Abstract
The tempo and intensity of retroviral neuropathogenesis are dependent on the capacity of the virus to invade the central nervous system. For murine leukemia viruses, an important determinant of neuroinvasiveness is the virus-encoded protein glycosylated Gag, the function of which in the virus life cycle is not known. While this protein is dispensable for virus replication, mutations which prevent its expression slow the spread of virus in vivo and restrict virus dissemination to the brain. To further explore the function of this protein, we compared two viruses, CasFrKP (KP) and CasFrKP41 (KP41), which differ dramatically in neurovirulence. KP expresses high early viremia titers, is neuroinvasive, and induces clinical neurologic disease in 100% of neonatally inoculated mice, with an incubation period of 18 to 23 days. In contrast, KP41 expresses early viremia titers 100- fold lower than those of KP, exhibits attenuated neuroinvasiveness, and induces clinical neurologic disease infrequently, with a relatively long incubation period. The genomes of these two viruses differ by only 10 nucleotides, resulting in differences at five residues, all located within the N-terminal cytoplasmic tail of glycosylated Gag. In this study, using KP as the parental virus, we systematically mutated each of the five amino acid residues to those of KP41 and found that substitution mutation of two membrane-proximal residues, E53 and L56, to K and P, respectively produced the greatest effect on early viremia kinetics and neurovirulence. These mutations disrupted the KP sequence E53FLL56, the leucine dipeptide of which suggests the possibility that it may represent a sorting signal for glycosylated Gag. Supporting this idea was the finding that alteration of this sequence motif increased the level of cell surface expression of the protein, which suggests that analysis of the intracellular trafficking of glycosylated Gag may provide further clues to its function.
Neonatal inoculation of CasBrE, an ecotropic murine retrovirus originally isolated from wild mice (16, 17), causes a neurologic disease manifested by noninflammatory spongiform degeneration primarily in areas of the central nervous system involved in motor function (1, 4, 16). However, both the field isolate and a molecular clone of the virus (clone 15-1) cause neurologic disease in only a small fraction of neonatally inoculated mice with a long incubation period of 6 to 12 months. This appeared to be a consequence of the minimal neuroinvasiveness of CasBrE (27). Introduction into the CasBrE genome of ∼0.5 kb of viral sequences from a strain of Friend murine leukemia virus (MuLV) between a KpnI site in the R region of the viral long terminal repeat (LTR) and a PstI site at the 3′ end of the viral leader sequence resulted in a virus, CasFrKP (KP), exhibiting dramatically increased neuroinvasiveness (31), a commensurate shortening of the incubation period to 18 to 24 days, and increased incidence of disease (100%). The basis of this increased neuroinvasiveness has not been fully defined. However, neuroinvasiveness in this model is a property of viruses which spread rapidly during the first week after neonatal inoculation. The importance of these early time points appears to be related to a progressive loss of susceptibility of the brain to virus infection which is observed as a function of age (10, 11, 18), this resistance being complete by postnatal days 10 to 12. This age-dependent restriction appears to be manifested at the level of the brain-capillary endothelial cell (22), which is the portal of entry of the virus into the brain parenchyma (3, 12). We have postulated that neuroinvasiveness in this model is determined by the capacity of the virus to attain high titers in peripheral nonneuronal tissues before the age-dependent restriction to brain infection is complete.
During the mapping of viral sequences which influence neuroinvasiveness, we constructed a chimeric virus, CasFrKP41 (KP41), which differs from KP in only 10 nucleotides. The 10 nucleotides are located 5′ of the initiation codon for the Gag structural proteins, the sequence of KP being derived from Friend MuLV and the sequence of KP41 being derived from CasBrE. These differences are contained within an open reading frame of a viral protein, glycosylated Gag, the two viruses differing at five amino acid residues located near the N terminus of the protein. KP41 spreads more slowly than KP in vivo, and in contrast to the short incubation period of KP, KP41 induces neurologic disease in only 18% of inoculated mice, with an incubation period of >125 days (31). Curiously, however, KP and KP41 spread with equal kinetics in vitro in Mus dunni cells (31).
Glycosylated Gag is a highly conserved viral protein expressed by replication-competent MuLV and feline leukemia virus (13, 14, 25, 33). It is translated from an initiation codon in frame and upstream of the start codon for pr65gag, the precursor of core proteins of the virus. Thus, the coding sequence of glycosylated Gag is identical to that of pr65gag except for a unique N-terminal sequence, which in the case of the KP virus is 88 residues in length. Unlike pr65gag, which is a cytosolic protein processed by the viral protease, glycosylated Gag is translated into the endoplasmic reticulum as a type II integral membrane protein (NcytoCexo), gp85gag, the C-terminal half being cleaved by a cellular protease and secreted (15). The N-terminal half of the molecule is integrated into the cell membrane by a signal/anchor domain and is displayed at the plasma membrane (15). The five amino acids which differ between KP and KP41 are located within the predicted N-terminal cytoplasmic tail.
Glycosylated Gag is dispensable for virus replication (9, 31, 34), but mutants of the virus KP, in which the expression of glycosylated Gag was knocked out, exhibit slower spread than wild-type virus in the mouse, accompanied by a loss of neuroinvasiveness (29). Interestingly, the kinetics of virus spread in vitro was indistinguishable from that of KP. In this respect, these glycosylated Gag-null mutants resemble the virus KP41, in which the slowing of virus spread was also seen in vivo but not in vitro (31). In the case of KP41, however, there is a sequence alteration within an otherwise intact glycosylated Gag open reading frame. This finding suggested that the sequence differences between KP and KP41 may provide clues of the function of this enigmatic protein.
