Abstract
Hematopoietic stem cell transplantation (HSCT) has many potential applications beyond current standard indications, including treatment of autoimmune disease, gene therapy, and transplant tolerance induction. However, severe myelosuppression and other toxicities after myeloablative conditioning regimens have hampered wider clinical use. To achieve donor hematopoietic stem cell (HSC) engraftment, it appears essential to establish niches for the donor HSCs by depleting the host HSCs. To date, this has been achievable only by nonselective treatments such as irradiation or chemotherapeutic drugs. An approach that is capable of more selectively depleting host HSCs is needed to widen the clinical application of HSCT. Here, we show in a clinically relevant nonhuman primate model that selective inhibition of B cell lymphoma 2 (Bcl-2) promoted hematopoietic chimerism and renal allograft tolerance after partial deletion of HSCs and effective peripheral lymphocyte deletion while preserving myeloid cells and regulatory T cells. Although Bcl-2 inhibition alone was insufficient to induce hematopoietic chimerism, the addition of a Bcl-2 inhibitor resulted in promotion of hematopoietic chimerism and renal allograft tolerance despite using only half of the dose of total body irradiation previously required. Selective inhibition of Bcl-2 is therefore a promising approach to induce hematopoietic chimerism without myelosuppression and has the potential to render HSCT more feasible for a variety of clinical indications.
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) has become the standard of care for various malignant and nonmalignant hematologic diseases. Although HSCT has been used for other conditions, such as autoimmune diseases (1), genetic disorders (2), or the induction of transplant tolerance (3–5), its wider clinical application has been limited by the nonselective conditioning therapies associated with this approach. To reliably achieve hematopoietic stem cell (HSC) engraftment, the recipient must undergo strenuous conditioning consisting of total body irradiation (TBI) or chemotherapeutic medications (such as cyclophosphamide, busulfan, or fludarabine). Such treatments are often associated with severe side effects, including pancytopenia, infections, infertility, and even death. If HSC engraftment was accomplished more safely, then HSCT could be more readily applied in a host of nonmalignant conditions.
Although we have achieved long-term immunosuppression-free renal allograft survival in human transplant recipients through transient chimerism induction (3, 6), pancytopenia and other undesirable toxicities associated with the HSCT conditioning regimen have prevented wider application of this approach. Therefore, developing a safer conditioning regimen without myelosuppressive complications is critically important. In an effort to develop a treatment regimen that reliably induces chimerism without causing nonselective myelosuppression, Cippà et al. (7) recently reported that inhibition of B cell lymphoma 2 (Bcl-2) promotes mixed chimerism in mice while avoiding conventional myelosuppressive conditioning. In this murine study, however, a nonclinically available Bcl-2 inhibitor (Bcl-2i), ABT-737, which inhibits not only Bcl-2 but also Bcl-xL, was used. Thus, it was not known whether inhibition of Bcl-2 alone can also promote hematopoietic chimerism. Because venetoclax, which selectively inhibits Bcl-2 alone, is the only U.S. Food and Drug Administration (FDA)–approved Bcl-2i, we undertook the current study to evaluate in a preclinical nonhuman primate (NHP) model the effect of venetoclax on peripheral lymphocytes and bone marrow progenitors and to determine its efficacy in inducing mixed chimerism and renal allograft tolerance. We found that Bcl-2i effectively depletes peripheral lymphocytes and partially deletes HSCs while preserving regulatory T (Treg) cells and myeloid cells. Although Bcl-2i alone was insufficient to induce hematopoietic chimerism, the addition of Bcl-2i to the conditioning regimen resulted in promotion of hematopoietic chimerism, necessitating only half the previously administered irradiation dose. Moreover, the hematopoietic chimerism promoted by this conditioning regimen resulted in long-term immunosuppression-free survival of major histocompatibility complex (MHC)–mismatched renal allografts cotransplanted with HSCs.
RESULTS
Selective Bcl-2 inhibition with venetoclax effectively induces apoptosis of peripheral lymphocytes and bone marrow HSCs while preserving myeloid cells
With eventual clinical application in mind, we chose, for this study, venetoclax, a highly selective, FDA-approved Bcl-2i with demonstrated safety and efficacy in clinical trials for chronic lymphocyte leukemia and other hematologic malignancies (8, 9). To evaluate the effect of Bcl-2i in the induction of apoptosis, we cultured peripheral blood cells and bone marrow cells with various concentrations of venetoclax for 24 hours. The in vitro assay showed a dose-dependent activation of caspase-3/7 (Fig. 1A) and cell apoptosis (Fig. 1B) of T, B, and natural killer (NK) cells, whereas no activation of caspase-3/7 in either granulocytes or monocytes was observed (Fig. 1A). Although granulocytes appeared to have weak annexin binding (apoptotic), propidium iodide (PI; dead) became weakly positive only with very high–dose Bcl-2i (Fig. 1B). No evidence of apoptosis was observed in monocytes (Fig. 1B). Although HSC depletion has never been reported in murine genetic modification models, in our studies in NHPs, weak-to-moderate activation of caspase-3/7 was observed in HSCs (CD34+CD90+CD45RA−) and multipotent progenitors (MPPs; CD34+CD90−CD45RA−). Dose-dependent apoptosis/cell death (annexin+PI+) was also observed in both HSCs and hematopoietic progenitors (Fig. 1B). In vivo, the administration of Bcl-2i (10 mg/kg) for 5 days resulted in marked depletion of T, B, and NK cells without depleting granulocytes and monocytes (Fig. 1C). Significant reduction of HSCs (to 42.5 ± 16.6% of pretreatment counts, P < 0.0001) and MPPs (to 54.6 ± 10.2%, P < 0.0001) in bone marrow was observed after administering Bcl-2i (Fig. 1D). Colony-forming units (CFUs) were also decreased to 31.6 ± 26.0% (P < 0.001) of pretreatment values (Fig. 1D). Considering from the effects of 3-gray (Gy) TBI, where >99% deletion of HSCs was achieved (fig. S1), the reduction of HSCs by Bcl-2i was moderate.
Fig. 1. Bcl-2 inhibition affects peripheral lymphocyte and bone marrow cell frequencies in vitro and in vivo.
