Abstract
The hydrated sodium salt of 1,2,3,4-tetrahydroquinolinedithiocarbamate (1) has been successfully synthesized and characterized using IR, NMR, and X-ray single crystal analysis. The υC–S and thioureide υC–N bands appeared at 1484 cm−1 and 968 cm−1, respectively, in Na(H2O)3+(thqdtc)– • H2O. The notable NCS2 carbon signal emerged at 212 ppm, credited to unique nitrogen and sulfur-induced deshielding effects. Compound 1 crystallizes in the monoclinic system, P21/c space group, with dimensions a = 14.4297(4) Å, b = 6.1534(2) Å, c = 17.6701(4) Å, β = 108.7340(10)°, V = 1485.83(7) Å3, and Z = 4. The structure of 1 exhibits a supramolecular architecture through secondary interactions, such as weak intermolecular interactions that link the molecules into a linear polymeric chain. The incorporation of heterocyclic rings in the dithiocarbamate ligands leads to the formation of an intriguing supramolecular architecture, as confirmed by BVS analysis results. The BVS value of sodium does not agree well with the formal oxidation state due to the interactions of anions, cations, coordinated and uncoordinated water molecules.
Keywords: Heterocyclic dithiocarbamate, Coordination chemistry, Spectral analysis, Structural analysis, Hydrogen bonding, Bond valence sum analysis
Highlights
-
•
Dithiocarbamate is versatile in organic and inorganic fields.
-
•
Hydrated sodium salt of dithiocarbamate has broad applications.
-
•
It tends to form linear polymeric chains effectively.
-
•
Uncoordinated water is a vital factor.
1. Introduction
Dithiocarbamate anions find motivation in their utility across a diverse range of applications, spanning medicine, agriculture, organic synthesis, and even as synthetic precursors for metal sulfide nanoparticles [[1], [2], [3], [4], [5], [6], [7]]. Hydrated sodium ions play a crucial role in various biological functions of aquatic animals. Understanding hydrated sodium ions is challenging due to their biological mechanisms and the stabilization energy arising from the presence of multiple hydrates in these ions. Numerous clusters of hydrated sodium ions have been reported. The coordination geometry of these clusters is an intriguing feature, owing to sodium's coordination ability with water [8]. Many theoretical studies further support sodium atoms' hydrate coordination ability, leading to a variety of coordination environments for sodium ions. Alkali metal ions play a significant role in the biological functions of specific enzymes. The π–interactions of alkali metal cations are particularly interesting due to their aromatic residue sensing nature. R. Alan Howie and colleagues (2008) investigated a series of dithiocarbamate salts with hydrogen bonding functionality. The dtc ligand possesses a delocalized π–orbital system, allowing electron density transfer from nitrogen to sulfur [9].
Alkali metal dithiocarbamates exhibit significant pi interactions due to the presence of the NCS2 moiety and water molecules. Among alkali metal dithiocarbamates, sodium, lithium, rubidium, and cesium metal dithiocarbamates have been reported in the literature. Alkali metal dithiocarbamates are water-soluble ionic compounds, resulting in the formation of many hydrated alkali metal dithiocarbamates. The coordination environment of alkali metals is influenced by water molecules. In this study, we synthesized a water-soluble hydrated sodium ionic compound with a dithiocarbamate moiety. To the best of our knowledge, few sodium salts of dithiocarbamates, such as Na[S2CN(CH2CH2OH)2] • 3H2O, Na[S2CN(CH2CH2OH)CH2CH2CH3] • 2H2O [9], Na2[S2CNH(CH2)2HNCS2)] • 6H2O [10], and Na2[S2CNCH2C6H5CH2CH2N(CH2C6H5)CS2] [11], have been reported.
The influence of cations on the supramolecular aggregation patterns of dithiocarbamate anions, functionalized with hydrogen bonding capacity, stems from the prevalence of charge–assisted O–H/S interactions [9]. However, the literature lacks a polymeric structure of hydrated sodium ions among these structures. The sodium salt of 1,2,3,4–tetrahydroquinolinedithiocarbamate, hydrated sodium ions, leads to a polymeric structure without dithiocarbamate coordination. This structure possesses a unique structural motif compared to the reported hydrated sodium salts of dithiocarbamates. In this paper, we present sodium 1,2,3,4–tetrahydroquinolinedithiocarbamate tetrahydrate as an ideal subject for investigation, as sodium does not interact with the ligand's delocalized π–orbital system. The chemical composition of the present structure is illustrated in Scheme 1.