In this study, we systematically mutated each of the residues which differed between KP and KP41 and identified a motif located in the membrane-proximal portion of the cytoplasmic tail of the protein which influenced kinetics of virus spread in the mouse as well as neuroinvasiveness. This motif appears to consist of two leucines preceded by a glutamic acid. Interestingly, this motif also influenced the level of expression of glycosylated Gag at the plasma membrane of infected cells although it did not appear to affect synthesis of the protein. This finding suggests that this motif may function as a sorting signal.
MATERIALS AND METHODS
Construction of chimeric viruses.
The viral DNA of KP (30) consists of the genome of CasBrE clone 15-1 (27) with the ∼0.5-kb segment of the leader sequence between KpnI (position 32 of the CasBrE genome) in the R region of the LTR and a PstI site (position 566 of the CasBrE genome) upstream of the start codon for pr65gag from Friend MuLV clone FB29 (35). All nucleotide positions designated henceforth are based on the nucleotide sequence of the virus KP. The techniques used to modify KP were based on those used in a previous study (30), with modifications. Briefly, a 1.1-kb ClaI-PstI fragment of the permuted genome of KP, containing the LTR and 5′ leader sequence, was subcloned into the vector pSP72 (Promega). A BsaAI site was introduced by using five overlapping oligonucleotides spanning a 175-nucleotide segment from BstXI (position 390) to PstI (position 565). A T→C mutation was introduced at position 462 to generate the BsaAI site. This point mutation was located within the coding sequence of glycosylated Gag and changed the codon for Y36 from TAT to TAC, which was silent. This mutation did not alter the neurovirulence of KP (data not shown).
This BsaAI site was then used as the 5′ boundary for the generation of chimeric genomes, using a series of five overlapping oligonucleotides (three on the top strand and two on the bottom) spanning 103 nucleotides from BsaAI to PstI. Using the annealing conditions described previously (30), we were unable to generate mutants without deletions. This appeared to be a consequence of the relatively high GC content of this sequence (60%). To overcome this problem, we annealed and ligated the oligonucleotides at 80°C by using the thermophilic Pfu DNA ligase (Stratagene) for 30 min followed by snap cooling in liquid nitrogen. The sample was phenol-chloroform extracted, and the ligated product was gel purified by using MetaPhor agarose (FMC BioProducts, Rockland, Maine). This fragment was then ligated into the BsaAI- and PstI-digested KP subclone, and both upper and lower strands were sequenced from ∼50 bp upstream of the BsaAI site through the polylinker region of Psp72. The ClaI-PstI fragment was then excised and introduced into the ClaI- and PstI-digested full-length genome of KP. These constructions were then transfected into M. dunni fibroblasts (19, 20) as described previously (27), and virus stocks were collected after five passages. Titers for all stocks used for this study were between 2 × 106 and 3 × 106 focus-forming units/ml.
Mice, virus inoculations, and clinical evaluations.
IRW (inbred Rocky Mountain White) mice bred and raised at Rocky Mountain Laboratories were used exclusively in this study. These mice are highly susceptible to central nervous system disease induced by a variety of neurovirulent retroviruses (27). Neonatal mice (1 to 2 days postnatally) were inoculated intraperitoneally (i.p.) with 30 μl of the virus stocks containing 6 × 104 to 9 × 104 focus-forming units of infectivity. Virus stocks were assayed for infectivity by using a focal immunoassay described previously (10). Once the mice were 13 days postinoculation (dpi), they were monitored daily for signs of neurologic disease as described previously (12). After 30 dpi, mice were examined at least twice a week until approximately 120 dpi, when the experiments were terminated. The day at which the first signs of neurologic disease were seen (abnormal abduction reflex and tremor) was considered the onset of disease. The disease usually progressed to paralysis of hind and forelimbs associated with wasting, but the mice were generally killed before the terminal stages of disease were reached in accordance with the policy of the Rocky Mountain Laboratories Animal Care and Use Committee.
Quantification of viruses.
For viremia titrations, neonatally inoculated mice were bled at 5 dpi by axillary vessel incision under methoxyflurane inhalation anesthesia. Viremia titers were determined by a focal immunoassay (10) using the SU (surface protein; gp70env)-specific monoclonal antibody 667 (23). Foci of infection were developed with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G, using 3-amino-9-ethylcarbazole as a substrate, and counted under a Nikon SMZ-10 dissecting microscope.
For determination of viral burden in the brain and spleen, mice were anesthetized by inhalation of methoxyflurane and exsanguinated by axillary vessel incision. Brains and spleens were taken from infected mice at 20 dpi along with age-matched uninfected controls and immediately frozen in liquid nitrogen. Cytosolic extracts from brain and spleen were prepared on ice by making 10% (wt/vol) homogenates in extract buffer (0.5% Nonidet P-40 in 0.01 M Tris base, 0.15 M NaCl, 0.001 M EDTA [pH 7.4], leupeptin [0.5 μg/ml], aprotinin [1 μg/ml], pepstatin A [0.7 μg/ml], Pefabloc [24 μg/ml]) with 20 strokes of a Dounce homogenizer. Nuclei and debris were removed by centrifugation as described previously (28). Viral protein was quantified in these extracts with an antigen capture enzyme-linked immunosorbent assay (ELISA) (37) using the anti-CA (capsid) monoclonal antibody R-187 (7) as the capture antibody and rabbit anti-CA antiserum (21) as the detecting antibody. For standardization of the assay, a Triton X-100 extract of sucrose density gradient-purified KP virus was included on each ELISA plate, and results were expressed as micrograms of total viral protein per gram of tissue.
