Abstract
Tolerance to both real and simulated haemorrhage varies between individuals. Exaggerated low-frequency (~0.1 Hz) oscillations in mean arterial pressure and brain blood flow [indexed via middle cerebral artery velocity (MCAv)] have been associated with improved tolerance to reduced central blood volume. The mechanism for this association has not been explored. We hypothesized that inducing low-frequency oscillations in arterial pressure and cerebral blood velocity would attenuate reductions in cerebral blood velocity and oxygenation during simulated haemorrhage. Fourteen subjects (11 men and three women) were exposed to oscillatory (0.1 and 0.05 Hz) and non-oscillatory (0 Hz) lower-body negative pressure profiles with an average chamber pressure of −60 mmHg (randomized and counterbalanced order). Measurements included arterial pressure and stroke volume via finger photoplethysmography, MCAv via transcranial Doppler ultrasound, and cerebral oxygenation of the frontal lobe via near-infrared spectroscopy. Tolerance was higher during the two oscillatory profiles compared with the 0 Hz profile (0.05 Hz, P = 0.04; 0.1 Hz, P = 0.09), accompanied by attenuated reductions in stroke volume (P < 0.001) and cerebral oxygenation of the frontal lobe (P ≤ 0.02). No differences were observed between profiles for reductions in mean arterial pressure (P = 0.17) and MCAv (P = 0.30). In partial support of our hypothesis, cerebral oxygenation, but not cerebral blood velocity, was protected during the oscillatory profiles. Interestingly, more subjects tolerated the oscillatory profiles compared with the static 0 Hz profile, despite similar arterial pressure responses. These findings emphasize the potential importance of haemodynamic oscillations in maintaining perfusion and oxygenation of cerebral tissues during haemorrhagic stress.
Keywords: cerebral blood flow, cerebral oxygen saturation, oscillatory lower-body negative pressure
1 |. INTRODUCTION
Haemorrhage accounts for 30–40% of deaths resulting from traumatic injury in the civilian setting (Kauvar, Lefering, & Wade, 2006) and 90% of potentially survivable deaths on the battlefield (Eastridge et al., 2012). During haemorrhage, blood is shunted preferentially to vital organs to ensure tissue survival under this stress (Chen et al., 1984; Forsyth, Hoffbrand, & Melmon, 1970). As the brain is one of the most essential and metabolically active organs of the body (Rowell, 1993), reflex physiological mechanisms are activated to reduce any disruption of blood flow and oxygen/nutrient supply to this organ. These mechanisms include the preferential shunting of blood to the brain (Chen et al., 1984), cerebral autoregulation and associated vasodilatation (MacKenzie et al., 1979), and increased oxygen extraction (Chen et al., 1984). The maintenance of blood flow and oxygenation to the brain is paramount to survival of neural tissues and crucial in reducing long-term complications associated with survival from haemorrhagic injuries.
Lower-body negative pressure (LBNP) has been shown to be an appropriate model for studying the physiological responses to the early ‘pre-shock’ stages of haemorrhage in healthy, conscious human subjects (Hinojosa-Laborde et al., 2014; Johnson et al., 2014; van Helmond et al., 2015, 2016). Both blood loss and LBNP elicit similar responses in key haemodynamic parameters, including heart rate, stroke volume, cardiac output, arterial pressure and cerebral blood velocity (Hinojosa-Laborde et al., 2014; Johnson et al., 2014; Rickards et al., 2015). Typically, LBNP is applied by stepwise reductions in chamber pressure until the subject reaches ‘presyncope’, which is commonly defined as a systolic blood pressure ≤80 mmHg and/or the subject experiences symptoms such as dizziness, visual disturbances, nausea, or other subjective sensations of impending syncope. In studies using maximal LBNP to presyncope, it has been demonstrated that there is a continuum of tolerance among healthy human subjects (Kay & Rickards, 2016; Levine, Giller, Lane, Buckey, & Blomqvist, 1994; Lightfoot & Tsintgiras, 1995; Rickards, Ryan, Cooke, & Convertino, 2011), which has been attributed, in part, to protection of absolute blood flow (Levine et al., 1994; Lieshout, Wieling, Karemaker, & Secher, 2003) in either the anterior (Levine et al., 1994; Rickards et al., 2011) or the posterior circulation (Kay & Rickards, 2016) of the brain. However, accumulating evidence suggests that the pattern of cerebral blood flow during simulated haemorrhagic stress might also be related to tolerance.
Results from recent studies indicate that low-frequency oscillations (within the 0.04–0.15 Hz range) in brain blood flow and arterial pressure are associated with increased tolerance to central hypovolaemia (Lucas, Lewis, Sikken, Thomas, & Ainslie, 2013; Rickards et al., 2011). The observation of oscillations in blood pressure during haemodynamic challenges such as haemorrhage and orthostatic stress were first published >60 years ago. In studies on haemorrhaged dogs with varying magnitudes of blood loss, a low-frequency oscillatory pattern in blood pressure was observed and was attributed to a baroreflex-mediated mechanism (Guyton & Harris, 1951; Madwed & Cohen, 1991). These low-frequency oscillations in arterial pressure are also evident in humans subjected to graded head-up tilt (Cooke et al., 1999) and LBNP (Cooke, Rickards, Ryan, Kuusela, & Convertino, 2009; Zamir, Goswami, Liu, Salmanpour, & Shoemaker, 2011). Although the study of arterial pressure oscillations has been extensive, only recently has this phenomenon been linked to tolerance to central hypovolaemia. Rickards et al. (2011) reported that subjects with high tolerance to LBNP exhibited higher low-frequency amplitude in both cerebral blood velocity [as measured by middle cerebral artery velocity (MCAv)] and mean arterial pressure (MAP) compared with subjects who had low tolerance. Likewise, when breathing rate was paced at 0.1 Hz, Lucas et al. (2013) reported increases in tolerance to the central hypovolaemic challenge of head-up tilt plus LBNP, along with an attenuated rate of decline in MCAv and MAP compared with the spontaneous breathing conditions.
The possible mechanisms underlying the relationship between increased low-frequency arterial pressure oscillations and improved tolerance to central hypovolaemia have yet to be studied, but might be linked to increased cerebral blood flow and improved cerebral oxygen delivery. To study the effects of these oscillations, we used oscillatory lower-body negative pressure (OLBNP) at frequencies of 0.1 and 0.05 Hz, which correspond to the frequencies of arterial pressure oscillations caused by sympathetic outflow (Stauss, 2007) and myogenic activity (Tzeng, Chan, Willie, & Ainslie, 2011). Forced oscillations in arterial pressure at these frequencies with OLBNP have also been shown to increase cerebral blood velocity oscillations (Hamner, Cohen, Mukai, Lipsitz, & Taylor, 2004). We hypothesized that inducing low-frequency oscillations in arterial pressure and cerebral blood velocity would attenuate the decrease in cerebral blood velocity and oxygenation during simulated haemorrhagic stress.
2 |. METHODS
2.1 |. Ethical approval
The experimental protocol was reviewed and approved by the institutional review board at the University of North Texas Health Science Center (protocol no. 2016–049) in accordance with the standards set by the Declaration of Helsinki, except for registration in a database. All subjects provided informed, written consent before inclusion in the study.
2.2 |. Subjects
Young, healthy subjects were recruited to participate in this study conducted at the University of North Texas Health Science Center. Subjects participated in two sessions in the laboratory, a familiarization session and an experimental session. Upon arrival for the familiarization session, subjects were briefed regarding the aims of the research study, the experimental protocol and associated risks, and given opportunities to ask questions. After written consent, subjects completed a health history questionnaire, and height, weight, sex, and age were recorded. Female subjects took a urine pregnancy test to ensure they were not pregnant. This was followed by seated and standing 12-lead ECG and blood pressure assessments, which were subsequently reviewed by a physician. After these medical screening assessments, subjects were placed in the LBNP chamber (VUV Analytics, Austin, TX, USA) while it was turned on to familiarize them with the sensation of the experimental protocol.
At least 24 h after the familiarization session, subjects returned to the laboratory for the experimental session. Female subjects were tested during the first 4 days of their menstrual cycle, corresponding to the early follicular/low-hormone phase. For 24 h before experimentation, subjects refrained from caffeine, alcohol, dietary supplements, medication and exercise. Upon arrival in the laboratory, subject height, weight and age were again recorded, and female subjects completed a urine pregnancy test to ensure they were still not pregnant. Subjects were then invited to use the restroom before instrumentation to account for potential confounding effects of bladder distension on sympathetic nerve activity (Fagius & Karhuvaara, 1989).
2.3 |. Instrumentation
All subjects were placed in the LBNP chamber in the supine position, with their iliac crest aligned with the opening of the chamber; they were sealed into the chamber with heavy-duty plastic and a neoprene band around the waist. Subjects were instrumented for continuous ECG recording in a standard lead II configuration (shielded leads, cable and amplifier; AD Instruments, Bella Vista, NSW, Australia). A photoplethysmography blood pressure monitor (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) was attached via a finger cuff for continuous measurement of arterial pressure and calculation of stroke volume. An oral/nasal cannula or face mask was used for continuous recording of end-tidal carbon dioxide (etCO2) and calculation of respiratory rate via a gas analyser (ML206 Gas Analyzer; AD Instruments). Cerebral blood velocity was measured in the middle cerebral artery (MCA) through the temporal window with a transcranial Doppler (TCD) ultrasound probe (2 MHz; ST3; Spencer Technologies, Seattle, WA, USA) placed on either side of the head. Cerebral oxygen saturation (ScO2) and muscle oxygenation (SmO2) were measured by placing a near-infrared spectroscopy (NIRS) sensor (OxiplexTS; ISS, Champaign–Urbana, IL, USA) over one of the frontal lobes and over the flexor carpi ulnaris muscle of the forearm. Efforts were made to measure MCAv and ScO2 on the same side of the head. An indwelling catheter was placed in an antecubital fossa vein for blood sampling.
