Abstract
Introduction: Glioblastoma and astrocytoma, grade 4, are the most common and aggressive brain tumors. Several biomarkers, such as the isocitrate dehydrogenase mutation (IDH-1), alpha-thalassemia/mental retardation, and the X-linked mutation (ATRX), enable more accurate glioma classification and facilitate patient management. This study aimed to determine the prognostic value of clinical and molecular factors (IDH, TP53, and ATRX mutations). We also studied the relationship between these molecular markers and the overall survival (OS) of 126 patients with grade 4 glioblastoma/astrocytoma.
Methods: The immunohistochemical study was conducted using antibodies namely, IDH1, R132H, p53, and ATRX. Statistical tests were used to investigate factors that might influence overall survival using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY).
Results: The median age at diagnosis was 51.5 years. Patients with a Karnofsky performance score (KPS) <70 presented less favorable survival outcomes compared to those with a KPS ≥70. The median OS for patients was found to be 11.17 months. Expression of IDH1 R132H was found in 13.5% of patients, p53 overexpression was identified in 55.6% of cases, and loss of ATRX expression was detected in 11.9%. The group of patients with IDH mutant/ATRX mutant/p53 wild-type had the best prognosis (OS = 27.393 months; p = 0.015). Our results were in line with previous studies.
Conclusion: The clinical value of IDH and ATRX mutations in prognostic assessment was confirmed (p ≤0.05). The overexpression of p53 had no significant impact on OS (p = 0.726). Therefore, p53 alone cannot predict survival in glioblastoma patients. Based on the results, these biomarkers may be a potential therapeutic target to prolong patient survival, hence the need for further investigations.
Keywords: clinicopathological parameters, atrx, p53, idh, glioma
Introduction
Astrocytoma and glioblastoma, grade 4, are the most common and aggressive brain tumors, accounting for 14.5% of all central nervous system tumors and 48.6% of malignant central nervous system tumors [1]. Their incidence increases with age and other factors such as ionizing radiation, inherited genetic disorders, and race or ethnicity [2].
Several alterations can contribute to gliomagenesis, including isocitrate dehydrogenase mutation (IDH-1), alpha-thalassemia/mental retardation, X-linked mutation (ATRX), and tumor protein p53 mutation (TP53). These mutations are of diagnostic and/or prognostic interest [2].
IDH-1 catalyzes the oxidative decarboxylation of isocitrate to alpha-ketoglutarate. In contrast, the mutant form of IDH-1 leads to the formation of 2-hydroxyglutarate, an oncometabolite involved in gliomagenesis by blocking dioxygenase functions in DNA and histone demethylation [3]. Several clinical trials investigating IDH-1 inhibitors among patients with gliomas are underway; however, their efficacy is not always approved [4].
The ATRX gene is located on Xq21.1 and encodes a protein involved in chromatin rearrangement [5]. The ATRX mutation is found in around 75% of grade 4 astrocytomas and rarely in IDH wild-type glioblastomas (only 3%) [6, 7]. In addition, patients with ATRX mutations have better overall survival (OS) than patients with wild-type ATRX [2,8,9].
The TP53 gene, located on 17p13.1, is a tumor suppressor involved in cell cycle and apoptosis. The mutant form of p53 contributes to tumor cell proliferation and facilitates the malignant transformation of astrocytic tumors. The presence of this mutation is often associated with the IDH-1 mutation (65%-90% of cases) [10-12]. In contrast, the TP53 mutation is present in only 30% of IDH wild-type glioblastomas [10]. Alteration of p53 expression leads to increased malignancy in glial cells by enhancing their proliferation, invasion, and resistance to chemotherapy [11]. In their study, Wang et al. reported that TP53 mutations are associated with a poor prognosis [12]. In astrocytomas, IDH-1 and TP53 mutations are also associated with the presence of the ATRX mutation [13]. Despite recent advances in treatment modalities, the prognosis remains dismal, with the OS ranging from 12 to 18 months [14].
This study aimed to examine the main clinicopathologic parameters and their association with patients' OS. We also determined the prognostic value of the IDHR132H mutation in association with p53 overexpression and ATRX loss in a series of 126 glioma patients.
Materials and methods
Patients and tissue samples
The present retrospective study includes 126 patients with grade 4 glioma diagnosed and treated between 2016 and 2020 at the University Hospital of Casablanca, Casablanca, Morocco. Tissue samples were obtained by neurosurgical intervention, fixed in formalin, and embedded in paraffin (FFPE).
