Abstract
Specific sleep characteristics have been associated with cognitive decline, Alzheimer’s disease and related dementias; however, studies examining the association between multidimensional sleep (a more comprehensive integration of sleep parameters) and cognitive decline are lacking. Among 2,811 older men without dementia, those with none, 1–2, and 3–5 “poor” self-reported sleep health dimensions had an adjusted 10-year change score of global cognition (3MS) of 2.9, 4.0 and 3.5 points (p-trend=0.05), and in executive function (Trails B) completion time of 36.7, 42.7, and 46.7 seconds (p-trend<0.01), respectively. In conclusion, a multidimensional measure of sleep health was associated with greater cognitive decline.
Keywords: sleep health, elderly, cognitive decline, Alzheimer’s disease
INTRODUCTION
Increasing evidence suggests that individual sleep disturbances evaluated objectively such as extreme sleep duration, poor sleep quality, and disrupted circadian rhythms are associated with poor cognition and risk of developing Alzheimer’s disease and related dementias in older adults [1–4]. However, objective assessment of sleep can be challenging and difficult to scale in large population studies, while self-reported measures have the advantage of being practical in real-world settings. Additionally, most studies have only looked at a global sleep score (ex. PSQI) or have focused on the effects of specific sleep characteristics or disorders, failing to capture the multidimensional nature of sleep [5]. To address this gap, the concept of “sleep health” has emerged, emphasizing multiple dimensions of sleep and their interrelationships to improve overall health and functioning [5]. A simple, and easily quantifiable, aggregate measure of self-reported sleep health has been developed, including characteristics representing sleep satisfaction, alertness, timing, efficiency, and duration [5], and has been shown to be closely related to health outcomes in older adults including physical functioning and frailty, depression, and mortality [6–9]. Among these studies, two have examined a gradient effect, and found that an increased number of poor sleep health dimensions (SHD) was associated with higher risk of depression and mortality [8,9]. However, to our knowledge, no study has investigated the association between this multidimensional measure of sleep health and cognitive outcomes in older adults, and whether more poor SHD are related to a more significant cognitive decline remain unknown. Hence, in this study, we aimed to examine the longitudinal association between a multidimensional sleep health assessment and subsequent cognitive decline over 10–12 years in community-dwelling older men without dementia.
METHODS
In this original study, we examined men enrolled in an ancillary study to the Osteoporotic Fractures in Men Study (MrOS), the MrOS Sleep Study [10,11]. Briefly, during the MrOS baseline examination (2000–2002), 5,994 community-dwelling men (≥65 years old) were recruited at six clinical centers in the United States. To participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement. Among them, 3,135 underwent a comprehensive sleep assessment between 2003 and 2005 (Sleep Visit 1). We excluded 188 men with incomplete data of significant cognitive impairment (Modified Mini-Mental State Examination (3MS) score <80 or taking medication for dementia) at Sleep Visit 1, and 136 men lacking follow-up data, leading to a final sample of 2,811 participants with multiple cognitive assessments over 10–12 years (Supplementary Figure 1). All men provided written informed consent and the study was approved by the Institutional Review Board at each site.
Sleep characteristics over the past year were self-reported and were categorized as “good” or “poor” based on the five sleep dimensions of the Satisfaction, Alertness, Timing, Efficiency, and Duration (SATED) scale [5]. Poor Satisfaction was defined as getting fewer hours of sleep than needed to feel rested. Poor Alertness was assessed by excessive daytime sleepiness and was defined by the standard cutoff score of > 10 on the Epworth Sleepiness Scale [12,13]. Poor Timing was assessed by mid-sleep time, which is calculated as the midpoint of the in-bed interval. Mid-sleep time was categorized based on octiles, and the first and eighth octiles were combined to define the “poor timing” dimension as extreme chronotypes have been linked to poor cognitive outcomes [14,15]. Since efficiency was defined as the ease of falling asleep and returning to sleep, here we used sleep latency as a measure of efficiency. Poor Latency was defined as reporting taking 30 minutes or more to fall asleep. Participants who reported a sleep duration of <7 hours or >8 hours were classified as having a Poor Duration. A multidimensional measure of sleep health was derived by summing the number of “poor” dimensions, with total scores ranging from 0 to 5 and higher scores indicating poorer sleep health. The scores were categorized, according to the number of poor sleep health dimensions, into two categories (≥1 versus 0) and three categories (0, 1–2, 3–5) to decipher a potential dose-response effect.