Pulse-chase analysis.
Subconfluent chronically infected M. dunni cells were pulsed for 10 min with Easy Tag Express (Dupont NEN) containing [35S]methionine and [35S]cysteine at a concentration of 200 μCi/ml in methionine-cystine-free high-glucose Dulbecco modified Eagle medium (GIBCO) supplemented with 2 mM l-glutamine (GIBCO) and 5% dialyzed fetal calf serum (GIBCO). Monolayers were washed once and incubated for various times in Dulbecco modified Eagle medium (containing methionine [30 μg/ml] and cystine [63 μg/ml]) plus 10% undialyzed fetal calf serum. Cells were lysed with 0.5% sodium dodecyl sulfate (SDS) in 0.05 M Tris-HCl (pH 8.0), scraped from the flasks, and boiled for 5 min. Lysates were diluted fourfold with a buffer containing 1.25% Nonidet P-40, 1.25% sodium deoxycholate, 0.0125 M sodium phosphate (pH 7.2), 2 mM EDTA, and the protease inhibitors at the concentrations listed above. Lysates were immunoprecipitated as described previously (5) with rabbit anti-peptide 4210 (15, 29) reactive with the extreme N terminus of glycosylated Gag. Immunoprecipitates were recovered with immobilized protein A (Pierce), eluted by boiling for 5 min in sample buffer containing 2% SDS and 5% 2-mercaptoethanol, and resolved by electrophoresis on 9% polyacrylamide gels (PAGE). Quantification was performed with a PhosphorImager (Molecular Dynamics).
Flow cytometry.
Flow cytometry was carried out on both spleen cell suspensions and suspensions of M. dunni cells. The latter were grown in tissue culture flasks, from which they were removed with saline-trypsin-EDTA. Infected and uninfected live cells were stained as described previously (15), using the following primary antibodies: monoclonal antibodies 667 (anti-SU) (23) and 34 (anti-Gag MA [matrix] protein) (8) for M. dunni cells and biotinylated monoclonal antibodies 667 and 34 for mouse spleen cells. The mouse primary and biotinylated antibodies were detected by using fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin (Cappel) and fluorescein isothiocyanate-streptavidin (GIBCO), respectively. Dead cells were stained with propidium iodide and gated out. Controls for specificity included cells infected with the virus KPgg−, a KP mutant in which glycosylated Gag expression is knocked out (15), uninfected cells, and infected cells in the absence of primary antibodies. Cells were analyzed with a FACStar fluorescence-activated cell sorter (Becton Dickinson), and the data were collected in the log mode. Ten thousand cells were analyzed per virus.
Statistical analysis.
The unpaired Student’s t test was used for the assessment of differences.
RESULTS
The kinetics of neurologic disease is strongly influenced by the amino acid sequence of the cytoplasmic tail of glycosylated Gag.
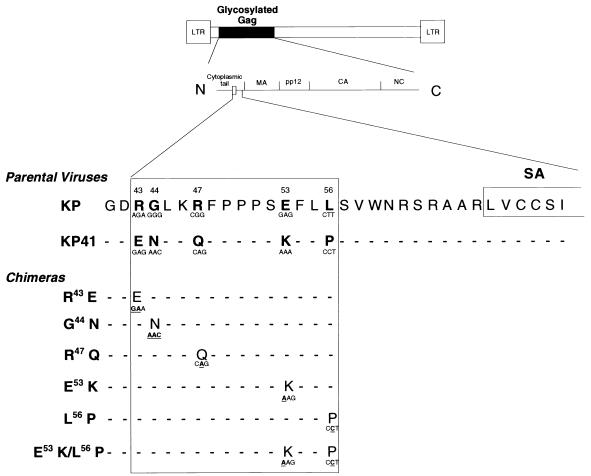
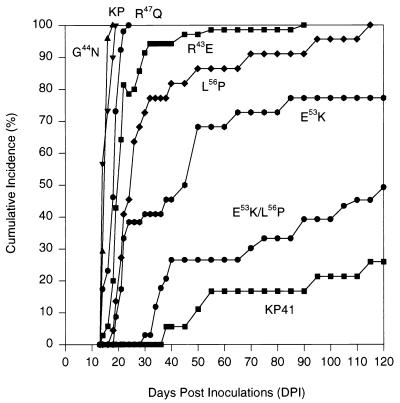
The amino acid sequence differences between KP and KP41 are located in cytoplasmic tail of glycosylated Gag just N terminal of the predicted transmembrane domain (24) (Fig. 1). We constructed a series of chimeric viruses in which each of the five residues of KP was changed to that of KP41 (Fig. 1). All viruses were otherwise identical to KP. Neonatal IRW mice were inoculated i.p. with comparable concentrations of virus (see Materials and Methods) and followed for signs of neurologic disease (Fig. 2). The viruses G44N and R47Q induced neurologic disease within 17 to 25 days in 100% of inoculated mice, kinetics which were similar to that of the parent virus KP. The viruses R43E, L56P, and E53K were somewhat slower, with E53K being the slowest. Because the E53 and L56 mutations appeared to produce a phenotype intermediate between those of KP and KP41, we made the double mutant E53K/L56P to determine whether the effect might be additive. The neurovirulence of this virus was nearly as blunted as that of KP41 (Fig. 2), with clinical neurologic disease being observed in only 49% of inoculated mice during the 120-day period of observation. Thus, a dramatic effect on incubation period was produced by mutation of both membrane-proximal residues, E53 and L56, although lengthening was also noted when either E53 or L56 was mutated individually.