Demographic data, cerebral magnetic resonance imaging (MRI), location of the tumor, type, extent of resection, Karnofsky performance score (KPS), and treatment characteristics were collected.
Immunohistochemistry (IHC)
An immunohistochemical study was carried out using three antibodies: IDHR132H (clone H09, 1:20 dilution, ph9) from Dianova (BIOZOL, Berlin, Germany), p53 (clone DO-7, ready to use, pH9) from Dako (Agilent Technologies, Inc, Santa Clara, CA), and ATRX (clone BSB-108, ready to use, pH9) from Bio SB (Bio SB, Inc., Santa Barbara, CA). For each stain used, there was an appropriate control tissue to ensure that the stains worked correctly.
Tissue sections of 4 µm thickness each were obtained using a microtome. The IHC staining followed the standard procedure of deparaffinization, antigen retrieval, and rehydration. These steps were performed using EnVision FLEX Target Retrieval Solution ((Agilent Technologies, Inc) at a high pH (x50). Endogenous peroxidase was blocked with EnVision FLEX Peroxidase Reagent (Agilent Technologies, Inc) for five minutes.
The slides were incubated with a primary antibody for 30 minutes, and then the visualization reagent (EnVision FLEX/HRP) containing dextran polymers, coupled with peroxidase molecules and biotinylated secondary antibody molecules, was applied for 20 minutes. 3,3′-diaminobenzidine tetrahydrochloride (DAB) was used for primary antibody detection. As a result, visible brown products were perceived. Later, hematoxylin was applied.
Slide examination
Slides were observed by two neuropathologists. Cases were considered mutant p53 if >10% of neoplastic cells showed nuclear staining. ATRX immunoreactivity was almost totally absent or completely retained; thus, cases with less than 10% of the stained nucleus were defined as ATRX loss.
Statistical analysis
The statistical study was carried out using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY) (chi-squared (χ2) test and Fisher's exact test). The OS was estimated using the Kaplan-Meier method, and differences in survival within groups were examined using the log-rank test (Mantel-Cox). The influence of various parameters on survival outcomes was tested by univariate analysis. Results were considered statistically significant when p-values were less than 0.05.
Results
Patient characteristics
The study included 126 cases of gliomas. The description of prognostic and clinicopathological factors is shown in Table 1.
Table 1. Demographic data, tumor characteristics, and treatment strategies of patients.
KPS: Karnofsky performance score
The data have been represented as N
| Clinicopathological characteristics | Age ≤40 years: 31/126 (24.6%) | Age >40 years: 95/126 (75.4%) |
| Female | 14 | 37 |
| Male | 17 | 58 |
| KPS at admission ≤70 | 3 | 26 |
| KPS at admission >70 | 28 | 69 |
| KPS at last evaluation ≤70 | 23 | 90 |
| KPS at last evaluation >70 | 8 | 5 |
| Frontal localization | 17 | 39 |
| Temporal localization | 7 | 31 |
| Parietal/Occipital localizations | 7 | 25 |
| Subtotal resection | 13 | 51 |
| Gross total resection | 18 | 44 |
| Postoperative radiotherapy | 26 | 82 |
| Postoperative chemotherapy | 24 | 70 |
| Death | 27 | 94 |
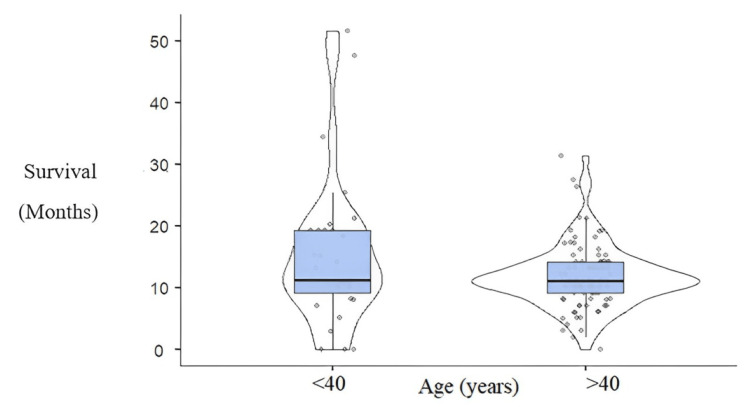
Patients' median age at the time of diagnosis was 51.5 years (SD±16.144), of whom 75.4% were older than 40 years. Young patients <40 years had an OS greater than patients >40 years (p = 0.004) (Figure 1).
