Summary
Background
Microplastic (MP) pollution has emerged as a significant environmental concern worldwide. While extensive research has focused on their presence in marine organisms and ecosystems, their potential impact on human health, particularly on the circulatory system, remains understudied. This project aimed to identify and quantify the mass concentrations, polymer types, and physical properties of MPs in human thrombi surgically retrieved from both arterial and venous systems at three anatomically distinct sites, namely, cerebral arteries in the brain, coronary arteries in the heart, and deep veins in the lower extremities. Furthermore, this study aimed to investigate the potential association between the levels of MPs and disease severity.
Methods
Thrombus samples were collected from 30 patients who underwent thrombectomy procedures due to ischaemic stroke (IS), myocardial infarction (MI), or deep vein thrombosis (DVT). Pyrolysis–gas chromatography mass spectrometry (Py-GC/MS) was employed to identify and quantify the mass concentrations of the MPs. Laser direct infrared (LDIR) spectroscopy and scanning electron microscopy (SEM) were used to analyse the physical properties of the MPs. Demographic and clinical information were also examined. A rigorous quality control system was used to eliminate potential environmental contamination.
Findings
MPs were detected by Py-GC/MS in 80% (24/30) of the thrombi obtained from patients with IS, MI, or DVT, with median concentrations of 61.75 μg/g, 141.80 μg/g, and 69.62 μg/g, respectively. Among the 10 target types of MP polymers, polyamide 66 (PA66), polyvinyl chloride (PVC), and polyethylene (PE) were identified. Further analyses suggested that higher concentrations of MPs may be associated with greater disease severity (adjusted β = 7.72, 95% CI: 2.01–13.43, p < 0.05). The level of D-dimer in the MP-detected group was significantly higher than that in the MP-undetected group (8.3 ± 1.5 μg/L vs 6.6 ± 0.5 μg/L, p < 0.001). Additionally, LDIR analysis showed that PE was dominant among the 15 types of identified MPs, accounting for 53.6% of all MPs, with a mean diameter of 35.6 μm. The shapes of the polymers detected using LDIR and SEM were found to be heterogeneous.
Interpretation
This study presents both qualitative and quantitative evidence of the presence of MPs, and their mass concentrations, polymer types, and physical properties in thrombotic diseases through the use of multimodal detection methods. Higher concentrations of MPs may be associated with increased disease severity. Future research with a larger sample size is urgently needed to identify the sources of exposure and validate the observed trends in the study.
Funding
This study was funded by the SUMC Scientific Research Initiation Grant (SRIG, No. 009-510858038), Postdoctoral Research Initiation Grant (No. 202205230031-3), and the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (No. 2020LKSFG02C).
Keywords: Microplastics, Thrombus, Stroke, Myocardial infarction, Deep vein thrombosis, Py-GC/MS
Research in context.
Evidence before this study
Microplastics (MPs) are not only present in all ecosystems, but are also being increasingly detected in various human body systems. A recent finding detected a single particle of low-density polyethylene (LDPE) in aortic thrombosis. However, it remains unclear whether MPs accumulate in thrombi in much smaller arteries and veins in humans, how concentrated they are, and what specific types and physical properties of MPs are involved. Moreover, the potential association between MPs and disease severity has not been investigated.
Added value of this study
This study presented evidence of the mass concentrations and types of MPs in thrombi from multiple anatomically distinct locations, including cerebral arteries in the brain, coronary arteries in the heart, and deep veins in the lower extremities, through pyrolysis-gas chromatography mass spectrometry (Py-GC/MS). By using laser direct infrared (LDIR) spectroscopy and scanning electron microscopy (SEM), this study further demonstrated that MPs with varying particle numbers, sizes, shapes, and densities accumulated in thrombi surgically retrieved from patients with ischaemic stroke. Moreover, this study identified a possible association between the levels of MPs and disease severity, shedding light on a previously understudied aspect of MP pollution.
Implications of all the available evidence
Our findings suggest that MPs of different concentrations, polymer types, and physical properties are present in human thrombi, and that higher levels of MPs may be associated with disease severity. This study highlights the importance of using multimodal detection methods to more accurately investigate the impact of MPs on human health.
Introduction
The increasing use of plastics has become a growing topic of concern and debate regarding their impact on both the environment and human health.1 Plastics have fuelled global demand for their ease of production, low production costs, versatility, durability, and resistance to corrosion. However, these same attributes lead to an extended environmental presence. After plastics are discarded, they break down into small microplastics (MPs) and nanoplastics, which then become atmospheric, terrestrial, and aquatic pollutants.
MP pollution can be classified into two types: primary and secondary.2 Primary MPs result from a wide range of consumables such as cosmetics and medical devices, with sizes less than 5 mm, and are directly released into the environment. Secondary MPs are the result of continuous physical and chemical fragmentation processes of larger plastic materials.3 The most abundant plastic polymers of MPs in the environment include polyethylene (PE), nylon (PA), polypropylene (PP), polyethylene terephthalate (PET), polystyrene (PS), and polyvinyl chloride (PVC).4,5 Due to the ubiquity of MPs in the environment and in everyday products, human exposure to MPs is unavoidable.6 In fact, MPs have been detected not only in various ecosystems but also in the human body, including sputum,7 lungs,8 heart,9 liver,10 blood,11 endometrium,12 testis,13 amniotic fluid and placenta.14,15 As such, MP pollutants have sparked growing concern due to their widespread presence and potential health implications.
A pioneering study by Leslie and colleagues detected, for the first time, the presence of MPs in the blood of 22 healthy adult volunteers and identified five types of MP polymers.11 Subsequently, another research group explored the accumulation of particles in 26 thrombi from patients with aortic dissection or arterial embolism of the lower extremities.16 They found that 16 (61.5%) of the thrombi contained a total of 87 particles, including largely iron compounds and pigment particles, and one single low-density polyethylene MP.16 Thrombus generation is known to be multifactorial in nature, with a combination of environmental and genetic risk factors.17 These findings suggest that MPs may serve as a potential risk factor associated with vascular health. However, considering that only one MP particle was identified with Raman spectroscopy,16 the true extent of their presence and consequences for thrombogenesis in the circulatory system remain understudied. In particular, the mass concentrations and various types of MPs involved in arterial and venous thrombotic diseases are currently unknown. In this regard, there is an urgent need for employing different methods to detect and analyse MPs in a more comprehensive manner.
The strong impact of MPs on ecosystems and human health has prompted the scientific community to develop advanced detection methods. Chemical characterisation of MPs using Raman spectroscopy or Fourier transform infrared spectroscopy (FTIR) is common practice in the field. Raman spectroscopy produces a molecular fingerprint spectrum based on the interaction between radiation and the material, whereas FTIR provides an infrared spectrum based on the change in dipole moment.18 Although Raman spectroscopy has good spatial resolution (as fine as 1 μm) and wide spectral coverage, it is limited by weak signals, fluorescence interference, and its dependence on material properties such as colour, biofouling, and degradation.19 On the other hand, FTIR, with a spatial resolution of 10–20 μm, may be limited by low throughput, laborious processing, and the need for skilled personnel, especially when dealing with weathered MPs.20 Recently, laser direct infrared (LDIR) spectroscopy, a fully automated approach in infrared chemical imaging using quantum cascade laser (QCL) technology to identify MPs in the size range of 20–500 μm, has greatly streamlined the characterisation of MPs by size, shape, and polymer type.21 In contrast, pyrolysis - gas chromatography mass spectrometry (Py-GC/MS) is an emerging thermal analytical technique for simultaneously identifying and quantifying MPs in complex samples. In Py-GC/MS, MPs are thermally decomposed (pyrolysed) under an inert atmosphere, separated on a gas chromatographic (GC) column, and finally characterised by mass spectrometry (MS).11,20,22 This method is independent of particle size and mechanical preselection, and allows the characterisation of polymer types in complex samples.
In this study, we aimed to utilise multimodal methods to analyse and quantify the mass concentrations, polymer types, and physical properties of MPs in thrombi retrieved from 30 patients diagnosed with ischaemic stroke, myocardial infarction, or deep vein thrombosis. We focused on the characterisation of MPs in thrombi located in intracranial arteries, coronary arteries, and deep veins, which have not been investigated thus far. We also attempted to investigate potential associations between MPs and clinical indicators or disease severity in these thrombotic conditions. The findings of this study may contribute new evidence regarding the potential health risks of MPs in thrombotic diseases.
Methods
Ethics statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College (SUMC) under Approval No. B2023063, and was conducted according to the revised Declaration of Helsinki (version 2013).
Participants
Participants were patients who underwent arterial or venous thrombectomy due to ischaemic stroke (IS), myocardial infarction (MI), or deep vein thrombosis (DVT) at the First Affiliated Hospital of SUMC, Shantou, China. Patients who met the following inclusion criteria were enrolled in the study: 1) had a thrombus that could be fully removed from vessels during surgery and promptly transferred into the sample bottle; 2) had no artificial implants, such as vascular stents, artificial grafts, or bones; 3) had no history of taking diagnostic or therapeutic agents carried by nanomaterials; and 4) provided consent for thrombectomy and participation in this study. Patients with any of the following conditions were excluded from the study: 1) had contraindications for the surgery, such as severe active bleeding or significant bleeding tendency, severe organ dysfunctions, uncontrollable blood pressure ≥180/100 mmHg, or an allergy to contrast agents; 2) had artificial implants or had taken diagnostic or therapeutic drugs that were carried by nanomaterials; and 3) refused to undergo interventional thrombectomy. After screening, a total of 30 patients (16 with IS, 5 with MI, and 9 with DVT) were included in this study from May to October 2023. Patient demographic information, medical history, and blood parameters such as full blood count, coagulation test, electrolyte panel, and lipid profile were collected on admission.