2.4 |. Protocol
After instrumentation, baseline measurements of respiration rate were collected for 2 min. Subjects were then coached to breathe in time with a metronome as follows: (i) if spontaneous respiration rate was ≥10 breaths min−1, the metronome was set at the subject’s spontaneous rate; or (ii) if the subject’s spontaneous respiration rate was <10 breaths min−1, the metronome was set to pace breathing at 10 breaths min−1. This breathing rate was maintained throughout all conditions. Pacing the breathing at a rate of ≥10 breaths min−1 ensured that breathing rate did not influence low-frequency oscillations in MAP and MCAv (i.e. 10 breaths min−1 is a frequency of 0.17 Hz).
After successfully pacing breathing, a 5 min baseline period began. One of three OLBNP profiles was then used to assess the role of arterial pressure oscillations on cerebral blood velocity and cerebral tissue oxygenation during central hypovolaemia (Figure 1). The order of the profiles was randomized and counter-balanced between subjects. The three profiles were as follows: (i) a 0 Hz (static) hypovolaemic profile, with the LBNP chamber pressure progressively reduced to −60 mmHg over 1 min and held at that pressure for a further 9 min period; (ii) a 0.1 Hz hypovolaemic profile, with chamber pressure progressively reduced to −60 mmHg for 1 min, after which pressure oscillated between −30 mmHg for 5 s and −90 mmHg for 5 s over 9 min (amplitude of 60 mmHg; 54 cycles over 9 min); and (iii) a 0.05 Hz hypovolaemic profile, with chamber pressure progressively reduced to −60 mmHg for 1 min, after which pressure oscillated between −30 mmHg for 10 s and −90 mmHg for 10 s over 9 min (amplitude of 60 mmHg; 27 cycles over 9 min). A 5 min recovery period intervened between each profile, with a 10 min recovery at the end of the experiment. Subjects were continuously monitored during the entire protocol, and LBNP was released if systolic arterial pressure fell below 80 mmHg or the subject reported symptoms such as visual disturbances, dizziness, light-headedness or nausea at any time.
FIGURE 1.
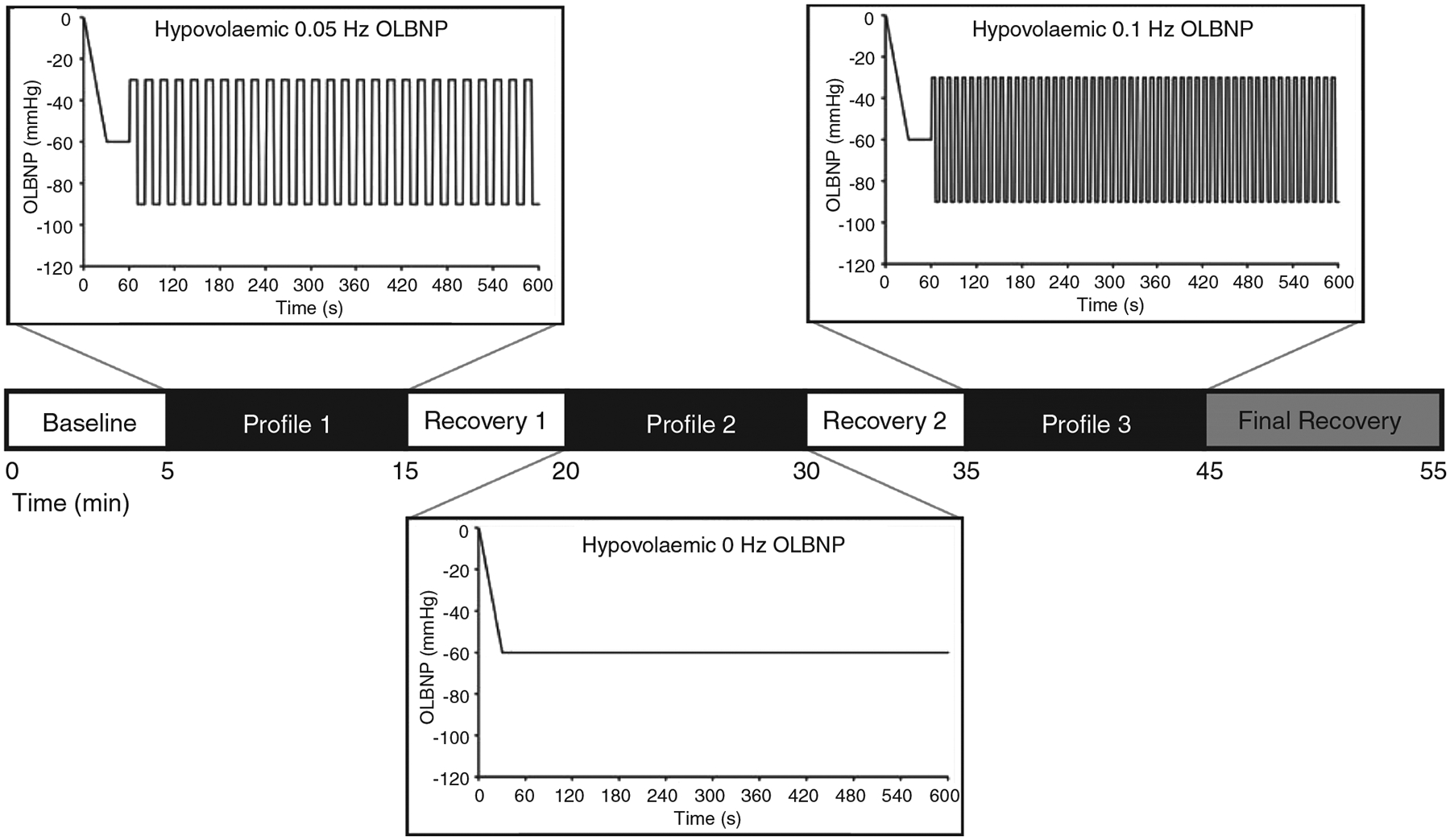
A representative experimental time line. Each profile was 10 min in duration, including a 1 min transition from 0 to −60 mmHg lower-body negative pressure (LBNP) and 9 min of oscillations (OLBNP). Each recovery period was 5 min in duration, and 10 min at the end of the experimental protocol. The order of profiles was randomized and counterbalanced
2.5 |. Blood sample collection and analysis
Seven 10 ml venous blood samples were taken over the course of the experiment to include baseline, the end of each of the three oscillatory profiles and the end of each recovery period (Figure 1). Whole blood samples were collected into a chilled syringe and placed into EDTA tubes treated with glutathione (1.23 mg glutathione per 1 ml whole blood) as a preservative. Samples were centrifuged at 4°C for 15 min at 483 g (1500 r.p.m.). Plasma was extracted and placed in Eppendorf tubes, then snap frozen in liquid nitrogen and stored in a freezer at −80°C for subsequent analysis. Noradrenaline was measured in duplicate with an enzyme-linked immunosorbent assay (BA E-6200; Rocky Mountain Diagnostics, Colorado Springs, CO, USA). Duplicate samples with a coefficient of variation <15% were included in the final analysis.
2.6 |. Data analysis
Continuous waveform recordings of ECG, arterial pressure, stroke volume, MCAv, ScO2 and end-tidal gases were sampled and recorded at 1000 Hz (PowerLab/Labchart; AD Instruments) for later analysis using specialized software (WinCPRS; Absolute Aliens, Turku, Finland). Baseline measurements were averaged over the entire 5 min period. The time frame used for analysis of time-domain data from the LBNP profiles was the final 1 min preceding the end of each LBNP profile or presyncope if the subject did not tolerate the entire protocol. This time frame corresponded to the maximal response during LBNP. The time frame used for frequency-domain analyses was ≥4 min and a maximum of 9 min if subjects tolerated the entire protocol; this extended time frame was required for accuracy of frequency-domain metrics. The final 1 min of each 5 min recovery period was also used for time-domain analysis.
R-waves were detected from the ECG signal and used to determine heart rate. Systolic and diastolic arterial pressures and systolic and diastolic cerebral blood velocities were then marked from the Finometer and TCD traces. MAP and mean MCAv were calculated as the area under the arterial pressure and cerebral blood velocity waveforms with the WinCPRS software. Stroke volume was recorded directly from the Finometer via PowerLab/LabChart. Cardiac output was subsequently calculated as heart rate multiplied by stroke volume, and total peripheral resistance was then calculated as MAP divided by cardiac output. Integrated cardiac baroreflex sensitivity was assessed by dividing the change in heart rate from baseline by the change in MAP from baseline (Mancia & Mark, 2011).
Oscillatory patterns of arterial blood pressures, cerebral blood velocities and etCO2 were determined using fast Fourier power spectral analysis, as previously described from our laboratory (Rickards, Sprick, Colby, Kay, & Tzeng, 2015). Data were made equidistant by interpolating linearly and resampling at 5 Hz. Data were then passed through a finite low-pass impulse response filter with a cut-off frequency of 0.5 Hz. A minimum of 4 min of data was used for fast Fourier transformation with a Hanning window, using the Welch method to compute power spectra. The bands used for frequency analysis were 0.04–0.07 and 0.07–0.15 Hz, corresponding to the very low (VLF), and low (LF) frequencies. Spectral power was expressed as the integrated area within the VLF and LF ranges.