Figure 1. Overall survival in age-related groups (p=0.004).
Survival has been represented by the number of months and age in two categories (≤40 years and >40 years).
(p <0.05)
In our cohort, 75 patients were male (59.5%), while 51 patients were female (40.4%) (male/female sex ratio of 1.47:1) (Table 1). Performance status was defined as good if the KPS was >70 and poor if the KPS was <70. Seventy-seven percent of patients had a KPS at admission >70, and 10.3% had a KPS at the last evaluation >70 (Table 1).
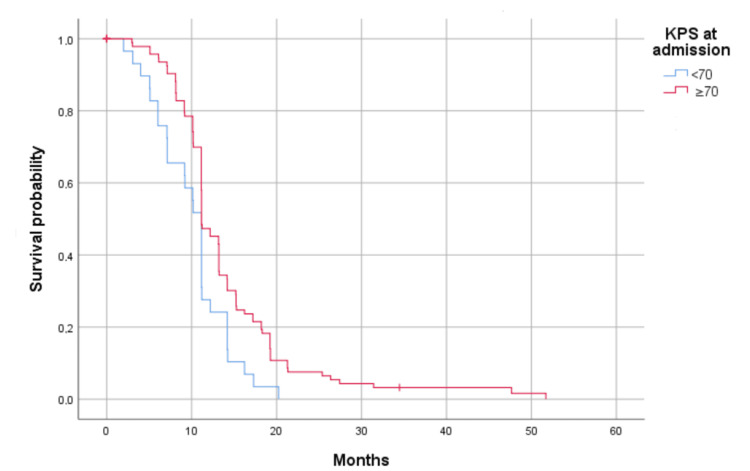
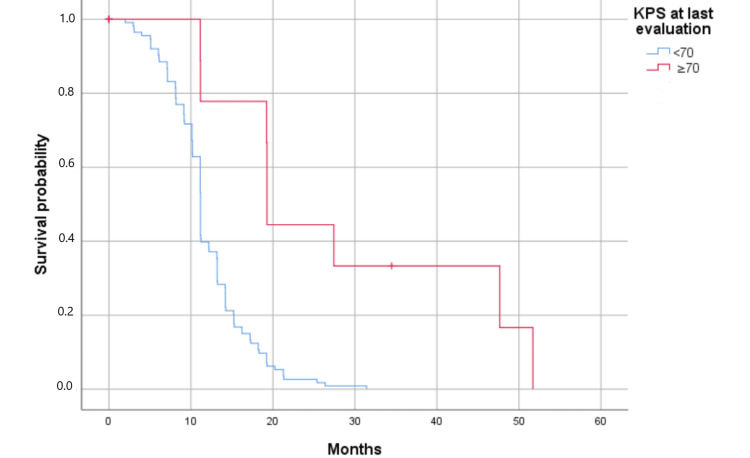
Survival analysis showed significant influences of KPS at admission (Figure 2) and KPS at the last evaluation (Figure 3) on OS in univariate analysis (p = 0.008 and p = 0.000, respectively). Furthermore, patients with KPS <70 presented less favorable survival outcomes compared to those with KPS ≥70.
Figure 2. Kaplan-Meier curves showing the significant association of KPS with the overall survival of glioblastoma patients; KPS at admission (p = 0.008).
Survival has been represented by probability (percentage) and months.
KPS at admission (p = 0.008)
(p <0.05)
KPS: Karnofsky performance score
Figure 3. Kaplan-Meier curves showing the significant association of KPS with the overall survival of glioblastoma patients; KPS at last evaluation (p = 0.000).
Survival has been represented by probability (percentage) and months.
KPS at last evaluation (p = 0.000)
(p <0.05)
KPS: Karnofsky performance score
The most common locations were the frontal lobe (44.4%), followed by the temporal lobe (30.2%); 25.4% of patients had parietal and occipital locations (Table 1). All patients had a surgical resection; 50.8% of cases received a subtotal resection; and 49.2% underwent a gross total resection (Table 1).
One hundred and eight received radiation, comprising 85.7% of our sample population, and 94 (74.6% of cases) received chemotherapy with temozolomide according to the Stupp protocol (Table 1). These therapies showed a distinct effect on OS (7.1 months versus 11.2 months), confirming their importance in the management of glioblastoma (p = 0.000).