FIG. 1.
Schematic diagram of a typical MuLV showing the location of glycosylated Gag. The predicted signal/anchor (SA) sequence of glycosylated Gag is located at the junction of cytoplasmic tail and MA. MA through NC are luminal. The region of the genome of the virus KP which was manipulated in this study is shown as a box in the cytoplasmic tail of glycosylated Gag. Numbers above the sequences denote amino acid positions. The five residues which differ between KP and KP41 are in bold. Residues of KP were mutated to those of KP41, generating six chimeric viruses, one of which had two mutations. The changes in the codons relative to KP are shown in bold and are underlined. The codons of the chimeras did not always correspond to those of KP41 since attempts were made to minimize the changes in the nucleotide sequence of the KP genome.
FIG. 2.
Cumulative incidence for neurologic disease induced by the parental viruses KP and KP41 as well as the six chimeric viruses over a 120-day period of observation. Mice were inoculated i.p. as neonates and followed for evidence of clinical neurologic disease. Mice were scored as positive at the first physical sign, which was generally an abnormality of reflex abduction of the hind limbs when mice were picked up by the tail. The numbers of mice followed for each group are as follows: KP, 30; KP41, 37; E53K/L56P, 39; R43E, 70; G44N, 24; R47Q, 30; E53K, 28; and L56P, 27.
Relative neuroinvasiveness of the chimeric viruses.
Glycosylated Gag-null mutants exhibit slower spread of virus in the spleen (9) and loss of neuroinvasiveness (29). However, by 2 to 3 weeks postinoculation, virus in the spleen reaches the same titers as does the wild-type virus (29). To evaluate the influence of the sequence of the cytoplasmic tail of glycosylated Gag on neuroinvasiveness, viral protein content of the brain and spleen was quantified by ELISA 20 days after inoculation of KP, KP41, and the double mutant E53K/L56P (Table 1). At this time point, the brain homogenates from KP41 and E53K/L56P exhibited sevenfold-lower levels of viral protein than KP. In contrast, the viral protein contents of splenic homogenates were similar. Thus, both KP41 and E53K/L56P viruses exhibited a loss of neuroinvasiveness but maintained the capacity to spread in the spleen to levels comparable to that of KP, at least by 20 dpi.
TABLE 1.
Quantitation of viral protein in the brain and spleen
| Virus | Mean viral protein (μg of total viral protein/g of tissue) ± SDa
|
No. of mice | |
|---|---|---|---|
| Brain | Spleenc | ||
| KP | 120 ± 39b | 8,320 ± 4,390 | 3 |
| KP41 | 15 ± 7.7 | 7,700 ± 2,570 | 4 |
| E53K/L56P | 17 ± 9.0 | 6,980 ± 2,530 | 5 |
Measured by antigen capture ELISA in 10% homogenates of brain and spleen harvested 20 days after neonatal i.p. inoculation. Values were extrapolated from standard curves of purified virus.
Statistically different from values for KP41 and E53K/L56P (P < 0.001).
The values are not statistically different from each other (P > 0.05).
Effects on viremia titers during the first week postinoculation.
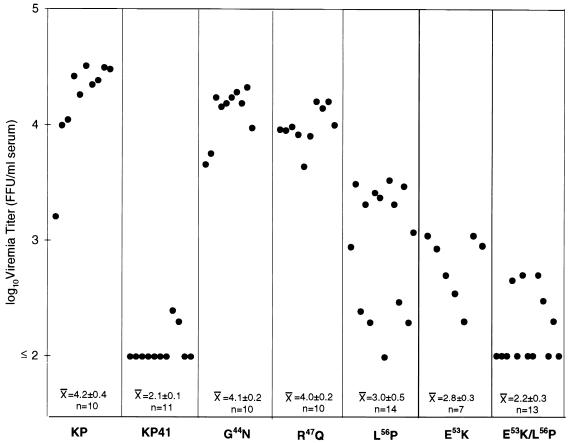
Previous studies have shown that in this system, early viremia kinetics are a reliable predictor of neuroinvasiveness (11, 31). Viruses which reached the highest titers in the serum during the first week postinoculation were found also to reach the highest levels in the brain. We therefore bled mice 5 days after i.p. inoculation of KP, KP41, and selected chimeric viruses and determined virus titers in the serum by focus assay (Fig. 3). The results segregated into essentially three groups. The highest-titer viruses, which resembled KP, consisted of the mutants at the membrane distal sites G44 and R47. The intermediate group consisted of the two viruses with mutations at E53 and L56. The low-titer group consisted of KP41 and the virus carrying the double mutation. Although there was considerable overlap, the titers of both E53K and L56P viruses were significantly different from those of KP and KP41 (P < 0.001 by unpaired Student’s t test). These results indicated that residues E53 and L56 were both important for expression of rapid early viremia kinetics and confirmed the earlier observations suggesting that neuroinvasiveness was a function of early viremia kinetics.
