Abstract
The market for large molecule biologic drugs has grown rapidly, including antisense oligonucleotide (ASO) drugs. ASO drugs work as single-stranded synthetic oligonucleotides that reduce production or alter functions of disease-causing proteins through various mechanisms, such as mRNA degradation, exon skipping, and ASO-protein interactions. Since the first ASO drug, fomivirsen, was approved in 1998, the U.S. Food and Drug Administration (FDA) has approved 10 ASO drugs to date. Although ASO drugs are efficacious in treating some diseases that are untargetable by small-molecule chemical drugs, concerns on adverse drug reactions (ADRs) and toxicity cannot be ignored. Illustrative of this, mipomersen was recently taken off the market due to its hepatotoxicity risk. This paper reviews ADRs and toxicity from FDA drug labeling, preclinical studies, clinical trials, and postmarketing real-world studies on the 10 FDA-approved ASO drugs, including fomivirsen and pegaptanib, mipomersen, nusinersen, inotersen, defibrotide, eteplirsen, golodirsen, viltolarsen, and casimersen. Unique and common ADRs and toxicity for each ASO drug are summarized here. The risk of developing hepatotoxicity, kidney toxicity, and hypersensitivity reactions co-exists for multiple ASO drugs. Special precautions need to be in place when certain ASO drugs are administrated. Further discussion is extended on studying the mechanisms of ADRs and toxicity of these drugs, evaluating the existing physiologic and pathologic states of patients, optimizing the dose and route of administration, and formulating personalized treatment plans to improve the clinical utility of FDA-approved ASO drugs and discovery and development of new ASO drugs with reduced ADRs.
SIGNIFICANCE STATEMENT
The current review provides a comprehensive analysis of unique and common ADRs and the toxicity of FDA-approved ASO drugs. The information can help better manage the risk of severe hepatotoxicity, kidney toxicity, and hypersensitivity reactions in the usage of currently approved ASO drugs and the discovery and development of new and safer ASO drugs.
Introduction
If a drug at an administered dose results in a plasma drug concentration over the minimal toxic concentration, it may cause adverse drug reactions (ADRs) and toxicity. This is also the case for next-generation therapeutics, such as antisense oligonucleotide (ASO) drugs. ASOs are a class of biologic entities with a fast-growing pace of development in recent years, with an innovative and promising approach for treating both rare and common human diseases. ASO drugs are therapeutic agents capable of reducing the abundance of proteins that cause human diseases (Roberts et al., 2020). ASO drugs employ single-stranded chains of synthetic oligonucleotides that block the ability of mRNA molecules to be translated into proteins through a variety of mechanisms (Crooke, 2017). Compared with small molecule-based chemical drugs, ASO drugs have shown distinct advantages in targeting diseases at the genetic level and may become highly useful in routine clinical practice to treat a diverse number of human diseases and conditions.
Although ASO drugs are highly promising pharmacotherapies, ADRs and toxicity are equally concerning in the development of ASO drugs as more conventional therapeutic agents. The safety assessment of ASOs has already revealed that this class of drugs has some shared ADRs with varying levels of severity. Some ASO drugs have shown certain toxicity levels during clinical trials, such as in the case of mipomersen and hepatotoxicity (Wong and Goldberg, 2014). The U.S. Food and Drug Administration (FDA) withdrew mipomersen in 2019 from the market after only 5 years after use approval due to its hepatotoxic liability. Therefore, it is critical to understand what types of ADRs and toxicities can be caused by ASO or ASO drug candidates and understand the underlying molecular mechanisms. The purpose of this review is to provide critical analysis of both frequently encountered and idiosyncratic ADRs and toxicities among the 10 FDA-approved ASO drugs. The article will cover a brief historical perspective on ASO drugs, recent fundamental discoveries, current challenges and knowledge gaps, and future perspective directions in consideration of ADRs and toxicity of the 10 FDA-approved ASO drugs.
Brief Historical Perspective
The development of ASOs as therapeutic agents spans more than 30 years since ASOs were first demonstrated to influence RNA processing and modulate the translation of proteins from mRNAs back in 1978 (Stephenson and Zamecnik, 1978). It took a subsequent 20 years for the first ASO drug, fomivirsen (Table 1), to be approved by the FDA in 1998 (Marwick, 1998). After a slow pace during the following 18 years, the FDA approved only two new ASO drugs (pegaptanib in 2004 and mipomersen in 2013). Since then, the pace has markedly quickened, and the FDA has approved a total of seven new drugs (defibrotide, eteplirsen, nusinersen, inotersen, golodirsen, viltolarsen, and casimersen) up to the present time. Currently, more than 100 ASOs are in the clinical drug development pipelines, and many more are in various preclinical development stages (Dhuri et al., 2020). In the coming years, ASO drugs may become frequently used for routine clinical treatment of human diseases, and perhaps they can become as prominent in their clinical utility as small-molecule chemical drugs.
TABLE 1.
FDA-approved ASO drugs between 1998 and 2021
| ASO Drug | Brand Name | Company for Manufacture | Disease to Be Treated | Therapeutic Target | FDA Approval Date |
|---|---|---|---|---|---|
| ASO Drugs Targeting mRNAs and Pre-mRNAs | |||||
| Fomivirsen | Vitravene | Isis Pharmaceuticals | Cytomegalovirus retinitis | Cytomegalovirus IE2mRNA | NDA 20-96108/26/1998a |
| Mipomersen | Kynamro | Genzyme | Homozygous familial hypercholesterolemia | Apolipoprotein B mRNA | NDA 20356801/29/2013b |
| Eteplirsen | Exondys 51 | Sarepta Therapeutics | Duchenne muscular dystrophy | Exon 51 dystrophinpre-mRNA | NDA 20648809/19/2016 |
| Nusinersen | Spinraza | Biogen | Spinal muscular atrophy | Survival motor neuron-2(SMN-2)mRNA | NDA 20953112/23/2016 |
| Inotersen | Tegsedi | Ionis Pharmaceuticals | Polyneuropathy of amyloidosis | Transthyretin (TTR)mRNA | NDA 21117210/05/2018 |
| Golodirsen | Vyondys 53 | Sarepta Therapeutics | Duchenne muscular dystrophy | Exon 53 dystrophinpre-mRNA | NDA 21197012/12/2019 |
| Viltolarsen | Viltepso | Nippon Shinyaku Co | Duchenne muscular dystrophy | Exon 53 dystrophinpre-mRNA | NDA 21215408/12/2020 |
| Casimersen | Amondys 45 | Sarepta Therapeutics | Duchenne muscular dystrophy | Exon 45 dystrophinpre-mRNA | NDA 21302602/25/2021 |
| ASO Drugs Targeting Proteins | |||||
| Pegaptanib | Macugen | Eyetech Pharmaceuticals | Neovascular macular degeneration | Vascular endothelial growth factor | NDA 21-75612/17/2004 |
| Defibrotide | Defitelio | Jazz Pharmaceuticals | Veno-occlusive disease | Multiple mechanisms of action | NDA20811403/30/2016 |
NDA, new drug application number.
aWithdrew in the European Union market in 2002 and in the United States in 2006.
bWithdrew in the U.S. market in 2019.
ADRs and toxicity are always of concern during ASO drug discovery and development. For example, when mipomersen was approved by the FDA, a black-box warning existed regarding the risk of liver enzyme (transaminase) elevation and hepatic steatosis. The drug was only prescribed to patients who participated in a risk evaluation and mitigation strategy (REMS) (Dixon et al., 2014). After further use for several years under restrictive guidelines, the FDA withdrew mipomersen from the market in 2019 due to an unacceptable risk of hepatotoxicity. Numerous class-wide and idiosyncratic ADRs and toxicities are known for other FDA-approved ASO drugs. Therefore, understanding why ADRs and toxicity occur at mechanistic and physiologic levels is prudent for developing future efficacious ASO drugs while avoiding such safety liabilities.
Key Recent Advances
A comprehensive analysis of ADRs and associated toxicity is provided for each of the following FDA-approved ASO drugs: fomivirsen, pegaptanib, mipomersen, defibrotide, eteplirsen, nusinersen, inotersen, golodirsen, viltolarsen, and casimersen.
