Abstract
Background
Osteoarthritis (OA) is a major cause of chronic pain. Non-steroidal anti-inflammatory drugs (NSAIDs) are analgesics commonly used for musculoskeletal pain; however, NSAIDs can increase the risk of certain adverse events, such as gastrointestinal bleeding, edema, heart failure, and hypertension.
Objective
The objective of this study was to characterize existing comorbidities among patients with OA. For patients with OA with and without a coexisting medical condition of interest (CMCOI), we estimated the prevalence of prescribing and dispensing NSAIDs pre-OA and post-OA diagnosis.
Methods
Data from three large administrative claims databases were used to construct an OA retrospective cohort. Databases leveraged were IBM MarketScan Medicare Supplemental Database (MDCR), IBM MarketScan Commercial Database (CCAE), and Optum’s de-identified Clinformatics® Data Mart Database (Optum CDM). The OA study population was defined to be those patients who had an OA diagnosis from an inpatient or outpatient visit with at least 365 days of prior observation time in the database during January 2000 through May 2021. Asthma, cardiovascular disorders, renal impairment, and gastrointestinal bleeding risks were the CMCOI of interest. Patients with OA were then classified as having or not having evidence of a CMCOI. For both groups, NSAID dispensing patterns pre-OA and post-OA diagnosis were identified. Descriptive analysis was performed within the Observational Health Data Sciences and Informatics framework.
Results
In each database, the proportion of the OA population with at least one CMCOI was nearly 50% or more (48.0% CCAE; 74.4% MDCR; 68.6% Optum CDM). Cardiovascular disease was the most commonly observed CMCOI in each database, and in two databases, nearly one in four patients with OA had two or more CMCOI (23.2% MDCR; 22.6% Optum CDM). Among the OA population with CMCOI, NSAID utilization post-OA diagnosis ranged from 33.0 to 46.2%. Following diagnosis of OA, an increase in the prescribing and dispensing of NSAIDs was observed in all databases, regardless of patient CMCOI presence.
Conclusions
This study provides real-world evidence of the pattern of prescribing and dispensing of NSAIDs among patients with OA with and without CMCOI, which indicates that at least half of patients with OA in the USA have a coexisting condition. These conditions may increase the risk of side effects commonly associated with NSAIDs. Yet, at least 32% of these patients were prescribed and dispensed NSAIDs. These data support the importance of shared decision making between healthcare professionals and patients when considering NSAIDs for the treatment of OA in patients with NSAID-relevant coexisting medical conditions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40266-024-01108-x.
Key Points
| Over half of all patients with osteoarthritis (OA) have evidence of a coexisting medical condition of interest (cardiovascular risk [to include hypertension], gastrointestinal bleeding risk, asthma, and renal impairment) that may put them at a higher risk of side effects associated with non-steroidal anti-inflammatory drug (NSAID) use. |
| Prescribing and dispensing NSAIDs to patients with OA with an NSAID-relevant coexisting medical condition of interest is substantial, with NSAID prescribing and dispensing increasing post-OA diagnosis. |
| This real-world study supports the importance of shared decision making between healthcare professionals and patients when considering NSAIDs for the treatment of OA in patients with NSAID-relevant coexisting medical conditions. |
Introduction
Osteoarthritis (OA) is a major cause of chronic pain, affecting over 32.5 million US adults [1–3]. The use of non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain is common [4–8]. Non-steroidal anti-inflammatory drugs are effective pain relievers, acting by inhibition of cyclooxygenase (COX) enzymes and thereby blockage of prostaglandin biosynthesis. However, inhibiting these enzymes can lead to an increase in certain adverse events [6, 8, 9]. Specifically, COX-1 inhibition increases the risk for gastrointestinal (GI) bleeding, vasoconstriction, decreased glomerular filtration rate, sodium retention, edema, and antiplatelet effects. Cyclooxygenase-2 inhibition leads to an increased risk for edema, heart failure, hypertension, and thrombotic events, such as myocardial infarction [10]. Based on this evidence, the labels for over-the-counter and prescription NSAIDs contain warnings about a higher risk of side effects for patients with coexisting medical conditions, such as asthma, cardiovascular (CV) disorders, GI bleeding, renal insufficiency, and liver disease. However, coexisting medical conditions, concomitant medications, and often the lack of awareness thereof contribute to treatment challenges that healthcare professionals (HCPs) face daily. Information from healthcare administrative claims databases that are representative of the majority of Americans can provide insights into the frequency of patients with OA with relevant comorbidities and their subsequent NSAID utilization, thereby providing important reminders to clinicians and individuals responsible for creating relevant treatment guidelines to consider comorbidities when prescribing, or providing guidance regarding, medication to manage chronic pain caused by OA. To characterize the current pattern of prescribed and dispensed non-aspirin NSAID utilization among patients with OA with and without coexisting medical conditions of interest (CMCOI) in the USA, individuals with a diagnosis of OA were identified in three healthcare claims databases and utilization of NSAIDs before and after OA diagnosis was determined.
