, and therefore, the importance of changes in lifestyle for the prevention of cognitive decline.
The most critical challenge in implementing the LatAm-FINGERS trial was harmonization, including defining eligibility criteria, outcomes, and intervention equivalent and compatibility with other World-Wide (WW) FINGERS studies. Harmonization of study procedures is essential to compare results across different populations. We divided the harmonization process into two steps: (1) an external harmonization with other WW-FINGERS studies; and (2) an internal harmonization that ensured the intervention was feasible and comparable across the 12 LA countries. Given the heterogeneity of countries in LA, achieving internal harmonization required a consensus process among researchers of the participating countries.
For external harmonization, the LatAm-FINGERS trial follows the FINGER model. Additionally, we harmonized the clinical trial with the US study (U.S. POINTER) because they also included Latinos and ethnically diverse populations and updated the intervention and outcomes based on the evolving science. The harmonization with U.S. POINTER was planned as a step toward the future, hoping that the results of the two trials can be analyzed together as an intervention in the Americas.
The main objective of this report is to present an overview of the trial design and discuss the different strategies and tools used for the multicultural harmonization of the LatAm-FINGERS.
2 ∣. METHODS
2.1 ∣. Trial overview
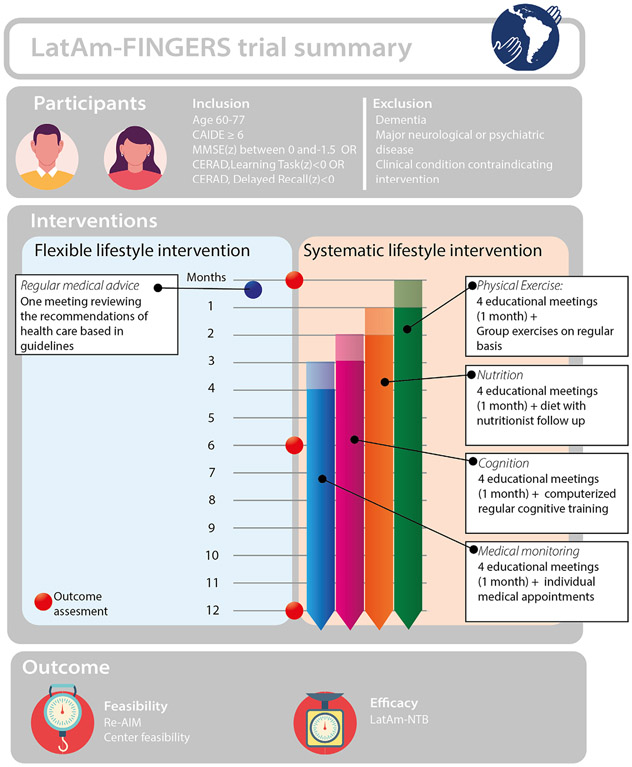
The LatAm-FINGERS was designed as a 1-year randomized controlled lifestyle intervention trial with two main objectives: (1) To investigate the feasibility of the FINGER multi-domain lifestyle intervention in the Latin American context; and (2) To investigate the efficacy of a regimented multidomain lifestyle intervention compared to a more flexible lifestyle intervention on global cognition. It is worth noting that with the determined feasibility, the LatAm-FINGERS team is working to extend the intervention to 2 years to better harmonize with FINGER and U.S. POINTER. The expected sample size is 1200 participants (100 subjects from each country). We adopted a non-competitive recruiting approach with a fixed target number of participants per center. Within the feasibility aim, we plan to measure each center’s capacity to implement the proposal, identify challenges during trial implementation, and propose strategies to overcome these barriers.7 LatAm-FINGERS initiative follows the RE-AIM (reach, effectiveness, adoption, implementation, and maintenance) framework to investigate the project’s effectiveness,feasibility, and sustainability.9 Table 1 shows an overview of the feasibility outcomes, including a dimension definition, metrics, and time evaluation instances. Finally, the main efficacy outcome of the study is the difference between the two groups in a global cognition composite (LatAm-Neuropsychological Test Battery).
TABLE 1.
RE-AIM (reach, effectiveness, adoption, implementation, and maintenance) framework description.