Fomivirsen (Vitravene)
Fomivirsen is the first FDA-approved ASO drug. Known commercially as Vitravene, it was manufactured by Isis Pharmaceuticals (now Ionis Pharma) and received FDA approval on August 26, 1998, for the treatment of cytomegalovirus (CMV) retinitis (Roehr, 1998). CMV retinitis is an infection that targets the light-sensing cells in the retina (Port et al., 2017). Fomivirsen is a phosphorothioate ASO drug that inhibits human CMV replication through an antisense mechanism (de Smet et al., 1999).
The commonly observed ADRs of Fomivirsen in clinical trials were increased intraocular pressure and ocular inflammation. Approximately 25% of patients experienced these ADRs during loading dosing (Perry and Balfour, 1999; Vitravene Study Group, 2002). Other ADRs of fomivirsen reported during the clinical trials included ophthalmic dysfunctions, such as anterior chamber inflammation, abnormal vision, cataract, decreased visual acuity, eye pain, and floaters. Fomivirsen was observed to also accumulate in the plasma at detectable concentrations, and also in the liver and kidneys. Some of the more frequent systemic side effects included anemia, diarrhea, abnormal pain, asthenia, fever, infection, nausea, headache, pneumonia, sepsis, rash, vomiting, sinusitis, and systemic CMV infection. Additional ADRs that occurred less frequently in patients included application site reactions, decreased peripheral vision, hypotony, corneal edema, eye irritation, retinal vascular disease, optic neuritis, vitreous opacity, and visual field defects.
Limited open-label clinical studies have been done on fomivirsen to evaluate its safety. However, systemic accumulation of fomivirsen was noted in animal studies following single or repeated intravitreal injections in monkeys at levels below 70 ng/ml in plasma and 350 ng/g in tissues. Metabolites of fomivirsen were detected in the liver, kidney, and plasma at a concentration of 14 ng/ml in plasma and 70 ng/g in tissues in monkeys treated with fomivirsen every other week for up to 3 months.
Fomivirsen was withdrawn from the European market in 2002 and the United States in 2006 due to the development of highly effective antiretroviral therapies for the same indication. In addition, the number of CMV cases dramatically decreased, and Ionis Pharmaceuticals stopped marketing the drug.
Pegaptanib Sodium (Macugen)
Pegaptanib sodium, sold under the name of Macugen, is manufactured by Eyetech Pharmaceuticals and was approved by the FDA on December 17, 2004, for the treatment of neovascular (wet) age-related macular degeneration (AMD) (Tobin, 2006). AMD can present itself in two forms, as blurred or no vision in the center of the visual field (Nowak, 2006). The number of AMD patients was estimated to be 196 million in 2020 and the projections are for 288 million cases by 2040 globally (Jonas et al., 2017). Macugen is an aptamer oligonucleotide with a three-dimensional conformation, enabling it to bind to extracellular vascular endothelial growth factor (VEGF) as an antagonist (Katz and Goldbaum, 2006). VEGF induces angiogenesis and increases vascular permeability and inflammation, which contribute to AMD. Pegaptanib inhibits VEGF, suppressing pathologic neovascularization.
ADRs were observed in two clinical trials with controlled, double-masked, randomized studies in approximately 1,200 patients with neovascular AMD (Chakravarthy et al., 2006). The patients were randomized and administered either 0.3 mg of Pegaptanib sodium or placebo once every 6 weeks for 8-9 treatments, intravitreally. The group treated with 0.3 mg Pegaptanib sodium exhibited a statistically significant reduction in the rate of vision decline as compared with the placebo group. The most frequently reported ADRs in the clinical studies included anterior chamber inflammation, blurred vision, cataract, conjunctival hemorrhage, corneal edema, eye discharge, eye irritation, eye pain, hypertension, increased intraocular pressure (IOP), ocular discomfort, punctate keratitis, reduced visual acuity, visual disturbance, vitreous floaters, and vitreous opacities (observed in 10–40% of patients). Approximately 6–10% of patients treated with pegaptanib sodium demonstrated ADRs that included ocular (blepharitis, conjunctivitis, photopsia, and vitreous disorder) and nonocular (bronchitis, diarrhea, dizziness, headache, nausea, and urinary tract infection) reactions. Approximately 1–5% of patients treated exhibited ocular side effects, such as allergic conjunctivitis, conjunctival edema, corneal abrasion, corneal deposits, corneal epithelium disorder, endophthalmitis, eye inflammation, eye swelling, eyelid irritation, meibomianitis, mydriasis, periorbital hematoma, retinal edema, and vitreous hemorrhage. Approximately 1–5% of patients reported some nonocular events, including arthritis, bone spurs, carotid artery occlusion, cerebrovascular accident, chest pain, contact dermatitis, contusion, diabetes mellitus, dyspepsia, hearing loss, pleural effusion, transient ischemic attack, urinary retention, vertigo, and vomiting. Serious ADRs related to the treatment with Pegaptanib sodium occurred in <1% of those treated, and these included endophthalmitis, retinal detachment, and iatrogenic traumatic cataract.
Warnings and precautions in the FDA-approved drug label for Macugen include endophthalmitis, increases in intraocular pressure, and anaphylaxis. Monitoring for infection, intraocular pressure, and the perfusion of the optic nerve head is recommended by the FDA.
ADRs of Pegaptanib in postmarketing settings were further compared with the premarketing clinical trials findings (Penedones et al., 2014). Both in pre- and postmarketing settings, ocular ADRs were more frequent than nonocular ADRs. In addition to the observed ADRs in the premarketing settings, the postmarketing findings have been typified by an increased number of events, such as retinal pigmented epithelium tears, thromboembolic events, and mortality.
Mipomersen (Kynamro)
Mipomersen was manufactured by Genzyme Corporation and approved by the FDA on January 29, 2013 (Hair et al., 2013), to reduce low-density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apo B), total cholesterol, and non–high-density lipoprotein-cholesterol (non–HDL-C) in patients with homozygous familial hypercholesterolemia (HoFH). Mipomersen is an ASO drug that inhibits APO B-100 synthesis used in combination with diet modification and lipid-lowering medications (Bell et al., 2011).
ADRs were described in four phase 3 clinical trials (ClinicalTrials.gov NCT00607373, NCT00706849, NCT00770146, and NCT00794664). These clinical trials were randomized, double-blinded, placebo-controlled studies with a total of 390 patients at a ratio of 2:1 for weekly subcutaneous injections of 200 mg of mipomersen or placebo for a median treatment of 25 weeks (Santos et al., 2015). The most common ADR, injection site reactions, occurred in 84% of patients and typically consisted of pain, local swelling, erythema, pruritus, and tenderness. Furthermore, flu-like symptoms, such as fatigue, influenza-like illness, chills, pyrexia, arthralgia, myalgia, or malaise were reported in approximately 30% of the patients within 2 days of treatment initiation (Reeskamp et al., 2019). Other less common ADRs (3–15%) included vascular disorder (hypertension), cardiac disorders (angina pectoris and palpitations), musculoskeletal and connective tissue disorders (pain in extremity and musculoskeletal pain), gastrointestinal disorders (nausea, vomiting, and abdominal pain), and nervous system and psychiatric disorders (headache and insomnia).
A black-box warning for the risk of hepatotoxicity, including elevation of transaminases and hepatic steatosis, was placed in the FDA-approved drug labeling of mipomersen. In clinical trials, 12% of the patients with HoFH treated with mipomersen compared with 0% of subjects treated with placebo had elevations in alanine transaminase (ALT) ≥3× the upper limits of normal (ULN), and 9% in the mipomersen group compared with 0% of placebo patients had at least one elevation in ALT ≥ 5 × ULN (Thomas et al., 2013). The FDA suggested that before initiating treatment with mipomersen, a full panel of liver function tests that include ALT, aspartate transaminase (AST), total bilirubin, and alkaline phosphatase should be conducted. Patients with moderate or severe hepatic impairment, or active liver diseases, including unexplained persistent elevations in serum transaminases, are contraindicated in the use of mipomersen.