Methods
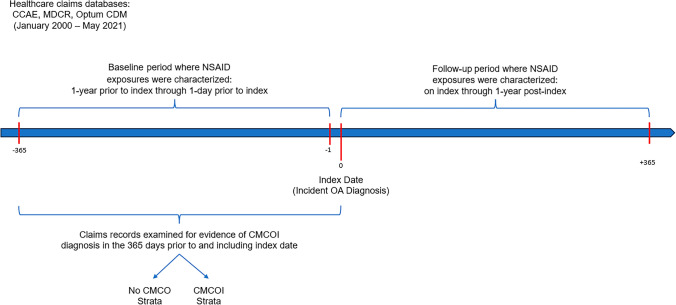
We followed a retrospective cohort design using real-world data from four administrative claims data sources. Figure 1 illustrates the overall design. Patients with OA (study population) were defined in all data sources during January 2000 through May 2021. A new OA diagnosis was considered the index date for follow-up and the follow-up continued for 365 days.
Fig. 1.
Study design. CCAE IBM MarketScan Commercial Database, CMCOI coexisting medical conditions of interest, MDCR IBM MarketScan Medicare Supplemental Database, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database, NSAIDs non-steroidal anti-inflammatory drugs, OA osteoarthritis,
Data Sources
Three large healthcare claims databases representing a large portion of the US population served as the source of de-identified patient-level information for this retrospective cohort study. The IBM® MarketScan® Commercial Claims and Encounters Database (CCAE) is a large US claims database representing individuals, their spouses, and dependents who are enrolled in private, employer-sponsored insurance health plans. The IBM® MarketScan® Medicare Supplemental Database represents the health services provided to retirees with primary or Medicare supplemental coverage through private health plans. The Optum de-identified Clinformatics® Data Mart Database (Optum CDM) consists of individuals with private health insurance, who are fully insured in commercial plans or in administrative services only and have Medicare Advantage (Medicare Advantage Prescription Drug) coverage. The versions of CCAE, MDCR, and Optum CDM used in this analysis contained data on more than 159 million, 10 million, and 91 million patient lives, respectively.
The major data elements of these three databases are adjudicated health insurance claims from outpatient pharmacy dispensing claims1 (coded with National Drug Codes), as well as inpatient and outpatient medical claims, including procedure codes and diagnosis codes (coded in the International Classification of Disease, Ninth Revision, Clinical Modification or the International Classification of Diseases, 10th Revision, Clinical Modification). All databases capture diagnoses and procedures submitted for office visits and hospitalizations, as well as prescription drug dispensing in retail and mail-order pharmacies. Patient-level information in all database records is de-identified and fully compliant with US patient confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996. These data sources have been standardized to the Observational Medical Outcomes Partnership Common Data Model (CDM) [11, 12]. Extract, transform, and load specifications for each data source are available at: https://ohdsi.github.io/ETL-LambdaBuilder/. The standardized data were assessed using a rigorous data quality process to evaluate conformance, completeness, and plausibility of the data [13].
Data Ethics and Availability
The New England Institutional Review Board (NEIRB) determined that analyses conducted with the Optum database (NEIRB 12-286) or the MarketScan databases (NEIRB 12-284) do not meet the definition of human subject research, and therefore institutional review board review and approval was not required. De-identified patient-level data were obtained through a commercial license agreement with each data holder and are therefore not immediately available for further dissemination.
Selection and Definition of CMCOI
The identification and selection of relevant CMCOI were informed by the review of US over-the-counter and prescription non-aspirin NSAID labels, with consideration as to which of these condition(s) are encountered in typical PCP settings as per other literature [14–22]. Through this exercise, CV risk (to include diseases such as hypertension, cerebrovascular disorders, and heart failure), GI bleeding risk, asthma, and renal impairment were deemed in scope for this study as CMCOI. Hepatic disorder, though a disease appearing within the warning and precautions section of NSAID labels, was omitted because warnings in prescription labels do not advise caution among individuals with pre-existing hepatic disease. Consequently, inclusion criteria were a diagnosis of any of the following conditions: cerebrovascular disease (ischemic and hemorrhagic stroke, transient ischemic attack, and others; coronary artery disease/ischemic heart disease [including myocardial infarction and complications and angina pectoris]; heart failure; hypertension); asthma (any diagnosis of asthma); renal impairment; and GI ulcer or hemorrhage. Thus, throughout the remainder of this report, a patient classified as having a CMCOI refers to the presence of one or more of these four conditions; it does not indicate the presence or absence of any other medical condition. A full list of codes defining each of these four CMCOI can be found in the Electronic Supplementary Material (ESM).