Survival outcomes after treatment were followed up to the time point when data were retrieved for analysis. The mortality rate at one month after surgery was 0%. The survival rate was 4%. The median OS for patients was found to be 11.17 months (confidence interval: 11.139-11.201). The rate of OS after 12 months of diagnosis was 41.26%. However, after two years of follow-up, the number of survivors was less than 4%.
Immunohistochemical and molecular characteristics
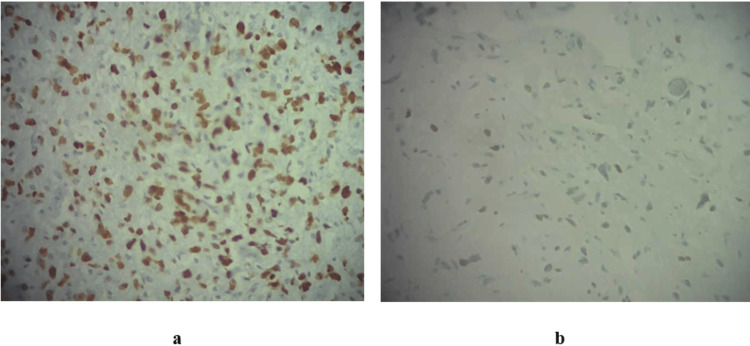
An IHC study was conducted using p53 and ATRX antibodies (Figure 4a). P53 immunostaining was >10% in 70 out of 126 patients (55.6%). ATRX expression was lost in 15 out of 126 patients (11.9%) (Figure 4b).
Figure 4. Glioblastoma labeled with two antibodies: p53 (a) and ATRX (b) (x400).
These microscopic observations show a nuclear expression of p53 (%) (a) and a loss of ATRX expression in a patient with astrocytoma, grade 4 (b).
Concerning the IDH-1 gene, the point mutation localized at codon 132 (CGT→CAT) was identified in 13.5% (17/126) of cases.
Clinical correlation of p53 status
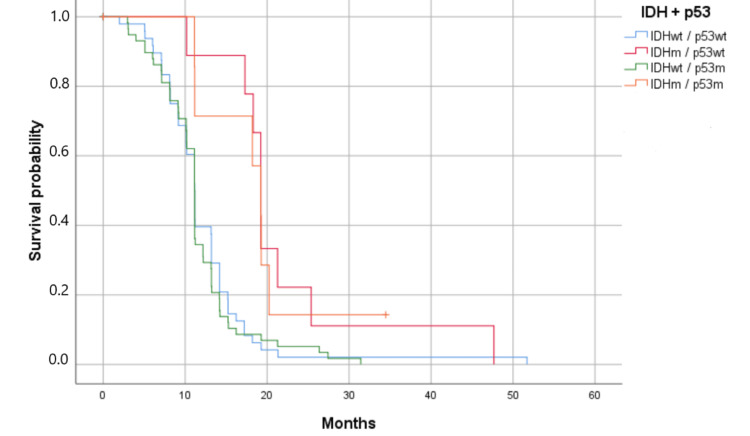
Associations of p53 with clinicopathological parameters were studied in 126 patients. For p53 immunostaining, cases were separated into four categories based on IDH and p53 status (Figure 5). Statistical analyses didn’t show an impact of clinical characteristics on p53 expression (p >0.05). Furthermore, p53 alone had no significant impact on survival (p = 0.726). On the other hand, the association between IDH and p53 statuses showed an impact on patients' OS (p = 0.001). Patients with IDH mutations had the best overall survival (19.230 months) (Figure 5).
Figure 5. Kaplan-Meier survival estimates by IDH mutation and p53 mutation (p = 0.001).
Survival has been represented by probability (percentage) and the number of months.
m: mutant; wt: wild-type
Patients with IDHm and p53wt had the best overall survival (p < 0.05).
Clinical correlation of ATRX status
ATRX status was analyzed in 126 patients; 15 of those cases (11.9%) presented an ATRX mutation. Patients with wild-type ATRX were significantly older (54 years versus 39 years, p = 0.000). The group of patients with the ATRX mutation had a KPS at admission of 90% vs. a KPS of 80% in the ATRX wild-type group (p = 0.278). The ATRX mutation group had a KPS at the last evaluation of 60% vs. a KPS of 40% in the ATRX wild-type group (p = 0.049).