Cognitive function was assessed using two cognitive tests administrated by trained staff: the 3MS and the Trail Making Test – Part B (Trails B). The 3MS is a global measurement of cognition, with components for orientation, concentration, language, praxis, and immediate and delayed memory. Scores range from 0 to 100, with higher scores representing better cognitive function [16]. Trails B is a timed test that measures attention, sequencing, visual scanning, and executive function [17]. Participants are given 300 seconds to complete the test, and higher completion times represent worse cognitive function. In addition to Sleep Visit 1, four repeated cognitive tests were implemented (n=2,740 in 2005–2006, n=2,489 in 2007–2009, n=1,019 in 2009–2012, and n=1,226 in 2014–2016).
Participants also completed examinations and questionnaires at Sleep Visit 1, including information about demographics, body mass index (BMI), smoking and alcohol use, physical activity [18], depressive symptoms [19], medical history, and medication use [20] (Supplemental Material).
Participant characteristics were first compared by presence of poor SHD using chi-square tests for categorical variables, t-tests for normally distributed continuous variables, and Wilcoxon rank sum tests for skewed continuous data. Random effects models were used to examine the association between multidimensional poor sleep health and change in cognitive function over the follow-up (Supplementary Material) [21]. Cube and log transformations were performed on 3MS and Trails B respectively to improve the normality of the distributions, and the results were back-transformed to the original scale. Covariates for model adjustment were selected based on potential biological plausibility. Models were minimally adjusted for age, race, and clinic site. These models were further adjusted by potentially confounding factors (education, BMI, smoking, alcohol use, physical activity, depressive symptoms, benzodiazepine or sleep medication use, history of diabetes mellitus, hypertension, stroke, cardiovascular disease, and Parkinson’s disease). Statistical tests were two-sided, and analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Among the 2,811 participants (mean age, 76.0 ±5.3 years), 33.7% did not report any poor SHD, 54.5% reported 1 to 2 poor SHD, and 11.8% reported three or more poor SHD. Of the individual dimensions, 18.1% of the participants had Poor Satisfaction, 12.3% suffered from Poor Alertness, 24.6% had Poor Timing, 20.9% had Poor Latency, and 38.6% had Poor Duration. Men with at least one poor SHD were more likely to be nonwhite, to smoke and drink alcohol, had less education and physical activity, had higher BMI and more depressive symptoms. They were also more likely to have a history of diabetes mellitus, stroke, and cardiovascular disease, and to take benzodiazepines and sleep medications (Table 1).
Table 1.