Scheme. 1.
Chemical composition of Na(H2O)3+(thqdtc)–• H2O.
2. Experimental
2.1. Chemicals
Generally, 1,2,3,4-tetrahydroquinoline (Alfa Aesar), carbon disulfide (Merck), sodium hydroxide, and solvents (SD Fine) were commercially available high-grade materials and used as received.
2.2. Spectroscopy
IR spectra were recorded using a Thermo Nicolet Avatar 330 FT–IR spectrophotometer (range: 400–4000 cm–1) with KBr pellets. NMR spectra were recorded (in CDCl3) on an AV–III 400 NMR spectrometer operating at 400 MHz.
2.3. X–ray crystallography
Details of the crystal data, data collection, and refinement parameters for Na(H2O)3+(thqdtc)– • H2O are summarized in Table 1. The intensity data were collected at ambient temperature (293(2) K) using a Bruker axs kappa apex2 CCD diffractometer with graphite monochromated MoKa radiation (λ = 0.71073 Å). The structure was solved using SIR–92 [12] and refined using a full matrix least square with SHELXL–97 [13]. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were refined isotropically. Bond distances and angles are provided in Table 2.
Table 1.
Crystal data, data collection and refinement parameters for Na(H2O)3+(thqdtc)– • H2O.
| Parameters | Na(H2O)3+(thqdtc)– • H2O |
|---|---|
| Empirical formula | C10H18NO4S2Na |
| FW | 303.36 |
| Crystal dimensions (mm) | 0.26 × 0.22 × 0.20 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 14.4297(4) |
| b/Å | 6.1534(2) |
| c/Å | 17.6701(4) |
| α/° | 90.00 |
| β/° | 108.7340(10) |
| γ/° | 90.00 |
| U/Å3 | 1485.83(7) |
| Z | 4 |
| DC/g cm−3 | 1.356 |
| μ/cm−1 | 0.392 |
| F(000) | 640 |
| λ/Å | MoKα (0.71073) |
| θ Range/° | 1.49–31.55 |
| Index ranges | −21 ≤ h ≤ 21, −9 ≤ k ≤ 9, −26 ≤ l ≤ 26 |
| Reflections collected | 5404 |
| Observed reflections Fo > 4′O(Fo) | 4026 |
| Weighting scheme | Calc. W = 1/(σ2(Fo2) + (0.0750p)2 + 0.1908p) where p = (Fo2 + 2Fc2)/3 |
| Number of parameters refined | 195 |
| Final R, Rw(obs, data) | 0.0403, 0.1171 |
| GOOF | 1.062 |
Table 2.
Bond distances (Å) and angles (°) for Na(H2O)3+(thqdtc)– • H2O.
| Bond distances (Å) | Bond angles (°) | ||||
|---|---|---|---|---|---|
| C8–N1 | 1.4735(18) | C5–C9–N1 | 117.50(13) | O3–Na1–O3i | 166.81(5) |
| C9–N1 | 1.4240(16) | C1–C9–N1 | 120.93(13) | O1–Na1–O3 | 95.00(5) |
| C10–N1 | 1.3463(17) | N1–C10–S2 | 118.96(9) | O2–Na1–O1 | 91.45(5) |
| C10–S2 | 1.7038(12) | N1–C10–S1 | 120.06(9) | O3–Na1–O1 | 102.69(5) |
| C10–S1 | 1.7164(12) | S2–C10–S1 | 120.98(8) | O1–Na1–O1i | 169.46(3) |
| O1–Na1 | 2.3612(13) | C10–N1–C9 | 125.48(10) | O3–Na1–O1i | 79.61(5) |
| O1i–Na1 | 2.3871(13) | C10–N1–C8 | 122.11(12) | O2–Na1–O2i | 169.65(5) |
| O2–Na1 | 2.3357(13) | C9–N1–C8 | 112.21(12) | O2i–Na1–Na1i | 47.75(3) |
| O2i–Na1 | 2.4998(15) | Na1–O1–Na1i | 81.71(3) | O2–Na1–Na1i | 52.39(4) |
| O3–Na1 | 2.3577(15) | Na1–O2–Na1i | 79.86(4) | O3–Na1–Na1i | 141.41(5) |
| O3i–Na1 | 2.3776(15) | Na1–O3–Na1i | 81.99(4) | O1i–Na1–Na1i | 132.74(4) |
| Na1–Na1i | 3.1063(2) | O2–Na1–O3 | 115.63(6) | O3i–Na1–Na1i | 48.73(4) |
| O2–Na1–O1i | 96.18(5) | O1–Na1–Na1i | 48.78(3) | ||
| O3–Na1–O1i | 80.54(5) | O2i–Na1–Na1i | 118.26(4) | ||
| O2–Na1–O3i | 77.05(5) | Na1i–Na1–Na1i | 164.18(5) | ||
Symmetry code: i = 2–x, 1/2+y, 1.5–z.
