Abstract
Two new adenovirus vector systems based on the tetracycline-regulated Tet-ON- (Gossen, M., et al., Science 268:1766–1769, 1995) and the RU 486-regulated progesterone antagonist (Wang, Y., et al., Proc. Natl. Acad. Sci. USA 91:8180–8184, 1994)-induced gene expression systems are described. We show that both systems permit a tight control of chloramphenicol acetyltransferase reporter gene expression in a variety of cell types, with induction levels of approximately 1,800-fold (Tet-ON system) and 600-fold (RU 486-regulated system), respectively. A significant advantage of our vector systems is that reporter protein expression can be adjusted over a wide range by varying the amount of inducer. The Tet-ON system is also shown to permit an efficient control of reporter gene expression in mice.
Recombinant adenovirus protocols typically make use of strong promoters to achieve high levels of production of recombinant protein (for reviews see references 1, 5, and 12). However, this sometimes leads to a problem, since constitutive gene expression is unphysiological and may interfere with signaling systems in the cell and lead to cellular toxicity. Thus, gene cassettes permitting regulated expression, on/off switches, and the possibility to fine tune the level of reporter gene expression are of value in experimental designs, such as basic studies of protein function, expression of cytotoxic proteins, and cancer treatment.
Here we describe the construction of two versatile adenovirus vector systems that allow for regulated reporter gene expression in gene transfer experiments. A major advantage with the adenovirus-mediated gene expression systems compared to conventional techniques is that they circumvent the need to establish stable transfected cell lines, a tedious task that has to be repeated with each cell type to be used.
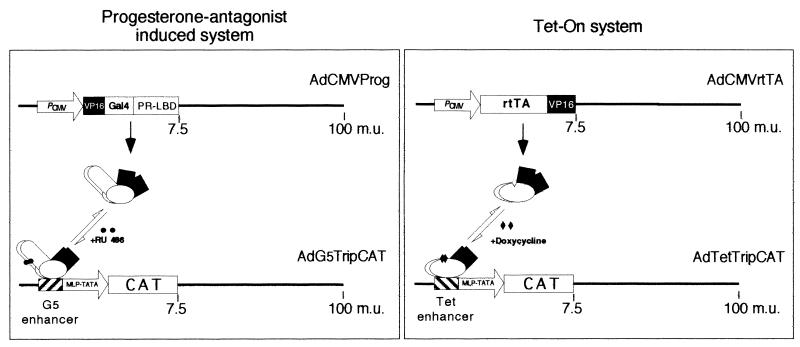
We have adopted a double-infection strategy to control reporter gene expression in our vector systems (Fig. 1). In the Tet-ON system the reverse tetracycline (Tet) repressor protein fused to the herpes simplex virus (HSV) VP16 transcriptional activation domain (rtTA) (4) was cloned behind the constitutively active cytomegalovirus (CMV) promoter and inserted into an adenovirus type 5 (Ad5) dl309 (9) vector (generating virus AdCMVrtTA; Fig. 1). The chloramphenicol acetyltransferase (CAT) reporter gene was cloned behind a Tet enhancer consisting of seven tandem Tet operator DNA binding sites fused to a minimal adenovirus major late promoter/tripartite leader construct (virus AdTetTripCAT; Fig. 1). Transcription of the CAT reporter gene is activated by the addition of doxycycline (DOX) to the culture medium (4).
FIG. 1.
Diagrams of the Tet-ON and the Prog systems. A mixed-infection strategy is used to introduce the activator and the CAT reporter genes to recipient cells. Reporter gene expression is then activated by the addition of the respective inducer. The Tet and Prog enhancer sequences were cloned upstream of a minimal major late TATA promoter element. We fused a cDNA encoding the major late tripartite leader immediately downstream of the transcription start site, as a 5′ noncoding sequence. The tripartite leader has been shown to function as an mRNA export signal (8) and a translational enhancer (15) in late-infected cells. The gene cassettes were inserted into the E1 region of Ad5 dl309 (9), making the recombinant adenoviruses replication deficient.
