Abstract
Background
Current evidence concerning bowel preparation before elective colorectal surgery is still controversial. This study aimed to compare the incidence of anastomotic leakage (AL), surgical site infections (SSIs), and overall morbidity (any adverse event, OM) after elective colorectal surgery using four different types of bowel preparation.
Methods
A prospective database gathered among 78 Italian surgical centers in two prospective studies, including 6241 patients who underwent elective colorectal resection with anastomosis for malignant or benign disease, was re-analyzed through a multi-treatment machine-learning model considering no bowel preparation (NBP; No. = 3742; 60.0%) as the reference treatment arm, compared to oral antibiotics alone (oA; No. = 406; 6.5%), mechanical bowel preparation alone (MBP; No. = 1486; 23.8%), or in combination with oAB (MoABP; No. = 607; 9.7%). Twenty covariates related to biometric data, surgical procedures, perioperative management, and hospital/center data potentially affecting outcomes were included and balanced into the model. The primary endpoints were AL, SSIs, and OM. All the results were reported as odds ratio (OR) with 95% confidence intervals (95% CI).
Results
Compared to NBP, MBP showed significantly higher AL risk (OR 1.82; 95% CI 1.23–2.71; p = .003) and OM risk (OR 1.38; 95% CI 1.10–1.72; p = .005), no significant differences for all the endpoints were recorded in the oA group, whereas MoABP showed a significantly reduced SSI risk (OR 0.45; 95% CI 0.25–0.79; p = .008).
Conclusions
MoABP significantly reduced the SSI risk after elective colorectal surgery, therefore representing a valid alternative to NBP.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04627-6.
Keywords: Colorectal surgery, Mechanical bowel preparation, Oral antibiotics, Anastomotic leakage, Surgical site infections
Introduction
Current practice and recommendations regarding bowel preparation before elective colorectal surgery to reduce the incidence of anastomotic leakage (AL) and surgical site infections (SSIs) remain controversial. Mechanical bowel preparation (MBP), once routinely used, may cause preoperative dehydration, electrolyte disturbance, and discomfort, and failed to demonstrate any clear benefit over no bowel preparation (NBP) [1–5]. European [6] and Italian [7] enhanced recovery after surgery (ERAS) societies’ guidelines currently recommend NBP, albeit leaving room for oral antibiotics (oA) alone or in combination with MBP [8]. At the same time, results of large retrospective population-based studies of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) suggested that MBP combined with oral antibiotics (MoABP) significantly decreased the rates of SSIs and overall morbidity (OM) compared to NBP [9–13], inducing four large North-American societies (The American Society of Colon and Rectal Surgeons, the Society of American Gastrointestinal and Endoscopic Surgeons, the American Society for Enhanced Recovery, and the Perioperative Quality Initiative) to recommend MoABP [14–16]. As a consequence, the use of MoABP is currently reported by 50% of Austrian–German [17] and by 80% of North American [18] surgeons. During the last 8 years, one RCT was launched comparing NBP with MoABP [19], two MoABP with oA [20, 21], and one MoABP with MBP for rectal cancer [22]. To the best of our knowledge, only one [22] of these trials recently completed the planned enrollment and none published its final results yet [23]. An interesting four-arm RCT comparing NBP with oA, MBP, and MoABP for colon resections [24] was recently closed before completion due to poor accrual. Meanwhile, one RCT comparing NBP with MoABP [25] failed to detect significant differences in SSIs and AL rates but was largely underpowered; oA showed a significant reduction of SSI rates in two RCTs, either alone [26, 27] or combined with MBP [26], and an international multicenter RCT comparing oA with MoABP [28] is currently still recruiting. Finally, one RCT reported that MoABP significantly reduced SSI rates compared to MBP after colorectal resections [29], and another that MoABP significantly reduced both SSI and AL rates compared to MBP after rectal resections [30].
Very recently, the European Association of Endoscopic Surgery, the European Society of ColoProctology, and the Society of American Gastrointestinal and Endoscopic Surgeons published a joint guideline [31] based on a previous systematic review and network meta-analysis [32], with a conditional recommendation for MoABP, supported by low-quality evidence due to variable adherence to preoperative intravenous antibiotic prophylaxis (PIVAP) and great heterogeneity regarding oA schedules [33].
The relevant heterogeneity of the available evidence induced the Italian ColoRectal Anastomotic Leakage (iCral) study group to estimate the effects of NBP in patients treated with PIVAP before elective colorectal surgery (treatment variable) in comparison to three other treatments (oA, MBP, MoABP) on a large dataset derived from two prospective multicenter open-label observational studies [34, 35]. Several recent studies of propensity score estimation showed that machine learning methods outperform logistic regression models with iterative variable sections in terms of bias reduction and mean-squared error [36] and may be advantageous in multiple treatment settings [37]. Therefore, a multi-treatment analysis based on machine learning procedures was used to compare four bowel preparation modalities before elective colorectal surgery.
Methods
Study design, participants, and setting
This was a secondary unplanned ad hoc multi-treatment re-analysis of two prospective cohorts of patients who had undergone colorectal surgery for malignant and benign diseases based on machine-learning procedures. A total of 8359 patients who underwent colorectal resection with anastomosis were enrolled in two consecutive studies upon explicit inclusion/exclusion criteria in 78 surgical centers in Italy from January 2019 to September 2021: iCral2 [34] and iCral3 [35].
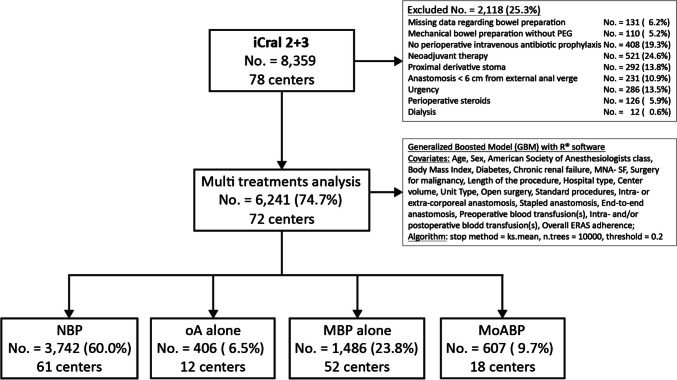
To control for data imbalance derived from several treatment confounders, the present analysis included 6241 patients (74.7%) out of 8359 available in the parent studies, based on explicit exclusion criteria (Fig. 1). Any record with missing information regarding preoperative bowel preparation or with MBP performed using anything different from polyethylene glycol (PEG) was excluded; patients treated without PIVAP were excluded considering its significant impact on the risk of SSIs [23]; delayed urgencies were excluded because this study is focused on elective resections; any anastomosis protected by a proximal stoma and patients treated with neo-adjuvant therapy, perioperative steroids, or dialysis were excluded because these treatments were impacting only on subgroups of subjects; patients treated by anterior resection with anastomosis at less than 6 cm from the anal verge and without protective stoma were excluded in relation to the significant impact of this procedure on the risk of AL. The study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology statement [39] and checklist (online supplemental material).
Fig. 1.
Study flowchart. PEG, polyethylene glycol; MNA-SF, mini nutritional assessment–short form [38]; ERAS, enhanced recovery after surgery; NBP, no bowel preparation; oA, oral antibiotics; MBP, mechanical bowel preparation; MoABP, mechanical bowel preparation and oral antibiotics
Four different treatment groups were considered: (a) no mechanical bowel preparation and no oral antibiotics (NBP; No. = 3742; 60.0%); (b) oral antibiotics alone (oA; No. = 406; 6.5%); (c) mechanical bowel preparation alone (MBP; No. = 1486; 23.8%); (d) mechanical bowel preparation and oral antibiotics (MoABP; No. = 607; 9.7%). All patients in the MBP and MoABP groups received products containing PEG on the day before surgery. Patients in the oAB and MoABP groups received several different oral antibiotic schedules, the majority of which contained metronidazole (Table 1).
Table 1.