FIG. 3.
Viremia titers 5 days after neonatal i.p. inoculation. Each point represents one mouse. The numbers at the bottom represent means ± standard deviations for each group. The chimeric viruses segregated into three groups: high titer resembling KP (G44N and R47Q); low titer resembling KP41 (E53K/L56P), and intermediate (E53K and L56P), which were statistically different (Student’s t test) from each other (P < 0.001). FFU, focus-forming units.
E53 and L56 influenced the relative level of expression of glycosylated Gag at the surface of infected cells.
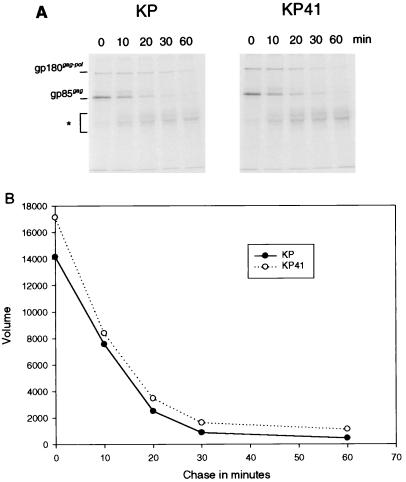
Previous studies with glycosylated Gag-null mutants indicated that neuroinvasiveness was dependent on the expression of this protein (29). Thus, it was of interest to determine whether there were differences in levels of expression of the protein among the viruses studied here. Initial studies focused on the synthesis and half-life of the gp85gag precursor of glycosylated Gag for KP and KP41. Chronically infected M. dunni cells were pulsed with [35S]methionine-cysteine for 10 min and chased for various times up to 1 h. Cell lysates were immunoprecipitated with an antiserum to a peptide at the extreme N terminus of the cytoplasmic tail, and the precipitates were resolved by SDS-PAGE (Fig. 4A). During the 10-min pulse, the levels of incorporation of the 35S label into gp85gag encoded by KP and KP41 were comparable, as were the rates of disappearance of label from the precursor during the chase period (Fig. 4B). Thus, the synthesis and half-lives of the protein appeared similar for these two viruses.
FIG. 4.
Pulse-chase analysis of gp85gag, the precursor of glycosylated Gag encoded by KP and KP41. M. dunni cells chronically infected with KP or KP41 were pulsed with [35S]methionine-cysteine for 10 min and chased in medium without radioisotope for 0 to 60 min before cell lysis. Lysates were subjected to immunoprecipitation with a rabbit antiserum to the N-terminal peptide 4210 of the glycosylated Gag cytoplasmic tail (29) and separation by SDS-PAGE (A). After the 10-min pulse (time zero), two proteins were labeled: gp85gag and gPr180gag-pol, which is a glycosylated gag-pol fusion protein (13). After 10 min of chase, two additional protein appeared: one slightly larger than gp85gag, which is likely a proteolytic cleavage product of gPr180gag-pol, and a diffuse protein ranging from 40 to 55 kDa (∗), which is the N-terminal cleavage product of gp85gag (15). (B) Quantification of the gp85gag bands for KP and KP41. The vertical axis represents arbitrary units of the volume measurements on a PhosphorImager.
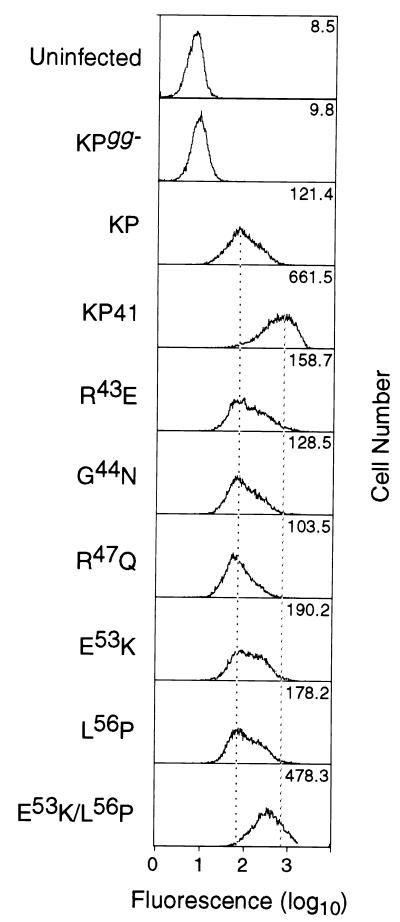
Cell surface expression of glycosylated Gag was quantified by flow cytometry with anti-MA monoclonal antibody 34, which recognizes a luminal epitope of glycosylated Gag (Fig. 5). We stained live M. dunni cells chronically infected with KP and KP41 as well as the chimeric viruses. Negative controls included both uninfected cells and cells infected with a glycosylated Gag-null mutant KPgg− (31). Surprisingly, there was a consistent five- to sevenfold higher level of cell surface glycosylated Gag expression in KP41-infected cells compared to KP-infected cells. Among the chimeric viruses analyzed, E53K/L56P expressed the highest amount of cell surface protein (nearly as high as that of KP41), whereas G44N and R47Q expressed low levels resembling that of KP (Fig. 5). The mean fluorescence intensities of R43E, E53K, and L56P were slightly (∼2-fold) higher than that of KP, a finding which was consistent in three separate experiments. These differences were not a reflection of general differences in viral protein expression, since the variation in levels of viral SU expression at the cell surface for all infected cells was ≤12% (data not shown).