In the progesterone antagonist-induced gene expression system (13) (hereafter referred to as the Prog system), a chimeric transactivator protein (13) consisting of the ligand binding domain of hPRB891 fused to the Gal4 DNA binding domain and the HSV VP16 transactivator domain was cloned behind a CMV promoter and inserted into an Ad5 dl309 (9) vector (generating virus AdCMVProg; Fig. 1). This human progesterone receptor mutant does not bind progesterone or other endogenous hormones but can still bind the progesterone antagonist RU 486 (13). The CAT reporter gene was cloned behind a Gal4 enhancer, consisting of five Gal4 DNA binding sites fused to a minimal major late promoter/tripartite leader construct (virus AdG5TripCAT; Fig. 1). Transcription of the CAT reporter gene is activated by the addition of RU 486 to the culture medium. More details about the cloning strategy and methods used in this article are available at www.bmc.uu.se/IMIM/res/GA.html.
One important characteristic expected of the virus vector systems is the possibility to fine tune reporter gene expression. This is important in several biological settings, such as studies of basic protein function in which too-high protein expression may be toxic or cause unphysiological perturbations of regulatory pathways in the cell. To test the induction potential of the two vector systems, HeLa cells were coinfected with 10 PFU per cell of the activator and reporter viruses belonging to each system. Reporter gene expression was induced at the start of infection by the addition of increasing amounts of RU 486 or DOX, respectively, and CAT gene expression was measured at 16 h postinfection by CAT enzyme assay (2). As shown in Fig. 2, CAT activity was undetectable in both systems in uninduced cells. The addition of increasing amounts of DOX or RU 486 resulted in a corresponding increase in CAT activity, with maximum levels of CAT obtained at 4 μM of the respective inducer. The same dose-response curves were observed in the tested range of 1 to 20 PFU per cell (data not shown). However, as expected, the total CAT activity decreased with a lower multiplicity of infection. Collectively, these results show that the Tet-ON and Prog systems fulfill the first criterion, namely, that the level of reporter gene expression can be controlled by varying the amount of inducer or the multiplicity of infection. The possibility of suppressing reporter gene expression during virus growth makes it possible to reconstruct recombinant adenoviruses expressing gene products that interfere with virus replication (data not shown).
FIG. 2.
Dose response for the Prog (A) and the Tet-ON (B) systems. HeLa cells were coinfected with 10 PFU of the respective activator and reporter viruses. Increasing amounts of inducer were added at the start of infection, and cells were harvested at 16 h postinfection. CAT activity was measured and quantitated by PhosphorImager scanning. Error bars indicate standard deviations calculated from three independent experiments.
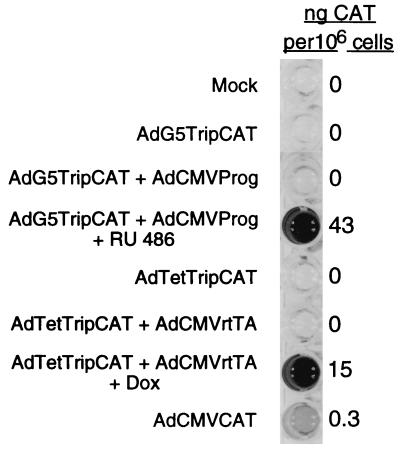
To quantitate reporter gene expression, HeLa cells were coinfected with 10 PFU per cell of activator and reporter viruses. The inducer was added at the start of infection, and CAT protein accumulation was quantitated at 16 h postinfection by CAT enzyme-linked immunosorbent assay (ELISA) (Boehringer Mannheim). As shown in Fig. 3, addition of the respective inducer resulted in dramatic induction of CAT protein expression in both the Prog and Tet-ON systems, with the Prog system consistently yielding a much higher level of protein expression. The background level of CAT expression was very low in both systems, making an accurate estimate of the fold induction difficult. However, assaying uninduced extracts at a 100-fold-higher concentration revealed that the fold induction was greater for the Tet-ON (approximately 1,800-fold) than for the Prog system (approximately 600-fold) (details available at www.bmc.uu.se/IMIM/res/GA.html). The levels of CAT protein production with both gene regulatory systems are impressive. Thus, the ratios of CAT protein produced with the Prog and Tet-On systems, compared to that of a recombinant adenovirus expressing CAT under the transcriptional control of the constitutively active CMV early promoter (AdCMVCAT), were 140 and 50, respectively (Fig. 3). To determine whether the Prog and Tet-ON systems could be used for high levels of protein production, we quantitated CAT expression in 293 cells, which support replication of the recombinant viruses. Infection with both systems resulted in an accumulation of approximately 1.5 μg of CAT protein per 106 cells at 24 h postinfection (data not shown). Since 293 cells can be grown in spinner cultures, this translates to quantities approximating 1 mg of CAT protein per liter of cells. It therefore appears realistic that these vector systems can be used for large-scale recombinant protein production. This alternative is attractive, since protein modifications specific for different cell types would be expected to occur correctly.