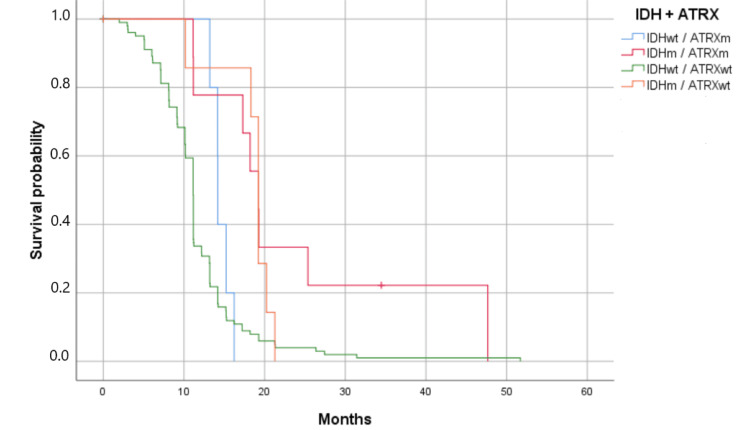
The median lifespan was 11.17 months for patients with ATRX wild-type, compared with 16.23 months for those with the ATRX mutation (p = 0.045). The association between IDH and ATRX status clearly shows an impact on patients' OS (p = 0.001). Patients with IDH mutations had the best OS (19.233 months) (Figure 6).
Figure 6. Kaplan-Meier survival estimates by IDH and ATRX mutations (p = 0.001).
Survival has been represented by probability (percentage) and the number of months.
m: mutant; wt: wildtype
Patients with the IDHm and the ATRXm had the best overall survival (p = 0.001).
Clinical correlation of p53 and ATRX status
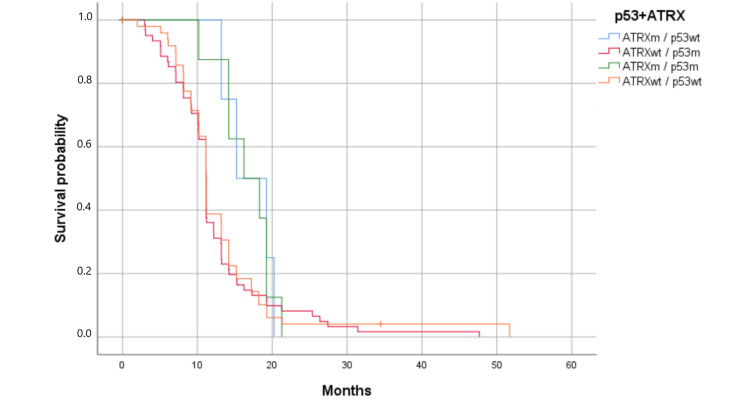
Cases were divided into four groups: Group A, ATRX mutant/p53 mutant; Group B, ATRX mutant/p53 wild-type; Group C, ATRX wild-type/p53 mutant; and Group D, ATRX wild-type/p53 wild-type. Kaplan-Meier curves showed that prognosis differed among the four groups. Group B had the best prognosis (OS = 16.23 months) and Group C had the poorest one (OS = 11.17 months) (Figure 7). The association of ATRX loss with p53 overexpression was not as robust in these tumors (p = 0.242).
Figure 7. Kaplan-Meier survival estimates by p53 and ATRX mutations (p = 0.242).
Survival has been represented by probability (percentage) and the number of months.
m: mutant; wt: wildtype
Patients with the ATRX mutation and p53 normal had the best survival; the association was not significant (p > 0.05).
Clinical correlation of p53, ATRX, and IDH status
The association of IDH, p53, and ATRX status was also studied in 126 patients. The statistical study revealed that the group of patients with IDH mutant/ATRX mutant/p53 wild-type had the best prognosis (OS = 27.393 months), and patients with IDH wild-type/ATRX wild-type/p53 mutant had the poorest one (OS = 11.255 months) (p = 0.015).
Discussion
Glioblastoma and astrocytoma, grade 4, are the most common primary aggressive brain tumors. The poor prognosis of these tumors is generally due to their ability to recur and resistance to therapies [15]. Recently, advances in molecular testing of gliomas have led to a better understanding of the pathogenesis and biology of these tumors. Classification of glial tumors into different prognostic groups enables us to establish appropriate protocols for better patient management [16]. Several studies have shown the relevance of numerous factors in the diagnosis and prognosis of glioblastoma patients, such as age, KPS and IDH, ATRX, and TP53 status. However, the impact of the latter remains subject to contradiction [15].