2.7 |. Statistical analysis
All data from the OLBNP protocols were analysed using a linear mixed model for repeated measures followed by Tukey’s post hoc tests (JMP Pro 10; SAS Institute Inc., Cary, NC, USA). A separate linear mixed model for repeated measures was used to analyse the baseline and recovery periods, followed by Tukey’s post hoc tests. Only the baseline and first and second recovery periods were included in this analysis because the final 10 min recovery period did not have a subsequent LBNP profile. A one-way repeated-measures ANOVA was performed on tolerance time with profile order as the factor to test for possible order effects. Unless otherwise stated, all data are presented as the mean ± SD, and exact P-values are reported for all comparisons.
3 |. RESULTS
Eighteen subjects were initially recruited to participate in this study. Four of these subjects were excluded due to technical difficulties in obtaining key outcome measurements (n = 2), self-exclusion due to anxiety from the experimental procedures (n = 1), and investigator withdrawal due to irregularity in the ECG recordings during the familiarization session (n = 1). As a result, 14 subjects completed the entire protocol and were included in the final analysis (11 male and three female; age, 26.2 ± 2.8 years; height, 175.6 ± 8.5 cm; and weight, 78.3 ± 15.8 kg). The average time between the familiarization session and experimental session was 34 ± 23 days, with a range of 8–77 days.
The average chamber pressure during each profile was: (i) 0 Hz, −61.6 ± 2.6 mmHg; (ii) 0.1 Hz, −57.4 ± 2.3 mmHg; and (iii) 0.05 Hz, −57.8 ± 2.1 mmHg. Tolerance to the two oscillatory profiles was higher compared with the static 0 Hz profile (0 Hz, 424.3 ± 146.1 s; 0.1 Hz, 502.9 ± 84.7 s; and 0.05 Hz, 515.9 ± 87.5 s; main effect, P = 0.03; see Figure 2 for post hoc test P-values), with no order effect (P = 0.98). This higher tolerance was accompanied by an attenuated decrease in ScO2 during the oscillatory profiles compared with the static profile (P = 0.003), despite similar reductions in mean MCAv across all profiles (P = 0.30; Table 1; Figure 3). Diastolic MCAv was similar across profiles, and there was an attenuated decrease in systolic MCAv for the 0.05 Hz profile only (Table 1). Note that, due to difficulties in finding and maintaining a TCD signal in one subject, 13 MCAv recordings were used for the final analysis. Respiration rate was successfully paced at >10 breaths min−1 for all subjects during each profile, and although etCO2 decreased during each of the profiles, it was not different between profiles (Table 1). Stroke volume was reduced with all profiles, but the percentage change in stroke volume was attenuated during the oscillatory profiles compared with the static 0 Hz profile, resulting in an attenuated increase in heart rate for these conditions (Figure 4). These differential stroke volume and heart rate responses between profiles resulted in similar reductions in cardiac output across the three profiles (P = 0.18; Figure 4). Likewise, there were no differences observed in the reduction of MAP (P = 0.17), the increase in total peripheral resistance (P = 0.75), or integrated baroreflex sensitivity (P = 0.46) between profiles (Figure 5; Table 1). Although no difference was observed in MAP, both systolic arterial pressure and pulse pressure were higher during the oscillatory profiles (Table 1; Figure 5). The attenuated reduction in pulse pressure with 0.1 and 0.05 Hz OLBNP may be related to the protected stroke volumes in these conditions. With regard to muscle oxygen saturation, the pattern of responses in SmO2 was similar to that of ScO2, with attenuated decreases during the oscillatory profiles (Table 1; Figure 5).
FIGURE 2.
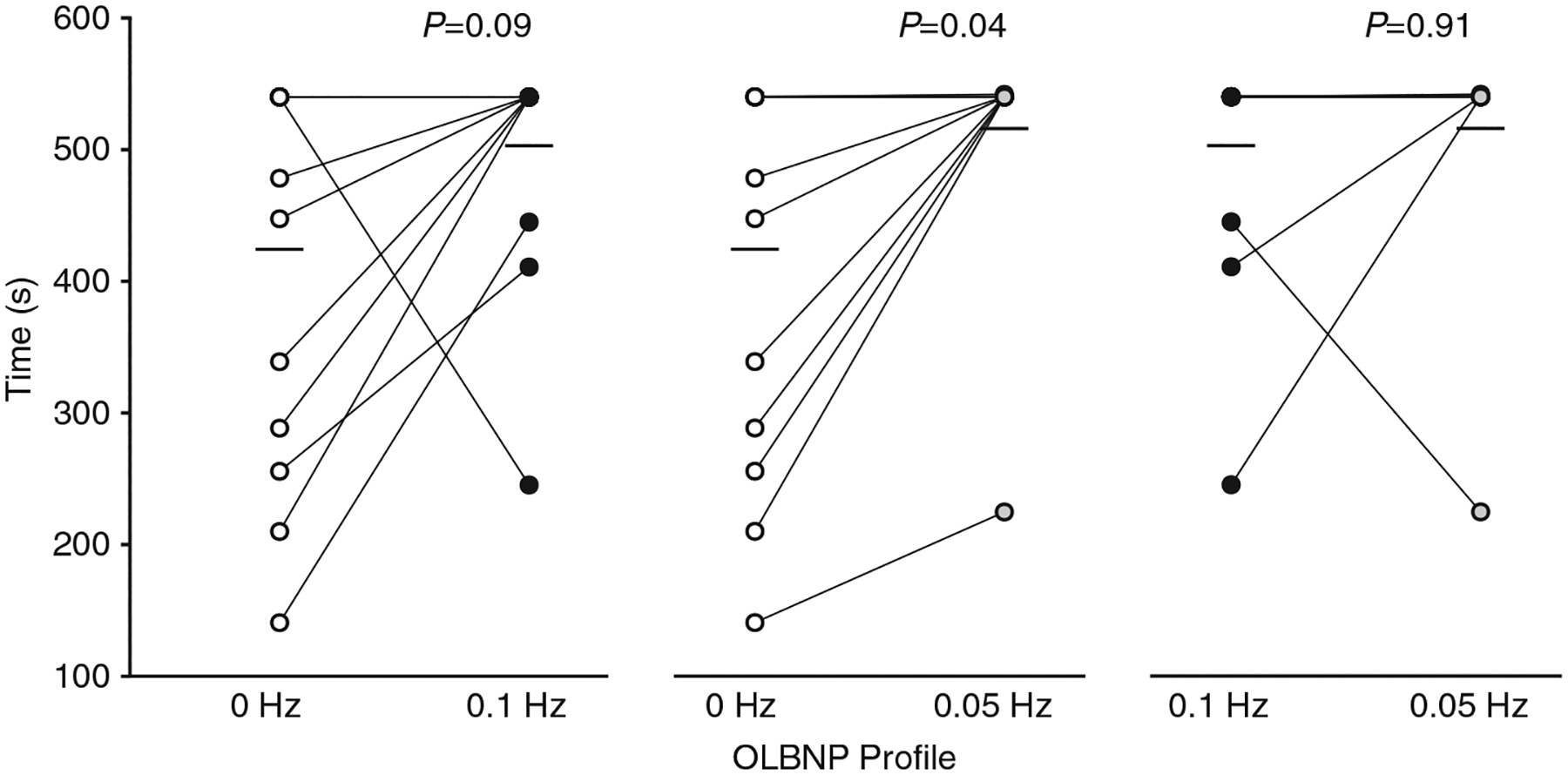
Tolerance to each oscillatory lower-body negative pressure (OLBNP) profile (time). Individual subject responses (circles) and mean responses (filled bars) are presented. Open circles, 0 Hz; black circles, 0.1 Hz; and grey circles, 0.05 Hz. Tolerance was higher for both the 0.1 and 0.05 Hz oscillatory profiles compared with the static 0 Hz profile. Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Owing to the 540 s cut-off for each of the profiles, subject data points may overlap. The number of subjects in each profile are as follows: 0 Hz profile, n = 14; 0.1 Hz profile, n = 14; and 0.05 Hz profile, n = 13
TABLE 1.
