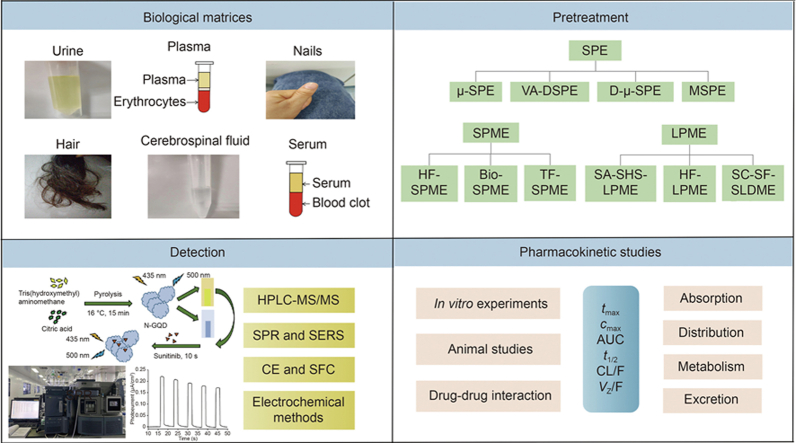
Abstract
Tyrosine kinase inhibitors (TKIs) have emerged as the first-line small molecule drugs in many cancer therapies, exerting their effects by impeding aberrant cell growth and proliferation through the modulation of tyrosine kinase-mediated signaling pathways. However, there exists a substantial inter-individual variability in the concentrations of certain TKIs and their metabolites, which may render patients with compromised immune function susceptible to diverse infections despite receiving theoretically efficacious anticancer treatments, alongside other potential side effects or adverse reactions. Therefore, an urgent need exists for an up-to-date review concerning the biological matrices relevant to bioanalysis and the sampling methods, clinical pharmacokinetics, and therapeutic drug monitoring of different TKIs. This paper provides a comprehensive overview of the advancements in pretreatment methods, such as protein precipitation (PPT), liquid-liquid extraction (LLE), solid-phase extraction (SPE), micro-SPE (μ-SPE), magnetic SPE (MSPE), and vortex-assisted dispersive SPE (VA-DSPE) achieved since 2017. It also highlights the latest analysis techniques such as newly developed high performance liquid chromatography (HPLC) and high-resolution mass spectrometry (HRMS) methods, capillary electrophoresis (CE), gas chromatography (GC), supercritical fluid chromatography (SFC) procedures, surface plasmon resonance (SPR) assays as well as novel nanoprobes-based biosensing techniques. In addition, a comparison is made between the advantages and disadvantages of different approaches while presenting critical challenges and prospects in pharmacokinetic studies and therapeutic drug monitoring.
Keywords: TKIs, Microextraction technique, HRMS methods, Pharmacokinetic studies, Therapeutic drug monitoring
Graphical abstract
Highlights
-
•
Progress and breakthough in microextraction and HRMS techniques for the determination of TKIs are discussed.
-
•
Practicality and convenience of biological matrices relevant in bioanalysis and the sampling methods for TKIs are concerned.
-
•
Pros and cons of different pretreatment and determination methods are comprehensively compared.
-
•
Prospects and challenges of therapeutic drug monitoring of TKIs in clinical application are outlined.
1. Introduction
The global burden of cancer as a significant public health issue is widely acknowledged; however, the lack of selectivity in most chemotherapeutics poses challenges due to their detrimental effects on both tumor cells and normal ones. This has necessitated the development of a new approach to targeted cancer therapy that focuses on leveraging biological mechanisms or pathways. Tyrosine kinase inhibitors (TKIs) are the first-line small molecule drugs in many cancer treatments [1], which effectively suppress the aberrant cell growth and proliferation caused by hyperactive tyrosine kinases through the inhibition of cell signal transduction pathways. Since its approval by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2001, imatinib has paved the way for an expanding repertoire of TKIs utilized in targeted therapy across a range of cancers covering hematological malignancies, solid tumors, hepatocellular carcinoma, melanoma, and breast cancer [2]. Undoubtedly, TKIs have dramatically improved long-term survival outcomes and the quality of life for patients. Notable examples among the widely employed TKIs include dasatinib, ibrutinib, idelalisib, ponatinib, trametinib, sunitinib, cobimetinib, dabrafenib, erlotinib, lapatinib, nilotinib, bosutinib, sorafenib, vemurafenib and so on [3]. The salient features are summarized in Table 1.
Table 1.
Characteristic features of representative tyrosine kinase inhibitors (TKIs).
| Compound | Indications | Usual dosage | Mechanism of action | cTarget (ng/mL) |
|---|---|---|---|---|
| Bosutinib | CML | 500 mg/day | BCR-ABL and Src | 150−600 (cmin−cmax) |
| Dasatinib | CML (Ph+) | 100 mg/day | BCR-ABL and Src | 1.5−50 (cmin−cmax) |
| Imatinib | CML (Ph+), GIST, and myelodysplasic syndrome | 400 mg/day 600 mg/day |
BCR-ABL, c-KIT, and PDGFR | 1000 (cmin) |
| ALL (Ph+) | ALL (Ph+) | 800 (cmin) | ||
| Nilotinib | CML (Ph+) | 400 mg/day | BCR-ABL | 800 (cmin) |
| Ponatinib | CML (Ph+) and T315I | 45 mg/day | BCR-ABL | 22 (cmin) |
| Ruxolitinib | Myelofibrosis |
15−20 mg b.i.d. |
JAKIs | 1160 (cmax) |
| Polycythemia vera | 10 mg b.i.d. | |||
| Crizotinib | NSCLC ALK positive | 500 mg/day | ALK and c-MET | 250 (cmin) |
| Afatinib | NSCLC EGFR positive | 40 mg/day | EGFR | 20−250 (cmin−cmax) |
| Erlotinib | NSCLC EGFR positive |
150 mg/day |
EGFR | 500 (cmin) |
| Pancreatic carcinoma | 100 mg/day | 700−1700 (cmin) | ||
| Gefitinib | NSCLC EGFR positive | 250 mg/day | EGFR | 200 (cmin) |
| Lapatinib | Breast cancer HER2 positive | 1250 mg/day | EGFR and HER2 | 300−600 (cmin) |
| Vandetanib | Medullar thyroid cancer RET positive | 300 mg/day | VEGFR, EGFR, and RET | 1000−1497 (cmin) |
| Axitinib | mRCC | 5 mg b.i.d. | VEGFR | 10−56.4 (cmin−cmax) |
| Sorafenib | HCC and mRCC | 400 mg b.i.d. | VEGFR, PDGFR, RAF, MEK, and ERK | 3750−5800 (cmin −cmax) |
| Sunitinib | mRCC and GIST | 50 mg/day (4/2) | FLT3, PDGFR, VEGFR, and c-kit | 37.5−100 (cmin− cmax) |
| Pazopanib | mRCC and Sarcoma | 800 mg/day | VEGFR | 20,000 (cmin) |
| Regorafenib | Metastatic colorectal cancer and GIST | 160 mg/day | VEGFR | 1000−2500 (cmin−cmax) |
| Dabrafenib | Melanoma BRAF positive | 150 mg b.i.d. | BRAF and MEK | 42.5 (cmin) |
cTarget: target concentrations; CML: chronic myeloid leukemia; BCR-ABL: breakpoint cluster region-abelson complex; Src: proto-oncogene tyrosine-protein kinase Src; cmax: maximum plasma/serum concentration; Ph+: Philadelphia chromosome positive; GIST: gastrointestinal stromal tumors; ALL: acute lymphoblastic leukemia; c-KIT: mast-stem cell growth factor receptor Kit; PDGFR: platelet-derived growth factor receptor; b.i.d.: bis in die; JAKIs: janus associate kinase inhibitors; NSCLC: non-small-cell lung cancer; ALK: anaplastic lymphoma kinase; c-MET: mesenchymal epithelial transformation factor kinase; EGFR: epidermal growth factor receptor; HER2: human epidermal growth factor receptor 2; RET: transfection rearrangement; VEGFR: vascular endothelial growth factor receptor; mRCC: metastatic renal cell carcinoma; HCC: hepatocellular carcinoma; RAF: rapidly accelerated fibrosarcoma kinases; MEK: mitogen-activated protein kinase; ERK: extracellular signal-regulated kinase; 4/2: 4-week dosing followed by 2-week rest; FLT3: fms-like tyrosine kinase 2; BRAF: RAF murine sarcoma viral oncogene homolog B kinase.
Although therapeutic activity has been proved in clinical trials involving patients with hematological malignancies and advanced solid tumors, certain TKIs (i.e., imatinib and its metabolite) exhibited considerable inter-individual variability in their concentrations, leaving patients with suppressed immune function susceptible to various infections, despite receiving theoretically effective cancer treatments. Additionally, it may lead to other side effects or adverse reactions [4,5]. Firstly, dose-dependent side effects such as anemia, diarrhea, rash, mucosal inflammation, renal impairment, and limb edema significantly impact the quality of life for patients [6,7]. Secondly, low plasma TKI concentrations indicate ineffective prescription drugs due to the insufficient levels resulting from inadequate molecular reactions or complete cytotoxic reactions. Furthermore, some TKIs (i.e., afatinib) may form multitudinous adducts with endogenous proteins, especially hemoglobin. The formation of protein adduct increases the risk of idiosyncratic drug interactions [8]. Unfortunately, there are no effective ways to control these side effects other than reducing the dosage or discontinuing therapy in severe cases. Accordingly, countries and regions have established recommended oral daily dosages (in milligram).
Over the past decade, therapeutic drug monitoring (TDM) of TKIs and their primary active metabolites has emerged as a promising approach to improve treatment efficacy while minimizing toxicity. Considering the crucial role of TKI level monitoring in clinical diagnosis, it is imperative to develop and establish pretreatment and detection technologies that encompass affordability, high sensitivity and efficiency, as well as easy-to-implement online monitoring for evaluating lower TKI values. However, since 2016, only one review concerning the pretreatment and determination methods has been published. In 2016, Suresha et al. [9] provided an overview of analytical methods for imatinib mesylate and demonstrated significant improvements in precision by validating the analytical procedure's suitability for its intended purpose. Despite the development and application of various pretreatment and determination methods to different TKIs, a comprehensive review on this topic is yet to be updated.
Given this, the urgent need for an up-to-date review on the biological matrices relevant to bioanalysis and sampling methods, pretreatment and detection techniques, clinical pharmacokinetics, and TDM of different TKIs is evident. In this review, we comprehensively provide the progress achieved since 2017. The pretreatment methods used for the purification and enrichment of TKIs includes not only conventional techniques like protein precipitation (PPT), protein precipitation (LLE), and others, but also advanced approaches including various microextraction methods, solid-phase extraction (SPE), micro-SPE (μ-SPE), magnetic SPE (MSPE), vortex-assisted dispersive SPE (VA-DSPE), and among others. In addition, the analytical methods employed for TKIs consist of liquid chromatography (LC) coupled with diverse detectors, electrochemical methods, capillary electrophoresis (CE), gas chromatography (GC), supercritical fluid chromatography (SFC), surface plasmon resonance (SPR) assays, and novel nanoprobes-based biosensing techniques. This review illustrates the development of different techniques applied in TKIs and facilitates a comparison of their advantages and disadvantages to identify critical points and prospects.
2. Biological matrices pertinent to bioanalysis and the sampling methods for TKIs
The bioanalysis of TKIs in biological matrices enables a deeper understanding of the absorption, distribution, metabolism, excretion (ADME), and other behaviors of pharmaceuticals in living organisms due to the narrow treatment windows and significant individual variation observed with certain TKI concentrations [10]. The instrument calibration and sample pre-concentration were conducted across various biological matrices, including urine, plasma, serum, whole blood, and alternative natural materials like nails, hair, and cerebrospinal fluid samples. Plasma is the preferred biological fluid for TKI monitoring due to its ability to better reflect changes in drug concentration in vivo and indicate the amount of drug consumed. Anticoagulants should be added during plasma collection followed by centrifugation and standing to obtain the supernatant for subsequent analysis [11,12]. Serum is a component of blood that lacks clotting factors. Literature search results show that serum samples are more commonly used than plasma samples in the analysis of TKIs [13,14]. Urine is another widely used biological matrix for studying drug dose recovery, drug urine clearance rate, and bioavailability [15,16]. TKIs and their relevant metabolites tend to be exhibit high concentrations in urine, making it a simple and non-invasive collection method. However, urine sample analysis results are influenced by physiological conditions with a limited detection window of two to three days. Moreover, alternative biological materials possess unique advantages over urine or standard blood tests despite being less frequently employed in actual testing [17]. The collection of TKIs in keratinized matrices of hair and nails is a non-invasive and challenging process to manipulate. TKIs are incorporated within the matrix with long-term stability, considering that nails grow slowly at a rate of approximately 38.1 mm per year [18]. Consequently, selecting a suitable biological matrix and determining a matching sample preparation method are crucial for ensuring reproducible recovery rates and accurate detection results.
The sampling method is another important factor that limits sample recovery and the reliability of experimental conclusions. Dried matrix spot (DMS) micro-sampling technology, which involves drying trace amounts of biological fluids (i.e., blood, urine, and saliva) on a paper substrate, has been successfully employed in monitoring toxic drugs and studying pharmacokinetics [[19], [20], [21]]. Compared to traditional sampling methods (i.e., venous blood collection and routine urine tests), DMS micro-sampling technology presents distinct advantages: minimal sample volume, convenient storage and transportation, and enhanced drug stability in the DMS matrix. This enables long-term preservation of samples at room temperature. For example, Mukai et al. [22] developed and validated a straightforward method for quantifying the concentrations of six TKIs in dried blood spots (DBS). Each DBS sample contained only 40 μL of blood, which was sufficient for analysis, and all TKIs remained stable at room temperature for 8−12 weeks. Both conversion equations showed good agreement between estimated plasma concentrations and measured plasma concentrations of bosutinib, imatinib and ponatinib. However, the direct quantification of TKIs at specific tissue sites cannot be achieved using DMS technology. Hence, microdialysis has been developed as a minimally invasive alternative to collect drug fractions at anatomical sites [23,24]. Besides, circulating tumor cell levels in patients have also been used to evaluate or predict worse tumor response prognosis in both TKI treatment and chemotherapy [25].
3. Pretreatment techniques
The field of modern analytical chemistry is confronted with an unprecedented complexity in terms of sample properties, encompassing the presence of multiphase objects, interference from intricate components, stringent concentration requirements, and variations in stability. These factors pose a multitude of challenges to the analysis process. In recent years, diverse innovative pretreatment methods have been developed alongside traditional approaches, including different microextraction techniques, methodologies based on novel materials, MSPE, and advancements in automation technology.
3.1. Traditional used pretreatment methods for TKIs
PPT method is a commonly employed approach for the preprocessing of biological samples in TKIs analysis. In the case of protein-rich materials, such as plasma and urine, it is necessary to employ different techniques for protein precipitation prior to analysis [26,27]. Typically, sample pre-purification involves a one-step simple protein precipitation using a protein precipitant followed by sample collection through centrifugation (∼15 min) [28], with a small volume of sample [29]. Commonly used protein precipitants include methanol, acetonitrile, dichloromethane, among others. Acetonitrile exhibits superior performance compared to methanol and other organic solvents like dichloromethane as it promotes enhanced protein precipitation and maximizes the recovery of strongly protein-bound imatinib [[30], [31], [32]].
Compared to PPT, LLE is a more productive sample preparation method for producing a relatively clean sample, thereby enhancing sensitivity and preventing the introduction of highly polar materials into the column and mass spectrometry (MS) systems [33]. Various solvents, including ethyl acetate [34,35], tert-butyl methyl ether [36], as well as a mixture of ethyl acetate and tert-butyl methyl ether (1:1, V/V) [37], have been reported for LLE of TKIs. Traditional LLE is characterized by its rapidity, ease of operation, and high accuracy [38]. Ni et al. [39] employed an extraction solvent consisting of ethyl acetate and tert-butyl methyl ether (1:1, V/V) for the extraction of six TKIs in human plasma. The method showed high accuracy levels (80%–120%) with relative standard deviation (RSD) less than 11.9%, and excellent extraction recovery (88.3%–103.6%, RSD < 12.7%). By utilizing effective LLE techniques, lower limit of quantification (LOQ) was achieved for apatinib (0.02 ng/mL), crizotinib (0.1 ng/mL), lapatinib (2.0 ng/mL), erlotinib (0.05 ng/mL), gefitinib (0.05 ng/mL), and icotinib (0.05 ng/mL).
In order to further improve the efficiency and recovery of analyte extraction, salt-out assisted liquid-liquid extraction (SALLE) has been developed for the pretreatment of TKIs in plasma samples used in clinical practices. This method integrates desalting, deproteinization, and target substance extraction into a single step. Commonly used salts include sodium chloride [40], ammonium acetate [41], among others. SALLE can be easily combined with capillary electrophoresis (CE) for online concentration and separation in a straightforward manner. The high organic solvent content (i.e., acetonitrile) in the upper phase allows for inline CE stacking of analytes within the capillary, even if the sample size exceeds 80% of the capillary volume. Consequently, it contributes to excellent peak efficiencies, good resolution, and high sensitivity [42]. Ahmed et al. [43] developed a SALLE-CE method for the analysis of four TKIs in human plasma. In their study, sodium chloride was added to induce phase separation between plasma-acetonitrile mixture resulting in efficient extraction of (60%–100%) from the upper phase while achieving excellent separation and significantly enhancing sensitivity (61–265 folds).
3.2. Evolution of liquid-phase microextraction (LPME) methods for TKIs
The conventional LLE methodologies typically necessitate a substantial quantity of organic solvents and are prone to the formation of emulsions. Consequently, LPME has emerged as a promising alternative technology that offers enhanced speed, cost-effectiveness, and environmental friendliness by using negligible extracting solvents without the need for laborious pretreatment steps. Unlike LLE, which is an equilibrium-based technique, the recovery of LPME relies on factors such as partition coefficients, sample volume, supported liquid membrane volume, and acceptor phase volume. Various configurations of LPME include salting-out-assisted switchable hydrophilicity solvent-based LPME (SA-SHS-LPME), hollow fiber LPME (HF-LPME), and stirring-controlled solidified floating solid-liquid drop microextraction (SC-SF-SLDME).
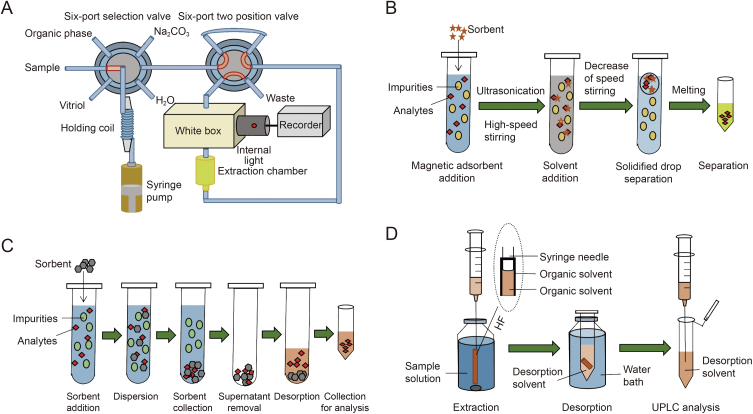
Switchable hydrophilicity solvents (SHSs), a kind of novel environmentally friendly solvent, have garnered significant attention for their applications in the extraction and purification of TKIs from various samples. During the SA-SHS-LPME process, the nonionic form of SHSs exhibits limited miscibility with water in the absence of CO2 but becomes completely miscible with water in the presence of CO2, enabling a switch between polar and nonpolar states (Fig. 1A). Hence, SA-SHS-LPME is an effective, rapid, robust and cost-effective technique that facilitates easy extraction of TKIs from polar samples without requiring dispersive solvents. Kakavandi et al. [15] developed SA-SHS-LPME for the separation of trace amounts of imatinib and its major metabolite in biological and environmental samples. In this study, triethylamine was selected as a hydrophobic compound, while protonated triethylamine carbonate served as a hydrophilic compound whose properties could be switched by the addition or elimination of CO2. After investigating several crucial recovery factors, high preconcentration factor (250) and recovery (97%–102%) were achieved with inter-day and intra-day precisions (RSD, n = 5) lower than 5%.