2.4. Synthesis of Na(H2O)3+(thqdtc)– • H2O
The sodium 1,2,3,4–tetrahydroquinoline-carbodithioate dihydrate was prepared using the following procedure [9,14]. A mixture of NaOH (50 mmol, 2.0 g) dissolved in a minimum quantity of water and 1,2,3,4–tetrahydroquinoline (50 mmol, 6.5 mL) was cooled to 5 °C in an ice bath. To this cold solution, CS2 (50 mmol, 3.0 mL) was added in small portions while stirring. After 15 min, the entire mass solidified and was air-dried and recrystallized from an acetone–petroleum ether mixture (boiling range: 40–60 °C). Shining yellowish microcrystalline substance was collected and dried. Recrystallization of the yellowish microcrystalline Na(H2O)3+(thqdtc)– • H2O from a CHCl3 solution yielded a pale yellow product. The pale yellow crystals were analyzed as Na(H2O)3+(thqdtc)– • H2O (Scheme 2).
Scheme. 2.
The preparation of Na(H2O)3+(thqdtc)–•H2O.
3. Results and discussion
In this paper, we aim to investigate the preparation and characterization using physical techniques such as IR, NMR, and single crystal analysis. Typically, dithiocarbamates exhibit high delocalization owing to the nature of pi electron movements from nitrogen to sulfur.
3.1. IR spectral studies
Dithiolate and dithiocarbamate ligands are very similar and peculiar; they exhibit high reactivity with alkali metals as well as other metals, forming compounds. In this study, the hydrated sodium salt of 1,2,3,4-tetrahydroquinolinedithiocarbamate (thqdtc) was formed due to the steric bulkiness of the dithiocarbamate ligand. Typically, the νC–N and νC–S stretching modes are significant absorption bands in dithiocarbamate complexes. The νC–N stretching mode has been utilized to gauge the contribution of the thioureide form (c) to the dithiocarbamate structure (Scheme 3).
Scheme. 3.
The resonance forms of NCS2.
The energy of the thioureide νC–N band falls in between the stretching frequencies associated with typical singly and doubly bonded carbon and nitrogen (Fig. 1). This can be best explained as the vibration of the polar C N band [15,16]. A νC–N band of moderate intensity, around 1450 cm−1, has been previously observed [17]. In Na(H2O)3+(thqdtc)– • H2O, the thioureide νC–N band at 1484 cm−1. Water bands are observed in the 3422–3250 cm−1 region in Na(thqdtc) • 4H2O. The νC–S bands appear at 968 cm−1 in Na(H2O)3+(thqdtc)– • H2O [18]. The C C Stretching, usually observed at 1641and1593 cm−1. The CH and CH2 stretching vibrations were assigned at 3008 cm−1 and 2936 cm−1, respectively.
Fig. 1.
IR spectrum of Na(H2O)3+(thqdtc)–• H2O.