Baseline characteristics by presence of poor sleep health dimensions in older men (n=2,811)
| No poor sleep health dimensions (n=946) |
At least one poor sleep health dimensions (n=1,865) | p-value | |
|---|---|---|---|
|
|
|||
| Characteristics | n (%) or mean (±SD) | n (%) or mean (±SD) | |
|
| |||
| Age (years) | 75.9 (±5.4) | 76.1 (±5.3) | 0.20 |
| Nonwhite race | 60 (6.3) | 203 (10.9) | <.0001 |
| Education | <.0001 | ||
| Less than high school | 27 (2.9) | 102 (5.5) | |
| High school | 118 (12.5) | 316 (16.9) | |
| College or higher | 801 (84.7) | 1447 (77.6) | |
| Body mass index (kg/m2) | 26.9 (±3.5) | 27.4 (±3.9) | <.0001 |
| Smoking | 12 (1.3) | 46 (2.5) | 0.03 |
| Alcohol use (drinks/week) | <.0001 | ||
| None | 363 (38.6) | 915 (49.3) | |
| 0–2 | 519 (55.2) | 841 (45.3) | |
| 3+ | 59 (6.3) | 101 (5.4) | |
| Physical Activity Scale for the Elderly score | 155.4 (±67.9) | 145.3 (±72.8) | <.001 |
| Geriatric Depression Scale score (0–15) | 1.2 (±1.5) | 1.9 (±2.2) | <.0001 |
| History of hypertension | 456 (48.2) | 928 (49.8) | 0.45 |
| History of diabetes mellitus | 97 (10.3) | 266 (14.3) | <.01 |
| History of stroke | 22 (2.3) | 81 (4.3) | <.01 |
| History of cardiovascular disease* | 224 (23.7) | 513 (27.5) | 0.03 |
| History of Parkinson’s disease | 10 (1.1) | 21 (1.1) | 0.87 |
| Benzodiazepine use | 19 (2.0) | 97 (5.2) | <.0001 |
| Sleep medication use | 11 (1.2) | 48 (2.6) | 0.01 |
Abbreviations: SD, standard deviation
Cardiovascular disease includes myocardial infarction, angina, and congestive heart failure.
The mean 3MS score and Trails B completion time at the initial assessment were 93.6 ±5.5 points and 117.2 ±50.7 seconds, respectively. Men with at least one poor SHD had lower cognition (93.4 ±4.5 points and 119.9 ±52.3 seconds) compared to those without any (94.1 ±4.4 points and 111.9 ±47.1 seconds). Repeat cognitive tests were performed at up to 4 follow-up timepoints (Supplementary Figure 1). As estimated by the adjusted random effects models, over 10-years, 3MS score decreased by 3.9 points and Trails B completion time increased by 43.5 seconds for those with at least one poor SHD compared to those with none (2.9-points decrease and 36.8-sec increase, respectively) (Table 2). Figure 1 shows estimated changes in 3MS and Trails B by number of poor SHD. The adjusted changes in 3MS scores were 2.9, 4.0 and 3.5 points (p-trend=0.05), and in Trails B completion times were 36.7, 42.7, and 46.7 seconds (p-trend<0.01), for those with none, 1–2, and 3–5 “poor” SHD, respectively. When individual SHD were examined, Poor Latency was associated with a decrease in 3MS score after multivariable adjustment (4.5-point and 3.3-point decrease for “poor” and “good” categories, respectively; Table 2), whereas Poor Timing was associated with an increase in Trails B completion time (48.7-second and 40.0-second increase for “poor” and “good” categories, respectively; Table 2).
Table 2.