Absolute cardiovascular, cerebrovascular and respiratory responses during each oscillatory lower-body negative pressure (OLBNP) profile
| Parameter | 0 Hz OLBNP | 0.l Hz OLBNP | 0.05 Hz OLBNP | Main effect P-value |
|---|---|---|---|---|
| Cardiovascular | ||||
| Heart rate (beats min−1) | 101.2 ± 16.3 | 79.8 ± 10.1* | 81.5 ± 14.1* | <0.001 |
| Systolic arterial pressure (mmHg) | 98.8 ± 11.7 | 105.5 ± 9.0* | 104.4 ± 8.1* | 0.03 |
| Diastolic arterial pressure (mmHg) | 66.0 ± 8.0 | 65.2 ± 5.6 | 65.3 ± 3.9 | 0.88 |
| Mean arterial pressure (mmHg) | 77.1 ± 8.6 | 79.7 ± 6.7 | 79.7 ± 4.9 | 0.28 |
| Integrated baroreflex sensitivity (ΔHR/ΔMAP; beats min−1 mmHg−1) | 6.2 ± 8.6 | 6.1 ± 8.4 | 3.1 ± 1.9 | 0.46 |
| Cerebrovascular | ||||
| ScO2 (%) | 65.2 ± 11.3 | 67.1 ± 11.3* | 66.6 ± 12.2* | 0.003 |
| Deoxygenated haemoglobin (μm) | 15.6 ± 4.2 | 14.9 ± 4.2* | 15.5 ± 4.1 | 0.02 |
| Oxygenated haemoglobin (μm) | 30.5 ± 10.0 | 31.9 ± 10.2* | 32.8 ± 11.6* | 0.008 |
| Systolic MCAv (cm s−1) | 65.4 ± 18.1 | 68.6 ± 18.7 | 72.4 ± 21.9* | 0.03 |
| Diastolic MCAv (cm s−1) | 34.0 ± 9.7 | 34.1 ± 10.0 | 37.1 ± 11.2 | 0.27 |
| Mean MCAv (cm s−1) | 43.2 ± 11.6 | 44.3 ± 12.6 | 47.3 ± 14.2 | 0.14 |
| Peripheral vasculature | ||||
| SmO2 (%) | 61.8 ± 6.8 | 65.8 ± 6.6* | 65.0 ± 6.4* | <0.001 |
| Deoxygenated haemoglobin (μm) | 30.1 ± 8.7 | 27.0 ± 7.7* | 28.1 ± 7.4* | <0.001 |
| Oxygenated haemoglobin (μm) | 48.2 ± 9.0 | 51.5 ± 10.3* | 52.1 ± 11.2* | <0.001 |
| Respiratory | ||||
| etCO2 (mmHg) | 28.8 ± 8.4 | 30.3 ± 8 | 28.8 ± 8.9 | 0.50 |
| Respiratory rate (breaths min−1) | 14.4 ± 4.3 | 14.1 ± 3.8 | 16.2 ± 5.9 | 0.22 |
Abbreviations: etCO2, end-tidal carbon dioxide; HR, heart rate; MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; ScO2 , cerebral oxygen saturation; and SmO2 , muscle oxygen saturation. Data were analysed with a mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD.
P = 0.001–0.06 versus 0 Hz.
FIGURE 3.
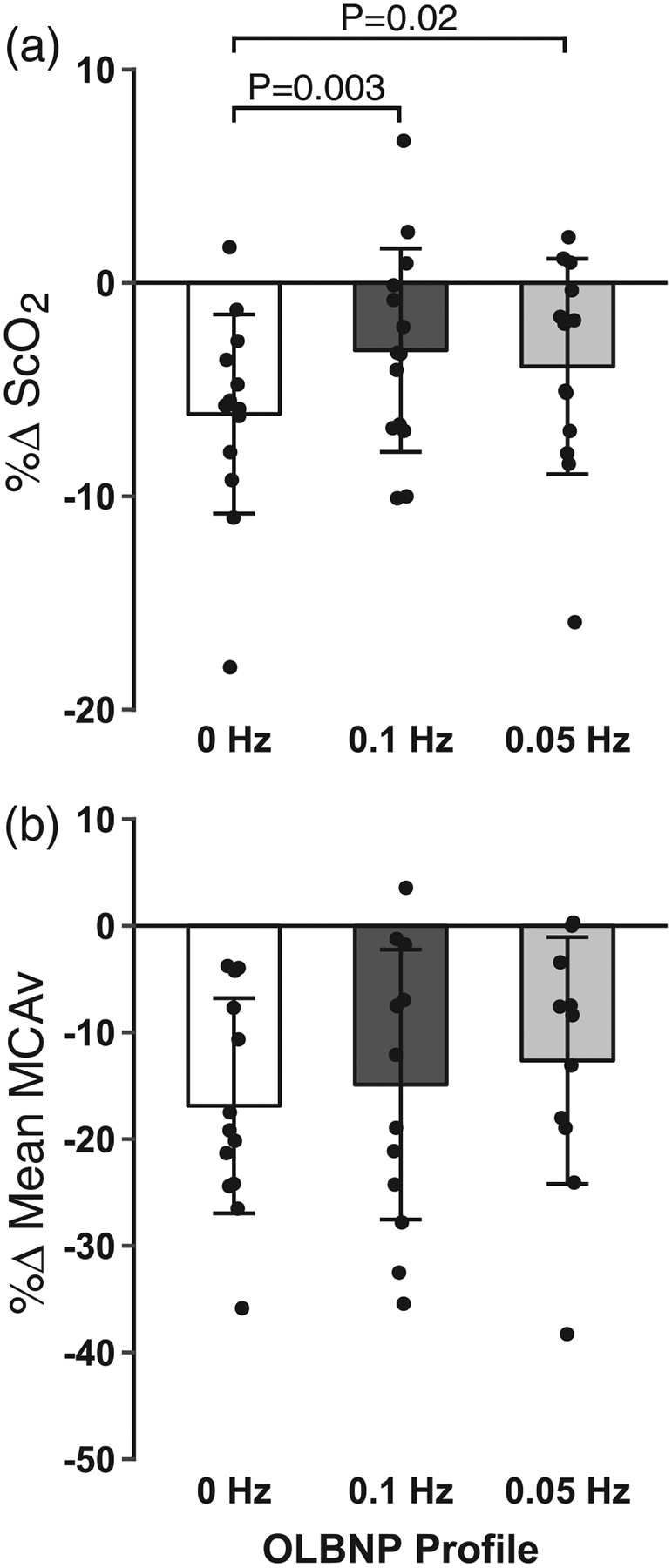
Mean and individual subject data for the percentage change in cerebral oxygen saturation (ScO2 ; a) and middle cerebral artery velocity (MCAv; b) during 0, 0.1 and 0.05 Hz oscillatory lower-body negative pressure (OLBNP). Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD
FIGURE 4.
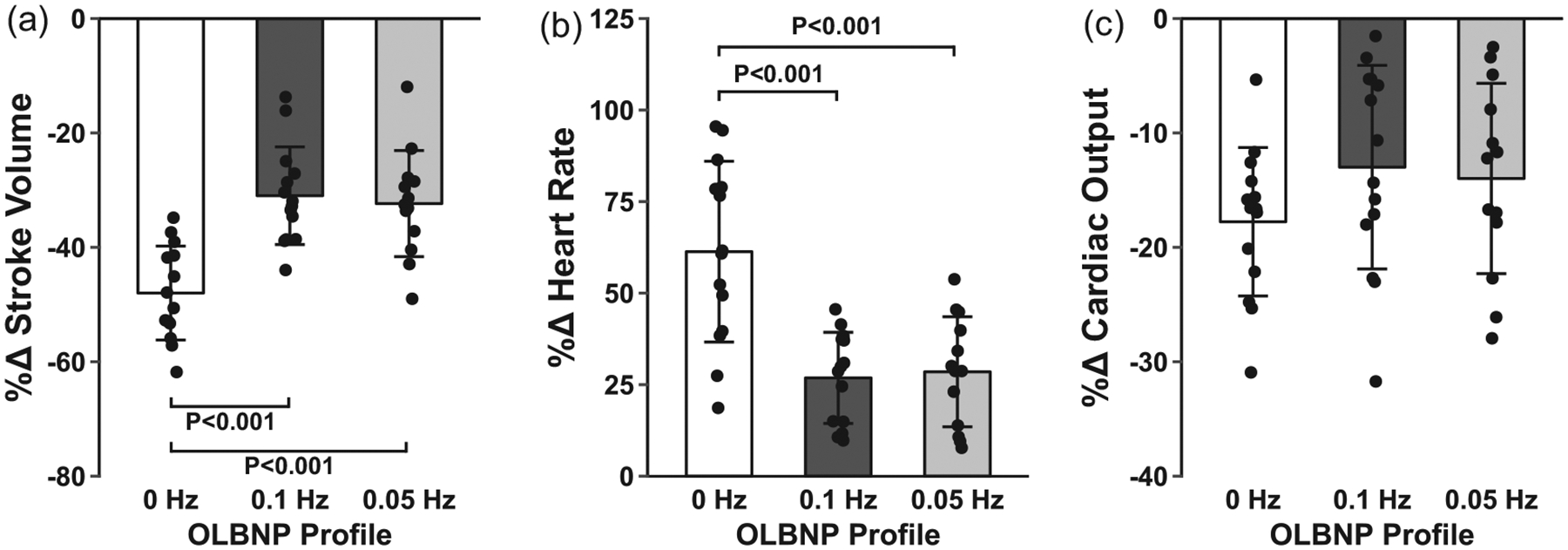
Mean and individual subject data for the percentage change in stroke volume (a), heart rate (b) and cardiac output (c) with 0, 0.1 and 0.05 Hz oscillatory lower-body negative pressure (OLBNP). Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD
FIGURE 5.
