Abstract
Cascade screening is the process of contacting relatives of people who have been diagnosed with certain hereditary conditions. Its purpose is to identify, inform, and manage those who are also at risk. We conducted a scoping review to obtain a broad overview of cascade screening interventions, facilitators and barriers to their use, relevant policy considerations, and future research needs. We searched for relevant peer-reviewed literature in the period 1990–2017 and reviewed 122 studies. Finally, we described 45 statutes and regulations related to the use and release of genetic information across the fifty states. We sought standardized best practices for optimizing cascade screening across various geographic and policy contexts, but we found none. Studies in which trained providers contacted relatives directly, rather than through probands (index patients), showed greater cascade screening uptake; however, policies in some states might limit this approach. Major barriers to cascade screening delivery include suboptimal communication between the proband and family and geographic barriers to obtaining genetic services. Few US studies examined interventions for cascade screening or used rigorous study designs such as randomized controlled trials. Moving forward, there remains an urgent need to conduct rigorous intervention studies on cascade screening in diverse US populations, while accounting for state policy considerations.
For precision medicine strategies to improve population health, targeted approaches must be considered, not only for disease treatment, but also for early diagnosis and prevention.1 Thus, an important component of precision medicine is the identification of people through genetic testing who are at high risk of disease because of pathogenic germline mutations. Once such people are identified, providers and patients can effectively manage high disease risk to reduce long-term morbidity and mortality.2–4 Following the diagnosis of certain genetic disorders, it is important to consider disseminating this information to blood relatives who also have an increased risk for the condition. This process is known as cascade screening.
While the term cascade screening is relatively new, the process has been standard practice since the inception of the clinical genetics field. Generally used for autosomal dominant conditions, the process involves offering genetic counseling and testing to at-risk relatives of an index patient (known as a proband) in a sequential manner based on the likelihood that they will test positive. Particular attention has been given to cascade testing for three conditions designated as tier 1 conditions by the Centers for Disease Control and Prevention: familial hypercholesterolemia, hereditary breast or ovarian cancer, and Lynch syndrome (a hereditary cancer disorder that puts people at heightened risk for colorectal and other cancers). There are guidelines for identifying probands with these conditions and for initiating cascade testing to ensure that at-risk adult relatives receive genetic counseling and testing so they can follow evidence-based management guidelines—which are known to reduce the burden of disease and are cost-effective. In the case of Lynch syndrome, following evidence-based guidelines reduces colorectal cancer rates by at least 56–62 percent and overall mortality by approximately 65 percent.5 Furthermore, testing twelve relatives per index case compared to six relatives improves the cost-effectiveness of genetic testing by decreasing the incremental cost-effectiveness ratio from $30,331 to $12,332 per life-year saved.6
While guidelines and professional organizations call for the implementation of cascade screening for certain clinically actionable genetic conditions, it remains unclear how genetic findings can be most effectively disseminated to relatives while respecting both federal and state privacy laws related to the disclosure of genetic information7 as well as the complex nature of families. The need to identify, develop, and implement best practices for cascade screening has recently been highlighted by the Cancer Moonshot Blue Ribbon Panel,8 the National Cancer Institute,9 and the Genomics and Population Health Action Collaborative.10
Scoping reviews are a useful method for investigating the current cascade screening literature. They are intended to provide a high-level overview of an area of interest, using qualitative and descriptive means rather than a quantitative synthesis of study outcomes. With a broad area such as cascade screening, involving different disorders and approaches, insights gained from a scoping review are valuable in identifying promising areas for more-focused investigations. In this review our objective was to examine barriers, facilitators, cost-effectiveness, implementation issues, registries, and policy interventions related to cascade screening in the peer-reviewed literature. We also identified state-level policies related to dissemination of genetic findings that could potentially limit the use of cascade screening approaches. This scoping review describes the literature on cascade screening, related state policies, and areas for future research.
Study Data And Methods
We performed a scoping review of the current US and non-US literature on cascade screening for genetic disorders. We searched Medline, Embase, Global Health, PsycInfo, CINAHL, and Scopus databases for literature published in the period 1990–2017 on five related subtopics: barriers or facilitators, cost-effectiveness, implementation, national health systems or registries, and policy interventions. Multiple coders reviewed and abstracted articles, and 20 percent of included articles were independently coded by two authors (double-coded) to ensure coding consistency. When inconsistencies were identified, a third author adjudicated the discrepancy (coding agreement: 86.4 percent).
