Abstract
Large animal models to study abdominal aortic aneurysms are sparse. The purpose of this model is to create reproducible, clinically significant infrarenal abdominal aortic aneurysms (AAA) in swine. To achieve this, we use a combination of balloon angioplasty, elastase and collagenase, and a lysyl oxidase inhibitor, called β-aminopropionitrile (BAPN), to create clinically significant infrarenal aortic aneurysms, analogous to human disease.
Noncastrated male swine are fed BAPN for 7 days prior to surgery to achieve a steady state in the blood. A midline laparotomy is performed and the infrarenal aorta is circumferentially dissected. An initial measurement is recorded prior to aneurysm induction with a combination of balloon angioplasty, elastase (500 units)/collagenase (8000 units) perfusion, and topical elastase application. Swine are fed BAPN daily until terminal procedure on either postoperative day 7, 14, or 28, at which time the aneurysm is measured, and tissue procured. BAPN + surgery pigs are compared to pigs that underwent surgery alone.
Swine treated with BAPN and surgery had a mean aortic dilation of 89.9% ± 47.4% at day 7, 105.4% ± 58.1% at day 14, and 113.5% ± 30.2% at day 28. Pigs treated with surgery alone had significantly smaller aneurysms compared to BAPN + surgery animals at day 28 (p < 0.0003). The BAPN + surgery group had macroscopic and immunohistochemical evidence of end stage aneurysmal disease.
Clinically significant infrarenal AAA can be induced using balloon angioplasty, elastase/collagenase perfusion and topical application, supplemented with oral BAPN. This model creates large, clinically significant AAA with hallmarks of human disease. This has important implications for the elucidation of AAA pathogenesis and testing of novel therapies and devices for the treatment of AAA. Limitations of the model include variation in BAPN ingested by swine, quality of elastase perfusion, and cost of BAPN.
Keywords: Medicine, Issue 153, Cardiovascular disease, Aortic aneurysms, cytokines, immunohistochemistry, macrophages, inflammation
Introduction
According to the Center for Disease Control (CDC), aortic aneurysms (AA) are a leading cause of death in the United States and represent a significant disease burden1. An aortic aneurysm is defined as a dilation of a discrete portion of the vessel lumen by over 50%2. A subset of AA in the abdomen, referred to as abdominal aortic aneurysms (AAA) are a growing concern. AAA remain clinically silent until impending rupture or dissection, with acute onset, severe abdominal pain generally being the only presenting symptom3,4. Rupture of AAA is almost always fatal with a mortality rate of 90%5. Open or endovascular surgery is the only therapeutic option for patients, and can be a highly morbid procedure. Importantly, AAA are one of the few cardiovascular diseases with no medical therapy for cure.
To date, much of the research on AAA pathogenesis has focused on rodent models, using elastase, which is an enzyme that degrades elastin found within the aortic media, to induce aneurysms.6,7 However, the clinical translatability of small animal models to human aneurysmal disease is restricted, as evaluation of structural changes in the aorta, and altered hemodynamics are limited due to size. Because of anatomical and size similarity, the porcine circulatory system correlates better with human biology than rodents8. Large animal models allow further understanding of cellular mechanisms of the disease process, can be used develop novel treatments at therapeutic doses for large mammals, and test mechanical repair devices, which would not be feasible in small animal models. Additionally, the acute nature of rodent models does not replicate the chronicity and pathologic characteristics of human aneurysmal disease.
The combination of elastase and a compound called β-aminopropionitrile (BAPN) has revolutionized murine AAA models, by creating aneurysms that are larger and contain sequela of chronic aneurysmal disease, including mural thrombus, dissection, and rupture9. BAPN is an inhibitor of lysyl oxidase, which is essential for collagen crosslinking, a crucial component of the aortic wall10,11,12. Lysyl oxidase activity decreases with aging and given the association of age and the chronic nature of complicated AA, BAPN has great potential to experimentally mimic the effects of aging9,13,14. The use of BAPN and its ability to replicate chronic disease in a subacute setting offers a novel advantage over alternative large animal models of AAA. Compared to other established porcine AAA models, this model creates the largest aneurysms with hallmarks of end-stage disease, and the results have been previously published8,11,15.
While conferring certain advantages, significant resources and investment are required to successfully complete this model that may deter some investigators. Among these resources include access to operating rooms, qualified surgeons and anesthesia providers, animal housing, and veterinary staff to assist with post-operative care. Additionally, the cost of BAPN may be prohibitively expensive for some labs.
Few large animal models exist to study the complex pathophysiology of AAA formation and translate to human disease. Large animal models of AAA are critical to help assess the viability of novel technologies and treatments for human disease. Therefore, the purpose of this study was to create a reproducible model of advanced stage infrarenal AAA in swine. The rationale for the use of BAPN and elastase swine model is to better understand the pathophysiology of AAA by mimicking the chronic nature and sequela of human aneurysmal disease in an acute or subacute setting, as well as to test novel therapies and devices for AAA treatment.
Protocol
Animal protocols were approved by the University of Virginia Institutional Animal Care and Use Committee (No. 3848).
NOTE: This model has been previously published by Cullen et al. and is a modified protocol described by Hynecek et al.8,15.
1. Animals
Use non-castrated male swine weighing 20–30 kg for the experiments.