Oral antibiotics schedules in the oA and MoABP groups
| Oral antibiotic(s) | Administration schedule | oA (406 pts.) | MoABP (607 pts.) | p* | ||
|---|---|---|---|---|---|---|
| No | % | No | % | |||
|
Metronidazole (500 mg) Paromomycin (250 mg) |
Started 2 days preop., TID Started 2 days preop., BID |
118 | 29.1 | 29 | 4.8 | .006 |
|
Metronidazole (500 mg) Cefazolin (2000 mg) |
Started 1 day preop., TID Started 1 day preop., OD |
76 | 18.7 | 50 | 8.2 | .102 |
|
Metronidazole (500 mg) Trimethoprim (160 mg)/sulfamethoxazole (800 mg) |
Started 1 day preop., TID Started 1 day preop., TID |
68 | 16.7 | 61 | 10.0 | .267 |
|
Metronidazole (500 mg) Neomicin plus bacitracin (300 mg) |
Started 1 day preop., TID Started 1 day preop., TID |
47 | 11.6 | 6 | 0.9 | .419 |
|
Metronidazole (500 mg) Amoxicilline (1000 mg) |
Started 3 days preop., BID Started 3 days preop., BID |
25 | 6.2 | 5 | 0.8 | .623 |
|
Metronidazole (250 mg) Ciprofloxacin (500 mg) |
Started 1 day preop., TID Started 1 day preop., BID |
20 | 4.9 | 21 | 3.5 | .823 |
|
Metronidazole (500 mg) Rifaximin (400 mg) |
Started 7 days preop., TID Started 7 days preop., BID |
5 | 1.2 | 9 | 1.5 | .963 |
|
Metronidazole (250 mg) Amoxicilline (1000 mg) |
Started 1 day preop., BID Started 1 day preop., BID |
0 | 0 | 50 | 8.2 | n.e |
|
Metronidazole (250 mg) Rifaximin (200 mg) |
Started 1 day preop., TID Started 1 day preop., BID |
3 | 0.8 | 0 | 0 | n.e |
|
Metronidazole (500 mg) Rifaximin (200 mg) |
Started 1 day preop., BID Started 1 day preop., BID |
0 | 0 | 68 | 11.2 | n.e |
|
Metronidazole (1000 mg) Rifaximin (400 mg) |
Started 1 day preop., TID Started 1 day preop., TID |
0 | 0 | 11 | 1.8 | n.e |
|
Metronidazole (500 mg) Paromomycin (500 mg) Rifaximin (400 mg) |
Started 1 day preop., BID Started 1 day preop., BID Started 1 day preop., BID |
0 | 0 | 126 | 20.8 | n.e |
| Paromomycin (250 mg) | Started 4 days preop., QID | 44 | 10.8 | 0 | 0 | n.e |
| Paromomycin (1000 mg) | Started 1 day preop., OD | 0 | 0 | 37 | 6.1 | n.e |
| Rifaximin (400 mg) | Started 1 day preop., TID | 0 | 0 | 102 | 16.8 | n.e |
| Amoxicillin (1000 mg) | Started 3 days preop., TID | 0 | 0 | 17 | 2.8 | n.e |
| Neomicin plus bacitracin (300 mg) | Started 1 day preop., TID | 0 | 0 | 15 | 2.5 | n.e |
*OD once daily, BID 2 times per day, TID 3 times per day, (QID) 4 times per day, preop., preoperatively, n.e., test not executable because there are cells with insufficient values
at test for proportions comparison, oA oral antibiotics
bMoABP mechanical bowel preparation plus oral antibiotics
Clinical data
The parent studies recorded both continuous and discrete variables related to biometric data, patient information, indication and type of surgical procedure, adherence to ERAS program items, and outcomes. Local investigators ensured data quality control, which was validated by the study coordinator, resolving any discrepancies through strict cooperation. Perioperative care was provided by local investigators, who were left free to decide on any complimentary imaging and/or any further action according to local criteria.
The descriptive variables considered in the 6241 patients are shown in Table 2. Continuous variables were categorized according to their median values to optimize the effectiveness of the analysis by reducing the number of unmatched cases.
Table 2.
Descriptive analysis of the variables considered in the 6241 patients before matching
| NBP | oA | MBP | MoABP | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. = 3742 | No. = 406 | No. = 1486 | No. = 607 | |||||||
| Variable | Pattern | No | % | No | % | No | % | No | % | p |
| Age (years) | ≤ 70 | 1863 | 49.8 | 203 | 50.0 | 882 | 59.4 | 342 | 56.3 | < .001 |
| > 70 | 1879 | 50.2 | 203 | 50.0 | 604 | 40.6 | 265 | 43.7 | ||
| Sex | Male | 1949 | 52.1 | 209 | 51.5 | 682 | 45.9 | 323 | 53.2 | < .001 |
| Female | 1793 | 47.9 | 197 | 48.5 | 804 | 54.1 | 284 | 46.8 | ||
| ASA class | I–II | 2402 | 64.2 | 255 | 62.8 | 1.028 | 69.2 | 407 | 67.1 | .003 |
| III | 1340 | 35.8 | 151 | 37.2 | 458 | 30.8 | 200 | 32.9 | ||
| Body mass index (Kg/m2) | ≤ 25.15 | 1803 | 48.2 | 234 | 57.6 | 765 | 51.5 | 323 | 53.2 | < .001 |
| > 25.15 | 1939 | 51.8 | 172 | 42.4 | 721 | 48.5 | 284 | 46.8 | ||
| Diabetes | Yes | 565 | 15.1 | 42 | 10.3 | 192 | 12.9 | 81 | 13.3 | .020 |
| No | 3177 | 84.9 | 364 | 89.7 | 1.294 | 87.1 | 526 | 86.7 | ||
| Chronic renal failure | Yes | 154 | 4.1 | 18 | 4.4 | 65 | 4.4 | 27 | 4.4 | .958 |
| No | 3588 | 95.9 | 388 | 95.6 | 1.421 | 95.6 | 580 | 95.6 | ||
| MNA-SF | ≤ 12 | 1971 | 52.7 | 166 | 40.9 | 883 | 59.4 | 309 | 50.9 | < .001 |
| > 12 | 1771 | 47.3 | 240 | 59.1 | 603 | 40.1 | 298 | 49.1 | ||
| Surgery for malignancy | Yes | 2713 | 72.5 | 312 | 76.8 | 992 | 66.8 | 427 | 70.3 | < .001 |
| No | 1029 | 27.5 | 94 | 23.2 | 494 | 33.2 | 180 | 29.7 | ||
| Diverticular disease | 535 | 52.0 | 60 | 63.8 | 142 | 28.7 | 107 | 59.4 | ||
| Endometriosis | 17 | 1.6 | 2 | 2.1 | 225 | 45.5 | 0 | 0.0 | ||
| Polyps | 214 | 20.8 | 18 | 19.1 | 47 | 9.5 | 17 | 9.5 | ||
| IBD | 142 | 13.8 | 6 | 6.4 | 16 | 3.3 | 22 | 12.2 | ||
| Other | 121 | 11.8 | 8 | 8.6 | 64 | 13.0 | 34 | 18.9 | ||
| Mini-invasive surgery | No | 431 | 11.5 | 51 | 12.6 | 281 | 18.9 | 62 | 10.2 | < .001 |
| Yes | 3311 | 88.5 | 355 | 87.4 | 1.205 | 81.9 | 545 | 89.8 | ||
| Laparoscopic | 2790 | 84.2 | 317 | 89.3 | 1.006 | 83.5 | 509 | 93.4 | ||
| Robotic | 344 | 10.4 | 15 | 4.2 | 129 | 10.7 | 17 | 3.1 | ||
| Converted | 177 | 5.4 | 23 | 6.5 | 70 | 5.8 | 19 | 3.5 | ||
| Standard procedure | Yes | 3225 | 86.2 | 371 | 91.4 | 1.251 | 84.2 | 488 | 80.4 | < .001 |
| Right colectomy | 1850 | 57.3 | 208 | 56.1 | 360 | 28.8 | 199 | 40.8 | ||
| Left colectomy | 1080 | 33.5 | 133 | 35.8 | 435 | 34.8 | 223 | 45.7 | ||
| Anterior resection | 295 | 9.2 | 30 | 8.1 | 456 | 36.4 | 66 | 13.5 | ||
| No | 517 | 13.8 | 35 | 8.6 | 235 | 15.8 | 119 | 19.6 | ||
| Transverse colectomy | 78 | 15.1 | 10 | 28.3 | 37 | 15.7 | 18 | 15.1 | ||
| Splenic flexure colectomy | 125 | 24.2 | 12 | 34.3 | 50 | 21.3 | 14 | 11.8 | ||
| Hartmann reversal | 84 | 16.3 | 4 | 11.5 | 63 | 26.8 | 12 | 10.1 | ||
| (Sub) total colectomy | 52 | 10.1 | 4 | 11.5 | 26 | 11.1 | 19 | 16.0 | ||
| Other | 178 | 34.3 | 5 | 14.4 | 59 | 25.1 | 56 | 47.0 | ||
| Anastomosis 1 | Intracorporeal | 2581 | 69.0 | 300 | 73.9 | 895 | 60.2 | 432 | 71.2 | < .001 |
| Extracorporeal | 1161 | 31.0 | 106 | 26.1 | 591 | 39.8 | 175 | 28.8 | ||
| Anastomosis 2 | Stapled | 3400 | 90.9 | 354 | 87.2 | 1.317 | 88.6 | 514 | 84.7 | < .001 |
| Handsewn | 342 | 9.1 | 52 | 12.8 | 169 | 11.4 | 93 | 15.3 | ||
| Anastomosis 3 | End-to-end | 1464 | 39.1 | 164 | 40.4 | 935 | 62.9 | 293 | 48.3 | < .001 |
| Other shape | 2278 | 60.9 | 242 | 59.6 | 551 | 37.1 | 314 | 51.7 | ||
| Operation length (minutes) | ≤ 175 | 1965 | 52.5 | 236 | 58.1 | 628 | 42.3 | 364 | 60.0 | < .001 |
| ˃ 175 | 1777 | 47.5 | 170 | 41.9 | 858 | 57.7 | 243 | 40.0 | ||
| Hospital type | Met./Ac | 2267 | 60.1 | 257 | 63.3 | 769 | 51.7 | 516 | 85.0 | < .001 |
| Local/Regional | 1475 | 39.4 | 149 | 36.7 | 717 | 48.3 | 91 | 15.0 | ||