FIG. 5.
Quantification by flow cytometry of glycosylated Gag expressed at the plasma membrane of M. dunni cells chronically infected with the viruses shown. Cells were stained in suspension with monoclonal antibody 34 (8), which reacts with a luminal epitope in the MA domain of glycosylated Gag (Fig. 1). Dead cells were stained with propidium iodide and gated out. Uninfected cells and cells infected with the glycosylated Gag-null mutant KPgg− (15) served as negative controls. Fluorescence intensities for KP and KP41 differed by five- to sevenfold. The chimeric viruses fell into three groups, as discussed in Results. All infected cells including KPgg− expressed high levels of viral SU protein (not shown), as revealed by staining with antibody 667 reactive with viral gp70 (23), the range of mean fluorescence intensity varying ≤12% for all of the viruses. The experiment was repeated three times.
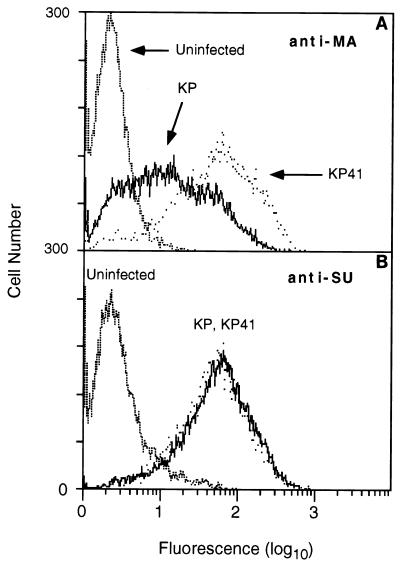
To determine whether these differences in cell surface expression of glycosylated Gag were also seen in vivo, spleen cells were harvested from mice 14 days after neonatal inoculation of KP and KP41 and similarly analyzed by flow cytometry with anti-MA antibody (Fig. 6). Although staining intensities were more heterogeneous and there appeared to be more overlap than seen in the M. dunni fibroblasts, the results clearly demonstrate that cell surface expression of the protein followed the same pattern, with KP41 > KP. No differences between KP and KP41 were seen in levels of SU protein expression at the cell surface. These results indicated that the residues in the cytoplasmic tail of glycosylated Gag which influenced virus spread in vivo also influenced the level of cell surface expression of the protein. High-level expression at the cell surface was associated with slower spread of virus in the mouse, loss of neuroinvasiveness, and lengthening incubation period for clinical disease. Curiously, this conclusion stands in stark contrast to the observation that elimination of glycosylated Gag expression altogether yielded a similar phenotype (31).
FIG. 6.
The differences in cell surface glycosylated Gag expressed by KP and KP41 were also observed in vivo. Mice were inoculated as neonates with KP or KP41, uninoculated mice serving as a negative control. Spleen cells were harvested at 14 dpi and stained with anti-MA antibody 34 (A) or anti-SU antibody 667 (B) as for Fig. 5. At least three mice were analyzed per group, and the experiment was repeated three times. Staining for glycosylated Gag was more heterogeneous than in M. dunni cells, and there was considerable overlap. Nevertheless, staining of KP41-infected cells was noticeably skewed to higher intensity compared to KP. In contrast, the levels of SU protein for KP and KP41-infected cells were identical.
DISCUSSION
In this study we used two chimeric murine retroviruses, KP and KP41, which differ in neurovirulence, to study the role of sequences in the 5′ end of the viral genome in pathogenesis. These two viral genomes differ by 10 nucleotides located 5′ of the initiation codon for pr65gag, the precursor of the viral core proteins. These sequence differences are located within the open reading frame of the transmembrane protein, glycosylated Gag, and change the coding sequence of the cytoplasmic tail of this protein at five amino acid residues. Comparison of the two parental viruses KP and KP41 revealed that the sequence differences influenced a number of in vivo phenotypes. KP was more neuroinvasive and induced disease with higher frequency and shorter incubation period than KP41. In addition, viremia titers at 5 days postinoculation were ≥100 times higher for KP than KP41. These results suggested that the sequence of the cytoplasmic tail of glycosylated Gag determined the kinetics of virus spread and the extent of virus dissemination in the mouse.
This region of the KP genome (Fig. 1) is highly charged, is proline rich, and contains a membrane-proximal pair of leucine residues. Although the three prolines are conserved in both KP and KP41, the charged residues and the C-terminal leucine of the leucine pair are not. We were unable to fully convert the KP phenotype to that of KP41 by mutating individually each of the five residues. Nevertheless, we did observe a partial effect when either E53 or L56 was mutated to the KP41 residue K or P, respectively. Induction of clinical disease was delayed, and the viremia titers at 5 dpi were intermediate between those of KP and KP41. Changing both residues in the double mutant E53K/L56P resulted in a virus with a phenotype very similar to that of KP41. Thus, viremia titers were statistically indistinguishable from that of KP41 at 5 days, the induction of clinical neurologic disease was markedly blunted, and viral burden in the brain for this double mutant was indistinguishable from that for KP41. These results suggested that the sequence E53FLL56 was involved in the rapid spread of virus in the mouse and dissemination of virus to the brain.
