Abstract
Objective
Despite pituitary neuroendocrine tumor (PitNET) being extra‐axial tumors without direct damage to brain tissue, patients with PitNET exhibit neuropsychological impairments. However, it remains unclear whether there are neuropsychological differences between PitNET and intra‐axial tumors that directly destroy the brain parenchyma. This prospective study aims to clarify this distinction to inform decision‐making for intracranial tumors of diverse origins.
Methods
A total of 146 patients with PitNET, 74 patients with glioma representing intra‐axial tumors, and 52 age‐, sex‐, and education‐matched healthy controls were recruited. All patients received standard treatment and postoperative rehabilitation. Clinical data were meticulously collected, and neuropsychological tests were administered to all participants both before and 3 months after surgery.
Results
Both PitNET and glioma patients experience the dual burden of cognitive and affective deficits. However, the feature of these deficits differs substantially. In PitNET patients, the deficits are relatively mild and focal, whereas in glioma patients, they are severe and extensive. Specifically, PitNET patients exhibit deficits in memory, anxiety, and negative affect. In contrast, glioma patients display deficits in executive function, attention, anxiety, positive/negative affect, and empathy. Notably, except for persistent memory deficits, the majority of neuropsychological scores declines in PitNET patients are restorable and can reach improvement within a short period after standard surgical therapy and perioperative management. Conversely, glioma patients not only fail to show improvements but also demonstrate worsening in terms of general cognition and memory postoperatively.
Interpretation
As an extra‐axial tumor, PitNET may exhibit distinctive cognitive and affective functioning compared to intra‐axial tumors, highlighting the need for specific treatment approaches for PitNET patients.
Introduction
Brain tumors cause not only neurological symptoms but also cognitive 1 , 2 and affective impairments 3 , 4 , 5 that reduce patient quality of life. 6 In the clinic, we focus most on neuropsychological changes associated with intra‐axial tumors, which directly destroy brain parenchyma 7 and result in obvious neuropsychological deficits. Among these, gliomas, the most prevalent primary brain tumors, have been extensively investigated. Compared with intra‐axial tumors, neuropsychological deficits resulting from extra‐axial brain tumors have historically received much less attention from researchers. For example, pituitary neuroendocrine tumors (PitNETs), the second most common primary brain tumors, originate in the sellar region. 8 , 9 These tumors compress surrounding brain tissue 10 , 11 and exhibit abnormal hormone secretion.
Prior researches have reported cognitive impairments in PitNET patients, 11 , 12 encompassing domains such as attention, 13 memory, 11 , 13 , 14 and executive function, 15 alongside affective disturbances like depression 16 and anxiety. 12 , 17 However, it remains unclear the distinction between neuropsychological status in PitNET patients and those with intra‐axial tumors, as well as the effect of the surgical intervention on their neuropsychological improvement, which limits selecting clinical management approaches for neuropsychological status in these brain tumors from different locations.
The present study aims to elucidate the characteristics of neuropsychological dysfunction in individuals diagnosed with PitNET, juxtaposing these findings with intra‐axial tumors, specifically glioma, which serves as a representative subset. Glioma, being not only the most common primary brain tumor but also the most prevalent intra‐axial tumor with a substantial population, has been extensively studied regarding the neuropsychological status of patients, yielding abundant related data. We employed a comprehensive neuropsychological test battery, considering the gold standard, 18 to probe cognitive and affective domains. Testing was performed twice, that is, pre‐ and postoperatively, shedding light on the impact of surgery on cognitive and affective functions. Additionally, we conducted an in‐depth analysis to discern any associations between various clinical factors and neuropsychological impairments.
Methods
Study design
We recruited individuals diagnosed with PitNET or glioma who sought treatment at Beijing Tiantan Hospital between July 2019 and October 2022. Healthy controls (HCs) were recruited from the local community. The inclusion/exclusion criteria, enrollment process, excluded patients, and testing timeline are detailed in Figure 1. Ultimately, our analysis included 146 patients with PitNET, 74 patients with gliomas, and 52 HCs. This prospective study received approval from the Medical Ethics Committee of our hospital, and all participants gave informed consent.
Figure 1.
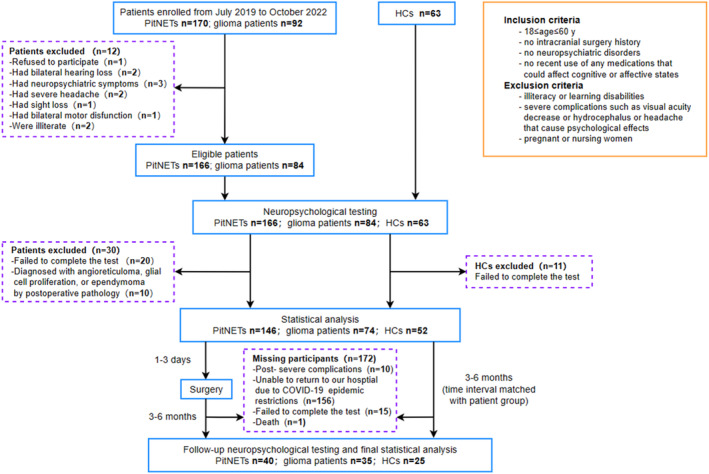
Flow diagram showing the participant enrollment and screening process, inclusion/exclusion criteria, study design, and testing timeline. HCs, healthy controls; PitNETs, pituitary neuroendocrine tumors.
Evaluation of clinical indicators
All patients received standard treatment and perioperative management. Postoperative endocrine reexamination was periodically monitored for PitNET patients, and hormone replacement therapy was conducted for pituitary insufficiency. We collected general demographic information, including sex, age, education level, and dominant hand. Additionally, we gathered clinical data, such as disease course, symptoms, radiological findings, endocrinal results, surgical approach, and pathological reports. Clinical symptoms encompassed both endocrine and neurological manifestations. Radiological data were obtained via T1‐weighted gadolinium‐enhanced and FLAIR sequences using a 3.0 Tesla scanner (TRIO; Siemens, Erlangen, Germany). 19 , 20 Endocrinological data for pituitary hormones were collected by analyzing fasting morning blood samples. All surgical procedures and pathological diagnoses were performed by experienced neurosurgeons and pathologists. Follow‐up assessments were recommended at 3, 6, and 12 months, and yearly thereafter. 9
Neuropsychological assessment
The neuropsychological assessment covered both cognitive (including general cognitive status, executive function, memory, and attention) and affective (including anxiety, depression, positive/negative affect, and empathy) domains. We administered a test battery comprising 10 scales: the mini–mental state examination (MMSE), 21 the Montreal Cognitive Assessment (MoCA), 22 the Frontal Assessment Battery (FAB), 23 the Wechsler Adult Intelligence Scale–Fourth Edition Digit Span Test (DST; including the Digit Span‐Forward [DST‐f], Digit Span‐Backward [DST‐b], and Digit Span‐Sort [DST‐s] tests), 24 the Trail Making Test‐part A (TMT‐A) and Trail Making Test‐part B (TMT‐B), 25 , 26 the Attentional Control Scale (ACS), 27 the Hamilton Anxiety Scale (HAMA), 28 the Beck Depression Inventory (BDI), 29 the Positive and Negative Affect Scale (PANAS) including the positive subscale (PANASp) and negative subscale (PANASn), 30 and the Interpersonal Reactivity Index (IRI). 31 , 32 All tests were conducted by a skilled psychologist in a quiet room, with an average duration of approximately 60 min. All patients underwent two assessments that took place 1–3 days before surgery and during a follow‐up visit after surgery. The HCs also completed two assessments at equivalent time points.