| Domain | Definition | Measures | Time pointa (in months) |
|---|---|---|---|
| Reach | Proportion of participants in the intervention. It is also the extent to which the target audience is reached by the intervention and any “spillover” effects on people not recruited. For example, any impact on the participants’ family and friends. | Number of evaluated subjects | −3/0 |
| Number of screening failures | −3/0 | ||
| Randomization rate: randomization/screened subjects | −3/0 | ||
| Statistical analysis of differences between included and excluded subjects | −3/0 | ||
| % of expected recruitment reach by country | −3/0 | ||
| Qualitative data: asking participants if they had shared any study information and whether receiving the intervention impacted family and friends such as joining in with diet or physical activities. | −3/0/4/6/12 | ||
| Efficacy | Degree of participants’ behavior changes | Changes in diet | 6/12 |
| Changes in physical activity | 6/12 | ||
| Changes in health metrics | 6/12 | ||
| Adoption | Characteristics of intervention settings and staff support delivery | Adequacy of facilities in all centers to perform the intervention | |
| Characteristics of physical exercise facilities/difficulties in the intervention development/identification of barriers and failures in setting up | −6/−3/0/4 | ||
| Characteristics of team meeting facilities/difficulties in its creation/identification of barriers and failures in setting up | −6/−3/0/4 | ||
| Report of difficulties or barriers in computer cognitive training adoption | −3/0/4/6/12 | ||
| % of randomized subjects to fail to computer adoption and change to pencil-paper intervention | −3/0/4 | ||
| Constitution of the interventionist and Intervention Leaders | −6/−3/0 | ||
| Implementation | Level of fidelity to the intervention protocol | Individual level | |
| % attendance to team meetings | 4/6/12 | ||
| % physical activity sessions completed | 4/6/12 | ||
| % cognitive training sessions completed | 4/6/12 | ||
| % telephone contacts completed | 4/6/12 | ||
| % health monitoring visits completed | 4/6/12 | ||
| Subject’s self-reported adherence to the intervention | 4/6/12 | ||
| Country level | |||
| % assistance at team meetings | 4/6/12 | ||
| % physical activity sessions completed | 4/6/12 | ||
| % cognitive training sessions completed | 4/6/12 | ||
| % telephone contacts completed | 4/6/12 | ||
| % health monitoring visits completed | 4/6/12 | ||
| Maintenance | Level of sustained use of the intervention at organizational and individual levels | %of subjects that completed 6-month evaluations | 6 |
| % of subjects that completed 12-month evaluations | 12 | ||
| Organizational level: center retention rate, | 4/6/12 | ||
| satisfaction ratings of the members at end of the trial | 12 | ||
| Individual level: Changes in outcomes at end of trial,satisfaction of subjects | 12 |
Zero represents the time of randomization, and negative values represent the time prior to it.
Consistent with eligibility criteria for FINGER and U.S. POINTER, LatAm-FINGERS participants are individuals at risk of dementia (advanced age, slightly below average cognitive performance, and poor cardiovascular risk profile). The inclusion criteria for the LatAm-FINGERS study are presented in Table 2.
TABLE 2.
Comparison between the main inclusion criteria from LatAm-FINGERS with FINGER and U.S. POINTER.
| FINGER | U.S. POINTER | LatAm FINGERS | |
|---|---|---|---|
| Age: 60–77 years | Age: 60–79 years | Age: 60–77 years | |
| CAIDE ≥ 6 |
|
CAIDE ≥ 6 | |
| MMSE (rawscore) ≥ 26/30 | TICSm ≥ 32 | At least one of the following: | MMSE (z score) between 0 and −1.5 |
| CERAD: learning task (raw score) ≥ 19 words | CDR ≤ 0.5, and CDR-Sum of Boxes ≤ 1 | CERAD, learning task (z scorea) <0 | |
| CERAD: delayed recall ≥ 75% | Lives in a U.S. POINTER intervention region | CERAD, delayed recall (z score) <0 | |
| No travel plans for more than 3 months | |||
| Reading and writing skills | |||
| ≥1 year of education | |||
| No significant physical disabilities that would interfere with intervention participation | No physical disabilities | ||
| Willing to complete all study-related activities for 24 months | Willing to complete all study-related activities for 12 months | ||
| Willing to be randomized to either lifestyle intervention group | |||
Abbreviations: CAIDE, cardiovascular risk factors, aging, and incidence of dementia–dementia risk score; CDR, Clinical Dementia Rating; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; LatAm-FINGERS, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline; LDL, low-density lipoprotein; MMSE, Mini-Mental State Examination; TICSm, modified Telephone Interview for Cognitive Status; U.S. POINTER, U.S. Study to Protect Brain Health through Lifestyle Intervention to Reduce Risk.
Z-scores were calculated using mean and standard deviation of healthy population of same age and education range.
Participants will be randomly assigned to one of two types of interventions. Randomization is performed in blocks of eight participants, and the allocation is generated by a computer.The multi-domain intervention, referred to as the Systematic Lifestyle Intervention (SLI), included aerobic exercise, adherence to the adapted Mediterranean diet (LatAm-MIND), social and cognitive training, and health counseling based on guidelines to control cardio-metabolic risk factors. The flexible intervention (FLI) group will receive regular health counseling. Participants will be evaluated at baseline, 6, and 12 months (see Figure 2).
FIGURE 2.
Trial summary showing key aspects of inclusion and exclusion criteria, interventions timeline, and outcomes. CAIDE, Cardiovascular Risk Factors, Aging, and Incidence of Dementia; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; LatAm-FINGERS, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline; MMSE, Mini-Mental State Examination; LatAm-NTB, Latin American Neuropsychological Test Battery; RE-AIM, reach, effectiveness, adoption, implementation, and maintenance
2.2 ∣. Biobank and magnetic resonance imaging
LatAm-FINGERS is collecting and storing serum, plasma, and DNA samples. A technology transfer program was created so that centers without technical skills might benefit from more experienced centers while developing local human resources. Local biobanks will store and manage the samples. In addition, a transport system to the nearest center will be available for centers that cannot store samples.