Sample collection
To prevent contamination of the thrombus samples with plastics, a plastic-free protocol was strictly adhered to throughout the experiment. Cotton laboratory coats and polymer-free nitrile gloves were worn. Thrombus samples from enrolled patients were collected into autoclaved glass bottles sealed with polytetrafluoroethylene (PTFE) lids following standard surgical procedures. The collected thrombus samples were immediately transferred along with ice packs to the laboratory for storage at −80 °C. They were subsequently shipped to Shanghai Weipu Testing Technology Group Co., Ltd., China.
Sample processing procedures for MP analysis using Py-GC/MS
The processing procedure of the thrombus samples for the analysis of MPs using Py-GC/MS was adopted from Ke et al.22 First, the thrombus sample was placed in a 100 mL beaker to measure the wet weight (WW), and then dried in a 60 °C oven (DHG-9070A, Shanghai, China) until a constant weight was achieved. The sample was then mixed with concentrated nitric acid (GR 65–68%, Shanghai Hushi Laboratorial Equipment Co., Ltd., China) three times the volume of the sample and allowed to stand for 48 h at room temperature to fully digest organic matter, followed by heating at 110 °C for approximately 3 h for further digestion of proteins. Then, the mixture was concentrated at 110 °C to approximately 1 mL, and the mass of the concentrate was recorded as the concentrate weight (CW). Next, the concentrate was transferred multiple times to a pyrolysis cup using a single-use glass Pasteur pipette (150 mm, Wertheim, Germany) and left to dry completely at 110 °C. After drying, the weight of the sample on the pyrolysis cup was recorded as the loading weight (LW). The total tested mass (TTM) was calculated as follows: TTM = LW/CW∗WW (unit: gram).
Analysis of MPs by Py-GC/MS
The analysis was performed using a pyrolysis unit coupled with gas chromatography mass spectrometry (Py-GC/MS).13,22 Briefly, a pyrolysis cup containing the concentrate described above was subjected to pyrolysis at 550 °C using a Frontier Lab EGA/PY-3030D instrument (Fukushima, Japan). Next, the pyrolysed products were directly injected into a GC-2030 instrument (Shimadzu Corp., Kyoto, Japan) equipped with an Rtx-5MS column (30 m × 0.25 mm × 0.25 μm) (Restek, USA) for separation. The column was heated from 40 °C (2 min) at a rate of 20 °C/min to 320 °C (14 min), resulting in a total programme time of 30 min. During the process, helium was used as the carrier gas at a linear velocity of 36.1 cm/s. The mass spectra were acquired using a QP2020-Plus mass spectrometer (Shimadzu Corp., Kyoto, Japan) in conjunction with a gas chromatography system. The ion source temperature was set at 230 °C and the mass range was 29–600 m/z. The identification of MPs was performed using LabSolutions software 4.45.
The 10 types of target polymers included polyamide 66 (PA66), polyethylene (PE), polyvinyl chloride (PVC), polystyrene (PS), polyethylene terephthalate (PET), polymethylmethacrylate (PMMA), polycarbonate (PC), polyamide 6 (PA6), polypropylene (PP), and polybutylene adipate terephthalate (PBAT). Each polymer was identified based on its characteristic components and ions,22 as well as the corresponding retention time, by matching with the National Institute of Standards and Technology (NIST) spectral library. NIST20 was utilised in this study. The quantification of the detected MPs was performed based on the calibration curves of the corresponding standards (Table S1).
Sample treatment for MP analysis by LDIR and SEM
To further analyse the particle number, size and morphology, we performed laser direct infrared (LDIR) spectroscopy and scanning electron microscopy (SEM) on three thrombus samples from the IS group. Due to the small size of the thrombi in the MI group, they were used up for Py-GC/MS analysis. The MP detected in the DVT group was PA66, which could not be accurately distinguished from natural proteins using LDIR (which is a prerequisite before SEM) (Fig. S1); therefore, these samples were also excluded from further analysis.
The processing of thrombus samples for MP analysis using LDIR was modified from Liu et al.23 The thrombus sample was digested with 68% nitric acid at a volume three times that of the sample at room temperature for 12 h. Next, the mixture was heated on a graphite electric heating plate at 60 °C for 3 h to dissolve proteins, followed by vacuum filtration through a 304 stainless steel mesh (500 μm-pore-diameter). The flow through in a glass container underwent another filtration through a stainless-steel mesh (13 μm-pore-diameter) so that the MPs remained on the mesh. After the glass container was rinsed with filtered analytical grade Milli-Q® water and pumped and filtered three times, the resulting mesh containing the MPs was fully immersed in filtered anhydrous ethanol. This was followed by ultrasonic vibration for 30 min at a power of 40 kHz to disperse the particles from the mesh into the ethanol. After sonication, the mesh was removed from the glass container, and the ethanol solution containing MPs in the glass container was concentrated to approximately 150 μL by drying in an infrared drying oven (Model: WA70-1, Hangzhou Qiwei Instrument, Co., Ltd., China) and then loaded onto a low-e microscope slide (MY2108LD34, Agilent Technologies Co., Ltd., China). Following complete ethanol evaporation, the sample was analysed using the Agilent 8700 LDIR spectrometer (Agilent Technologies Co., Ltd., China). Then, the sample was sputter-coated for analysis of the LDIR-detected MPs via SEM.
Analysis of MPs by LDIR and SEM
The Agilent 8700 LDIR spectrometer (Agilent Technologies Co., Ltd., China) in particle analysis mode, was used to determine the abundance and size distribution of MPs, following similar methods employed in previous studies.9,24,25 The sample was analysed by using an automatic testing method and compared with the microplastic spectrum library (Microplastics Starter 1.0_1_1_1_2, Agilent Technologies Co., Ltd., China) to identify polymer types. The spectral resolution and scanning wavelength range were 1 cm−1 and 975–1800 cm−1, respectively. Particles in the size range of 20–500 μm with a matching degree >0.65 were considered to be positively detected. To monitor potential MP contamination from the reagents and instruments, and to correct instrument performance, a whole procedural control was included. Briefly, 20 mL of filtered Milli-Q® water in a sample container underwent the same treatment as the thrombus samples and was analysed in parallel with each other using LDIR. Two MPs were detected in the procedural control (Fig. S2). To quantify the number of individual types of MPs in the samples, the number of each polymer type was corrected by subtracting the count in the whole procedural control from the total particle count in the thrombus sample. The LDIR-detected MPs were further scanned by an Apreo 2C scanning electron microscope (Thermo Fisher Scientific, USA) to identify their morphologies.
Quality insurance and quality control
To ensure meticulous quality control, a plastic-free principle was strictly followed throughout the experiment. During the entire experimental procedure, cotton laboratory coats and polymer-free nitrile gloves were worn. Considering that laboratory reagents could be a potential source of extraneous MP contamination,26 before use, all reagents and solutions, such as anhydrous ethanol, nitric acid, and analytical grade Milli-Q® water, underwent vacuum filtration three times through a PTFE membrane (0.45 μm-pore-diameter). The filtered reagents and solutions were used in subsequent processes only if the background detection of MPs using Py-GC/MS was blank (Fig. S3). All glassware used in the procedure was rinsed three times with filtered anhydrous ethanol before use. Furthermore, for Py-GC/MS, comprehensive quality control was performed by managing background contamination, conducting a spiking recovery experiment, and establishing limits of detection and quantification. A similar whole procedural control was performed for MP detection using LDIR.
Controlling for background contamination
To minimise the possibility of contamination, a rigorous quality control system (QCS) consisting of five blank and two negative controls was designed to examine critical checkpoints, encompassing sampling and Py-GC/MS measurements. Five blank controls were set up to control the catheters and guidewires, sample container and instrument: 1) catheters and guidewires were surgical consumables in direct contact with the IS, MI, or DVT thrombus during thrombectomy; 2) the sample container was a glass bottle (20 mL) identical to the one used to store the thrombus; and 3) the instrument was the equipment where the samples were processed and measured. In the three catheter and guidewire blanks, catheters (Rebar18, Medtronic, USA) and guidewires (Synchro, Stryker, USA) that had been in contact with the thrombi were randomly selected from the enrolled patients and collected in a new glass bottle following thrombus collection. Analytical grade Milli-Q® water previously filtered through a PTFE membrane (0.45 μm-pore-diameter) was added to the bottle to fully cover the catheters and guidewires, for ultrasonic vibration. Similarly, in the sample container blank, a new glass bottle was sonicated with analytical grade Milli-Q® water. Then, the sonicated analytical grade Milli-Q® water from these blank controls was concentrated and loaded onto a pyrolysis cup for Py-GC/MS measurement in parallel with the processed thrombus samples. In the instrument blank, to eliminate interference from the instrument itself or standards, the instrument without loading any substances or after running the last standard was measured. Two kinds of negative controls were used to control the sampling and whole procedural processes. The sampling process involved collecting thrombi from the patients. A standardised procedure mimicking thrombus collection was performed. In the final step, the same sterile saline as that used for infusion during thrombectomy and analytical grade Milli-Q® water mimicking the human blood system with which the surgical tools came into contact, was collected into a glass bottle instead of a thrombus and used as a sampling control. In the whole procedural control, analytical grade Milli-Q® water was used as the studied subject. The sampling and whole procedural controls underwent the same pretreatment and analytical processes as the thrombus samples. Three repeats were set for each control and pooled as one per control for Py-GC/MS measurements.
Recovery experiment
To determine the recoveries of the polymers in thrombi using this method, a spiking recovery test was carried out. Given the ubiquitous presence of MPs in the environment and their inevitable occurrence in biological tissue samples, known concentrations of 10 types of MP solutions were added to three thrombus subsamples to conduct the recovery test. Recovery (%) = (Mass of MPs in spiked sample - Mass of MPs in sample)/Spiked amount × 100%. The recoveries ranged from 80.8% to 107.8%, indicating the adequate performance of the method in extracting, identifying, and quantifying the concentrations of the 10 target MPs in this study (Table S2), which was in line with previous studies that employed the same method.22,23
Limits of detection and quantification
The limit of detection (LOD) and quantification (LOQ) were determined to be three and ten times of the procedural blank signal for each polymer, respectively (Table S3).22 The LOD represents the minimum concentration of a target polymer that can be reliably detected, while the LOQ is the lowest concentration of a polymer that can be accurately quantified using the Py-GC/MS system.
