Abstract
We previously showed that vaccinia virus infection of BSC40 cells was blocked by soluble heparin, suggesting that cell surface heparan sulfate mediates vaccinia virus binding (C.-S. Chung, J.-C. Hsiao, Y.-S. Chang, and W. Chang, J. Virol. 72:1577–1585, 1998). In this study, we extended our previous work and demonstrated that soluble A27L protein bound to heparan sulfate on cells and interfered with vaccinia virus infection at a postbinding step. In addition, we investigated the structure of A27L protein that provides for its binding to heparan sulfate on cells. A mutant of A27L protein, named D-A27L, devoid of a cluster of 12 amino acids rich in basic residues, was constructed. In contrast to the soluble A27L protein, purified D-A27L protein was inactive in all of our assays, including binding to heparin in vitro, binding to heparan sulfate on cells, and the ability to block virus infection. These data demonstrated that the N-terminal region acts as a glycosaminoglycan (GAG)-binding domain critical for A27L protein binding to cells. Previously A27L protein was thought to be involved in fusion of virus-infected cells induced by acid treatment. When we investigated whether cell surface GAGs also participate in A27L-dependent fusion, our results indicated that soluble A27L protein blocked cell fusion, whereas D-A27L protein did not. Taken together, the results therefore demonstrated that A27L-mediated cell fusion is triggered by its interaction with cell surface GAGs through the N-terminal domain.
Virus entry is a complicated process and often involves multiple stages with various viral and cellular factors (23, 40). Especially for enveloped viruses, it is generally accepted that viruses first bind to cell surface receptors to trigger a conformational change of the virus-receptor complexes (4). Subsequently, a second set of cellular molecules, named coreceptors or fusion factors, come into contact with viruses to initiate membrane fusion between the virion envelope and plasma membrane (28). Identification of these cell surface molecules has been time-consuming and labor-intensive; however, isolation of genes encoding these molecules has proved critical for revealing the mechanistic complexity of virus entry processes. For human immunodeficiency virus entry, recent data indicated that not only does cellular CD4 need to interact with viral envelope protein, but also a second accessory molecule such as chemokine receptor-5 (CCR5) plays essential roles in virus-induced fusion. Interactions of gp120 with both CD4 and CCR5 are necessary to induce conformational changes to expose the fusion peptide located at the N terminus of gp41 for membrane fusion (1–3, 6, 7, 15).
Previous studies regarding herpes simplex virus (HSV) have also indicated complicated entry mechanisms (4). HSV is much larger and contains many membrane proteins on virions. Multiple viral proteins such as gB, -C, -D, -H, and -L were shown to be involved in virus entry (10, 18–20, 29). Some of these proteins, such as gB and gC, are now known to bind to glycosaminoglycans (GAGs) on cells, and the presence of gD allows further interactions of bound virions to bind to a coreceptor, HVEM, for fusion (8, 25, 38, 39). Although how fusion is triggered is still not yet known, identification of these cellular molecules provides a critical basis for future studies of HSV fusion mechanisms.
Since vaccinia virus has a wide host range, it is conceivable that certain cellular components that are ubiquitously expressed on cells mediate virus entry. Our previous data suggested that recombinant A27L protein bound to heparan sulfate on BSC40 cells. In addition, BSC40 cells treated with sodium chlorate to block sulfation of GAG side chains became more resistant to vaccinia virus infection (12). We interpreted these results as indicating A27L protein interaction with the negative charges of sulfates on GAGs. We therefore looked for amino acid sequences on A27L protein that are basic or positively charged and suitable for interaction with sulfates (31). A stretch of 12 amino acids (aa) from aa 21 to 32 (STKAAKKPEAKR) rich in lysine and arginine is, in principle, ideal for charge interactions. This region was also present in the soluble A27L protein, which contained aa 21 to 84 and was shown to bind to cells (Fig. 1A) (12). The A27L hydropathy plot translated from the open reading frame predicts a membrane protein of 110 aa with a signal peptide from aa 1 to 20. Previously, the A27L protein on virions was purified, and the sequencing data indicated that the mature form starts at serine of aa 21, suggesting processing of the signal peptide (37). This placed the arginine- and lysine-rich region described above at the most external region of the N terminus for easy accessibility. Furthermore, several monoclonal antibodies (MAbs) that recognized overlapping epitopes within this region were able to neutralize vaccinia virus infection (27). It is likely that this N-terminal region modulates the functions of A27L protein in virus entry.