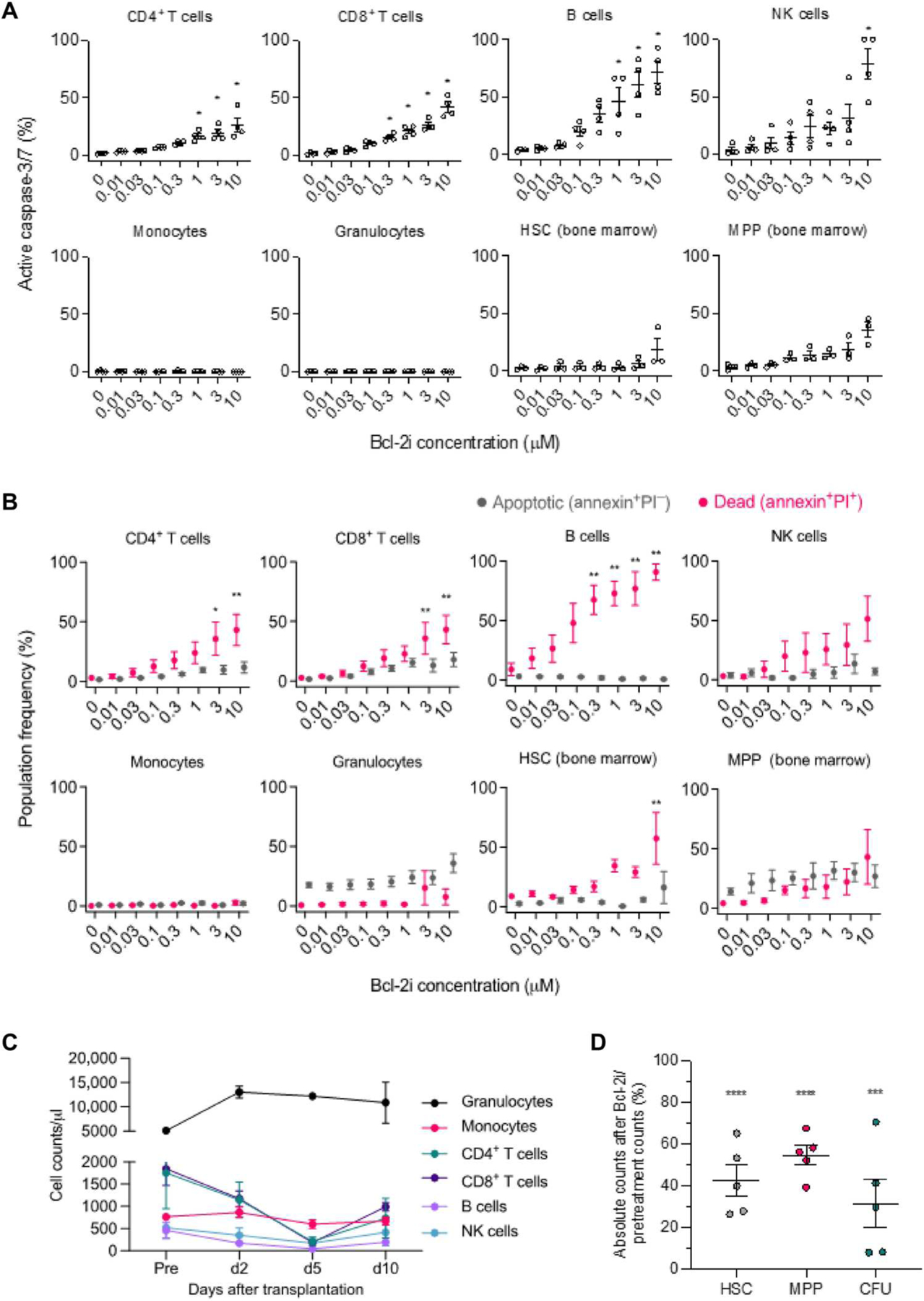
(A) Analysis for active caspase-3/7 after Bcl-2i treatment. Peripheral blood cells and bone marrow cells were cultured with various concentrations of Bcl-2i for 24 hours (n = 4, peripheral blood; n = 3, bone marrow; each sample was obtained from different NHPs). Dose-dependent activation of caspase-3/7 was measured by flow cytometry in peripheral blood–derived CD4+ T cells, CD8+ T cells, B cells (CD3−CD20+), NK cells (CD3−CD16+NKG2a+), granulocytes, and monocytes. Dose-dependent activation of caspase-3/7 was also measured in HSCs (CD34+CD90+CD45RA−) and MPPs (CD34+CD90−CD45RA−) isolated from bone marrow. (B) Analysis for annexin V binding and PI incorporation after Bcl-2i treatment. Peripheral blood cells and bone marrow cells were evaluated for apoptosis (annexin+PI−) and death (annexin+PI+) over 24 hours in culture with Bcl-2i by flow cytometry (n = 4, peripheral blood; n = 3, bone marrow; each sample was obtained from different NHPs). (C and D) Three cynomolgus monkeys were treated with Bcl-2i (10 mg/kg) for 5 days. (C) Peripheral blood cell counts were measured at the indicated time points. (D) Bone marrow aspiration was performed before and 5 days after Bcl-2i treatment, and absolute counts of HSCs, MPPs, and CFUs were evaluated. Each value represents cell counts on day 5 relative to pretreatment values (%) (n = 5, each sample was obtained from different NHPs). Data are presented as means ± SE. Data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Bcl-2 inhibition deleted conventional T, B, and NK cells while enriching Treg cell frequencies after CKBMT
On the basis of the above in vitro and in vivo studies of selective Bcl-2 inhibition, we hypothesized that venetoclax could promote hematopoietic chimerism induction while further lowering the requirement for toxic nonselective myelosuppressive treatments, such as TBI, in our conditioning regimen for combined kidney and bone marrow transplantation (CKBMT). Our original effective and reasonably tolerated conditioning regimen consisted of 3-Gy TBI, 7-Gy local thymic irradiation, and pretransplant antithymocyte globulin (ATG). This was followed, after MHC-mismatched CKBMT on day 0, by costimulatory blockade (CB) with anti-CD154 monoclonal antibody (mAb) and a 28-day course of cyclosporine A (CyA) (group A) (10, 11). In the current study, the TBI dose was reduced to 1.5 Gy without (group B) or with Bcl-2i (10 mg/kg per day from days −4 to +6) (group C), respectively. Group D received Bcl-2i but no TBI (Fig. 2A and Table 1).
Fig. 2. Conditioning regimens with Bcl-2i deplete conventional T cells, B cells, and NK cells without affecting Treg cells.