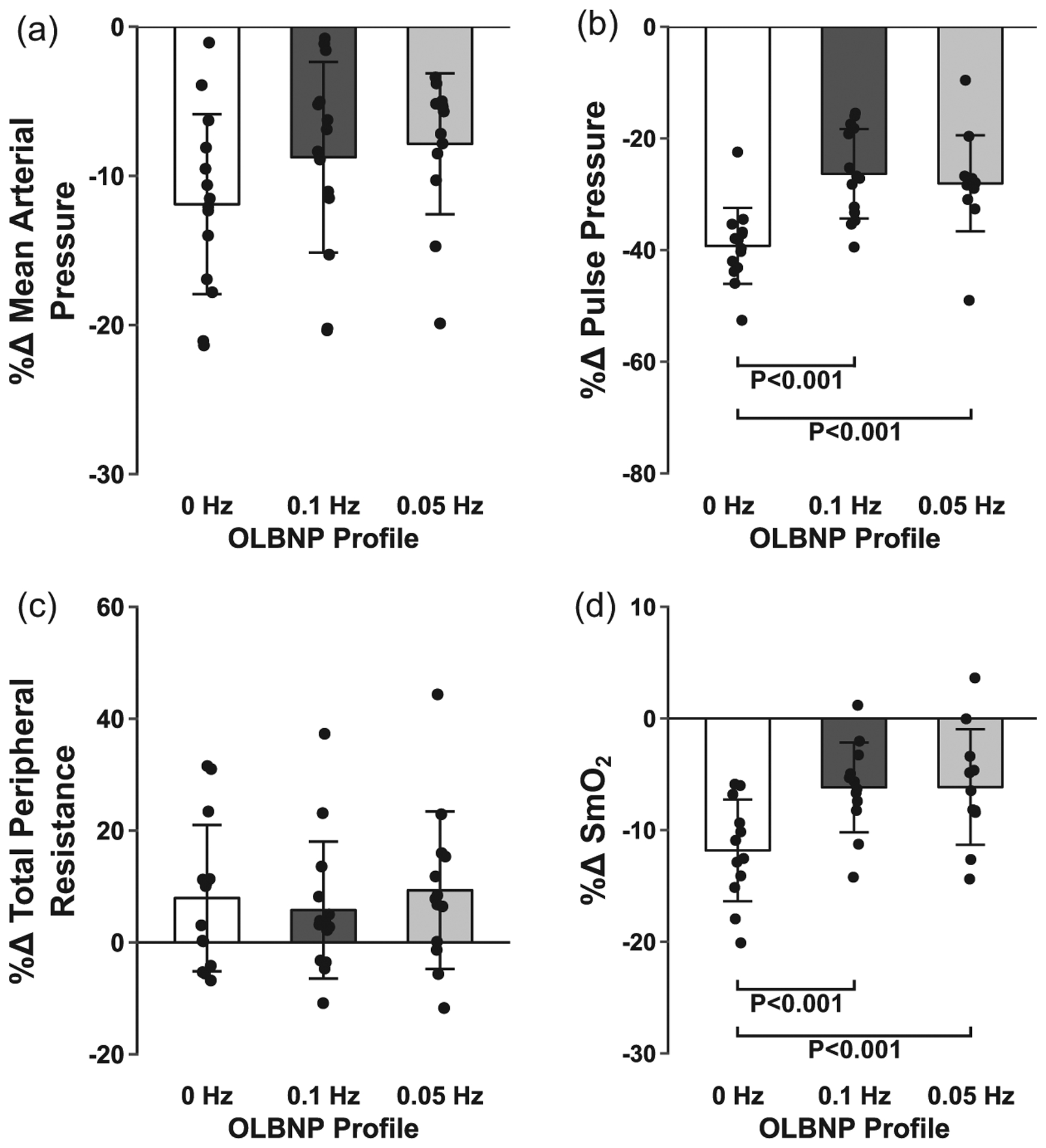
Mean and individual subject data for the percentage change in mean arterial pressure (a), pulse pressure (b), total peripheral resistance (c) and muscle oxygen saturation (SmO2; d) with 0, 0.1 and 0.05 Hz oscillatory lower-body negative pressure (OLBNP). Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD
Blood samples were collected successfully in a subset of subjects for the analysis of venous plasma concentrations of noradrenaline (n = 11). Circulating noradrenaline concentrations reflected the response of heart rate and pulse pressure and were lower during the oscillatory profiles compared with 0 Hz (Figure 6), but did not return to baseline concentrations during each recovery period (Table 2).
FIGURE 6.
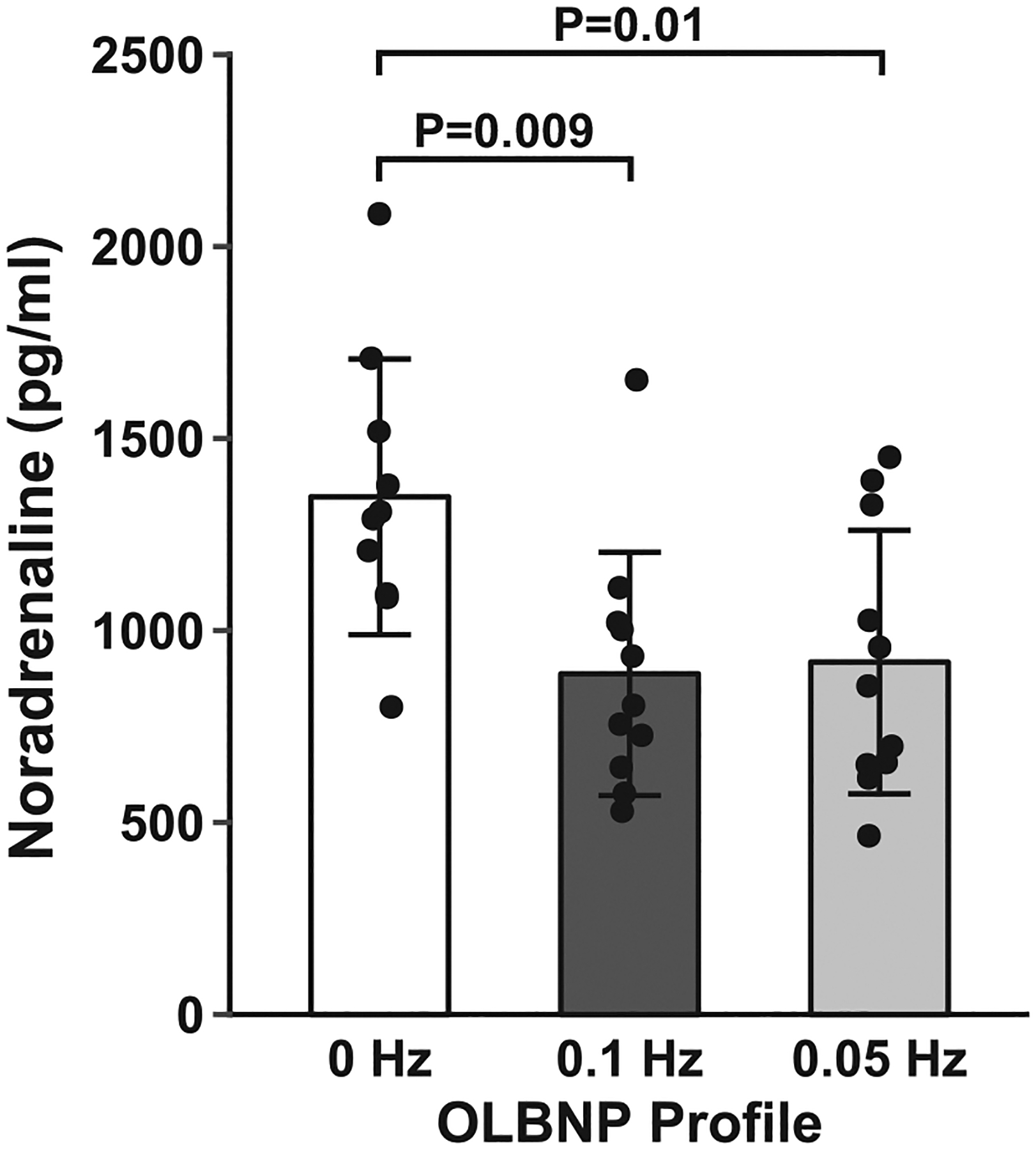
Mean and individual subject data for the plasma noradrenaline responses to 0, 0.1 and 0.05 Hz oscillatory lower-body negative pressure (OLBNP). Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD
TABLE 2.
Absolute cardiovascular, cerebrovascular and respiratory responses for baseline and during the two recovery periods intervening between the oscillatory lower-body negative pressure (OLBNP) profiles
| Parameter | Baseline | Recovery 1 | Recovery 2 | Main effect P-value |
|---|---|---|---|---|
| Cardiovascular | ||||
| Heart rate (beats min−1) | 63.4 ± 9.5 | 59.8 ± 12.6 | 60.1 ± 12.9 | 0.08 |
| Systolic arterial pressure (mmHg) | 122.5 ± 8.6 | 128.1 ± 13.1* | 127.7 ± 12.7* | 0.03 |
| Diastolic arterial pressure (mmHg) | 67.7 ± 5.3 | 67.3 ± 14.6 | 71.7 ± 4.6 | 0.37 |
| Mean arterial pressure (mmHg) | 87.4 ± 6.3 | 93.0 ± 7.9* | 91.8 ± 7.9* | 0.009 |
| Cerebrovascular | ||||
| ScO2 (%) | 69.2 ± 10.9 | 69.4 ± 11.3 | 69.4 ± 11.5 | 0.95 |
| Deoxygenated haemoglobin (μM) | 13.8 ± 3.8 | 13.9 ± 3.7 | 14.1 ± 3.7 | 0.69 |
| Oxygenated haemoglobin (μM) | 32.9 ± 11.0 | 33.8 ± 12.2 | 33.9 ± 12.3 | 0.36 |
| Systolic MCAv (cm s−1) | 82.0 ± 22.9 | 80.5 ± 20.9 | 80.6 ± 18.8 | 0.46 |
| Diastolic MCAv (cm s−1) | 36.9 ± 8.0 | 37.6 ± 10.0 | 38.0 ± 10.6 | 0.79 |
| Mean MCAv (cm s−1) | 51.2 ± 11.5 | 52.2 ± 13.1 | 52.8 ± 13.4 | 0.66 |
| Peripheral vasculature | ||||
| SmO2 (%) | 70.1 ± 6.3 | 71.8 ± 5.7* | 72.1 ± 6.5* | 0.003 |
| Deoxygenated haemoglobin (μM) | 23.0 ± 6.6 | 22.4 ± 5.7 | 21.9 ± 5.6 | 0.42 |
| Oxygenated haemoglobin (μM) | 55.1 ± 14.5 | 57.6 ± 12.1 | 57.3 ± 12.9 | 0.16 |
| Respiratory | ||||
| etCO2 (mmHg) | 36.7 ± 3.3 | 35.5 ± 5.4 | 33.9 ± 6.7* | 0.07 |
| Respiratory rate (breaths min−1) | 14.7 ± 3.8 | 14.3 ± 3.3 | 15.2 ± 4.0 | 0.30 |
| Blood samples | ||||
| Noradrenaline (pg ml−1) | 477.0 ± 239.2 | 725.9 ± 398.8* | 673.9 ± 324.7 | 0.03 |
Abbreviations: etCO2, end-tidal carbon dioxide; MCAv, middle cerebral artery velocity; ScO , cerebral oxygen saturation; and SmO2 , muscle oxygen saturation. Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD.
