Abstract
Objective
Mutations in the glucocerebrosidase (GBA1) gene and subthalamic nucleus deep brain stimulation (STN‐DBS) are independently associated with cognitive dysfunction in persons with Parkinson's disease (PwP). We hypothesized that PwP with both GBA1 mutations and STN‐DBS are at greater risk of cognitive dysfunction than PwP with only GBA1 mutations or STN‐DBS, or neither. In this study, we determined the pattern of cognitive dysfunction in PwP based on GBA1 mutation status and STN‐DBS treatment.
Methods
PwP who are GBA1 mutation carriers with or without DBS (GBA1+DBS+, GBA1+DBS−), and noncarriers with or without DBS (GBA1−DBS+, GBA1−DBS−) were included. Using the NIH Toolbox, cross‐sectional differences in response inhibition, processing speed, and episodic memory were compared using analysis of variance with adjustment for relevant covariates.
Results
Data were available for 9 GBA1+DBS+, 14 GBA1+DBS−, 17 GBA1−DBS+, and 26 GBA1−DBS− PwP. In this cross‐sectional study, after adjusting for covariates, we found that performance on the Flanker test (measure of response inhibition) was lower in GBA1+DBS+ PwP compared with GBA1−DBS+ PwP (P = 0.030).
Interpretation
PwP who carry GBA1 mutations and have STN‐DBS have greater impaired response inhibition compared with PwP with STN‐DBS but without GBA1 mutations. Longitudinal data, including preoperative scores, are required to definitively determine whether GBA1 mutation carriers respond differently to STN‐DBS, particularly in the domain of response inhibition.
Introduction
Deep brain stimulation (DBS) is a well‐established treatment for persons with Parkinson's disease (PwP). Studies have suggested that genetic subtyping of PwP may be useful in understanding differential cognitive and motor outcomes of DBS. 1 In a nonrandomized study, we previously demonstrated that PwP with mutations in the glucocerebrosidase (GBA1) gene may be particularly susceptible to global cognitive deficit after subthalamic nucleus DBS (STN‐DBS). 2 PwP with GBA1 mutations have reduced activity of the glucocerebrosidase (GCase) enzyme resulting in impaired sphingolipid metabolism 3 with more rapid accumulation and spread of Lewy body pathology compared with non‐GBA1 PwP. 4 Clinically, GBA1 PwP are at increased risk for cognitive impairment and progress to dementia faster compared with non‐GBA1 patients. 4 , 5 , 6 , 7 , 8 Importantly, STN‐DBS itself can impair cognition, with a negative impact on verbal fluency, 9 executive control of action, 10 and inhibitory control. 11 This may be due to a micro‐lesional effect 12 , 13 and/or unintended current spread into adjacent associative and limbic subregions 14 or other nearby nuclei such as the substantia nigra. 15 Given that GBA1 mutations and STN‐DBS are independently associated with cognitive dysfunction in PwP, we hypothesized that PwP with both GBA1 mutations and STN‐DBS are at greater risk of cognitive dysfunction than PwP with only GBA1 mutations or STN‐DBS, or neither. The specific cognitive domains associated with cognitive dysfunction in PwP based on GBA1 and DBS status, alone or in combination, remain unknown.
In this cross‐sectional study, we used the NIH Toolbox to compare response inhibition (Flanker Inhibitory Control and Attention Test), processing speed (Pattern Comparison Processing Speed Test), and episodic memory (Picture Sequence Memory Test) in PwP who are GBA1 carriers with or without STN‐DBS (GBA1+DBS+, GBA1+DBS−), and noncarriers with or without STN‐DBS (GBA1−DBS+, GBA1−DBS−). These tests were selected since response inhibition 16 , 17 and processing speed 18 may be impaired by STN‐DBS (regardless of genetic status), and the combined effects of GBA1 mutations and STN‐DBS on these cognitive domains in PwP are unknown. The episodic memory task involves a visual memory component, and PwP with GBA1 mutations are more likely to have visual memory dysfunction compared with nonmutation carriers. 5 , 8 Given our prior findings that GBA1+DBS+ PwP are at greatest risk of global cognitive dysfunction compared with PwP without GBA1 mutations and/or those without DBS, 2 we hypothesized that GBA1+DBS+ PwP would be the most impaired group in one or more of the above cognitive domains.