The correlation between the kinetics and extent of virus spread in vivo and the level of cell surface expression of the glycosylated Gag protein was a consistent and striking observation. Expression of glycosylated Gag at the cell surface was five- to sevenfold greater for KP41-infected cells than for KP-infected cells, and this difference was observed both in M. dunni cells in vitro and in spleen cells from inoculated mice. This phenotype also appeared to map to the E53FLL56 sequence. The chimeric viruses in which either E53 or L56 was mutated exhibited approximately twofold-higher levels of cell surface glycosylated Gag than KP, and the level of the double mutant E53K/L56P was nearly as high as that of KP41. The levels expressed by the double mutant, however, never quite reached that of KP41, indicating some contribution by one or all of the other three residues which differed between these two viruses (R43, G44, and R47). Interestingly, the R43E mutant exhibited slightly increased levels of cell surface glycosylated Gag (Fig. 5), and mice inoculated with this virus showed a demonstrable lengthening of incubation period compared to KP (Fig. 2), suggesting the possible importance of this residue.
What then is the nature of the effect of E53FLL56 on cell surface expression? It is possible that nucleotide sequence differences in this region of the genome alter RNA secondary structure and perhaps affect translation of the protein. The pulse-chase analysis of the glycosylated Gag precursor, gp85gag, for KP and KP41 failed to reveal any differences in either the synthesis or half-life of this protein, which suggests that the difference in cell surface expression was a consequence of posttranslational effects, perhaps related to differences in proteolytic processing, degradation, or protein trafficking. Previously, we have reported that glycosylated Gag of the virus KP is proteolytically cleaved in the extracellular domain (15). Pulse-chase analysis (Fig. 4A) indicates that the cleavage of the protein encoded by KP41 was indistinguishable from that of KP. Thus, differential cleavage would not appear to account for the differences in cell surface expression. The E53FLL56 sequence resembles the dihydrophobic trafficking signals which have been identified in the cytoplasmic domains of a variety of transmembrane proteins (reviewed in references 32 and 36). The minimum requirement for this motif is LZ, where Z is any nonaromatic hydrophobic residue. In addition, in the major histocompatibility complex class II invariant chain (26) and the cation-independent mannose-6-phosphate receptor (6), closely associated acidic residues also appear to contribute to the sorting function of this leucine-based motif. This motif appears to influence both endocytosis from the cell surface as well as the direct sorting of proteins from the trans-Golgi to the endosomal compartment in nonpolarized cells. Mutations in this motif often result in increased levels of cell surface expression (2), due presumably to the dominant influence of bulk flow of integral membrane proteins from the trans-Golgi to the cell surface in the absence of specific trafficking signals in their cytoplasmic tails.
Whether or not the E53FLL56 sequence represents a trafficking signal, the results presented here indicate that the function of glycosylated Gag is influenced by the sequence of its cytoplasmic tail, which in turn influences levels of cell surface expression. In this study, high-level cell surface expression was associated with an attenuated phenotype in vivo, which inevitably leads one to the conclusion that lower levels translate to more activity. However, the attenuated in vivo phenotypes of KP41 and the double mutant E53K/L56P (slow early viremia kinetics and loss of neuroinvasiveness) were similar to, albeit less complete than, those of the glycosylated Gag-null mutants described previously (29, 31). This suggests two possible scenarios: (i) glycosylated Gag functions at the cell surface and the concentration of protein at this site is critical, high levels being inhibitory; and (ii) glycosylated Gag does not function at the cell surface but instead functions intracellularly. In the latter case, high levels of cell surface expression may simply be a surrogate marker for low-level intracellular accumulation of the protein. If so, then the relationships presented here would suggest that high intracellular partitioning of the protein translates to more activity. We currently favor the latter hypothesis and are approaching the question by comparing the fate of cell surface and intracellular forms of glycosylated Gag protein expressed by KP and KP41.
ACKNOWLEDGMENTS
We thank Diane Brooks for assistance with flow cytometry. We also thank Gary Hettrick and Robert Evans of the RML Graphics Department for figure reproductions.
R. Fujisawa is a recipient of a JSPS Research Fellowship for Japanese Biological and Behavioral Researchers at NIH (69607).