Each of the four CMCOI was defined using one or more Systematized Nomenclature of Medicine (SNOMED) standard concepts from the Observational Medical Outcomes Partnership Vocabulary (version 5.3). The SNOMED concepts used to define each of the four CMCOI were manually reviewed to ensure that the included concepts aligned with the clinical definition of the CMCOI of interest. For example, the definition of GI bleeding risks includes GI ulcers, GI hemorrhage, descendent diagnosis codes of these two concepts, as well as anticoagulants and corticosteroids whose use is associated with such conditions.
Study Population
Following Williamson et al., patients with OA were defined as patients with an OA diagnosis from an inpatient or outpatient visit with at least 365 days of prior observation time in the database during January 2000 through May 2021 [23]. In validation studies, this algorithm defining patients with OA was reported to have 0.78 sensitivity (95% confidence interval 0.75–0.81) and 0.95 specificity (95% confidence interval 0.94–0.96) [24]. All concepts/codes used to define OA can be found in the ESM.
Once the OA study population had been identified, each patient’s electronic claim records were examined for evidence of a CMCOI in the 365 days prior to and including the index date (OA diagnosis date). Patients with OA were then classified into a dichotomous scheme of having no evidence of a CMCOI or having a CMCOI, with patients classified as having a CMCOI if they had a diagnosis of or prescription consistent with at least one of the listed conditions among the four CMCOI within the past 365 days. For both groups, non-aspirin NSAID dispensing patterns were characterized for the baseline period, defined to be 1 year prior to but not including the index date, and for the follow-up period, defined to be 1 year post and including the index date. The non-aspirin NSAIDs described in this study include all branded and generic prescription drugs marketed in the USA that contain non-aspirin NSAID ingredients, including, but not limited to, celecoxib, diclofenac, etodolac, fenoprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, naproxen/naproxen sodium, and sulindac. Prescribed drugs were captured on the ingredient (molecular) level, include drugs of any dosage form, and include both single and multi-ingredient products. An exhaustive list of all drug concepts included in the study can be found in the ESM. The descriptive analysis was performed within the Observational Health Data Sciences and Informatics framework, allowing for replication across the three databases [11].
Statistical Analyses
All patient characteristics were described using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The statistical analysis and figure generation were done via the R programming language.
Results
OA Population Characteristics
Across all databases, the mean age of patients was 61.4 years. Patients in the MDCR database were older (mean age 75.4 years) than patients in the other databases (CCAE: 52.8 years; Optum CDM: 64.4 years). The MDCR database had the largest fraction of patients aged older than 80 years (29.3%) compared with the other databases (CCAE: 0%; Optum CDM: 14.0%). The age distributions of those patients with OA with at least one CMCOI was observed to be more skewed towards older patients relative to those with no observed CMCOI.
Female patients accounted for 58.8% of the patients across all databases (CCAE: 57.6%; MDCR: 61.0%; Optum CDM: 59.2%). In general, a greater proportion of male patients than female patients had a CMCOI. The sex and age distributions for the total OA population, the OA population with no evidence of a CMCOI, and the OA population with an observed CMCOI are presented in Table 1.
Table 1.