Baseline characteristics by presence of poor sleep health dimensions in older men (n=2,811)
| 3MS score |
Trails B time |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 1 |
Model 2 |
|||||||
| n | Change/year | p-value | Change/year | p-value | Change/year | p-value | Change/year | p-value | ||
|
| ||||||||||
| Any poor sleep health dimensions | 0.005 | 0.007 | 0.005 | 0.008 | ||||||
| No | 946 | −0.29 | −0.29 | 3.43 | 3.68 | |||||
| Yes | 1,865 | −0.38 | −0.39 | 4.08 | 4.35 | |||||
|
| ||||||||||
| Sleep health dimensions | ||||||||||
|
| ||||||||||
| Satisfaction | 0.88 | 0.84 | 0.91 | 0.94 | ||||||
| Good | 2,301 | −0.36 | −0.36 | 4.02 | 4.24 | |||||
| Poor | 510 | −0.35 | −0.35 | 4.05 | 4.26 | |||||
| Alertness | 0.65 | 0.61 | 0.10 | 0.12 | ||||||
| Good | 2,466 | −0.36 | −0.36 | 3.96 | 4.17 | |||||
| Poor | 345 | −0.33 | −0.33 | 4.54 | 4.76 | |||||
| Timing | 0.07 | 0.07 | 0.003 | 0.003 | ||||||
| Good | 2,121 | −0.34 | −0.34 | 3.79 | 4.00 | |||||
| Poor | 690 | −0.41 | −0.41 | 4.63 | 4.87 | |||||
| Latency | 0.003 | 0.005 | 0.12 | 0.12 | ||||||
| Good | 2,223 | −0.33 | −0.33 | 3.85 | 4.07 | |||||
| Poor | 588 | −0.45 | −0.45 | 4.32 | 4.57 | |||||
| Duration | 0.63 | 0.70 | 0.82 | 0.86 | ||||||
| Good | 1,726 | −0.35 | −0.35 | 3.87 | 4.12 | |||||
| Poor | 1,085 | −0.36 | −0.36 | 3.93 | 4.16 | |||||
Abbreviations: 3MS, Modified Mini-Mental State Examination; Trails B, Trail Making Test – Part B
P-value for the beta coefficient of sleep health predictor*time. Change in cognition is presented as average change per year, calculated using the coefficients derived from the random-effects models (beta coefficient for time + beta coefficient for sleep health predictor*time).
Model 1: Adjusted for age, race, and clinic site.
Model 2: Model 1 + education, body mass index, smoking, alcohol use, physical activity, depressive symptoms, benzodiazepine or sleep medication use, history of diabetes mellitus, hypertension, stroke, cardiovascular disease, and Parkinson’s disease.
Figure 1. Changes in cognitive functions over 10 years by number of poor sleep health dimensions (n=2,811).
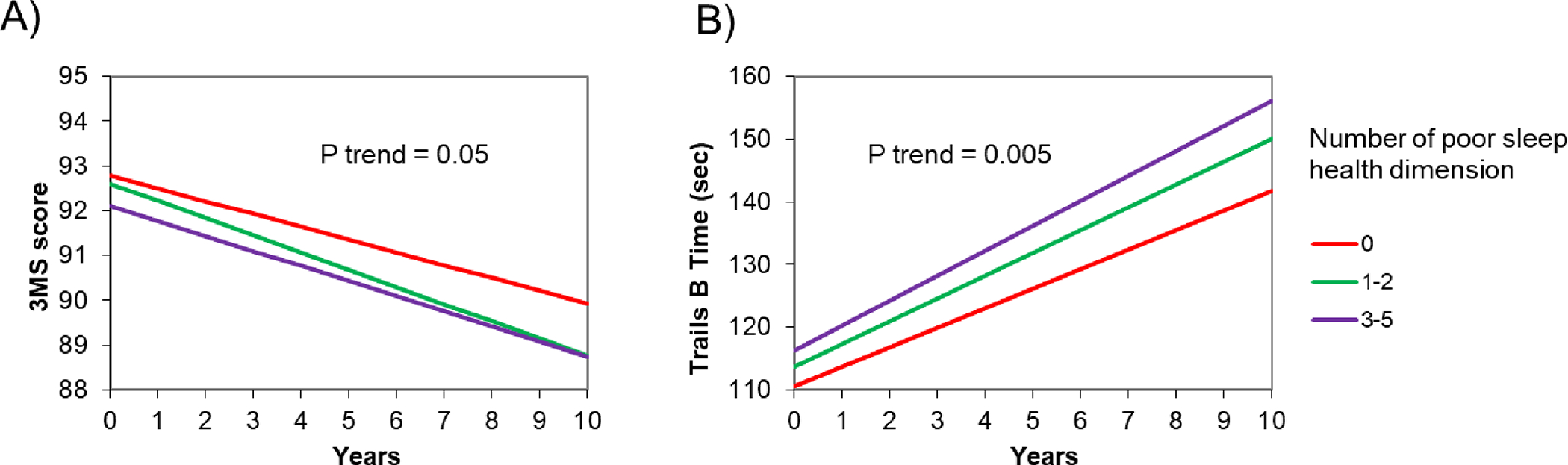
Abbreviations: 3MS, Modified Mini-Mental State Examination; Trails B, Trail Making Test – Part B.