Fig. 1.
Schematic diagram of different microextraction methods of tyrosine kinase inhibitors (TKIs): (A) salting-out-assisted switchable hydrophilicity solvent-based liquid-phase microextraction (SA-SHS-LPME), (B) stirring-controlled solidified floating solid-liquid drop microextraction (SC-SF-SLDME), (C) dispersive microsolid-phase extraction (D-μ-SPE) [54], and (D) hollow-fiber solid-phase microextraction (HF-SPME). UPLC: ultra-performance liquid chromatography tandem mass spectrometry. Reprinted from Ref. [54] with permission.
In order to improve droplet stability, HF-LPME has been developed, which uses a hydrophobic porous hollow fiber to support and protect the liquid membranes and form an extraction interface. This technique can yield high enrichment multiples and prevent contamination from large molecules or particles. Moreover, it can be easily modified utilizing nanostructures for a wider range of applications. Moghaddam et al. [44] used nanostructures such as graphite, graphene oxide (GO), or nitrogen-doped GO to modify HF-LPME for the extraction of imatinib and sunitinib from biological fluids. In this study, a low-voltage electro-membrane extraction device (6.0 V) was fabricated. After evaluating and optimizing various essential factors related to normalized recovery, 1-octanol with a concentration of 0.01 % (m/V) GO was selected as the supported liquid membrane, and the pH of the acceptor and the donor phases were set at 2.8 and 7.9 respectively, with an extraction time of 17.0 min.
In recent years, the development of solidified floating organic drop microextraction has effectively addressed the challenges associated with collecting small microdroplets by solidifying the extraction solvent. This technique offers advantages such as low consumption of organic solvents, high enrichment factor, as well as compatibility with reversed-phase chromatography [45]. However, the overall duration of extraction procedure is prolonged due to the mandatory centrifugation step required for collecting the extraction phase in these methods, which impedes automation [46]. To overcome these remaining challenges, an advanced SC-SF-SLDME technique is proposed as a serviceable sample treatment approach. The schematic diagram illustrating the SC-SF-SLDME procedure is presented in Fig. 1B. The SC-SF-SLDME procedure, as applied by Ghazaghi et al. [47], offers a facile and efficient extraction and preconcentration method for four TKIs in human serum and cerebrospinal fluid, eliminating the need for time-consuming centrifugation steps and disperser solvents. The synthesized magnetic carbon nanotube (CNT)-nickel hybrid and 1-undecanol were utilized as the nano-adsorbent and organic solvent, respectively. The former acted as both an extractor and a coalescence facilitator between organic droplets, enabling easy recollection through hydrophobic force. The proposed method demonstrated a short extraction time of 5 min, while the performance was evaluated by determining the mentioned TKIs in human serum and cerebrospinal fluid samples with good recoveries ranging from 93% to 98% (Table 2) [15,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43],47].
Table 2.
Liquid-phase sample preparation techniques for tyrosine kinase inhibitors (TKIs) in different biological material.
| Biological material | Analytes | Pretreatment method | Extraction process | Sample size | Recovery | Monitoring metabolites | Samples from patients | Potential for clinical application | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Fresh human urine and plasma | 2 TKIs | SA-SHS-LPME | Transfer 10 mL of sample solution containing 50 μg/L of imatinib and N-desmethyl imatinib to a 15 mL centrifuge tube, add 600 μL of the P-TEA-C and 1.5 g of NaCl to each tube and centrifuge (2 min), add 2.0 ml 10 M NaOH solution and centrifuge (4,000 r/min, 5 min), and collect the TEA phase which floated on the surface and dry it (evaporating under nitrogen) | 4 mL | 97.0%–102% | No | No | No | [15] |
| Human plasma | 2 TKIs | LLE | Add 0.5 mL of ethyl acetate to the samples, vortex for 1 min, stand for 1 min (room temperature) and centrifuge (12,000 g, 10 min, 4 °C), and dry upper organic phase (vacuum volatilization, room temperature) | 3 mL | 89.6%–92.9% for imatinib and 87.7%–91.9% for N-demethyl-imatinib | Yes | Yes | Yes | [33] |
| Human plasma | 3 TKIs | LLE | Add 1.2 mL of ethyl acetate to each tube, vortex (5 min) and centrifuge (15,000 r/min, 10 min, 4 °C), and dry upper organic phase (evaporating using a Thermo Electron RVT4104 refrigerated vapor trap, 35 °C) | 300 μL | 53.2%–67.9% for imatinib, 63.7%–71.8% for dasaitinb, and 82.6%–103.4% for nilotinib | No | Yes | Yes | [34] |
| Human plasma | 2 TKIs | LLE | Add 100 μL of 10% ammonia solution to each tube and vortex (30 s), add 5 mL of ethyl acetate, vortex (3 min) and centrifuge (1,690 g, 10 min), and dry upper organic phase (evaporating under nitrogen, 37 °C) | 500 μL | 78.93%–96.54% | Yes | Yes | Yes | [35] |
| Artificial human serum | 2 TKIs | LLE | Add 20 μL of 1 M NaOH solution to adjust the pH, add 0.8 mL of tert-butyl methyl ether into aliquots of serum, vortex (2 min) and centrifuge (10,000 g, 5 min), collect upper organic phase and extract the aqueous part once more with 4 mL of tert-butyl methyl ether, and dry the combined upper organic phase under vacuum in an Eppendorf Vacufuge Concentrator | 0.5 mL | ≥83% | Yes | No | No | [36] |
| Human plasma | 7 TKIs | LLE | Add 50 μL of acetonitrile to the samples and vortex for 20 s, add 1 mL of ethyl acetate and tert-butyl methyl ether (1:1, V/V) to each tube, vortex (30 s) and centrifuge (10,000 g, 10 min), and dry upper organic phase (evaporating, 40 °C) | n.d. | ≥70% | No | No | Yes | [37] |
| Human plasma | 6 TKIs | LLE | Vortex samples (a few seconds), add 700 μL of ethyl acetate to each tube, vortex (30 s) and centrifuge (10,000 r/min, 5 min), and dry upper organic phase (evaporating under nitrogen flow) | 150 μL | n.d. | Yes | Yes | Yes | [38] |
| Human plasma | 6 TKIs | LLE | Add 500 μL ethyl acetate:tert-butyl methyl ether (1:1, V/V) to each tube, vortex (1 min) and centrifuge (10,000 g, 5 min, 4 °C), and dry upper organic phase (evaporating under a stream of nitrogen, 40 °C) | 20 μL | 88.3%–103.6% | No | Yes | Yes | [39] |
| Rat plasma | 1 TKIs | SALLE | Dissolve 350 mg of NaCl in 1 mL of sunitinib-spiked plasma followed by the addition of 500 μL of acetonitrile, vortex (1 min) for phase separation, and suck out the upper layer (200 μL) | 1 mL | 87.4%–123.32% | No | No | No | [40] |
| Human plasma | 12 TKIs | SALLE | Add 200 μL of acetonitrile and then add 100 μL of 5 M ammonium acetate solution. Next, vortex (3 min) and centrifuge (12,000 g, 10 min, 4 °C) | 100 μL | 83.19%–112.04% | No | Yes | Yes | [41] |
| Human plasma | 3 TKIs | On-line SALLE | Mix relevant volumes of plasma solution and three TKIs stock solutions in Eppendorf tube to obtain a final volume of 80 μL (1 h, room temperature) and place the Eppendorf tube in the CE plastic vials | n.d. | n.d. | No | No | Yes | [42] |
| Human plasma | 4 TKIs | SALLE | Add 3 mL of acetonitrile to 2 mL of standard solution and vortex (1 min), add 0.1 g of 2% NaCl (m/V) and vortex (1 min), and stand (3 min) | n.d. | n.d. | No | Yes | Yes | [43] |
| Human serum and cerebrospinal fluid | 4 TKIs | SC-SF-SLDME | Adjust the pH of samples to 11.0, add appropriate amount of NaCl with final concentration 10% (m/V), add 1.5 mg of MNi-CNT hybrid and immerse in an ultrasonic water bath for 30 s, insert 30 mL of 1-undecanol and stir with glass-coated magnet (600 r/min for 5 min, 300 r/min for 5 min), ice bath, collect 1-undecanol, and elute the MNi-CNT using acetonitrile | 2 mL | 84.0%–98.5% | No | No | No | [47] |
n.d.: not discovered. SA-SHS-LPME: salting-out-assisted switchable hydrophilicity solvent-based liquid-phase microextraction; P-TEA-C: protonated triethylamine carbonate; LLE: liquid-liquid extraction; SALLE: salt-out assisted LLE; CE: capillary electrophoresis; SC-SF-SLDME: stirring-controlled solidified floating solid-liquid drop microextraction; MNi-CNT: magnetic carbon nanotube-nickel hybrid.
3.3. SPE
3.3.1. Traditional used materials for SPE of TKIs
Recently, SPE has emerged as a routine sample preparation technique due to its enhanced purification ability, reduced matrix effect, and decreased consumption of organic solvents compared to protein precipitation and LLE. This method relies on the selective distribution of TKIs between the solid packing material and liquid mobile phase, with all extraction procedures described in accordance with Nernst's distribution law. The SPE offers a wide range of applications in the analysis of 17 TKIs and 2 metabolites in human plasma [48]. Generally, the SPE process consists of four steps: column preparation, sample loading, column post-wash, and sample desorption. During the process, an aqueous sample is passed through an immobilized phase and subsequently extracted using suitable organic solvents. The commonly used SPE columns for TKIs encompass reversed-phase adsorbents, which are utilized for the extraction of nonpolar or weakly polar compounds from aqueous solutions (i.e., C18) [49]; exchange adsorbents, which are employed for the extraction of ionic compounds from aqueous solutions (i.e., SCX) [50]; a column designed specifically for extracting macromolecular proteins and peptides from aqueous solution (i.e., C4) [51]; as well as plate suitable for high throughput processing of microsample sizes (i.e., 96-well micro-elution plate) [48]. The application of various SPE columns in the extraction and purification process of TKIs in recent years is summarized. For example, Maher et al. [52] conducted sample preparation using C18 cartridges prior to a bioanalytical ultra-performance liquid chromatography tandem MS (UPLC-MS/MS) method for comparative pharmacokinetic profiles of selected irreversible TKIs in rat plasma, achieving excellent extraction recovery of over 92.42% (neratinib) and 89.73% (pelitinib). The matrix effect observed for neratinib and pelitinib at concentrations of 0.5, 5, 50, 150 ng/mL was found to be less than 5.79% and 3.44%, respectively, indicating that the matrix effect of plasma samples on the analysis results was negligible with the applied methodology.
3.3.2. From traditional to novel materials in SPE
In recent years, molecular imprinted polymers (MIPs) have been extensively used in SPE for the highly selective adsorption of TKIs and their metabolites, yielding remarkable outcomes. The structurally tailored recognition sites within synthetic polymers exhibit exceptional specificity towards the target molecule (template) [53]. By utilizing a thermo-sensitive monomer, MIPs-SPE demonstrates superior selectivity and enables precise control over adsorption and desorption processes through temperature modulation. Kazemi et al. [16] synthesized a new thermos-sensitive MIPs as a solid phase extractant for the selective separation of imatinib mesylate in urine and plasma samples. The polymerization of N-vinylcaprolactam was used as a thermo-sensitive monomer, while 1-vinyl-2-pyrrolidone and methyl methacrylate were used as functional monomers. The newly developed MIP-based SPE exhibited exceptional uniformity in terms of size, shape, and efficiency, which can be attributed to the successful molecular imprinting of functional monomers with suitable templates. The thermos-sensitive MIPs-SPE indicated superior selectivity towards imatinib mesylate compared to other compounds and could be effectively applied for determination of trace levels of imatinib mesylate with a recovery exceeding 90%.
3.3.3. μ-SPE
In contrast to the traditional micrometer-sized sorbents used in SPE, nano-materials employed in μ-SPE offer a significantly reduced diffusion pathway, thereby contributing to enhanced extraction dynamics and increased capacity. A schematic illustration of the μ-SPE procedure is depicted in Fig. 1C [54]. Following extraction, the solid adsorbent containing the target analyte is separated, and subsequently desorbed using a desorption solvent without involving evaporation or reconstitution steps [[54], [55]]. Moreover, μ-SPE provides analyte enrichment and reduces analytical time, enabling the use of smaller sample quantities. Koller et al. [55] applied a straightforward μ-SPE technology as a sample cleanup procedure using mixed-mode cation exchange sorbent for the extraction of 11 TKIs from human plasma samples. The authors compared various extraction methods of μ-SPE with protein precipitation. Although protein precipitation was an admittedly simple approach for the pretreatment of conventional biological substrates such as plasma, it caused ion suppression in electrospray ionization due to being unable to remove all endogenous compounds that interfere with subsequent analysis. Compared to protein precipitation, μ-SPE achieved the elimination of over 91% of early eluting and 96% of late eluting endogenous phospholipids. The proposed approach enabled simultaneous monitoring of 11 TKIs in plasma while ensuring highly effective removal of endogenous phospholipids, resulting in high extraction recoveries (82%–114%, RSD < 13%), negligible matrix effect (85%–118%, RSD < 15%), and an exceptional overall process efficiency (80%–118%, RSD = 7%).
3.3.4. VA-DSPE
The VA-DSPE method not only retains the advantages of SPE, but also presents shorter extraction time and higher efficiency. The sorbent plays a crucial role in enhancing the extraction efficiency in the VA-DSPE process. Metal-organic frameworks (MOFs), which are novel hybrid inorganic-organic micro-porous crystalline materials self-assembled through coordination bonds between metal ions and organic linkers, exhibit great potential in sorption-related fields [56]. VA-DSPE based on MOFs displays excellent extraction efficiency due to its unique high surface area, large mesoporous pores, and excellent chemical and solvent stability. Qi et al. [57] successfully developed a novel VA-DSPE method for extracting imatinib mesylate from rat plasma using MIL-101(Cr) as the sorbent. The initial mobile phase (0.1% formic acid:methanol (6:4, V/V)) was used as an elution solvent, allowing simple recovery of imatinib mesylate after adsorption. Direct extraction of imatinib mesylate from protein-rich rat plasma was achieved solely relying on the porous structure of MIL-101(Cr), without any additional pretreatment procedures. After systematic optimization of the VA-DSPE conditions, a lower LOQ (1 ng/mL) was achieved with a mean recovery exceeding 81.2%.
3.3.5. MSPE
MSPE, being an emerging SPE technology, has received widespread attention due to its rapid separation process and excellent adsorption efficiency. By incorporating an external magnetic field, it greatly simplifies the sample pretreatment procedure by eliminating the need for filtration and centrifugation while also circumventing issues associated with adsorbent packing in SPE [58,59]. Moreover, most magnetic adsorbents can be readily recycled and reused, resulting in substantial cost savings and environmental benefits [60].
There are various types of magnetic materials, including classical core-shell structures, hybrid and doped magnetic materials, as well as magnetic nanoparticles (MNPs). Among them, Fe3O4 is the most indispensable and widely used material. Existing literature has demonstrated that commercial MNPs can effectively facilitate the enrichment of 15 TKIs in plasma samples [61]. In contrast, modified sorbents or synthetic composite materials exhibit enhanced adsorption capacity and superior extraction selectivity. Recently, GO hybrid materials have aroused extensive interests due to their excellent adsorptive properties, enabling quantitative recoveries with reduced eluent solvent volumes compared to traditional sorbents. Arvand and Masouleh [62] reported a robustness method for extraction of imatinib by Fe3O4/GO nanocomposite in wastewater. After optimizing the conditions, the relative recoveries of imatinib reached 88.4% with the utilization of 5 mL of eluent solvents. Considering that the magnetism and dispersibility of Fe3O4 nanoparticles (NPs) may deteriorate due to air oxidation, it is essential to apply proper coating or grafting techniques (i.e., copolymerization of chitosan-grafted poly(N-vinylcaprolactam)) on their surface in order to maintain stability. Sahebi et al. [63] used chitosan grafted onto Fe3O4@poly(N-vinylcaprolactam) as a dual-responsive sorbent, sensitive to both temperature and pH, for the detection of imatinib mesylate in bio-samples. The NPs were subjected to various characterization techniques and parameters including sorbent dosage, sample pH, extraction and desorption time, as well as elution solvent type and quantity. The LOQ achieved by this approach was 1.0 ng/mL with inter/intraday precision ranging 3.4%–6.7% and 5.1–7.7%, respectively. In addition, ionic liquids, especially those containing multiple active nitrogen atoms, are employed for the modification of Fe3O4 NPs to achieve adjustable solubility and minimize environmental pollution. Sahebi et al. [64] synthesized novel MNPs modified with an ionic liquid (Fe3O4@1,4-diazabicyclo octane) through quaternization reaction for MSPE of imatinib mesylate from human plasma samples. Ionic liquid sorbents possess distinctive physical and chemical properties such as nonvolatility, thermal stability, and chemical stability that facilitate easy recycling without causing environmental contamination. The proposed method was validated under optimized conditions, yielding satisfactory extraction recovery values (93%–102%, RSD < 5.9%), linearity (0.7–2500 ng/mL), and lower LOQ (0.21 ng/mL).
To enhance the selectivity of sample preparation techniques, MIPs have been used as the sorbents in MSPE. Upon removal of the template from MIPs, cavities are formed that are complementary to target molecules, enabling specific selection and recognition. In a study by Pirdadeh-Beiranvand et al. [65], a novel magnetic molecularly imprinted nanofiber (MMIN) was constructed and used as the sorbent for MSPE in the selective extraction of nilotinib. The electrospun precursor nanofibers containing nilotinib were used as templates. The MMIN nanocomposite exhibited good selectivity with minimal interference from other compounds investigated and demonstrated high adsorption capacity, resulting in recoveries in the range of 90.0%–98.0%.
Apart from Fe3O4 NPs, graphitic carbon nitride (g-C3N4) has also been used as an adsorbent due to its exceptional performance, including good biocompatibility, a large specific surface area, and remarkable post-synthesis ability. Considering the heterocyclic and benzene structure of the TKIs, magnetic g-C3N4 (MCN) exhibits great potential for high adsorption capacity through π−π conjugation, hydrogen bond interaction, electrostatic interaction, hydrophobic effect, etc.. Liu et al. [66] developed a novel MSPE method to detect three TKIs in urine. The MCN/boron imidazole framework-20 (BIF-20) sorbent was fabricated by combining MCN with BIF-20, which synergistically leveraged both magnetic response and outstanding adsorption capacity. The MSPE performance remained stable even after three cycles with good adsorption of TKIs at levels of 148.33, 283.25, and 188.17 mg/g, respectively. The analytical method based on MCN/BIF-20 MSPE exhibited high sensitivity with limit of detection (LOD) of 2.2–3.4 ng/mL, wide linear range (12.5–500 ng/mL), good precision (RSDs < 3.9%), as well as good accuracy (90.35%–98.69%).
3.4. Solid phase microextraction (SPME)
3.4.1. Development of new SPME technology
The SPME is a miniaturized equilibrium technique, characterized by an exceptionally small extraction phase volume compared to the sample volume. This feature results in lower operating costs, reduced time consumption, as well as the potential for partial or complete automation, thereby enhancing reproducibility and enabling online hyphenation. Various SPME configurations have been developed, including biocompatible-SPME (Bio-SPME), HF-SPME, and thin-film SPME (TF-SPME).
Coupling the SPME equipment directly to the mass spectrometer instrumentation can effectively compensate for the inherent limitations (i.e., poor sensitivity at trace levels and narrow linear dynamic range) of pure ambient MS when analyzing complex matrices like blood and urine [67]. Nevertheless, it remains a challenge to develop coatings that do not induce toxic reactions in the system [68]. The absence of protein attachment or adsorption makes Bio-SPME ideal for nano-electrospray ionization (ESI) applications [69]. Additionally, ion suppression is reduced due to decreased introduction of compounds such as phospholipids and salts through Bio-SPME, achieved by free-concentration and a washing step after extraction. Gómez-Ríos et al. [70] directly coupled Bio-SPME fibers with MS for rapid quantitation of imatinib in biofluids. Total sample preparation was completed within 2 min, allowing selection from a wide range of sample volumes (10–1500 μL). Additionally, low limits of detection (LODs) at a ng/mL level with good accuracy (≥90%) were obtained across different matrix including phosphate-buffered saline (PBS), urine, and whole blood.