The acquisition of baseline magnetic resonance imaging (MRI) is planned for all participants. A complex harmonization procedure was performed to ensure external harmonization with other neuroimaging cohorts (Alzheimer’s Disease Neuroimaging Initiative [ADNI]), and internal harmonization among centers to enable data sharing across databases while considering regional feasibility. MRI acquisition protocol follows ADNI3 specifications for 3T scanners but also allows for a basic protocol with the same resolution in the centers in which 3T is unavailable. So far, 10 centers are performing MRI studies, 8 with 3T MRI scanners and 2 with 1.5T MRI scanners. Four centers are using Siemens-manufactured scanners, four are using Phillips scanners (with one of the centers using both Siemens and Phillips), and two are using GE scanners. Images are uploaded to the Laboratory of Neuroimaging (LONI) platform,10 where quality control is also performed. In addition, the Global Alzheimer’s Association Interactive Network (GAAIN)11 will be used for data sharing. Applications for the study data may be submitted 2 years after the study is completed. The decision to grant access to data will be based on scientific merit as evaluated by a LatAm-FINGERS steering committee. A collaboration with the U.S. POINTER MRI project is planned to analyze and compare images from both cohorts.
2.3 ∣. Harmonization of the study protocol
One key issue of study design was harmonizing eligibility criteria, outcomes, and intervention to make them comparable and consistent with other WW-Fingers studies.
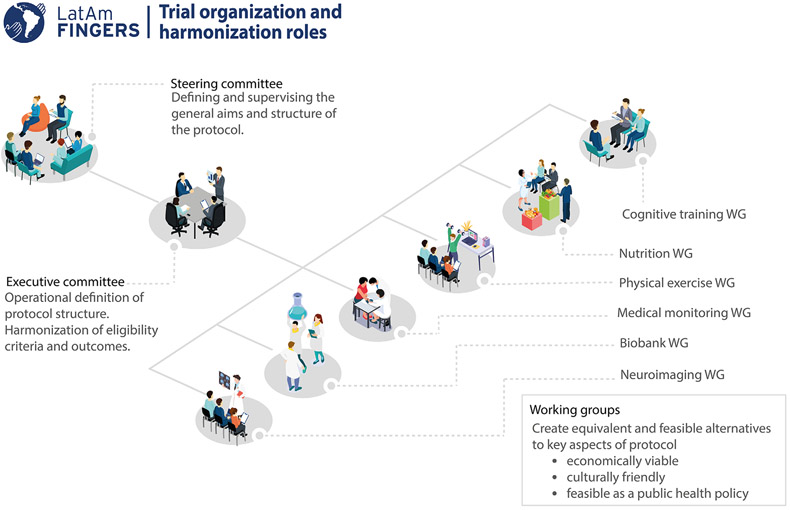
The organizational structure of the trial was assigned specific roles to overcome the challenge of harmonization: (1) the steering committee defined and supervised the general aims and structure of the protocol, (2) the executive committee organized the operational aspects and harmonization of the protocol, and (3) working groups (WG) including specialists from the lifestyle domains (nutrition, cognitive training, physical exercise, and health monitoring) developed specific components of the interventions. These WG included at least one member of each participating center (Figure 3).
FIGURE 3.
Organizational chart showing the different roles in the harmonization process. LatAm-FINGERS, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline; WG, working group
2.4. ∣. Eligibility harmonization
2.4.1 ∣. External harmonization of eligibility criteria
The original FINGER and U.S. POINTER protocols were used as models to adapt and create LatAm-FINGERS eligibility criteria, outcomes, and interventions. In addition, adaptations were made to accommodate differences between the LA population and the Finnish and US populations regarding race and ethnicity, culture, socio-economic status, language, lifestyle, and baseline medical conditions.
The FINGER inclusion criteria were used to include persons with cognitive performance at the mean level or slightly lower than expected for age according to Finnish population norms. On the other hand, the U.S. POINTER inclusion criteria were designed to identify at-risk individuals who may benefit most from a multimodal lifestyle intervention. Thus, U.S. POINTER criteria underscore modifiable risk factors for dementia and rely less on cognitive requirements (see Table 2).
2.4.2 ∣. Internal harmonization of eligibility criteria
In addition to being different from the Finnish and US populations, LA has internal diversity among countries. The region gathers two languages (Spanish and Portuguese); multiple dialects; and various racial, ethnic, and cultural features, making the selection of a unified set of inclusion criteria a highly complex task.