In a separate search, we identified laws in the fifty states and the District of Columbia regulating the collection, use, and release of genetic information that could affect implementation of cascade screening.11,12 A survey of genetic privacy laws across all fifty states by Scott Smith and colleagues12 formed the basis for our work. We found that seventy-seven of the laws described in their survey had been amended between January 1, 2011, and October 26, 2017.We qualitatively described forty-five laws pertaining to disclosure of protected genetic health information. Additional details on our methods, along with specific search strategies for each database, are in online appendix exhibits 1–5.13
Our study had several limitations. First, although we searched multiple databases and leveraged broad search criteria, it is possible that relevant studies were not identified or were misclassified during the inclusion process. Second, while 20 percent of the articles were double-coded, it is possible that some misclassification of codes occurred. Third, cascade screening is contingent on the adequate identification of probands, but that was not investigated in this review as such studies were outside its purview. Fourth, the policy search was not intended to be a comprehensive and systematic assessment of all state laws that affect genetic information privacy. The survey by Smith and colleagues,12 on which we based our search, was checked for updates. As examples, we independently reviewed laws in Pennsylvania, Ohio, and Oregon laws, but no attempts were made to find new laws passed in the period 2011–17 for the remaining forty-seven states and the District of Columbia. Pennsylvania, Ohio, and Oregon have different laws and demonstrate the variation in state laws that regulate the collection, use, and release of genetic information. Other laws may exist that restrict the disclosure of genetic information.
Study Results
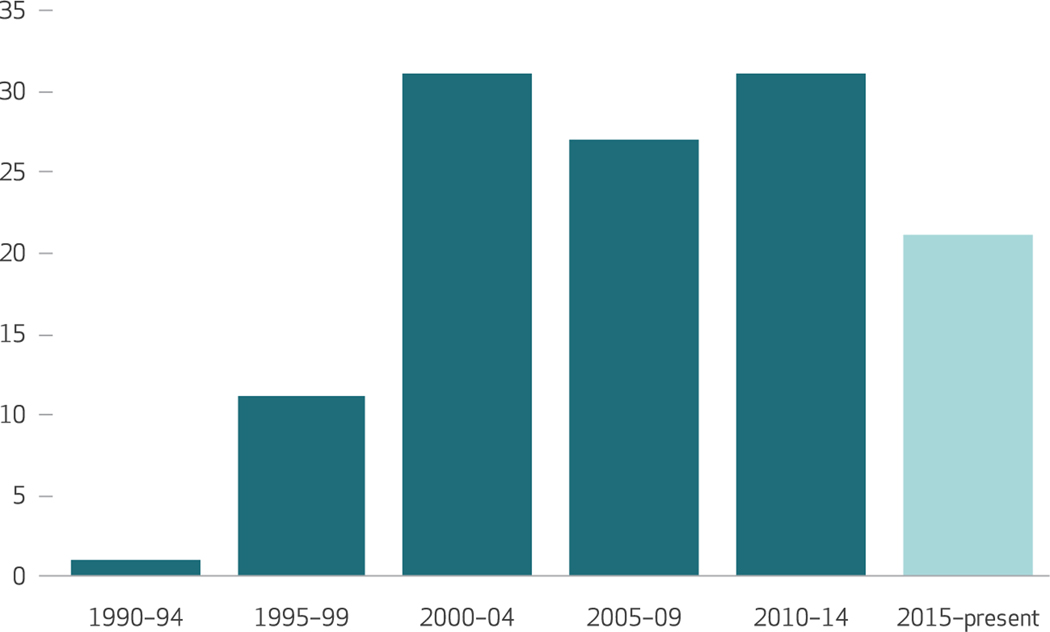
We included 122 articles in our study and abstracted them. (For more information about the included studies, see appendix exhibits 3, 6, and 7.)13 The included studies pertained to twenty-five different genetic disorders, of which the most common were familial hypercholesterolemia, in 35 studies (28.7 percent); hereditary breast and ovarian cancer, in 16 studies (13.1 percent); and Lynch syndrome, in 14 studies (11.5 percent). The number of publications on cascade screening increased between 1990 and 2004, and it held steady through 2014 (exhibit 1). Below, we organize our study findings by key questions.
Exhibit 1. Numbers of cascade screening studies included in the analysis, by publication date since 1990.
SOURCE Authors’ analysis. NOTES The latest five-year period (2015–19) has not yet ended, so the data for that period are incomplete. The bar shows data for January 1, 2015–August 1, 2017.
WHAT INTERVENTIONS HAVE BEEN TESTED AND FOUND TO BE EFFECTIVE?