FIG. 3.
Stringent control of reporter gene expression. HeLa cells were coinfected with 10 PFU of the respective activator and reporter viruses. The inducer (4 μM) was added at the start of infection, and cell extracts were prepared at 16 h postinfection. CAT protein expression was quantitated by using a CAT ELISA kit (Boehringer Mannheim). The mean value of CAT protein expression, calculated from seven independent experiments, is shown.
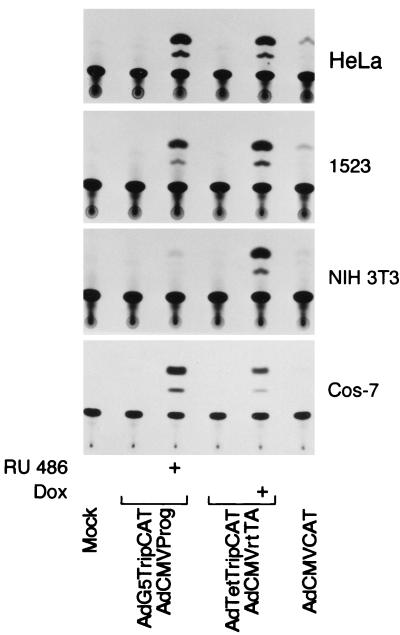
An important feature expected of our vector systems is the potential to function in a broad range of cell types. In theory, the major limitation of our experimental approach would be whether the recipient cell expresses the adenovirus receptor and coreceptors and thus permits virus uptake. To test the host range of the viral vectors we infected Cos-7 (simian virus 40-transformed African green monkey kidney cells), NIH 3T3 (mouse fibroblasts), and 1523 cells (human foreskin fibroblasts). As shown in Fig. 4, in all cells we observed a high induction combined with a background equal to that of uninfected extracts. The Tet-ON system functioned reproducibly in all cell types tested. In contrast, the Prog system induced CAT expression efficiently in all cell types except NIH 3T3 cells. Since the Tet-ON system functioned in this cell type, the failure of the Prog system was not due to virus uptake but was more likely caused by the inability of RU 486 to activate the progesterone receptor in this cell type.
FIG. 4.
The Tet-ON and the Prog systems work with similar efficiencies in several cell types. HeLa cells, Cos-7 cells, NIH 3T3 cells, and 1523 cells were coinfected with 10 PFU per cell of activator and reporter viruses belonging to the respective systems. Inducer (4 μM) was added at the start of infection, and CAT activity was measured at 16 h postinfection. For comparison, CAT expression induced by infection with 20 PFU of AdCMVCAT is shown.
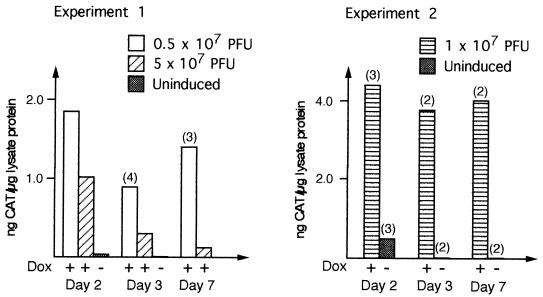
To determine whether the high levels of induction of CAT protein expression observed in cell lines also were reproduced in an in vivo model system, we injected BALB/c mice intramuscularly with mixtures of AdCMVrtTA and AdTetTripCAT. The results from two independent experiments are summarized in Fig. 5. Injected muscles and also lung and liver samples from the same animals were analyzed for CAT expression at various times postinjection. Only muscle biopsies were found to be positive for CAT, suggesting that the viruses did not disseminate from the site of injection (data not shown). Although the amounts of CAT protein detected in the muscle biopsies varied between individual animals (Fig. 5), DOX treatment consistently gave a high level of induction of CAT expression. The observed variability is likely due mainly to two factors: experimental difficulties in (i) injecting small volumes and in (ii) excision of an equal size of muscle tissue. However, the results summarized in Fig. 5 demonstrate that the adenovirus Tet-ON vector system permits efficient control of reporter gene expression also in an animal model system.