Methods
This prospective cross‐sectional study was approved by the Institutional Review Board (IRB) at Rush University Medical Center and at Rutgers‐Robert Wood Johnson Medical School, and informed consent was obtained from each participant. PwP were recruited based on convenience sampling from the Rush Movement Disorders clinic between July 2016 and March 2019 and at Rutgers University between July 2020 and March 2023. Furthermore, PwP with known GBA1 mutations were invited to participate. PwP were recruited if they carried a clinical diagnosis of PD by United Kingdom Parkinson Disease Society Brain Bank criteria, 19 and STN‐DBS PwP met standard criteria for implantation. 20 PwP with unknown GBA1 status were fully sequenced for GBA1 as previously described. 21
Demographics and clinical data were collected in all PwP including baseline age, age at disease onset, sex, years since DBS procedure, most recent Unified Parkinson's Disease Rating Scale (UPDRS) Part‐III or International Parkinson and Movement Disorder Society revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS Part‐III) scores, and levodopa equivalent daily dose (LEDD). 22 UPDRS scores were converted to MDS‐UPDRS scores where necessary. 23 A cross‐sectional evaluation of cognition was performed using the NIH Toolbox. PwP completed three measures within the NIH Toolbox Cognitive Battery: (1) Flanker Inhibitory Control and Attention Test, to test response inhibition, (2) Pattern Comparison Processing Speed Test, to test processing speed, and (3) the Picture Sequence Memory Test, to test episodic memory. 24 DBS PwP were at least 1 year post implantation and in their optimally programmed state. All DBS PwP completed the evaluation in the ON medication/ON stimulation state, except for 1 GBA1+DBS PwP who completed the evaluation in the OFF medication/ON stimulation state (skipped Carbidopa/Levodopa 12.5/50 mg). Non‐DBS PwP were tested in the ON medication state. Details regarding the test administration protocol have been previously described. 25
For each cognitive test, a fully adjusted T‐score was calculated. This score compares the score of the test‐taker to those in the NIH Toolbox nationally representative normative sample, while adjusting for key demographic variables collected during the Toolbox national norming study. 26 These variables include age, gender, 3 races (white, black, other), ethnicity (Hispanic vs. non‐Hispanic), and educational attainment. The T‐score is devised such that the mean is 50 and standard deviation is 10. 26
Age, age of onset, disease duration, and MDS‐UPDRS were compared using one‐way analysis of variance (ANOVA). For these variables, given the unequal sample sizes among groups, we performed Levene's test for homogeneity of variances, which was not significant (P > 0.05). However, Levene's test was significant (P < 0.05) when comparing years of DBS and LEDD, so Kruskal–Wallis test was performed. Sex was compared using the chi‐squared test. GBA1 mutations were subcategorized according to severity: risk variant, mild, or severe. 27 The number of GBA1 PwP with risk variant, mild, and severe mutations was compared between the DBS and non‐DBS groups using the chi‐squared test.
For our cognitive tests (dependent variables), given the unequal sample sizes among groups, we performed Levene's test for homogeneity of variances, which was significant (P < 0.05) for the Flanker test and the Pattern Comparison Processing Speed Test, but not the Picture Sequence Memory Test (P > 0.05). To account for this, a weighted least squares (WLS) adjustment was performed for Flanker scores and Pattern Comparison Processing Speed scores. Cognitive scores were compared using ANOVA with post hoc Bonferroni adjustment for pairwise comparisons (P < 0.05). Additional analysis was performed using analysis of covariance adjusting for disease duration, MDS‐UPDRS Part III, and LEDD, using Bonferroni adjustment for pairwise comparisons (P < 0.05). Sex and age were already accounted for in the fully adjusted T score as described above.
Results
Baseline characteristics
A total of 66 PwP (9 GBA1+DBS+, 14 GBA1+DBS−, 17 GBA1−DBS+, and 26 GBA1−DBS−) were enrolled. Mean age, age of onset, sex, and MDS‐UPDRS Part III scores were not significantly different among the 4 groups (Table 1). MDS‐UPDRS Part III scores were consistent with historic values for PwP. 28 LEDD scores were > 50% lower in PwP with DBS compared to those without DBS, which can be considered a surrogate measure of effective STN‐DBS response. 2 When examining GBA1 PwP, the number of PwP with risk variant, mild, or severe mutations was not significantly different between the DBS and non‐DBS groups (P = 0.9). All GBA1 PwP were heterozygous mutation carriers, and thus, no PwP had Gaucher's disease (GD). Pattern Comparison Processing Speed scores were missing for 1 GBA1+DBS+ PwP. Picture Sequence Memory test scores were missing for 2 GBA1+DBS+, 1 GBA1−DBS+, and 1 GBA1−DBS− PwP.