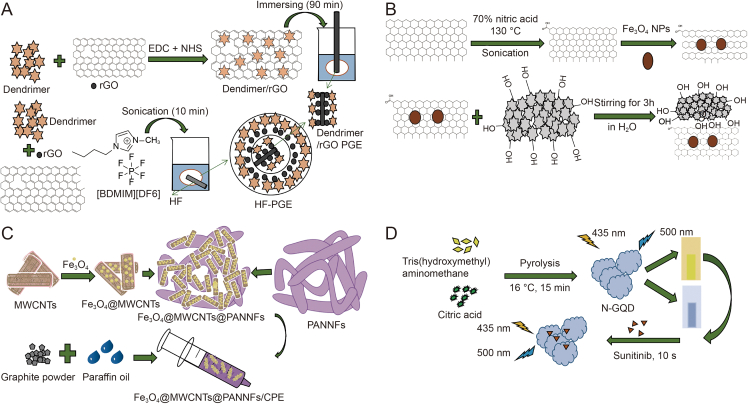
In order to further improve enrichment efficiency and reduce LOD by minimizing matrix effects, HF-SPME based on nanocomposites, such as dendrimer/reduced GO (rGO), has been developed for simultaneous separation, extraction, and preconcentration of TKIs (Fig. 1D). The incorporation of dendrimers within hollow fiber cavities enables effective target binding and subsequent entrapment within the fiber cavities for preconcentration, thereby dramatically mitigating sample matrix interference [71]. Besides, rGO shows superior electrical conductivity (8-fold higher) compared to other conductive nanomaterials such as GO [72]. Undoubtedly, this method offers a straightforward and efficient approach for optimizing design parameters with minimal experimental runs. Hatamluyi et al. developed the HF-SPME method for simultaneous microextraction and determination of imatinib using dendrimer-assisted rGO as an adsorbent (Fig. 2A) [73]. To determine the optimal adsorbent conditions, various parameters including the type, quantity, and ratio of dendrimer and rGO were optimized using a Taguchi L25 orthogonal array design. The comparison results demonstrated that the hollow fiber-modified pencil graphite sensor offered a broader linear range (0.01–200 μmol/L), hihger sensitivity, and lower LOD (7.39 nmol/L).
Fig. 2.
Synthesis procedure of diverse nanomaterials: (A) dendrimer/reduced graphene oxide (Dendrimer/rGO) [73], (B) honey coated magnetic multi-walled carbon nanotubes (Honey@magnetic-CNTs) composite [17], (C) Fe3O4@multiwalled carbon nanotubes@polyacrylonitrile nanofibers/carbon paste electrode (Fe3O4@MWCNTs@PANNFs/CPE) [128], and (D) nitrogen-doped graphene quantum dots (N-GQDs) [150]. EDC: N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride; NHS: N-hydroxysuccinimide; BDMIM: 1-butyl-2,3 dimethylimidazolium hexafluorophosphate; HF: hollow fiber; PGE: pencil graphite electrode; NPs: nanoparticles. Reprinted from Refs. [[17], [73], [128], [150]] with permission.
Furthermore, the utilization of SPME methods with high accuracy and matrix resistance is advantageous for the rapid and high-throughput analysis of TKIs in intricate matrix such as biofluids. TF-SPME has been recognized as an effective and sensitive method that employs a thin film with a high surface-area-to-volume ratio as the extraction phase for extracting and purifying various compounds in biological media [74]. This method capitalizes on its large extraction capacity, short equilibrium time, and minimal sample pre-treatment requirements. Recently, the use of nanostructured materials such as electrospinning in TF-SPME remarkably enhances the extraction efficiency due to their excellent stability and distinctively large surface area. Khodayari et al. [14] fabricated a novel and efficient sorbent (electrospun polyfam/Co-MOF-74 composite nanofibers) and applied it in the TF-SPME method for simultaneous extraction of three anticancer drugs (TKIs) from wastewater and biological samples. MOFs were employed to further modify the electrospun nanofibers, resulting in enhanced extraction efficiency toward target analytes due to their higher surface area and remarkable porosity. The extraction recoveries ranged from 60.5% to 92.7%, with a limited amount (3.3 mg) of sorbent used and high reusability (16 consecutive adsorption/desorption cycles), while also minimizing organic solvent consumption (500 μL) in this method.
3.4.2. Dispersive μ-SPE (D-μ-SPE) or dispersive SPME (DSPME)
Since the sorbent is thoroughly mixed with the samples using techniques such as ultrasounds, vortex, or emulsification, the D-μ-SPE mode enables improved extraction efficiency by promoting increased interaction between the sorbent and analytes. Nanotechnology has introduced diverse nanomaterials as nano-adsorbents for rapid extraction kinetics, high extraction efficiencies, and reduced use of toxic organic solvents. Among these materials, MNPs have gained significant popularity [75]. Magnetite (Fe3O4), owing to its unique physical and chemical properties, has been extensively employed and is usually modified and coated with different materials to increase extraction capacity and selectivity. For example, Ghazaghi et al. [76] prepared NPs (Fe3O4@NixSiOy) with uniform size to selectively extract four TKIs from complex matrices. The cost-effective modified material silicate was applied as a coating on the surface of nano Fe3O4 to enhance chemical stability and biocompatibility. The Fe3O4@NixSiOy displayed strong affinity towards the TKIs and could be easily separated using magnetic methods from serum and cerebrospinal fluid samples, achieving recoveries ranging from 94.6% to 98.6% (Table 3) [13,14,17,[48], [49], [50], [51], [52],55,57,61,63,65,70,73,76].
Table 3.
Solid-phase extraction (SPE) methods for tyrosine kinase inhibitors (TKIs) in different matrix.
| Analytes | Matrix | Sorbent | Eluent | Sample size | Recovery (%) | Refs. |
|---|---|---|---|---|---|---|
| Dasatinib, erlotinib, and nilotinib | Human plasma, serum, and urine samples | Fe3O4/MPA NPs | Methanol containing 1 × 10−3 mol/L imidazole | 1.0 mL | 90.0–97.3 | [13] |
| Sorafenib, dasatinib, and erlotinib hydrochloride | Wastewater and biological samples | Electrospun polyfam/Co-MOF-74 | Alkaline methanol | 20 mL | 78.5–92.7 | [14] |
| Sunitinib | Human blood, hair, urine, and urine | Honey@magnetic-CNTs | Methanol | n.d. | 81–102 | [17] |
| 17 TKIs and 2 metabolites | Human plasma | Oasis® MCX | Acetonitrile:methanol:25% ammonia (57:38:5, V/V/V) and 8% formic acid | 300 μL | 20–30 | [48] |
| Dasatinib, imatinib, and nilotinib | Rat plasma | Strata® C18-E (55 μm, 70 Å; 200 mg/3 mL) | Methanol | n.d. | 94.37 | [49] |
| Imatinib, dasatinib, and nilotinib | Human plasma | Versaplate-SCX (25 mg) | Acetonitrile:methanol (1:1, V/V) with 5% ammonia solution | 200 μL | 96–114 | [50] |
| Imatinib, nilotinib, and lapatinib | Human plasma | RapidFire 300 | n.d. | n.d. | n.d. | [51] |
| Neratinib and pelitinib | Rat plasma | C18 Bond Elut cartridges | Methanol | n.d. | >89.73 | [52] |
| 11 TKIs | Human plasma | PRiME μ-SPE MCX | 5% NH4OH in methanol:water (1:1, V/V) solution | 300 μL | 82–114 | [55] |
| Imatinib mesylate | Rat plasma | MIL-101(Cr) | 0.1% formic acid in water:methanol (6:4. V/V) solution | 100 μL | >81.2 | [57] |
| 15 TKIs | Human plasma | HLB magnetic particles | Acetonitrile | 100 μL | 91.28–102.7 | [61] |
| Imatinib | Human plasma and urine samples | Fe3O4@PNVCL-COOH NPs | n.d. | 200 μL | >81.4 | [63] |
| Nilotinib | Human serum | MMIN | Methanol | n.d. | 90.0–98.0 | [65] |
| Imatinib | Human urine and whole blood | Biocompatible coating/C18-SCX | 1–4 μL of methanol (0.1% FA), 1.3 kV | 10 μL–1.5 mL | n.d. | [70] |
| Imatinib | Human serum and urine samples | Dendrimer/rGO NPs | n.d. | 2.0 mL | 93.15–102.23 | [73] |
| 4 TKIs | Human serum and cerebrospinal fluid samples | Fe3O4@NixSiOy NPs | n.d. | 1.5 mL | 90.0–98.6 | [76] |
n.d.: not discovered. SCX: strong cation exchange; μ-SPE: micro-solid phase extraction; NPs: nanoparticles; MPA: melamine-phytate super-molecular aggregate; rGO: dendrimer/reduced graphene oxide; FA: formic acid; Co-MOF-74: Co-metal organic framework-74; CNTs: magnetized carbon nanotubes; HLB: hydrophile-lipophile balance; PNVCL: poly(N-vinylcaprolactam); MMIN: magnetic molecularly imprinted nanofiber.
In order to prevent aggregation in the sample solution and enhance the chemical stability, a novel bio-inspired adsorbent of D-μ-SPE was developed by modifying magnetite with melamine-phytate super-molecular aggregate (MPA). Melamine, a heterocyclic aromatic compound used in N-doped carbons, and phytic acid, a biocompatible and nontoxic material serving as a crosslinker between carbon frameworks, were chemically reacted to form phosphorus and nitrogen modifications [77,78]. The resulting supramolecular aggregate was utilized as a novel magnetic sorbent to prevent pore collapse. In the work conducted by Adlnasab et al. [13], Fe3O4/MPA was applied in D-μ-SPE for the extraction of trace quantities of dasatinib, erlotinib, and nilotinib. The magnetic sorbent facilitated rapid separation of these drugs from human plasma, serum, and urine samples using a methanol eluent containing imidazole. After optimization, an extraction time of 2 min was determined to be the most effective. Through validation, this method proved to be an accurate and efficient technique for extracting three TKIs from human plasma, serum, and urine samples with a high pre-concentration factor (500) and satisfactory recoveries (90.0%–97.3%).
The DSPME technique has gained recognition for its ability to extract, purify, and preconcentrate various analytes from real samples. It offers the advantages of not requiring a fiber, eliminating limitations associated with sorbent placement on the fiber, and reducing extraction and desorption time [79]. The type and quantity of adsorbents are crucial factors in DSPME procedures that influence extraction efficiency. Magnetized CNTs have garnered significant research attention because of their excellent thermal and electrical properties. Hooshmand and Es'haghi [17] presented a novel DSPME approach based on a newly-synthesized nanocomposite for the efficient microextraction and determination of sunitinib from human samples. This nanocomposite named honey coated magnetic multi-walled CNTs (Honey@magnetic-CNTs), could be effectively separated by applying an external magnetic field (Fig. 2B) [17]. Although ecological hazards associated with CNTs have been reported, the presence of harmless honey as a precursor on the surface of CNT NPs did not result in the production of toxic byproducts. Under optimized conditions, the applied nanocomposite showed good performance and fast extraction (within 10 min) of analytes from biological samples (e.g., hair, plasma, urine, and nail). The LOD and LOQ were 1.58 and 5.28 ng/mL respectively, with RSD value of 3.15%.
3.5. Summary of pretreatment techniques
Considering the complexity of the biological matrix, it is necessary to carefully consider the suitability of sample pretreatment methods for TKIs in order to meet the requirements of determination methods. LLE and SALLE have been widely used because of their wide extraction range, simple equipment, and mild extraction conditions. However, they are prone to emulsification and require a large amount of organic solvent. To address these issues, novel techniques such as SA-SHS-LPME, HF-LPME, and SC-SF-SLDME have been developed for good purification effect and high enrichment and extraction efficiency. Nevertheless, if LPME employs chlorine-containing toxic organic compounds as extractants, it still raises concerns. SPE, including μ-SPE, VA-DSPE, D-μ-SPE, and MSPE, are commonly employed techniques in TKI pretreatment due to their wide selection of different types of columns and diverse sorbents available. Remarkably, innovative microextraction technologies such as SA-SHS-LPME, HF-LPME, SC-SF-SLDME, Bio-SPME, HF-SPME, and TF-SPME have been developed for better efficiency in extracting analytes while minimizing the use of organic solvents according to green chemistry principles. Besides, the synthesis of novel materials such as MPA, MCN, and MIPs has significantly expanded their applicability in effectively and selectively purifying multiple TKIs from complicated matrices. Furthermore, DSPME overcomes the limitations of sorbent placement on fibers and reduces extraction and desorption time without requiring a fiber. Prospectively, pretreatment methods are expected to advance towards higher sensitivity and selectivity, the development of environmentally friendly solvents for synthesis, and seamless online automation integration with analytical equipment.
4. Analytical methods
4.1. LC-based methods
Currently, LC-based techniques are the most popular approaches for TDM of TKIs, where TKIs can be separated with different mobility in stationary phases driven by mobile phase.
4.1.1. Optimization of chromatographic conditions
For better separation efficiency and quantitative effect of target substances, it is necessary to optimize the consistency of stationary phase and mobile phase, additives and other factors in the process of establishing an high performance liquid chromatography (HPLC) method. Among them, significant advancements have been made in the development of stationary phases in recent years. Commonly used stationary phases for TKIs include C18, C8, phenyl, XDB-CN, and hydrophilic interaction chromatography (HILIC) columns. Each packing column possesses distinct characteristics and application ranges. Among these reversed-phase columns, the C18 column is the most widely utilized with over 90% representation among the 85 literature sources analyzed. This preference can be attributed to its high intercolumn reproducibility and suitability for the separation of acidic, neutral, and basic compounds [37,66]. Conventional LC often requires significant time and organic solvent consumption for target separation. In order to reduce separation time, researchers have undertaken the development of chromatographic materials with smaller particles (<2 μm). Consequently, ultra-high performance liquid chromatography (UHPLC) has emerged as a promising technique due to its improved detection capability, increased throughput, as well as reduced analysis time [5]. For example, Merienne et al. [48] developed an UHPLC analytical tool to quantify 17 TKIs and 2 metabolites in human plasma. This high-throughput assay enables rapid determination of 19 TKIs in less than 5 min per run and has been routinely used for TDM, pharmacokinetic studies, and threshold definition.
In addition to optimizing stationary phases, it is crucial to compare and optimize mobile phases when establishing an appropriate HPLC method. To enhance separation effect and peak shape, influencing factors such as pH (i.e., formic acid) [33], buffer solution (i.e., ammonium formate) [3], as well as elution mode (i.e., isocratic elution or gradient elution) [80] should be focused on according to the characteristics of samples and TKIs.
4.1.2. LC coupled with MS detectors
Recently, LC-MS has emerged as the predominant method for routine TDM and pharmacodynamics studies of TKIs. LC-MS/MS offers distinct advantages including high sensitivity, specificity, and high throughput capabilities. This paper provides a comprehensive summary of LC-MS methods used for TKIs since 2017.
4.1.2.1. Development of ion sources
Different ionization types, such as ESI, heated electrospray ionization (HESI) and electrospray chemical ionization (ESCI) are used in LC-MS techniques, each with its own characteristics and advantages. The ESI mode is employed in high coverage MS, making it suitable for the analysis of large molecules or non-volatile substances [2,55]. Considering the acidity and alkalinity of the samples, most TKIs are experimentally analyzed in positive ESI mode [50,52,81]. However, accurate measurement of plasma levels of TKIs remains challenging even under optimized MS conditions, due to electron multiplier saturation in the detector. The implementation of in-source collision-induced dissociation (CID) proves to be valuable in reducing excessive ion accumulation that frequently leads to MS damage and enables better control over the linear range of quantification [82]. Besides, the HESI source has been employed to improve the high saturation and associated instability observed in ESI source analysis. The ionization efficiency, sensitivity, and reproducibility of HESI sources are improved by the application of gasification chamber heating. Moreover, atmospheric pressure chemical ionization (APCI) is a commonly employed soft ionization technique that serves as a complementary method to ESI. Hence, ESCI or ESI-APCI is frequently used for the analysis of small molecular polar and non-polar compounds that are challenging to ionize using electrospray but can generate singly charged ions through higher temperature chemical ionization. Maher et al. [83] developed an UHPLC-ESCI-MS/MS method for investigating the pharmacokinetic interaction between imatinib and vitamin preparations in rats. The linear response for imatinib ranged from 1 to 500 ng/mL. The proposed study revealed that orally consuming vitamin preparations could potentially alter the pharmacokinetic profile of imatinib, highlighting the crucial importance of TDM for quantitatively evaluating imatinib-vitamin interactions.
4.1.2.2. Evolution of mass analyzers
Mass analyzers used in the determination of TKIs mainly include triple quadruple (QqQ), ion trap (IT), Q-TRAP, time of flight (TOF), orbitrap, as well as their tandem configurations. A comprehensive summary of the advantages and disadvantages of different mass analyzers can be found in Table 4.
Table 4.
Comparison of the advantages and disadvantages for different detectors.
| Detectors | Advantages | Disadvantages |
|---|---|---|
| Low resolution mass spectrometer | Cascade function and strong qualitative ability ; multiple scanning modes, such as SRM, MRM and neutral loss ; capacity of providing the mass charge ratio of substances and their fragments ; and higher sensitivity. |
High cost and need regular maintenance ; low resolution ; and less quantitative ability for untargeted unknowns. |
| High resolution mass spectrometer | Good resolution for high m/z ions ; accuracy of mass to charge ratio at PPM level ; faster scan speed of TOF ; and wider application range by cascade with Q MS. |
Quantification is not as accurate as QqQ ; expensive equipment and operational complexity ; and limited ion capacity of Orbitrap. |
| Light absorption-based detector | Low detection limit (ng) and low noise ; high sensitivity and wide linear range ; non-destructive sample ; and fast, only 10 ms per image. |
Requiring UV absorption and the limits in operating wavelength and mobile phase types. |
| Refraction and scattering based detector | General purpose detector ; the response is independent of the optical properties of the sample ; not affected by sample volatility and functional groups ; and mobile phase evaporation at low temperature and high sensitivity to thermal instability and volatile compounds. |
Incompatible with gradient stripping ; low selectivity ; and signal conversion is required for linkage to HPLC. |
| Electrochemical detector | High sensitivity (general ng level) ; selective detector ; simple equipment with low cost ; and easy automatic operation. |
Baselines are prone to drift ; susceptible to interference from impurities and dissolved oxygen in the mobile phasence ; and the electrode has limited lifetime and is sensitive to changes in temperature and flow rate. |
SRM: selective response monitoring; MRM: multiple response monitoring; PPM: part per million; TOF: time-of flight; Q MS: quadruple mass spectrometry; QqQ: triple quadruple; UV: ultraviolet; HPLC: high performance liquid chromatography.
Today, QqQ analyzers have become the most widely used analyzers for TKIs in LC-MS on account of the availability of robust mass spectrometers and their exceptional quantitative capacities [12,[84], [85], [86]]. In the cascade process of triple-quadrupole, specific precursor ions are selected in Q1, and product ions formed by CID are scanned with Q3 using multiple reaction monitoring (MRM) mode [26,[87], [88], [89]]. The precursor-to-product ion transitions of the common TKIs subjected to MRM detection were as follows: m/z 394.29⟶278.19 for erlotinib [90], m/z 408.2⟶339.1 for anlotinib, m/z 494.3⟶394.1 for imatinib, and 488.2⟶401.1 for dasatinib [31], among others.