| Unit type | Colorectal/oncologic | 470 | 12.6 | 22 | 5.4 | 490 | 33.0 | 144 | 23.7 | < .001 |
| General | 3272 | 87.4 | 384 | 94.6 | 996 | 67.0 | 463 | 76.3 | ||
| Center volume | < 4 cases/month | 887 | 23.7 | 136 | 33.5 | 449 | 30.2 | 221 | 36.4 | < .001 |
| ≥ 4 cases/month | 2855 | 76.3 | 270 | 66.5 | 1.037 | 69.8 | 386 | 63.6 | ||
| Preoperative BT(s) | Yes | 234 | 6.2 | 17 | 4.2 | 68 | 4.6 | 26 | 4.3 | .023 |
| No | 3508 | 93.8 | 389 | 95.8 | 1.418 | 95.4 | 581 | 95.7 | ||
| Intra/postoperative BT(s) | Yes | 242 | 6.5 | 15 | 3.7 | 95 | 6.4 | 43 | 7.1 | .141 |
| No | 3500 | 93.5 | 391 | 96.3 | 1.391 | 93.6 | 564 | 92.9 | ||
| Overall ERAS adherence (%) | ≤ 73.68 | 1271 | 34.0 | 88 | 21.7 | 1.108 | 74.6 | 209 | 34.4 | < .001 |
| ˃ 73.68 | 2471 | 66.0 | 318 | 78.3 | 378 | 25.4 | 398 | 65.6 | ||
| Nutritional screening | 2780 | 74.3 | 301 | 74.1 | 914 | 61.5 | 410 | 67.6 | ||
| Prehabilitation | 1730 | 46.2 | 228 | 56.2 | 276 | 18.6 | 183 | 30.2 | ||
| Counseling | 2751 | 73.5 | 276 | 68.0 | 733 | 49.3 | 471 | 77.6 | ||
| Immune enhancing nutrition | 1271 | 34.0 | 217 | 53.5 | 268 | 18.0 | 113 | 18.6 | ||
| Antithrombotic prophylaxis | 3585 | 95.8 | 388 | 95.6 | 1.385 | 93.2 | 550 | 90.6 | ||
| Preoperative carbohydrates load | 2505 | 66.9 | 256 | 63.1 | 517 | 34.8 | 326 | 53.7 | ||
| No preanesthesia | 3265 | 87.3 | 293 | 77.2 | 867 | 58.3 | 448 | 73.8 | ||
| Standard anesthesia protocol | 3188 | 85.2 | 396 | 97.5 | 934 | 62.9 | 584 | 96.2 | ||
| Normothermia | 3572 | 95.5 | 398 | 98.0 | 1.211 | 81.5 | 576 | 94.9 | ||
| Goal-directed fluid therapy | 3084 | 82.4 | 359 | 88.4 | 900 | 60.6 | 539 | 88.8 | ||
| PONV prophylaxis | 3370 | 90.1 | 392 | 96.6 | 1.143 | 76.9 | 543 | 89.5 | ||
| Multimodal analgesia | 3448 | 92.1 | 402 | 99.0 | 1.142 | 76.9 | 573 | 94.4 | ||
| No nasogastric tube | 3376 | 90.2 | 391 | 96.3 | 1.127 | 75.8 | 491 | 80.9 | ||
| Minimally invasive surgery | 3311 | 88.5 | 355 | 87.4 | 1.205 | 81.1 | 545 | 89.8 | ||
| No drains | 1525 | 40.7 | 242 | 59.6 | 171 | 11.5 | 178 | 29.3 | ||
| Urinary catheter < 24–48 h | 3096 | 82.7 | 380 | 93.6 | 832 | 56.0 | 484 | 79.7 | ||
| Early mobilization | 2391 | 63.9 | 373 | 91.9 | 391 | 26.3 | 469 | 77.3 | ||
| Early oral feeding | 2286 | 61.1 | 352 | 86.7 | 431 | 29.0 | 374 | 61.6 | ||
| Pre-discharge check | 3275 | 87.5 | 345 | 85.0 | 848 | 57.1 | 503 | 82.9 | ||
NBP no bowel preparation, oA oral antibiotics alone, MBP mechanical bowel preparation alone, MoABP mechanical bowel preparation and oral antibiotics, ASA American Society of Anesthesiologists, MNA-SF mini nutritional assessment–short form, IBD inflammatory bowel disease, Intracorporeal, anastomosis performed under visual control through the scope, Extracorporeal, anastomosis performed under direct visual control through an open access, Met./Ac., Metropolitan/Academic, BT blood transfusion, ERAS: Enhanced recovery after surgery, PONV postoperative nausea/vomiting, p chi square independence test with three degrees of freedom
Outcomes
All the outcomes were calculated at 60 days after surgery. Any adverse event was recorded and graded [40, 41], as well as any reoperation, readmission, or death.
The primary endpoints were AL, defined according to the international consensus criteria [42], SSIs, according to the criteria of the Centers for Disease Control and Prevention/National Healthcare Safety Network (CDC/NHSN) [43], and overall morbidity (OM; any adverse event). The secondary endpoints were superficial and/or deep incisional surgical site infections (sdiSSIs), defined as specific complications including purulent drainage from superficial incisions, positive culture of fluid or tissue from superficial incisions, pain or tenderness, localized swelling, redness, heat, and/or infections involving deep fascial and muscle layers without fascial dehiscence; deep wound dehiscence; abdominal collection/abscess, defined as any intraperitoneal postoperative collection altering the normal postoperative course, requiring either medical, radiological, endoscopic, or surgical intervention [43]; major morbidity (any adverse event grade > II); reoperation (any unplanned operation); mortality (any death).
Ethics
Both studies were conducted in accordance with the Declaration of Helsinki and guidelines for good clinical practice E6 (R2). All enrolled patients signed a consent to be included in the studies. The study protocols were approved by the ethics committee of the coordinating center (Marche Regional Ethics Committee (CERM) 2018/334 released on 11/28/2018 for iCral2 and 2020/192 released on 07/30/2020 for iCral3) and registered at ClinicalTrials.gov (NCT03771456 for iCral2 and NCT04397627 for iCral3). Subsequently, all other centers were authorized to participate in their local ethics committees. Both studies were approved for planned primary and any unplanned secondary analyses; therefore, no further authorization for the current analysis was requested. Individual participant-level anonymized datasets were made available upon reasonable request by contacting the study coordinator.
Statistical analysis
Sample sizes were calculated and reported in the respective core papers [34, 35]. Events per variable guideline were followed [44]. There were no missing data in the database of 6241 patients. The target of estimands was represented by the average treatment effect in the true population of interest (ATT) answering the question “How would the average outcome(s) change if anyone receiving the reference treatment (NBP) had instead received another treatment?” A machine-learning technique, named the Generalized Boosted Model (GBM), was used to estimate the propensity score weights for the binary comparisons between the reference treatment and the other treatment arms. GBM estimation involves an iterative process with multiple regression trees to capture complex and nonlinear relationships between treatment assignment and the covariates without over-fitting the data [37]. The choice of GBM is due to a better balance of the features [37] and to an enhanced bias reduction [35] compared to other multinomial logistic regression models such as inverse probability weighting (IPWT). The analysis was performed using the “twang library” (Toolkit for Weighting and Analysis of Nonequivalent Groups,) of the software “R©” (Version 4.2.2, The R Foundation© for Statistical Computing, Vienna, Austria, 2022). As GBM works iteratively estimating the propensity scores according to the minimization of the distance of the weighted distributions of the covariates given the baseline treatment, balance comparisons have been estimated by performing 10,000 iterations and using the Kolmogorov–Smirnov (KS.mean) metrics with a threshold of 0.2 (a KS-mean difference less than 0.2 typically indicates a negligible difference between the means of the groups) [37]. The KS.mean was preferred based on the availability of a large sample size allowing comparison of the entire distribution rather than just of the mean.