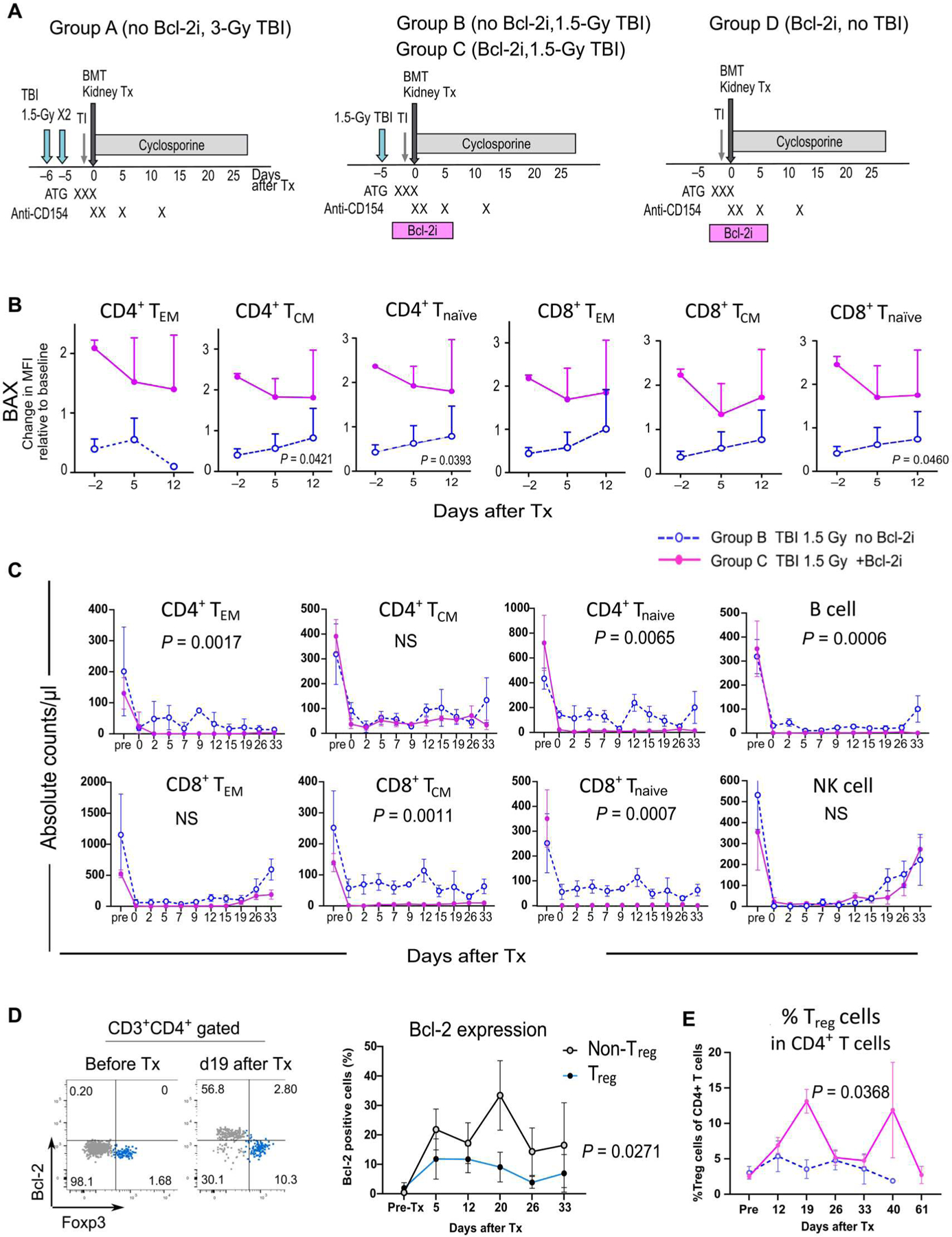
(A) Our original conditioning regimen (group A) included 3-Gy TBI in addition to thymic irradiation, ATG, CB (costimulatory blockade; with anti-CD154 mAb), and a 28-day course of CyA. In groups B and C, TBI was reduced to half (1.5 Gy) without or with Bcl-2i, respectively. In group D, TBI was removed from the regimen. (B) BAX expression on T cells isolated from groups B and C was measured by flow cytometry in the peritransplant period. Expression is shown relative to baseline (pretreatment). Tnaïve, naïve T cells, CD3+CD95−CD45RA+; TEM, effector memory T cells, CD3+CD95+CD28−; TCM, central memory T cells, CD3+CD95+CD28+. MFI, mean fluorescence intensity. (C) Absolute counts of various peripheral lymphocytes in group B (blue dotted lines) and group C (magenta lines) are shown, including naïve T cells, TEM, TCM, B cells (CD3−CD20+), and NK cells (CD3−CD16+NKG2a+) (n = 4, group B; n = 5, group C; each sample was obtained from different NHPs). (D) Representative flow cytometry dot plots show Bcl-2 expression on Foxp3− and Foxp3+ cells among CD3+CD4+ T cells (left panels). Bcl-2 expression was measured longitudinally in Treg cells and non-Treg CD4+ T cells (right panel) (n = 4, each sample was obtained from different NHPs). (E) The frequency of Treg cells among CD4+ T cells was measured in group C (magenta line) and group B (blue dotted line) recipients (%) (n = 5, group B; n = 6, group C; each sample was obtained from different NHPs). Data are presented as means ± SE. Data were analyzed using mixed-model ANOVA for repeated measure. NS, not significant. Tx, transplantation.
Table 1.
Conditioning regimen, percent chimerism, and renal allograft survival
| n | TBI (Gy) | Bcl-2i | Chimerism max (%) and duration |
Renal allograft survival (days) | |||
|---|---|---|---|---|---|---|---|
| Lymphoid | Myeloid | days | |||||
| A¶ | 8 | 3 | − | 1.6 ± 1.0 | 51.6 ± 19.5 | 61.3 ± 14.8 | 1300*, 1167*, 837†, 771*, 401†, 373†, 206§, 58‡ |
| B | 5 | 1.5 | − | 1.1 ± 1.9 | 0.8 ± 0.2 | 14.0 ± 3.2 | >1318†, 176‡, 167‡, 100‡, 58‡ |
| C¶ | 6 | 1.5 | + | 14.8 ± 4.4 | 67.7 ± 6.3 | 81.1 ± 10.2 | >1993║, >1657║, >635║, >593║, 313§, 127‡ |
| D | 2 | 0 | + | <1.0 | <1.0 | 0 | 142‡, 120‡ |
End of study without rejection.
Chronic rejection.
Acute rejection.
Euthanized because of urinary obstruction without rejection.
Now in progress without rejection.
Fine-Gray subdistribution hazards model showing that the risk of developing chronic rejection is significantly higher (P < 0.0001) in group A compared with that in group C.
We compared group B and C recipients to evaluate how peripheral lymphocytes were deleted by the conditioning regimen containing Bcl-2i. During the peri-transplant period, Bcl-2–associated X protein (BAX) was consistently higher in naïve and memory CD4 and CD8 T cells of group C recipients compared with that of group B recipients (Fig. 2B). Higher BAX was associated with more substantial depletion of CD4+ and CD8+ naïve and memory T cells, NK cells, and B cells (Fig. 2C). Bcl-2 expression on Treg cells was lower (P = 0.027) than that of non-Treg conventional T cells (CD3+CD4+Foxp3−) after transplant (Fig. 2D), and this was associated with a significant (P = 0.036) enrichment of Treg cells among CD4+ T cells in the group C recipients (Fig. 2E).
Bcl-2 inhibition promotes hematopoietic chimerism without pancytopenia
In the group receiving 3-Gy TBI conditioning (group A), seven of the eight recipients developed myeloid lineage–dominant chimerism (maximum of 51.6 ± 19.5% in the myeloid lineages versus 1.6 ± 1.0% in the lymphoid lineages) for 1 to 2 months (Fig. 3A and Table 1). In group B, which received half-dose (1.5-Gy) TBI but no Bcl-2i, very few donor chimeric cells were detectable by flow cytometry (Fig. 3A and Table 1). Adding Bcl-2i (group C) resulted in significantly (P < 0.0001) superior chimerism in both myeloid and lymphoid lineages (Fig. 3A). Chimerism was not induced in the two recipients treated with Bcl-2i but no TBI (Table 1 and Fig. 3A), indicating that minimal TBI treatment is still required to induce chimerism in MHC-mismatched CKBMT. Of particular relevance to the anticipated clinical application of this regimen, no granulocytopenia, thrombocytopenia, or anemia was observed in group C recipients (Fig. 3B). This stands in contrast to the pancytopenia observed in group A recipients treated with 3-Gy TBI without Bcl-2i. Because group B recipients failed to develop chimerism, granulocyte counts after CKBMT were lower than those in group C recipients.
Fig. 3. Chimerism and CBC after CKBMT differ on the basis of conditioning regimen.
