Abstract
Background
Breast cancer (BC) treatment has recently been revolutionized by the introduction of newer targeted agents, that helped tailoring therapies around the single patient. Along with increased survival rates, a careful evaluation of diet, lifestyle habits, physical activity, emotional and psychological experiences linked to the treatment journey, is now mandatory. However, a true proposal for an omnicomprehensive and “integrative” approach is still lacking in literature.
Methods
A scientific board of internationally recognized specialists throughout different disciplines designed a shared proposal of holistic approach for BC patients.
Results
A narrative review, containing information on BC treatment, endocrinological and diet aspects, physical activity, rehabilitation, integrative medicine, and digital narrative medicine, was developed.
Conclusions
In the context of a patient-centered care, BC treatment cannot be separated from a patient’s long-term follow-up and care, and an organized interdisciplinary collaboration is the future in this disease’s cure, to make sure that our patients will live longer and better.
Trial Registration
NCT05893368: New Model for Integrating Person-based Care (PbC) in the Treatment of Advanced HER2-negative Breast Cancer (PERGIQUAL). Registration date: 29th May 2023.
Keywords: Breast cancer, Multidisciplinary approach, Tailored oncology, Cancer prevention, Oncology and metabolism, Personalized medicine, Integrative oncology
Introduction
Breast cancer (BC) represents the first most prevalent neoplasm worldwide and one of the leading cause of death in the female gender [1]. The proportion of intrinsic (e.g. random genomic alterations arising during DNA replication) and extrinsic (e.g. mutations that arise owing to environmental exposure) factors that determine its development is still a matter of debate. However, analysis of mutational fingerprints suggested that up to 65% of BCs present extrinsic mutational signatures, indicating considerable contribution of environmental exposures/lifestyle habits in the development of this disease [2].
Obesity is a risk factor for cancer development and associated with cancer-related mortality in different tumors, including BC [3]. Although the epidemiological association among obesity and lifestyle habits (as alcohol, smoking, and sedentary behavior) with many forms of cancer is clear enough, the biological relationship between carcinogenesis and these risk factors remains poorly understood [4].
In addition, several environmental pollutants (Cadmium, BBA, etc.), which are released after industrial processes and absorbed into certain plants and water, have recently been considered as additional risk factors for several different hormonal responsive cancers [5, 6]. Indeed, our group has demonstrated as Cadmium might alter breast cancer responsiveness in vitro, (Bimonte et al. Endocrine, in revision) leading to even more complex pathogenetic mechanisms in the hormonal carcinogenesis and therapeutical approach.
The treatment landscape of BC is constantly evolving, with pharmacological interventions which have reached impressive results both in early and advanced settings [7•]. According to molecular features (e.g. estrogen receptor, HER2 status, PDL1 expression, and BRCA mutation), defined sequences and/or combination of target therapy, anti-hormonal therapy, immunotherapy, and/or chemotherapy can be offered to patients [8–19]. The development of new treatments unavoidably led to different toxicity profiles, which means that there is a true need of an organized network of professional healthcare figures involved in the patients’ treatment path. Moreover, improved survival outcomes recently translated into particular attention for quality of life (QoL) [20, 21].
Evidences of an integrated and omnicomprehensive approach are still lacking in literature, since available data are only focused on prevention or provide only a few useful hints for breast cancer patients [22, 23].
Therefore, a holistic approach might be a quite powerful and effective tool, beside pharmacological intervention, not only to help tailoring the treatment for each patient but also to improve QoL. Herein, we provide a narrative review on an “integrative medicine” model, based on an interdisciplinary approach that might help achieving improvement in survival and QoL outcomes for women affected by BC.
Methods
This narrative review is the result of an intense collaboration between specialists across different disciplines, which shared their scientific and clinical experience to develop information and hints on all aspects of a BC patient treatment journey. Several detailed paragraphs have been developed by specific experts to explore the latest available data for each discipline and suggest how to properly manage a BC patient.
Nutrition and Metabolism in Breast Cancer
Patients undergoing BC treatments, such as Chemotherapy (CHT), Antibody–Drug Conjugates (ADCs), and Radiation Therapy (RT), face nutritional and metabolic challenges. CHT often carries nutrition related symptoms, such as nausea and vomiting [24]. Antibody–Drug Conjugates (ADCs) and target therapies may also cause similar effects and altered taste or smell perception as well as oral mucositis [25]. Damage to taste receptor cells, along with zinc and other heavy metal chelation, contributes to loss of taste. Radiation therapy (RT) frequently damages salivary glands, altering the composition and quantity of saliva, favoring xerostomia. All these side effects, among others, may lead to malnutrition [26] that negatively affects QoL and survival, contributing to other conditions such as cardiovascular diseases and treatment-related acute events [27]. CHT can also disrupt glucose metabolism [27, 28], leading in some patients to insulin resistance, which contributes to increased body weight, generally characterized by excessive fat mass with loss of muscle mass, a condition known as sarcopenic obesity (SO) [27]. This condition is further exacerbated by reduced PA due to treatment-induced side effects like asthenia and fatigue [26]. Moreover, the use of corticosteroids and cytotoxic agents affects the maintenance of proper weight and body composition as well, increasing the risk of SO. Also estrogen deprivation due to adjuvant endocrine treatments for Hormone Receptor Positive (HR +) BC and/or CHT can affect body composition, with alteration in skeletal, adipose, and muscle tissues, leading to sarcopenia, obesity and osteoporosis [29]. Obesity at diagnosis and body weight gain after treatment are frequent and have been linked to higher BC recurrence and mortality rates and to lower QoL [30].
On the other hand, osteoporosis, a known side effect of adjuvant hormonal therapy for breast cancer, increases the risk of fragility fractures, impacting long-term morbidity and QoL. In addition to that, the decline in muscle strength and quality due to sarcopenia, possibly caused by the mechanisms previously described, can also affect the risk of falls and consequent fragility fractures necessitating a comprehensive treatment approach, including nutritional interventions [31]. This is particularly important for HR + BC patients, where managing nutritional intake is vital due to the significant concern of hormonal imbalances that might contribute to the previously described mechanisms. Interestingly, several studies have found a link between BC, diet and life-style. The latest studies have focused on the identification of risk factors that could contribute to unfavorable outcome of BC, thus in 2018 the World Cancer Research Fund has produced a report showing the latest and significant evidence to improve the overall survival through lifestyle recommendations [32]. Indeed, lifestyle improvement has a significant impact on BC management after initial diagnosis, both in term of therapeutical efficacy and QoL [33]. Despite the overall nutritional healthy choices, the maintenance of a normal body weight is mandatory, since it is well documented that obesity is linked to worst outcomes [34, 35]. For these reasons, particular attention must be paid to meeting the protein and caloric needs as part of managing weight and body composition, as mentioned before, a crucial aspect of BC treatment. Achieving and maintaining an optimal body weight in BC patients is a priority for the whole care team both by correct healthy diet but also by appropriate PA. The principles of the MeDi, with its balanced approach to macro and micronutrient intake, are highly recommended for their beneficial effects on glucose and insulin levels, as well as overall metabolic health [36, 37•]. In contrast the Western Diet, with high consumption of fats, activating pathways involving insulin and IGF-1 which, when combined with sedentary life, could worsen outcomes of BC patients [38]. The role of MeDi is due to the food of which this nutritional pattern is composed. MeDi includes a daily consumption of vegetables and fruits that allows an optimal intake of polyphenols and fiber [39, 40]. Interestingly, a recent review indicated that a higher fruit, vegetable, and fiber intake and a moderate soy/isoflavone consumption were associated with beneficial outcomes in BC survivors [40]. A small consumption of dairy product is contemplated in the MeDi pattern, but dairy products are often avoided by women due to the fear of a potential worsening of the disease. Interestingly, some studies have indicated that the consumption of a modest amount may reduce the risk of BC; nonetheless, some dairy products contain a high percentage of saturated fatty acids with pro-carcinogenic effects. No recommendations can also be made for the soy products [41, 42]. Soy foods contain isoflavones that have weak estrogen-like activity, but it has anti-carcinogenic and antioxidant properties Thus, at the present time, it is recommended to follow MeDi indications [41, 42]. Carbohydrates should be present but whole grain bread and whole meal products should be chosen to decrease the glycemic index [43•]. MeDi limits the intake of meat and animal food. Even though few studies have evaluated in BC survivors whether red and processed meat increase or worsen cancer, it is advised to follow MeDi indication and limit this type of food.