-
In order to maximize the proportion of weight-based doses of BAPN ingested, provide pigs with divided meals of standard chow and 0.15 g/kg of BAPN mixed with whole-milk plain yogurt or wet dog food. Start BAPN administration 7 days prior to the index operation to achieve steady state in the blood, and daily during post-operative course.
NOTE: BAPN has numerous side effects if ingested in large quantities. Isolation precautions for staff including cap, gowns, gloves, and shoe covers should be worn whenever interacting with animals fed BAPN or handling BAPN.
Make pigs nil per os (NPO) the night prior to surgery.
2. Anesthesia
Induce general anesthesia (GA) using tiletamine-zolzepam 6 mg/kg, xylazine (2 mg/kg), and atropine sulfate (0.04 mg/kg) administered intramuscularly.
Intubate the pig using a standard endotracheal tube (ETT) and Miller blade.
Obtain peripheral intravenous (IV) access using a 16 or 18 gauge IV in an ear vein and secure in place with tape.
Connect the ETT to anesthesia machine and maintain GA using inhaled isoflurane (0.2 mg/kg).
Apply electrocardiogram (EKG) leads and pulse oximetry to monitor vital signs during surgery. Take oral temperature at the beginning of the case. Place an electrocautery pad on dependent portion of the pig.
Be sure one member of your staff is continually monitoring and regularly recording vital signs to ensure the pig is appropriately sedate, ventilated, and oxygenated, as well as to identify and appropriately intervene upon any hemodynamic instability during the surgery.
3. Surgical technique
-
Perform sterilization of the surgical area using sterile gauze, povidone-iodine and 70% isopropyl alcohol. Drape the pig in the usual sterile fashion. Take a blood sample prior to incision.
NOTE: At this point, all equipment, including instruments, balloons, wires, etc. must be sterile.
Using an eleven blade or Bovie electrocautery, perform a midline laparotomy to enter the abdominal cavity.
-
Displace the abdominal viscera cephalad to the pig’s left to expose the retroperitoneum. Cover the bowel with a moist blue towel to avoid desiccation. Make a sharp incision to enter the retroperitoneum, allowing access to the inferior vena cava (IVC) and infrarenal abdominal aorta.
NOTE: Identification and protection of the ureters bilaterally is crucial at this portion of the case. Swine retroperitoneal anatomy (including the course of the ureters) grossly mirrors that of humans, with subtle variations detailed below.
-
Circumferentially dissect the aorta from the renal vessels, inferiorly to the aortic trifurcation. Take care to avoid IVC and lumbar artery injury. Once the entire infrarenal aorta is exposed, use calipers to measure the aortic diameter at the mid portion of the infrarenal aorta.
NOTE: Unlike humans, swine have an aortic trifurcation, not bifurcation.
Identify the caudal mesenteric artery on the anterior portion of the infrarenal aorta, which usually lies a few centimeters proximal to the aortic trifurcation. This artery does not exist in humans. Dissect, clamp, and transect this artery. At this point, administer 5000 units of unfractionated heparin sulfate intravenously.
Cannulate the caudal mesenteric artery with a 0.018 in stainless steel wire guide from a micropuncture introducer set. Serially dilate the artery over the wire with a 5 French (Fr) and then a 7 Fr introducer.
Leaving the 7 Fr introducer in place, replace the 0.018 in wire with a 0.035 in guidewire, and then remove the 7 Fr introducer, ensuring hemostasis with a finger over the cannulation site as the introducer is removed. Insert a 0.035 in guide wire until approximately 30 cm of wire remains or resistance is encountered.
Insert a 16 mm percutaneous transluminal angioplasty balloon over the wire into the infrarenal aorta and position it at the midpoint of the dissected aorta. Inflate the balloon while intermittently measuring the diameter of the dilated aorta with calipers until maximal dilation is approximately 80% greater than your baseline measurement.
After 10 min of reperfusion, cross clamp the aorta just distal to the renal vessels and proximal to the aortic trifurcation. Identify and clamp the previously dissected lumbar vessels to isolate the infrarenal aorta from systemic circulation. This is important to avoid systemic perfusion of elastase, which can cause a septic response in the acute post-operative period.
-
Reintroduce the 7 Fr introducer over the wire and remove the wire. Flush the isolated aortic segment with saline assuring no leakage of fluid. Connect the elastase (500 units) and collagenase (8000 units) solution to the introducer and perfuse 30 mL into the isolated aorta under constant manual pressure for 10 min. The entire 30 mL of solutions should be introduced to the isolated segment.
NOTE: A well perfused aortic segment should be taut without leakage from the aortic wall or from the cannulation site. A vessel loop may be wrapped just proximal to the cannulation site to ensure no elastase escapes. Over the course of 10 min, elastase/collagenase solution can be observed “weeping” through the aortic wall.
-
After 10 min, irrigate the solution from the aortic lumen with saline. Remove the introducer and ligate the caudal mesenteric artery stump. Release all clamps (lumbar clamps first, followed by distal clamp, then proximal clamp).
NOTE: Restrict the clamp time to no more than 10 min in order to prevent spinal cord ischemia. Have a repair stitch (5–0 polypropylene) loaded in case of bleeding from the caudal mesenteric artery stump after the cross clamps are released.
Soak a 2 cm × 5 cm piece of surgical gauze with 20 mL of undiluted elastase (27 units/mL) and wrap around the intervened aorta for 10 min. Take a measure of the aorta after all interventions with a caliper.