Statistical analysis
We conducted the statistical analysis using SPSS software (ver. 22.0; IBM Corp., Armonk, NY, USA). We calculated the z‐standardized score for each subject: first calculate the mean (μ) and standard deviation (s) of the scores for each domain in the first test of HCs, and then calculate z‐standardized score = (original score − μ)/s. Continuous variables are presented as the mean ± standard deviation. Data with an approximately normal distribution and satisfying the assumption of homogeneity of variance were subjected to an analysis of variance, followed by Bonferroni's post hoc test. Heterogeneous data were analyzed using Welch's analysis of variance, and pairs of data were compared using Tamhane's T2 test. We used Student's t‐tests to compare two sets of data. For continuous variables with a non‐Gaussian distribution, we used the Kruskal–Wallis H test to compare more than two groups, and the Mann–Whitney U test to compare two groups. Categorical variables, expressed as the number of cases (percentage), were subjected to the chi‐squared test or Fisher's exact test. In addition, after matching for parameters such as age and education level, we conducted Pearson and Spearman correlation analyses (for homogeneous and heterogeneous data, respectively) of the neuropsychological scores and clinical indicators (including disease course, tumor size, and suprasellar extension of PitNET). For individual‐level analyses, we conducted z‐tests to compare the mean standardized z‐scores for each cognitive and affective scale of preoperative PitNET patients to the HCs. A mean z‐score ± 1 SD of HCs was considered within the normal range, while a z‐score greater or below this range indicated abnormality. 11 The level of statistical significance was set at p < 0.05.
Results
Demographic and clinical data
Table 1 provides an overview of the demographic and clinical characteristics of the study population. No significant differences were observed among PitNET patients, glioma patients, and HCs with respect to sex, age, education level, or dominant hand. The mean tumor size of PitNET was significantly smaller than that of glioma (6.25 ± 7.03 vs. 35.85 ± 33.11 cm3, p < 0.001).
Table 1.
Demographic and clinical characteristics of the participants.
| Values | PitNET (n = 146) | Glioma (n = 74) | HCs (n = 52) | p‐value |
|---|---|---|---|---|
| Sex (M/F) | 71:75 (48.63%:51.37%) | 40:34 (54.05%:45.95%) | 19:33 (36.54%:63.46%) | 0.146 |
| Age (y) | 39.26 ± 9.45 | 40.64 ± 10.94 | 36.54 ± 10.08 | 0.201 |
| Education (y) | 13.82 ± 3.54 | 13.58 ± 3.11 | 12.75 ± 3.21 | 0.193 |
| Dominant hand (R/L) | 138:8 (94.52%:5.48%) | 66:8 (89.19%:10.81%) | 49:3 (94.23%:5.77%) | 0.317 |
| Disease course (mos) | 18.79 ± 27.43 | 10.83 ± 23.38 | – | <0.001 |
| Symptom | ||||
| Visual field defect/hypopsia | 52 (35.62%) | 4 (5.41%) | – | <0.001 |
| Amenorrhea/decreased libido | 51 (34.93%) | – | – | – |
| Acromegaly | 45 (30.82%) | – | – | – |
| Cushing syndrome | 6 (4.11%) | – | – | – |
| Headache | 48 (32.88%) | 13 (17.57%) | – | 0.015 |
| Dizzy | 18 (12.33%) | 17 (22.97%) | – | 0.044 |
| Epilepsy | – | 34 (45.95%) | – | – |
| Nausea/vomit | – | 10 (13.51%) | – | – |
| Language disorder | – | 4 (5.41%) | – | – |
| Motor/sense dysfunction | – | 13 (17.57%) | – | – |
| Functional/nonfunctional | 61:85 (41.78%:58.22%) | – | – | – |
| Location | ||||
| Sellar region | 146 (100%) | – | – | – |
| Frontal lobe | – | 40 (54.05%) | – | – |
| Insular | – | 17 (22.97%) | – | – |
| Parietal lobe | – | 11 (14.86%) | – | – |
| Temporal lobe | – | 6 (8.11%) | – | – |
| Occipital lobe | – | 0 | – | – |
| Laterality (R/L) | – | 35:39 (47.30%:52.70%) | – | – |
| Dominant hemisphere tumor | – | 37 (50.00%) | – | – |
| Tumor size (cm3) | 6.25 ± 7.03 | 35.85 ± 33.11 | – | <0.001 |
| Suprasellar extension | 96 (65.75%) | – | – | – |
| Suprasellar extension (mm) | 11.47 ± 5.68 | – | – | – |
| Surgical approach | ||||
| Endonasal transsphenoidal | 140 (95.89%) | – | – | – |
| Transcranial | 6 (4.11%) | 74 (100%) | – | – |
| Pathology | ||||
| Gonadotroph adenoma | 52 (35.62%) | – | – | – |
| Somatotroph adenoma | 39 (26.71%) | – | – | – |
| Corticotroph adenoma | 27 (18.49%) | – | – | – |
| Lactotroph adenoma | 16 (10.96%) | – | – | – |
| Null cell adenoma | 11 (7.53%) | – | – | – |
| Thyrotroph adenoma | 1 (0.68%) | – | – | – |
| WHO I | – | 6 (8.11%) | – | – |
| WHO II | – | 39 (52.70%) | – | – |
| WHO III | – | 20 (27.03%) | – | – |
| WHO IV | – | 9 (12.16%) | – | – |
Boldface type indicates statistically significant differences.
“–”, not applicable; HCs, healthy controls; L, left; PitNET, pituitary neuroendocrine tumor; R, right.
Preoperative comparison of PitNET patients, glioma patients, and HCs
Table 2 presents the preoperative cognitive and affective scores for the three groups in our study. Patients with PitNET exhibited a notable decline in memory test performance compared to HCs (DST, p = 0.020; DST‐s, p = 0.006), although no deficits were observed in general cognitive function, executive function, or attention. Individual‐level analysis revealed memory deficits in 42 (28.76%) PitNET patients. Patients with glioma showed poorer executive function (FAB, p = 0.001) and attention (ACS, p < 0.001; attention focusing subscale, p < 0.001; attention diversion subscale, p < 0.001) than HCs. No deficits in general cognitive function or memory were observed in the glioma group. Patients with PitNET showed better executive function (FAB, p = 0.002) and attention (ACS, p < 0.001; attention focusing subscale, p < 0.001; attention diversion subscale, p < 0.001) than those in the glioma group.
Table 2.