As mentioned, it is not possible to find an association between specific nutrients and BC, except for alcohol. Alcohol is the only nutrient with demonstrated negative effects on BC, confirmed in every study [44, 45]. This risk is not associated to the type of alcohol consumed and the patients’ age, since ethanol promotes tumor growth and metastasis formation, and apparently increases the effects of estrogens on breast tissue through several pathways [46].
It is also important to note that BC patients may also autonomously modify their diets, following anecdotal advices and common beliefs or online and social media sources [47], to try cope with physical and psychological consequences of their condition. Orthorexia nervosa (ON) is a condition where patients become overly preoccupied with eating “healthy” foods, often leading to dangerous exclusions potentially resulting in malnutrition [48]. ON risk has been found higher among BC patients compared to healthy controls [49]. In this context, clinical nutrition specialist plays a crucial role in addressing nutritional issues and ensuring comprehensive care, guiding patients in becoming aware of their specific needs, offering tailored advice and support.
To sum up, nutritional assessment and therapy is essential for addressing the adverse effects of BC treatments and enhancing their efficacy, as well as improving patient outcomes. The comprehensive management of BC necessitates an integrative approach that combines structured nutritional strategies and professional counseling for lifestyle modifications. This holistic approach is crucial for enhancing the therapeutic efficacy and improving the overall outcomes for patients, particularly in the context of HR + BC. Nutritional care extends beyond merely defining a proper diet. It involves assisting patients in finding personalized strategies to optimize their nutrition within their daily lives and monitoring their nutritional status to identify early signs of malnutrition or related conditions such as sarcopenia, obesity, or symptoms of eating disorders. Promptly addressing these conditions through early interventions is vital for effectively treatment or management, highlighting the importance of nutritional care in BC treatment.
Integrative Treatments in Early and Advanced Disease
QoL during and beyond cancer treatments is a key aspect in the large population of BC survivors, either for the physical and psychological burden of the disease experience and the numerous side effects and symptoms [50, 51], even with late onsets, potentially affecting patients’ adherence to anticancer treatments [52–54].
Most of the early and late side effects are not completely assessed and properly met among BC patients/survivors, who may turn to “alternative medicine” practitioners outside the comprehensive cancer centers, with potential risks for drug interactions, unproven treatments and eventually threats to their health [55].
Worldwide, an estimated 33–47% of oncological patients use complementary, alternative, or integrative medicine during their therapeutic process, while more than 80% of BC survivors reported using integrative therapies during and beyond cancer treatments [56–58].
The Integrative oncology, according to the definition approved by a panel of multi-disciplinary experts, is a “patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and/or lifestyle modifications from different traditions alongside conventional cancer treatments”. Accordingly, it combines lifestyle counselling, body-mind activities, and complementary therapies with anticancer standard care, to improve management of symptoms, adherence to the treatment protocols, improve QoL, clinical outcomes across the cancer continuum, and empower people preventing cancer and become active participants before, during, and beyond oncological treatment” [59].
Despite the widespread use of integrative treatments and the available evidence about their safety and efficacy [60], facilities and services providing those treatments inside the BC centers are lacking [61], at least in Europe, while many of the National Cancer Institute designated comprehensive cancer centers already offer some integrative therapies in the United States.
A holistic model should include a comprehensive assessment for each patient after cancer diagnosis, either waiting for surgery or neoadjuvant chemotherapy, with a preliminary psycho-oncological distress evaluation and a brief interview about lifestyles, to identify psychological and/or physical needs that may affect patient’s compliance to treatments.
To be accepted by the scientific community as a reliable answer to the multiple unmet needs of BC patients, the integrative model must be safe, rational and evidence based. Moreover, the specific interventions should be prescribed by integrative oncology practitioners rather than on demand by the patient, who might otherwise hold incorrect information from unreliable sources often failing to distinguish between “integrative/complementary” and “alternative” cures.
According to the SIO/ASCO clinical guidelines, complementary approaches for BC patients should include acupuncture, massage therapy, mindfulness-based protocols, and music therapy, tailored to the clinical needs summarized in the following lines.
Acupuncture represents a reliable, cost-effective, and safe procedure for symptom management, if performed properly by a specialized practitioner. It can be recommended for chronic pain, fatigue, nausea/vomiting and hot flashes, as an option to avoid or reduce pharmacological treatments, or for other conditions (fatigue, hot flashes, and CHT-induced peripheral neuropathy) for which conventional treatments are ineffective, not available or burdened by remarkable side effects.
Even though acupuncture alone is debated for cancer pain, it could be recommended in frail patients with comorbidities and when side effects of drugs, such as opioids, are relevant. According to the Society for Integrative Oncology-ASCO guidelines about integrative medicine for pain management in oncology, acupuncture should be recommended for aromatase inhibitor-related joint pain in breast cancer patients and survivors [62]. CHT-induced peripheral neuropathy (CIPN) is a challenging symptom, and the role of acupuncture is still uncertain [63]; nevertheless, some randomized studies showed promising results [64–66], and acupuncture might be considered in selected patients to treat CIPN symptoms according to the ESMO guidelines [67]. Among the massage therapy procedures, reflexology is a safe and well tolerated technique which can be used to alleviate pain, anxiety [68] and sleep disorders that are poorly controlled by pharmacological treatments [69].
Mindfulness-based stress reduction (MBSR) techniques have been shown to reduce distress and improve primary insomnia and psychological well-being in patients with cancer [70, 71]. In randomized trials based on MBSR programs women reported decreased fatigue, depression, anxiety, fear of recurrence and health related QoL [72, 73]. Mindfulness and hypnosis may improve QoL, fatigue and anxiety even in advanced breast cancer patients [74].
Music therapy has been shown beneficial on depression, pain, and cancer-related fatigue during and after treatments, either through active (e.g., singing, using percussive instruments, and playing with sounds) and passive (e.g., listening) techniques [75, 76]. Music therapy may also be recommended for reducing anxiety in the perioperative setting, among patients undergoing mammographic screening, during radiation, and CHT sessions [77]. In the advanced stages of the disease, integrative approaches could represent an additional tool for the oncology health-care providers, who struggle with managing pain, complex side effects or toxicities, and drug interactions using conventional treatments. According to the 5th ESO-ESMO international consensus guidelines, complementary therapies have the potential to reduce disease symptom burden and/or side-effects of anticancer therapies and therefore improve the QoL of advanced breast cancer patients [74].