Irrigate the abdomen with saline, replace the bowel, and close the abdomen in three layers. BAPN inhibits wound healing, so in order to minimize the risks of wound breakdown and fascial dehiscence, use suture with a long absorptions time and be sure to take judiciously small sized bites of tissue at every layer. Utilize a running synthetic absorbable monofilament 1 looped Polydioxanone (PDS) suture for the fascia, a running braided absorbable 2–0 suture for the deep dermal later, and a running subcuticular absorbable monofilament suture (4–0) for the skin.
4. Postoperative care
Utilize 0.2 mg/kg subcutaneous buprenorphine-SR for post-operative analgesia. Evaluate each pig three times daily for the first three postoperative days for signs of pain and discomfort and administer additional analgesia if identified.
Administer postoperative antibiotics (1 g cephalexin intramuscularly) on POD 1–3.
Socially house animals after POD 3.
5. Aortic tissue procurement
Perform tissue procurement on either POD 7, 14, or 28.
-
Induce GA as described in steps 2.1–2.5 above.
NOTE: Terminal aortic tissue procurement does not need to be sterile.
5.3. Reopen the previous midline laparotomy incision, being cognizant of adhered bowel to the anterior abdominal wall. Reflect the bowel to expose the retroperitoneum and aorta similar to step 3.3 above.
Dissect the aorta until the aneurysm is exposed and measure the external diameter of the aneurysmal segment with calipers. Calculate aortic dilation (%) using the following equation: [(harvest infrarenal diameter - initial operative infrarenal diameter) × 100%]. Once the measurement is attained, administer a lethal dose of pentobarbital-phenytoin (e.g., Euthasol) via injection into the IVC.
Dissect the aorta from the trifurcation to the suprarenal aorta and explant the aneurysmal segment with a control segment of untreated aorta. Place the sample in either liquid nitrogen or formalin for histologic evaluation.
Representative Results