P = 0.004–0.06 versus baseline.
Systolic and mean arterial pressures were also higher in the recovery periods compared with baseline (Table 2). The etCO2 was lower than baseline only during the second recovery period. Importantly, both ScO2 and MCAv were similar to baseline during each recovery period preceding the next OLBNP profile (P ≥ 0.66).
Frequency-domain analysis confirmed that oscillating the pressure within the LBNP chamber resulted in an increase in oscillations in MAP, MCAv and etCO2 at the corresponding frequency (Table 3).
TABLE 3.
Frequency analysis of mean arterial pressure (MAP), middle cerebral artery velocity (MCAv) and end-tidal CO2 (etCO2) in the low-frequency and very low-frequency ranges
| Parameter | Low frequency (0.07–0.15 Hz) | Very low frequency (0.04–0.07 Hz) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 Hz | 0.1 Hz | 0.05 Hz | Main effect P-value | 0 Hz | 0.1 Hz | 0.05 Hz | Main effect P-value | |
| MAP power (mmHg2) | 4.3 ± 2.7 | 9.5 ± 7.8* | 2.6 ± 1.2† | 0.004 | 3.7 ± 3.3 | 2.9 ± 2.2 | 24.3 ± 15.7*† | <0.001 |
| MCAv power [(cm s−1)2] | 2.0 ± 2.3 | 4.6 ± 3.6* | 1.2 ± 1.0† | 0.003 | 0.8 ± 0.6 | 0.8 ± 0.7 | 2.3 ± 1.6*† | 0.002 |
| etCO2 power (mmHg2) | 0.09 ± 0.1 | 0.18 ± 0.1* | 0.14 ± 0.2 | 0.02 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.06 ± 0.04*† | 0.001 |
Data were analysed with a linear mixed model for repeated measures followed by Tukey’s post hoc tests. Values are reported as means ± SD.
P = 0.001–0.07 versus 0 Hz.
P = 0.001–0.07 versus 0.1 Hz.
4 |. DISCUSSION
In this study, we sought to assess the effects of low-frequency oscillations in arterial pressure on cerebral blood flow and oxygenation during simulated haemorrhage. The key findings of our study are as follows: (i) inducing oscillations at 0.05 and 0.1 Hz via OLBNP increased tolerance to central hypovolaemia; (ii) this increase in tolerance with OLBNP was associated with an attenuation in the decrease in cerebral oxygenation but not in cerebral blood velocity in the MCA; and (iii) differential responses in stroke volume and heart rate between profiles resulted in similar cardiac output and arterial pressure responses between conditions.
In the clinical setting, increased variability in arterial pressure and cerebral blood flow is associated with poor clinical outcomes, such as stroke (Rickards & Tzeng, 2014). However, in previous studies using LBNP or LBNP plus head-up tilt to presyncope, enhanced oscillations in arterial pressure and cerebral blood velocity (spontaneous or induced) were associated with greater tolerance to this stress (Lucas et al., 2013; Rickards et al., 2011; Rickards, Ryan, Cooke, Lurie, & Convertino, 2007). Rickards et al. (2011) reported that subjects who exhibited high tolerance to presyncopal LBNP had higher amplitude in spontaneously occurring low-frequency arterial pressure and MCAv oscillations (0.04–0.15 Hz) compared with low-tolerant subjects. Likewise, Lucas et al. (2013) had subjects breath at 0.1 Hz (6 breaths min−1) to induce low-frequency oscillations in arterial pressure and MCAv, which resulted in higher tolerance to combined head-up tilt and LBNP compared with the spontaneous breathing conditions. In the present study, we were also able to induce oscillations in arterial pressure and cerebral blood velocity via OLBNP and demonstrated a similar increase in tolerance to central hypovolaemia. Using an external, non-physiological source to induce haemodynamic oscillations, such as the OLBNP stimulus used in the present study, may allow for isolation of the mechanism underlying the improvement in tolerance to central hypovolaemia independent of respiratory-driven oscillations.
The finding that low-frequency oscillations protected against reductions in cerebral oxygenation during central hypovolaemia, despite similar reductions in mean MCAv, is intriguing. As the brain is sensitive to ischaemia and hypoxia, development of interventions that maintain cerebral oxygenation during a hypovolemic challenge may be beneficial within the clinical setting. In our study, measurements of cerebral oxygenation were taken over the frontal lobe with NIRS. The MCA, along with the anterior cerebral artery, supplies blood to the anterior regions of the brain (i.e. the frontal lobe). No difference was observed in blood velocity through the MCA between conditions; therefore, we assume that there was no difference in delivery of oxygen to the frontal lobe. The sample volume measured via NIRS for assessment of cerebral oxygenation is derived predominantly from the venous circulation (75%; 20% arterial and 5% capillary; Madsen & Secher, 1999) and is calculated as the quotient of oxygenated haemoglobin concentration over total haemoglobin (oxygenated plus deoxygenated haemoglobin concentrations). As we observed attenuated decreases in oxygenated haemoglobin concentration and overall cerebral oxygen saturation with the 0.1 and 0.05 Hz OLBNP profiles compared with the 0 Hz condition (Table 1), the oscillatory conditions appear to be modifying the amount of oxygen extracted from the arterial blood into the tissues compared with the non-oscillatory condition. Although speculative, this might reflect a decreased metabolic demand in the neural tissues during exposure to oscillatory blood delivery. The pattern of responses observed in ScO2 was also observed in SmO2, indicating attenuated decreases in peripheral tissue oxygenation; however, because there was no measurement of delivery of blood to the periphery in our study, this might be attributable to a decrease in either oxygen delivery or oxygen extraction.
The effects of oscillatory blood flow on tissue oxygenation have not been assessed extensively; however, there is evidence from computational models to suggest that oscillatory flow is beneficial for tissue oxygenation (Tsai & Intaglietta, 1993). It might be that brief, rhythmic increases in arterial pressure during the oscillatory stimulus provide a pump-like effect that improves tissue perfusion via temporary increases in the pressure gradient down the vascular tree (Salvi et al., 2018; see an example of the rhythmic pressure oscillations from the present study in Figure 7). The increase in MCAv power at each frequency might also have been amplified in this study because etCO2 oscillated at the same frequency as the OLBNP (see Table 3), and it is known that cerebral vessel calibre is very sensitive to alterations in arterial PCO2. In our study, it is important to note the inherent limitation of measuring velocity as an index of cerebral blood flow via transcranial Doppler ultrasound and the subsequent interpretation of oxygen delivery. As TCD-derived measurements do not account for vessel diameter, potential changes in vessel diameter, and therefore flow and oxygen delivery, might be occurring with increased oscillations, but this effect is masked with assessment of velocity only. Future studies will seek to address this limitation with measurements of flow through the extracranial arteries (e.g. internal carotid artery, vertebral artery) that supply the intracranial circulation.
FIGURE 7.