Twenty covariates potentially affecting the four-treatments variable assignments [45] were included in the model (Fig. 1).
For the outcome analysis, weighted logistic regression models for both primary and secondary endpoints defined as dichotomous variables, according to the baseline treatment (NBP) and the other three treatment arms (oA, MBP, and MoABP), were estimated using the “svyglm library” (Survey General Linear Models) of the software “R©” (Version 4.2.2, The R Foundation© for Statistical Computing, Vienna, Austria, 2022). The logistic regression models for the endpoints were adjusted considering the same 20 covariates used in the weight estimation, using a “doubly robust” estimation of the treatment effects [37]. Considering that the primary endpoints were not independent, having been selected based on available evidence [23], a Sidak–Bonferroni adjustment for multiple comparisons/outcomes was applied, calculating α = 0.012. Statistical significance, therefore, was accepted for p values < 0.012. All the instructions used with the software “R©” are available upon reasonable request to the study coordinator.
Results
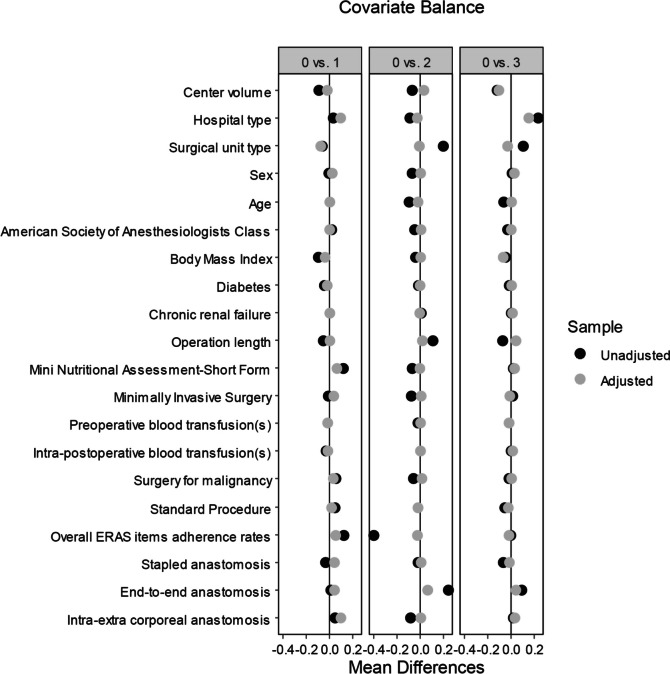
The population of 6241 patients included data deriving from 72 (92.3%) of the original 78 centers. NBP group included data deriving from 61 (84.7%), oA from 12 (16.7%), MBP from 52 (72.2%), and MoABP from 18 (25.0%) of the 72 centers. All the 20 covariates included in the model showed an optimal balance among treatment groups (Fig. 2).
Fig. 2.
Love plot of covariates’ Kolmogorov–Smirnov mean differences before and after adjustment using a machine learning technique, comparing the reference treatment (no bowel preparation, named “0” in the figure) with the other 3 treatments (oral antibiotics alone, named “1”; mechanical bowel preparation alone, named “2”; mechanical bowel preparation and oral antibiotics, named “3”); ERAS, enhanced recovery after surgery
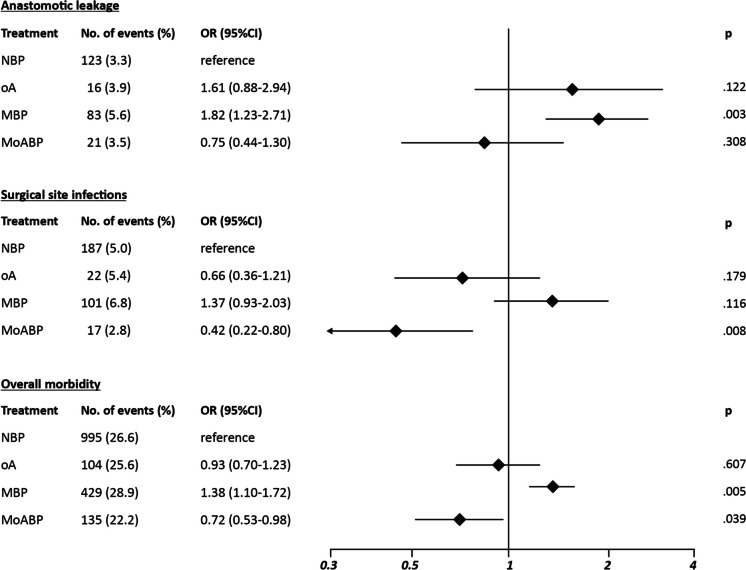
The multi-treatment weighted logistic regression analysis for primary endpoints (Fig. 3) showed the AL risk (3.3% after NBP) to be significantly higher after MBP (5.6%; OR 1.82; 95% CI 1.23–2.71; p = 0.003) and comparable after oA (3.9%) and MoABP (3.5%). The SSI risk (5.0% after NBP) was significantly lower after MoABP (2.8%; OR 0.42; 95% CI 0.22–0.80; p = 0.008) and comparable after oA (5.4%) and MBP (6.8%). The OM risk (26.6% after NBP) was significantly higher after MBP (28.9%; OR 1.38; 95% CI 1.10–1.72; p = 0.005), comparable after oA (25.6%) and MoABP (22.2%).
Fig. 3.
Multi-treatment weighted logistic regression analysis for primary endpoints (log scale); NBP, no bowel preparation; oA, oral antibiotics alone; MBP, mechanical bowel preparation alone; MoABP, mechanical bowel preparation and oral antibiotics
Concerning secondary endpoints (Table 3), no significant differences were recorded concerning the risk of deep wound dehiscence, abdominal collection/abscess, reoperation, and mortality. The risk of sdiSSI (3.3% after NBP) was significantly reduced after MoABP (1.7%; OR 0.29; 95% CI 0.14–0.60; p = 0.001), and the risk of major morbidity (5.3% after NBP) was significantly higher after oA (7.6%; OR 2.07; 95% CI 1.31–3.28; p = 0.002).
Table 3.
Multi-treatment weighted logistic regression analysis for secondary endpoints
| Endpoint/treatment |
NBP (No. = 3742) OR (95% CI); p |
oA (No. = 406) OR (95% CI); p |
MBP (No. = 1486) OR (95% CI); p |
MoABP (No. = 607) OR (95% CI); p |
|---|---|---|---|---|
| sdiSSIs |
3.3% Reference |
2.5% 0.67 (0.33–1.40); p = .285 |
4.9% 1.29 (0.81–2.07); p = .289 |
1.7% 0.29 (0.14–0.60); p = .001 |
| Deep wound dehiscence |
0.2% Reference |
0.7% 3.08 (0.84–11.2); p = .089 |
0.3% 0.75 (0.19–2.96); p = .678 |
0.2% 0.50 (0.06–4.13); p = .521 |
| Abdominal collection/abscess |
1.7% Reference |
0.7% 0.35 (0.08–1.51); p = .157 |
1.8% 1.53 (0.81–2.91); p = .190 |
1.0% 0.54 (0.15–1.88); p = .332 |
| Major morbidity |
5.3% Reference |
7.6% 2.07 (1.31–3.28); p = .002 |
6.7% 1.04 (0.72–1.52); p = .825 |
4.9% 0.71 (0.46–1.12); p = .140 |
| Reoperation |
4.6% Reference |
5.4% 1.48 (0.86–2.53); p = .158 |
6.2% 1.26 (0.86–1.85); p = .230 |
4.5% 0.76 (0.47–1.22); p = .250 |
| Mortality |
0.9% Reference |
0.5% 0.86 (0.21–3.48); p = .833 |
1.0% 1.38 (0.61–3.11); p = .439 |
0.3% 0.62 (0.11–3.38); p = .578 |
NBP no bowel preparation, oA oral antibiotics alone, MBP mechanical bowel preparation alone, MoABP mechanical bowel preparation and oral antibiotics, sdiSSIs superficial and/or deep incisional surgical site infections
All the details regarding the multi-treatment machine learning adjusted comparisons are reported in the online supplemental material.