Another FDA warning is hepatic steatosis. During clinical trials in patients with HoFH and hyperlipidemia, the median absolute increase in hepatic fat was 10% after 26 weeks of treatment compared with baseline (0%). The increase in hepatic fat (hepatic steatosis) is a risk factor for advanced liver diseases, including steatohepatitis and cirrhosis (Nassir et al., 2015). Because of the risk of hepatotoxicity, mipomersen is only available through a restricted program under a REMS, called the Kynamro REMS (Gupta, 2015). Mipomersen is no longer available after its distribution was terminated in 2019 due to the risk of hepatotoxicity (Lui et al., 2021).
Defibrotide Sodium (Defitelio)
Defibrotide sodium, manufactured by Jazz Pharmaceuticals and sold under the name Defitelio, was approved by the FDA on March 30, 2016, for the treatment of adult or pediatric patients with hepatic veno-occlusive disease (VOD) and renal or pulmonary dysfunction following hematopoietic stem-cell transplantation (Choy, 2016). Defibrotide is a mixture of single- and double-stranded phosphodiester oligonucleotides (9–80, average 50 nucleotides) extracted through controlled depolymerization of porcine gut mucosa (Kornblum et al., 2006). Although the mechanism of action has not been fully elucidated, in vitro studies showed that defibrotide sodium reduces hepatic sinusoidal endothelial cell activation and protects them from damage. Also in vitro, it promotes hydrolysis of fibrin clots by enhancing the enzymatic activity of factors such as plasmin and tissue plasminogen activator (Richardson et al., 2017). The clinically recommended dosage of defibrotide is 6.25 mg/kg given every 6 hours as an intravenous infusion over 2 hours, which should be dosed based on the baseline body weight of the patient.
ADRs of defibrotide were defined based on interventional and expanded clinical trial studies, including NCT00272948, NCT00358501, and NCT00628498 (Kessler-Icekson et al., 1988; Corbacioglu et al., 2012; Richardson et al., 2016). Patients with VOD received either defibrotide at 6.25 mg/kg intravenously every 6 hours or placebo (given as a 2-hour infusion) for at least 21 days up to 60 days. The most common ADRs included hypotension, diarrhea, vomiting, nausea, and epistaxis (incidence > 10%). The most common ADRs with an incidence of 5% or greater included hypotension (11%) and pulmonary alveolar hemorrhage (7%).
The warnings and precautions in the FDA-approved drug label of Defitelio include hemorrhage and hypersensitivity reactions. As previously stated, since Defibrotide increases the in vitro activity of fibrinolytic enzymes, it may increase the risk of bleeding in patients with VOD after hematopoietic stem-cell transplantation. Hypersensitivity reactions occurred in less than 2% of patients and included rashes, urticaria, and angioedema. Patients on defibrotide treatment should be monitored for both bleeding and hypersensitivity reactions.
Eteplirsen (Exondys 51)
Eteplirsen is manufactured by Sarepta Therapeutics and received an accelerated FDA-approval on September 19, 2016 (Syed, 2016), to treat Duchenne muscular dystrophy (DMD). DMD is the most common type of muscular dystrophy, affecting 7.1 per 100,000 males and 2.8 per 100,000 in the general global population (Crisafulli et al., 2020). Muscle weakness usually begins around the age of 4 and worsens quickly, and most are unable to walk by the age of 12 (Bushby et al., 2010). DMD is caused by mutations of the dystrophin gene, encoding dystrophin protein responsible for connecting the actin cytoskeleton of each muscle fiber to the underlying extracellular matrix (Elangkovan and Dickson, 2021). Although there is no known cure, some medications may help to improve symptoms, including steroids, anticonvulsants, and immunosuppressants (Beytía et al., 2012). Recently, ASO-mediated exon-skipping therapies have been developed as precision medicine for the treatment of DMD (Matsuo, 2021). Eteplirsen is an ASO drug indicated for the treatment of DMD in patients who have a confirmed mutation of the dystrophin gene that is circumvented by ASO-induced skipping of exon 51 during spicing of the dystrophin pre-mRNA. Exondys 51, as eteplirsen is commercially known, binds to exon 51 of dystrophin pre-mRNA, resulting in exclusion of this exon during mRNA processing (Lim et al., 2017). The clinical dose recommended for Exondys 51 is 30 mg/kg once weekly given as 35- to 60-minute intravenous infusion.
Eteplirsen’s ADRs were reported in a phase 2 clinical study with 12 participants (NCT01396239). This was a double-blind placebo-controlled randomized trial (Mendell et al., 2013). The participating patients were randomized to weekly intravenous infusions of 30 and 50 mg/kg eteplirsen, or placebo for 24 weeks. The most common ADRs of eteplirsen were balance disorder, vomiting, and contact dermatitis. These ADRs had an incidence at least 25% higher than placebo in all 12 DMD patients treated with either dose of eteplirsen (Mendell et al., 2013). Because of the small numbers of patients in this study, these ADR frequencies may not reflect the frequencies observed in practice. In a follow-up phase 3 clinical trial (NCT02255552) with 109 patients who received at least 30 mg/kg/week of eteplirsen for up to 144 weeks, at least 10% of patients reported vomiting, contusion, excoriation, arthralgia, rash, catheter site pain, and respiratory tract infection. Less than 1% of patients had bronchospasm, cough, dyspnea, fever, flushing, hypersensitivity reaction, hypotension and urticaria from postmarketing surveillance and case reports. Transient erythema, facial flushing, and elevated temperature had been reported on days of eteplirsen infusion. Because only three clinical studies had evaluated eteplirsen, the information for monitoring parameters is lacking. There are no animal data available to assess the use of eteplirsen during pregnancy and the effect of the drug on milk production.
A warning and precaution for hypersensitivity reaction has been placed in the FDA-approved drug label of eteplirsen. In the clinical trials, some patients treated with eteplirsen presented with hypersensitivity reactions, including rash, pyrexia, flushing, urticaria, bronchospasm, dyspnea, and cough. The FDA recommends following the institute-appropriate medical treatment of these ADRs. Another recommendation is to consider slowing drug infusion or interrupting eteplirsen therapy altogether if hypersensitivity reactions arise.
Nusinersen (Spinraza)
Nusinersen is manufactured by Biogen and was approved by the FDA on December 23, 2016, for the treatment of spinal muscular atrophy (SMA) in pediatric and adult patients (Hoy, 2017). SMA is a rare neuromuscular disorder that results in the loss of motor neurons and progressive muscle wasting. Reported prevalence of SMA is approximately 1–3 per 100,000 among different populations (Bertolin et al., 2019; Ito et al., 2022). Nusinersen is the first FDA-approved drug to treat SMA (Son and Yokota, 2018). This is a survival motor neuron-2 (SMN2)-directed ASO drug designed to treat SMA caused by mutations in chromosome 5q that lead to SMN protein deficiency (Claborn et al., 2019). Nusinersen is initiated as four loading doses and a maintenance dose. The first three doses are administered at 14-day intervals, and the fourth one is administered 30 days after the third dose. Maintenance doses are given every 4 months after the fourth loading dose.
ADRs were determined in numerous clinical trials, including NCT01494701, NCT01703988, NCT01839656, NCT02193074, NCT02292537, NCT01780246, and NCT02052791 (Darras et al., 2019). The ADRs were analyzed from 323 infants and children who either received nusinersen at 12 mg per administration at 14-day intervals or underwent sham procedures (Darras et al., 2019). The most common ADRs reported in approximately 20% of treated patients were vomiting, constipation, back pain, headache, pyrexia, and lower respiratory tract infections. ADRs of lower frequency included decreased weight, seasonal allergy symptoms, falls, epistaxis, flatulence, upper respiratory tract congestion, urinary tract infection, and ear infection.