Table 1.
Demographics and clinical characteristics according to GBA and DBS status
| GBA1+DBS+ | GBA1+DBS− | GBA1−DBS+ | GBA1−DBS− | |
|---|---|---|---|---|
| n | 9 | 14 | 17 | 26 |
| Demographics | ||||
| Age baseline | 60.4 (7.50) | 58.8 (7.6) | 59.5 (16.8) | 59.8 (6.2) |
| Age onset | 48.3 (10.40) | 43.4 (12.6) | 49.4 (11.7) | 47.5 (7.5) |
| Disease duration, years | 12.1 (7.8) | 15.4 (12.0) | 10.1 (13.8) | 12.3 (7.0) |
| Sex M/F | 5/4 | 8/6 | 12/5 | 16/10 |
| Years of DBS | 2.0 (1.7) | 0.0 (0.0) | 1.9 (1.3) | 0.0 (0.0) |
| MDS‐UPDRS‐III | 28.9 (11.1) (n = 8) | 24.6 (11.8) | 27.7 (8.8) | 27.2 (9.5) |
| LEDD | 440.4 (301.2) | 912.8 (650.3) | 385.9 (265.9) | 953.3 (693.6) |
| GBA1 mutation (n) | E326K (2), T369M (1), N370S (2), L279P (1), L444P (2), P134T (1) | E326K (3), T369M (1), G364R (1), G202R (1), D140H (1), H255Q (1), R159W (1), N370S (3), L444P (2) | ||
| GBA1 mutation severity | ||||
| Risk variant (%) | 3 (33.3) | 4 (28.6) | ||
| Mild (%) | 2 (22.2) | 4 (28.6) | ||
| Severe (%) | 4 (44.4) | 6 (42.8) | ||
Comparison of NIH toolbox cognitive measures according to GBA1 and DBS status
Using ANOVA, Flanker scores were significantly different between GBA1+DBS+ vs. GBA1−DBS− PwP (P = 0.041) and GBA1+DBS+ vs. GBA1−DBS+ groups (P = 0.010) (Tables 2 and 3, Fig. 1). Of note, when excluding the GBA1+DBS+ PwP who performed the Flanker test in OFF medication/ON stimulation state, significance was retained when comparing the GBA1+DBS+ vs. GBA1−DBS+ groups only (P = 0.019).
Table 2.
Performance on NIH toolbox measures according to GBA1 and DBS status
| GBA1+DBS+ | GBA1+DBS− | GBA1−DBS+ | GBA1−DBS− | |
|---|---|---|---|---|
| N | 9 | 14 | 17 | 26 |
| Flanker score (SE) | 29.88 (3.52) | 37.14 (1.56) | 46.30 (3.56) | 40.35 (1.28) |
| PCPS score (SE) | 44.55 (10.84) (n = 8) | 44.36 (3.96) | 37.39 (3.07) | 41.89 (2.59) |
| PSMT Score (SE) | 44.04 (4.79) (n = 7) | 47.29 (3.39) | 47.73 (3.07) | 49.84 (2.53) (n = 25) |
PCPS, pattern comparison processing speed; PSMT, picture sequence memory test; WLS, weighted least square.
Table 3.
Pairwise comparisons without adjustment for covariates
| Measure | Overall | GBA1+DBS+ vs. GBA1−DBS+ | GBA1+DBS+ vs. GBA1−DBS− | GBA1+DBS+ vs. GBA1+DBS− | GBA1+ DBS− vs. GBA1−DBS− | GBA1−DBS+ vs. GBA1−DBS− | GBA1+DBS− vs. GBA1−DBS+ |
|---|---|---|---|---|---|---|---|
| Flanker score | 0.007 | 0.010 | 0.041 | 0.382 | 0.711 | 0.722 | 0.129 |
| PCPS score | 0.513 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| PSMT score | 0.739 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
Above P values are reflective of Bonferroni adjustment for pairwise comparisons. Of note, the comparison between PD patients in the GBA1+DBS+ vs. GBA1−DBS+ groups remained statistically significant (P = 0.030) after adjustment for covariates. PCPS, pattern comparison processing speed; PSMT, picture sequence memory test.