DELIVERY OF CASCADE SCREENING:
Several studies examined who should be involved in the delivery of cascade screening. Among these studies, two UK studies used a genetic registry–based approach for cascade screening. These registries were maintained by two university hospitals14 and the North West Regional Genetic Family Register service;15 both studies reported grant funding through National Health Services. With the registry-based approach, registry providers (that is, a trained nurse or genetic counselor)14,15 identified and contacted relatives with the proband’s consent. Relatives’ primary care providers were also contacted to facilitate disclosure to relatives. This approach was acceptable to probands with X-linked genetic disorders and chromosome translocations15 and was effective in identifying new cases of familial hypercholesterolemia.14 Ninety-nine percent of probands (259 of 262) agreed to participate, and 81 percent (230 of 285) of first-degree relatives contacted either were tested or were already known to be affected.14 In a separate study, including a genetics specialist on the care team increased genetic testing in family members from 0.13 to 1.05 relatives tested per proband in a low-resource South African setting.16
In contrast to these findings, two studies in which the proband was responsible for contacting relatives showed low genetic testing uptake for familial hypercholesterolemia (23 percent, or 52 of 225)17 and low transfer of information from probands to relatives about the presence of BRCA1/2 mutations in the family (54 percent of the relatives were aware of the BRCA1/2 mutation in their family).18 In both of these studies, the authors recommended that clinical staff members contact relatives directly with probands’ consent. Furthermore, three-quarters of probands in one study felt that it was appropriate for clinical staff members to contact relatives about testing.15 Considering what type of provider should lead cascade screening, a study in England found that a cascade screening program for familial hypercholesterolemia led by a primary care provider (that is, primary care was responsible for the entire adult care pathway) was less costly than a specialist-led model or a dual model in which primary care providers could refer patients to lipidologists, but may provide insufficient patient support.19
BUILDING COMMUNICATION SKILLS:
Looking to improve proband-initiated cascade screening,20 several studies focused on building probands’ communication skills through the use of written materials, education, and telephone counseling. However, this approach was not associated with increased family communication or genetic screening among relatives,20,21 except in cases where the genetic condition conferred high risk to offspring.21 Taken together, these studies suggest that there are remaining challenges to proband-initiated cascade screening and suggest the potential value of including a provider-based approach to cascade screening.
In addition to examining family communication, several studies considered the optimal media for imparting information. For example, one study found that 40 percent of relatives who received family outreach through a mailed letter underwent genetic testing, compared to only 23 percent in the control group, whose members did not receive letters.22 Of note, no relatives voiced concerns about privacy related to receiving the mailed letters.22 Another study found that in-person counseling was preferred to online counseling, but testing uptake among the two counseling approaches was similar. Genetic counselors reported advantages to online counseling, including saving an estimated 7.6 percent time savings and 10.2 percent cost savings. However, they stated that clearer webcam images, better sound quality, and other technical improvements would be needed to improve their satisfaction with online delivery of genetic counseling.23
COST-EFFECTIVENESS:
Simulation modeling studies and cost analyses examined the cost-effectiveness of and costs related to different cascade screening strategies. Eleven studies found that genetic testing of relatives was cost-effective, compared with standard clinical screening. For example, genetic testing was found to be more cost-effective than serum iron studies in identifying people at increased risk for hereditary hemochromatosis.24 While four studies found that, compared to active cascade screening, population-based genetic screening was more efficacious (that is, it identified more carriers), it was less efficient (that is, more people were genotyped per detected mutation) and more costly (as in the case of familial hypercholesterolemia).25 Furthermore, studies reported that the effectiveness of genetic testing increased as more relatives were identified.6,26 In some cases, cost-effectiveness depended on the condition27 or the cost of treating the condition.28 For example, cascade screening for autosomal recessive disorders identified only a small percentage of affected people unlike autosomal dominant disorders such as Lynch syndrome.29 Overall, all but five of the thirty-eight studies that examined costs found that the costs of cascade screening were acceptable—according to the authors’ definitions, which varied across studies.
WHAT ARE THE BARRIERS TO AND FACILITATORS OF CASCADE SCREENING?
The most commonly cited barriers to cascade screening were low understanding or knowledge related to cascade screening among probands or relatives (cited in six studies), limited communication skills among probands (five), low knowledge of or interest in cascade screening among primary care providers (four), costs or limited insurance coverage of genetic testing (three), depression or anxiety in probands or relatives (three), and geographic barriers to receiving genetic services (three). Studies also found less communication and cascade screening uptake among male than among female relatives and among more distant than among first-degree relatives. Parents’ desire for information to better understand their children’s risk was the most commonly cited facilitator of pursuing screening (cited in four studies).