After administration of nusinersen therapy is associated with thrombocytopenia and coagulation abnormalities. In the clinical studies (Mercuri et al., 2018; Darras et al., 2019), 16% nusinersen-treated patients with normal or elevated platelet levels at baseline developed a platelet level below the lower limits of normal. Because of the risk of thrombocytopenia and coagulation abnormalities, patients treated with nusinersen may be at increased risk of bleeding complications. Nusinersen is present in and excreted by the kidney. Renal toxicity, including potentially fatal glomerulonephritis, was observed after administration of nusinersen. In the same clinical studies, 58% of Nusinersen-treated patients had elevated urine protein, compared with 34% sham-control group. The mechanism of renal and liver injury is most likely due to drug accumulation with repeated doses, because subcutaneously or intravenously administered ASOs bind to plasma proteins that are filtered at the glomerulus and then reabsorbed in the proximal tubule (Frazier, 2015). Given these safety findings, warnings and precautions for thrombocytopenia, coagulation abnormalities, and renal toxicity have been placed in the FDA-approved drug label of nusinersen. Platelet count, coagulation tests, and quantitative spot urine protein testing at baseline and prior to each dose of nusinersen are highly recommended by the FDA.
The ADRs identified in the clinical trials have been further confirmed from postmarketing and case reports, including angioedema, aseptic meningitis, hydrocephalus, hypersensitivity reaction, maculopapular rash, meningitis, serious infection, and skin rash (Mendonça et al., 2021). Long-term animal studies to evaluate carcinogenic potential of nusinersen were not performed. There are not sufficient data to evaluate nusinersen-associated developmental risks in pregnant women and no adverse events were observed in animal studies of fetal development. In intrathecal toxicity studies in juvenile primates, administration of nusinersen resulted in brain histopathology at the moderate and high doses and acute, transient deficits in lower spinal reflexes at the high dose in each study.
An observational clinical trial (NCT04419233) is expected to recruit 50 participants to evaluate the safety of nusinersen in the postmarketing setting in China and is estimated to be completed in January 2023. Another observational clinical trial (NCT04317794) is also in recruitment phases with an expected 145 participants to evaluate the safety of nusinersen in the postmarketing setting in Korea. The estimated completion date of the study is July 2027. The data released from these studies will provide information for ADRs and toxicity in diverse populations.
Inotersen (Tegsedi)
Inotersen is manufactured by Ionis Pharmaceuticals and was approved by the FDA on October 5, 2018, to treat the polyneuropathy of hereditary transthyretin (TTR)-mediated amyloidosis (hATTR-PN) in adults (Keam, 2018). hATTR-PN is a rare disease with an estimated 10,000 afflicted patients worldwide (Schmidt et al., 2018), but without treatment. This is an inherited, progressive, and fatal disease. Current options for the treatment of the disease include liver transplantation and TTR stabilizers of tafamidis and diflunisal (Luigetti et al., 2020). Recently, more promising gene-silencing therapies have been developed, including patisiran, the first FDA-approved siRNA drug (Zhang et al., 2021), and the ASO drug inotersen. Inotersen works by degrading mutant and wild-type TTR mRNAs by binding to the TTR mRNAs, therefore reducing serum TTR and TTR protein deposits in tissues (Gales, 2019). The clinically recommended dose for Inotersen is 284 mg once weekly subcutaneously. The injection should be given on the same day every week.
ADRs were observed in a phase 2/3 clinical trial (NCT01737398). This clinical trial was an international, randomized, double-blinded, placebo-controlled study to assess the efficacy and safety of inotersen (Benson et al., 2018). During the clinical trial, 173 adult patients with polyneuropathy caused by hATTR-PN were randomly assigned in a 2:1 ratio to receive weekly subcutaneous injections of inotersen (300 mg) or placebo for 64 weeks. The most common ADRs occurring in approximately 20% of inotersen-treated patients and more frequently than with placebo were nausea, fever, fatigue, thrombocytopenia, injection site reactions, and headaches. The less common ADRs (<20%) included peripheral edema, chills, anemia, vomiting, myalgia, decreased renal function, arrhythmia, arthralgia, presyncope, decreased appetite, paresthesia, dyspnea, elevated liver function test, orthostasis, influenza-like illness, contusion, bacterial infection, eosinophilia, and dry mouth.
The FDA mandated the inclusion of a black box warning in the drug’s packing insert describing the safety concerns with the use of inotersen. The most serious concerns are thrombocytopenia and glomerulonephritis. In the clinical trial of NCT01737398, inotersen caused low platelet count, which can lead to sudden and unpredictable life-threatening thrombocytopenia (Benson et al., 2018). Inotersen-associated thrombocytopenia was also reported in the clinical trials of the NEURO-TTR (NCT04136184) and NEURO-TTR open-label extension (Narayanan et al., 2020). The key strategies to prevent thrombocytopenia include monitoring the platelet count and withholding therapy if the patient develops signs or symptoms of thrombocytopenia (unusual or prolonged bleeding, neck stiffness, or atypical headache). Caution should be used when using antiplatelet drugs or anticoagulants concomitantly with inotersen because of the risk of bleeding. Inotersen may also cause glomerulonephritis that may result in dialysis-dependent renal failure. In the same clinical trial, glomerulonephritis occurred in 3% of inotersen-treated patients, compared with no patient on placebo (Benson et al., 2018). The mechanism of action for glomerulonephritis can be explained by accumulation of ASOs in proximal tubule cells, which leads to increased tubular proteinuria. Urine protein-to-creatinine ratio greater than 5 times the ULN occurred in 15% of inotersen-treated patients, compared with 8% of patients on placebo. Therefore, inotersen should generally not be initiated in patients with a urine protein-to-creatinine ratio of 1,000 mg/g or greater (Benson et al., 2018). Because of the risks of thrombocytopenia and glomerulonephritis, inotersen is available only through a restricted distribution program called the Tegsedi REMS.
Other warnings and precautions have been indicated in the FDA-approved drug label of inotersen, including stroke and cervicocephalic arterial dissection, inflammatory and immune effects, liver injury, hypersensitive reactions, and reduced serum vitamin A levels. In the clinical trial, stroke and cervicocephalic arterial dissection occurred within 2 days of the first inotersen dose. Patients also experienced symptoms of cytokine release (e.g., nausea, vomiting, muscular pain, and weakness) and a high sensitivity C-reactive protein level greater than 100 mg/L (Benson et al., 2018). The liver is a site where ASOs accumulates. In the clinical studies, 8% of Inotersen-treated patients had increased ALT levels at least three times the ULN, compared with 3% of patients on placebo. Furthermore, 3% of inotersen-treated patients had ALT values at least eight times the ULN, compared with no patients on placebo. Monitoring ALT, AST, and total bilirubin at baseline and every 4 months during treatment with inotersen is recommended.
From postmarketing and case reports, <1% of patients reported autoimmune hepatitis, cerebrovascular accident, coronary artery dissection, hepatobiliary disease, hypersensitivity reaction, immune thrombocytopenia, lower back pain, paraplegia, speech disturbance, vasculitis, and weight loss.
A worldwide clinical trial for pregnancy outcome in women treated with inotersen is underway (NCT 04270058) and is expected to be completed in 2030. A data collection clinical trial (NCT04850105) with an estimated 240 participants currently under recruitment is aimed at further characterizing the long-term safety of inotersen in patients with hATTR-PN under real-world conditions. Aforementioned studies will provide more evidence on how to manage safety concerns of Inotersen.
Golodirsen (Vyondys 53)
Golodirsen is manufactured by Sarepta Therapeutics and was approved by the FDA on December 12, 2019 (Heo, 2020). Golodirsen is indicated for the treatment of DMD in patients with a mutation in the dystrophin gene, which can be treated by ASO-induced skipping of exon 53 in the dystrophin pre-mRNA. Golodirsen is designed to bind to exon 53 of dystrophin pre-mRNA, thus excluding the exon during processing. The clinically recommended dose is 30 mg/kg once weekly in 35- to 60-minute infusion intravenously.
ADRs were reported in a phase 1/2 clinical trial (NCT02310906). This was a randomized, placebo-controlled dose-titration study (part 1) followed by open-label evaluation (part 2) with 39 participants to assess safety and tolerability of golodirsen (Frank et al., 2020). The common ADRs observed in at least 20% of patients included headaches, pyrexia, fall, abdominal pain, nasopharyngitis, cough, vomiting, and nausea. Less common ADRs reported in approximately 5% of treated patients were administration site pain, diarrhea, dizziness, ligament sprain, contusion, influenza, oropharyngeal pain, rhinitis, skin abrasions, ear infections, seasonal allergies, tachycardia, site-related reaction, constipation, and bone fractures (Frank et al., 2020). An extension study (NCT03532542) to evaluate golodirsen in patients with DMD is under enrolling stages by invitation in a phase 3 study with an estimated 260 participants and is expected to be completed in August 2026.