Panel A represents the estimated change in 3MS score over 10 years by number of poor sleep health dimensions. Panel B represents the estimated change in Trails B test time over 10 years by number of poor sleep health dimensions.Adjusted for age, race, clinic site, education, body mass index, smoking, alcohol use, physical activity, depressive symptoms, benzodiazepine or sleep medication use, history of diabetes mellitus, hypertension, stroke, cardiovascular disease, and Parkinson’s disease.
DISCUSSION
Among older community-dwelling men without dementia, having a higher number of poor self-reported SHD was associated with greater cognitive decline, especially on executive function, after consideration of a number of potential confounders. Analyses of individual SHD suggested that Poor Latency was associated with a greater decline in global cognition, whereas Poor Timing was related to a greater decline in executive function.
Although no previous studies have investigated the relationship between multidimensional sleep health and cognitive aging, our results are consistent with prior literature linking multidimensional sleep health to adverse health outcomes such as physical impairment, depression, and mortality in community-dwelling older adults [6–9]. We contribute to the literature by showing that increment in the number of poor SHD is associated with greater cognitive decline over 10 years even after accounting for covariates. Our findings are also in line with other studies reporting associations between specific sleep characteristics and cognitive decline [22–24]. More precisely, we found that only Poor Latency was associated with global cognition when studying individual SHD. In the literature, mixed findings have been reported on the relationship between sleep latency and cognition [5,25–29]. Our study highlights the importance of studying sleep latency together with other dimensions of sleep health. For executive function, an association was found with Poor Timing only, which corroborates a growing body of research suggesting circadian dysfunctions as markers of neurodegenerative diseases 30].
We found that the increased number of poor sleep health dimensions was a stronger predictor of decline in executive function than global cognition. This result is consistent with prior research showing that the prefrontal cortex area, an important structure for executive function [31], may be particularly vulnerable to sleep disturbances [32,33]. Several biological mechanisms underlying the relationship between sleep and cognitive aging have been proposed. Indeed, poor sleep alters the clearance process via the glymphatic system, leading to the accumulation of amyloid-beta which is implicated in the neurodegenerative process of Alzheimer’s disease [34]. It has also been linked with an increased risk of cardiovascular diseases and risk factors [35–37], all of which are known to be associated with an increased risk of cognitive decline and dementia [38,39]. Finally, poor sleep could be an early marker of dementia, caused by the degeneration of neurons that promote wakefulness and sleep [40].
Overall, these results highlight the fact that considering individual SHD separately may underestimate the effects of sleep on cognition, reinforcing the importance of examining sleep in its multidimensional aspect. The SATED scale is a simple measure and easy to assess on a large scale, making it a promising tool at the population level to evaluate sleep health as an early marker of cognitive decline in late life. If poor multidimensional sleep health is confirmed to be a risk factor for dementia, interventions to improve sleep health may provide new opportunities for slowing down cognitive decline or reducing the risk of dementia in older adults.
Strengths of this study include a population of community-dwelling older men who were not selected based on sleep problems or cognitive function, its longitudinal design with a 10-year follow-up with high retention, a multidimensional measure of sleep health, and the consideration of a large number of possible confounders including information about demographics, lifestyle, comorbidities, and medication. Some limitations also need to be acknowledged. Generalizability of the results may be limited for women, non-White, and younger populations because this study involves mainly White older men. Sleep measures were self-reported which can lead to a lack of accuracy in responses; however, validated measures such as the Epworth Sleepiness Scale were used. The cognitive battery of tests was somewhat limited. Future longitudinal studies are needed to examine objective measures of multidimensional sleep health and cognitive decline in older adults. Finally, this is an observational study, and therefore causal relationship between multidimensional sleep health and cognitive decline cannot be assumed.