Discussion
To the best of our knowledge, this is the first multi-treatment propensity score weighting analysis performed using the machine-learning weighted/adjusted regression model to assess different bowel preparation methods before elective colorectal surgery. When conclusive evidence from randomized trials is lacking or when researchers need to assess treatment effects based on real-life data, multiple treatments propensity score weighting analysis based on machine-learning methods performed on data from prospective observational studies offers an alternative approach for estimating treatment effects. The machine learning GBM model adopted in this study provides an improvement in bias reduction and external validity (not reducing the sample size analyzed) in comparison with propensity score-matching analyses between the ATT and the other treatments (three in the present study) and enhances bias reduction in comparison with IPWT [36, 37].
The main finding of the present analysis is that MoABP, compared to NBP, showed a significantly lower SSI risk, with no significant difference concerning the AL risk and a borderline reduction of the OM risk (Fig. 3). As the severity of complications comprised into OM rates may be skewed between groups and not captured by aggregate analysis, a detailed list of adverse events is reported in Table S4 in online supplemental material. This finding remained consistent with the analysis of secondary endpoints, with a significant reduction of the sdiSSI risk, without any significant difference regarding the risks of major morbidity, mortality, and reoperation (Table 3). Although the only available, though largely underpowered, randomized trial comparing NBP with MoABP [25] failed to detect any significant difference regarding SSI rates in the two arms, our results support the findings of the ACS-NSQIP retrospective series [9–13], the North American societies guidelines [14–16], and the most recent European guideline [31] towards the recommendation of MoABP in elective colorectal surgery. However, since both oA and MBP determine deep alterations of gut microbiota with possible impact on SSIs and AL rates [46], and considering that an optimal oral antibiotics administration schedule is far from being established in clinical practice (Table 1), the results of ongoing randomized trials comparing oA alone for colon resection [28] and MBP for rectal resections [22] with MoABP are eagerly awaited.
At the same time, no significant differences were recorded for all the primary endpoints concerning oA (Fig. 3), whereas it determined a significantly higher major morbidity risk (Table 3), possibly linked to a higher, though not significant, rate of major deep wound dehiscence, sdiSSIs, anastomotic leakage, and cardiac dysfunction events (Table S4 in online supplemental material).
Finally, MBP determined significantly higher AL and OM risks (Fig. 3), confirming the available evidence from randomized trials [1–4] and the findings of a recent propensity score-matched comparison of NBP vs. MBP alone performed on a more limited number of cases derived by the iCral database [5]. Considering that MBP alone was still used in nearly one-quarter of our cases, a de-implementation strategy or, according to the preference of some surgeons for a clean colon, a shift towards MoABP is highly advisable.
The main strength of the present study is represented by a large number of prospectively enrolled patients in a well-defined time-lapse in a large number of centers, treated by mini-invasive surgery in more than 80% of cases, representing a wide sample of surgical units performing colorectal resections in Italy. Although the multicenter nature of the data may be a definite source of clustering bias, it is undoubtedly representative of real-life clinical practice. Another strength is represented by its methodology (Fig. 1): (a) a reasoned selection of patients from the parent database was performed upon explicit criteria, limiting data imbalance; (b) the inclusion of 20 covariates into the model allowed to account for the potential clustering bias of multicenter data, for any confounder due to different perioperative pathways, to surgical approach and techniques, to blood transfusion-related morbidity [47], and to patient-related factors; (c) evaluation of the treatments effect through a weighted-adjusted regression model including the same 20 covariates [48]. Although the treatment groups were significantly unbalanced before GBM weighting (Table 2) concerning several well-known risk factors for the endpoints (i.e.,: age, sex, ASA class, nutritional status, minimally invasive surgery, type of resection, type and caseload of the recruiting center), the machine-learning generalized boosted model used in this study markedly improves bias reduction minimizing the distance of the weighted distributions of the 20 covariates (Fig. 2) compared to alternative methods such as IPWT [36, 37].
However, this study has several limitations, and its results should be interpreted with caution: (a) a relevant heterogeneity of oral antibiotic schedules (Table 1), as within and between previously published RCT and related meta-analyses [33]; (b) the exclusion criteria applied to the parent database (Fig. 1) practically excluded any resection performed for low rectal cancer, making the results not applicable to this subgroup of patients; (c) several aspects of health-acquired infections preventive bundle (preoperative whole-body bathing, hair removal, and skin decontamination) and single surgeon’s experience [49] were not measured in the parent studies; (d) finally, further bias from residual unknown factors and potential measurement errors by the participating investigators may have had an impact on the results.
Conclusions
This multi-treatment machine learning analysis, despite the limitations mentioned above, showed that mechanical bowel preparation combined with oral antibiotics significantly reduced the SSI risk after elective colorectal surgery.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
*Collaborators (clinical investigators) of the iCral study group are Paolo Ciano1, MD, Michele Benedetti1, MD, Leonardo Antonio Montemurro1, MD, Marco Clementi2, 3, MD, Elisa Bertocchi4, MD, Gaia Masini4, MD, Amedeo Altamura5, MD, Francesco Rubichi5, MD, Marco Migliore6, MD, Daniele Parlanti7, MD, Gabriele Vago7, MD, Antonio Sciuto8, MD, Ugo Pace9, MD, Andrea Fares Bucci9, MD, Michele Simone10, MD, Diletta Cassini11, MD, Lorenzo Pandolfini12, MD, Alessandro Falsetto12, MD, Ferdinando Ficari13, MD, Francesco Giudici13, MD, Fabio Cianchi13, MD, Alberto Patriti14, MD, Marcella Lodovica Ricci14, MD, Walter Siquini15, MD, Alessandro Cardinali15, MD, Stefano D’Ugo16, MD, PhD, FEBS, FACS, Marcello Spampinato16, MD, PhD, FEBS (HPB), Stefano Scabini17, MD, Alessandra Aprile17, MD, Domenico Soriero17, MD, Marco Caricato18, MD, FACS, Gabriella Teresa Capolupo18, MD, FACS, Giusto Pignata19, MD, Jacopo Andreuccetti19, MD, Ilaria Canfora19, MD, Andrea Liverani20, MD, Giuseppe Lamacchia20, MD, Claudia Franceschilli20, MD,Roberto Campagnacci21, MD, Angela Maurizi21, MD, Pierluigi Marini22, MD, Grazia Maria Attinà22, MD, Ugo Elmore23, MD, Francesco Puccetti23, MD, Francesco Corcione24, MD, Umberto Bracale24, MD, Roberto Peltrini24, MD, Roberto Santoro25, MD, Pietro Amodio25, MD, Massimo Carlini26, MD, FACS, Domenico Spoletini26, MD, PhD, FACS, Rosa Marcellinaro26, MD, Antonio Giuliani27, MD, Giovanni Del Vecchio27, MD, Mario Sorrentino28, MD, Massimo Stefanoni28, MD, Giovanni Ferrari29, MD, Pietro Maria Lombardi29, MD, Alberto Di Leo30, MD, Lorenzo Crepaz30, MD, Augusto Verzelli31, MD, Andrea Budassi31, MD, Giuseppe Sica32, MD, Giulia Bagaglini32, MD, Stefano Rausei33, MD, Silvia Tenconi33, MD, Davide Cavaliere34, MD, Leonardo Solaini34, MD, Giorgio Ercolani34, MD, Gian Luca Baiocchi35, MD, FACS, Sarah Molfino35, MD, Marco Milone36, MD, Giovanni Domenico De Palma36, MD, Giovanni Ciaccio37, MD, Paolo Locurto37, MD, Giovanni Domenico Tebala38, MD, Antonio Di Cintio38, MD, Luigi Boni39, MD, FACS, Elisa Cassinotti39, MD, Stefano Mancini40, MD, Andrea Sagnotta40, MD, PhD, Mario Guerrieri41, MD, Monica Ortenzi41, MD, Roberto Persiani42, MD, Alberto Biondi42, MD, Andrea Lucchi43, MD, FACS, Giulia Vitali43, MD, Dario Parini44, MD, Maurizio De Luca44, MD, Antonino Spinelli45, MD, Francesco Carrano45, MD, Michele Genna46, MD, Francesca Fior46, MD, Vincenzo Bottino47, MD, Antonio Ferronetti47, MD, Andrea Coratti48, MD, Giuseppe Giuliani48, MD, Roberto Benigni48, MD, Dario Scala49, MD, Battistino Puppio49, MD, Alessio Vagliasindi49, MD, Andrea Muratore50, MD, Patrizia Marsanic50, MD, Nicoletta Sveva Pipitone Federico50, MD, Maurizio Pavanello51, MD, Carlo Di Marco51, MD, Umberto Rivolta52, MD, Camillo Leonardo Bertoglio52, MD, PhD, Micaela Piccoli53, MD, FACS, Francesca Pecchini53, MD, Carlo Talarico54, MD, Vincenzo Greco54, MD, Alessandro Carrara55, MD, Michele Motter55, MD, Giuseppe Tirone55, MD, Mauro Totis56, MD, Nicolò Tamini56, MD, Franco Roviello57, MD, Riccardo Piagnerelli57, MD, Alessandro Anastasi58, MD, Giuseppe Canonico58, MD, Gianluca Guercioni59, MD, Simone Cicconi59, MD, Giuseppe Maria Ettorre60, MD, Marco Colasanti60, MD, Mauro Montuori61, MD, Enrico Pinotti61, MD, Pierpaolo Mariani62, MD, Roberta Carminati62, MD, Nicolò de Manzini63, MD, Edoardo Osenda63, MD, Annibale Donini64, MD, Luigina Graziosi64, MD, Mariano Fortunato Armellino65, MD, Ciro De Martino65, MD, Lucio Taglietti66, MD, Arianna Birindelli66, MD, Gabriele Anania67, MD, Matteo Chiozza67, MD, Mariantonietta Di Cosmo68, MD, Daniele Zigiotto68, MD, Carlo Vittorio Feo69, MD, Fioralba Pindozzi69, MD, Paolo Millo70, MD, Manuela Grivon70, MD, Corrado Pedrazzani71, MD, Cristian Conti71, MD, Silvio Guerriero72, MD, Lorenzo Organetti72, MD, Andrea Costanzi73, MD, Michela Monteleone73, MD, Nereo Vettoretto74, MD, Emanuele Botteri74, MD, Federico Marchesi75, MD, Giorgio Dalmonte75, MD, Massimo Basti76, MD, Diletta Frazzini76, MD, Graziano Longo77, MD, Simone Santoni77, MD, Moreno Cicetti78, MD, Gabriele La Gioia78, MD, Giuseppe Brisinda79, MD, Stefano Berti80, MD.