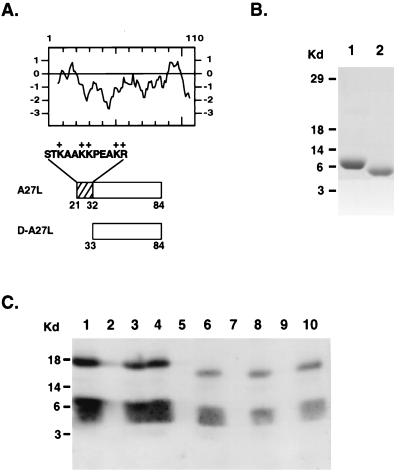
FIG. 1.
(A) Hydropathy plot of A27L protein containing 110 aa translated from the open reading frame. The ectodomains expressed in A27L and D-A27L proteins were shown as boxed regions, and the lysine- and arginine-rich region (aa 21 to 32) was drawn as a striped box. Individual amino acid sequences from positions 21 to 32 were shown on the top of the striped area. The numbers of amino acids shown for the A27L and D-A27L proteins are identical to the numbering of amino acid sequences in the hydropathy plot. The boxed regions are aligned with the hydropathy plot to demonstrate that the A27L protein contains a hydrophilic ectodomain immediately adjacent to the signal peptide of aa 1 to 20. (B) Purification of D-A27L protein. Construction of D-A27L protein was similar to that of A27L protein, as described previously (13). In brief, PCR primers corresponding to different A27L DNA sequences were used to amplify the DNA fragment containing the ectodomains of D-A27L protein. The DNA was cloned into pET21a(+) and transformed into BL21(DE3), and the resulting recombinant D-A27L protein contains a T7 tag at the N terminus and a hexahistidine tag at the C terminus to allow purification with a nickel column. Both soluble A27L protein (lane 1) and D-A27L protein (lane 2) were purified as described above, separated by sodium dodecyl sulfate-polyacrylamide (12%) gel electrophoresis (SDS-PAGE), and stained with Coomassie brilliant blue. (C) D-A27L protein does not bind to the heparin column in vitro. Purified A27L (lanes 1 to 5) and D-A27L (lanes 6 to 10) proteins were incubated with heparin-Sepharose beads (lanes 4, 5, 9, and 10) or control Sepharose beads (lanes 2, 3, 7, and 8) at 4°C for 60 min, and the supernatant was collected after a brief centrifugation. The beads were washed three times, and the volumes of the bound (lanes 2, 4, 7, and 9) or supernatant (lanes 3, 5, 8, and 10) samples were adjusted so that an equal proportion of each sample was loaded on a 12% polyacrylamide SDS-PAGE gel. Purified A27L and D-A27L proteins were shown as controls in lanes 1 and 6, respectively. The gel was transferred for Western blot analysis with a MAb specific for A27L protein (1:500). The two bands in each lane represent the dimeric (top) and the monomeric (bottom) forms of the proteins. Kd, kilodaltons.
A mutant construct, D-A27L, was generated to express soluble proteins in bacteria. D-A27L encodes the open reading frame sequences from aa 33 to 84. Compared with our original A27L ectodomain construct, D-A27L protein lacks aa 21 to 32 from the N terminus (Fig. 1A). Both A27L and D-A27L proteins contain a hexahistidine tag and were purified with nickel columns (Fig. 1B). To test if this deletion removed the ability of A27L protein to bind to GAGs, both A27L and D-A27L proteins were incubated with heparin CL6B beads, respectively, in vitro, and after washing, the bound fractions and the flowthrough were collected and analyzed by Western blotting with a serum specific for A27L protein (Fig. 1C). In the control, soluble A27L protein bound to heparin beads (lane 4), and very little of the protein was detected in the flowthrough (lane 5). This binding was specific to heparin, since A27L protein did not bind to beads alone (lane 2). In contrast, D-A27L protein did not bind to either control beads (lane 7) or heparin beads (lane 9). All of the D-A27L protein was detected in both of the flowthrough fractions (lanes 8 and 10). The results therefore demonstrated that the region of aa 21 to 32 is necessary for A27L protein binding to heparin beads in vitro.