Despite the excellent quantitative performance and high sensitivity of the QqQ analyzer in ion-selective mode, its limited ability to quantitatively analyze untargeted unknowns restricts application. In comparison to QqQ, IT is more suitable for qualitative research on molecular structure due to its multilevel cascade capability, which enables the provision of local structural information of molecules. Additionally, the resolution of local high-resolution mode (zoom scan) in IT is several times higher (6000–9000) than that of quadrupole MS, making it ideal for determining ion mass number. Dutta et al. [91] have developed and validated a stability-indicating method using LC-IT-MSn for the determination of nintedanib in bulk drug. The proposed structure of nintedanib degradant products was elucidated, and a predicted fragmentation pattern was established. Q-TRAP presents a promising alternative to QqQ for TKI determination, combining the high sensitivity of traditional QqQ with the superior ion accumulation ability of linear IT technology [92,93]. The use of MS3 in comparison to MS2 enables significantly improved sensitivity and selectivity [94].
However, the limited resolution of QqQ analyzer makes it susceptible to interference from compounds with similar m/z values, thereby restricting the quantification of untargeted unknowns in complex matrices. To improve resolution and selectivity, high-resolution MS (HRMS) techniques such as TOF MS, orbitrap MS, and their tandem technologies have been developed. We present a comparison of the advantages and disadvantages of different analyzers in Table 4. The Q-TOF MS technique combines the high-resolution capabilities of TOF MS with the excellent quantitative abilities of quadruple MS (QMS), making it an ideal method for accurately determining the molecular formula of unknown compounds. In a study by Johnsirani et al. [95], LC-Q-TOF-MS with gas-phase fragmentation reactions was used to investigate the degradation profile of sorafenib tosylate. Under MS/MS-CID conditions, prominent ion peaks at m/z 447 (m/z 465 for H2O), m/z 406 (m/z 447 for C2H3N), m/z 425 (m/z 465 for 2HF) and m/z 386 (m/z 406 for HF) were observed as precursor ions of sorafenib tosylate. The method demonstrated satisfactory LOD (0.62 μg/mL) and LOQ (1.97 μg/mL), with robustness in parameters maintained during five replicate injections of standard drug solution (100 g/mL) and stressed samples.
Although TOF/MS systems enable rapid full-scan data acquisition, the baselines tend to be noisier, limiting their application in low m/z ions. Orbitrap MS presents a promising alternative to TOF MS for the analysis of TKIs due to its superior resolution for low m/z ions. To meet the increasing demand for quantification with higher selectivity, sensitivity, and throughput, the Q-Orbitrap MS system has been developed. Ni et al. [39] employed the HPLC-Q-Orbitrap MS/MS method for simultaneous detection and quantitation of six TKIs in human plasma. The Q-Orbitrap MS system was operated in selected ion monitoring (SIM) mode to achieve excellent sensitivity. Data were acquired using Xcalibur (Thermo Scientific) by recording ion currents of precursor and product ions (m/z) corresponding to apatinib (m/z 398.1975/212.0818), crizotinib (m/z 450.1258/260.1506), erlotinib (m/z 394.1761/336.1343), gefitinib (m/z 447.1593/128.1070), icotinib (m/z 392.1604/304.1081), lapatinib (m/z 581.1420/458.1066), and imatinib (m/z 494.2662/394.1662) in positive ion mode. The method achieved an attractive lower LOQ of 0.02–2.0 ng/mL and was successfully applied for apatinib monitoring in plasma samples from patients with non-small cell lung cancer.
4.1.2.3. Matrix effects in MS methods and elimination/reduction methods
The matrix effect interferences encompass endogenous substances originating from the analyte itself and exogenous substances derived from the external environment during method establishment, which can significantly impact the LOD, LOQ, linearity, accuracy, and precision of the method. These effects are manifested as ion inhibition or ion enhancement. Therefore, comprehending and mitigating matrix effects in the process of method development is crucial [96]. Methods to eliminate matrix effects include selecting appropriate sample pretreatment methods, optimizing chromatographic separation and MS analysis conditions, compensating with a matrix standard solution for correction purposes, and employing suitable internal standards. Among these strategies, the utilization of an internal standard is widely favored due to its ability to counterbalance variations caused by sample loading volume, mobile phase composition, and detector response for more precise outcomes [97]. Kocan et al. [98] presented an LC-MS/MS bioanalytical assay for the quantitation of lapatinib in end-stage renal disease patients undergoing hemodialysis. To evaluate matrix effects, extracted human plasma samples were fortified with both lapatinib and lapatinib-d4. The average values of the matrix factor for the low and high quality control (QC) levels were 1.03 (0.950–1.09, coefficient of variation (CV) = 4.65%, n = 8) and 1.00 (0.945–1.05, CV = 43.11%, n = 8), respectively, indicating no significant matrix effects observed using this method. Lapatinib exhibited an overall recovery of approximately 85% within a dynamic range of 2.50–1000 ng/mL. However, the synthesis or cost of certain internal isotopes poses limitations on their practical application.
4.1.3. LC coupled with ultraviolet (UV)/PDA
Given the expensive nature of LC-MS/MS instrumentation, which requires frequent maintenance and has special requirements that are not commonly available, it limits the ease of operation and suitability of the mobile phase. Therefore, the HPLC-UV/PDA method emerges as a rapid, economical, simple, accurate, precise, and reliable analytical technique for routine quantification of TKIs in clinical samples. Structurally speaking, TKIs exhibit characteristic absorption ranges at the wavelength of 230–326 nm for imatinib [[99], [100], [101], [102], [103], [104], [105]], 260–325 nm for dasatinib [[106], [107], [108], [109], [110]], 235–265.5 nm for sorafenib [30,102,111], 265–385 nm for nintedanib [112,113], 277 nm for sunitinib [114], etc. Sakhi et al. [30] developed a sensitive HPLC/UV method for the determination of sorafenib in standard solutions and spiked plasma. The analysis was carried out at a wavelength of 235 nm with a retention time of 6.43 min, resulting in well-resolved chromatograms with distinct peak separation. The LOD achieved the required level of sensitivity at 5 ng/mL, while the LOQ was set at 7.5 ng/mL.
4.1.4. Quality by design (QbD)-mediated LC methods
Recently, the implementation of QbD principles offers an apparent advantage in comprehensively understanding the performance of methods for estimating various analytes in bulk drugs, pharmaceutical formulations, and bioanalytical samples. Hence, the application of QbD approach in analytical method development of TKIs has gained popularity in pharmaceutical practice due to its ability to improve process stability while reducing time and cost during process design and development [115]. Sharma et al. [116] developed a robust analytical method employing QbD paradigms to effectively separate and quantify the degradation products of sorafenib tosylate in biological matrix, using a mobile phase independent of buffer. Factor screening and quality risk assessment studies were used for selecting critical method parameters, and systematic optimization was carried out using a 2 factor-3 level-13 run face-centered cubic design. The validation studies confirmed the high efficiency (98.15%–101.68%) and sensitivity (LOD and LOQ of 0.002 and 0.007 μg/mL, respectively) of the developed method in human plasma matrix. The selectivity and specificity of the systematically developed method were further confirmed by the absence of any significant alterations in the retention time of sorafenib tosylate and its degradation products. Due to the lack of a comprehensive database for reversed-phase HPLC parameters, such as different chromatographic columns, mobile phases, and pH levels, etc., an investigation into QbD was conducted to efficiently optimize these parameters. Waghmare et al. [117] implemented QbD-based approach for reversed-phase HPLC analysis to assess drug compatibility/estimation in plasma with its constituents. The parameter was optimized using a 3-level factorial design approach, and the best results were obtained under the following conditions: C18 column, ammonium format buffer consisting of acetonitrile (28.19:71.81, V/V), and buffer with pH 3. This optimized method was successfully applied to a pharmacokinetic study, demonstrating high recovery (99.91%), specificity (99.94%), precision (RSD < 0.543%), and robustness (RSD < 0.500%) (Table S1) [2,3,5,8,12,[15], [16], [17],[26], [27], [28], [29], [30], [31], [32], [33], [34], [35],[37], [38], [39],41,[48], [49], [50],52,54,55,61,63,64,76,80,83,84,86,[88], [89], [90], [91], [92], [93], [94], [95],[97], [98], [99], [100], [101], [102], [103], [104], [105],109,[111], [112], [113], [114],116,117].
4.2. Development of electrochemical methods for TKIs
In recent years, significant progress has been made in electrochemical methods (i.e., voltammetry, potentiometry, and electro-Fenton process) due to advancements in nanophase materials. These methods demonstrate high accuracy, good reproducibility, as well as good selectivity by excluding interference. Among them, voltammetry stands out as a promising alternative to classical approaches due to its wider application range achieved by electrode construction with various properties including glassy carbon electrode (GCE), carbon paste electrode (CPE), pencil graphite lead, screen-printed carbon electrode (SPCE), paste electrode (PE), Pd@Pt electrode, indium tin oxide (ITO), etc..
4.2.1. Voltammetry
The utilization of GCEs in electrochemical sensors for the detection of target molecules is widespread due to their cost-effectiveness and stable electrochemical window. However, bare carbon electrodes exhibit weak electrochemical signals, posing challenges in meeting requirements for highly sensitive detection. To address this issue, surface modification of GCEs with rGO/NPs composites is conducted to enhance electrical conductivity and increase the electrochemically active surface area. Wu et al. [118] synthesized rGO/Ag-NPs composites by a one-step hydrothermal method and used them for surface modification of GCEs in the electrochemical detection of imatinib. The enhanced oxidation peak response (4.52 μA) suggested that the excellent electrical conductivity of Ag-NPs improved sensing performance. Under optimal conditions, the electrochemical sensor demonstrated a wide linear range of 10 nM-0.28 mM for imatinib detection, along with a low LOD of 1.1 nM. The sensor exhibited excellent anti-interference performance and reproducibility. Besides, the utilization of Ag-NPs, known for their electrochemical catalytic properties, was proposed to improve the sensitivity of detection. The study conducted by Tahernejad-Javazmi et al. [119] presented an amplified biosensor based on Au-NPs/rGO/double-stranded (ds)-DNA/GCE for the detection of dasatinib in aqueous solution, tablets, and urine samples. Differential pulse voltammetry was employed to detect the guanine oxidation peak current of ds-DNA as the signal for dasatinib within a concentration range of 0.03–5.5 μM. The proposed method provided a significantly higher sensitivity (LOD = 0.009 μM) compared to the HPLC method (LOD = 0.03 μM), with an improvement factor of 30-fold. Moreover, the Au-NPs/rGO/ds-DNA/GCE demonstrated satisfactory reproducibility with a low RSD of 3.1% (n = 7), without any noticeable interference.
In addition to GO, CNTs and their related composites have emerged as prominent carbon nanomaterials at the nanoscale due to their exceptional conductivity, resistance to surface fouling, and high specific surface area. Moreover, when dispersed in Nafion (NafionNT), they can form uniform and stable films with excellent reproducibility and remarkable electrocatalytic properties. Vercelli et al. [120] investigated the responses of sunitinib at modified electrodes using cyclic voltammetry determinations. The peak current values of sunitinib at NafionNT/GCE electrodes were found to be three times higher than those observed at bare GCE electrodes, which could potentially be attributed to the formation of H-aggregates resulting from sunitinib aggregation. Different pulse voltammetry analysis demonstrated good reproducibility (RSD = 6%, n = 3) in micromolar concentrations when applied to human plasma samples. Chen et al. [121] reported the first simultaneous determination of imatinib using a modified GCE with carboxylated multiwalled CNTs (MWCNTs-COOH) and a NiO–ZnO composite (NiO–ZnO/MWCNT-COOH/GCE). The composite exhibited excellent analyte absorption and good electrical conductivity. This proposed method was successfully applied for the analysis of imatinib in human serum and urine samples, yielding satisfactory results including a linear range of 0.015–2.0 μM, an LOD of 2.4 nM (signal-to-noise ratio (S/N) = 3), and a sensitivity of 9.64 μA/μM/cm2.
Compared to various metals/metal oxide nanomaterials, MOFs have attracted the interest of researchers due to their intriguing properties as a remarkable class of hybrid organic-inorganic microporous crystalline materials. The excellent adsorption capacity resulting from ultra-high porosity and uniform and tunable pore size has led to an increased accumulation of TKIs at the surface of MOF-modified electrodes [122]. Rezvani Jalal et al. [123] reported the in-situ growth of HKUST-1 framework on conductive GO nanoribbons (GONRs)-modified GCE (HKUST-1/GONRs/GCE) for the determination of imatinib in urine and serum samples. This strategy effectively addressed the poor conductivity issue associated with MOFs by the synergistic effect between GONRs and HKUST-1 framework. The achieved electrochemical performance showed promise with linear dynamic ranges of 0.04–1.0 and 1.0−80 μmol/L, as well as a LOD at 6 nmol/L for imatinib determination.
Over the past decades, CPEs have become another widely used electrode materials for the fabrication of electrochemical sensors due to their rapid surface renewal, chemical inertness, and low residual current. To enhance analytical performance, various chemical modifications have been applied to CPEs, including Al2W3O12-NPs/CPE [124], ZnO-NPs/ionic liquid/CPE [125], CPE modified with MMWCNTs and polyacrylonitrile nanofibers (PAN-NFs) (MMWCNTs/PAN-NFs/CPE) [126], CPE modified by PAN-NFs and nickel-zinc-ferrite (Ni0.5Zn0.5Fe2O4) (PAN-NFs/Ni0.5Zn0.5Fe2O4/CPE) [127], etc.. Ghapanvari et al. developed Fe3O4/MWCNTs/PAN-NFs/CPE via bulk modification approach to detect an important anticancer drug imatinib in urine samples (Fig. 2C) [128]. The facilitated electrochemical oxidation was ascribed to the good electrocatalytic behavior of Fe3O4/MWCNTs/PAN-NFs composite material. Compared to the previously reported nine works, the modified CPE showed superior linear dynamic range (0.0017−0.8500 μM) and a lower LOD (0.4 nM).
Although not as widely employed as GCE and CPE, pencil graphite lead is also used as a classical electrode for TKI detection due to its advantageous features such as low cost, simplicity, as well as the possibility of in situ measurements [73]. In order to further reduce expenses and fabricate miniaturized electrochemical sensors, disposable SPCEs, based on thick-film technology, provide attractive opportunities in the detection of different TKIs [129]. However, the sensitivity of this method is consistently limited by catalytic activity. The utilization of appropriate support materials with high surface area can facilitate better distribution of NPs and prevent aggregation, thereby improving sensitivity. In the work of Kalambate et al. [130], a highly sensitive electrochemical sensing platform based on Pd@Pt/MWCNT was developed for the determination of anticancer drugs dasatinib from urine and blood serum samples. The electrochemical behavior of dasatinib was evaluated using cyclic voltammetry, and the determination was performed through adsorptive stripping square wave voltammetry. The established sensor showed several advantages, including ease of preparation, high sensitivity (1.3 μA/μM), selectivity (tolerance limit < 5%), and long-term stability (retaining 95.5% of its initial response over a period of 30 days). In recent years, metal nanocomposites and high electrical conductivity carbon have been proposed for the fabrication of various electrochemical sensors. Moghaddam et al. [131] used 1-hexyl-3-methylimidazolium tetrafluoroborate (mim-BF4−) in PE (Fe3O4-SWCNTs/mim-BF4−/PE) to enhance the sensing performance of dasatinib, an anticancer drug. Under optimal conditions, the oxidation current of dasatinib was increased by approximately 5.58 times and its oxidation potential was reduced by about 120 mV. The modified sensor exhibited satisfactory recovery of 99.58%–103.6% with a LOD of 0.7 nM and a linear dynamic range spanning from 0.001 to 220 μM.
4.2.2. Potentiometry and electro-fenton process
A non-aqueous potentiometric titration method serves as a cost-effective alternative for analyzing organic substances with poor solubility in water, weak acids, or bases that cannot be directly determined in water, and strong acids or bases that cannot be titrated step by step in water. This method enables more accurate determination results while avoiding moisture interference. Rele and Tiwatane [132] developed a rapid, accurate, and sensitive non-aqueous potentiometric titration method to quantitatively determine and validate imatinib mesylate in pharmaceutical dosages. The proposed method showed satisfactory precision (RSD < 1%, n = 6), strict linearity within the range of 20%–100% (r2 > 0.999), and percentage recovery of 100.087%–100.692% under optimized conditions. This simple, rapid, and accurate analysis method holds great practical implications for the pharmaceutical industry and research institutions.
Electro-Fenton process, which combines the characteristics of electrochemical and Fenton reactions, is widely recognized as one of the most prominent electrochemical advanced oxidation processes due to its exceptional oxidation capacity and low energy consumption. In this process, H2O2 is generated in situ while the catalyst undergoes continuous electrochemical regeneration. Yang et al. [133] conducted a comparative study on the oxidative degradation and mineralization of imatinib using a graphene-modified carbon felt (CF) cathode and a raw CF cathode within the framework of the electro-Fenton process. Under identical operating conditions, it was observed that the graphene-modified CF cathode achieved complete mineralization with higher total organic carbon removal compared to the raw CF cathode (75%) after an 8 h treatment period. In addition, the intermediates formed during mineralization exhibited greater toxicity than imatinib, whereas the solution toxicity was entirely eliminated following treatment with a graphene-based CF cathode.
4.3. Development of spectroscopic methods for TKIs
4.3.1. SPR methods
Considering that TDM has been suggested as the optimal way to minimize the risk of adverse reactions in oncology patients, there has been significant interest in miniaturizing point-of-care testing devices over the past few decades. Sensing technologies that enable rapid and reliable determination of the TKIs in small sample volumes at microliter levels, allowing testing to be conducted at patients' bedside or in medical practices, present a highly promising alternative to conventional HPLC methods. The SPR-based platform offers real-time monitoring of interactions with TKI molecules by immobilizing receptor molecules on the sensor surface. Tartaggia et al. [134] investigated the TDM of imatinib at clinically relevant concentrations using an SPR sensing platform in human plasma. A selective receptor, single-stranded (ssDNA) aptamer, was employed to avoid interference from components of human plasma samples. The sample preparation procedure achieved a recovery of 48.8%–52.8%. Within the concentration range of 400–6000 ng/mL, a lower LOQ (LLOQ) of 400 ng/mL was achieved with precision within 12.0% and accuracy of 97.1%–101.5%.
4.3.2. Surface-enhanced Raman spectroscopy (SERS)
The application of SERS offers a distinct advantage in the detection of trace specific components in complex mixed systems, as it can greatly reduce fluorescence interference while amplifying the signal by 10n times. Recently, a fast and label-free analytical platform based on SERS and multivariate calibration has been employed for measuring low concentrations of compounds in body fluids, showing a good correlation with HPLC-MS data. Fornasaro et al. [135] proposed a point-of-care approach using SERS and partial least-squares regression (PLSR) for fast and cost-effective quantitation of imatinib in human plasma. The optimal PLSR model consisted of three latent variables ranging from 123 to 5000 ng/mL for imatinib, with a standard error of prediction (SEP) at 510 ng/mL. The novel SERS method presented simplicity, short analysis time, and good sensitivity (LOQ = 636 ng/mL), which aligns well with LC-MS/MS methods during clinical verifications, providing an affordable protocol for medical professionals to dose imatinib.
4.3.3. Evolution of novel NPs for TKIs
Conventional spectrophotometric methods, such as UV spectrophotometry based on conjugated structure and fluorescence spectrophotometry based on fluorescence properties [136,137], are simple and sensitive techniques for the detection of TKIs. Assays based on ion-pair associates [138] and 96-microwell-based spectrophotometric assay [139,140] have been reported for the convenient and accurate determination of TKIs in pharmaceutical formulations. However, traditional spectrophotometric methods may face challenges in distinguishing certain TKI structures (i.e., vandetanib, dasatinib, and sorafenib) due to their similar UV-visible characteristics [141]. For this reason, novel univariate [142] and multivariate validated chemometric methods [143] have been developed to simultaneously estimate TKIs in a mixture without the need for separation. Currently, biosynthesized semiconductor quantum dots (QDs) have exhibited superior performance in determining TKIs. Zhang et al. [144] presented a facile and eco-friendly strategy that used microalgae cells as bio-reactors to produce CdSe QDs as fluorescent nanoprobe for imatinib detection. The biosynthesized CdSe QDs had an approximate size range of 5–6 nm and exhibited superior optical properties. A low LOD of 0.014 μg/mL was obtained within the linear range of 10.43–417.30 μg/mL, providing new insights into the potential conversion of toxic metals into fluorescent QDs in the environment.