WHAT ARE THE CONSIDERATIONS WHEN ALIGNING INTERVENTIONS WITH PRIVACY POLICY?
A 2011 report presents state-level laws that govern genetic testing or the use and release of genetic data by key stakeholders.12 Some states may have more laws pertaining to genetic testing and data sharing than others. (For genetic disclosure laws by state, see appendix exhibit 8.)13 For example, the 2011 report did not find any Pennsylvania laws governing genetic testing or the use and release of genetic data. Our subsequent review of Pennsylvania laws concluded that the state has no specific genetic privacy laws, nor does it have a general health care privacy law.
In contrast, states such as Ohio,30 Connecticut,31 and Kentucky32 have specific disclosure restrictions for health insurers. These restrictions ensure that insurers are barred from using genetic health information in processing applications for health care coverage. Oregon has a broad genetic privacy law that applies to everyone and restricts the ways in which protected genetic health information can be obtained and disclosed.33 The law requires that all people and entities (for example, health care providers and insurers) obtain patients’ consent (as defined by a second law)34 before obtaining protected genetic health information. Furthermore, the second law34 prohibits any person from disclosing the identity of someone for whom a genetic test was performed. Exceptions exist for medical repositories or registries and research, if the information is deidentified (as defined by another law).35 Twenty states in addition to Oregon that were included in the 2011 report12 currently have laws that require patient consent, and many more states have other protections for personal genetic health information. Laws vary and have implications for cascade screening by state.
WHAT TOPICS NEED ADDITIONAL RESEARCH?
Several gaps in the current literature suggest areas for additional research. Seventy-four percent of the studies in our review did not include information on participants’ race or ethnicity. Among the twenty-two studies that did so, seventeen (77 percent) included primarily non-Hispanic white populations. No studies focused specifically on black, Asian American, or Hispanic study populations, and only one aforementioned study in South Africa focused on cascade screening in a limited-resource public health facility.16 Among the thirty-three studies that reported the composition of their sample by sex, all but three had primarily female study populations. There remains a clear need to increase the diversity of research populations to improve the generalizability of study findings.
Future research should incorporate implementation outcomes to increase understanding of how to effectively implement cascade screening strategies in clinical and public health settings. Fifty-one (41.8 percent) studies reported health outcomes such as genetic test results. However, collecting additional outcomes (for example, how well the interventions are implemented and whether the strategies are sustainable) will be important if cascade screening strategies are to be effectively implemented in clinical and public health settings.
Study design and context are also important factors to consider. We found that the randomized controlled trial—the most rigorous study design—was the least used (in only two studies). (For more information about the designs of the included studies, see appendix exhibit 6.)13 Moving forward, there remains a need to incorporate more rigorous designs to test the implementation of different cascade screening strategies, to determine which approach will be most effective. In addition to study design, location of study has implications for relevance in a US policy context. Eighty-five (69.7 percent) of our studies on cascade screening were conducted outside the US, and thus their results might not be generalizable to the US. Location matters at the state level, too: Given the variation that exists in state laws, state-specific intervention studies may be needed.
Discussion
This review and complementary policy search provides insights into the current knowledge base about and implications for cascade screening delivery in the US.
LITERATURE REVIEW IMPLICATIONS
The current literature suggests that cascade screening programs may be a cost-effective way to identify people with certain hereditary disorders within certain health care settings. However, many current screening programs have suboptimal cascade screening uptake among relatives (that is, relatives are not notified, counseled, or tested), which reduces these programs’ effectiveness. In particular, proband-initiated discussions about cascade screening with relatives was generally not an effective way to increase genetic testing rates in relatives, although this is the most commonly used method in genetics practice today.17,18 A lack of communication about genetic information and poor communication between the proband and relatives about cascade screening may contribute to low genetic counseling and testing uptake by family members.18 Direct contact between trained providers and at-risk relatives could help overcome barriers related to patient communication with relatives.17,18,22 This could be particularly helpful among more distant (second- or third-degree)18,36–38 or male18,20,36,39,40 relatives who are eligible for testing but have lower uptake. While provider-initiated cascade screening with proband consent appears to be the most effective approach, busy providers and their staff members could find it challenging to contact all at-risk relatives. Public health approaches (such as mandatory reporting of clinically actionable genetic diseases, bidirectional reporting between registries and medical facilities, and centralized cascade screening conducted by state departments of health) could help facilitate providers’ contacting at-risk relatives. Furthermore, studies to determine why provider contact is more effective than proband contact may provide insights useful for designing future interventions.