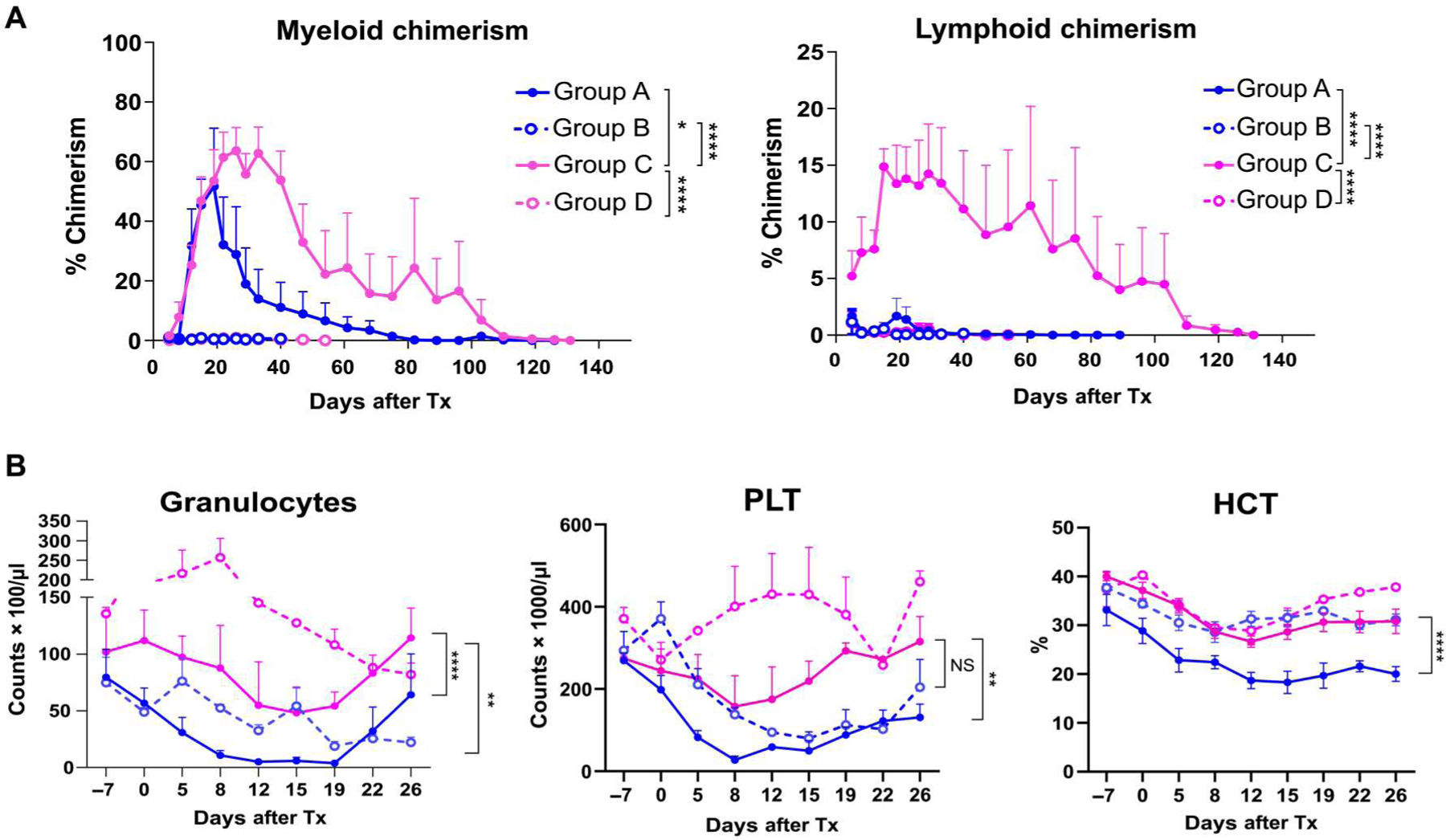
(A) Hematopoietic chimerism after CKBMT. Peripheral blood chimerism was determined by flow cytometry using H38 expression. Both myeloid (left) and lymphoid (right) chimerism were measured (n = 6, group A; n = 3, group B; n = 6, group C; n = 2, group D; each sample was obtained from different NHPs). (B) CBC after CKBMT. Granulocytes, platelets (PLT), and hematocrit (HCT) were measured for groups A, B, C, and D (n = 6, group A; n = 4, group B; n = 6, group C; n = 2, group D; each sample was obtained from different NHPs). Mixed-model ANOVA for repeated measure was used for data analysis. Data are presented as means ± SE. *P < 0.05, **P < 0.01, and ****P < 0.0001.
Promotion of chimerism through Bcl-2 inhibition induced robust long-term immunosuppression-free renal allograft survival
With the 3-Gy TBI conditioning regimen (group A), six of the eight recipients achieved immunosuppression-free long-term (>300 days) survival (Fig. 4, A and B). Although allograft tolerance was stable without rejection (fig. S2) in half of these recipients, the remaining three grafts eventually failed because of development of donor-specific antibody (DSA) and chronic rejection (Fig. 4C and Table 1). In group B, only low degrees of chimerism were achieved, and four of the five recipients developed acute rejection within 6 months (Fig. 4, A, B, and D, and Table 1). The remaining recipient in this group is now alive but has developed DSA and borderline rejection with C4d staining (fig. S3). In group C, higher degrees of mixed chimerism resulted in more stable allograft tolerance, with five of the six recipients achieving long-term survival (>300 days) (Fig. 4, A and B, and Table 1) without rejection (Fig. 4E) and no C4d deposition (fig. S4). Kaplan-Meier analysis showed significant differences between groups B and C in both renal allograft survival (P < 0.05) and rejection-free allograft survival (P < 0.01). There were no differences in renal allograft survival by the degree of MHC disparity (haplotype versus full mismatch) in group B and C recipients (fig. S5). The Fine-Gray subdistribution hazards model (12) showed that the risk of developing chronic rejection was significantly higher (P < 0.0001) in group A compared with that in group C (Table 1). With no TBI (group D), both recipients failed to achieve long-term survival (Fig. 4, A and B) because of acute rejection (Fig. 4F). As reported previously (11, 13), we have performed skin transplantation to evaluate the specificity of long-term unresponsiveness after treatment and transient hematopoietic chimerism in our immunosuppression-free renal allograft recipients. Observations in the current study remained consistent, with now three recipients accepting kidney donor skin grafts for >6 weeks while promptly (<10 days) rejecting third party skin allografts placed 11 to 14 months after immunosuppression withdrawal (Fig. 4G and fig. S6).
Fig. 4. Bcl-2i plus TBI extends renal allograft survival in combined CKBMT.