All statistical analyses were performed using Fisher exact test or chi squared test as appropriate. Data values are reported as mean aortic dilation (%) ± standard deviation (%). Statistical significance was set P < 0.05. The combination of BAPN and surgery providing elastase treatment (surgery/elastase) creates more robust and reproducible AAA in swine at day 28 compared to those treated with surgery and elastase alone (mean aortic dilation (%) ± standard deviation (%): 113.5% ± 30.2% (n = 8) versus 59.7% ± 29.2% (n = 12); P < .01) as shown in Figure 1. AAA grew progressively larger as time progressed (mean aortic dilation (%) ± standard deviation (%) of 86.9% ± 47.4% (n = 4), 105.4% ± 58.1% (n = 5), and 113.5% ± 30.2% (n = 8) at 7, 14, and 28 day harvest time points, respectively, Figure 1). Evidence of chronic aneurysmal disease is evident in animals treated with BAPN and surgery/elastase, including intraluminal thrombus and atherosclerosis (Figure 2). Histologic evaluation demonstrated significantly increased elastin fragmentation and collagen alteration in BAPN-treated swine AAA than surgery/elastase alone (Figure 3 and Figure 4, respectively).
Figure 1: β-Aminopropionitrile (BAPN) treatment increases swine abdominal aortic aneurysm (AAA) size.
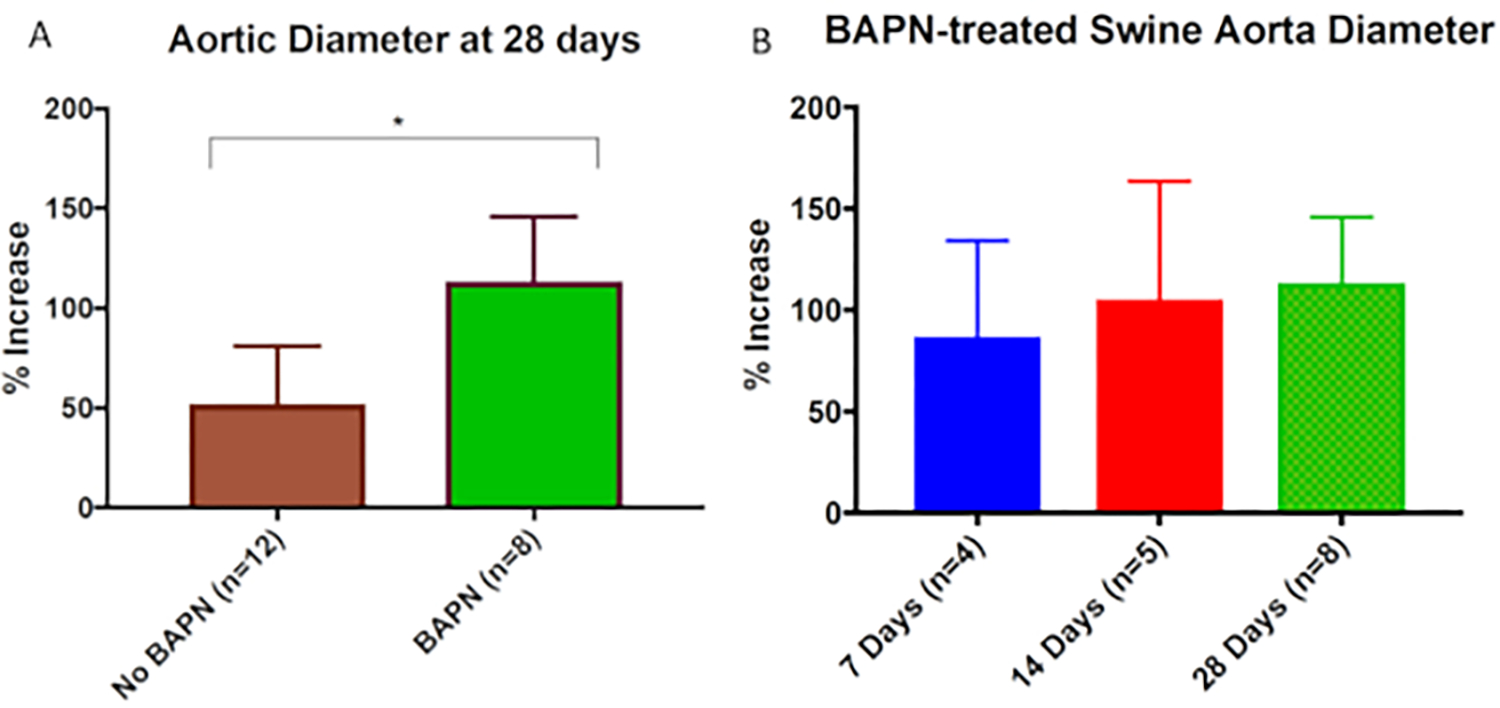
(A) BAPN + surgery/elastase swine had significantly higher mean aortic dilation compared with non-BAPN-treated (surgery/elastase alone) swine at 28 days (113.5% ± 30.2% vs. 59.7% ± 29.2%; P < .01). (B) BAPN-treated swine showed mean aortic dilation of 86.9% ± 47.4%, 105.4% ± 58.1%, and 113.5% ± 30.2% at 7, 14, and 28-day harvest time points, respectively. This figure was published by Cullen et al.15 and reproduced here with permission.
Figure 2: Sample photographs of porcine abdominal aortic aneurysms (AAA).
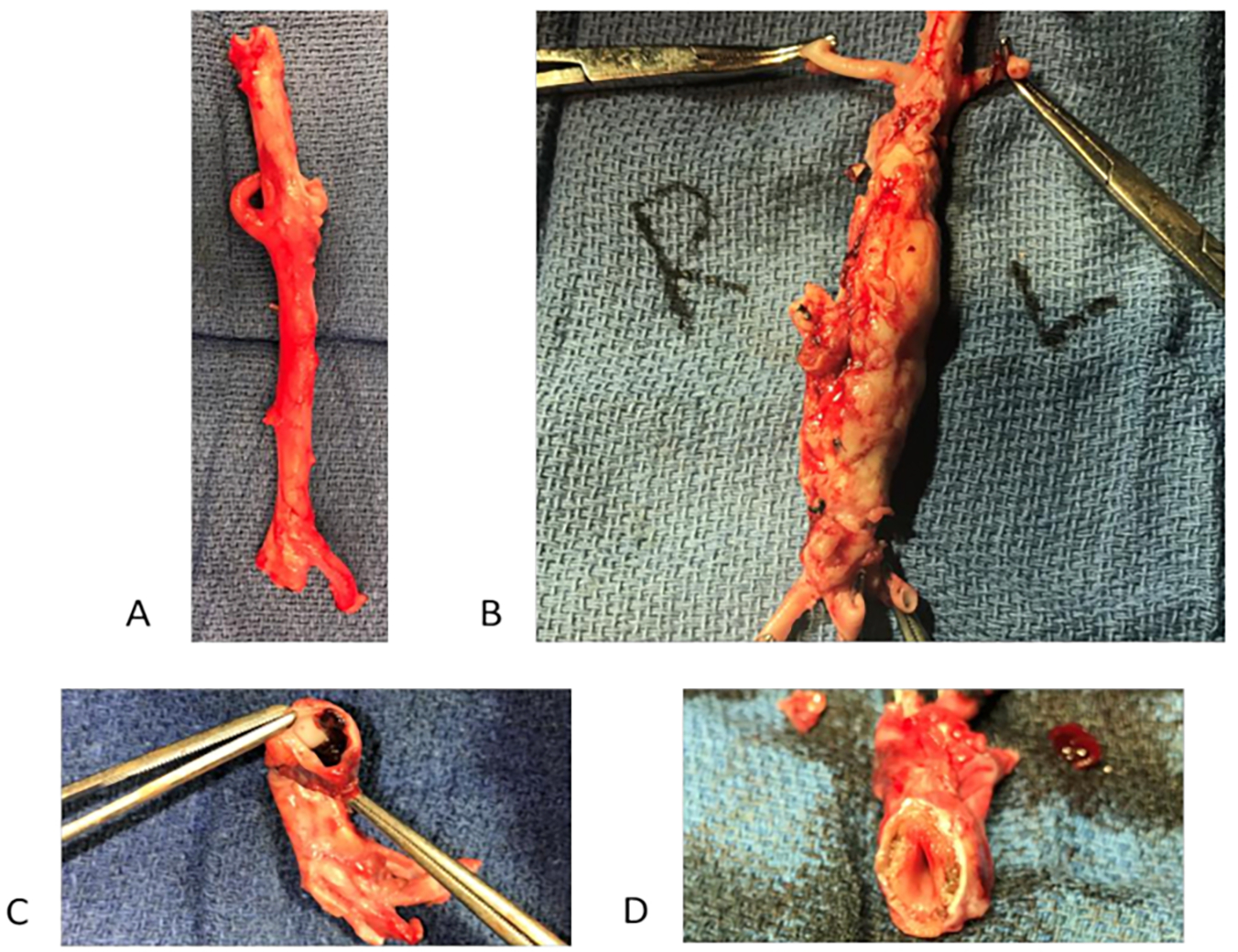
(A) Control abdominal aorta (no treatment with BAPN or elastase). (B) Infrarenal AAA formed on post-operative day (POD) 28 after treatment with surgery/elastase, and BAPN (C) Intraluminal thrombus in AAA on POD 28 in surgery/elastase and BAPN treated animals (D) Atherosclerosis in infrarenal AAA on POD 28 in surgery/elastase and BAPN treated animals.