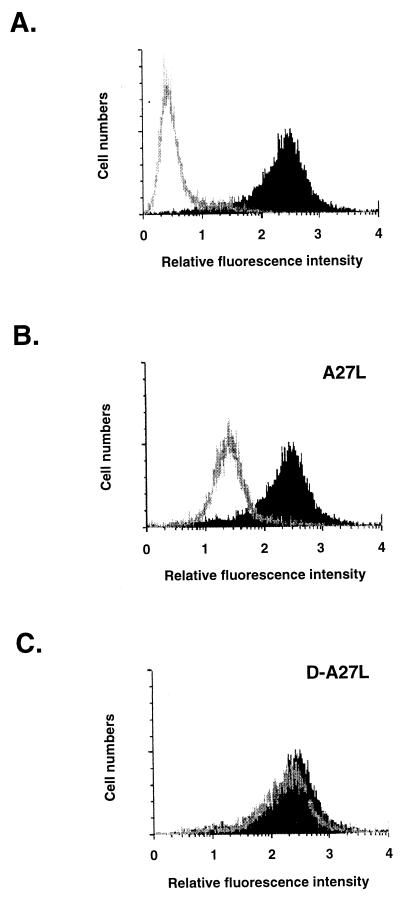
We extended the in vitro analysis described above to determine if soluble A27L protein binding to heparan sulfate on cells is also mediated by the same N-terminal domain. We monitored the ability of D-A27L protein to compete with soluble A27L protein for cell binding by using fluorescence-activated cell sorter analysis. Biotinylated A27L protein bound specifically to HeLa cells and revealed a significant shift in fluorescence staining on cell surfaces (Fig. 2A). The amount of A27L protein bound to cells was significantly reduced when soluble heparin was added as a competitor, but not with chondroitin sulfate or dermantan sulfate (data not shown). When cells were incubated with 10-fold excessive amounts of unlabeled A27L protein to compete with biotinylated A27L protein for cell binding, the fluorescence staining detected was significantly reduced (Fig. 2B). When D-A27L protein was used as a cold competitor, no decrease in fluorescence was observed, indicating that D-A27L protein did not compete with A27L protein for cell binding (Fig. 2C). Failure of D-A27L protein to bind to heparin beads correlates with the protein’s inability to bind to cells, confirming that the N-terminal domain is necessary for A27L interaction with heparan sulfate on cells. This was not only restricted to HeLa cells, since other cell lines, such as BSC40 and mouse L cells, were also tested and similar results were obtained (data not shown).
FIG. 2.
D-A27L protein did not compete with biotinylated A27L protein for cell binding. (A) HeLa cells were incubated with phosphate-buffered saline alone (gray line in panel A), biotinylated A27L protein alone (shaded areas in panels A, B, and C), or biotinylated A27L protein in the presence of 10-fold excess of cold A27L protein (gray line in panel B) or D-A27L protein (gray line in panel C) at 4°C for 30 min. Cells were washed three times, and phycoerythrin-conjugated streptavidin (1:100) was added for another 30-min incubation. After washing, cells were analyzed by a fluorescence-activated cell sorter (excitation, 488 nm; emission, 578 nm).