3.2. NMR spectral studies
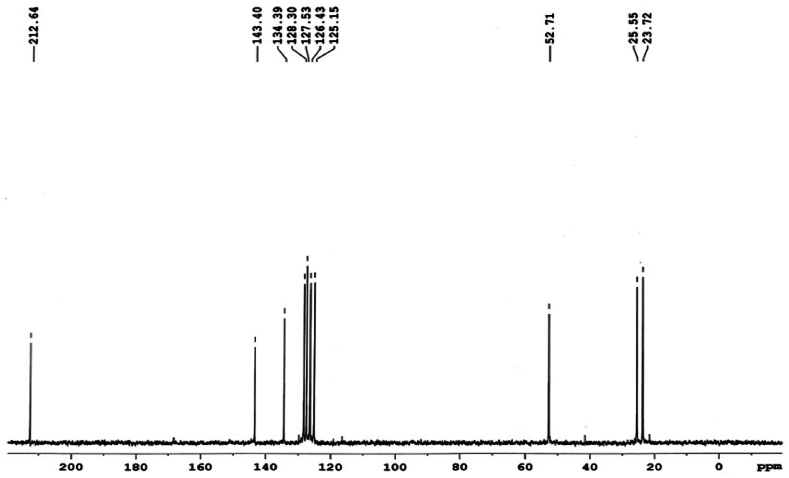
Free 1,2,3,4–tetrahydroquinoline exhibits signals at 3.29 and 6.46 ppm for protons at C-2 and C-8, respectively. The 1H NMR spectrum of Na(H2O)3+(thqdtc)– • H2O (Fig. 2) reveals that protons at C-2 and C-8 undergo strong deshielding, resulting in signals at 4.3 and 7.2 ppm, respectively. The observed deshielding of the protons at C-2 and C-8 in Na(H2O)3+(thqdtc)– • H2O is attributed to the release of electrons from the NR2 groups, causing a high electron density towards the sulfur via the thioureide π–system [19]. Other proton signals remain relatively unaffected in Na(H2O)3+(thqdtc)– • H2O due to their distance from the thioureide π–system. A quintet observed around 2.10 and a triplet observed around 2.75 ppm are attributed to the protons at C-3 and C-4, respectively. A multiplet observed in the region of 7.12–7.22 ppm is assigned to the protons at C-5, C-6, and C-7 in thqdtc. Free 1,2,3,4–tetrahydroquinoline displays a signal at 41.9 ppm due to the C-2 carbon. In the case of Na(H2O)3+(thqdtc)– • H2O, C-2 carbon is deshielded, resulting in signal at 52.7 ppm. The downfield shift of the C-2 carbon signal in the case of Na(thqdtc)•4H2O is due to a reduction in the electron density in their vicinity, contributing to a significant thioureide structure Nδ+ Cδ– in the salt. In Na(H2O)3+(thqdtc)– • H2O, the aromatic carbons of thqdtc are observed in the region of 125.2–143.4 ppm. The important NCS2 carbon signal is found at 212.6 ppm due to the deshielding effects of nitrogen and sulfur in the thqdtc ligand (Fig. 3). This result indicates that the dithiocarbamate anion is not coordinated with sodium metal.
Fig. 2.
1H NMR spectrum of Na(H2O)3+(thqdtc)–• H2O.
Fig. 3.
13C NMR spectrum of Na(H2O)3+(thqdtc)–• H2O.
3.3. Single crystal X–ray analysis of Na(H2O)3+(thqdtc)–• H2O
The single crystal X–ray structure of Na(H2O)3+(thqdtc)– • H2O reveals four formula units per unit cell (Figure S1), and the [Na(H2O)3+] units aggregate into linear polymeric chains (Fig. 4). Fig. 5 illustrates that one of the four water molecules in Na(H2O)3+(thqdtc)– • H2O acts as a bridging unit between [Na(H2O)3+] and thqdtc (O3–H3b … O1w–H1d … S1).
Fig. 4.
Linear polymeric chain formed by [Na(H2O)3+] (symmetry code: i = 2–x, 1/2+y, 1.5–z).
Fig. 5.
ORTEP diagram of Na(H2O)3+(thqdtc)–• H2O.
The Na+ ion is coordinated by six water oxygen atoms at distances ranging from 2.3357(13) to 2.4998(15) Å, forming a distorted octahedron. The O–Na–O bond angles range from 74.31(5) to 169.65(6)%. No Na–S bonds are formed; instead, S1 and S2 accept four oxygen and two hydrogen bonds from the water molecules, respectively, with S⋯H distances in the range of 2.41(3)–2.70(3) Å and S⋯O distances from 3.2743(15) to 3.4423(18) Å (Table 3). The C10–S1 bond length is 1.7164(12) Å, and the C10–S2 bond length is 1.7038(12) Å, showing a slight difference.
Table 3.