Statistical analysis
Descriptive statistics were used to analyse the demographic characteristics of the participants. The mean (standard deviation, SD) and median (interquartile range, IQR) were used to describe continuous variables with normal and skewed distributions, respectively. Numbers and frequencies (%) were used to present categorical variables. The Unpaired t-test or Mann–Whitney U test was used to analyse differences in MP concentrations of different locations. Multiple linear regression was used to examine the associations between the concentrations of total MPs in the thrombus and clinical indicators. Age and biological sex were adjusted in the linear model. Spearman's correlation analysis was used to evaluate the correlation between the size of the thrombus and the concentration of MPs. All the statistical analyses were performed using SPSS version 27.0 or GraphPad Prism 9. When the two-sided p value was below 0.05, the result was considered statistically significant.
Role of funders
The funding sources had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Demographic characteristics
The demographic characteristics of the patients are shown in Table 1 and Table S4. The median age of the enrolled patients was 65.2 years, with 16 patients (53.3%) being male. Among the patients, eight (26.7%) had a history of smoking, and 3 (10%) had a history of alcohol consumption. Ten (33.3%) patients were diagnosed with hypertension, and 9 (30%) patients had type II diabetes. Seventeen (56.7%) patients preferred to drink both boiled water and tea, 9 (30%) preferred boiled water only, and 4 (13.3%) preferred tea only. Half of the patients used plastic bags more than 11 times per day, 36.7% used them 4–10 times per day, and 13.3% used them less than 3 times per day. Only 1 (3.3%) patient used plastic bottles for eating or drinking more than 11 times per day, 11 (36.7%) used them 4–10 times per day, and 18 (60%) used them less than 3 times per day. Twenty-four (80%) of the patients never consumed takeaway foods, while 20% ate them 1–3 times per week. Five (16.7%) patients wore masks for more than 2 h per day, 9 (30%) wore them for 30 min to 2 h per day, and 16 (53.3%) wore them for less than 30 min per day. Twelve (40%) of the patients came from rural areas, and 18 (60%) came from urban areas. Two (6.7%) patients lived above the 8th floor, 10 (33.3%) lived between the 5th and the 8th floor, and 18 (60%) lived below the 5th floor.
Table 1.
Demographic characteristics of the participants in this study (n = 30).
| Characteristics | Total (n = 30) | MP-undetected (n = 6) | MP-detected (n = 24) |
|---|---|---|---|
| Age (year) | 65.2 ± 9.0 | 66.8 ± 11.5 | 64.8 ± 8.4 |
| Biological sex [n (%)] | |||
| Female | 14 (46.7%) | 2 (33.3%) | 12 (50%) |
| Male | 16 (53.3%) | 4 (66.7%) | 12 (50%) |
| Smoking [n (%)] | |||
| Yes | 8 (26.7%) | 2 (33.3%) | 6 (25%) |
| No | 22 (73.3%) | 4 (66.7%) | 18 (75%) |
| Drinking [n (%)] | |||
| Yes | 3 (10%) | 1 (16.7%) | 2 (8.3%) |
| No | 27 (90%) | 5 (83.3%) | 22 (91.7%) |
| Hypertension [n (%)] | |||
| Yes | 10 (33.3%) | 3 (50%) | 7 (29.2%) |
| No | 20 (66.7%) | 3 (50%) | 17 (70.8%) |
| Diabetes [n (%)] | |||
| Yes | 9 (30%) | 3 (50%) | 6 (33.3%) |
| No | 21 (70%) | 3 (50%) | 18 (66.7%) |
| Water drinking preference [n (%)] | |||
| Boiled water | 9 (30%) | 2 (33.3%) | 7 (29.2%) |
| Tea | 4 (13.3%) | 0 (0%) | 4 (16.7%) |
| Both | 17 (56.7%) | 4 (66.7%) | 13 (54.2%) |
| Frequency of using plastic bags [n (%)] | |||
| ≥11 times/day | 15 (50%) | 2 (33.3%) | 13 (54.2%) |
| 4–10 times/day | 11 (36.7%) | 4 (66.7%) | 7 (29.2%) |
| ≤3 times/day | 4 (13.3%) | 0 (0%) | 4 (16.7%) |
| Frequency of using plastic bottles for eating or drinking [n (%)] | |||
| ≥11 times/day | 1 (3.3%) | 0 (0%) | 1 (4.2%) |
| 4–10 times/day | 11 (36.7%) | 2 (33.3%) | 9 (37.5%) |
| ≤3 times/day | 18 (60%) | 4 (66.7%) | 14 (58.3%) |
| Frequency of eating takeaway foods [n (%)] | |||
| Never | 24 (80%) | 4 (66.7%) | 20 (83.3%) |
| 1–3 times/week | 6 (20%) | 2 (33.3%) | 4 (16.7%) |
| Frequency of mask wearing within 2 weeks [n (%)] | |||
| ≥2 h/day | 5 (16.7%) | 0 (0%) | 5 (20.8%) |
| 30 min-2 hours/day | 9 (30%) | 1 (16.7%) | 8 (33.3%) |
| ≤30 min/day | 16 (53.3%) | 5 (83.3%) | 11 (45.8%) |
| Residential area [n (%)] | |||
| Rural | 12 (40%) | 2 (33.3%) | 10 (41.7%) |
| Urban | 18 (60%) | 4 (66.7%) | 14 (58.3%) |
| Floor of house [n (%)] | |||
| ≥9th | 2 (6.7%) | 0 (0%) | 2 (8.3%) |
| 5th–8th | 10 (33.3%) | 1 (16.7%) | 9 (37.5%) |
| 1st–4th | 18 (60%) | 5 (83.3%) | 13 (54.2%) |
Clinical characteristics
The clinical indicators are summarised in Table 2 and Table S5. The mean systolic and diastolic blood pressures were 128.5 mmHg and 80.5 mmHg, respectively. The median (interquartile range, IQR) blood glucose level was 7.6 (6.0, 10.1) mmol/L. The mean values for total cholesterol and low-density lipoprotein cholesterol were 4.2 mmol/L and 2.5 mmol/L, respectively. The median levels of high-density lipoprotein cholesterol, triglyceride, lipoprotein(a), and homocysteine, were 0.9 (0.8, 1.1) mmol/L, 1.1 (0.9, 1.8) mmol/L, 130 (53.2, 276.3) mmol/L, and 9.9 (7.5, 12) μmol/L, respectively. The median platelet count was 225 × 109 (190.8 × 109, 299.5 × 109)/L. The median prothrombin time, thrombin time, and activated partial thromboplastin time were 11.5 (10.8, 12.2) sec, 18.0 (17.6, 18.8) sec, and 27.3 (25.5, 28.3) sec, respectively. The mean fibrinogen level was 3.7 g/L. D-dimer is a marker for hypercoagulability and is associated with thrombotic events in patients with arterial and venous thrombosis.27 The median log-transformed D-dimer level was 7.9 μg/L, which was significantly greater in the MP-detected group (8.3 ± 1.5 μg/L) than in the MP-undetected group (6.6 ± 0.5 μg/L, Unpaired t-test, p < 0.001).
Table 2.
Clinical indicators of the participants in this study (n = 30).
| Indicators | Total (n = 30) | MP-undetected (n = 6) | MP-detected (n = 24) | p value |
|---|---|---|---|---|
| SBP (mmHg) [mean, SD] | 128.5 ± 21.0 | 126.5 ± 26.1 | 129.0 ± 20.2 | 0.803 |
| DBP (mmHg) [mean, SD] | 80.5 ± 16.3 | 78.8 ± 24.0 | 80.9 ± 14.4 | 0.845 |
| Glucose (mmol/L) [median, IQR] | 7.6 (6.0, 10.1) | 7.4 (6.0, 9.5) | 8.2 (5.8, 10.9) | 0.860 |
| TC (mmol/L) [mean, SD] | 4.2 ± 1.1 | 4.2 ± 0.8 | 4.2 ± 1.1 | 0.966 |
| LDL (mmol/L) [mean, SD] | 2.5 ± 0.8 | 2.6 ± 0.7 | 2.5 ± 0.8 | 0.651 |
| HDL (mmol/L) [median, IQR] | 0.9 (0.8, 1.1) | 0.9 (0.8, 0.9) | 0.9 (0.8, 1.1) | 0.561 |
| TG (mmol/L) [median, IQR] | 1.1 (0.9, 1.8) | 1.5 (1.0, 1.9) | 1.1 (0.9, 1.6) | 0.347 |
| Lp(a) (mmol/L) [median, IQR] | 130.0 (53.2, 276.3) | 121.2 (73.9, 554.6) | 130.0 (47.3, 244.6) | 0.595 |
| Hcy (μmol/L) [median, IQR] | 9.9 (7.5, 12.0) | 11.1 (7.5, 16.6) | 9.7 (7.0, 11.4) | 0.296 |
| Platelets (109/L) [median, IQR] | 225.0 (190.8, 299.5) | 274.0 (203.8, 451.0) | 224.5 (190.0, 288.3) | 0.230 |
| PT (sec) [median, IQR] | 11.5 (10.8, 12.2) | 11.3 (10.7, 11.9) | 11.5 (10.7, 12.7) | 0.494 |
| TT (sec) [median, IQR] | 18.0 (17.6, 18.8) | 17.7 (17.6, 18.2) | 18.2 (17.6, 19.2) | 0.210 |
| APTT (sec) [median, IQR] | 27.3 (25.5, 28.3) | 27.9 (27.3, 30.0) | 26.7 (25.2, 27.9) | 0.093 |
| Fibrinogen (g/L) [mean, SD] | 3.7 ± 1.1 | 4.2 ± 0.9 | 3.6 ± 1.2 | 0.209 |
| D-dimer (μg/L) [mean, SD] | 7.9 ± 1.5 | 6.6 ± 0.5 | 8.3 ± 1.5 | <0.001 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TG, triglyceride; Lp(a), lipoprotein(a); Hcy, homocysteine; PT, prothrombin time; TT, thrombin time; APTT, activated partial thromboplastin time; MD, mean difference.