Figure 3: Elastin fragmentation is increased in β-aminopropionitrile (BAPN)-treated swine abdominal aortic aneurysm (AAA).
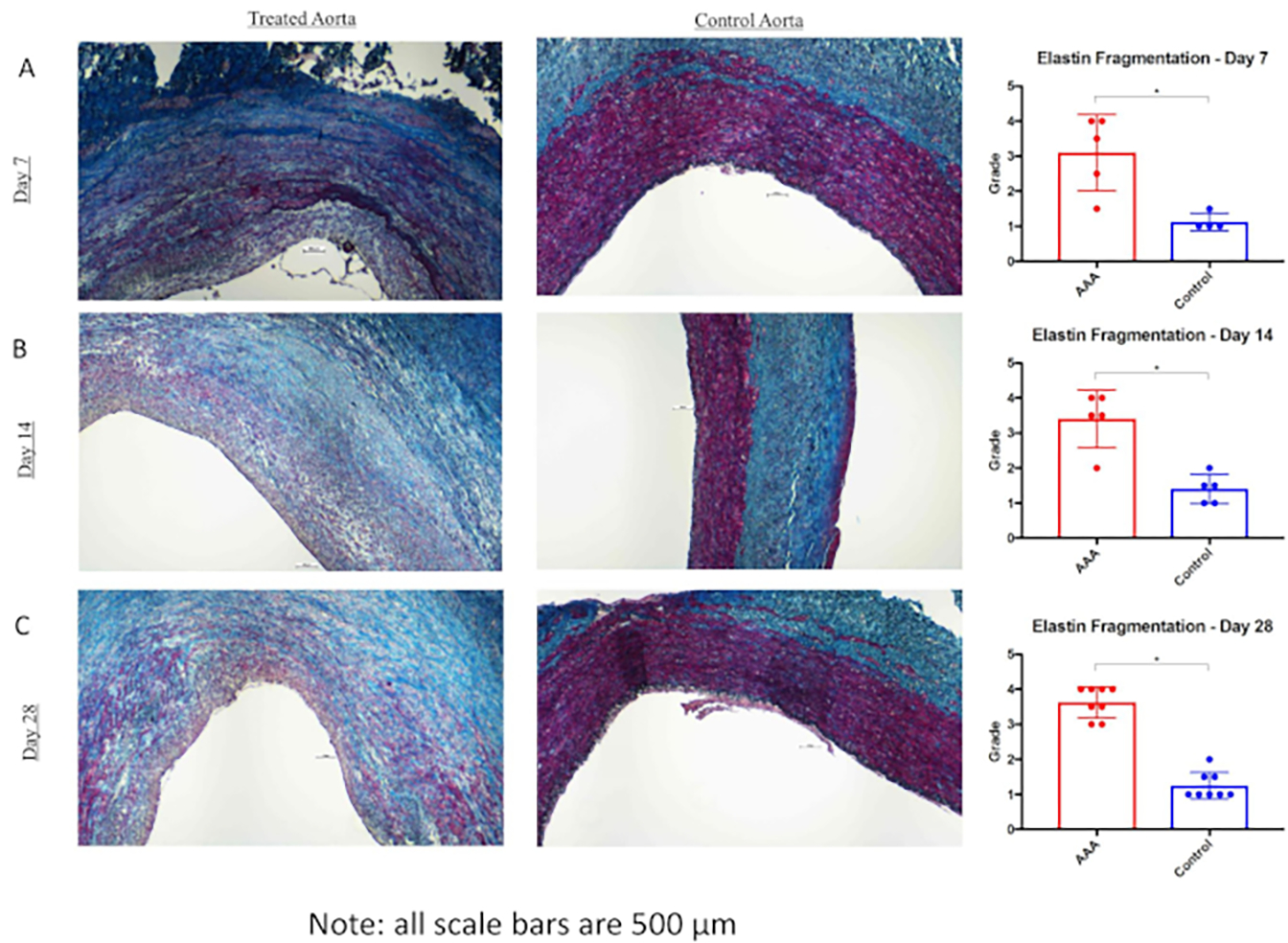
van Gieson staining in infrarenal aorta and suprarenal aorta at 7 days (A), 14 days (B), and 28 days (C). Far right, Elastin (black) fragmentation as measured by independent reviewers of infrarenal aorta versus suprarenalaorta at 7, 14, and 28 days. Scale bar represents 500 μm; 4x lens objective. *P < 0.05. This figure was published by Cullen et al.15 and reproduced here with permission.
Figure 4: Collagen is altered in β-aminopropionitrile (BAPN)-treated swine abdominal aortic aneurysm (AAA).