Cognitive and affective functioning of the participants.
| Values | PitNET (n = 146) | Glioma (n = 74) | HCs (n = 52) | F score | p‐value |
|---|---|---|---|---|---|
| General cognitive function | |||||
| MMSE | −0.00 ± 1.12 | −0.40 ± 1.42 | 0.00 ± 1.00 | – | 0.111 |
| MoCA | −0.27 ± 0.97 | −0.11 ± 1.09 | 0.00 ± 1.00 | – | 0.148 |
| Executive function | |||||
| TMT‐B | 0.22 ± 1.11 | 0.65 ± 1.52 | 0.00 ± 1.00 | – | 0.050 b |
| FAB | −0.24 ± 1.35 | −1.24 ± 2.35 | 0.00 ± 1.00 | – | <0.001 b c |
| Memory | |||||
| DST | −0.43 ± 0.91 | −0.22 ± 1.14 | 0.00 ± 1.00 | 4.013 | 0.019 a |
| DST‐f | −0.27 ± 0.87 | −0.11 ± 1.02 | 0.00 ± 1.00 | – | 0.080 |
| DST‐b | −0.36 ± 0.98 | −0.25 ± 1.17 | 0.00 ± 1.00 | – | 0.121 |
| DST‐s | −0.38 ± 0.83 | −0.14 ± 0.98 | 0.00 ± 1.00 | – | 0.005 a |
| Attention | |||||
| TMT‐A | 0.16 ± 0.98 | 0.24 ± 1.17 | 0.00 ± 1.00 | – | 0.459 |
| ACS | 0.01 ± 1.29 | −1.48 ± 0.87 | 0.00 ± 1.00 | 63.044 | <0.001 b c |
| Attention focusing | −0.12 ± 1.07 | −1.62 ± 1.02 | 0.00 ± 1.00 | – | <0.001 b c |
| Attention diversion | 0.13 ± 1.28 | −0.87 ± 0.83 | 0.00 ± 1.00 | 27.381 | <0.001 b c |
| Anxiety | |||||
| HAMA | 0.97 ± 1.54 | 1.06 ± 1.50 | 0.00 ± 1.00 | – | <0.001 a b |
| Mental | 0.57 ± 1.34 | 0.71 ± 1.38 | 0.00 ± 1.00 | – | 0.010 a b |
| Physical | 1.32 ± 1.89 | 1.24 ± 1.75 | 0.00 ± 1.00 | – | <0.001 a b |
| Depression | |||||
| BDI | 0.18 ± 1.13 | 0.23 ± 1.08 | 0.00 ± 1.00 | – | 0.531 |
| Affection | |||||
| PANAS | 0.07 ± 0.95 | −0.01 ± 0.96 | 0.00 ± 1.00 | 0.252 | 0.778 |
| PANASp | −0.36 ± 1.02 | −0.47 ± 0.97 | 0.00 ± 1.00 | – | 0.021 b |
| PANASn | 0.61 ± 1.24 | 0.63 ± 1.33 | 0.00 ± 1.00 | – | 0.003 a b |
| Empathy | |||||
| IRI | 0.04 ± 1.09 | −0.63 ± 0.90 | 0.00 ± 1.00 | 11.342 | <0.001 b c |
| Perspective taking | 0.13 ± 1.13 | −0.08 ± 1.06 | 0.00 ± 1.00 | – | 0.226 |
| Fantasy | 0.17 ± 1.06 | −0.23 ± 0.83 | 0.00 ± 1.00 | 4.966 | 0.008 c |
| Empathy concern | 0.05 ± 1.03 | −1.06 ± 0.67 | 0.00 ± 1.00 | – | <0.001 b c |
| Personal distress | −0.24 ± 1.29 | −0.24 ± 1.36 | 0.00 ± 1.00 | – | 0.469 |
Boldface type indicates statistically significant differences.
“–”, not applicable; ACS, Attentional Control Scale; BDI, Beck Depression Inventory; DST: Digit Span Test; DST‐b, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Backward; DST‐f, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Forward; DST‐s, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Sort; FAB, Frontal Assessment Battery; HAMA, Hamilton Anxiety Scale; HCs, healthy controls; IRI, Interpersonal Reactivity Index; MMSE, mini‐mental state examination; MoCA, Montreal Cognitive Assessment; PANAS, Positive and Negative Affect Scale; PANASn, Positive and Negative Affect Scale‐negative affect; PANASp, Positive and Negative Affect Scale‐positive affect; PitNET, pituitary neuroendocrine tumor; TMT‐A, Trail‐Making Test part A; TMT‐B, Trial‐Making Test‐part B.
Significant differences between PitNET patients and HCs (p < 0.05).
Significant differences between glioma patients and HCs (p < 0.05).
Significant differences between PitNET and glioma patients (p < 0.05).
Turning to affective domains, the PitNET group showed more anxiety (HAMA, p < 0.001; mental subscale, p = 0.037; physical subscale, p < 0.001) and negative affect (PANASn, p = 0.003) than the HCs, although no deficits were observed concerning depression or empathy. Individual‐level analysis showed anxiety in 87 (59.59%) PitNET patients, along with negative affect in 52 (35.61%) PitNET patients. On the other hand, patients with gliomas also exhibited more anxiety (HAMA, p < 0.001; mental subscale, p = 0.009; physical subscale, p < 0.001) and negative affect (PANASn, p = 0.014) than HCs. Moreover, there was a noticeable reduction in positive affect (PANASp, p = 0.019) and empathy (IRI, p = 0.003, empathic concern subscale, p < 0.001) within the glioma group in comparison to the HCs. Nevertheless, depression was not observed in the glioma group. Additionally, it is worth noting that patients with PitNET showed a higher capacity for empathy compared to the glioma group (IRI, p < 0.001, fantasy subscale, p = 0.006, empathic concern subscale, p < 0.001).
Preoperative versus postoperative results
The cognitive and affective changes before and after surgery in both the PitNET and glioma groups are summarized in Tables 3 and 4, respectively. In comparison to the preoperative data, the PitNET group exhibited noteworthy postoperative enhancements in general cognitive function scores (MMSE, p = 0.045; MoCA, p = 0.034) and executive function scores (TMT‐B, p < 0.001; FAB, p = 0.025). Improved scores were also observed on the DST‐b memory test (p = 0.045) and the TMT‐A attention test (p = 0.001). Additionally, the PitNET group showed reduced levels of anxiety (HAMA, p = 0.002; mental subscale, p = 0.004; physical subscale, p = 0.015) and negative affect (PANASn subscale; p = 0.045) following surgery. However, no statistically improved scores were observed in the domains of positive affect, depression, or empathy within the PitNET patient group. Conversely, there were no statistically improved scores detected in either cognitive or affective domains within the glioma group following the surgical intervention.
Table 3.
Comparison between preoperative and postoperative cognitive and affective functioning in participants with PitNET.