For TKIs with weak fluorescence, micelles have been used to increase the fluorescence intensity. Common surfactant-based micellar formations include carboxymethyl cellulose (CMC) [145], cremophor RH 40 (Cr RH 40) [146], kolliphor RH 40 [147], etc.. Apart from the spectrofluorimetric method based on micellars, resonance light scattering (RLS) spectroscopy techniques are more widely adopted due to their rapid response without requiring any preparation steps [148]. Pirdadeh-Beiranvand et al. [149] constructed a Ni0.5Zn0.5Fe2O4-NPs-decorated poly nanofiber as an RLS probe for determining sunitinib in serum samples. Nitrogen (N) functionalization and doping are suggested to improve the fluorescence efficiency and quantum yield of NPs. For example, Kashani et al. synthesized nitrogen-doped graphene quantum dots (N-GQDs) and employed them in a fluorescence sensor for determining sunitinib (Fig. 2D) [150]. This sensor used bottom-up strategies, wherein a one-step pyrolysis of citric acid served as the carbon source and tris(hydroxymethyl)aminomethane acted as a surface passivation agent. The N-GQDs-based sensor exhibited low toxicity and photostability, with a high quantum yield of 78% and LOD of 0.03 mmol/L in the pH-sensitive range of 3.00–10.00. Moreover, nitrogen-doped carbon dots (N-CDs) have also emerged as popular fluorescence nanoprobes owing to their wonderful fluorescence properties, cost-effective preparation, as well as excellent biocompatibility. Forough et al. [151] prepared N-CDs for the detection of sunitinib in rat plasma. Citric acid and urea were used as the carbon and nitrogen sources respectively, resulting in a high fluorescence quantum yield (15.9%) obtained within a short time of only 3 min. Notably, these N-CDs exhibited satisfactory stability under various environmental conditions such as pH levels, ionic strength, and UV radiation exposure while maintaining good solvent compatibility to avoid any medium-related issues. The method provided a low LOD of 30 ng/mL within the linear range of 0.1–7 μg/mL, meeting the requirements for monitoring sunitinib treatment.
4.4. CE methods for TKIs
Among the newly developed CE methods, capillary zone electrophoresis (CZE) is more popular in terms of flexibility and efficiency compared to traditional CE methods. In-column field-amplified sample injection (FASI) method has been adopted to enhance the sensitivity and improve quantification performance because of the short optical path length within the detection cell. A novel analytical platform is presented for sensitive and rapid analysis of imatinib mesylate in biological media, requiring a minimal volume of both sample and solvent. Forough et al. [151] described an FASI-CE-UV method for the assay of imatinib mesylate in human plasma, and the developed method was extensively validated according to U.S. FDA guidelines. Within 15 min, significant variables affecting the proposed method were evaluated for maximum extraction performance. The verification results showed that the method exhibited excellent linearity (R2 = 0.998) over concentrations ranging from 25 to 1500 ng/mL (LOD = 6.24 ng/mL), with mean precision (RSD = 6.83%–7.71%) and mean recoveries (98%–106%). Hence, the validated method was successfully applied for the determination of imatinib mesylate in patient's plasma samples.
Due to its low sample and background electrolyte consumption, short analysis time, and high separation efficiency, CZE has emerged as a powerful and widely accepted technique for the analysis of TKIs in various dosage forms [43]. Gonzalez et al. [152] developed, validated, and compared three analytical methods, namely UHPLC, CZE, and sequential injection analysis (SIA), for the determination of dasatinib in tablet dosage form. The LOD and LOQ values obtained from the CZE methods (0.831 μg/mL and 2.517 μg/mL) were lower than those achieved with the SIA methods (2.7 μg/mL and 8.9 μg/mL), but higher than those obtained using UHPLC (0.006 μg/mL and 0.019 μg/mL). Beyond dispute, the CZE method is more cost-effective compared to UHPLC because CZE method does not require costly equipment and high maintenance costs. All three methods demonstrated suitable selectivity and nearly 100% recovery for the determination of dasatini. However, techniques utilizing optical absorbance detection may have limited specificity and can be affected by co-migrating species. When coupled online with MS, CZE-MS offers enhanced selectivity and specificity, making it a valuable two-dimensional analysis platform, especially for complex biological samples. Zhao et al. [36] used CZE-MS/MS with field-amplified sample stacking for the quantification of imatinib and related compounds. After collecting precursor ions with appropriate m/z values using QqQ/MS followed by fragment scanning and quantification using TOF MS, they achieved significantly reduced background noise levels while improving overall sensitivity and selectivity. The recovery exceeded 83% for imatinib and its major metabolite, reaching 90% for imatinib specifically. In comparison to conventional CZE, S/N were increased at least 10-fold, resulting in the LODs of 0.2–4 ng/mL for all three analytes.
4.5. SFC
SFC, an analytical technique that integrates the characteristics of GC and LC, has been developed as a complementary method for analyzing TKIs using supercritical carbon dioxide (SC-CO2) as the major constituent of the mobile phase. Compared to conventional mobile phases, it offers advantages such as low surface tension, relatively low critical temperature, and moderate critical pressure. To evaluate the limited bioavailability of imatinib mesylate, which exhibits lower solubility during fasting compared to feeding conditions, a SFC-based model was developed and its correlation with the solubility of imatinib mesylate was established [153]. Sodeifian et al. [154] measured the solubility of sunitinib malate in SC-CO2 using SFC with experimental correlations and thermodynamic modeling. The dissolved mole fractions ranged from 0.5 × 10−5 to 8.56 × 10−5, and the measurements were conducted at pressures ranging from 120 to 270 bar and temperatures ranging from 34.85−64.85 ℃. This model gave the best correlation among the semi-empirical models with average absolute relative deviation (AARD%), Radj., and F-values of 12.16%, 0.9899, and 383.64, respectively. Moreover, although an array of counterfeit TKI products may be economically appealing, they pose a significant risk to patients. Therefore, it is imperative to establish an effective method for screening suspected TKIs in light of the potential harm caused by counterfeit drugs. Zhang et al. [155] developed and validated a rapid analytical method using SFC-MS for the simultaneous identification and determination of 11 TKIs. In this study, six different columns were evaluated, including Torus high density diol (DIOL), Torus 2-picolylamine, Torus diethylamine, Torus 1-aminoanthracene, Acquity Ethylene Bridged Hybrid (BEH), and XBridge BEH C18. Among them, an Acquity UPC2 Torus DIOL column (3.0 mm × 100 mm, 1.7 μm) was selected as the optimal choice for achieving the best separation efficiency. The calibration curves showed excellent linearity within the dynamic range 1–600 μg/mL (R2 > 0.9995) for all TKIs tested. This method was successfully applied to identify afatinib powder (0.94 mg/mg), osimertinib powder (0.92 mg/mg), and osimertinib capsule (57 mg per capsule).
4.6. Other methods
In addition to the aforementioned detection techniques, conductometry, immunoassay, high performance thin layer chromatography (HPTLC), and other detection methods have also been developed and applied in this field. The conductometric analysis is suitable for routine quality control analysis of TKIs pharmaceuticals due to its cost-effectiveness and rapid analytical procedures [156]. For further improvement of the specificity and sensitivity, antibody-based immunoassays are chosen in many clinical analysis and testing [157]. The determination of imatinib was performed by Saita et al. [158] using a highly sensitive sandwich enzyme-linked immunosorbent assay (ELISA) that employed two anti-imatinib antibodies for ultra-specific discrimination. This assay demonstrated a strong correlation with the HPLC method (y = 0.983x + 0.081, R2 = 0.948) and showed lower cross-reactivity with metabolites compared to competitive ELISAs. Under optimized conditions, a satisfactory linear detection of 64 pg/mL−8 ng/mL, with a LOQ of 64 pg/mL using this sandwich ELISA were achieved for 100 mL samples. In addition, HPTLC offers significant advantages over other LC-MS/MS techniques in terms of its ability to simultaneously analyze multiple samples, cost-effectiveness, high-throughput screening capabilities, as well as minimal mobile phase volume [159,160]. Sharma et al. [161] reported an HPTLC densitometric method for the quantification of sorafenib tosylate and studied its impact on plasma. Chromatographic separation was performed using a solvent system composed of toluene:n-hexane:isopropyl alcohol (7:2:1, V/V/V) with Rf values of 0.3. Linearity in validation studies was established within the range of 20–800 ng/band (LOD = 3.44 ng/band; LOQ = 10.44 ng/band), along with a remarkable recovery of 99.66% (RSD < 2%) and precision (<20%).
4.7. Summary of determination methods
Considering the exceptional selectivity, throughput, and outstanding quantitative performance in detecting trace substances within biological matrix components, LC-MS/MS emerged as the most widely adopted technology for TKIs and their derivatives analysis. The advancement of diverse HRMS instrumentation types (i.e., TOF-MS, RP ≥ 20,000, mass accuracy ≤ 3 ppm, scan rate up to 500 Hz; Orbitrap-MS, resolving power (RP) ≥ 100,000; mass accuracy ≤ 2 ppm; scan rate up to 40 Hz) has further promoted profound research on TKIs within complex matrix by combining higher mass spectral RP with superior mass accuracy. Moreover, electrochemical methods utilizing various electrodes such as GCE, CPE, pencil graphite lead, SPCE, PE, ITO, and Pd@Pt electrode have been successfully employed for TKI determination without requiring expensive instruments of facility maintenance. Other unconventional biosensing methodologies like SPR, SERS, and RLS based on novel NPs have also been promising alternatives to LC-MS/MS equipment. Furthermore, the absence of organic solvents in CE results in lower operating costs while maintaining high separation efficiency. Additionally, SFC methods generally exhibit enhanced column efficiency and shorter separation.
5. Clinical pharmacokinetics of TKIs
TKIs can function as competitive inhibitors of adenosine triphosphate (ATP) binding to tyrosine kinase, and they can also act as tyrosine analogues to obstruct tyrosine kinase activity and suppress cell proliferation. Consequently, numerous anti-tumor drugs have been developed [[162], [163], [164]]. First-generation TKIs (e.g., gefitinib, erlotinib, and lapatinib), which are all synthetic low molecular weight phenyl-quinazolines, have gained extensive usage in the treatment of patients with metastatic non-small cell lung cancer due to their anticancer efficacy against epidermal growth factor receptor (EGFR)-mutated cancers. To overcome resistance towards first-generation TKIs, researchers have developed second-generation EGFR TKIs (e.g., afatinib, dacomitinib, vandetanib, neratinib, pelitinib, and canertinib). They exert actions through irreversible binding to the EGFR tyrosine kinase. Despite promising preclinical data, few drugs other than afatinib and dacomitinib have shown marginal improvement in clinical efficacy among patients. Moreover, second-generation EGFR TKIs irreversibly inhibit wild-type EGFR, leading to more profound adverse effects. As a result, third-generation EGFR TKIs (e.g., osimertinib, rociletinib, and olmutinib) are actively undergoing clinical development with a specific focus on targeting EGFR-T790 M (a tumor-mutated gene), while preserving wild-type EGFR to minimize the occurrence of toxic side effects.
TKIs are a revolutionary class of small-molecule targeted anti-tumor drugs capable of traversing cell membranes and inhibiting the signaling pathway responsible for cancer cell growth and division within tumor cells [165,166]. Some TKIs also exhibit anti-angiogenic properties. They demonstrate remarkable efficacy in treating chronic myelogenous leukemia, Philadelphia chromosome (Ph)-positive acute lymphoblastic leukemia, and so on, with their utilization steadily increasing [167,168]. However, certain patients may experience varying degrees of adverse reactions during treatment. To gain a better understanding of the factors influencing drug exposure, we compare and summarize the pharmacokinetic profile of these TKIs and their similarities. And the absorption, distribution, metabolism and excretion (ADME) of the available TKIs are reviewed. The pharmacokinetic parameters assessed included time to reach maximum plasma/serum concentration (tmax), maximum plasma/serum concentration (cmax), area-under-the-curve plasma/serum concentrations (AUC), elimination half-life (t1/2), apparent oral clearance (CL/F), and apparent volume of distribution (VZ/F).
Over the past three decades, there has been significant development in the field of kinase inhibitors, leading to the approval of numerous receptor TKIs [[169], [170], [171], [172], [173]]. In terms of absorption, most TKIs rapidly reach their peak plasma concentration within a short time frame (1.5−6 h) [[174], [175], [176], [177]]. The bioavailability of lapatinib and nilotinib is notably enhanced by food intake, whereas imatinib and sunitinib are unaffected by food consumption [178,179]. The non-proportional increase in exposure to sorafenib and nilotinib at higher doses may be attributed to various factors such as solubility characteristics, saturation at absorption sites, and interactions with transporters [180,181]. TKIs exhibit extensive tissue distribution, characterized by a large distribution volume, high degree of protein binding, and prolonged terminal half-life. The majority of TKIs undergo primary metabolism mediated by cytochrome P450 (CYP) 3A4, with other CYP enzymes and uridine diphospho-glucuronosyltransferase (UGT) playing a minor role in the case of sorafenib and dasatinib [171]. Some TKIs are thought to have no impact on CYP enzyme activity due to insufficient data [182]. Regarding excretion, most TKIs are predominantly eliminated through feces, with only a small fraction excreted in urine. Severe liver impairment affects the pharmacokinetics of imatinib and lapatinib but not gefitinib (Table 5) [[165], [166], [167], [168],170,171,[173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184]]. Furthermore, these drugs can inhibit their own metabolizing enzymes and transporters, leading to unpredictable homeostatic metabolism and drug-drug interactions.
Table 5.
Pharmacokinetic parameters of the individual tyrosine kinase inhibitors (TKIs).
| TKIs | Dose (mg) | n | cmax (ng/mL) | tmax (h) | t1/2 (h) | AUC0−24 (ng·h/mL) | CL/F (mL/min) | VZ/F (L) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Afatinib | 40 | 30 | 25.2a | 3.98a | 26.9a | 324a | 952a | 2220a | [165] |
| Axitinib | 5 | 8 | 29.2a | 2.22a | 10.6a | 136a,c | n.d. | n.d. | [166] |
| Bosutinib | 400 | 6 | 87.9 ± 30.8a | 3.50b | 39.70 ± 8.49a | 2190 ± 661a | 248 ± 80.9a | 14,300 ± 6060a | [167] |
| Crizotinib | 250 | 6 | 109a | 3b | 94a | 2777a,c | n.d. | n.d. | [168] |
| Dabrafenib | 150 | n.d. | 2160a | 2a | 8.4a | 12120c | n.d. | n.d. | [170] |
| Dasatinib | 100 | 12 | 56a | 1.5b | 4.3a | 218a | n.d. | 4224a | [171] |
| Ponatinib | 45 | 22 | 54.7a | 6b | 24.2b | 1273a,c | 590a | 1242a | [173] |
| Regorafenib | 160 | 8 | 2509a | n.d. | n.d. | 27027a | n.d. | n.d. | [174] |
| Ruxolitinib | 25 | n.d. | 1450 ± 718a | n.d. | 2.51 ± 0.638a | 4020 ± 1220c | 3766 ± 151.5a | n.d. | [175] |
| Vandetanib | 300 | 16 | 179a | 6b | 184a | 22200a,c | 225a | n.d. | [176] |
| Pazopanib | 800 | 10 | 19400a | 3.5a | 31.1a | 275.1a | n.d. | n.d. | [177] |
| Imatinib | 400 | n.d. | 1907.5 ± 355.0a | 3.1 ± 2.0a | 14.8 ± 5.8a | 24.8 ± 7.4a | 208.3 ± 120a | 236.0 ± 76.5a | [178] |
| Sunitinib | 37.5 | 4 | 276 | 1.8 | 55.9 | 2981.7 | n.d. | n.d. | [179] |
| Sorafenib | 50 | 15 | 460a | n.d. | 29a | 11040a,c | n.d. | n.d. | [180] |
| Nilotinib | 400 | 15 | 1595a | n.d. | 17a | n.d. | n.d. | n.d. | [181] |
| Erlotinib | 150 | 38 | 1320a | n.d. | n.d. | 19.4a | n.d. | n.d. | [182] |
| Lapatinib | 1000 | 3 | 820b | 2b | 24b | 4900b,d | n.d. | n.d. | [183] |
| Gefitinib | 500 | 18 | 167.5 | 3 | 34 | 5044c | n.d. | n.d. | [184] |
a Values are reported as mean ± standard deviation (SD). b Values are reported as median (range). c The area under concentration-time curve (AUC) from time zero to infinity. d AUC0−12: AUC for 12 h. n.d.: not discovered. cmax: maximum plasma/serum concentration; tmax: time to reach maximum plasma/serum concentration; t1/2: elimination half-life; AUC0−24: AUC from 0 h to 24 h; CL/F: apparent oral clearance; VZ/F: apparent volume of distribution.
6. TDM of TKIs
TKIs are a class of targeted therapies for malignant diseases that competitively bind to ATP at their binding sites, thereby inhibiting tyrosine kinases involved in various pathways, including tumor cell growth and proliferation, as well as apoptosis inhibition and angiogenesis promotion [185,186]. Imatinib, nilotinib, and dasatinib represent first-line treatments for Ph-positive chronic myeloid leukemia (CML) [171,178,181]. However, patients treated with these drugs face the risk of developing renal or hepatic impairment at any stage of their disease. Additionally, some TKIs can inhibit their own transporters and metabolic enzymes leading to unpredictable steady-state disposal and metabolism [187]. Therefore, it is necessary to gather more data on the potential impact of these impairments on the pharmacokinetics of these drugs in order to provide practical guidance regarding the implications for patient management.
TDM measures drug concentrations in biological fluids to aid prescribing and can help predict clinical outcomes of dosing regiments when the obtained concentrations are compared to the range of treatment [186]. TDM is recommended under the following situations: significant pharmacokinetic deterioration; predictable correlation between plasma concentration and clinical endpoints (with defined treatment ranges); and limited availability of treatment options [188,189]. The optimization of therapeutic drug activity can be achieved by individualizing doses based on serum drug concentration measurements, particularly when the drug exhibits interindividual variability in pharmacokinetics leading to inadequate treatment or excessive exposure. According to the recommendations of European LeukemiaNet, the clinical response (breakpoint cluster region-abelson complex 1 (BCR-ABL1) transcript level) of CML patients should be evaluated at 3 (≤10%), 6 (≤1%), and 12 months (≤0.1%) following initiation of these TKI therapies [190]. By incorporating a total of 38 studies (28 imatinib, 7 nilotinib, and 3 dasatinib) into a systematic review, García-Ferrer et al. [191] found that TDM proved valuable in predicting the effectiveness of imatinib when the cmin reached a threshold of 1000 ng/mL, which aligns with established guidelines. They recommended maintaining imatinib levels within the range of 1000–1500 ng/mL as higher concentrations did not yield improved efficacy. TDM represents a novel approach to optimizing dosage in TKI therapy for patients with CML, leading to faster and more effective clinical responses. Miura [192] conducted a review of exposure-response relationships for three TKIs based on their previous studies. For example, maintaining high cmax or c2 (>50 ng/mL) with dasatinib is recommended to obtain clinical response, while lower plasma concentration (c0 < 2.5 ng/mL) can help avoid pleural effusion. Treatments should be individualized primarily for patients with susceptible genotypes. Other TKIs are used in various pathologies [187], such as gefitinib, erlotinib, afatinib, and crizotinib for non-small cell lung cancer treatment and lapatinib and regorafenib for human epidermal growth factor-positive breast cancer and metastatic colorectal cancer treatment respectively. Different thresholds have been identified for the TKIs; however, available studies on them are limited and sometimes contradictory; thus further monitoring studies are required.
7. Conclusions and future perspectives
TKIs have emerged as the first-line small molecule drugs in numerous cancer treatments, dramatically improving long-term survival prognosis and quality of life. However, despite the theoretical effectiveness of these cancer therapies, considerable inter-individual variations in the concentration of specific TKIs can compromise patients' immune function and render them vulnerable to various infections. Hence, it is crucial to conduct efficient analysis of TKIs with high sensitivity and assess their clinical pharmacokinetics for TDM. These endeavors are essential for evaluating the roles of TKIs in personalized precision medicine and TDM. This review provides a comprehensive summary and comparison of pretreatment and determination methods for TKIs in biological samples since 2017, with a particular focus on pharmacokinetic studies and TDM.