Demographic characteristics of the study OA population stratified by CMCOI status
| CCAE | MDCR | Optum CDM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No CMCOI | CMCOIa | Total | No CMCOI | CMCOI | Total | No CMCOI | CMCOI | Total | |
| n | 3,695,622 | 3,406,452 | 7,102,074 | 706,321 | 2,052,400 | 2,758,721 | 2,509,846 | 5,471,987 | 7,981,833 |
| % OA population | 52.0% | 48.0% | 100.0% | 25.6% | 74.4% | 100.0% | 31.4% | 68.6% | 100.0% |
| Age, mean (SD), years | 50.7 (10.3) | 55.0 (7.9) | 52.8 (9.5) | 73.7 (7.2) | 75.9 (7.9) | 75.4 (7.8) | 56.6 (14.2) | 67.9 (12.3) | 64.4 (14.0) |
| 18–24 | 1.9% | 0.4% | 1.1% | 0% | 0% | 0% | 1.3% | 0.1% | 0.5% |
| 25–44 | 20.4% | 9.6% | 15.2% | 0.0% | 0.0% | 0.0% | 17.1% | 3.8% | 8.0% |
| 45–64 | 75.2% | 86.7% | 80.7% | 1.9% | 1.6% | 1.7% | 49.6% | 31.1% | 36.9% |
| 65–79 | 1.6% | 3.2% | 2.4% | 77.2% | 66.2% | 69.0% | 26.8% | 46.6% | 40.4% |
| ≥80 | 0% | 0.0% | 0.0% | 20.9% | 32.2% | 29.3% | 4.6% | 18.3% | 14.0% |
| Female | 59.0% | 56.1% | 57.6% | 64.6% | 59.8% | 61.0% | 59.2% | 59.2% | 59.2% |
CCAE IBM MarketScan Commercial Database, CMCOI coexisting medical conditions of interest, MDCR IBM MarketScan Medicare Supplemental Database, OA osteoarthritis, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database, SD standard deviation
aThe CMCOI population refers to those patients observed to have one or more of cardiovascular risk, gastrointestinal bleeding risk, asthma, or renal impairment present
CMCOI Prevalence in the OA Population
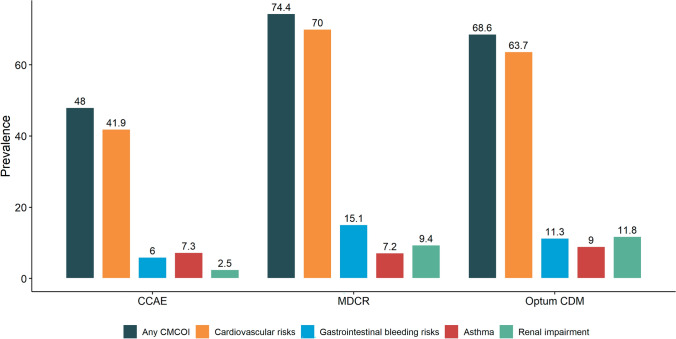
The proportion of patients with OA with a CMCOI was approximately half (48.0%) in CCAE (Fig. 2). For the two databases noted to have older OA populations, a greater proportion of patients had at least one CMCOI: 74.4% in MDCR and 68.6% in Optum CDM.
Fig. 2.
Coexisting medical conditions of interest (CMCOI) prevalence in the osteoarthritis population. CMCOIs are not mutually exclusive. In particular, one patient can have multiple CMCOIs. CCAE IBM MarketScan Commercial Database, MDCR IBM MarketScan Medicare Supplemental Database, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database
Cardiovascular risk was the most commonly identified CMCOI across the three databases; 41.9% of patients with OA in the CCAE database had some CV disease compared to approximately two thirds of patients with OA in the MDCR (70.0%) and Optum CDM (63.7%) databases (Fig. 2). Comparatively, the prevalence of GI bleeding risk factors, asthma, and renal disease was lower in all three databases relative to CV diseases. While few patients with OA in the CCAE database (6.0%) had GI bleeding risk factors, the prevalence of GI bleeding risk factors among the OA population was approximately twice as high in the two other databases (MDCR: 15.1%; Optum CDM: 11.3%). Across all databases, the prevalence of asthma within the OA population was low (7.2% to 9.0%). Few patients in the CCAE database (2.5%) had renal disease; however, similar percentages of individuals in MDCR (9.4%) and Optum CDM (11.8%) databases had renal impairment.
Among the patients with OA, the MDCR database had the highest proportion of patients with one (51.2%) or two (19.1%) CMCOI, while Optum CDM had the highest proportion of patients with three (4.2%) or at least four (0.5%) CMCOI. In comparison, the CCAE (privately insured) database, which consists of the youngest population relative to the other databases, had the lowest proportion of patients with one (39.0%), two (7.9%), three (1.0%), or at least four (0.09%) CMCOI. The cardinality of CMCOI within the OA population of each of the three databases is presented in Table 2.
Table 2.