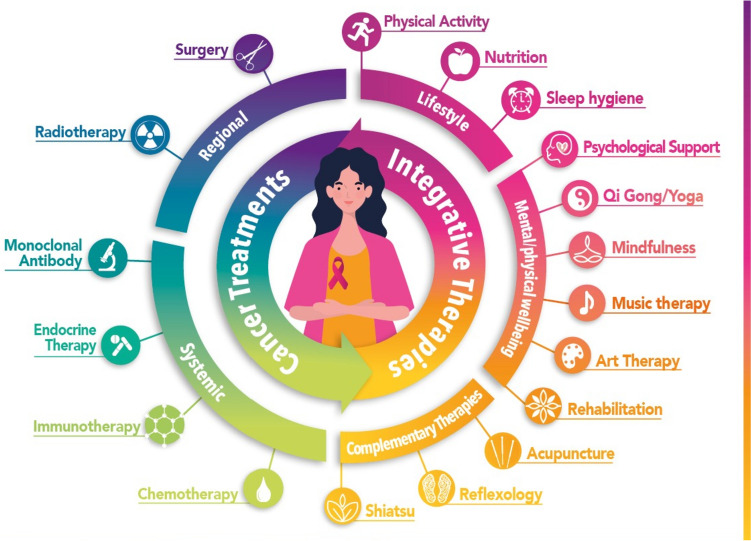
Figure 1 shows our proposal for a holistic model of cancer care continuum, in which integrative and supportive approaches are combined with anticancer treatments.
Fig. 1.
Integrative oncological approach for early and advanced breast cancer
The Role of Rehabilitation, Physical Activity, and Exercise
Rehabilitation and physical activity might be part of a unique therapeutical pattern, even though they have quite different aims. In fact, while rehabilitation might play a pivotal role soon after surgery, physical activity may play an important role for the maintenance of optimal body composition and quality of life during or after pharmacological treatments.
Indeed, it is estimated that 60–90% of cancer patients report some degree of mobility and independence problems; therefore, cancer rehabilitation, performed in its various forms and settings, may help enhancing physical, psychological functioning, and social interactions, providing a better QoL [78, 79].
During the disease, due to BC itself or to medical/surgical treatments, patients experience a series of impairments and consequent disabilities that require intervention for pain control, mobility improvement, lymphedema, and restoration of independence in activities of daily living (ADL) [80].
Rehabilitation is a multidisciplinary process that involves different healthcare professionals, such as a physiatrist, physical therapist, and occupational therapist [81, 82]. The care plan begins with a multidimensional assessment, which identifies the problems in terms of impairments and disabilities, proceeding to formulating an individual rehabilitation plan (IRP) based on a biopsychosocial approach, according to the WHO-International Classification of Functioning, Disability, and Health (ICF) framework [83]. For practical purposes, it has proposed ICF Core Sets, namely, the salient ICF categories/codes of functioning to be rated in all patients with BC to guide multidisciplinary assessments of impairments of body structure (e.g., increased volume of upper limb treated with lymphadenectomy) and/or body functions (e.g., pain of upper limb treated with lymphadenectomy), limitations in ADL and restrictions in social participation (e.g., lifting and carrying objects), including the contextual factors involved in the management of this population (e.g., caregiver burden) [83]
Table 1 depicts an example of the operative procedure of the rehabilitation approach for a BC patient. In fact, it is possible using the ICF, to individually approach rehabilitation of the patients, depending on the individual health and health-related domains regarding individually functioning and disability.
Table 1.
Example of rehabilitation procedures for BC patients
| ICF code | ICF category | Outcome measure | Rehabilitation intervention | Expected outcome |
|---|---|---|---|---|
| b28014 | Sensation of pain in upper limb | Numerical Rating Scale (NRS) | Analgesics and transcutaneous electrical nerve stimulation (TENS) | Pain relief |
| b710 | Mobility of joint functions | Active and Passive Range of Motion (ROM) | Flexibility exercises (e.g., ROM exercises and joint mobilization) | Mobility and motility improvements |
| b730 | Muscle power functions | Manual muscle testing (MMT) | Resistance/strengthening exercise | Improvement of muscle strength |
| s730 | Structure of upper extremity | Tape measure circumference | Complete decongestive therapy (CDT) | Reduction of upper limb circumference |
| d430 | Lifting and carrying objects | Disabilities of the Arm, Shoulder, and Hand (DASH) Questionnaire | Flexibility exercises, Resistance/strengthening exercise, Occupational Therapy (OT) | Improvement in performing daily activities involving the upper limb (e.g., lifting a cup or carrying a box from one room to another) |
| d445 | Hand and arm use | DASH Questionnaire, Barthel Index (BI) | Flexibility exercises, resistance/strengthening exercise, OT | Improvement in performing daily activities involving the upper limb (e.g., turning door handles or catching an object) |
| d640 | Doing housework | Eastern Cooperative Oncology Group (ECOG) Performance Status, BI | Flexibility exercises, resistance/strengthening exercise, OT | Improvement in managing household (e.g., washing clothes, storing food and disposing of garbage, tidying rooms, closets and drawers; collecting, washing, drying, folding and ironing clothes) |
| e310 | Immediate family | Caregiver Strain Index (CSI) | Educational program to improve skills in meal support, nursing care, welfare care, or symptom management | Reduction of the level of stress of family caregiver (e.g., parents and partner) |
| e580 | Health services, systems, and policies | Rehabilitation Complexity Scale (RCS) | Care coordination | Facilitation of the access to multidisciplinary health services to better manage health-related issues |
Rehabilitation interventions are indicated in several moments of the care pathway of BC patients. For instance, primary prevention refers to measures designed to avoid the development of risk factors or disease occurrence, with PA that is highly recommended for these purposes [84, 85].
A specific role is recognized in rehabilitation for women undergoing surgery, namely, “pre-habilitation” [86]. This approach should be implemented as soon as the surgical indication has been established to improve not only the short-medium term functional items but also the surgical outcomes in terms of clinical, functional, psychological, and post-operative complications [87, 88]. Pre-habilitation has also a key role in women who will undergo radio- and/or CHT, which is known to lead to significant musculoskeletal impairments, including widespread pain (e.g., joint pain and myalgia), muscle wasting or cachexia, bone fragility, and cardiorespiratory deconditioning [89, 90].
Rehabilitation programs are both useful in hospitalized and outpatient settings, as reported by several studies with significant improvements in functioning, cognitive status, QoL, and social participation [91].
The COVID-19 pandemic has led to the rapid development of telerehabilitation for BC patients, which in a recent systematic review provided not otherwise possible access to care and reduced cancer-related disability [92].