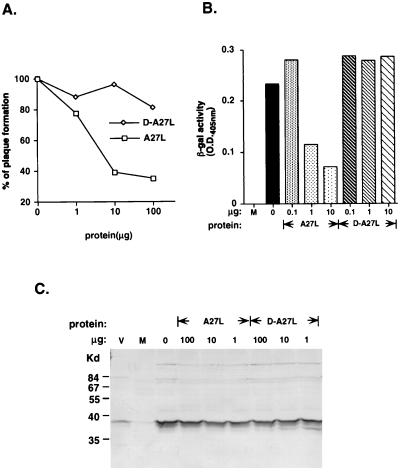
A27L protein is an envelope protein on the surface of intracellular mature viruses, and antibodies against A27L protein neutralized vaccinia virus infection (30–32, 35). If A27L protein indeed mediates vaccinia virus binding to heparan sulfate on cells, it is likely that soluble A27L protein would interfere with vaccinia virus infection. With purified D-A27L protein, the involvement of the GAG-binding domain in virus infection could be assessed. BSC40 cells were infected with vaccinia virus in the presence of soluble A27L or D-A27L protein, and plaque numbers were determined 3 days later. As shown in Fig. 3A, soluble A27L protein blocked plaque formation in a dosage-dependent manner, with 23% inhibition at the 1-μg protein level. Inhibition was saturated at 61 and 66% for 10 and 100 μg, respectively. D-A27L protein did not have any significant effect on virus infection, indicating the GAG-binding domain is essential for this inhibitory effect.
FIG. 3.
Soluble A27L protein but not D-A27L protein blocked vaccinia virus entry at the postbinding step. (A) Plaque reduction by soluble A27L protein. BSC40 cells were incubated first with various amounts of soluble A27L or D-A27L protein (1, 10, or 100 μg in 200 μl) at 4°C for 30 min and subsequently infected with vaccinia virus (600 PFU) for another 4°C for 30 min. Cells were washed with citrate buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl [pH 3.0]) for 1 min, washed with phosphate-buffered saline, and overlaid with agar. Plaque numbers were determined 3 days later. (B) Viral early gene expression was reduced by A27L protein. Cells were incubated with soluble A27L or D-A27L protein (0.1, 1, or 10 μg in 100 μl) at 4°C for 30 min and subsequently infected with vMJ360 at a multiplicity of infection of 5 PFU per cell for another 4°C for 30 min. Cells were washed twice with phosphate-buffered saline and harvested at 2 h postinfection. Lysates were prepared for β-galactosidase (β-gal) assays as described before (3). O.D., optical density. (C) Vaccinia virus binding to cells was not affected by A27L protein. BSC40 cells were preincubated with soluble proteins (1, 10, or 100 μg) at 4°C for 30 min and subsequently infected with vaccinia virus at a multiplicity of infection of 10 PFU per cell at 4°C for 30 min. Cells were washed, lysed in sodium dodecyl sulfate-containing buffer, and loaded onto 10% polyacrylamide sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel for Western blot analysis with an antiserum against vaccinia virions (V). M, lysate from mock-infected cells.
We then checked if soluble A27L protein blocked virus infection at an early stage. Cells were infected with a recombinant vaccinia virus, vMJ360, in the presence of soluble A27L or D-A27L protein (14). Cell lysates were prepared at 2 h postinfection and assayed for lacZ expression from an early viral promoter (Fig. 3B). Incubation of cells with 1 μg of soluble A27L protein blocked approximately 51% of viral infection. With 10 μg of A27L protein, blockage of viral infection increased to 60% inhibition. The results appeared consistent with the observations in Fig. 3A. D-A27L protein did not inhibit viral gene expression from the 0.1- to 10-μg range tested. Presumably, D-A27L protein did not bind to cells and was ineffective in blocking virus infection. A27L protein required the N-terminal sequences in order to inhibit virus infection, suggesting that A27L protein interferes with the virus entry process.
The ability of A27L protein to block vaccinia virus infection suggested two possible mechanisms: it could block vaccinia virus adsorption, or it could block virus fusion or penetration. For envelope viruses, binding and fusion are two distinct steps, and soluble viral proteins that interfere with either process have been previously described (17, 26, 38, 39). We performed virus binding assays to quantitate the amount of vaccinia virions bound to cells in the presence of soluble A27L protein. BSC40 cells were incubated with soluble protein and infected with vaccinia virus. Cells were then immediately harvested, and lysates were prepared for Western blot analysis with an antiserum against vaccinia virions (Fig. 3C). The amount of viruses bound to cells appeared unchanged, regardless of the concentration of soluble A27L protein used. This indicated that addition of soluble A27L protein did not reduce vaccinia virus binding to cells and instead blocked virus infection at the fusion or penetration stage. This result may not be too surprising, since A27L protein was shown to induce cell fusion of vaccinia virus-infected cells by acid treatment (31, 32). In addition, MAb C3 against A27L protein blocked cell fusion without affecting virus binding (32, 35). However, it was not known how A27L protein triggers fusion, and no cell surface components involved in fusion have been identified.