Figure 1.
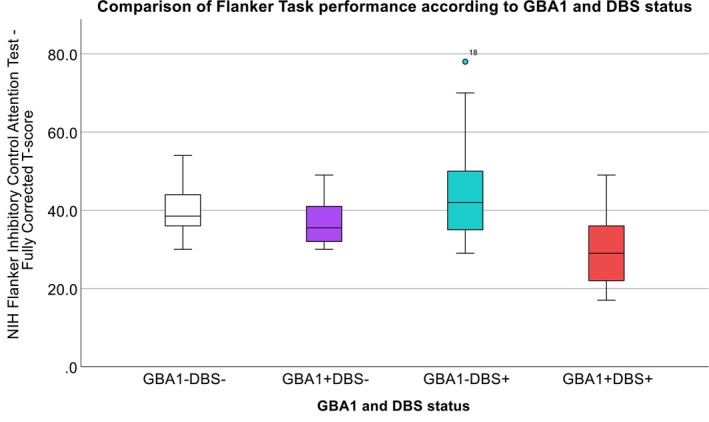
Comparison of Flanker Task performance according to GBA1 and DBS status. In persons with Parkinson's disease (PwP), Flanker scores were significantly different between GBA1+DBS+ (red) vs. GBA1−DBS− (white) groups (P = 0.041). Flanker scores were also significantly different between GBA1+DBS+ (red) vs. GBA1−DBS+ (teal) groups (P = 0.010). After adjustment for covariates, group differences in Flanker scores remained significant when comparing GBA1+DBS+ vs. GBA1−DBS+ PwP only (P = 0.030).
After adjusting for covariates including disease duration, MDS‐UPDRS Part III, and LEDD, differences between group Flanker scores remained significant when comparing GBA1+DBS+ vs. GBA1−DBS+ PwP only (P = 0.030). Flanker scores and Picture Sequence Memory Test scores were lower in GBA1+DBS+ PwP compared with GBA1+DBS− PwP (Table 2), but these differences were not statistically significant. Overall, group comparisons of Pattern Comparison Processing Speed and Picture Sequence Memory test scores were not statistically different among the four groups (Table 3).
Discussion
In this study, we aimed to define the pattern of cognitive dysfunction in PwP based on GBA1 and STN‐DBS status. Pal et al 2 and Mangone et al 29 have previously demonstrated that GBA1 PwP with STN‐DBS are at risk of cognitive decline when examining global cognitive function. In this cross‐sectional study, we found that GBA1 PwP with STN‐DBS may be more prone to impaired response inhibition compared with PwP with STN‐DBS but without GBA1 mutations.
Several studies have identified the STN as playing an important role in response inhibition, a process involved in inhibiting choices and actions especially in the face of competing alternatives. 30 STN‐DBS results in more impulsive decision making in high conflict tasks (such as Flanker or Stroop tasks). STN‐DBS PwP may have a faster reaction time potentially leading to more errors. 30 Several brain regions have been implicated in this process 31 including the inferior frontal cortex (IFC) 32 and structures within the medial prefrontal cortex (mPFC), such as the anterior cingulate cortex (ACC) and the presupplementary motor area (pre‐SMA). 33 GBA1 mutations cause a deficiency in glucocerebrosidase (GCase) enzyme activity, and both the ACC and frontal cortex, regions involved in response inhibition, may have reduced GCase activity in GBA1 carriers. 34 , 35 Consequently, it is conceivable that GBA1 carriers are at higher risk of worsening impulse control after STN‐DBS than PwP without GBA1 mutations. Anatomic and tractography studies would be useful in further exploring these associations but these data were not available in this study. However, as DBS manufacturers increasingly incorporate imaging with DBS programming, 36 these associations may be examined with greater ease in future studies.
Strengths of our study include use of validated measures from the NIH Toolbox cognition battery, comparison of several cognitive domains among the groups of interest, and comparable numbers of GBA1 PwP with risk variant, mild, and severe mutations when comparing DBS and non‐DBS groups. Limitations of this study include small sample size, cross‐sectional evaluation, and some missing data. Flanker and Picture Sequence Memory Test scores were lower in GBA1+DBS+ PwP vs. GBA1+DBS− PwP, but did not achieve statistical significance, likely due to insufficient power. Also, we did not have preoperative cognitive scores, so we are not able to definitively determine whether the between group differences are related to DBS or are simply a reflection of baseline differences according to mutation status.