The main objective of inclusion criteria harmonization was to achieve a homogenous selection of participants across countries. Furthermore, considering the results of the FINGER trial,12 the selected criteria attempted to target the population that would benefit the most from the intervention. For that purpose, we converted the results of neuropsychological tests to z scores. For the normalization process, we used the means and standard deviations from healthy populations from each country using the corresponding age and educational range. With this criterion, the conversion to z value was performed as follows: subtracting the mean normal performance (for that age and education in that country) from the individual score and dividing the result by the standard deviation (of the same “healthy” population). Barriers to these processes included the absence of normative data for many LA countries for commonly used cognitive tests, including the Mini-Mental State Examination (MMSE)13 and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD).14 In these cases, the normative data from the country with more similarity in terms of education, language, and culture were used. We chose countries with the same language; similar levels of formal education; and, if possible, cultural affinity, which is many times given by geographical proximity (e.g., this was the case of Uruguay, which used the normative values of Argentina). Finally, exclusion criteria were designed to avoid including people with dementia and to exclude participants who could not tolerate the intervention. Exclusion criteria are dementia diagnosis, MMSE < 20, any medical conditions that could interfere with the safety of the patient during the intervention, significant neurologic disease or psychiatric disorders, body mass index (BMI) > 40 kg/m2, stroke in the last 2 years, sensory deprivation or severe communication difficulties, illiteracy, use of psychoactive medications within the last 3 months, active enrollment in another clinical trial, and history of alcohol or substance abuse or dependence within the past 2 years.
2.5 ∣. Outcomes definitions
Outcomes selection aimed to reproduce the results from FINGER in the LA population. Hence, the outcomes from the original trial that showed significant effects were included. Furthermore, to harmonize our data with the U.S. POINTER, the outcomes were also aligned with this study to permit a cross-study comparison. Finally, we also included local measures of interest (see Table 3).
TABLE 3.
LatAm-FINGERS outcome harmonization with the FINGER and U.S. POINTER studies.
| Outcomes LatAm-FINGERS |
Type | Tests | FINGER | U.S. POINTER |
New measures |
|
|---|---|---|---|---|---|---|
| Primary LatAm-NTB | Cognitive | Free and cued selective reminding test | X | |||
| Logical memory | X | X | ||||
| Wechsler Memory IV Digit Span (forward, backward, sequencing) | X | X | ||||
| Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale-revised | X | X | ||||
| Category Fluency Test (CFT) | X | X | ||||
| Phonological fluency | X | |||||
| Stroop Test 40 stimulus short version | X | |||||
| Concept Shifting Test (CST) | X | |||||
| TMT shifting score (Parts B-A) | X | X | ||||
| Mini-Mental State Examination | X | X | ||||
| Secondary | Cognitive processing speed | Concept Shifting Test (Condition A) | X | |||
| Stroop Test 40 stimulus short version (Condition 2) | X | |||||
| Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale-Revised | X | X | ||||
| Cognitive memory | Craft Story immediate and delayed recall | X | X | |||
| Cognitive executive | Concept Shifting Test (Condition C) | X | ||||
| TMT shifting score (Parts B-A) | X | X | ||||
| Wechsler Memory IV Digit Span (forward, backward, sequencing) | X | X | ||||
| Category fluency | X | X | ||||
| Stroop Test 40 stimulus short version (Condition 2) | X | |||||
| Cognitive | BrainHQ assessment (this comes with the BrainHQ package) | X | ||||
| Global/functional | Clinical Dementia Rating Scale Sum of Boxes (CDR-SoB) | X | X | |||
| The Lawton Instrumental Activities of Daily Living Scale (IADL) | X | X | ||||
| Lifestyle (U.S Pointer Screening) | Physical Activity Questionnaire (U.S. POINTER) | X | ||||
| IPAQ | X | |||||
| Food Frequency Questionnaire (U.S. POINTER) | X | |||||
| Dietary habits (U.S. POINTER) | X | |||||
| Cognitive Activity Questionnaire (U.S. POINTER) | X | |||||
| Exploratory | Miscellaneous | Cardiovascular risk | Framingham riskscore | X | ||
| Sleep habits | Pittsburgh Sleep Quality Index | X | X | |||
| Metabolic | Blood pressure, lipids, HDL, cholesterol, fasting glucose, HbA1c, anthropometry (height, weight, waist circumference, and BMI) | X | X | |||
| Mood | Mood: Geriatric Depression Scale (GDS) | X | ||||
| QoL | SF-12 | X (SF-36) | X | |||
| Physical performance | SPPB (Short Physical Performance Battery) | X | X | |||
| Physical performance | Auto-report of falls | X | X | |||
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; IPAQ, International Physical Activity Questionnaire; LatAm-FINGERS, Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline; LatAm-NTB, Latin American Neuropsychological Test Battery; QoL, quality of life; TMT, Trail-Making Test; U.S. POINTER, U.S. Study to Protect Brain Health through Lifestyle Intervention to Reduce Risk.
2.6 ∣. Harmonization of the interventions
The members of the WG were provided with the details of the FIN-GER and U.S. POINTER interventions. Each member was asked to look for equivalent and feasible alternatives in their own country, considering that the proposed intervention should be affordable, easy to access within their own country, culturally friendly (i.e., incorporating regional or deep-rooted aspects of their society to facilitate adherence), and feasible as a public health policy for their region in the future. In the second stage, the WG members were required to reach a consensus among the 12 countries and decide how to harmonize the interventions. Finally, the protocol for each domain was presented to the executive and steering committees for approval.