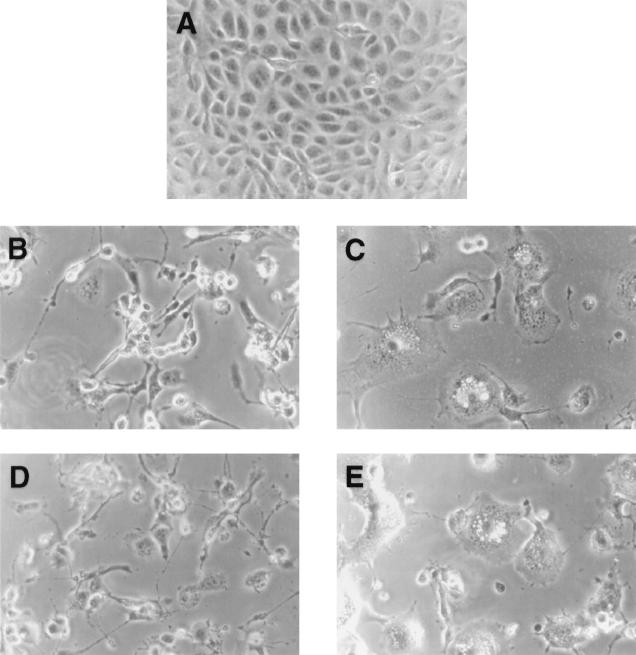
Since the N-terminal region of A27L protein acts as a GAG-binding domain, it provides a clue for understanding the mechanism of A27L-dependent cell fusion. We obtained a recombinant virus, WR32-7/Ind14K, in which expression of A27L protein was regulated by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to the culture medium (33, 34). Cell fusion was strictly dependent on A27L protein expression and was readily observed around 18 h postinfection in the presence of IPTG (34). This virus allowed us to determine fusion formation without acid treatment and provided a more convenient assay. BSC40 cells infected by WR32-7/Ind14K developed cell fusion at neutral pH when IPTG was added to the culture medium (Fig. 4C), whereas no fusion was observed when IPTG was omitted (Fig. 4B). When these infected cells were incubated with soluble A27L protein, cell fusion was significantly blocked (Fig. 4D). Incubation of D-A27L protein with infected cells had no inhibitory effect, and cell fusion developed to a similar extent to the control infected cells (Fig. 4E). Taken together, removal of the GAG-binding domain of D-A27L protein eliminated its biological activities in both cell binding and cell fusion. This suggests that cell binding through GAG interaction is essential for A27L protein to mediate cell fusion.
FIG. 4.
Soluble A27L protein blocked fusion of cells infected by WR32-7/Ind14K. BSC40 cells were either mock infected (A) or were infected with a recombinant virus, WR32-7/Ind14K, at a multiplicity of infection of 5 PFU per cell in the presence (C to E) or in the absence (B) of IPTG. After infection, cells were incubated with medium alone (A to C) or medium containing A27L protein (D) or D-A27L protein (E) for another 24 h and photographed.
This finding may provide an explanation for several previous observations. For example, several MAbs that recognize epitopes within this region may neutralize virus infection due to interrupting virus and GAG interactions (13, 27). Mutations or deletions within the N-terminal sequences that were known to inactivate A27L functions may also be due to an inability to interact with GAGs (21, 22).
What is the role of A27L protein in vaccinia virus binding and fusion? It is apparent that A27L protein and GAG interaction could act as the initial stage of virus infection, since the charge interactions through GAGs were often described as nonspecific and with low affinity (4). It seems likely that other high-affinity interactions between cells and bound viruses will be required to provide specificity. Incubation of cells with soluble heparin blocked virus binding to cells, whereas soluble A27L protein did not (Fig. 3C) (12). This would suggest that perhaps there are multiple viral proteins mediating virus binding to GAGs. Blocking of virus adsorption could only be achieved when all of these interactions were simultaneously blocked by heparin. This would explain the failure of soluble A27L protein to inhibit virus binding. This may also explain that A27L protein is not essential for virus infection, since binding to GAGs may be shared by other viral membrane proteins, similar to gB and gC in HSV-1 (10, 24, 25).