Attempts to address communication and knowledge gaps among key stakeholders—including probands, their relatives, and providers—remain important, since knowledge about and awareness of cascade screening is associated with disclosure of genetic results to relatives, screening uptake by relatives,41 and providers’ recommendations of testing.42
In addition to communication barriers, geographic barriers42–44 can reduce access to genetic services. Online genetic counseling for cascade screening can help overcome barriers posed by geographic location. Such counseling was found to save costs and time in one study.23 Other counseling methods that are not dependent on geography—such as remote conferencing using telephone45 and video46—also have been used with probands.
POLICY SEARCH IMPLICATIONS
While studies indicated that patients may accept cascade screening–related clinical services and active outreach offered to family members, state statutes and regulations as well as federal laws such as the Health Insurance Portability and Accountability Act (HIPAA) of 1996 must be considered before such approaches are tested or adopted within a given state.
To comply with HIPAA, covered entities (health care providers, insurers, and health care clearinghouses) must establish the appropriate safeguards. However, HIPAA allows covered entities to disclose protected health information without patient consent to a public health authority to prevent or control disease, injury, or disability as long as that body is legally authorized to receive such reports. Similarly, in the context of genetic disorders,47 HIPAA may allow a public health authority to conduct surveillance, investigations, and interventions (screenings) using protected genetic health information disclosed by a health care provider if the authority is able to request such information about a particular genetic disorder.7,48 However, general state disease-control laws must be considered, because they might not authorize health departments to conduct cascade screening.
In addition, some state laws go beyond the protections HIPAA establishes and may allow or further restrict the disclosure of personal genetic health information. Because state laws governing the protection of personal genetic health information vary, the need different state-level approaches to developing cascade screening programs will continue.
RESEARCH GAPS IN THE LITERATURE
Most of the cascade screening studies we examined were conducted outside of the US, particularly in the Netherlands and the UK. Because the US has distinct national and state policies related to genetic testing and health privacy, additional research that accounts for the US policy and health care context is needed. In addition to variation in policies, variation in costs over time should be considered. Our review included cost analyses that were conducted in the period 1996–2017 both inside and outside the US. The cost-effectiveness of cascade screening strategies should be reevaluated periodically to take into account the decreasing costs of genetic testing and treatment (for example, as generic drugs for disease treatment become available).
Another research gap concerns the diversity of populations studied. Similar to a recent review of translational genomic research,49 our study found very low reporting of the racial and ethnic composition of study populations. Thus, we have little information about the associations between race and ethnicity and cascade screening. When race and ethnicity are known, studies have documented disparities in the use of genetic testing along racial and ethnic lines, including greater barriers for testing such as low knowledge about testing, concerns about genetic testing misuse, suboptimal insurance coverage, and high levels of medical mistrust.50 To ensure that cascade screening does not worsen such disparities, researchers might consider including diverse study populations, measuring equity, and reporting the racial/ethnic composition of their study populations.
Although the studies we examined addressed a variety of outcomes (such as clinical, cost, and patient preference), we found only limited measuring of implementation outcomes.51 Future research collecting information on such outcomes could provide additional data to inform the translation of cascade screening strategies into unique clinical and public health contexts and their adaptation for those contexts.52 While health systems are well positioned to drive cascade screening, collaborations with public health settings that leverage state or national registries might help coordinate the translation of cascade screening to populations. For example, the use of collaboratives such as the Lynch Syndrome Screening Network53 could increase the implementation of cascade screening.
Finally, few of the studies in our review included a comparator group or used randomized controlled methods. Moving forward, studies that more rigorously test the efficacy and implementation of cascade screening programs and related interventions could be useful for identifying the most effective delivery strategies.
Conclusion
This scoping review did not find standardized processes to optimize cascade screening across geographical regions and policy contexts. We did identify gaps in the current literature, as well as modifiable barriers related to the delivery of cascade screening. More specifically, strategies that address geographic barriers to receipt of genetic services and communication barriers between probands and relatives may be useful for improving cascade screening uptake. There remains an urgent need to conduct rigorous intervention research to identify effective strategies for cascade screening in the US, accounting for variation in state-level policies related to the screening. This work will be important for moving the field of precision medicine forward by identifying high-risk people and connecting them to genetic services to improve their health outcomes.