Warnings and precautions for hypersensitivity reactions and renal toxicity have been placed in the FDA-approved drug label of golodirsen. Hypersensitivity reactions occurred in golodirsen-treated patients in the clinical trials with phenotypes including rash, pruritus, pyrexia, dermatitis, urticaria, and skin exfoliation. If a hypersensitivity reaction occurs, patient care should be managed following the institute appropriate medical treatment and slower infusion rates or interruption of golodirsen therapy should be considered. Renal toxicity was not observed in the clinical studies; however, it was reported in preclinical studies with different species of animals receiving Golodirsen. Mice administered either intravenous or subcutaneous injections of Golodirsen developed renal tubular degeneration, as well as degeneration of the transitional epithelium of the urinary bladder at all tested doses. Tubular degeneration by intravenous administration was accompanied by increases in serum urea nitrogen. Primates after intravenous administration of golodirsen also developed kidney dysfunction with tubular basophilia, dilatation, and mononuclear cell infiltration. The FDA recommends monthly proteinuria monitoring by dipstick urinalysis and monitoring of serum cystatin C every 3 months.
Sarepta Therapeutics is currently conducting a placebo-controlled, postmarketing confirmation trial named ESSENCE (NCT02500381) to support the accelerated approval. The trial is expected to be concluded in April 2024.
Viltolarsen (Viltepso)
Viltolarsen, sold under the name of Viltepso and manufactured by Nippon Shinyaku Co, was granted accelerated approval by the FDA on July 12, 2020, based on its capacity to increase dystrophin levels in skeletal muscle in patients with DMD (Dhillon, 2020). Continued approval is dependent upon verification of the clinical benefits in confirmatory trials. Viltolarsen is an ASO drug also indicated for the treatment of DMD in patients who have a confirmed mutation of the dystrophin gene that is treatable by ASO-induced skipping of exon 53. Viltolarsen is designed to bind to exon 53 of dystrophin pre-mRNA, resulting in exclusion of this exon during mRNA processing. This exclusion leads to the increase in dystrophin production, which helps keep muscle cells intact. The clinically recommended dose is 80 mg/kg once weekly in a 60-minute infusion intravenously.
ADRs were reported in a phase 2 clinical trial (NCT02740972). This is a multicenter, two-period, randomized, placebo-controlled study with 16 participants (Clemens et al., 2020). The initial period of the clinical study (4 weeks) consisted of patients being randomized (double-blind), with administration via an injection with viltolarsen or placebo. All patients then received 20 weeks of open-label viltolarsen (40 mg/kg or 80 mg/kg) once a week. The most common observed ADRs included injection site reaction, upper respiratory tract infection, cough, and pyrexia (observed in >15% of patients treated). Other less common ADRs included contusion, arthralgia, diarrhea, vomiting, and abdominal pain. Several clinical studies to assess the safety and tolerability of viltolarsen are under recruiting status, including NCT 04956289 (phase 2, 20 participants, to be completed in May 2024), NCT04060199 (phase 3, 74 participants, to be completed in November 2024), NCT04768062 (phase 3, 74 participants, to be completed in June 2026), and NCT04687020 (phase 4, 16 participants, to be completed in November 2031).
A warning and precaution for renal toxicity has been placed in the FDA drug label of viltolarsen. Although kidney toxicity was not directly observed in the clinical studies, preclinical studies reported potential risk of renal toxicity. Viltolarsen was administered to juvenile male mice via subcutaneous injection on postnatal day 7 and intravenous weekly injections from postnatal day 14 to 70. It was administered at doses of 0, 15, 60, 240, and 1,200 mg/kg. Renal toxicity was observed at the highest dose. In mice at the 240 and 1,200 mg/kg doses, there was a dose-dependent increase in deaths caused by renal toxicity, as well as a dose-dependent incidence and severity of renal tubular effects, such as degeneration. Reduced body weight and delayed sexual maturation were also observed at the highest dose. At 60 mg/kg, no renal toxicity was observed, and plasma exposure was similar to that in humans at 80 mg/kg. Long-term animal studies to assess the carcinogenic potential of viltolarsen were not conducted. There were also no adequate data on the developmental risk associated with the use of Viltolarsen in pregnant women. In addition, no adverse effect on embryofetal development was observed in animal studies. Although renal toxicity was not observed in clinical studies, renal function should be monitored in patients taking viltolarsen, as renal toxicity and fatal glomerulonephritis were reported in preclinical studies.
The postmarket data are not available for viltolarsen due to the short length of time since its approval for use.
Casimersen (Amondys 45)
Developed by Sarepta Therapeutic Inc., casimersen is the newest FDA-approved ASO drug that belongs to the phosphorodiamidate morpholino oligomer subclass. The FDA approved the drug on February 25, 2021 (Shirley, 2021), and its primary function is helping people who are suffering from DMD with a confirmed mutation on exon 45 of the DMD gene. Casimersen is designed to bind pre-mRNA of exon 45 of the DMD pre-mRNA, leading to skipping of the exon during mRNA processing. Therefore, patients can produce shorter functional dystrophin. Casimersen is approved under the provisions of accelerated approval regulations based on its capacity to increase dystrophin production in skeletal muscle of patients with DMD. The clinically recommended dose of casimersen 45–30 mg/kg once weekly in 35- to 60-minute infusions intravenously with 0.2-micron filter.
ADRs were reported in an initiated phase 1 clinical trial with 12 participants at therapeutic escalating doses (NCT02530905). The clinical trial was a multicenter, randomized, double-blind, placebo-controlled, dose-titration study (Wagner et al., 2021). The recruited patients were all males, ages 7–21 years, who were diagnosed with DMD and confirmed genetic mutation amenable for treatment involving ASO-induced exon 45 skipping. The patients were randomized 2:1 to receive ascending doses of casimersen at 4, 10, 20, and 30 mg/kg or placebo once a week through intravenous infusion for 12 weeks. After that, all patients received casimersen at 30 mg/kg in an open-label extension period for up to 132 weeks. The common ADRs observed (>20% patients) included upper respiratory tract infections, cough, pyrexia, headache, arthralgia, and oropharyngeal pain. In addition to the observed common ADRs, other less common ADRs (<10% patients) included ear pain, nausea, ear infection, post-traumatic pain, dizziness, and light-headedness. An extension study to evaluate casimersen in patients with DMD is underway with an estimated enrollment of 260 participants (NCT03532542), which is expected to be completed in 2026. Another double-blind, placebo-controlled, multicenter study to evaluate the efficacy and safety of casimersen is also ongoing (so called ESSENCE, NCT02500381), which has 222 estimated participants and is expected to be completed in 2024.
A warning and precaution for kidney toxicity has been placed in the FDA-approved drug label of casimersen. Although kidney toxicity was not shown in the clinical trial, kidney toxicity was reported in preclinical studies, including potentially fatal glomerulonephritis. In the preclinical studies, intravenous administration to juvenile male rats led to renal tubular degeneration and necrosis at the tested doses. Therefore, kidney function should be monitored when patients are treated with casimersen. Meanwhile, serum cystatin C, glomerular filtration rate, urine dipstick, and urine protein-to-creatinine ratio are recommended to be measured before starting Amondys 45 in the drug label. Further, patients with renal dysfunction are recommended to be closely monitored during treatment with casimersen.
Because the drug received FDA approval very recently, the postmarket safety data are not available. To evaluate ADRs and toxicity of casimersen, more data and information must be collected and/or generated.