There have been reports suggesting that A27L protein was required in virus and cell fusion. A MAb against A27L, C3, was reported to block vaccinia virus infection without affecting virus binding (32). Dequenching analysis to demonstrate that intracellular mature virus enters cells by membrane fusion revealed that virus and cell membrane fusion was blocked by MAb C3 (16). Since we do not know the epitope that MAb C3 recognizes, it is unclear how MAb C3 blocks virus fusion. Inhibition of virus infection by soluble A27L protein without affecting virus attachment was also consistent with a role in fusion. We speculate that soluble A27L protein bound to GAGs on cells, created a steric hindrance, and interfered with conformational changes of virus-GAG complexes, which otherwise could have been converted into fusiongenic intermediates. In this model, we do not think the A27L protein itself serves as a fusion protein. Viral fusion proteins such as hemagglutinin (HA2) and gp41 contain hydrophobic amino acids at the N terminus which often were described as fusion peptides (9, 11). There is no similar structure on A27L protein which may serve as the fusion peptide. In addition, vaccinia virus cell fusion could be mediated by multiple viral proteins. Other proteins, such as F13L, B5R, and A34R, were also shown to be required for cell fusion of virus-infected cells (5, 41, 42). With few conserved structures among these viral proteins, it appeared less likely that they all contain fusion peptides to participate in the fusion process. Rather, it may indicate that these viral proteins, including A27L protein, somehow regulate the activity of the fusion machinery. Besides, there are several viral proteins known to interact with A27L protein, such as A13L, A14L, and A17L proteins, and their roles in fusion need to be determined in the future (30, 36).
In conclusion, our study revealed the importance of the N-terminal domain of A27L protein in the vaccinia virus adsorption to GAGs on cells. This interaction also controls the ability of A27L protein to trigger cell fusion. Our mapping of the GAG-binding domain allowed us to begin dissecting the mechanistic complexity involved in virus entry. The study of individual membrane proteins should enable us to identify other viral and cellular proteins which participate in the vaccinia virus entry process.
Acknowledgments
We thank G. Smith and J. F. Rodriguez for providing the recombinant WR32-7/Ind14K virus. We also thank Douglas Platt for carefully reviewing the manuscript.
This work was supported by grants from Academia Sinica and the National Science Council (NSC87-2311-B-001-117) of the Republic of China.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Baiocchi M, Olivetta E, Chelucci C, Santarcangelo A C, Bona R, d’Aloja P, Testa U, Komatsu N, Verani P, Federico M. Human immunodeficiency virus (HIV)-resistant CD4+ UT-7 megakaryocytic human cell line becomes highly HIV-1 and HIV-2 susceptible upon CXCR4 transfection: induction of cell differentiation by HIV-1 infection. Blood. 1997;89:2670–2678. [PubMed] [Google Scholar]
- 3.Balter M. A second coreceptor for HIV in early stages of infection. Science. 1996;272:1740. doi: 10.1126/science.272.5269.1740. [DOI] [PubMed] [Google Scholar]
- 4.Bentz J. Viral fusion mechanisms. Boca Raton, Fla: CRC Press; 1993. [Google Scholar]
- 5.Blasco R, Moss B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-dalton outer envelope protein. J Virol. 1991;65:5910–5920. doi: 10.1128/jvi.65.11.5910-5920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradbury J. HIV-1-resistant individuals may lack HIV-1 coreceptor. Lancet. 1996;348:463. doi: 10.1016/S0140-6736(05)64540-0. [DOI] [PubMed] [Google Scholar]
- 7.Broder C C, Dimitrov D S. HIV and the 7-transmembrane domain receptors. Pathobiology. 1996;64:171–179. doi: 10.1159/000164032. [DOI] [PubMed] [Google Scholar]
- 8.Bryne K M, Horohov D W, Kousoulas K G. Glycoprotein B of bovine herpesvirus-1 binds heparin. Virology. 1995;209:230–235. doi: 10.1006/viro.1995.1248. [DOI] [PubMed] [Google Scholar]
- 9.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 10.Cai W, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988;62:2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. . (Erratum, 62:4438.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 12.Chung C-S, Hsiao J-C, Chang Y-S, Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czerny C P, Johann S, Holzle L, Meyer H. Epitope detection in the envelope of intracellular naked orthopox viruses and identification of encoding genes. Virology. 1994;200:764–777. doi: 10.1006/viro.1994.1240. [DOI] [PubMed] [Google Scholar]