In conclusion, PwP who carry GBA1 mutations and have STN‐DBS have greater impaired response inhibition compared with PwP with STN‐DBS but without GBA1 mutations. The pattern of cognitive dysfunction according to GBA1 status, mutation severity, and DBS implantation warrants further examination in a larger cohort of PwP.
Author Contributions
Gian Pal (GP) was responsible for the research project conception, organization, execution, statistical analysis, editing, and critique. Ahmad Almelegy (AA) was responsible for data collection, writing of the first draft of the manuscript, and editing and critique of the manuscript. Srujanesh Gunda (SG) was involved in study design, data organization, and the editing of the manuscript. Steven Buyske (SB) was involved in study design, data analysis, and the editing of the manuscript. Marc Rosenbaum (MR), Sepehr Sani (SS), Mitra Afshari (MA), and Leo Verhagen Metman (LVM) were involved in study design, data collection, and the editing of the manuscript. Christopher G. Goetz (CGG), Deborah Hall (DH), and M. Maral Mouradian (MMM) were responsible for the study design and the editing of the manuscript.
Conflict of Interest
None.
Acknowledgements
We would like to acknowledge our PwP who contributed to this research program.
Funding Information
This research was supported by the Parkinson Disease Foundation and the National Institute of Neurological Disorders and Stroke K23‐NS097625‐06. The Rush Parkinson's Disease and Movement Disorder Program is a designated Clinical Center of Excellence of the Parkinson's Foundation. The Robert Wood Johnson Medical School is designated as a Center for Advanced Research funded by the American Parkinson Disease Association.
[Correction added on 21 February 2024, after first online publication: The tenth author's name was corrected to M. Maral Mouradian.]
Funding Statement
This work was funded by National Institute of Neurological Disorders and Stroke grant K23‐NS097625‐06; Parkinson Disease Foundation; American Parkinson Disease Association ; Clinical Center of Excellence of the Parkinson's Foundation.
References
- 1. Artusi CA, Dwivedi AK, Romagnolo A, et al. Association of Subthalamic Deep Brain Stimulation with Motor, functional, and pharmacologic outcomes in patients with monogenic Parkinson disease: a systematic review and meta‐analysis. JAMA Netw Open. 2019;2(2):e187800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pal G, Mangone G, Hill EJ, et al. Parkinson disease and subthalamic nucleus deep brain stimulation: cognitive effects in GBA mutation carriers. Ann Neurol. 2022;91(3):424‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alcalay RN, Levy OA, Waters CC, et al. Glucocerebrosidase activity in Parkinson's disease with and without GBA mutations. Brain. 2015;138(Pt 9):2648‐2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winder‐Rhodes SE, Evans JR, Ban M, et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community‐based incident cohort. Brain. 2013;136(Pt 2):392‐399. [DOI] [PubMed] [Google Scholar]
- 5. Alcalay RN, Caccappolo E, Mejia‐Santana H, et al. Cognitive performance of GBA mutation carriers with early‐onset PD: the CORE‐PD study. Neurology. 2012;78(18):1434‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brockmann K, Srulijes K, Pflederer S, et al. GBA‐associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407‐411. [DOI] [PubMed] [Google Scholar]
- 7. Malek N, Weil RS, Bresner C, et al. Features of GBA‐associated Parkinson's disease at presentation in the UK tracking Parkinson's study. J Neurol Neurosurg Psychiatry. 2018;89(7):702‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zokaei N, McNeill A, Proukakis C, et al. Visual short‐term memory deficits associated with GBA mutation and Parkinson's disease. Brain. 2014;137(Pt 8):2303‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parsons TD, Rogers SA, Braaten AJ, Woods SP, Troster AI. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta‐analysis. Lancet Neurol. 2006;5(7):578‐588. [DOI] [PubMed] [Google Scholar]
- 10. Jahanshahi M. Effects of deep brain stimulation of the subthalamic nucleus on inhibitory and executive control over prepotent responses in Parkinson's disease. Front Syst Neurosci. 2013;7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto‐striato‐subthalamic‐pallidal network for goal‐directed and habitual inhibition. Nat Rev Neurosci. 2015;16(12):719‐732. [DOI] [PubMed] [Google Scholar]