Drug-Specific and Class-Wide ADRs and Toxicities among All FDA-Approved ASO Drugs
Due to different properties of absorption, distribution, metabolism, and excretion of each ASO drug involving different target organs and tissues, some specific ADRs are unique to particular ASO drugs (Table 2). Just to name a few, ocular ADRs are mainly found in fomivirsen and pegaptanib, which are directly administered into the eyes by intravitreal injection. On the other hand, although each FDA-approved ASO drug has a unique oligonucleotide sequence, they are also administered by different routes of delivery to target different organs and tissues. Some ADRs are found to occur across the spectrum of ASO drugs in general, which are also summarized in Table 2. A common ADR is application site reaction, which is likely caused by injection. Headache, pyrexia, and fever may reflect ADRs that are mediated by immune responses to the ASO drugs. Cough may be caused by respiratory infection, whereas vomiting and nausea are common gastrointestinal ADRs to the ASO drugs. Some common ADRs, such as headache, fever, flu-like symptoms, abdominal pain, nausea, and fatigue, can be treated easily with nonprescription drugs.
TABLE 2.
Common ADRs of the FDA-approved ASO drugs
| ASO Drug | Common ADRs | Shared Common ADRs by Multiple ASO Drugs |
|---|---|---|
| Fomivirsen | Increased intraocular pressure and ocular inflammation. | Injection site reactions Headache Pyrexia Fever Respiratory infection Cough Vomiting Nausea |
| Pegaptanib | Anterior chamber inflammation, blurred vision, cataract, conjunctival hemorrhage, corneal edema, eye discharge, eye irritation, eye pain, hypertension, increased intraocular pressure, ocular discomfort, punctate keratitis, reduced visual acuity, visual disturbance, vitreous floaters, and vitreous opacities. | |
| Mipomersen | Injection site reactions and flu-like symptoms. | |
| Defibrotide | Hypotension, diarrhea, vomiting, nausea, and epistaxis. | |
| Eteplirsen | Lower respiratory infection, pyrexia, constipation, headache, vomiting, and back pain. | |
| Nusinersen | Injection site reactions, nausea, headache, fatigue, thrombocytopenia, and fever. | |
| Inotersen | Balance disorder, vomiting, and contact dermatitis. | |
| Golodirsen | Headaches, pyrexia, fall, abdominal pain, nasopharyngitis, cough, vomiting, and nausea. | |
| Viltolarsen | Injection site reaction, upper respiratory tract infection, cough, and pyrexia. | |
| Casimersen | Upper respiratory tract infections, cough, pyrexia, headache, arthralgia, and oropharyngeal pain. |
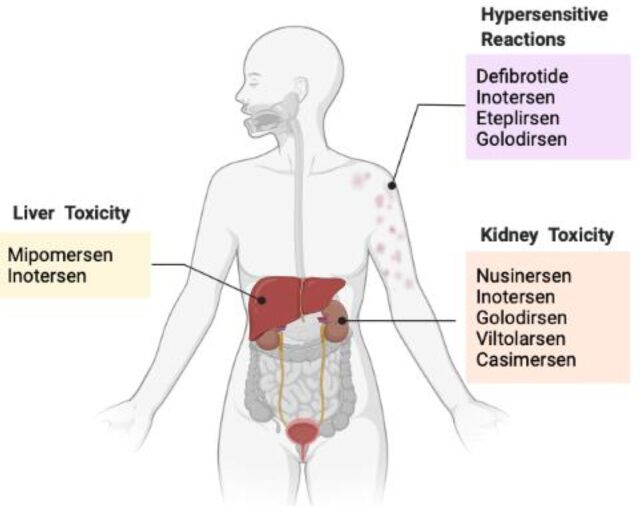
The major safety concerns for the FDA-approved ASO drugs are severe hepatotoxicity, renal toxicity, and hypersensitivity reactions, which are commonly manifested with several ASO drugs (Fig. 1 and Table 3). In particular, kidney toxicity has been indicated in nusinersen, inotersen, golodirsen, viltolarsen, and casimersen, which are the ASO drugs developed for treatment of muscular dystrophy. Monitoring kidney function is critical while under treatment with these ASO drugs. Hypersensitivity reactions are listed as a precaution in defibrotide, inotersen, eteplirsen, and golodirsen. If a hypersensitivity reaction occurs, patients should carefully follow the institute-appropriate medical treatment, and as a last resource or in cases of severe manifestation of these adverse reactions, discontinuing treatment is recommended. Risk of hepatotoxicity with elevation of ALT and AST and hepatic steatosis has been indicated in mipomersen and inotersen, which are the ASO drugs targeting hepatic gene expression. Because of severity of hepatotoxicity, mipomersen has been discontinued from the market in 2019. Inotersen has numerous warnings and precautions on its black-box label. Because of the risks of thrombocytopenia and glomerulonephritis, inotersen is only available through a restricted distribution program.
TABLE 3.
Warnings and precautions for each ASO drug in the FDA-approved drug labeling
| ASO Drug | FDA Warnings and Precautions | Shared Warnings and Precautions by Multiple ASO Drugs |
|---|---|---|
| Fomivirsen | Ocular inflammation Increased intraocular pressure |
Hepatotoxicity Kidney toxicity Hypersensitive reactions |
| Pegaptanib | Endophthalmitis Increased intraocular pressure |
|
| Mipomersen | Black box: Risk of hepatotoxicity Elevation of ALT and AST Hepatic steatosis |
|
| Defibrotide | Hemorrhage Hypersensitive reactions |
|
| Nusinersen | Thrombocytopenia Coagulation abnormalities Renal toxicity |
|
| Inotersen | Black box: Thrombocytopenia Glomerulonephritis and renal toxicity Elevation of ALT and AST Stroke and cervicocephalic arterial dissection Inflammatory and immune effects Hypersensitive reactions Reduced serum vitamin A |
|
| Eteplirsen | Hypersensitive reactions | |
| Golodirsen | Renal toxicity Hypersensitive reactions |
|
| Viltolarsen | Kidney toxicity | |
| Casimersen | Kidney toxicity |
Fig. 1.
Shared warnings of toxicity in multiple FDA-approved ASO drugs. Hypersensitive reactions, such as rash and urticaria, are shown in defibrotide, inotersen, eteplirsen, and golodirsen. Hepatotoxicity is indicated after treatment with mipomersen and inotersen. Kidney toxicity is shown with the treatment of nusinersen, inotersen, golodirsen, viltolarsen, and casimersen.
Potential Mechanisms of the Common ADRs and Toxicity of the ASO Drugs
Although each ASO drug has a specific mechanism and a unique panoply of ADRs and toxicity, they also share some common mechanisms of toxicity as a class of drugs. ASO can cause toxicity via two possible mechanisms, via either hybridization dependent off-target effects or RNAse-H1 dependent reduction in unintended off-target RNAs (Burel et al., 2016). The hybridization dependent off-target effects can occur due to the binding of ASOs to complementary sites of non-selective RNAs that have a homologous sequence to the intended target RNA (Kamola et al., 2017). Due to their various chemical functional groups, ASOs may produce nonspecific interactions with plasma proteins, undesired organ targeting, and possible binding with other small molecule–based drugs that can result in toxicity. Studies have shown that hepatotoxicity of ASOs is mediated by the RNase-H1-dependent reduction in unintended off-target RNAs in mice (Burel et al., 2016).
The mechanism of liver toxicity of mipomersen has been linked to the potential of the drug to induce endoplasmic reticulum stress (Hashemi et al., 2014). The induced stress impairs very low-density lipoprotein assembly via proteasomal and non-proteasomal pathways, which might induce a reduction of apo B mRNA levels, therefore causing drug-induced reduction of very low-density lipoprotein, which increases the risk of hepatic steatosis (Bell et al., 2011; Rinaldi and Wood, 2018). Preclinical studies established that hepatic Kupffer cells and renal proximal tubular epithelium receive the highest distribution of mipomersen. The liver showed increased incidence of minimal cytoplasmic vacuolation at high exposure levels of mipomersen in addition to proinflammatory signals. In the kidneys, minimal slight degeneration of the tubular epithelium was observed in monkeys.