In this study, we examined associations between clinicopathological parameters and overall survival in 126 patients with grade 4 glioblastoma/astrocytoma. After follow-up, only 4% of patients were still alive, with other studies suggesting a five-year relative survival of less than 5% [17].
Our results showed that sex, tumor site, and surgical approach did not affect the prognosis. Young age (<40) and KPS at the last evaluation (≥70) were associated with significantly better patient survival (19.27 months vs. 11.17 months, p = 0.000). This result is in line with other studies suggesting that low KPS is an independent risk factor for death in these patients [18].
In addition, we evaluated the association between the expression of IDH, ATRX, and p53 and clinicopathological, radiological, and therapeutic data. We investigated the prognostic value of these biomarkers and their impact on overall survival parameters.
The IDH1R132H mutation has been detected in 13.5% of cases, particularly in young patients, compared to 10% and 12.9% in previous studies [19-21]. In line with several studies, this mutation was associated with a significant increase in OS for patients [17, 22, 23].
P53 nuclear staining ≥10% predicted the TP53 mutation with 84.8% sensitivity and 96.7% specificity [24], [25]. Discrepancies between the two tests could be explained by the presence of TP53 sequence alterations in intronic regions not covered by the primers used for sequencing. In addition, weak or negative immunostaining for p53 could be misinterpreted, particularly in the case of nonsense mutations that do not result in p53 overexpression and therefore will not be detected by immunohistochemistry. The overexpression of p53 was identified in 55.6% (70/126) of patients, compared to 48.4% and 50.8% in the literature [26, 9]. Contrary to some studies that reported that TP53 mutations were associated with reduced median survival, we didn’t observe any association between p53 status and patient survival [26, 27]. Kaplan-Meier analysis showed that patients with p53 overexpression had slightly poorer overall survival than patients without this mutation, even though there was no statistical significance. Our result was in line with previous studies [13, 26].
Ramamoorthy and Smith have shown that in the lack of ATRX, the histone variant macroH2A1.1 binds to tankyrase 1 polymerase, preventing it from localizing on telomeres and resolving cohesion, thus promoting recombination between sister telomeres. Forced resolution of this event induces genomic instability and hinders cell growth [28].
Regarding the detection of ATRX mutation, several studies suggest that gene panel next-generation sequencing analysis (NGS) was less sensitive than IHC in the detection of loss of ATRX expression [29]. This may be explained by epigenetic mechanisms resulting in the loss of ATRX or the presence of mutations in intronic or promoter regions not covered by the designed amplicons [29, 30]. However, the two techniques cannot be compared since NGS shows the mutation at the gene level, whereas IHC shows protein expression. Loss of ATRX was identified in 11.9% of cases, compared to 15.3% in another study [26], and has been significantly associated with better overall survival in high-grade glioma patients. This result is consistent with other studies [8, 9]. Patients with the ATRX mutation were significantly younger (39 years versus 54 years, p = 0.000) and had a KPS of 90% at admission vs. a KPS of 80% in the ATRX wild-type group (p = 0.278). The KPS at the last evaluation was 60% vs. a KPS of 40% in the ATRX wild-type group (p = 0.049).
In their studies, Zhou et al. and Xie et al. showed that the association of ATRX and IDH mutations, particularly in young patients, had a better prognosis and was associated with a high survival rate in grade 4 astrocytoma [8, 9]. Our study also highlighted the prognostic role of the ATRX mutation in a similar group. In our cohort, most patients who were IDHR132H mutants also had a loss of ATRX. However, other patients may have alternative mutations other than R132H, such as R132C and R132G. Thus, it would be interesting to explore other mutations that may affect the IDH gene, although they are in the minority.
Thus, testing for the ATRX mutation may have utility in the clinical management of high-grade glioma patients. As reported in a previous study [24], we observed that the association of ATRX loss with either TP53 mutation or p53 overexpression was not robust in these tumors (p = 0.242). Despite these findings, several limitations exist in this study, including its retrospective nature. Hence, this investigation is limited to the constraints of such studies. Furthermore, the sample size was small.
Conclusions
In summary, this study identified genetic alterations in IDH, ATRX, and TP53. In addition, we investigated the relationships between ATRX, p53, and IDH1 expression and clinicopathological parameters in high-grade gliomas.