After the conclusion of the rehabilitation phase, the patient can start a physical activity program, along with a dedicated nutrition and exercise plan. Indeed, PA is well recognized as a safe, effective, and feasible intervention strategy that can reduce the number and severity of BC treatment-related side effects and symptoms (e.g. pain, fatigue, sleep disturbances, and cognitive impairment, neurological disorders), with a clear positive impact on survival [93, 94]. It was demonstrated that patients meeting or exceeding the minimum PA guidelines of ≥ 150 min of moderate to vigorous PA (MVPA) per week before and at 1 and 2 years after BC diagnosis experienced statistically significant reductions in hazards of recurrence and mortality [93]. Moreover, PA has also been reported to reduce the likelihood of metastatic spreading [95] and is associated with numerous other health benefits among BC survivors, including weight loss or maintenance, reduced depression and anxiety, management of post-treatment symptoms, mitigation of cancer-related fatigue, improved social support, and QoL [94]. The potential biological mechanisms with protective effects on BC include a reduction of sex and metabolic hormones, adipokines and oxidative stress, improvement of the immune function [95, 96]. Most health benefits induced by PA are associated with body mass index (BMI) and weight loss, indicating that the level of adiposity and the percentage of fat mass are critical indicators in determining the effect of exercise [97] Moreover, postmenopausal women treated with aromatase inhibitors (AIs) might experience severe arthralgias, osteopenia and osteoporosis, depression, hypertension, and sexual dysfunction [98], reported to be grade 3 or higher in up to one third of BC patients. This affects QoL [99] and might be positively modulated by a correct PA approach. Despite this knowledge, the level of PA is low among BC patients, with more than one-third of this population that prefers spending prolonged time sitting or participating in little or no leisure-time PA (LTPA) [100, 101]. Therefore, it is essential to introduce a qualified kinesiologist as part of the clinical team who can help start and maintain a safe exercise program by operating on behavior change strategies and administering an adapted PA (APA) plan [102]. Exercise prescription based on Frequency, Intensity, Time, Type (FITT) parameters should be preceded by an assessment of all components of health-related fitness (i.e., cardiorespiratory fitness, muscular strength and endurance, body composition, and flexibility) to create a personalized exercise program considering the individual needs of patients based on general recommendations [103].
The most recent PA guidelines for BC survivors emphasize the importance to “avoid inactivity” and be as physically active as possible, and recommend performing moderate-intensity aerobic training at least three times per week, for at least 30 min, two or more days a week of resistance training using two sets of 8 to 15 repetitions at least 60% of one repetition maximum (1RM), and daily stretching of major muscle groups, with specific exercise modifications based on health and cancer status and treatment-related side effects [103].
Despite most of the available literature has proposed effective exercise programs dealing mostly with aerobic exercise and/or resistance training using hand-held or machine weights or therabands, a growing body of studies has investigated the effects of innovative practices for BC patients including Pilates, Yoga, water-based exercise, Tai Chi, dragon boating, recreational sports, wall/rock climbing, and triathlon [103, 104], suggesting a potential additive positive effect on several issues.
A recent systematic review on the role of yoga in symptoms management for cancer survivors reported improved fatigue and QoL, whereas further research is needed to confirm the effects on improving sleep, depressive symptoms, anxiety/distress, and cancer-related cognitive change [105].
Similarly, mind–body approaches as Pilates and Tai Chi showed significant effects in females with BC in terms of functional capacity, fatigue, flexibility, and QoL improvement compared to a control group [106]. Even body composition, and QoL, as well as muscular, memory, and cognitive functions were improved [107, 108]. As suggested by Costa and colleagues [109], in the management of BC patients, it is crucial to verify the impact of a supervised group exercise intervention to improve aspects related to health, physical functioning, and QoL.
Rogers et al. [110] reported that 3-month PA intervention benefits on health persisted 9 months after the end of the intervention. Adherence and long-term benefits to both PA unsupervised and supervised physical exercise (PE) programs may represent a major issue for cancer survivors.
An intervention that comprises a supervised in-person PE program and active lifestyle recommendations is advisable in BC patients as it appears to be more effective than unsupervised or home-based programs (i.e., more attention, motivation, and reinforcement) [103, 109].
However, some factors such as on-site appointments, travel, time constraints, and lack of trained professionals can increase barriers to accessing physical activity for patients [111]. For these reasons, online PA interventions and wearables or smartphone applications may represent an additional support in the promotion of PA and PE in BC patients [111, 112].
Even though it is difficult to provide a general and definitive exercise prescription for BC patients and survivors, it is reasonable to propose a supervised multi-component PE intervention preferably in person (in condition of poor accessibility, an online supervised intervention may be a valid alternative) that is focused not only on aerobic exercise or a combination between aerobic and resistance exercise, but rather on improving all the components of physical fitness as well as on meditation, posture, core strength, and breathing techniques.
e-PROMs and Digital Narrative Medicine: A Novel Person-Based Framework for Integrative Oncology
A comprehensive assessment of patient needs, habits, resources, difficulties, and expectations is key to foster Integrative Oncology.
Integrating therapies with a dietary plan, exercise programs, and all the different non-pharmacological options requires appropriate methodologies to detect the unique and unrepeatable subjectivity of everyone, associated not only with the psychological profile but also with the social and environmental context of reference.
The emerging paradigm of person-centered medicine has led to a change in the approach and management of health needs, introducing new models and tools for patient engagement in the care process. An important change is represented using the Patient Reported Outcome Measures (PROMs-e-PROMs), related to health status reported directly by the patient, without the mediation of the clinical interview that often leads to underestimating effects [113].
A randomized study conducted in patients undergoing CHT showed clinical benefits, including improved tumor survival, when symptoms reported systematically by the patient using a web-based monitoring system during treatment are taken into consideration in addition to standard reference parameters [114]. A review by ESMO has shown the benefits and provided indications for optimal use [115•].
However, PROMs and e-PROMS remain anchored to a purely clinical model. They do not enter the emotional and social territory of the specific subject that influences quality of life. PROMs gather information and not experiences; they identify mostly problems and not the bio-psychosocial resources of each individual [116].
For Integrative Oncology, the ICF serves as a reference framework not only for the rehabilitation but also for the development of and appropriate listening model. For each individual, the ICF distinguishes between capacity and performance. Capacity is the functioning of a patient assessed with clinical instruments, while performance is the lived experience of the person related to the global environment. Performance can be better or worse than capacity, depending on the personal, family, social, and environmental resources available. Detecting the specific mix of these resources is fundamental.
For this objective, personalized listening can be strengthened by the introduction of narrative medicine [117, 118] which enrich the point of view expressed in a standardized manner, favoring a person-centered approach rather than a disease-centered one.
The development of digital technologies represents an opportunity for the diffusion of narrative medicine in clinical practice, facilitating the listening of subjective needs and demands.
In 2016, the first digital platform designed for constructing narrative medicine pathways was launched in Italy. The platform's functionalities leverage the potential of narrative medicine for doctor-patient communication while preserving the confidentiality of health data. The IRCCS National Cancer Institute “Regina Elena” in Rome was the first to conduct pilot studies in the field of oncology, integrating digital narrative diaries into clinical practice for surgical and chemotherapy/radiotherapy-treated patients.
The results in terms of feasibility and usefulness have been very positive. The strength, as perceived by both clinicians and patients, lies in the ability to share relevant aspects of the patient's experiences and needs that would otherwise go unnoticed [119•, 120].
A new framework called PbC (Person-based Care) has been proposed to integrate e-PROMS and digital narrative medicine in the ongoing Pergiqual study (NCT 05893368) at the Fondazione Policlinico Gemelli in Rome. The approach involves integrating digital narrative listening and quality of life questionnaires (EORTC QLQ C-30 and B-23) to assess the impact of the disease and treatments, with the aim of personalizing the care pathway. The model includes an initial phase of narrative listening using a prompts-guided digital diary, followed by a baseline evaluation using e-PROMs of QoL before the start of therapy. This is followed by ongoing narrative monitoring throughout the course of therapy and follow-up, integrated with repeated assessments of quality of life using e-PROMs over time.