Rates of detection in human thrombi
A total of 30 patients were enrolled in the study, including 16 patients with ischaemic stroke (IS), 5 with myocardial infarction (MI), and 9 with deep vein thrombosis (DVT). Using Py-GC/MS, the detection rates of MPs in thrombi from patients with IS, MI, and DVT were 81.3% (13/16), 40% (2/5), and 100% (9/9), respectively, yielding an overall detection rate of 80% (24/30). Among the 10 types of microplastic polymers, three were detected: polyamide 66 (PA66), polyvinyl chloride (PVC), and polyethylene (PE). There was a heterogeneous distribution of polymers in the samples. PA66, PVC, and PE were detected in the IS and MI samples, whereas only PA66 was detected in the DVT samples. Specifically, PA66 was detected in 81.3% of the IS thrombi, 40% of the MI thrombi, and 100% of the DVT thrombi. PVC was detected in 37.5% of the IS thrombi and 20% of the MI thrombi. PE was detected in 25% of the IS thrombi and 20% of the MI thrombi. PVC and PE were not detected in the DVT thrombi (Table 3, Fig. S4). The spectra of 10 standards as positive controls (Fig. S5), 13 IS samples with detected MPs (Figs. S6 and S7), 2 MI samples with detected MPs (Fig. S8), 9 DVT samples with detected MPs (Fig. S9), and 6 samples without detectable MPs (Figs. S10–S15) were provided. Crucially, a vigorous quality control process was conducted to exclude potential contamination from environmental MPs. This process involved the inclusion of five different types of blank controls, comprising catheters and guidewires that had come into contact with IS, MI, or DVT thrombi, containers used for storing thrombi, and the Py-GC/MS instrument. Additionally, two types of negative controls, consisting of sampling and whole procedural controls, were also included. Notably, no peaks corresponding to MPs were detected in the spectra of any of the quality control groups in Py-GC/MS, which ruled out contamination from environmental MPs (Fig. S16).
Table 3.
Rates of MP detection in IS, MI, and DVT thrombi (n = 30).
| Variables | Rate of detection (%) |
||
|---|---|---|---|
| IS (n = 16) | MI (n = 5) | DVT (n = 9) | |
| Any MPs | 81.3% (13/16) | 40% (2/5) | 100% (9/9) |
| PA66 | 81.3% (13/16) | 40% (2/5) | 100% (9/9) |
| PVC | 37.5% (6/16) | 20% (1/5) | 0% (0/9) |
| PE | 25% (4/16) | 20% (1/5) | 0% (0/9) |
Abbreviations: MP, microplastics; IS, ischaemic stroke; MI, myocardial infarction; DVT, deep vein thrombosis; PA66, polyamide 66; PVC, polyvinyl chloride; PE, polyethylene.
Locations of MP-detected thrombi
The thrombi containing MPs were located differently. In the IS group, 10 thrombi (76.1%) were located in the anterior circulation (AC), which derives blood from the internal carotid arteries (ICAs) and supplies the majority of the cerebral hemispheres. Three (23.1%) were located in the posterior circulation (PC), which derives from the vertebral arteries to supply the brainstem, cerebellum, and posterior portion of the cerebral hemispheres. Within the AC, 3 thrombi were restricted to the ICA, while 7 thrombi involved the middle cerebral artery (MCA), a distal branch of the ICA. In the MI group, one thrombus was located in the left coronary artery, while the other was in the right coronary artery. In the DVT group, 2 thrombi (22.2%) were located in the popliteal-femoral vein, while 7 (77.8%) were located in the ilio-femoral vein.
MP concentrations and disease severity
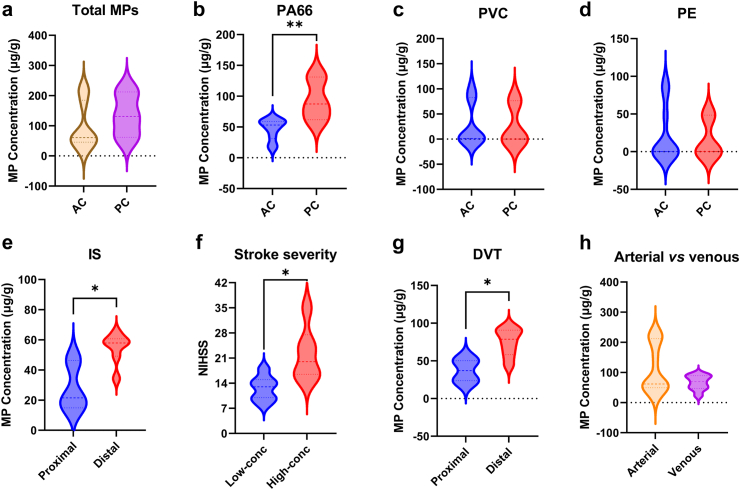
The concentrations of total MPs detected in the IS thrombi ranged from 14.88 to 221.8 μg/g, with a median concentration of 61.75 μg/g. The concentrations of PA66, PVC, and PE are provided in Table 4. There was no significant association between the concentration of MPs and the size of thrombi (Table S6). Although not statistically significant, the concentration of total MPs in the PC was greater than that in the AC [131.1 (61.8, 212.4) μg/g vs 60.68 (45.5, 184.8) μg/g, Mann–Whitney U test, p > 0.05] (Fig. 1a). However, the level of PA66 was greater in the PC than in the AC (93.4 ± 35.1 μg/g vs 46.6 ± 17.4 μg/g, Unpaired t-test, p < 0.01), while the levels of PVC and PE were similar at both locations (Fig. 1b–d). Within the AC, when MP-detected thrombi reached further to the MCA, the concentration of MPs was significantly greater than that when the MPs were restricted proximally to the ICA (54.7 ± 10.1 μg/g vs 27.6 ± 16.6 μg/g, Mann–Whitney U test, p < 0.05) (Fig. 1e). The National Institutes of Health Stroke Scale (NIHSS) is a standardised tool for quantifying stroke severity (the maximum score is 42). In patients with IS who had a high concentration of MPs, the NIHSS score was significantly higher than that in patients with a low concentration of MPs (22.0 ± 7.5 vs 12.6 ± 3.5, Unpaired t-test, p < 0.05) (Fig. 1f). Moreover, linear regression analysis indicated a positive association between the NIHSS score and the concentration of MPs in thrombi (adjusted β = 7.72, 95% CI: 2.01–13.43, p < 0.05) (Table S7). In the MI samples, the concentrations of total detected MPs were 49.3 μg/g in the left coronary artery, and 234.3 μg/g in the right coronary artery. In the DVT samples, the MP concentrations ranged from 23.8 to 94.2 μg/g, with a mean of 66.4 μg/g. Similarly, the more distally the MP-thrombi were, the greater the concentration of MPs (74.8 ± 18.6 μg/g vs 37.0 ± 18.7 μg/g, Unpaired t-test, p < 0.01) (Fig. 1g). Overall, the concentration of MPs in the arterial system (IS and MI) was not significantly different from that in the venous system (DVT) [61.8 (49.3, 212.4) μg/g vs 69.6 (47.2, 89.5) μg/g, Mann–Whitney U test, p > 0.05] (Fig. 1h).
Table 4.
Concentrations of MPs in thrombi obtained from patients with IS, MI, and DVT.
| Variables | Concentration (μg/g) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| IS |
MI |
DVT |
|||||||
| Total | PA66 | PVC | PE | Total | PA66 | PVC | PE | PA66 | |
| Minimum | 14.9 | 14.9 | 2.5 | 47.5 | 49.3 | 49.3 | 14.4 | 134.3 | 23.8 |
| Maximum | 221.8 | 131.1 | 104.3 | 90.6 | 234.3 | 85.6 | 14.4 | 134.3 | 94.2 |
| Mean | 105.5 | 57.4 | 59.6 | 77.0 | 141.8 | 67.5 | 14.4 | 134.3 | 66.4 |
| SD | 75.7 | 29.2 | 42.9 | 22.2 | 130.8 | 25.7 | 0.0 | 0.0 | 24.1 |
| SEM | 21.0 | 8.1 | 17.5 | 11.1 | 92.5 | 18.2 | 0.0 | 0.0 | 8.0 |
| Percentile | |||||||||
| 25% | 53.6 | 40.4 | 7.1 | 47.7 | 49.3 | 49.3 | 14.4 | 134.3 | 47.2 |
| 50% | 61.8 | 58.0 | 79.5 | 64.9 | 141.8 | 67.5 | 14.4 | 134.3 | 69.6 |
| 75% | 192.9 | 63.4 | 88.4 | 88.2 | 234.3 | 85.6 | 14.4 | 134.3 | 89.5 |
| IQR | 139.3 | 23.0 | 81.3 | 40.5 | 185.0 | 36.3 | 0.0 | 0.0 | 42.3 |
Abbreviations: SD, standard deviation; SEM, standard error of the mean; IQR, interquartile range.
Fig. 1.