From the 1General Surgery Unit, Sandro Pertini Hospital, ASL Roma 2, Roma; 2General Surgery Unit, University of L’Aquila, L’Aquila; 3Department of Applied Clinical Sciences and Biotechnology, University of L’Aquila, L’Aquila; 4General Surgery Unit, IRCCS Sacro Cuore Don Calabria Hospital, Negrar di Valpolicella (VR); 5General Surgery Unit, Cardinale G. Panico Hospital, Tricase (LE); 6Oncologic Surgery Unit, Candiolo Cancer Institute, FPO-IRCCS, Candiolo (TO); 7General Surgery Unit, Infermi Hospital, Rimini; 8General Surgery Unit, ASL Napoli 2 Nord, Pozzuoli (NA); 9Colorectal Surgical Oncology, Istituto Nazionale per lo Studio e la Cura dei Tumori, “Fondazione Giovanni Pascale IRCCS-Italia”, Naples; 10Department of Surgical Oncology, IRCCS Istituto Tumori “Giovanni Paolo II”, Bari; 11General Surgery Unit, ASST Ovest Milanese, Legnano (MI); 12General Surgery Unit, Santa Maria Annunziata & Serristori Hospital, Florence; 13General Surgery and IBD Unit, Careggi University Hospital, Firenze; 14Department of Surgery, Marche Nord Hospital, Pesaro e Fano (PU); 15General Surgery Unit, S. Lucia Hospital, Macerata; 16General Surgery Unit, “V. Fazzi” Hospital, Lecce; 17General & Oncologic Surgery Unit, IRCCS “San Martino” National Cancer Center, Genova; 18Colorectal Surgery Unit, Policlinico Campus BioMedico, Roma; 192nd General Surgery Unit 2, Spedali Civili di Brescia; 20General Surgery Unit, Regina Apostolorum Hospital, Albano Laziale (RM); 21General Surgery Unit, “C. Urbani” Hospital, Jesi (AN); 22General & Emergency Surgery Unit, San Camillo-Forlanini Hospital, Roma; 23 23Gastrointestinal Surgery Unit, IRCCS San Raffaele Scientific Institute Università Vita-Salute San Raffaele, Milano; 24Minimally Invasive General and Oncologic and Surgery Unit, “Federico II” University, Napoli; 25General Oncologic Surgery Unit, Belcolle Hospital, Viterbo; 26General Surgery Unit, S. Eugenio Hospital, ASL Roma 2, Roma; 27General Surgery Unit, S. Carlo Hospital, Potenza; 28General Surgery Unit, Latisana-Palmanova Hospital, Friuli Centrale University, Udine; 29General Oncologic and Mininvasive Surgery Unit, Great Metropolitan Niguarda Hospital, Milano; 30General and Minimally Invasive Surgery Unit, San Camillo Hospital, Trento; 31General Surgery Unit, Profili Hospital, Fabriano (AN); 32Minimally Invasive Surgery Unit, Policlinico Tor Vergata University Hospital, Roma; 33General Surgery Unit, Gallarate Hospital (VA); 34General & Oncologic Surgery Unit, AUSL Romagna, Forlì (FC); 35General Surgery Unit 3, Department of Clinical and Experimental Sciences, University of Brescia, Brescia; 36General & Endoscopic Surgery Unit, “Federico II” University, Napoli;37General Surgery Unit, S. Elia Hospital, Caltanissetta; 38General Surgery Unit, S. Maria Hospital, Terni; 39General Surgery Unit, Fondazione IRCCS Ca’ Granda, Policlinico Maggiore Hospital, Milano; 40General & Oncologic Surgery Unit, San Filippo Neri Hospital, ASL Roma 1; 41Surgical Clinic, Torrette Hospital, University of Ancona; 42General Surgery Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Roma; 43General Surgery Unit, “Ceccarini” Hospital, Riccione (RN); 44General Surgery Unit, S. Maria della Misericordia Hospital, Rovigo; 45Department of Biomedical Sciences, Humanitas University, Pieve Emanuele (MI) and IRCCS Humanitas Research Hospital, Rozzano (MI); 46General Surgery Unit, University Hospital, Verona; 47General & Oncologic Surgery Unit, Evangelico Betania Hospital, Napoli; 48General and Emergency Surgery Unit, Misericordia Hospital, Grosseto; 49Abdominal Oncologic Surgery Unit, IRCCS CROB Basilicata Referral Cancer Center, Rionero in Vulture (PZ); 50General Surgery Unit, “E. Agnelli” Hospital, Pinerolo (TO); 51General Surgery Unit, AULSS2 Marca Trevigiana, Conegliano Veneto (TV); 52General Surgery Unit, Fornaroli Hospital, ASST Ovest Milanese, Magenta (MI); 53General Surgery Unit, Civil Hospital, Baggiovara (MO); 54General Surgery Unit, Villa dei Gerani Hospital, Vibo Valentia (VV); 551st General Surgery Unit, S. Chiara Hospital, Trento; 56Colorectal Surgery Unit, San Gerardo Hospital, ASST Monza; 57General & Oncologic Surgery Unit, AOU Senese, Siena; 58General Surgery Unit, San Giovanni di Dio Hospital, Firenze; 59General Surgery Unit, “C. e G. Mazzoni” Hospital, Ascoli Piceno; 60General & Transplant Surgery Unit, San Camillo-Forlanini Hospital, Roma; 61General & Mininvasive Surgery Unit, S. Pietro Hospital, Ponte San Pietro (BG); 62General Surgery Unit, Pesenti Fenaroli Hospital, Alzano Lombardo (BG); 63Surgical Clinic, University of Trieste, Trieste; 64General & Emergency Surgery Unit, University of Perugia, Perugia; 65General & Emergency Surgery Unit, S. Giovanni di Dio e Ruggi d’Aragona Hospital, Salerno; 66General Surgery Unit, ASST Valcamonica, Esine (BS); 67General & Laparoscopic Surgery Unit, University Hospital, Ferrara; 68General & Upper GI Surgery Unit, University Hospital, Verona; 69General Surgery Unit, Delta Hospital, University of Ferrara, Lagosanto (FE); 70General Surgery Unit, “U. Parini” Regional Hospital, Aosta; 71General & HPB Surgery Unit, University Hospital, Verona; 72General Surgery Unit, “A. Murri” Hospital, Fermo; 73General Surgery Unit, S. Leopoldo Hospital, Merate (LC); 74General Surgery Unit, Spedali Civili of Brescia, Montichiari (BS); 75Surgical Clinic, University of Parma; 76General Surgery Unit, Spirito Santo Hospital, Pescara; 77General Surgery Unit, Policlinico Casilino, Roma; 78General Surgery Unit, S. Maria della Misericordia Hospital, Urbino (PU); 79General
Surgery Unit, San Giovanni di Dio Hospital, Crotone; 80General Surgery Unit, ASL 5 Liguria POLL, La Spezia; Italy. α Study coordinator.