Because of the relatively narrow therapeutic window for some TKI concentrations and significant individual variability, biological analysis of TKIs in biological matrices can enhance our understanding of ADME behavior in biota. Relevant biological matrices used in bioanalysis include urine, plasma, serum, whole blood, and cerebrospinal fluid samples. Although less commonly employed in practical tests, non-invasive alternative biomaterials such as hair and nails offer unique advantages like long-term stability that are also incorporated into the substrate of biological analysis. Besides, unconventional sampling methods such as DBS microsampling techniques with smaller sample sizes, easier storage and transportation, and improved drug stability within the matrix; as well as microdialysis techniques enabling in-situ sampling are utilized to improve sample recovery and ensure the reliability of experimental conclusions.
The utilization of various TKIs can be optimized through an effective pretreatment process, leading to enhanced recovery in complex sample matrix due to better purification and enrichment capabilities. The introduction of novel materials like MPA, MCN, and MIPs has greatly improved extraction efficiency and selectivity. Besides, progress in microextraction methods such as SA-SHS-LPME, HF-LPME, SC-SF-SLDME, Bio-SPME, HF-SPME, and TF-SPME have achieved superior enrichment effects while minimizing the use of organic solvents (μL), aligning with contemporary environmentally friendly principles. Additionally, emerging methods such as μ-SPE, D-μ-SPE, and MSPE have overcome limitations associated with sorbent placement on fibers for the preconcentration of diverse TKIs in complex matrices.
Generally, LC-MS/MS is the most widely used technology in TKI detection. With the development of HRMS instrumentation, it has become possible to discover unknown chemicals without prior hypotheses or authentic standards, thanks to its combination of high mass spectral RP and high mass accuracy. In order to meet the requirements of TDM testing at the patient's bedside or in medical practices, nonconventional biosensing methodologies such as electrochemical methods (i.e., voltammetry, potentiometry, and electro-Fenton process) with various electrodes (i.e., GCE, CPE, pencil graphite lead, SPCE, PE, ITO, and Pd@Pt electrode), SPR, SERS, as well as RLS have been promising alternatives to large and expensive equipment. Moreover, the emergence of novel NPs (i.e., CdSe-QDs, N-GQDs, N-CDs, Au-NPs/rGO/ds-DNA, HKUST-1/GONRs, Fe3O4-SWCNTs/mim-BF4-, etc.) offers the potential for facile, eco-friendly, specific, and sensitive determination of TKIs. Furthermore, CE along with SFC has been developed as a complementary analytical technique for TKIs due to their remarkably low injection volumes, relatively short analysis time, and moderate conditions. From a perspective point of view, equipment features such as high throughput for accurate analysis, rapid detection through miniaturization and reduced solvent consumption along with automation combined with high sensitivity hold great promise in the development trends of determination technology.
This review provides an overview of the current knowledge on pharmacokinetic aspects of available TKIs, including in vitro experiments, animal studies, and drug-drug interaction studies. However, translating information from mass-balanced studies using single-dose targeted TKI in healthy volunteers to clinical oncology practice is challenging. In daily administration for clinical oncology practice, self-inhibition mechanisms significantly alter pharmacokinetic outcomes and correlations of claimed drug interactions, making their disposal and metabolism under homeostasis pharmacokinetics complex and unpredictable. Therefore, TDM is a novel strategy that provides a way to optimize drug dosing for enhanced and expedited clinical responses. It can provide recommended target values for c0 and cmax of various targeted TKIs, and personalized drug administration schedules tailored to patients with distinct genotypes, thereby offering effective recommendations for subsequent clinical drug treatments. We strongly advocate adjusting the dosage of TKIs based on the desired concentration levels in order to maximize efficacy while minimizing the occurrence of adverse events, especially during prolonged TKI administration.
Despite the significant progress achieved, several challenges persist in the development and clinical application of TKIs. 1) Despite the utilization of various sampling methods, invasive methods such as intravenous blood collection still dominate as the primary means of sample acquisition, posing inconvenience to cancer patients requiring multiple samples over an extended period and with compromised immune systems. 2) Drug resistance poses the initial significant challenge in conducting clinical trials. Following clinical use, nearly all targeted anticancer drugs eventually develop resistance, which involves a multitude of complex mechanisms. 3) Targeted cancer drugs exhibit effectiveness only among a limited number of patients, thus low efficiency becomes another major obstacle for these drugs. 4) Given that clinicians cannot possibly be aware of all potentially clinically significant drug interactions, this underscores the necessity for management systems and practical identification. 5) The TKI therapeutic approach presents new challenges in achieving a balance between TKI activity and managing drug side effects to optimize patient quality of life; therefore, educating patients and physicians on recognizing early toxicities associated with drugs is crucial.
Given the potential of biomarkers to guide treatment decisions, optimize combinations, and minimize toxicity, it is imperative for future trials to investigate the optimal patient selection criteria for various TKIs. Additionally, understanding resistance mechanisms and developing strategies to assess and overcome resistance to newly developed TKIs are crucial areas of research. Moreover, drawing insights from combination therapy with other small molecule anticancer drugs during clinical trials, adopting a joint sequential therapy strategy is essential in identifying effective combination therapy pathways and advancing the next generation of TKI drugs. Lastly, considering the complexity and diversity of clinical samples as well as real-time clinical detection requirements, there is a need for miniaturized portable devices that offer high sensitivity and specificity in selective identification along with efficient pre-processing technologies and detection strategies enabling rapid and accurate high-throughput detection.
CRediT author statement
Lan Chen: Writing - Original draft preparation; Yuan Zhang: Conceptualization, Writing - Reviewing and Editing; Yi-Xin Zhang, Wei-Lai Wang, De-Mei Sun: Writing - Reviewing and Editing; Peng-Yun Li: Visualization, Supervision; Xue-Song Feng: Conceptualization, Validation; Yue Tan: Supervision, Conceptualization.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of Liaoning Province, China (Grant No.: 2023-MS-172).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.11.006.
Contributor Information
Xue-Song Feng, Email: xsfeng@cmu.edu.cn.
Yue Tan, Email: tanyue2006@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Cardoso E., Guidi M., Blanchet B., et al. Therapeutic drug monitoring of targeted anticancer protein kinase inhibitors in routine clinical use: A critical review. Ther. Drug Monit. 2020;42:33–44. doi: 10.1097/FTD.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 2.Verougstraete N., Stove V., Verstraete A.G., et al. Quantification of eight hematological tyrosine kinase inhibitors in both plasma and whole blood by a validated LC-MS/MS method. Talanta. 2021;226 doi: 10.1016/j.talanta.2021.122140. [DOI] [PubMed] [Google Scholar]
- 3.Huynh H.H., Pressiat C., Sauvageon H., et al. Development and validation of a simultaneous quantification method of 14 tyrosine kinase inhibitors in human plasma using LC-MS/MS. Ther. Drug Monit. 2017;39:43–54. doi: 10.1097/FTD.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 4.Farag S., Verheijen R.B., Martijn Kerst J., et al. Imatinib pharmacokinetics in a large observational cohort of gastrointestinal stromal tumour patients. Clin. Pharmacokinet. 2017;56:287–292. doi: 10.1007/s40262-016-0439-7. [DOI] [PubMed] [Google Scholar]
- 5.Xu R., Lin Q., Qiu X., et al. UPLC-MS/MS method for the simultaneous determination of imatinib, voriconazole and their metabolites concentrations in rat plasma. J. Pharm. Biomed. Anal. 2019;166:6–12. doi: 10.1016/j.jpba.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Xia Y., Chen S., Luo M., et al. Correlations between imatinib plasma trough concentration and adverse reactions in Chinese patients with gastrointestinal stromal tumors. Cancer. 2020;126:2054–2061. doi: 10.1002/cncr.32751. [DOI] [PubMed] [Google Scholar]
- 7.Yang J.C., Reguart N., Barinoff J., et al. Diarrhea associated with afatinib: An oral ErbB family blocker. Expert Rev. Anticancer Ther. 2013;13:729–736. doi: 10.1586/era.13.31. [DOI] [PubMed] [Google Scholar]
- 8.Lu X., Liu S., Yang X., et al. Determination of tyrosine kinase inhibitor afatinib in rat plasma using LC-MS/MS and its application to in vivo pharmacokinetic studies of afatinib liposomes. J. Pharm. Biomed. Anal. 2019;164:181–186. doi: 10.1016/j.jpba.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 9.Suresha D.N., Pramila T., Tamizh Mani T. Method development and validation of Imatinib Mesylate-Review. Int. J. Pharm. Pharm. Anal. 2016;1:1–11. [Google Scholar]
- 10.Li N., Zhang T., Chen G., et al. Recent advances in sample preparation techniques for quantitative detection of pharmaceuticals in biological samples. Trends Analyt. Chem. 2021;142 [Google Scholar]
- 11.Li H., Zhang D., Cheng X., et al. A validated 2D-LC-UV method for simultaneous determination of imatinib and N-desmethylimatinib in plasma and its clinical application for therapeutic drug monitoring with GIST patients. Curr. Pharm. Anal. 2022;18:122–131. [Google Scholar]
- 12.Zhang M., Liu X., Chen Z., et al. Method development and validation for simultaneous determination of six tyrosine kinase inhibitors and two active metabolites in human plasma/serum using UPLC-MS/MS for therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2022;211 doi: 10.1016/j.jpba.2021.114562. [DOI] [PubMed] [Google Scholar]
- 13.Adlnasab L., Ezoddin M., Shojaei R.A., et al. Ultrasonic-assisted dispersive micro solid-phase extraction based on melamine-phytate supermolecular aggregate as a novel bio-inspired magnetic sorbent for preconcentration of anticancer drugs in biological samples prior to HPLC-UV analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018;1095:226–234. doi: 10.1016/j.jchromb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Khodayari P., Jalilian N., Ebrahimzadeh H., et al. Trace-level monitoring of anti-cancer drug residues in wastewater and biological samples by thin-film solid-phase micro-extraction using electrospun polyfam/Co-MOF-74 composite nanofibers prior to liquid chromatography analysis. J. Chromatogr. A. 2021;1655 doi: 10.1016/j.chroma.2021.462484. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi Kakavandi N., Asadi T., Jannat B., et al. Method development for determination of imatinib and its major metabolite, N-desmethyl imatinib, in biological and environmental samples by SA-SHS-LPME and HPLC. Biomed. Chromatogr. 2021;35 doi: 10.1002/bmc.5088. [DOI] [PubMed] [Google Scholar]
- 16.Kazemi A., Ahmad Panahi H., Safaeijavan R. Thermosensitive molecularly imprinted poly(1-vinyl-2-pyrrolidone/methyl methacrylate/N-vinylcaprolactam) for selective extraction of imatinib mesylate in human biological fluid. J. Sep. Sci. 2020;43:614–621. doi: 10.1002/jssc.201900535. [DOI] [PubMed] [Google Scholar]
- 17.Hooshmand S., Es’haghi Z. Hydrophilic modified magnetic multi-walled carbon nanotube for dispersive solid/liquid phase microextraction of sunitinib in human samples. Anal. Biochem. 2018;542:76–83. doi: 10.1016/j.ab.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 18.da Silva A.T.M., Bessa C.D.P.B., Borges W. de S., et al. Bioanalytical methods for determining ecstasy components in biological matrices: A review. Trends Analyt. Chem. 2018;108:323–346. [Google Scholar]
- 19.Boons C.C.L.M., Timmers L., Janssen J.J.W.M., et al. Feasibility of and patients’ perspective on nilotinib dried blood spot self-sampling. Eur. J. Clin. Pharmacol. 2019;75:825–829. doi: 10.1007/s00228-019-02640-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Jung S.Y., Choi M.Y., et al. Development of a dried blood spot sampling method towards therapeutic monitoring of radotinib in the treatment of chronic myeloid leukaemia. J. Clin. Pharm. Ther. 2020;45:1006–1013. doi: 10.1111/jcpt.13124. [DOI] [PubMed] [Google Scholar]
- 21.Tuzimski T., Petruczynik A. Review of chromatographic methods coupled with modern detection techniques applied in the therapeutic drugs monitoring (TDM) Molecules. 2020;25 doi: 10.3390/molecules25174026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukai Y., Yoshida T., Kondo T., et al. Development and validation of a simple method for simultaneously measuring the concentrations of BCR-ABL and bruton tyrosine kinase inhibitors in dried blood spot (DBS): A pilot study to obtain candidate conversion equations for predicting plasma concentration based on DBS concentration. Ther. Drug Monit. 2022;44:762–770. doi: 10.1097/FTD.0000000000000997. [DOI] [PubMed] [Google Scholar]
- 23.Longuespée R., Theile D., Fresnais M., et al. Approaching sites of action of drugs in clinical pharmacology: New analytical options and their challenges. Br. J. Clin. Pharmacol. 2021;87:858–874. doi: 10.1111/bcp.14543. [DOI] [PubMed] [Google Scholar]
- 24.Portnow J., Badie B., Markel S., et al. A neuropharmacokinetic assessment of bafetinib, a second generation dual BCR-Abl/Lyn tyrosine kinase inhibitor, in patients with recurrent high-grade gliomas. Eur. J. Cancer. 2013;49:1634–1640. doi: 10.1016/j.ejca.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamminga M., de Wit S., Schuuring E., et al. Circulating tumor cells in lung cancer are prognostic and predictive for worse tumor response in both targeted-and chemotherapy, Transl. Lung Cancer Res. 2019;8:854–861. doi: 10.21037/tlcr.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye Z., Wu L., Zhang X., et al. Quantification of sorafenib, lenvatinib, and apatinib in human plasma for therapeutic drug monitoring by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2021;202 doi: 10.1016/j.jpba.2021.114161. [DOI] [PubMed] [Google Scholar]
- 27.Allard M., Khoudour N., Rousseau B., et al. Simultaneous analysis of regorafenib and sorafenib and three of their metabolites in human plasma using LC-MS/MS. J. Pharm. Biomed. Anal. 2017;142:42–48. doi: 10.1016/j.jpba.2017.04.053. [DOI] [PubMed] [Google Scholar]
- 28.Chokshi A., Gajjar A., Bhanushali P., et al. Quantification of antileukemic drug Dasatinib in human plasma: Application of a sensitive liquid chromatographic method. J. Chem. Metrol. 2021;15:152–162. [Google Scholar]
- 29.Kleigrewe K., Söhnel A.C., Humpf H.U. A new high-performance liquid chromatography-tandem mass spectrometry method based on dispersive solid phase extraction for the determination of the mycotoxin fusarin C in corn ears and processed corn samples. J. Agric. Food Chem. 2011;59:10470–10476. doi: 10.1021/jf2026814. [DOI] [PubMed] [Google Scholar]
- 30.Sakhi M., Khan A., Khan I., et al. A new sensitive HPLC/UV method for simultaneous determination of paclitaxel, sorafenib and omeprazole in standard solutions and spiked plasma: Application to in-vitro and in-vivo evaluation of paclitaxel polymeric nanoformulations. Trop. J. Pharm. Res. 2021;20:1949–1959. [Google Scholar]
- 31.Wang Z., Lian L., Dong Y., et al. Determination of anlotinib, a tyrosine kinase inhibitor, in rat plasma by UHPLC-MS/MS and its application to a pharmacokinetic study. J. Anal. Methods Chem. 2019;2019 doi: 10.1155/2019/5016757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaragal R.R., Kumar D., Mutnuri S. Development of UPLC-MS/MS method for analyzing phorate: Application to wastewater treatment. J. Iran. Chem. Soc. 2020;17:2923–2931. [Google Scholar]
- 33.Zhuang W., Qiu H.-B., Chen X.-M., et al. Simultaneous quantification of imatinib and its main metabolite N-demethyl-imatinib in human plasma by liquid chromatography-tandem mass spectrometry and its application to therapeutic drug monitoring in patients with gastrointestinal stromal tumor. Biomed. Chromatogr. 2017;31 doi: 10.1002/bmc.4022. [DOI] [PubMed] [Google Scholar]
- 34.Zeng J., Cai H.L., Jiang Z.P., et al. A validated UPLC-MS/MS method for simultaneous determination of imatinib, dasatinib and nilotinib in human plasma. J. Pharm. Anal. 2017;7:374–380. doi: 10.1016/j.jpha.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurjar M., Mehta P., Sharma J., et al. An HPLC method for simultaneous quantification of sunitinib and its active metabolite, SU12662, using hydrophilic interaction chromatography principle. Bioanalysis. 2020;12:75–85. doi: 10.4155/bio-2019-0188. [DOI] [PubMed] [Google Scholar]
- 36.Zhao T., Wang L., Chen D.D.Y. Quantification of imatinib and related compounds using capillary electrophoresis-tandem mass spectrometry with field-amplified sample stacking. Electrophoresis. 2020;41:1843–1850. doi: 10.1002/elps.202000118. [DOI] [PubMed] [Google Scholar]
- 37.Ezzeldin E., Iqbal M., Herqash R.N., et al. Simultaneous quantitative determination of seven novel tyrosine kinase inhibitors in plasma by a validated UPLC-MS/MS method and its application to human microsomal metabolic stability study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020;1136 doi: 10.1016/j.jchromb.2019.121851. [DOI] [PubMed] [Google Scholar]
- 38.Jolibois J., Schmitt A., Royer B. A simple and fast LC-MS/MS method for the routine measurement of cabozantinib, olaparib, palbociclib, pazopanib, sorafenib, sunitinib and its main active metabolite in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019;1132 doi: 10.1016/j.jchromb.2019.121844. [DOI] [PubMed] [Google Scholar]
- 39.Ni M., Zhou J., Li H., et al. Simultaneous determination of six tyrosine kinase inhibitors in human plasma using HPLC-Q-Orbitrap mass spectrometry. Bioanalysis. 2017;9:925–935. doi: 10.4155/bio-2017-0031. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z., Zhang C., Yu X., et al. Microwave-assisted solid-phase synthesis of nitrogen-doping carbon dot with good solvent compatibility and its sensing of sunitinib. Anal. Bioanal. Chem. 2021;413:6435–6447. doi: 10.1007/s00216-021-03609-9. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L., Wang S., Chen M., et al. Simultaneous and rapid determination of 12 tyrosine kinase inhibitors by LC-MS/MS in human plasma: Application to therapeutic drug monitoring in patients with non-small cell lung cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021;1175 doi: 10.1016/j.jchromb.2021.122752. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed O.S., Ladner Y., Xia J., et al. A fully automated on-line salting-out assisted liquid-liquid extraction capillary electrophoresis methodology: Application to tyrosine kinase inhibitors in human plasma. Talanta. 2020;208 doi: 10.1016/j.talanta.2019.120391. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed O.S., Ladner Y., Montels J., et al. Coupling of salting-out assisted liquid-liquid extraction with on-line stacking for the analysis of tyrosine kinase inhibitors in human plasma by capillary zone electrophoresis. J. Chromatogr. A. 2018;1579:121–128. doi: 10.1016/j.chroma.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Moghaddam A.Z., Bameri A.E., Ganjali M.R., et al. A low-voltage electro-membrane extraction for quantification of imatinib and sunitinib in biological fluids. Bioanalysis. 2021;13:1401–1413. doi: 10.4155/bio-2021-0138. [DOI] [PubMed] [Google Scholar]