Supplementary Material
Acknowledgments
Amy Sturm is on the scientific advisory boards for Genome Medical and Clear Genetics. Heather Zierhut is a senior adviser for GeneMatters, LLC. Heather Hampel is on the scientific advisory board for InVitae Genetics and Genome Medical; has stock in Genome Medical; has been the principal investigator of a study supported by Myriad Genetic Laboratories, Inc.; and has done consulting work for Beacon Lab Benefit Solutions. The authors acknowledge the contributions and efforts of Martha Knuth, of the Stephen B. Thacker CDC Library, in developing and implementing search strategies, deduplicating records, and reviewing the “Study Data And Methods” section of the manuscript for accuracy; and of Stephen D. Morgan in aiding in full-text abstraction of a subset of included articles. The authors are very grateful for the wide-ranging support provided by Siobhan Addie, of the National Academies of Sciences, Engineering, and Medicine, in the development and preparation of the manuscript. The authors appreciate the expert legal support provided by Rachel Hulkower, of the Public Health Law Program of the Centers for Disease Control and Prevention (CDC). Finally, the authors thank Chris Hammond, from the Office of the General Counsel of the Department of Health and Human Services (HHS) for his legal advice and independent review of Pennsylvania laws. The policy search does not constitute a systematic update of state laws related to genetic information privacy. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Agency for Healthcare Research and Quality, the CDC, the National Cancer Institute, or HHS.
Contributor Information
Megan C. Roberts, Cancer Prevention Fellow in the Division of Cancer Control and Population Sciences, National Cancer Institute, in Rockville, Maryland.
W. David Dotson, senior coordinating scientist in the Office of Public Health Genomics, Centers for Disease Control and Prevention (CDC), in Atlanta, Georgia..
Christopher S. DeVore, Public Health Fellow in the Office of Public Health Preparedness and Response, CDC, and a master of public health candidate at the Rollins School of Public Health, Emory University, in Atlanta.
Erica M. Bednar, genetic counselor in the Department of Clinical Cancer Genetics and the Cancer Prevention and Control Platform at the University of Texas MD Anderson Cancer Center, in Houston, Texas.
Deborah J. Bowen, professor of bioethics and humanities at the University of Washington, in Seattle.
Theodore G. Ganiats, director of the National Center for Excellence in Primary Care Research, Agency for Healthcare Research and Quality, in Rockville, Maryland.
Ridgely Fisk Green, Carter Consulting, Inc., contractor in the Office of Public Health Genomics, CDC, and at Carter Consulting, in Atlanta..
Georgia M. Hurst, the director of ihavelynchsyndrome.org, in Evanston, Illinois.
Alisdair R. Philp, genetic counselor and a clinical assistant professor at the University of Kansas Hospitals and Clinics, in Westwood.
Charité N. Ricker, genetic counselor and clinical instructor at the University of Southern California, in Los Angeles.
Amy C. Sturm, professor at the Genomic Medicine Institute, Geisinger, in Danville, Pennsylvania.
Angela M. Trepanier, associate professor (clinician educator) at the Center for Molecular Medicine and Genetics, Wayne State University, in Detroit, Michigan.
Janet L. Williams, director, Research Genetic Counselors, at the Genomic Medicine Institute, Geisinger, in Danville, Pennsylvania
Heather A. Zierhut, an assistant professor in genetics, cell biology, and development at the College of Biological Sciences, University of Minnesota Twin Cities, in Minneapolis.
Katherine A. Wilemon, CEO of the Familial Hypercholesterolemia Foundation, in Pasadena, California.
Heather Hampel, associate director of the Division of Human Genetics and of biospecimen research, and a professor of internal medicine, all at the Ohio State University Comprehensive Cancer Center, in Columbus..