In summary, a multidimensional measure of sleep health was significantly associated with long-term decline in both global cognition and executive function among community-dwelling older men without dementia. Sleep health as a simple, self-reported measure might be a valuable risk marker of future cognitive decline in late life.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study staff and all the men who participated in MrOS Sleep Study.
FUNDING
Y.L. is supported by National Institute on Aging (NIA) 1R00AG056598. K.Y. is supported in part by (NIA) R35AG071916. The MrOS Study is supported by National Institutes of Health funding. The following institutes provided support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, R01 AG066671, and UL1 TR002369). The National Heart, Lung, and Blood Institute (NHLBI) provided funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Footnotes
CONFLICT OF INTEREST
Y.L is an Editorial Board Member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data supporting the findings of this study are openly available at https://mrosonline.ucsf.edu.
REFERENCES
- 1.Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, Kivimäki M, Dugravot A, Singh-Manoux A (2021) Association of sleep duration in middle and old age with incidence of dementia. Nat Commun 20;12(1):2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lysen TS, Luik AI, Ikram MK, Tiemeier H, Ikram MA (2020) Actigraphy-estimated sleep and 24-hour activity rhythms and the risk of dementia. Alzheimers Dement 16(9):1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, Song Y, Stone K (2014) Associations of Objectively and Subjectively Measured Sleep Quality with Subsequent Cognitive Decline in Older Community-Dwelling Men: The MrOS Sleep Study. Sleep 1;37(4):655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Yaffe K (2011) Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 70(5):722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buysse DJ (2014) Sleep Health: Can We Define It? Does It Matter? Sleep 1;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tighe CA, Brindle RC, Stahl ST, Wallace ML, Bramoweth AD, Forman DE, Buysse DJ (2021) Multidimensional Sleep Health and Physical Functioning in Older Adults. Gerontol Geriatr Med 20;7:23337214211016224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen TY, Lee S, Buxton OM (2022) Multidimensional sleep health is associated with physical frailty in a national sample of Taiwanese community-dwelling older adults: Sex matters. Sleep Health 8(5):528–35. [DOI] [PubMed] [Google Scholar]
- 8.Furihata R, Hall MH, Stone KL, Ancoli-Israel S, Smagula SF, Cauley JA, Kaneita Y, Uchiyama M, Buysse D (2017) An Aggregate Measure of Sleep Health Is Associated With Prevalent and Incident Clinically Significant Depression Symptoms Among Community-Dwelling Older Women. Sleep 1;40(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace ML, Stone K, Smagula SF, Hall MH, Simsek B, Kado DM, Redline S, Vo TN, Buysse D (2018) Which Sleep Health Characteristics Predict All-Cause Mortality in Older Men? An Application of Flexible Multivariable Approaches. Sleep 1;41(1):zsx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus S, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study — A large observational study of the determinants of fracture in older men. Contemp Clin Trials 1;26(5):569–85. [DOI] [PubMed] [Google Scholar]
- 11.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 1;26(5):557–68. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–5. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW (1992) Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 15(4):376–81. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Ren Y, Hou T, Liang X, Dong Y, Wang Y, Cong L, Wang X, Qin Y, Ren J, Sindi S, Tang S, Du Y, Qiu C (2022) Associations of sleep timing and time in bed with dementia and cognitive decline among Chinese older adults: A cohort study. J Am Geriatr Soc 70(11):3138–51. [DOI] [PubMed] [Google Scholar]
- 15.Suh SW, Han JW, Lee JR, Byun S, Kwon SJ, Oh SH, Lee KH, Han G, Hong JW, Kwak KP, Kim BJ, Kim SG, Kim JL, Kim TH, Ryu SH, Moon SW, Park JH, Seo J, Youn JC, Lee DY, Lee DW, Lee SB, Lee JJ, Jhoo JH, Kim KW (2018) Sleep and cognitive decline: A prospective nondemented elderly cohort study. Ann Neurol 83(3):472–82. [DOI] [PubMed] [Google Scholar]