The results of the Pergiqual study will enable the multidisciplinary group to develop a dedicated digital environment that utilizes e-PROMs and narrative medicine in a cohesive patient journey.
Conclusions
Since novel treatment strategies led to impressive survival outcomes for BC patients, QoL in now another key mandatory endpoint in clinical practice.
The chronicization of the disease, increasingly evident for many biological BC subtypes, requires now a multidisciplinary approach to address all the potential sequelae of the disease or therapies side effects, which could affect long-term QoL.
Approaching the patient with a holistic view is a major challenge today. The integrative therapies consider the patient as a whole, addressing not only the physical aspects but also emotional well-being.
This holistic approach can contribute to better QoL, and therefore survival, outcomes.
The “electronic health” (eHealth) technologies are now aiding clinicians to overcome physical barriers and distances, but more work is needed to ascertain and optimize effectiveness, measure long-term effects.
Moreover, in recent years, liquid biopsy and circulating biomarkers helped address medical needs by providing a non-invasive tool for patient in course of treatments and during follow-up. Although their role in BC prognosis has now been deeply evaluated [121], it is still not clear wheter this new non-invasive approach could be useful to address molecular benefits of holistic approaches on QoL and how can we integrate these techniques in machine-learning programmes [122].
Our manuscript focused on the need of an integrated management, from oncologic intervention to nutrition, physical activity, and rehabilitation. Interesting new models and tools for patient engagement in the care process, such as digital narrative medicine, can capture the patient’s experiences and its emotional needs. Continuous evaluation of new approaches will further help improving the management of this tumor but also detect early discomfort and maintain well-being of all our patients.
Author Contribution
Conceptualization: FA. and MS. Data curation: FA, MS, and RA. Methodology: FA, MS, and RA. Project administration: FA, MS, and RA. Supervision: FA, MS, and RA. Validation: FA, MS, RAME, CL, BV, CC, MS, BV, MA, BZM, IG, BC, FE, BA, PI, and SG. Original draft: FA, MS, RA ME, CL, BV, CC, MS, BV, MA, BZM, IG, BC, FE, BA, PI, and SG. Review and editing: FA, MS, and RA.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. AR was supported by a fellowship by Italian Ministry of Research (grant number PON ARS01 00693) to SM. LC was supported by a fellowship by Italian Ministry of Research (grant number PON). Research was also supported by co-funding of the European Union-NRRP–Mission 4 Component 2 Investment 1.3-NextGenerationEU Project PE 00000003-ON Foods-Research and innovation network on food and nutrition Sustainability, Safety and Security-Working ON Food–CUP D93C22000890001-RTDA: EM.
Data Availability
As a review article, all data and materials are directly available from the authors upon specific request.
Declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiralerspong S, Goodwin PJ. obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Solis MA, Maya-Nuñez G, Casas-González P, et al. Effects of the lifestyle habits in breast cancer transcriptional regulation. Cancer Cell Int. 2016;13(16):7. doi: 10.1186/s12935-016-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiocchetti M, Bastari G, Cipolletti M, et al. The peculiar estrogenicity of diethyl phthalate: modulation of estrogen receptor Α activities in the proliferation of breast cancer cells. Toxics. 2021;9:237. doi: 10.3390/toxics9100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darbre PD. Endocrine disrupting chemicals and breast cancer cells. Adv Pharmacol. 2021;92:485–520. doi: 10.1016/bs.apha.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Sarhangi N, Hajjari S, Heydari SF, et al. Breast cancer in the era of precision medicine. Mol Biol Rep. 2022;49(10):10023–10037. doi: 10.1007/s11033-022-07571-2. [DOI] [PubMed] [Google Scholar]
- 8.Schmid P, Cortes J, Pusztai L, McArthur H, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 9.Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 10.von Minckwitz G, Huang C-S, Mano MS, Loibl S, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 11.Masuda N, Lee S-J, Ohtani S, Im Y-H, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Procter M, de Azambuja E, Zardavas D, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geyer CE, Jr, Garber JE, Gelber RD, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33(12):1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston SRD, Toi M, O’Shaughnessy J, Rastogi P, et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. The Lancet Oncology. 2022 doi: 10.1016/S1470-2045(22)00694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortes J, Rugo HS, Cescon DW, Im S-A, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217–226. doi: 10.1056/NEJMoa2202809. [DOI] [PubMed] [Google Scholar]
- 16.Emens LA, Adams S, Barrios CH, Diéras V, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32(8):983–993. doi: 10.1016/j.annonc.2021.05.355. [DOI] [PubMed] [Google Scholar]
- 17.Swain SM, Miles D, Kim S-B, Im Y-H, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 18.Hurvitz SA, Hegg R, Chung W-P, Im S-A, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2022 doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 19.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 20.Di Lauro V, Barchiesi G, Martorana F, et al. Health-related quality of life in breast cancer patients treated with CDK4/6 inhibitors: a systematic review. ESMO Open. 2022;7(6):100629. doi: 10.1016/j.esmoop.2022.100629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertz S, Benjamin C, Girvalaki C, et al. Progression-free survival and quality of life in metastatic breast cancer: the patient perspective. Breast. 2022;65:84–90. doi: 10.1016/j.breast.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JC, Ligibel JA. Lifestyle interventions for breast cancer prevention. Curr Breast Cancer Rep. 2018;10(3):202–208. doi: 10.1007/s12609-018-0281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orman A, Johnson DL, Comander A, Brockton N. Breast cancer: a lifestyle medicine approach. Am J Lifestyle Med. 2020;14(5):483–494. doi: 10.1177/1559827620913263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayl AE, Meyers CA. Side-effects of chemotherapy and quality of life in ovarian and breast cancer patients. Curr Opin Obs Gynecol. 2006;18:24–28. doi: 10.1097/01.gco.0000192996.20040.24. [DOI] [PubMed] [Google Scholar]
- 25.De Cicco P, Catani MV, Gasperi V, Sibilano M, et al. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019;11(7):1514. doi: 10.3390/nu11071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boltong A, Aranda S, Keast R, Wynne R, et al. A prospective cohort study of the effects of adjuvant breast cancer chemotherapy on taste function, food liking, appetite and associated nutritional outcomes. PLoS ONE. 2014;9:e103512. doi: 10.1371/journal.pone.0103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buch K, Gunmalm V, Andersson M, Schwarz P, et al. Effect of chemotherapy and aromatase inhibitors in the adjuvant treatment of breast cancer on glucose and insulin metabolism-a systematic review. Cancer Med. 2019;8:238–245. doi: 10.1002/cam4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, et al. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic, and lifestyle factors. J Clin Oncol. 2005;23:774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ee C, Cave A, Vaddiparthi V, Naidoo D, Boyages J. Factors associated with weight gain after breast cancer: results from a community-based survey of Australian women. Breast. 2023;S0960–9776(23):00012–17. doi: 10.1016/j.breast.2023.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12(4):282–294. doi: 10.1007/s12282-022-01355-z. [DOI] [PubMed] [Google Scholar]
- 31.Wong RMY, Wong H, et al. The relationship between sarcopenia and fragility fracture-a systematic review. Osteoporos Int. 2019;30(3):541–553. doi: 10.1007/s00198-018-04828-0. [DOI] [PubMed] [Google Scholar]
- 32.World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report 2018. Available at https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018
- 33.Campbell JN, Barton C, Cutress RI, Copson ER. Impact of obesity, lifestyle factors and health interventions on breast cancer survivors. Proc Nutr Soc. 2023;82(1):47–57. doi: 10.1017/S0029665122002816. [DOI] [PubMed] [Google Scholar]