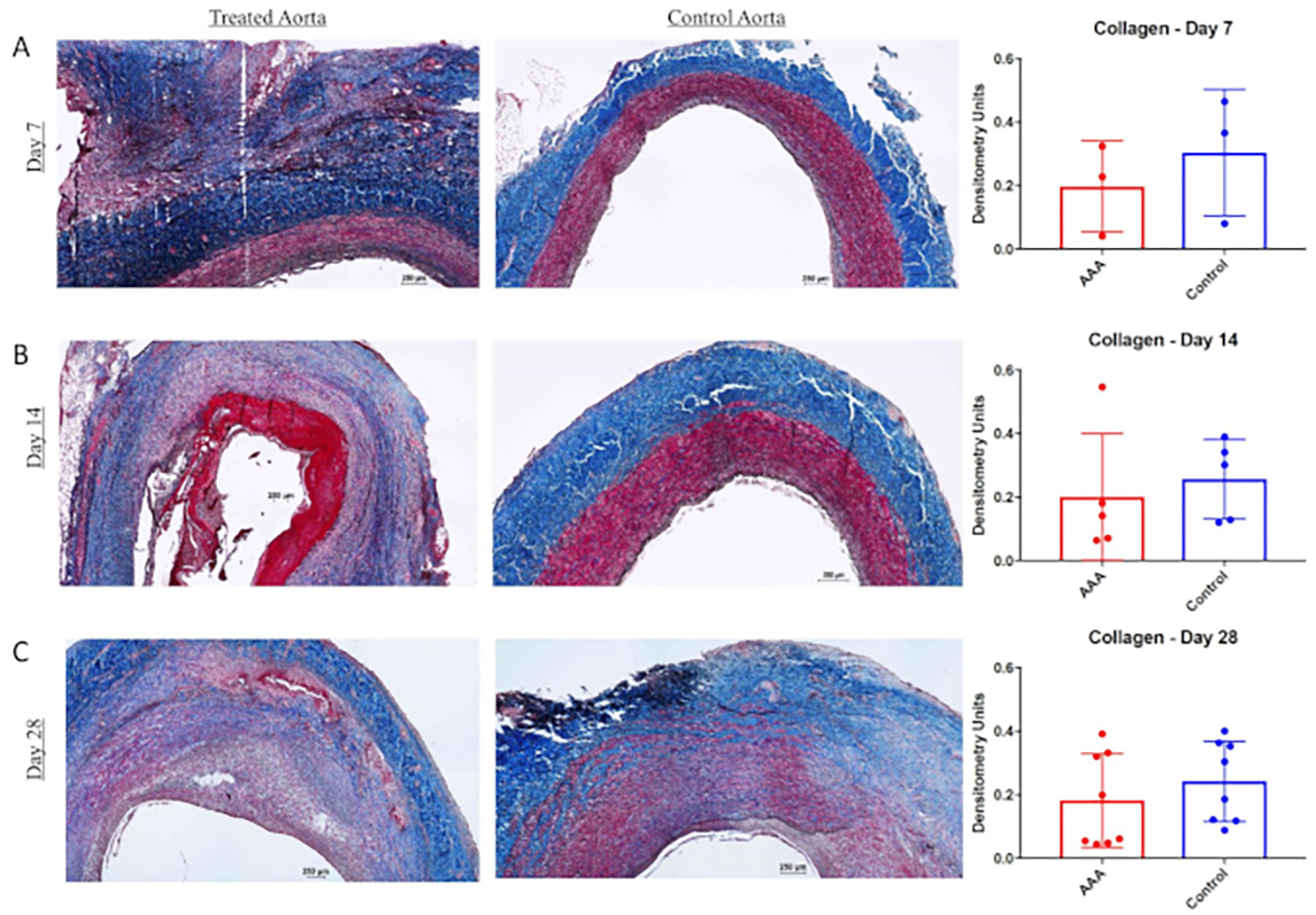
Masson trichrome and van Gieson staining in infrarenal aorta and suprarenal aorta at 7 days (A), 14 days (B), and 28 days (C). Far right, Collagen (blue) content within the wall of infrarenal versus suprarenal aorta as measured by densitometry units at 7, 14, and 28 days. Scale bar represents 250 mm; 4x lens objective. This figure was published by Cullen et al.15 and reproduced with permission.
Discussion
A novel model of infrarenal AAA in swine was created using a combination of balloon angioplasty, perfusion and topical elastase, and dietary as BAPN. Using this model, aortic dilation of >100% was achieved with gross and histologic characteristics of chronic human aneurysmal disease. This model provides a gateway to further understand the complex pathophysiology of AAA and translate potential therapies to human disease.
Prior models of AAA in swine have been achieved with modest success. Marinov et al. used elastase perfusion alone and saw some histologic changes including elastin disruption, but were not able to attain the phenotype that defines an aneurysm (>50% dilation)16. Given the durability of the porcine aorta, more than one intervention is needed to attain clinically significant aneurysms, which was originally described by Hynecek et al. using a combination of elastase and collagenase perfusion and balloon angioplasty8. They saw mean aortic diameter of 73% as well as histologic changes of aneurysmal disease, including endothelial loss, neutrophil infiltration, and elastin disruption.