References
- 1.Khoury MJ, Iademarco MF, Riley WT. Precision public health for the era of precision medicine. Am J Prev Med. 2016;50(3):398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Final recommendation statement: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing [Internet]. Rockville (MD): USPSTF; 2013. Dec [cited 2018 Mar 26]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic [Google Scholar]
- 4.Goldberg AC, Hopkins PN, Toth PP, Ballantyne CM, Rader DJ, Robinson JG, et al. Familial hypercholesterolemia: screening, diagnosis, and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011; 5(3, Suppl):S1–8. [DOI] [PubMed] [Google Scholar]
- 5.Järvinen HJ, Aarnio M, Mustonen H, Aktan-Collan K, Aaltonen LA, Peltomäki P, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5): 829–34. [DOI] [PubMed] [Google Scholar]
- 6.Mvundura M, Grosse SD, Hampel H, Palomaki GE. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med. 2010;12(2): 93–104. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Beyond the HIPAA privacy rule: enhancing privacy, improving health through research. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 8.National Cancer Institute. Cancer Moonshot Blue Ribbon Panel report 2016 [Internet]. Bethesda (MD): NCI; 2016. [cited 2018 Mar 26]. Available from: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf [Google Scholar]
- 9.Helzlsouer K, Shelburne N, Breslau E, Umar A. Approaches to identify and care for individuals with inherited cancer syndromes [Internet]. Bethesda (MD): National Cancer Institute; 2017. Jun 20 [cited 2018 Apr 5]. Available from: https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-17–041.html [Google Scholar]
- 10.National Academies of Sciences, Engineering, and Medicine. Action collaboratives: Genomics and Population Health Action Collaborative [Internet]. Washington (DC): National Academies; [cited 2018 Mar 26]. Available from: http://www.nationalacademies.org/hmd/Activities/Research/GenomicBasedResearch/Innovation-Collaboratives/Genomics-and-Population-Health.aspx [Google Scholar]
- 11.Habte L, Marblestone CN, Forde JM. Privacy issues in the sharing of genetic information. Milwaukee (WI): Foley and Lardner LLP; 2014. Sep. (White Paper). [Google Scholar]
- 12.Smith S, Nielson PS, Kennedy B. Genetic privacy laws: 50 state survey. J Health Life Sci L. 2011;5(1):75–93. [Google Scholar]
- 13. [To access the appendix, click on the Details tab of the article online.]
- 14.Bhatnagar D, Morgan J, Siddiq S, Mackness MI, Miller JP, Durrington PN. Outcome of case finding among relatives of patients with known heterozygous familial hypercholesterolaemia. BMJ. 2000;321(7275): 1497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright C, Kerzin-Storrar L, Williamson PR, Fryer A, Njindou A, Quarrell O, et al. Comparison of genetic services with and without genetic registers: knowledge, adjustment, and attitudes about genetic counselling among probands referred to three genetic clinics. J Med Genet. 2002;39(12):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoeman M, Apffelstaedt JP, Baatjes K, Urban M. Implementation of a breast cancer genetic service in South Africa—lessons learned. S Afr Med J. 2013;103(8):529–33. [DOI] [PubMed] [Google Scholar]
- 17.Marks D, Thorogood M, Neil SM, Humphries SE, Neil HA. Cascade screening for familial hypercholesterolaemia: implications of a pilot study for national screening programmes. J Med Screen. 2006;13(3): 156–9. [DOI] [PubMed] [Google Scholar]
- 18.Sermijn E, Goelen G, Teugels E, Kaufman L, Bonduelle M, Neyns B, et al. The impact of proband mediated information dissemination in families with a BRCA1/2 gene mutation. J Med Genet. 2004;41(3):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pears R, Griffin M, Watson M, Wheeler R, Hilder D, Meeson B, et al. The reduced cost of providing a nationally recognised service for familial hypercholesterolaemia. Open Heart. 2014;1(1):e000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery SV, Barsevick AM, Egleston BL, Bingler R, Ruth K, Miller SM, et al. Preparing individuals to communicate genetic test results to their relatives: report of a randomized control trial. Fam Cancer. 2013;12(3):537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodgson J, Metcalfe S, Gaff C, Donath S, Delatycki MB, Winship I, et al. Outcomes of a randomised controlled trial of a complex genetic counselling intervention to improve family communication. Eur J Hum Genet. 2016;24(3):356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006; 43(8):665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otten E, Birnie E, Ranchor AV, van Langen IM. Online genetic counseling from the providers’ perspective: counselors’ evaluations and a time and cost analysis. Eur J Hum Genet. 2016;24(9):1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Serag HB, Inadomi JM, Kowdley KV. Screening for hereditary hemochromatosis in siblings and children of affected patients. A cost-effectiveness analysis. Ann Intern Med. 2000;132(4):261–9. [DOI] [PubMed] [Google Scholar]
- 25.Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HA. Cost effectiveness analysis of different approaches of screening for familial hypercholesterolaemia. BMJ. 2002;324(7349):1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladabaum U, Wang G, Terdiman J, Blanco A, Kuppermann M, Boland CR, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med. 2011;155(2):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabater-Molina M, García-Molina E, Tovar I, Ruiz-Espejo F, Gimeno JR, Valdés M. Cost-effectiveness of genetic studies in inherited heart diseases. Cardiogenetics. 2013;3(1): 28–30. [Google Scholar]
- 28.Marang–van de Mheen PJ, ten Asbroek AH, Bonneux L, Bonsel GJ, Klazinga NS. Cost-effectiveness of a family and DNA based screening programme on familial hypercholesterolaemia in the Netherlands. Eur Heart J. 2002;23(24):1922–30. [DOI] [PubMed] [Google Scholar]
- 29.Morris JK, Law MR, Wald NJ. Is cascade testing a sensible method of screening a population for autosomal recessive disorders? Am J Med Genet A. 2004;128A(3):271–5. [DOI] [PubMed] [Google Scholar]
- 30.Rev Ohio. Code Ann., section 3904.01, 0.13. [Google Scholar]
- 31.Conn. Gen. Stat., sections 38a-988 and 38a–988a.