- 34.Meyer D, Pastor-Villaescusa B, Michel S, Hauner H, Hauner D. Associations between circulating obesity-related biomarkers and prognosis in female breast cancer survivors: a systematic review of observational data in women enrolled in lifestyle intervention trials. BMC Cancer. 2022;22(1):1187. doi: 10.1186/s12885-022-10274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lake B, Damery S, Jolly K. Effectiveness of weight loss interventions in breast cancer survivors: a systematic review of reviews. BMJ Open. 2022;12(10):e062288. doi: 10.1136/bmjopen-2022-062288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caprara G, Tieri M, Fabi A, et al. Results of the ECHO (Eating habits CHanges in Oncologic patients) survey: an Italian cross-sectional multicentric study to explore dietary changes and dietary supplement use, in breast cancer survivors. Front Oncol. 2021;11:705927. doi: 10.3389/fonc.2021.705927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porciello G, Montagnese C, Crispo A, Grimaldi M, Libra M, Vitale S, Palumbo E, Pica R, Calabrese I, Cubisino S, Falzone L, Poletto L, Martinuzzo V. Mediterranean diet and quality of life in women treated for breast cancer: a baseline analysis of DEDiCa multicentre trial. PLoS ONE. 2020;15(10):e0239803. doi: 10.1371/journal.pone.0239803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jochems HJS, Van Osch FHM, Bryan TR, et al. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: a systematic review of current epidemiological literature. BMJ Open. 2018;8(2):e014530. doi: 10.1136/bmjopen-2016-014530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi RE, Pericleous M, Mandair D, Whyand T, Caplin ME. The role of dietary factors in prevention and progression of breast cancer. Anticancer Res. 2014;34(12):6861–6875. [PubMed] [Google Scholar]
- 40.Hardt L, Mahamat-Saleh Y, Aune D, Schlesinger S. Plant-based diets and cancer prognosis: a review of recent research. Curr Nutr Rep. 2022;11(4):695–716. doi: 10.1007/s13668-022-00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jabbari M, Pourmoradian S, Eini-Zinab H, Mosharkesh E, Hosseini Balam F, Yaghmaei Y, Yadegari A, Amini B, Moghadam DA, Barati M, Hekmatdoost A. Levels of evidence for the association between different food groups/items consumption and the risk of various cancer sites: an umbrella review. Int J Food Sci Nutr. 2022;73(7):861–874. doi: 10.1080/09637486.2022.2103523. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Chung M, Park Y. Association of fermented products with risk of cancer recurrence and mortality among breast cancer survivors: a prospective cohort study. Nutr Cancer. 2023;12:1–11. doi: 10.1080/01635581.2023.2186259. [DOI] [PubMed] [Google Scholar]
- 43.Sasanfar B, Toorang F, Mohebbi E, Zendehdel K, Azadbakht L. Dietary carbohydrate quality and risk of breast cancer among women. Nutr J. 2021;20(1):93. doi: 10.1186/s12937-021-00752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freudenheim JL. Alcohol’s effects on breast cancer in women. Alcohol Res. 2020;40(2):11. doi: 10.35946/arcr.v40.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald JA, Goyal A, Terry MB. Alcohol intake and breast cancer risk: weighing the overall evidence. Curr Breast Cancer Rep. 2013;5(3). 10.1007/s12609-013-0114-z. [DOI] [PMC free article] [PubMed]
- 46.De Cicco P, et al. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019;11:1514. doi: 10.3390/nu11071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford KL. CE Orsso, N Kiss, SB Johnson, SA Purcell, A Gagnon, A Laviano, CM Prado Dietary choices after a cancer diagnosis: a narrative review. Nutrition. 2022;103–104:111838. doi: 10.1016/j.nut.2022.111838. [DOI] [PubMed] [Google Scholar]
- 48.Donini LM, Barrada JR, Barthels F, et al. A consensus document on definition and diagnostic criteria for orthorexia nervosa. Eat Weight Disord. 2022;27(8):3695–3711. doi: 10.1007/s40519-022-01512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aslan H, Aktürk Ü. Demographic characteristics, nutritional behaviors, and orthorexic tendencies of women with breast cancer: a case-control study. Eat Weight Disord. 2020;25(5):1365–1375. doi: 10.1007/s40519-019-00772-y. [DOI] [PubMed] [Google Scholar]
- 50.Mogal HD, Howard-McNatt M, Dodson R, Fino NF, Clark CJ. Quality of life of older African American breast cancer survivors: a population-based study. Support Care Cancer. 2017;25(5):1431–1438. doi: 10.1007/s00520-016-3539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenlon D, Frankland J, Foster CL, et al. Living into old age with the consequences of breast cancer. Eur J Oncol Nurs. 2013;17(3):311–316. doi: 10.1016/j.ejon.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Coughlin SS, Yoo W, Whitehead MS, Smith SA. Advancing breast cancer survivorship among African-American women. Breast Cancer Res Treat. 2015;153(2):253–261. doi: 10.1007/s10549-015-3548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page AE, Adler NE (Eds.). Cancer care for the whole patient: meeting psychosocial health needs. National Academies Press. 2008. [PubMed]
- 54.Friese CR, Harrison JM, Janz NK, et al. Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer. 2017;123(11):1925–1934. doi: 10.1002/cncr.30547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grant SJ, Hunter J, Seely D, Balneaves LG, Rossi E, Bao T. Integrative oncology: international perspectives. Integr Cancer Ther. 2019;18:1534735418823266. doi: 10.1177/1534735418823266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187–203. doi: 10.1177/1534735411423920. [DOI] [PubMed] [Google Scholar]
- 57.Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. JNCI Monographs. 2014;50:346–358. doi: 10.1093/jncimonographs/lgu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez G, McQuade J, Cohen L, et al. Integrative oncology physician consultations at a comprehensive cancer center: analysis of demographic, clinical and patient reported outcomes. J Cancer. 2017;8(3):395. doi: 10.7150/jca.17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witt CM, Balneaves LG, Cardoso MJ, et al. A comprehensive definition for integrative oncology. J Natl Cancer Inst Monogr. 2017;52. 10.1093/jncimonographs/lgx012. [DOI] [PubMed]
- 60.Gannotta R, Malik S, Chan AY, et al. Integrative Medicine as a Vital Component of Patient Care. Cureus. 2018;10(8):e3098. doi: 10.7759/cureus.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magno S, Filippone A, Accetta C, et al. Lifestyle counselling and access to integrative treatments in Italian breast centres: Senonetwork national survey. Br J Surg. 2022;109(10):1013–1014. doi: 10.1093/bjs/znac166. [DOI] [PubMed] [Google Scholar]
- 62.Mao JJ, Ismaila N, Bao T, et al. Integrative medicine for pain management in oncology: Society for Integrative Oncology-ASCO Guideline. J Clin Oncol. 2022;40(34):3998–4024. doi: 10.1200/JCO.22.01357. [DOI] [PubMed] [Google Scholar]
- 63.Ju ZY, Wang K, Cui HS, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;12:CD012057. doi: 10.1002/14651858.CD012057.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Molassiotis A, Suen LKP, Cheng HL, et al. A randomized assessor-blinded wait-list-controlled trial to assess the effectiveness of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Integr Cancer Ther. 2019;18:1534735419836501. doi: 10.1177/1534735419836501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wardley AM. ACUFOCIN: Randomized clinical trial of ACUpuncture plus standard care versus standard care alone for chemotherapy induced peripheral neuropathy (CIPN); (12003). In. ASCO Virtual Scientific Program: American Societyof Clinical Oncology 2020. J Clin Oncol. 2020;38(15):12003–12003. doi: 10.1200/JCO.2020.38.15_suppl.12003. [DOI] [Google Scholar]
- 66.Bao T, Patil S, Chen C, et al. Effect of acupuncture vs sham procedure on chemotherapy-induced peripheral neuropathy symptoms: a randomized clinical trial. JAMA Netw Open. 2020;3:e200681. doi: 10.1001/jamanetworkopen.2020.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMOeEONSeEANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020; 31 (10). 10.1016/j.annonc.2020.07.003. [DOI] [PubMed]
- 68.Tsay SL, Chen HL, Chen SC, et al. Effects of reflexotherapy on acute postoperative pain and anxiety among patients with digestive cancer. Cancer Nurs. 2008;31:109–115. doi: 10.1097/01.NCC.0000305694.74754.7b. [DOI] [PubMed] [Google Scholar]
- 69.Magno S, Cappai E, Dentale F, et al. Effects of plantar reflexology on sleep and quality of life in patients with breast cancer undergoing chemotherapy and hormonal therapies. J Clin Oncol. 2017;35(15). 10.1200/JCO.2017.35.15_suppl.e21697.