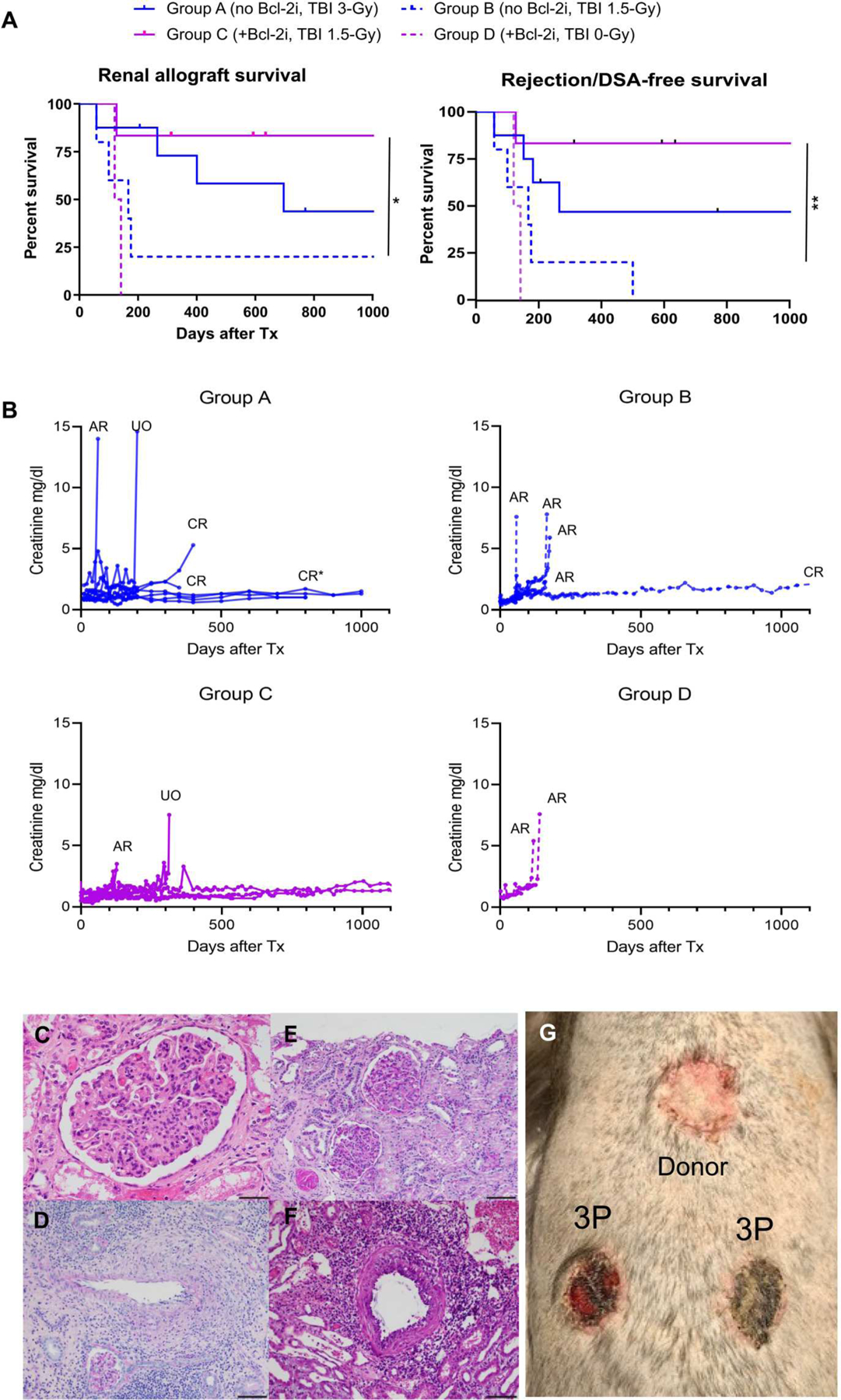
(A) Renal allograft survival rates. Renal allograft survival (left) and rejection/DSA-free survival (right) were measured in groups A to D (n = 8, group A; n = 5, group B; n = 6, group C; n = 2, group D). Kaplan-Meier analysis was used to estimate survival time distributions, and Mantel-Cox log-rank test was used to compare between-group differences.*P < 0.05 and **P < 0.01. (B) Serum creatinine after CKBMT. Serum creatinine (milligrams per deciliter) was measured longitudinally after CKBMT (AR, acute rejection; CR, chronic rejection; UO, urinary obstruction). CR* indicates a recipient euthanized on day 837 because of extensive proteinuria despite normal serum creatinine (n = 8, group A; n = 5, group B; n = 6, group C; n = 2, group D). (C to F) Representative histopathologic findings in groups A to D. (C) A day 837 renal allograft biopsy from a group A recipient shows extensive transplant glomerulopathy with glomerulitis and peritubular capillaritis consistent with chronic active antibody-mediated rejection. H&E, 40×; scale bar, 50 μm. (D) A day 112 renal allograft biopsy from a group B recipient shows acute cellular rejection with transmural arteritis without C4d or DSA [Banff T cell–mediated rejection (TCMR) type III]. PAS, 10×; scale bar, 200 μm. (E) A day 804 allograft biopsy from a group C recipient shows no evidence of rejection. PAS, 20×; scale bar, 100 μm. (F) Shown is an image of the kidney from an autopsy (day 120) of a goup D recipient. The image shows evidence of Banff TCMR type III. H&E, 20×; scale bar, 100 μm. (G) Three group C recipients underwent skin transplantation 1 year after CKBMT from the kidney donor and third party (3P) donors. A representative photo taken at 3 weeks shows specific acceptance of the skin graft from the bone marrow and kidney donor (donor) and rejection of the 3P skin.
DISCUSSION
Adequate depletion of host HSCs to create sufficient niches in host bone marrow appears to be essential for achieving allogeneic HSC engraftment (14, 15). Because low-dose TBI is required to induce hematopoietic chimerism, even in autologous bone marrow transplantation, a physical rather than immunological requirement is implied (15). To date, this has been reproducibly accomplished using TBI and chemotherapeutic drugs, both of which are associated with dose-dependent myelosuppression and genotoxicity (16, 17). Identifying less toxic alternative modalities for more selective HSC depletion is therefore essential to increase the utility of HSCT for clinical applications. The murine studies by Cippà et al. (7, 18), showing successful engraftment of allogeneic HSCs without myelosuppressive treatments, provide encouraging observations. However, the precise mechanism by which Bcl-2 inhibition might induce hematopoietic chimerism without myeloablative treatment has not been clarified, because selective inhibition of Bcl-2 has not been reported to delete HSCs, even in murine studies. Although previous studies with Bcl-2–deficient mice have demonstrated selective effects on thymocytes and the peripheral lymphoid compartment, no abnormality has been observed in HSC function (19).
Therefore, to clarify a role for Bcl-2i in HSC engraftment, we initiated a series of preclinical studies in NHPs to evaluate the effect of selective Bcl-2i with venetoclax on lymphocytes and HSCs both in vitro and in vivo. The results of these studies showed dose-dependent induction of apoptosis among T, B, and NK cells, whereas granulocytes and monocytes were largely spared. Venetoclax also induced dose-dependent cell death in vitro and partial deletion of HSCs and MPPs in vivo, although the response of the latter was weaker than for lymphocytes. We found that partial deletion of HSCs and MPPs yielded substantial promotion of hematopoietic chimerism, permitting us to reduce the TBI dose by half. Moreover, post-CKBMT Bcl-2 expression was noted to increase mainly in conventional T cells, whereas it remained minimal in Treg cells. As observed in clinical trials (9), where Bcl-2i effectively deleted leukemic cells overexpressing Bcl-2, we observed that Bcl-2i preferentially deleted conventional T cells that express higher Bcl-2 while preserving Treg cells expressing minimal Bcl-2. This observation was also supported by studies by Ludwig et al. (20), which demonstrated that Treg cells are less dependent on Bcl-2 for their survival. Treg cell enrichment by Bcl-2i possibly provides an additional mechanism favoring hematopoietic chimerism.
Promoting mixed chimerism by Bcl-2i resulted in improved immunosuppression-free renal allograft survival. In the group receiving the original protocol with 3-Gy TBI but without Bcl-2i (group A), one of the eight animals experienced acute rejection, and three of the eight developed chronic rejection, reducing long-term, rejection-free allograft survival to 50%. When the TBI dose was decreased to half in group B, four of the five recipients lost their kidney allografts because of acute rejection. By adding Bcl-2i to the regimen in group C, superior rejection-free allograft survival was achieved consistently with the higher degree of lymphoid chimerism, which has been found to be relevant to allograft tolerance (21). Enrichment of Treg cells by Bcl-2i may be an important mechanism for promoting renal allograft tolerance, because Treg enrichment has been consistently observed in peripheral blood and renal allografts in our preclinical and clinical studies (3, 22–24).