Hydrogen–bonding geometric parameters for Na(H2O)3+(thqdtc)– • H2O [(Å), (%)].
| H–bond | D⋅⋅⋅H | H⋅⋅⋅A | D⋅⋅⋅A | D―H⋅⋅⋅A |
|---|---|---|---|---|
| O1–H1A⋅⋅⋅S1 | 0.84(3) | 2.45(3) | 3.2793(14) | 168(2) |
| O1–H1B⋅⋅⋅S1 | 0.71(3) | 2.70(3) | 3.3137(12) | 146(3) |
| O1W–H1C⋅⋅⋅S1 | 0.82(4) | 2.65(4) | 3.4423(18) | 162(4) |
| O1W–H1D⋅⋅⋅S1 | 0.85(3) | 2.59(3) | 3.3959(17) | 159(3) |
| O2–H2A⋅⋅⋅O1W | 0.85(3) | 2.23(3) | 2.974(3) | 147(3) |
| O2–H2B⋅⋅⋅S2 | 0.89(3) | 2.41(3) | 3.2743(15) | 165(3) |
| O3–H3A⋅⋅⋅S2 | 0.82(3) | 2.58(3) | 3.2954(17) | 147(3) |
| O3–H3B⋅⋅⋅O1W | 0.77(3) | 2.22(3) | 2.865(2) | 142(2) |
The four S1⋯H–O hydrogen bonds are responsible for the lengthening of the C10–S1 bonds. It has been previously reported [20] that in the case of disodium 1,4–piperazinecarbodithioate hexahydrate, the C–S bond lengths are influenced by the number of hydrogen bonds accepted by the sulfur atoms. Since C10 is involved in three bonds [C10–N1, C10–S1, and C10–S2] with a partial double bond character, the bond angles around C10 are very close to 120°.
In contrast, the N atom is surrounded by one partial double bond [N1–C10] and two single bonds [N1–C8 and N1–C9]; thus, the C8–N1–C9 angle [112.21(12)%] is markedly smaller than the other two. This is a common feature of the dithiocarbamates [21,22], as well as dithiocarbamates with organic cations [23,24].
A helical-like grid architecture is formed by a [Na(H2O)3+] unit and another unit of [Na(H2O)3+] moieties, which are linear polymeric chains with a larger number of [Na(H2O)3+] moieties, as depicted in Figure S2. A net-like architecture (Figure S3) is also formed through the dtc organic ligand, water molecules, and [Na(H2O)3+] units. The dithiocarbamate reveals that the bulkiness of the pendant group in the dithiocarbamate part of the compound plays an important role in the construction of the supramolecular architecture through water O in [Na(H2O)3+]/S interactions.
As anticipated, complex 1 features interesting intermolecular dtc–H(benzyl)/dtc–H(benzyl) and dtc–C(benzyl)/dtc–H(benzyl) secondary bonding, leading to the formation of a layer-like architecture (Figure S4). These dtc–H(H8B)/dtc–H(H8B) and dtc–C(C8)/dtc–H(H8B) contacts, with dimensions of 2.3717 (1) Å and 2.892(2) Å, respectively, are comparable to the sum of the van der Waals radii of the respective elements (rvdw (H) = 1.2 Å and rvdw (C) = 1.70). In this structure, there are four O–H/O hydrogen bonding contacts and O–H/S interactions that exclusively serve to stabilize the layers.
Tetrahydroquinoline dithiocarbamate complexes would generally involve the coordination of the dithiocarbamate ligands to the metal ions (zinc, nickel, or iron, see Fig. 6) [[25], [26], [27]]. The dithiocarbamate ligands coordinate in a bidentate manner through the sulfur atoms, forming chelate complexes. The exact coordination geometry, bonding angles, and other structural features can vary based on factors such as the specific metal ion (transition metals), oxidation state (+2), ligand environment, and experimental conditions. Zinc and iron typically engage with dithiocarbamate, resulting in tetrahedral or square planar geometries, aligning with their distinctive (d10, d6) electronic configurations. Interestingly, both zinc and iron exhibit a preference for the tetrahedral geometry. Conversely, nickel, with its d8 electronic configuration, predominantly adopts the square planar geometry, presenting a noteworthy structural variation in dithiocarbamate complexes.
Fig. 6.
Delineating the structural geometry of tetrahydroquinoline dithiocarbamate (thqdtc) with various metals.