The possible mechanism of kidney toxicity of the ASO drugs is the accumulation of the oligonucleotide within the lysosomes of the proximal tubules, resulting in physiologic perturbation of tubular absorptive capacity and increased tubular proteinuria (Frazier, 2015). This was mainly associated to ASO ionic interactions with extracellular, cell-surface, or intracellular proteins. Repeated dosing results in ASOs accumulation in these tissues, which reaches steady-state levels, and the degree of toxicity then depends on the concentrations and inherent potency of the accumulated ASO. At high doses, the prominence of granules in epithelial cells correlates with an increased incidence or severity of degradation in the kidney; this is mostly a result of lysosomal breakdown.
Current Challenges, Knowledge Gaps, and Perspective on Future Directions
Several FDA-approved ASO drugs target rare diseases. A limitation of the safety data is the very limited number of patients included in clinical trials, which is understandable given the low frequency of these diseases. However, common and less common ADRs and severe toxicity have emerged for this class of drugs. The listed ADRs, warnings, and precautions in the FDA-approved drug labels are supported, in part, by preclinical studies. Although some of the ADRs have been observed in clinical studies, they may not be entirely reflective of real-world conditions when ASO drugs are used to target diseases on a worldwide scale. Hence, it is critical to further extend the observations from clinical trials to examine patient samples in the postmarketing real-world settings to validate the findings on ADRs and toxicity from the preclinical and clinical trials in premarketing settings.
Another current challenge and knowledge gap is to understand inclusive mechanisms causing hepatotoxicity, renal toxicity, and hypersensitivity reactions by the ASO drugs. Although some speculations on mechanisms for hepatotoxicity and renal toxicity have been proposed, the precise molecular and biochemical events at the genetic level involving development of toxicity are unclear. Numerous questions need to be urgently addressed considering the rapid pace of discovery and development of ASO drugs. Going forward, the following questions should be addressed: What are the distinct features of ASO drug–induced toxicity when compared with other small molecule–based drugs? What are the underlying molecular events occurring during ASO drug–induced toxicity? What is the capacity for liver and kidney to repair damage and/or develop toleranceto ASO drug–induced toxicity? What pharmacological interventions can be applied to mitigate the risk of ASO drug–induced toxicity? Does ASO drug–induced toxicity impair function of the liver and kidneys as it relates to absorption, distribution, metabolism, and excretion of other small molecule–based drugs, altering their therapeutic efficacy and ADRs (drug–drug interactions)? These questions can lead to numerous perspective future directions to be addressed pertinent to the success of ASO-based drug discovery and development for treating myriad devastating diseases.
Abbreviations
- ADR
adverse drug reaction
- ALT
alanine aminotransferase
- AMD
age-related macular degradation
- ASO
antisense oligonucleotide
- AST
aspartate aminotransferase
- CMV
cytomegalovirus
- DMD
Duchenne muscular atrophy
- FDA
U.S. Food and Drug Administration
- HoFH
homozygous familial hypercholesterolemia
- REMS
risk evaluation and mitigation strategy
- SMA
spinal muscular atrophy
- TTR
transthyretin
- ULN
upper limit of normal
- VEGF
vascular endothelial growth factor
- VOD
veno-occlusive disease
Authorship Contributions
Participated in research design: Alhamadani, Zhang, Parikh, Wu, Rasmussen, Bahal, Zhong, Manautou.
Performed data analysis: Alhamadani, Zhang, Parikh, Wu.
Wrote or contributed to the writing of the manuscript: Alhamadani, Zhang, Parikh, Wu, Rasmussen, Bahal, Zhong, Manautou.
Footnotes
This study was partly supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R35-GM140862] (to X.Z.) and the University of Connecticut Research Excellence Program (to J.E.M.).
References
- Bell DA, Hooper AJ, Burnett JR (2011) Mipomersen, an antisense apolipoprotein B synthesis inhibitor. Expert Opin Investig Drugs 20:265–272. [DOI] [PubMed] [Google Scholar]
- Benson MDWaddington-Cruz MBerk JLPolydefkis MDyck PJWang AKPlanté-Bordeneuve VBarroso FAMerlini GObici L, et al. (2018) Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med 379:22–31. [DOI] [PubMed] [Google Scholar]
- Bertolin C, Querin G, Martinelli I, Pennuto M, Pegoraro E, Sorarù G (2019) Insights into the genetic epidemiology of spinal and bulbar muscular atrophy: prevalence estimation and multiple founder haplotypes in the Veneto Italian region. Eur J Neurol 26:519–524. [DOI] [PubMed] [Google Scholar]
- Beytía MdeL, Vry J, Kirschner J (2012) Drug treatment of Duchenne muscular dystrophy: available evidence and perspectives. Acta Myol 31:4–8. [PMC free article] [PubMed] [Google Scholar]
- Burel SAHart CECauntay PHsiao JMachemer TKatz MWatt ABui H-HYounis HSabripour M, et al. (2016) Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res 44:2093–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby KFinkel RBirnkrant DJCase LEClemens PRCripe LKaul AKinnett KMcDonald CPandya S, et al. ; DMD Care Considerations Working Group (2010) Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 9:77–93. [DOI] [PubMed] [Google Scholar]
- Choy M (2016) Pharmaceutical approval update. P&T 41:416–441. [PMC free article] [PubMed] [Google Scholar]
- Claborn MK, Stevens DL, Walker CK, Gildon BL (2019) Nusinersen: a treatment for spinal muscular atrophy. Ann Pharmacother 53:61–69. [DOI] [PubMed] [Google Scholar]
- Clemens PRRao VKConnolly AMHarper ADMah JKSmith ECMcDonald CMZaidman CMMorgenroth LPOsaki H, et al. ; CINRG DNHS Investigators (2020) Safety, tolerability, and efficacy of viltolarsen in boys with Duchenne Muscular dystrophy amenable to exon 53 skipping: a phase 2 randomized clinical trial. JAMA Neurol 77:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbacioglu SCesaro SFaraci MValteau-Couanet DGruhn BRovelli ABoelens JJHewitt ASchrum JSchulz AS, et al. (2012) Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet 379:1301–1309. [DOI] [PubMed] [Google Scholar]
- Crisafulli S, Sultana J, Fontana A, Salvo F, Messina S, Trifirò G (2020) Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J Rare Dis 15:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke ST (2017) Molecular mechanisms of antisense oligonucleotides. Nucleic Acid Ther 27:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras BT, Farrar MA, Mercuri E, Finkel RS, Foster R, Hughes SG, Bhan I, Farwell W, Gheuens S (2019) An integrated safety analysis of infants and children with symptomatic spinal muscular atrophy (SMA) treated with nusinersen in seven clinical trials. CNS Drugs 33:919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Smet MD, Meenken CJ, van den Horn GJ (1999) Fomivirsen - a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul Immunol Inflamm 7:189–198. [DOI] [PubMed] [Google Scholar]
- Dhillon S (2020) Viltolarsen: first approval. Drugs 80:1027–1031. [DOI] [PubMed] [Google Scholar]
- Dhuri K, Bechtold C, Quijano E, Pham H, Gupta A, Vikram A, Bahal R (2020) Antisense oligonucleotides: an emerging area in drug discovery and development. J Clin Med 9:2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DL, Sisson EM, Butler M, Higbea A, Muoio B, Turner B (2014) Lomitapide and mipomersen: novel lipid-lowering agents for the management of familial hypercholesterolemia. J Cardiovasc Nurs 29:E7–E12. [DOI] [PubMed] [Google Scholar]
- Elangkovan N, Dickson G (2021) Gene therapy for Duchenne muscular dystrophy. J Neuromuscul Dis 8:S303–S316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DESchnell FJAkana CEl-Husayni SHDesjardins CAMorgan JCharleston JSSardone VDomingos JDickson G, et al. ; SKIP-NMD Study Group (2020) Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 94:e2270–e2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier KS (2015) Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist’s perspective. Toxicol Pathol 43:78–89. [DOI] [PubMed] [Google Scholar]