Proportiona of the osteoarthritis population in CCAE, MDCR, and Optum CDM databases with 0, 1, 2, 3 or at least 4 CMCOI
| Number of CMCOI | CCAE (%) | MDCR (%) | Optum CDM (%) |
|---|---|---|---|
| 0 | 52.0 | 25.6 | 31.4 |
| 1 | 39.0 | 51.2 | 45.9 |
| 2 | 7.9 | 19.1 | 17.9 |
| 3 | 1.0 | 3.8 | 4.3 |
| 0.1 | 0.4 | 0.5 |
CCAE IBM MarketScan Commercial Database, CMCOI coexisting medical conditions of interest, MDCR IBM MarketScan Medicare Supplemental Database, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database
aProportions may not sum to 100% because of rounding
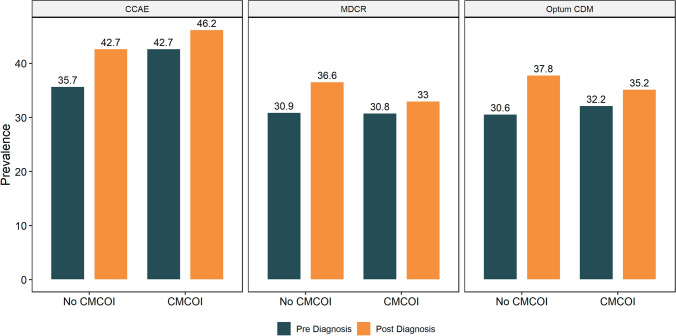
Dispensing Patterns of Any NSAID by CMCOI Status
Among patients with OA with CMCOI, NSAID dispensing increased post-index across all three databases, with a range from 30.8% (MDCR) to 42.7% (CCAE) at baseline and 33.0% (MDCR) to 46.2% (CCAE) during the follow-up (Fig 3). In contrast, among patients with no CMCOI, 30.6% (Optum CDM) to 35.6% (CCAE) were dispensed NSAIDs at baseline and 36.6% (MDCR) to 42.7% (CCAE) post-index.
Fig. 3.
Prescribing and dispensing of non-steroidal anti-inflammatory drugs among patients with osteoarthritis, stratified by coexisting medical conditions of interest (CMCOI) status and database. CCAE IBM MarketScan Commercial Database, MDCR IBM MarketScan Medicare Supplemental Database, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database
Following a diagnosis of OA, an increase in the prescribing and dispensing of NSAIDs was observed in all databases, regardless of patient CMCOI presence (Fig. 3). Across all three databases, increases in prescribing and dispensing of NSAIDs ranged from 2.1 percentage points to 7.3 percentage points following a diagnosis of OA, with prescribing and dispensing of NSAIDs increasing to a lesser magnitude among the CMCOI versus no CMCOI strata (average increase 2.9 vs 6.6 percentage points, respectively).
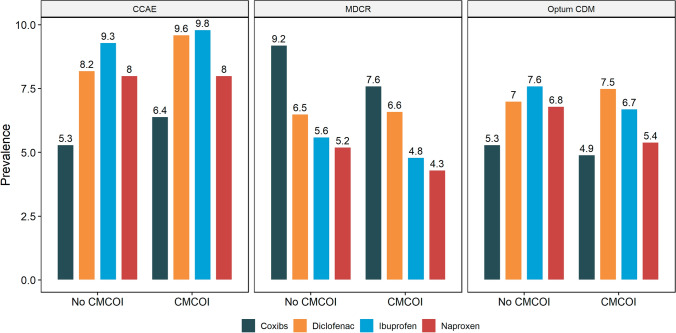
Prescribing and Dispensing of Specific NSAIDs Among the OA Population
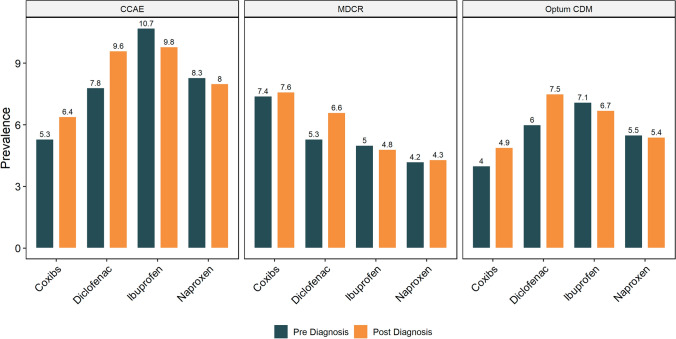
Among patients with OA with CMCOI, the pattern of dispensing of specific NSAIDS during the follow-up was similar in the CCAE and Optum CDM databases; ibuprofen and diclofenac were most frequently prescribed and dispensed and COX-2 inhibitors (coxibs) least frequently (Fig. 4). In contrast, in the MDCR database, coxibs were the most commonly prescribed and dispensed NSAID during the follow-up.
Fig. 4.
Prescribing and dispensing of non-steroidal anti-inflammatory drug by coexisting medical conditions of interest (CMCOI) status post-osteoarthritis diagnosis. CCAE IBM MarketScan Commercial Database, Coxibs COX-2 inhibitors, MDCR IBM MarketScan Medicare Supplemental Database, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database
Among patients with OA with CMCOI, post-OA diagnosis prescribing and dispensing of coxibs increased to a greater extent in the CCAE (+1.0%) and Optum CDM (+0.9%) databases than the MDCR (+0.2%) database (Fig. 5). Prescribing and dispensing of diclofenac increased at similar levels across the MDCR (+1.2%), Optum CDM (+1.4%), and the CCAE (+1.7%) databases after diagnosis. Across all three databases, prescribing and dispensing of ibuprofen decreased (CCAE: − 0.8%; MDCR − 0.2%; Optum CDM: − 0.4%) and prescribing and dispensing of naproxen remained similar (CCAE: − 0.2%; MDCR: +0.1%; Optum CDM: − 0.1%) after diagnosis.