Concentrations of MPs in human thrombi from different vascular locations. (a) Concentrations of total MPs in the AC (n = 10) and PC (n = 3) of the IS group. (b–d) Concentrations of PA66, PVC, and PE in the AC (n = 10) and PC (n = 3) of the IS group. Unpaired t-test, ∗∗p < 0.01. (e) Concentrations of MPs in the proximal (n = 3) and distal (n = 7) AC. Mann–Whitney U test, ∗p < 0.05. (f) NIHSS score, which reflects stroke severity, of patients with IS with low (n = 7) or high (n = 6) levels of MPs. Unpaired t-test, ∗p < 0.05. (g) Concentrations of MPs in the proximal (n = 2) and distal (n = 7) veins of DVT samples. Unpaired t-test, ∗p < 0.05. (h) Concentrations of MPs in arterial (n = 15) and venous (n = 9) thrombotic diseases. Abbreviations: AC, anterior circulation; PC, posterior circulation; NIHSS, National Institutes of Health Stroke Scale.
Physical properties of MPs
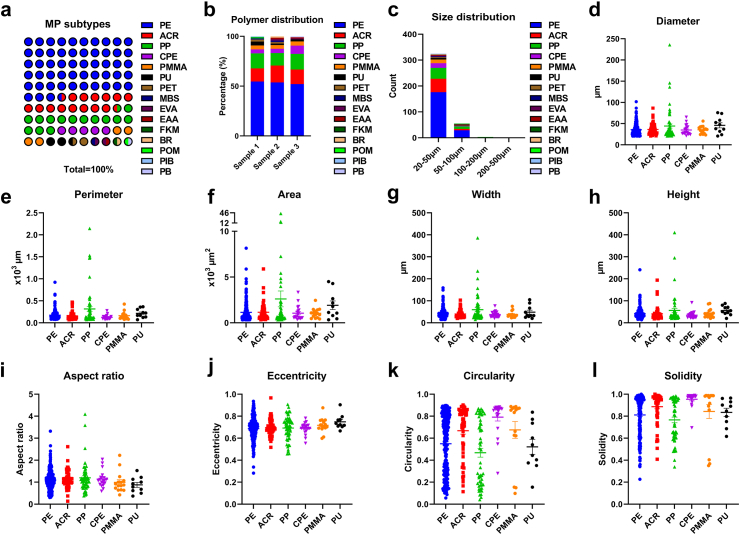
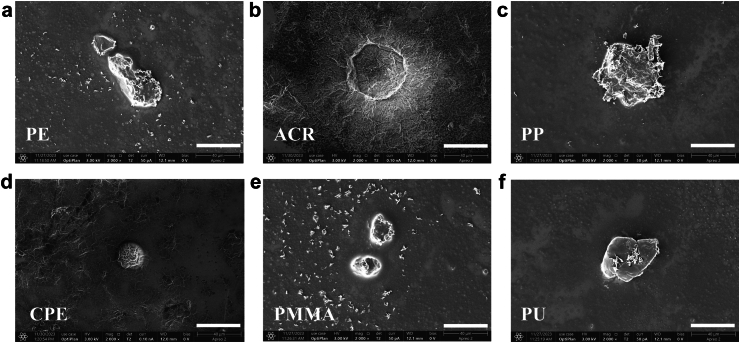
To characterise the physical properties of the IS thrombi, LDIR spectroscopy was used. Among all 384 particles detected by LDIR spectroscopy, 66.4% were non-MPs, while 33.6% were MPs. A total of 15 different types of MP polymers were detected, with PE being the most dominant type, making up 53.6% of the total MPs. This was followed by acrylate polymer (ACR, 14.8%), polypropylene (PP, 14.3%), chlorinated polyethylene (CPE, 5.2%), polymethyl methacrylate (PMMA, 3.9%), and polyurethane (PU, 2.6%), and the remaining 9 types of polymers accounted for 5.5% of the total MPs (Fig. 2a, Table S8). The polymer distributions across the samples were similar (Fig. 2b). The majority (84.6%) of the MPs ranged from 20 to 50 μm in size, 14.3% ranged from 50 to 100 μm, and 1.1% ranged from 100 to 500 μm (Fig. 2c). The mean diameters of the six most abundant polymers were 35.6 μm, 36.2 μm, 44.3 μm, 34.8 μm, 35.5 μm, and 45.9 μm (Fig. 2d). The perimeter, area, width, height, and aspect ratio (width/height) are presented in Fig. 2e–i. The eccentricity (distance to focus/distance to directrix), circularity [4π·area/(perimeter)2], and solidity (area/convex area), were also calculated, revealing substantial heterogeneity in the shapes and densities of the detected MPs (Fig. 2j–l). A third detection technique, SEM imaging, was employed to further determine the high-resolution morphological structure of the six most abundant polymers that were present in the thrombi as pre-scanned by LDIR spectroscopy (Fig. 3, Fig. S17). Under SEM imaging, fragment-shaped MPs and spherical-shaped MPs were detected.
Fig. 2.
LDIR imaging analysis of the physical properties of MPs in IS thrombi. (a) Type and abundance of MP polymers detected by LDIR (n = 3). (b) Polymer distribution of the detected MPs. (c) Size distribution of the detected MPs (d–l) Diameter, perimeter, area, width, height, aspect ratio, eccentricity, circularity and solidity of PE, ACR, PP, CPE, PMMA, and PU. Abbreviations: PE, polyethylene; ACR, acrylate polymer; PP, polypropylene; CPE, chlorinated polyethylene; PMMA, polymethyl methacrylate; PU, polyurethane.
Fig. 3.
SEM imaging of MPs in IS thrombi. (a–f) Representative SEM images of PE, ACR, PP, CPE, PMMA, and PU. Scale bar: 40 μm.
Discussion
In this study, we combined multimodal methods for detecting MPs in human thrombi samples, and investigated their presence, mass concentrations, and polymer types in human thrombi from both arterial and venous systems at three anatomically distinct sites: cerebral arteries in the brain, coronary arteries in the heart, and deep veins in the lower extremities. In addition, this study revealed that MPs with different particle numbers, sizes, shapes, and densities accumulated in thrombi obtained from patients with ischaemic stroke. Moreover, this study identified a possible association between the levels of MPs and disease severity.
The discovery of MPs in different human body systems, including the respiratory,7,8 cardiovascular,9,11,16 reproductive,12,14,15 and digestive systems,22 has raised concerns about their potential health hazards for humans. This has prompted the scientific community to intensify efforts in developing different analytical methods to identify and characterise MPs under various conditions. However, there are several challenges associated with analysing MPs in biological samples, such as the complexity of the samples, variable properties of the targeted polymers, and the costs and expertise required for analysis. In this study, three analytical methods were employed to investigate MPs in human thrombi samples, namely, Py-GC/MS, LDIR, and SEM. Py-GC/MS is a critical mass spectrometry technique for analysing MPs. The operation process begins with sample placement in an anaerobically heated environment such as an inert gas. The samples are then thermally degraded at a present temperature to thermally decomposed products, which are separated on a chromatographic column and finally characterised using mass spectrometry.28 This method does not require mechanical preselection of particles, and allows the detection of bulk amounts of micro- and nanoplastics in complex environments without practical lower limits on particle sizes. However, the lower limit of detection is constrained by the total polymer mass in the samples. That is, only when MP particles exceed a critical mass that defines the limit of detection and limit of quantification can they be properly detected and quantified.20 In addition, the most significant limitation associated with Py-GC/MS is that it is a destructive process and does not provide information on the number and physical properties of MPs. In contrast, spectroscopy-based approaches such as FTIR and Raman spectroscopy can identify and count MP particles without damaging samples. FTIR is an absorption spectroscopic technique widely used to identify organic materials. When a molecule absorbs an infrared photon, it changes from its fundamental vibrational state to an excited vibrational state. Then, the infrared spectra are further analysed via spectral library searches against known standard spectra.20 LDIR has recently been employed as an alternative to FTIR for the analysis of MPs. Compared to FTIR, it has the advantage of having a preliminary scan and eventually detects areas with actual particles, hence accelerating the identification process.21 In fact, LDIR has been combined with other methods to detect MPs in human samples, such as FTIR in human sputum samples,7 SEM in human lung,29 cardiac and blood samples,9 and Py-GC/MS in human testis and semen samples.13 One downside of LDIR is that only one spectrum is recorded when two particles are adjacent to each other, while μFTIR has a better chance to discriminate between individual particles.30 Another limitation of LDIR is that the recorded infrared band is narrower. As less information is collected, it is more susceptible to misidentification.30 Similar to FTIR, because the spatial resolution of LDIR is 10–20 μm, the minimum detection limit is typically set to 20 μm such that particles smaller than 20 μm are not within the scope of detection.7,9,21 Raman spectroscopy, another vibrational technique, can identify MPs with sizes down to 360 nm.31 Raman spectroscopy generates a molecular fingerprint spectrum based on the shift of electromagnetic radiation scattered by the molecules in a sample, known as the Raman effect.32 A significant drawback of Raman spectroscopy is its poor signal detectability and lack of repeatability.33 Obtaining reliable quantitative measurements using Raman spectroscopy can be challenging, especially when the target molecules do not emit strong Raman signals or are not Raman active. Additionally, if the sample is exposed to intense laser radiation or contains impurities that cause fluorescence, the Raman spectrum may be overshadowed.34 Furthermore, Raman spectroscopy is greatly limited by its reliance on material properties such as colour, biofouling, and degradation.19 SEM is a promising technique that can be used to analyse the surface morphology of MPs with particle sizes as small as 1 nm.35 However, its shortcomings, such as complicated pretreatment processes, low work efficiency, and high cost, are also obvious.36 Therefore, each analytical approach has limitations in practical applications. Multiple methods should be combined to ensure more accurate analysis of MPs in biological samples.