Based on the results presented, the ATRX and IDH mutations conferred a survival advantage for glioma patients. The clinical management of glioma patients should therefore be targeted according to molecular features. These findings may be a potential therapeutic target for high-grade glial tumors, hence the need for further investigation.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Asmae Squalli Houssaini, Salma Lamrabet, Nadia Senhaji, Mehdi Karkouri
Acquisition, analysis, or interpretation of data: Asmae Squalli Houssaini, Nadia Senhaji, Jean Paul Nshizirungu, Mohammed Sekal, Mehdi Karkouri, Sanae Bennis, Hajar Mahfoudi, Samira Elfakir
Critical review of the manuscript for important intellectual content: Asmae Squalli Houssaini, Salma Lamrabet, Nadia Senhaji, Jean Paul Nshizirungu, Mohammed Sekal, Mehdi Karkouri, Sanae Bennis, Hajar Mahfoudi, Samira Elfakir
Drafting of the manuscript: Nadia Senhaji
Supervision: Sanae Bennis
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Updates in IDH-wildtype glioblastoma. Melhem JM, Detsky J, Lim-Fat MJ, Perry JR. Neurotherapeutics. 2022;19:1705–1723. doi: 10.1007/s13311-022-01251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epidemiology of glioblastoma multiforme-literature review. Grochans S, Cybulska AM, Simińska D, Korbecki J, Kojder K, Chlubek D, Baranowska-Bosiacka I. Cancers (Basel) 2022;14:2412. doi: 10.3390/cancers14102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The effects of 2-hydroxyglutarate on the tumorigenesis of gliomas. Reiter-Brennan C, Semmler L, Klein A. Contemp Oncol (Pozn) 2018;22:215–222. doi: 10.5114/wo.2018.82642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molecular and circulating biomarkers in patients with glioblastoma. Senhaji N, Squalli Houssaini A, Lamrabet S, Louati S, Bennis S. Int J Mol Sci. 2022;23:7474. doi: 10.3390/ijms23137474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Schwartzentruber J, Korshunov A, Liu XY, et al. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 6.Mutant ATRX: uncovering a new therapeutic target for glioma. Haase S, Garcia-Fabiani MB, Carney S, et al. Expert Opin Ther Targets. 2018;22:599–613. doi: 10.1080/14728222.2018.1487953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Koschmann C, Calinescu AA, Nunez FJ, et al. Sci Transl Med. 2016;8:328. doi: 10.1126/scitranslmed.aac8228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and genetic features of brainstem glioma in adults: a report of 50 cases in a single center. Zhou C, Zhao H, Yang F, Huangfu L, Dong C, Wang S, Zhang J. J Clin Neurol. 2021;17:220–228. doi: 10.3988/jcn.2021.17.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omics-based integrated analysis identified ATRX as a biomarker associated with glioma diagnosis and prognosis. Xie Y, Tan Y, Yang C, et al. Cancer Biol Med. 2019;16:784–796. doi: 10.20892/j.issn.2095-3941.2019.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glioblastoma multiforme: novel therapeutic targets. Muir M, Gopakumar S, Traylor J, Lee S, Rao G. Expert Opin Ther Targets. 2020;24:605–614. doi: 10.1080/14728222.2020.1762568. [DOI] [PubMed] [Google Scholar]
- 11.Gain-of-function (GOF) mutant p53 as actionable therapeutic target. Schulz-Heddergott R, Moll UM. Cancers (Basel) 2018;10:188. doi: 10.3390/cancers10060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gain of function of mutant TP53 in glioblastoma: prognosis and response to temozolomide. Wang X, Chen JX, Liu JP, You C, Liu YH, Mao Q. Ann Surg Oncol. 2014;21:1337–1344. doi: 10.1245/s10434-013-3380-0. [DOI] [PubMed] [Google Scholar]
- 13.The importance of IDH1, ATRX and WT-1 mutations in glioblastoma. Gülten G, Yalçın N, Baltalarlı B, Doğu G, Acar F, Doğruel Y. Pol J Pathol. 2020;71:127–137. doi: 10.5114/pjp.2020.97020. [DOI] [PubMed] [Google Scholar]