- 14.Davison A J, Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989;210:749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- 15.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 16.Doms R W, Blumenthal R, Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J Virol. 1990;64:4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller A O, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller A O, Santos R E, Spear P G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989;63:3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller A O, Spear P G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci USA. 1987;84:5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerber S I, Belval B J, Herold B C. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology. 1995;214:29–39. doi: 10.1006/viro.1995.9957. [DOI] [PubMed] [Google Scholar]
- 21.Gong S, Lai C, Dallo S, Esteban M. A single point mutation of Ala-25 to Asp in the 14,000-Mr envelope protein of vaccinia virus induces a size change that leads to the small plaque size phenotype of the virus. J Virol. 1989;63:4507–4514. doi: 10.1128/jvi.63.11.4507-4514.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong S C, Lai C F, Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178:81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- 23.Haywood A M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994;68:1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herold B C, Visalli R J, Susmarski N, Brandt C R, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulfate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 25.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer H, Osterrieder N, Czerny C-P. Identification of binding sites for neutralizing monoclonal antibodies on the 14-kDa fusion protein of orthopox viruses. Virology. 1994;200:778–783. doi: 10.1006/viro.1994.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 29.Muggeridge M I, Wilcox W C, Cohen G H, Eisenberg R J. Identification of a site on herpes simplex virus type 1 glycoprotein D that is essential for infectivity. J Virol. 1990;64:3617–3626. doi: 10.1128/jvi.64.8.3617-3626.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez D, Rodriguez J-R, Esteban M. The vaccinia virus 14-kilodalton fusion protein forms a stable complex with the processed protein encoded by the vaccinia virus A17L gene. J Virol. 1993;67:3435–3440. doi: 10.1128/jvi.67.6.3435-3440.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez J F, Esteban M. Mapping and nucleotide sequence of the vaccinia virus gene that encodes a 14-kilodalton fusion protein. J Virol. 1987;61:3550–3554. doi: 10.1128/jvi.61.11.3550-3554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez J F, Janeczko R A, Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985;56:482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez J F, Smith G L. Inducible gene expression from vaccinia virus vectors. Virology. 1990;177:239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez J F, Smith G L. IPTG-dependent vaccinia virus: identification of a virus protein enabling virion envelopment by Golgi membrane and ingress. Nucleic Acids Res. 1990;18:5347–5351. doi: 10.1093/nar/18.18.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez J F, Paez E, Esteban M. A 14,000-Mr enveloped protein of vaccinia virus is involved in cell fusion and forms covalently linked trimers. J Virol. 1987;61:395–404. doi: 10.1128/jvi.61.2.395-404.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez J R, Risco C, Carrascosa J L, Esteban M, Rodriguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15 kilodalton envelope protein. J Virol. 1997;71:1821–1833. doi: 10.1128/jvi.71.3.1821-1833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi T, Oie M, Ichihashi Y. N-terminal amino acid sequences of vaccinia virus structural proteins. Virology. 1994;202:844–852. doi: 10.1006/viro.1994.1406. [DOI] [PubMed] [Google Scholar]
- 38.Tal-Singer R, Peng C, de Leon M P, Abrams W R, Banfield B W, Tufaro F, Cohen G H, Eisenberg R J. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce De Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wimmer E. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 41.Wolffe E J, Isaacs S N, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67:4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. . (Erratum, 67:5709–5711.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolffe E J, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3904–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]