Recent evidence based on Raman spectroscopy has shown the accumulation of iron compounds, pigment particles, and one MP in thrombi collected from patients with aortic dissection or arterial embolism of the lower extremities.16 A total of 87 particles were identified in the thrombi through Raman spectroscopy, but only one particle was identified as low-density polyethylene (LDPE), a type of MP, while most of the particles were iron compounds and pigment particles. Considering the pigmented nature of the samples and the inherent limitations of Raman spectroscopy, the true extent of MP presence in the thrombi might have been underestimated. Moreover, the mass concentrations and polymer types of MPs in thrombi located in much smaller arteries and veins, and their relevance to disease severity remain unknown. To this end, our study retrieved thrombi from intracranial arteries in patients with IS, coronary arteries in patients with MI, and deep veins in patients with DVT, and subsequently measured the mass concentrations and 10 types of MPs using Py-GC/MS. MPs were present in 80% (24/30) of the thrombi collected from patients with IS, MI, and DVT, with mean concentrations of 61.8 μg/g, 141.8 μg/g, and 69.6 μg/g, respectively. Multiple types of MP polymers, namely, PA66, PVC, and PE, were detected among the 10 target polymer types. PA66, also known as nylon 66, is widely used in the manufacturing of consumer goods, textiles, electrical parts, automotive components, plastic bags, and packaging materials. Peng et al. reported the widespread detection of polyamide MPs in indoor dust, sludge, fish guts and gills, fishery sediment, freshwater sediment, and marine sediment, with concentrations ranging from 0.725 to 321 mg/kg.37 PVC, commonly used in the building and construction, automotive, piping, cable, and household goods industries, is known for its strength, durability, lightweight nature, and versatility.38 PE, the most common form of waste in the environment, is widely used in packaging, construction, and transportation. It is commonly found in shopping bags, plastic bottles, milk jugs, films, and toiletry.39 No significant differences were found in the investigated lifestyle habits involving the use of plastic products between participants with and without MPs detected in their thrombi. Considering that MPs have been detected in various foods, including salts, beer, daily drinking water, fishery products, and crop plants,40, 41, 42, 43, 44 and that they can be released from daily necessities such as toothbrushes,45 the sources of particle exposure for our participants may vary. It would be helpful to identify the exposure sources in future studies with larger sample sizes and thorough investigations into daily aspects involving the consumption or use of MP-contaminated products.
In the IS group, the concentration of MPs was significantly greater in thrombi located further from the ICA towards the MCA, and this difference was associated with disease severity. Similar findings were observed in DVT, where the concentration of MPs increased as the MP-thrombi reached more distal locations. Further analyses suggested a possible dose-dependent relationship between the MP concentration and disease severity. Additionally, the D-dimer level in the MP-detected group was significantly greater than that in the MP-undetected group. D-dimer is the degradation product of crosslinked fibrin and its levels reflect ongoing activation of the haemostatic and thrombolytic system.46 Our results resonate with a preclinical study that demonstrated enhanced thrombus formation in a hamster model exposed to a high concentration of MPs.47 It has been proposed that MP exposure could increase the activation of oxidative stress pathways.48 Additionally, other underlying mechanisms include apoptosis, pyroptosis, inflammation, and interactions between MPs and multiple cellular components.49 MPs may also directly interact with immune cells and endothelial cells, leading to endothelial activation, inflammation, and atherogenesis. Endothelial dysfunction induced by MP stimulation is related to thrombosis.50 Throughout the formation and progression of thrombi, circulating MPs may become trapped in developing thrombi. These sequestered MPs may further enhance the deposition of platelets and fibrin in thrombi.16,51 Overall, the results of preclinical research support that MPs may be associated with D-dimer and thrombotic events in our study participants. Further research is needed to fully understand the complex interactions and underlying pathways involved.
Although Py-GC/MS offers unparalleled advantages in quantifying MPs by mass concentrations and types without practical limits on the size of the MPs, as supported by the identification and quantification of 4 (PET, PE, PS, and PP) out of 5 types of target polymers in blood samples in Leslie et al.‘s work,11 and 3 (PA66, PVC, and PE) out of 10 types of target polymers in our study, Py-GC/MS is a destructive procedure that results in the loss of important physical data such as particle number, size, and shape. Therefore, we utilised LDIR spectroscopy to obtain such data from a subset of available IS thrombi. Using LDIR, we identified 15 different types of polymers in the thrombi. Consistent with the Py-GC/MS findings, PE was found to be abundant, constituting 53.6% of the total identified MPs. Py-GC/MS analysis indicated the widespread presence of PA66 in the thrombi. However, due to interference from natural proteins,52 and the limited detection capabilities for sizes less than 20 μm, we were unable to accurately distinguish PA66 using LDIR. This suggests that LDIR is not optimal for characterising PA66 in protein-rich biological samples, and relying solely on LDIR to measure MPs in such samples might underestimate certain types of MPs such as PA66 and their potential risks. PVC was detected using Py-GC/MS analysis but not by LDIR, possibly because the LDIR instrument has limitations in reliably identifying particles smaller than 20 μm, while PVC particles in thrombi might be smaller than that size.53 In a similar vein, exclusive reliance on LDIR for detection could lead to an underestimation of the risks associated with certain polymers such as PVC. Some polymers such as PMMA and PET were detected by LDIR, but not by Py-GC/MS, probably because their mass fell below the limit of quantification in Py-GC/MS. On the other hand, polymers such as PU, ACR, and CPE were detected by LDIR, but not by Py-GC/MS, because they were not within the scope of detection for Py-GC/MS.
Recently, Wu et al. used Raman spectroscopy to determine the presence of MPs (<20 μm) in thrombi, while in our study using LDIR, we predominantly observed MPs between 20 μm and 50 μm in size. Significant differences in MP size have also been observed in other biological samples. For instance, Amato-Lourenço et al. reported a mean MP size of 3.92 ± 0.67 μm in human lung tissues using Raman spectroscopy,54 whereas Jenner et al. reported a mean MP length of 223.10 ± 436.16 μm (range 12–2475 μm) and a mean MP width of 22.21 ± 20.32 μm (range 4–88 μm) in the same type of tissues using μFTIR.8 These discrepancies could be attributed to differences in MP exposure, or the use of different detection methods. It is important to note that Raman spectroscopy and FTIR typically involve manual spot observation, selection, and counting, which can introduce selection bias. Additionally, Raman spectroscopy can be heavily influenced by fluorescence and may cause UV degradation of MPs in the samples.55 Nevertheless, with a typical lower detection limit of down to 1 μm, Raman spectroscopy remains a valuable technique for identifying small MPs that cannot be characterised effectively by FTIR or LDIR. Our result is in line with the report by Yang et al., where most MPs detected in human heart tissue and blood were under 100 μm in diameter.9 In a recent study, Yang et al. investigated the prevalence of MPs in heart tissues (pericardia, epicardial adipose tissues, pericardial adipose tissues, myocardia, and atrial appendages) as well as pre- and postoperative venous blood samples from patients undergoing cardiac surgery, by means of LDIR and SEM. Across the five types of heart tissues, nine types of MPs were discovered, with diameters ranging from 20 to 469 μm, and median MP counts ranging from 34 to 2875. Most MPs detected in heart tissues were smaller than 100 μm, while a small fraction of the total MPs (0.25%) ranged from 300 to 500 μm. In parallel, MPs ranging from 20 to 184 μm in size were detected in every venous blood sample, with 67% of the MPs falling within the range of 30–50 μm.9 Similarly, in another recent study, a high abundance of MPs between 20.34 μm and 307.29 μm in size were identified in the human placenta, a unique vascular organ with two separate circulatory systems.24 More specifically, 80.29% of the MPs ranged from 20 to 100 μm, 15.22% ranged from 100 to 200 μm, and 4.49% ranged from 200 to 500 μm.24 Another study by Rotchell et al. reported that MPs in human saphenous vein tissues had a mean width of 41.27 ± 62.80 μm (range 7–300 μm) and a mean length of 119.59 ± 226.82 μm (range 16–1074 μm).56 In our study, 84.6% of the MPs ranged from 20 to 50 μm, 14.3% ranged from 50 to 100 μm, and 1.1% exceeded 100 μm. Collectively, our findings indicate that MPs larger than 100 μm may exist in the circulatory system, and that smaller MPs may translocate more easily into the circulatory system. This raises further questions regarding how these MPs reach the bloodstream and subsequently accumulate in tissues and organs. The three major exposure routes of MPs include ingestion, inhalation, and dermal exposure.57 The sources of dietary MP exposure in humans include seafood, drinking water, salts, honey, and beer, among others.58,59 For instance, Barboza et al. recently reported the accumulation of MPs, predominantly <100 μm (67% in fragment shape), in the dorsal muscle of three species of highly consumed fish.60 The gastrointestinal tract is a potential source of MPs that may penetrate into the circulatory system. It has been speculated that MPs smaller than 150 μm may translocate from the gut cavity to the circulatory system, albeit with limited absorption.61 Inhalation is another possible route through which MPs are deposited in the body. Recent studies have shown the accumulation of MPs larger than 100 μm in human sputum (median size: 75.43 μm; interquartile range: 44.67–210.64 μm) and lung tissues (11.11% of MPs larger than 100 μm).7,29 However, further studies are needed to explore whether and how inhaled MPs enter the bloodstream. In vivo studies on the effects of MPs on rodents via dermal penetration are rather limited, but large MPs are unlikely to penetrate the skin barrier.62 Recently, it was noted that medical procedures such as invasive cardiac surgery and intravenous infusion may allow direct access of MPs and nanoplastics to the bloodstream.9,56,63 In our study, sterile saline used for infusion during surgery and catheters and guidewires that had been in contact with the thrombi were analysed using Py-GC/MS to investigate any MP contamination from the medical procedure, and none of the 10 target MPs were detected. Thus, the MPs detected in the thrombi of 24 participants might have derived from other exposure sources rather than from the surgery itself. Finally, in this study, SEM, the third analytical technique, was used and provided clear morphological information that was not obtainable with Py-GC/MS or LDIR. Both LDIR and SEM imaging revealed that the shapes of the MPs in the thrombi were heterogeneous. Previously, only one irregularly block-shaped MP particle (LDPE) in thrombi, and several kinds of fragmented or irregularly shaped MPs including LDPE, polystyrene-co-acrylonitrile (PSAN), polyvinyl alcohol (PVA), polyethylene-co-acrylic acid (PEAA), and polyamine 6 (PA) in human whole blood, were identified by Raman spectroscopy.16,64 Our findings expand our understanding that not only irregular MPs but also spherical MPs can accumulate in human thrombi. Preclinical studies have not yet reached a clear conclusion regarding the shape-dependent effects of MP toxicity. Some studies found that spherical particles seemed to be more harmful,65,66 while others reported that irregular MPs could have stronger adverse effects.67, 68, 69 The discrepancies in these findings could be attributed to various factors, such as the different types of MPs and doses used in those studies. In summary, our multimodal findings emphasise the importance of using combinations of different analytical methods to complement each other, allowing for a more comprehensive and accurate assessment of MPs in biological samples.