- 16.Teng EL, Chui HC (1987) The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48(8):314–8. [PubMed] [Google Scholar]
- 17.Reitan RM (1958) Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept Mot Skills 8(3):271–6. [Google Scholar]
- 18.Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46(2):153–62. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh J, Yesavage J (1986) Geriatric Depression Scale: recent evidence and development of a shorter version. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press. pp 165–173 [Google Scholar]
- 20.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P (1994) Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10(4):405–11. [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH (1982) Random-effects models for longitudinal data. Biometrics 38(4):963–74. [PubMed] [Google Scholar]
- 22.Xu W, Tan CC, Zou JJ, Cao XP, Tan L (2020) Sleep problems and risk of all-cause cognitive decline or dementia: an updated systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 1;91(3):236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastião YV, Wen Y, Schwartz S, Borenstein A, Wu Y, Morgan D, Anderson W (2017) Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep 1;40(1). [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Sun D, Tan Y (2018) A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders. Sleep Breath 1;22(3):805–14. [DOI] [PubMed] [Google Scholar]
- 25.Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE (2012) What sleep characteristics predict cognitive decline in the elderly? Sleep Med 1;13(7):886–92. [DOI] [PubMed] [Google Scholar]
- 26.Brewster GS, Varrasse M, Rowe M (2015) Sleep and Cognition in Community-Dwelling Older Adults: A Review of Literature. Healthcare 3(4):1243–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL (2006) Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci 61(4):405–10. [DOI] [PubMed] [Google Scholar]
- 28.Miyata S, Noda A, Iwamoto K, Kawano N, Okuda M, Ozaki N (2013) Poor sleep quality impairs cognitive performance in older adults. J Sleep Res 22(5):535–41. [DOI] [PubMed] [Google Scholar]
- 29.Saint Martin M, Sforza E, Barthélémy JC, Thomas-Anterion C, Roche F (2012) Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep Med 1;13(9):1146–52. [DOI] [PubMed] [Google Scholar]
- 30.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K (2019) Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 18(3):307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuster J (2008) The Prefrontal Cortex, fifth edition, Academic Press, London. [Google Scholar]
- 32.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D (2000) Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9(4):335–52. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K, Nasrallah I, Hoang TD, Lauderdale DS, Knutson KL, Carnethon MR, Launer LJ, Lewis CE, Sidney S (2016) Sleep Duration and White Matter Quality in Middle-Aged Adults. Sleep 1;39(9):1743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M (2013) Sleep drives metabolite clearance from the adult brain. Science 18;342(6156):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y (2018) Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev 39:25–36. [DOI] [PubMed] [Google Scholar]
- 36.Itani O, Jike M, Watanabe N, Kaneita Y (2017) Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med 32:246–56. [DOI] [PubMed] [Google Scholar]
- 37.Bock J, Covassin N, Somers V (2022) Excessive daytime sleepiness: an emerging marker of cardiovascular risk. Heart Br Card Soc 31;heartjnl-2021–319596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Ortega V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 8;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR, Lewis CE, Lloyd-Jones DM (2020) Cardiovascular risk factors and accelerated cognitive decline in midlife: The CARDIA Study. Neurology 18;95(7):e839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh J, Eser RA, Ehrenberg AJ, Morales D, Petersen C, Kudlacek J, Dunlop SR, Theofilas P, Resende EDPF, Cosme, Alho EJL, Spina S, Walsh CM, Miller BL, Seeley WW, Bittencourt JC, Neylan TC, Heinsen H, Grinberg LT(2019) Profound degeneration of wake-promoting neurons in Alzheimer’s disease. Alzheimers Dement 15(10):1253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are openly available at https://mrosonline.ucsf.edu.