However, prior models do not address a fundamental issue with all AAA models: how to replicate a chronic disease process in an acute or subacute setting. Most elastase models of AAA in mice show peak dilation at approximately 2 weeks followed by regression thereafter, whereas human disease evolves chronically over years. The key to this question may rest in the use of BAPN, a lysyl oxidase inhibitor preventing collagen crosslinking. BAPN has an “aging” effect, and combined with elastase treatment, it has been shown to simulate chronic aneurysm growth. In a murine model by Lu et al., mice were observed 100 days post-operatively, and demonstrated evidence of end-stage AAA with thrombus formation and spontaneous rupture9. The novelty and significance of our porcine AAA model is in the use of BAPN, which replicates this chronic disease process in a subacute setting and a more translatable animal species. Pigs fed a diet of BAPN combined with balloon angioplasty, elastase perfusion, and topical elastase application showed more robust aneurysms with evidence of end-stage disease, including mural thrombus, atherosclerosis, and rupture compared to those treated with surgery and elastase alone (Figure 1). This model augments and improves upon the prior model by Hynecek et al. by creating larger aneurysms with sequela of chronic disease8.
Although BAPN is essential to replicate the chronicity of AAA, surgical intervention provides the initial insult to the aorta to induce aneurysm formation. BAPN without surgery or elastase use has been examined, but did not show any significant aortic dilation11. For non-surgically trained investigators, the induction of AAA in swine via laparotomy can be daunting. Each step is fraught with potential complications, from bowel and ureteral injuries to arterial or venous bleeding requiring repair to post-operative wound infections. The investigator must be prepared for contingencies in order to survive the pig to its goal end point. A true team effort is required, including an experienced surgeon well versed in abdominal anatomy, provision of exceptional anesthesia including attention to vital signs and fluid status, and attentive post-operative care. Our team has experienced all of the above complications and acted accordingly whether with repair of enterotomy or caval injuries or antibiotics for infections. However, an unforeseen complication involved the degree to which BAPN impaired incisional wound healing in the pigs. Around 3 weeks post-operatively, some pigs exhibited breakdown of their incisions with occasional fascial dehiscence requiring take-back to the operating theater for revision and debridement. Careful monitoring of incisions post-operatively as well as closure in multiple layers is advised to prevent this complication.
The critical portion of the surgery involves cannulation of aorta via the caudal mesenteric artery, which can be frustrating, given its small size. The use of a micropuncture wires has aided us in this cannulation. This step is essential as the cannulation wire allows access to the aorta for the balloon and perfusion cannula. Balloon angioplasty prior to perfusion is essential in our experience, as the balloon dilation hypothetically creates endothelial disruption allowing elastase perfusion to more readily enter the aortic media. Adequate perfusion is defined as a taut segment of aorta without leakage or escape of fluid around the catheter or from the aortic wall. Achieving adequate perfusion of elastase, while limiting total aortic cross clamp time to no longer than 10 min, is essential for good aneurysm formation while simultaneously avoiding ischemic complications. Limiting total aortic cross clamp time to less than 20 min for the entire procedure and allowing adequate time for reperfusion in between balloon dilation and elastase perfusions avoids the dreaded spinal ischemia complication. If adequate perfusion is not obtained, there is likely a leak from somewhere in the perfused segment of the aorta, usually an inadvertent aortotomy from the dissection or retrograde leak from the cannulation site. It is crucial to repair any defect in the perfused segment to allow adequate perfusion of elastase. A vessel loop may be wrapped just proximal to aortic cannulation site to prevent retrograde flow of elastase. Any lumbar artery should also be temporarily clamped during perfusion to avoid elastase entering the systemic circulation, which may cause a septic response in the swine.
Logical next steps for this model include testing novel therapies for the medical treatment of AAA. As mentioned previously, there are no known medical therapies to attenuate or regress aortic aneurysm growth and current definitive care involves open surgical or endovascular approaches. Prior study has defined the roles of proinflammatory cytokines, Interleukin-1β (IL-1 β) and Interleukin-6 (IL-6) in the pathogenesis of descending thoracic aortic aneurysms and AAA, and inhibition of these receptors may provide potential therapeutic avenues for the treatment of these diseases17,18,19. These studies have only been done in murine models so the next steps should involve large animal models. Additionally, a large animal descending thoracic aortic aneurysm is another avenue for future study. Due to differing embryologic origins, there are inherent differences in the wall composition of the thoracic and abdominal aorta, leading to differing pathophysiology of aneurysms in these two segments20.
There are a few limitations of this model. First, since multiple interventions are employed, it is difficult to determine which intervention contributes most to aneurysm formation. The amount of pressure required to achieve an adequately elastase-perfused aortic segment is difficult to measure, and may vary. This could affect the amount of elastase entering the aortic media and subsequent aneurysm formation from one pig to the next. We are currently exploring a strategy to address this. BAPN was mixed in with the pig’s food and swine intake in the perioperative period may vary, altering the amounts of BAPN each pig ingests. Finally, this model requires many resources and investment to be successful. This includes operating rooms, surgeons and anesthesia providers, animal housing, post-operative care, and purchase of BAPN, which can be prohibitively expensive. Each lab should carefully evaluate their resources and funding prior to attempting this model.
Overall, despite certain limitations, swine AAA with sequela of chronic disease can be created reproducibly using a combination of BAPN, balloon angioplasty, elastase perfusion and topical elastase application. This has important implications for translational research applicable to human disease.
Materials.