| Values | Pre‐operation (n = 40) | Post‐operation (n = 40) | t/Z | p‐value |
|---|---|---|---|---|
| General cognitive function | ||||
| MMSE | 0.06 ± 1.09 | 0.35 ± 0.60 | −2.002 | 0.045 |
| MoCA | −0.29 ± 0.95 | 0.07 ± 0.98 | −2.125 | 0.034 |
| Executive function | ||||
| TMT‐B | 0.46 ± 1.31 | −0.18 ± 1.08 | −3.929 | <0.001 |
| FAB | −0.47 ± 1.82 | 0.20 ± 0.68 | −2.235 | 0.025 |
| Working memory | ||||
| DST | −0.45 ± 0.85 | −0.26 ± 0.97 | −1.721 | 0.093 |
| DST‐f | −0.42 ± 0.87 | −0.33 ± 0.91 | −0.697 | 0.490 |
| DST‐b | −0.32 ± 0.86 | −0.08 ± 1.01 | −2.006 | 0.045 |
| DST‐s | −0.35 ± 0.84 | −0.23 ± 0.91 | −0.919 | 0.358 |
| Attention | ||||
| TMT‐A | 0.15 ± 0.95 | −0.25 ± 0.84 | −3.347 | 0.001 |
| ACS | −0.22 ± 1.51 | 0.01 ± 1.37 | −1.261 | 0.215 |
| Attention focusing | −0.27 ± 1.18 | −0.12 ± 1.06 | −0.924 | 0.361 |
| Attention diversion | −0.08 ± 1.49 | 0.13 ± 1.54 | −1.174 | 0.247 |
| Anxiety | ||||
| HAMA | 0.97 ± 1.37 | 0.20 ± 1.50 | −3.046 | 0.002 |
| Mental | 0.41 ± 1.10 | −0.12 ± 1.27 | −2.843 | 0.004 |
| Physical | 1.61 ± 1.99 | 0.72 ± 1.71 | −2.427 | 0.015 |
| Depression | ||||
| BDI | 0.30 ± 1.41 | −0.09 ± 0.88 | −1.467 | 0.142 |
| Affection | ||||
| PANAS | −0.02 ± 0.91 | −0.07 ± 1.02 | 0.317 | 0.753 |
| PANASp | −0.36 ± 0.97 | −0.13 ± 1.06 | −1.600 | 0.118 |
| PANASn | 0.46 ± 1.07 | 0.06 ± 1.10 | −2.005 | 0.045 |
| Empathy | ||||
| IRI | 0.13 ± 1.19 | 0.17 ± 1.06 | −0.249 | 0.805 |
| Perspective taking | 0.13 ± 1.11 | 0.00 ± 1.11 | −0.941 | 0.347 |
| Fantasy | 0.30 ± 1.16 | 0.44 ± 0.99 | −0.673 | 0.505 |
| Empathy concern | 0.28 ± 0.97 | 0.24 ± 0.75 | −0.582 | 0.561 |
| Personal distress | −0.32 ± 1.50 | −0.35 ± 1.47 | −0.103 | 0.918 |
Boldface type indicates statistically significant differences.
ACS, Attentional Control Scale; BDI, Beck Depression Inventory; DST: Digit Span Test; DST‐b, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Backward; DST‐f, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Forward; DST‐s, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Sort; FAB, Frontal Assessment Battery; HAMA, Hamilton Anxiety Scale; IRI, Interpersonal Reactivity Index; MMSE, mini‐mental state examination; MoCA, Montreal Cognitive Assessment; PANAS, Positive and Negative Affect Scale; PANASn, Positive and Negative Affect Scale‐negative affect; PANASp, Positive And Negative Affect Scale‐positive affect; PitNET, pituitary neuroendocrine tumor; TMT‐A, Trail‐Making Test‐part A; TMT‐B, Trial‐Making Test‐part B.
Table 4.
Comparison between preoperative and postoperative cognitive and affective functioning in participants with glioma.
| Values | Pre‐operation (n = 35) | Post‐operation (n = 35) | t/Z | p value |
|---|---|---|---|---|
| General cognitive function | ||||
| MMSE | −0.33 ± 1.50 | −0.31 ± 1.85 | −0.738 | 0.460 |
| MoCA | −0.13 ± 1.24 | −0.34 ± 1.50 | −0.431 | 0.666 |
| Executive function | ||||
| TMT‐B | 0.87 ± 1.72 | 0.67 ± 1.54 | −0.118 | 0.906 |
| FAB | −1.69 ± 2.78 | −2.01 ± 3.94 | −0.525 | 0.599 |
| Working memory | ||||
| DST | −0.38 ± 1.26 | −0.30 ± 1.42 | −0.361 | 0.720 |
| DST‐f | −0.24 ± 1.15 | −0.26 ± 1.26 | −0.021 | 0.983 |
| DST‐b | −0.38 ± 1.25 | −0.15 ± 1.38 | −1.178 | 0.239 |
| DST‐s | −0.28 ± 1.04 | −0.31 ± 1.09 | −0.218 | 0.828 |
| Attention | ||||
| TMT‐A | 0.39 ± 1.45 | 0.57 ± 1.51 | −0.527 | 0.598 |
| ACS | −1.41 ± 0.77 | −1.56 ± 1.03 | −0.984 | 0.325 |
| Attention focusing | −1.49 ± 0.78 | −1.32 ± 1.13 | −0.056 | 0.955 |
| Attention diversion | −0.89 ± 0.83 | −1.27 ± 0.83 | −1.953 | 0.051 |
| Anxiety | ||||
| HAMA | 1.00 ± 1.53 | 0.72 ± 2.00 | −0.948 | 0.343 |
| Mental | 0.62 ± 1.44 | 0.38 ± 1.61 | −0.618 | 0.537 |
| Physical | 1.28 ± 1.76 | 1.03 ± 2.26 | −0.866 | 0.387 |
| Depression | ||||
| BDI | 0.08 ± 1.09 | 0.14 ± 1.00 | −0.592 | 0.554 |
| Affection | ||||
| PANAS | −0.02 ± 1.00 | −0.36 ± 1.08 | 1.456 | 0.155 |
| PANASp | −0.47 ± 1.02 | −0.70 ± 1.14 | −0.762 | 0.446 |
| PANASn | 0.61 ± 1.52 | 0.39 ± 1.30 | −0.814 | 0.416 |
| Empathy | ||||
| IRI | −0.60 ± 0.78 | −0.72 ± 1.05 | 0.647 | 0.522 |
| Perspective taking | −0.04 ± 1.08 | −0.29 ± 1.05 | 0.967 | 0.341 |
| Fantasy | −0.08 ± 0.76 | −0.34 ± 1.28 | 1.317 | 0.197 |
| Empathy concern | −1.23 ± 0.56 | −1.17 ± 0.74 | −0.458 | 0.650 |
| Personal distress | −0.18 ± 1.42 | −0.03 ± 1.14 | −0.569 | 0.573 |
ACS, Attentional Control Scale; BDI, Beck Depression Inventory; DST: Digit Span Test; DST‐b, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Backward; DST‐f, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Forward; DST‐s, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Sort; FAB, Frontal Assessment Battery; HAMA, Hamilton Anxiety Scale; IRI, Interpersonal Reactivity Index; MMSE, mini‐mental state examination; MoCA, Montreal Cognitive Assessment; PANAS, Positive and Negative Affect Scale; PANASn, Positive and Negative Affect Scale‐negative affect; PANASp, Positive and Negative Affect Scale‐positive affect; TMT‐A, Trail‐Making Test‐part A; TMT‐B, Trial‐Making Test‐part B.
To mitigate the potential influence of practice effects, HCs underwent a second assessment after the initial evaluation, with the same time interval as that used for the other two groups (Table S1). This additional assessment revealed no statistically significant differences, except for the mental subscale of the HAMA (p = 0.015).
Postoperative comparison of PitNET patients, glioma patients, and HCs
The postoperative cognitive and affective scores of PitNET and glioma patients, along with the results of the second assessment of HCs, are summarized in Table 5. There were no significant differences in the time interval between the two neuropsychological tests among the three groups (p = 0.333).
Table 5.