Fig. 5.
Prescribing and dispensing of specific non-steroidal anti-inflammatory drugs by the coexisting medical conditions of interest population, pre-osteoarthritis and post-osteoarthritis diagnosis. CCAE IBM MarketScan Commercial Database, Coxibs COX-2 inhibitors, MDCR IBM MarketScan Medicare Supplemental Database, Optum CDM Optum’s de-identified Clinformatics® Data Mart Database
Discussion
This study provides real-world evidence to describe the current pattern of NSAID utilization among patients with OA with and without CMCOI. Findings indicate that around half of patients with OA in the USA, men and women alike, have a coexisting condition that may increase their risk of NSAID side effects. Furthermore, the percentage of patients with OA with underlying conditions, as well as the total number of coexisting CMCOI, was higher in databases consisting of older populations. Yet NSAIDS are prescribed and dispensed to at least 32% of these patients. These data also suggest that similar trends of prescribing and dispensing NSAIDs were observed among those with and without CMCOI.
The real-world data presented in this study suggests that CV diseases are the most common underlying conditions among patients diagnosed with OA, with at least 42% of patients with OA having a CV risk disorder, with the inclusion of hypertension contributing to this high proportion. The frequency of asthma, GI bleeding risk, or renal disease were at least four-fold lower than CV disorders among patients with OA in any of the databases analyzed. Nonetheless, ibuprofen, diclofenac, naproxen, and/or coxibs were each prescribed and dispensed to ~4% to ~10% of patients with OA with CMCOI, with the percent of prescribing and dispensing any NSAID varying from 30.6% to 46.2%. Among older adults (i.e., MDCR database) with an OA diagnosis, coxibs were most commonly prescribed and dispensed regardless of the presence or absence of a CMCOI, in line with previous observations [22]. Although nonselective and selective NSAIDs are effective at managing pain, each is associated with increased risks of specific side effects. These factors should be considered when prescribing NSAIDs to patients with OA with underlying risks.
Estimates of relative risk of NSAID-related asthma, CV, GI bleeding, and renal impairment events vary depending on dose, duration, and source of exposure information (e.g., survey of frequency of use, electronic medical records of dispensing); study design (e.g., observational, randomized clinical); indication; and comparator (e.g., no use, placebo, aspirin/acetaminophen). There has been some discussion in the literature and at regulatory body meetings, but no consensus exists on the extent to which relative risks vary by NSAID use. Data from meta-analyses and systematic reviews identify that NSAID-related increases in relative risks for CV disease range from 1.22 to 2.26 [25], for GI bleeding from 1.09 to 18.45 [26, 27], and for acute kidney disease from 1.05 to 4.10 [28, 29]. Information available on the US Food and Drug Administration website, which is directed to healthcare professionals, reminds them of the increased risks for serious CV events (10–50%) associated with NSAID use, regardless of an individual’s history of or risk factors for CV disease [30].
Strengths and Limitations of Analysis
A study protocol and analysis plan were established prior to conduct of the current study. The choice of the three healthcare claims databases supported evaluation of data representative of a broad range of the US primary HCP population. Of note, two of these databases (MDCR and Optum CDM) include privately insured individuals with Medicare Supplemental or Medicare Advantage, who are presumably healthier than the general nonworking Medicare population. Across the three databases, healthcare claims for a visit to a HCP, whether a well-care visit or a visit to monitor a chronic condition such as OA or one of the CMCOI, within the specified timeframe were analyzed. Recognizing the size of the three databases evaluated, no statistical analyses were planned as small mathematical differences could qualify as statistically significant while not being clinically relevant. Healthcare claims also included retail and mail-order dispensing of prescription drugs; however, it must be noted that the actual use of medication could not be confirmed in the databases. Prescribing and dispensing of any NSAIDs were present during the baseline period for both those patients with OA with a CMCOI and those with no evidence of a CMCOI, which may be potentially attributable to the use of such medications to manage OA symptoms prior to receiving a formal OA diagnosis. Nonetheless, the use of any NSAIDs was observed to have increased for both OA subpopulations across all three databases. Furthermore, the designation of a patient having a CMCOI was determined by examining the patient’s medical records up to and including the date of the OA diagnosis and, therefore, the prevalence of CMCOI among the OA population may be an underestimate, as a patient could develop a CMCOI at a later time following the OA diagnosis. Last, use of over-the-counter medications, including NSAIDs, are not captured in such databases, possibly resulting in an underestimation of the prevalence of NSAID use.