3.4. Bond valence sum
Bond valence sum analysis is an important tool to confirm the oxidation states of metals in metal complexes, ionic liquids, or salts. It is also helpful in checking the quality or precision of crystallographic data. In some cases, the R value is low or high depending on the accuracy of crystal data; this includes anisotropic thermal parameters and any disordered molecules. The calculated BVS value should agree with the corresponding oxidation state of the molecules, reflecting the accuracy of the crystal data. If the calculated BVS value does not agree with the oxidation state, it indicates that the oxidation state is wrongly identified, the metal may have been chosen incorrectly, or there may be issues with other interactions or imprecise crystal data [28].
The provided tools calculate the oxidation state of metals in metal complexes using the following equations: Zj = ∑ Sij, Sij = exp[(R0- Rij)/b)], where Zj represents the oxidation state, Sij is the sum of individual bond valences, and b is a constant (0.37). Additionally, the EXPO2014 software [29] is employed to determine bond valence sums for the obtained ionic liquids, yielding details such as atoms, bond distances, and bond valence values, presented in a Table 4.
Table 4.
Bond Valence sum of Na in Na(H2O)3+(thqdtc)– • H2O.
In general, the oxidation state of sodium metal is considered as 1. However, the BVS analysis yielded values of 1.251 and 1.0670 for sodium metal, which do not align well with its formal oxidation state. These results suggest a charge imbalance in sodium metals likely caused by the influence of coordinated and uncoordinated water molecules, as well as the dithiocarbamate anion. Notably, the two BVS values diverge due to the precision of the data.
4. Conclusions
The synthesis of the hydrated sodium salt of 1,2,3,4–tetrahydroquinolinedithiocarbamate was achieved through a reaction involving 1,2,3,4–tetrahydroquinoline and carbon disulfide with sodium hydroxide. Comprehensive characterization was conducted utilizing IR, NMR, and single crystal X–ray structural analysis. Significant findings from this study indicate that no coordination bond forms between the dithiocarbamate ligand and sodium metal. This is attributed to the pronounced repulsion between sulfur and nitrogen in dithiocarbamate. The structural analysis reveals the composition comprising one hydrated sodium anion, one dithiocarbamate cation, and an uncoordinated water molecule. Interestingly, the [Na(H2O)3+] cation was found to construct a supramolecular architecture, influenced by the bulkiness of the tetrahydrouinoline group within the dithiocarbamate segment of the compound. This structural aspect plays a pivotal role in shaping the supramolecular architecture.
Furthermore, bond valence sum analysis provided additional support for the formation of this distinctive supramolecular architecture, emphasizing the imbalance in the formal oxidation state of sodium. These results underscore the intriguing nature of the synthesized compound and its potential for various applications and further research.
Data availability
Crystallographic data have been deposited (CCDC 2280947) with the Cambridge Crystallographic Centre. A copy of the data can be obtained free of charge, and applications can be directed to CCDC, 12 Union Road, Cambridge CBZ 1FZ UK.
CRediT authorship contribution statement
Srinivasan N: Writing – original draft, Investigation, Conceptualization. S. Thirumaran: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:S. Thirumaran reports was provided by Department of Chemistry, Annamalai University, Annamalainagar 608 002, India. N. Srinivasan reports a relationship with Department of Chemistry, Annamalai University, Annamalainagar 608 002, India. that includes:. N. Srinivasan If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28642.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cvek B., Dvorak Z. Targeting of nuclear factor-kappaB and proteasome by dithiocarbamate complexes with metals. Curr. Pharmaceut. Des. 2007;13:3155–3167. doi: 10.2174/138161207782110390. [DOI] [PubMed] [Google Scholar]