2.6.1 ∣. Physical intervention
The physical component is the most challenging aspect of the multi-domain intervention in terms of adherence due to the intensity with which this intervention is proposed in the FINGER and U.S. POINTER trials. To reduce the challenge of implementing this intervention in the different countries, no restrictions were made on the type of associations, agreements, or collaborations with physical activity clubs, gyms, or physical activity instructors reached by each participating center.
Emphasis was made on the standardization of the physical activity program in terms of the general category of the proposed exercise, intensity, and frequency. The LatAm-FINGERS Physical Activity Program (LatAm-FINGERS PAP) includes five phases: warm-up; exercises for coordination, stretching, and balance; resistance and muscle strength training; aerobic exercise; and relaxation activities (see the supporting information for a detailed description of the LatAm-FINGERS PAP).
Physical activity is encouraged to be performed in groups to promote socialization and adherence and must be supervised by qualified personnel to ensure compliance and avoid adverse events. The protocol has certain flexibility as we encouraged each center to look for equivalent exercises that include the LatAm-FINGERS PAP guidelines and are popular within their culture (e.g., local dances such as salsa or tango). Assistance for this intervention is registered by the intervention leader, who is in contact with the physical activity trainers. The adherence information is included in the Research Electronic Data Capture (REDCap) database.
2.6.2 ∣. Nutritional intervention
Diet has a robust idiosyncratic component and is associated with local factors, such as the availability of ingredients, cost, and cooking habits in a region.The nutrition intervention of LatAm-FINGERS is based on the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, which is a hybrid between the Mediterranean and the DASH diets that have shown beneficial effects on dementia risk. The original FINGER trial15 included a Mediterranean-like diet (Healthy Nordic diet), so at this point, LatAm harmonized more closely with the U.S. POINTER trial and created the LatAm-MIND diet.
The nutritional WG was in charge of drawing parallels between locally available products (e.g., tropical fruits and foods of animal origin) with similar nutritional characteristics to those in the MIND diet. The objective was to find substitutes for products unavailable (e.g., marine fish in landlocked countries) or too expensive for the regional economy (e.g., dried fruits and nuts). A “mosaic” program was constructed with interchangeable food blocks to give participants 16 different meal alternatives within the LatAm-MIND diet.
Regarding harmonization within LA, the main nutritional components of the LatAm-MIND diet (i.e., amount of saturated fat per day and amount and type of animal protein) were the same across countries. However, each country can adapt the specific ingredients to comply with the particularities of their regional dietary habits.
Adherence to this intervention is an important challenge, so strategies to follow up were included. To monitor compliance with the diet, participants complete a daily food registry to encourage self-monitoring and adherence to the proposed meals. The intervention team reviews these records at team meetings and follow-up phone calls and gives feedback to participants. This information is included in the project’s database, where an adherence score per intervention is calculated.
2.6.3 ∣. Cognitive intervention
The cognitive training includes computerized cognitive exercises that will be implemented using BrainHQ (Posit Science Corporation), a computerized program for cognitive training.16 This program has 13 unique brain-training exercises organized into six categories: attention, processing speed, memory, social skills, intelligence, and visuospatial abilities. Participants will be encouraged to perform at least 4 weekly sessions, with a minimum of 25 minutes per session.
Implementation is facilitated by using different platforms: a web application or a mobile app. Many participants are not familiarized with the use of computers, but most of them use cell phones.17 During the harmonization process, a concern was raised about the adherence of low-educated people to computerized cognitive rehabilitation. This issue was solved by dedicating time in each team meeting to learning how to use the program. The program also has other strategies to increment adherence. The adaptation of baseline parameters is a critical feature.
Medical monitoring intervention
Medical monitoring is a standard of care intended to be homogeneous worldwide. For this reason, the harmonization process was straight-forward in this case. The medical intervention will be carried out by the clinician interventionist (i.e., general practitioner, family doctor, geriatrician, or neurologist).
2.7 ∣. Database harmonization and data sharing
Data harmonization was a priority in the design of the study protocol. The aims of the data harmonization process were (1) to make data sharing feasible across the WW-FINGERS network, (2) to share data among the study Centers, and (3) to give access to researchers worldwide.
For this, we coordinated with WW-FINGERS, a standard data dictionary with critical aspects of the data collection. We used the REDCap18,19 system to create the trial dataset and share the data dictionary. In addition, a plan for data sharing with researchers external to the network was made to ensure the third objective, in which they could have access after a 2-year period depending on the steering committee’s approval of the project.
3 ∣. RESULTS
3.1 ∣. Study progress
LatAm-FINGERS recruitment started on December 2021, and as of November 11, 2022, 1549 participants had been screened, and 815 were randomized into the intervention (Figure 1).