Our study has several limitations. Firstly, although we have expanded the scope of MP detection by Py-GC/MS from 5 types of MP polymers in blood samples, as reported by Leslie and colleagues,11 to 10 types in our thrombi samples, there were other types of polymers that were beyond the scope of our Py-GC/MS detection system. Similarly, since the detection limit of LDIR is 20–500 μm, there may be significant amounts or diversity of MP particles that were missed in the analysis. Therefore, further efforts are needed to expand the Py-GC/MS spectral library and incorporate Raman spectroscopy to identify possible undetected MP polymers in the samples and to gain a better understanding of their associations with the formation of thrombi. Secondly, the sample size used in our study, particularly for thrombi from patients with MI and thrombi analysed using LDIR and SEM, was relatively small. Although we observed trends in the concentrations and types of MPs based on the location of the thrombi, this observation was based on a very low number of thrombi from nearly all the sites. Therefore, a larger number of thrombi should be collected from a larger sample size to validate the observed trends in this study. Equally important is to increase the sample size in other centres to examine whether our single-centre results can be generalised to other populations and settings. Thirdly, we were unable to identify specific exposure risk factors associated with the presence of MPs in thrombi. Furthermore, an inherent limitation of our observational study is the inability to establish a cause-and-effect relationship between the presence and concentration of MPs and the occurrence of thrombotic events. Additional research is required to understand the potential sources and pathways of MP exposure, whether a cause-and-effect relationship truly exists, and whether there are conjoint effects with other environmental factors involved in thrombus formation. Fourthly, we did not measure the concentrations of MPs in the blood of our patients. Consequently, the correlation between the levels of MPs in the blood and those in thrombi is unknown. Despite these limitations, we report the discovery of MPs within intracranial and coronary arterial thrombi and deep vein thrombi, by means of multimodal detection methods. This study provides valuable qualitative and quantitative evidence of MPs in thrombi from multiple pathological sites. Additionally, by comparing and contrasting different analytical methods for characterising MPs in our thrombi samples, we emphasise that although some of the findings appear mutually exclusive, they may complement each other and provide a more comprehensive understanding of the presence of MPs, not only in our thrombi samples, but also likely in other types of biological samples. Overall, our results contribute to the growing body of knowledge on the potential health hazards associated with MPs and highlight the need for further research in this area.
Contributors
All the authors have read and approved the final version of the manuscript. T.W. contributed to the design of the research and analysis of the data, and took the lead in writing the manuscript. Z.Y. contributed to the implementation of the research, data analysis, and writing of the manuscript. X.L., Y.C., X.H., J.F., R.S., and W.L. performed thrombectomy. Y.X. directed the project. T.W., Z.Y., and S.G. accessed and verified the data. W.Z. directed the project, and critically revised the manuscript. S.G. conceived and planned the project, performed data analysis, and revised the manuscript.
Data sharing statement
The data are available upon request from the authors.
Declaration of interests
The authors declare no competing financial interests.
Acknowledgements
This study was funded by the SUMC Scientific Research Initiation Grant (SRIG, No. 009-510858038), Postdoctoral Research Initiation Grant (No. 202205230031-3), and the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (No. 2020LKSFG02C). We would like to thank Prof. Qingying Zhang for her statistical assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105118.
Contributor Information
Weiduan Zhuang, Email: zhuangweiduan@sina.cn.
Shaowei Guo, Email: 07swguo1@stu.edu.cn.
Appendix A. Supplementary data
References
- 1.Ali N., Katsouli J., Marczylo E.L., Gant T.W., Wright S., Bernardino de la Serna J. The potential impacts of micro-and-nano plastics on various organ systems in humans. eBioMedicine. 2024;99 doi: 10.1016/j.ebiom.2023.104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith M., Love D.C., Rochman C.M., Neff R.A. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018;5(3):375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gewert B., Plassmann M.M., MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts. 2015;17(9):1513–1521. doi: 10.1039/c5em00207a. [DOI] [PubMed] [Google Scholar]
- 4.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ Sci Eur. 2016;28(1):2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campanale C., Massarelli C., Savino I., Locaputo V., Uricchio V.F. A detailed review study on potential effects of microplastics and additives of concern on human health. Int J Environ Res Public Health. 2020;17(4):1212. doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kutralam-Muniasamy G., Shruti V.C., Perez-Guevara F., Roy P.D. Microplastic diagnostics in humans: "The 3Ps" progress, problems, and prospects. Sci Total Environ. 2023;856(Pt 2) doi: 10.1016/j.scitotenv.2022.159164. [DOI] [PubMed] [Google Scholar]
- 7.Huang S., Huang X., Bi R., et al. Detection and analysis of microplastics in human sputum. Environ Sci Technol. 2022;56(4):2476–2486. doi: 10.1021/acs.est.1c03859. [DOI] [PubMed] [Google Scholar]
- 8.Jenner L.C., Rotchell J.M., Bennett R.T., Cowen M., Tentzeris V., Sadofsky L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y., Xie E., Du Z., et al. Detection of various microplastics in patients undergoing cardiac surgery. Environ Sci Technol. 2023;57(30):10911–10918. doi: 10.1021/acs.est.2c07179. [DOI] [PubMed] [Google Scholar]
- 10.Horvatits T., Tamminga M., Liu B., et al. Microplastics detected in cirrhotic liver tissue. eBioMedicine. 2022;82 doi: 10.1016/j.ebiom.2022.104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leslie H.A., van Velzen M.J.M., Brandsma S.H., Vethaak A.D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 12.Sun J., Sui M., Wang T., Teng X., Sun J., Chen M. Detection and quantification of various microplastics in human endometrium based on laser direct infrared spectroscopy. Sci Total Environ. 2024;906 doi: 10.1016/j.scitotenv.2023.167760. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q., Zhu L., Weng J., et al. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. 2023;877 doi: 10.1016/j.scitotenv.2023.162713. [DOI] [PubMed] [Google Scholar]
- 14.Ragusa A., Svelato A., Santacroce C., et al. Plasticenta: first evidence of microplastics in human placenta. Environ Int. 2021;146 doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 15.Halfar J., Cabanova K., Vavra K., et al. Microplastics and additives in patients with preterm birth: the first evidence of their presence in both human amniotic fluid and placenta. Chemosphere. 2023;343 doi: 10.1016/j.chemosphere.2023.140301. [DOI] [PubMed] [Google Scholar]
- 16.Wu D., Feng Y., Wang R., et al. Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J Adv Res. 2023;49:141–150. doi: 10.1016/j.jare.2022.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crous-Bou M., Harrington L.B., Kabrhel C. Environmental and genetic risk factors associated with venous thromboembolism. Semin Thromb Hemost. 2016;42(8):808–820. doi: 10.1055/s-0036-1592333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elert A.M., Becker R., Duemichen E., et al. Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters? Environ Pollut. 2017;231(Pt 2):1256–1264. doi: 10.1016/j.envpol.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Araujo C.F., Nolasco M.M., Ribeiro A.M.P., Ribeiro-Claro P.J.A. Identification of microplastics using Raman spectroscopy: latest developments and future prospects. Water Res. 2018;142:426–440. doi: 10.1016/j.watres.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 20.Mariano S., Tacconi S., Fidaleo M., Rossi M., Dini L. Micro and nanoplastics identification: classic methods and innovative detection techniques. Front Toxicol. 2021;3 doi: 10.3389/ftox.2021.636640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiting Q.T., O'Connor K.F., Potter P.M., Al-Abed S.R. A high-throughput, automated technique for microplastics detection, quantification, and characterization in surface waters using laser direct infrared spectroscopy. Anal Bioanal Chem. 2022;414(29-30):8353–8364. doi: 10.1007/s00216-022-04371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke D., Zheng J., Liu X., et al. Occurrence of microplastics and disturbance of gut microbiota: a pilot study of preschool children in Xiamen, China. eBioMedicine. 2023;97 doi: 10.1016/j.ebiom.2023.104828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S., Guo J., Liu X., et al. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: a pilot prospective study. Sci Total Environ. 2023;854 doi: 10.1016/j.scitotenv.2022.158699. [DOI] [PubMed] [Google Scholar]
- 24.Zhu L., Zhu J., Zuo R., Xu Q., Qian Y., An L. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Sci Total Environ. 2023;856(Pt 1) doi: 10.1016/j.scitotenv.2022.159060. [DOI] [PubMed] [Google Scholar]
- 25.Song X., Chen T., Chen Z., et al. Micro(nano)plastics in human urine: a surprising contrast between Chongqing's urban and rural regions. Sci Total Environ. 2024;917 doi: 10.1016/j.scitotenv.2024.170455. [DOI] [PubMed] [Google Scholar]