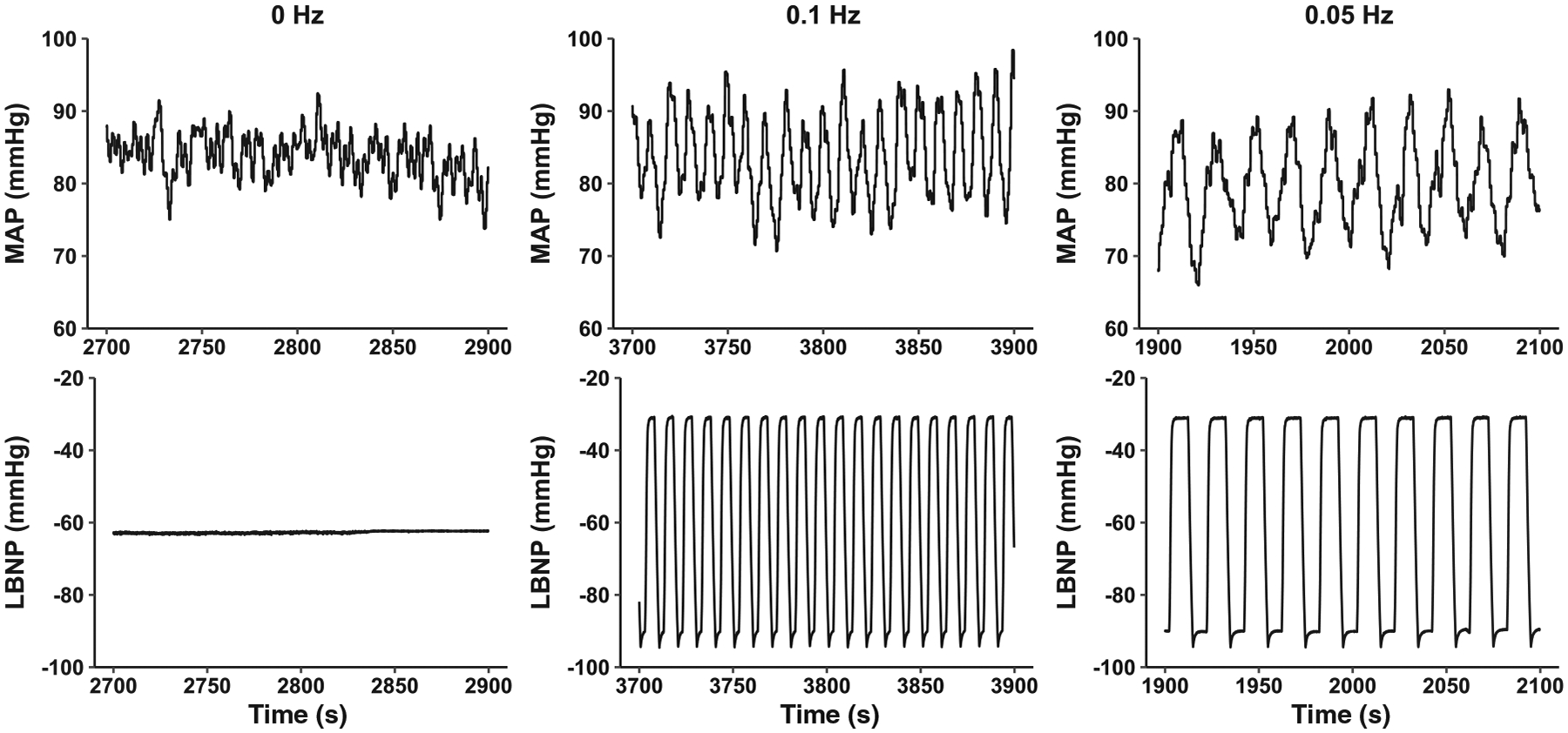
Representative trace of mean arterial pressure (MAP) during each lower-body negative pressure (LBNP) profile
During hypovolaemia, the baroreflex is activated in order to maintain arterial pressure and perfusion of the vital organs. Integrated cardiac baroreflex sensitivity was not different across the three LBNP profiles, and although MAP and cardiac output decreased with LBNP, these reductions were also not different between conditions. This suggests that each OLBNP profile induced a similar cardiovascular challenge, and appropriate compensatory mechanisms were engaged. Interestingly, however, the mechanisms underlying these compensatory responses were different for the static versus oscillatory conditions. The reduction in stroke volume was attenuated for both the 0.1 and the 0.05 Hz oscillations compared with the non-oscillatory 0 Hz condition; this might be due to improved venous return during the upstroke of the oscillations (i.e. during the transient −30 mmHg LBNP phase). The attenuated decrease in stroke volume with both oscillatory profiles (0.1 and 0.05 Hz) resulted in lower circulating noradrenaline and a reduced heart rate response. It has also been reported previously that pulse pressure is the primary arterial pressure for baroreceptor activation (Ead, Green, & Neil, 1952). Greater pulse pressures would result in greater distension of the baroreceptors and, overall, reduce baroreflex-mediated sympathetic activation. As such, it might be that the protection of pulse pressure during the oscillatory protocols (0.1 and 0.05 Hz) reduced sympathetic outflow (lower heart rate and noradrenaline responses) due to greater distension of the baroreceptors. In comparison, greater stroke volume and pulse pressure reductions during the 0 Hz profile led to higher circulating noradrenaline and an increased heart rate response. Taken together, these findings suggest a role of low-frequency haemodynamic oscillations in reducing the cardiovascular burden in maintaining blood flow to vital organs during haemorrhagic stress.
4.1 |. Methodological considerations
In addition to the limitation of measuring cerebral blood velocity and not flow, there are other methodological considerations that should be discussed. The use of NIRS for measuring cerebral oxygen saturation may be influenced by skin blood flow. Davie & Grocott (2012) assessed three commercially available NIRS systems for possible contamination from skin blood flow and reported varying effects of skin contamination for each device. The effectiveness of these devices in measuring cerebral tissue oxygenation relies largely on the number of light emitters and detectors and the distance between the emitters and detectors. Of the tested NIRS systems, the EQUANOX Classic (Nonin Medical Inc.) had the greatest number of light emitters, with only two emitters and two detectors, whereas the FORE-SIGHT (CAS Medical Systems Inc.) had the greatest distance between emitters and detectors at 50 mm. No one system had both multiple emitters/detectors and a distance between emitters and detector of >40 mm. Although the ISS OxiplexTS used in our study has not been assessed systematically for skin contamination, it has 2 × 4 light emitters at varying distances from the detector (20.6, 25.8, 30.4 and 35.5 mm) and a maximal distance of 35.5 mm between emitters and the detector. This configuration allows for deep penetration of light into the cerebral tissue for monitoring of oxygenation and multiple shallow penetration depths for the system to filter out the contribution of superficial tissue layers, including the skin. It is also important to note that room temperature was the same between conditions because all profiles were conducted in the same experimental session, ruling out differences in ambient temperature on skin blood flow responses between conditions. Furthermore, although it is known that skin blood flow decreases during LBNP (Gagnon, Brothers, Ganio, Hastings, & Crandall, 2014), our OLBNP profiles were designed to oscillate around the same chamber pressure, resulting in similar reductions in cardiac output, mean arterial pressure and MCA velocity in each condition; therefore, skin blood flow reductions were likely very similar. Given these factors, skin contamination in the cerebral oxygenation measurement should be minimal, although this cannot be ruled out completely as a confounding factor without thoroughly assessing our NIRS device for skin contamination in similar experimental conditions.
Our experimental design included all OLBNP conditions (0, 0.05 and 0.1 Hz) within the same experimental session. Although this could result in one condition affecting the cardiovascular responses to the next, the order of the conditions was randomized a priori to control for any confounding order effects. In fact, of the 14 subjects included in the analysis, the 0 Hz profile was completed first by five subjects, second by six subjects, and third by three subjects; the 0.1 Hz profile was completed first by five subjects, second by four subjects, and third by five subjects; and the 0.05 Hz profile was completed first by four subjects, second by four subjects and third by six subjects. Furthermore, there was no order effect when assessed statistically (P = 0.98). It is also important to note that the time for recovery was only 5 min between each profile. This amount of time allowed for most of the haemodynamic responses to return to approximately baseline values (Table 3); however, plasma noradrenaline concentrations and arterial pressure remained elevated compared with baseline. This limitation will be addressed in future studies by conducting each OLBNP protocol on a different day.
Although the rate of breathing was paced at each subject’s spontaneous rate during the OLBNP protocols, the depth of breathing was not controlled, and etCO2 decreased between 5.6 and 7.1 mmHg from baseline during all conditions (see Table 1). This reduction in etCO2 (reflecting arterial PCO2) might have reduced the diameter of the blood vessels being measured, resulting in changes in blood flow that might not be detected via assessment of velocity with TCD ultrasound. Recent studies have shown a decrease in MCA diameter during hypocapnia, but only with decreases in etCO2 greater than occurred in our study (Coverdale, Gati, Opalevych, Perrotta, & Shoemaker, 2014; Verbree et al., 2014). Importantly, the magnitude of hypocapnia was similar between all conditions, allowing us to make appropriate comparisons for cerebral blood velocity between groups.
4.2 |. Conclusions
We assessed the effects of low-frequency oscillations on cerebral blood velocity and oxygenation during simulated haemorrhagic stress. Inducing these oscillations during central hypovolaemia resulted in a protection of frontal lobe cerebral oxygenation, but with no protection of arterial pressure or blood velocity in the anterior cerebral circulation. Importantly, inducing low-frequency oscillations in arterial pressure and cerebral blood velocity was associated with increased tolerance to this simulated haemorrhagic stress.
New Findings.
• What is the central question of this study?
Do low-frequency oscillations in arterial pressure and cerebral blood velocity protect cerebral blood velocity and oxygenation during central hypovolaemia?
• What is the main finding and its importance?
Low-frequency oscillations in arterial pressure and cerebral blood velocity attenuate reductions in cerebral oxygen saturation but do not protect absolute cerebral blood velocity during central hypovolaemia. This finding indicates the potential importance of haemodynamic oscillations in maintaining cerebral oxygenation and therefore viability of tissues during challenges to cerebral blood flow and oxygen delivery.
ACKNOWLEDGEMENTS
We thank the subjects for their time and willingness to participate in this study, Dr Albert Yurvati for his assistance with subject medical reviews, and Daniel Cooley for his assistance during experiments.
Funding information
Funding for this study was provided by the American Heart Association (AHA Grant-in-Aid 17GRNT33671110; principal investigator C.A.R.) and a University of North Texas Health Science Center Institute of Cardiovascular and Metabolic Diseases Junior Faculty Seed Grant (principal investigator C.A.R.).
Footnotes
COMPETING INTERESTS
None declared.