- 2.Li H., Lai C.S., Wu J., Ho P.C., de Vos D., Tiekink E.R.T. Cytotoxicity, Qualitative Structure-Activity Relationship (QSAR), and anti-tumor activity of bismuth dithiocarbamate complexes. J. Inorg. Biochem. 2007;101:809–816. doi: 10.1016/j.jinorgbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Szolar O.H.J. Environmental and pharmaceutical analysis of dithiocarbamates. Anal. Chim. Acta. 2007;582:191–200. doi: 10.1016/j.aca.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Grainger R.S., Innocenti P. New applications of dithiocarbamates in organic synthesis. Heteroat. Chem. 2007;18:568–571. doi: 10.1002/hc.20336. [DOI] [Google Scholar]
- 5.Fan D., Afzaal M., Malik M.A., Nguyen C.Q., O'Brien P., Thomas P.J. Using coordination chemistry to develop new routes to semiconductor and other materials. Coord. Chem. Rev. 2007;251:1878–1888. doi: 10.1016/j.ccr.2007.03.021. [DOI] [Google Scholar]
- 6.Vickers M.S., Cookson J., Beer P.D., Bishop P.T., Thiebaut B. Dithiocarbamate ligand stabilised gold nanoparticles. J. Mater. Chem. 2006;16:209–215. doi: 10.1039/B509173J. [DOI] [Google Scholar]
- 7.Koh Y.W., Lai C.S., Du A.Y., Tiekink E.R.T., Loh K.P. Growth of bismuth sulfide nanowire using bismuth trisxanthate single source precursors. Chem. Mater. 2003;15:4544–4554. doi: 10.1021/cm021813k. [DOI] [Google Scholar]
- 8.Wang P., Shi R., Su Y., Tang L., Huang X., Zhao J. Hydrated sodium ion clusters [Na+(H2O)n (n = 1–6)]: an ab initio study on structures and non–covalent interaction. Front. Chem. 2019;7:624–634. doi: 10.3389/fchem.2019.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alan Howie R., de Lima Geraldo M., Menezes Daniele C., Wardell James L., Wardell Solange M.S. V., Youngce David J., Tiekink Edward R.T. He influence of cation upon the supramolecular aggregation patterns of dithiocarbamate anions functionalised with hydrogen bonding capacity—the prevalence of charge-assisted O–H⋯S interactions. CrystEngComm. 2008;10:1626–1637. doi: 10.1039/B809730E. [DOI] [Google Scholar]
- 10.Vrable V., Gergely S., Lokaj J., Kello E., Garaj J. Structure of disodium ethylenebisdithiocarbamate hexahydrate. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1987;43:2293–2295. doi: 10.1107/S0108270187088012. [DOI] [Google Scholar]
- 11.Reyes–Martınez Reyna, Hopfl Herbert, Godoy–Alcantar Carolina, Medrano Felipe, Tlahuext Hugo. Comparative analysis of M–O, M–S and cation–p(arene) interactions in the alkali metal (Na+,K+,Rb+,Cs+) bis–dithiocarbamate salts of N,N’–dibenzyl–1,2–ethylene-diamine. CrystEngComm. 2009;11:2417–2424. doi: 10.1039/B907160A. [DOI] [Google Scholar]
- 12.Altomare A., Burla M.C., Camalli M., Cascavano G., Giacovazzo G., Guagliardi A., Polidori G. A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1994;27:435. doi: 10.1107/S002188989400021X. [DOI] [Google Scholar]
- 13.Sheldrick G.M. University of Gottingen; Gottingen, Germany: 1997. SHELXL–97, Program for Crystal Structure Solution and Refinement. [Google Scholar]
- 14.Garg B.S., Garg R.K., Reddy M.J. Synthesis and spectral characterization of zinc(II), cadmium(II) and mercury(II) with tetrahydroquinoline and isoquinoline dithiocarbonates. Indian J. Chem. A. 1993;32:697–700. http://nopr.niscair.res.in/handle/123456789/43948 [Google Scholar]
- 15.Ronconi L., Giovagnini L., Marzano C., Effio F.B., Graziani R., Pilloni G., Fregona D. Gold dithiocarbamate Derivatives as potential Antineoplastic agents: design, spectroscopic properties, and in vitro antitumor activity. Inorg. Chem. 2005;44:1867–1881. doi: 10.1021/ic048260v. [DOI] [PubMed] [Google Scholar]