In the current study, we successfully reduced the requirement for TBI by 50%, thereby avoiding pancytopenia; however, complete elimination of TBI could not be achieved by Bcl-2 inhibition alone. If further experience suggests that avoidance of even low-dose TBI is preferable, other synergistic treatments should evaluated to assess their ability to deplete more HSCs. For example, Mcl-1, another Bcl-2 family protein, has been shown to deplete HSCs (25). We are now testing dual inhibition of Mcl-1 and Bcl-2 without TBI in MHC-mismatched bone marrow transplantation. The preliminary results have been promising, with successful induction of chimerism without any TBI (26). Another possible approach would be to use anti-CD117 antibody, which targets C-kit exclusively expressed on HSCs (27). Although initial clinical studies have been disappointing, revealing limited chimerism induction by anti-CD117 alone, even in patients with severe combined immune deficiency (28), the combination of anti-CD117 antibody and Bcl-2i would appear to be a reasonable approach, because HSCs need two signals through both C-kit and Bcl-2 to survive (29).
Because transient chimerism has been shown to be sufficient to induce renal allograft tolerance in our approach (3, 30), CyA was discontinued at 4 weeks after transplantation. Although withdrawal from immunosuppression has been consistently followed by disappearance of chimerism, we consider it advantageous for clinical application because the risk of graft-versus-host disease is eliminated. If more prolonged chimerism or full hematopoietic chimerism is deemed necessary for other hematologic indications, then the regimen can be modified accordingly.
The current studies are limited in the following respects. First, the optimal dose, duration, and timing of Bcl-2i remain to be defined. In the current study, Bcl-2i was administered from days −4 to 6, based on the conditioning regimen used in the murine model, but Bcl-2i could potentially delete donor HSCs infused on day 0. Therefore, in preliminary experiments, we withheld Bcl-2i treatments on days 0 to 2. However, chimerism could not be induced with this modification. We hypothesize that the host lymphocytes recovered too rapidly after stopping Bcl-2i because of its short half-life (19 to 26 hours), which interfered with the engraftment of donor stem cells. Second, although thymic irradiation, ATG, and CB were all required for the previous regimen (10, 11, 13), these requirements need to be reevaluated for the Bcl-2i regimen. Last, although we did not find differences in renal allograft survival by the degree of MHC disparity (haplotype versus full mismatch) in this study, its effects on chimerism and allograft tolerance induction also remain to be concluded with more animals.
In conclusion, the current study establishes that selective inhibition of Bcl-2 with venetoclax can promote hematopoietic chimerism without myelosuppression, leading to stable long-term immunosuppression-free renal allograft survival in a clinically relevant NHP transplant model. This approach not only removes a major hurdle to wider application of our approach for induction of renal allograft tolerance but also advances the design of less toxic conditioning regimens, which can be applied in conjunction with HSCT to treat a wide variety of disorders.
MATERIALS AND METHODS
Study design
Cynomolgus monkeys were used in this study because they share many immunologic and clinical characteristics with humans, which provide the most valid option for preclinical testing of reagents that may later be used in humans and for revealing unanticipated toxicity of new therapeutic protocols. The primary outcome of interest was days to graft rejection (or graft loss). Because there were no censored animals, all rejection times can be observed, and two group comparisons of four to eight animals per group were made using exact Wilcoxon tests. We used exact tests with these necessarily small sample sizes (n ≤ 5) to avoid the necessity of making large sample approximations or unverifiable parametric assumptions. With these sample sizes, statistical significance can be attained when complete, or nearly complete, separation of groups is observed. Table S1 presents the minimal detectable effect sizes for a power of 80% at a two-sided α level of 0.05, assuming that, on some transformed scale, such as the log scale, test statistic for testing difference in mean days to graft rejection follows a t distribution with 2n − 2 df. The detectable effect size by each sample size is the true mean difference between groups, on the transformed scale, divided by the common within group SD. Note that when analyzing the data with the exact test for small (n < 5) sample size to target complete (or near complete) separation (effect size > 2), it is unnecessary to transform the data because the results of the Wilcoxon test are invariant to monotonic change of scale.
Outcomes may also be dichotomized as either success or failure such as graft survival to 400 days or chronic rejection positive or negative. We used the Fisher’s exact test to compare the proportion of successes between treatment groups. Table S2 presents the power of the Fisher’s exact test, at a one-sided α level of 0.05, for the comparison of the five animals per group over a range of true success rates in the two experimental groups. Thus, for example, if the true success rate is 10% in one group but 90% in the other, then we have an 82% probability of detecting a significant difference in the observed success rates. The power analysis, which relies on rigorous nonparametric statistical tests, shows that we can attain statistical significance with four to nine transplants per group (table S2).
Animals and pair selections
Cynomolgus donor and recipient monkeys weighing 4 to 8 kg were used for this study (Charles River Primates). Donors and recipients were paired on the basis of ABO blood type compatibility and MHC mismatching (data file S1). MHC characterization was performed as previously described (31, 32). All surgical procedures and postoperative care of animals were performed in accordance with National Institute of Health (NIH) guidelines for the care and use of primates and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee (protocol no. 2019 N000128).
Combined kidney and bone marrow transplantation
The rationale for using TBI in our NHP model is that NHPs have proved to be very resistant to cyclophosphamide, which necessitates high toxic dosages to achieve chimerism (100 to 120 mg/kg in humans versus 200 mg/kg in NHPs) (33). Thus, TBI will continue to be used in our NHP model with the expectation that, if TBI can be successfully reduced or eliminated from the NHP protocol, then the same will be true for cyclophosphamide in humans.
Recipients of the original regimen (group A) were conditioned with TBI (1.5 Gy/day on days −6 and −5, relative to the day of CKBMT), local thymic irradiation (7 Gy on day −1), and equine-derived anti-human thymocyte globulin [Atgam; Pharmacia & Upjohn Co.; 50 mg/kg per day (previously determined to be equivalent to 15 mg/kg in humans) intravenously on days −2, −1, and 0]. After CKBMT, the recipients were also treated with anti-CD154 mAb (NIH Nonhuman Primate Reagent Resource) 20 mg/kg per day intravenously on days 0, 2, 7, and 12. CyA (Novartis) was administered 2 to 6 mg/kg per day intramuscularly on days 0 to 27 and adjusted to maintain trough concentrations of 150 to 250 ng/ml. No immunosuppression was administered after day 28 (Fig. 2A). In group B, the dose of TBI was reduced to 1.5 Gy on day −5 without a Bcl-2i. For group C, TBI was also reduced to 1.5 Gy, and a Bcl-2i, venetoclax (ABT-199, Selleckchem; 10 mg/kg per day intramuscularly on days −4 to +6), was added (Fig. 2A). Controls were group D (no TBI) (Table 1).
Bone marrow transplantation
Donor bone marrow cells were obtained by multiple aspirations from the iliac crests, humerus head, and vertebral bones under general anesthesia. If the donor animal was euthanized, then the donor bone marrow cells were harvested from the vertebral bones after euthanasia. Donor bone marrow cells were washed and resuspended with normal saline and infused intravenously (1.0 × 108 to 3.0 × 108 mononuclear cells per kg).
Kidney transplantation
Kidney transplantation was performed as previously detailed (34). Briefly, through a midline incision, the kidney allograft was transplanted intraperitoneally by anastomosing renal vein and artery to the vena cava and abdominal aorta, respectively. Ureterovesical anastomosis was performed by the Lich-Gregoir technique. Unilateral native nephrectomy was performed simultaneously. Postoperatively, the kidney allograft was monitored by ultrasound (Mindray) until the remaining native kidney was removed by day 50. Subsequently, kidney allograft function was monitored by daily urine output and serum creatinine measurement once or twice a week.