- Gales L (2019) Tegsedi (inotersen): an antisense oligonucleotide approved for the treatment of adult patients with hereditary transthyretin amyloidosis. Pharmaceuticals (Basel) 12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Adamis AP, Cunningham ET, Goldbaum M, Guyer DR, Katz B, Patel M; VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group (2006) Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 113:1508.e1–1508.e15. [DOI] [PubMed] [Google Scholar]
- Gupta S (2015) LDL cholesterol, statins and PCSK 9 inhibitors. Indian Heart J 67:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair P, Cameron F, McKeage K (2013) Mipomersen sodium: first global approval. Drugs 73:487–493. [DOI] [PubMed] [Google Scholar]
- Hashemi N, Odze RD, McGowan MP, Santos RD, Stroes ESG, Cohen DE (2014) Liver histology during Mipomersen therapy for severe hypercholesterolemia. J Clin Lipidol 8:606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YA (2020) Golodirsen: first approval. Drugs 80:329–333. [DOI] [PubMed] [Google Scholar]
- Hoy SM (2017) Nusinersen: first global approval. Drugs 77:473–479. [DOI] [PubMed] [Google Scholar]
- Ito M, Yamauchi A, Urano M, Kato T, Matsuo M, Nakashima K, Saito K (2022) Epidemiological investigation of spinal muscular atrophy in Japan. Brain Dev 44:2–16. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Cheung CMG, Panda-Jonas S (2017) Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) 6:493–497. [DOI] [PubMed] [Google Scholar]
- Kamola PJMaratou KWilson PARush KMullaney TMcKevitt TEvans PRidings JChowdhury PRoulois A, et al. (2017) Strategies for in vivo screening and mitigation of hepatotoxicity associated with antisense drugs. Mol Ther Nucleic Acids 8:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Goldbaum M (2006) Macugen (pegaptanib sodium), a novel ocular therapeutic that targets vascular endothelial growth factor (VEGF). Int Ophthalmol Clin 46:141–154. [DOI] [PubMed] [Google Scholar]
- Keam SJ (2018) Inotersen: first global approval. Drugs 78:1371–1376. [DOI] [PubMed] [Google Scholar]
- Kessler-Icekson G, Schlesinger H, Leger JJ, Leger J, Braverman Y, Binah O (1988) Ventricular myosin of the shrew Crocidura russula, correlation with contractile properties. J Mol Cell Cardiol 20:1069–1073. [DOI] [PubMed] [Google Scholar]
- Kornblum N, Ayyanar K, Benimetskaya L, Richardson P, Iacobelli M, Stein CA (2006) Defibrotide, a polydisperse mixture of single-stranded phosphodiester oligonucleotides with lifesaving activity in severe hepatic veno-occlusive disease: clinical outcomes and potential mechanisms of action. Oligonucleotides 16:105–114. [DOI] [PubMed] [Google Scholar]
- Lim KR, Maruyama R, Yokota T (2017) Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Devel Ther 11:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui DTW, Lee ACH, Tan KCB (2021) Management of Familial Hypercholesterolemia: Current Status and Future Perspectives. J Endocr Soc 5:bvaa122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigetti M, Romano A, Di Paolantonio A, Bisogni G, Sabatelli M (2020) Diagnosis and treatment of hereditary transthyretin amyloidosis (hATTR) polyneuropathy: current perspectives on improving patient care. Ther Clin Risk Manag 16:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick C (1998) First “antisense” drug will treat CMV retinitis. JAMA 280:871. [PubMed] [Google Scholar]
- Matsuo M (2021) Antisense oligonucleotide-mediated exon-skipping therapies: precision medicine spreading from duchenne muscular dystrophy. Japan Med Assoc J 4:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell JRRodino-Klapac LRSahenk ZRoush KBird LLowes LPAlfano LGomez AMLewis SKota J, et al. ; Eteplirsen Study Group (2013) Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 74:637–647. [DOI] [PubMed] [Google Scholar]
- Mendonça RH, Polido GJ, Matsui C, Silva AMS, Solla DJF, Reed UC, Zanoteli E(2021) Real-world data from nusinersen treatment for patients with later-onset spinal muscular atrophy: a single center experience. J Neuromuscul Dis 8:101–108. [DOI] [PubMed] [Google Scholar]
- Mercuri EDarras BTChiriboga CADay JWCampbell CConnolly AMIannaccone STKirschner JKuntz NLSaito K, et al. ; CHERISH Study Group (2018) Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med 378:625–635. [DOI] [PubMed] [Google Scholar]
- Narayanan PCurtis BRShen LSchneider ETami JAPaz SBurel SATai LJMachemer TKwoh TJ, et al. (2020) Underlying immune disorder may predispose some transthyretin amyloidosis subjects to inotersen-mediated thrombocytopenia. Nucleic Acid Ther 30:94–103. [DOI] [PubMed] [Google Scholar]
- Nassir F, Rector RS, Hammoud GM, Ibdah JA (2015) Pathogenesis and prevention of hepatic steatosis. Gastroenterol Hepatol (N Y) 11:167–175. [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ (2006) Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 58:353–363. [PubMed] [Google Scholar]
- Penedones A, Mendes D, Alves C, Batel Marques F (2014) Safety monitoring of ophthalmic biologics: a systematic review of pre- and postmarketing safety data. J Ocul Pharmacol Ther 30:729–751. [DOI] [PubMed] [Google Scholar]
- Perry CM, Balfour JA (1999) Fomivirsen. Drugs 57:375–380, discussion 381. [DOI] [PubMed] [Google Scholar]
- Port AD, Orlin A, Kiss S, Patel S, D’Amico DJ, Gupta MP (2017) Cytomegalovirus Retinitis: A Review. J Ocul Pharmacol Ther 33:224–234. [DOI] [PubMed] [Google Scholar]
- Reeskamp LF, Kastelein JJP, Moriarty PM, Duell PB, Catapano AL, Santos RD, Ballantyne CM (2019) Safety and efficacy of mipomersen in patients with heterozygous familial hypercholesterolemia. Atherosclerosis 280:109–117. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Grupp SA, Pagliuca A, Krishnan A, Ho VT, Corbacioglu S (2017) Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome with multiorgan failure. Int J Hematol Oncol 6:75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PGRiches MLKernan NABrochstein JAMineishi STermuhlen AMArai SGrupp SAGuinan ECMartin PL, et al. (2016) Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 127:1656–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi C, Wood MJA (2018) Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14:9–21. [DOI] [PubMed] [Google Scholar]
- Roberts TC, Langer R, Wood MJA (2020) Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19:673–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehr B (1998) Fomivirsen approved for CMV retinitis. J Int Assoc Physicians AIDS Care 4:14–16. [PubMed] [Google Scholar]
- Santos RD, Raal FJ, Catapano AL, Witztum JL, Steinhagen-Thiessen E, Tsimikas S (2015) Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler Thromb Vasc Biol 35:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Waddington-Cruz M, Botteman MF, Carter JA, Chopra AS, Hopps M, Stewart M, Fallet S, Amass L (2018) Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve 57:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley M (2021) Casimersen: first approval. Drugs 81:875–879. [DOI] [PubMed] [Google Scholar]
- Son HW, Yokota T (2018) Recent advances and clinical applications of exon inclusion for spinal muscular atrophy. Methods Mol Biol 1828:57–68. [DOI] [PubMed] [Google Scholar]
- Stephenson ML, Zamecnik PC (1978) Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci USA 75:285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YY (2016) Eteplirsen: first global approval. Drugs 76:1699–1704. [DOI] [PubMed] [Google Scholar]
- Thomas GS, Cromwell WC, Ali S, Chin W, Flaim JD, Davidson M (2013) Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 62:2178–2184. [DOI] [PubMed] [Google Scholar]
- Tobin KA (2006) Macugen treatment for wet age-related macular degeneration. Insight 31:11–14. [PubMed] [Google Scholar]
- Vitravene Study Group. (2002) Safety of intravitreous fomivirsen for treatment of cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol 133:484–498. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Kuntz NL, Koenig E, East L, Upadhyay S, Han B, Shieh PB (2021) Safety, tolerability, and pharmacokinetics of casimersen in patients with Duchenne muscular dystrophy amenable to exon 45 skipping: a randomized, double-blind, placebo-controlled, dose-titration trial. Muscle Nerve 64:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E, Goldberg T (2014) Mipomersen (kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P&T 39:119–122. [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Bahal R, Rasmussen TP, Manautou JE, Zhong XB (2021) The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol 189:114432. [DOI] [PMC free article] [PubMed] [Google Scholar]