Author contribution
Marco Catarci (iCral study group coordinator) and Stefano Guadagni (corresponding author) share the first authorship of this paper and are fully accountable for the contents of the submitted manuscript. Conceptualization: Marco Catarci and Stefano Guadagni; investigation and data curation: Marco Catarci, Stefano Guadagni, Francesco Masedu, Giacomo Ruffo, Massimo Giuseppe Viola, Felice Borghi, Gianluca Garulli, Felice Pirozzi, Paolo Delrio, Raffaele De Luca, Gianandrea Baldazzi, Marco Scatizzi; formal analysis, methodology, resources, and software: Marco Catarci, Stefano Guadagni, Francesco Masedu; validation and visualization: Marco Catarci, Stefano Guadagni, Francesco Masedu, Giacomo Ruffo, Massimo Giuseppe Viola, Felice Borghi, Gianluca Garulli, Felice Pirozzi, Paolo Delrio, Raffaele De Luca, Gianandrea Baldazzi, Marco Scatizzi; writing—review and editing: Marco Catarci, Stefano Guadagni, Francesco Masedu, Giacomo Ruffo, Massimo Giuseppe Viola, Felice Borghi, Gianluca Garulli, Felice Pirozzi, Paolo Delrio, Raffaele De Luca, Gianandrea Baldazzi, Marco Scatizzi. All authors reviewed and approved the manuscript before submission.
Funding
Open access funding provided by Università degli Studi dell’Aquila within the CRUI-CARE Agreement.
Data availability
All the datasets and all the instructions used with the software “R©” are available upon reasonable request to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Marco Catarci (Study coordinator)
The collaborators of the “Italian ColoRectal Anastomotic Leakage (iCral) study group” are listed in the acknowledgments.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefano Guadagni, Email: stefano.guadagni@univaq.it.
The Italian ColoRectal Anastomotic Leakage (iCral) study group:
Paolo Ciano, Michele Benedetti, Leonardo Antonio Montemurro, Marco Clementi, Elisa Bertocchi, Gaia Masini, Amedeo Altamura, Francesco Rubichi, Marco Migliore, Daniele Parlanti, Gabriele Vago, Antonio Sciuto, Ugo Pace, Andrea Fares Bucci, Michele Simone, Diletta Cassini, Lorenzo Pandolfini, Alessandro Falsetto, Ferdinando Ficari, Francesco Giudici, Fabio Cianchi, Alberto Patriti, Marcella Lodovica Ricci, Walter Siquini, Alessandro Cardinali, Stefano D’Ugo, Marcello Spampinato, Stefano Scabini, Alessandra Aprile, Domenico Soriero, Marco Caricato, Gabriella Teresa Capolupo, Giusto Pignata, Jacopo Andreuccetti, Ilaria Canfora, Andrea Liverani, Giuseppe Lamacchia, Claudia Franceschilli, Roberto Campagnacci, Angela Maurizi, Pierluigi Marini, Grazia Maria Attinà, Ugo Elmore, Francesco Puccetti, Francesco Corcione, Umberto Bracale, Roberto Peltrini, Roberto Santoro, Pietro Amodio, Massimo Carlini, Domenico Spoletini, Rosa Marcellinaro, Antonio Giuliani, Giovanni Del Vecchio, Mario Sorrentino, Massimo Stefanoni, Giovanni Ferrari, Pietro Maria Lombardi, Alberto Di Leo, Lorenzo Crepaz, Augusto Verzelli, Andrea Budassi, Giuseppe Sica, Giulia Bagaglini, Stefano Rausei, Silvia Tenconi, Davide Cavaliere, Leonardo Solaini, Giorgio Ercolani, Gian Luca Baiocchi, Sarah Molfino, Marco Milone, Giovanni Domenico De Palma, Giovanni Ciaccio, Paolo Locurto, Giovanni Domenico Tebala, Antonio Di Cintio, Luigi Boni, Elisa Cassinotti, Stefano Mancini, Andrea Sagnotta, Mario Guerrieri, Monica Ortenzi, Roberto Persiani, Alberto Biondi, Andrea Lucchi, Giulia Vitali, Dario Parini, Maurizio De Luca, Antonino Spinelli, Francesco Carrano, Michele Genna, Francesca Fior, Vincenzo Bottino, Antonio Ferronetti, Andrea Coratti, Giuseppe Giuliani, Roberto Benigni, Dario Scala, Battistino Puppio, Alessio Vagliasindi, Andrea Muratore, Patrizia Marsanic, Nicoletta Sveva Pipitone Federico, Maurizio Pavanello, Carlo Di Marco, Umberto Rivolta, Camillo Leonardo Bertoglio, Micaela Piccoli, Francesca Pecchini, Carlo Talarico, Vincenzo Greco, Alessandro Carrara, Michele Motter, Giuseppe Tirone, Mauro Totis, Nicolò Tamini, Franco Roviello, Riccardo Piagnerelli, Alessandro Anastasi, Giuseppe Canonico, Gianluca Guercioni, Simone Cicconi, Giuseppe Maria Ettorre, Marco Colasanti, Mauro Montuori, Enrico Pinotti, Pierpaolo Mariani, Roberta Carminati, Nicolò de Manzini, Edoardo Osenda, Annibale Donini, Luigina Graziosi, Mariano Fortunato Armellino, Ciro De Martino, Lucio Taglietti, Arianna Birindelli, Gabriele Anania, Matteo Chiozza, Mariantonietta Di Cosmo, Daniele Zigiotto, Carlo Vittorio Feo, Fioralba Pindozzi, Paolo Millo, Manuela Grivon, Corrado Pedrazzani, Cristian Conti, Silvio Guerriero, Lorenzo Organetti, Andrea Costanzi, Michela Monteleone, Nereo Vettoretto, Emanuele Botteri, Federico Marchesi, Giorgio Dalmonte, Massimo Basti, Diletta Frazzini, Graziano Longo, Simone Santoni, Moreno Cicetti, Gabriele La Gioia, Giuseppe Brisinda, and Stefano Berti
References
- 1.Miettinen RP, Laitinen ST, Mäkelä JT, Pääkkönen ME (2000) Bowel preparation with oral polyethylene glycol electrolyte solution vs. no preparation in elective open colorectal surgery: prospective, randomized study. Dis Colon Rectum 43:669–675 [DOI] [PubMed] [Google Scholar]
- 2.Bucher P, Gervaz P, Soravia C, Mermillod B, Erne M, Morel P (2005) Randomized clinical trial of mechanical bowel preparation versus no preparation before elective left-sided colorectal surgery. Br J Surg 92:409–414 [DOI] [PubMed] [Google Scholar]
- 3.Guenaga KF, Matos D, Wille-Jorgensen P (2011) Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 9:CD001544 [DOI] [PMC free article] [PubMed]
- 4.Rollins KE, Javanmard-Emamghissi H, Lobo DN (2018) Impact of mechanical bowel preparation in elective colorectal surgery: a meta-analysis. World J Gastroenterol 24:519–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catarci M, Guadagni S, Masedu F et al (2024) Mechanical bowel preparation in elective colorectal surgery: a propensity score-matched analysis of the Italian colorectal anastomotic leakage (iCral) study group prospective cohorts. Updates Surg 76(1):107–117 [DOI] [PubMed] [Google Scholar]
- 6.Gustafsson UO, Scott MJ, Hubner M et al (2019) Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS©) society recommendations: 2018. World J Surg 43:659–695 [DOI] [PubMed] [Google Scholar]
- 7.Ficari F, Borghi F, Catarci M et al (2019) Enhanced recovery pathways in colorectal surgery: a consensus paper by the Associazione Chirurghi Ospedalieri Italiani (ACOI) and the PeriOperative Italian Society (POIS). G Chir 40(4 Suppl.):1–40 [PubMed] [Google Scholar]
- 8.Ljungqvist O, Lobo DN (2022) Bowel preparation for colorectal surgery: have all questions been Answered? JAMA Surg 157(1):41–42 [DOI] [PubMed] [Google Scholar]
- 9.Kim EK, Sheetz KH, Bonn J et al (2014) A statewide colectomy experience: the role of full bowel preparation in preventing surgical site infection. Ann Surg 259:310–314 [DOI] [PubMed] [Google Scholar]
- 10.Scarborough JE, Mantyh CR, Sun Z, Migaly J (2015) Combined mechanical and oral antibiotic bowel preparation reduces incisional surgical site infection and anastomotic leak rates after elective colorectal resection: an analysis of colectomy-targeted ACS NSQIP. Ann Surg 262:331–337 [DOI] [PubMed] [Google Scholar]
- 11.Garfinkle R, Abou-Khalil J, Morin N et al (2017) Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum 60:729–737 [DOI] [PubMed] [Google Scholar]
- 12.Koller SE, Bauer KW, Egleston BL et al (2018) Comparative effectiveness and risks of bowel preparation before elective colorectal surgery. Ann Surg 267:734–742 [DOI] [PubMed] [Google Scholar]
- 13.Midura EF, Jung AD, Hanseman DJ et al (2018) Combination oral and mechanical bowel preparations decreases complications in both right and left colectomy. Surgery 163:528–534 [DOI] [PubMed] [Google Scholar]