Blood chemistry and complete blood cell count measurement
Blood chemistries including creatinine and blood urea nitrogen were measured once or twice a week. Chemistries were measured using the Catalyst Dx Chemistry Analyzer (IDEXX Laboratories). Complete blood cell counts were also measured weekly using HemaTrue (HESKA).
Skin transplantation
Three-centimeter-diameter full-thickness grafts were taken from the abdomen of the kidney donor or the third party animals. Skin was then cryopreserved using RPMI 1640 medium supplemented with 10% dimethyl sulfoxide and 30% fetal bovine serum and placed in the −80°C deep freezer. On the day of skin transplant, the donor and the third party skins were thawed in a warm water bath and washed twice with saline. The skin grafts were transplanted to full-thickness graft beds on the recipient’s posterior thorax. The grafts were sutured in place using 4–0 Nylon suture (Ethicon) and covered with a gauze and protective dressing using fiberglass orthopedic casting tapes (3M). The dressing was removed after 7 days, after which the grafts were inspected daily. Rejection was considered complete when the entire graft lost signs of viability.
Evaluation of in vitro effects of Bcl-2i on peripheral blood cells and hematopoietic stem cells
Whole blood or bone marrow cells were lysed to remove red blood cells. Remaining cells were seeded into a six-well plate (1 × 106 cells per well) with RPMI 1640 supplemented with 10% fetal bovine serum (R&D Systems), 1× penicillin-steptmycin-glutamine (Thermo Fisher Scientific), 1 mM sodium pyruvate (Corning), and 1× nonessential amino acids (Thermo Fisher Scientific). Cells were treated with the different final concentrations of Bcl-2i and incubated for 24 hours in a 5%CO2/air environment at 37°C. Cells were collected and stained for surface markers, followed by annexin (Thermo Fisher Scientific) or CellEvent active caspase-3/7 (Thermo Fisher Scientific) staining according to the manufacturer’s instructions.
CFU assay
CFU assays were performed in MethoCult H4434 (STEMCELL Technologies) following the manufacturer’s instructions. Briefly, after lysing red cells in ammonium chloride lysis buffer, bone marrow cells were mixed in 1.5 ml of complete MethoCult H4434 and seeded into 35-mm petri dishes at 2.5 × 104 cells per dish. The assay was performed in quadruplicate, and hematopoietic colonies were scored after incubating for 14 days (37°C, 5% CO2) using microscopy.
Flow cytometric analyses
Flow cytometry analysis was performed by the following procedure. Peripheral blood mononuclear cells were suspended with flow cytometry medium (0.1% bovine serum albumin and 0.1% sodium azide in phosphate-buffered saline) and labeled with fluorochrome-conjugated antibodies (1:100 dilution). Cells were incubated for 30 min at 4°C and washed with flow cytometry medium. Intracellular staining was performed using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s instruction. Anti-cleaved caspase antibodies were conjugated with Alexa Fluor 488 or phycoerythrin using the Zenon Rabbit IgG Labeling Kits (Invitrogen) according to the manufacturer’s instruction. Stained samples was evaluated using FACSverse (BD Biosciences) or Accuri flow cytometers (BD Pharmingen), and analysis was conducted using FlowJo software (Tree Star Inc.). Antibodies used in this study are listed in table S1.
Detection of hematopoietic chimerism
To provide accurate evaluation of post–bone marrow transplant hematopoietic chimerism, we selected recipient/donor pairs on the basis of the reactivity to H38 (anti–HLA-BW6 antibody, One Lambda), where monkeys positive for HLA-BW6 were selected as the donor, and monkeys negative for HLA-BW6 were selected as the recipient. Peripheral blood cells were first stained with H38. Cells were incubated for 30 min at 4°C and then washed twice. Cell-bound mAb was detected with fluorescein isothiocyanate–conjugated goat (Fab_2) anti-mouse immunoglobulin G (IgG) + IgM mAb (Biosource), which was incubated for 30 min at 4°C, followed by two washes and analysis on a FACScan (Becton Dickinson). In all experiments, the percentage of cells stained for H38 expression was determined from a single-color fluorescence histogram and compared with those obtained from donor and pretreatment frozen recipient cells, which were used as positive and negative controls. By using forward and 90° light scatter (FSC and SSC, respectively) dot plots, we gated lymphocyte (FSC- and SSC-low), granulocyte (SSC-high), and monocyte (FSC-high but SSC-low) populations, and chimerism was determined separately for each population. Nonviable cells were excluded by PI (Thermo Fisher Scientific) staining.
Histopathological analyses
Protocol renal biopsies were obtained every 2 to 4 months in recipients with stable function and whenever a rise in serum creatinine occurred. Tissue was processed for light microscopy, and a portion was frozen for immunofluorescence staining. Other organs obtained surgically (lymph nodes, native kidney, and spleen) were similarly processed. After euthanasia of a monkey, a complete autopsy was performed for histopathologic examination of the renal allograft, lymph nodes, heart, lung, liver, pancreas, thymus, and skin. Allograft hematoxylin and eosin (H&E) and Periodic acid–Schiff (PAS)–stained samples were scored by current Banff criteria (35), including C4d deposition by immunohistochemistry (36).
Statistical analysis
Raw, individual-level data are presented in data file S2. Statistical analysis was performed with GraphPad Prism 7.01 (GraphPad Software Inc.) and SAS 9.4 (SAS Institute). Mixed-model analysis of variance (ANOVA) for repeated measures, followed by pair-wise post hoc t test as needed, was used to compare chimerism, complete blood count, and T cell subsets. Kaplan-Meier analysis was used to estimate survival time distributions, and Mantel-Cox log-rank test was used to compare between-group differences. The Fine-Gray subdistribution hazards model (12) was used to evaluate risk of developing rejection. P values lower than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments:
We acknowledge A. D’Attilio, M. Hogan, and M. Duggan for veterinary supervision. We also thank D. H. Sachs, J. C. Madsen, and J. F. Markmann for critical reading and comments and A. Adams for editorial analysis and comment.
Funding:
The present work was supported, in part, by grants from the National Institute of Allergy and Infectious Disease (NIAID): RO1AI155714 (to T.K.), 1R56AI139679-01 (to T.K.), 1R21AI126289-02, and RO1AI155714 (to T.K.). We also appreciate the support by the Pablo & Almudena Legorreta Research Foundation.