Demographic, clinical, cognitive, and affective comparison among the participants at follow‐up.
| Values | PitNET (n = 40) | Glioma (n = 35) | HCs (n = 25) | F score | p value |
|---|---|---|---|---|---|
| Sex (M/F) | 20:20 (50.00%:50.00%) | 16:19 (45.71%:54.29%) | 9:16 (36.00%:64.00%) | – | 0.541 |
| Age (y) | 38.25 ± 8.70 | 39.31 ± 11.07 | 35.36 ± 10.16 | – | 0.504 |
| Education (y) | 12.88 ± 3.30 | 13.37 ± 2.81 | 12.76 ± 3.55 | 0.700 | |
| Dominant hand (R/L) | 39:1 (97.50%:2.50%) | 30:5 (85.71%:14.29%) | 24:1 (96.00%:4.00%) | 0.140 | |
| Time interval (mos) | 3.48 ± 1.74 | 4.94 ± 3.93 | 3.36 ± 0.64 | – | 0.333 |
| General cognitive function | |||||
| MMSE | 0.33 ± 0.60 | −0.30 ± 1.90 | 0.24 ± 0.93 | – | 0.060 |
| MoCA | 0.05 ± 0.98 | −0.35 ± 1.53 | 0.55 ± 0.96 | – | 0.014 b |
| Executive function | |||||
| TMT‐B | −0.18 ± 1.08 | 0.67 ± 1.54 | −0.61 ± 0.71 | – | 0.003 b |
| FAB | 0.14 ± 0.76 | −1.75 ± 3.91 | 0.24 ± 0.63 | – | <0.001 b c |
| Memory | |||||
| DST | −0.28 ± 0.98 | −0.22 ± 1.40 | 0.61 ± 1.01 | 5.182 | 0.007 a b |
| DST‐f | −0.34 ± 0.92 | −0.26 ± 1.24 | 0.17 ± 0.95 | – | 0.149 |
| DST‐b | −0.08 ± 1.02 | −0.05 ± 1.35 | 0.54 ± 1.09 | – | 0.065 |
| DST‐s | −0.26 ± 0.90 | −0.23 ± 1.06 | 0.74 ± 1.05 | 9.154 | <0.001 a b |
| Attention | |||||
| TMT‐A | −0.27 ± 0.85 | 0.46 ± 1.47 | −0.33 ± 0.95 | – | 0.022 b |
| ACS | −0.00 ± 1.39 | −1.48 ± 0.95 | 0.19 ± 1.18 | 20.826 | <0.001 b c |
| Attention focusing | −0.11 ± 1.07 | −1.24 ± 1.10 | 0.04 ± 0.92 | – | <0.001 b c |
| Attention diversion | 0.10 ± 1.41 | −1.21 ± 0.77 | 0.26 ± 1.44 | – | <0.001 b c |
| Anxiety | |||||
| HAMA | 0.19 ± 1.51 | 0.67 ± 2.05 | −0.54 ± 0.68 | – | 0.033 b |
| Mental | −0.15 ± 1.27 | 0.38 ± 1.66 | −0.71 ± 0.52 | – | 0.008 b |
| Physical | 0.76 ± 1.72 | 0.94 ± 2.28 | 0.03 ± 1.05 | – | 0.133 |
| Depression | |||||
| BDI | −0.08 ± 0.89 | 0.16 ± 1.03 | −0.35 ± 0.51 | – | 0.197 |
| Affection | |||||
| PANAS | −0.08 ± 1.03 | −0.28 ± 1.05 | −0.64 ± 1.00 | 1.925 | 0.151 |
| PANASp | −0.13 ± 1.07 | −0.65 ± 1.14 | −0.53 ± 0.87 | 3.027 | 0.053 |
| PANASn | 0.05 ± 1.11 | 0.45 ± 1.31 | −0.30 ± 0.72 | – | 0.248 |
| Empathy | |||||
| IRI | 0.13 ± 1.04 | −0.69 ± 1.00 | −0.30 ± 1.08 | 6.545 | 0.002 c |
| Perspective taking | −0.03 ± 1.10 | −0.22 ± 0.96 | −0.14 ± 0.83 | – | 0.793 |
| Fantasy | 0.42 ± 1.00 | −0.37 ± 1.25 | 0.08 ± 1.23 | 4.127 | 0.019 c |
| Empathy concern | 0.23 ± 0.75 | −1.19 ± 0.75 | 1.19 ± 2.50 | – | <0.001 b c |
| Personal distress | −0.40 ± 1.45 | 0.03 ± 1.13 | 0.04 ± 1.04 | – | 0.530 |
Boldface type indicates statistically significant differences.
“–”, not applicable; ACS, Attentional Control Scale; BDI, Beck Depression Inventory; DST: Digit Span Test; DST‐b, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Backward; DST‐f, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Forward; DST‐s, Wechsler Adult Intelligence Scale—Fourth Edition Digit Span‐Sort; FAB, Frontal Assessment Battery; HAMA, Hamilton Anxiety Scale; HCs, healthy controls; IRI, Interpersonal Reactivity Index; MMSE, mini‐mental state examination; MoCA, Montreal Cognitive Assessment; PANAS, Positive and Negative Affect Scale; PANASn, Positive and Negative Affect Scale‐negative affect; PANASp, Positive and Negative Affect Scale‐positive affect; PitNET, pituitary neuroendocrine tumor; TMT‐A, Trail‐Making Test‐part A; TMT‐B, Trial‐Making Test‐part B.
Significant differences between PitNET patients and HCs (p < 0.05).
Significant differences between glioma patients and HCs (p < 0.05).
Significant differences between PitNET and glioma patients (p < 0.05).
In consonance with the presurgical evaluation, the PitNET group continued to exhibit diminished postoperative memory performance in comparison to the HCs (DST, p = 0.016; DST‐s, p = 0.001). Diverging from the presurgical assessment, the glioma group not only persisted in displaying poorer executive function (TMT‐B, p = 0.003; FAB, p = 0.002) and attention (TMT‐A, p < 0.001; attention focusing subscale, p < 0.001; attention diversion subscale, p < 0.001) than the HCs after surgery but also manifested new deficits in general cognitive function (MoCA, p = 0.014) and memory (DST, p = 0.014; DST‐s, p < 0.001). Furthermore, mirroring the presurgical evaluation, the PitNET group continued to demonstrate superior executive function (FAB, p = 0.002) and attention (ACS, p < 0.001; attention focusing subscale, p < 0.001; attention diversion subscale, p < 0.001) relative to the glioma group postoperatively.
Regarding the affective domains, the PitNET group did not exhibit any postoperative deficit in any affective test. Conversely, the glioma group continued to display increased anxiety levels (HAMA, p = 0.032; mental subscale, p = 0.006) and lower levels of empathy compared to the HCs (empathic concern subscale, p < 0.001). Additionally, the PitNET group maintained a higher level of empathy (IRI, p = 0.001; fantasy subscale, p = 0.015; empathic concern subscale, p < 0.001) than the glioma group postoperatively.
Correlation analysis
Correlations between clinical variables and cognitive/affective functions in the PitNET and glioma groups are presented in Figures 2 and 3. In the PitNET group, some cognitive and affective functions were associated with tumor size rather than disease course. Specifically, larger tumor size was correlated with worse attention, more anxiety, abnormal affect, and low empathy. In contrast, in the glioma group, some cognitive and affective functions were associated with disease course rather than tumor size. Specifically, a longer disease course was correlated with worse memory, poor executive function, abnormal affect, and low empathy.
Figure 2.