- 14.Holubar SD, Hedrick T, Gupta R et al (2017) American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on prevention of postoperative infection within an enhanced recovery pathway for elective colorectal surgery. Perioper Med (Lond) 6:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmichael JC, Keller DS, Baldini G et al (2017) Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 60:761–784 [DOI] [PubMed] [Google Scholar]
- 16.Migaly J, Bafford AC, Francone TD et al (2019) The American Society of Colon and Rectal Surgeons clinical practice guidelines for the use of bowel preparation in elective colon and rectal surgery. Dis Colon Rectum 62:3–8 [DOI] [PubMed] [Google Scholar]
- 17.Willis MA, Keller PS, Sommer N et al (2023) Adherence to fast-track measures in colorectal surgery-a survey among German and Austrian surgeons. Int J Colorectal Dis 38:80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McChesney SL, Zelhart MD, Green RL et al (2020) Current U.S. pre-operative bowel preparation trends: a 2018 survey of the American Society of Colon and Rectal Surgeons Members. Surg Infect. (Larchmt)21:1–8 [DOI] [PubMed]
- 19.Panaiotti L, Olkina A, Petrov A, Lankov T, Karachun A (2020) Mechanical bowel preparation with oral antibiotics vs no preparation before elective colon resection for colon cancer. Eur J Surg Oncol 46:e92 [Google Scholar]
- 20.Phillips B (2024) Neomycin and metronidazole hydrochloride with or without polyethylene glycol in reducing infection in patients undergoing elective colorectal surgery. NCT03042091. ClinicalTrials.gov – NIH – US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03042091
- 21.Kennedy E (2024) Mechanical bowel prep randomized study. NCT04931173. ClinicalTrials.gov – NIH – US National Library of Medicine. https://clinicaltrials.gov/ct2/show/ NCT04931173
- 22.Assistance Publique (2024) - Hôpitaux de Paris. Mechanical bowel preparation and oral antibiotics before rectal cancer surgery (PREPACOL2). NCT03491540. ClinicalTrials.gov – NIH – US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03491540
- 23.Willis MA, Toews I, Soltau SLV, Kal JC, Meerpohl JJ, Vilz TO (2023) Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst Rev 2:CD014909 [DOI] [PMC free article] [PubMed]
- 24.Assistance Publique (2024) - Hôpitaux de Paris. Mechanical bowel preparation and oral antibiotics before colon cancer surgery (COLONPREP). NCT03475680. ClinicalTrials.gov – NIH – US National Library of Medicine. https://clinicaltrials.gov/ct2/show/ NCT03475680
- 25.Koskenvuo L, Lehtonen T, Koskensalo S et al (2019) Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 394:840–848 [DOI] [PubMed] [Google Scholar]
- 26.Futier E, Jaber S, Garot M,: COMBINE study group et al (2022) Effect of oral antimicrobial prophylaxis on surgical site infection after elective colorectal surgery: multicentre, randomised, double-blind, placebo-controlled trial. BMJ 379:e071476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espin Basany E, Solís-Peña A, Pellino G et al (2020) Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 5(8):729–738 [DOI] [PubMed] [Google Scholar]
- 28.Pellino G, Solís-Peña A, KraP M, Huguet BM, Espín-Basany E (2021) Preoperative oral antibiotics with versus without mechanical bowel preparation to reduce surgical site infections following colonic resection: protocol for an international randomized controlled trial (ORALEV2). Colorectal Dis 23(8):2173–2181 [DOI] [PubMed] [Google Scholar]
- 29.Frountzas M, Michalopoulou V, Georgiou G et al (2024) The impact of mechanical bowel preparation and oral antibiotics in colorectal cancer surgery (MECCA Study): a prospective randomized clinical trial. J Clin Med 13(4):1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koskenvuo L, Lunkka P, Varpe P, Hyöty M, Satokari R, Haapamäki C, Lepistö A, Sallinen V (2024) Morbidity after mechanical bowel preparation and oral antibiotics prior to rectal resection: the MOBILE2 randomized clinical trial. JAMA Surg e240184. 10.1001/jamasurg.2024.0184. Epub ahead of print [DOI] [PMC free article] [PubMed]
- 31.Antoniou SA, Huo B, Tzanis AA et al (2023) EAES, SAGES, and ESCP rapid guideline: bowel preparation for minimally invasive colorectal resection. Surg Endosc 37(12):9001–9012 [DOI] [PubMed] [Google Scholar]
- 32.Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL (2022) Strategies for antibiotic administration for bowel preparation among patients undergoing elective colorectal surgery: a network meta-analysis. JAMA Surg 157(1):34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN (2019) The role of oral antibiotic preparation in elective colorectal surgery: a meta-analysis. Ann Surg 270:43–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catarci M, Ruffo G, Viola MG et al (2022) ERAS program adherence-institutionalization, major morbidity and anastomotic leakage after elective colorectal surgery: the iCral2 multicenter prospective study. Surg Endosc 36:3965–3984 [DOI] [PubMed] [Google Scholar]
- 35.Italian ColoRectal Anastomotic Leakage (iCral) study group (2023) Patient-reported outcomes, return to intended oncological therapy and enhanced recovery pathways after colorectal surgery: a prospective multicenter observational investigation by the Italian ColoRectal Anastomotic Leakage (iCral 3) study group. Ann Surg Open 4(1):e267 [Google Scholar]
- 36.Lee BK, Lessler J, Stuart EA (2010) Improving propensity score weighting using machine learning. Stat Med 29(3):337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaffrey DF, Beth Ann Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF (2013) A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 32(19):3388–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiser MJ, Bauer JM, Ramsch C et al (2009) Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 13(9):782 [DOI] [PubMed] [Google Scholar]
- 39.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457 [DOI] [PubMed] [Google Scholar]
- 40.Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katayama H, Kurokawa Y, Nakamura K et al (2016) Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 46(6):668–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahbari NN, Weitz J, Hohenberger W et al (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147(3):339–351 [DOI] [PubMed] [Google Scholar]
- 43.Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332 [DOI] [PubMed] [Google Scholar]
- 44.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49(12):1373–1379 [DOI] [PubMed] [Google Scholar]
- 45.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T (2006) Variable selection for propensity score models. Am J Epidemiol 163(12):1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williamson AJ, Alverdy JC (2021) Influence of the microbiome on anastomotic leak. Clin Colon Rectal Surg 34(6):439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catarci M, Guadagni S, Masedu F, Montemurro LA, Ciano P, Benedetti M, Delrio P, Garulli G, Pirozzi F, Scatizzi M, Leakage ICA, (iCral) study group (2023) blood transfusions and adverse events after colorectal surgery: a propensity-score-matched analysis of a hen-egg issue. Diagnostics (Basel) 13(5):952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hullsiek KH (2002) Propensity score modeling strategies for the causal analysis of observational data. Biostatistics 3(2):179–193 [DOI] [PubMed] [Google Scholar]
- 49.García-Granero E, Navarro F, Cerdán Santacruz C et al (2017) Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: an institutional analysis of 800 patients. Surgery 162(5):1006–1016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the datasets and all the instructions used with the software “R©” are available upon reasonable request to the corresponding author.