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| Arrow Ergo Pack System | Arrow | CDC-21242-X1A | Just need 7 Fr dilator |
| Atlas PTA Balloon dilation catheter | Bard | AT-120184 | 16 mm × 4 cm × 120 cm |
| Bovie electrocautery | Bovie Medical | A2350 | |
| Collagenase Type 1 (5 gm) | Worthington | LS004196 | |
| Crile Needle drviers | MFI medical | 61–2201 | |
| DeBakey Atraumatic Forceps | MFI medical | 52–4977 | |
| DeBakey Peripheral Vascular Clamp | Medline | MDS1318119 | |
| Glidewire | Terumo Interventional Systems | GS3506 | outer Wire diameter 0.035 mm, Length 150 cm |
| GraphPad Prism 6 | GraphPad Software Inc. La Jolla, Calif) | statistical software | |
| Metzenbaum Scissors | MFI medical | 61–0004 | |
| Mayo-Hegar Needle Holder | tiger medical | N407322 | |
| Micropuncture Introducer Set | Cook | G47946 | |
| Mixter Forceps, Standard Grade, Right angle | Cole-Parmer | UX-10818–16 | |
| Monocryl suture | Ethicon | Y496G-BX | 4–0 monocryl |
| PDS II suture | Ethicon | D8926 | Number 1 looped |
| Porcine Pancreatic Elastase | Sigma-Aldrich | E0258–50 MG | |
| Satinsky Vascular Clamps | Medline | MDs5632515 | |
| Suction canister | Cardinal Health | 65651212 | |
| Schuco Aspirator | MFI medical | S430A | |
| Vicryl suture | Ethicon | J789D-SD | 2–0 vicryl |
| Yankauer Suction tube | Sklarcorp | 07–1801 |
Acknowledgments
We thank Anthony Herring and Cindy Dodson for their knowledge and technical expertise.
Sources of Funding:
Funding for this study was provided by the National Heart, Lung, and Blood Institute of the National Institute of Health under Award No. T32HL007849 and Grant Nos. R01HL081629–07 (G.R.U.) and R01HL124131–01 (G.R.U.).
Footnotes
Disclosures
None
Video Link
The video component of this article can be found at https://www.jove.com/video/60169/
References
- 1.WISQARS. Leading Causes of Death Reports, National and Regional, 1999 – 2016. https://webappa.cdc.gov/sasweb/ncipc/leadcause.html (2018).
- 2.Erbel R, et al. Diagnosis and management of aortic dissection. European Heart Journal. 22 (18), 1642–1681, (2001). [DOI] [PubMed] [Google Scholar]
- 3.Cameron J Current Surgical Therapy 11th edition. Vol. 11th (ed Cameron) 777–783, Elsevier Saunders; (2014). [Google Scholar]
- 4.Dalman RL, Mell M Overview of abdominal aortic aneurysm. https://www.uptodate.com/contents/overview-of-abdominal-aortic-aneurysm (2017).
- 5.Pearce WH, Zarins CK, Bacharach JM Atherosclerotic Peripheral Vascular Disease Symposium II: controversies in abdominal aortic aneurysm repair. Circulation. 118 (25), 2860–2863, (2008). [DOI] [PubMed] [Google Scholar]
- 6.Daugherty A, Cassis LA Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 24 (3), 429–434, (2004). [DOI] [PubMed] [Google Scholar]
- 7.Anidjar S, et al. Elastase-induced experimental aneurysms in rats. Circulation. 82 (3), 973–981 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Hynecek RL, et al. The creation of an infrarenal aneurysm within the native abdominal aorta of swine. Surgery. 142 (2), 143–149, (2007). [DOI] [PubMed] [Google Scholar]
- 9.Lu G, et al. A novel chronic advanced stage abdominal aortic aneurysm murine model. Journal of Vascular Surgery. 66 (1), 232–242 e234, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrow MV, Simpson CF, Miller EJ Lathyrism: a review. The Quarterly Review of Biology. 49 (2), 101–128 (1974). [DOI] [PubMed] [Google Scholar]
- 11.Coulson WF, Linker A, Bottcher E Lathyrism in swine. Archives of Pathology & Laboratory Medicine. 87 (4), 411–417 (1969). [PubMed] [Google Scholar]
- 12.McCallum HM Experimental Lathyrism in Mice. The Journal of Pathology and Bacteriology. 89 625–636 (1965). [DOI] [PubMed] [Google Scholar]
- 13.Behmoaras J, et al. Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta. Rejuvenation Research. 11 (5), 883–889, (2008). [DOI] [PubMed] [Google Scholar]
- 14.Davies I, Schofield JD Connective tissue ageing: the influence of a lathyrogen (beta-aminopropionitrile) on the life span of female C57BL/Icrfat mice. Experimental Gerontology. 15 (5), 487–494 (1980). [DOI] [PubMed] [Google Scholar]
- 15.Cullen JM, et al. A novel swine model of abdominal aortic aneurysm. Journal of Vascular Surgery. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinov GR, et al. Can the infusion of elastase in the abdominal aorta of the Yucatan miniature swine consistently produce experimental aneurysms? Journal of Investigative Surgery. 10 (3), 129–150 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Pope NH, et al. Interleukin-6 Receptor Inhibition Prevents Descending Thoracic Aortic Aneurysm Formation. Annals of Thoracic Surgery. 100 (5), 1620–1626, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston WF, et al. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 33 (2), 294–304, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston WF, et al. Inhibition of interleukin-1beta decreases aneurysm formation and progression in a novel model of thoracic aortic aneurysms. Circulation. 130 (11 Suppl 1), S51–59, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS Regional heterogeneity within the aorta: relevance to aneurysm disease. Journal of Thoracic and Cardiovascular Surgery. 136 (5), 1123–1130, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]