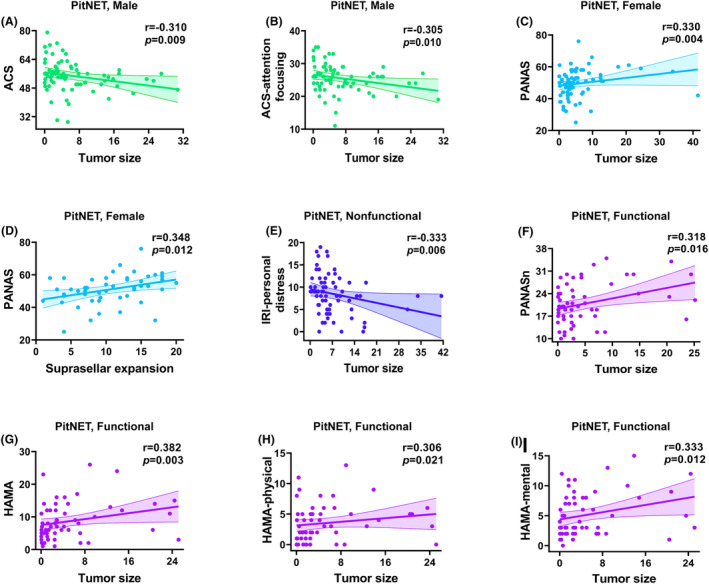
Correlations of clinical variables with cognitive and affective deficits in PitNET patients (A–I). Only correlations significant at p < 0.05 are shown. PitNET, pituitary neuroendocrine tumors; ACS, Attentional Control Scale; HAMA, Hamilton Anxiety Scale; IRI, Interpersonal Reactivity Index; PANAS, Positive and Negative Affect Scale; PANASn, Positive and Negative Affect Scale‐negative affect; PANASp, Positive and Negative Affect Scale‐positive affect.
Figure 3.
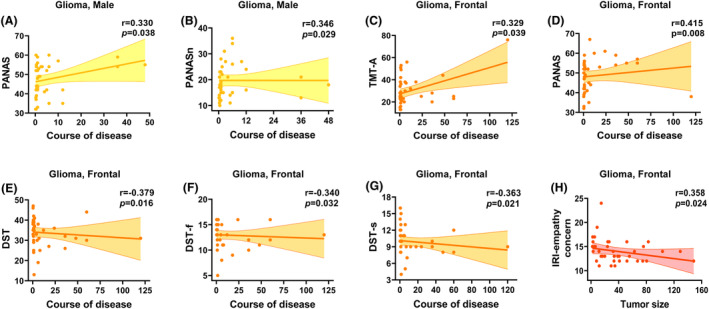
Correlations of clinical variables with cognitive and affective deficits in glioma patients (A–H). Only correlations significant at p < 0.05 are shown. DST, Wechsler Adult Intelligence Scale–Fourth Edition Digit Span Test; DST‐f, Wechsler Adult Intelligence Scale–Fourth Edition Digit Span‐Forward; DST‐s, Wechsler Adult Intelligence Scale–Fourth Edition Digit Span‐Sort; IRI, Interpersonal Reactivity Index; PANAS, Positive and Negative Affect Scale; PANASn, Positive and Negative Affect Scale‐negative affect; PANASp, Positive and Negative Affect Scale‐positive affect; TMT‐A, Trail Making Test‐part A.
Discussion
In the current study, we investigated the neuropsychological difference between PitNET and glioma by enrolling 272 participants (including 146 PitNET patients, 74 glioma patients, and 52 HCs). Our findings revealed that neuropsychological impairments in PitNET share both similarities and distinctions with those in glioma patients. Moreover, the response to surgical intervention for neuropsychological impairments differed significantly between PitNET and glioma patients. To the best of our knowledge, this is the first study to comprehensively evaluate the neuropsychological status of PitNET patients using intra‐axial tumor patients as a disease control.
Mild and focal cognitive and affective impairments in PitNET compared to glioma
Although both PitNET and glioma patients experience the dual burden of cognitive and affective deficits, the nature of these deficits differs substantially. In PitNET patients, the deficits are relatively mild and focal compared to HCs, whereas in glioma patients, they are severe and extensive compared to HCs. Specifically, PitNET patients exhibited no deficits in most cognitive domains (including general cognitive function, executive function, and attention) except for the memory domain. In contrast, patients with gliomas showed deficits in executive function and attention but not memory. In terms of affective domains, PitNET patients exhibited anxiety (both mental and physical anxiety) and negative affect, with no deficits in terms of depression, positive affect, or empathy. Conversely, patients with gliomas not only showed anxiety (mental and physical) and negative affect as PitNET patients but also exhibited little positive affect and low empathy.
It is worth noting that our study included various types of PitNET as well as different grades and locations of glioma. Previous studies reported both commonalities and distinctions in neuropsychological characteristics among these subtypes. For example, patients with prolactin adenoma presented deficits in verbal/nonverbal memory and attention. 13 Patients with somatotroph adenoma showed deficits in executive function, 6 , 15 attention, 14 and depression. 12 , 16 Patients with corticotroph adenoma exhibited deficits in memory, executive function, and depression. 33 Patients with nonfunctional adenoma displayed deficits in memory and attention. 11 Regarding glioma, previous studies reported memory and executive deficits in patients with frontal glioma, 34 , 35 impaired empathy ability in those with insular glioma, 36 verbal working memory deficits in those with low‐grade glioma, 37 and anxiety and depression in those with high‐grade glioma. 38 Although each subtype has unique feature, our study mainly focuses on delineating the neuropsychological distinctions between PitNET and glioma patients. The aim is to facilitate the initial screening and assessment of neuropsychological status rather than individualized assessment. Consequently, we include all PitNET and glioma subtypes collectively for analysis and comparison, which may explain some inconsistencies in our results compared to previous findings. Additionally, the methods used to assess neuropsychological domains varied among studies. Each scale has a specific focus, which may also account for some inconsistent results.
Restorable cognitive and affective impairments in PitNET but not glioma patients after surgery
The difference in neuropsychological resilience between PitNET and glioma patients is striking. PitNET patients showed statistically significant improvements of presurgery anxiety and negative affect at the 3 months follow‐up. Additionally, surgical intervention also ameliorated the initially low preoperative scores of many cognitive scales in PitNET patients, bringing them closer to the levels observed in healthy controls. This observation aligns with prior studies on PitNET patients, which also reported improvements in some cognitive domains following surgery. 39 , 40 This rapid short‐term recovery underscores the positive effect of standard surgical therapy and perioperative management on neuropsychological functioning in PitNET patients. Thus, an aggressive and appropriate treatment strategy is warranted for PitNET patients because the impairments were reversible. Conversely, glioma patients not only failed to show any improvement but also exhibited worse general cognition and memory postoperatively. Surgery plays a limited role of improving neuropsychological status due to irreversible impairments. We should explore alternative treatment approaches to ameliorate neuropsychological status.
Persistent memory deficits in PitNET patients
Additionally, it is noteworthy that no statistical improvement was observed in memory deficits in PitNET patients. Butterbrod et al. 11 also reported continuous postoperative memory deficits in PitNET patients at the 3 months follow‐up. These findings suggest that memory deficits may be irreversible during the short‐term period compared to other cognitive deficits in PitNET patients. The neural mechanisms underlying this memory deficit remain unclear. We infer that it may attribute to the PitNET tumor compressing surrounding frontal lobe or the hippocampal structure within the temporal lobe, which being associated with memory functioning. 41 We also do not preclude the possibility that the memory deficits may reach an improvement at a long‐term follow‐up observation, spanning 1 or 2 years. In a word, memory deficits require more extended care and long‐term management scheme.