Implications
Based on these data, there appears to be an opportunity to increase awareness among doctors and other prescribers about the high frequency of CMCOIs in patients diagnosed with OA. These patients may benefit from alternative analgesic options and/or a closer HCP follow-up to monitor for potential NSAID-related side effects.
Conclusions
These data show the prevalence of CMCOI among the OA population. Even more so, these data highlight the elevated prevalence of multiple CMCOI among older patients. Supported by this evidence, physicians and prescribers can play a critical role in identifying patients with non-aspirin NSAID-relevant CMCOI and should regularly monitor those patients for the development of side effects. Alternative analgesics and nonpharmacologic treatment modalities may provide an important alternative for patients with OA. These findings highlight the importance of shared decision making between the healthcare professional and patient when selecting the optimal analgesic for their OA pain.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CCAE
IBM MarketScan Commercial Database
- CDM
Common Data Model
- CMCOI
Coexisting medical condition of interest
- CV
Cardiovascular
- ETL
Extract, transform, and load
- GI
Gastrointestinal
- HCP
Healthcare professionals
- HIPAA
Health Insurance Portability and Accountability Act
- MDCR
IBM MarketScan Medicare Supplemental Database
- NDC
National Drug Codes
- NEIRB
New England Institutional Review Board
- NSAID
Nonsteroidal anti-inflammatory drugs
- OA
Osteoarthritis
- OHDSI
Observational Health Data Sciences and Informatics
- OMOP
Observational Medical Outcomes Partnership
- Optum CDM
Optum de-identified Clinformatics® Data Mart Database
- SD
Standard deviations
- SNOMED
Systematized Nomenclature of Medicine
Declarations
Funding
Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division, provided funding for the study and for support by an independent medical writer.
Conflicts of Interest/Competing Interests
All authors were employees of or consultants to Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division or Janssen Research and Development, LLC, a subsidiary of Johnson & Johnson.
Ethics Approval
The New England Institutional Review Board (NEIRB) determined that analyses conducted with the Optum database (NEIRB 12-286) or the MarketScan databases (NEIRB 12-284) do not meet the definition of human subject research, and therefore institutional review board review and approval were not required.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
De-identified patient-level data were obtained through a commercial license agreement with each data holder and are therefore not immediately available for further dissemination.
Code Availability
Not applicable.
Authors’ Contributions
JI: protocol/study design, study execution, data review and analysis, critical review and revision of manuscript, final approval of version to be published, agreement to be accountable for all aspects of work and that questions are appropriately investigated and resolved. AS: data review and analysis, critical review and revision of manuscript, final approval of version to be published, agreement to be accountable for all aspects of work and that questions are appropriately investigated and resolved. KEB: drafting manuscript, data review and analysis, critical review and revision of manuscript, final approval of version to be published, agreement to be accountable for all aspects of work and that questions are appropriately investigated and resolved. KW: protocol/study design, data review and analysis, critical review and revision of manuscript, final approval of version to be published, agreement to be accountable for all aspects of work and that questions are appropriately investigated and resolved. RW: protocol/study design, data review and analysis, drafting manuscript, critical review and revision of manuscript, final approval of version to be published, agreement to be accountable for all aspects of work and that questions are appropriately investigated and resolved. AM: protocol/study design, data review and analysis, critical review and revision of manuscript, final approval of version to be published, agreement to be accountable for all aspects of work and that questions are appropriately investigated and resolved. All authors had full access to the study data and had final responsibility to submit the manuscript for publication.
Footnotes
Accordingly, throughout the remainder of the paper, the phrase NSAID “prescribing and dispensing” and “NSAID dispensing” will be used to refer to an NSAID outpatient pharmacy dispensing claim.
References
- 1.Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. OA affects over 32.5 million US adults. Page last reviewed: July 27, 2020. Available from: https://www.cdc.gov/arthritis/index.htm. Accessed 17 Feb 2023.
- 2.Mayo Clinic. Patient care & health information: diseases & conditions. Osteoarthritis. Available from: https://www.mayoclinic.org/diseases-conditions/osteoarthritis/symptoms-causes/syc-20351925. Accessed 17 Feb 2023.