REFERENCES
- Chen RY, Fan FC, Schuessler GB, Simchon S, Kim S, & Chien S (1984). Regional cerebral blood flow and oxygen consumption of the canine brain during hemorrhagic hypotension. Stroke, 15, 343–350. 10.1161/01.Str.15.2.343 [DOI] [PubMed] [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KUO, & Eckberg DL (1999). Human responses to upright tilt: A window on central autonomic integration. The Journal of Physiology, 517, 617–628. 10.1111/j.1469-7793.1999.0617t.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke WH, Rickards CA, Ryan KL, Kuusela TA, & Convertino VA (2009). Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. The Journal of Physiology, 587, 4987–4999. 10.1113/jphysiol.2009.177352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverdale NS, Gati JS, Opalevych O, Perrotta A, & Shoemaker JK (2014). Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. Journal of Applied Physiology, 117, 1090–1096. 10.1152/japplphysiol.00285.2014 [DOI] [PubMed] [Google Scholar]
- Davie SN, & Grocott HP (2012). Impact of extracranial contamination on regional cerebral oxygen saturation: A comparison of three cerebral oximetry technologies. Anesthesiology, 116, 834–840. 10.1097/ALN.0b013e31824c00d7 [DOI] [PubMed] [Google Scholar]
- Ead HW, Green JH, & Neil E (1952). A comparison of the effects of pulsatile and non-pulsatile blood flow through the carotid sinus on the reflexogenic activity of the sinus baroceptors in the cat. The Journal of Physiology, 118, 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, … Blackbourne LH (2012). Death on the battlefield (2001–2011): Implications for the future of combat casualty care. The Journal of Trauma and Acute Care Surgery, 73(6 Suppl 5), S431–S437. 10.1097/TA.0b013e3182755dcc [DOI] [PubMed] [Google Scholar]
- Fagius J, & Karhuvaara S (1989). Sympathetic activity and blood pressure increases with bladder distension in humans. Hypertension, 14, 511–517. [DOI] [PubMed] [Google Scholar]
- Forsyth RP, Hoffbrand BI, & Melmon KL (1970). Redistribution of cardiac output during hemorrhage in the unanesthetized monkey. Circulation Research, 27, 311–320. 10.1161/01.Res.27.3.311 [DOI] [PubMed] [Google Scholar]
- Gagnon D, Brothers RM, Ganio MS, Hastings JL, & Crandall CG (2014). Forehead versus forearm skin vascular responses at presyncope in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 307, R908–R913. 10.1152/ajpregu.00204.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, & Harris JW (1951). Pressoreceptor-autonomic oscillation: A probable cause of vasomoter waves. The American Journal of Physiology, 165, 158–166. 10.1152/ajplegacy.1951.165.1.158 [DOI] [PubMed] [Google Scholar]
- Hamner JW, Cohen MA, Mukai S, Lipsitz LA, & Taylor JA (2004). Spectral indices of human cerebral blood flow control: Responses to augmented blood pressure oscillations. The Journal of Physiology, 559, 965–973. 10.1113/jphysiol.2004.066969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, … Convertino VA (2014). Validation of lower body negative pressure as an experimental model of hemorrhage. Journal of Applied Physiology, 116, 406–415. 10.1152/japplphysiol.00640.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, van Helmond N, Curry TB, van Buskirk CM, Convertino VA, & Joyner MJ (2014). Reductions in central venous pressure by lower body negative pressure or blood loss elicit similar hemodynamic responses. Journal of Applied Physiology, 117, 131–141. 10.1152/japplphysiol.00070.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauvar DS, Lefering R, & Wade CE (2006). Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. The Journal of Trauma, Injury, Infection and Critical Care, 60(6 Suppl), S3–S11. 10.1097/01.ta.0000199961.02677.19 [DOI] [PubMed] [Google Scholar]
- Kay VL, & Rickards CA (2016). The role of cerebral oxygenation and regional cerebral blood flow on tolerance to central hypovolemia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 310, R375–R383. 10.1152/ajpregu.00367.2015 [DOI] [PubMed] [Google Scholar]
- Levine BD, Giller CA, Lane LD, Buckey JC, & Blomqvist CG (1994). Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation, 90, 298–306. [DOI] [PubMed] [Google Scholar]
- Lieshout JJV, Wieling W, Karemaker JM, & Secher NH (2003). Syncope, cerebral perfusion, and oxygenation. Journal of Applied Physiology, 94, 833–848. 10.1152/japplphysiol.00260.2002 [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, & Tsintgiras KM (1995). Quantification of tolerance to lower body negative pressure in a healthy population. Medicine & Science in Sports & Exercise, 27, 697–706. [PubMed] [Google Scholar]
- Lucas SJ, Lewis NC, Sikken EL, Thomas KN, & Ainslie PN (2013). Slow breathing as a means to improve orthostatic tolerance: A randomized sham-controlled trial. Journal of Applied Physiology, 115, 202–211. 10.1152/japplphysiol.00128.2013 [DOI] [PubMed] [Google Scholar]
- MacKenzie ET, Farrar JK, Fitch W, Graham DI, Gregory PC, & Harper AM (1979). Effects of hemorrhagic hypotension on the cerebral circulation. I. Cerebral blood flow and pial arteriolar caliber. Stroke, 10, 711–718. 10.1161/01.Str.10.6.711 [DOI] [PubMed] [Google Scholar]
- Madsen PL, & Secher NH (1999). Near-infrared oximetry of the brain. Progress in Neurobiology, 58, 541–560. 10.1016/S0301-0082(98)00093-8 [DOI] [PubMed] [Google Scholar]
- Madwed JB, & Cohen RJ (1991). Heart rate response to hemorrhage-induced 0.05-Hz oscillations in arterial pressure in conscious dogs. American Journal of Physiology-Heart and Circulatory Physiology, 260, H1248–H1253. 10.1152/ajpheart.1991.260.4.H1248 [DOI] [PubMed] [Google Scholar]
- Mancia G, & Mark AL (2011). Arterial baroreflexes in humans. In Terjung R (Ed.), Comprehensive physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc. [Google Scholar]
- Rickards CA, Johnson BD, Harvey RE, Convertino VA, Joyner MJ, & Barnes JN (2015). Cerebral blood velocity regulation during progressive blood loss compared with lower body negative pressure in humans. Journal of Applied Physiology, 119, 677–685. 10.1152/japplphysiol.00127.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickards CA, Ryan KL, Cooke WH, & Convertino VA (2011). Tolerance to central hypovolemia: The influence of oscillations in arterial pressure and cerebral blood velocity. Journal of Applied Physiology, 111, 1048–1058. 10.1152/japplphysiol.00231.2011 [DOI] [PubMed] [Google Scholar]
- Rickards CA, Ryan KL, Cooke WH, Lurie KG, & Convertino VA (2007). Inspiratory resistance delays the reporting of symptoms with central hypovolemia: Association with cerebral blood flow. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 293, R243–R250. 10.1152/ajpregu.00087.2007 [DOI] [PubMed] [Google Scholar]
- Rickards CA, Sprick JD, Colby HB, Kay VL, & Tzeng YC (2015). Coupling between arterial pressure, cerebral blood velocity, and cerebral tissue oxygenation with spontaneous and forced oscillations. Physiological Measurement, 36, 785–801. 10.1088/0967-3334/36/4/785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickards CA, & Tzeng YC (2014). Arterial pressure and cerebral blood flow variability: Friend or foe? A review. Frontiers in Physiology, 5, 120. 10.3389/fphys.2014.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB (1993). Human cardiovascular control. New York, NY: Oxford University Press. [Google Scholar]
- Salvi P, Faini A, Castiglioni P, Brunacci F, Montaguti L, Severi F, … Parati G (2018). Increase in slow-wave vasomotion by hypoxia and ischemia in lowlanders and highlanders. Journal of Applied Physiology, 125, 780–789. 10.1152/japplphysiol.00977.2017 [DOI] [PubMed] [Google Scholar]
- Stauss HM (2007). Identification of blood pressure control mechanisms by power spectral analysis. Clinical and Experimental Pharmacology and Physiology, 34, 362–368. 10.1111/j.1440-1681.2007.04588.x [DOI] [PubMed] [Google Scholar]
- Tsai AG, & Intaglietta M (1993). Evidence of flowmotion induced changes in local tissue oxygenation. International Journal of Microcirculation, Clinical and Experimental, 12, 75–88. [PubMed] [Google Scholar]
- Tzeng YC, Chan GS, Willie CK, & Ainslie PN (2011). Determinants of human cerebral pressure–flow velocity relationships: New insights from vascular modelling and Ca2+ channel blockade. The Journal of Physiology, 589, 3263–3274. 10.1113/jphysiol.2011.206953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helmond N, Johnson BD, Curry TB, Cap AP, Convertino VA, & Joyner MJ (2015). Coagulation changes during lower body negative pressure and blood loss in humans. American Journal of Physiology-Heart and Circulatory Physiology, 309, H1591–H1597. 10.1152/ajpheart.00435.2015 [DOI] [PubMed] [Google Scholar]
- van Helmond N, Johnson BD, Curry TB, Cap AP, Convertino VA, & Joyner MJ (2016). White blood cell concentrations during lower body negative pressure and blood loss in humans. Experimental Physiology, 101, 1265–1275. 10.1113/ep085952 [DOI] [PubMed] [Google Scholar]
- Verbree J, Bronzwaer A-SGT, Ghariq E, Versluis MJ, Daemen MJAP, van Buchem MA, … van Osch MJP (2014). Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. Journal of Applied Physiology, 117, 1084–1089. 10.1152/japplphysiol.00651.2014 [DOI] [PubMed] [Google Scholar]
- Zamir M, Goswami R, Liu L, Salmanpour A, & Shoemaker JK (2011). Myogenic activity in autoregulation during low frequency oscillations. Autonomic Neuroscience: Basic and Clinical, 159, 104–110. 10.1016/j.autneu.2010.07.029 [DOI] [PubMed] [Google Scholar]