- 70.Hoffman CJ, Ersser SJ, Hopkinson JB, et al. Effectiveness of mindfulness-based stress reduction in mood, breast- and endocrine-related quality of life, and well-being in stage 0 to III breast cancer: a randomized, controlled trial. J Clin Oncol. 2012;30(12):1335–1342. doi: 10.1200/JCO.2010.34.0331. [DOI] [PubMed] [Google Scholar]
- 71.Henderson VP, Clemow L, Massion AO, et al. The effects of mindfulness-based stress reduction on psychosocial outcomes and quality of life in early-stage breast cancer patients: a randomized trial. Breast Cancer Res Treat. 2012;131:99–109. doi: 10.1007/s10549-011-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wurtzen H, Dalton SO, Elsass P, et al. Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer. 2013;49:1365–1373. doi: 10.1016/j.ejca.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 73.Lengacher CA, Reich RR, Paterson CL, et al. Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2016;34:2827–2834. doi: 10.1200/JCO.2015.65.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou K, Li X, Li J, et al. A clinical randomized controlled trial of music therapy and progressive muscle relaxation training in female breast cancer patients after radical mastectomy: results on depression, anxiety and length of hospital stay. Eur J Oncol Nurs. 2015;19:54–59. doi: 10.1016/j.ejon.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Zhang Y, Fan Y, Tan XS, Lei X. Effects of music intervention on the physical and mental status of patients with breast cancer: a systematic review and meta-analysis. Breast Care. 2018;13(3):183–190. doi: 10.1159/000487073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Magno S, Filippone A, Scaldaferri A. Evidence-based usefulness of integrative therapies in breast cancer. Transl Cancer Res. 2018;7:S379–S389. doi: 10.21037/tcr.2018.02.06. [DOI] [Google Scholar]
- 78.Pergolotti M, Alfano CM, Cernich AN, et al. A health services research agenda to fully integrate cancer rehabilitation into oncology care. Cancer. 2019;125(22):3908–3916. doi: 10.1002/cncr.32382. [DOI] [PubMed] [Google Scholar]
- 79.Lyons KD, Padgett LS, Marshall TF, et al. Follow the trail: Using insights from the growth of palliative care to propose a roadmap for cancer rehabilitation. CA Cancer J Clin. 2019;69(2):113–126. doi: 10.3322/caac.21549. [DOI] [PubMed] [Google Scholar]
- 80.Kline-Quiroz C, Nori P, Stubblefield MD. Cancer rehabilitation: acute and chronic issues, nerve injury, radiation sequelae, surgical and chemo-related, Part 1. Med Clin North Am. 2020;104(2):239–250. doi: 10.1016/j.mcna.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 81.Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer rehabilitation: an overview of current need, delivery models, and levels of care. Phys Med Rehabil Clin N Am. 2017;28(1):1–17. doi: 10.1016/j.pmr.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Sharma R, Molinares-Mejia D, Khanna A, Maltser S, Ruppert L, Wittry S, Murphy R, Ambrose AF, Silver JK. Training and practice patterns in cancer rehabilitation: a survey of physiatrists specializing in oncology care. PM R. 2020;12(2):180–185. doi: 10.1002/pmrj.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brach M, Cieza A, Stucki G, et al. ICF Core Sets for breast cancer. J Rehabil Med. 2004;(44 Suppl):121–7. 10.1080/16501960410016811. [DOI] [PubMed]
- 84.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo W, Fensom GK, Reeves GK, et al. Physical activity and breast cancer risk: results from the UK Biobank prospective cohort. Br J Cancer. 2020;122:726–732. doi: 10.1038/s41416-019-0700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Santa Mina D, van Rooijen SJ, Minnella EM, et al. Multiphasic prehabilitation across the cancer continuum: a narrative review and conceptual framework. Front Oncol. 2020;10:598425. doi: 10.3389/fonc.2020.598425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brahmbhatt P, Sabiston CM, Lopez C, Chang E, Goodman J, Jones J, McCready D, Randall I, Rotstein S, Santa MD. Feasibility of prehabilitation prior to breast cancer surgery: a mixed-methods study. Front Oncol. 2020;25(10):571091. doi: 10.3389/fonc.2020.571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toohey K, Hunter M, McKinnon K, Casey T, Turner M, Taylor S, Paterson C. A systematic review of multimodal prehabilitation in breast cancer. Breast Cancer Res Treat. 2023;197(1):1–37. doi: 10.1007/s10549-022-06759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv. 2018;12(1):64–73. doi: 10.1007/s11764-017-0645-9. [DOI] [PubMed] [Google Scholar]
- 90.Di Leone A, Terribile D, Magno S, et al. Neoadjuvant chemotherapy in breast cancer: an advanced personalized multidisciplinary prehabilitation model (APMP-M) to optimize outcomes. J Pers Med. 2021;11(5):324. doi: 10.3390/jpm11050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leclerc AF, Foidart-Dessalle M, Tomasella M, et al. Multidisciplinary rehabilitation program after breast cancer: benefits on physical function, anthropometry and quality of life. Eur J Phys Rehabil Med. 2017;53(5):633–642. doi: 10.23736/S1973-9087.17.04551-8. [DOI] [PubMed] [Google Scholar]
- 92.Brick R, Padgett L, Jones J, et al. The influence of telehealth-based cancer rehabilitation interventions on disability: a systematic review. J Cancer Surviv. Published online February 2022;1–26. 10.1007/s11764-022-01181-4. [DOI] [PMC free article] [PubMed]
- 93.Cannioto RA, Hutson A, Dighe S, et al. Physical activity before, during, and after chemotherapy for high-risk breast cancer: relationships with survival. J Natl Cancer Inst. 2021;113(1):54–63. doi: 10.1093/jnci/djaa046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caperchione CM, Sabiston CM, Stolp S, et al. A preliminary trial examining a 'real world' approach for increasing physical activity among breast cancer survivors: findings from project MOVE. BMC Cancer. 2019;19(1):272. doi: 10.1186/s12885-019-5470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz-Vozmediano J, Löhnchen S, Jurado L, et al. Influence of a multidisciplinary program of diet, exercise, and mindfulness on the quality of life of stage IIA-IIB breast cancer survivors. Integr Cancer Ther. 2020;19:1534735420924757. doi: 10.1177/1534735420924757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Boer MC, Wörner EA, Verlaan D, van Leeuwen PAM. The mechanisms and effects of physical activity on breast cancer. Clin Breast Cancer. 2017;17(4):272–278. doi: 10.1016/j.clbc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 97.Hong BS, Lee KP. A systematic review of the biological mechanisms linking physical activity and breast cancer. Phys Act Nutr. 2020;24(3):25–31. doi: 10.20463/pan.2020.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honma N, Makita M, Saji S, Mikami T, et al. Characteristics of adverse events of endocrine therapies among older patients with breast cancer. Support Care Canc: Off J Multinatl Assoc Support Care Canc. 2019;27(10):3813–3822. doi: 10.1007/s00520-019-04674-8. [DOI] [PubMed] [Google Scholar]