Table 4 describes some baseline characteristics of the sample. It must be noted that Puerto Rico is not included in this table because they haven’t recruited participants as of the date of this publication. Regarding the sample’s demographic characteristics, we must highlight the overrepresentation of women (from a minimum of 64% in Mexico to 85% in Colombia). This sex distribution does not match the sex distribution rates of the region.20 This result is consistent across centers and cannot be attributed to recruitment strategies because the different centers used different recruitment strategies. Therefore, the discrepancy in sex recruitment may be a limitation to the generalization of our findings compared to the LA population. However, this information will be essential to design a specific recruitment plan to engage men.
TABLE 4.
Demographic characteristics by country (n = 815).
| Overall, n = 815 |
Argentina, n = 75 |
Bolivia, n = 53 |
Chile, n = 96 |
Colombia, n = 85 |
Costa Rica, n = 63 |
Ecuador, n = 41 |
Mexico, n = 85 |
Peru, n = 81 |
Dominican R., n = 52 |
Uruguay, n = 100 |
Brazil, n = 84 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Womena | 74% | 71% | 60% | 74% | 85% | 70% | 73% | 64% | 81% | 71% | 79% | 73% |
| Age (years)b | 67.46 (4.76) | 67.36 (4.50) | 65.92 (4.30) | 68.62 (4.67) | 65.67 (4.11) | 65.86 (3.85) | 66.85 (4.50) | 70.36 (4.72) | 65.88 (4.29) | 68.83 (4.43) | 67.46 (5.14) | 68.23 (4.89) |
| Education (years)b | 12.60 (3.80) | 15.03 (1.73) | 13.85 (2.79) | 12.15 (3.38) | 11.48 (4.02) | 13.75 (3.35) | 12.60 (3.61) | 10.22 (3.74) | 14.48 (2.19) | 6.85 (3.87) | 14.07 (2.50) | 12.80 (3.60) |
| Ethnicitya | ||||||||||||
| White | 32% | 96% | 7.50% | 2.10% | – | 32% | – | – | 8.60% | 9.60% | 100% | 58% |
| Mestizo | 56% | 2.70% | 81% | 92% | 95% | 32% | 98% | 100% | 89% | 52% | – | 1.20% |
| Native American | 0.60% | – | 3.80% | – | 1.20% | – | – | – | – | 1.90% | – | 1.20% |
| Mulato | 3.50% | – | – | – | 1.20% | 23% | – | – | – | 17% | – | 6.00% |
| Black | 2.20% | – | – | – | – | – | – | – | – | 13% | – | 13% |
| Other | 4.20% | – | 1.90% | 4.20% | 2.40% | 8.90% | 2.40% | – | 2.50% | 3.80% | – | 20% |
| Missing race data | 1.10% | 1.30% | 5.70% | 2.10% | – | 3.60% | – | – | – | 1.90% | – | – |
| MMSEb | 27.32 (1.68) | 28.21 (1.52) | 27.40 (0.79) | 26.42 (1.91) | 27.17 (1.69) | 27.69 (1.90) | 28.10 (1.01) | 26.59 (1.88) | 28.02 (1.30) | 27.12 (1.86) | 27.29 (0.73) | 27.27 (2.01) |
Abbreviations: n, number of subjects; MMSE: Mini-Mental State Examination. aRelative frequency over the site’s total (%).
Mean (standard deviation).
The mean (standard deviation) age is 67.46 (4.76) years, the level of education is 12.6 (3.8) years, and the MMSE score is 27.32 (1.68). Education level is very heterogeneous, and it will be interesting to analyze how adherence to the intervention and effectiveness is affected by this variable. Regarding ethnicity, the diversity of the LA population is reflected in self-reported ethnicity: 56% of the participants self-reported being Mestizo, and 32% as White. Other reported ethnicities included Mulatto (3.5%), Black (2.2%), and Asian (0.7%).
It is well known that lifestyle interventions tend to improve cardiovascular risk factors. The preliminary recruitment results show a wide opportunity for improvement in cardiovascular risk factors: 16% are current smokers, 46% have high systolic blood pressure (>130 mmHg), 22% have diastolic hypertension (>85 mmHg), 81% of participants were overweight (BMI ≥ 25 kg/m2), 36% had low high-density lipoprotein (<40 mg/dL in men or 50 mg/dL in women), 36% had hypertriglyceridemia (>150 mg/dL), and 62% of had central obesity (waist circumference > 88 cm for women or 102 cm for men). According to current diagnostic criteria, these results indicate a metabolic syndrome prevalence of 39% in our sample (see Table 5).21
TABLE 5.
Global metabolic syndrome data.
| Characteristic | N a | n(%) |
|---|---|---|
| Overweight (BMI ≥ 25) | 557 | 451 (81%) |
| Current smokers | 456 | 73 (16%) |
| High waist circumferenceb | 555 | 344 (62%) |
| High triglycerides (>150 mg/dL) | 622 | 224 (36%) |
| Abnormal HDL (< 40 mg/dL in men or < 50 mg/dL in women) | 631 | 227 (36%) |
| High fasting glucose (> 100 mg/dL) | 628 | 226 (36%) |
| High systolic blood pressure (> 130 mmHg) | 561 | 258 (46%) |
| High diastolic blood pressure (> 85 mmHg) | 559 | 123 (22%) |
| Metabolic syndrome diagnosis (2005 criteria) | 482 | 188 (39%) |
Evaluated subjects.