Factors impacting cognitive and affective functioning in PitNET patients
The microstructural changes in the brain are different between PitNET and glioma. PitNET has a relatively mild and noninvasive impact on brain tissue. PitNET tends to indirectly influence brain function via the abnormal secretion of hormones (excessive or insufficient) 15 , 42 , 43 or compressing the surrounding brain tissue. 44 In contrast, glioma tends to result in the destructive infiltration of the cortical and subcortical structures that are crucial for cognition. 7 These different microstructural changes may explain the milder cognitive and affective impairments in PitNET patients compared to glioma patients.
Regarding the rapid postsurgical improvements observed in PitNET patients, it is plausible to speculate that tumor resection reduces secretions from active tumor cells and relieves the compression on surrounding brain tissue. Conversely, due to the growth of glioma merging with normal brain tissue, the inevitable brain tissue resection following the surgical intervention results in the limited improvement of neuropsychological impairments and may even lead to further neuropsychological deficits. Another possible factor may be the fact that PitNET is mainly operated through endonasal transsphenoidal approach while glioma is operated transcranially. Nevertheless, a previous study found that no statistically neuropsychological difference was observed between endonasal transsphenoidal and transcranial approach in PitNET patients. 39 Therefore, we infer that the surgical approach may be not the primary cause of postoperative neuropsychological differences.
In addition, cognitive and affective deficits in brain tumor patients are related to various clinical factors, such as tumor size, tumor growth rates, 1 abnormal neurotransmitter activity, 45 headaches, 46 and epilepsy. 47 In our study, we observed that cognitive and affective impairments were primarily associated with larger tumor size in PitNET patients, rather than the duration of the disease course. Conversely, in glioma patients, cognitive and affective impairments were more strongly linked to the duration of the disease course rather than tumor size. Our findings indicate that it is crucial to provide additional attention and care to neuropsychological problems in PitNET patients with larger tumors and glioma patients with longer disease course.
Limitations
Several limitations of this study should be acknowledged. First, because of COVID‐19‐pandemic‐related restrictions on mobility, some patients were lost to follow‐up. Although this limited the data that could be collected, we believe that the obtained data are adequate to assess postsurgical cognitive and affective status. Second, the average follow‐up duration is relatively short (3 months). A long‐term follow‐up study is therefore needed in the future. Despite these limitations, our results provide valuable insights into the cognitive and affective functioning of PitNET patients.
Conclusion
Despite PitNET being an extra‐axial tumor, PitNET patients experience both cognitive and affective impairments. These impairments are distinctive from those in patients with glioma. PitNET patients display mild and focal neuropsychological deficits compared with the severe and extensive dysfunction in glioma (intra‐axial tumors). Moreover, cognitive and affective impairments are restorable in PitNET but not in glioma patients. The scores of many neuropsychological scales can obtain significant amelioration in PitNET patients after standard surgical intervention and perioperative management within a short‐term period (3 months) except for memory. Memory deficits may prove challenging to restore during a short period and require long‐term attention and care. Additionally, some cognitive and affective impairments in PitNET patients may be related to larger tumor size, which suggests that it is important to pay additional attention to neuropsychological problems in PitNET patients with larger tumor. Our findings establish a theoretical foundation for more precise neuropsychological therapies for PitNET patients, and guide different neuropsychological management approaches for intra‐ and extra‐axial tumors.
Author Contributions
We would like to thank all authors who contributed to the study. Conception and design: Pinan Liu, Xingchao Wang, and Hanlu Tang. Methodology: Hanlu Tang and Xingchao Wang. Data acquisition: Hanlu Tang, Yehong Fang, Zhixu Bie, Ruolin Yang, and Heyuan Jia. Writing—original draft preparation: Hanlu Tang and Yehong Fang. Writing review and editing: Pinan Liu, Bo Wang, Zhijun Yang, and Zhixian Gao. Supervision: Pinan Liu and Xingchao Wang. We also thank all participants who took part in the study.
Conflict of Interest
No authors have any potential conflicts of interest to disclose.
Supporting information
Table S1.
Acknowledgments
This work was supported by the Beijing Municipal Science and Technology Project (2022) (Grant Number: Z221100007422041, to P.L.)
Funding Statement
This work was funded by Beijing Municipal Science and Technology Project (2022) grant Z221100007422041.
Contributor Information
Xingchao Wang, Email: wangxc@mail.ccmu.edu.cn.
Pinan Liu, Email: pinanliu@ccmu.edu.cn.
References
- 1. Ali FS, Hussain MR, Gutiérrez C, et al. Cognitive disability in adult patients with brain tumors. Cancer Treat Rev. 2018;65:33‐40. doi: 10.1016/j.ctrv.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 2. Posti JP, Bori M, Kauko T, et al. Presenting symptoms of glioma in adults. Acta Neurol Scand. 2015;131(2):88‐93. doi: 10.1111/ane.12285 [DOI] [PubMed] [Google Scholar]
- 3. Acquaye AA, Lin L, Vera‐Bolanos E, Gilbert MR, Armstrong TS. Hope and mood changes throughout the primary brain tumor illness trajectory. Neuro‐Oncology. 2016;18(1):119‐125. doi: 10.1093/neuonc/nov101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324‐333; discussion 333–334. doi: 10.1097/00006123-200008000-00011 [DOI] [PubMed] [Google Scholar]
- 5. Mattoo SK, Bhansali AK, Gupta N, Grover S, Malhotra R. Psychosocial morbidity in acromegaly: a study from India. Endocrine. 2008;34(1–3):17‐22. doi: 10.1007/s12020-008-9112-8 [DOI] [PubMed] [Google Scholar]
- 6. Solomon E, Brănișteanu D, Dumbravă A, et al. Executive functioning and quality of life in acromegaly. Psychol Res Behav Manag. 2019;12:39‐44. doi: 10.2147/prbm.S183950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krishna S, Kakaizada S, Almeida N, Brang D, Hervey‐Jumper S. Central nervous system plasticity influences language and cognitive recovery in adult glioma. Neurosurgery. 2021;89(4):539‐548. doi: 10.1093/neuros/nyaa456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daly AF, Beckers A. The epidemiology of pituitary adenomas. Endocrinol Metab Clin N Am. 2020;49(3):347‐355. doi: 10.1016/j.ecl.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 9. Melmed S. Pituitary‐tumor Endocrinopathies. N Engl J Med. 2020;382(10):937‐950. doi: 10.1056/NEJMra1810772 [DOI] [PubMed] [Google Scholar]
- 10. Wijethilake N, MacCormac O, Vercauteren T, Shapey J. Imaging biomarkers associated with extra‐axial intracranial tumors: a systematic review. Front Oncol. 2023;13:1131013. doi: 10.3389/fonc.2023.1131013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butterbrod E, Gehring K, Voormolen EH, et al. Cognitive functioning in patients with nonfunctioning pituitary adenoma before and after endoscopic endonasal transsphenoidal surgery. J Neurosurg. 2019;133:709‐716. doi: 10.3171/2019.5.Jns19595 [DOI] [PubMed] [Google Scholar]