- 3.Young RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–e422. doi: 10.1097/j.pain.0000000000002291. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, Mathieson S, Kobayashi S, Shaheed CA, Nogueira LAG, Simic M, et al. Prevalence of nonsteroidal antiinflammatory drugs prescribed for osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2023;75(11):2345–2358. doi: 10.1002/acr.25157. [DOI] [PubMed] [Google Scholar]
- 5.Overton C, Nelson AE, Neogi T. Osteoarthritis treatment guidelines from six professional societies: similarities and differences. Rheum Dis Clin North Am. 2022;48(3):637–657. doi: 10.1016/j.rdc.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osani MC, Vaysbrot EE, Zhou M, McAlindon TE, Bannuru RR. Duration of symptom relief and early trajectory of adverse events for oral nonsteroidal antiinflammatory drugs in knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2020;72(5):641–651. doi: 10.1002/acr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2020;72(2):149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Costa BR, Pereira TV, Saadat P, Rudnicki M, Iskander SM, Bodmer NS, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. 2021;375:n2321. doi: 10.1136/bmj.n2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper C, Chapurlat R, Al-Daghri N, Herrero-Beaumont G, Bruyère O, Rannou F, et al. Safety of oral non selective non steroidal anti inflammatory drugs in osteoarthritis: what does the literature say? Drugs Aging. 2019;36(Suppl. 1):15–24. doi: 10.1007/s40266-019-00660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler TO, Durham CO, Planton J, Edlund BJ. Use of nonsteroidal-anti-inflammatory drugs in the older adult. J Am Assoc Nurse Pract. 2014;26:414–423. doi: 10.1002/2327-6924.12139. [DOI] [PubMed] [Google Scholar]
- 11.Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015;216:574–578. [PMC free article] [PubMed] [Google Scholar]
- 12.Voss EA, Makadia R, Matcho A, Ma Q, Knoll C, Schuemie M, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc. 2015;22(3):553–564. doi: 10.1093/jamia/ocu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blacketer C, Defalco FJ, Ryan PB, Rijnbeek PR. Increasing trust in real-world evidence through evaluation of observational data quality. J Am Med Inform Assoc. 2021;28(10):2251–2257. doi: 10.1093/jamia/ocab132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rui P, Okeyode T. National ambulatory medical survey: 2015 state and national summary tables. Available from: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2015_namcs_web_tables.pdf. Accessed 24 Nov 2023.
- 15.Centers for Disease Control and Prevention. National Center for Environmental Health. Asthma data, statistics, and surveillance: most recent asthma data. Available from: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed 24 Nov 2023.
- 16.Centers for Disease Control and Prevention. National Center for Health Statistics. Percentage of heart attack for adults aged 18 and over (95% confidence intervals), United States, 2019–2021. National health interview survey. Available from: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html. Accessed 24 Nov 2023.
- 17.Centers for Disease Control and Prevention. National Center for Health Statistics. Percentage of coronary heart disease for adults aged 18 and over (95% confidence intervals), United States, 2019–2021. National health interview survey. Available from: https://wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html. Accessed 24 Nov 2023.
- 18.Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the United States: a population-based study. J Clin Gastroenterol. 2015;49:690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 19.Newman D, Tong M, Levine E, Kishore S. Prevalence of multiple chronic conditions by US.. state and territory, 2017. PLoS ONE. 2020;15(5):e0232346. doi: 10.1371/journal.e0232346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boersma P, Black LI, Ward BW. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis. 2020;17:200130. doi: 10.5888/pcd17.200130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelaya CE, Dahlhamer JM, Lucas JW, Connor EM. Chronic pain and high-impact chronic pain among US adults, 2019. NCHS Data Brief, no 390. Hyattsville, MD: National Center for Health Statistics; 2020. Available from: https://www.chromeextension://efaidnbmnnnibpcajpcglclefindmkaj/. https://www.cdc.gov/nchs/data/databriefs/db390-H.pdf. Accessed 24 Nov 2023. [PubMed]
- 22.Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9(1):143–150. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson T, Green ME, Birtwhistle R, Khan S, Garies S, Wong ST, et al. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med. 2014;12(4):367–372. doi: 10.1370/afm.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha S, Dave AJ, Losina E, Katz JN. Diagnostic accuracy of administrative data algorithms in the diagnosis of osteoarthritis: a systematic review. BMC Med Inform Decis Mak. 2016;16:82. doi: 10.1186/s12911-016-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drug: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Regalt A, Nicotra F, on behalf of the investigators of the Safety of Non-Steroidal Anti-Inflammatory Drugs (SOS) Project et al. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project) Drug Saf. 2012;35(12):1127–1146. doi: 10.2165/11633470-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coxib and traditional NSAID Trialists’ (CNT) Collaboration Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomized trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45:531–539. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Donnan PT, Bell S, Guthrie B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2017;18:256. doi: 10.1186/s12882-017-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. Drug safety and availability. FDA Drug Safety Communication: FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm451800.htm. Accessed 24 Nov 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.