- 26.Kutralam-Muniasamy G., Shruti V.C., Perez-Guevara F., Roy P.D., Elizalde-Martinez I. Common laboratory reagents: are they a double-edged sword in microplastics research? Sci Total Environ. 2023;875 doi: 10.1016/j.scitotenv.2023.162610. [DOI] [PubMed] [Google Scholar]
- 27.Pulivarthi S., Gurram M.K. Effectiveness of D-dimer as a screening test for venous thromboembolism: an update. N Am J Med Sci. 2014;6(10):491–499. doi: 10.4103/1947-2714.143278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prata J.C., da Costa J.P., Duarte A.C., Rocha-Santos T. Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends Anal Chem. 2019;110:150–159. [Google Scholar]
- 29.Wang S., Lu W., Cao Q., et al. Microplastics in the lung tissues associated with blood test index. Toxics. 2023;11(9):759. doi: 10.3390/toxics11090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primpke S., Godejohann M., Gerdts G. Rapid identification and quantification of microplastics in the environment by quantum cascade laser-based hyperspectral infrared chemical imaging. Environ Sci Technol. 2020;54(24):15893–15903. doi: 10.1021/acs.est.0c05722. [DOI] [PubMed] [Google Scholar]
- 31.Xu G., Cheng H., Jones R., et al. Surface-enhanced Raman spectroscopy facilitates the detection of microplastics <1 μm in the environment. Environ Sci Technol. 2020;54(24):15594–15603. doi: 10.1021/acs.est.0c02317. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S., Qi Y., Tan S.P.H., Bi R., Olivo M. Molecular fingerprint detection using Raman and infrared spectroscopy technologies for cancer detection: a progress review. Biosensors (Basel) 2023;13(5):557. doi: 10.3390/bios13050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranska M., Schutze W., Schulz H. Determination of lycopene and beta-carotene content in tomato fruits and related products: comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal Chem. 2006;78(24):8456–8461. doi: 10.1021/ac061220j. [DOI] [PubMed] [Google Scholar]
- 34.Dong D., Zhao C. Limitations and challenges of using Raman spectroscopy to detect the abiotic plant stress response. Proc Natl Acad Sci U S A. 2017;114(28):E5486–E5487. doi: 10.1073/pnas.1707408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim W.J., Hong S.H., Eo S.E. Identification methods in microplastic analysis: a review. Anal Methods. 2017;9(9):1384–1391. [Google Scholar]
- 36.Huang Z., Hu B., Wang H. Analytical methods for microplastics in the environment: a review. Environ Chem Lett. 2023;21(1):383–401. doi: 10.1007/s10311-022-01525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng C., Tang X., Gong X., Dai Y., Sun H., Wang L. Development and application of a mass spectrometry method for quantifying nylon microplastics in environment. Anal Chem. 2020;92(20):13930–13935. doi: 10.1021/acs.analchem.0c02801. [DOI] [PubMed] [Google Scholar]
- 38.Miliute-Plepiene J., Fråne A., Almasi A.M. Overview of polyvinyl chloride (PVC) waste management practices in the nordic countries. Clean Eng Technol. 2021;4 [Google Scholar]
- 39.Romani V.P., Martins V.G., Goddard J.M. Radical scavenging polyethylene films as antioxidant active packaging materials. Food Control. 2020;109 [Google Scholar]
- 40.Lee H., Kunz A., Shim W.J., Walther B.A. Microplastic contamination of table salts from Taiwan, including a global review. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-46417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liebezeit G., Liebezeit E. Synthetic particles as contaminants in German beers. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2014;31(9):1574–1578. doi: 10.1080/19440049.2014.945099. [DOI] [PubMed] [Google Scholar]
- 42.Alberghini L.A.-O., Truant A., Santonicola S.A.-O., Colavita G.A.-O., Giaccone V. Microplastics in fish and fishery products and risks for human health: a review. Int J Environ Res Public Health. 2022;20(1):789. doi: 10.3390/ijerph20010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo Y., Li L., Feng Y., et al. Quantitative tracing of uptake and transport of submicrometre plastics in crop plants using lanthanide chelates as a dual-functional tracer. Nat Nanotechnol. 2022;17(4):424–431. doi: 10.1038/s41565-021-01063-3. [DOI] [PubMed] [Google Scholar]
- 44.Lin Y.D., Huang P.H., Chen Y.W., et al. Sources, degradation, ingestion and effects of microplastics on humans: a review. Toxics. 2023;11(9):747. doi: 10.3390/toxics11090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang C., Gopalan S., Zhang X., Xu L., Niu J., Naidu R. Raman imaging to identify microplastics released from toothbrushes: algorithms and particle analysis. Environ Pollut. 2023;337 doi: 10.1016/j.envpol.2023.122510. [DOI] [PubMed] [Google Scholar]
- 46.Rajendran V., Gopalan S., Varadaraj P., et al. Course of COVID-19 based on admission D-dimer levels and its influence on thrombosis and mortality. J Clin Med Res. 2021;13(7):403–408. doi: 10.14740/jocmr4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemmar A., Hoylaerts M.F., Hoet P.H., Vermylen J., Nemery B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol Appl Pharmacol. 2003;186(1):38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- 48.Hu M., Palic D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu X., Wang C., Duan X., Liang B., Genbo Xu E., Huang Z. Micro- and nanoplastics: a new cardiovascular risk factor? Environ Int. 2023;171 doi: 10.1016/j.envint.2022.107662. [DOI] [PubMed] [Google Scholar]
- 50.Becker R.C. Thrombogenesis in atrial fibrillation: contributing mechanisms and natural history. J Thromb Thrombolysis. 2008;26(3):262–264. doi: 10.1007/s11239-008-0278-y. [DOI] [PubMed] [Google Scholar]
- 51.Santilli F., Marchisio M., Lanuti P., Boccatonda A., Miscia S., Davì G. Microparticles as new markers of cardiovascular risk in diabetes and beyond. Thromb Haemost. 2016;116(2):220–234. doi: 10.1160/TH16-03-0176. [DOI] [PubMed] [Google Scholar]
- 52.Barth A. Infrared spectroscopy of proteins. Biochim Biophys Acta. 2007;1767(9):1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Fischer I., Schmitt W.F., Porth H.-C., Allsopp M.W., Vianello G. Ullmann's encyclopedia of industrial chemistry. 2014. Poly(vinyl chloride) pp. 1–30. [Google Scholar]
- 54.Amato-Lourenco L.F., Carvalho-Oliveira R., Junior G.R., Dos Santos Galvao L., Ando R.A., Mauad T. Presence of airborne microplastics in human lung tissue. J Hazard Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126124. [DOI] [PubMed] [Google Scholar]
- 55.Silva A.B., Bastos A.S., Justino C.I.L., da Costa J.P., Duarte A.C., Rocha-Santos T.A.P. Microplastics in the environment: challenges in analytical chemistry - a review. Anal Chim Acta. 2018;1017:1–19. doi: 10.1016/j.aca.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 56.Rotchell J.M., Jenner L.C., Chapman E., et al. Detection of microplastics in human saphenous vein tissue using μFTIR: a pilot study. PLoS One. 2023;18(2) doi: 10.1371/journal.pone.0280594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lett Z., Hall A., Skidmore S., Alves N.J. Environmental microplastic and nanoplastic: exposure routes and effects on coagulation and the cardiovascular system. Environ Pollut. 2021;291 doi: 10.1016/j.envpol.2021.118190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez A., Rodriguez-Viso P., Domene A., Orozco H., Velez D., Devesa V. Dietary microplastics: occurrence, exposure and health implications. Environ Res. 2022;212(Pt A) doi: 10.1016/j.envres.2022.113150. [DOI] [PubMed] [Google Scholar]
- 59.van der Laan L.J.W., Bosker T., Peijnenburg W. Deciphering potential implications of dietary microplastics for human health. Nat Rev Gastroenterol Hepatol. 2023;20(6):340–341. doi: 10.1038/s41575-022-00734-3. [DOI] [PubMed] [Google Scholar]
- 60.Barboza L.G.A., Lopes C., Oliveira P., et al. Microplastics in wild fish from north east atlantic ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci Total Environ. 2020;717 doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- 61.Barboza L.G.A., Dick Vethaak A., Lavorante B., Lundebye A.K., Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar Pollut Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 62.Larese Filon F., Mauro M., Adami G., Bovenzi M., Crosera M. Nanoparticles skin absorption: new aspects for a safety profile evaluation. Regul Toxicol Pharmacol. 2015;72(2):310–322. doi: 10.1016/j.yrtph.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Li P., Li Q., Lai Y., et al. Direct entry of micro(nano)plastics into human blood circulatory system by intravenous infusion. iScience. 2023;26(12) doi: 10.1016/j.isci.2023.108454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guan Q., Jiang J., Huang Y., et al. The landscape of micron-scale particles including microplastics in human enclosed body fluids. J Hazard Mater. 2023;442 doi: 10.1016/j.jhazmat.2022.130138. [DOI] [PubMed] [Google Scholar]
- 65.Jaikumar G., Brun N.R., Vijver M.G., Bosker T. Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ Pollut. 2019;249:638–646. doi: 10.1016/j.envpol.2019.03.085. [DOI] [PubMed] [Google Scholar]
- 66.Schwarzer M., Brehm J., Vollmer M., et al. Shape, size, and polymer dependent effects of microplastics on daphnia magna. J Hazard Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.128136. [DOI] [PubMed] [Google Scholar]
- 67.Vardi A., Schatz D., Beeri K., et al. Dinoflagellate-cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Curr Biol. 2002;12(20):1767–1772. doi: 10.1016/s0960-9822(02)01217-4. [DOI] [PubMed] [Google Scholar]
- 68.An D., Na J., Song J., Jung J. Size-dependent chronic toxicity of fragmented polyethylene microplastics to daphnia magna. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2021.129591. [DOI] [PubMed] [Google Scholar]
- 69.Frydkjaer C.K., Iversen N., Roslev P. Ingestion and egestion of microplastics by the cladoceran daphnia magna: effects of regular and irregular shaped plastic and sorbed phenanthrene. Bull Environ Contam Toxicol. 2017;99(6):655–661. doi: 10.1007/s00128-017-2186-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.