- 12. Sievers C, Dimopoulou C, Pfister H, et al. Prevalence of mental disorders in acromegaly: a cross‐sectional study in 81 acromegalic patients. Clin Endocrinol. 2009;71(5):691‐701. doi: 10.1111/j.1365-2265.2009.03555.x [DOI] [PubMed] [Google Scholar]
- 13. Bala A, Łojek E, Marchel A. Cognitive functioning of patients with a PRL‐secreting pituitary adenoma: a preliminary report. Neurology. 2016;86(8):731‐734. doi: 10.1212/wnl.0000000000002252 [DOI] [PubMed] [Google Scholar]
- 14. Sievers C, Sämann PG, Pfister H, et al. Cognitive function in acromegaly: description and brain volumetric correlates. Pituitary. 2012;15(3):350‐357. doi: 10.1007/s11102-011-0326-z [DOI] [PubMed] [Google Scholar]
- 15. Shan S, Fang L, Huang J, Chan RCK, Jia G, Wan W. Evidence of dysexecutive syndrome in patients with acromegaly. Pituitary. 2017;20(6):661‐667. doi: 10.1007/s11102-017-0831-9 [DOI] [PubMed] [Google Scholar]
- 16. Szcześniak D, Jawiarczyk‐Przybyłowska A, Rymaszewska J. The quality of life and psychological, social and cognitive functioning of patients with acromegaly. Adv Clin Exp Med. 2015;24(1):167‐172. doi: 10.17219/acem/38156 [DOI] [PubMed] [Google Scholar]
- 17. Pivonello R, Auriemma RS, Delli Veneri A, et al. Global psychological assessment with the evaluation of life and sleep quality and sexual and cognitive function in a large number of patients with acromegaly: a cross‐sectional study. Eur J Endocrinol. 2022;187(6):823‐845. doi: 10.1530/eje-22-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305‐1309. doi: 10.1200/jco.2005.04.6086 [DOI] [PubMed] [Google Scholar]
- 19. Le Fèvre C, Sun R, Cebula H, et al. Ellipsoid calculations versus manual tumor delineations for glioblastoma tumor volume evaluation. Sci Rep. 2022;12(1):10502. doi: 10.1038/s41598-022-13739-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pallud J, Taillandier L, Capelle L, et al. Quantitative morphological magnetic resonance imaging follow‐up of low‐grade glioma: a plea for systematic measurement of growth rates. Neurosurgery. 2012;71(3):729‐739; discussion 739–740. doi: 10.1227/NEU.0b013e31826213de [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 22. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 23. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11):1621‐1626. doi: 10.1212/wnl.55.11.1621 [DOI] [PubMed] [Google Scholar]
- 24. Blackburn HL, Benton AL. Revised administration and scoring of the digit span test. J Consult Psychol. 1957;21(2):139‐143. doi: 10.1037/h0047235 [DOI] [PubMed] [Google Scholar]
- 25. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19(5):393‐394. doi: 10.1037/h0044509 [DOI] [PubMed] [Google Scholar]
- 26. Tombaugh TN. Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203‐214. doi: 10.1016/s0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 27. Derryberry D, Reed MA. Anxiety‐related attentional biases and their regulation by attentional control. J Abnorm Psychol. 2002;111(2):225‐236. doi: 10.1037//0021-843x.111.2.225 [DOI] [PubMed] [Google Scholar]
- 28. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50‐55. doi: 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- 29. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561‐571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 30. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063‐1070. doi: 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 31. Davis MH. A multidimensional approach to individual differences in empathy. J Pers Soc Psychol. 1980;10(85):85‐103. [Google Scholar]
- 32. Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44(1):113‐126. [Google Scholar]
- 33. Zarino B, Verrua E, Ferrante E, et al. Cushing's disease: a prospective case‐control study of health‐related quality of life and cognitive status before and after surgery. J Neurosurg. 2019;1‐11. doi: 10.3171/2019.8.Jns19930 [DOI] [PubMed] [Google Scholar]
- 34. Mu YG, Huang LJ, Li SY, et al. Working memory and the identification of facial expression in patients with left frontal glioma. Neuro‐Oncology. 2012;14(Suppl 4):iv81‐iv89. doi: 10.1093/neuonc/nos215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang X, Zhang G, Wang Y, et al. Alteration of default mode network: association with executive dysfunction in frontal glioma patients. J Neurosurg. 2022;1‐10. doi: 10.3171/2022.8.Jns22591 [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Gu X, Fan J, et al. Recovery of empathetic function following resection of insular gliomas. J Neuro‐Oncol. 2014;117(2):269‐277. doi: 10.1007/s11060-014-1380-y [DOI] [PubMed] [Google Scholar]
- 37. Teixidor P, Gatignol P, Leroy M, Masuet‐Aumatell C, Capelle L, Duffau H. Assessment of verbal working memory before and after surgery for low‐grade glioma. J Neuro‐Oncol. 2007;81(3):305‐313. doi: 10.1007/s11060-006-9233-y [DOI] [PubMed] [Google Scholar]
- 38. Ribeiro M, Benadjaoud MA, Moisy L, et al. Symptoms of depression and anxiety in adults with high‐grade glioma: a literature review and findings in a group of patients before chemoradiotherapy and one year later. Cancer. 2022;14(21):5192. doi: 10.3390/cancers14215192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Tong X, Zou Y, Tian X, Mao Z, Sun Z. The impact on cognitive functions of patients with pituitary adenoma before and after surgery. Neurol Sci. 2017;38(7):1315‐1321. doi: 10.1007/s10072-017-2980-z [DOI] [PubMed] [Google Scholar]
- 40. Hendrix P, Griessenauer CJ, Hans E, Simgen A, Oertel J , Karbach J. Cognitive function surrounding resection of nonfunctioning pituitary adenomas with suprasellar extension: a prospective matched‐control study. J Clin Neurosci. 2017;40:109‐114. doi: 10.1016/j.jocn.2017.02.028 [DOI] [PubMed] [Google Scholar]
- 41. Desgranges B, Baron JC, Eustache F. The functional neuroanatomy of episodic memory: the role of the frontal lobes, the hippocampal formation, and other areas. NeuroImage. 1998;8(2):198‐213. doi: 10.1006/nimg.1998.0359 [DOI] [PubMed] [Google Scholar]
- 42. Crespo I, Webb SM. Perception of health and cognitive dysfunction in acromegaly patients. Endocrine. 2014;46(3):365‐367. doi: 10.1007/s12020-014-0236-8 [DOI] [PubMed] [Google Scholar]
- 43. Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol. 2013;9(6):357‐365. doi: 10.1038/nrendo.2013.78 [DOI] [PubMed] [Google Scholar]
- 44. Meskal I, Gehring K, Rutten GJ, Sitskoorn MM. Cognitive functioning in meningioma patients: a systematic review. J Neuro‐Oncol. 2016;128(2):195‐205. doi: 10.1007/s11060-016-2115-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarter M, Bruno JP, Parikh V. Abnormal neurotransmitter release underlying behavioral and cognitive disorders: toward concepts of dynamic and function‐specific dysregulation. Neuropsychopharmacology. 2007;32(7):1452‐1461. doi: 10.1038/sj.npp.1301285 [DOI] [PubMed] [Google Scholar]
- 46. Begasse de Dhaem O, Robbins MS. Cognitive impairment in primary and secondary headache disorders. Curr Pain Headache Rep. 2022;26(5):391‐404. doi: 10.1007/s11916-022-01039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perrine K, Kiolbasa T. Cognitive deficits in epilepsy and contribution to psychopathology. Neurology. 1999;53(5 Suppl 2):S39‐S48. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