- 45.Liu H., Zhang M., Wang X., et al. Extraction and determination of polybrominated diphenyl ethers in water and urine samples using solidified floating organic drop microextraction along with high performance liquid chromatography. Microchim. Acta. 2012;176:303–309. [Google Scholar]
- 46.Cruz-Vera M., Lucena R., Cárdenas S., et al. One-step in-syringe ionic liquid-based dispersive liquid-liquid microextraction. J. Chromatogr. A. 2009;1216:6459–6465. doi: 10.1016/j.chroma.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 47.Ghazaghi M., Mousavi H.Z., Shirkhanloo H., et al. Stirring-controlled solidified floating solid-liquid drop microextraction as a new solid phase-enhanced liquid-phase microextraction method by exploiting magnetic carbon nanotube-nickel hybrid. Anal. Chim. Acta. 2017;951:78–88. doi: 10.1016/j.aca.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Merienne C., Rousset M., Ducint D., et al. High throughput routine determination of 17 tyrosine kinase inhibitors by LC-MS/MS. J. Pharm. Biomed. Anal. 2018;150:112–120. doi: 10.1016/j.jpba.2017.11.060. [DOI] [PubMed] [Google Scholar]
- 49.Alzoman N.Z., Maher H.M., Shehata S.M., et al. UPLC-MS/MS study of the effect of dandelion root extract on the plasma levels of the selected irreversible tyrosine kinase inhibitors dasatinib, imatinib and nilotinib in rats: Potential risk of pharmacokinetic interactions. Biomed. Chromatogr. 2019;33 doi: 10.1002/bmc.4674. [DOI] [PubMed] [Google Scholar]
- 50.Wojnicz A., Colom-Fernández B., Steegmann J.L., et al. Simultaneous determination of imatinib, dasatinib, and nilotinib by liquid chromatography-tandem mass spectrometry and its application to therapeutic drug monitoring. Ther. Drug Monit. 2017;39:252–262. doi: 10.1097/FTD.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 51.Vrobel I., Janečková H., Faber E., et al. Ultrafast online SPE-MS/MS method for quantification of 3 tyrosine kinase inhibitors in human plasma. Ther. Drug Monit. 2016;38:516–524. doi: 10.1097/FTD.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 52.Maher H.M., Alzoman N.Z., Shehata S.M., et al. Comparative pharmacokinetic profiles of selected irreversible tyrosine kinase inhibitors, neratinib and pelitinib, with apigenin in rat plasma by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2017;137:258–267. doi: 10.1016/j.jpba.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 53.Li J., Zhao L., Wei C., et al. Preparation of restricted access media molecularly imprinted polymers for efficient separation and enrichment ofloxacin in bovine serum samples. J. Sep. Sci. 2019;42:2491–2499. doi: 10.1002/jssc.201900103. [DOI] [PubMed] [Google Scholar]
- 54.Khezeli T., Daneshfar A. Development of dispersive micro-solid phase extraction based on micro and nano sorbents. Trends Analyt. Chem. 2017;89:99–118. [Google Scholar]
- 55.Koller D., Vaitsekhovich V., Mba C., et al. Effective quantification of 11 tyrosine kinase inhibitors and caffeine in human plasma by validated LC-MS/MS method with potent phospholipids clean-up procedure. Application to therapeutic drug monitoring. Talanta. 2020;208 doi: 10.1016/j.talanta.2019.120450. [DOI] [PubMed] [Google Scholar]
- 56.Li N., Zhu Q., Yang Y., et al. A novel dispersive solid-phase extraction method using metal-organic framework MIL-101 as the adsorbent for the analysis of benzophenones in toner. Talanta. 2015;132:713–718. doi: 10.1016/j.talanta.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 57.Qi C., Cai Q., Zhao P., et al. The metal-organic framework MIL-101(Cr) as efficient adsorbent in a vortex-assisted dispersive solid-phase extraction of imatinib mesylate in rat plasma coupled with ultra-performance liquid chromatography/mass spectrometry: Application to a pharmacokinetic study. J. Chromatogr. A. 2016;1449:30–38. doi: 10.1016/j.chroma.2016.04.055. [DOI] [PubMed] [Google Scholar]
- 58.Chen L., Zhang M., Fu F., et al. Facile synthesis of magnetic covalent organic framework nanobeads and application to magnetic solid-phase extraction of trace estrogens from human urine. J. Chromatogr. A. 2018;1567:136–146. doi: 10.1016/j.chroma.2018.06.066. [DOI] [PubMed] [Google Scholar]
- 59.Chen L., He Y., Lei Z., et al. Preparation of core-shell structured magnetic covalent organic framework nanocomposites for magnetic solid-phase extraction of bisphenols from human serum sample. Talanta. 2018;181:296–304. doi: 10.1016/j.talanta.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 60.Jiang H., Li N., Cui L., et al. Recent application of magnetic solid phase extraction for food safety analysis. Trends Analyt. Chem. 2019;120 [Google Scholar]
- 61.Li G., Zhao M., Zhao L. Development and validation of an UPLC-MS/MS method for simultaneous determination of fifteen targeted anti-cancer drugs in human plasma and its application in therapeutic drug monitoring. J. Pharm. Biomed. Anal. 2022;212 doi: 10.1016/j.jpba.2021.114517. [DOI] [PubMed] [Google Scholar]
- 62.Arvand M., Masouleh A.N. Magnetic solid-phase extraction of imatinib and doxorubicin as cytostatic drugs by Fe3O4/graphene oxide nanocomposite. J. Iran. Chem. Soc. 2017;14:1673–1682. [Google Scholar]
- 63.Sahebi H., Pourmortazavi S.M., Zandavar H., et al. Chitosan grafted onto Fe3O4@poly(N-vinylcaprolactam) as a new sorbent for detecting Imatinib mesylate in biosamples using UPLC-MS/MS. Analyst. 2019;144:7336–7350. doi: 10.1039/c9an01654f. [DOI] [PubMed] [Google Scholar]
- 64.Sahebi H., Konoz E., Ezabadi A., et al. Sensitive determination of imatinib mesylate in human plasma using DABCO-based ionic liquid-modified magnetic nanoparticles. Chromatographia. 2020;83:1009–1019. [Google Scholar]
- 65.Pirdadeh-Beiranvand M., Afkhami A., Madrakian T. Magnetic molecularly imprinted electrospun nanofibers for selective extraction of nilotinib from human serum. Anal. Bioanal. Chem. 2020;412:1629–1637. doi: 10.1007/s00216-020-02393-2. [DOI] [PubMed] [Google Scholar]
- 66.Liu D., Peng J., Chen L., et al. Solid phase extraction-based magnetic carbon nitride/metal organic framework composite with high performance liquid chromatography for the determination of tyrosine kinase inhibitors in urine samples. Anal. Methods. 2020;12:4798–4805. doi: 10.1039/d0ay01243b. [DOI] [PubMed] [Google Scholar]
- 67.Souza Silva E.A., Risticevic S., Pawliszyn J. Recent trends in SPME concerning sorbent materials, configurations and in vivo applications. Trends Analyt. Chem. 2013;43:24–36. [Google Scholar]
- 68.Souza-Silva É.A., Reyes-Garcés N., Gómez-Ríos G.A., et al. A critical review of the state of the art of solid-phase microextraction of complex matrices III. Bioanalytical and clinical applications. Trends Analyt. Chem. 2015;71:249–264. [Google Scholar]
- 69.Bojko B., Cudjoe E., Gómez-Ríos G.A., et al. SPME: Quo vadis? Anal. Chim. Acta. 2012;750:132–151. doi: 10.1016/j.aca.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 70.Gómez-Ríos G.A., Reyes-Garcés N., Bojko B., et al. Biocompatible solid-phase microextraction nanoelectrospray ionization: An unexploited tool in bioanalysis. Anal. Chem. 2016;88:1259–1265. doi: 10.1021/acs.analchem.5b03668. [DOI] [PubMed] [Google Scholar]
- 71.Miodek A., Mejri-Omrani N., Khoder R., et al. Electrochemical functionalization of polypyrrole through amine oxidation of poly(amidoamine) dendrimers: Application to DNA biosensor. Talanta. 2016;154:446–454. doi: 10.1016/j.talanta.2016.03.076. [DOI] [PubMed] [Google Scholar]
- 72.Zhou M., Wang Y., Zhai Y., et al. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chemistry. 2009;15:6116–6120. doi: 10.1002/chem.200900596. [DOI] [PubMed] [Google Scholar]
- 73.Hatamluyi B., Es’haghi Z. A layer-by-layer sensing architecture based on dendrimer and ionic liquid supported reduced graphene oxide for simultaneous hollow-fiber solid phase microextraction and electrochemical determination of anti-cancer drug imatinib in biological samples. J. Electroanal. Chem. 2017;801:439–449. [Google Scholar]
- 74.Jerath A., Yang Q.J., Pang K.S., et al. Tranexamic acid dosing for cardiac surgical patients with chronic renal dysfunction: A new dosing regimen. Anesth. Analg. 2018;127:1323–1332. doi: 10.1213/ANE.0000000000002724. [DOI] [PubMed] [Google Scholar]
- 75.Cui Y., Liu S., Wei K., et al. Magnetic solid-phase extraction of trace-level mercury(II) ions using magnetic core-shell nanoparticles modified with thiourea-derived chelating agents. Microchim. Acta. 2015;182:1337–1344. [Google Scholar]
- 76.Ghazaghi M., Mousavi H.Z., Shirkhanloo H., et al. Ultrasound assisted dispersive micro solid-phase extraction of four tyrosine kinase inhibitors from serum and cerebrospinal fluid by using magnetic nanoparticles coated with nickel-doped silica as an adsorbent. Microchim. Acta. 2016;183:2779–2789. [Google Scholar]
- 77.Xiong W., Kang J.H., Jung Y. Preparation of nitrogen-doped porous carbon from melamine-formaldehyde resins crosslinked by phytic acid. Int. J. Electrochem. Sci. 2018;13:852–862. [Google Scholar]
- 78.Ghorbani M., Aghamohammadhassan M., Chamsaz M., et al. Dispersive solid phase microextraction. Trends Analyt. Chem. 2019;118:793–809. [Google Scholar]
- 79.Jo S., Jeong H., Bae S.R., et al. Modified platinum electrode with phytic acid and single-walled carbon nanotube: Application to the selective determination of dopamine in the presence of ascorbic and uric acids. Microchem. J. 2008;88:1–6. [Google Scholar]
- 80.Liu Y., Liu H., Xia Z., et al. Simultaneous and rapid determination of six tyrosine kinase inhibitors in patients with non-small cell lung cancer using HPLC-MS/MS. Int. J. Anal. Chem. 2021;2021 doi: 10.1155/2021/5524361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Q., Li Z., Xu K., et al. Intracellular concentration and transporters in imatinib resistance of gastrointestinal stromal tumor. Scand. J. Gastroenterol. 2019;54:220–226. doi: 10.1080/00365521.2019.1577488. [DOI] [PubMed] [Google Scholar]
- 82.Hirasawa T., Kikuchi M., Shigeta K., et al. High-throughput liquid chromatography/electrospray ionization-tandem mass spectrometry method using in-source collision-induced dissociation for simultaneous quantification of imatinib, dasatinib, bosutinib, nilotinib, and ibrutinib in human plasma. Biomed. Chromatogr. 2021;35 doi: 10.1002/bmc.5124. [DOI] [PubMed] [Google Scholar]
- 83.Maher H.M., Alzoman N.Z., Shehata S.M. Ultra-performance LC-MS/MS study of the pharmacokinetic interaction of imatinib with selected vitamin preparations in rats. Bioanalysis. 2018;10:1099–1113. doi: 10.4155/bio-2018-0043. [DOI] [PubMed] [Google Scholar]
- 84.Wen J., Bao S., Cai Y., et al. A reliable and stable method for determination of brigatinib in rat plasma by UPLC-MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;1068–1069:84–89. doi: 10.1016/j.jchromb.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Son H., Kang W. Quantitative determination of bilobetin in rat plasma by HPLC-MS/MS and its application to a pharmacokinetic study. Biomed. Chromatogr. 2020;34 doi: 10.1002/bmc.4784. [DOI] [PubMed] [Google Scholar]
- 86.Pasquini B., Orlandini S., Furlanetto S., et al. Quality by Design as a risk-based strategy in pharmaceutical analysis: Development of a liquid chromatography-tandem mass spectrometry method for the determination of nintedanib and its impurities. J. Chromatogr. A. 2020;1611 doi: 10.1016/j.chroma.2019.460615. [DOI] [PubMed] [Google Scholar]
- 87.Rudzki P.J., Gniazdowska E., Buś-Kwaśnik K. Quantitative evaluation of the matrix effect in bioanalytical methods based on LC-MS: A comparison of two approaches. J. Pharm. Biomed. Anal. 2018;155:314–319. doi: 10.1016/j.jpba.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 88.Veerman G.D.M., de Bruijn P., Dingemans A.C., et al. To quantify the small-molecule kinase inhibitors ceritinib, dacomitinib, lorlatinib, and nintedanib in human plasma by liquid chromatography/triple-quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2021;193 doi: 10.1016/j.jpba.2020.113733. [DOI] [PubMed] [Google Scholar]
- 89.Iacuzzi V., Zanchetta M., Gagno S., et al. A LC-MS/MS method for therapeutic drug monitoring of sorafenib, regorafenib and their active metabolites in patients with hepatocellular carcinoma. J. Pharm. Biomed. Anal. 2020;187 doi: 10.1016/j.jpba.2020.113358. [DOI] [PubMed] [Google Scholar]
- 90.Maher H.M., Alzoman N.Z., Shehata S.M., et al. Validated UPLC-MS/MS method for the quantification of dasatinib in plasma: Application to pharmacokinetic interaction studies with nutraceuticals in Wistar rats. PLoS One. 2018;13 doi: 10.1371/journal.pone.0199208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dutta D., Das S., Seijas J.A., et al. Validated stability-indicating HPTLC method for nintedanib & characterization of degradants by LC-MSn. The 23rd International Electronic Conference on Synthetic Organic Chemistry. November 15–December 15, 2019 doi: 10.3390/ecsoc-23-06502. [DOI] [Google Scholar]
- 92.He Y., Zhou L., Gao S., et al. Development and validation of a sensitive LC-MS/MS method for simultaneous determination of eight tyrosine kinase inhibitors and its application in mice pharmacokinetic studies. J. Pharm. Biomed. Anal. 2018;148:65–72. doi: 10.1016/j.jpba.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukai Y., Yoshida T., Kondo T., et al. Novel high-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification of BCR-ABL and Bruton’s tyrosine kinase inhibitors and their three active metabolites in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020;1137 doi: 10.1016/j.jchromb.2019.121928. [DOI] [PubMed] [Google Scholar]
- 94.Abdelhameed A.S., Attwa M.W., Al-Shaklia N.S., et al. A highly sensitive LC-MS/MS method to determine novel Bruton’s tyrosine kinase inhibitor spebrutinib: Application to metabolic stability evaluation. R. Soc. Open Sci. 2019;6 doi: 10.1098/rsos.190434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnsirani P., Wani A.A., Bharatam P.V., et al. LC-ESI-QTOF-MS analysis utilizing gas-phase fragmentation reactions subjected to ESI-IS-CID and ESI-CID-MS/MS conditions to study the degradation behaviour of sorafenib tosylate: NMR and in vitro cytotoxicity and apoptosis detection studies of hydrolytic degradation products. J. Pharm. Biomed. Anal. 2020;177 doi: 10.1016/j.jpba.2019.112881. [DOI] [PubMed] [Google Scholar]
- 96.Roosendaal J., Groenland S.L., Rosing H., et al. Determination of the absolute bioavailability of oral imatinib using a stable isotopically labeled intravenous imatinib-d8 microdose. Eur. J. Clin. Pharmacol. 2020;76:1075–1082. doi: 10.1007/s00228-020-02888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pirro E., De Francia S., De Martino F., et al. A new HPLC-UV validated method for therapeutic drug monitoring of tyrosine kinase inhibitors in leukemic patients. J. Chromatogr. Sci. 2011;49:753–757. doi: 10.1093/chrsci/49.10.753. [DOI] [PubMed] [Google Scholar]
- 98.Kocan G.P., Huang M., Li F., et al. A sensitive LC-MS-MS assay for the determination of lapatinib in human plasma in subjects with end-stage renal disease receiving hemodialysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018;1097–1098:74–82. doi: 10.1016/j.jchromb.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Hasin F., Islam M.T., Ahmad M.F., et al. Validation of assay method for the estimation of imatinib mesylate in tablet dosage form by HPLC. Eur. J. Biomed. Pharm. Sci. 2017;4:74–81. [Google Scholar]
- 100.Coban O., Degim Z. Development and validation of highly selective method for the determination of imatinib mesylate and dexketoprofen trometamol combination in three different media. Braz. J. Pharm. Sci. 2020;56 [Google Scholar]
- 101.Shah P., Shah N., Shah R. Method development and validation of a stability indicating RP-HPLC method for assay determination of imatinib in imatinib mesylate tablets dosage form. Int. J. Curr. Pharm. Rev. Res. 2015;6:4453–4468. [Google Scholar]
- 102.Alhazmi H.A., Moraya D.A., Alahdal E., et al. Ultrafast monolithic HPLC method for simultaneous quantification of the anticancer agents, imatinib and sorafenib: Application to tablet dosage forms. Trop. J. Pharm Res. 2018;17:1127–1134. [Google Scholar]
- 103.Alaejos Á.C., Cabrera S.J., Rodríguez B.C., et al. Validation and comparison of two analytical methods for imatinib therapeutic drug monitoring. Chromatographia. 2021;84:589–596. [Google Scholar]
- 104.Kuna A.K., Seru G., Radha G.V. Analytical method development and validation for the estimation of Imatinib mesylate and its dimer impurity in pharmaceutical formulation by reverse-phase high-performance liquid chromatography. Asian J. Pharm. Clin. Res. 2018;11:136–139. [Google Scholar]
- 105.Zhou Y., Lai J., Qiu F., et al. Development and validation of an HPLC-UV method for routine trough plasma concentration monitoring of imatinib in Chinese patients with gastrointestinal stromal tumor. J. Chin. Pharm. Sci. 2020;29:637–648. [Google Scholar]
- 106.Lakka N.S., Kuppan C., Srinivas K.S., et al. Separation and characterization of new forced degradation products of dasatinib in tablet dosage formulation using LC-MS and stability-indicating HPLC methods. Chromatographia. 2020;83:947–962. [Google Scholar]
- 107.Mohan B., Ravi Kumar D., Sharma R.S.K., et al. Validated RP-HPLC method for estimation of related impurities in dasatinib. Eurasian J. Analyt. Chem. 2020;15:51–58. [Google Scholar]
- 108.Patel D.M., Patel D., Patel A., et al. Method development and validation for simultaneous estimation of benidipine hydrochloride and metoprolol succinate in tablet. J. Drug Delivery Ther. 2019;9:28–33. [Google Scholar]
- 109.Sankar P.R., Anusha S. Development and validation of RP-HPLC method for the determination of dasatinib in the tablet dosage form. Int. J. Pharm. Sci. Res. 2019;10:4531–4537. [Google Scholar]
- 110.Sojitra C., Tehare A., Dholakia C., et al. Development of chromatographic method for determination of impurities in solid dispersion of dasatinib. Braz. J. Anal. Chem. 2018;5:19–29. [Google Scholar]
- 111.Ravi Sankar P., Viswanath A., Eswarudu M.M., et al. Development and validation of RP-HPLC method for the determination of sorafenib in pharmaceutical dosage form. Int. J. Pharm. Sci. Rev. Res. 2021;69:15–23. [Google Scholar]
- 112.Shukla S.K., Kadry H., Bhatt J.A., et al. Statistical optimization and validation of a novel ultra-performance liquid chromatography method for estimation of nintedanib in rat and human plasma. Bioanalysis. 2020;12:159–174. doi: 10.4155/bio-2019-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kumar R., Munipalli V.K., Singh R.M., et al. Validated RP-HPLC method for determination and quantification of nintedanib in pharmaceutical formulation. J. Adv. Pharmacol. 2020;1:38–47. [Google Scholar]
- 114.Lalitha R., Gandla K., Shruthi P.V.T., et al. Method development and validation of sunitinib in bulk and pharmaceutical dosage form by RP-HPLC method. Int. J. Pharmacogn. Chem. 2020;1:70–73. [Google Scholar]
- 115.Jayagopal B., Murugesh S. QbD-mediated RP-UPLC method development invoking an FMEA-based risk assessment to estimate nintedanib degradation products and their pathways. Arab. J. Chem. 2020;13:7087–7103. [Google Scholar]