(women ≥ 88 cm, men ≥ 102 cm).
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein.
4 ∣. DISCUSSION
LA includes genetic, cultural, sociodemographic, and economic diversity, which translates into diverse lifestyles. Nevertheless, it shares a common problem, a growing number of individuals living with dementia.22 The FINGER intervention was effective in reducing cognitive decline in Finland. However, this intervention needs to be adapted to LA countries because of population characteristics, lifestyle, and health-care use differences. Therefore, the central objective of LatAm-FINGERS is to create a multi-domain intervention as effective as that tested in the Finnish study, that will be feasible in our region and, if successful, will be a candidate to become a health policy for LA.
LatAm-FINGERS faced a significant challenge to combine the region’s diversity into a single healthy lifestyle intervention feasible across LA. This report presents the results of that effort that were achieved by effective teamwork. Unlike most clinical trials, created by a single group and applied by centers worldwide, the LatAm-FINGERS initiative included members of all participating centers in the protocol design. We brought together specialists from 12 countries, transforming diversity into an advantage and generating a unified cultural perspective. With a north–south extension of 10,759 km, separated by two languages, multiple dialects, and enormous cultural diversity, we underscored the characteristics that united us and achieved the remarkable result of unifying the efforts toward a common objective. This is the first non-pharmacological trial across 12 LA countries in dementia and an unprecedented extensive collaboration in the fields of aging and neuroscience research. We hope this is the first step toward multiple collaborations within our region and worldwide.
Furthermore, LatAm-FINGERS is building a larger dataset with outcomes on people at risk for dementia in LA. With longitudinal follow-up, it has already grown to be the region’s largest cognitive, imaging, plasma, and genetic database of individuals at risk for dementia. This is a significant contribution because the lack of information on dementia in LMIC countries has been identified and stressed in the literature.5 The most recent analysis of population-attributable factors for dementia risk factors was published with data that was already 10 years old at the time of analysis.2
5 ∣. CONCLUSION
This report described the study design and discussed the different strategies and tools used for the multicultural harmonization of the LatAm-FINGERS. However, it must be stated that the goals of LatAm-FINGERS may be more comprehensive than the efficacy of the intervention. This project contributes to narrowing the gap between dementia research in LMIC and high-income countries. The project also aspires to achieve the collaborative effort of a region that shares interests, risk factors, and culture but is as vast as 13% of the planet’s populated surface. LatAm-FINGERS aspires to be the foundation stone of dementia teamwork, demonstrating the value of collaboration.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic Review: The authors reviewed the prevalence of dementia worldwide, and the significant incidence of modifiable risk factors for dementia in low- and middle-income countries (LMIC). The evidence on multi-domain interventions for preventing cognitive decline and dementia was applied to their study design and aims.
Interpretation: This study describes the design of the first non-pharmacological multicenter randomized clinical trial to prevent cognitive impairment in Latin America (LA). Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline (LatAm-FINGERS) overcame a significant challenge: to combine the region’s diversity into a multi-domain risk reduction intervention feasible across LA while preserving the original FINGER design.
Future Directions: LatAm-FINGERS’ goals have outgrown the original ones (feasibility and efficacy of the intervention). This project contributes to narrowing the gap between dementia research in LMIC and high-income countries and aspires to be the foundation stone of dementia teamwork, demonstrating the value of Latin American collaboration.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of all the members of the LatAm-FINGERS Consortium. The LatAm-FINGERS project is entirely funded by the Alzheimer’s Association: ALZ Strategic Initiatives SG-21-715176-LATAM FINGERS.