- 12. Lefaucheur R, Derrey S, Martinaud O, et al. Early verbal fluency decline after STN implantation: is it a cognitive microlesion effect? J Neurol Sci. 2012;321(1):96‐99. [DOI] [PubMed] [Google Scholar]
- 13. Tröster AI, Jankovic J, Tagliati M, Peichel D, Okun MS. Neuropsychological outcomes from constant current deep brain stimulation for Parkinson's disease. Mov Disord. 2017;32(3):433‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coenen VA, Honey CR, Hurwitz T, Rahman AA, McMaster J, Bürgel U, Mädler B Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson's disease. Neurosurgery 2009;64(6):1106–1114; discussion 14–5. [DOI] [PubMed] [Google Scholar]
- 15. Blomstedt P, Hariz MI, Lees A, et al. Acute severe depression induced by intraoperative stimulation of the substantia nigra: a case report. Parkinsonism Relat Disord. 2008;14(3):253‐256. [DOI] [PubMed] [Google Scholar]
- 16. Obeso I, Wilkinson L, Rodriguez‐Oroz MC, Obeso JA, Jahanshahi M. Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict‐induced slowing in Parkinson's disease. Exp Brain Res. 2013;226(3):451‐462. [DOI] [PubMed] [Google Scholar]
- 17. Witt K, Pulkowski U, Herzog J, et al. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol. 2004;61(5):697‐700. [DOI] [PubMed] [Google Scholar]
- 18. Williams AE, Arzola GM, Strutt AM, Simpson R, Jankovic J, York MK. Cognitive outcome and reliable change indices two years following bilateral subthalamic nucleus deep brain stimulation. Parkinsonism Relat Disord. 2011;17(5):321‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Defer GL, Widner H, Marie RM, Remy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT‐PD). Mov Disord. 1999;14(4):572‐584. [DOI] [PubMed] [Google Scholar]
- 21. Nichols WC, Pankratz N, Marek DK, et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72(4):310‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith C. Levodopa dose equivalency: a systematic review [Powerpoint Slides] 2010. [cited 2021 04/11/2021]. https://silo.tips/download/levodopa‐dose‐equivalency‐a‐systematic‐review.
- 23. Hentz JG, Mehta SH, Shill HA, Driver‐Dunckley E, Beach TG, Adler CH. Simplified conversion method for unified Parkinson's disease rating scale motor examinations. Mov Disord. 2015;30(14):1967‐1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weintraub S, Dikmen SS, Heaton RK, et al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: validation in an adult sample. J Int Neuropsychol Soc. 2014;20(6):567‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S54‐S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slotkin J, Kallen M, Griffith J, Magasi S, Salsman J, Nowinski C. NIH toolbox. Technical Manual [Google Scholar]. 2012.
- 27. Cilia R, Tunesi S, Marotta G, et al. Survival and dementia in GBA‐associated Parkinson's disease: the mutation matters. Ann Neurol. 2016;80(5):662‐673. [DOI] [PubMed] [Google Scholar]
- 28. Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty‐six‐month outcomes. Neurology. 2012;79(1):55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangone G, Bekadar S, Cormier‐Dequaire F, et al. Early cognitive decline after bilateral subthalamic deep brain stimulation in Parkinson's disease patients with GBA mutations. Parkinsonism Relat Disord. 2020;76:56‐62. [DOI] [PubMed] [Google Scholar]
- 30. Zavala B, Zaghloul K, Brown P. The subthalamic nucleus, oscillations, and conflict. Mov Disord. 2015;30(3):328‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion‐weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27(14):3743‐3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120(2):329‐355. [DOI] [PubMed] [Google Scholar]
- 33. Cavanagh JF, Wiecki TV, Cohen MX, et al. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14(11):1462‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuo S‐H, Tasset I, Cheng MM, et al. Mutant glucocerebrosidase impairs α‐synuclein degradation by blockade of chaperone‐mediated autophagy. Sci Adv. 2022;8(6):eabm6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muñoz SS, Petersen D, Marlet FR, Kücükköse E, Galvagnion C. The interplay between glucocerebrosidase, α‐synuclein and lipids in human models of Parkinson's disease. Biophys Chem. 2021;273. [DOI] [PubMed] [Google Scholar]
- 36. Merola A, Romagnolo A, Krishna V, et al. Current directions in deep brain stimulation for Parkinson's disease—directing current to maximize clinical benefit. Neurol Ther. 2020;9(1):25‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]