- 32.Ky. Rev. Stat. Ann., section 304.12–085. [Google Scholar]
- 33.Or. Rev. Stat., section 192.531 et seq. [Google Scholar]
- 34.2015 ORS 192.535.
- 35.2015 ORS 192.539
- 36.Finlay E, Stopfer JE, Burlingame E, Evans KG, Nathanson KL, Weber BL, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagoel L, Dishon S, Almog R, Silman Z, Bisland-Becktell S, Rennert G. Proband family uptake of familial-genetic counselling. Psychooncology. 2000;9(6):522–7. [DOI] [PubMed] [Google Scholar]
- 38.Ishii N, Arai M, Koyama Y, Ueno M, Yamaguchi T, Kazuma K, et al. Factors affecting encouragement of relatives among families with Lynch syndrome to seek medical assessment. Fam Cancer. 2011;10(4): 649–54. [DOI] [PubMed] [Google Scholar]
- 39.Cody N, Green A, McDevitt T, Lynch SA. Cascade screening in BRCA1/2 mutation carriers. Ir Med J. 2008; 101(5):140–2. [PubMed] [Google Scholar]
- 40.Maxwell SJ, Molster CM, Poke SJ, O’Leary P. Communicating familial hypercholesterolemia genetic information within families. Genet Test Mol Biomarkers. 2009;13(3):301–6. [DOI] [PubMed] [Google Scholar]
- 41.McClaren BJ, Aitken M, Massie J, Amor D, Ukoumunne OC, Metcalfe SA. Cascade carrier testing after a child is diagnosed with cystic fibrosis through newborn screening: investigating why most relatives do not have testing. Genet Med. 2013;15(7): 533–40. [DOI] [PubMed] [Google Scholar]
- 42.Klitzman R, Thorne D, Williamson J, Marder K. The roles of family members, health care workers, and others in decision-making processes about genetic testing among individuals at risk for Huntington disease. Genet Med. 2007;9(6):358–71. [DOI] [PubMed] [Google Scholar]
- 43.Bernhardt BA, Zayac C, Pyeritz RE. Why is genetic screening for autosomal dominant disorders under-used in families? The case of hereditary hemorrhagic telangiectasia. Genet Med. 2011;13(9):812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saxena A, Phadke SR. Feasibility of thalassaemia control by extended family screening in Indian context. J Health Popul Nutr. 2002;20(1):31–5. [PubMed] [Google Scholar]
- 45.Schwartz MD, Valdimarsdottir HB, Peshkin BN, Mandelblatt J, Nusbaum R, Huang AT, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014; 32(7):618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradbury A, Patrick-Miller L, Harris D, Stevens E, Egleston B, Smith K, et al. Utilizing remote real-time videoconferencing to expand access to cancer genetic services in community practices: a multicenter feasibility study. J Med Internet Res. 2016;18(2):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veenstra DL, Roth JA, Garrison LP Jr, Ramsey SD, Burke W. A formal risk-benefit framework for genomic tests: facilitating the appropriate translation of genomics into clinical practice. Genet Med. 2010;12(11): 686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HHS.gov. The HIPAA privacy rule [Internet]. Washington (DC): Department of Health and Human Services; 2015. [last reviewed 2015 Apr 16; cited 2018 Mar 26]. Available from: https://www.hhs.gov/hipaa/for-professionals/privacy/index.html [Google Scholar]
- 49.Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19(8): 858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suther S, Kiros GE. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11(9):655–62. [DOI] [PubMed] [Google Scholar]
- 51.Proctor EK, Landsverk J, Aarons G, Chambers D, Glisson C, Mittman B. Implementation research in mental health services: an emerging science with conceptual, methodological, and training challenges. Adm Policy Ment Health. 2009;36(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA. 2016;315(18):1941–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch Syndrome Screening Network [home page on the Internet]. Lansing (MI): Lynch Syndrome Screening Network; 2014. [last reviewed 2014 Jan; cited 2018 Mar 30]. Available from: https://www.lynchscreening.net/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.