- 116.Sharma T., Khurana R.K., Jain A., et al. Development of a validated liquid chromatographic method for quantification of sorafenib tosylate in the presence of stress-induced degradation products and in biological matrix employing analytical quality by design approach. Biomed. Chromatogr. 2018;32 doi: 10.1002/bmc.4169. [DOI] [PubMed] [Google Scholar]
- 117.Waghmare S.A., Sumithra M. QbD based development and validation of RP-HPLC method for nintedanib esylate: Application to bioanalytical and stability study in plasma. Anal. Chem. Lett. 2021;11:392–408. [Google Scholar]
- 118.Wu Z., Liu J., Liang M., et al. Detection of imatinib based on electrochemical sensor constructed using biosynthesized graphene-silver nanocomposite. Front. Chem. 2021;9 doi: 10.3389/fchem.2021.670074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tahernejad-Javazmi F., Shabani-Nooshabadi M., Karimi-Maleh H. Gold nanoparticles and reduced graphene oxide-amplified label-free DNA biosensor for dasatinib detection. New J. Chem. 2018;42:16378–16383. [Google Scholar]
- 120.Vercelli B., Crotti S., Agostini M. Voltammetric responses at modified electrodes and aggregation effects of two anticancer molecules: Irinotecan and sunitinib. New J. Chem. 2020;44:18233–18241. [Google Scholar]
- 121.Chen H., Luo K., Li K. A facile electrochemical sensor based on NiO-ZnO/MWCNT-COOH modified GCE for simultaneous quantification of imatinib and itraconazole. J. Electrochem. Soc. 2019;166:B697–B707. [Google Scholar]
- 122.Hassan Pour B., Haghnazari N., Keshavarzi F., et al. High sensitive electrochemical sensor for imatinib based on metal-organic frameworks and multiwall carbon nanotubes nanocomposite. Microchem. J. 2021;165 [Google Scholar]
- 123.Rezvani Jalal N., Madrakian T., Afkhami A., et al. In situ growth of metal-organic framework HKUST-1 on graphene oxide nanoribbons with high electrochemical sensing performance in imatinib determination. ACS Appl. Mater. Interfaces. 2020;12:4859–4869. doi: 10.1021/acsami.9b18097. [DOI] [PubMed] [Google Scholar]
- 124.Mirsadeghi S., Zandavar H., Rahimi M., et al. Photocatalytic reduction of imatinib mesylate and imipenem on electrochemical synthesized Al2W3O12 nanoparticle: Optimization, investigation of electrocatalytic and antimicrobial activity. Colloids Surf. A Physicochem. Eng. Asp. 2020;586 [Google Scholar]
- 125.Alavi-Tabari S.A.R., Khalilzadeh M.A., Karimi-Maleh H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018;811:84–88. [Google Scholar]
- 126.Ghapanvari M., Madrakian T., Afkhami A. 2018. Sensitive determination of imatinib in human biological samples by differential pulse voltammetry based on carbon paste electrode modified by MMWCNTs/PAN NFs. [Google Scholar]
- 127.Yarahmadi A., Madrakian T., Afkhami A., et al. Electrochemical determination of sunitinib in biological samples using polyacrylonitrile nanofibers/nickel-zinc-ferrite nanocomposite/carbon paste electrode. J. Electrochem. Soc. 2019;166:B1268–B1275. [Google Scholar]
- 128.Ghapanvari M., Madrakian T., Afkhami A., et al. A modified carbon paste electrode based on Fe3O4@multi-walled carbon nanotubes@polyacrylonitrile nanofibers for determination of imatinib anticancer drug. J. Appl. Electrochem. 2020;50:281–294. [Google Scholar]
- 129.Rodríguez J., Castañeda G., Lizcano I. Electrochemical sensor for leukemia drug imatinib determination in urine by adsorptive striping square wave voltammetry using modified screen-printed electrodes. Electrochim. Acta. 2018;269:668–675. [Google Scholar]
- 130.Kalambate P.K., Li Y., Shen Y., et al. Mesoporous Pd@Pt core-shell nanoparticles supported on multi-walled carbon nanotubes as a sensing platform: Application in simultaneous electrochemical detection of anticancer drugs doxorubicin and dasatinib. Anal. Methods. 2019;11:443–453. [Google Scholar]
- 131.Moghaddam A., Zamani H.A., Karimi-Maleh H. A new electrochemical platform for dasatinib anticancer drug sensing using Fe3O4-SWCNTs/ionic liquid paste sensor. Micromachines. 2021;12 doi: 10.3390/mi12040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rele R.V., Tiwatane P. A non-aqueous potentiometric titration method for validation of imatinib mesylate from pharmaceutical dosages. Asia. J. Rese. Chem. 2019;12:307–310. [Google Scholar]
- 133.Yang W., Zhou M., Oturan N., et al. Electrocatalytic destruction of pharmaceutical imatinib by electro-Fenton process with graphene-based cathode. Electrochim. Acta. 2019;305:285–294. [Google Scholar]
- 134.Tartaggia S., Meneghello A., Bellotto O., et al. An SPR investigation into the therapeutic drug monitoring of the anticancer drug imatinib with selective aptamers operating in human plasma. Analyst. 2021;146:1714–1724. doi: 10.1039/d0an01860k. [DOI] [PubMed] [Google Scholar]
- 135.Fornasaro S., Bonifacio A., Marangon E., et al. Label-free quantification of anticancer drug imatinib in human plasma with surface enhanced Raman spectroscopy. Anal. Chem. 2018;90:12670–12677. doi: 10.1021/acs.analchem.8b02901. [DOI] [PubMed] [Google Scholar]
- 136.Ravisankar P., Anusha S., Srinivasa Babu P. Development and validation of UV-spectrophotometric method for determination of dasatinib in bulk and pharmaceutical dosage form and its degradation behaviour under various stress conditions. Int. J. Pharm. Sci. Rev. Res. 2020;53:45–50. [Google Scholar]
- 137.Ravisankar P., Babu P.S., Taslim S.M., et al. Development and validation of UV-spectrophotometric method for determination of sorafenib in pharmaceutical dosage form and its degradation behaviour under various stress conditions. Int. J. Pharm. Sci. Rev. Res. 2019;56:12–17. [Google Scholar]
- 138.Souri E., Amoon E., Shabani Ravari N., et al. Spectrophotometric methods for determination of sunitinib in pharmaceutical dosage forms based on ion-pair complex formation. Iran. J. Pharm. Res. 2020;19:103–109. doi: 10.22037/ijpr.2020.1101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Darwish I.A., Khalil N.Y., Darwish H.W., et al. Spectrophotometric and computational investigations of charge transfer complexes of chloranilic acid with tyrosine kinase inhibitors and application to development of novel universal 96-microwell assay for their determination in pharmaceutical formulations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;252 doi: 10.1016/j.saa.2021.119482. [DOI] [PubMed] [Google Scholar]
- 140.Darwish I.A., Darwish H.W., Khalil N.Y., et al. Experimental and computational evaluation of chloranilic acid as an universal chromogenic reagent for the development of a novel 96-microwell spectrophotometric assay for tyrosine kinase inhibitors. Molecules. 2021;26 doi: 10.3390/molecules26030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abdelhameed A.S., Attwa M.W., Attia M.I., et al. Development of novel univariate and multivariate validated chemometric methods for the analysis of dasatinib, sorafenib, and vandetanib in pure form, dosage forms and biological fluids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022;264 doi: 10.1016/j.saa.2021.120336. [DOI] [PubMed] [Google Scholar]
- 142.Abdelhameed A.S., Hassan E.S., Attwa M.W., et al. Simple and efficient spectroscopic-based univariate sequential methods for simultaneous quantitative analysis of vandetanib, dasatinib, and sorafenib in pharmaceutical preparations and biological fluids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;260 doi: 10.1016/j.saa.2021.119987. [DOI] [PubMed] [Google Scholar]
- 143.Belal F., Ibrahim F., Sheribah Z.A., et al. New spectrophotometric/chemometric assisted methods for the simultaneous determination of imatinib, gemifloxacin, nalbuphine and naproxen in pharmaceutical formulations and human urine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;198:51–60. doi: 10.1016/j.saa.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Z., Chen J., Yang Q., et al. Eco-friendly intracellular microalgae synthesis of fluorescent CdSe QDs as a sensitive nanoprobe for determination of imatinib. Sens. Actuat. B Chem. 2018;263:625–633. [Google Scholar]
- 145.Zidan D.W., Hassan W.S., Elmasry M.S., et al. A novel spectrofluorimetric method for determination of imatinib in pure, pharmaceutical preparation, human plasma, and human urine. Luminescence. 2018;33:232–242. doi: 10.1002/bio.3406. [DOI] [PubMed] [Google Scholar]
- 146.Darwish H.W., Bakheit A.H., Al-Shakliah N.S., et al. Development of innovative artificial neural networks for simultaneous determination of lapatinib and foretinib in human urine by micellar enhanced synchronous spectrofluorimetry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;238 doi: 10.1016/j.saa.2020.118438. [DOI] [PubMed] [Google Scholar]
- 147.Darwish H.W., Bakheit A.H., Al-Shakliah N.S., et al. Experimental and computational evaluation of kolliphor RH 40 as a new fluorescence enhancer in development of a micellar-based spectrofluorimetric method for determination of lapatinib in tablets and urine. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salem H., Abo Elsoud F.A., Heshmat D., et al. Resonance Rayleigh scattering technique-using erythrosine B, as novel spectrofluorimetric method for determination of anticancer agent nilotinib: Application for capsules and human plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021;251 doi: 10.1016/j.saa.2021.119428. [DOI] [PubMed] [Google Scholar]
- 149.Pirdadeh-Beiranvand M., Afkhami A., Madrakian T. Ni0.5Zn0.5Fe2O4 nanoparticles-decorated poly (vinyl alcohol) nanofiber as resonance light scattering probe for determination of sunitinib in serum samples. Talanta. 2020;218 doi: 10.1016/j.talanta.2020.121190. [DOI] [PubMed] [Google Scholar]
- 150.Kashani H.M., Madrakian T., Afkhami A. Highly fluorescent nitrogen-doped graphene quantum dots as a green, economical and facile sensor for the determination of sunitinib in real samples. New J. Chem. 2017;41:6875–6882. [Google Scholar]
- 151.Forough M., Farhadi K., Eyshi A., et al. Rapid ionic liquid-supported nano-hybrid composite reinforced hollow-fiber electromembrane extraction followed by field-amplified sample injection-capillary electrophoresis: An effective approach for extraction and quantification of Imatinib mesylate in human plasma. J. Chromatogr. A. 2017;1516:21–34. doi: 10.1016/j.chroma.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 152.Gonzalez A.G., Taraba L., Hraníček J., et al. Determination of dasatinib in the tablet dosage form by ultra high performance liquid chromatography, capillary zone electrophoresis, and sequential injection analysis. J. Sep. Sci. 2017;40:400–406. doi: 10.1002/jssc.201600950. [DOI] [PubMed] [Google Scholar]
- 153.Sodeifian G., Razmimanesh F., Ali Sajadian S. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids. 2019;146:89–99. [Google Scholar]
- 154.Sodeifian G., Razmimanesh F., Ali Sajadian S. Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC-CO2): Experimental correlations and thermodynamic modeling. J. Mol. Liq. 2020;297 [Google Scholar]
- 155.Zhang S., Jin W., Yang Y. Simultaneous identification and determination of eleven tyrosine kinase inhibitors by supercritical fluid chromatography-mass spectrometry. Anal. Methods. 2019;11:2211–2222. [Google Scholar]
- 156.Alhazmi H.A., Ali Bokar Nasib A., Ali Musleh Y., et al. Application of drug-metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib. Open Chem. 2020;18:798–807. [Google Scholar]
- 157.Smy L., Sadler A.J., McMillin G.A. Evaluation of imatinib concentrations in samples submitted for BCR-ABL1 or imatinib testing-evidence to support therapeutic drug monitoring for dose optimization? Ther. Drug Monit. 2020;42:559–564. doi: 10.1097/FTD.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 158.Saita T., Yamamoto Y., Hosoya K., et al. An ultra-specific and sensitive sandwich ELISA for imatinib using two anti-imatinib antibodies. Anal. Chim. Acta. 2017;969:72–78. doi: 10.1016/j.aca.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 159.Dutta D., Das S., Ghosh M. November 15–December 15, 2018. Validated HPTLC method for the determination of nintedanib in bulk drug, 22nd International Electronic Conference on Synthetic Organic Chemistry. [Google Scholar]
- 160.Bhole R., Zombade T., Bonde C.G., et al. Identification and characterization of degradation products by using Ms-Ms studies for developed and validated stability indicating HPTLC method for estimation of Nintedanib Esylate in pharmaceutical dosage form. Eurasian J. Anal. Chem. 2019;14:60–70. [Google Scholar]
- 161.Sharma T., Kaur Khurana R., Borges B., et al. An HPTLC densitometric method for simultaneous quantification of sorafenib tosylate and chrysin: Analytical method development, validation and applications. Microchem. J. 2021;162 [Google Scholar]
- 162.Westover D., Zugazagoitia J., Cho B.C., et al. Mechanisms of acquired resistance to first-and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 2018;29:i10–i19. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pottier C., Fresnais M., Gilon M., et al. Tyrosine kinase inhibitors in cancer: Breakthrough and challenges of targeted therapy. Cancers. 2020;12 doi: 10.3390/cancers12030731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jiao Q., Bi L., Ren Y., et al. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer. 2018;17 doi: 10.1186/s12943-018-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wind S., Schnell D., Ebner T., et al. Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin. Pharmacokinet. 2017;56:235–250. doi: 10.1007/s40262-016-0440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith B.J., Pithavala Y., Bu H., et al. Pharmacokinetics, metabolism, and excretion of [14C] axitinib, a vascular endothelial growth factor receptor tyrosine kinase inhibitor in humans. Drug Metab. Dispos. 2014;42:918–931. doi: 10.1124/dmd.113.056531. [DOI] [PubMed] [Google Scholar]
- 167.Abbas R., Hsyu P.-H. Clinical pharmacokinetics and pharmacodynamics of bosutinib. Clin. Pharmacokinet. 2016;55:1191–1204. doi: 10.1007/s40262-016-0391-6. [DOI] [PubMed] [Google Scholar]
- 168.Johnson T.R., Tan W., Goulet L., et al. Metabolism, excretion and pharmacokinetics of [14C] crizotinib following oral administration to healthy subjects. Xenobiotica. 2015;45:45–59. doi: 10.3109/00498254.2014.941964. [DOI] [PubMed] [Google Scholar]
- 169.van Erp N.P., Gelderblom H., Guchelaar H.J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat. Rev. 2009;35:692–706. doi: 10.1016/j.ctrv.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 170.Puszkiel A., Noé G., Bellesoeur A., et al. Clinical pharmacokinetics and pharmacodynamics of dabrafenib. Clin. Pharmacokinet. 2019;58:451–467. doi: 10.1007/s40262-018-0703-0. [DOI] [PubMed] [Google Scholar]
- 171.Levêque D., Becker G., Bilger K., et al. Clinical pharmacokinetics and pharmacodynamics of dasatinib. Clin. Pharmacokinet. 2020;59:849–856. doi: 10.1007/s40262-020-00872-4. [DOI] [PubMed] [Google Scholar]
- 172.Verheijen R.B., Beijnen J.H., Schellens J.H.M., et al. Clinical pharmacokinetics and pharmacodynamics of pazopanib: Towards optimized dosing. Clin. Pharmacokinet. 2017;56:987–997. doi: 10.1007/s40262-017-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Narasimhan N.I., Dorer D.J., Niland K., et al. Effects of food on the pharmacokinetics of ponatinib in healthy subjects. J. Clin. Pharm. Ther. 2013;38:440–444. doi: 10.1111/jcpt.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hulin A., Stocco J., Bouattour M. Clinical pharmacokinetics and pharmacodynamics of transarterial chemoembolization and targeted therapies in hepatocellular carcinoma. Clin. Pharmacokinet. 2019;58:983–1014. doi: 10.1007/s40262-019-00740-w. [DOI] [PubMed] [Google Scholar]
- 175.Ogama Y., Mineyama T., Yamamoto A., et al. A randomized dose-escalation study to assess the safety, tolerability, and pharmacokinetics of ruxolitinib (INC424) in healthy Japanese volunteers. Int. J. Hematol. 2013;97:351–359. doi: 10.1007/s12185-013-1280-5. [DOI] [PubMed] [Google Scholar]
- 176.Johansson S., Read J., Oliver S., et al. Pharmacokinetic evaluations of the co-administrations of vandetanib and metformin, digoxin, midazolam, omeprazole or ranitidine. Clin. Pharmacokinet. 2014;53:837–847. doi: 10.1007/s40262-014-0161-2. [DOI] [PubMed] [Google Scholar]
- 177.Hurwitz H.I., Dowlati A., Saini S., et al. Phase I trial of pazopanib in patients with advanced cancer. Clin. Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 178.Peng B., Lloyd P., Schran H. Clinical pharmacokinetics of imatinib. Clin. Pharmacokinet. 2005;44:879–894. doi: 10.2165/00003088-200544090-00001. [DOI] [PubMed] [Google Scholar]
- 179.Montero A.J., Kwon D., Flores A., et al. A phase I clinical, pharmacokinetic, and pharmacodynamic study of weekly or every three week ixabepilone and daily sunitinib in patients with advanced solid tumors. Clin. Cancer Res. 2016;22:3209–3217. doi: 10.1158/1078-0432.CCR-15-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lathia C., Lettieri J., Cihon F., et al. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother. Pharmacol. 2006;57:685–692. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 181.Tian X., Zhang H., Heimbach T., et al. Clinical pharmacokinetic and pharmacodynamic overview of nilotinib, a selective tyrosine kinase inhibitor. J. Clin. Pharmacol. 2018;58:1533–1540. doi: 10.1002/jcph.1312. [DOI] [PubMed] [Google Scholar]
- 182.Fukudo M., Ikemi Y., Togashi Y., et al. Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin. Pharmacokinet. 2013;52:593–609. doi: 10.1007/s40262-013-0058-5. [DOI] [PubMed] [Google Scholar]
- 183.Thiessen B., Stewart C., Tsao M., et al. A phase I/II trial of GW572016 (lapatinib) in recurrent glioblastoma multiforme: Clinical outcomes, pharmacokinetics and molecular correlation, Cancer Chemother. Pharmacol. 2010;65:353–361. doi: 10.1007/s00280-009-1041-6. [DOI] [PubMed] [Google Scholar]
- 184.Swaisland H.C., Ranson M., Smith R.P., et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin. Pharmacokinet. 2005;44:1067–1081. doi: 10.2165/00003088-200544100-00005. [DOI] [PubMed] [Google Scholar]
- 185.Arora A., Scholar E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 186.Josephs D.H., Fisher D.S., Spicer J., et al. Clinical pharmacokinetics of tyrosine kinase inhibitors: Implications for therapeutic drug monitoring. Ther. Drug Monit. 2013;35:562–587. doi: 10.1097/FTD.0b013e318292b931. [DOI] [PubMed] [Google Scholar]
- 187.Herviou P., Thivat E., Richard D., et al. Therapeutic drug monitoring and tyrosine kinase inhibitors. Oncol. Lett. 2016;12:1223–1232. doi: 10.3892/ol.2016.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lewis R., Brüggemann R., Padoin C., et al. September 11–12, 2015. Triazole antifungal therapeutic drug monitoring, Sixth European Conference on Infections in Leukaemia Meeting, Sophia Antipolis, France. [Google Scholar]
- 189.Ghiculescu R.A. Therapeutic drug monitoring: Which drugs, why, when and how to do it. Aust. Prescr. 2008;31:42–44. [Google Scholar]
- 190.Baccarani M., Deininger M.W., Rosti G., et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872–884. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.García-Ferrer M., Wojnicz A., Mejía G., et al. Utility of therapeutic drug monitoring of imatinib, nilotinib, and dasatinib in chronic myeloid leukemia: A systematic review and meta-analysis. Clin. Ther. 2019;41:2558–2570.e7. doi: 10.1016/j.clinthera.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 192.Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol. Pharm. Bull. 2015;38:645–654. doi: 10.1248/bpb.b15-00103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.