FIG. 5.
Inducible expression of CAT in vivo. Mice were injected intramuscularly with the indicated PFUs of the Tet-ON system. Muscle extracts were analyzed for CAT protein expression by CAT ELISA. The results are presented as nanograms of CAT protein per microgram of total lysate protein. Bars based on mean values derived from extracts prepared from multiple animals are indicated, with the number of animals used shown within brackets at the top. Control animals not injected or receiving DOX only showed no CAT protein expression (data not shown).
During our work we have become aware of other research groups that have adopted similar experimental strategies to construct adenovirus vectors for inducible gene expression (for example see references 6, 7, 10, 11, and 14). Most of these reports are based on recombinant adenoviruses expressing the Tet-OFF system (3). This system does not, contrary to results with our Tet-ON or Prog systems, allow for the rapid induction of reporter gene expression since the tetracycline needs to be washed out or consumed for gene activation. This may be critical in studies in which kinetic parameters play a role. It is also worth pointing out that our Prog and Tet-ON systems may very well be used in conjunction with each other, permitting independent control of expression of two different proteins in an experimental set up (data not shown).
Acknowledgments
We thank Lars-Gunnar Larsson for the kind gift of 1523 cells and Neil Portwood for much help during the initial stages of this project. RU 486 was kindly provided by ROUSSEL-UCLAF.
This work was supported by the Swedish Cancer Society.
REFERENCES
- 1.Berkner K L. Expression of heterologous sequences in adenoviral vectors. Curr Top Microbiol Immunol. 1992;158:39–66. doi: 10.1007/978-3-642-75608-5_3. [DOI] [PubMed] [Google Scholar]
- 2.Bondesson M, Öhman K, Mannervik M, Fan S, Akusjärvi G. Adenovirus E4 open reading frame 4 protein autoregulates E4 transcription by inhibiting E1A transactivation of the E4 promoter. J Virol. 1996;70:3844–3851. doi: 10.1128/jvi.70.6.3844-3851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 5.Graham F L, Prevec L. Adenovirus-based expression vectors and recombinant vaccines. In: Ellis R W, editor. Vaccines: new approaches to immunological problems. Boston, Mass: Butterworth-Heinemann; 1992. pp. 363–390. [DOI] [PubMed] [Google Scholar]
- 6.Harding T C, Geddes B J, Murphy D, Knight D, Uney J B. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nat Biotechnol. 1998;16:553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- 7.Hu S-X, Ji W, Zhou Y, Logothetis C, Xu H-J. Development of an adenovirus vector with tetracycline-regulatable human tumor necrosis factor α gene expression. Cancer Res. 1997;57:3339–3343. [PubMed] [Google Scholar]
- 8.Huang W, Flint S J. The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA export. J Virol. 1998;72:225–235. doi: 10.1128/jvi.72.1.225-235.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones N, Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978;13:181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- 10.Massie B, Couture F, Lamoureux L, Mosser D D, Guilbault C, Jolicoeur P, Bélanger F, Langelier Y. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J Virol. 1998;72:2289–2296. doi: 10.1128/jvi.72.3.2289-2296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neering S J, Hardy S F, Minamoto D, Spratt S K, Jordan C T. Transduction of primitive human hematopoietic cells with recombinant adenovirus vectors. Blood. 1996;4:1147–1155. [PubMed] [Google Scholar]
- 12.Trapnell B C, Gorzilla M. Gene therapy using adenoviral vectors. Curr Opin Biotechnol. 1994;5:617–625. doi: 10.1016/0958-1669(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, O’Malley B W, Jr, Tsai S Y, O’Malley B W. A regulatory system for use in gene transfer. Proc Natl Acad Sci USA. 1994;91:8180–8184. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida Y, Hamada H. Adenovirus-mediated inducible gene expression through tetracycline-controllable transactivator with nuclear localization signal. Biochem Biophys Res Commun. 1997;230:426–430. doi: 10.1006/bbrc.1996.5975. [DOI] [PubMed] [Google Scholar]
- 15.Yueh A, Schneider R J. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 1996;15:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]