- 14.Assessment of pre-operative measurements of tumor size by MRI methods as survival predictors in wild type IDH glioblastoma. Palpan Flores A, Vivancos Sanchez C, Roda JM, et al. Front Oncol. 2020;10:1662. doi: 10.3389/fonc.2020.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Increased signal intensity in FLAIR sequences in the resection cavity can predict progression and progression-free survival in gliomas. Sarbu N, Oleaga L, Valduvieco I, Pujol T, Berenguer J. Neurocirugia (Astur) 2016;27:269–276. doi: 10.1016/j.neucir.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Association between IDH1/2 mutations and brain glioma grade. Deng L, Xiong P, Luo Y, Bu X, Qian S, Zhong W, Lv S. Oncol Lett. 2018;16:5405–5409. doi: 10.3892/ol.2018.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Impact of Immunohistochemical profiling of glioblastoma multiforme on clinical outcomes: real-world scenario in resource limited setting. Kumar N, Elangovan A, Madan R, et al. Clin Neurol Neurosurg. 2021;207:106726. doi: 10.1016/j.clineuro.2021.106726. [DOI] [PubMed] [Google Scholar]
- 18.Prognostic factors of patients with gliomas - an analysis on 335 patients with glioblastoma and other forms of gliomas. Liang J, Lv X, Lu C, et al. BMC Cancer. 2020;20:35. doi: 10.1186/s12885-019-6511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The 2021 WHO classification of tumors of the central nervous system: a summary. Louis DN, Perry A, Wesseling P, et al. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Retrospective and randomized analysis of influence and correlation of clinical and molecular prognostic factors in a mono-operative series of 122 patients with glioblastoma treated with STR or GTR. Salvati M, Bruzzaniti P, Relucenti M, et al. Brain Sci. 2020;10:91. doi: 10.3390/brainsci10020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EGFR amplification and IDH mutations in glioblastoma patients of the northeast of Morocco. Senhaji N, Louati S, Chbani L, et al. Biomed Res Int. 2017;2017:8045859. doi: 10.1155/2017/8045859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinicopathological correlation of glioma patients with respect to immunohistochemistry markers: a prospective study of 115 patients in a tertiary care hospital in North India. Dahuja G, Gupta A, Jindal A, Jain G, Sharma S, Kumar A. Asian J Neurosurg. 2021;16:732–737. doi: 10.4103/ajns.AJNS_377_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Targeting therapeutic vulnerabilities with PARP inhibition and radiation in IDH-mutant gliomas and cholangiocarcinomas. Wang Y, Wild AT, Turcan S, et al. Sci Adv. 2020;6:0. doi: 10.1126/sciadv.aaz3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Utility of ATRX immunohistochemistry in diagnosis of adult diffuse gliomas. Ikemura M, Shibahara J, Mukasa A, et al. Histopathology. 2016;69:260–267. doi: 10.1111/his.12927. [DOI] [PubMed] [Google Scholar]
- 25.Revisiting TP53 mutations and immunohistochemistry-a comparative study in 157 diffuse gliomas . Takami H, Yoshida A, Fukushima S, et al. Brain Pathol. 2015;25:256–265. doi: 10.1111/bpa.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Immunohistochemical analysis of ATRX, IDH1 and p53 in glioblastoma and their correlations with patient survival. Chaurasia A, Park SH, Seo JW, Park CK. J Korean Med Sci. 2016;31:1208–1214. doi: 10.3346/jkms.2016.31.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The p53 pathway in glioblastoma. Zhang Y, Dube C, Gibert M Jr, et al. Cancers (Basel) 2018;10:297. doi: 10.3390/cancers10090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loss of ATRX suppresses resolution of telomere cohesion to control recombination in ALT cancer cells. Ramamoorthy M, Smith S. Cancer Cell. 2015;28:357–369. doi: 10.1016/j.ccell.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.A custom DNA-based NGS panel for the molecular characterization of patients with diffuse gliomas: diagnostic and therapeutic applications. Tirrò E, Massimino M, Broggi G, et al. Front Oncol. 2022;12:861078. doi: 10.3389/fonc.2022.861078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The efficacy of immunohistochemistry in the diagnosis of molecular genetic alterations in central nervous system gliomas: next-generation sequencing of 212 mutations in 112 patients. Daoud EV, Chkheidze R, Yell PC, Hatanpaa KJ, Raisanen JM, Cai C. Clin Neuropathol. 2022;41:35–40. doi: 10.5414/NP301381. [DOI] [PubMed] [Google Scholar]