Footnotes
Competing interests: T.K. is an inventor on a patent entitled “Method to induce hematopoietic chimerism” (WO 2020/227647). T.R.S. is a member of the Data and Safety Monitoring Board (DSMB) for Bluebird Bio, the DSMB, and adjudication committee for Syneos Health. T.R.S. is a member of the Scientific Advisory Board for Qihan Biotech and Ossium Health. The other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCE AND NOTES
- 1.Hügle T, Daikeler T, Stem cell transplantation for autoimmune diseases. Haematologica 95, 185–188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan RA, Gray D, Lomova A, Kohn DB, Hematopoietic stem cell gene therapy: Progress and lessons learned. Cell Stem Cell 21, 574–590 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DSC, Hertl M, Goes NB, Wong W, Williams WW Jr., Colvin RB, Sykes M, Sachs DH, HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 358, 353–361 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R, Ildstad ST, Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci. Transl. Med. 4, 124ra128 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S, Induced immune tolerance for kidney transplantation. N. Engl. J. Med. 365, 1359–1360 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, Tolkoff-Rubin N, Preffer F, Crisalli K, Gao B, Wong W, Morris H, LoCascio SA, Sayre P, Shonts B, Williams WW Jr., Smith RN, Colvin RB, Sykes M, Cosimi AB, Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am. J. Transplant. 14, 1599–1611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cippà PE, Gabriel SS, Chen J, Bardwell PD, Bushell A, Guimezanes A, Kraus AK, Wekerle T, Wüthrich RP, Fehr T, Targeting apoptosis to induce stable mixed hematopoietic chimerism and long-term allograft survival without myelosuppressive conditioning in mice. Blood 122, 1669–1677 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW, ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, Kipps TJ, Anderson MA, Brown JR, Gressick L, Wong S, Dunbar M, Zhu M, Desai MB, Cerri E, Heitner Enschede S, Humerickhouse RA, Wierda WG, Seymour JF, Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH, Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59, 256–262 (1995). [PubMed] [Google Scholar]
- 11.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, Winn HJ, Colvin RB, Sachs DH, Cosimi AB, CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am. J. Transplant. 4, 1391–1398 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Fine JP, Gray RJ, A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94, 496–509 (1999). [Google Scholar]
- 13.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, Ko DSC, Bartholomew A, Kimikawa M, Hong HZ, Abrahamian G, Colvin RB, Cosimi AB, Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation 68, 1767–1775 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D, Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 318, 1296–1299 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita Y, Sachs DH, Sykes M, Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood 83, 939–948 (1994). [PubMed] [Google Scholar]
- 16.Howell SJ, Shalet SM, Spermatogenesis after cancer treatment: Damage and recovery. J. Natl. Cancer Inst. Monogr., 12–17 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Wallace WH, Thomson AB, Saran F, Kelsey TW, Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. Biol. Phys. 62, 738–744 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Cippà PE, Gabriel SS, Kraus AK, Chen J, Wekerle T, Guimezanes A, Wüthrich RP, Fehr T, Bcl-2 inhibition to overcome memory cell barriers in transplantation. Am. J. Transplant. 14, 333–342 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ, Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75, 229–240 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Ludwig LM, Hawley KM, Banks DB, Thomas-Toth AT, Blazar BR, McNerney ME, Leverson JD, LaBelle JL, Venetoclax imparts distinct cell death sensitivity and adaptivity patterns in T cells. Cell Death Dis. 12, 1005 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaiss CC, Oura T, Sasaki H, Dehnadi A, Matsunami M, Rosales IA, Cosimi AB, Kawai T, Importance of hematopoietic mixed chimerism for induction of renal allograft tolerance in nonhuman primates. Transplantation 103, 689–697 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, LoCascio SA, Morokata T, Dey BR, Tolkoff-Rubin NT, Preffer F, Bonnefoix T, Kattleman K, Spitzer TR, Sachs DH, Sykes M, Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am. J. Transplant. 11, 1236–1247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sprangers B, DeWolf S, Savage TM, Morokata T, Obradovic A, LoCascio SA, Shonts B, Zuber J, Lau SP, Shah R, Morris H, Steshenko V, Zorn E, Preffer FI, Olek S, Dombkowski DM, Turka LA, Colvin R, Winchester R, Kawai T, Sykes M, Origin of enriched regulatory T cells in patients receiving combined kidney-bone marrow transplantation to induce transplantation tolerance. Am. J. Transplant. 17, 2020–2032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotta K, Aoyama A, Oura T, Yamada Y, Tonsho M, Huh KH, Kawai K, Schoenfeld D, Allan JS, Madsen JC, Benichou G, Smith RN, Colvin RB, Sachs DH, Cosimi AB, Kawai T, Induced regulatory T cells in allograft tolerance via transient mixed chimerism. JCI insight 1, e86419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno SI, Akashi K, Korsmeyer SJ, Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307, 1101–1104 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Hirose DMT, Lassiter G, Kawai T, paper presented at 2021 American Transplant Congress, Boston, MA, USA, 4 to 9 June 2021. [Google Scholar]
- 27.Czechowicz A, Palchaudhuri R, Scheck A, Hu Y, Hoggatt J, Saez B, Pang WW, Mansour MK, Tate TA, Chan YY, Walck E, Wernig G, Shizuru JA, Winau F, Scadden DT, Rossi DJ, Selective hematopoietic stem cell ablation using CD117-antibody-drug-conjugates enables safe and effective transplantation with immunity preservation. Nat. Commun. 10, 617 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal R, Dvorak CC, Kwon H-S, Long-Boyle JR, Prohaska SS, Brown JW, Le A, Guttman-Klein A, Weissman IL, Cowan MJ, Logan AC, Weinberg KI, Parkman R, Roncarolo M-G, Shizuru JA, Non-genotoxic anti-CD117 antibody conditioning results in successful hematopoietic stem cell engraftment in patients with severe combined immunodeficiency. Blood 134, 800 (2019). [Google Scholar]
- 29.Domen J, Weissman IL, Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J. Exp. Med. 192, 1707–1718 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Hoshino T, Fujioka S, Shimuzu K, Tanabe K, Okawa T, Ota K, Cosimi AB, Sachs DH, Mixed chimerism and immune tolerance induction by low-stress pretreatment before kidney transplantation in monkeys. Nihon Rinsho Meneki Gakkai Kaishi 18, 670–674 (1995). [PubMed] [Google Scholar]
- 31.O’Connor SL, Blasky AJ, O’Connor DH, Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics 59, 449–462 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pendley CJ, Becker EA, Karl JA, Blasky AJ, Wiseman RW, Hughes AL, O’Connor SL, O’Connor DH, MHC class I characterization of Indonesian cynomolgus macaques. Immunogenetics 60, 339–351 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sogawa H, Boskovic S, Nadazdin O, Abrahamian G, Colvin RB, Sachs DH, Cosimi AB, Kawai T, Limited efficacy and unacceptable toxicity of cyclophosphamide for the induction of mixed chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 86, 615–619 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosimi AB, Delmonico FL, Wright JK, Wee SL, Preffer FI, Jolliffe LK, Colvin RB, Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-CD4 monoclonal antibody. Surgery 108, 406–413 (1990). [PubMed] [Google Scholar]
- 35.Loupy A, Haas M, Roufosse C, Naesens M, Adam B, Afrouzian M, Akalin E, Alachkar N, Bagnasco S, Becker JU, Cornell LD, Clahsen-van Groningen MC, Demetris AJ, Dragun D, Duong van Huyen JP, Farris AB, Fogo AB, Gibson IW, Glotz D, Gueguen J, Kikic Z, Kozakowski N, Kraus E, Lefaucheur C, Liapis H, Mannon RB, Montgomery RA, Nankivell BJ, Nickeleit V, Nickerson P, Rabant M, Racusen L, Randhawa P, Robin B, Rosales IA, Sapir-Pichhadze R, Schinstock CA, Seron D, Singh HK, Smith RN, Stegall MD, Zeevi A, Solez K, Colvin RB, Mengel M, The Banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 20, 2318–2331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adam BA, Smith RN, Rosales IA, Matsunami M, Afzali B, Oura T, Cosimi AB, Kawai T, Colvin RB, Mengel M, Chronic antibody-mediated rejection in nonhuman primate renal allografts: Validation of human histological and molecular phenotypes. Am. J. Transplant. 17, 2841–2850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