APPENDIX
LatAm-FINGERS Collaborators
Juan Andrés Abin-Carriquiry
Katherine Aguero
Karen Aleman
Alejandra Amestoy
Josselyn Arce
Florencia Arredondo
Micaela Arruabarrena
Lucy Baldeón
Valquíria de Oliveira Barbara
Isabella Maria Bello Avolio
Rodrigo Beltran
Yaritza I Berrios López
Laura Bocos
Eliane de Oliveira Borca
Jacinta Bravo
Paula Schimidt Brum
Pablo Burgos
Cristina Cañadas
Moisés Capriles
María Agostina Carello
Douglas Cerqueira Ferdinando
Valeria Contrera
Eliane Correa Miotto
Lucia Cortabarria
Sebastian Cortes Barquero
Nicolás Corvalán
Letícia Costa da Silva
Nathalia Crespi Pardelli
Leandro Dansilio
João Victor de Faria Rocha
Janice Diaz-Monge
Carlos Doren
Lilian Viana dos Santos Azevedo
Gabriela Espinoza Cruz
Camila Fabres
Alex Fernandez
Lucia Fernandez
Rodolfo Ferrando
Laís Soares Figueiredo
Beatriz Figueiredo e Silva
Beatriz Flores Blanco
Margarita Garcia Fontes
Natalia Gattini
Oscar Hidalgo Mora
Greta Keller
Oriana Lara
Luis Diego Leal Chaves
Alice Leites
Jose Lema
Luzia Lima Carreira
André Luis Lopes
Fiorella Lopez
Beatriz Marcela Mar
Francesca Mariani
Melissa Martinez
Carlos Martinez Canyazo
Mercedes Menendez
Barbara Meneses
Silvia Stahl Merlin
Silvia Montes
Paula Mora Vargas
Lara Mora Villalobos
Jamileth More
Maira Moreno
Yleana Muñoz
Pamela Muro
Nury Navarro
Alberto Nuñez-Duque
Ana Luisa Pedrosa de Menezes
Pedro Peguero
Cristiane Peixoto
Raphaella Araújo Pena
Flávia Pinheiro Machado
Junea Senra Portilho
Natalia Pozo
Fabian Preciozzi
Gabriel Puelma
Ana Laura Reyes
Javiera Rodriguez
Adriana Rueda
Liza E. San Miguel-Montes
Katherine Sanchez
Sofía Santero
Carina Silva Correa
Jerusa Smid
Adalberto Studart-Neto
Eduardo Sturzeneker Trés
Ramon Suarez
Roberto Superchi
Leonel Tadao Takada
Maren Torheim
Emma Valles
Yanina Varela
Daniella Vela
Ana Vigil Martínez
Maria Aquimara Zambone Magalhåes
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All human subjects provided informed consent.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Mattap SM, Mohan D, McGrattan AM, et al. The economic burden of dementia in low- and middle-income countries (LMICs): a systematic review. BMJ Glob Health. 2022;7(4). Published online first: Epub Date. doi: 10.1136/bmjgh-2021-007409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. Published online first: Epub Date. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019;7(5):e596–e603. Published online first: Epub Date. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews FE, Stephan BC, Robinson L, et al. A two decade dementia incidence comparison from the Cognitive function and ageing studies I and II. Nat Commun. 2016;7:11398. Published online first: Epub Date. doi: 10.1038/ncomms11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribeiro F, Teixeira-Santos AC, Caramelli P, Leist AK. Prevalence of dementia in Latin America and Caribbean countries: systematic review and meta-analyses exploring age, sex, rurality, and education as possible determinants. Ageing Res Rev. 2022;81:101703. Published online first: Epub Date. doi: 10.1016/j.arr.2022.101703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergara RC, Zitko P, Slachevsky A, San Martin C, Delgado C. Population attributable fraction of modifiable risk factors for dementia in Chile. Alzheimers Dement (Amst). 2022;14(1):e12273. Published online first: Epub Date. doi: 10.1002/dad2.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suemoto CK, Mukadam N, Brucki SMD, et al. Risk factors for dementia in Brazil: differences by region and race. Alzheimer’s Dement. 2023;19(5):1849–1857. doi: 10.1002/alz.12820 [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16(7):1078–1094. Published online first: Epub Date. doi: 10.1002/alz.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43(3):337–350. Published online first: Epub Date. doi: 10.1016/j.amepre.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Irimia A, Hobel SM, et al. The LONI QC system: a semi-automated, web-based and freely-available environment for the comprehensive quality control of neuroimaging data. Front Neuroinform. 2019;13:60. Published online first: Epub Date. doi: 10.3389/fninf.2019.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toga AW, Neu SC, Bhatt P, Crawford KL, Ashish N. The Global Alzheimer’s Association Interactive Network. Alzheimers Dement. 2016;12(1):49–54. Published online first: Epub Date. doi: 10.1016/j.jalz.2015.06.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. Published online first: Epub Date. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. Published online first: Epub Date. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. Published online first: Epub Date. doi: 10.1212/wnl.39.9.1159 [DOI] [PubMed] [Google Scholar]
- 15.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657–665. Published online first: Epub Date. doi: 10.1016/j.jalz.2012.09.012 [DOI] [PubMed] [Google Scholar]
- 16.Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt FW. Speed of processing training results in lower risk of dementia. Alzheimers Dement. 2017;3(4):603–611. Published online first: Epub Date. doi: 10.1016/j.trci.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crivelli L, Quiroz YT, Calandri IL, et al. Working group recommendations for the practice of teleneuropsychology in Latin America. Arch Clin Neuropsychol. 2022;37(3):553–567. Published online first: Epub Date. doi: 10.1093/arclin/acab080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. Published online first: Epub Date. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. Published online first: Epub Date. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comisión Económica para América Latina y el Caribe (CEPAL), Panorama Social de América Latina, 2018. LC/PUB.2019/3-P, Santiago, 2019. [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. Published online first: Epub Date. doi: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 22.Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e25.[Published onlinefirst: Epub Date]. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.