- 16.Baggio R., Frigerio A., Halac E.B., Vega D., Perec M. Anionic halide and isothiocyanate adducts of zinc and cadmium dithiocarbamates. J. Chem. Soc. Dalton Trans. 1992:549–554. doi: 10.1039/DT9920000549. [DOI] [Google Scholar]
- 17.Aravamudan G., Brown D.H., Venkappayya D. Some metal complexes of morpholine-4-carbodithioate. J. Chem. Soc. A. 1971:2744. doi: 10.1039/J19710002744. [DOI] [Google Scholar]
- 18.Bonati F., Ugo R. Tin(IV) dithiocarbamates and related compounds. J. Organomet. Chem. 1967;10:257–268. doi: 10.1016/S0022-328X(00)93085-7. [DOI] [Google Scholar]
- 19.Srinivasan N., Thirumaran S., Ciattini S. Effect of position of methyl substituent in piperidinedithiocarbamate on the ZnS4N chromophore: synthesis, spectral, valence-bond parameters and single crystal X-ray structural studies on bis(2-methylpiperidinecarbodithioato-S,S’)-(pyridine)zinc(II) and bis(4-methylpiperidinecarbodithioato-S,S‧)(pyridine)zinc(II) J. Mol. Struct. 2009;936:234–238. doi: 10.1016/j.molstruc.2009.08.001. [DOI] [Google Scholar]
- 20.Legros J.P., Troy D., Galy J. Structure of disodium 1,4-piperazinedicarbodithioate hexahydrate, 2Na+.C6H8N2S42-.6H2O. Acta Crystallogr. C. 1984;40:801–804. doi: 10.1107/S0108270184005783. [DOI] [Google Scholar]
- 21.Vrabel V., Gergely S., Lokaj J., Kello E., Garg J. Structure of disodium ethylenebisdithiocarbamate hexahydrate. Acta Crystallogr. C. 1987;43:2293–2295. doi: 10.1107/S0108270187088012. [DOI] [Google Scholar]
- 22.Ymen I., Acta Crystallogr C. Structure of sodium diisopropyldithiocarbamate pentahydrate. Na[C7H14NS2].5H2O. 1983;39:874–877. doi: 10.1107/S010827018300668X. [DOI] [Google Scholar]
- 23.Reyes–Martinez R., Hopfl H., Godoy–Alcantar C., Medrano F., Tlahuext H. Comparative analysis of M–O, M–S and cation–π(arene) interactions in the alkali metal (Na+, K+, Rb+, Cs+) bis-dithiocarbamate salts of N,N′-dibenzyl-1,2-ethylenediamine. CrystEngComm. 2009;11:2417–2424. doi: 10.1039/B907160A. [DOI] [Google Scholar]
- 24.Alan Howie R., de Lima G.M., Menezes D.C., Wardell J.L., Wardell S.M.S.V., Young D.J., Tiekink E.R.T. The influence of cation upon the supramolecular aggregation patterns of dithiocarbamate anions functionalised with hydrogen bonding capacity—the prevalence of charge-assisted O–H⋯S interactions. CrystEngComm. 2008;10:1626–1637. doi: 10.1039/B809730E. [DOI] [Google Scholar]
- 25.Srinivasan Narayanaswamy, Thirumaran Subbiah. Synthesis of ZnS nanoparticles from pyridine adducts of zinc(II) dithiocarbamates. Compt. Rendus Chem. 2014;17:964–970. doi: 10.1016/j.crci.2013.10.009. [DOI] [Google Scholar]
- 26.Valarmathi P., Srinivasan N., Thirumaran S. Synthesis and spectral studies of Ni(II) heterocyclic dithiocarbamates with S2PN chromophore: X-ray crystal structures of bis(1,2,3,4-tetrahydroquinolinecarbodithioato- S,S′)nickel(II) and 1,2,3,4-tetrahydroquinolinecarbodithioato-S,S′)(thiocyanato-N)(triphenylphosphine)nickel(II) J. Sulfur Chem. 2011;32:583. doi: 10.1080/17415993.2011.628991. [DOI] [Google Scholar]
- 27.Saeed S., Hussain R., Butcher Ray J. Growth of semiconducting iron sulfide thin films by chemical vapor deposition from air-stable single-source metal organic precursor for photovoltaic application. J. Coord. Chem. 2014;67:1693–1701. doi: 10.1080/00958972.2014.918265. [DOI] [Google Scholar]
- 28.Wood R.M., Palenik G.J. Bond valence sums in coordination chemistry. Sodium−Oxygen complexes. Inorg. Chem. 1999;38:3926–3930. doi: 10.1021/ic9903331. [DOI] [PubMed] [Google Scholar]
- 29.Altomare A., Cuocci C., Giacovazzo C., Moliterni A., Rizzi R., Corriero N., Falcicchio A. EXPO2013: a kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013;46:1231–1235. doi: 10.1107/S0021889813013113. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystallographic data have been deposited (CCDC 2280947) with the Cambridge Crystallographic Centre. A copy of the data can be obtained free of charge, and applications can be directed to CCDC, 12 Union Road, Cambridge CBZ 1FZ UK.