REFERENCES
- 1.Andrews J M, Gardner M B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974;33:285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 3.Baszler T V, Zachary J F. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglial cell infection: ultrastructural findings. Lab Invest. 1990;63:612–623. [PubMed] [Google Scholar]
- 4.Brooks B R, Swarz J R, Johnson R T. Spongiform polioencephalomyelopathy caused by a murine retrovirus. I. Pathogenesis of infection in newborn mice. Lab Invest. 1980;43:480–486. [PubMed] [Google Scholar]
- 5.Buller R S, Ahmed A, Portis J L. Identification of two allelic forms of an endogenous murine retroviral env gene linked to the Rmcf locus. J Virol. 1987;61:29–34. doi: 10.1128/jvi.61.1.29-34.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H J, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. J Biol Chem. 1997;272:7003–7012. doi: 10.1074/jbc.272.11.7003. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of Friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K, Cloyd M, Britt W, Portis J, Collins J, Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology. 1981;112:131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- 9.Corbin A, Prats A C, Darlix J L, Sitbon M. A nonstructural gag-encoded glycoprotein precursor is necessary for efficient spreading and pathogenesis of murine leukemia viruses. J Virol. 1994;68:3857–3867. doi: 10.1128/jvi.68.6.3857-3867.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czub M, Czub S, McAtee F J, Portis J L. Age-dependent resistance to murine retrovirus-induced spongiform neurodegeneration results from central nervous system-specific restriction of virus replication. J Virol. 1991;65:2539–2544. doi: 10.1128/jvi.65.5.2539-2544.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czub M, McAtee F J, Portis J L. Murine retrovirus-induced spongiform encephalomyelopathy: host and viral factors which determine the length of the incubation period. J Virol. 1992;66:3298–3305. doi: 10.1128/jvi.66.6.3298-3305.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czub S, Lynch W P, Czub M, Portis J L. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab Invest. 1994;70:711–723. [PubMed] [Google Scholar]
- 13.Edwards S A, Fan H. Gag-related polyproteins of Moloney murine leukemia virus: evidence for independent synthesis of glycosylated and unglycosylated forms. J Virol. 1979;30:551–563. doi: 10.1128/jvi.30.2.551-563.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans L H, Dressler S, Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core protein of Friend murine leukemia virus. J Virol. 1977;24:865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisawa R, McAtee F J, Zirbel J H, Portis J L. Characterization of glycosylated Gag expressed by a neurovirulent murine leukemia virus: identification of differences in processing in vitro and in vivo. J Virol. 1997;71:5355–5360. doi: 10.1128/jvi.71.7.5355-5360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner M B, Henderson B E, Officer J E, Rongey R W, Parker J C, Oliver C, Estes J D, Huebner R J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973;51:1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartley J W, Rowe W P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new “amphotropic” class. J Virol. 1976;19:19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman P M, Ruscetti S K, Morse H C., III Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part I. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981;1:275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- 19.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lander M R, Moll B, Rowe W P. Procedure for culture of cells from mouse tail biopsies: brief communication. J Natl Cancer Inst. 1978;60:477–478. [PubMed] [Google Scholar]
- 21.Lynch W P, Portis J L. Murine retrovirus-induced spongiform encephalopathy: disease expression is dependent on postnatal development of the central nervous system. J Virol. 1993;67:2601–2610. doi: 10.1128/jvi.67.5.2601-2610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch W P, Robertson S J, Portis J L. Induction of focal spongiform neurodegeneration in developmentally restricted mice by implantation of murine retrovirus-infected microglia. J Virol. 1995;69:1408–1419. doi: 10.1128/jvi.69.3.1408-1419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAtee F J, Portis J L. Monoclonal antibodies specific for wild mouse neurotropic retrovirus: detection of comparable levels of virus replication in mouse strains susceptible and resistant to paralytic disease. J Virol. 1985;56:1018–1022. doi: 10.1128/jvi.56.3.1018-1022.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neil J C, Smart J E, Hayman M J, Jarrett O. Polypeptides of feline leukemia virus: a glycosylated gag-related protein is released into culture fluids. Virology. 1980;105:250–253. doi: 10.1016/0042-6822(80)90173-7. [DOI] [PubMed] [Google Scholar]
- 26.Pond L, Kuhn L A, Teyton L, Schutze M P, Tainer J A, Jackson M R, Peterson P A. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J Biol Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- 27.Portis J L, Czub S, Garon C F, McAtee F J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990;64:1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portis J L, Czub S, Robertson S, McAtee F J, Chesebro B. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J Virol. 1995;69:8070–8075. doi: 10.1128/jvi.69.12.8070-8075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portis J L, Fujisawa R, McAtee F J. The glycosylated gag protein of MuLV is a determinant of neuroinvasiveness: analysis of second site revertants of a mutant MuLV lacking expression of this protein. Virology. 1996;226:384–392. doi: 10.1006/viro.1996.0666. [DOI] [PubMed] [Google Scholar]
- 30.Portis J L, Perryman S, McAtee F J. The R-U5-5′ leader sequence of neurovirulent wild mouse retrovirus contains an element controlling the incubation period of neurodegenerative disease. J Virol. 1991;65:1877–1883. doi: 10.1128/jvi.65.4.1877-1883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portis J L, Spangrude G J, McAtee F J. Identification of a sequence in the unique 5′ open reading frame of the gene encoding glycosylated Gag which influences the incubation period of neurodegenerative disease induced by a murine retrovirus. J Virol. 1994;68:3879–3887. doi: 10.1128/jvi.68.6.3879-3887.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandoval I V, Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994;4:292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 33.Schultz A M, Rabin E H, Oroszlan S. Posttranslational modification of Rauscher leukemia virus precursor polyproteins encoded by the gag gene. J Virol. 1979;30:255–266. doi: 10.1128/jvi.30.1.255-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartzberg P, Colicelli J, Goff S P. Deletion mutants of Moloney murine leukemia virus which lack glycosylated gag protein are replication competent. J Virol. 1983;46:538–546. doi: 10.1128/jvi.46.2.538-546.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitbon M, Sola B, Evans L, Nishio J, Hayes S F, Nathanson K, Garon C F, Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986;47:851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- 36.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 37.Wehrly K, Chesebro B. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods. 1997;12:288–293. doi: 10.1006/meth.1997.0481. [DOI] [PubMed] [Google Scholar]