- 99.Ugras SK, Rahman RL. Hormone replacement therapy after breast cancer: Yes. No or maybe. Mol Cell Endocrinol. 2021;525:111180. doi: 10.1016/j.mce.2021.111180. [DOI] [PubMed] [Google Scholar]
- 100.Cao C, Friedenreich CM, Yang L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 2022;8(3):395–403. doi: 10.1001/jamaoncol.2021.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dorri S, Asadi F, Olfatbakhsh A, Kazemi A. A systematic review of electronic health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast cancer (Tokyo, Japan) 2020;27(1):25–46. doi: 10.1007/s12282-019-00982-3. [DOI] [PubMed] [Google Scholar]
- 102.Murri A, Vitucci D, Tranchita E, et al. “OPERATION PHALCO”—adapted physical activity for breast cancer survivors: is it time for a multidisciplinary approach? Cancers. 2022;15(1):34. doi: 10.3390/cancers15010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eyigor S, Kanyilmaz S. Exercise in patients coping with breast cancer: an overview. World J Clin Oncol. 2014;5(3):406–411. doi: 10.5306/wjco.v5.i3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: a review of the evidence base and future directions for research. Cancer. 2019;125(12):1979–1989. doi: 10.1002/cncr.31979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B. Effects of pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: a randomized controlled study. Eur J Phys Rehabil Med. 2010;46(4):481–487. [PubMed] [Google Scholar]
- 107.Sprod LK, Janelsins MC, Palesh OG, et al. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J Cancer Surviv: Res Pract. 2012;6(2):146–154. doi: 10.1007/s11764-011-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Janelsins MC, Davis PG, Wideman L, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11(3):161–170. doi: 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riani Costa LA, Barreto RF, de Leandrini SMM, et al. The influence of a supervised group exercise intervention combined with active lifestyle recommendations on breast cancer survivors’ health, physical functioning, and quality of life indices: study protocol for a randomized and controlled trial. Trials. 2021;22(1):934. doi: 10.1186/s13063-021-05843-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rogers LQ, Courneya KS, Oster RA, et al. Physical activity intervention benefits persist months post-intervention: randomized trial in breast cancer survivors. J Cancer Surviv: Res Pract. 2023 doi: 10.1007/s11764-022-01329-2. [DOI] [PubMed] [Google Scholar]
- 111.Kang H, Moon M. Effects of digital physical activity interventions for breast cancer patients and survivors: a systematic review and meta-analysis. Healthc Inform Res. 2023;29(4):352–366. doi: 10.4258/hir.2023.29.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phillips SM, Penedo FJ, Collins LM, et al. Optimization of a technology-supported physical activity promotion intervention for breast cancer survivors: Results from Fit2Thrive. Cancer. 2022;128(5):1122–1132. doi: 10.1002/cncr.34012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial [published correction appears in J Clin Oncol. 2019;37(6):528] J Clin Oncol. 2016;34(6):557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Maio M, Basch E, Bryce J, Perrone F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol. 2016;13(5):319–325. doi: 10.1038/nrclinonc.2015.222. [DOI] [PubMed] [Google Scholar]
- 115.Di Maio M, Basch E, Denis F, Ripamonti CI, Santini D, on behalf of ESMO Guidelines Committee The role of patient reported outcomes measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann Oncol. 2022;33(9):878–892. doi: 10.1016/j.annonc.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Carfora L, Foley CM, Hagi-Diakou P, et al. Patients’ experiences and perspectives of patient-reported outcome measures in clinical care: a systematic review and qualitative meta-synthesis. PLoS One. 2022;17(4):e0267030. doi: 10.1371/journal.pone.0267030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ciotti S, Bianconi F, Saraceni VM, Vulpiani MC, Rinonapoli G, Caraffa A, Zampolini M. Narrative medicine in amyotrophic lateral sclerosis and a rehabilitation project based on international classification of functioning, disability and health. Am J Phys Med Rehabil. 2018;97(11):832–838. doi: 10.1097/PHM.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 118.Istituto Superiore di Sanità – Centro Nazionale Malattie Rare. Consensus Conference: “Linee di indirizzo per l’utilizzo della medicina narrativa in ambito clinico- assistenziale, per le malattie rare e cronico-degenerative”. Sole24Ore Sanità, Milano. 2015. www.iss.it/cnmr.
- 119.• Cercato MC, Colella E, Fabi A, Bertazzi I, Giardina BG, Di Ridolfi P, Mondati M, Petitti P, Bigiarini L, Scarinci V, Franceschini A, Servoli F, Terrenato I, Cognetti F, Sanguineti G, Cenci C. Narrative medicine: feasibility of a digital narrative diary application in oncology. J Int Med Res. 2022;50(2):3000605211045507. 10.1177/03000605211045507. This study paved the way for a new concept of quality of life measurement. The digital narrative medicine permits to go beyond the classic PROMs, as it helps combining the physical symptoms with emotional distress to provide a better tailored psychophysical profile of our patients. [DOI] [PMC free article] [PubMed]
- 120.Cercato MC, Vari S, Maggi G, Faltyn W, Onesti CE, Baldi J, Scotto di Uccio A, Terrenato I, Molinaro C, Scarinci V, Servoli F, Cenci C, Biagini R, Ferraresi V. Narrative medicine: a digital diary in the management of bone and soft tissue sarcoma patients. Preliminary Results of a Multidisciplinary Pilot Study. J Clin Med. 2022;11(2):406. 10.3390/jcm11020406. [DOI] [PMC free article] [PubMed]
- 121.Mazzitelli C, Santini D, Corradini AG, et al. Liquid biopsy in the management of breast cancer patients: where are we now and where are we going. Diagnostics (Basel) 2023;13(7):1241. doi: 10.3390/diagnostics13071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ling L, Aldoghachi AF, Chong ZX, et al. Addressing the clinical feasibility of adopting circulating mirna for breast cancer detection, monitoring and management with artificial intelligence and machine learning platforms. Int J Mol Sci. 2022;23(23):15382. doi: 10.3390/ijms232315382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As a review article, all data and materials are directly available from the authors upon specific request.